Abstract
For more than a century, antibody has been used for passive parenteral immunization against viral and bacterial pathogens. This approach has been successful for prevention of viral respiratory infection and has led to testing of intranasal or aerosol delivery of antibody to passively immunize the respiratory tract mucosal surface. Mucosal delivery may be advantageous because it allows the antibody to neutralize the virus particles before they initiate infection and because it concentrates the antibody where viral replication takes place. Animal studies have shown the feasibility of passive intranasal immunization against a number of respiratory tract viruses. Development of nasal antibody treatments for humans is under way, and early clinical studies have confirmed that this approach is safe and can be used to prevent respiratory tract disease. Polyclonal human immunoglobulin from pooled plasma preparations can be used to provide broad protection against a number of different pathogens, while monoclonal antibodies or their fragments can be used to target specific viruses.
Acute viral infections of the respiratory tract are responsible for a substantial share of human disease worldwide. Medical interventions against viral respiratory tract disease include vaccination, treatment with antiviral drugs, and treatment of disease symptoms. Vaccine development has been hampered by antigenic variation among virus strains, lack of effective or long-lasting immune responses, and, in some cases, vaccine-induced immunopotentiation of disease. Few antiviral drugs are available for respiratory tract infections, in part because the mild nature of most infections does not warrant the risk of side effects. Only one drug (amantadine and the related drug rimantadine) is licensed for prophylaxis (of influenza). Antiviral drugs have limited activity against more serious disease (61, 97).
Passive immunization with antibody represents an additional, less frequently considered option that combines beneficial elements of vaccination and drug treatment. Passive immunization can be used for prophylaxis or therapy, its duration of action can be longer than that of drugs, it is effective immediately, and it has few side effects. The use of passive parenteral immunization against viral and bacterial infections in humans began more than a century ago but became less important following the discovery of antibiotics and the development of new vaccines (34, 35, 61). Originally, serum from immunized animals was used for treatment, and both hypersensitivity reactions against and increased clearance of the foreign antibody were considerable problems. More recently, preparations of purified human immunoglobulin G (IgG), which are minimally immunogenic in humans, have become available for passive parenteral immunization (35). Human monoclonal antibodies or murine monoclonal antibodies, which can be humanized to reduce immunogenicity, may be used to provide a high level of neutralizing activity with narrow specificity.
Today, passive parenteral immunization with human blood-derived antibodies is in widespread use for prophylaxis and therapy of infectious diseases with known and unknown causes, including hepatitis A and B, rabies, tick-borne encephalitis, varicella, respiratory syncytial virus (RSV) infection, and Kawasaki syndrome (34, 35, 65). The first monoclonal antibody for prophylaxis of an infectious disease (RSV infection) was licensed in 1998. Antibody may be most effective against viral infections when given prophylactically rather than therapeutically. Nevertheless, as discussed below, therapeutic antibody treatment may have benefits in selected infections.
The effectiveness of antibody delivery to mucosal surfaces, including the respiratory tract, is under investigation. This strategy may be most useful for treatment of the upper airways, where secretory antibody is most important for protection against viral infection. In this article, we discuss the role of antibody in respiratory tract immunity and review the results of studies testing the antiviral activity of passive intranasal immunization with antibody in humans and animals.
ANTIBODY AS A MEDIATOR OF RESPIRATORY TRACT IMMUNITY
Roles of Immunoglobulins G and A in Antiviral Immunity
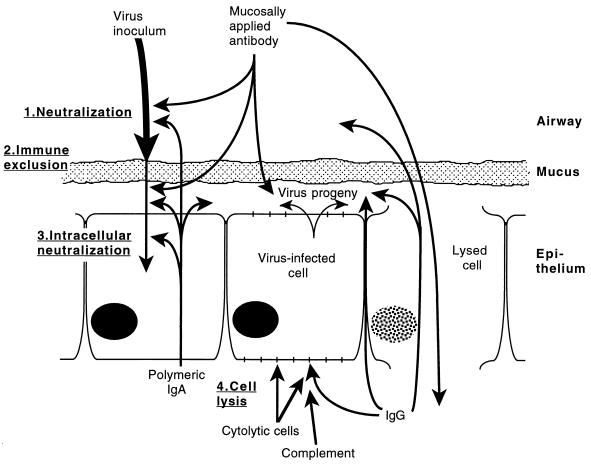
Antibody in mucosal secretions is thought to have two major activities against viral pathogens: (i) immune exclusion, which prevents virus from reaching host target cells, and (ii) direct neutralization of viral infectivity (Fig. 1). Immune exclusion is a hypothetical barrier to infection that combines the activities of antibody and the mucus blanket that covers the epithelium of the respiratory tract (12, 63). Mucus provides a physical barrier that restricts access of the virus to epithelial cells. Antibody cross-links and agglutinates virus particles, further reducing their ability to penetrate mucus. Once trapped in mucus, virus particles are cleared from the respiratory tract as ciliary activity moves the mucus to the nasopharynx (18, 84). Immune exclusion is thought to be most important for preventing virus from establishing an infection, but it could also help prevent the spread of infection within the respiratory tract via secretions. The extent to which immune exclusion is active against viral infection is not known, but lack of ciliary activity is associated with severe and chronic respiratory tract infections, suggesting the importance of the mucous barrier (76).
FIG. 1.
Potential mechanisms of protection against viral infection of the respiratory tract mucosa. Following inoculation, virus particles encounter neutralizing antibody (step 1), which reaches the mucosal surface naturally by transepithelial transport (mostly polymeric IgA) or transudation (mostly IgG) or artificially by nose drop, spray, or aerosol delivery. Immune exclusion (step 2) occurs when virus particles are cross-linked by antibody, trapped in mucus, and removed by mucociliary movement. Antibody can diffuse through mucus to neutralize progeny virus and virus particles that pass through the mucus blanket. Virus neutralization may occur intracellularly during transepithelial transport of polymeric IgA (step 3). At the basolateral surface of infected epithelial cells, specific IgG may bind to virus-encoded membrane proteins, allowing cell lysis by complement or antibody-dependent cytolytic cells (step 4). Virus-infected cells may also be lysed by specific cytolytic T lymphocytes. Cell lysis may allow additional movement of immune system mediators across the epithelium in both directions.
Neutralization occurs when binding of antibody to virus particles prevents them from infecting target cells, either by preventing the interaction of ligands on the viral surface with cell receptors or by impeding internalization or uncoating intracellularly. Virus neutralization is defined in vitro, using cultured cells, but the process is probably critical to the protective activity of antibody in vivo. Neutralizing antibody can limit the initial viral infection but can also be important for elimination of an established infection. Once cells are infected, other mediators of immunity, including innate immunity, specific antibody in serosal fluids, antibody-dependent cellular cytotoxicity, and cytolytic T cells, can also become involved in clearance. Infection can also result in loss of integrity of the epithelial barrier, allowing lymphocytes and systemic antibody to reach the mucosal surface.
In the upper airways, IgA is the major antibody isotype found in secretions (53, 92). The presence of secretory antibody correlates with resistance against a number of respiratory viruses, including parainfluenza virus type 1 (96), influenza virus (51), and RSV (62, 68, 110). IgA is produced in monomeric and polymeric forms, with the principal form being dimeric (63, 113). IgA polymers bind to specific basolateral receptors on epithelial cells and are endocytosed, transported to the apical cell surface, and released into mucosal secretions (14, 66, 98). Transported IgA remains associated with a portion of the cellular receptor, termed the secretory component, that protects the IgA molecule against proteolytic cleavage (15, 106). This transport process appears to be the principal mechanism by which antibody reaches upper-airway secretions (13). In lower-respiratory-tract secretions, IgA and IgG are both present in high concentrations. Antibody probably reaches these secretions in large part by diffusion between cells or through breaks in the epithelium (7).
As described below, a series of animal studies involving passive mucosal immunization have demonstrated that antibody in respiratory tract secretions can prevent, diminish, or cure viral infections. Despite the high prevalence of IgA in natural secretions, IgG antibody was used in most of these studies because it is more readily available. This raises the issue of whether IgA is superior to IgG for respiratory tract protection. IgA has a number of theoretical advantages. Being polymeric, IgA is better at agglutinating antigens than are monomeric antibodies such as IgG, which may facilitate immune exclusion (72). IgA is also less likely to participate in inflammatory processes, since it does not fix complement efficiently (67, 105). Finally, IgA may have a longer duration of activity as a result of secretory component-mediated protection against proteolysis.
Studies have suggested that the mechanisms of virus neutralization by IgG and IgA may be different in some cases. In vitro, polymeric IgA and IgM were found to interfere with early stages of influenza virus infection, preventing binding and penetration of target cells (73, 103). IgG and monomeric IgA (produced by reduction of secretory IgA) interfered with later stages of virus replication (103). An in vivo study, on the other hand, showed equivalent activities of monoclonal IgG and IgA antibodies in protection of the lower respiratory tract of mice against Sendai virus infection (58). The relative efficacy of IgG and IgA in the upper airways has not been addressed experimentally.
An additional feature of IgA is its ability to neutralize virus intracellularly while it is undergoing transepithelial transport. Intracellular neutralization of Sendai and influenza viruses by polymeric IgA has been demonstrated in vitro in experiments with polarized monolayers of epithelial cells (56, 57). Intraepithelial neutralization in mice was suggested by a study in which parenteral but not mucosal delivery of specific IgA protected the animals against rotavirus challenge (16).
Induction of Immunity in Respiratory Tract Lymphoid Tissue
Traditional vaccines and DNA vaccines are usually delivered parenterally to stimulate circulating antibody responses and cell-mediated immunity. More recently, the importance of secretory immunity has been recognized, along with the realization that mucosal responses are elicited most strongly when antigen is delivered to a mucosal surface (60). The oral, nasal, rectal, and vaginal routes of antigen delivery are effective in inducing mucosal and in many cases systemic immune responses. Nasal delivery has been found to be particularly effective for induction of mucosal and systemic immunity (42, 107, 112).
The understanding of mucosal immunity has evolved largely through studies of the intestinal tract, but similar processes appear to be present at most mucosal sites. Induction of mucosal immunity begins when antigen contacts lymphoid cells in or below the epithelium (55). This can occur when viruses infect and kill epithelial cells, but antigen can also cross intact epithelia via specialized antigen-transporting epithelial cells, termed M cells (71). In the gut, M cells are associated with lymphoid follicles in the small intestine, colon, and rectum (9, 50, 74); similar cells are found overlying lymphoid follicles in the respiratory tract (8, 49). Antigen that crosses the epithelium is processed in mucosal lymphoid tissue, eventually leading to production of activated B lymphoblasts, which take up residence at mucosal sites and differentiate into IgA-secreting plasma cells (60). It appears that most polymeric IgA transported into secretions is produced locally by subepithelial plasma cells (63).
In the lower respiratory tract, lymphoid follicles are found at sites of airway bifurcation (8). In the human nasopharynx, lymphoid follicles are found in organized structures such as the tonsils and adenoids (49). Rodents do not have tonsils or similar structures but instead have lymphoid tissue collected under the epithelium at the back of the nasal passages (49, 99). While antigen transport at these sites is believed to lead to secretory immune responses, a significant amount of soluble antigen can cross the nasal epithelium at other sites, leading to systemic immunity or tolerance (49).
Active versus Passive Intranasal Immunization To Prevent Human Viral Diseases
Ideally, a vaccine will provide cost-effective long-term protection, but this is often an elusive goal for a number of reasons. Many vaccines are poorly immunogenic or stimulate immunity with a short duration of activity. In some cases, a strong but inappropriate immune response is elicited, which can be ineffective or harmful, as was found with formalin-inactivated RSV (46, 47) and measles (70) vaccines. Even if effective for the general population, vaccines can elicit poor responses in the very young, the elderly, or immunosuppressed individuals. When effective vaccination is not possible, passive immunization may be a practical alternative, particularly when the period of virus exposure is known or the populations at greatest risk of disease can be identified.
Traditionally, serum from immunized horses or other animals has been used for parenteral passive immunization (48). These high-titer preparations are effective but elicit an immune response that does not allow them to be used repeatedly or for a long duration. This problem has been partially addressed by the use of F(ab′)2 fragments of equine globulin, eliminating the Fc region, which is responsible for immune-complex formation. Commercially available preparations of IgG purified from pooled human plasma have been available for over 50 years. Formulations without IgG aggregates (called intravenous immune globulin [IVIG]), which are designed for intravenous use, were introduced more recently (35). IVIG preparations have significant levels of antibody against common human pathogens and can be used to treat a variety of specific infectious diseases as well as diseases of unknown etiology. While IVIG is broadly reactive, the level of antibody against any particular species or strain of pathogen may be too low to be effective. Greater activity can be achieved by screening plasma donors to increase the titer of antibody in plasma pools or, potentially, by immunizing plasma donors.
The highest specific activity against a pathogen is provided by monoclonal antibodies, but reactivity across serotypes or antigenic variants might be limited. Therefore, it may be necessary to use a mixture of several different monoclonal antibodies to protect against a significant number of virus strains. Unlike plasma-derived antibodies, the structure of monoclonal antibodies can be altered through genetic engineering techniques. Recent advances allow humanization of mouse monoclonal antibodies, selection of active human antibody fragments from combinatorial libraries, construction of novel antibody fragments, and selection of antibody isotype (10).
Immunoprophylaxis of the respiratory tract can be achieved by either parenteral or mucosal delivery of antibody. Parenteral delivery has the advantage of providing relatively long-lasting immunity. Mucosal delivery provides shorter-term protection but has several advantages. For treatment of the upper airways, antibody can be delivered by nose drops or spray, methods that can be performed in the home and require modest amounts of antibody. Delivery of antibody into the lungs is possible with small-particle aerosol generators, which have become simpler to use in recent years. Safety concerns are fewer with mucosally delivered antibody, since the respiratory tract mucosa is regularly exposed to foreign material. Immunity against nonhuman antibody will develop after long-term use, but problems associated with parenteral treatment, such as serum sickness, would not be expected. With mucosal delivery, viral contamination of blood products may be of less concern and other risks associated with parenteral delivery, such as fluid overload, are eliminated. Finally, antibody present on mucosal surfaces is more likely to encounter virus at an early stage of infection and, in some cases, could prevent the initiation of infection. The duration of protection following passive mucosal immunization has yet to be firmly established, but a nose drop or aerosol dose of antibody probably remains active for longer than 24 h (as shown by studies described below).
Treatment of Established Infections
There are few options available for treatment of viral infections once symptoms of the disease have manifested themselves. Ribavirin and amantadine (or rimantadine) have limited activities against RSV infection and influenza, respectively (97). Passive antibody immunization is a potentially attractive alternative to antiviral drugs for therapy. Animal studies show that parenteral or mucosal delivery of antibody can suppress viral replication, but it is not clear whether this would be sufficient to cause a significant reduction in disease once infection is well established. As described below, a combination of passive immunization and treatment with an anti-inflammatory agent may be a useful approach to immunotherapy.
PASSIVE NASAL IMMUNIZATION STUDIES WITH ANIMAL MODELS OF VIRAL INFECTION
Influenza Virus
Animal models of infection have allowed testing of nasally delivered antibody for protection of the respiratory tract against a variety of viruses (Table 1). One of the first to be tested was influenza virus. In humans, influenza virus is transmitted primarily in aerosols generated by coughing or sneezing (69). The virus remains viable in aerosol particles that are small enough (less than 5 μm) to be inhaled into the smaller airways of the lungs. As a result, infection is initiated throughout the entire respiratory tract. Although the currently licensed vaccines for influenza virus are parenterally delivered, it has become clear in recent years that secretory antibody plays a major role in protection against infection (86). Furthermore, secretory IgA cross-protects against heterologous antigenic drift viruses better than circulating IgG does (51).
TABLE 1.
Animal studies of prophylactic mucosal antibody treatment for respiratory viruses
| Virus | Animal model | Treatmenta | Outcome | Reference(s) |
|---|---|---|---|---|
| Influenza virus | Mouse | Human gamma globulin | Decreased mortality | 2, 3 |
| Mouse | Goat antiserum in liposomes | Decreased mortality | 115 | |
| Mouse | Mouse lung wash | Virus titers reduced | 101 | |
| Mouse | Human IgG or F(ab′)2 fragments | Decreased mortality | 85 | |
| Mouse | Monoclonal IgA (i.v.)b | Virus titers reduced | 87 | |
| Sendai virus | Mouse | Monoclonal IgA or IgG | IgG and IgA both reduced virus titers | 58, 59 |
| RSV | Cotton rat | Human IgG | Virus titers reduced | 78 |
| Cotton rat | Monoclonal IgG | Virus titers reduced | 5 | |
| Mouse | Human IgG, lung or nose-only delivery | Virus titers reduced | 31 | |
| Mouse | Humanized monoclonal IgG | Virus titers reduced | 104 | |
| Mouse | Monoclonal IgA | Virus titers reduced | 111 | |
| Mouse | CDR peptidec | Virus titers reduced | 11 | |
| Rhesus monkey | Monoclonal IgA, nose-only delivery | Virus titers reduced | 114 |
Intranasal treatment with aspiration into the lungs, unless nose-only delivery is indicated.
Polymeric IgA injected intravenously (i.v.) was transported into lung secretions.
Complementarity-determining region (CDR) of a virus-neutralizing monoclonal antibody.
In mice, human gamma globulin delivered intranasally before or after influenza virus challenge protects against lethal infection (2). Intranasal treatment is very efficient, requiring an antibody dose of 50 mg/kg for protection, while parenteral delivery requires 2,000 to 3,000 mg/kg for a similar effect (3). Liposome encapsulation of antibody enhances its efficacy (115). F(ab′)2 fragments of human IgG are as protective as intact antibody when delivered intranasally, showing that complement fixation or other Fc region-dependent processes are not required for activity (85). The protective capacity of secretory IgA isolated from respiratory tract washings of influenza virus hemagglutinin-immunized mice has also been assessed (101). Intranasal delivery of 600 ng of IgA 3 h before viral challenge provides almost complete protection against pulmonary infection with the influenza virus strain bearing the hemagglutinin used for immunization and partial protection against viruses with a hemagglutinin from an antigenic drift strain.
Monoclonal antibodies to influenza virus have been used to demonstrate the protective effect of secretory antibody in the upper respiratory tract. Renegar and Small (87) compared the protective effects of monomeric IgA, polymeric IgA, and IgG monoclonal antibodies against influenza virus hemagglutinin. The antibodies were delivered parenterally in that study, but polymeric IgA appeared in nasal secretions subsequently, presumably as a result of transepithelial transport. In contrast, little IgG or monomeric IgA could be detected in nasal secretions following parenteral delivery. When the mice were challenged with influenza virus 4 h after injection of the antibody, polymeric IgA was much more protective than IgG against upper respiratory tract infection. Replication of virus in the lower respiratory tract, where IgG might have been more active, was not examined.
Sendai Virus
Sendai virus (mouse parainfluenza virus type 1) is a paramyxovirus that is indigenous to mice. In humans, parainfluenza virus type 1 infection is confined largely to the nose and throat, but in mice, Sendai virus infects the lower respiratory tract if delivered into the lungs via the nasal route. Mazanec et al. (59) tested whether intranasally delivered monoclonal IgA antibodies protect mice against Sendai virus challenge. A panel of monoclonal IgA antibodies was produced by orally immunizing mice with Sendai virus plus cholera toxin adjuvant and fusing immune spleen cells with myeloma cells. Ascitic fluids containing two neutralizing antibodies were pooled for testing in mice, and a nonspecific monoclonal IgA myeloma antibody was used as a control. Antibody (50 μl given once or twice) was delivered via the intranasal route to the lungs of mice, which were challenged by the same route with Sendai virus 30 min to 24 h later. An additional dose of antibody was delivered 4 or 48 h after the challenge. Titer determination of virus in lung tissue homogenates 3 days after challenge showed a reduction in virus replication in specific antibody-treated mice compared to controls.
In a subsequent study, the same group studied the importance of antibody isotype and polymeric form (58). Passive intranasal immunizations with IgG1, IgG2a, IgG2b, and IgA monoclonal antibodies, as well as monomeric and polymeric fractions of monoclonal IgA, were compared in mice. Hybridoma ascitic fluid was diluted to contain equivalent anti-Sendai virus activity, as determined by an enzyme-linked immunosorbent assay, rather than equivalent antibody concentration. When antibody was delivered intranasally to anesthetized mice 1 h before and 4 and 24 h after intranasal challenge with Sendai virus, no significant differences were found between the protective activities of IgA and IgG, nor was there any difference between monomeric and polymeric IgA. Virus replication was assessed in the lungs but not in the upper airways. Taken together, the results of these studies with influenza and parainfluenza viruses suggest that the antibody isotype may not be critical to the efficacy of topically administered antibodies but that IgA is the principal mediator of mucosal defense after active immunization.
Respiratory Syncytial Virus
RSV, another paramyxovirus, is the major cause of lower respiratory tract disease in human infants and young children worldwide (20, 75). Outbreaks of RSV occur each year during the winter months, causing mild upper airway disease in most healthy children and adults. In about 40% of children experiencing their first RSV infection, however, the virus spreads to the lower respiratory tract, causing bronchiolitis or pneumonia, and approximately 1% of these cases result in hospitalization (64). In the United States, RSV infection results in the hospitalization of 91,000 children per year (38). At highest risk of lower respiratory disease are infants with certain underlying conditions such as premature birth, bronchopulmonary dysplasia, or congenital heart disease (20, 64). Also at high risk are the elderly and people with deficient immunity (1, 26).
It has been over 40 years since RSV was identified as a human pathogen, yet there is still no licensed vaccine against it. One reason for this is the generally poor human immune response to RSV. Adult volunteers, for instance, could be reinfected with RSV as soon as 2 months after a previous infection (33). Another barrier to vaccine development is the potential for vaccine-enhanced illness. When a formalin-inactivated vaccine against RSV was tested during the 1960s, vaccine recipients suffered more severe disease during the following RSV season than those who received a placebo (46, 47). The reason for this is not yet completely understood, but in mice, immunization with formalin-inactivated RSV preferentially stimulates the Th2 subclass of helper T cells, which leads to greater inflammatory responses in the lungs upon infection with RSV (4, 23, 30, 109).
RSV replicates in respiratory tract tissues of a number of animal species, which has allowed the testing of a variety of passive-immunization strategies. In mice and cotton rats, RSV replicates in the upper and lower airways when the inoculum is delivered intranasally and the animals are anesthetized to allow aspiration of the fluid into the lungs. Passive parenteral immunization with antibody to RSV reduces virus replication in both nasal and pulmonary tissues, although higher doses of antibody are required to protect the nose (80–82, 93). Protection has been demonstrated in mice and cotton rats with transfer of a variety of antibody types, including cotton rat immune serum antibody, monoclonal antibodies to either of the two RSV major surface glycoproteins, and IVIG (25, 29, 45, 80, 81, 102, 104, 108). Although standard IVIG products contain sufficient antibody to RSV to protect animals against infection, a high-titer product (RSVIG), purified from the plasma of selected donors, has been developed for passive immunization of high-risk human infants (39, 95). Parenterally delivered RSVIG is highly protective in cotton rat and mouse models of RSV infection (29, 94). In human trials, monthly intravenous infusion of large doses of RSVIG (750 mg/kg) resulted in a significant reduction in hospitalization (41%) and reduced morbidity in premature infants or those with bronchopulmonary dysplasia (32, 77). RSVIG is marketed by MedImmune, Inc., in the United States and Canada under the name RespiGam.
RSV causes an exclusively mucosal infection. The virus infects the host via the mucosal surface, and replication is confined largely to the epithelial cells (20). In tissue culture, RSV particles bud from the apical membranes of polarized cells, suggesting that viral progeny are shed into secretions in vivo (89). For these reasons, the protective effect of mucosally delivered antibody has been studied. Intranasal (or topical) antibody delivery was first tested in a cotton rat model of RSV infection (78). Cotton rats were anesthetized and treated intranasally with IVIG at 100 mg/kg. Anesthesia was used to ensure that antibody was delivered into the lungs. One or more days after IVIG treatment, the cotton rats were challenged intranasally with RSV. Virus titers in lung tissue homogenates were determined 4 days after the challenge. The protective effect of intranasal IVIG treatment was similar to that of parenteral treatment but required a dose many times lower. The lungs were protected against infection when antibody was delivered as many as 7 days before virus challenge.
The RSV surface protein responsible for cell-to-cell fusion, designated the F glycoprotein, is highly conserved across different strains belonging to the two antigenic subgroups (A and B) of RSV (6). Many monoclonal antibodies to the F glycoprotein neutralize numerous RSV strains and have potential as agents for passive immunization of high-risk infants. A number of monoclonal antibodies against the F glycoprotein are protective when delivered intranasally. In a study with cotton rats, eight different monoclonal antibodies against the F glycoprotein were delivered intranasally prior to virus challenge (5). Most protected against challenge when used at doses as low as 0.1 mg/kg. Delivery of two monoclonal antibodies together resulted in an additive effect, with protection occurring at total antibody doses of 0.01 mg/kg.
RSHZ19 is a monoclonal antibody under development by SmithKline Beecham for passive immunization of humans (104). To produce RSHZ19, the DNA coding for the complementarity-determining regions of a mouse monoclonal antibody against F glycoprotein was cloned and expressed within a human variable-region framework. The resulting humanized IgG1 antibody retains the binding properties of the mouse antibody and neutralizes a variety of RSV strains. In a mouse model of RSV infection, intranasal delivery of 25 μg of RSHZ19 1 day before challenge completely suppressed RSV replication in the lungs. Replication in the nose was not assessed.
MEDI-493 (palivizumab) is a humanized monoclonal antibody against RSV that was developed by MedImmune, Inc. Although designed for parenteral injection, it could potentially be delivered intranasally as well. After the protective level of circulating MEDI-493 in cotton rats had been determined (45), a small-scale human study showed that monthly dosing with 10 to 15 mg/kg is sufficient to maintain the target level of neutralizing antibody (100). In a large-scale clinical trial, treatment of high-risk infants with monthly intramuscular injections of 15 mg of MEDI-493 per kg reduced hospitalization for RSV infection by 55% compared to placebo-treated controls, a highly significant effect (P < 0.001) (44). MEDI-493 was licensed for RSV prophylaxis by the U.S. Food and Drug Administration in June 1998, and is marketed under the trade name Synagis.
HNK20 is a mouse monoclonal IgA antibody against the RSV F glycoprotein and was developed specifically for intranasal passive immunization (111). The rationale for the development of HNK20 was that delivery of antibody by nose drop to high-risk infants could protect the site of initial infection, preventing subsequent spread of infection into the lungs. IgA was chosen as the preferred isotype since it is dominant in upper-airway secretions and is less likely to elicit inflammatory responses at mucosal surfaces.
HNK20 was chosen from a panel of monoclonal antibodies produced by using lymphocytes from the lungs of RSV-immune mice (111). The use of lung lymphocytes maximized the production of IgA-secreting hybridomas. HNK20 was found to neutralize a variety of RSV clinical isolates belonging to antigenic subgroups A and B, with a concentration of about 0.1 μg/ml being required for a 50% reduction in virus plaque numbers. When HNK20 is delivered intranasally to mice 1 to 24 h prior to RSV challenge, virus titers in lung homogenates 4 days later are reduced by up to 2 log10 units. The reduction is maximal when a dose of 0.5 mg/kg is used. Virus titers in nasal tissue homogenates are reduced if antibody is delivered 1 h, but not 24 h, prior to virus challenge (111).
While rodent models have been useful for demonstrating the protective effects of mucosal antibody against RSV, they are less useful for examining the proximal spread of infection within the respiratory tract, because the antibody and virus are generally delivered to the entire respiratory tract. To examine the effect of HNK20 on virus spread from the upper to the lower respiratory tract, we developed a rhesus monkey model of RSV infection (114). In this model, the virus inoculum is delivered in small-volume nose drops (125 μl per nostril) to replicate natural RSV infection of the upper respiratory tract. The monkeys are only lightly sedated during delivery to maintain the gag reflex and prevent aspiration of the inoculum into the lower airways. HNK20 or placebo is delivered in the same manner to mimic nose drop treatment of human infants.
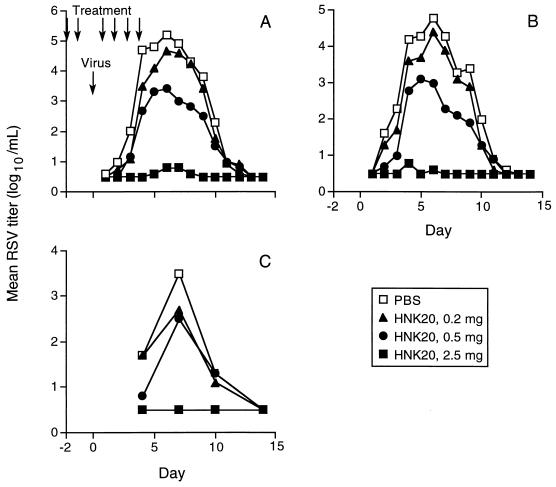
This model was used to test the protective effect of daily HNK20 nose drop treatment (114). The monkeys (six per group) received daily treatments with one of several doses of HNK20 or saline control for 2 days. On the third day, antibody treatment was omitted and intranasal RSV challenge was given instead. On the day after challenge, the daily HNK20 or placebo treatment was resumed and continued for the next 3 days. Nose and throat swab specimens were collected daily, and the RSV titers were determined. In control animals, the virus was first detected in nose and throat samples within 1 to 2 days after challenge, and the levels peaked at 5 log10 units/ml after 6 days (Fig. 2). The virus was almost undetectable in monkeys treated with the highest dose of HNK20 (0.5 mg/kg), while lower doses had intermediate effects. Bronchoalveolar lavages were performed every 3 to 4 days following challenge. The mean virus titer in lavage fluid from control monkeys reached 3.5 log10 units/ml, while no virus was detected in lavage fluid from monkeys treated with the highest dose of HNK20 (Fig. 2). All the monkeys developed neutralizing serum antibody to RSV and were resistant to rechallenge 50 days later.
FIG. 2.
Mean RSV titers in nasal swab (A), throat swab (B), and bronchoalveolar lavage (C) specimens collected from monkeys for 14 days after intranasal virus challenge. The monkeys were treated with nose drops containing 0.2, 0.5, or 2.5 mg of monoclonal IgA HNK20 or an equal volume of phosphate-buffered saline (PBS) placebo. The treatments were given once daily for 2 days before and 4 days after virus challenge, as indicated by the arrows. Samples in which no virus was detected (less than 1 log10 unit/ml) were assigned a titer of 0.5 log10 unit/ml. Reprinted from reference 114 with permission of the publisher.
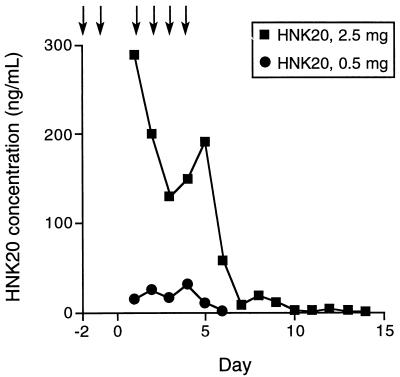
Analysis of the HNK20 concentration in samples of mucosal secretions collected in this and other experiments suggests that protective levels of antibody are maintained in the nose for over 24 h after treatment (Fig. 3). HNK20-treated monkeys developed antibody to mouse IgA in serum and secretions, but this did not significantly affect the rate of HNK20 clearance from mucosal secretions. HNK20 was equally effective in naive and HNK20-immunized monkeys in an experiment in which antibody was delivered intranasally 1 h before RSV challenge (unpublished data).
FIG. 3.
Mean concentration of monoclonal antibody HNK20 in nasal swab specimens collected from monkeys treated with nose drops containing 2.5 or 0.5 mg of HNK20. The antibody was delivered once daily as indicated by arrows. HNK20 was quantitated by enzyme-linked immunosorbent assay. Reprinted from reference 114 with permission of the publisher.
PASSIVE NASAL IMMUNIZATION STUDIES WITH HUMANS
Common respiratory viruses of humans include influenza A and B viruses, RSV, parainfluenza virus types 1 to 4, adenoviruses, rhinoviruses, enteroviruses, and coronaviruses. The effectiveness of passive mucosal immunization against these different viruses is determined by factors such as the site of infection, the pathogenesis and severity of disease, and the extent of antigenic variation among the virus strains. RSV, which initiates infection in the nasopharynx, may be susceptible to nose drop delivery of antibody, while influenza virus, which infects the lower respiratory tract directly, would most probably require aerosol delivery of antibody (although nose drop treatment might reduce upper-airway symptoms and virus transmission). Parainfluenza viruses behave more like RSV, although only type 3 is associated with severe lower respiratory tract disease (19). The major cold viruses, particularly rhinoviruses, could potentially be prevented by nose drop treatment, but the self-limiting nature of the disease would have to be weighed against the cost of treatment in determining the value of treatment, and application may have the greatest value in high-risk individuals (e.g., asthmatics). Moreover, passive immunization against these viruses with virus-specific neutralizing antibodies is impractical due to the profusion of antigenic types (particularly for rhinoviruses).
Timing of treatment is another important consideration. Because of ciliary clearance of mucosal surfaces, passive mucosal immunization of the respiratory tract would be short-lived, particularly in the nose. Thus, a regimen of frequent, perhaps daily, treatments would be required. For prevention of respiratory tract infections by RSV or parainfluenza virus type 3, prophylactic nose drop treatment would be justified, given the severity of the disease, its seasonality, and the fact that high-risk individuals can be identified. For other viruses, antibody prophylaxis might be appropriate when an individual is at higher than usual risk for infection, such as occurs during a virus outbreak or exposure to others with known viral infections.
Immunoglobulin Treatment
A number of studies have examined prophylaxis with intranasally delivered antibody in humans (Table 2). Buthala et al. tested whether intranasal treatment with gamma globulin would reduce upper respiratory infection or disease in volunteers challenged with coxsackievirus A-21, an enterovirus that causes cold-like symptoms in adults (17). Seronegative subjects were divided into two groups of 10 for nose drop treatment with gamma globulin (containing virus-neutralizing activity with a titer of 1:1,024) or placebo. The subjects received six daily treatments of 0.3 ml per nostril, starting 1 day before virus challenge and continuing until 4 days after challenge. There was an interval of 1 to 2 h between challenge and treatment. During the 15 days following challenge, the symptoms and signs of respiratory tract infection were lower in gamma globulin-treated subjects than in placebo-treated subjects, but the differences were not statistically significant. Significant differences were found in the volume of nasal secretions and in body temperature. The peak virus titer in nasal washes was delayed and somewhat lower in the gamma globulin-treated subjects.
TABLE 2.
Human studies of intranasal antibody treatment for prevention of viral infection of the respiratory tract
| Virus or disease | Treatment | Delivery method | Outcome | Reference |
|---|---|---|---|---|
| Coxsackie virus | Gamma globulin | Nose drops | Decreased virus shedding and disease | 17 |
| Influenza virus | Gamma globulin | Aerosol | Trend toward decreased illness | 27 |
| Rhinovirus | MAba to virus receptor | Nose drops | Trend toward decreased virus shedding and disease | 36 |
| Upper RTa infection | Human IgA (IgAbulin) | Nasal spray | Trend toward decreased upper RT illness | 41 |
| Upper RT infection | Human IgA (IgAbulin) | Nasal spray | No effect on upper RT illness | 52 |
| Upper RT infection | Human IgA (IgAbulin) | Nose drops | Decrease in upper RT infections | 28 |
| Upper RT infection | Human IgA (IgAbulin) | Nasal spray | Decrease in rhinitis | 37 |
| RSV | IgG (IVIG) | Aerosol | Treatment safe | 88 |
| RSV | MAb IgA | Nose drops | Treatment safe, trend toward decreased RT illness | Unpublished |
RT, respiratory tract; MAb, monoclonal antibody.
A similar study was carried out to determine whether intranasal gamma globulin treatment protects against influenza A virus challenge (27). Human gamma globulin (with a hemagglutination inhibition titer of 1:20) or albumin placebo was delivered by aerosol to volunteers. The volunteers were treated twice, once orally and once nasally, with each treatment delivering 3 ml of aerosolized liquid. The influenza virus challenge was delivered intranasally 1 h after the second aerosol treatment. Of 32 placebo recipients, 12 developed signs or symptoms or illness, while 3 of 16 gamma globulin recipients became ill. The seroconversion rate, as a measure of virus infection, was also lower in the gamma globulin-treated subjects. None of the differences, however, were statistically significant.
More recently, a human globulin product containing mainly IgA (IgAbulin) has become available. Two studies have tested whether nasal treatment with this human IgA product prevents upper respiratory tract infection in athletes. In one study, 15 members of the Swedish cross-country ski team self-administered IgA in a nasal spray at least twice daily for 17 days during the 1992 winter Olympic games (41). None of the skiers developed apparent respiratory tract infection during this period. A control group of skiers was not included in the study, but over the same period, 9 of 19 team coaches developed respiratory tract infections. During a competition the preceding year, 5 of 13 skiers and 4 of 12 coaches developed respiratory tract infections in the absence of IgA treatment. A similar study, using canoeists as test subjects, found no significant reduction in upper respiratory tract infections following prophylactic nose drop treatment with IgA (52).
Two recent studies have shown the value of IgAbulin for intranasal passive immunization of children against respiratory tract infections. In one study, children were treated twice each day for 90 days with nose drops containing IgA or albumin placebo (28). Upper respiratory tract infections were significantly reduced in IgA recipients on days 0 to 30 (P < 0.012) and days 30 to 60 (P < 0.0036) of treatment. In the second study, children were randomized into two groups of 20 to receive nasal spray treatment with IgA or human albumin placebo for 8 weeks during the winter (37). A volume of 0.2 ml (containing 9 mg of antibody or 4 mg of albumin) was given twice per day, divided between the two nostrils. Parents recorded symptoms of upper respiratory tract infection daily. Over the treatment period, children receiving IgA experienced 42% fewer days with rhinitis (etiology undefined) than did children receiving placebo, a significant difference (P = 0.004). The children were also monitored for an additional 8 weeks after the treatment had ended. During this period, there was no difference in the frequency of rhinitis between the two groups. These important studies provide the best evidence to date that prophylactic nasal antibody treatment is both practical and effective.
Monoclonal Antibody Treatment
Rhinoviruses are the most frequent cause of the common cold. While over 100 serotypes of rhinovirus have been identified, 90% of them use intercellular cell adhesion molecule 1 (ICAM-1) to bind to host cells (24). Blockage of the interaction of virus with this cellular receptor provides a potential avenue for prophylaxis that is independent of serotype. A murine IgG1 monoclonal antibody developed against ICAM-1 prevented infection of cultured cells by multiple serotypes of rhinovirus (21). In chimpanzees, nose drop delivery of 250 μg of this antibody, termed rhinovirus receptor monoclonal antibody (RRMA), prior to intranasal challenge with rhinovirus reduced the antibody response to the virus, suggesting reduced replication (22).
Two placebo-controlled clinical studies of RRMA were reported in 1988 (36). In the first study, 15 subjects were treated every 8 h for 3 days with nose drops containing 15 μg of RRMA. Another group of 15 subjects was treated similarly with placebo. Rhinovirus challenge was administered twice on the second day, each time 1 h after antibody treatment. Neither virus shedding nor development of illness over the following days was affected by RRMA treatment. As a result, the dose and frequency of RRMA treatment were increased in a second study. In this study, 10 intranasal treatments with 100 μg of RRMA or placebo were given at 1-h intervals before and just after rhinovirus challenge. Additional treatments were given at 6-h intervals for the following 36 h. Although there was no difference between the two groups in the rate of infection, several beneficial effects were attributable to RRMA treatment, including delays in onset of virus shedding and illness and reductions in symptoms and nasal mucus production. This study helped lay the groundwork for more recent work in which receptor interaction was blocked by intranasal treatment with soluble forms of ICAM-1 (43, 54).
Nose drop prophylaxis with murine IgA monoclonal antibody HNK20 has been tested in clinical studies. HNK20 was shown to be free of adverse effects in small-scale trials involving daily intranasal administration to adults, to healthy infants, and to high-risk infants. An in-patient study was conducted to assess the prophylactic activity of HNK20 in normal adult volunteers who were challenged intranasally with wild-type RSV subgroup A virus 1 h after administration of the first dose of study medication. During the phase of acute virus infection (days 5 to 8 days after challenge), mean daily virus shedding was lower in HNK20-treated subjects than in placebo-treated subjects.
A controlled clinical trial was subsequently conducted with more than 600 infants with risk factors for severe RSV in Australia, New Zealand, Argentina, and South Africa. When the entire study population was considered, treatment with HNK20 did not result in a significant decrease in the incidence of hospitalization for RSV lower respiratory tract infection. However, among infants younger than 4 months at study entry (younger than 7 months of age throughout the winter), treatment with HNK20 was associated with a 42% reduction in RSV hospitalization. Since the trial was not powered to detect differences in subgroups and since only 30% of the total number of subjects were in the younger-than-4-month age group, these differences in RSV hospitalization showed statistical trends only.
POTENTIAL FOR THERAPEUTIC NASAL ANTIBODY TREATMENT TO REDUCE DISEASE
Once a viral respiratory infection has established itself, intranasal administration of a specific antibody might limit the progression of infection by neutralizing virus particles as they are released from infected cells. This, in turn, might shorten the course of infection. Animal studies show that intranasal passive immunization can reduce further replication of virus in the respiratory tract, but it is not clear whether this translates into a reduction in disease, since the host immune response can continue to cause tissue damage.
In animal studies, RSV infection has been the primary target for therapeutic intranasal passive immunization. These studies show that intranasal delivery of neutralizing antibody, either IVIG or monoclonal antibody, reduces RSV replication in the lungs of cotton rats and mice when delivered 1 to 5 days after virus challenge (5, 25, 29, 78, 79, 104, 111, 116). The effect of therapeutic treatment is similar to that of prophylactic treatment, although somewhat higher doses of antibody are required. However, there is little information about the effect of therapeutic treatment on disease. In a study in which monoclonal antibody RSHZ19 was delivered parenterally 3 days after RSV infection of cotton rats, histopathologic testing showed that lung damage was reduced in treated animals (116). In another study, parenteral IVIG reduced lung damage if delivered 1 day, but not 5 days, after infection, even though the virus levels were reduced in both instances (29).
In humans, therapeutic passive immunization against respiratory viruses would be started after the appearance of disease symptoms. The animal experiments described above suggest that passive immunization against an acute viral infection at this stage may be too late to reverse the disease process, even if virus replication is reduced. This conclusion is supported by human studies in which parenteral delivery of IVIG or RSVIG for RSV lower respiratory tract infection had no effect on the subsequent length of hospitalization (40, 90, 91).
A more effective approach might be treatment combining antibody to reduce virus replication with an agent to reverse the inflammatory response resulting from infection. Prince and Porter (83) tested this strategy for prevention of disease due to parainfluenza virus type 3 infection in cotton rats. Pulmonary inflammation in cotton rats following infection with parainfluenza virus type 3 was greatly reduced by intranasal treatment with the glucocorticosteroid triamcinolone acetonide, but virus titers in lung homogenates rose 10-fold. Intranasal IVIG treatment reduced the pulmonary titers of virus but had no effect on histopathologic findings. A combination of the two agents maintained the beneficial aspects of each such that inflammation and virus titers were both reduced.
CONCLUSIONS
The lack of success with vaccination or antiviral drug development against a number of respiratory viruses, as well as the emergence of new viruses, has led to renewed interest in prophylactic passive immunization. Intranasal antibody prophylaxis has shown great promise against viral respiratory tract infections in animals and is just beginning to show clear efficacy in human clinical studies. The marginal effects of nasal antibody treatment in some trials may be the result of low levels of virus-specific antibody in the Ig preparations used in many of these studies. This problem can be addressed, as it was for parenteral RSV antibody prophylaxis, by screening plasma donors to increase the titer of virus-neutralizing activity in IgG preparations. The effectiveness of intranasal antibody treatment might also be enhanced by using monoclonal antibodies and formulations optimized to maintain high levels of antibody in the nasal compartment. Monoclonal antibodies offer many advantages including high specific activity, purity, and ease of manufacture, and clinical studies indicate that prophylactic nasal delivery of monoclonal antibodies is both safe and practical. Further clinical studies are required to determine whether nasal prophylaxis with serum-derived or monoclonal antibodies will prove to be effective at an acceptable cost and treatment frequency.
Evidence to date suggests that once symptoms of respiratory tract disease manifest themselves it may be too late for antibody treatment to alter the course of illness. Treatment with antibody plus an anti-inflammatory agent may be an effective alternative approach for passive therapeutic immunization.
REFERENCES
- 1.Agius G, Dindinaud G, Biggar R J, Peyre R, Vaillant V, Ranger S, Poupet J Y, Cisse M F, Castets M. An epidemic of respiratory syncytial virus in elderly people: clinical and serological findings. J Med Virol. 1990;30:117–127. doi: 10.1002/jmv.1890300208. [DOI] [PubMed] [Google Scholar]
- 2.Akerfeldt S, Geijer S, Holubars E, Fuchs G, Brundin M. Prophylactic and therapeutic antiviral effect of human gamma globulin. Biochem Pharmacol. 1972;21:503–509. doi: 10.1016/0006-2952(72)90323-1. [DOI] [PubMed] [Google Scholar]
- 3.Akerfeldt S, Lofberg E, Fuchs G. Antiviral effect of human gamma globulin in mice. Comparison between the efficacy of local and systemic administration. Biochem Pharmacol. 1973;22:2911–2917. doi: 10.1016/0006-2952(73)90159-7. [DOI] [PubMed] [Google Scholar]
- 4.Alwan W H, Kozlowska W J, Openshaw P J. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal G P, Hatfield J, Young J F, Top F H, Prince G A, Horswood R L, Hemming V, Hensen S. Efficacy of passively administered monoclonal antibodies against respiratory syncytial virus infection in cotton rats. Vaccines. 1991;91:283–288. [Google Scholar]
- 6.Beeler J A, van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol. 1989;63:2941–2950. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell D Y, Haseman J A, Spock A, McLennan G, Hook G E R. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis. 1981;124:72–79. doi: 10.1164/arrd.1981.124.1.72. [DOI] [PubMed] [Google Scholar]
- 8.Bienenstock J, Befus D. Gut- and bronchus-associated lymphoid tissue. Am J Anat. 1984;170:437–445. doi: 10.1002/aja.1001700316. [DOI] [PubMed] [Google Scholar]
- 9.Bockman D E, Cooper M D. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix and Peyer’s patches. An electron microscopic study. Am J Anat. 1973;136:455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- 10.Borrebaeck C A K. Antibody engineering. A practical guide. W. H. New York, N.Y: Freeman & Co.; 1992. [Google Scholar]
- 11.Bourgeois C, Bour J B, Aho L S, Pothier P. Prophylactic administration of a complementarity-determining region derived from a neutralizing monoclonal antibody is effective against respiratory syncytial virus infection in BALB/c mice. J Virol. 1998;72:807–810. doi: 10.1128/jvi.72.1.807-810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braley J F, Dawson C A, Moore V L. Immunologic block against antigen absorption from isolated perfused rabbit lungs. J Immunol. 1978;121:926–929. [PubMed] [Google Scholar]
- 13.Brandtzaeg P. Immune functions of human nasal mucosa and tonsils in health and disease. In: Bienenstock J, editor. Immunology of the lung and upper respiratory tract. New York, N.Y: McGraw-Hill Book Co.; 1984. pp. 28–95. [Google Scholar]
- 14.Brandtzaeg P. Role of J chain and secretory component in receptor-mediated glandular and hepatic transport of immunoglobulins in man. Scand J Immunol. 1985;22:111–146. doi: 10.1111/j.1365-3083.1985.tb01866.x. [DOI] [PubMed] [Google Scholar]
- 15.Brown W R, Newcomb R W, Ishizaka K. Proteolytic degradation of exocrine and serum immunoglobulins. J Clin Investig. 1970;49:1374–1380. doi: 10.1172/JCI106354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns J W, Siadat-Pajouh M, Krishnaney A A, Greenberg H B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 17.Buthala D A, Damiano R, Eidson E E. Studies on coxsackie A-21 (COE) virus-infected volunteers: effect of local therapy with gamma globulin. Ann N Y Acad Sci. 1970;173:794–807. [Google Scholar]
- 18.Cohen A B, Gold W M. Defence mechanisms of the lungs. Annu Rev Physiol. 1975;37:325–350. doi: 10.1146/annurev.ph.37.030175.001545. [DOI] [PubMed] [Google Scholar]
- 19.Collins P L, Chanock R M, McIntosh K. Parainfluenza viruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1205–1241. [Google Scholar]
- 20.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1313–1351. [Google Scholar]
- 21.Colonno R J, Callahan P L, Long W J. Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses. J Virol. 1986;57:7–12. doi: 10.1128/jvi.57.1.7-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colonno R J, Tomassini J E, Callahan P L. Isolation and characterization of a monoclonal antibody which blocks attachment of human rhinoviruses. In: Brinton M, Rueckert R, editors. Positive strand RNA viruses. New York, N.Y: Alan R. Liss, Inc.; 1987. pp. 93–102. [Google Scholar]
- 23.Connors M, Giese N A, Kulkarni A B, Firestone C-Y, Morse H C, Murphy B R. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couch R B. Rhinoviruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 713–734. [Google Scholar]
- 25.Crowe J E, Murphy B R, Chanock R M, Williamson R A, Barbas C F, Burton D R. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc Natl Acad Sci USA. 1994;91:1386–1390. doi: 10.1073/pnas.91.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Englund J A, Sullivan C J, Jordan M C, Dehner L P, Vercellotti G M, Balfour H H. Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med. 1988;109:203–208. doi: 10.7326/0003-4819-109-3-203. [DOI] [PubMed] [Google Scholar]
- 27.Fruchtman M H, Mauceri A A, Wigley F M, Waldman R H. Aerosol administration of human gamma globulin as prophylaxis against influenza virus challenge. Clin Med. 1972;79:17–20. [Google Scholar]
- 28.Giraudi V, Riganti C, Torales M R, Sedola H, Gaddi E. Upper respiratory infections in children: response to endonasal administration of IGA. Int J Pediatr Otorhinolaryngol. 1997;39:103–110. doi: 10.1016/s0165-5876(96)01472-3. [DOI] [PubMed] [Google Scholar]
- 29.Graham B S, Davis T H, Tang Y-W, Gruber W C. Immunoprophylaxis and immunotherapy of respiratory syncytial virus-infected mice with respiratory syncytial virus-specific immune serum. Pediatr Res. 1993;34:167–172. doi: 10.1203/00006450-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Graham B S, Henderson G S, Tang Y-W, Lu X, Neuzil K M, Colley D G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 31.Graham B S, Tang Y-W, Gruber W C. Topical immunoprophylaxis of respiratory syncytial virus (RSV)-challenged mice with RSV-specific immune globulin. J Infect Dis. 1995;171:1468–1474. doi: 10.1093/infdis/171.6.1468. [DOI] [PubMed] [Google Scholar]
- 32.Groothuis J R, Simoes E A F, Levin M J, Hall C B, Long C E, Rodriguez W J, Arrobio J, Meissner H C, Fulton D R, Welliver R C, Tristram D A, Siber G R, Prince G A, Van Raden M, Hemming V G. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 33.Hall C B, Walsh E E, Long C E, Schnabel K C. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 34.Hammarstrom L, Gardulf A, Hammarstrom V, Jannson A, Lindberg K, Smith C I E. Systemic and topical immunoglobulin treatment in immunocompromised patients. Immunol Rev. 1994;139:43–70. doi: 10.1111/j.1600-065x.1994.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 35.Hammarstrom L, Smith C I E. The use of intravenous IgG as prophylaxis and for treatment of infections. Infection. 1990;18:314–324. doi: 10.1007/BF01647018. [DOI] [PubMed] [Google Scholar]
- 36.Hayden F G, Gwaltney J M, Colonno R J. Modification of experimental rhinovirus colds by receptor blockade. Antiviral Res. 1988;9:233–247. doi: 10.1016/0166-3542(88)90055-1. [DOI] [PubMed] [Google Scholar]
- 37.Heikkinen T, Ruohola A, Ruuskanen O, Waris M, Uhari M, Hammarstrom L. Intranasally administered immunoglobulin for the prevention of rhinitis in children. Pediatr Infect Dis J. 1998;17:367–372. doi: 10.1097/00006454-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Heilman C A. Respiratory syncytial and parainfluenza viruses. J Infect Dis. 1990;161:402–406. doi: 10.1093/infdis/161.3.402. [DOI] [PubMed] [Google Scholar]
- 39.Hemming V G, Prince G A, Groothuis J R, Siber G R. Hyperimmune globulins in prevention and treatment of respiratory syncytial virus infections. Clin Microbiol Rev. 1995;8:22–33. doi: 10.1128/cmr.8.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemming V G, Rodriguez W, Kim H W, Brandt C D, Parrott R H, Burch B, Prince G A, Baron P A, Fink R J, Reaman G. Intravenous immunoglobulin treatment of respiratory syncytial virus infections in infants and young children. Antimicrob Agents Chemother. 1987;31:1882–1886. doi: 10.1128/aac.31.12.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemmingsson P, Hammarstrom L. Nasal administration of immunoglobulin as effective prophylaxis against infections in elite cross-country skiers. Scand J Infect Dis. 1993;25:783–785. doi: 10.3109/00365549309008580. [DOI] [PubMed] [Google Scholar]
- 42.Hirabayashi Y, Kurata H, Funato H, Nagamine T, Aizawa C, Tamura S, Shimada K, Kurata T. Comparison of intranasal inoculation of influenza HA vaccine combined with cholera toxin B subunit with oral or parenteral vaccination. Vaccine. 1990;8:243–248. doi: 10.1016/0264-410x(90)90053-o. [DOI] [PubMed] [Google Scholar]
- 43.Huguenel E D, Cohn D, Dockum D P, Greve J M, Fournel M A, Hammond L, Irwin R, Mahoney J, McClelland A, Muchmore E, Ohlin A C, Scuderi P. Prevention of rhinovirus infection in chimpanzees by soluble intercellular adhesion molecule-1. Am J Respir Crit Care Med. 1997;155:1206–1210. doi: 10.1164/ajrccm.155.4.9105055. [DOI] [PubMed] [Google Scholar]
- 44.IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 45.Johnson S, Oliver C, Prince G A, Hemming V G, Pfarr D S, Wang S-C, Dormitzer M, O’Grady J, Koenig S, Tamura J K, Woods R, Bansal G, Couchenour D, Tsao E, Hall W C, Young J F. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 46.Kapikian A Z, Mitchell R H, Chanock R M, Shvedoff R A, Stewart C E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 47.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 48.Krause R M, Dimmock N J, Morens D M. Summary of antibody workshop: the role of humoral immunity in the treatment and prevention of emerging and extant infectious diseases. J Infect Dis. 1997;176:549–559. doi: 10.1086/514074. [DOI] [PubMed] [Google Scholar]
- 49.Kuper C F, Koornstra P J, Hameleers D M H, Biewenga J, Spit B J, Duijvestijn A M, van Breda Vriesman P J C, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 50.Langman J M, Rowland R. The number and distribution of lymphoid follicles in the human large intestine. J Anat. 1986;149:189–194. [PMC free article] [PubMed] [Google Scholar]
- 51.Liew F Y, Russell S M, Appleyard G, Brand C M, Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984;14:350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- 52.Lindberg K, Berglund B. Effect of treatment with nasal IgA on the incidence of infectious disease in world-class canoeists. Int J Sports Med. 1996;17:235–238. doi: 10.1055/s-2007-972838. [DOI] [PubMed] [Google Scholar]
- 53.Lorin M I, Gaerlan P F, Mandel I D. Quantitative composition of nasal secretions in normal subjects. J Lab Clin Med. 1972;80:275–281. [PubMed] [Google Scholar]
- 54.Marlin S D, Staunton D E, Springer T A, Stratowa C, Sommergruber W, Merluzzi V J. A soluble form of intercellular adhesion molecule-1 inhibits rhinovirus infection. Nature. 1990;344:70–72. doi: 10.1038/344070a0. [DOI] [PubMed] [Google Scholar]
- 55.Mayrhofer G. Epithelial disposition of antigen. In: Goldie R, editor. Immunopharmacology of epithelial barriers. London, United Kingdom: Academic Press, Inc.; 1994. pp. 19–70. [Google Scholar]
- 56.Mazanec M B, Coudret C L, Fletcher D R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazanec M B, Kaetzel C S, Lamm M E, Fletcher D, Nedrud J G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazanec M B, Lamm M E, Lyn D, Portner A, Nedrud J G. Comparison of IgA versus IgG monoclonal antibodies for passive immunization of the murine respiratory tract. Virus Res. 1992;23:1–12. doi: 10.1016/0168-1702(92)90063-f. [DOI] [PubMed] [Google Scholar]
- 59.Mazanec M B, Nedrud J G, Lamm M E. Immunoglobulin A monoclonal antibodies protect against Sendai virus. J Virol. 1987;61:2624–2626. doi: 10.1128/jvi.61.8.2624-2626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGhee J R, Kiyono H. New perspectives in vaccine development: mucosal immunity to infections. Infect Agents Dis. 1993;2:55–73. [PubMed] [Google Scholar]
- 61.McIntosh K, Halonen P, Ruuskanen O. Report of a workshop on respiratory viral infections: epidemiology, diagnosis, treatment, and prevention. Clin Infect Dis. 1993;16:151–164. doi: 10.1093/clinids/16.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McIntosh K, Masters H B, Orr I, Chao R K, Barkin R M. The immunologic response to infection with respiratory syncytial virus in infants. J Infect Dis. 1978;138:24–32. doi: 10.1093/infdis/138.1.24. [DOI] [PubMed] [Google Scholar]
- 63.McNabb P C, Tomasi T B. Host defense mechanisms at mucosal surfaces. Annu Rev Microbiol. 1981;35:477–496. doi: 10.1146/annurev.mi.35.100181.002401. [DOI] [PubMed] [Google Scholar]
- 64.Meissner H C. Economic impact of viral respiratory disease in children. J Pediatr. 1994;124:S17–S21. doi: 10.1016/S0022-3476(94)70186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meissner H C, Schlievert P M, Leung D Y M. Mechanisms of immunoglobulin action: observations on Kawasaki syndrome and RSV prophylaxis. Immunol Rev. 1994;139:109–123. doi: 10.1111/j.1600-065x.1994.tb00859.x. [DOI] [PubMed] [Google Scholar]
- 66.Mestecky J, McGhee J R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 67.Mestecky J, McGhee J R, Elson C O. Intestinal IgA system. Immunol Allergy Clin North Am. 1988;8:349–368. [Google Scholar]
- 68.Mills J, Van Kirk J E, Wright P F, Chanock R M. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J Immunol. 1971;107:123–130. [PubMed] [Google Scholar]
- 69.Murphy B R, Webster R G. Orthomyxoviruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1397–1445. [Google Scholar]
- 70.Nader P R, Horwitz M S, Rousseau J. Atypical exanthem following exposure to natural measles: eleven cases in children previously inoculated with killed vaccine. J Pediatr. 1968;72:22–28. doi: 10.1016/j.jpeds.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 71.Neutra M R, Kraehenbuhl J-P. Transepithelial transport and mucosal defence. I. The role of M cells. Trends Cell Biol. 1992;2:134–138. doi: 10.1016/0962-8924(92)90099-9. [DOI] [PubMed] [Google Scholar]
- 72.Newcomb R W, Sutoris C A. Comparative studies on human and rabbit exocrine IgA antibodies to an albumin. Immunochemistry. 1974;111:623–632. doi: 10.1016/0019-2791(74)90219-5. [DOI] [PubMed] [Google Scholar]
- 73.Outlaw M C, Dimmock N J. Mechanisms of neutralization of influenza virus on mouse tracheal epithelial cells by mouse monoclonal polymeric IgA and polyclonal IgM directed against the viral haemagglutinin. J Gen Virol. 1990;71:69–76. doi: 10.1099/0022-1317-71-1-69. [DOI] [PubMed] [Google Scholar]
- 74.Owen R L, Piazza A J, Ermak T H. Ultrastructural and cytoarchitectural features of lymphoreticular organs in the colon and rectum of adult BALB/c mice. Am J Anat. 1991;190:10–18. doi: 10.1002/aja.1001900103. [DOI] [PubMed] [Google Scholar]
- 75.Parrott R H, Kim H W, Arrobio J O, Hodes D S, Murphy B R, Brandt C D, Camargo E, Chanock R M. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973;98:289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- 76.Pedersen H, Mygind N. Absence of axonemal arms in nasal mucosa cilia in Kartagener’s syndrome. Nature. 1976;262:494–495. doi: 10.1038/262494a0. [DOI] [PubMed] [Google Scholar]
- 77.PREVENT Study Group. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 78.Prince G A, Hemming V G, Horswood R L, Baron P A, Chanock R M. Effectiveness of topically administered neutralizing antibodies in experimental immunotherapy of respiratory syncytial virus infection in cotton rats. J Virol. 1987;61:1851–1854. doi: 10.1128/jvi.61.6.1851-1854.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prince G A, Hemming V G, Horswood R L, Baron P A, Murphy B R, Chanock R M. Mechanism of antibody-mediated viral clearance in immunotherapy of respiratory syncytial virus infection of cotton rats. J Virol. 1990;64:3091–3092. doi: 10.1128/jvi.64.6.3091-3092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prince G A, Hemming V G, Horswood R L, Chanock R M. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 1985;3:193–206. doi: 10.1016/0168-1702(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 81.Prince G A, Horswood R L, Camargo E, Koenig D, Chanock R M. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun. 1983;42:81–87. doi: 10.1128/iai.42.1.81-87.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prince G A, Horswood R L, Chanock R M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985;55:517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prince G A, Porter D D. Treatment of parainfluenza virus type 3 bronchiolitis and pneumonia in a cotton rat model using topical antibody and glucocorticosteroid. J Infect Dis. 1996;173:598–608. doi: 10.1093/infdis/173.3.598. [DOI] [PubMed] [Google Scholar]
- 84.Proctor D F, Lundqvist G. Clearance of inhaled particles from the human nose. Arch Intern Med. 1973;131:132–139. [PubMed] [Google Scholar]
- 85.Ramisse F, Deramoudt F X, Szatanik M, Bianchi A, Binder P, Hannoun C, Alonso J M. Effective prophylaxis of influenza A virus pneumonia in mice by topical passive immunotherapy with polyvalent human immunoglobulins or F(ab′)2 fragments. Clin Exp Immunol. 1998;111:583–587. doi: 10.1046/j.1365-2249.1998.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Renegar K B, Small P A. Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Renegar K B, Small P A. Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 88.Rimensberger P C, Schaad U B. Clinical experience with aerosolized immunoglobulin treatment of respiratory syncytial virus infection in infants. Pediatr Infect Dis J. 1994;13:328–330. doi: 10.1097/00006454-199404000-00018. [DOI] [PubMed] [Google Scholar]
- 89.Roberts S R, Compans R W, Wertz G W. Respiratory syncytial virus matures at the apical surface of polarized epithelial cells. J Virol. 1995;69:2667–2673. doi: 10.1128/jvi.69.4.2667-2673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodriguez W J, Gruber W C, Groothuis J R, Simoes E A, Rosas A J, Lepow M, Kramer A, Hemming V. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics. 1997;100:937–942. doi: 10.1542/peds.100.6.937. [DOI] [PubMed] [Google Scholar]
- 91.Rodriguez W J, Gruber W C, Welliver R C, Groothuis J R, Simoes E A F, Meissner H C, Hemming V G, Hall C B, Lepow M L, Rosas A J, Robertsen C, Kramer A A the Respiratory Syncytial Virus Immune Globulin Study Group. Respiratory syncytial virus (RSV) immune globulin intravenous therapy for RSV lower respiratory tract infection in infants and young children at high risk for severe RSV infections. Pediatrics. 1997;99:454–461. doi: 10.1542/peds.99.3.454. [DOI] [PubMed] [Google Scholar]
- 92.Rossen R D, Schade A L, Butler W T, Kasel J A. The proteins in nasal secretion: a longitudinal study of the gammaA-globulin, gammaG-globulin, albumin, siderophilin, and total protein concentrations in nasal washings from adult male volunteers. J Clin Investig. 1966;45:768–776. doi: 10.1172/JCI105391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sami I R, Piazza F M, Johnson S A, Darnell M E R, Ottolini M G, Hemming V G, Prince G A. Systemic immunoprophylaxis of nasal respiratory syncytial virus infection in cotton rats. J Infect Dis. 1995;171:440–443. doi: 10.1093/infdis/171.2.440. [DOI] [PubMed] [Google Scholar]
- 94.Siber G R, Leombruno D, Leszczynski J, McIver J, Bodkin D, Gonin R, Thompson C M, Walsh E E, Piedra P A, Hemming V G, Prince G A. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J Infect Dis. 1994;169:1368–1373. doi: 10.1093/infdis/169.6.1368. [DOI] [PubMed] [Google Scholar]
- 95.Siber G R, Leszczynski J, Pena-Cruz V, Ferren-Gardner C, Anderson R, Hemming V G, Walsh E E, Burns J, McIntosh K, Gonin R, Anderson L J. Protective activity of a human respiratory syncytial virus immune globulin prepared from donors screened by microneutralization assay. J Infect Dis. 1992;165:456–463. doi: 10.1093/infdis/165.3.456. [DOI] [PubMed] [Google Scholar]
- 96.Smith C B, Purcell R H, Bellanti J A, Chanock R M. Protective effect of antibody to parainfluenza type I virus. N Engl J Med. 1966;275:1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- 97.Snell N J C. New developments in the treatment of viral respiratory tract infections. Exp Opin Investig Drugs. 1997;6:1001–1008. doi: 10.1517/13543784.6.8.1001. [DOI] [PubMed] [Google Scholar]
- 98.Solari R, Kraehenbuhl J-P. The biosynthesis of secretory component and its role in the transepithelial transport of IgA dimer. Immunol Today. 1985;6:17–20. doi: 10.1016/0167-5699(85)90163-X. [DOI] [PubMed] [Google Scholar]
- 99.Spit B J, Hendriksen E G J, Bruijntes J P, Kuper C F. Nasal lymphoid tissue in the rat. Cell Tissue Res. 1989;255:193–198. doi: 10.1007/BF00229081. [DOI] [PubMed] [Google Scholar]
- 100.Subramanian K N, Weisman L E, Rhodes T, Ariagno R, Sanchez P J, Steichen J, Givner L B, Jennings T L, Top F H, Carlin D, Connor E. Safety, tolerance and pharmacokinetics of a humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. Pediatr Infect Dis J. 1998;17:110–115. doi: 10.1097/00006454-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 101.Tamura S, Funato H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21:1337–1344. doi: 10.1002/eji.1830210602. [DOI] [PubMed] [Google Scholar]
- 102.Taylor G, Stott E J, Bew M, Fernie B F, Cote P J, Collins A P, Hughes M, Jebbett J. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology. 1984;52:137–142. [PMC free article] [PubMed] [Google Scholar]
- 103.Taylor H P, Dimmock N J. Mechanism of neutralization of influenza virus by secretory IgA is different from that of monomeric IgA or IgG. J Exp Med. 1985;161:198–209. doi: 10.1084/jem.161.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tempest P R, Bremner P, Lambert M, Taylor G, Furze J M, Carr F J, Harris W J. Reshaping a human monoclonal antibody to inhibit human respiratory syncytial virus infection in vivo. Bio/Technology. 1991;9:266–271. doi: 10.1038/nbt0391-266. [DOI] [PubMed] [Google Scholar]
- 105.Tomasi T B, Grey H M. Structure and function of immunoglobulin A. Prog Allergy. 1972;16:81–213. [PubMed] [Google Scholar]
- 106.Underdown B J, Dorrington K J. Studies on the structural and conformational basis for the relative resistance of serum and secretory immunoglobulin A to proteolysis. J Immunol. 1974;112:949–959. [PubMed] [Google Scholar]
- 107.Walker R I. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine. 1994;12:387–400. doi: 10.1016/0264-410x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 108.Walsh E E, Schlesinger J J, Brandriss M W. Protection from respiratory syncytial virus infection in cotton rats by passive transfer of monoclonal antibodies. Infect Immun. 1984;43:756–758. doi: 10.1128/iai.43.2.756-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Waris M E, Tsou C, Erdman D D, Zaki S R, Anderson L J. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Watt P J, Robinson B S, Pringle C R, Tyrrell D A J. Determinants of susceptibility to challenge and the antibody response of adult volunteers given experimental respiratory syncytial virus vaccines. Vaccine. 1990;8:231–236. doi: 10.1016/0264-410x(90)90051-m. [DOI] [PubMed] [Google Scholar]
- 111.Weltzin R, Hsu S A, Mittler E S, Georgakopoulos K, Monath T P. Intranasal monoclonal immunoglobulin A against respiratory syncytial virus protects against upper and lower respiratory tract infections in mice. Antimicrob Agents Chemother. 1994;38:2785–2791. doi: 10.1128/aac.38.12.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weltzin R, Kleanthous H, Guirakhoo F, Monath T P, Lee C K. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine. 1997;15:370–376. doi: 10.1016/s0264-410x(97)00203-x. [DOI] [PubMed] [Google Scholar]
- 113.Weltzin R, Lucia-Jandris P, Michetti P, Fields B N, Kraehenbuhl J P, Neutra M R. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989;108:1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weltzin R, Traina-Dorge V, Soike K, Zhang J Y, Mack P, Soman G, Drabik G, Monath T P. Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection. J Infect Dis. 1996;174:256–261. doi: 10.1093/infdis/174.2.256. [DOI] [PubMed] [Google Scholar]
- 115.Wong J P, Stadnyk L L, Saravolac E G. Enhanced protection against respiratory influenza A infection in mice by liposome-encapsulated antibody. Immunology. 1994;81:280–284. [PMC free article] [PubMed] [Google Scholar]
- 116.Wyde P R, Moore D K, Hepburn T, Silverman C L, Porter T G, Gross M, Taylor G, Demuth S G, Dillon S B. Evaluation of the protective efficacy of reshaped human monoclonal antibody RSHZ19 against respiratory syncytial virus in cotton rats. Pediatr Res. 1995;38:543–550. doi: 10.1203/00006450-199510000-00012. [DOI] [PubMed] [Google Scholar]