Abstract
Background
STRIVE was a prospective, 4-year, multicenter, observational, open-label, single-arm study of natalizumab treatment in anti-JC virus antibody-negative patients with early relapsing-remitting multiple sclerosis (RRMS).
Objective
Study objectives examined the effects of natalizumab on cognitive processing speed, confirmed disability improvement (CDI), and patient-reported outcomes (PROs).
Methods
Clinical and PRO secondary endpoints were assessed annually over 4 years in STRIVE. The Symbol Digit Modalities Test (SDMT) was used as a measure of cognitive processing speed. PROs were assessed using the Multiple Sclerosis Impact Score (MSIS-29) and the Work Productivity and Activity Impairment Questionnaire (WPAI).
Results
At all four annual assessments, the proportion of patients in the intent-to-treat (ITT) population (N = 222) who exhibited clinically meaningful improvement in their SDMT score from baseline (i.e., change ≥ 4 points) ranged from 41.9 to 54.0%. The cumulative probability of CDI at 4 years in patients in the ITT population with a baseline Expanded Disability Status Scale score ≥ 2 (N = 133) was 43.9%. Statistically significant reductions in the mean change from screening in the MSIS-29 physical and psychological scores, indicating improved quality of life, were observed over all 4 years (P ≤ 0.0012 for all). A statistically significant decrease from screening in the impact of MS on regular activities, signifying an improvement in this WPAI measure, was also observed over all 4 years of the study.
Conclusion
These results further extend our knowledge of the effectiveness, specifically regarding improvements in cognitive processing speed, disability and PROs, of long-term natalizumab treatment in early RRMS patients.
Clinicaltrials.gov
NCT01485003 (5 December 2011)
Correction to: CNS Drugs (2022) 36:977–993 10.1007/s40263-022-00950-0
The article “Improvements in Cognitive Processing Speed, Disability, and Patient‑Reported Outcomes in Patients with Early Relapsing‑Remitting Multiple Sclerosis Treated with Natalizumab: Results of a 4‑year, Real‑World, Open‑Label Study”, written by Jai Perumal, Roumen Balabanov, Ray Su, Roger Chang, Laura J. Balcer, Steven L. Galetta, Robin L. Avila, Danette Rutledge, Robert J. Fox was originally published Online First without Open Access. After publication in volume 36, issue 9, pages 977–993 the author decided to opt for Open Choice and to make the article an Open Access publication. With the author(s)’ decision to opt for Open Choice the copyright of the article changed on 27th December 2022 to The Author(s) 2022 and the article is forthwith distributed under a Creative Commons Attribution NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Key Points
| Prospective real-world studies examining the effect of long-term natalizumab treatment on cognitive processing speed, disability, and patient-reported outcomes in patients with early relapsing-remitting multiple sclerosis are limited. |
| Over 4 years of treatment with natalizumab in STRIVE, patients with early relapsing-remitting multiple sclerosis experienced improvements in cognitive processing speed, disability, and patient-reported outcomes. |
| These results highlight the importance of assessing multiple outcomes measures, including clinical and patient-reported outcomes, to more completely capture the overall impact of a disease-modifying therapy on patients with relapsing-remitting multiple sclerosis. |
Introduction
For patients with relapsing-remitting multiple sclerosis (RRMS), natalizumab is a highly efficacious treatment with respect to clinical, radiological, and patient-reported outcomes measures, as demonstrated in the phase 3 pivotal trial [1–4]. Data from the 10-year interim analysis of the TYSABRI Observational Program (TOP), the largest ongoing prospective real-world study of natalizumab-treated RRMS patients, support these clinical trial findings and demonstrate long-term safety and clinical disease control with respect to relapses and disability [5]. Outside of TOP, the only other long-term prospective observational study examining the safety and effectiveness of natalizumab in RRMS patients is the 4-year STRIVE study [6]. STRIVE complements and extends the results of TOP by capturing radiological outcomes as well as additional clinical and patient-reported outcomes (PROs) not collected in TOP. In addition, with the growing evidence of the importance of initiating disease-modifying therapy (DMT) early in the disease course, STRIVE only enrolled patients within 3 years of their RRMS diagnosis. Also, STRIVE only enrolled anti-JC virus (JCV) antibody negative patients, i.e., those with an estimated PML risk of 1/10,000 [7]. STRIVE, therefore, provides clinicians and patients with practical benefit-risk information when considering treatment options for patients in early stages of RRMS.
The results of STRIVE’s primary endpoint (i.e., the proportion of patients achieving No Evidence of Disease Activity (NEDA) in years 1 and 2 and clinical NEDA in years 3 and 4) as well as some of its secondary endpoints (i.e., annualized relapse rate (ARR), MRI, 24-week confirmed disability worsening (CDW)) and safety were recently published [6]. In the intent-to-treat (ITT) population 56.1% and 73.6% of patients achieved NEDA in years 1 and 2, respectively, and 84.6% and 91.9% of patients achieved clinical NEDA in years 3 and 4, respectively. The ARR decreased by 90.0%, from 1.41 (95% confidence interval (CI): 1.19, 1.66) in the year prior to natalizumab initiation to 0.14 (95% CI 0.10, 0.21) at the end of the study (P < 0.0001). The cumulative probability of CDW at year 4 was 19.3%. The median number of gadolinium-enhancing (Gd+) lesions and new or newly enlarging T2 lesions at each annual assessment was 0, indicating that over half of the patients did not exhibit new radiological activity. Safety outcomes in STRIVE were consistent with the established safety profile of natalizumab [6].
The present analysis of STRIVE examines the effects of natalizumab on additional secondary endpoints included in the study design (i.e., cognitive processing speed, 24-week confirmed disability improvement (CDI), and PROs) over 4 years. Additional exploratory analyses at 4 years assessed how patients who experienced clinically meaningful improvements on cognitive processing speed or CDI performed on other clinical/radiological outcomes and PROs. Together these findings from STRIVE extend our knowledge of the effectiveness and improvements in cognition, disability, and PROs of prolonged natalizumab treatment in early RRMS patients.
Methods
Study Design and Patients
STRIVE (Study of Tysabri in Early Relapsing-Remitting Multiple Sclerosis in Anti-JC Virus Antibody Negative Patients; clinicaltrials.gov NCT01485003) was a prospective, 4-year, multicenter, observational, open-label, single-arm study conducted at 45 sites in the USA from February 2012 to November 2018 [6]. Patients in STRIVE received 300 mg of natalizumab intravenously every 4 weeks.
Details of the study design, inclusion and exclusion criteria, patient disposition, and baseline characteristics of the ITT and 4-year natalizumab completer populations, as well as the study’s primary and related secondary endpoint results, have been published [6]. Briefly, eligible patients were aged 18–65 years with a documented diagnosis of RRMS (McDonald 2010 criteria) [8], a disease duration ≤ 3 years, and an Expanded Disability Status Scale (EDSS) score ≤ 4.0, as well as a negative test for anti-JCV antibodies within 6 months prior to screening or at the baseline visit. Patients were either naïve to DMTs or had been treated with a DMT for ≤ 36 months prior to the date of informed consent. Patients were excluded, however, if they had prior natalizumab treatment [6].
A patient who completed the 4-year study on natalizumab was termed a “4-year natalizumab completer.” If a patient permanently discontinued natalizumab treatment but chose to remain in the study, data were collected on the reasons for discontinuation. In such cases, the investigative site continued to follow up with the patient as per the protocol schedule of assessments through month 48. As these patients completed the study, they were considered “study completers” along with the 4-year natalizumab completers. If a patient withdrew from the study, the participating neurologist documented the reason for early withdrawal on the study exit form and conducted the final assessments, after which no further data were collected.
All patients provided written informed consent prior to enrollment, and approval was granted by the Copernicus Group IRB #1 (reference number IRB00001313) at 17 study sites and, at the rest of the study sites, by independent ethics committees. The study was performed in accordance with Good Clinical Practice guidelines.
Clinical Outcome Measures
Cognitive Processing Speed
The Symbol Digit Modalities Test (SDMT), which involves matching geometric designs and numerical responses over a set time [9], was used as a measure of cognitive processing speed. Responses could be written or oral. The SDMT score reflects the number of correct matches, with a higher score indicating better cognitive processing speed. A clinically meaningful improvement in SDMT score was defined as an increase of ≥ 4 points [9]. SDMT scores were assessed at baseline and yearly thereafter.
Confirmed Disability Improvement (CDI)
EDSS assessments were conducted at the baseline visit and every 6 months (± 1 month) thereafter. CDI was defined as a ≥ 1.0-point decrease in EDSS score from a baseline EDSS score ≥ 2.0, confirmed 24 weeks later. CDI was not assessed in patients with baseline EDSS scores < 2.0. Maintenance of EDSS improvement referred to a patient’s EDSS score remaining below baseline from the time of CDI to the end of the study.
No Evidence of Disease Activity (NEDA)
NEDA was calculated at year 4 as a composite measure of no relapses or 24-week CDW, no Gd+ lesions, and no new or newly enlarging T2 lesions. CDW was defined as a ≥ 1.5-point increase from a baseline EDSS score of 0.0, a ≥ 1.0-point increase from a baseline EDSS score of 1.0 to < 6.0, or a ≥ 0.5-point increase from a baseline score ≥ 6.0, confirmed 24 weeks later. Clinical NEDA was calculated at year 4 as a composite measure of no relapses or 24-week CDW. MRI NEDA was calculated at year 4 as a composite measure of no Gd+ lesions and no new or newly enlarging T2 lesions.
Patient-Reported Outcomes (PROs)
Quality of Life (QoL)
Patient quality of life (QoL) was assessed using the Multiple Sclerosis Impact Scale (MSIS-29), a brief, self-reported measurement that assesses the physical (20 questions) and psychological (nine questions) impact of MS [10]. The MSIS-29 yields two separate scores (physical and psychological) on a scale of 0–100, with lower scores representing better QoL. Scores on the MSIS-29 can also be divided into five categories: “no problems” (0–19), “few problems” (20–39), “moderate problems” (40–59), “quite a few problems” (60–79), and “extreme problems” (80–100). A change in the MSIS-29 physical or psychological score resulting in a downward or upward move of at least one category was considered an improvement or worsening, respectively, as described previously [11]. Patients completed the MSIS-29 at screening and yearly thereafter.
Capacity for Work
Capacity for work was measured by the Work Productivity and Activity Impairment Questionnaire (WPAI), which quantitatively assesses absenteeism (i.e., hours missed) and presenteeism (i.e., hours worked) as well as the impact of MS on work productivity and regular activities over the preceding 7 days [12]. Patients were asked to answer specific questions based on whether they were currently employed at the time of completing the questionnaire. Currently employed patients were asked to reflect on the past week and provide the number of hours missed from work, both related and unrelated to MS, as well as how many hours they actually worked. Employed patients also rated the impact of MS on work productivity and regular activities on a scale from 0 (i.e., MS had no effect) to 10 (i.e., MS completely prevented productivity/activities). Patients who were not currently employed at the time of completing the questionnaire were only asked to rate the impact of MS on regular activities as described above. WPAI outcomes were evaluated at screening and yearly thereafter.
Statistical Analyses
Analyses except for CDI were conducted in the full ITT population, defined as all enrolled patients who completed informed consent and received one or more doses of natalizumab in STRIVE. In general, continuous variables were analyzed using summary statistics and categorical variables were analyzed using frequency distributions. Missing data were not imputed. Statistical significance was defined as P < 0.05.
To assess the robustness of the results and any potential attrition bias linked to treatment effectiveness, sensitivity analyses on SDMT, MSIS-29, and WPAI scores were performed using the data from all patients who completed the 4-year study on natalizumab (termed “4-year natalizumab completers”). Changes from baseline and screening in SDMT score and PROs, respectively, were analyzed via a Wilcoxon signed-rank test in both the ITT population and 4-year natalizumab completers. The one exception to this was the percent change of patients currently employed compared to screening (i.e., WPAI question 1: Are you currently employed [working for pay]?) which was analyzed via a chi-square test because the responses (i.e., no vs. yes) were categorical variables. The cumulative probability of CDI was estimated using the Kaplan-Meier method in ITT patients with a baseline EDSS score ≥ 2.
Exploratory analysis was conducted to assess how patients who experienced a clinically meaningful improvement in their SDMT score (i.e., ≥ 4) at year 4 did on specific patient-reported (i.e., MSIS-29 and WPAI) and clinical/radiological (i.e., NEDA, clinical NEDA, MRI NEDA) outcomes at that timepoint. In particular, the percentage of patients who worsened, remained stable, or improved on their MSIS-29 physical and psychological scores was calculated using the categorical change as described in Sect. 2.3.1. For the WPAI measures related to hours of work missed per week and the impact on regular activities due to MS, an increase, no change, or reduction indicated a worsening, stability, or improvement in the measure, respectively. NEDA outcomes were assessed as described previously [6].
An additional analysis was conducted to assess how patients who experienced CDI performed on specific patient-reported (i.e., MSIS-29 and WPAI) and clinical/radiological (i.e., NEDA, clinical NEDA, MRI NEDA) outcomes as described above.
Results
Patient Enrollment and Baseline Characteristics
The STRIVE ITT population, defined as all enrolled patients who completed informed consent and received one or more doses of natalizumab, consisted of 222 patients. Of the 155 patients in the ITT population who completed the study, 105 were treated with natalizumab over the entire 4 years of STRIVE (i.e., the 4-year natalizumab completers) [6]. The main reason for natalizumab discontinuation was seroconversion to anti-JC virus antibody-positive status/elevated index/progressive multifocal leukoencephalopathy risk (n = 27). Reported lack of efficacy was listed as a reason for discontinuation of natalizumab in only eight patients [6].
At baseline, patients in the ITT population (N = 222) had a median (range) of 1 (0–12) relapses in the prior year, 0 (0–71) Gd+ lesions, and a median (range) EDSS score of 2.0 (0–6.5) [6]. The mean (SD) SDMT score at baseline was 52.0 (14.0) (Table 1). The mean (SD) MSIS-29 physical and psychological score at screening was 42.2 (19.0) and 22.0 (9.3), respectively, in the ITT population (Table 1). Most patients (69.7%) were employed, reporting an average (SD) of 5.4 (12.7) hours missed from work per week because of MS and a median (range) score of 2 (0–10) on work productivity affected by MS at screening. The median (range) score on regular activities affected by MS for ITT patients regardless of employment status at screening was 3 (0–10) (Table 1).
Table 1.
SDMT and PRO scores at baseline/screening in the STRIVE ITT population and 4-year natalizumab completers
| Assessment | ITT population (N = 222) | 4-year natalizumab completers (n = 105) |
|---|---|---|
| SDMT | ||
| Mean (SD) | 52.0 (14.0)a | 51.3 (14.5) |
| Median (range) | 53.0 (9.0–97.0) | 53.0 (9.0–96.0) |
| MSIS-29 physical score | ||
| Mean (SD) | 42.2 (19.0)b | 40.0 (18.1)c |
| Median (range) | 37.0 (20.0–100.0) | 34.0 (20.0–93.0) |
| MSIS-29 psychological score | ||
| Mean (SD) | 22.0 (9.3)b | 20.7 (9.0)c |
| Median (range) | 21.0 (9.0–45.0) | 17.0 (9.0–45.0) |
| WPAId | ||
| Percentage of patients currently employed | 69.7%e | 72.8%c |
| Hours missed from work week because of MSf | ||
| Mean (SD) | 5.4 (12.7)g | 4.3 (10.7)h |
| Median (range) | 0.0 (0.0–80.0) | 0.0 (0.0–49.0) |
| Hours missed from work per week for non-MS reasons | ||
| Mean (SD) | 2.9 (5.6)i | 3.6 (6.1)j |
| Median (range) | 0 (0.0–30.0) | 0 (0.0–30.0) |
| Hours worked per week | ||
| Mean (SD) | 33.1 (16.3)k | 33.2 (15.9)l |
| Median (range) | 39 (0–84) | 36.5 (0.0–84.0) |
| Work productivity affected by MSm | ||
| Mean (SD) | 2.5 (2.5)n | 2.4 (2.4)h |
| Median (range) | 2 (0–10) | 1 (0–9) |
| Regular activities affected by MSo | ||
| Mean (SD) | 3.7 (3.0)e | 3.5 (3.0)c |
| Median (range) | 3 (0–10) | 3 (0–10) |
ITT intent-to-treat, MS multiple sclerosis, MSIS-29 Multiple Sclerosis Impact Scale, PRO patient-reported outcome, SD standard deviation, SDMT Symbol Digits Modality Test, WPAI Work Productivity and Activity Impairment Questionnaire
an = 221
bn = 217
cn = 103
dThe questions on the impact of MS on regular activities were answered regardless of employment status. The remaining WPAI questions were only answered if the patient was employed at the time of questionnaire completion
en = 218
fQuestion posed: “How many hours did you miss from work because of problems associated with MS?”
gn = 144
hn = 69
in = 147
jn = 75
kn = 146
ln = 74
mQuestion posed: “How much did MS affect your productivity while you were working? Scale is from 0 (no effect) to 10 (completely prevented working)”
nn = 136
oQuestion posed: “How much did MS affect your ability to do your regular daily activities other than work at a job? Scale is from 0 (no effect) to 10 (completely prevented daily activities)”
Baseline characteristics of the 4-year natalizumab completers (n = 105) were similar to those of the ITT population. Patients had a median (range) of 1 (0–4) relapses in the prior year, 0 (0–71) Gd+ lesions, and a median (range) EDSS score of 2.0 (0–4.0) [6]. The mean (SD) SDMT score at baseline was 51.3 (14.5) (Table 1).
The mean (SD) MSIS-29 physical and psychological score at screening was 40.0 (18.1) and 20.7 (9.0), respectively, in the 4-year natalizumab completer population (Table 1). Most patients (72.8%) were employed, reporting an average (SD) of 4.3 (10.7) hours missed from work per week because of MS and a median (range) score of 1 (0–9) on work productivity affected by MS at screening. The median (range) score on regular activities affected by MS for 4-year completers regardless of employment status at screening was 3 (0–10) (Table 1).
Clinical Outcomes
Cognitive Processing Speed
In the ITT population, the percentage of patients with a clinically meaningful improvement in their SDMT score from baseline (i.e., SDMT score change ≥ 4) ranged from 41.9% (80 of 191) to 54.0% (94 of 174) across the four annual assessments (Table 2). Similar results were observed in the 4-year natalizumab completers, with the percentage of patients experiencing a clinically meaningful improvement in their SDMT score from baseline ranging from 44.8% (47 of 105) to 63.8% (67 of 105) across the four annual assessments (Table 2). Of the 4-year natalizumab completers who experienced a clinically meaningful improvement in year 1 (44.8%; n = 47), 83.0% (39 of 47) maintained that improvement at year 4 (see Table 2).
Table 2.
Change in SDMT scores in the ITT population and 4-year natalizumab completers
| 1 year | 2 years | 3 years | 4 years | |
|---|---|---|---|---|
| ITT population | (n = 191) | (n = 158) | (n = 157) | (n = 174) |
| Mean change from baseline in SDMT score (95% CI) | 2.3 (0.8, 3.7) | 4.3 (2.4, 6.2) | 3.6 (2.1, 5.2) | 4.6 (2.9, 6.2) |
| P value | 0.0006 | < 0.0001 | < 0.0001 | < 0.0001 |
| Patients with a clinically meaningful improvement from baseline in SDMT score, n (%) | 80 (41.9) | 78 (49.4) | 85 (54.1) | 94 (54.0) |
| 4-year natalizumab completers | (n = 105) | (n = 101) | (n = 105) | (n = 105) |
| Mean change from baseline in SDMT score (95% CI) | 3.1 (1.3, 4.8) | 4.2 (1.9, 6.5) | 4.2 (2.3, 6.0) | 5.7 (3.6, 7.8) |
| P value | 0.0005 | < 0.0001 | < 0.0001 | < 0.0001 |
| Patients with a clinically meaningful improvement from baseline in SDMT score, n (%) | 47 (44.8) | 50 (49.5) | 60 (57.1) | 67 (63.8) |
| Patients with maintenance of clinically meaningful improvement in SDMT score, n (%)a | 47 (100) | 39 (83.0) | 38 (80.9) | 39 (83.0) |
CI confidence interval, ITT intent-to-treat, SDMT Symbol Digit Modalities Test
aDenominator for years 2–4 is the number of patients with a clinically meaningful improvement from baseline in SDMT score in year 1 (n = 47)
Exploratory analyses were conducted to determine whether the change in the SDMT score varied depending on the patients’ SDMT scores at baseline. Patients were categorized into three subgroups based on the baseline SDMT score quartiles (i.e., those with baseline SDMT scores either below the lowest quartile (< 25%), within the interquartile range (≥ 25% to < 75%), or above or equal to the highest quartile (≥ 75%)]. In the ITT population, a higher proportion of patients with baseline SDMT scores below the lowest quartile (< 25%) experienced clinically meaningful improvement in their SDMT scores than the other two quartile subgroups (i.e., ≥ 25% to < 75% and ≥ 75%) over the course of the study (Table 3). The differences were most evident between the lowest (< 25%) and highest (≥ 75%) quartile subgroups at years 1 (i.e., 56.1% (23 of 41) vs. 27.3% (15 of 55)) and 2 (i.e., 66.7% (22 of 33) vs. 34.9% (15 of 43)) (Table 3). Consistent results were found in the 4-year natalizumab completers.
Table 3.
Change in SDMT scores in quartile subgroups of the ITT population and 4-year natalizumab completers
| Population | Quartile subgroup | 1 year | 2 years | 3 years | 4 years |
|---|---|---|---|---|---|
| ITT | < 25% | ||||
| N | 41 | 33 | 32 | 37 | |
| Mean change from baseline in SDMT score (95% CI) | 6.3 (1.9, 10.7) | 9 (4.4, 13.6) | 9 (5.2, 12.7) | 7.9 (4.3, 11.6) | |
| P value | 0.0002 | 0.0003 | < 0.0001 | < 0.0001 | |
| Patients with a clinically meaningful improvement from baseline in SDMT score, n (%) | 23 (56.1) | 22 (66.7) | 24 (75) | 21 (56.8) | |
| ≥ 25% to < 75% | |||||
| N | 95 | 82 | 79 | 88 | |
| Mean change from baseline in SDMT score (95% CI) | 2 (0.3, 3.7) | 3.9 (1.2, 6.6) | 2.7 (1.1, 4.4) | 3.7 (1.4, 6) | |
| P value | 0.0137 | 0.0034 | 0.0003 | < 0.0001 | |
| Patients with a clinically meaningful improvement from baseline in SDMT score, n (%) | 42 (44.2) | 41 (50) | 37 (46.8) | 46 (52.3) | |
| ≥ 75% | |||||
| N | 55 | 43 | 46 | 49 | |
| Mean change from baseline in SDMT score (95% CI) | – 0.2 (– 2.5, 2.1) | 1.4 (– 1.3, 4.1) | 1.4 (– 2, 4.8) | 3.5 (0.3, 6.7) | |
| P value | 0.8785 | 0.0717 | 0.0163 | 0.0017 | |
| Patients with a clinically meaningful improvement from baseline in SDMT score, n (%) | 15 (27.3) | 15 (34.9) | 24 (52.2) | 27 (55.1) | |
| 4-year natalizumab completers | < 25% | ||||
| N | 23 | 22 | 23 | 23 | |
| Mean change from baseline in SDMT score (95% CI) | 6.5 (2.6, 10.4) | 10.5 (4.7, 16.4) | 8.7 (3.6, 13.8) | 10.3 (4.7, 15.8) | |
| P value | 0.0011 | 0.0011 | 0.0009 | 0.0002 | |
| Patients with a clinically meaningful improvement from baseline in SDMT score, n (%) | 15 (65.2) | 16 (72.7) | 17 (73.9) | 16 (69.6) | |
| ≥ 25% to < 75% | |||||
| N | 53 | 51 | 53 | 53 | |
| Mean change from baseline in SDMT score (95% CI) | 2.9 (0.3, 5.5) | 3 (– 0.1, 6.1) | 2.7 (0.7, 4.8) | 5.1 (2.4, 7.7) | |
| P value | 0.0158 | 0.0519 | 0.0009 | < 0.0001 | |
| Patients with a clinically meaningful improvement from baseline in SDMT score, n (%) | 24 (45.3) | 25 (49) | 25 (47.2) | 32 (60.4) | |
| ≥ 75% | |||||
| N | 29 | 28 | 29 | 29 | |
| Mean change from baseline in SDMT score (95% CI) | 0.7 (– 2, 3.4) | 1.4 (– 2.4, 5.3) | 3.1 (– 0.7, 7) | 3.3 (– 0.8, 7.4) | |
| P value | 0.5976 | 0.0603 | 0.0039 | 0.0068 | |
| Patients with a clinically meaningful improvement from baseline in SDMT score, n (%) | 8 (27.6) | 9 (32.1) | 18 (62.1) | 19 (65.5) |
ITT intent-to-treat, SDMT Symbol Digit Modalities Test
CDI
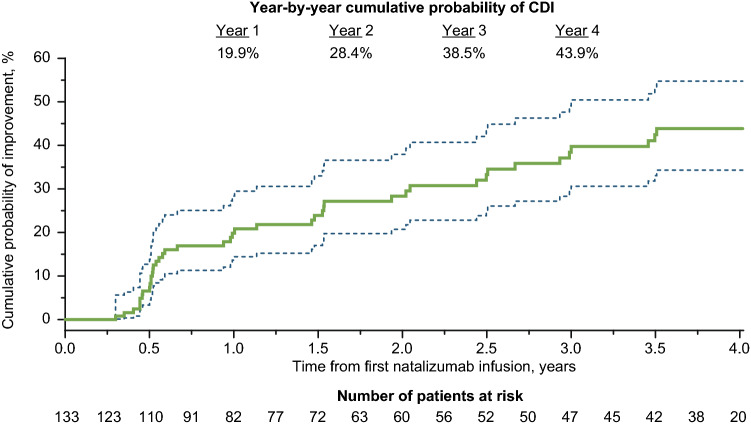
The cumulative probability of CDI at 4 years in patients in the ITT population with a baseline Expanded Disability Status Scale score ≥ 2 (N = 133) was 43.9% (Fig. 1). Of the ITT patients with a baseline EDSS score ≥ 2, 32.3% (43 of 133) patients experienced 24-week CDI at some point during the study. Baseline demographics, disease characteristics, and PROs of these two patient groups (i.e., ITT patients with baseline EDSS score ≥ 2.0 and those who achieved CDI during the study) were generally similar (Table 4). Of the patients who achieved CDI, 62.8% (27 of 43) exhibited a reduction in EDSS score ≥ 1.5, and 44.2% (19 of 43) exhibited a reduction ≥ 2.0. Most of the patients who experienced CDI (79.1%; 34 of 43) maintained the improvement for the remainder of the 4-year study period.
Fig. 1.
Cumulative probability for time to confirmed disability improvement (CDI) over 4 years in the intent-to-treat population. Time point shown is for onset of Expanded Disability Status Scale score decrease, which was confirmed 24 weeks later. Dashed lines show the 95% confidence interval
Table 4.
Baseline characteristics of ITT patients with baseline EDSS score ≥ 2.0 and those with baseline EDSS score ≥ 2.0 who experienced CDI
| Characteristic | Patients with baseline EDSS score ≥ 2.0 (n = 133) | Patients with baseline EDSS score ≥ 2.0 who achieved CDI (n = 43) |
|---|---|---|
| Age, mean (SD), y | 33.9 (8.6) | 32.9 (8.8) |
| Female, n (%) | 91 (68.4) | 26 (60.5) |
| Number of relapses in the past 12 months, mean (SD) | 1.5 (0.9) | 1.6 (1.0) |
| EDSS score, mean (SD) | 2.8 (0.8) | 2.9 (0.9) |
| MS duration, mean (SD), y | 1.6 (0.8) | 1.6 (0.9) |
| T2 lesion volume, mean (SD), cc | 8.4 (10.9) | 7.1 (8.0) |
| Number of Gd+ lesions, mean (SD) | 2.6 (7.5)b | 4.0 (12.0)b |
| Prior MS treatment, n (%) | 68 (51.1) | 18 (41.9) |
| SDMT score, mean (SD) | 49.2 (14.9) | 50.6 (13.2) |
| MSIS-29, mean (SD)c | ||
| Physical score | 47.8 (20.0) | 42.0 (17.7) |
| Psychological score | 23.8 (9.6) | 22.0 (9.3) |
| WPAI, mean (SD) | ||
| Work missed/week due to MS, hd | 8.0 (15.1)e | 9.7 (14.4)e |
| Regular activities affected by MSf | 4.3 (2.9)g | 3.8 (3.2)g |
CDI confirmed disability improvement, EDSS Expanded Disability Status Scale, Gd+ gadolinium- enhancing, ITT intent-to-treat, MS multiple sclerosis, MSIS-29 Multiple Sclerosis Impact Scale, SD standard deviation, SDMT Symbol Digit Modalities Test, WPAI Work Productivity and Activity Impairment Questionnaire
aP values for continuous variables are based on t-test; P values for sex and prior disease-modifying therapy use are based on chi-squared test
bData were missing for 14 patients (four patients with CDI)
cData were missing for three patients (one patient with CDI)
dQuestion posed: “How many hours did you miss from work because of problems associated with MS?”
eData were missing for 56 patients (13 patients with CDI)
fQuestion posed: “How much did MS affect your ability to do your regular daily activities other than work at a job? Scale is from 0 (no effect) to 10 (completely prevented daily activities)”
gData were missing for two patients (one patient with CDI)
PROs
QoL: MSIS-29
Statistically significant reductions in the mean change from screening in the MSIS-29 physical and psychological scores, which signify better QoL, were observed over all 4 years of the study in the ITT population with similar results seen in the 4-year natalizumab completers (Table 5). For example, at year 4, the mean change from screening in the MSIS-29 physical and psychological scores (95% CI; P value) in the ITT population was − 4.7 (−6.9, − 2.4; < 0.0001) and − 2.6 (−3.8, − 1.4; < 0.0001), respectively. Similarly, in the 4-year natalizumab completers the mean change from screening in the MSIS-29 physical and psychological scores (95% CI; P value) at year 4 was − 4.5 (− 7.2, − 1.7; 0.0003) and −2.3 (− 3.8, − 0.7; 0.0125), respectively.
Table 5.
Change in MSIS-29 scores in the ITT population and 4-year natalizumab completers
| 1 year | 2 years | 3 years | 4 years | |
|---|---|---|---|---|
| ITT population | (n = 189) | (n = 155) | (n = 153) | (n = 176) |
| Mean change from screening in MSIS-29 physical score (95% CI) | – 3.9 (– 5.8, – 2.1) | – 3.8 (– 6.2, – 1.5) | – 4.8 (– 7.0, – 2.5) | – 4.7 (– 6.9, – 2.4) |
| P value | < 0.0001 | 0.0005 | < 0.0001 | < 0.0001 |
| Mean change from screening in MSIS-29 psychological score (95% CI) | – 1.7 (– 2.8, – 0.6) | – 2.0a (– 3.2, – 0.7) | – 2.3 (– 3.6, – 1.0) | – 2.6b (– 3.8, – 1.4) |
| P value | 0.0012 | 0.0009 | 0.0005 | < 0.0001 |
| 4-year natalizumab completers | (n = 103) | (n = 99) | (n = 99) | (n = 104) |
| Mean change from screening in MSIS-29 physical score (95% CI) | – 3.1 (– 5.6, – 0.6) | – 3.4 (– 6.2, – 0.6) | – 5.0 (– 7.6, – 2.5) | – 4.5 (– 7.2, – 1.7) |
| P value | 0.0069 | 0.0197 | 0.0002 | 0.0003 |
| Mean change from screening in MSIS-29 psychological score (95% CI) | – 1.5 (– 2.8, – 0.2) | – 1.6a (– 3.2, 0.0) | – 2.2 (– 3.0, – 0.5) | – 2.3a (– 3.8, – 0.7) |
| P value | 0.0133 | 0.0310 | 0.0077 | 0.0125 |
CI confidence interval, ITT intent-to-treat, MSIS-29 Multiple Sclerosis Impact Scale
aData missing in one patient
bData missing in two patients
At 4 years, most patients in the ITT population were stable or exhibited improvement on both the physical (88.5%, 154 of 174) and psychological (91.3%, 157 of 172) components of the MSIS-29 relative to screening, with similar results seen among the 4-year natalizumab completers (Fig. 2).
Fig. 2.
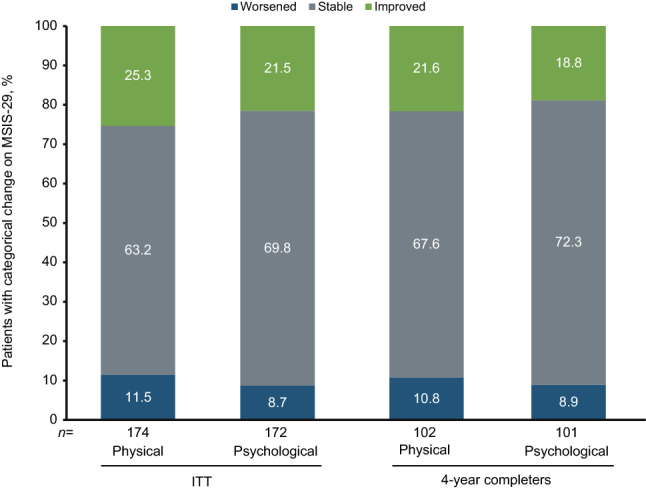
Proportion of patients experiencing worsening, stability, or improvement in Multiple Sclerosis Impact Scale (MSIS-29) scores at year 4 compared with baseline in the intent-to-treat (ITT) and 4-year completer populations
Capacity for work: WPAI
There were no statistically significant differences in the percent change of patients who were employed at each annual assessment compared to screening in either the ITT or the 4-year natalizumab completer populations (Table 6). Of the patients who were employed at screening and subsequently completed all four annual assessments, 74.2% (72 of 97) and 76.9% (50 of 65) maintained their employment throughout the study in the ITT and 4-year natalizumab completer cohorts, respectively.
Table 6.
Mean change from screening in WPAI results in the ITT population and 4-year natalizumab completers
| 1 year | 2 years | 3 years | 4 years | |
|---|---|---|---|---|
| ITT population | ||||
| (n = 186) | (n = 153) | (n = 151) | (n = 173) | |
| Percent change of patients currently employed (95% CI) | – 1.4 (– 10.5, 7.6) | 4.1 (– 5.1, 13.4) | 1.1 (– 8.3, 10.6) | 2.5 (– 6.5, 11.6) |
| P value | 0.754 | 0.386 | 0.815 | 0.585 |
| (n = 125) | (n = 110) | (n = 105) | (n = 126) | |
| Hours missed from work per week because of MS (95% CI)a | – 1.7 (– 3.6, 0.2) | – 2.8 (– 5.3, – 0.4) | – 2.5 (– 5.2, 0.1) | – 2.5 (– 4.7, – 0.3) |
| P value | 0.0550 | 0.0153 | 0.0618 | 0.0260 |
| (n = 125) | (n = 110) | (n = 107) | (n = 126) | |
| Hours missed from work per week for non-MS reasons (95% CI) | 0.6 (– 1.1, 2.2) | – 0.7 (– 2.4, 0.9) | 1.1 (– 1.4, 3.6) | 0.5 (– 1.6, 2.6) |
| P value | 0.9878 | 0.4135 | 0.7883 | 0.8756 |
| (n = 123) | (n = 112) | (n = 108) | (n = 125) | |
| Hours worked per week (95% CI) | – 1.2 (– 4.3, 1.9) | 2.6 (– 0.8, 6.0) | 0.8 (– 2.6, 4.2) | 1.3 (– 2.2, 4.8) |
| P value | 0.5498 | 0.0648 | 0.6086 | 0.3671 |
| (n = 116) | (n = 105) | (n = 103) | (n = 121) | |
| Work productivity affected by MS (95% CI)b | 0.0 (– 0.4, 0.5) | 0.0 (– 0.7, 0.6) | – 0.6 (– 1.2, 0.0) | – 0.5 (– 1.0, 0.0) |
| P value | 0.8974 | 0.6389 | 0.0337 | 0.0172 |
| (n = 187) | (n = 151) | (n = 153) | (n = 173) | |
| Regular activities affected by MS (95% CI)c | – 0.4 (– 0.8, 0.0) | – 0.6 (– 1.1, – 0.1) | – 0.9 (– 1.4, – 0.4) | – 0.9 (– 1.4, – 0.5) |
| P value | 0.0321 | 0.0070 | 0.0004 | < 0.0001 |
| 4-year natalizumab completers | ||||
| (n = 101) | (n = 97) | (n = 97) | (n = 102) | |
| Percentage of patients currently employed (95% CI) | – 5.5 (– 18.0, 7.1) | 1.4 (– 10.8, 13.6) | – 3.7 (– 16.2, 8.8) | – 1.2 (– 13.5, 11.0) |
| P value | 0.392 | 0.821 | 0.560 | 0.842 |
| (n = 67) | (n = 71) | (n = 67) | (n = 74) | |
| Hours missed from work per week because of MS (95% CI)a | – 1.0 (– 3.2, 1.2) | – 1.3 (– 3.3, 0.8) | – 1.4 (– 3.8, 1.0) | – 0.8 (– 3.0, 1.4) |
| P value | 0.1904 | 0.3980 | 0.1770 | 0.5148 |
| (n = 68) | (n = 71) | (n = 68) | (n = 74) | |
| Hours missed from work per week for non-MS reasons (95% CI) | 0.9 (– 1.7, 3.6) | – 1.8 (– 3.7, 0.0) | 1.3 (– 2.2, 4.9) | 1.0 (– 2.5, 4.4) |
| P value | 0.8957 | 0.0842 | 0.9744 | 0.8364 |
| (n = 67) | (n = 74) | (n = 68) | (n = 74) | |
| Hours worked per week (95% CI) | – 3.1 (– 7.4, 1.2) | 1.4 (– 2.6, 5.3) | 0.8 (– 3.2, 4.8) | 1.1 (– 3.6, 5.8) |
| P value | 0.3084 | 0.2148 | 0.6382 | 0.6338 |
| (n = 63) | (n = 68) | (n = 65) | (n = 71) | |
| Work productivity affected by MS (95% CI)b | – 0.1 (– 0.7, 0.5) | – 0.2 (– 1.0, 0.6) | – 0.8 (– 1.6, 0.0) | – 0.5 (– 1.2, 0.2) |
| P value | 0.8738 | 0.4562 | 0.0610 | 0.0867 |
| (n = 103) | (n = 96) | (n = 99) | (n = 103) | |
| Regular activities affected by MS (95% CI)c | – 0.3 (– 0.8, 0.3) | – 0.5 (– 1.1, 0.1) | – 0.9 (– 1.5, – 0.2) | – 1.0 (– 1.6, – 0.4) |
| P value | 0.2994 | 0.1075 | 0.0054 | 0.0017 |
The questions on the impact of MS on regular activities were answered regardless of employment status. The remaining WPAI questions were only answered if the patient was employed at the time of questionnaire completion
CI confidence interval, ITT intent-to-treat, MS multiple sclerosis, WPAI Work Productivity and Activity Impairment Questionnaire
aQuestion posed: “How many hours did you miss from work because of problems associated with MS?”
bQuestion posed: “How much did MS affect your productivity while you were working? Scale is from 0 (no effect) to 10 (completely prevented working)”
cQuestion posed: “How much did MS affect your ability to do your regular daily activities other than work at a job? Scale is from 0 (no effect) to 10 (completely prevented daily activities)”
In the ITT population, irrespective of employment status, a statistically significant decrease in the impact of MS on regular activities was observed over all 4 years of the study (year 1: – 0.4, P = 0.0321; year 2: – 0.6, P = 0.0070; year 3: – 0.9, P = 0.0004; year 4: – 0.9, P < 0.0001) compared with the impact at screening (Table 6). For those employed ITT patients, there was also a statistically significant decrease in the impact of MS on work productivity in years 3 (– 0.6, P = 0.0337) and 4 (– 0.5, P = 0.0172) compared with the impact at screening (Table 6). The number of hours missed per week due to MS was also significantly reduced in years 2 (– 2.8, P = 0.0153) and 4 (– 2.5, P = 0.0260) compared with screening (Table 6). These changes all indicate improvements on these WPAI measures.
Although similar results were observed in the 4-year natalizumab completers, statistically significant decreases compared with screening were observed only with respect to the impact of MS on regular activities in years 3 (–0.9, P = 0.0054) and 4 (–1.0, P = 0.0017; Table 6).
Exploratory Analyses
PROs and NEDA Status in Patients with Clinically Meaningful Improvement in Their SDMT Score at 4 Years (ITT Population)
An exploratory analysis using the STRIVE ITT population was conducted to determine how patients with clinically meaningful improvement in their SDMT score at year 4 did on other outcome measures (i.e., PROs and NEDA) at that timepoint. Most patients who experienced a clinically meaningful improvement in their SDMT score (i.e., ≥ 4) at year 4 also exhibited stability or improvement on their MSIS-29 physical (92.4%, 85 of 92) and psychological (92.3%, 84 of 91) categories (Fig. 3). For those patients who were employed at that time and completed the WPAI questionnaire, most (90.8%, 49 of 54) were stable or had improvements with respect to the number of work hours missed/week due to MS (Fig. 3). Irrespective of employment status, 56.7% (51 of 90) of patients who experienced clinically meaningful improvement on their SDMT score from baseline at year 4 also exhibited an improvement on their ability to do regular activities affected by MS (Fig. 3).
Fig. 3.
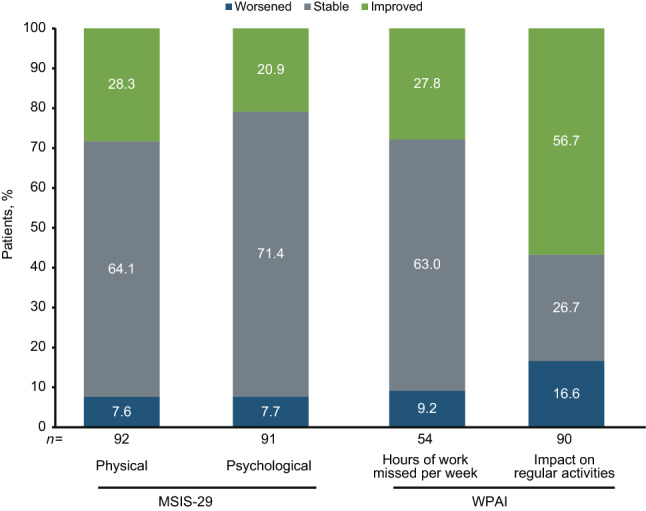
Proportion of patients with clinically meaningful improvements on their SDMT (Symbol Digit Modalities Test) score (≥ 4) who exhibited worsening, stability, or improvement on patient-reported outcomes at year 4. MSIS-29 is categorical change in physical and psychological scores. For WPAI, hours of work missed/week and impact on regular activities are due to multiple sclerosis (MS) (impact on regular activities assessed in all patients regardless of employment status). MSIS-29 Multiple Sclerosis Impact Scale, WPAI Work Productivity and Activity Impairment Questionnaire
The majority of patients who experienced clinically meaningful improvement in their SDMT score at year 4 also achieved NEDA (70.1%, 54 of 77), Clinical NEDA (89.3%, 75 of 84), and MRI NEDA (81.8%, 63 of 77) (Fig. 4).
Fig. 4.
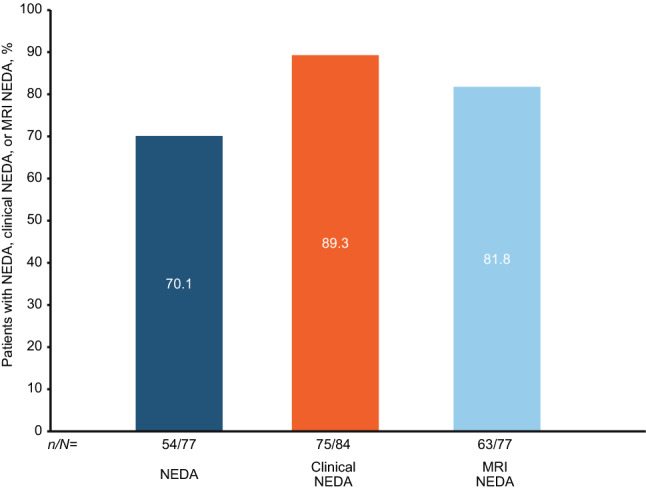
Proportion of patients with clinically meaningful improvements on their SDMT score (≥ 4) who achieved NEDA, Clinical NEDA, or MRI NEDA at year 4. NEDA was defined as no relapses or 24-week CDW, no Gd+ lesions, and no new or newly enlarging T2 lesions (CDW was defined as a ≥ 1.5-point increase from a baseline EDSS score of 0.0, a ≥ 1.0-point increase from a baseline EDSS score of 1.0 to < 6.0, or a ≥ 0.5-point increase from a baseline score ≥ 6.0, confirmed 24 weeks later). Clinical NEDA was defined as no relapses or 24-week CDW. MRI NEDA was defined as no Gd+ lesions and no new or newly enlarging T2 lesions. CDW confirmed disability worsening; EDSS Expanded Disability Status Scale; Gd+ gadolinium-enhancing; MRI magnetic resonance imaging; NEDA No Evidence of Disease Activity
PROs and NEDA Status in Patients Who Experienced CDI (ITT Population with Baseline EDSS Score ≥ 2)
An exploratory analysis using the STRIVE ITT population was conducted to determine how patients with CDI did on other outcome measures (i.e., PROs and NEDA) at year 4. Most patients who experienced CDI also exhibited stability or improvement on their MSIS-29 physical (97.3%, 36 of 37) and psychological (94.6%, 35 of 37) categories at year 4 (Fig. 5). For those patients who were employed at that time and completed the WPAI questionnaire, all (100%, 23 of 23) were stable or had improvements with respect to the number of work hours missed/week due to MS (Fig. 5). Irrespective of employment status, 61.1% (22 of 36) of patients who experienced CDI also exhibited an improvement on their ability to perform regular activities impacted by MS at year 4 (Fig. 5).
Fig. 5.
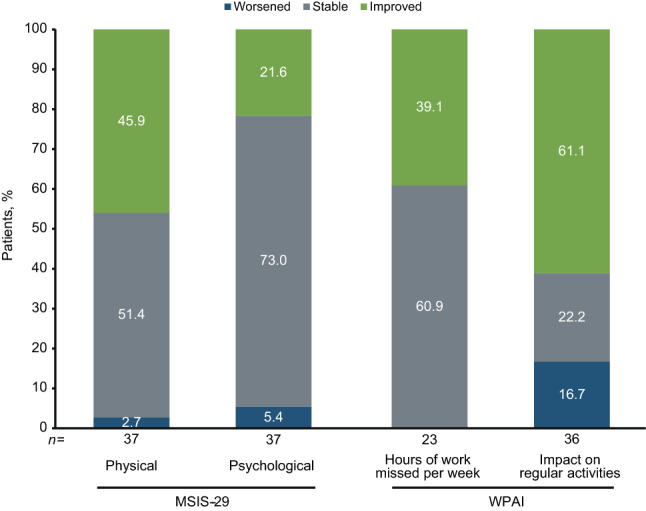
Proportion of ITT patients with confirmed disability improvement who exhibited worsening, stability, or improvement on patient-reported outcomes at year 4. MSIS-29 is categorical change in physical and psychological scores. For WPAI, hours of work missed/week and impact on regular activities are due to MS (impact on regular activities assessed in all patients regardless of employment status). ITT Intent-to-treat; MSIS-29 Multiple Sclerosis Impact Scale; WPAI Work Productivity and Activity Impairment Questionnaire
The majority of patients who experienced CDI also achieved NEDA (67.7%, 23 of 34), Clinical NEDA (88.9%, 32 of 36) and MRI NEDA (79.4%, 27 of 34) at year 4 (Fig. 6).
Fig. 6.
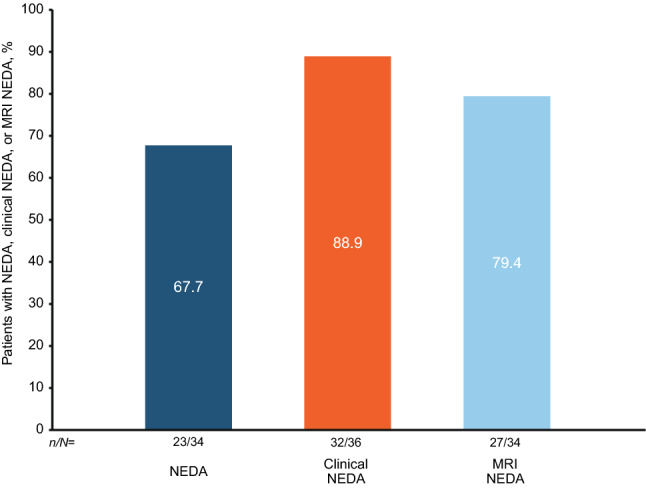
Proportions of ITT patients with confirmed disability improvement who achieved NEDA, Clinical NEDA, or MRI NEDA at year 4. NEDA was defined as no relapses or 24-week CDW, no Gd+ lesions, and no new or newly enlarging T2 lesions (CDW was defined as a ≥ 1.5-point increase from a baseline EDSS score of 0.0, a ≥ 1.0-point increase from a baseline EDSS score of 1.0 to < 6.0, or a ≥ 0.5-point increase from a baseline score ≥ 6.0, confirmed 24 weeks later). Clinical NEDA was defined as no relapses or 24-week CDW. MRI NEDA was defined as no Gd+ lesions and no new or newly enlarging T2 lesions. CDW confirmed disability worsening; EDSS Expanded Disability Status Scale; Gd+ gadolinium-enhancing; ITT Intent-to-treat; MRI magnetic resonance imaging; NEDA No Evidence of Disease Activity
Discussion
In this analysis of real-world data from STRIVE, natalizumab treatment was associated with improvements in cognitive processing speed, disability, and PROs. Given the physical, cognitive, and psychosocial burdens associated with MS, these findings highlight the importance of assessing patients using both clinical measures as well as PROs to more completely capture the overall impact of DMTs on outcomes that are meaningful to patients.
Cognitive change is common in adults with MS, with the prevalence ranging from 35–65% depending on the sample studied and the criteria applied [13]. Cognitive processing speed is one of the most commonly affected cognitive domains in MS with processing deficits found in newly diagnosed RRMS patients [13]. In STRIVE, > 40% of natalizumab-treated patients in the ITT and 4-year natalizumab completer populations exhibited a clinically meaningful improvement in SDMT score at each annual assessment. Among the 4-year natalizumab completers, 83% of patients who exhibited a clinically meaningful improvement in their SDMT score at 1 year maintained that clinically meaningful improvement at year 4. Interestingly, in both the ITT and 4-year natalizumab completer populations, a higher proportion of patients with baseline SDMT scores below the lowest quartile (< 25%) experienced clinically meaningful improvement in their SDMT scores than the other two quartile subgroups over the course of the study. The reasons for these differences are unknown, but may be, in part, due to a significantly higher proportion of patients in the lowest quartile subgroup (< 25%) having ≥ 1 Gd+ lesions at baseline than the other two subgroups (data not shown). Further research is needed. Several prior studies have examined the effect of natalizumab on cognitive processing speed [2, 14–18]; however, unlike STRIVE, these studies examined the effects over only 1 [14, 17] or 2 [2, 15, 16, 18] years. STRIVE is the first study to show maintenance of a clinically meaningful improvement in SDMT score over 4 years of natalizumab treatment. Although a previous study reported a SDMT learning/practice effect in natalizumab-treated MS patients [18], the study design differed from that of STRIVE, since in the previous study patients completed their SDMT assessments monthly for approximately 2 years while in STRIVE SDMT assessments were conducted yearly. The timing of the SDMT assessments in STRIVE aligns with the guidance of a recent National MS Society expert panel that recommended a baseline cognitive screening with the SDMT, as a minimum, as an integral part of disease monitoring followed by annual reassessments for all adults diagnosed with MS [13]. In addition to its impact on cognitive processing speed, natalizumab has also previously been shown to improve other cognitive outcomes, including memory attention, and executive function [15, 19].
Historically phase 3 clinical trial outcomes in RRMS focused on limiting disability worsening. The first published data that focused on improvements in disability were from a post hoc analysis of natalizumab’s phase 3 AFFIRM study [4]. CDI has since become a common secondary endpoint included in phase 3 RRMS clinical trials [20–22]. This outcome measure is also commonly used in observational studies. The largest multinational observational trial for natalizumab, TOP, found that the nearly one-quarter of natalizumab-treated patients experienced CDI in the 10-year interim analysis [23]. Furthermore, patients who initiated natalizumab treatment earlier in their disease course were more likely to experience CDI and patients without prior DMT use had the highest probability of CDI [23]. Based on these data one would expect patients in STRIVE to be more likely to experience CDI since STRIVE was designed to study patients who were treatment-naive or earlier in their disease course (≤ 3 years of diagnosis). This may explain why the cumulative probability of CDI at 4 years in STRIVE was higher than that seen in the TOP 5-year interim analysis [24]. Of the patients in STRIVE who experienced CDI, 44.2% (19 of 43) exhibited a reduction of ≥ 2.0 EDSS points. Furthermore, 79.1% (34 of 43) patients who experienced CDI maintained the improvement for the remainder of the 4-year study period. Currently there is limited literature looking at the comparative effectiveness of high-efficacy monoclonal antibody DMTs with respect to CDI. However, one publication, which looked at the comparative effectiveness of alemtuzumab versus natalizumab, found that the cumulative hazard of 6-month disability improvement was significantly higher with natalizumab than alemtuzumab [25].
PROs are of increasing importance to clinicians, patients, payers, and regulators, as they provide a broader assessment of the impact of MS and MS therapies on patients than just clinical and radiological measures alone [26]. Natalizumab treatment was associated with improvements in QoL that were maintained over the course of the study. MSIS-29 physical and psychological scores were significantly lower at all 4-yearly time points during natalizumab treatment than at screening in both the ITT population and the 4-year natalizumab completers. These findings, which demonstrate an improvement in QoL, support and extend the results of other shorter clinical and real-world studies examining the effect of natalizumab treatment on various QoL measures, including MSIS-29 [11, 17, 27], Short Form-36 or Short-Form-12 [4, 11, 27], and NeuroQoL [28].
MS can severely impair patients’ ability to perform normal daily activities as well as their ability to work and continue to work. In the ITT population, the impact of MS on regular activities was significantly reduced from screening over all 4 years. Employed patients also missed significantly fewer hours of work due to MS in years 2 and 4 compared with screening, and the impact of MS on work productivity was significantly reduced in years 3 and 4. Although the 4-year natalizumab completers had similar results to the ITT population, a statistically significant decrease was observed only with respect to the impact of MS on regular daily activities in years 3 and 4. The reason for this difference in unknown.
To our knowledge there has only been one other observational study conducted in Italy where patients completed WPAI questionnaires at enrollment and after 1 year of natalizumab treatment [17]. In contrast to STRIVE, the Italian study found statistically significant reductions in absenteeism (i.e., hours of work missed) and work productivity loss (i.e., impact of MS on work productivity) after 1 year of natalizumab treatment. The discrepancy in findings may be due to differences in the inclusion criteria. Unlike STRIVE, patients in the Italian study were required to be working full time with no time off work planned in the following year for any reason and must have reported a loss of at least 1 working hour due to MS during the previous week [17].
Little is known of how the improvements in cognitive processing speed and disability seen with natalizumab treatment correspond to effects on PROs and NEDA. Therefore, exploratory analyses were conducted to determine how patients who experience clinically meaningful improvement in their SDMT scores or CDI perform on other patient-reported and clinical outcomes. In both analyses, it was found that the majority of patients who experienced clinically meaningful improvement in their SDMT scores or CDI also exhibited stability or improvement on their PROs. Furthermore, the exploratory analyses showed that a majority of these patients also achieved NEDA, Clinical NEDA, and MRI NEDA. Hence, these results demonstrate that a proportion of patients can experience improvements in several clinical/radiological outcomes and PROs with meaningful impact on patients’ daily lives.
There are several limitations of the STRIVE study, including the lack of a comparator/reference group, the open-label study design, missing data, and a potential bias for sustained improvement because of regression to the mean in this early-in-disease and mostly treatment-naïve patient cohort. The absence of a reference group along with the failure to collect patients’ baseline education level also made it infeasible to calculate z-scores. Therefore, interpretation and generalization of these results should be performed with caution. Nevertheless, the mean change from baseline and the percentage of patients with clinically meaningful improvement in SDMT included in the current article are outcome measurements consistent with those in other published articles [29, 30]. In addition, the SDMT, which does not provide a comprehensive assessment of overall cognitive function, can be impacted by other factors, such as cognitive-mediated eye-movement issues [31]. Finally, the exploratory analyses were not prespecified and, therefore, further studies are needed to confirm those results.
To assess the robustness of the results and address any potential attrition bias, sensitivity analyses on SDMT and PRO outcomes were performed based on the data from patients who completed the 4-year study on natalizumab. The results of these sensitivity analyses were similar to those of the ITT population. In addition, reported lack of efficacy was only listed as a reason for treatment discontinuation in eight patients, whereas seroconversion to anti-JCV antibody-positive status/elevated index/PML risk was the most common reason (n = 27) for treatment discontinuation [6], which is consistent with other observational studies.
Conclusion
These results from STRIVE highlight the benefits of long-term natalizumab treatment early in the RRMS disease course with respect to CDI, cognitive processing speed, and PROs, including the impacts of MS on physical and psychological QoL and on patients’ abilities to work and perform regular activities. These findings further the understanding of improvements in other clinical outcomes with meaningful impact on MS patients’ daily lives while being treated with natalizumab.
The original article has been corrected.
Acknowledgements
Alexandra D’Agostino, PhD, of Ashfield MedComms, an Inizio Company (Middletown, CT, USA) wrote the first draft of the manuscript based on input from authors. Joshua Safran and Celia Nelson of Ashfield MedComms copyedited and styled the manuscript per journal requirements. The authors gratefully acknowledge the STRIVE investigators, listed below, for their efforts and contributions, as well as the STRIVE patients. STRIVE investigators: Bridget Bagert, MD, Roumen Balabanov, MD, Margaret Burnett, MD, Claudia Chaves, MD, Stanley Cohan, MD, PhD, Joanna Cooper, MD, Eric Eggenberger, DO, John Foley, MD, Edward Fox, MD, PhD, Robert Fox, MD, Dennis Garwacki, MD, Lawrence Goldstick, MD, Benjamin Greenberg, MD, MHS, Mark Gudesblatt, MD, Craig Herrman, MD, Jonathan Howard, MD, John Huddlestone, MD, Mark Janicki, MD, Jeffrey Kaplan, MD, George Katsamakis, MD, Amos Katz, MD, Mariko Kita, MD, Lauren Krupp, MD, Ellen Lathi, MD, Kermit Lloyd, MD, Kenneth Mankowski, DO, Tamara Miller, MD, Stephen Newman, MD, Scott Newsome, DO, Allan Perel, MD, Jai Perumal, MD, John Puente, MD, Marcus Rice, MD, Emily Riser, MD, Peter Riskind, MD, PhD, Teri Schreiner, MD, MPH, Christopher Sheppard, MD, Scott Silliman, MD, Jason Silversteen, DO, Jacob Sloane, MD, PhD, Charles Smith, MD, Ben Thrower, MD, Robert Tillett, MD, Carlo Tornatore, MD.
Declarations
Funding
This study was sponsored by Biogen. Biogen provided funding for medical writing support in the development of this manuscript and paid the open access fee.
Conflicts of interest
Jai Perumal has received fees from Acorda, Biogen, Genzyme, and Teva. Roumen Balabanov has received consulting fees from Biogen, Sanofi, and Teva and grant/research support from Biogen. Ray Su and Roger Chang were employees of Biogen at the time of these analyses and may hold stock and/or stock options in Biogen. Robin L. Avila, and Danette Rutledge are employees of and may hold stock and/or stock options in Biogen. Laura Balcer is editor-in-chief of the Journal of Neuro-Ophthalmology. Steven Galetta has received consulting fees from Biogen and Genentech. Robert J. Fox has received personal consulting fees from AB Science, Biogen, Celgene, EMD Serono, Genentech, Genzyme, Greenwich Biosciences, Immunic, Janssen, Novartis, Sanofi, and TG Therapeutics; has served on advisory committees for AB Science, Biogen, Genzyme, Immunic, Janssen, Novartis, Sanofi, and TG Therapeutics; and has received clinical trial contract and research grant funding from Biogen, Novartis, and Sanofi.
Ethics approval
Approval was granted by the Copernicus Group IRB #1 (reference number IRB00001313) at 17 study sites and, at the rest of the study sites, by an independent ethics committee. The study was performed in accordance with Good Clinical Practice guidelines.
Consent to participate
All patients provided written informed consent prior to enrollment in STRIVE.
Consent for publication
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available. The authors fully support sharing whenever possible. Requests for de-identified data should be made to Biogen via established company data-sharing policies and processes detailed on the website http://clinicalresearch.biogen.com/.
Code availability
Not applicable.
Author contributions
Study conception: JP, RB, LB, SG, RJF. Data collection: JP, RB, LB, SG, RJF. Statistical analysis: RS, RC. All authors were involved in critically reviewing the manuscript for intellectual content and provided final approval of the submitted version. All authors agree to be accountable for this work.
Footnotes
The article was published without final corrections being incorporated. The final article with all corrections is republished here. The original article has also been updated.
References
- 1.Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock-Guttman B, Galetta SL, Giovannoni G, Havrdova E, Hutchinson M, Kappos L, et al. Additional efficacy endpoints from pivotal natalizumab trials in relapsing-remitting MS. J Neurol. 2012;259(5):898–905. doi: 10.1007/s00415-011-6275-7. [DOI] [PubMed] [Google Scholar]
- 3.Phillips JT, Giovannoni G, Lublin FD, O’Connor PW, Polman CH, Willoughby E, et al. Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler. 2011;17(8):970–979. doi: 10.1177/1352458511399611. [DOI] [PubMed] [Google Scholar]
- 4.Rudick RA, Miller D, Hass S, Hutchinson M, Calabresi PA, Confavreux C, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007;62(4):335–346. doi: 10.1002/ana.21163. [DOI] [PubMed] [Google Scholar]
- 5.Butzkueven H, Kappos L, Wiendl H, Trojano M, Spelman T, Chang I, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP) J Neurol Neurosurg Psychiatry. 2020;91(6):660–668. doi: 10.1136/jnnp-2019-322326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perumal J, Balabanov R, Su R, Chang R, Balcer L, Galetta S, et al. Natalizumab in early relapsing-remitting multiple sclerosis: a 4-year, open-label study. Adv Ther. 2021;19(1):116. doi: 10.1007/s12325-021-01722-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tysabri Prescribing Information . TYSABRI® (natalizumab) [prescribing information] Cambridge: Biogen, Inc; 2021. [Google Scholar]
- 8.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R, et al. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 2017;23(5):721–733. doi: 10.1177/1352458517690821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29) a new patient-based outcome measure. Brain. 2001;124(5):962–973. doi: 10.1093/brain/124.5.962. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson JJ, Kern DM, Agarwal SS, Zeidman R, Rajagopalan K, Kamat SA, et al. Impact of natalizumab on patient-reported outcomes in multiple sclerosis: a longitudinal study. Health Qual Life Outcomes. 2012;10:155. doi: 10.1186/1477-7525-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 13.Kalb R, Beier M, Benedict RH, Charvet L, Costello K, Feinstein A, et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler. 2018;24(13):1665–1680. doi: 10.1177/1352458518803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow S, O'connor P, Polman C, Goodman A, Kappos L, Lublin F, et al. Evaluation of the symbol digit modalities test (SDMT) and MS neuropsychological screening questionnaire (MSNQ) in natalizumab-treated MS patients over 48 weeks. Mult Scler. 2010;16(11):1385–1392. doi: 10.1177/1352458510378021. [DOI] [PubMed] [Google Scholar]
- 15.Gudesblatt M, Wissemann K, Zarif M, Bumstead B, Fafard L, Wilken J, et al. Improvement in Cognitive Function as Measured by NeuroTrax in Patients with Relapsing Multiple Sclerosis Treated with Natalizumab: A 2-Year Retrospective Analysis. CNS Drugs. 2018;32(12):1173–1181. doi: 10.1007/s40263-018-0553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iaffaldano P, Viterbo RG, Paolicelli D, Lucchese G, Portaccio E, Goretti B, et al. Impact of natalizumab on cognitive performances and fatigue in relapsing multiple sclerosis: a prospective, open-label, two years observational study. PLoS ONE. 2012;7(4):e35843. doi: 10.1371/journal.pone.0035843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capra R, Morra VB, Mirabella M, Gasperini C, Scandellari C, Totaro R, et al. Natalizumab is associated with early improvement of working ability in relapsing-remitting multiple sclerosis patients: WANT observational study results. Neurol Sci. 2021;42(7):2837–45. [DOI] [PubMed]
- 18.Roar M, Illes Z, Sejbaek T. Practice effect in Symbol Digit Modalities Test in multiple sclerosis patients treated with natalizumab. Mult Scler Relat Disord. 2016;10:116–122. doi: 10.1016/j.msard.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Mattioli F, Stampatori C, Bellomi F, Scarpazza C, Capra R. Natalizumab significantly improves cognitive impairment over three years in MS: pattern of disability progression and preliminary MRI findings. PLoS ONE. 2015;10(7):e0131803. doi: 10.1371/journal.pone.0131803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–1839. doi: 10.1016/S0140-6736(12)61768-1. [DOI] [PubMed] [Google Scholar]
- 21.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 22.Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 23.Wiendl H, Spelman T, Butzkueven H, Kappos L, Trojano M, Su R, et al. Real-world disability improvement in patients with relapsing–remitting multiple sclerosis treated with natalizumab in the Tysabri Observational Program. Mult Scler J. 2021;27(5):719–28. [DOI] [PubMed]
- 24.Butzkueven H, Kappos L, Pellegrini F, Trojano M, Wiendl H, Patel RN, et al. Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results. J Neurol Neurosurg Psychiatry. 2014;85(11):1190–1197. doi: 10.1136/jnnp-2013-306936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalincik T, Brown JWL, Robertson N, Willis M, Scolding N, Rice CM, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol. 2017;16(4):271–281. doi: 10.1016/S1474-4422(17)30007-8. [DOI] [PubMed] [Google Scholar]
- 26.D'Amico E, Haase R, Ziemssen T. Review: Patient-reported outcomes in multiple sclerosis care. Mult Scler Relat Disord. 2019;33:61–66. doi: 10.1016/j.msard.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Foley JF, Nair KV, Vollmer T, Stephenson JJ, Niecko T, Agarwal SS, et al. Long-term natalizumab treatment is associated with sustained improvements in quality of life in patients with multiple sclerosis. Patient Prefer Adherence. 2017;11:1035. doi: 10.2147/PPA.S134865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hersh CM, Kieseier B, de Moor C, Miller DM, Campagnolo D, Williams JR, et al. Impact of natalizumab on quality of life in a real-world cohort of patients with multiple sclerosis: Results from MS PATHS. Mult Scler J Exp Transl Clin. 2021;7(2):20552173211004634. doi: 10.1177/20552173211004634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benedict RH, Cohan S, Lynch SG, Riester K, Wang P, Castro-Borrero W, et al. Improved cognitive outcomes in patients with relapsing-remitting multiple sclerosis treated with daclizumab beta: results from the DECIDE study. Mult Scler. 2018;24(6):795–804. doi: 10.1177/1352458517707345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benedict RHB, Tomic D, Cree BA, Fox R, Giovannoni G, Bar-Or A, et al. Siponimod and cognition in secondary progressive multiple sclerosis: EXPAND secondary analyses. Neurology. 2021;96(3):e376–e386. doi: 10.1212/WNL.0000000000011275. [DOI] [PubMed] [Google Scholar]
- 31.Pavisian B, Patel VP, Feinstein A. Cognitive mediated eye movements during the SDMT reveal the challenges with processing speed faced by people with MS. BMC Neurol. 2019;19(1):340. doi: 10.1186/s12883-019-1543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available. The authors fully support sharing whenever possible. Requests for de-identified data should be made to Biogen via established company data-sharing policies and processes detailed on the website http://clinicalresearch.biogen.com/.