Abstract
Objective:
This analysis was designed to present a summary of available evidence that will inform practice and guide future research for photobiomodulation (PBM) after titanium implant placement procedures.
Materials and methods:
A systematic review was performed according to the Cochrane Collaboration and in line with Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) criteria. Two investigators screened the titles and abstracts, and reviewed articles for risk of bias. Online databases searched included PubMed, Embase, Scopus, and Web of Science. Terms were specific to the effects of PBM on dental implant stability.
Results:
Eight hundred fifty-six studies were identified, and 15 studies fulfilled the inclusion criteria. Light sources included both laser and light emitting diode (LED) devices. Wavelengths ranged from 618 to 1064 nm. The meta-analysis concluded that all 15 published studies were able to safely apply PBM near dental implants without adverse events. Laser and LED wavelengths that reported significant results included 618, 626, 830, 940 (2 × ), and 1064 nm.
Conclusions:
The use of adjunctive PBM can be safely prescribed after surgical placement of titanium implants. Six groups reported statistical significance for improving implant stability (four laser diode, two LED) in wavelengths ranging from 618 to 1064 nm. The amount of time spent delivering PBM was not a variable that differentiated whether a study reported significant results.
Keywords: photobiomodulation, phototherapy, laser, osseointegration, dental implant, tissue regeneration
Introduction
Photobiomodulation (PBM) therapy is a nonthermal nonionizing treatment of red and near-infrared light.1–3 PBM applications involve light wavelengths between 600 and 1000 nm. Incorporating PBM into a surgical procedure can promote wound healing, provide analgesia, and reduce inflammation and edema. Several groups have published PBM protocols that can significantly improve tissue regeneration and dental implant stability.4–6
There are several proposed generalized biologic mechanisms of action for PBM. These fall within the categories of intracellular, cell membrane receptors, and extracellular components. Intracellular mechanisms involve the absorption of PBM wavelengths by cytochrome c oxidase and photodissociation of nitric oxide located within the mitochondria.7,8 PBM initiates a cascade that leads to the enhancement of enzyme activity,7 electron transport,9 increasing mitochondrial respiration, and increasing adenosine triphosphate (ATP) production.8 The second proposed mechanism is the cell membrane receptor that involves the activity of photosensitive ion transporters on cell membranes, Opsins 2–4, TRPV1, AHR, and P2X7. The third type of mechanism is defined by extracellular components that predictably upregulate the transforming growth factor (TGF)-β1 immediately after PBM treatment.2–5,10,11
It is understood that PBM light wavelengths work in conjunction with the hemoglobin coefficient of absorption12–15 and can photoactivate latent TGF-β1 through a redox-mediated physiochemical process.16–21 This is notable considering that TGF-β1 is a pluripotent mesenchymal stem cell19,22,23 with the potential to impact re-epithelialization, inflammation, angiogenesis, and granulation tissue formation during wound healing.17,19,22,24 It is also well understood that TGF-β1 can be predictably upregulated by specific PBM wavelengths 600–1000 nm.16–18,20,21 This mechanism is supported by evidence that TGF-β1 will present immediately after PBM treatment in the degranulating platelets of freshly wounded tissues.16,20 Therefore, PBM can directly affect keratinocyte function and migration, which is essential to wound re-epithelialization.22
Titanium dental implant placement is a routine surgical procedure with predictable outcomes. A surgeon must consider the restorative treatment plan, implant size, and the alveolar ridge anatomy.25,26 Surgeons must precisely plan implants in an ideal three-dimensional position to avoid inadvertent bone loss on adjacent sites.27–29 Dental implant surgery relies primarily on the osseointegration potential at the titanium surface, which is engineered to promote tissue regeneration.30,31
Titanium implant placement can be evaluated by resonance frequency analysis (RFA) at various time points after surgery. RFA is a noninvasive protocol used to measure implant stability, which can clinically assess implant integration.30 Acquiring the implant stability quotient (ISQ) can help determine the implant stability value on a linear scale of 1–100. The ISQ can be measured beginning at the time of placement to correlate ISQ values with the healing cascade of osseointegration.32
Multiple research groups have provided guidance for PBM applications after titanium implant placement.33,34 Despite these findings, there are no validated evidence-based protocols for PBM to improve implant stability. Clinicians do not have guidance on how to select a laser system or how to prescribe an optimal dose. These are critical for all clinicians to prescribe an effective dose and achieve a predictable result safely. PBM settings can be safely optimized for the varying optical properties of human tissues and titanium.35,36 This review and meta-analysis aim to present a summary of available evidence that will inform practice and guide future research for PBM after titanium implant placement procedures.
Materials and Methods
This systematic literature review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Fig. 1).37 The PICO question formulated was as follows: In patients receiving titanium implant placement surgery (P), does the amount of time prescribed for PBM (I) compared with placebo therapy (C) have an effect on implant stability of titanium implants (O)? The study protocol was registered with PROSPERO (Registration No. CRD42022341395).
FIG. 1.
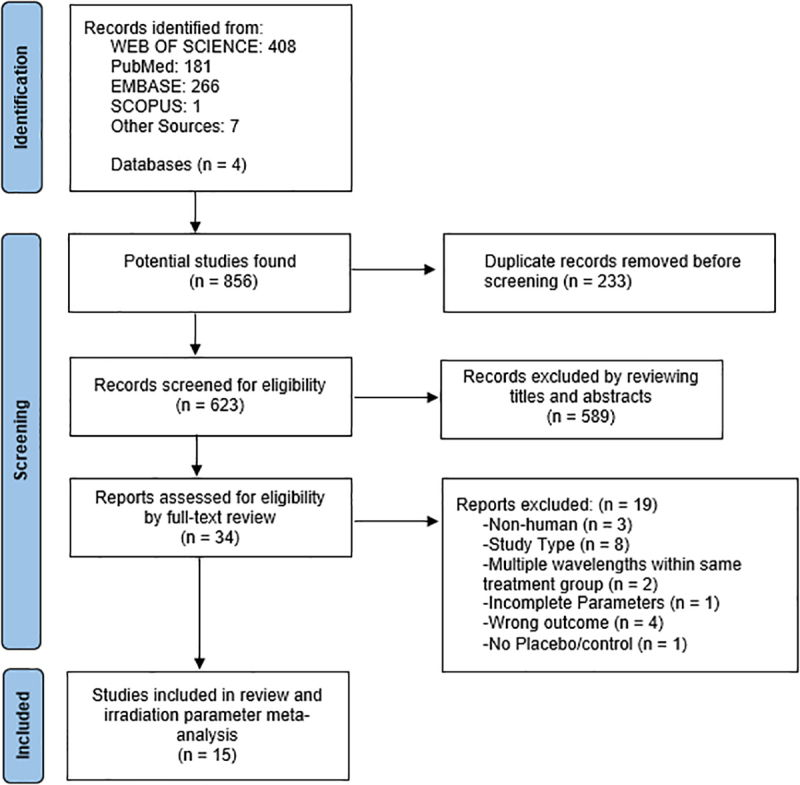
PRISMA flow diagram.
Search strategy
A detailed systematic review of the literature was conducted between January 1, 1967, and July 1, 2022, in the following databases: Elsevier Embase, Scopus, The National Library of Medicine PubMed (MEDLINE), and Web of Science. The search terms included keywords and medical subject headings: dental implant (including full size, mini-implant), ISQ, RFA, PBM, low-level laser therapy, laser dentistry, LLLT, and PBM. The comprehensive search strategy can be retrieved via PROSPERO (Registration No. CRD42022341395).
Two reviewers (D.S. and J.P.) conducted the title, abstract, and full-text screenings after prescreening standardization in selection criteria. Two reviewers (D.S. and B.L.) performed the revised Cochrane risk-of-bias tool for randomized trials (RoB 2).38 Authors of the included studies were not contacted to clarify issues regarding missing data for irradiation parameters. This highlighted the limitation caused by a lack of standardized reporting for PBM.
Studies that passed the title/abstract screening and reported the quantity of dose delivery in time (sec) were reviewed for irradiation parameter dosing analysis (D.S., J.C., and T.C.Z.). Data sets were examined for appropriateness when more than one publication reported the same patient group. Disagreements for study eligibility or irradiation parameter reporting were resolved through an open debate between reviewers until an agreement was reached or through settlement by an arbitrator (J.P.F.).
Additional electronic manual searches were conducted to ensure a thorough screening process. Manual searches were performed in the following journals: Journal of Dental Research, Journal of Clinical Periodontology, Journal of Periodontology, Clinical Oral Implants Research, The International Journal of Oral & Maxillofacial Implants, Journal of Oral and Maxillofacial Surgery, and International Journal of Periodontics and Restorative Dentistry. Systematic reviews were retrieved and processed for full-text screening, including Chen et al.39 and Costa et al.40
Study selection criteria
Inclusion criteria
Human prospective randomized controlled trials (RCTs).
RCTs comparing PBM therapy versus placebo as an adjunct therapy after titanium implant placement surgery.
Test groups using a single PBM system and the same laser wavelength throughout the treatment.
Reporting the following PBM irradiation parameters: wavelength (nm), treatment time (sec), number of contact points per visit, and the total amount of visits.
Reporting of adverse events, safety, and efficacy.
Statistical analysis.
Exclusion criteria
Nonhuman studies.
Study type: cohort, case control, case series, expert opinion, review).
Inadequate site standardization.
Using multiple laser systems at different wavelengths for the same group or the same PBM system at varying wavelengths.
No placebo or control.
Non-English language.
Quality assessment of studies
The Cochrane risk-of-bias tool for RCTs (Cochrane RoB 2.0) was utilized to assess bias. Risk categories included (1) risk of bias arising from the randomization process, (2) risk of bias due to deviations from the intended interventions (effect of assignment to intervention; effect of adhering to intervention), (3) missing outcome data, (4) risk of bias in the measurement of the outcome, (5) risk of bias in the selection of the reported result, and (6) overall risk of bias. Responses were stratified as either a “low risk of bias” or a potential marker “for a risk of bias.” Two reviewers (D.S., B.L.) independently performed the assessment (Table 1). Disagreement was resolved by discussion among the reviewers. Unresolved debates were settled through arbitration (J.P.F.).
Table 1.
RoB2: Risk-of-Bias Assessment
| No. | Author | Randomization process | Derivations from the intended intervention | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall risk of bias |
|---|---|---|---|---|---|---|---|
| 1 | Abohabib | + | + | + | + | + | Low RoB |
| 2 | Bozkaya | + | + | + | + | + | Low RoB |
| 3 | Ekizer | + | + | + | + | + | Low RoB |
| 4 | Elsyad | + | ? | + | + | + | Some concern |
| 5 | Garcia | + | + | + | + | + | Low RoB |
| 6 | Gokmenoglu | ? | ? | + | + | + | Low RoB |
| 7 | Kinalski | + | + | + | + | + | Low RoB |
| 8 | Lobato | + | + | + | + | + | Low RoB |
| 9 | Matys (2019) | + | + | + | + | + | Low RoB |
| 10 | Matys (2020) | + | + | + | + | + | Low RoB |
| 11 | Memarian | + | ? | + | + | + | Some concern |
| 12 | Osman | + | + | + | - | + | High RoB |
| 13 | Palled | + | + | + | + | + | Low RoB |
| 14 | Sleem | ? | - | + | + | ? | High RoB |
| 15 | Torkzaban | + | + | + | + | + | Low RoB |
(+) is the symbol of “low risk of bias.” (?) is the symbol for “some concern.” (-) is the symbol for “high risk of bias.”
Data extraction
An electronic data extraction database (MS EXCEL) was created by the author (D.S.). Studies were itemized by two reviewers (D.S., J.P.) according to year, author, title, laser wavelength, exposure time (sec), output power, dose/energy density, total dose per point, site of clinical application, statistical significance, and frequency of visits. Exposure time analysis was conducted by two reviewers (D.S., J.C.) and was limited to author, site of clinical application, wavelength (nm), laser power (W), and exposure time (sec).
Data synthesis
Irradiation parameter analysis was conducted by three reviewers (D.S., J.C., T.C.Z.). It included the following where applicable: site of clinical application, wavelength (nm), laser power (W), beam area spot size (cm2), exposure time (sec), energy dose (J), fluence (J/cm2), points per treatment, and the number of sessions (Tables 2 and 3).
Table 2.
Summary of Characteristics of 15 Articles
| Author | Year | System type (laser, LED) | Wavelength (nm) | Laser power (W) | Beam area/spot size (cm2) | Treatment time (sec) - per point | Treatment time (sec) - per visit | |
|---|---|---|---|---|---|---|---|---|
| 1 | Abohabib | 2018 | Laser | 940 | 1.7 | 2.83a | 60 | 60a |
| 2 | Bozkaya | 2021 | Laser | 830 | 0.126 | 0.0028 | 3 | 60 |
| 3 | Ekizer | 2016 | LED | 618 | — | — | 1200b | 1200b |
| 4 | Elsyad | 2019 | Laser | 940 | 0.25b | 0.004 | 60 | 360 |
| 5 | Garcia | 2012 | Laser | 830 | 0.086b | 0.0028 | 3 | 60a |
| 6 | Gokmenoglu | 2014 | LED | 626 | 0.185 | 4.8 | 1200b | 1200a |
| 7 | Kinalski | 2021 | Laser | 808 | 0.05b | 0.4 | 80 | 480a |
| 8 | Lobato | 2019 | Laser | 808 | 0.05b | 0.4 | 83 | 498a |
| 9 | Matys | 2019 | Laser | 635 | 0.1a | 0.5024 | 40 | 80a |
| 10 | Matys | 2020 | Laser | 635 | 0.1a | 0.5024 | 40 | 80a |
| 11 | Memarian | 2018 | Laser | 810 | 0.05 | 1 | 400 | 400a |
| LED | 626 | 0.185b | 4.8 | 1200b | 1200 | |||
| 12 | Osman | 2017 | Laser | 910 | 0.7 | — | 60 | 60a |
| 13 | Palled | 2021 | Laser | 1064 | 0.033a | 0.28a | 60 | 60a |
| 14 | Sleem | 2019 | Laser | 830 | 0.1b | 0.28 | 30 | 60b |
| 15 | Torkzaban | 2017 | Laser | 940 | 0.1b | 0.28 | 40 | 80a |
(—) indicates a value that cannot be derived from other reported values.
Indicates value was not reported in the literature and calculated by the authors with values given in the article.
Indicates value that was initially misreported and corrected by the authors' calculations.
LED, light emitting diode.
Table 3.
Summary of Characteristics of 15 Articles
| Author | Energy dose (J) - per point | Energy dose (J) - per visit | Fluence (J/cm2)- per point | Clinical site: extraoral (E)/intraoral (I), contact (C)/noncontact (NC) | Contact points - per visit | Appointments (no.) | Appointment days | Statistical significant (yes, no) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Abohabib | 102 | 102a | 36 | I | 1 | 4 | D0, 7, 14, 21 | Yes |
| 2 | Bozkaya | 0.3 | 6 | 135 | I, NC | 20 | 7 | D0, 3, 5, 7, 9, 12, 15 | Yes |
| 3 | Ekizer | — | — | — | E- LED headset | 1 | 21 | D0–21 | Yes |
| 4 | Elsyad | 15a | 90 | 3750a | I, NC | 6 | 3 | D0, D3, D7 | Yes |
| 5 | Garcia | 0.25 | 5 | 92.1 | I, C | 20 | 7 | D0, 3, 5, 7, 9, 11, 14 | No |
| 6 | Gokmenoglu | 222 | 222a | 46.2 | E- LED headset | 1 | 9 | D0, 4, 6, 9, 11, 13, 15, 17, 21 | Yes |
| 7 | Kinalski | 4a | 24a | 11 | I, NC | 6 | 1 | D1 | No |
| 8 | Lobato | 4.15b | 24.9b | 10.375a | I, C | 6 | 2 | D0 (2 × ) | No |
| 9 | Matys | 4 | 8a | 8 | I, C | 2 | 6 | D(-1), 0, 2, 4, 7, 14 | No |
| 10 | Matys | 4 | 8a | 8 | I, C | 2 | 5 | D1, 2, 4, 7, 14 | No |
| 11 | Memarian | 20a | 20a | 20 | I | 1 | 5 | D0, 3, 7, 10, 14 | No |
| 222 | 222b | 46.2 | E- LED headset | 1 | 5 | D0, 3, 7, 10, 14 | No | ||
| 12 | Osman | 42a | 42a | — | I, NC | 1 | 6 | D0, 3, 6, 9, 12, 14 | No |
| 13 | Palled | 2 | 2a | 7.07 | I, NC | 1 | 4 | D0, 2 week, 6 week, 12 week | Yes |
| 14 | Sleem | 3b | 6b | 10.71b | I, NC | 2 | 8 | D1, 5, 10, 15, 20, 25, 30, 35 | No |
| 15 | Torkzaban | 4 | 8 | 14.18 | I, C | 2 | 7 | D1, 2, 4, 6, 8, 10, 12 | No |
(—) indicates a value that cannot be derived from other reported values.
Indicates value was not reported in the literature and calculated by the authors with values given in the article.
Indicates value that was initially misreported and corrected by the authors' calculations.
Notations were indicated if data were misreported or corrected and if the value was not reported and added by synthesis (Tables 2 and 3). The value of fluence (J/cm2) was calculated as [(power × time)/area spot size]; energy (J) as (power × time); power density (W/cm2) as (power/area spot size); and spot size (cm2) as (π(radius1 × radius2)), noting that many laser diode beams are elliptical and not round. The mean, median, mode, and upper/lower quartile ranges were generated and plotted.
Results
The literature search process is detailed in Fig. 1. A total of 15 out of 856 articles were identified that fulfilled the inclusion reporting criteria. This group included both laser diode and light emitting diode (LED) devices. Reported wavelengths ranged from 618 to 1064 nm. None of the 15 published studies reported negative adverse events because of PBM in human subjects. Six studies reported statistical significance for the application of PBM to improve implant stability (Table 4). Four included laser devices with the following wavelengths: 830, 940 (2 × ), and 1064 nm (Figs. 2 and 3). Two reported LED devices with wavelengths, 618 and 626 nm (Tables 2 and 3). The prescribed value for time was not a variable that differentiated whether a study reported significant results.
Table 4.
Summary of Characteristics of Statistically Significant Studies
| Author | Year | System (laser, LED) | Wavelength (nm) | Laser power (W) | Beam area/spot size (cm2) | Treatment time (sec) - per point | Energy dose (J) - per point | Fluence (J/cm2)- per point | Contact points - per visit | Appointments (no.) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abohabib | 2018 | Laser | 940 | 1.7 | 2.83a | 60 | 102 | 36 | 1 | 4 |
| Bozkaya | 2021 | Laser | 830 | 0.126 | 0.0028 | 3 | 0.3 | 135 | 20 | 7 |
| Ekizer | 2016 | LED | 618 | — | — | 1200b | — | — | 1 | 21 |
| Elsyad | 2019 | Laser | 940 | 0.25b | 0.004 | 60 | 15a | 3750a | 6 | 3 |
| Gokmenoglu | 2014 | LED | 626 | 0.185 | 4.8 | 1200b | 222 | 46.2 | 1 | 9 |
| Palled | 2021 | Laser | 1064 | 0.033a | 0.28a | 60 | 2 | 7.07 | 1 | 4 |
(—) Indicates a value that cannot be derived from other reported values.
Indicates value was not reported in the literature and calculated by the authors with values given in the article.
Indicates a value that was initially misreported and corrected by the authors' calculations.
FIG. 2.
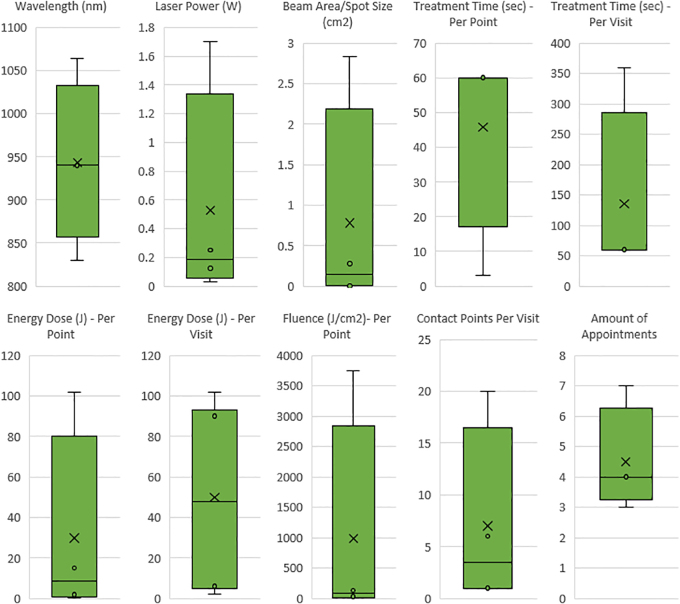
Statistically significant laser parameters: Bozkaya et al. (830 nm), Abohabib et al. (940 nm), ELsyad et al. (940 nm), Palled et al. (1064 nm). Box and whisker plot diagrams indicate inner points, outer points, mean markers, mean lines, and upper and lower quartile ranges for all reported PBM settings. Wavelengths (830–1064 nm), laser power (0.033–1.7 W), beam area/spot size (0.0028–2.83 cm2), treatment time per point (3–60 sec), treatment time per visit (60–360 sec), energy dose per point (0.3–102 J), energy dose per visit (2–102 J), fluence per point (7.07–3750 J/cm2), contact points per visit (1–20), and number of appointments (4–7).
FIG. 3.
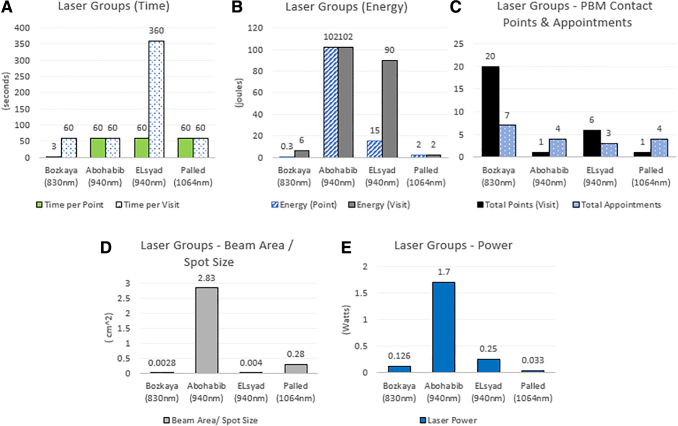
Statistically significant laser parameters: Bozkaya et al. (830 nm), Abohabib et al. (940 nm), ELsyad et al. (940 nm), Palled et al. (1064 nm). (A) Time, (B) energy, (C) PBM contact points and appointment intervals, (D) beam area/spot size, and (E) power.
Assessment of the risk of bias
The risk of bias in each study was summarized according to the Cochrane risk-of-bias 2.0 classification from 1999.41 Ten studies were considered low risk of bias. Three were rated as having a moderate risk of bias. Two were considered a high risk of bias. Details can be found in Table 1.
Irradiation parameter analysis for time (sec)
Fifteen studies reported the treatment time (in sec) spent for each clinical application of PBM (Tables 2 and 3). Two groups reported wavelengths at 635 nm for 80 sec of PBM per visit. Six studies reported wavelengths between 808 and 830 nm, from 60 to 498 sec per visit. Four studies reported wavelengths within 910–940 nm, from 60 to 360 sec of PBM per visit. One group reported a 1064 nm wavelength for 60 sec of PBM per visit. Three groups reported LED wavelengths within 618–626 nm at 1200 sec per visit.
All laser groups
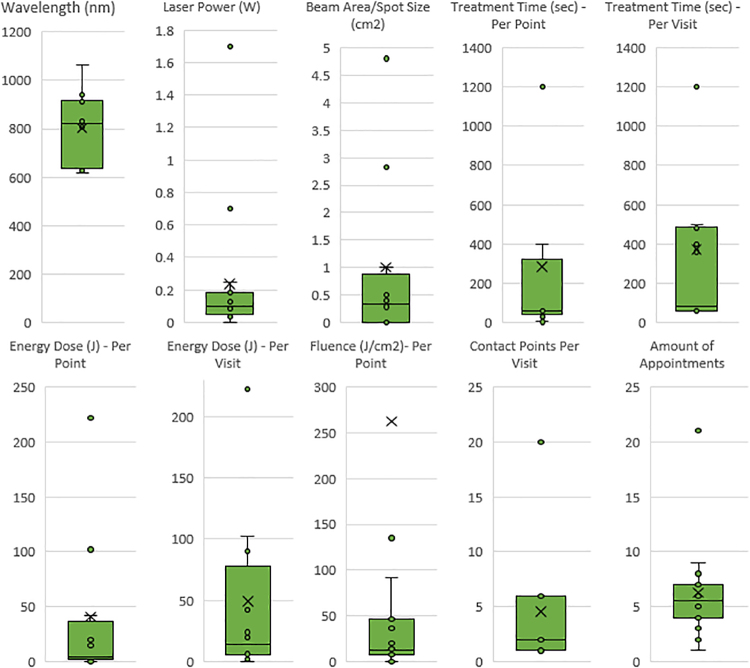
A total of 12 studies reported irradiation parameters for laser devices between 635 and 1064 nm (Tables 2 and 3 and Fig. 4). The two most common reported wavelengths were 940 nm (3 × ) and 830 nm (3 × ). The range of laser power was from 0.033 to 1.7 W. Beam area spot size ranged from 0.0028 to 2.83 cm2. Treatment time per point ranged from 3 to 400 sec. Energy dose ranged from 0.25 to 102 J. Fluence ranged from 4 to 3750 J/cm2. All laser groups reported intraoral laser placement at the time of PBM application. The total number of clinical applications ranged from 1 to 20 per appointment. The number of visits ranged from 1 to 21 days.
FIG. 4.
Meta-analyses of all the laser and LED parameters for 15 reported studies. Box and whisker plot diagrams indicate inner points, outer points, mean markers, mean line, and upper and lower quartile ranges for all reported PBM settings. Wavelengths (618–1064 nm), laser power (0.033–1.7 W), beam area/spot size (0.0028–4.8 cm2), treatment time per point (3–1200 sec), treatment time per visit (60–1200 sec), energy dose per point (0.25–222 J), energy dose per visit (2–222 J), fluence per point (7.07–3750 J/cm2), contact points per visit (1–20), and number of appointments (1–21). LED, light emitting diode; PBM, photobiomodulation.
Statistically significant laser groups
Four laser studies reported statistical significance for PBM after dental implant placement (Figs. 2 and 3).34,42–44 Wavelengths ranged from 830 to 1064 nm, with the most commonly reported at 940 nm (2 × ). The range of laser power (W) was from 0.033 to 1.7 W. Beam area spot size ranged 0.0028–2.83 cm2. Treatment time per point ranged from 3 to 60 sec. Energy dose ranged from 0.3 to 102 J per point. Fluence ranged from 36 to 3750 J/cm2 per point. Contact points ranged from 1 to 20 per appointment. The number of visits ranged from 1 to 7 days, with 4 days being the most common value.
All LED groups: irradiation parameter analysis
Three studies reported irradiation parameters for LED devices (Tables 2 and 3).45–47 Wavelengths ranged from 618 to 626 nm. Six hundred twenty-six nanometers was the most common reported wavelength (2 × ). Two of these groups reported a laser power (W) of 0.185 W. Two groups reported beam area spot size at 4.8 cm2. Treatment time per point was reported at 1200 sec for all three groups. Two groups reported energy dose at 222 J per point. Two groups provided values for fluence at 46.2 J/cm2 per point. All groups reported extraoral headset mounted device placement at the time of PBM application. All groups prescribed 1 contact point per visit. The number of appointment intervals ranged from 5 to 21 days.
Discussion
The present study's findings determined that prescribing PBM after implant placement can significantly improve implant stability, as reported in six studies (Figs. 2 and 3 and Table 4). Activating a PBM device near a titanium implant (directly in contact with tissue) is safe and will not cause an adverse event. This analysis also determined that the quantity of time spent applying PBM is not correlated to significant differences reported for implant stability. This is due to the heterogeneity of PBM devices, light wavelengths, clinical protocols, and varying implant dimensions (Tables 2 and 3). PBM devices were placed at different anatomic locations, including extraoral and internal contact points.
The present meta-analysis evaluated the application of PBM postimplant placement. We determined that activating a laser directly in contact with soft tissue is safe and will not cause a negative or adverse event. All wavelengths evaluated in this study are within the red and near-infrared spectra (600–1000 nm). The only exception is the study by Palled et al., which utilized a 1064 nm device.42
The transfer of energy from the PBM device to the clinical site is an area of interest. This can be influenced both by the tissue optical properties and the beam area spot size of the light source. Tissue optical properties vary from person-to-person, and are characterized by absorption (μa) and scattering (μs).12,48 PBM settings can activate a biologic reaction by adjusting for specific tissue optical properties and the beam area spot size. This delivery of optimal PBM dose is received at the intended target site as fluence (J/cm2).10,41,49,50
Prescribing PBM treatment to reach an intended penetration depth can be achieved by absorption of specific biomolecular targets such as hemoglobin.15 Asimov et al. have suggested methods that maximize these targets through a wide variety of wavelengths by targeting oxygen coupling/decoupling of hemoglobin. A clinician can prescribe an effective PBM treatment by accurately calculating irradiation parameters to reach a specific depth. Thus, allowing the clinician to maximize the effect of a particular wavelength to create a desired biologic reaction at a specific depth of penetration.15
The surgical placement of titanium implants has become a common procedure in contemporary dentistry. Prescribing PBM after implant placement can significantly affect implant stability. Muslim et al. proposed the idea that light wavelength can produce optical transmittance and reflectance at the interface of titanium.51 Titanium implants are highly amorphous and porous. Surface porosities are considered voids that transmit light from the PBM device.51 The amount of light transmitted is dependent on the PBM wavelength profile that interacts with the optical properties of tissue. It is theorized that energy can transmit through the tissues and distribute through the porous voids on the titanium surface. This will allow the energy to further transmit and reflect through the titanium implant body and stimulate the bone tissue in direct contact with the titanium implant body.
There was no correlation between the studies with significant increases in implant stability and the amount of time spent applying PBM. Time spent applying PBM ranged from 3 to 1200 sec (Table 4). This is due to the high heterogeneity of clinical protocols included in this analysis. Several concerns included a wide range of PBM wavelengths, a nonstandardized selection of laser/LED devices, different surgical-site locations, and a wide variety of implant sizes. Despite these findings, this does not disqualify time as an important variable when prescribing PBM. Future clinical studies (for PBM and implant stability) would need to compare similar treatment protocols (wavelength, laser power, fluence) to determine optimal prescriptions for time.
Reporting significance for implant stability was possible for both LED and laser PBM devices. There was wide heterogeneity in how studies prescribed the device placement at different anatomic locations. All LED (618–626 nm) protocols placed the device externally (extraoral) to the surgical site. The laser devices (635–1064 nm) utilized internal (intraoral) contact points. There was no regularity of placing the device in contact versus noncontact (fixed distance) between protocols. There were also variations in the number of contact points per protocol. We could not correlate any of these factors with significantly improving implant stability.
Prescribing PBM during titanium osseointegration can result in significant improvements in implant stability (Fig. 2 and Table 4). Continued PBM application has a direct impact on the healing cascade of bone wound healing that takes place over a surgical site. This specific mechanism is induced by the presence of hemoglobin coefficients stimulated by specific PBM wavelengths in the red and near-infrared range,15 thus inducing the bone healing cascade with fibrin localization, tissue vascularization, and trabecular activity.52
Limitations
There currently needs to be a universal consensus for naming PBM in literature. Several variations exist in how this therapy is defined and cataloged across databases. This includes nomenclature for PBM, low-level laser therapy, PBM, LLLT, and laser stimulation. This inconsistency has limited the potential for groups to locate data and further develop a research protocol to develop clinical care.
The broad clinical heterogeneity for qualifying studies impacted reporting for wavelength, beam area/spot size, location of implant placement, and size of titanium implants (full size and mini-implant). These factors directly affect the depth of penetration and amount of light received. Standardizing the anatomical location of PBM in future studies is necessary. This is also true when considering different sizes of titanium implants and bone tissue densities. Significant efforts are needed to standardize how PBM is prescribed after titanium implant placement.
Conclusions
This is the first known review and irradiation parameter analysis to focus on the significant effects of PBM to improve implant stability. The 15 studies from this meta-analysis support the use of PBM after the placement of titanium implants. It is noted that PBM can be applied to titanium implant surgical sites without adverse negative events. Six out of 15 groups reported statistically significant improvements of implant stability for multiple types of PBM devices (Fig. 2 and Table 4). Results include LED and laser PBM, including a diverse group of wavelengths.
The results of this review and meta-analysis of PBM irradiation parameters after titanium implant placement must be interpreted with caution due to the heterogeneity of all studies. The specific clinical protocols of each reported study should be followed when prescribing PBM for future research. This includes adhering to specific devices, the prescription of clinical contact points, and the total number of PBM visits. There needs to be more standardization in the literature with reporting PBM settings. Future research collaborations can develop future prescription dosing protocols by thoroughly documenting the clinical applications of PBM. This should include the following irradiation parameters at a minimum: wavelength (nm), laser power (W), beam area/spot size (cm2), treatment time (sec), power density (W/cm2), energy dose (J), fluence (J/cm2), clinical application, amount of contact points per dose, and the total number of clinical appointments.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors' Contributions
D.S.: Contributed to the conception, design, prescreening standardization, title, abstract, full-text screening, Cochrane risk-of-bias 2 analysis, data acquisition and interpretation, irradiation parameter dosing analysis, exposure time analysis, electronic data conversion form, article drafter, and critically revised the article. J.P.: Contributed to the conception, design, prescreening standardization, title, abstract, and full-text screening. B.L.: Contributed to Cochrane risk-of-bias 2 analysis, and data interpretation, and critically revised the article. H.S.: Contributed to the conception, design, data interpretation, and critically revised the article. J.C.: Contributed to the irradiation parameter dosing analysis, exposure time analysis, and creation of electronic data conversion form. T.C.Z.: Contributed to the design, interpretation, data analysis, irradiation parameter dosing analysis, and critically revised the article. J.P.F.: Contributed to the conception, design, data acquisition, interpretation, data analysis, arbitrator for RoB2 and irradiation parameter dosing analysis, and critically revised the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
D.S. was supported by the National Institutes of Dental & Craniofacial Research (NIDCR) of the National Institutes of Health grant number T90DE030854 and the Center for Innovation & Precision Dentistry (CiPD) at the University of Pennsylvania. D.S. was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880. T.Z. was supported by the National Institutes of Health grant numbers R01 EB028778, R01 EB032821, and PO1 CA08797. B.L. was supported by the Thouron Award (London, United Kingdom).
References
- 1. López-Ramírez M, Vílchez-Pérez MÁ, Gargallo-Albiol J, et al. Efficacy of low-level laser therapy in the management of pain, facial swelling, and postoperative trismus after a lower third molar extraction. A preliminary study. Lasers Med Sci 2012;27(3):559–566. [DOI] [PubMed] [Google Scholar]
- 2. Eshghpour M, Ahrari F, Takallu M.. Is low-level laser therapy effective in the management of pain and swelling after mandibular third molar surgery? J Oral Maxillofac Surg 2016;74(7):1322.e1–e8. [DOI] [PubMed] [Google Scholar]
- 3. Chung H, Dai T, Sharma SK, et al. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 2012;40(2):516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gholami L, Asefi S, Hooshyarfard A, et al. Photobiomodulation in periodontology and implant dentistry: Part 1. Photobiomodul Photomed Laser Surg 2019;37(12):739–765. [DOI] [PubMed] [Google Scholar]
- 5. Gholami L, Asefi S, Hooshyarfard A, et al. Photobiomodulation in periodontology and implant dentistry: Part 2. Photobiomodul Photomed Laser Surg 2019;37(12):766–783. [DOI] [PubMed] [Google Scholar]
- 6. Rosero KAV, Sampaio RMF, Deboni MCZ, et al. Photobiomodulation as an adjunctive therapy for alveolar socket preservation: A preliminary study in humans. Lasers Med Sci 2020;35(8):1711–1720. [DOI] [PubMed] [Google Scholar]
- 7. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J Biol Chem 2005;280(6):4761–4771. [DOI] [PubMed] [Google Scholar]
- 8. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg 2005;23(4):355–361. [DOI] [PubMed] [Google Scholar]
- 9. Peplow PV, Chung TY, Baxter GD. Laser photostimulation (660nm) of wound healing in diabetic mice is not brought about by ameliorating diabetes. Lasers Surg Med 2012;44(1):26–29. [DOI] [PubMed] [Google Scholar]
- 10. Zhu TC, Finlay JC, Dimofte A, et al. Light dosimetry at tissue surfaces for oblique incident circular fields. Proc SPIE Int Soc Opt Eng 2004;5315:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang YY, Sharma SK, Carroll J, et al. Biphasic dose response in low level light therapy—An update. Dose Response 2011;9(4):602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandell JL, Zhu TC. A review of in-vivo optical properties of human tissues and its impact on PDT. J Biophotonics 2011;4(11–12):773–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng DY, Chan AK, Dalci O, et al. A pilot study of laser energy transmission through bone and gingiva. J Am Dent Assoc 2018;149(8):704–711. [DOI] [PubMed] [Google Scholar]
- 14. Jacques SL. Optical properties of biological tissues: A review. Phys Med Biol 2013;58(11):R37–R61. [DOI] [PubMed] [Google Scholar]
- 15. Asimov M, Asimov R, Rubinov A. Efficiency of Laser Action on Hemoglobin and Oxyhemoglobin in Skin Blood Vessels. The International Society for Optics and Photonics (SPIE). Conference Proceedings. 1998. [Google Scholar]
- 16. Arany PRC, Chen AC-H, Hunt TH, et al. Role of ROS-mediated TGF beta activation in laser photobiomodulation. In: Proceedings SPIE 7165, Mechanisms for Low-Light Therapy IV, 71650C. 2009. [Google Scholar]
- 17. Tang E, Khan I, Andreana S, et al. Laser-activated transforming growth factor-β1 induces human β-defensin 2: Implications for laser therapies for periodontitis and peri-implantitis. J Periodontal Res 2017;52(3):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hosseinpour S, Fekrazad R, Arany PR, et al. Molecular impacts of photobiomodulation on bone regeneration: A systematic review. Prog Biophys Mol Biol 2019;149:147–159. [DOI] [PubMed] [Google Scholar]
- 19. Finnson KW, Arany PR, Philip A. Transforming growth factor beta signaling in cutaneous wound healing: Lessons learned from animal studies. Adv Wound Care (New Rochelle) 2013;2(5):225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arany PR, Nayak RS, Hallikerimath S, et al. Activation of latent TGF-beta1 by low-power laser in vitro correlates with increased TGF-beta1 levels in laser-enhanced oral wound healing. Wound Repair Regen 2007;15(6):866–874. [DOI] [PubMed] [Google Scholar]
- 21. Arany PR, Cho A, Hunt TD, et al. Photoactivation of endogenous latent transforming growth factor-β1 directs dental stem cell differentiation for regeneration. Sci Transl Med 2014;6(238):238ra69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramirez H, Patel SB, Pastar I. The role of TGFβ signaling in wound epithelialization. Adv Wound Care (New Rochelle) 2014;3(7):482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janssens K, ten Dijke P, Janssens S, et al. Transforming growth factor-beta1 to the bone. Endocr Rev 2005;26(6):743–774. [DOI] [PubMed] [Google Scholar]
- 24. Bonewald LF, Mundy GR. Role of transforming growth factor-beta in bone remodeling. Clin Orthop Relat Res 1990(250):261–276. [PubMed] [Google Scholar]
- 25. Branemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl 1977;16:1–132. [PubMed] [Google Scholar]
- 26. Albrektsson T, Wennerberg A. On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res 2019;21 Suppl 1:4–7. [DOI] [PubMed] [Google Scholar]
- 27. Kois JC, Kan JY. Predictable peri-implant gingival aesthetics: Surgical and prosthodontic rationales. Pract Proced Aesthet Dent 2001;13(9):691–698; quiz 700, 21–22. [PubMed] [Google Scholar]
- 28. Tarnow DP, Cho SC, Wallace SS. The effect of inter-implant distance on the height of inter-implant bone crest. J Periodontol 2000;71(4):546–549. [DOI] [PubMed] [Google Scholar]
- 29. Saadoun AP, LeGall M, Touati B. Selection and ideal tridimensional implant position for soft tissue aesthetics. Pract Periodontics Aesthet Dent 1999;11(9):1063–1072; quiz 74. [PubMed] [Google Scholar]
- 30. Oates TW, Valderrama P, Bischof M, et al. Enhanced implant stability with a chemically modified SLA surface: A randomized pilot study. Int J Oral Maxillofac Implants 2007;22(5):755–760. [PubMed] [Google Scholar]
- 31. Fiorellini JP, Nevins ML, Norkin A, et al. The effect of insulin therapy on osseointegration in a diabetic rat model. Clin Oral Implants Res 1999;10(5):362–368. [DOI] [PubMed] [Google Scholar]
- 32. Torkzaban P, Kasraei S, Torabi S, et al. Low-level laser therapy with 940nm diode laser on stability of dental implants: A randomized controlled clinical trial. Lasers Med Sci 2018;33(2):287–293. [DOI] [PubMed] [Google Scholar]
- 33. Matys J, Flieger R, Świder K, et al. A clinical trial of photobiomodulation effect on orthodontic microscrews stability using a 635nm red laser light. Photobiomodul Photomed Laser Surg 2020;38(10):607–613. [DOI] [PubMed] [Google Scholar]
- 34. MA EL, Abdraboh AE, Aboelnagga MM, et al. Effect of low-level laser irradiation on stability and marginal bone of narrow implants retaining overdentures in moderately controlled diabetic patients. J Oral Implantol 2019;45(5):391–397. [DOI] [PubMed] [Google Scholar]
- 35. Kinalski MA, Agostini BA, Bergoli CD, et al. Influence of low-level laser therapy on implant stability in implants placed in healed sites: A randomized controlled trial. Int J Implant Dent 2021;7(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ng D, Chan AK, Papadopoulou AK, et al. The effect of low-level laser therapy on orthodontically induced root resorption: A pilot double blind randomized controlled trial. Eur J Orthod 2018;40(3):317–325. [DOI] [PubMed] [Google Scholar]
- 37. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 39. Chen Y, Liu C, Chen X, et al. Clinical evidence of photobiomodulation therapy (PBMT) on implant stability and success: A systematic review and meta-analysis. BMC Oral Health 2019;19(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Costa ACD, Maia TAC, Silva PGD, et al. Effects of low-level laser therapy on the orthodontic mini-implants stability: A systematic review and meta-analysis. Prog Orthod 2021;22(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dimofte A, Finlay JC, Zhu TC. A method for determination of the absorption and scattering properties interstitially in turbid media. Phys Med Biol 2005;50(10):2291–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palled V, Rao J, Singh RD, et al. Assessment of the healing of dental implant surgical site following low-level laser therapy using bioclinical parameters: An exploratory study. J Oral Implantol 2021;47(3):230–235. [DOI] [PubMed] [Google Scholar]
- 43. Abohabib AM, Fayed MM, Labib AH. Effects of low-intensity laser therapy on the stability of orthodontic mini-implants: A randomised controlled clinical trial. J Orthod 2018;45(3):149–156. [DOI] [PubMed] [Google Scholar]
- 44. Bozkaya S, Uraz A, Guler B, et al. The stability of implants and microbiological effects following photobiomodulation therapy with one-stage placement: A randomized, controlled, single-blinded, and split-mouth clinical study. Clin Implant Dent Relat Res 2021;23(3):329–340. [DOI] [PubMed] [Google Scholar]
- 45. Memarian J, Ketabi M, Amini S. The effect of low-level laser 810nm and light-emitting diode photobiomodulation (626 nm) on the stability of the implant and inflammatory markers interleukin-1 beta and prostaglandin E2, around implants. Dent Res J (Isfahan) 2018;15(4):283–288. [PMC free article] [PubMed] [Google Scholar]
- 46. Gokmenoglu C, Ozmeric N, Erguder I, et al. The effect of light-emitting diode photobiomodulation on implant stability and biochemical markers in peri-implant crevicular fluid. Photomed Laser Surg 2014;32(3):138–145. [DOI] [PubMed] [Google Scholar]
- 47. Ekizer A, Türker G, Uysal T, et al. Light emitting diode mediated photobiomodulation therapy improves orthodontic tooth movement and miniscrew stability: A randomized controlled clinical trial. Lasers Surg Med 2016;48(10):936–943. [DOI] [PubMed] [Google Scholar]
- 48. Wang HW, Zhu TC, Putt ME, et al. Broadband reflectance measurements of light penetration, blood oxygenation, hemoglobin concentration, and drug concentration in human intraperitoneal tissues before and after photodynamic therapy. J Biomed Opt 2005;10(1):14004. [DOI] [PubMed] [Google Scholar]
- 49. Dimofte A, Finlay JC, Liang X, et al. Determination of optical properties in heterogeneous turbid media using a cylindrical diffusing fiber. Phys Med Biol 2012;57(19):6025–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ebrahimi T, Moslemi N, Rokn A, et al. The influence of low-intensity laser therapy on bone healing. J Dent (Tehran) 2012;9(4):238–248. [PMC free article] [PubMed] [Google Scholar]
- 51. Muslim N, Soon YW, Lim CM, Voo NY. Influence of sputtering power on properties of titanium thin films deposited by RF magnetron sputtering. ARPN J Eng Appl Sci 2015;10(16):7184–7189. [Google Scholar]
- 52. Evian CI, Rosenberg ES, Coslet JG, et al. The osteogenic activity of bone removed from healing extraction sockets in humans. J Periodontol 1982;53(2):81–85. [DOI] [PubMed] [Google Scholar]