Abstract
Ventricular arrhythmias are common following left ventricular assist device implantation (LVAD), and the effects of ventricular tachycardia (VT) ablation on thrombosis and embolic events are unknown. We aimed to assess LVAD thrombosis, stroke, and embolic event rates after VT ablation. Left ventricular assist device implantation patients from two academic centers who underwent endocardial VT ablation between 2009 and 2016 were compared to a control group with VT who were not ablated and followed for one year. The primary composite outcome was confirmed or suspected LVAD thrombosis, stroke, or other embolic event. Survival analysis was conducted with Kaplan-Meier curves, log-rank tests, and Cox regression. Forty-three LVAD patients underwent VT ablation, and 73 LVAD patients had VT but were not ablated. Patients who were ablated were more likely have VT prior to LVAD (p = 0.04), monomorphic VT (p < 0.01), and to be on antiarrhythmics (p < 0.01). Fifty-eight percent of the patients in the ablation group experienced the primary composite outcome (11% had confirmed device thrombosis [DT], 41% suspected DT, 39% had a stroke or embolic event) compared to 30% in the control group (12% with confirmed DT, 11% with suspected DT, 14% with stroke or embolic event) (p = 0.002). In multivariable regression, ablation was an independent predictor of the primary composite outcome (hazard ratios, 2.24; 95% confidence interval, 1.09–4.61; p = 0.03). Patients with LVADs referred for endocardial VT ablation had elevated rates of DT and embolic events.
Keywords: LVAD, ventricular tachycardia, ablation, thrombosis
Left ventricular assist devices (LVAD) provide continuous unloading of the failing left ventricle (LV) and have been shown to dramatically improve survival and quality of life in patients with end-stage heart failure.1–3 Ventricular arrhythmias (VA) are common after LVAD implantation with rates of appropriate defibrillation therapy ranging from 22% to 52%.4–7 The first postoperative month, in particular, is associated with a high VA burden with up to 75% of VA occurring within 4 weeks of device implantation.5 Continuous ventricular unloading allows for adequate end-organ perfusion under variable loading conditions, and thus VA which are often lethal in patients with heart failure are acutely well tolerated in patients supported with an LVAD.7–10 Prolonged and intractable VA, however, are often poorly tolerated owing to deleterious effects on right ventricular function.11
Ventricular arrhythmia in patients supported with LVADs are often refractory to antiarrhythmic drug therapy, and endocardial ventricular tachycardia (VT) ablation is becoming more common.11–14 When successful at eliminating VA, ablation is associated with improved 1-year survival.15 Endocardial radiofrequency (RF) ablation, however, is known to activate platelets and the coagulation cascade necessitating full anticoagulation during the procedure.16 Evidence is mounting on the frequency of silent cerebral embolic events following endocardial VT and premature ventricular contraction (PVC) ablation in non-heart failure patients.17
Despite improvements in pump design over the years, cardioembolic events and pump thrombosis remain a significant source of morbidity and mortality for LVAD patients.18,19 Although rates of pump thrombosis have dramatically improved with the development of the HeartMate 3 (Abbott, Chicago, IL), pump thrombosis remain elevated for the HeartMate II (Abbott, Chicago, IL) and HeartWare HVAD (Medtronic, Minneapolis, MN) pumps.19–21 Stroke rates remain unacceptably high regardless of the pump type, although the HeartMate 3 appears to have a lower late stroke rate.21 Stroke rates and pump thrombosis with the HVAD can be mitigated with more aggressive antiplatelet therapy, tighter anticoagulation targets, and blood pressure control, although a similar association has not been shown with the other pump types.19,22
This study aimed to investigate the ramifications of endocardial VT ablation on LVAD device thrombosis and cardioembolic rates.
Methods
Patient Population
The data, analytic methods, and study materials will be made available to other researches for purposes of reproducing the results or replicating the procedure. Requests to access the dataset from qualified researchers may be sent to the corresponding author. All LVAD (HeartMate II or HeartWare HVAD) patients who underwent catheter ablation for VA from 2009 to 2016 at the University of Chicago and Columbia University were included in the treatment arm. The control arm included patients who had an LVAD (Heartmate II or HVAD) placed from 2010 to 2016 at the University of Chicago and had an electrophysiology (EP) consult for sustained VA after LVAD placement and did not undergo catheter ablation. Institutional review board approval at both centers was obtained. Ventricular arrhythmia was defined as >30 seconds of VA, device therapy for VA including either antitachycardia pacing or implantable cardioverter-defibrillator shock, or VA requiring external shock. Patients were excluded if they had an EP study or mapping, but no ablation was performed. Data regarding demographics, medical comorbidities, medication use, and device therapy were collected at the time of VT ablation for the intervention arm and initial EP consult for the control group. The presence of VT storm (defined as >3 episodes of VT requiring device therapy or incessant VT requiring escalation of intravenous antiarrhythmic drugs within a 24-hour time period) was recorded for both groups if it was present from 2 months before ablation to 1 year after ablation and either present on or within 1 year after the initial EP consult for VA. Patients were followed for one year after ablation or EP consult. Echocardiographic parameters and right heart catheterization hemodynamics were recorded from the study closest to ablation or EP consult, and right heart dysfunction was defined as a pulmonary artery pulsatility index <2.
The primary composite endpoint was a combination of confirmed thrombosis (defined as thrombosis seen on autopsy, thrombus visualized intraoperatively during device explant, or identified during device disassembling after explant), suspected thrombosis (clinical suspicion of device thrombus), and the addition of at least one of the following: lactate dehydrogenase (LDH) >2.5 the upper limit of normal, positive RAMP study (LV end-diastolic dimension slope of >−0.16 cm/stage during incremental LVAD speed increase), or power spike of >10 watts or >2 watts above the baseline power), and cardioembolism (stroke or other systemic emboli). Secondary outcomes included death, transplant, and recurrent VT after ablation.
Electrophysiology Study and Ablation
Patients were referred both as inpatients and outpatients due to recurrent VA refractory to appropriate medical therapy, and the procedures were performed either at Columbia University or the University of Chicago. Therapeutic anticoagulation with Coumadin was uninterrupted for the procedure or the patients received therapeutic heparin periprocedurally. Heparin was administered during the procedure to maintain a goal activated clotting time greater than 300 seconds prior to any left-sided instrumentation. Left ventricular assist device controller parameters were continuously monitored during the procedure. All initial cases were endocardial ablations only. Access to the LV, either trans-septal or retrograde access, was done at the operator’s discretion. Three-dimensional electroanatomic endocardial voltage maps were created using commercially available mapping systems. Intracardiac echocardiography was used at the operators discretion. Ventricular arrhythmias were induced with programmed stimulation when feasible and activation, entrainment mapping, or pace mapping were performed as appropriate. The primary endpoint for ablation was termination and noninducibility of the clinical VT whenever possible; additional substrate modification and targeting of nonclinical VAs were performed at the discretion of the primary operator.
Statistical Methods
Categorical variables were presented as numbers and percentages and analyzed using a Chi-square test or, if there were small expected numbers, a Fisher exact test. Continuous variables were presented as means with SD and analyzed with t-tests if they were normally distributed. Non-normally distributed continuous variables are reported as medians with interquartile ranges (IQR) and differences between groups compared with the Wilcoxon rank sum test. Statistical significance was determined by a two-sided p ≤ 0.05.
Time to the primary outcome was analyzed with Kaplan-Meier survival analysis and log-rank tests. Univariable Cox proportional hazard regression was performed to identify predictors of the primary composite outcome and results reported as hazard ratios (HR) with p values and 95% confidence intervals (CI). Any predictor with a p ≤ 0.20 was included for multivariable Cox regression. Predictors demonstrating significant multicollinearity were excluded. Any predictor with a p ≤ 0.05 was considered significant in the multivariable model, and all significant predictors were assessed for interaction with all other significant predictors and reported if significant interaction effects were found. The proportionality assumption was tested for the final multivariable model. Stata 14.2 (StataCorp, College Station, TX) was used for data analysis.
Results
Baseline Characteristics
Forty-three LVAD patients (22 University of Chicago, 21 Columbia University) with refractory VT underwent endocardial VT ablation between 2009 and 2016, and they were compared to 73 patients with VT warranting electrophysiology consultation who did not undergo ablation. Baseline characteristics of the cohort are summarized in Table 1. The ablation arm and control arm were similar for most variables, although the ablation arm had more patients meeting the formal definition of VT storm (63% vs. 26%, p < 0.001), more monomorphic VT (100% vs. 74%, p <0.001) and had a history of VT prior to LVAD implantation (67% vs. 47%, p = 0.04). There was no difference in the rates of right ventricular dysfunction, defined using either hemodynamic variables or echocardiographic assessment between the two groups. The ablation arm was more likely to be on an antiarrhythmic medication, whereas the control arm was more likely to be on inotropic therapy. The University of Chicago and Columbia cohorts were similar with the notable exception of more patients with VT storm ablated at Columbia University (see Table 1, Supplemental Digital Content 1, http://links.lww.com/ASAIO/A491).
Table 1.
Baseline Characteristics
| Ablation (n = 43) |
No Ablation (n = 73) |
P Value | |
|---|---|---|---|
| General Characteristics | |||
| Age, years, mean ± SD | 62.8 ± 10.4 | 60.5 ± 11.4 | 0.28 |
| Male, n (%) | 39 (91) | 60 (82) | 0.21 |
| LVAD Characteristics | |||
| Duration of LVAD to time of first VT, days, median (IQR) | 18 (5–55) | 41 (9–231) | 0.64 |
| Destination, n (%): | |||
| BTT | 11 (26) | 19 (26) | 0.96 |
| DT | 32 (74) | 54 (74) | |
| LVAD Type, n (%): | 0.98 | ||
| HMII | 36 (84) | 61 (84) | |
| HVAD | 7 (16) | 12 (16) | |
| LVAD Speed (RPM) | |||
| HMII | 8989 ± 374 | 9164 ± 469 | 0.15 |
| HVAD | 2730 ± 42 | 2681 ± 170 | 0.70 |
| Inflow Cannula Angle (degrees) | 61.6 ± 29.4 | 72.3 ± 19.8 | 0.053 |
| Origin of Cardiomyopathy | 0.27 | ||
| Ischemic, n (%) | 28 (65) | 32 (44) | |
| Nonischemic, n (%) | 15 (35) | 41 (56) | |
| Medical History | |||
| Hypertension, n (%) | 20 (47) | 43 (59) | 0.20 |
| Hyperlipidemia, n (%) | 15 (35) | 49 (67) | 0.001 |
| Atrial Fibrillation, n (%) | 21 (49) | 46 (63) | 0.14 |
| DM, n (%) | 11 (26) | 32 (44) | 0.05 |
| COPD, n (%) | 8 (19) | 17 (23) | 0.55 |
| PAD, n (%) | 7 (16) | 3 (4) | 0.04 |
| CVA, n (%) | 6 (14) | 11 (15) | 0.87 |
| Prior Sternotomy, n (%) | 22 (51) | 25 (34) | 0.07 |
| VT Storm, n (%) | 27 (63) | 19 (26) | <0.001 |
| VT Prior to LVAD, n (%) | 29 (67) | 34 (47) | 0.04 |
| VT Ablation Prior to LVAD, n (%) | 3 (7) | 3 (4) | 0.67 |
| Monomorphic VT, n (%) | 43 (100) | 54 (74) | <0.001 |
| ICD Shock, n (%) | 31 (72) | 44 (60) | 0.20 |
| Therapeutic Anticoagulation, n (%) | 28 (65) | 44 (60) | 0.67 |
| Hemodynamic RV Dysfunction (PAPi < 2), n (%) | 11 (26) | 28 (38) | 0.16 |
| Echocardiographic RV Dysfunction (Moderate or Greater), n (%) | 33 (77) | 54 (74) | 0.74 |
| Left Ventricular End Diastolic Dimension (cm) ± SD | 6.5 ± 1.2 | 6.7 ± 1.5 | 0.58 |
| Mean Arterial Blood Pressure (mmHg) ± SD | 84 ± 12 | 85 ± 17 | 0.78 |
| Medical and Device Therapy | |||
| Beta Blocker, n (%) | 29 (67) | 29 (40) | 0.004 |
| ACE/ARB, n (%) | 6 (14) | 20 (27) | 0.09 |
| Mineralocorticoid Antagonist, n (%) | 18 (42) | 25 (34) | 0.41 |
| ICD, n (%) | 42 (98) | 71 (97) | 1 |
| CRT, n (%) | 32 (74) | 46 (63) | 0.21 |
| Amiodarone, n (%) | 37 (86) | 47 (64) | 0.01 |
| Lidocaine, n (%) | 14 (33) | 9 (12) | 0.008 |
| Mexilitine, n (%) | 12 (28) | 8 (11) | 0.02 |
| Dofetilide, n (%) | 1 (2) | 0 (0) | 0.37 |
| Sotalol, n (%) | 3 (7) | 0 (0) | 0.05 |
| Esmolol, n (%) | 6 (14) | 0 (0) | 0.002 |
| Any Antiarrhythmic, n (%) | 41 (95) | 50 (68) | < 0.001 |
| Inotropes, n (%) | 4 (9) | 20 (27) | 0.03 |
| Vasoactive Medications, n (%) | 6 (14) | 3 (4) | 0.08 |
Baseline characteristics in the control group and those undergoing ablation.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BTT, bridge to transplant; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; CVA, stroke; DM, diabetes mellitus; DT, destination therapy; HMII, HeatMate II; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; LVAD, left ventricular assist device; PAD, peripheral arterial disease; PAPi, pulmonary artery pulsatility index; RV, right ventricle; VT, ventricular tachycardia.
Device Thrombosis and Embolism
Left ventricular assist device patients in the ablation group had increased rates of the composite outcomes confirmed and suspected thrombosis (HR, 2.63; 95% CI, 1.28–5.42; p = 0.01; ablation n = 17, control n = 13) and the primary composite outcome of confirmed or suspected thrombosis and stroke or embolic event (HR, 2.46; 95% CI, 1.28–4.70; p = 0.01; ablation n = 20, control n = 17) (Figures 1 and 2). The median time to the first event of the composite primary outcome was 72 days (Q1 = 32 days, Q3 = 200 days, IQR, 168 days). There was no difference in the rate of confirmed device thrombosis between those who were and were not ablated (HR, 0.85; 95% CI, 0.21–3.41; p = 0.82; ablation n = 3, control n = 6). Those who underwent ablation had a higher rate of suspected device thrombosis (HR, 3.95; 95% CI, 1.60–9.81; p < 0.01; ablation n = 14, control n = 7) and stroke or other embolic event (HR, 2.93; 95% CI, 1.20–7.20; p = 0.02; ablation n = 12, control n = 8) (Figure 3). There was no difference in rate of the primary composite outcome between those who had recurrent VA after ablation and those who did not (HR, 1.82; 95% CI, 0.73–4.52; p = 0.20; recurrent VA after ablation n = 12/25, no recurrent VA after ablation n = 8/18). A trans-septal approach was utilized in 76% of patients in the University of Chicago cohort, and there was no difference in the primary composite endpoint when compared to patients who had a retrograde approach. The center of ablation (Columbia University or The University of Chicago) did not impact the rate of the composite endpoint (Table 2).
Figure 1.
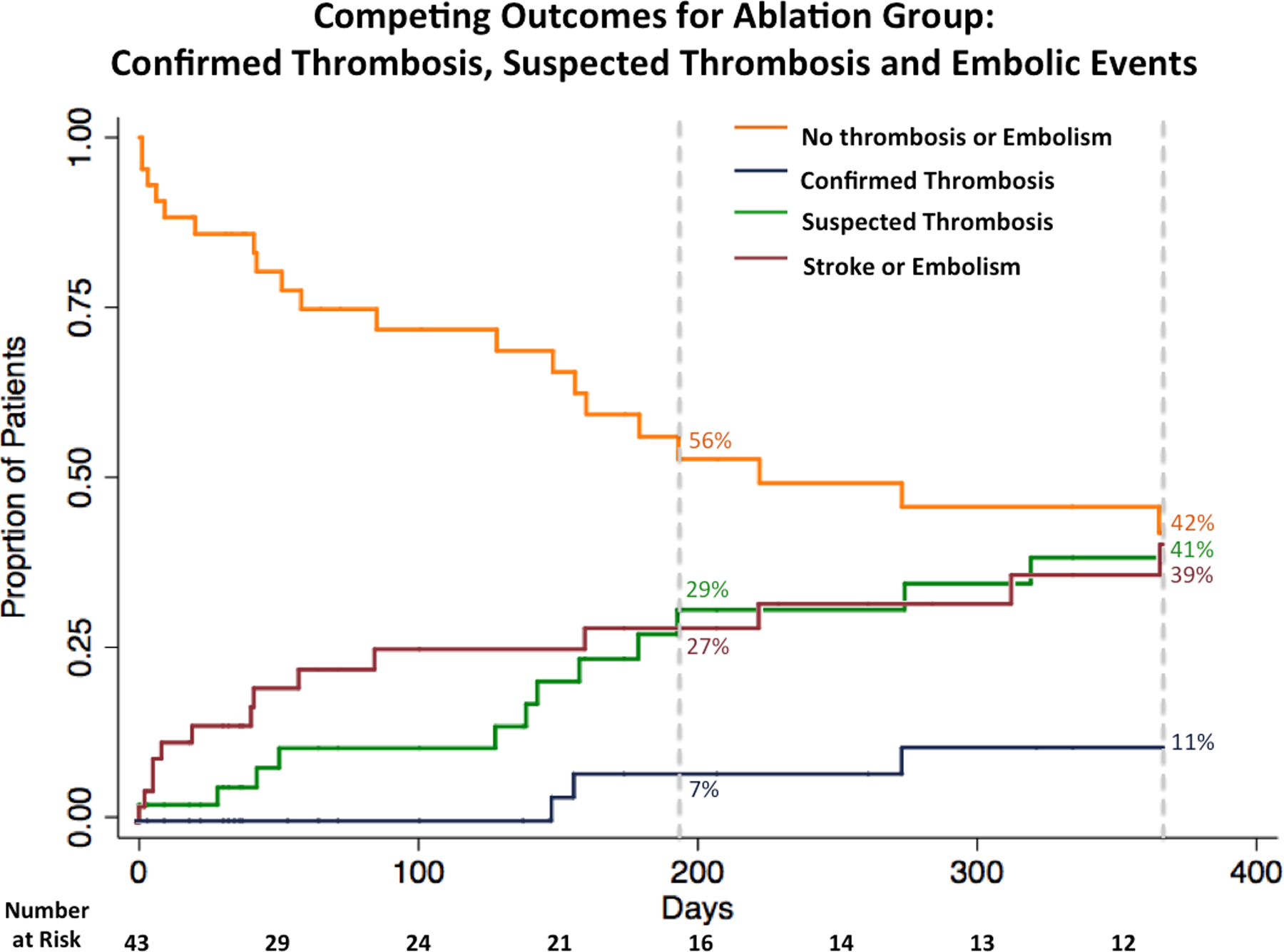
Competing outcomes for the ablation group. Kaplan-Meier survival curve for the composite outcome of confirmed or suspected thrombosis and stroke or embolism and Kaplan-Meier event curves for the individual outcomes confirmed thrombosis, suspected thrombosis, and stroke or embolism.
Figure 2.
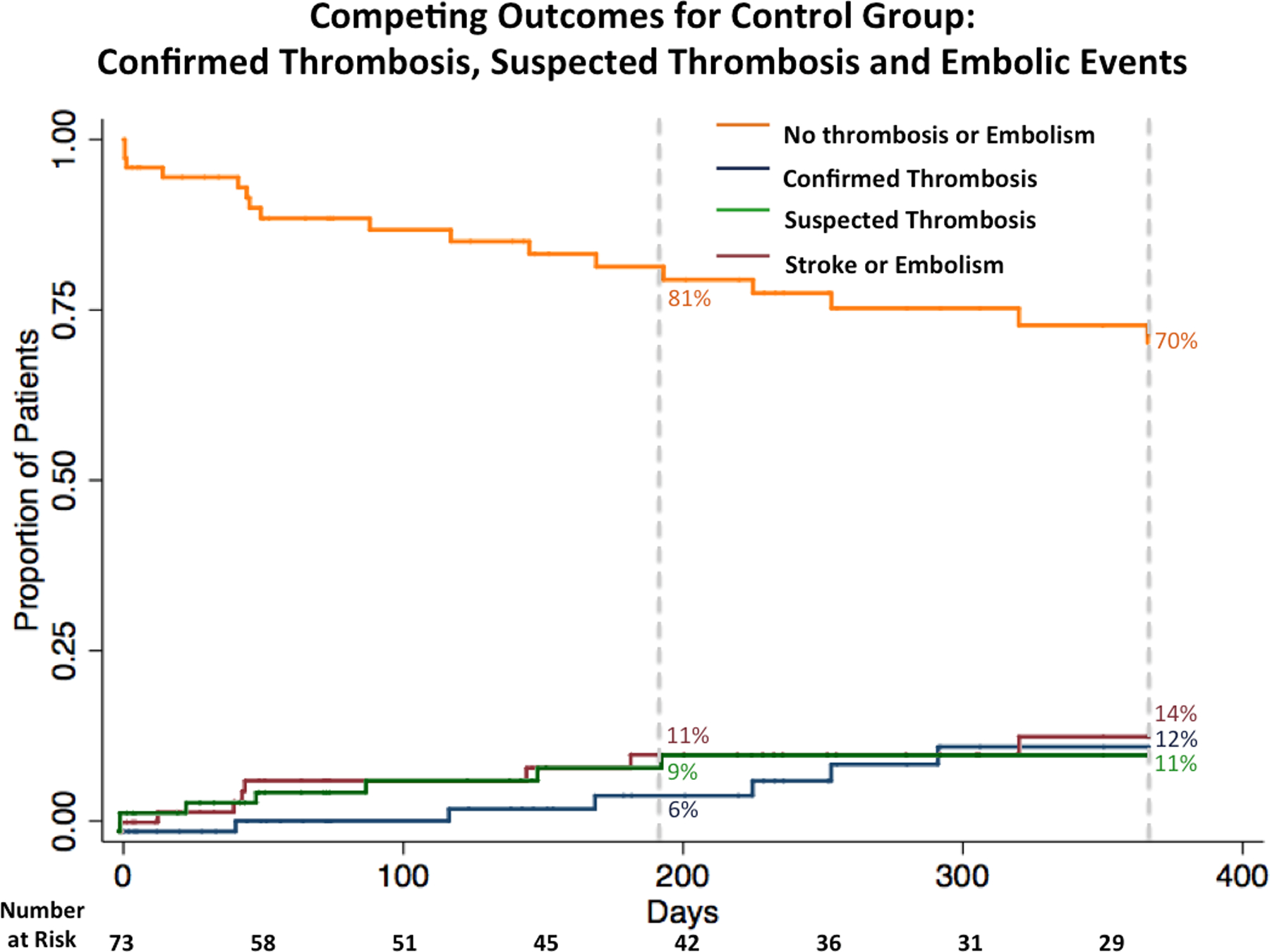
Competing outcomes for the control group. Kaplan-Meier survival curve the composite outcome of confirmed or suspected thrombosis and stroke or embolism and Kaplan-Meier event curves for the individual outcomes confirmed thrombosis, suspected thrombosis, and stroke or embolism.
Figure 3.
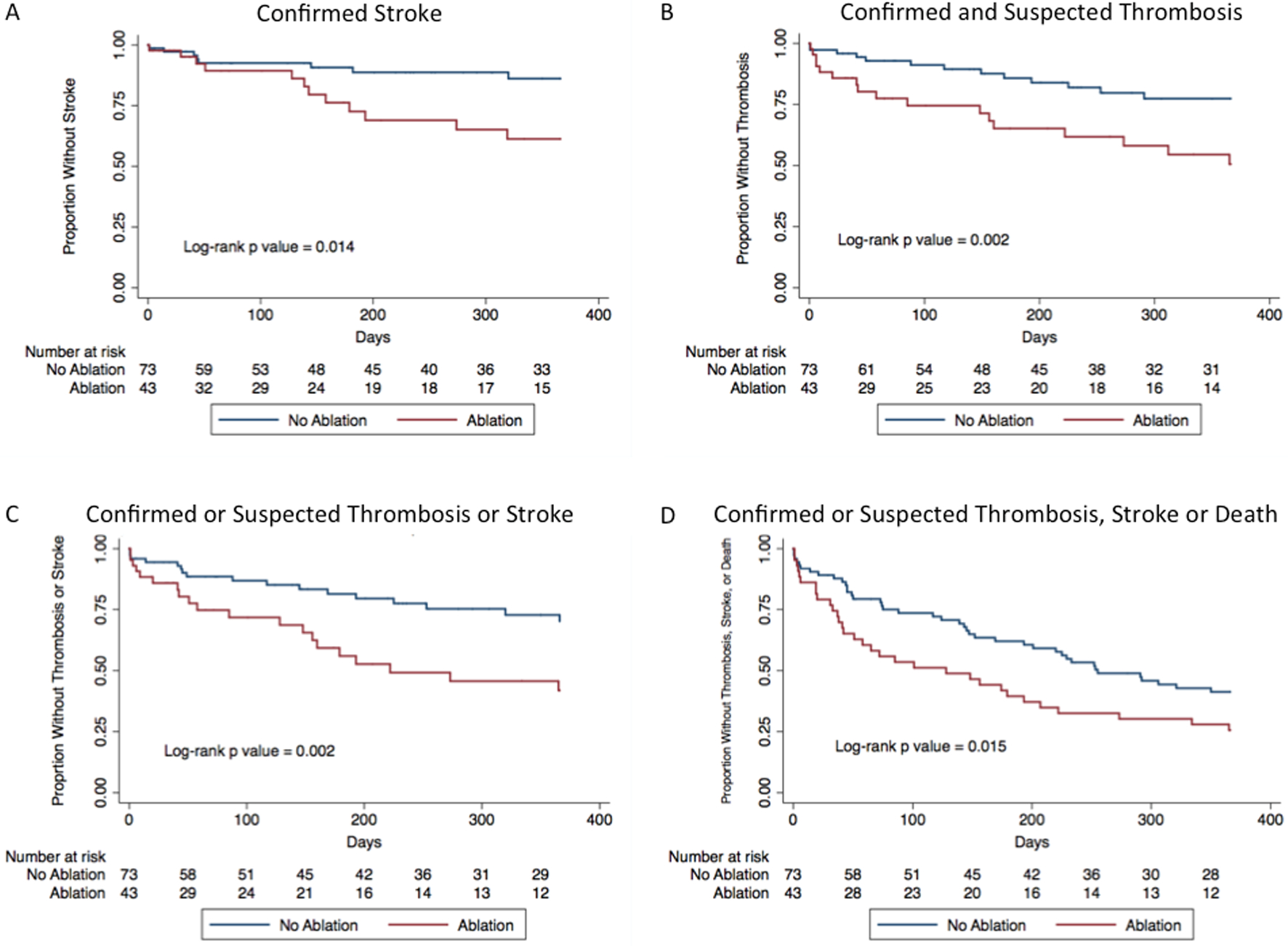
Kaplan-Meier survival curves for individual and composite outcomes between the ablation group and control group. A: Kaplan-Meier curves for stroke or embolic event; (B) Kaplan-Meier curves for confirmed and suspected device thrombosis; (C) Kaplan-Meier curves for the primary composite outcome of confirmed or suspected thrombosis and stroke or embolic event; and (D) Kaplan-Meier curves of the composite outcome of confirmed or suspected thrombosis, stroke or embolic event, and death.
Table 2.
Predictors of Confirmed or Suspected Thrombosis or Cardioembolism
| Variable | Univariate | Multivariate | |
|---|---|---|---|
| Ablation | HR | 2.46 | 2.07 |
| CI | 1.28–4.70 | 0.96–4.48 | |
| P | 0.007 | 0.06 | |
| Time of Ventricular Tachycardia from LVAD (days) | HR | 1.00 | |
| CI | 0.999–1.001 | ||
| P | 0.82 | ||
| Type of LVAD (HeartWare) | HR | 1.33 | |
| CI | 0.61–2.90 | ||
| P | 0.48 | ||
| Age (Per 1 Year Increase) | HR | 0.96 | 0.94 |
| CI | 0.93–0.99 | 0.91–0.97 | |
| P | 0.004 | 0.001 | |
| Sex (Male) | HR | 0.98 | |
| CI | 0.41–2.35 | ||
| P | 0.96 | ||
| Bridge to Transplant | HR | 1.10 | |
| CI | 0.53–2.28 | ||
| P | 0.79 | ||
| Non-Ischemic | HR | 1.11 | |
| Cardiomyopathy | CI | 0.58–2.11 | |
| P | 0.76 | ||
| Hypertension | HR | 1.07 | |
| CI | 0.56–2.05 | ||
| P | 0.84 | ||
| Hyperlipidemia | HR | 1.05 | |
| CI | 0.55–2.01 | ||
| P | 0.89 | ||
| Atrial Fibrillation | HR | 0.55 | 0.66 |
| CI | 0.29–1.06 | 0.32–1.36 | |
| P | 0.07 | 0.26 | |
| Diabetes | HR | 1.04 | |
| CI | 0.54–2.02 | ||
| P | 0.91 | ||
| Chronic Obstructive | HR | 0.87 | |
| Pulmonary Disease | CI | 0.40–1.91 | |
| P | 0.73 | ||
| Peripheral Arterial Disease | HR | 3.45 | 3.88 |
| CI | 1.51–7.88 | 1.58–9.52 | |
| P | 0.003 | 0.003 | |
| Stroke | HR | 1.60 | |
| CI | 0.70–3.64 | ||
| P | 0.27 | ||
| Prior Sternotomy | HR | 0.78 | |
| CI | 0.40–1.53 | ||
| P | 0.47 | ||
| Antiarrhythmic Use | HR | 1.17 | |
| CI | 0.53–2.55 | ||
| P | 0.70 | ||
| Beta Blocker | HR | 1.58 | * |
| CI | 0.81–3.07 | ||
| P | 0.18 | ||
| Angiotensin Converting | HR | 0.913 | |
| Enzyme Inhbitor / Angio- | CI | 0.42–2.0 | |
| Tensin Receptor Blocker | P | 0.82 | |
| Spironolactone | HR | 1.11 | |
| CI | 0.57–2.16 | ||
| P | 0.76 | ||
| Implantable Cardioverter | HR | 0.64 | |
| Defibrillator | CI | 0.09–4.67 | |
| P | 0.66 | ||
| Cardiac Resynchronization | HR | 1.29 | |
| Therapy | CI | 0.64–2.61 | |
| P | 0.48 | ||
| Inotrope Use | HR | 1.25 | |
| CI | 0.57–2.74 | ||
| P | 0.58 | ||
| Vasoactive Use | HR | 3.29 | 1.75 |
| CI | 0.96–11.22 | 0.47–6.53 | |
| P | 0.06 | 0.40 | |
| Ventricular Tachycardia | HR | 1.15 | |
| Prior to LVAD | CI | 0.59–2.21 | |
| P | 0.69 | ||
| Ventricular Tachycardia | HR | 1.08 | |
| Ablation Prior to LVAD | CI | 0.26–4.51 | |
| P | 0.91 | ||
| Monomorphic | HR | 1.95 | |
| Ventricular Tachycardia | CI | 0.69–5.52 | |
| P | 0.21 | ||
| Implantable Cardioverter | HR | 1.29 | |
| Defibrillator Shock | CI | 0.65–2.56 | |
| P | 0.47 | ||
| Ventricular Tachycardia | HR | 1.86 | 1.87 |
| Storm | CI | 0.97–3.55 | 0.89–3.94 |
| P | 0.06 | 0.10 | |
| Right Heart Failure | HR | 0.72 | |
| (Pulmonary Artery | CI | 0.36–1.44 | |
| Pulsatility Index < 2) | P | 0.35 | |
| Year Ablation | HR | 1.28 | 1.06 |
| Performed | CI | 1.03–1.60 | 0.85–1.33 |
| P | 0.026 | 0.58 | |
| Ablation Performed | HR | 1.54 | |
| at Columbia | CI | 0.70–3.38 | |
| P | 0.28 |
Univariable and multivariable Cox regression for predictors of suspected or confirmed device thrombosis and stroke or embolic event. Interaction effects between all statistically significant variables in the multiregression analysis were checked and were not significant.
Excluded from multivariate analysis due to multicollinearity with ablation.
CI, confidence interval; HR, hazard ratio; LVAD, left ventricular assist device.
Significant univariable predictors of the primary composite outcome of confirmed or suspected thrombosis and stroke or embolic event included ablation, age, peripheral artery disease (PAD), vasoactive use, VT storm, and beta-blocker use (Table 2). Beta-blocker use was excluded from the multivariable model due to collinearity with ablation. In a multivariable model including the remaining predictors, ablation (HR, 2.24; 95% CI, 1.09–4.61; p = 0.03) and PAD (HR, 4.02; 95% CI, 1.65–9.82; p = 0.02) increased the risk of the composite endpoint while increased age decreased the risk (HR, 0.94; 95% CI, 0.91–0.97; p < 0.01).
Of the 21 patients diagnosed with suspected device thrombosis, 18 (85%) had an LDH > 2.5 times the upper limit of normal and 7 (33%) had a positive RAMP study (Figure 4). Left ventricular assist device power was available for 15 of the 21 patients with a suspected device thrombosis and 8 (53%) had a power spike. Four patients (19%) were treated with lytics, 1 (5%) was transplanted, and 11 (52%) were dead by the end of the follow-up period.
Figure 4.
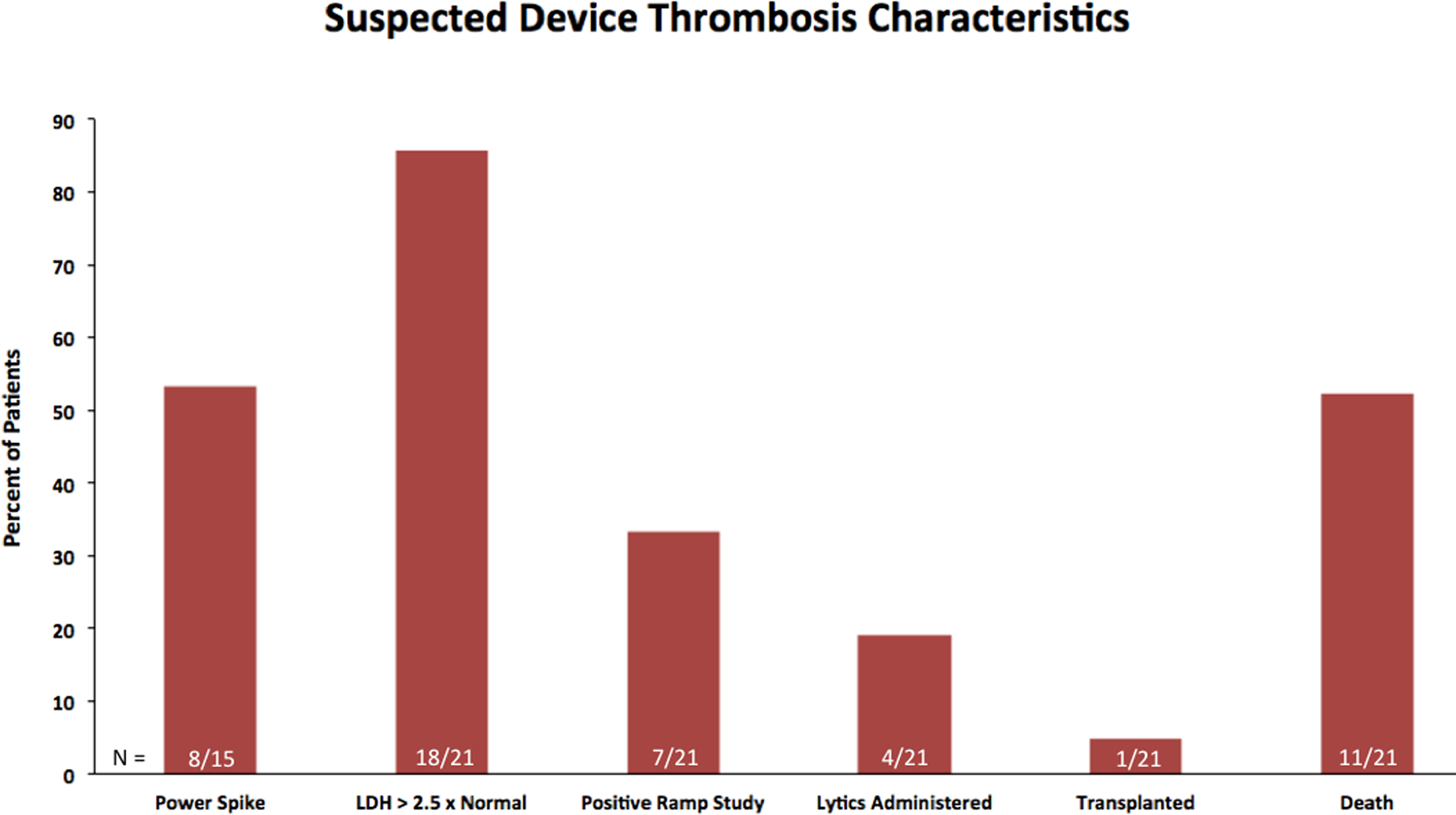
Bar graph for the clinical characteristics and outcomes for those with a suspected device thrombosis. LDH, lactate dehydrogenase.
Recurrent Ventricular Tachycardia and Death
There was no difference in the rate of death between those who were ablated and those who were not (HR, 1.19; 95% CI, 0.67–2.13; p = 0.56; ablation n = 19, control n = 28). There was an increase in the secondary endpoint of confirmed or suspected thrombosis, stroke or embolic event, and death with ablation (HR, 1.67; 95% CI, 1.05–2.66; ablation n = 32, control n = 41).
Recurrent VT in the control group, after initial EP consultation, occurred in 43 patients (58%). Recurrent VT after ablation occurred in 25 patients (58%). Those who had recurrent VA after ablation had an increase risk of death (HR, 2.81; 95% CI, 1.01–7.85; p = 0.05; recurrent VA after ablation n = 14/25, no recurrent VA after ablation n = 5/18) compared to those who were free of VA for one year after ablation. There was no difference in the rates of suspected or confirmed thrombosis between those who were VT free after ablation compared to those who had recurrent VT (44% vs. 48%, p = 1.0). Similarly, there was no difference between the rates of CVA between those who were free from VT after ablation compared to those who had recurrent VT after ablation (28% vs. 40%, p = 0.53).
Anticoagulation
Only one of 43 patients who were ablated was not on either therapeutic Coumadin or a heparin drip immediately before and after the procedure (Table 3). Patients in both the control arm and ablation arm had similar rates of therapeutic international normalized ratio (INR) at the time of the initial EP consult or the day of VT ablation, respectively (p = 0.67). Evidence of subtherapeutic anticoagulation within the preceding 30 days was examined for any patient who experienced the primary composite outcome. Data were available for 35 of the 37 patients who had the primary composite outcome. Nineteen (54%) had evidence of at least one subtherapeutic INR within 30 days of the event, 11 (31%) had no subtherapeutic INRs, and 5 (14%) had the outcome within 30 days of LVAD implantation. There was no difference in the rates of subtherapeutic anticoagulation between the intervention and control groups (p = 0.57).
Table 3.
Ablation Characteristics
| University of Chicago (n = 22) |
Columbia (n = 21) |
P Value | |
|---|---|---|---|
| Site of Ablation | |||
| LV Ablation, n (%) | 19 (86) | 21 (100) | 0.23 |
| RV Ablation | 5 (23) | 1 (5) | 0.19 |
| Ablation Near Inflow Cannula | 8 (36) | 13 (62) | 0.09 |
| Anticoagulation | |||
| Therapeutic Anticoagulation on day of procedure | 21 (95) | 21 (100) | 1.0 |
| Ablation Study Details * | |||
| Time in VT during ablation in minutes, median (IQR) | 30 (23–85) | NR | NA |
| Longest Sustained VT During Ablation in minutes, median (IQR) | 16 (8.7–36.8) | NR |
Procedural characteristics of ablation.
Data available for 15 of 22 patients.
IQR, interquartile range; LV, left ventricle; NA, not applicable; NR, not reported; RV, right ventricle; VT, ventricular tachycardia.
Discussion
In this study, we explored the impact of endocardial VT ablation on the rates of LVAD device thrombosis and cardioembolic events. Our main findings are as follows: VT ablation is associated with high rates of device thrombosis (suspected and confirmed) and cardioembolism. Furthermore, the rate of device thrombosis and stroke was nearly double compared to LVAD patients with VT who did not undergo ablation as well as expected rates from clinical trials (see Table 2, Supplemental Digital Content 1, http://links.lww.com/ASAIO/A491).
Ventricular arrhythmia in patients supported with LVADs is often well tolerated when they occur in short salvos, although prolonged or refractory VA often has deleterious effects on the unsupported right ventricle.23 Intrinsic myocardial scar, surgical scar as well as mechanical irritation of the ventricular myocardium from the inflow cannula together with electrolyte imbalance and catecholamine surges in the perioperative setting are thought to drive many of the VA that occur following LVAD implantation.24,25 Postimplantation VA is associated with increased rehospitalization rates and nearly a threefold increased rate of death.23,26,27 Ventricular arrhythmia in patients supported with LVADs is often refractory to pharmacotherapy with as few as one in five patients achieving sustained termination of VA with conservative management alone.26 Catheter-based ablation approaches are becoming increasingly more common for LVAD patients owing to the high rates of recurrent VA and poor efficacy of antiarrhythmic drug therapy.28 Periprocedural complication rates are low following endocardial VT ablation with arrhythmia-free survival rates reported as high as 76%.13,25 While studies to date have focused on the feasibility and efficacy of endocardial VT ablation in LVAD patients, few studies to date have reported on the durable safety of this approach and its impact on LVAD performance and function.
Here, we reported the largest, multicenter, cohort to date exploring the long-term safety of endocardial VT ablation in LVAD patients. Although patients in the study were not randomized, we utilized a control population of LVAD recipients with a VT burden sufficient to warrant electrophysiology consultation who did not undergo ablation to further contextualize our observations. Compared to those who did not undergo ablation, patients who underwent ablation had a near doubling of the rate of confirmed or suspected device thrombosis or cardioembolic event. While it may be possible that patients undergoing this procedure are characterized by a higher burden of vascular disease than those not chosen to undergo this procedure, these findings of an increased hazard have a plausible explanation. Interestingly, as demonstrated in the Kaplan-Meyer analysis, the risk of confirmed or suspected pump thrombosis increase immediately after ablation although there appears to be a delayed effect on stroke risk with the curves diverging closer to 120 days after ablation. The delayed stroke events may be related to a time-dependent need for a critical mass of fibrin deposition and platelet activation within the rotor and stators (presumed source of cardioembolism) needed to lead to a clinically significant effect on cerebral circulation.
Endocardial ablation is known to be thrombogenic from coagulation and tissue necrosis induced by hyperthermia as well as activation of both platelets and the coagulation cascade by RF ablation.16 To mitigate risk, ablation is performed on full anticoagulation with targeted activated clotting times, temperature feedback catheters, and catheter irrigation. Importantly, we explored the role of anticoagulation in the development of the thromboembolic events in our cohort and found that the vast majority of patients were on therapeutic anticoagulation at the time of the procedure. Historically, the rates of clinically relevant thromboembolic complications of left-sided VT ablation with RF ablation was thought to be a modest 2.8%.29,30 More recently, subclinical cerebral embolic thrombosis rates as high as 58% have been reported following LV endocardial ablation for VT and symptomatic PVCs in a group of 19 patients who underwent protocolized brain magnetic resonance imagings before and after ablation,17 although this may be an underestimation if one considers only the patients undergoing ablation of VA.
Left ventricular assist devices are similarly thrombogenic with device thrombosis rates three months after implantation ranging between 0% and 8.4% depending on pump type.18,31,32 Thrombosis risk factors include those intrinsic to the pump itself such as small stator gap size, heat generation and lack of heat dissipation, shear stress forces and cannula malposition, factors related to the patient such as a hypercoagulable state, low blood flow states or atrial fibrillation and factors related to management such as suboptimal anticoagulation.33,34 Ventricular tachycardia leads to low flow states and thus may also promote device thrombosis. In patients supported with LVADs, VT ablation theoretically carries additional risk as long durations of induced VT during an electrophysiology study promotes stasis of flow in the LVAD inflow cannula and stators which might promote LVAD device thrombosis and increase the risk of ingestion of thrombus originating in the static LV. Patients who underwent endocardial VT ablation had a near doubling of their rates of confirmed or suspected DT or cardioembolism despite more favorable inflow cannula positioning in this group compared to controls. Steep inflow cannula angulations have been shown to increase thrombosis rates and are associated with worse unloading and overall prognosis.34,35 In multivariable Cox regression analysis, ablation remained an independent predictor of LVAD thrombosis or embolism.
Alternative methods of cell injury, including cryotherapy, may lead to less activation of platelets and the coagulation cascade and warrant further evaluation.30 Animal studies comparing cryolesions to RF energy suggest that cryoablation is associated with less thrombosis and smaller thrombus volumes.30 Currently, endocardial cryoablation is primarily used in the thin atrial myocardium for atrial fibrillation ablation or for surgical ablation, but recent animal models with novel delivery systems have suggested potentially expanding cryoablation to endocardial VT ablation.36 Alternatively, epicardial VT ablation at the time of LVAD implantation or shortly after implantation has recently been studied with promising safety and efficacy outcomes.37–40
We also reported that patients with no recurrent VA after ablation have improved survival compared to those with recurrent VA suggesting that further strategies to optimize ablation and patient selection may provide significant benefit in this patient population.15
We believe that the findings of the current paper should be viewed as an opportunity for improvement in the fields of advanced heart failure and electrophysiology. Furthermore, prospective studies investigating the thrombogenic potential of individual components of the ablation procedure might allow the community to identify aspects of the procedure that increase risk and allow for modifications that will improve outcomes for patients supported with LVADs. It remains to be seen if procedural details such as the time in sustained VT during the procedure, anticoagulation targets, amount of irrigation, or the frequency and characteristics of RF applications can be modified to mitigate risk.
Limitations
This is a retrospective, nonrandomized study and thus is limited by incomplete data for some of the variables and a control group that is not identical to the treatment arm. Additionally, while we attempted to account for confounding factors by incorporating a control group of LVAD patients with VT who underwent evaluation by the electrophysiology service, given the retrospective nature and nonrandomized design, we are unable to account for all confounders, and patients who underwent VT ablation may be different from those who did not in an unmeasured way. Multivariate analysis was used to assess the potential interaction of the variables, although it is possible that our sample size was too small to fully assess the impact of each variable. Despite every effort to choose a control arm as closely matched to the ablation arm, intuitively the ablation arm likely represented a sicker population. Furthermore, our control arm was derived from a single institution while our treatment arm was drawn from two institutions, which may have further contributed to uncontrolled confounders. In addition to potential differences in patient-specific factors, procedural techniques and threshold for intervention are also likely different between the two groups. Although the group that ultimately underwent ablation may have represented a sicker population with a higher VT burden, there was no difference in the rates of RV failure between the control group and the ablation arm, and VT storm did not predict thrombosis on multiple regression analysis. It is important to point out that the control arm had a higher rate of inotropic support, which may be reflective of masked underlying RV dysfunction in the control arm or, alternatively, this may be reflective of the lower rates of VT storm in this cohort. The majority of events for the primary endpoint represented suspected device thrombosis for both the ablation arm and the control arm. The retrospective nature of this analysis likely underestimated the true incidence of confirmed thrombosis as pathology reports and manufacturer review of explanted pumps were not available for all of the patients. Additionally, postmortem evaluation of pumps for the presence of thrombus was not performed on all patients and several patients with highly suspected thrombosis based on noninvasive assessment were not offered device exchange because of comorbidities that were felt to make re-operation high risk. Additionally, although there was no difference in the rates of subtherapeutic anticoagulation in the ablation arm compared to the control arm among patients who had an event, the anticoagulation status among those who did not have an event is less clearly defined. This study does represent the largest cohort to date of LVAD patients undergoing endocardial VT ablation, and the multicenter design of the study mitigates much of institutional and procedural bias.
Conclusions
Endocardial VT ablation in LVAD patients with refractory VT is associated with high rates of device thrombosis and cardioembolic events. A prospective study is warranted to better define the causal relationship between ablation and thrombosis in patients supported with LVADs as well as to evaluate alternative ablation methods to mitigate risk.
Supplementary Material
Disclosure:
Dr. Garan is supported by the National Institutes of Health Grant No. KL2TR001874 and has received honoraria from Abiomed (Danvers, MA). Dr. Uriel is a consultant to Medtronic and Abbott. Dr. Jeevanandam is a consultant to Abbott. Dr. Sayer is a consultant to Abbott. The other authors have no conflicts of interest to report.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s Web site (www.asaiojournal.com).
References
- 1.Slaughter MS, Rogers JG, Milano CA, et al. ; HeartMate II Investigators: Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 361: 2241–2251, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Aaronson KD, Slaughter MS, Miller LW, et al. ; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators: Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 125: 3191–3200, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Rose EA, Gelijns AC, Moskowitz AJ, et al. ; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group: Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 345: 1435–1443, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bedi M, Kormos R, Winowich S, McNamara DM, Mathier MA, Murali S: Ventricular arrhythmias during left ventricular assist device support. Am J Cardiol 99: 1151–1153, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Andersen M, Videbaek R, Boesgaard S, Sander K, Hansen PB, Gustafsson F: Incidence of ventricular arrhythmias in patients on long-term support with a continuous-flow assist device (HeartMate II). J Heart Lung Transplant 28: 733–735, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Ambardekar AV, Allen LA, Lindenfeld J, et al. : Implantable cardioverter-defibrillator shocks in patients with a left ventricular assist device. J Heart Lung Transplant 29: 771–776, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Garan AR, Yuzefpolskaya M, Colombo PC, et al. : Ventricular arrhythmias and implantable cardioverter-defibrillator therapy in patients with continuous-flow left ventricular assist devices: need for primary prevention? J Am Coll Cardiol 61: 2542–2550, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Oz MC, Rose EA, Slater J, Kuiper JJ, Catanese KA, Levin HR: Malignant ventricular arrhythmias are well tolerated in patients receiving long-term left ventricular assist devices. J Am Coll Cardiol 24: 1688–1691, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Busch MC, Haap M, Kristen A, Haas CS: Asymptomatic sustained ventricular fibrillation in a patient with left ventricular assist device. Ann Emerg Med 57: 25–28, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Fasseas P, Kutalek SP, Kantharia BK: Prolonged sustained ventricular fibrillation without loss of consciousness in patients supported by a left ventricular assist device. Cardiology 97: 210–213, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Garan AR, Iyer V, Whang W, et al. : Catheter ablation for ventricular tachyarrhythmias in patients supported by continuous-flow left ventricular assist devices. ASAIO J 60: 311–316, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen CT, Kay GN, Kalman J, et al. ; EP-Europace,UK: EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm 11: e166–e196, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Dandamudi G, Ghumman WS, Das MK, Miller JM: Endocardial catheter ablation of ventricular tachycardia in patients with ventricular assist devices. Heart Rhythm 4: 1165–1169, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Osaki S, Alberte C, Murray MA, et al. : Successful radiofrequency ablation therapy for intractable ventricular tachycardia with a ventricular assist device. J Heart Lung Transplant 27: 353–356, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Moss JD, Flatley EE, Beaser AD, et al. : Characterization of ventricular tachycardia after left ventricular assist device implantation as destination therapy. JACC Clin Electrophysiol 3: 1412–1424, 2017. 10.1016/j.jacep.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 16.Anfinsen OG, Gjesdal K, Brosstad F, et al. : The activation of platelet function, coagulation, and fibrinolysis during radiofrequency catheter ablation in heparinized patients. J Cardiovasc Electrophysiol 10: 503–512, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Whitman IR, Gladstone RA, Badhwar N, et al. : Brain emboli after left ventricular endocardial ablation. Circulation 135: 867–877, 2017. [DOI] [PubMed] [Google Scholar]
- 18.Starling RC, Moazami N, Silvestry SC, et al. : Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 370: 33–40, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Teuteberg JJ, Slaughter MS, Rogers JG, et al. ; ADVANCE Trial Investigators: The HVAD Left ventricular assist device: Risk factors for neurological events and risk mitigation strategies. JACC Heart Fail 3: 818–828, 2015. [DOI] [PubMed] [Google Scholar]
- 20.Mehra MR, Uriel N, Naka Y, et al. ; MOMENTUM 3 Investigators: A fully magnetically levitated left ventricular assist device - final report. N Engl J Med 380: 1618–1627, 2019. [DOI] [PubMed] [Google Scholar]
- 21.Colombo PC, Mehra MR, Goldstein DJ, et al. : Comprehensive analysis of stroke in the long-term cohort of the MOMENTUM 3 Study. Circulation 139: 155–168, 2019. [DOI] [PubMed] [Google Scholar]
- 22.Najjar SS, Slaughter MS, Pagani FD, et al. ; HVAD Bridge to Transplant ADVANCE Trial Investigators: An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 33: 23–34, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Garan AR, Levin AP, Topkara V, et al. : Early post-operative ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant 34: 1611–1616, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Griffin JM, Katz JN: The burden of ventricular arrhythmias following left ventricular assist device implantation. Arrhythm Electrophysiol Rev 3: 145–148, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacher F, Reichlin T, Zado ES, et al. : Characteristics of ventricular tachycardia ablation in patients with continuous flow left ventricular assist devices. Circ Arrhythm Electrophysiol 8: 592–597, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Raasch H, Jensen BC, Chang PP, et al. : Epidemiology, management, and outcomes of sustained ventricular arrhythmias after continuous-flow left ventricular assist device implantation. Am Heart J 164: 373–378, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Yoruk A, Sherazi S, Massey HT, et al. : Predictors and clinical relevance of ventricular tachyarrhythmias in ambulatory patients with a continuous flow left ventricular assist device. Heart Rhythm 13: 1052–1056, 2016. [DOI] [PubMed] [Google Scholar]
- 28.Santangeli P, Rame JE, Birati EY, Marchlinski FE: Management of ventricular arrhythmias in patients with advanced heart failure. J Am Coll Cardiol 69: 1842–1860, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Zhou L, Keane D, Reed G, Ruskin J: Thromboembolic complications of cardiac radiofrequency catheter ablation: A review of the reported incidence, pathogenesis and current research directions. J Cardiovasc Electrophysiol 10: 611–620, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Khairy P, Chauvet P, Lehmann J, et al. : Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation 107: 2045–2050, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Maltais S, Kilic A, Nathan S, et al. ; PREVENT Study Investigators: PREVENtion of heartmate ii pump thrombosis through clinical management: The PREVENT multi-center study. J Heart Lung Transplant 36: 1–12, 2017. [DOI] [PubMed] [Google Scholar]
- 32.Mehra MR, Naka Y, Uriel N, et al. ; MOMENTUM 3 Investigators: A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med 376: 440–450, 2017. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein DJ, John R, Salerno C, et al. : Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 32: 667–670, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Chivukula VK, Beckman JA, Prisco AR, et al. : Left ventricular assist device inflow cannula angle and thrombosis risk. Circ Heart Fail 11: e004325, 2018. [DOI] [PubMed] [Google Scholar]
- 35.Imamura T, Nguyen A, Chung B, et al. : Association of inflow cannula position with left ventricular unloading and clinical outcomes in patients with heartmate ii left ventricular assist device. ASAIO J 65: 331–335, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berte B, Sacher F, Wielandts JY, et al. : A new cryoenergy for ventricular tachycardia ablation: A proof-of-concept study. Europace 19: 1401–1407, 2017. [DOI] [PubMed] [Google Scholar]
- 37.Shabari F, Yousef J, Cohn W, et al. : Open chest epicardial ablation of ventricular tachycardia early after left ventricular assist device implantation. J Heart Lung Transplant 32: S272, 2013. [Google Scholar]
- 38.Patel M, Rojas F, Shabari FR, et al. : Safety and feasibility of open chest epicardial mapping and ablation of ventricular tachycardia during the period of left ventricular assist device implantation. J Cardiovasc Electrophysiol 27: 95–101, 2016. [DOI] [PubMed] [Google Scholar]
- 39.Moss JD, Vohra A, Shin JH, et al. : Detailed endocardial mapping around the left ventricular assist device inflow cannula facilitates successful ablation of ventricular tachycardia. J Heart Lung Transplant 34: S215–S216, 2015. [Google Scholar]
- 40.Moss JD, Oesterle A, Raiman M, et al. : Feasibility and utility of intraoperative epicardial scar characterization during left ventricular assist device implantation. J Cardiovasc Electrophysiol 30: 183–192, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


