Abstract
Background
The role of neutrophil–lymphocyte ratio (NLR) as a predictor for survival in single fraction SBRT-treated non-small cell lung cancer (NSCLC) patients remains unclear. We performed an observational cohort study to determine the role of pretreatment NLR in predicting survival of early-stage NSCLC patients after single fraction SBRT.
Methods
A single-institution database of peripheral early-stage NSCLC patients treated with SBRT from February 2007 to May 2022 was queried. Optimal threshold of neutrophil–lymphocyte ratio (NLR) was defined based on maximally selected rank statistics. Cox multivariable analysis (MVA), Kaplan–Meier, and propensity score matching were performed to evaluate outcomes.
Results
A total of 286 patients were included for analysis with median follow up of 19.7 months. On Cox multivariate analysis, as a continuous variable, NLR was shown to be an independent predictor of OS (adjusted hazards ratio [aHR] 1.06, 95% CI 1.02–1.10, p = 0.005) and PFS (aHR 1.05, 95% CI 1.01–1.09, p = 0.013). In addition, NLR was associated with DF (aHR 1.11, 95% CI 1.05–1.18, p < 0.001). Maximally selected rank statistics determined 3.28 as the cutoff point of high NLR versus low NLR. These findings were confirmed upon propensity matching.
Conclusions
Pretreatment NLR is an independent predictor for survival outcomes of peripheral early-stage NSCLC patients after single fraction SBRT.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-10719-3.
Keywords: Non-small cell lung cancer, Neutrophil–lymphocyte ratio, Stereotactic body radiation therapy, Survival
Introduction
Lung cancer is the leading cause of cancer-related deaths in the United States, with 235,760 new cases and 131,880 deaths in 2021 [1]. Standard of care for patients with early-stage non-small cell lung cancer (NSCLC) is surgical resection [2]. For patients medically inoperable, stereotactic body radiation therapy (SBRT) is utilized as definitive treatment [3]. Various lung SBRT dose fractionation regimens are employed globally with single-fraction SBRT found to be equally effective as multi-fraction regimens [4–11].
For several malignancies, neutrophil–lymphocyte ratio (NLR) has been associated with survival outcomes of patients treated with SBRT [12–14]. In NSCLC, previous studies have demonstrated that NLR predicts overall survival (OS) [15–20], and one study linked NLR and local recurrence [21]. No study previously reported on NLR and single fraction SBRT. To investigate the correlation of NLR to outcomes, we performed a single-institution, observational cohort study involving patients with peripheral early-stage NSCLC who underwent predominantly single-fraction SBRT.
Materials and Methods
Our cohort study was approved by the Roswell Park Comprehensive Cancer Center Institutional Review Board (EDR 171,710). It follows the Strengthening the Reporting of Observations Studies in Epidemiology (STROBE) reporting guideline.
The cohort database was selected from NSCLC patients treated with SBRT at Roswell Park Comprehensive Cancer Center between February 2007 to May 2022. Consecutive patients with peripheral early-stage NSCLC (T1-2N0M0) and a complete blood count within six months of the start of SBRT treatment were included. Patients were excluded from the analysis if they had missing NLR data. Patients treated with SBRT regimens of more than 3 fractions were excluded as these regimens (i.e., 5 fractions) were reserved for patients with centrally located lesions with higher risk of toxicity which could affect survival [22–25].
Other clinically relevant variables such as age, gender, race, Karnofsky Performance Status (KPS), histology (adenocarcinoma, squamous cell carcinoma, NSCLC not otherwise specified), primary cancer site, T-stage, radiation fractions, smoking status, year of treatment, and tumor location were obtained from the electronic health record (EHR). All missing values were coded as unknown. Patient race was separated as White, African American, American Indian/Alaska Native, Asian, Hispanic, and unknown or declined to answer. Non-white patients were grouped together as a single category because of the small subgroup sample sizes.
Primary outcomes were overall survival (OS) and progression-free survival (PFS). OS was determined from the time interval encompassing the start of treatment to the last known follow-up or death (from any cause). PFS was determined from the time of the start of the treatment to any tumor recurrence, the last known follow up, or death. The secondary outcomes were local failure (LF), nodal failure (NF), and distant failure (DF). Secondary outcomes were determined from the time between the start of treatment to a failure at same cancer site, thoracic nodal station, or extra thoracic or contralateral lung failure, respectively. All tumor recurrences were determined through multidisciplinary discussion based on radiographic findings and, when available, biopsy results of metastatic sites. For patients with multiple failure events either synchronously or metachronously during their follow up period, all failure events were counted separately for analysis.
Statistical analysis
To visualize the relationship between patient survival and pre-treatment NLR as a continuous variable, a nonlinear Cox regression model with restricted cubic splines (RCS) was performed as previously shown [26]. RCS is a smooth, piecewise polynomial function that visualizes the association between a variable and an outcome without any prior assumption in the association. The model was constructed for OS and PFS using 3 knots at the 10th, 50th, and 90thpercentiles based on the lowest Akaike information criterion [27].
Cox multivariable analysis (MVA) was used to investigate the prognostic role of pre-treatment NLR as a continuous variable in early-stage peripheral NSCLC patients, with the addition of clinically relevant variables (age, gender, race, KPS, histology, site of cancer, T-stage, fractions of radiation, smoking status, year of radiation treatment, and tumor location). Furthermore, Fine-Gray competing risk MVA was also performed to evaluate secondary outcomes (LF, NF, and DF). Kaplan–Meier method and log-rank tests were used to examine the univariate association between OS and PFS with pre-treatment NLR after dichotomization. An optimal cutoff for high versus low NLR was obtained by using an outcome-based process by maximizing the log-rank test statistic and survival differences as previously described [26, 28, 29]. The cutoff was searched between the NLR quantiles of 0.1 and 0.9. The optimal cutoff was analyzed for both OS and PFS, and patients were then stratified into two cohorts, high versus low pre-treatment NLR, by above versus below the optimal cutoff. Based on optimal cutoff, 1- and 3-year survival and tumor controls were calculated for analysis. Note that the searching of optimal cutoff by the log-rank statistic is conditional on the overall significant association between NLR and the survival outcomes. Therefore, multiple testing during the cutoff searching is not an issue.
To limit selection bias, propensity score matching was performed using the optimal cutoff value calculated for NLR. The two cohorts, high and low NLR, were matched based on the previous variables listed above. Matching was based on nearest neighbor method in a 1:1 ratio with no replacement using a caliper distance of 0.2 [30]. Furthermore, Cox and Fine-Gray regression models were performed to evaluate OS, PFS, and secondary outcomes after matching. Logistic regression was performed to identify any related variables to high versus low NLR. A subgroup analysis was performed among patients treated with single-fraction SBRT to see whether our findings would be consistent.
All tests were two-sided and p values less than 0.05 were considered statistically significant. Adjusted hazard ratios (aHR) and 95% confidence intervals (CI) were reported for analysis. Data analyses were done using R (version 4.1.2, R Project for Statistical Computing, Vienna, Austria).
Results
A total of 286 patients (164 female [57.3%]; median [IQR] age 76 [69–81] years) were included in our analysis (Table 1). Most patients had adenocarcinoma (160, 55.9%) or squamous cell carcinoma (93, 32.5%). The majority of tumors were clinical stage T1 (235, 82.2%). SBRT prescriptions with heterogeneity correction were 27 Gy in 1 fraction (211, 72.8%) and 54 Gy in 3 fractions (75, 26.2%). The median NLR was 3.06 ([IQR] 2.21–4.33). There were 15 local failures (5.2%), 27 nodal failures (9.4%), and 50 distant failures (17.5%). The median follow up was 19.7 months ([IQR] 9.78–35.48).
Table 1.
Baseline characteristics
| Patients, No. (%) | |||
|---|---|---|---|
| All (n = 286) | Low NLR (n = 158) | High NLR (n = 128) | |
| Age | |||
| < 65 | 48 (16.8) | 27 (17.1) | 21 (16.4) |
| ≥ 65 | 238 (83.2) | 131 (82.9) | 107 (83.6) |
| Gender | |||
| Male | 122 (42.7) | 61 (38.6) | 61 (47.7) |
| Female | 164 (57.3) | 97 (61.4) | 67 (52.3) |
| Race | |||
| White | 271 (94.8) | 148 (93.7) | 123 (96.1) |
| Other | 15 (5.2) | 10 (6.3) | 5 (3.9) |
| KPS | |||
| 70–100 | 178 (62.2) | 101 (63.9) | 77 (60.2) |
| < 70 | 108 (37.8) | 57 (36.1) | 51 (39.8) |
| Histology | |||
| Adenocarcinoma | 160 (55.9) | 92 (58.2) | 68 (53.1) |
| Squamous Cell | 93 (32.5) | 47 (29.7) | 46 (35.9) |
| NSCLC (NOS) | 33 (11.5) | 19 (12.0) | 14 (10.9) |
| Site | |||
| Left | 140 (49.0) | 80 (50.6) | 60 (46.9) |
| Right | 146 (51.0) | 78 (49.4) | 68 (53.1) |
| T staging | |||
| 1 | 235 (82.2) | 136 (86.1) | 99 (77.3) |
| 2 | 51 (17.8) | 22 (13.9) | 29 (22.7) |
| Fractions | |||
| 1 | 211 (72.8) | 117 (74.1) | 94 (73.4) |
| 3 | 75 (26.2) | 41 (25.9) | 34 (26.6) |
| Smoking Status | |||
| Current | 79 (27.6) | 50 (31.6) | 29 (22.7) |
| Former | 188 (65.7) | 97 (61.4) | 91 (71.1) |
| Never | 19 (6.6) | 11 (7.0) | 8 (6.3) |
| Year of radiation | |||
| 2013 or earlier | 220 (76.9) | 125 (79.1) | 95 (74.2) |
| 2013 or later | 66 (23.1) | 33 (20.9) | 33 (25.8) |
N number, KPS Karnofsky performance, NOS not otherwise specified
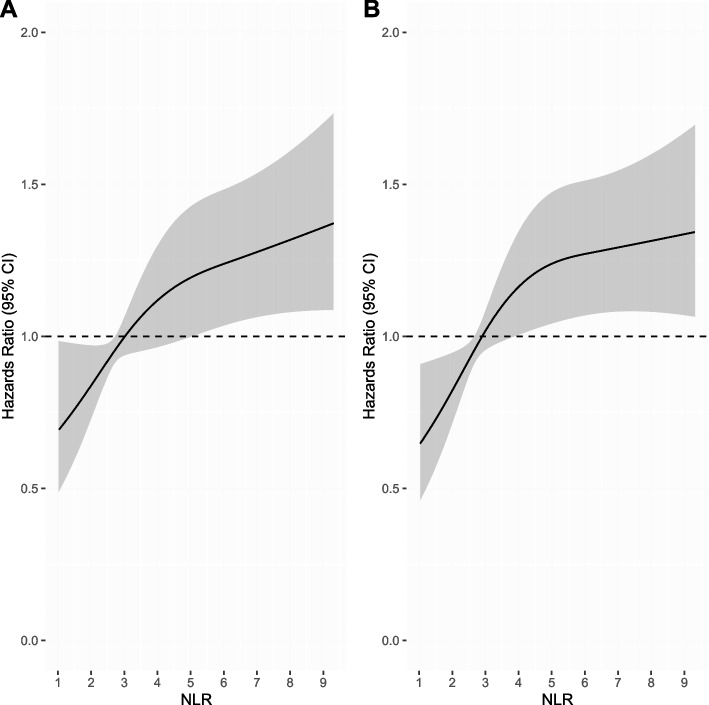
The nonlinear Cox regression model showed worsening OS and PFS without plateau in a continuous fashion as NLR increased (Fig. 1). On Cox MVA, as a continuous variable, elevated NLR was associated with poorer OS (adjusted hazards ratio [aHR] 1.06, 95% CI 1.02–1.10, p = 0.005) and PFS (aHR 1.05, 95% 1.01–1.09, p = 0.01; Table 2). In addition, age and KPS showed an expected association with OS, and KPS also showed an expected association with PFS (Table 2). Fine-Gray competing risk MVA indicated that elevated NLR was significantly related to increased likelihood of DF (aHR 1.11, 95% CI 1.05–1.18, p < 0.001), but not related to NF (aHR 1.08, 95% CI 0.97–1.21, p = 0.16; eTable 1). The number of LF events were too few to evaluate by MVA.
Fig. 1.
Nonlinear Cox regression for overall (A) and progression-free (B) survival based on neutrophil–lymphocyte ratio (NLR) as a continuous variable
Table 2.
Cox multivariate analysis for overall and progression-free survival outcomes
| Overall Survival | Progression-Free Survival | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| NLR | 1.06 (1.02–1.10) | 0.005a | 1.05 (1.01–1.09) | 0.01a |
| Age | ||||
| < 65 | 1 [Reference] | NA | 1 [Reference] | NA |
| ≥ 65 | 2.56 (1.47–4.46) | < 0.001a | 1.47 (0.92–2.36) | 0.11 |
| Gender | ||||
| Male | 1 [Reference] | NA | 1 [Reference] | NA |
| Female | 0.91 (0.66–1.26) | 0.57 | 0.83 (0.60–1.13) | 0.24 |
| Race | ||||
| White | 1 [Reference] | NA | 1 [Reference] | NA |
| Other | 1.22 (0.59–2.51) | 0.6 | 1.26 (0.62–2.56) | 0.52 |
| KPS | ||||
| 70–100 | 1 [Reference] | NA | 1 [Reference] | NA |
| < 70 | 1.59 (1.12–2.24) | 0.009a | 1.43 (1.03–1.99) | 0.03a |
| Histology | ||||
| Adenocarcinoma | 1 [Reference] | NA | 1 [Reference] | NA |
| Squamous Cell | 1.16 (0.81–1.65) | 0.42 | 1.11 (0.80–1.56) | 0.53 |
| NSCLC (NOS) | 1.21 (0.72–2.02) | 0.47 | 0.93 (0.55–1.55) | 0.77 |
| Site | ||||
| Left | 1 [Reference] | NA | 1 [Reference] | NA |
| Right | 0.93 (0.66–1.30) | 0.66 | 0.87 (0.63–1.21) | 0.41 |
| T staging | ||||
| 1 | 1 [Reference] | NA | 1 [Reference] | NA |
| 2 | 1.45 (0.99–2.12) | 0.06 | 1.39 (0.96–2.01) | 0.09 |
| Fractions | ||||
| 1 | 1 [Reference] | NA | 1 [Reference] | NA |
| 3 | 1.37 (0.97–1.94) | 0.07 | 1.34 (0.97–1.87) | 0.08 |
| Smoking Status | ||||
| Current | 1 [Reference] | NA | 1 [Reference] | NA |
| Former | 0.80 (0.54–1.17) | 0.24 | 0.75 (0.52–1.07) | 0.11 |
| Never | 0.80 (0.35–1.82) | 0.6 | 0.67 (0.30–1.50) | 0.32 |
| Year of radiation | ||||
| 2013 or earlier | 1 [Reference] | NA | 1 [Reference] | NA |
| 2013 or later | 0.76 (0.54–1.07) | 0.12 | 0.84 (0.60–1.17) | 0.3 |
aHR adjusted hazards ratio, CI confidence interval, KPS Karnofsky performance, NOS not otherwise specified
astatistically significant
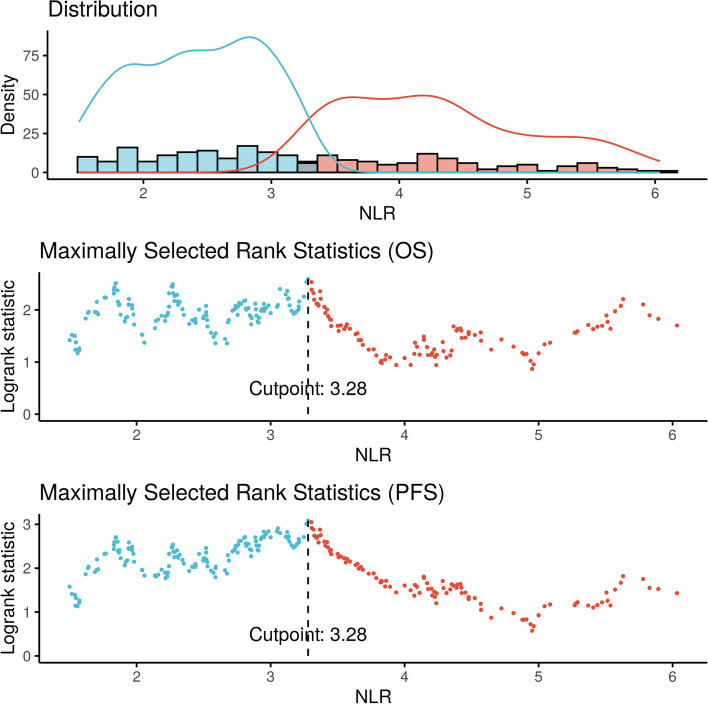
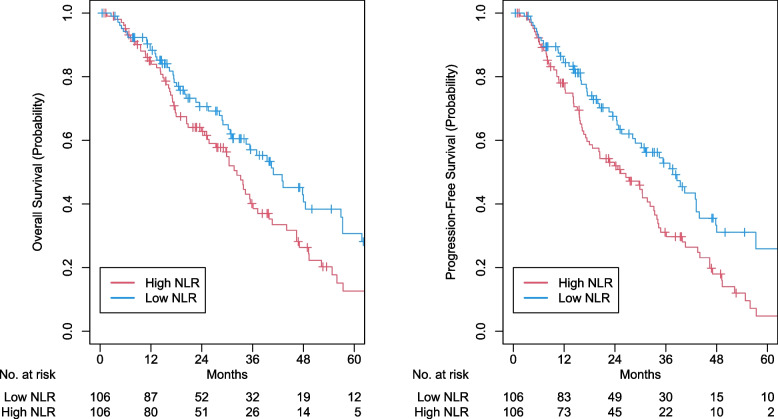
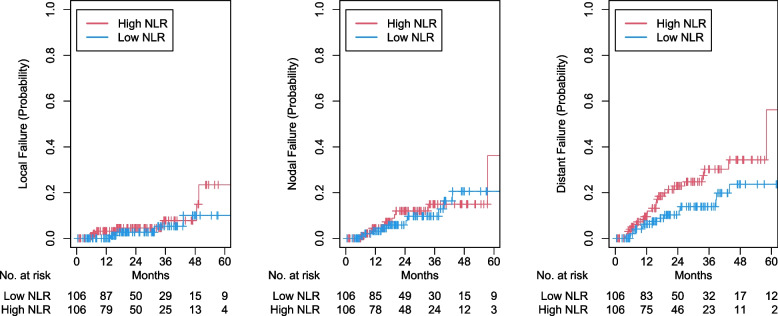
Determined by maximally selected rank statistics, the optimal cutoff value of NLR was 3.28 (Fig. 2). There were 131 patients and 161 patients in the high (≥ 3.28) and low NLR groups, respectively. After propensity matching, high NLR was associated with worse OS (2-year OS 62.9% vs 70.6%; aHR 1.50, 95% CI 1.05–2.15, p = 0.027), and PFS (2-year PFS 52.0% vs 67.6%; aHR 1.68, 95% CI 1.19–2.38, p = 0.003), and DF (2-year DF 22.9% vs 10.3%; aHR 1.97, 95% CI 1.01–3.83, p = 0.045; eTable 2). However, high NLR was not associated with NF (2-year NF 12.0% vs 5.8%; aHR 1.22, 95% CI 0.52–2.87, p = 0.65) and LF (2-year LF 4.5% vs 2.6%; aHR 1.57, 95% CI 0.572–0.792, p = 0.43). Kaplan- Meier curves were generated for OS, PFS, LF, NF, and DF for high versus low NLR (Fig. 3 and 4). In logistic regression, there were no statistically significant variables related to NLR (eTable3). Comparisons between our survival outcomes and those in other published studies analyzing NLR in patients treated with SBRT are described in Table 3.
Fig. 2.
Distribution of neutrophil–lymphocyte ratio (NLR) and threshold assessment using maximum log-rank test statistic
Fig. 3.
Kaplan–Meier curves for high versus low neutrophil–lymphocyte ratio (NLR) for overall survival (OS) and progression-free survival (PFS) after propensity score matching
Fig. 4.
Cumulative incidence curves for high versus low neutrophil–lymphocyte ratio (NLR) for local failure, nodal failure, and distant failure after propensity score matching
Table 3.
Characteristics of studies on the role of pre-treatment NLR on NSCLC patients treated with SBRT
| Author | Year | Region | # pts | Median OS (months) | 3- year OS (%) | Median NLR (range) | NLR cutoff | OS | Tumor recurrence | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High NLR | Low NLR | HR (95% CI) | P value | HR (95% CI) | P value | Type | |||||||
| Current | 2022 | USA | 286 | 23.48 | 40.1 | 57.1 | 3.06 (2.21–4.33) | 3.28 | 1.06 (1.02–1.10) | 0.005a | 1.08 (0.97–1.21) | 0.16 | nodal |
| 1.11(1.05–1.18) | < 0.001a | distant | |||||||||||
| AduQuaye | 2022 | Canada | 61 | 36 | NR | NR | 3.42 (0.27–13.69) | NR | 1.26 (1.04–1.53) | 0.017a | 1.05(1–1.1) | 0.021a | local |
| Kotha | 2021 | USA | 389 | 31.5 | NR | NR | 3.0 (0.4–42) | 4 | 1.44 (1.12–1.86) | 0.01a | NR | NR | NR |
| Sebastian | 2019 | USA | 156 | 32.9 | NR | NR | 3.6 (0.2–41.8) | 3.6 | 1.91 (1.09–3.33) | 0.023a | 1.21 (0.42–3.49) | 0.73 | local |
| 1.30 (0.61–2.74) | 0.5 | regional nodal | |||||||||||
| 1.95 (0.81–4.72) | 0.14 | distant | |||||||||||
| Luo | 2018 | China | 63 | NR | NR | NR | 2.47 (0.86–7.29) | 2.06 | 1.489 (1.096–2.021)b | 0.011b | NR | NR | NR |
| Shaverdian | 2016 | USA | 118 | NR | 61.0 | 92.0 | 2.79(0.95–11.6) | 2.18 | 1.477 (NR) | 0.008a | 0.816 (NR) | 0.456 | locoregional |
| 1.255 (NR) | 0.438 | distant | |||||||||||
| Giuliani | 2016 | Canada | 122 | 43.7 | 41.2 | 66.2 | 3.0 (0.3–22.0) | 3 | 1.22 (1.08–1.38) | 0.001a | NR | NR | NR |
| Cannon | 2015 | USA | 59 | 43 | NR | NR | 2.8 (0.5–33.0) | 2.98 | NR | 0.005a | NR | 0.937 | nonlocal |
pts patients, NR not reported
astatistically significant; bcalculated with univariate analysis (instead of multivariate)
Among those treated with single-fraction SBRT (n = 211) on Cox MVA, NLR as a continuous variable remained statistically significant for OS (aHR 1.09, 95% CI 1.03–1.15, p = 0.001) and PFS (aHR 1.08, 95% CI 1.03–1.14, p = 0.002). On Fine-Gray competing risk MVA, NLR was not statistically significant for nodal failure (aHR 1.06, 95% CI 0.91–1.25, p = 0.4). Number of local (n = 10) and distant failures (n = 27) were too few to analyze in MVA.
Discussion
Pre-treatment NLR was significantly associated with distant failure, progression-free, and overall survival. This is the first study to show that pre-treatment NLR was a statistically significant predictor of increased distant failure and poor PFS in early-stage NSCLC patients treated with single fraction SBRT.
Neutrophils can either stimulate or suppress the cytotoxic T-cell response. The balance between stimulation and suppression may be related to the ratio of neutrophils to lymphocytes [31]. As a biomarker of systemic inflammation, NLR has been shown to serve a prognostic role in various cancers [12–14]. In NSCLC patients treated with either surgical or non-surgical methods, NLR has been shown to be a prognostic factor [32, 33].
Our findings are consistent with previous studies demonstrating high NLR was associated with worse overall survival outcomes in NSCLC patients treated with SBRT [15–21]. On Cox multivariate analysis, high NLR was continuously associated with poor OS and PFS. After propensity matching in our study, patients with a NLR greater than 3.28 were also significantly more likely to have an inferior OS and PFS outcomes after radiation therapy. Our optimal cutoff value of 3.28 was similar to prior studies (range: 2.06–4.00) that analyzed the role of NLR on prognosis of early-stage NSCLC patients after SBRT [15–21]. Our study is the first to describe an association with NLR and distant failure which contrasts with prior smaller studies [16, 18]. Additionally we are the first to show NLR is associated with poor PFS outcomes as a continuous variable and dichotomous variable with a cutoff of 3.28.
An association with PFS and distant failure suggests neutrophils could serve as a therapeutic target for intervention in high NLR patients to improve disease outcomes. Interventions that block TGFβ activity or enhance type I interferon activity at the tumor microenvironment could facilitate neutrophil anti-tumor cytotoxicity [34]. Emerging clinical data suggest radiation plays a key role in the reactivation of the anti-tumor immune response [35].
The immunomodulatory effect of single fraction SBRT on increasing intra-tumor and peripheral blood effector T cells has been shown in humans [36, 37]. Thus, following radiation effector T Cells flood into the tumor. Over the course of 4 weeks, these T-Cell are reduced and suppressor T-Cell numbers increase. Therefore, it is logical that another radiation fraction too close to the first fraction may wipe out the effector T-Cell population. Preclinical models also show a significant benefit to having a long period (10 days) between radiation treatments [38]. Comparison between 1 and 3 fraction SBRT regimens was not the purpose of this study, since such comparison has been already published [6]. However, as shown on Tables 2 and 3 fraction SBRT cohorts had a trend toward worse OS (P = 0.07) and PFS (P = 0.08). In current practice, the only lung SBRT regimen with such an interval between treatments is single fraction where the time to the next treatment is infinite. The use of single fraction SBRT in our cohort may thus explain why pre-treatment NLR was a statistically significant predictor of increased distant failure and poor PFS.
In ongoing clinical trials, PACIFIC-4 (NCT03833154) and SWOG 1914 (NCT04214262), adjuvant immunotherapy is being studied as a novel therapeutic to improve outcomes following SBRT in early-stage NSCLC. Possibly, immunotherapy may have the greatest benefit in patients at risk for poor outcomes with SBRT alone such as those with high NLR. Given these findings, consideration should be made for NLR to be tracked in SBRT trials.
Limitations
Our study has limitations inherent in retrospective reviews including that some patients, especially those who came from a distance, had limited follow up. Too few local failures occurred in our patient cohort for analysis. Additionally, pre-treatment NLR data was collected at a single point in time, neglecting dynamic changes in NLR prior to or after treatment. Although treatment for adenocarcinoma and squamous cell NSCLC is different, separate cohorts for each histology were too small for any further analysis.
Conclusions
In our single-institution study, NLR was an independent, adverse prognostic factor for poor distant recurrence, progression-free, and overall survival in peripheral early-stage NSCLC patients treated with single fraction SBRT.
Supplementary Information
Additional file 1: eTable 1. Fine-Gray multivariate analysis for nodal and distant failure recurrences. eTable 2. Characteristics of NSCLC patients after propensity score matching (n=214). eTable 3. Logistic regression of NSCLC patient cohort to identify related variables to NLR.
Acknowledgements
Not applicable.
Authors’ contributions
K.H., S.P., S.M., and A.I. wrote the main manuscript text. K.H., S.M., and H.Y. prepared all figures. K.H. and S.M. prepared all tables. All authors edited substantial portions of the manuscript, provided the interpretation of the data, and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Cancer Institute Cancer Center Support Grant (P30CA016056). This project is supported in part by funding from the National Cancer Institute of the National Institutes of Health under Award number: R25CA181003. Sponsors had no role in the preparation of this manuscript.
Availability of data and materials
Data cannot be shared publicly because of protected health information. Data are available from the Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Research data will be shared upon request to the corresponding author.
Declarations
Ethics approval and consent to participate
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Roswell Park Comprehensive Cancer Center (EDR-171710). A waiver of consent was obtained from the Institutional Review Board of Roswell Park Comprehensive Cancer Center due to the retrospective nature of the study making consent impractical and contacting patients to obtain consent would pose a greater risk than the waiver.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Karen Huang and Sharan Prasad contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(5):497–530. doi: 10.6004/jnccn.2022.0025. [DOI] [PubMed] [Google Scholar]
- 3.Prezzano KM, Ma SJ, Hermann GM, Rivers CI, Gomez-Suescun JA, Singh AK. Stereotactic body radiation therapy for non-small cell lung cancer: a review. World Journal of Clinical Oncology. 2019;10(1):14–27. doi: 10.5306/wjco.v10.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings MA, Ma SJ, Hermann G, et al. Comparison of single- and five-fraction regimens of stereotactic body radiation therapy for peripheral early-stage non-small-cell lung cancer: a two-institution propensity-matched analysis. Clin Lung Cancer. 2018;19(6):511–517. doi: 10.1016/j.cllc.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Ma SJ, Syed YA, Rivers CI, Gomez Suescun JA, Singh AK. Comparison of single- and five-fraction schedules of stereotactic body radiation therapy for central lung tumours: a single institution experience. J Radiother Pract. 2017;16(2):148–154. doi: 10.1017/S1460396917000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma SJ, Serra LM, Syed YA, Hermann GM, Gomez-Suescun JA, Singh AK. Comparison of single- and three-fraction schedules of stereotactic body radiation therapy for peripheral early-stage non-small-cell lung cancer. Clin Lung Cancer. 2018;19(2):e235–e240. doi: 10.1016/j.cllc.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh AK, Gomez-Suescun JA, Stephans KL, et al. One versus three fractions of stereotactic body radiation therapy for peripheral stage I to II non-small cell lung cancer: a randomized, multi-institution, phase 2 trial. Int J Radiat Oncol Biol Phys. 2019;105(4):752–759. doi: 10.1016/j.ijrobp.2019.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Videtic GM, Hu C, Singh AK, et al. A randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer: NRG oncology RTOG 0915 (NCCTG N0927) Int J Radiat Oncol Biol Phys. 2015;93(4):757–764. doi: 10.1016/j.ijrobp.2015.07.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tjong MC, Louie AV, Singh AK, et al. Single-fraction stereotactic ablative body radiotherapy to the lung - the knockout punch. Clin Oncol (R Coll Radiol) 2022;34(5):e183–e194. doi: 10.1016/j.clon.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Bartl AJ, Mahoney M, Hennon MW, et al. Systematic review of single-fraction stereotactic body radiation therapy for early stage non-small-cell lung cancer and lung oligometastases: how to stop worrying and love one and done. Cancers (Basel). 2022;14(3):790. doi: 10.3390/cancers14030790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma SJ, Cummings M, Serra LM, et al. Three- Versus Five-Fraction Regimens of Stereotactic Body Radiotherapy for Peripheral Early-Stage Non-Small-Cell Lung Cancer: A Two-Institution Propensity Score-Matched Analysis. Clin Lung Cancer. 2018;19(3):e297–e302. doi: 10.1016/j.cllc.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Reddy AV, Hill CS, Sehgal S, et al. High neutrophil-to-lymphocyte ratio following stereotactic body radiation therapy is associated with poor clinical outcomes in patients with borderline resectable and locally advanced pancreatic cancer. J Gastrointest Oncol. 2022;13(1):368–379. doi: 10.21037/jgo-21-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills M, Reddy AV, Richardson L, Richardson KM, Kersh CR. Pre-treatment Neutrophil-to-Lymphocyte Ratio (NLR) Predicts Survival in Patients with Malignant Adrenal Lesions treated with Stereotactic Body Radiotherapy (SBRT) Int J Radiat Oncol Biol Phys. 2019;105(1, Supplement):E569. [Google Scholar]
- 14.Park Y, Chang AR. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Hepatocellular Carcinoma Treated with Stereotactic Body Radiotherapy. Korean J Gastroenterol. 2022;79(6):252–259. doi: 10.4166/kjg.2022.021. [DOI] [PubMed] [Google Scholar]
- 15.Cannon NA, Meyer J, Iyengar P, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol. 2015;10(2):280–285. doi: 10.1097/JTO.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 16.Shaverdian N, Veruttipong D, Wang J, Schaue D, Kupelian P, Lee P. Pretreatment Immune Parameters Predict for Overall Survival and Toxicity in Early-Stage Non-Small-Cell Lung Cancer Patients Treated With Stereotactic Body Radiation Therapy. Clin Lung Cancer. 2016;17(1):39–46. doi: 10.1016/j.cllc.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Kotha NV, Cherry DR, Bryant AK, Nalawade V, Stewart TF, Rose BS. Prognostic utility of pretreatment neutrophil-lymphocyte ratio in survival outcomes in localized non-small cell lung cancer patients treated with stereotactic body radiotherapy: Selection of an ideal clinical cutoff point. Clin Transl Radiat Oncol. 2021;28:133–140. doi: 10.1016/j.ctro.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebastian N, Wu T, Bazan J, et al. Pre-treatment neutrophil-lymphocyte ratio is associated with overall mortality in localized non-small cell lung cancer treated with stereotactic body radiotherapy. Radiother Oncol. 2019;134:151–157. doi: 10.1016/j.radonc.2019.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo H, Ge H, Cui Y, et al. Systemic inflammation biomarkers predict survival in patients of early stage non-small cell lung cancer treated with stereotactic ablative radiotherapy - a single center experience. J Cancer. 2018;9(1):182–188. doi: 10.7150/jca.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giuliani M, Sampson LR, Wong O, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes on outcomes in lung stereotactic body radiotherapy. Curr Oncol. 2016;23(4):e362–368. doi: 10.3747/co.23.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aduquaye M, Dube S, Bashir B, et al. Impact of pre-treatment NLR and other hematologic biomarkers on the outcomes of early-stage non-small-cell lung cancer treated with stereotactic body radiation therapy. Curr Oncol. 2022;29(1):193–208. doi: 10.3390/curroncol29010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrugia M, Yu H, Ma SJ, et al. Right atrial dose is associated with worse outcome in patients undergoing definitive stereotactic body radiation therapy for central lung tumors. Cancers (Basel). 2022;14(6):1391. doi: 10.3390/cancers14061391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrugia M, Ma SJ, Hennon M, et al. Exceeding radiation dose to volume parameters for the proximal airways with stereotactic body radiation therapy is more likely for ultracentral lung tumors and associated with worse outcome. Cancers (Basel). 2021;13(14):3463. doi: 10.3390/cancers13143463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma SJ, Mix M, Rivers C, Hennon M, Gomez J, Singh AK. Mortality following single-fraction stereotactic body radiation therapy for central pulmonary oligometastasis. J Radiosurg SBRT. 2017;4(4):325–330. [PMC free article] [PubMed] [Google Scholar]
- 25.Syed YA, Ma SJ, Gomez Suescun JA. Unilateral vocal cord paralysis in squamous cell lung cancer treated with stereotactic body radiation therapy. J Radiother Pract. 2016;15(4):405–407. doi: 10.1017/S1460396916000388. [DOI] [Google Scholar]
- 26.Ma SJ, Yu H, Yu B, et al. Association of pack-years of cigarette smoking with survival and tumor progression among patients treated with chemoradiation for head and neck cancer. JAMA Netw Open. 2022;5(12):e2245818. doi: 10.1001/jamanetworkopen.2022.45818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 28.Ma SJ, Yu H, Khan M, et al. Defining the optimal threshold and prognostic utility of pre-treatment hemoglobin level as a biomarker for survival outcomes in head and neck cancer patients receiving chemoradiation. Oral Oncol. 2022;133:106054. doi: 10.1016/j.oraloncology.2022.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma SJ, Yu H, Khan M, et al. Evaluation of optimal threshold of neutrophil-lymphocyte ratio and its association with survival outcomes among patients with head and neck cancer. JAMA Netw Open. 2022;5(4):e227567. doi: 10.1001/jamanetworkopen.2022.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma SJ, Yu H, Khan M, et al. Evaluation of optimal threshold of neutrophil-lymphocyte ratio and its association with survival outcomes among patients with head and neck cancer. JAMA Netw Open. 2022;5(4):e227567–e227567. doi: 10.1001/jamanetworkopen.2022.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non–small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137(2):425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 33.Gu XB, Tian T, Tian XJ, Zhang XJ. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep. 2015;5:12493. doi: 10.1038/srep12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granot Z. Neutrophils as a therapeutic target in cancer. Front Immunol. 2019;10:1710. doi: 10.3389/fimmu.2019.01710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boustani J, Grapin M, Laurent PA, Apetoh L, Mirjolet C. The 6th R of radiobiology: reactivation of anti-tumor immune response. Cancers (Basel). 2019;11(6):860. doi: 10.3390/cancers11060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow J, Hoffend NC, Abrams SI, Schwaab T, Singh AK, Muhitch JB. Radiation induces dynamic changes to the T cell repertoire in renal cell carcinoma patients. Proc Natl Acad Sci U S A. 2020;117(38):23721–23729. doi: 10.1073/pnas.2001933117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh AK, Winslow TB, Kermany MH, et al. A pilot study of stereotactic body radiation therapy combined with cytoreductive nephrectomy for metastatic renal cell carcinoma. Clin Cancer Res. 2017;23(17):5055–5065. doi: 10.1158/1078-0432.CCR-16-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore C, Hsu CC, Chen WM, et al. Personalized Ultrafractionated Stereotactic Adaptive Radiotherapy (PULSAR) in preclinical models enhances single-agent immune checkpoint blockade. Int J Radiat Oncol Biol Phys. 2021;110(5):1306–1316. doi: 10.1016/j.ijrobp.2021.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: eTable 1. Fine-Gray multivariate analysis for nodal and distant failure recurrences. eTable 2. Characteristics of NSCLC patients after propensity score matching (n=214). eTable 3. Logistic regression of NSCLC patient cohort to identify related variables to NLR.
Data Availability Statement
Data cannot be shared publicly because of protected health information. Data are available from the Institutional Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Research data will be shared upon request to the corresponding author.