Abstract
Oral treprostinil has been shown to improve exercise capacity and delay disease progression in patients with pulmonary arterial hypertension (PAH), but its effects on hemodynamics are not well-characterized. The FREEDOM-EV trial was a Phase III, international, placebo-controlled, double-blind, event-driven study in 690 participants with PAH who were taking a single oral PAH therapy. FREEDOM-EV demonstrated a significantly reduced risk for clinical worsening with oral treprostinil taken three times daily and did not uncover new safety signals in PAH patients. Sixty-one participants in the FREEDOM-EV trial volunteered for a hemodynamics sub- study. Pulmonary artery compliance (PAC), a ratio of stroke volume to pulmonary pulse pressure, significantly increased from Baseline to Week 24 in the oral treprostinil group compared with the placebo group (geometric mean 26.4% active vs. −6.0% placebo; ANCOVA p=0.007). There was a significant increase in cardiac output in the oral treprostinil group compared to the placebo group (geometric mean 11.3% active vs. −6.4% placebo; ANCOVA p=0.005) and a corresponding significant reduction in pulmonary vascular resistance (PVR) (geometric mean −21.5 active vs. −1.8% placebo; ANCOVA p=0.02) from Baseline to Week 24. These data suggest that increased compliance contributes to the physiological mechanism by which oral treprostinil improves exercise capacity and delays clinical worsening for patients with PAH.
Keywords: FREEDOM-EV, Right-heart catherization, PVR, Hemodynamics, Cardiac output
FREEDOM-EV demonstrated a significantly reduced risk for clinical worsening in patients with pulmonary arterial hypertension (PAH) who took oral treprostinil three times daily [1]. Prospective hemodynamics studies of oral treprostinil, however, are limited [2]. A subset of participants in the FREEDOM-EV trial consented to a right heart catheterization (RHC) at Baseline and study Week 24. Some of the results were previously reported in abstract form [3].
1. Methods
Sixty-one participants of the FREEDOM-EV (NCT01560624) study underwent two RHCs (34 oral treprostinil, 27 placebo) [1]. All participants were on a stable dose of oral PAH monotherapy at study entry. The baseline RHC was performed prior to the first study drug dose; follow-up RHC was performed within 72 h of Week 24 assessments. When possible, RHC was performed 3–6 h after study drug administration. For this optional sub-study, investigator assessments were accepted without a core lab review; no reproducibility requirements for thermodilution or pressures were specified. Pulmonary artery compliance (capacitance) was calculated as [(cardiac output/heart rate)/(systolic pulmonary artery pressure – diastolic pulmonary artery pressure)]. Heart rate was collected once during each RHC.
Six participants with missing or mismatched CO measurement methodology, Fick (direct or indirect) or thermodilution, at baseline and follow up were not included in CO-based analyses. The treatment groups were compared using analysis of covariance with change from baseline for each log-transformed hemodynamic parameter as the dependent variable, treatment as a fixed effect, and log-transformed baseline hemodynamic parameter as a covariate.
2. Results
2.1. Demographics and baseline characteristics
The sub-study treatment groups were generally balanced (Table 1), but those treated with oral treprostinil had greater median height and weight than placebo treated participants (p = 0.005 and 0.007, respectively). Although median time since diagnosis was significantly longer in the oral treprostinil group compared to placebo (9.7 vs 2.9 months, p = 0.05), background PAH therapies and time on those therapies were similar. The 54 participants with matched cardiac output measures had similar demographics (Supplemental Table E1).
Table 1.
Baseline demographics for all participants in the FREEDOM-EV hemodynamic sub-study.
| Placebo (n = 27) | Oral Treprostinil (n = 34) | Overall (n = 61) | p- valuea | |
|---|---|---|---|---|
|
| ||||
| Mean Age ± SD (years) | 40.1 ± 14.6 | 44.1 ± 14.4 | 42.3 ± 14.5 | 0.30 |
| Female/Male | 23/4 | 23/11 | 46/15 | 0.14 |
| Race, n (%) | 0.14 | |||
| White | 18 (66.7) | 27 (79.4) | 45 (73.8) | |
| Asian | 6 (22.2) | 7 (20.6) | 13 (21.3) | |
| Black or African American | 3 (11.1) | 0 (0.0) | 3 (4.9) | |
| Median Weight (kg) (IQR) | 58.0 (52.0–79.2) | 75.0 (63.2–92.5) | 68.0 (56.0–86.0) | 0.007 |
| Median Height (cm) (IQR) | 159 (154–164) | 164 (160–170) | 162 (155–167)) | 0.005 |
| Geographic Region n (%) | 0.11 | |||
| North America | 5 (18.5) | 12 (35.3) | 17 (27.9) | |
| South and Latin America | 17 (63.0) | 12 (35.3) | 29 (47.5) | |
| Asia-Pacific | 5 (18.5) | 8 (23.5) | 13 (21.3) | |
| Europe | 0 (0.0) | 2 (5.9) | 2 (3.3) | |
| Median Time Since Diagnosis (months), (IQR) | 2.9 (0.1–9.6) | 9.7 (3.4–39.1) | 6.6 (1.9–16.1) | 0.05 |
| Background PAH Therapy n (%) | 1.00 | |||
| PDE5-I or sGC Stimulator | 23 (85.2) | 29 (85.3) | 52 (85.2) | |
| ERA | 4 (14.8) | 5 (14.7) | 9 (14.8) | |
| Median Time on Background PAH Therapyb (months), (IQR) | 6.2 (2.6–10.0) | 6.5 (3.5–12.4) | 6.4 (2.9–10.4) | 0.67 |
| Risk Stratification by number of low-risk criteria met, n (%)c | 0.90 | |||
| 0 | 3 (11) | 4 (12) | ||
| 1 | 12 (44) | 11 (33) | ||
| 2 | 7 (26) | 11 (33) | ||
| 3 | 5 (19) | 7 (21) | ||
p-values were calculated using Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical variables.
Imputed first day of month for participants missing day of start.
Low-risk criteria defined as WHO functional class I or II, 6MWD greater than 440 m, and/or N-terminal pro–brain natriuretic peptide less than 300 pg/ml. Risk criteria met were only counted for subjects with all three measures available. Abbreviations: ERA, endothelin receptor antagonist; PAH, pulmonary arterial hypertension; PDE5-I, phosphodiesterase type 5 inhibitor; sGC, soluble guanylate cyclase.
2.2. Hemodynamic outcomes
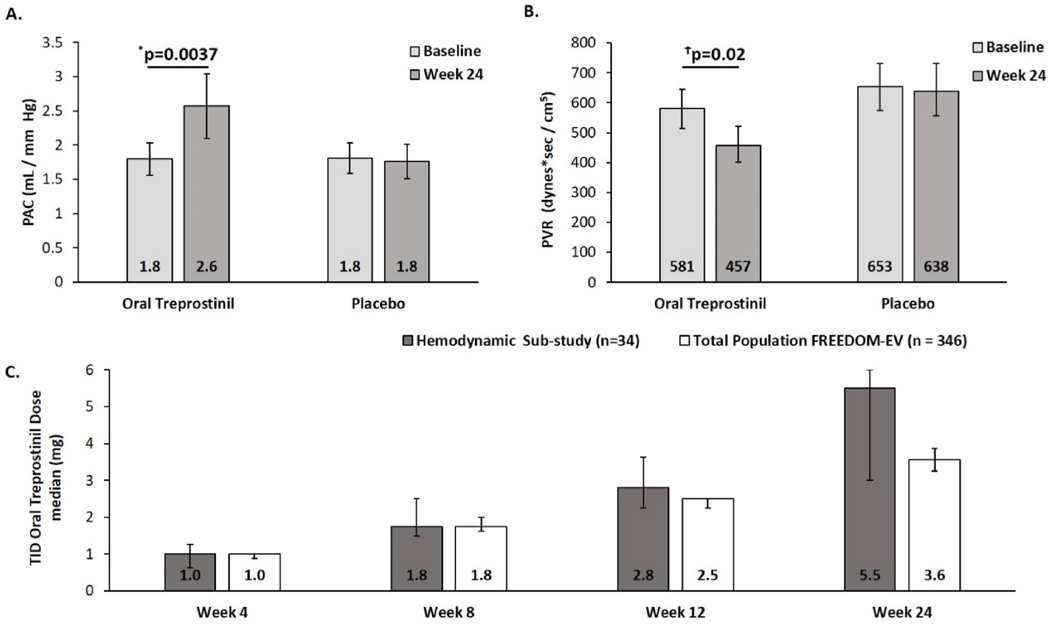
Mean PAC increased 44% at Week 24 (raw mean, Fig. 1a) in treprostinil treated patients compared to stable measurements for placebo; in a model with log-transformed PAC that adjusts for baseline, the increase in geometric mean was 26% (Table 2) in treprostinil treated patients compared to a 6% decrease in the geometric mean for placebo (Table 2). CO increased for oral treprostinil participants relative to placebo (Table 2), and PVR decreased (Table 2, Fig. 1b). No significant changes were found in PAWPm, PAPm, SAPm, RAPm, SvO2, or SaO2. Analyses based on the raw data are shown in the supplement (Table E2). In patients receiving oral treprostinil, NT-proBNP was not statistically lower at Week 24 (Table 2).
Fig. 1.
FREEDOM-EV Hemodynamic Sub-study. A) Mean (unadjusted) pulmonary arterial compliance increased by 44% from baseline to Week 24 in the oral treprostinil (n = 30) group versus 0% in the placebo (n = 24) group. Values represent means; error bars represent mean ± standard error B) Geometric mean (unadjusted) pulmonary vascular resistance dropped 21% from baseline to Week 24 in the oral treprostinil (n = 30) group versus 2% in the placebo (n = 24) group. Values represent geometric means; error bars represent geometric mean multiplied/divided by geometric standard error. C) Comparison of median dose for the hemodynamic sub-study to the total population of FREEDOM-EV over time. Error bars represent the 95% confidence interval. *p-value is obtained from the analysis of covariance with change from baseline in raw PAC as the dependent variable, treatment as fixed effect, and baseline raw PAC as a covariate. Ϯp-value is obtained from the analysis of covariance with change from baseline in log-transformed PVR as the dependent variable, treatment as fixed effect, and log-transformed baseline PVR as a covariate. PAC: pulmonary artery compliance; TID: three times a day; PVR: pulmonary vascular resistance.
Table 2.
Summary of hemodynamic parameters.
| Placebo |
Oral Treprostinil |
p-valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | Week 24 | % Change (95% CI) | n | Baseline | Week 24 | % Change (95% CI) | ||
|
| |||||||||
| PAC (mL/mmHg) | 24 | 1.5 | 1.4 | − 6.0 (− 19.7–9.9) | 30 | 1.5 | 1.9 | 26.4 (9.9–45.5) | 0.007 |
| PVR (dynes*sec/cm5) | 24 | 653 | 638 | − 1.8 (− 15.0–13.4) | 30 | 581 | 457 | − 21.5 (− 31.0–− 10.8) | 0.02 |
| CO (L/min) b | 24 | 4.5 | 4.3 | − 6.4 (− 14.3–2.3) | 30 | 4.9 | 5.4 | 11.3 (2.9–20.5) | 0.005 |
| CI (L/min/m2) | 22 | 2.8 | 2.6 | − 8.1 (− 16.0–0.7) | 26 | 2.9 | 3.1 | 7.8 (− 0.8 – 17.3) | 0.01 |
| RAPm (mmHg) | 24 | 7.0 | 6.7 | − 1.1 (− 19.8–22.0) | 33 | 6.8 | 6.6 | − 4.2 (− 19.9–14.5) | 0.82 |
| PAPm (mmHg) | 27 | 46.7 | 44.4 | − 4.9 (− 13.4–4.4) | 34 | 47.1 | 43.1 | − 8.2 (− 15.6–− 0.2) | 0.57 |
| PAWPm (mmHg) | 26 | 8.1 | 7.9 | − 8.0 (− 22.0–8.6)- | 33 | 9.5 | 10.0 | 9.9 (− 5.1 – 27.3) | 0.12 |
|
SAPm (mmHg)
SVR (dynes*sec/cm5) |
27 24 |
85.4 1366 |
85.4 1448 |
− 0.6 (− 5.6 – 4.6) 6.7 (− 4.2 – 18.7) |
33 29 |
88.9 1313 |
89.1 1203 |
0.7 (− 3.9 – 5.5) − 8.9 (− 17.3–0.5) |
0.72 0.03 |
| SvO2 (%) | 21 | 65.2 | 69.1 | 5.2 (0.4–10.2) | 26 | 66.9 | 67.5 | 1.4 (− 2.7 – 5.8) | 0.25 |
| SaO2 (%) | 26 | 95.7 | 95.4 | − 0.23 (− 1.6 – 1.2) | 32 | 94.9 | 94.7 | − 0.4 (− 1.6 – 0.9) | 0.89 |
| NT-proBNP (ng/mL) | 27 | 360 | 374 | 5.1 (− 27.0–51.3) | 33 | 334 | 277 | − 18.0 (− 41.0–14.0) | 0.32 |
Hemodynamic parameters are expressed as geometric means. Only subjects with both baseline and Week 24 hemodynamic measures were included. Systemic arterial pressure measurements were noninvasive.
p-value, % change in the geometric mean, and its associated 95% CI are obtained from the analysis of covariance with change from baseline in log-transformed data for each hemodynamic parameter as the dependent variable, treatment as fixed effect, and log-transformed baseline hemodynamic parameter as a covariate.
Thermodilution measures were used for 17 placebo and 19 treprostinil participants, the remaining 7 and 11 participants were measured using Fick. Abbreviations: PAC, pulmonary artery compliance, CO, cardiac output; CI, cardiac index; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance; PAWPm, pulmonary artery wedge pressure mean; PAPm, pulmonary artery pressure mean; SAPm, systemic arterial pressure mean; RAPm, right atrial pressure mean; SaO2, arterial oxygen saturation; SvO2, mixed venous oxygen saturation; NT-proBNP, N-terminal proB-type natriuretic peptide.
Median oral treprostinil doses (Fig. 1c) at Week 24 were higher in the hemodynamic sub-study (5.5 mg TID, CI 3.0–6.0 mg) as compared to 3.6 mg TID (CI 3.3–3.9 mg) in the parent FREEDOM-EV study. The adverse event profile in sub-study participants was similar to the overall FREEDOM-EV population, despite the higher average oral treprostinil dose achieved (data not shown) [1].
3. Discussion
This FREEDOM-EV hemodynamic sub-study is the first prospective, placebo-controlled study to examine the effects of oral treprostinil taken three times daily in PAH patients. Treprostinil treatment resulted in a significant increase in PAC and CO with an associated reduction in PVR compared to the placebo group at Week 24. These data can be explained by the known pulmonary vasodilator effect of prostacyclin therapies [4–6]. Compliance, a ratio of stroke volume to pulmonary pulse pressure, represents the pulsatile component of afterload in PAH in contrast to the static component of resistance. In PAH, RV failure occurs with a combined increase in PVR and decrease in PAC [7]. Lankhaar et al. proposed that patients may have larger changes in compliance than resistance at earlier stages of disease [8]. In a bivariate analysis, PAC at diagnosis was the sole independent predictor of mortality (over PVR or CI) and was the most discriminating variable in receiver-operator curves [9]. Interestingly, the large improvement in compliance we observed with oral treprostinil resulted in PAC values within the lowest quartile of risk in that study. Recent registry work from the Italian network of investigators did not assess compliance but did suggest that large reductions in PVR correlate with improvements in RV function and risk scores [10,11].
Treatment groups were generally well-balanced at baseline and similar to the parent study [1]. The sub-study participants achieved a higher median dose of oral treprostinil by Week 24 (5.5 mg TID) than the parent study (3.6 mg TID). The hemodynamic improvements described here may explain the marked reductions in NT-pro-BNP observed in the entire FREEDOM-EV population [1].
Previous data on the hemodynamic effects of oral treprostinil are limited [2]. An open-label study investigated the safety and tolerability of transitioning patients from parenteral treprostinil infusion to oral treprostinil, and found no difference in CO, CI, PVR, SVR at Week 24 [2]. Three patients had late clinical worsening with increased PVR after Week 24 and returned to parenteral drug. Because the primary goal of that study was a successful transition from parenteral to oral treprostinil in a very select group of PAH patients, it is not surprising that hemodynamic parameters were largely unchanged.
Prospective hemodynamic studies of prostacyclin receptor agonists have had mixed results. In a phase II study of 61 PAH patients on mono- or combination therapy, ralinepag demonstrated significant improvements in PVR, SVR and PAPm compared to placebo at Week 22 [12]. A post-hoc analysis of the study correlated ralinepag plasma levels with ralinepag dose and with improvements in PVR and 6MWD [13]. The phase II selexipag study of 43 participants (approximately 70% on background monotherapy and 30% combination) demonstrated a reduction in PVR and increase in CI at Week 17 [14]. Conversely, in the larger TRITON study, selexipag as part of an initial triple therapy regimen did not result in hemodynamic benefit as compared to placebo (initial macitentan and tadalafil) participants who had a 50% reduction in PVR [15].
In summary, in patients with PAH, addition of oral treprostinil to approved oral monotherapy improved PAC, PVR, and CO. These improvements combined with the anti-platelet and anti-inflammatory properties of oral treprostinil may have contributed to the significant reduction in risk-adjusted clinical worsening in FREEDOM-EV [1,4–6]. These data suggest that increased compliance contributes to the physiological mechanism by which oral treprostinil reduces NT-pro-BNP and delays disease progression for patients with PAH [1,16].
Supplementary Material
Acknowledgement
We would like to acknowledge Natalie Patzlaff, PhD (United Therapeutics Corp.) for assistance in drafting and editing this manuscript.
Funding source
United Therapeutics Corp. funded this study.
Role of the funding source
United Therapeutics Corp. funded and supported design, management, data analyses and medical writing for this study.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: RJW has received research support and travel support for associated research presentations from United Therapeutics, and consulting fees from Bayer and Merck. TRPZ has research grants from Janssen, Bayer, and United Therapeutics, is on the advisory board of Janssen, Bayer, and Ferrer, and received lecture honoraria for Actelion/Janssen, Bayer, Pfizer, and Ferrer. DJ, RG, MB, AO, and LH are employees of United Therapeutics. Akram Khan has recieved research support from United Theraputics, Johnson & Johnson, Ely Lilly, 4D Medical.
ABBREVIATIONS
- CI
cardiac index
- CO
cardiac output
- HR
heart rate
- NT-proBNP
N-terminal proB-type natriuretic peptide
- PAC
pulmonary artery compliance
- PAPd
pulmonary artery pressure diastolic
- PAPm
pulmonary artery pressure mean
- PAPs
pulmonary artery pressure systolic
- PAWPm
pulmonary artery wedge pressure mean
- PVR
Pulmonary vascular resistance
- RAPm
right atrial pressure mean
- RV
right ventricle
- SAPd
systemic arterial pressure diastolic
- SAPm
systemic arterial pressure mean
- SAPs
systemic arterial pressure systolic
- SaO2
arterial oxygen saturation
- SvO2
mixed venous oxygen saturation
- SVR
systemic vascular resistance
Footnotes
CRediT authorship contribution statement
Akram Khan: Conceptualization, Contributed patients to the study and data acquisition, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. R. James White: Conceptualization, Contributed patients to the study and data acquisition, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Gisela Meyer: Contributed patients to the study and data acquisition, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Tomas R. Pulido Zamudio: Contributed patients to the study and data acquisition, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Carlos Jerjes-Sanchez: Contributed patients to the study and data acquisition, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Dana Johnson: Formal analysis, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Rob Grover: Supervision, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Meredith Broderick: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Aliou Ousmanou: Supervision, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Louis Holdstock: Supervision, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Evangelos Michelakis: Contributed patients to the study and data acquisition, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2022.106744.
References
- [1].White RJ, Jerjes-Sanchez C, Meyer GMB, Pulido T, Sepulveda P, Wang KY, Grünig E, Hiremath S, Yu Z, Gangcheng Z, Yip WLJ, Zhang S, Khan A, Deng CQ, Grover R, Tapson VF, Svetliza GN, Lescano AJ, Bortman GR, Diez FA, Botta CE, Fitzgerald J, Feenstra E, Kermeen FD, Keogh AM, Williams TJ, Yousseff PP, Ng BJ-H, Smallwood DM, Dwyer NB, Brown MR, Lang IM, Steringer-Mascherbauer R, Arakaki JSO, Campos FTAF, de A Correa R. de Souza R, Meyer GMB, Moreira MAC, Yoo HHB, Lapa MS, Swiston J, Hirani N, Mehta S, Michelakis E, Sepulveda PA, Blancaire MMZ, Liu J, Shuyang Z, Pan L, Chunde B, Qun Y, Xiaoshu C, Zaixin Y, Li X, Hua Y, Gangcheng Z, Zhu X, Chen Y, Zhaozhong C, Yang Y, Daxin Z, Jieyan S, Nielsen-Kudsk JE, Carlsen J, Bourdin A, Hachulla E, Dromer C, Chaouat A, Reynaud-Gauber M, Seronde M-F, Klose H, Halank M, Hoffken G, Ewert R, Rosenkranz S, Grunig E, Kruger U, Kronsbein J, Hauptmeier BM, Koch A, Held M, Lange TJ, Neurohr C, Wilkens H, Wirtz HRW, Konstantinides S, Argyropoulou-Pataka P, Orfanos S, Hiremath S, Kerkar PG, Suresh PV, Baxi HA, Oomman A, Abhaichand RK, Arjun PKE, Chopra V, Mehrotra R, Rajput RK, Sawhney JPS, Bimalendu S, Sharma KH, Sastry BKS, Kramer MR, Segel MJ, Ben-Dov I, Berkman N, Yigla M, Adir Y, D’Alto M, Vizza CD, Scelsi L, Vitulo P, Pulido TR, Jerjes-Sanchez C, Boonstra A, Vonk MC, Sobkowicz B, Mularek-Kubzdela T, Torbicki A, Podolec P, Teik LS, Yip WLJ, Chang H-J, Kim H-K, Park J-B, Chang S-A, Kim D-K, Chung W-J, Song J-M, Nissell M, Hjalmarsson C, Rundqvist B, Huang W-C, Cheng C-C, Hsu C-H, Hsu HH, Wang K-Y, Coghlan JG, Kiely DG, Pepke-Zaba JW, Lordan JL, Corris PA, Cadaret L, Hansdottir S, Oudiz RJ, Badesch DB, Mathier M, Schilz R, Hill N, Waxman A, Markin CJ, Zwicke DL, Fisher M, Franco V, Sood N, Park MH, Allen R, Feldman JP, Balasubramanian V, Seeram VK, Bajwa A, Thompson AB, Migliore C, Elwing J, McConnell JW, Mehta JP, Rahaghi FF, Rame JE, Khan A, Patel B, Oren RM, Klinger JR, Alnuaimat H, Allen S, Harvey W, Eggert MS, Hage A, Miller CE, Awdish RLA, Cajigas H, Grinnan D, Trichon BH, McDonough C, White RJ, Rischard F, Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind placebo-controlled clinical trial, Am. J. Respir. Crit. Care 201 (2019) 707–717, 10.1164/rccm.201908-1640oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chakinala MM, Feldman JP, Rischard F, Mathier M, Broderick M, Leedom N, Laliberte K, White RJ, Transition from parenteral to oral treprostinil in pulmonary arterial hypertension, J. Heart Lung Transplant 36 (2017) 193–201, 10.1016/j.healun.2016.06.019. [DOI] [PubMed] [Google Scholar]
- [3].White MGPT, Diaz JS, Khan A, Grover Deng R, Broderick OA, Holdstock ME, Treatment with oral treprostinil Improves hemodynamics in participants with PAH, in: 14th PVRI 14th Annual World Congress on Pulmonary Vascular Disease, Lima, Peru, 2020. https://pvrinstitute.org/en/professionals/learning/2020/2/15/97-treatment-with-oral-treprostinil-improves-hemodynamics-in-participants-with-pah/. [Google Scholar]
- [4].Whittle BJ, Silverstein AM, Mottola DM, Clapp LH, Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: treprostinil is a potent DP1 and EP2 agonist, Biochem. Pharmacol 84 (2012) 68–75, 10.1016/j.bcp.2012.03.012. [DOI] [PubMed] [Google Scholar]
- [5].Falcetti E, Flavell DM, Staels B, Tinker A, Haworth Sheila G., Clapp LH, IP receptor-dependent activation of PPARγ by stable prostacyclin analogues, Biochem. Biophys. Res. Commun 360 (2007) 821–827, 10.1016/j.bbrc.2007.06.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lombroso M, Nicosia S, Paoletti R, Whittle BJR, Moncada S, Vane JR, The use of stable prostaglandins to investigate prostacyclin (PGI2)-binding sites and PGI2- sensitive adenylate cyclase in human platelet membranes, Prostaglandins 27 (1984) 321–333, 10.1016/0090-6980(84)90083-2. [DOI] [PubMed] [Google Scholar]
- [7].Saouti N, Westerhof N, Postmus PE, Vonk-Noordegraaf A, The arterial load in pulmonary hypertension, Eur. Respir. Rev 19 (2010) 197–203, 10.1183/09059180.00002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lankhaar J-W, Westerhof N, Faes TJC, Gan CT-J, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A, Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension, Eur. Heart J 29 (2008) 1688–1695, 10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- [9].Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD, Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension, J. Am. Coll. Cardiol 47 (2006) 799–803, 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- [10].Badagliacca R, Raina A, Ghio S, D’Alto M, Confalonieri M, Correale M, Corda M, Paciocco G, Lombardi C, Mule M`, Poscia R, Scelsi L, Argiento P, Sciomer S, Benza RL, Vizza CD, Influence of various therapeutic strategies on right ventricular morphology, function and hemodynamics in pulmonary arterial hypertension, J. Heart Lung Transplant 37 (2018) 365–375, 10.1016/j.healun.2017.08.009. [DOI] [PubMed] [Google Scholar]
- [11].Badagliacca R, D’Alto M, Ghio S, Argiento P, Bellomo V, Brunetti ND, Casu G, Confalonieri M, Corda M, Correale M, D’Agostino C, Michele LD, Galgano G, Greco A, Lombardi C, Manzi G, Mercurio V, Mule M`, Paciocco G, Papa S, Romeo E, Scelsi L, Stolfo D, Vitulo P, Naeije R, Vizza CD, Risk reduction and hemodynamics with initial combination therapy in pulmonary arterial hypertension, Am. J. Respir. Crit. Care 203 (2021) 484–492, 10.1164/rccm.202004-1006oc. [DOI] [PubMed] [Google Scholar]
- [12].Torres F, Farber H, Ristic A, McLaughlin V, Adams J, Zhang J, Klassen P, Shanahan W, Grundy J, Hoffmann I, Cabell C, Subías PE, Sood N, Keogh A, D’Souza G, Rubin L, Efficacy and safety of ralinepag, a novel oral IP agonist, in PAH patients on mono or dual background therapy: results from a phase 2 randomised, parallel group, placebo-controlled trial, Eur. Respir. J 54 (2019), 1901030, 10.1183/13993003.01030-2019. [DOI] [PubMed] [Google Scholar]
- [13].Farber H, Snood N, Preston I, Adams J, Grundy J, King C, Klassen P, Tapson V, McLaughlin V, Oudiz R, Ralinepag plasma levels correlate with improvements in functional and hemodynamic parameters in patients with pulmonary arterial hypertension, J. Heart Lung Transplant 38 (2019) S208, 10.1016/j.healun.2019.01.506. [DOI] [Google Scholar]
- [14].Simonneau G, Torbicki A, Hoeper MM, Delcroix M, Karlócai K, Galiè N, Degano B, Bonderman D, Kurzyna M, Efficace M, Giorgino R, Lang IM, Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension, Eur. Respir. J 40 (2012) 874–880, 10.1183/09031936.00137511. [DOI] [PubMed] [Google Scholar]
- [15].Chin KM, Sitbon O, Doelberg M, Feldman J, Gibbs JSR, Grünig E, Hoeper MM, Martin N, Mathai SC, McLaughlin VV, Perchenet L, Poch D, Saggar R, Simonneau G, Galie N, Three- versus two-drug therapy for patients ` with newly diagnosed pulmonary arterial hypertension, J. Am. Coll. Cardiol 78 (2021) 1393–1403, 10.1016/j.jacc.2021.07.057. [DOI] [PubMed] [Google Scholar]
- [16].Jing Z-C, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, Torbicki A, Xu K-F, Yehle D, Laliberte K, Arneson C, Rubin LJ, Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial, Circulation 127 (2013) 624–633, 10.1161/circulationaha.112.124388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.