Abstract
Orthostatic hypotension is an unusually large decrease in blood pressure on standing that increases the risk of adverse outcomes even when asymptomatic. Improvements in haemodynamic profiling with continuous blood pressure measurements have uncovered four major subtypes: initial orthostatic hypotension, delayed blood pressure recovery, classic orthostatic hypotension, and delayed orthostatic hypotension. Clinical presentations are varied and range from cognitive slowing with hypotensive unawareness or unexplained falls to classic presyncope and syncope. Establishing whether symptoms are due to orthostatic hypotension requires careful history taking, a thorough physical examination, and supine and upright blood pressure measurements. Management and prognosis vary according to the underlying cause, with the main distinction being whether orthostatic hypotension is neurogenic or non-neurogenic. Neurogenic orthostatic hypotension might be the earliest clinical manifestation of Parkinson’s disease or related synucleinopathies, and often coincides with supine hypertension. The emerging variety of clinical presentations advocates a stepwise, individualised, and primarily non-pharmacological approach to the management of orthostatic hypotension. Such an approach could include the cessation of blood pressure lowering drugs, adoption of lifestyle measures (eg, counterpressure manoeuvres), and treatment with pharmacological agents in selected cases.
Introduction
An unusually large decrease in blood pressure on standing (orthostatic hypotension) is a physical sign and a final common pathway of various conditions that affect blood pressure homoeostasis. Orthostatic hypotension is very common and increases with age, affecting about one in five community-dwelling adults older than 60 years and one in four people in long-term care.1 Up to a quarter of patients presenting with unexplained syncope and severe orthostatic intolerance have orthostatic hypotension.2 Orthostatic hypotension negatively affects quality of life, can lead to traumatic falls, shortens life expectancy, and increases the risk of various, predominantly cardiovascular, conditions.3–5
Standing blood pressure can often be increased sufficiently to improve orthostatic tolerance by removing or reducing factors that trigger or exacerbate orthostatic hypotension, such as hypovolaemia and medications, or by preventing physical deconditioning. Chronic orthostatic hypotension can be a feature of neurological disorders that affect the baroreflex and cause impaired vasoconstriction during standing; in such contexts, the condition is termed neurogenic orthostatic hypotension. Neurogenic orthostatic hypotension is most frequently encountered in patients with neurodegenerative disorders that involve intracellular accumulation of misfolded α-synuclein in nerve tissue (ie, synucleinopathies), spinal cord injuries, or small fibre neuropathies caused by diabetes, amyloidosis, toxic agents, autoimmune diseases, or paraneoplastic diseases.3,6–10 Orthostatic hypotension with non-neurogenic causes (eg, hypovolemia, polypharmacy, heart failure, arrhythmias, or advanced valvular disease) is more frequent in the general population,11 but can coexist with and aggravate neurogenic orthostatic hypotension.12
The increased use of advanced haemodynamic profiling with continuous blood pressure measurements has uncovered four major subtypes of impaired orthostatic blood pressure regulation (initial orthostatic hypotension, delayed blood pressure recovery, classic orthostatic hypotension, and delayed orthostatic hypotension) and has aided clinical recognition of the varied presentation of orthostatic hypotension.13–15 Evidence for the prognostic implications of the emerging orthostatic hypotension subgroups is increasing.4,5 Neurogenic orthostatic hypotension might represent the earliest clinical manifestation of Parkinson’s disease or related synucleinopathies, which is of particular interest as it could be used as a biomarker to assess the effects of future disease-modifying therapies.16 In this Review, we critically review the key physiological changes induced by standing, the main clinical features of the four subtypes of orthostatic hypotension, and the implications of these four subtypes for ancillary testing, long-term prognosis, and treatment; we also present the latest insights into a stepwise diagnostic and individualised management approach.
Clinical presentation and classification of orthostatic hypotension
Orthostatic hypotension occurs because of an inability to maintain normotension against the effects of gravity on cardiovascular haemodynamics in the upright posture (figure 1). Orthostatic hypotension typically occurs when the compensatory responses are insufficient or overwhelmed, often due to failure of the arterial baroreflex.
Figure 1: Steady-state circulatory adjustments to the upright posture.
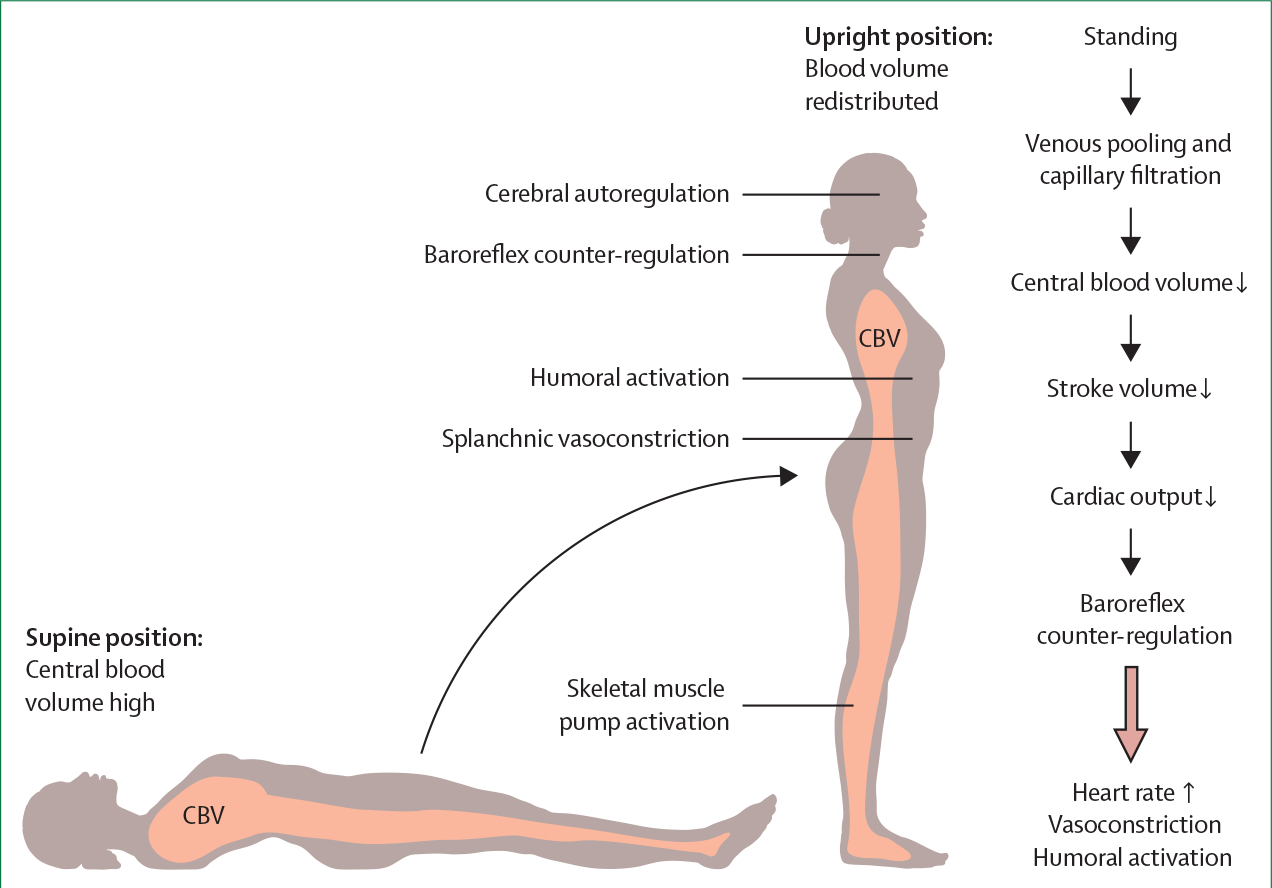
Orthostatic stress results in a shift of blood from the chest to the distensible venous capacitance system below the diaphragm. This venous pooling rapidly reduces central blood volume, and this reduction is compounded by increased capillary filtration of plasma secondary to the increased hydrostatic pressure in the legs. The reduction in central blood volume decreases cardiac filling and stroke volume (the volume pumped by each heartbeat). In healthy individuals, heart rate increases on standing, but not enough to compensate for the reduction in stroke volume, and so cardiac output (the product of stroke volume and heart rate) decreases. The key circulatory adjustments to the upright posture (large arrow) are arterial baroreflex-mediated constriction of arterioles and splanchnic venous capacitance vessels, with a subsequent increase in systemic vascular resistance that compensates for the decrease in cardiac output, therefore maintaining normotension (mean arterial pressure=cardiac output × systemic vascular resistance). This response is augmented by increased skeletal and abdominal muscle tone, which increases venous return through the skeletal muscle pump. In healthy individuals, mean blood pressure changes little, systolic blood pressure decreases only slightly, if at all, and diastolic blood pressure increases. Humoral activation, particularly increases in plasma renin activity and vasopressin release, also supports the circulation during standing for long durations. Cerebral autoregulation helps to maintain cerebral blood flow over various perfusion pressures. CBV=central blood volume.
The resultant orthostatic hypotension can present in various ways: as an asymptomatic measurement result, based on orthostatic vital signs;3,6 with orthostatic symptoms (eg, lightheadedness or dizziness) that are relieved upon assumption of a supine posture;3,17 with orthostatic signs (cognitive slowing) without symptoms (ie, hypotensive unawareness);17–19 as recurrent unexplained falls;5,20,21 or with overt loss of consciousness (syncope).17,22 The clinical presentation of orthostatic hypotension can include more than one symptom, and transitioning between symptoms is possible depending on the underlying cause or aggravating factors. Notably, patients might be vague about symptoms, might refer only to fatigue, cognitive impairment, leg weakness, or gait impairment, or might report no symptoms at all.11,17,23,24 Figure 2 shows the four major subtypes of orthostatic hypotension that have been defined on the basis of the magnitude and timing of the blood pressure response to standing.13,15,22,25
Figure 2: Four major subtypes of orthostatic hypotension.
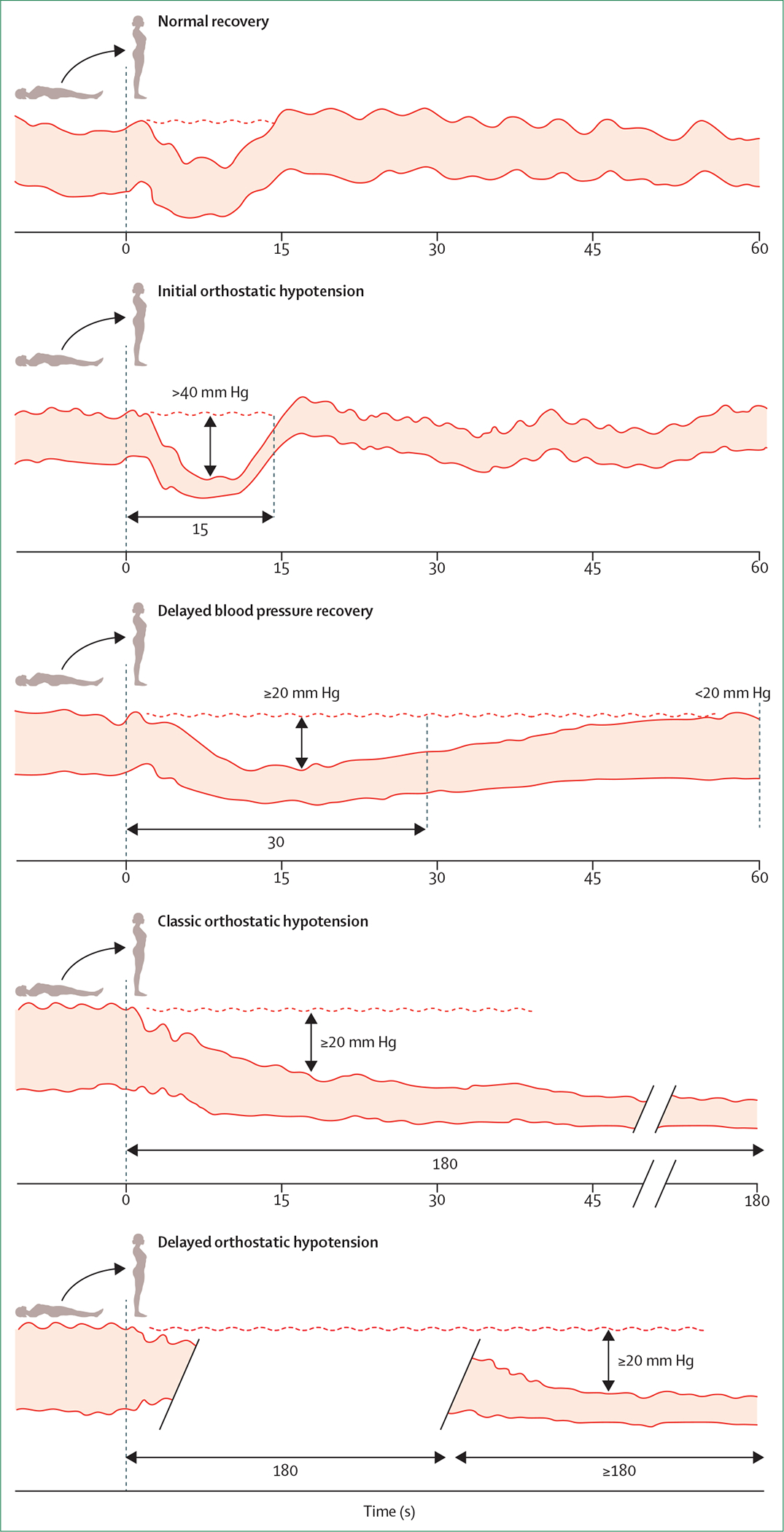
Schematics of continuous blood pressure curves showing normal recovery and the diagnostic criteria for initial orthostatic hypotension,25 delayed blood pressure recovery,15 classic orthostatic hypotension,25 and delayed orthostatic hypotension.25 Because around 95% of patients with orthostatic intolerance and unexplained syncope can be identified as having orthostatic hypotension by systolic blood pressure criteria alone,26 we display only the systolic blood pressure criteria. Individual blood pressure tracings were revised from ref 27. Note that a reduction in systolic blood pressure of ≥30 mm Hg is commonly used to define classic orthostatic hypotension in patients who have supine hypertension.6,15,22,28
Diagnostic investigations of suspected orthostatic hypotension
Medical history taking
Medical history taking is crucial for evaluating patients with orthostatic symptoms and distinguishing orthostatic hypotension from other conditions that cause orthostatic intolerance, particularly vasovagal syncope and postural tachycardia syndrome.15,22 Symptoms and signs of autonomic activation (eg, sweating and palpitations) could indicate postural tachycardia syndrome and vasovagal syncope, and the presence of emotional or pain triggers could indicate vasovagal syncope.22 A history of recurrent and transient orthostatic intolerance occurring immediately upon standing (typically within around five steps after standing up and walking) is highly specific for initial orthostatic hypotension.13,29,30 Classic orthostatic hypotension can provoke less immediate and more protracted symptoms than initial orthostatic hypotension owing to prolonged hypoperfusion of the brain, including the retina; such hypoperfusion can cause lightheadedness, blurry dimmed vision, muffled hearing, cognitive slowing, and eventually syncope (ie, transient loss of consciousness due to global cerebral hypoperfusion).3,17,22 Occasionally, transient ischaemic attacks can occur if the cerebral hypoperfusion is regional. Classic orthostatic hypotension, particularly if neurogenic, can also manifest with hypoperfusion of the shoulder and neck muscles (causing so-called coat hanger pain), the lungs (causing dyspnea due to ventilation–perfusion mismatch secondary to hypoperfusion of the lung apices), and rarely the myocardium (causing chest pain even with patent coronary arteries).3,17 These symptoms occur when the individual is standing or even sitting, but not when supine, and are persistent and recurrent. Orthostatic intolerance is typically more pronounced in the morning after overnight natriuresis, when the patient is plasma-volume-depleted, after cessation of exercise, after food or alcohol intake, when the ambient temperature is high, when the patient is standing after prolonged recumbency, and with physical deconditioning.3,17,22 The frequency, severity, and type of orthostatic intolerance does not seem to differ between patients with classic and delayed orthostatic hypotension.2,31,32
Orthostatic symptoms in neurogenic orthostatic hypotension correlate with the lowest blood pressure while standing rather than with the magnitude of the decrease in blood pressure.33 However, despite very low blood pressure while standing, up to half of all patients with neurogenic orthostatic hypotension do not report symptoms,18 although witnesses might observe slowing of cognition when the patient is standing.17 This hypotensive unawareness could partly explain the increased risk of falls in patients with neurogenic orthostatic hypotension compared with people of a similar age without orthostatic hypotension, as the patient might not take preventive measures and might not recall the loss of consciousness that caused the fall.19 Hypotensive unawareness is particularly common in patients with neurogenic orthostatic hypotension caused by a CNS synucleinopathy (ie, multiple system atrophy, Parkinson’s disease, or Lewy body dementia).18,24 Neuropsychological tests while the patient is upright yield poorer cognitive performance than tests while supine or sitting in patients with neurogenic orthostatic hypotension due to Parkinson’s disease34 and peripheral autonomic disorders.35 The association between cognitive impairment and orthostatic hypotension seems to be related to the extent of the hypotension, as shown by a post-hoc analysis of two randomised controlled trials of the cholinesterase inhibitor rivastigmine in patients with Parkinson’s disease dementia.36 These analyses revealed that patients with orthostatic hypotension whose blood pressure increased after treatment had a larger cognitive benefit than patients whose blood pressure did not increase, suggesting that the clinical benefit is explained by an antihypotensive effect rather than an anticholinergic effect.36
History taking should always include a full review of medications for unwanted cardiovascular effects and comorbidities associated with orthostatic hypotension. Special attention should be paid to the possibility that the presence of orthostatic hypotension is a sign of a more widespread neurological disorder that also affects the control of other organs. The initial evaluation should therefore extend to other autonomic symptoms (eg, constipation, erectile dysfunction, and bladder problems) and include a thorough neurological examination to look for signs of cognitive decline, parkinsonism, ataxia, or peripheral neuropathy.37 At least a quarter of all patients with pure autonomic failure are eventually diagnosed with a CNS synucleinopathy.38,39 Clinical features that suggest an increased risk for such a diagnosis include severe bladder problems, constipation, subtle motor deficits, and dream enactment behaviour (panel).38,39
Panel: Case study.
A 58-year-old woman presented with an 8-year history of lightheadedness when standing still or after climbing stairs, which was relieved by sitting or lying down. She reported heat intolerance and urinary retention with occasional incontinence (beginning 6 years previously, managed with intermittent self-catheterisation). She had dream enactment behaviour that had been witnessed, and had a mild broad-based gait. Her medical history was otherwise unremarkable and she was not taking any medication. Her neurological examination was normal. A cardiac evaluation revealed mild chronotropic incompetence but no structural abnormalities. A tilt-table test reproduced her typical symptoms within 3 min, coinciding with a progressive decrease in systolic blood pressure (from 159/112 mm Hg supine to 85/71 mm Hg tilted) but only a mild increase in heart rate (from 62 beats per min supine to 75 bpm tilted), giving a ratio of Δ(heart rate)/Δ(systolic blood pressure) of 0·2 beats per min/mm Hg. The severe orthostatic hypotension and mild increase in heart rate, combined with reduced heart-rate variability during deep breathing and absence of a blood pressure overshoot during the Valsalva manoeuvre, confirmed neurogenic orthostatic hypotension. Combined neurogenic orthostatic hypotension, dream enactment behaviour, and underactive bladder supported the diagnosis of possible prodromal multiple system atrophy, despite the absence of laryngeal stridor, apnoea, overt parkinsonism, hyper-reflexia, or brain MRI abnormalities. An interactive session identified leg-crossing and tensing of the abdominal and gluteal muscles as highly effective counter-manoeuvres, which the patient successfully applied to reduce symptoms. Additionally, she used bolus water drinking (500 mL) in advance of triggering events (eg, standing during social events) and taught herself to stand up gradually. She raised the head of her bed 20° to reduce supine hypertension and improve orthostatic hypotension. Over time, her lightheadedness worsened, and treatment with fludrocortisone (0·05 mg/day) and midodrine (5 mg three times a day) was started. Although fludrocortisone was kept at a stable dose, she was allowed to use midodrine as needed, as her symptoms varied: mild symptoms during winter while at home, but severe symptoms on hot summer days or when she was more physically active. At age 61 years she developed gait and limb ataxia and was diagnosed with multiple system atrophy of the cerebellar type.
Active bedside standing test
The bedside standing test examines basic physiology and is essential to diagnose and classify orthostatic hypotension.12,14,40 Blood pressure measurements with an automated oscillometric device or an upper-arm cuff and stethoscope are appropriate in ambulatory settings. For convenience, sitting-to-standing (rather than lying-to-standing) measurements are often used for the initial evaluation of orthostatic hypotension at the expense of a lower sensitivity.6,41,42 Accurate measurement with the arm cuff at heart-level while the patient is supine and standing is required in research protocols. An upright measurement with the arm vertical and parallel to the body will suffice in daily practice. Lying-to-standing measurements are more likely to detect orthostatic hypotension than sitting-to-standing measurements, but can be challenging in older patients or patients with balance or musculoskeletal problems.43
Screening for supine hypertension (ie, systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or both) is important,44 as it could affect management strategies, particularly in neurogenic orthostatic hypotension.6,45,46 For patients with spinal cord injury or extreme frailty who cannot perform a standing test, a passive seated orthostatic stress test is an alternative.47 For orthostatic blood pressure measurements, a duration of 5 min of supine rest is adequate. This period is sufficient for blood pressure stabilisation and enables evaluation of the presence of supine hypertension, yet is short enough to prevent the undesired enhancement of the initial, transient blood pressure decrease upon standing that is often seen after protracted supine rest.43
We advise three oscillometric blood pressure measurements, 1 min, 2 min, and 3 min after standing. The exact timing of measurements can vary when oscillometric devices are used, but this variation is acceptable when screening for classic orthostatic hypotension. Delayed blood pressure recovery can be assessed with an additional oscillometric measurement after 30 s of standing.41 Initial orthostatic hypotension occurs too soon after standing to be detected by conventional blood pressure measurements; instead, continuous beat-to-beat blood pressure monitoring would be required.14 However, such assessment is seldom needed, because initial orthostatic hypotension can often be established on the basis of history alone.13,29,30,48
We propose a stepwise diagnostic approach for patients with symptoms that could relate to orthostatic hypotension (ie, orthostatic intolerance, syncope, falls, or transient loss of consciousness), starting with a detailed history, physical examination, and a bedside standing test (figure 3). When the decreases in blood pressure and the symptoms are reproduced during bedside testing, the diagnosis of symptomatic orthostatic hypotension can be confirmed.22 If an abnormal decrease in blood pressure occurs with no symptoms during testing, a diagnosis of symptomatic orthostatic hypotension is likely if the history is suggestive,22 particularly in patients with neurodegenerative conditions that are associated with hypotensive unawareness.18,24 However, alternative causes of orthostatic intolerance, syncope, transient loss of consciousness, and falls should be considered if orthostatic hypotension is observed during testing but the presenting symptoms are less consistent with this condition.
Figure 3: Proposed flowchart for diagnostic investigation.
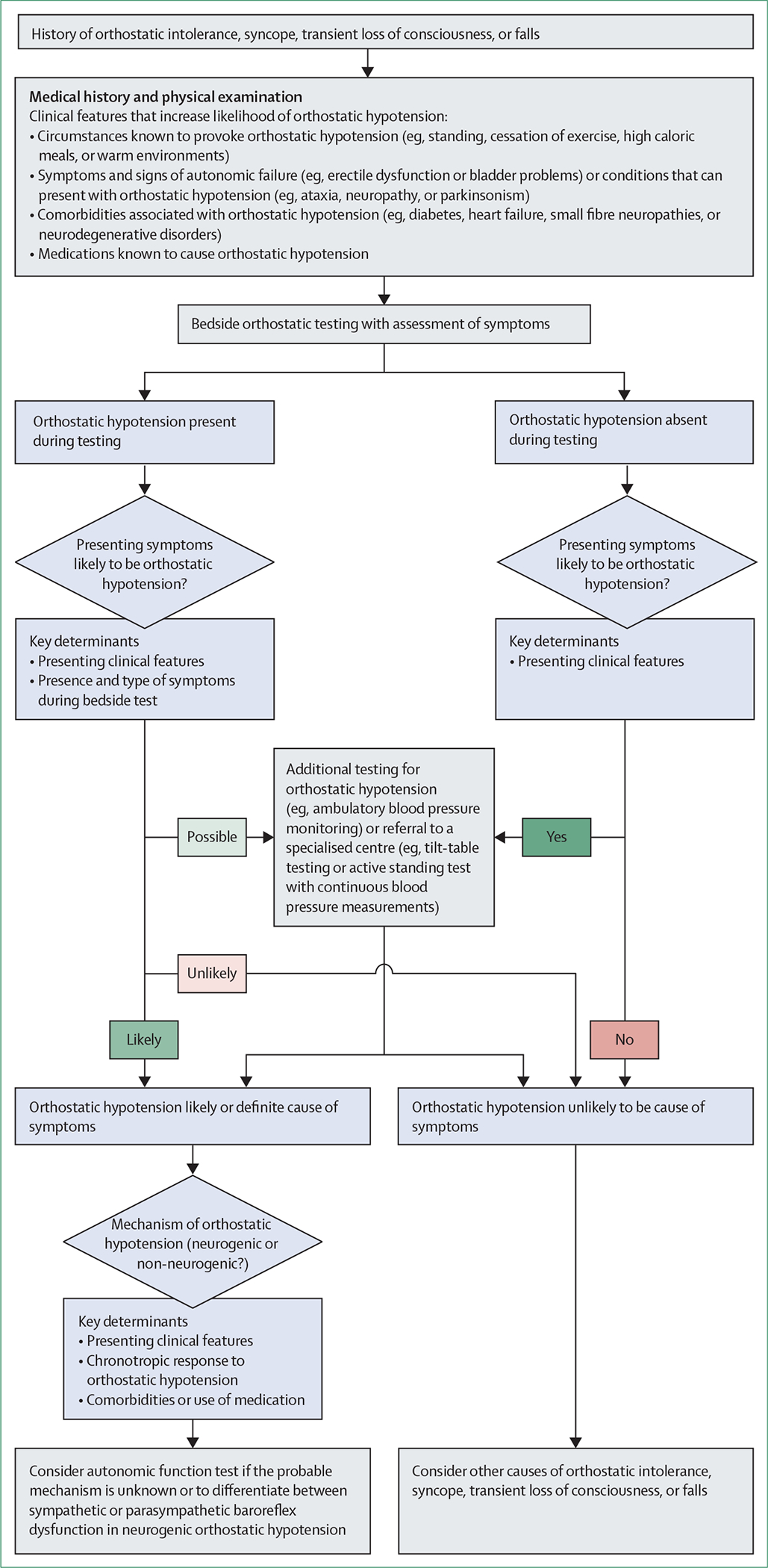
We propose that diagnosis is based on clinical presentation and whether orthostatic hypotension is reproduced during bedside testing, and if so, whether it is associated with symptoms.15,22 If a patient presents with symptoms that could relate to orthostatic hypotension, a detailed medical history and physical examination should be taken: to establish the circumstances of the events; to identify accompanying symptoms of autonomic failure or signs of neurological conditions that can present with orthostatic hypotension; to identify comorbidities associated with orthostatic hypotension; and to identify any drugs that might induce orthostatic hypotension. If the medical history supports possible orthostatic hypotension, a bedside active standing test should be done. If this test identifies orthostatic hypotension and the patient has their typical symptoms, a definitive diagnosis of symptomatic orthostatic hypotension can be made. If the standing test reveals orthostatic hypotension but the patient is asymptomatic on that occasion, the diagnosis is still likely to be orthostatic hypotension, particularly in patients with neurodegenerative conditions associated with hypotensive unawareness. If orthostatic hypotension is found but the presenting symptoms are not entirely consistent with orthostatic hypotension, alternative causes of orthostatic intolerance, syncope, transient loss of consciousness, and falls should be considered. If bedside screening does not show orthostatic hypotension despite a typical presentation, repeated measurements after meals or exercise and at different times of day, or ambulatory blood pressure monitoring, could be useful. If the clinical evaluation is inconclusive, referral to a specialised centre could be considered.15 For all patients with orthostatic hypotension, it is important to identify the probable mechanism (neurogenic or non-neurogenic)6 and to consider autonomic function testing.6,15,40
In patients who present without transient loss of consciousness or with falls without orthostatic intolerance, and in older patients (>60 years) who are frail and have comorbidities, it is crucial that the reported circumstances of the presenting symptoms correspond to triggers for orthostatic hypotension, and that red flags of cardiac syncope are excluded.22 Of note, a normal blood pressure response without symptoms during bedside testing cannot rule out the possibility of orthostatic hypotension. If bedside screening does not show orthostatic hypotension despite typical symptoms, repeated measurements after meals or exercise and at different times, or ambulatory blood pressure monitoring with a diary of activities, can be useful. Ambulatory blood pressure monitoring can provide information on how often blood pressure decreases and how low it becomes in daily life, document postprandial hypotension, and establish whether nocturnal hypertension is present. Such monitoring can also document other triggers for hypotension, such as specific physical activities.38 Self-measurements of blood pressure during symptoms might also be insightful.49,50 If a patient reports symptoms that persist during and after repeated blood pressure measurements with normal values, orthostatic hypotension can be excluded as a possible cause and other diagnoses (eg, inebriation syndrome) should be considered.51 However, orthostatic hypotension is still possible if the patient reports transient symptoms during bedside screening. Intermittent blood pressure measurements might not capture mild and transient decreases in orthostatic blood pressure, which are as frequent as classic orthostatic hypotension in Parkinson’s disease and can also cause orthostatic intolerance and syncope.52 A standing test with continuous blood pressure recordings can be helpful if a diagnosis cannot be established on the basis of history alone.14,15
Identifying the probable mechanism (neurogenic or non-neurogenic) while considering all clinical features (ie, symptoms and signs of neurological conditions that can present with orthostatic hypotension, comorbidities, and medication use) is important for all patients with orthostatic hypotension, whether symptomatic or not. A 2020 study showed the additional value of bedside testing to distinguish between neurogenic and non-neurogenic orthostatic hypotension.53 A diminished increase in heart rate despite a decrease in blood pressure (ie, Δ[heart rate]/Δ[systolic blood pressure] <0·5 beats per min/mm Hg) makes a neurogenic cause very likely.53,54 This distinction is valid only for patients without conditions (eg, atrial fibrillation) or medications (eg, β-blockers) that affect heart rate control.54
Autonomic function testing
For complex cases—particularly those in patients with considerable comorbidities, other autonomic symptoms, or conflicting results (eg, orthostatic hypotension without orthostatic intolerance)—referral to a centre with expertise in autonomic testing should be considered. Tilt table testing is particularly useful for the detection of delayed orthostatic hypotension, but can also identify conditions that mimic orthostatic hypotension, including reflex syncope or psychogenic pseudosyncope, whereas an active standing test with beat-to-beat blood pressure measurements can help to identify initial orthostatic hypotension.14,15 Other autonomic tests (eg, the Valsalva manoeuvre, deep breathing, and measurements of plasma norepinephrine concentration) also help to separate neurogenic from non-neurogenic causes of orthostatic hypotension and differentiate between sympathetic or parasympathetic baroreflex dysfunction.6,15,40 However, when neurogenic orthostatic hypotension is present, cardiovascular autonomic testing seems to be of limited value in differentiating between the parkinsonian form of multiple system atrophy and Parkinson’s disease.55 Assessing supine norepinephrine concentrations could be valuable to predict conversion in pure autonomic failure: patients who convert to multiple system atrophy have higher supine norepinephrine concentrations than patients who convert to Parkinson’s disease or Lewy body dementia.38,39 A 2021 cohort study showed that the presence of α-synuclein markers in the CSF can predict the conversion of pure autonomic failure to Parkinson’s disease, Lewy body dementia, or multiple system atrophy at an early stage.56
Prognosis of orthostatic hypotension
Observational evidence linking the four orthostatic hypotension patterns with adverse events varies. Initial orthostatic hypotension is under-reported in epidemiological studies evaluating the initial blood pressure response to standing, due to its requirement for specialised equipment for measurement; however, at least one large cohort study established that delayed blood pressure recovery, but not initial orthostatic hypotension, was associated with future falls.21 Delayed orthostatic hypotension is also less commonly studied in the literature than classic orthostatic hypotension, but has been associated with progression to classic orthostatic hypotension, the development of synucleopathies, and a higher risk of premature death.57 Substantially more evidence comes from population-based studies on classic orthostatic hypotension and delayed blood pressure recovery patterns measured with traditional upper arm sphygmomanometers. Multiple large, prospective cohort studies of community-dwelling adults have shown that both delayed blood pressure recovery and classic orthostatic hypotension are associated with future risk of adverse health outcomes, including frailty,47,58 falls,5,20,21,47,58–61 fractures,41,59,62 syncope,41 cognitive decline and dementia,61,63,64 depression,65 stroke,63,64,66,67 cardiovascular disease,66,68 and early death.4,5,41,69 One study showed that early orthostatic hypotension (applying classic orthostatic hypotension criteria within 1 min of standing) was more strongly related to individual long-term risks than later orthostatic hypotension (applying criteria in the 1–3 min range).41 A topic of active study is whether orthostatic hypotension, through hypoperfusion of the heart and brain, causes many of these outcomes or is merely a marker of frailty, or, in the case of neurogenic orthostatic hypotension, a marker of more widespread neurodegeneration.27,70–73 Whether orthostatic hypotension treatments can help to improve prognosis has not been studied. However, recognising the frequent comorbidities among people with orthostatic hypotension that might also augment their risk of adverse cardiovascular outcomes, especially diabetes and hypertension, is important. Strategies targeting a reduction in orthostatic hypotension should therefore be used with care to avoid exacerbating these comorbid conditions.
Neurogenic orthostatic hypotension has serious long-term implications. The 10-year mortality rate is 64% for patients with classic orthostatic hypotension and 50% for those with delayed orthostatic hypotension that progressed to classic orthostatic hypotension.57 In patients with delayed orthostatic hypotension that did not convert to classic orthostatic hypotension, mortality rates were similar to those of the general population of that age (7–9%).57 In patients with CNS synucleinopathies, the presence of neurogenic orthostatic hypotension is associated with shorter survival.74 Neurogenic orthostatic hypotension has been associated with end-organ damage, including renal failure, left ventricular hypertrophy, and cerebrovascular disease.45,46 Most of these studies, however, did not account for the presence of supine hypertension as a potential confounder.45 This omission is important because supine hypertension is common in patients with neurogenic orthostatic hypotension and is independently associated with target organ damage and worse survival,45 therefore emphasising the need for strategies that can treat neurogenic orthostatic hypotension without aggravating supine hypertension. Another potential confounder that affects adverse outcomes in neurogenic orthostatic hypotension is pharmacological treatments. A 2021 cohort study in 129 patients with multiple system atrophy challenged the safety of fludrocortisone and midodrine to treat orthostatic hypotension, as these drugs increased the risk of sudden death.75 Although this association might be explained by more severe autonomic dysfunction in patients receiving orthostatic hypotension treatment, the association between these treatments and mortality remained significant when accounting for markers of autonomic dysfunction (eg, blood pressure variability) and measures of disease severity.75
Non-pharmacological management of orthostatic hypotension
Non-pharmacological treatments are the foundation of orthostatic hypotension management, because pharmacological interventions do not restore normal baroreflex control and therefore do not offer tailored posture-dependent correction of blood pressure. Instead, pharmacological interventions increase blood pressure independent of posture, frequently provoking or aggravating supine hypertension. Lifestyle measures are therefore strongly recommended as they can avoid or reduce hypotensive episodes without inducing long-term hypertension. There are few high-quality prospective trials on the non-pharmacological management of orthostatic hypotension.76–80 Clinical trials usually assess a single approach across all participants, whereas in clinical practice, approaches tailored to the individual patient are recommended.81,82 Tailored approaches are particularly pertinent for orthostatic hypotension, which is a heterogeneous condition that requires personalised management. The effective, immediate, and marked circulatory responses in patients who are highly symptomatic enables these interventions to be evaluated with the individual patient as their own control (a n-of-1 trial design).82,83 We therefore advocate for an individual approach. The order of the non-pharmacological measures proposed here is based on evidence of efficacy in the literature, ease of adoption, risk–benefit ratio for each recommendation, and patient compliance and tolerability. Factors specific to individual patients favour particular approaches to improving symptoms and daily function. Such improvement might require a combination approach, although much benefit can be gained from each non-pharmacological approach independently.76
Education and counselling
Treatment goals should prioritise improvements in the patient’s symptoms and ability to complete activities of daily living rather than targeting a specific blood pressure. Simple descriptions of the underlying physiology of orthostatic hypotension—accompanied, if available, by leaflets, videos, and online resources (eg, Syncopedia)—can help patients, partners, and family members to understand and manage the condition.28,84 Counselling should encourage the early recognition of warning symptoms and avoidance of triggering events, such as standing for long periods, standing still after exercise, straining, eating large, carbohydrate-rich meals, drinking alcohol, and being in warm environments (including hot showers and saunas). Easy-to-apply lifestyle measures should be promoted, including minimising orthostatic stress in provoking situations (eg, sitting rather than standing) or slower changes of posture (eg, sitting on the edge of the bed before standing up) to counteract the initial decrease in blood pressure upon standing.85 Patients should avoid the supine position during the day and sleep with the head of the bed raised at night. Adapting the home environment, such as putting a chair in the bathroom to shower in a sitting position, can facilitate activities of daily living.
Medication review
All medications should be reviewed for unwanted cardiovascular effects and modified or discontinued if appropriate. Cessation or reduction in the dose or frequency of use of drugs, not only of hypertensive medications, are often needed. Of note, however, is that the treatment of uncontrolled hypertension can reduce the occurrence of non-neurogenic orthostatic hypotension.42,71,86 Nevertheless, specific classes of medication (eg, central or peripheral α-antagonists, and β-blockers) can exacerbate orthostatic hypotension by diminishing the compensatory cardiovascular responses needed to maintain blood pressure in the upright posture.
Physical countermanoeuvres
Physical countermanoeuvres should be considered a next step after education and medication review, as they effectively improve orthostatic tolerance and are easy to apply.80,87,88 These manoeuvres can end an episode of orthostatic hypotension if applied immediately when hypotensive symptoms present, and are also helpful as preventive measures when applied pre-emptively to increase blood pressure when standing. An interactive session assessing the efficacy and practicality of the various measures enables tailored therapy (figure 4).84 Ideally, patients learn to anticipate situations in which orthostatic hypotension is likely to occur and apply these measures automatically. However, physical counter-manoeuvres are not useful to avoid syncope in patients without prodromes, and could be difficult for patients who have motor disabilities or compromised balance as a result of being frail or having neurological conditions.
Figure 4: Physical countermanoeuvres to increase blood pressure in patients with orthostatic hypotension.
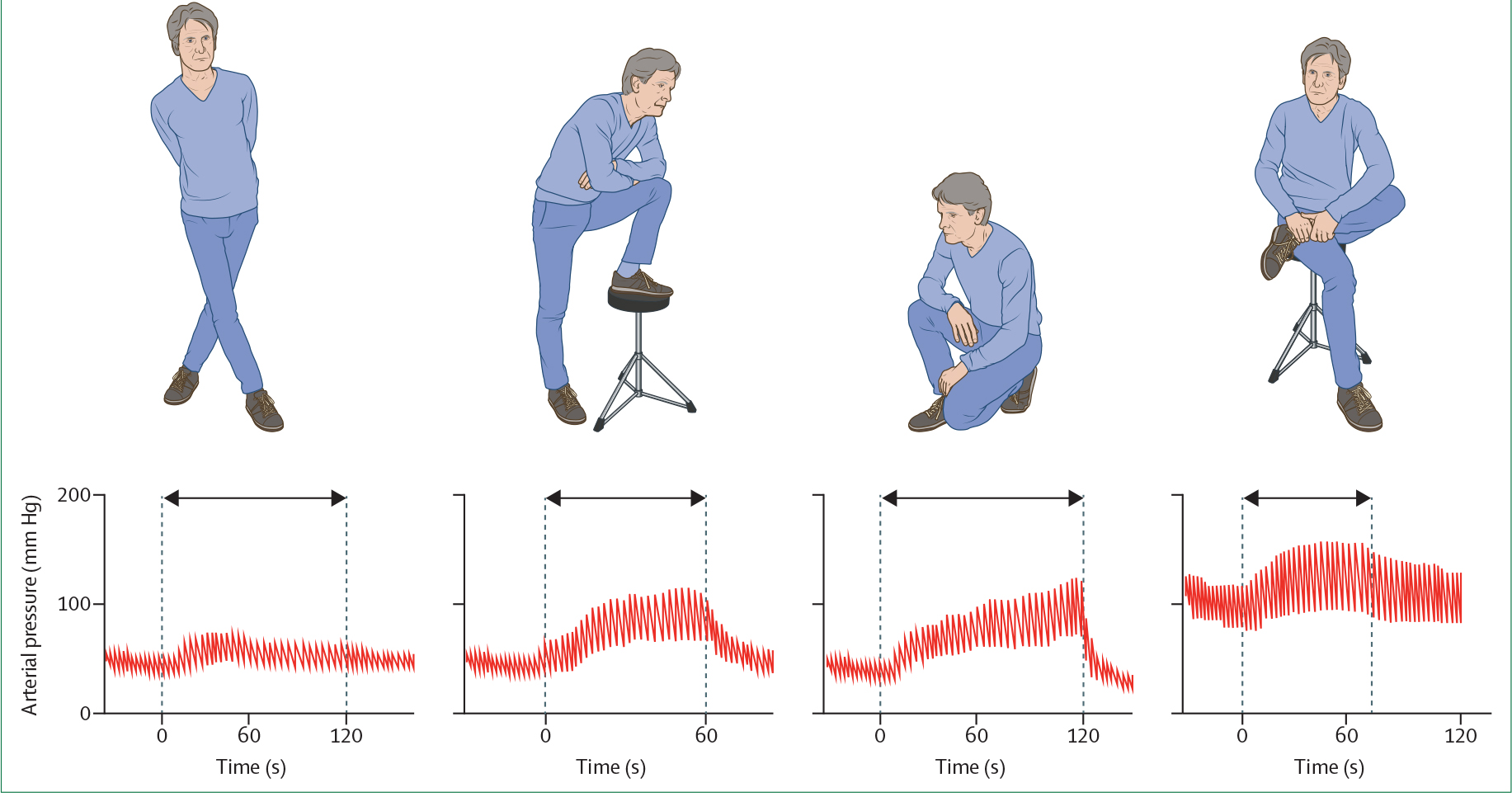
Red lines show the effects of crossing the legs, placing a foot on a chair, squatting when standing, and crossing the legs while seated on beat-to-beat arterial pressure measured at the finger in a middle-aged patient with pure autonomic failure and incapacitating neurogenic classic orthostatic hypotension. The arrows indicate the duration of the manoeuvres. Note the increase in blood pressure and pulse pressure. Blood pressure tracings are adapted from ref 84.
Bolus water drinking
As an additional measure, bolus water drinking can be recommended in advance of triggering events.76,80,89 Rapid ingestion of water (500 mL ingested in 2–3 min) elicits, within 5 to 10 min, an increase in blood pressure mediated by the sympathetic nervous system, and this effect lasts for 30–45 min. This pressor effect seems to be a spinal reflex triggered by acute reduction in osmolality in the portal vein (ie, osmopressor reflex), engaging residual sympathetic efferent nerves.
Eating frequent small meals and decreasing alcohol intake
Postprandial hypotension is defined as a decrease in systolic blood pressure of more than 20 mm Hg up to 30 min after a meal.11 Patients with neurogenic orthostatic hypotension and postprandial hypotension could reduce symptoms by eating smaller and more frequent meals with a lower carbohydrate content and avoiding alcohol.11 Conversely, in patients with coexisting supine hypertension, eating a sweet snack (around 400 kcal) or drinking a glass of wine before going to sleep has been shown to counteract supine hypertension.11,28,90
Increasing dietary salt intake
Traditionally, patients with orthostatic hypotension have been recommended to avoid a low-sodium diet and instructed to increase dietary salt intake to about 10 g NaCl per day, provided that there are no contraindications. Increases in salt intake can be achieved by adding salt liberally to meals or ingesting salt tablets. Fluid intake should be 2–3 L/day. Salt supplementation promotes plasma volume expansion, therefore improving orthostatic tolerance.91,92 Daily measurement of body weight is helpful to monitor this intervention. This approach should be undertaken cautiously in adults with supine or seated hypertension, and the relative improvement in orthostatic hypotension should be balanced against the potential to exacerbate hypertension.
Avoidance of physical deconditioning
For patients with orthostatic hypotension, lower-body strength training and moderate, non-strenuous activities should be encouraged. These activities are potentially beneficial by increasing plasma volume and increasing the efficacy of physical counterpressure manoeuvres. Exercise that is associated with minimal orthostatic stress (recumbent or semi-recumbent cycling or rowing exercise) is safe and well tolerated by patients with severe orthostatic hypotension. Notably, post-exercise hypotension can predispose susceptible individuals to syncope, so patients should be advised to take particular care in the first minutes after cessation of exercise. Swimming will not provoke symptoms, but getting out of the water can be problematic, especially in warm temperatures. While cycling, patients should try to avoid freewheeling as it could provoke symptoms. Tricycles that provide more stability are helpful. Hiking with a walking stick or pole could help patients to tolerate longer walking trails, as the poles can be used to bend forward and mitigate orthostatic stress.
Additional measures for severely affected patients
Elevation of the head-end of the bed to permit gravitational stress during sleep can improve orthostatic tolerance in patients with orthostatic hypotension. This technique ameliorates nocturnal hypertension and decreases nocturia and overnight depletion of plasma volume, therefore inducing a more favourable distribution of body fluids.92,93 Small tilting angles do not improve orthostatic tolerance;94 therefore, steeper angles (elevation of the head-end using 20–30 cm blocks) are recommended. In our experience, the beneficial effects can be so pronounced that patients might accept the discomfort. A hard pillow under the mattress at the level of the thighs can prevent the patient in supine sleep position from sliding down, and a footboard can be helpful. Another practical solution to enable head-up sleeping is an adjustable electric bed or mattress.
Compression stockings (worn to the upper thigh) require substantial effort to put on and have only a marginal effect on standing blood pressure.80 The abdomen is the most effective compression site to combat orthostatic hypotension, by diminishing splanchnic pooling. Elastic abdominal binders can be effective in highly symptomatic patients who are willing to attempt this intervention,80,95 but are uncomfortable to wear, especially in warm weather.
For some patients, a small, portable, and lightweight folding chair can be helpful to enable them to sit, and thereby ameliorate or end an episode, when presyncopal symptoms are triggered by standing. The seat height is an important determinant of the blood pressure response, with lower chairs having a more beneficial effect.88
Pharmacological management of orthostatic hypotension
The inability to control orthostatic intolerance with non-pharmacological measures is often explained by poor compliance. Patients with severe orthostatic hypotension, who remain symptomatic despite adherence to non-pharmacological therapy, should be considered for pharmacological treatment, which includes intravascular volume expansion and pressor drugs.11,28 Pharmacological treatment of neurogenic orthostatic hypotension is complex, and should be prescribed with caution because of the uncertainties regarding its long-term safety.75 We recommend considering referral to a specialised unit, particularly for patients with multimorbidity or when non-pharmacological measures and treatment with a single drug do not control symptoms. The mineralocorticoid agonist fludrocortisone induces an initial expansion of extracellular fluid volume, therefore raising blood pressure. Studies on the safety of this approach for patients with orthostatic hypotension are scarce, and the clinical effectiveness, particularly with long-term use, is uncertain.96 Doses greater than 0·2 mg/day are not recommended. Long-term use of fludrocortisone in high doses increases the risks of heart failure, renal fibrosis, and hospitalisation for any reason.11,28
Pressor agents, such as the α-adrenergic-receptor agonist midodrine and the synthetic norepinephrine precursor droxidopa, are short-acting and safer relative to mineralocorticoid agonists. The pressor response to droxidopa depends on the extent of postganglionic sympathetic denervation, with patients with low plasma norepinephrine concentrations before treatment having the most robust pressor response.97 Conversely, noradrenaline-reuptake inhibitors, such as atomoxetine, have a pressor effect when peripheral sympathetic neurons are functional, including in patients with preganglionic or premotor sympathetic lesions, such as in multiple system atrophy.97 The acetylcholinesterase inhibitor pyridostigmine exerts only modest pressor effects when used alone,98,99 but seems to have synergistic effects when combined with midodrine or atomoxetine.11,28 Postprandial hypotension can be treated with the α-glucosidase inhibitor acarbose11,28 or caffeine.100 The selection of one drug rather than another, or the use of combinations, depends on the presence of plasma volume depletion, the extent of noradrenergic denervation, the time of day at which the patient has symptoms, the presence and severity of supine hypertension, and the patients’ symptomatic response (table).
Table:
Pharmacological management of orthostatic hypotension
| Mechanism | Recommended dose | Side-effects | GRADE recommendation | FDA approval for orthostatic hypotension | |
|---|---|---|---|---|---|
|
| |||||
| Droxidopa101 | Short-acting synthetic norepinephrine precursor | 100–600 mg 3 times a day (morning, midday, and 3–4 h before going to bed) or tailored to the patients’ needs | Supine hypertension, headache, nausea, fatigue; use with caution in patients with congestive heart failure or chronic renal failure | Ila | Yes |
| Midodrine102 | Short-acting direct α1-adrenergic receptor agonist | 2·5–15 mg 2 or 3 times a day (morning, midday, and 3–4 h before going to bed or tailored to the patients’ needs) | Supine hypertension, piloerection (goose bumps), itchy scalp, urinary retention; use with caution in patients with congestive heart failure or renal failure | Ila | Yes |
| Fludrocortisone96 | Long-acting synthetic mineralocorticoid (volume expansion that increases sodium and water reabsorption) | 0·05–0·2 mg once a day; no benefit with doses higher than 0·2 mg/day | Supine hypertension, hypokalaemia, renal failure, oedema, end organ damage; use with caution in patients with congestive heart failure | IIb | No |
| Acarbose103 | Short-acting α-glycosidase inhibitor | 50–150 mg before meals to prevent postprandial hypotension | Abdominal gas, bloating | IIc | No |
| Atomoxetine97 | Short-acting norepinephrine reuptake inhibitor | 10–18 mg twice a day | Supine hypertension, insomnia, irritability, decreased appetite | IIc | No |
| Pyridostigmine98,99 | Short-acting acetylcholinesterase inhibitor | 30–60 mg 2 or 3 times a day | Abdominal cramps, diarrhoea, sialorrhoea, excessive sweating, urinary incontinence | IIc | No |
Evidence graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) recommendation.104
Supine hypertension is common in orthostatic hypotension and can be aggravated by the treatment of orthostatic hypotension, and aiming to avoid it is likely to result in orthostatic hypotension remaining undertreated. The risks of orthostatic hypotension in some patients can be greater than the risks of supine hypertension, which might not be similar in severity and scope to those of essential hypertension. When symptomatic, treatment of orthostatic hypotension should be prioritised, and moderate supine hypertension tolerated. However, severe supine hypertension is associated with increased risk of cardiovascular adverse events and shorter survival.11,45,46 When considerable supine hypertension persists in patients with neurogenic orthostatic hypotension, despite head-up sleeping and avoidance of long-acting antihypotensive drugs, treatment with short-acting antihypertensive agents before bed might be considered, with the caveat that such treatment could exacerbate orthostatic symptoms if the patients gets up from bed at night.6,89,90
Conclusions and future directions
Orthostatic hypotension is common, particularly among patients 60 years and older and patients with neurodegenerative disorders causing baroreflex dysfunction, spinal cord disorders, and small fibre neuropathies, and is associated with impaired quality of life and adverse outcomes. Management of orthostatic hypotension needs a stepwise, individualised approach consisting of precise history taking and measurement of orthostatic blood pressure and heart rate changes and, in dialogue with the patient, tailored therapy. The primary management approach is non-pharmacological.
Our understanding of many aspects of the management of orthostatic hypotension is poor, and more research is required. Studies with large-scale prospective population cohorts are needed to establish the prognosis of patients with all four subtypes of orthostatic hypotension. These studies should incorporate beat-to-beat blood pressure measurements with standardised standing protocols to fully characterise the initial response to standing. Specific investigations should also focus on the value of ambulatory blood pressure monitoring to identify orthostatic hypotension and supine hypertension during activities of daily living and thereby guide therapy. Advanced profiling of the parameters that determine blood pressure105 could considerably improve our understanding of the orthostatic hypotension subtypes and provide biomarker signatures of future adverse events. Efforts should be made to provide evidence-based recommendations for the treatment of neurogenic orthostatic hypotension. For non-pharmacological interventions, researchers could take advantage of multiple n-of-1 clinical trials. Long-term follow-up studies are needed to establish the safety of pharmacological treatment of neurogenic orthostatic hypotension. The evaluation of strategies to manage supine hypertension in patients with orthostatic hypotension, and the effect of these strategies on both orthostatic symptoms and long-term cardiovascular risks, are important pressing issues. Future treatments should ideally selectively increase blood pressure in periods of orthostatic stress. Potential avenues include posture-responsive interventions (eg, compression garments106 or spinal cord stimulation107) to slow and delay the occurrence of orthostatic hypotension, therefore offering more time to accommodate the symptoms. Additionally, biomarkers to predict the conversion of isolated neurogenic orthostatic hypotension to Parkinson’s disease and related synucleinopathies38,39,56 need validation to ascertain their utility and accelerate the development of disease-modifying therapies.16
Search strategy and selection criteria.
We searched PubMed and OVID for papers published in English from March 1, 2017, to March 1, 2022, using the keywords “orthostatic hypotension”, “initial orthostatic hypotension”, “postural hypotension”, or “orthostatic blood pressure”. The resulting articles were screened for relevance to the topic and rigour of their approach. We focused on seminal work, clinical studies with the highest level of evidence, and recent systematic reviews and meta-analyses.
Acknowledgments
We thank Rogier Trompert for preparing the illustrations in figures 1, 2, and 4.
Footnotes
Declaration of interests
HK is supported by grants from the Familial Dysautonomia Foundation, the Michael J Fox Foundation for Parkinson’s Research, the Multiple System Atrophy Coalition, the National Institutes of Health (NIH; R01HL103988 and U54NS065736), and the US Food and Drug Administration (FDR3731-01); receives royalties from Up to Date; receives consultancy and speakers’ fees from Med-IQ, Biogen, Biohaven Pharmaceuticals, Lundbeck, Pfizer, and Theravance Biopharma; and serves as the editor-in-chief of Clinical Autonomic Research. VEC is supported by grants from the Canadian Institutes of Health Research, the National Sciences and Engineering Research Council of Canada, the International Collaboration on Repair Discoveries, the Heart and Stroke Foundation of Canada, and the Craig H Neilsen Foundation. SPJ is supported by NIH grants K23HL135273 and R56HL153191. RDT reports consultancy and speakers’ fees from Union Chimique Belge, GlaxoSmithKline, Theravance, Novartis, and Zogenix; and grants from the Dutch National Epilepsy Fund, the Michael J Fox Foundation for Parkinson’s Research (MJFF-020200), Christelijke Vereniging voor de Verpleging van Lijders aan Epilepsie, Medtronic, New Life Wearables, and The Netherlands Organisation for Health Research and Development (114025101). All other authors declare no competing interests.
Contributor Information
Wouter Wieling, Department of Internal Medicine, Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands.
Horacio Kaufmann, Department of Neurology, New York University School of Medicine, New York, NY, USA.
Victoria E Claydon, Department of Biomedical Physiology and Kinesiology, Simon Fraser University, Burnaby, BC, Canada.
Veera K van Wijnen, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Mark P M Harms, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Stephen P Juraschek, Department of Medicine, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, MA, USA.
Roland D Thijs, Department of Neurology, Leiden University Medical Centre, Leiden, Netherlands; UCL Queen Square Institute of Neurology, University College London, London, UK; Stichting Epilepsie Instellingen Nederland, Heemstede, Netherlands.
References
- 1.Saedon NI, Pin Tan M, Frith J. The prevalence of orthostatic hypotension: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2020; 75: 117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torabi P, Ricci F, Hamrefors V, Sutton R, Fedorowski A. Classical and delayed orthostatic hypotension in patients with unexplained syncope and severe orthostatic intolerance. Front Cardiovasc Med 2020; 7: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palma JA, Kaufmann H. Epidemiology, diagnosis, and management of neurogenic orthostatic hypotension. Mov Disord Clin Pract 2017; 4: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedorowski A, Ricci F, Hamrefors V, et al. Orthostatic hypotension: management of a complex, but common, medical problem. Circ Arrhythm Electrophysiol 2022; 15: e010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mol A, Bui Hoang PTS, Sharmin S, et al. Orthostatic hypotension and falls in older adults: a systematic review and meta-analysis. J Am Med Dir Assoc 2019; 20: 589–97.e5. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol 2017; 264: 1567–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RM. Prevalence of orthostatic hypotension in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 2011; 17: 724–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Ke SJ, Qiu XP, Liu LB. Prevalence, risk factors, and prognosis of orthostatic hypotension in diabetic patients: a systematic review and meta-analysis. Medicine 2017; 96: e8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma 2006; 23: 1713–25. [DOI] [PubMed] [Google Scholar]
- 10.Palma JA, Gonzalez-Duarte A, Kaufmann H. Orthostatic hypotension in hereditary transthyretin amyloidosis: epidemiology, diagnosis and management. Clin Auton Res 2019; 29 (suppl 1): 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibao CA, Biaggioni I. Management of orthostatic hypotension, postprandial hypotension, and supine hypertension. Semin Neurol 2020; 40: 515–22. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann H, Norcliffe-Kaufmann L, Palma JA. Baroreflex dysfunction. N Engl J Med 2020; 382: 163–78. [DOI] [PubMed] [Google Scholar]
- 13.van Wijnen VK, Finucane C, Harms MPM, et al. Noninvasive beat-to-beat finger arterial pressure monitoring during orthostasis: a comprehensive review of normal and abnormal responses at different ages. J Intern Med 2017; 282: 468–83. [DOI] [PubMed] [Google Scholar]
- 14.Finucane C, van Wijnen VK, Fan CW, et al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin Auton Res 2019; 29: 427–41. [DOI] [PubMed] [Google Scholar]
- 15.Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness. Clin Auton Res 2021; 31: 369–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharabi Y, Vatine GD, Ashkenazi A. Parkinson’s disease outside the brain: targeting the autonomic nervous system. Lancet Neurol 2021; 20: 868–76. [DOI] [PubMed] [Google Scholar]
- 17.van Dijk JG, van Rossum IA, Thijs RD. Timing of circulatory and neurological events in syncope. Front Cardiovasc Med 2020; 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman R, Illigens BMW, Lapusca R, et al. Symptom recognition is impaired in patients with orthostatic hypotension. Hypertension 2020; 75: 1325–32. [DOI] [PubMed] [Google Scholar]
- 19.Tipton PW, Cheshire WP. Mechanisms underlying unawareness of neurogenic orthostatic hypotension. Clin Auton Res 2020; 30: 279–81. [DOI] [PubMed] [Google Scholar]
- 20.Hartog LC, Schrijnders D, Landman GWD, et al. Is orthostatic hypotension related to falling? A meta-analysis of individual patient data of prospective observational studies. Age Ageing 2017; 46: 568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finucane C, O’Connell MD, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J Am Geriatr Soc 2017; 65: 474–82. [DOI] [PubMed] [Google Scholar]
- 22.Brignole M, Moya A, de Lange FJ, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018; 39: 1883–948. [DOI] [PubMed] [Google Scholar]
- 23.Merola A, Romagnolo A, Rosso M, et al. Orthostatic hypotension in Parkinson’s disease: does it matter if asymptomatic? Parkinsonism Relat Disord 2016; 33: 65–71. [DOI] [PubMed] [Google Scholar]
- 24.Merola A, Romagnolo A, Rosso M, et al. Autonomic dysfunction in Parkinson’s disease: a prospective cohort study. Mov Disord 2018; 33: 391–97. [DOI] [PubMed] [Google Scholar]
- 25.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci 2011; 161: 46–48. [DOI] [PubMed] [Google Scholar]
- 26.Fedorowski A, Hamrefors V, Sutton R, et al. Do we need to evaluate diastolic blood pressure in patients with suspected orthostatic hypotension? Clin Auton Res 2017; 27: 167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Wijnen VK, Harms MPM, Wieling W. Orthostatic hypotension in the first minute after standing up: what is the clinical relevance and do symptoms matter? Hypertension 2018; 71: 816–18. [DOI] [PubMed] [Google Scholar]
- 28.Palma JA, Kaufmann H. Management of orthostatic hypotension. Continuum 2020; 26: 154–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Wijnen VK, Harms MP, Go-Schon IK, et al. Initial orthostatic hypotension in teenagers and young adults. Clin Auton Res 2016; 26: 441–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Twist DJL, Dinh T, Bouwmans EME, Kroon AA. Initial orthostatic hypotension among patients with unexplained syncope: an overlooked diagnosis? Int J Cardiol 2018; 271: 269–73. [DOI] [PubMed] [Google Scholar]
- 31.Tzur I, Barchel D, Khateb Z, Swarka M, Izhakian S, Gorelik O. Delayed versus classic orthostatic hypotension: clinical and prognostic implications. Blood Press 2020; 29: 209–19. [DOI] [PubMed] [Google Scholar]
- 32.Byun JI, Moon J, Kim DY, et al. Delayed orthostatic hypotension: severity of clinical symptoms and response to medical treatment. Auton Neurosci 2018; 213: 81–85. [DOI] [PubMed] [Google Scholar]
- 33.Palma JA, Gomez-Esteban JC, Norcliffe-Kaufmann L, et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord 2015; 30: 639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centi J, Freeman R, Gibbons CH, Neargarder S, Canova AO, Cronin-Golomb A. Effects of orthostatic hypotension on cognition in Parkinson disease. Neurology 2017; 88: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guaraldi P, Poda R, Calandra-Buonaura G, et al. Cognitive function in peripheral autonomic disorders. PLoS One 2014; 9: e85020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espay AJ, Marsili L, Mahajan A, et al. Rivastigmine in Parkinson’s disease dementia with orthostatic hypotension. Ann Neurol 2021; 89: 91–98. [DOI] [PubMed] [Google Scholar]
- 37.Cheshire WP Jr, Goldstein DS. The physical examination as a window into autonomic disorders. Clin Auton Res 2018; 28: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufmann H, Norcliffe-Kaufmann L, Palma JA, et al. Natural history of pure autonomic failure: a United States prospective cohort. Ann Neurol 2017; 81: 287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coon EA, Mandrekar JN, Berini SE, et al. Predicting phenoconversion in pure autonomic failure. Neurology 2020; 95: e889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheshire WP, Freeman R, Gibbons CH, et al. Electrodiagnostic assessment of the autonomic nervous system: a consensus statement endorsed by the American Autonomic Society, American Academy of Neurology, and the International Federation of Clinical Neurophysiology. Clin Neurophysiol 2021; 132: 666–82. [DOI] [PubMed] [Google Scholar]
- 41.Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med 2017; 177: 1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juraschek SP, Taylor AA, Wright JT Jr, et al. Orthostatic hypotension, cardiovascular outcomes, and adverse events: results from SPRINT. Hypertension 2020; 75: 660–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Twist DJL, Harms MPM, van Wijnen VK, et al. Diagnostic criteria for initial orthostatic hypotension: a narrative review. Clin Auton Res 2021; 31: 685–98. [DOI] [PubMed] [Google Scholar]
- 44.Fanciulli A, Jordan J, Biaggioni I, et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res 2018; 28: 355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palma JA, Redel-Traub G, Porciuncula A, et al. The impact of supine hypertension on target organ damage and survival in patients with synucleinopathies and neurogenic orthostatic hypotension. Parkinsonism Relat Disord 2020; 75: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE. Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol 2016; 15: 954–66. [DOI] [PubMed] [Google Scholar]
- 47.Shaw BH, Borrel D, Sabbaghan K, et al. Relationships between orthostatic hypotension, frailty, falling and mortality in elderly care home residents. BMC Geriatr 2019; 19: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Jong JSY, Blok MRS, Thijs RD, et al. Diagnostic yield and accuracy in a tertiary referral syncope unit validating the ESC guideline on syncope: a prospective cohort study. Europace 2021; 23: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen SW, Wang YK, Dou RH, et al. Characteristics of the 24-h ambulatory blood pressure monitoring in patients with Parkinson’s disease—the SFC BP multicentre study in China. J Hypertens 2020; 38: 2270–78. [DOI] [PubMed] [Google Scholar]
- 50.Ghazi L, Drawz PE, Pajewski NM, Juraschek SP. The association of orthostatic hypotension with ambulatory blood pressure phenotypes in SPRINT. Am J Hypertens 2021; 34: 511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palma JA, Norcliffe-Kaufmann L, Kaufmann H. An orthostatic hypotension mimic: the inebriation-like syndrome in Parkinson disease. Mov Disord 2016; 31: 598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fanciulli A, Campese N, Goebel G, et al. Association of transient orthostatic hypotension with falls and syncope in patients with Parkinson disease. Neurology 2020; 95: e2854–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fanciulli A, Kerer K, Leys F, et al. Validation of the neurogenic orthostatic hypotension ratio with active standing. Ann Neurol 2020; 88: 643–45. [DOI] [PubMed] [Google Scholar]
- 54.Norcliffe-Kaufmann L, Kaufmann H, Palma JA, et al. Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol 2018; 83: 522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leys F, Fanciulli A, Ndayisaba JP, Granata R, Struhal W, Wenning GK. Cardiovascular autonomic function testing in multiple system atrophy and Parkinson’s disease: an expert-based blinded evaluation. Clin Auton Res 2020; 30: 255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singer W, Schmeichel AM, Shahnawaz M, et al. Alpha-synuclein oligomers and neurofilament light chain predict phenoconversion of pure autonomic failure. Ann Neurol 2021; 89: 1212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibbons CH, Freeman R. Clinical implications of delayed orthostatic hypotension: a 10-year follow-up study. Neurology 2015; 85: 1362–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mol A, Slangen LRN, van Wezel RJA, Maier AB, Meskers CGM. Orthostatic blood pressure recovery associates with physical performance, frailty and number of falls in geriatric outpatients. J Hypertens 2021; 39: 101–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doyle K, Lavan A, Kenny RA, Briggs R. Delayed blood pressure recovery after standing independently predicts fracture in community-dwelling older people. J Am Med Dir Assoc 2021; 22: 1235–41.e1. [DOI] [PubMed] [Google Scholar]
- 60.Juraschek SP, Lipsitz LA, Beach JL, Mukamal KJ. Association of orthostatic hypotension timing with clinical events in adults with diabetes and hypertension: results from the ACCORD trial. Am J Hypertens 2019; 32: 684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angelousi A, Girerd N, Benetos A, et al. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens 2014; 32: 1562–71. [DOI] [PubMed] [Google Scholar]
- 62.Hamrefors V, Härstedt M, Holmberg A, et al. Orthostatic hypotension and elevated resting heart rate predict low-energy fractures in the population: The Malmö Preventive Project. PLoS One 2016; 11: e0154249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rawlings AM, Juraschek SP, Heiss G, et al. Association of orthostatic hypotension with incident dementia, stroke, and cognitive decline. Neurology 2018; 91: e759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Min M, Shi T, Sun C, et al. The association between orthostatic hypotension and cognition and stroke: a meta-analysis of prospective cohort studies. Blood Press 2020; 29: 3–12. [DOI] [PubMed] [Google Scholar]
- 65.Briggs R, Carey D, Kennelly SP, Kenny RA. Longitudinal association between orthostatic hypotension at 30 seconds post-standing and late-life depression. Hypertension 2018; 71: 946–54. [DOI] [PubMed] [Google Scholar]
- 66.Juraschek SP, Daya N, Appel LJ, et al. Orthostatic hypotension and risk of clinical and subclinical cardiovascular disease in middle-aged adults. J Am Heart Assoc 2018; 7: e008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehta T, McClure LA, White CL, Taylor A, Benavente OR, Lakshminarayan K. Effect of postural hypotension on recurrent stroke: Secondary Prevention of Small Subcortical Strokes (SPS3) study. J Stroke Cerebrovasc Dis 2019; 28: 2124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ricci F, Fedorowski A, Radico F, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J 2015; 36: 1609–17. [DOI] [PubMed] [Google Scholar]
- 69.Juraschek SP, Longstreth WT Jr, Lopez OL, et al. Orthostatic hypotension, dizziness, neurology outcomes, and death in older adults. Neurology 2020; 95: e1941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lipsitz LA. Orthostatic hypotension and falls. J Am Geriatr Soc 2017; 65: 470–71. [DOI] [PubMed] [Google Scholar]
- 71.Juraschek SP, Appel LJ, Miller ER 3rd, Mukamal KJ, Lipsitz LA. Hypertension treatment effects on orthostatic hypotension and its relationship with cardiovascular disease. Hypertension 2018; 72: 986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pilotto A, Romagnolo A, Scalvini A, et al. Association of orthostatic hypotension with cerebral atrophy in patients with Lewy body disorders. Neurology 2021; 97: e814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johansson M, Ricci F, Aung N, Sutton R, Melander O, Fedorowski A. Proteomic profiling for cardiovascular biomarker discovery in orthostatic hypotension. Hypertension 2018; 71: 465–72. [DOI] [PubMed] [Google Scholar]
- 74.Goldstein DS, Holmes C, Sharabi Y, Wu T. Survival in synucleinopathies: a prospective cohort study. Neurology 2015; 85: 1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pavy-Le Traon A, Foubert-Samier A, Ory-Magne F, et al. Ambulatory blood pressure and drug treatment for orthostatic hypotension as predictors of mortality in patients with multiple system atrophy. Eur J Neurol 2022; 29: 1025–34. [DOI] [PubMed] [Google Scholar]
- 76.Frith J, Newton JL. Combination non-pharmacologic intervention for orthostatic hypotension in older people: a phase 2 study. Age Ageing 2020; 49: 253–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eschlböck S, Wenning G, Fanciulli A. Evidence-based treatment of neurogenic orthostatic hypotension and related symptoms. J Neural Transm 2017; 124: 1567–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Logan A, Freeman J, Pooler J, et al. Effectiveness of non-pharmacological interventions to treat orthostatic hypotension in elderly people and people with a neurological condition: a systematic review. JBI Evid Synth 2020; 18: 2556–617. [DOI] [PubMed] [Google Scholar]
- 79.Loughlin EA, Judge CS, Gorey SE, et al. Increased salt intake for orthostatic intolerance syndromes: a systematic review and meta-analysis. Am J Med 2020; 133: 1471–78.e4. [DOI] [PubMed] [Google Scholar]
- 80.Newton JL, Frith J. The efficacy of nonpharmacologic intervention for orthostatic hypotension associated with aging. Neurology 2018; 91: e652–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greenhalgh T, Snow R, Ryan S, Rees S, Salisbury H. Six ‘biases’ against patients and carers in evidence-based medicine. BMC Med 2015; 13: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mirza RD, Punja S, Vohra S, Guyatt G. The history and development of N-of-1 trials. J R Soc Med 2017; 110: 330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med 2011; 8: 161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wieling W, Colman N, Krediet CT, Freeman R. Nonpharmacological treatment of reflex syncope. Clin Auton Res 2004; 14 (suppl 1): 62–70. [DOI] [PubMed] [Google Scholar]
- 85.de Bruïne ES, Reijnierse EM, Trappenburg MC, et al. Standing up slowly antagonises initial blood pressure decrease in older adults with orthostatic hypotension. Gerontology 2017; 63: 137–43. [DOI] [PubMed] [Google Scholar]
- 86.Juraschek SP, Hu JR, Cluett JL, et al. Effects of intensive blood pressure treatment on orthostatic hypotension : a systematic review and individual participant-based meta-analysis. Ann Intern Med 2021; 174: 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robinson LJ, Pearce RM, Frith J. Acceptability of non-drug therapies in older people with orthostatic hypotension: a qualitative study. BMC Geriatr 2018; 18: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wieling W, van Dijk N, Thijs RD, de Lange FJ, Krediet CT, Halliwill JR. Physical countermeasures to increase orthostatic tolerance. J Intern Med 2015; 277: 69–82. [DOI] [PubMed] [Google Scholar]
- 89.Jordan J, Diedrich A, Grassi G, Tank J. Living with severe orthostatic hypotension. J Hypertens 2016; 34: 1942–44. [DOI] [PubMed] [Google Scholar]
- 90.Jordan J, Tank J, Heusser K, Reuter H, Biaggioni I. What do we really know about supine hypertension in patients with orthostatic hypotension. Curr Opin Cardiol 2019; 34: 384–89. [DOI] [PubMed] [Google Scholar]
- 91.Williams ELRS, Schondorf R, Shen WK, Wieling W, Claydon VE. Salt supplementation in the management of orthostatic intolerance: vasovagal syncope and postural tachycardia syndrome. Auton Neurosci 2022; 237: 102906. [DOI] [PubMed] [Google Scholar]
- 92.Stock JM, Chelimsky G, Edwards DG, Farquhar WB. Dietary sodium and health: how much is too much for those with orthostatic disorders? Auton Neurosci 2022; 238: 102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wieling W, Raj SR, Thijs RD. Are small observational studies sufficient evidence for a recommendation of head-up sleeping in all patients with debilitating orthostatic hypotension? MacLean and Allen revisited after 70 years. Clin Auton Res 2009; 19: 8–12. [DOI] [PubMed] [Google Scholar]
- 94.Fan CW, Walsh C, Cunningham CJ. The effect of sleeping with the head of the bed elevated six inches on elderly patients with orthostatic hypotension: an open randomised controlled trial. Age Ageing 2011; 40: 187–92. [DOI] [PubMed] [Google Scholar]
- 95.Fanciulli A, Goebel G, Metzler B, et al. Elastic abdominal binders attenuate orthostatic hypotension in Parkinson’s disease. Mov Disord Clin Pract 2015; 3: 156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Veazie S, Peterson K, Ansari Y, et al. Fludrocortisone for orthostatic hypotension. Cochrane Database Syst Rev 2021; 5: CD012868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shibao CA, Palma JA, Celedonio JE, Martinez J, Kaufmann H, Biaggioni I. Predictors of the pressor response to the norepinephrine transporter inhibitor, atomoxetine, in neurogenic orthostatic hypotension. Hypertension 2021; 78: 525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry 2003; 74: 1294–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shibao C, Okamoto LE, Gamboa A, et al. Comparative efficacy of yohimbine against pyridostigmine for the treatment of orthostatic hypotension in autonomic failure. Hypertension 2010; 56: 847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Furukawa K, Suzuki T, Ishiguro H, et al. Prevention of postprandial hypotension-related syncope by caffeine in a patient with long-standing diabetes mellitus. Endocr J 2020; 67: 585–92. [DOI] [PubMed] [Google Scholar]
- 101.Biaggioni I, Arthur Hewitt L, Rowse GJ, Kaufmann H. Integrated analysis of droxidopa trials for neurogenic orthostatic hypotension. BMC Neurol 2017; 17: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wright RA, Kaufmann HC, Perera R, et al. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology 1998; 51: 120–24. [DOI] [PubMed] [Google Scholar]
- 103.Shibao C, Gamboa A, Diedrich A, et al. Acarbose, an alpha-glucosidase inhibitor, attenuates postprandial hypotension in autonomic failure. Hypertension 2007; 50: 54–61. [DOI] [PubMed] [Google Scholar]
- 104.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Dijk JG, Ghariq M, Kerkhof FI, et al. Novel methods for quantification of vasodepression and cardioinhibition during tilt-induced vasovagal syncope. Circ Res 2020; 127: e126–38. [DOI] [PubMed] [Google Scholar]
- 106.Okamoto LE, Diedrich A, Baudenbacher FJ, et al. Efficacy of servo-controlled splanchnic venous compression in the treatment of orthostatic hypotension: a randomized comparison with midodrine. Hypertension 2016; 68: 418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Squair JW, Berney M, Castro Jimenez M, et al. Implanted system for orthostatic hypotension in multiple-system atrophy. N Engl J Med 2022; 386: 1339–44. [DOI] [PubMed] [Google Scholar]


