Abstract
Anterior cruciate ligament (ACL) injury and ACL reconstruction (ACLR) surgery are common. Laboratory-based biomechanical assessment can evaluate ACL injury risk and rehabilitation progress after ACLR; however, lab-based measurements are expensive and inaccessible to most people. Portable sensors such as wearables and cameras can be deployed during sporting activities, in clinics, and in patient homes. Although many portable sensing approaches have demonstrated promising results during various assessments related to ACL injury, they have not yet been widely adopted as tools for out-of-lab assessment. The purpose of this review is to summarize research on out-of-lab portable sensing applied to ACL and ACLR and offer our perspectives on new opportunities for future research and development. We identified 49 original research articles on out-of-lab ACL-related assessment; the most common sensing modalities were inertial measurement units, depth cameras, and RGB cameras. The studies combined portable sensors with direct feature extraction, physics-based modeling, or machine learning to estimate a range of biomechanical parameters (e.g., knee kinematics and kinetics) during jump-landing tasks, cutting, squats, and gait. Many of the reviewed studies depict proof-of-concept methods for potential future clinical applications including ACL injury risk screening, injury prevention training, and rehabilitation assessment. By synthesizing these results, we describe important opportunities that exist for clinical validation of existing approaches, using sophisticated modeling techniques, standardization of data collection, and creation of large benchmark datasets. If successful, these advances will enable widespread use of portable-sensing approaches to identify ACL injury risk factors, mitigate high-risk movements prior to injury, and optimize rehabilitation paradigms.
Subject terms: Disease prevention, Risk factors, Biomedical engineering
Introduction
Anterior cruciate ligament (ACL) injury is common in sports, with an estimated 400,000 people injuring their ACL in the United States each year1, leading to over 129,000 ACL reconstruction (ACLR) surgeries2. Concerningly, nearly half of these patients are under 20 years of age, and they suffer from not only over 20% reinjury rates3,4 but also 50–80% knee osteoarthritis rates within a decade of injury5,6. Knee osteoarthritis can lead to chronic pain and significant disability requiring surgical treatments such as total knee arthroplasty.
Research using biomechanical assessments, defined as quantitative measurement of kinematics (i.e., motions) and kinetics (i.e., forces) during human movement, have demonstrated that ACL injury risk and knee function following ACLR are associated with biomechanical parameters such as dynamic knee valgus, and knee extension and abduction moments during jump-landing and gait7–11. Biomechanical assessment during dynamic movement is crucial because ACL injuries occur during movement and thus static observations are insufficient12,13. For this reason, clinical measures like the Landing Error Scoring System (LESS) score are proposed in an attempt to quantify ACL injury risk;14 however, they rely on human raters and binary scores, making them less objective and informative than quantitative Multiomic analyses kinematic and kinetic assessment. Similarly, static physical examinations such as the Lachman test and pivot shift test rely on subjective feelings and experiences of the examiners that reduce the assessment reliability15,16. Also, although ACLR patients’ readiness to return to sport is traditionally assessed in clinics via strength and hop tests, recent studies suggest that readiness could be more holistically assessed based on kinematics and kinetics during running17,18, squatting19,20, and single-leg drop vertical jump21.
Preventing injury is a major goal of biomechanical assessments which seek to identify individuals at high risk of injury and provide feedback to prevent high-risk movement patterns. Identifying those with high injury risk and training them to adopt less risky movement patterns can lead to a wide range of health, societal, and economic benefits, including reductions in injury rates, sports drop-out rates, knee osteoarthritis incidence, and financial costs associated with rehabilitation and symptom management22,23.
After an ACL injury, the goal of biomechanical assessment is to guide and monitor the progress of comprehensive rehabilitation to allow return to sport and other physical activities without reinjury. Rehabilitation following ACLR should be customized according to various individual factors, like the amount of healing present in the ACL graft, activity level, and personal preferences24. Periodic assessment of kinematics and kinetics during squatting and walking can provide valuable insights into patients’ recovery status25–27 and allow for customized physical rehabilitation protocols, thus accelerating recovery, lowering the risk of a secondary ACL injury, and helping athletes return to pre-injury sports level28,29.
Traditionally, biomechanical assessment requires optical motion capture and force plates. Although these devices are considered the gold standard for measurement, they confine the assessment to specialized motion laboratories, making evaluation inaccessible to a majority of people. Portable sensors, including inertial measurement units (IMUs), depth cameras, red-green-blue (RGB) cameras, electromyography (EMG), are more portable and less expensive than gait lab equipment, making them promising for out-of-lab assessment of pathologies like osteoarthritis30, atrial fibrillation31, and Parkinson’s disease32. Similarly, these sensors may offer tremendous opportunities for less expensive, widespread ACL injury risk screening and injury-prevention training. Recent portable-sensor-based assessment methods can be used in clinics or homes33–35, thus increasing accessibility and affordability, and potentially benefiting thousands of patients following ACLR.
Although accessibility of portable-sensor-based assessments can enable their broad ACL-related use, tools that are ready for at-scale, clinical assessment of kinematics and kinetics do not yet exist. Previous reviews report on wearable sensing (IMU, EMG, pedometer, goniometer, and pressure insole) for knee health, as opposed to all portable sensing studied in this review. In our manuscript, we also characterize depth cameras and RGB cameras. Also, prior reviews either did not specifically focus on ACL injury30,36–38, or were focused on a specific aspect of sensor performance such as asymmetry identification39. No study has comprehensively reviewed the utility of portable sensing in ACL-related assessment, and thus the ideal methods to use, which clinically relevant parameters to assess, and at what point in the clinical workflow they should be employed remain unclear. To this end, we undertook this review to summarize the existing portable-sensor-based ACL assessment literature, including current target motions, sensing approaches, modeling techniques, and clinical applications. We also offer our perspectives on (1) future work that is necessary to achieve greater clinical impact and (2) new opportunities that may enhance the validity, reproducibility, and generalizability of the assessment methods.
Results
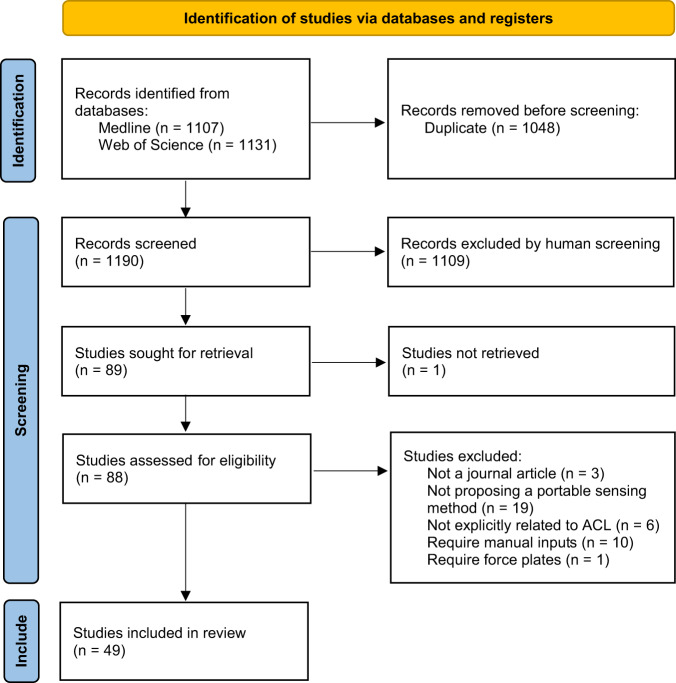
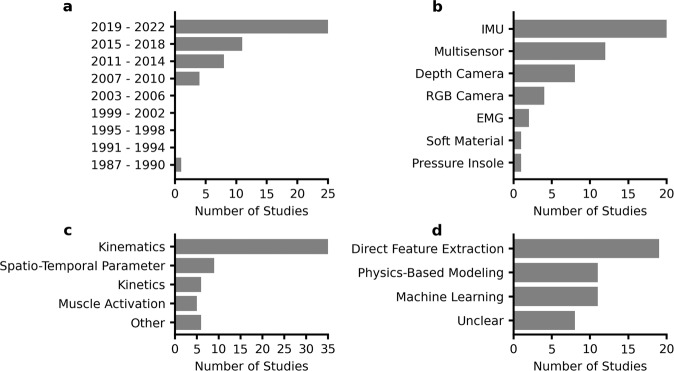
Our search yielded 1344 articles, of which 49 articles were included (Fig. 1), dating from 1990 to 2022. In all, 98% of articles were published since 2007 and 51% since 2019 (Fig. 2a). IMUs were the most common sensor used in isolation (22%), followed by depth cameras (16%), RGB cameras (8%), and EMG (4%) (Fig. 2b). Kinematic parameters were the dominant target (71%), followed by spatiotemporal parameters (18%), kinetics (12%), and muscle activation (10%) (Fig. 2c). The sum of percentages is greater than 100% because several studies targeted parameters in multiple categories. Direct feature extraction (37%) was the most common analysis approach, followed by physics-based modeling (24%), and machine learning (22%) (Fig. 2d). The majority of studies used custom methods in their analysis (71%), while the remaining studies (29%) used direct outputs from commercially-available systems. ACL injury risk screening (57%) and rehabilitation assessment (55%) were the most common clinical applications, with a smaller percentage focusing on injury prevention training (6%). The sum of percentages is greater than 100% because several studies targeted multiple clinical applications.
Fig. 1. PRISMA flow chart.
Search and study selection process for this review.
Fig. 2. Characteristics of the included studies.
a The number of articles has continually increased since 2007. b IMU was the most common sensor. “Multisensor” represents studies that used two or more sensing modalities. c Kinematics, including joint and segment angles, were most commonly estimated. Spatio-temporal parameters include timing of foot-ground contact, hop distance, and jump height, whereas kinetics include ground reaction force (GRF) and joint moments. All of the outcome measures used in each study were counted in the totals. d Direct feature extraction was the most common methodology.
Sensing approach
IMU-based and RGB-camera-based studies had diverse configurations in terms of the number and placement of sensors (Table 1). The configuration of IMUs ranged from using one IMU for capturing shank movement to using seven IMUs for capturing all the lower-body joint kinematics. IMU sensors were most commonly placed on the shank, followed by the thigh, foot, and waist. The configuration of RGB cameras ranged from using one camera for capturing single-plane kinematics to using four calibrated cameras for capturing 3-D kinematics. The configurations of depth cameras were consistent in that seven out of eight studies placed a depth camera in front of the subject.
Table 1.
Configurations of the included studies that only used one sensing modality.
| Sensor | Number | Placement | Paper |
|---|---|---|---|
| IMU | 1 | Shank | 55,77,84 |
| IMU | 1 | Waist | 68,85 |
| IMU | 1 | Ear | 64 |
| IMU | 1 | Wrist | 81 |
| IMU | 2 | Thigh and shank | 51,71 |
| IMU | 2 | Foot and shank | 69 |
| IMU | 2 | Both thighs | 78 |
| IMU | 2 | Both shanks | 57 |
| IMU | 3 or more | Multiple segments | 33,56,58,59,63,65,66,76,86 |
| Depth camera | 1 | Frontal plane | 34,61,88,131–134 |
| Depth camera | 1 | Sagittal plane | 70 |
| RGB camera | 1 | Frontal plane | 60 |
| RGB camera | 1 | Sagittal plane | 67 |
| RGB camera | 2 | Frontal and sagittal plane | 74 |
| RGB camera | 4 | Ceiling | 75 |
| EMG | 1 | Proximal to the patella | 83 |
| EMG | 3 | Vastus medialis and tibial tuberosity | 79 |
| Pressure insole | 2 | Foot | 80 |
| Soft fabric sensor | 1 | Above patella | 62 |
Included studies used six different types of multi-sensor combinations, and five of them involve IMU (Fig. 3). Seven studies designed algorithms to fuse multi-sensor data to estimate parameters35,40–45, whereas the remaining five studies independently used different sensors to estimate different parameters46–50. Some sensor combinations might be redundant and could potentially be simplified. For example, four grounded optoelectronic bars were used to detect the initial foot-ground contact during landing alongside a shank IMU44,45. The optoelectronic bars might be unnecessary because an IMU can estimate foot-ground collision by detecting the acceleration impulse51. Also, two studies simultaneously used a shank-worn IMU to measure tibial acceleration and a goniometer to measure knee flexion angle48,49. The goniometer, which needs four belts to be strapped to the knee, could be replaced by an additional IMU on the thigh.
Fig. 3. Studies used multiple sensing modalities.
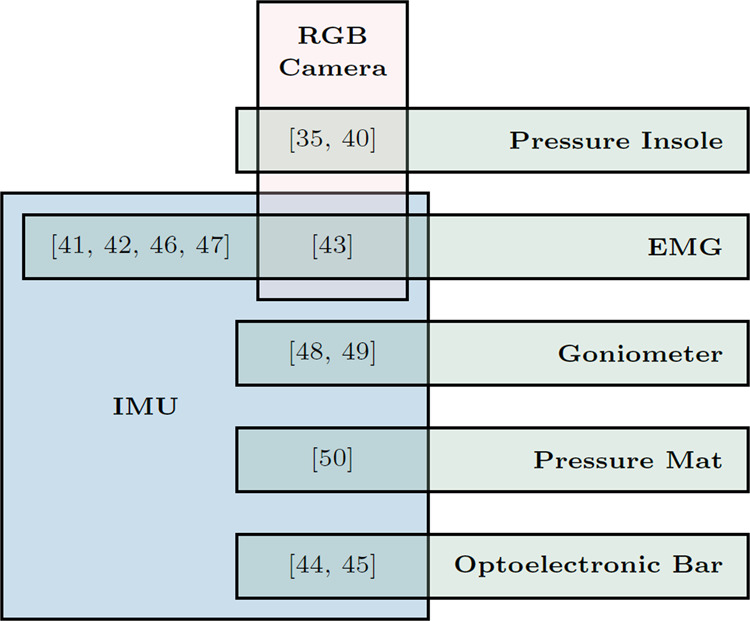
Eleven studies simultaneously used two sensing modalities and one study used three sensing modalities.
Target motions and biomechanical parameters
Jump-landing, cutting, gait, and squatting were the most commonly measured activities in our included sample. Many included studies estimated two primary kinematic parameters associated with ACL injury risk - knee flexion angle and abduction angle, mostly using IMUs, depth cameras, and RGB cameras (Table 2). Several studies estimated four primary parameters associated with ACL injury risk during jump-landing tasks, i.e., knee extension moment, knee separation distance, knee internal rotation, and vertical GRF during jump-landing tasks. Although knee abduction moment is a primary parameter that has been associated with ACL injury risk7,8 as well as knee osteoarthritis52,53, only one study estimated it during squatting35. Apart from primary parameters, other target parameters can provide insights into ACL injury risk and rehabilitation outcomes as well. For example, trunk kinematics during drop jump54 and the LESS score14 can be used to identify athletes with risky movement patterns. In addition, some “non-primary” target parameters are correlated with primary parameters, e.g., tibial acceleration with knee extension moment during drop landing (r = 0.72)55, thigh angular velocity with knee extension moment during single-leg forward hopping (r = 0.59)56, tibial angular velocity with knee extension moment during gait (r = 0.76)57, and tibial and thigh angular velocity with knee abduction moment during drop vertical jump (r = 0.28–0.51)58.
Table 2.
Target motions and biomechanical parameters of included studies.
| Category | Parameter | Jump-landing tasks | Cutting | Gait | Squat |
|---|---|---|---|---|---|
| Primary kinematic parameters | Knee flexion angle | 33,34,51,59,61–63,65–67,71,75,86,133 | 33,65–67,86,132 | 33,42,48,49,63 | 35,63 |
| Knee abduction angle | 33,34,63,65–67,75,86 | 33,65–67,86,132 | 33,63 | 35,63,134 | |
| Knee separation distance | 61,133 | – | – | – | |
| Knee internal rotation | 63,66,67 | 66,67 | 63 | 35,63 | |
| Primary kinetic parameters | Knee extension moment | 71 | – | – | 35 |
| Knee abduction moment | – | – | – | 35 | |
| Vertical GRF | 40,50,71 | – | 70 | – | |
| Additional kinematic parameters | Trunk kinematics | 33,51,59,65 | 33,65 | 33 | – |
| Hip kinematics | 33,63,75,86 | 33,86,132 | 33,63 | 35,63 | |
| Ankle kinematics | 33,63,75,86 | 33,86 | 33,63 | 35,63 | |
| Tibia kinematics | 45,55,56,58,59,75,77 | 132 | 46,48,49,57,84 | 45 | |
| Thigh kinematics | 56,78 | – | 84 | – | |
| Additional kinetic parameters | Hip kinetics | – | – | – | 35 |
| Ankle kinetics | – | – | – | 35 | |
| Anterior-posterior GRF | – | – | 70 | – | |
| Medio-lateral GRF | – | – | 70 | – | |
| EMG | Quadriceps EMG | – | – | 42,46,47 | 47,79 |
| Hamstring EMG | – | – | 42,47 | 47 | |
| Spatio-temporal parameters | Jump height | 85 | – | – | – |
| Hop distance | 69 | – | – | – | |
| Human contour | 60 | – | – | – | |
| Foot-ground contact | 50,51,69,133 | – | 46,64,68 | – | |
| Step count | – | – | 81 | – | |
| Categorical metrics | Injury risk rated by experts | – | – | – | 76 |
| Time after surgery | – | – | 43 | – | |
| Recovery status | – | – | 41,42 | – | |
| “LESS” score | 44,74,88,131 | – | – | – |
Three studies conducted injury prevention training during drop vertical jumps by combining visual feedback with wearable IMUs59, an RGB camera60, or a depth camera61. In a drop vertical jump, the subject drops off a box, lands with both feet on the ground, and then immediately performs a maximum height vertical jump. The first study trained subjects to control their knee flexion angle and trunk lean estimated by three IMUs, and the training outcomes included increased knee flexion angle, increased trunk lean, reduced thigh angular velocity, and reduced knee abduction moment59. The second study trained subjects to maximize the overlap between their body contour estimated by an RGB camera and the contour of an expert movement, and the training outcomes included reduced vertical ground reaction force and ankle dorsiflexion moment60. The third study trained subjects to increase their knee separation distance estimated by a depth camera, and the training outcomes included increased knee flexion angle and knee separation distance61. Although these training regimes have been demonstrated effective in modifying ACL injury risk factors, their utility in reducing real-world injury incidence rates have not been prospectively validated.
Accuracy and reliability
The validity of the sensing approaches proposed by 22 studies (45%) was examined against the gold standard from force plates, optical motion capture, and human raters. Three studies (6%) examined their validity against parameters measured by another portable sensing system, i.e. knee angles from goniometers62, knee angles from a commercial IMU system63, and step time asymmetry from pressure insoles64. The remaining 24 studies (49%) did not examine the validity of the estimated parameters. There were substantial differences in the accuracy metrics used across studies, making it challenging to compare the performance of different approaches. IMU-based studies reported root mean square errors (RMSEs) of 1.1–6.5 deg for knee flexion angle estimation33,51,65,66 and 3.3–10.9 deg for knee abduction angle estimation33,65,66. The accuracy of knee abduction angle estimation was poor considering the small knee abduction range of motion. The RMSE of knee flexion angle estimation was 6.8 deg when using eight calibrated RGB cameras35, while the RMSE was as low as 1.7 deg when using one single RGB camera and two reflective boards with Moiré patterns attached to the thigh and shank67. Three studies examined the reliability of the sensing approach, either within-day62,68 or between-day45 test–retest repeatability based on intraclass correlation coefficients (ICC). Excellent repeatability was observed in the knee flexion angle estimated by a soft fabric sensor (ICC ≥ 0.9)62.
Methodology of biomechanical parameter estimation
We categorized the methodologies for the analysis of the acquired data into three separate categories: (1) physics-based modeling that includes studies with kinematics reconstructed from raw sensor measurements and kinetics estimated via inverse dynamics or musculoskeletal models, (2) machine learning models to estimate subjects’ status or estimate parameters, and (3) direct feature extraction using investigator-defined parameters from the raw sensor data.
Eleven studies primarily used physics-based modeling. Integration of gyroscope data was combined with several drift compensation methods to estimate the sensor and body segment orientation in eight investigations42,45,51,59,63,65,66,69, and seven of these studies used the relative orientation between two segments to derive joint angles42,45,51,59,63,65,66. Only one study used musculoskeletal modeling, which estimated the GRF by simulating 25 artificial muscle-like actuators placed under each foot70.
Most of the machine learning studies focused on building classification models, whereas only one study built a regression model (linear regression) to predict kinetic parameters71. Two other studies implemented an existing deep-learning-based keypoint detection algorithm, OpenPose72,73, to estimate joint centers from 2-D videos; one of these studies used the keypoints to derive planar joint angles74 and the other predicted the “LESS” score75. Classification models including Support Vector Machines44,46,76, Linear Multinomial Logistic Regression76, Decision Trees44,76, Naive Bayes76, K Nearest Neighbors44,76, and Fuzzy Clustering41,43 were used to identify walking states, identify subjects at high ACL injury risk, and classify the time (e.g., 0–3 months, 3–6 months, and 6 months or more) after ACLR surgeries.
A few studies directly used raw sensor measurements for assessment40,48–50,55–58,62,64,68,77–80, for example, extracting peak acceleration and angular velocity from the thigh or shank IMU data for assessment40,55–58,77,78. Some of these direct measurements could provide useful insights as they are correlated with primary parameters (section 2.2), but this approach may be limited, compared to physics-based and machine learning approaches which directly estimate primary force-related measures. Characteristics of the IMU data (e.g., local peaks) can also be used to segment gait cycles and derive step time asymmetry64,68.
Experimental design
The number of subjects recruited in the included studies ranged from 9 to 169, with the median being 24 (Fig. 4a). Twenty-five studies (51%) did not exhibit biases across the sex of included subjects, in that the percentages of females were between 34% and 66% (Fig. 4b). Importantly, five studies (10%) focused on females44,45,48,49,61,71,81 (Fig. 4b), as females are more than twice as likely as males to have a first-time non-contact ACL injury82. However, four studies (8%) recruited only male subjects without providing a scientific rationale. Twenty-one studies (43%) recruited patients following ACLR. The time from ACLR to experimental testing were within 3 months46,57,64,68,79,83, 3–12 months35,40–43,46,50,56,57,63,64,78,80,84,85, or more than 12 months50,56,69,81 for the recruited patients. Eight studies (16%) recruited athletes at high risk of ACL injury, including basketball players44,45, soccer players75,86, and gymnasts61. Eight studies (16%) explicitly reported that their experiments were performed out of lab, including clinics40,63,64, hospitals68, soccer fields86, and unconstrained daily life46,80,81.
Fig. 4. Subjects characteristics of the included studies.
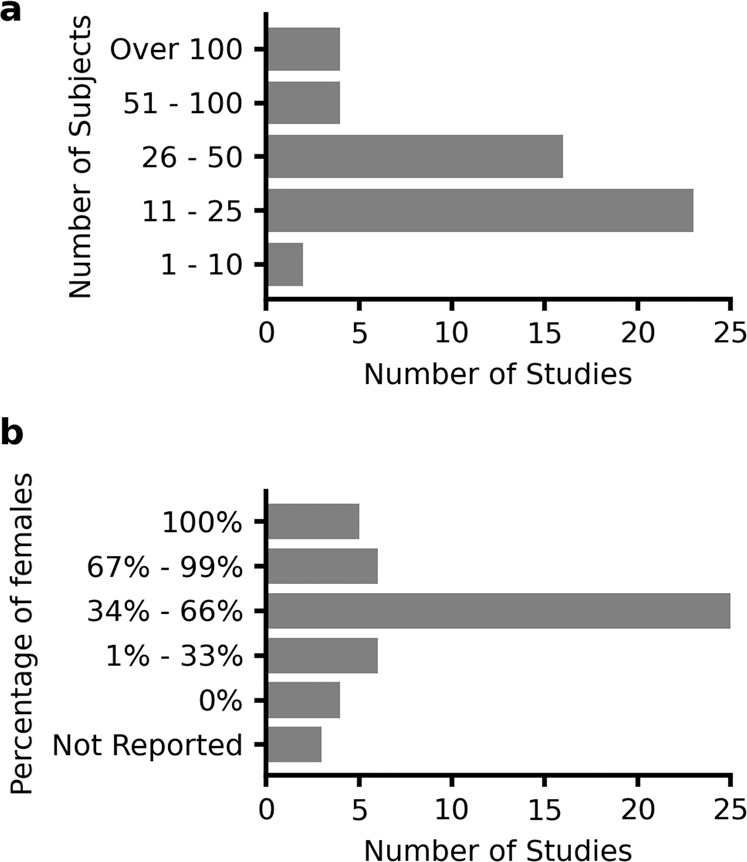
a Number of subjects and b percentage of female subjects.
None of the studies that estimated ACL injury risk factors prospectively evaluated their estimation results against subjects’ future injury occurrence. One study recruited thirteen basketball players, and one of them suffered from an ACL injury within 2 weeks after the first test session45. The injury was caused by an incorrect landing phase after a single-leg jump with a pivot-shift mechanism. Analysis of shank-worn IMU data revealed that the variances of her tibial orientation and acceleration were significantly larger than the 12 uninjured players during a countermovement jump test, indicating poor leg stability and load absorption capability. For rehabilitation assessment, six studies (12%) have used portable sensors to track post-surgery longitudinal changes of quadriceps EMG79,83, step count46,81, and level of gait asymmetry64,81.
Quality assessment
The upper quartile, median, and lower quartile of overall scores are 88%, 75%, and 68%, respectively (Table 3). Most of the studies clearly stated their aims (Q1, 94%), sufficiently described their methods (Q11, 82%), and adequately described their results (Q12, 92%). A few studies might have funding sources or conflicts of interest that may affect the authors’ interpretation of the results (Q19, 39%). Only a small portion of studies justified the sample size (Q3, 16%) or explicitly stated that subjects were recruited from multiple locations (Q5, 27%).
Table 3.
AXIS quality assessment results of the included studies.
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Q17 | Q18 | Q19 | Q20 | Overall (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 80 | 1 | 1 | 1 | 1 | 1 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 84 | 1 | 1 | 1 | 1 | 1 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 78 | 1 | 1 | 0 | 1 | 1 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 94 |
| 132 | 1 | 1 | 0 | 1 | 1 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 94 |
| 35 | 1 | 1 | 1 | 1 | 0 | 1 | NA | 1 | 1 | 0 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 88 |
| 40 | 1 | 1 | 1 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 0 | 1 | 88 |
| 48 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 88 |
| 49 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 88 |
| 56 | 1 | 1 | 1 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 0 | 1 | 1 | 88 |
| 66 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 88 |
| 68 | 1 | 1 | 1 | 1 | 0 | 1 | NA | 1 | 0 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 88 |
| 69 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 88 |
| 70 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 88 |
| 74 | 1 | 1 | 0 | 1 | 1 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 0 | 1 | 1 | 88 |
| 55 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 0 | 1 | 1 | 82 |
| 57 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 0 | 1 | 1 | 82 |
| 71 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 0 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 82 |
| 75 | 1 | 1 | 0 | 1 | 1 | 1 | NA | 1 | 0 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 0 | 1 | 82 |
| 77 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 0 | 1 | 82 |
| 86 | 1 | 1 | 0 | 1 | 0 | 0 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 82 |
| 33 | 1 | 1 | 1 | 1 | 0 | 1 | NA | 1 | 1 | 0 | 0 | 1 | NA | NA | 1 | 0 | 1 | 1 | 1 | 1 | 76 |
| 44 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 0 | 1 | 1 | NA | NA | 1 | 1 | 1 | 0 | 1 | 1 | 76 |
| 46 | 1 | 1 | 0 | 1 | 0 | 0 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 0 | 1 | 1 | 76 |
| 50 | 1 | 1 | 0 | 1 | 1 | 0 | NA | 1 | 1 | 0 | 0 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 76 |
| 59 | 1 | 1 | 0 | 0 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 0 | 1 | 76 |
| 61 | 1 | 1 | 0 | 1 | 1 | 1 | NA | 1 | 0 | 0 | 0 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 76 |
| 65 | 1 | 1 | 0 | 0 | 0 | 0 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 76 |
| 83 | 1 | 1 | 0 | 1 | 0 | 0 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 0 | 1 | 76 |
| 85 | 1 | 1 | 0 | 1 | 0 | 0 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 0 | 1 | 76 |
| 88 | 1 | 1 | 0 | 1 | 0 | 0 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 0 | 1 | 76 |
| 81 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 0 | 1 | 72 |
| 34 | 1 | 1 | 0 | 1 | 0 | 0 | NA | 1 | 1 | 0 | 0 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 71 |
| 58 | 1 | 1 | 0 | 0 | 0 | 0 | NA | 1 | 1 | 0 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 71 |
| 79 | 1 | 0 | 0 | 1 | 0 | 1 | NA | 0 | 0 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 1 | 1 | 71 |
| 131 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 1 | 1 | 1 | NA | NA | 1 | 1 | 0 | 0 | 0 | 1 | 71 |
| 133 | 1 | 1 | 0 | 1 | 0 | 0 | NA | 1 | 1 | 0 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 0 | 1 | 71 |
| 63 | 1 | 1 | 0 | 1 | 1 | 0 | NA | 1 | 1 | 0 | 1 | 0 | NA | NA | 1 | 0 | 1 | 1 | 0 | 1 | 65 |
| 67 | 1 | 1 | 0 | 1 | 0 | 0 | NA | 1 | 1 | 0 | 1 | 1 | NA | NA | 1 | 1 | 1 | 0 | 0 | 1 | 65 |
| 41 | 0 | 0 | 0 | 1 | 1 | 0 | NA | 1 | 0 | 0 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 0 | 1 | 59 |
| 43 | 1 | 1 | 0 | 1 | 1 | 0 | NA | 1 | 1 | 0 | 0 | 1 | NA | NA | 1 | 1 | 0 | 0 | 0 | 1 | 59 |
| 45 | 1 | 1 | 0 | 0 | 0 | 0 | NA | 0 | 0 | 1 | 1 | 1 | NA | NA | 1 | 1 | 1 | 0 | 1 | 1 | 59 |
| 76 | 1 | 1 | 0 | 0 | 0 | 1 | NA | 0 | 0 | 0 | 1 | 1 | NA | NA | 1 | 1 | 1 | 1 | 0 | 1 | 59 |
| 51 | 1 | 1 | 0 | 0 | 0 | 0 | NA | 1 | 1 | 0 | 0 | 1 | NA | NA | 1 | 1 | 0 | 0 | 1 | 1 | 53 |
| 134 | 0 | 0 | 0 | 1 | 0 | 1 | NA | 1 | 1 | 0 | 1 | 1 | NA | NA | 1 | 1 | 0 | 0 | 0 | 1 | 53 |
| 62 | 1 | 1 | 0 | 0 | 0 | 0 | NA | 0 | 0 | 1 | 0 | 1 | NA | NA | 1 | 1 | 0 | 1 | 0 | 1 | 47 |
| 42 | 1 | 0 | 0 | 1 | 1 | 0 | NA | 0 | 1 | 0 | 0 | 0 | NA | NA | 1 | 0 | 0 | 1 | 1 | 1 | 47 |
| 47 | 0 | 1 | 0 | 0 | 0 | 0 | NA | 1 | 0 | 0 | 0 | 0 | NA | NA | 1 | 1 | 1 | 0 | 0 | 1 | 35 |
| 64 | 1 | 1 | 0 | 0 | 0 | 0 | NA | 1 | 0 | 0 | 1 | 0 | NA | NA | 1 | 1 | 0 | 0 | 0 | 0 | 35 |
1 = positive response, 0 = negative response or unclear, and NA = not applicable.
Quality of reporting: Q1, Q4, Q10, Q11, Q12, Q16, and Q18; Quality of study design: Q2, Q3, Q5, Q8, Q17, Q19, and Q20; Potential biases: Q6, Q7, Q9, Q13, Q14, and Q15.
NA was excluded for computing the overall score of each study.
Readiness for deployment
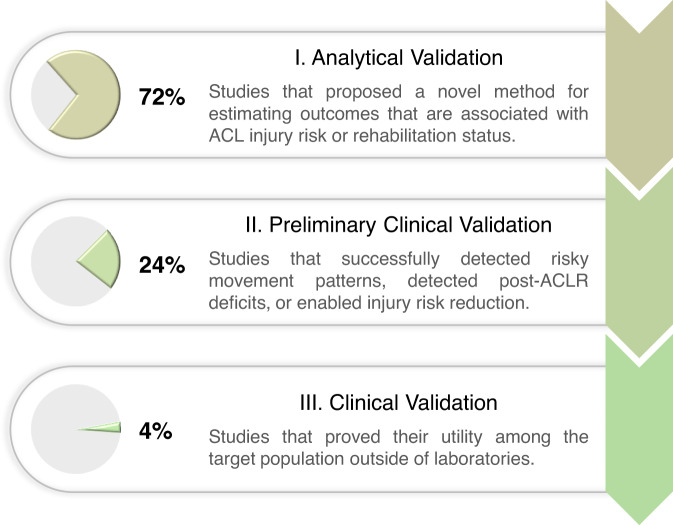
We adapted the V3 framework87 to categorize the included studies into three stages based on their readiness for deployment (Fig. 5). The original V3 framework was not well-suited for our review as most of the included studies used existing portable sensing hardware and thus do not require stage I - “hardware verification”. Our framework omitted this stage and used stage II - “analytical validation” and stage III - “clinical validation” of the V3 with an additional level (preliminary clinical validation) in between to allow more fine-grained distinction of the levels of clinical validation that were performed.
Fig. 5. Categorization of the included studies into three stages based on their readiness for deployment.
Studies in stage I provided proof-of-concept evidence, studies in stage II demonstrated their clinical utilities, and studies in stage III proved their clinical utilities.
72% of the included studies are in stage I, analytical validation, as they proposed a novel method in-laboratory and associated its outcome with ACL injury risk or rehabilitation status. 24% of the included studies are in stage II, preliminary clinical validation, as they demonstrated their clinical utility. These studies successfully used portable sensors to detect deficits in patients following ACLR40,46,48,50,80, identify subjects with high-risk movement patterns44,51,76,86,88, and enable injury prevention training that led to significant reductions in ACL injury risk factors59–61. However, before the deployment of these studies, further clinical validation is needed to validate their test-retest reliability as well as effectiveness in accelerating the post-ACLR recovery, or predicting and reducing real-world injury incidence rate. 4% of the included studies are in stage III, clinical validation, as they have proved their utility among the target population (e.g., athletes, patients following ACLR) outside of laboratories (e.g., sports fields, clinics). The only two studies in this stage monitored the longitudinal changes of quadriceps EMG and performed feedback training during rehabilitation exercises among an ACLR cohort79,83, and one of them was published in 199083. Randomized controlled trials showed that EMG biofeedback accelerated the recovery of quadriceps strength and knee range of motion in early-stage rehabilitation.
Discussion
The use of portable sensing for ACL injury risk stratification and rehabilitation has been increasing at an accelerated rate, with 98% of our reported studies occurring since 2007 and 51% since 2019 (Fig. 2). These studies have been dominated by the use of IMUs (43%) as well as depth and RGB cameras (24%). Such portable technologies for assessing mechanics have primarily been leveraged to collect kinematic data at the knee during jump-landing tasks, and, to a lesser extent, during cutting and gait motions. While these portable sensing studies have laid important groundwork for a variety of ACL-related biomechanical assessment tasks, future validation studies are needed to characterize the validity and reliability of such approaches, prior to the clinical deployment. This review highlights current knowledge gaps and future opportunities for the underlying technologies as well as their clinical applications. In the following, we provide our perspective on how to improve existing modeling methods and achieve broader clinical impact.
Using portable sensing to estimate traditional parameters or identify new parameters associated with ACL injury risk represents a significant research opportunity. Jump-landing tasks were the most popular dynamic motion among included studies and have been established as being well suited for identifying athletes at greater risk of ACL injury7. Cutting motions were less popular, but could be complementary to jump-landing tasks because knee kinematics and kinetics were significantly different during these two motions when assess in-lab11,89. Thus, simultaneous evaluation of jump-landing and cutting movement quality would provide a broader array of real-world conditions for more reliable injury risk screening. Systematic reviews of lab-based assessments have established that the knee angles, knee moments, and vertical GRF during jump-landing tasks and cutting are primary parameters for understanding ACL injuries and rehabilitation7,8. Among these primary parameters, knee angles were estimated by many of the reviewed studies; however, only one included study estimated knee extension moment71 and no study estimated knee abduction moment during jump-landing tasks or cutting. Recent research has shown that knee abduction moment during gait can be estimated from simulated 2-D video data using neural networks90 or from real 2-D video data using neural networks and musculoskeletal simulation91. Future research should test these methods for dynamic activities relevant to ACL injury risk screening, such as jump-landing and cutting motions.
A particular challenge with studies to establish new metrics for injury risk screening, however, is the low occurrence of ACL injuries that makes it challenging to pair pre-injury mechanical patterns to injury occurrence. Previous studies recruited hundreds to thousands of athletes from high-risk sports (typically young female basketball and football players) to validate traditional ACL injury risk factors such as joint laxity measured using a knee arthrometer92 and knee abduction moment measured by marker-based motion capture and force plates10. In contrast, despite the portable nature of the sensing approaches, the included studies estimated parameters with 9–169 subjects (the median is 24), and only 16% recruited athletes. Consequently, ACL injury incidence was rare among the included studies (only one study reported an incident45), suggesting the need for future studies that validate clinical utility through prospective evaluation of the estimated parameters against real injury occurrence. One solution to scarcity of data from injured athletes is the creation of a standardized pipeline of sensor deployment, data processing, archiving, and data sharing, which could enable multi-center data collection from a large number of athletes in the real world. While all portable sensors will make multi-center more accessible, deployment of such a multi-center effort is particularly convenient for RGB and depth cameras, which can passively collect data from multiple subjects without the burden of donning and doffing the sensors. Furthermore, such datasets will enable the identification and prospective validation of novel parameters that may more accurately predict ACL injury risk than traditional parameters.
The prospective studies of ACL injury risk mentioned previously will be pivotal for providing targets for preventative training. It has been shown that young athletes can be trained to adopt less risky posture and reduce injury risk9. However, effective training typically requires multiple sessions per week during both pre-season and in-season93 along with verbal feedback from expert coaching staff based on subjective visual observations94. In reality, with practical constraints such as demanding exercise protocols for athletes and limited availability of coaches knowledgeable in injury prevention, such training and feedback are challenging to implement. Prior studies have demonstrated that the subjectivity of such feedback may also lead to athletes experiencing considerable variability between recommendations amongst coaches95. In contrast to the subjective verbal feedback from human observations, the visual feedback enabled by portable sensors is tangible, quantifiable, and more importantly, objective59–61. Further, the capabilities for automatic tracking of training and progress provided by portable sensing technologies could also open the door to new strategies, such as gamification, to motivate and engage athletes in completing injury prevention programs96,97.
Current efforts focus on the paradigm described above for testing specific movements, providing feedback, and athletes learning to change that movement. However, lab-based methods cannot measure risk factors during practice or game-play; thus real-world changes to risky behaviors in response to training are unknown. Portable sensors are now commonly used to measure athletes’ position in field sports using GPS, or movements using IMUs98, from which ACL injury risk factors can be extracted. Longitudinal tracking of risk factor improvements during practice and game-play provides a novel avenue to determine the effectiveness of injury prevention training. Additionally, in-game and in-practice tracking allows data collection in a much larger volume in terms of the duration and the number of athletes compared to traditional in-lab environments. The sheer scale of data may overcome relatively lower quality of portable sensors and enable the identification of novel risk factors that were not possible in a solely lab-based environment.
Patients following ACLR need 4–12 months of rehabilitation to restore movement quality99,100. After rehabilitation, patients’ readiness to return to sport is commonly determined by whether their inter-limb symmetry in muscle strength, hop distance, and hop task completion time are larger than 90%101–103. While these tests are beneficial since they can be implemented in clinics, previous studies reported that strength tests lack functional relevance to sporting situations104. Furthermore, it was reported that since hop tests only indirectly assess knee function and loading, they may mask asymmetry in lower limb biomechanics103,105–107. Hop tests are also sensitive to small alterations in the test procedures108. Apart from strength and hop test measures, asymmetrical knee kinematics and kinetics during gait and double-leg squat as well as improper single-leg squat mechanics such as increased knee abduction angle may reveal dysfunctional movement patterns26,27,109. One barrier impeding the adoption of parameters during squatting and gait into return-to-sport decision-making is their reliance on force plates and marker-based motion capture from specialized laboratories. Many studies have proposed portable-sensing methods to estimate knee kinematics (Table 2). Also, as previously mentioned, portable sensors coupled with neural networks and musculoskeletal simulation have shown promise at predicting knee kinetics71,90,91. Using these technologies to develop assessments that are fast and accurate enough for clinical deployment could enable better return-to-sport decision making and potentially lower the risk of reinjury. Clinical validation studies could start with young athletes3,4, since their high reinjury rate could increase statistical power. Additionally, the ability to track biomechanical changes over time will both inform the rehabilitation approaches of clinicians and promote long-term patient engagement.
According to the AXIS assessment results (Table 3), the quality of study design is generally high; however, there is a risk of bias due to sample size and representativeness, which adversely affects the validity and generalizability. Specifically, the number of subjects is low (the median being 24) in most of the studies, and most studies did not provide justifications for the sample size (84%). Further, many studies recruited healthy subjects from a smaller biased pool (e.g., university students) (82%), and many other studies recruited patients from a single clinic or hospital, an inherent source of bias (62%). Few studies examined the test-retest repeatability of portable sensing. Also, accuracy metrics were not well-defined and thus hard to interpret in several studies, for example, it was unclear whether normalized RMSE (NRMSE) was obtained by normalizing RMSE over each trial, all the trials of each subject, or all the trials of all the subjects33,63. Additionally, metrics of different measures may not be directly compared, for example, RMSE and R2 of the entire biomechanical parameter profile should not be compared against those of specific time points (e.g., initial contact, peak, or midstance)66. These limitations highlight the opportunity of establishing the validity and reliability of sensor-based measures for ACL-related assessments.
Most of the included studies used direct feature extraction and IMU data integration with drift compensation to estimate parameters; however, they cannot provide insights into GRF or joint kinetics. In contrast, machine learning, despite being computationally expensive and prone to overfitting in low-data regimes110, has shown its potential for estimating kinetics in an end-to-end manner111–113 or in combination with musculoskeletal modeling91. Few included studies have attempted to use machine learning to estimate kinetic parameters associated with an ACL injury, so this field remains unstudied and represents important future opportunities; here we offer a few ideas.
Using transfer learning to augment training data for machine learning models in conditions with limited data. For example, machine learning models can be pre-trained on large corpora of data collected during easier-to-measure motions (e.g., walking) and/or data synthesized from readily available sources (e.g. ref. 114). Subsequently, those models can be fine-tuned on data collected during drop vertical jump or cutting where massive data collection is more difficult.
Incorporation of machine learning to enhance physics-based modeling. For example, physics-based models simplify the human body using parameterized formulas, where parameters were traditionally determined empirically or using population averages. Alternatively, these parameters can be learned from collected data using machine learning115,116.
Extraction of 3-D joint angles from camera data. Calculation of 3D joint kinematics requires collection of ≥3 non-collinear markers per rigid segment. Body keypoint detection algorithms employed by the included studies only extract joint centers, making derivation of 3-D joint angles (flexion, abduction, and internal rotation) impossible. Future studies may consider employing novel algorithms for extracting body meshes117–120 or conventional biomechanical markersets that can be used to derive 3-D angles.
Machine learning models have a strong dependency on datasets, so it can be challenging to generalize the results to new datasets. To prevent overfitting and guarantee the models’ reliability and generalizability, we suggest future studies follow the general recommendations provided to biomechanists by Halilaj and colleagues110. Here we offer a few additional recommendations for ACL-related assessment.
Use the target population for model training and testing. If the dataset is from a general population cohort, the model may not generalize to an athlete cohort who are stronger and faster, or to a patient cohort with pathological movement patterns.
Use a large and representative dataset, for example, a dataset collected from multiple laboratories with a balanced distribution in sex, height, weight, and age of included subjects. A small dataset collected by one single operator using one set of devices can suffer from biases such as biased marker positioning121. If the model is trained on such datasets, whether those biases are inherited by the model needs to be investigated.
Do not test the model using the data from the subjects that were involved in model training, unless additionally training data can be easily acquired from new subjects in practical applications.
Benchmark datasets are curated, publicly available sets of data for enabling objective comparisons between studies and rigorous selection of state-of-the-art methods122. They have already proven their fundamental importance in research areas such as computer vision. Although many included studies estimated the same biomechanical parameter during the same type of motion (Table 2), significant differences exist in their recruited cohorts of subjects, sources of ground truth, and metrics of validity. It is therefore difficult to impossible to truly compare the validity, sensitivity to parameter changes, or test-retest repeatability between these methods. Although a few comprehensive biomechanical datasets containing multiple portable sensors have been published123–125, none have focused on ACL-specific tasks. To make it easier for future researchers to select the optimal sensing approach and estimation method, we here call for collection and publication of a benchmark dataset, ideally following a standardized pipeline (section 3.1.1) and open science principles to ensure its findability, accessibility, interoperability, and reusability126. Such an ideal dataset should contain simultaneously collected ground-truth kinematics, GRF, and multiple portable sensor data (Table 1 and Fig. 3) during a range of ACL-specific motions (Table 2). A dataset that includes repeated measures on the same or separate days will enable assessment of measurement reliability, and thus calculation of the minimum detectable change which is crucial for clinical use on an individual basis127.
We are unable to aggregate or statistically compare the performance of portable sensing approaches because the included studies reported different accuracy metrics and investigated different motions and biomechanical parameters. As a result, the validity and reliability of portable sensing for ACL-related assessment aggregated across the included studies cannot be computed. Our review of the literature demonstrates a lack of consensus in portable sensing performance and points to the need for benchmark datasets with ground-truth measurements, which would enable objective comparisons between studies. Another limitation is that we did not formulate clinical research questions following the patient, intervention, comparison, outcome (PICO) framework128, which may limit the clinical impact of our review. This is because we attempted to include technology-focused studies that would otherwise be excluded by the PICO framework due to not strictly following clinical research principles. For example, 72% of the included studies are in the analytical validation stage, and most of them focused on technology development without designing controlled experiments or recruiting a patient cohort. We believe that assessing the successes and gaps in current technical studies could pave the way for more clinical validation studies that could fit the PICO framework.
In this manuscript, we summarize the state of using portable sensors to enable a range of ACL-related biomechanical assessments. Through these studies, we showed that portable sensing can potentially be used to monitor patient progress through the rehabilitation process and train athletes to reduce injury risk factors. However, despite their promising results, the validity and reliability of these portable sensing methods are not well-established. Thus, we highlight numerous opportunities that exist in the validation and benchmarking of portable sensing approaches for estimating various biomechanical parameters during injury risk screening, injury prevention training, and rehabilitation assessment. We also highlight two important opportunities for future research in: (1) exploring sophisticated modeling techniques to enable more accurate assessment and (2) standardizing data collection and processing methods to pave the way for procurement of large benchmark datasets, and multi-center trials for clinical validation. The sheer amount of portable sensor data may enable large-scale prospective studies for the identification of new ACL injury risk factors, leading to novel targets for preventative training. The capabilities for automatic tracking of training and progress provided by portable sensing technologies could open the door to new strategies, such as gamified platforms, to motivate and engage athletes and patients in completing training programs. If successful, these advances will enable widespread use of portable-sensing approaches to estimate ACL injury risk factors, mitigate high-risk movements, customize rehabilitation paradigms for improved long-term health outcomes, and quantify return-to-sport readiness.
Methods
Literature search approach
Our scoping review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-2020) guidance129. We searched articles published up to 6 March 2022 from the following databases: Medline (1950-) and Web of Science Core Collection (1950-). The search focused on retrieving articles that included: (1) ACL, (2) portable sensing approaches such as IMU, EMG, video, and pressure insole, and (3) clinical applications such as injury risk screening (Table 4). We only considered articles written in English and those contained at least one term from each of the three categories above either in their title, abstract, or keywords.
Table 4.
Specific search terms used for the literature review.
| General | Specific terms |
|---|---|
| ACL | ACL OR anterior cruciate ligament |
| AND | |
| Sensing OR feedback | pressure insol* OR force insol* OR acceleromete* OR gyroscop* OR IMU OR IMUs OR inertial measurement uni* OR inertial senso* OR electromyogra* OR EMG OR video OR cellphon* OR smartphon* OR camer* OR depth OR Kinect OR wearabl* OR portabl* OR field-based OR out of lab OR *n field OR feedback OR biofeedback |
| AND | |
| Clinical application | injury risk OR risk factor OR risk metrics OR risk mitigation OR risk reduction OR injury prevention OR screening OR rehabilitation OR rehab OR trainin* OR retrainin* OR return to spor* OR return to play OR secon* injury OR reinjur* |
*Denotes wildcard matching.
Inclusion and exclusion criteria
Two authors (T.T. and A.A.G.) independently reviewed titles, abstracts, and keywords of all the retrieved articles. Inclusion/exclusion disagreements were resolved by full-text review and discussion to reach consensus. We excluded dissertations, theses, conference proceedings, and conference abstracts. We also excluded articles whose primary purpose was not development, validation, or use of portable sensing for ACL-related assessment. In addition, we excluded articles that did not involve human subjects, articles that required human raters or manual labeling for qualitative assessment, and articles that used force plates and marker-based motion capture measurements as input data for assessment. Articles were not excluded if those measurements were used as the gold standard to determine the validity of portable sensing approaches.
Outcome extraction
We carefully read and extracted the following outcomes from the included articles: area of application (i.e., injury risk screening, injury prevention training, rehabilitation assessment), sensing approach (e.g., IMU, RGB camera, EMG), target motion (i.e., jump-landing tasks, cutting, gait, squatting), target biomechanical parameter (e.g., knee flexion angle, vertical GRF), category of the parameter (e.g., kinematics, kinetics), methodology of estimation (i.e., physics-based modeling, machine learning, direct feature extraction), developer of the method (i.e., academic laboratories, commercial companies), number of subjects, sex ratio, involvement of athletes, experiment site (e.g., laboratory, clinics), validation (e.g., validated against force plates and optical motion capture), and repeatability (e.g., test-retest reliability). We also assessed whether included articles validated their clinical utility in detecting patients’ recovery status, accelerating rehabilitation, predicting future ACL injury occurrences, or enabling feedback training for injury risk reduction. Some terminologies were unified or simplified if they depicted the same fundamental measurement. For example, knee flexion angle, knee extension angle, and sagittal plane knee angle were unified as knee flexion angle.
Quality assessment
The Appraisal tool for Cross-Sectional Studies (AXIS) was used to assess the quality of the included studies130. AXIS includes 20 questions (Supplementary Table 1), with seven related to quality of reporting, seven related to quality of study design, and six related to potential biases. Two authors (T.T. and A.A.G.) independently assessed each study by scoring each question with 1 (positive response), 0 (negative response or unclear), or NA (not applicable to this study). Disagreements were resolved by review and discussion to reach consensus. The overall quality of each study was reported as the percentage of positive responses over positive plus negative responses (excluding NA). The AXIS tool does not provide cut-off values for high or low study quality, and we did not exclude studies based on the assessment results.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China under grant 52250610217, the Joe and Clara Tsai Foundation through the Wu Tsai Human Performance Alliance, the Philips Healthcare, and the U.S. National Institutes of Health (NIH) under grant R01 AR077604, R01 EB002524, R01 AR079431, and P41 EB027060.
Author contributions
A.S.C. and P.B.S. oversaw the development of the review. All the authors conceptualized the study. T.T. and A.A.G. conducted the literature search. T.T., A.A.G., and B.F. prepared the first draft of the manuscript. All authors participated in drafting the manuscript, critically reviewed drafts of the manuscript, and approved the final version to be submitted.
Data availability
No new or unpublished data is included within the study.
Code availability
All code relating to summary figure development is available on request to T.T.
Competing interests
Peter Shull is an Associate Editor of npj Digital Medicine. Other authors (T.T., A.A.G., B.F., K.G.S., S.L.S., S.D.U., J.L.H., S.L.D., and A.S.C.) declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41746-023-00782-2.
References
- 1.Murray, M. M. The ACL Handbook: Knee Biology, Mechanics, and Treatment (eds Murray, M. M., Vavken, P. & Fleming, B.) p. 19–28 (Springer New York, 2013).
- 2.Mall NA, et al. Incidence and trends of anterior cruciate ligament reconstruction in the united states. Am. J. Sports Med. 2014;42:2363–2370. doi: 10.1177/0363546514542796. [DOI] [PubMed] [Google Scholar]
- 3.Webster KE, Feller JA. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am. J. Sports Med. 2016;44:2827–2832. doi: 10.1177/0363546516651845. [DOI] [PubMed] [Google Scholar]
- 4.Barber-Westin S, Noyes FR. One in 5 athletes sustain reinjury upon return to high-risk sports after acl reconstruction: a systematic review in 1239 athletes younger than 20 years. Sports Health. 2020;12:587–597. doi: 10.1177/1941738120912846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimori M, et al. Articular cartilage injury of the posterior lateral tibial plateau associated with acute anterior cruciate ligament injury. Knee Surg. Sports Traumatol. Arthrosc. 2008;16:270–274. doi: 10.1007/s00167-007-0458-x. [DOI] [PubMed] [Google Scholar]
- 6.Muthuri S, McWilliams D, Doherty M, Zhang W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2011;19:1286–1293. doi: 10.1016/j.joca.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Pedley JS, et al. Utility of kinetic and kinematic jumping and landing variables as predictors of injury risk: a systematic review. J. Sci. Sport Exerc. 2020;2:287–304. doi: 10.1007/s42978-020-00090-1. [DOI] [Google Scholar]
- 8.Hughes G. A review of recent perspectives on biomechanical risk factors associated with anterior cruciate ligament injury. Res. Sports Med. 2014;22:193–212. doi: 10.1080/15438627.2014.881821. [DOI] [PubMed] [Google Scholar]
- 9.Alentorn-Geli E, et al. Prevention of non-contact anterior cruciate ligament injuries in soccer players. part 2: a review of prevention programs aimed to modify risk factors and to reduce injury rates. Knee Surg. Sports Traumatol. Arthrosc. 2009;17:859–879. doi: 10.1007/s00167-009-0823-z. [DOI] [PubMed] [Google Scholar]
- 10.Hewett TE, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am. J. Sports Med. 2005;33:492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 11.Kristianslund E, Krosshaug T. Comparison of drop jumps and sport-specific sidestep cutting: implications for anterior cruciate ligament injury risk screening. Am. J. Sports Med. 2013;41:684–688. doi: 10.1177/0363546512472043. [DOI] [PubMed] [Google Scholar]
- 12.Lu T-W, Chang C-F. Biomechanics of human movement and its clinical applications. Kaohsiung J. Med. Sci. 2012;28:S13–S25. doi: 10.1016/j.kjms.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. Am. J. Sports Med. 2006;34:299–311. doi: 10.1177/0363546505284183. [DOI] [PubMed] [Google Scholar]
- 14.Padua DA, et al. The landing error scoring system (less) is a valid and reliable clinical assessment tool of jump-landing biomechanics: the jumpacl study. The Am. J. Sports Med. 2009;37:1996–2002. doi: 10.1177/0363546509343200. [DOI] [PubMed] [Google Scholar]
- 15.Lam M-H, et al. Knee stability assessment on anterior cruciate ligament injury: clinical and biomechanical approaches. BMC Sports Sci., Med. Rehabil. 2009;1:1–9. doi: 10.1186/1758-2555-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange T, et al. The reliability of physical examination tests for the diagnosis of anterior cruciate ligament rupture–a systematic review. Man. Ther. 2015;20:402–411. doi: 10.1016/j.math.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Pairot-de Fontenay B, et al. Running biomechanics in individuals with anterior cruciate ligament reconstruction: a systematic review. Sports Med. 2019;49:1411–1424. doi: 10.1007/s40279-019-01120-x. [DOI] [PubMed] [Google Scholar]
- 18.Knurr KA, et al. Running biomechanics before injury and 1 year after anterior cruciate ligament reconstruction in division i collegiate athletes. Am. J. Sports Med. 2021;49:2607–2614. doi: 10.1177/03635465211026665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batty LM, Feller JA, Hartwig T, Devitt BM, Webster KE. Single-leg squat performance and its relationship to extensor mechanism strength after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2019;47:3423–3428. doi: 10.1177/0363546519878432. [DOI] [PubMed] [Google Scholar]
- 20.Batty LM, et al. Single-leg squat after anterior cruciate ligament reconstruction: an analysis of the knee valgus angle at 6 and 12 months. Orthop. J. Sports Med. 2020;8:2325967120946328. doi: 10.1177/2325967120946328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King E, et al. Whole-body biomechanical differences between limbs exist 9 months after acl reconstruction across jump/landing tasks. Scand. J. Med. Sci. Sports. 2018;28:2567–2578. doi: 10.1111/sms.13259. [DOI] [PubMed] [Google Scholar]
- 22.Maffulli N, Longo UG, Gougoulias N, Loppini M, Denaro V. Long-term health outcomes of youth sports injuries. Br. J. Sports Med. 2010;44:21–25. doi: 10.1136/bjsm.2009.069526. [DOI] [PubMed] [Google Scholar]
- 23.Poulsen E, et al. Knee osteoarthritis risk is increased 4-6 fold after knee injury-a systematic review and meta-analysis. Br. J. Sports Med. 2019;53:1454–1463. doi: 10.1136/bjsports-2018-100022. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, et al. Graft maturity of the reconstructed anterior cruciate ligament 6 months postoperatively: a magnetic resonance imaging evaluation of quadriceps tendon with bone block and hamstring tendon autografts. Knee Surg. Sports Traumatol. Arthrosc. 2015;23:661–668. doi: 10.1007/s00167-014-3302-0. [DOI] [PubMed] [Google Scholar]
- 25.Salem GJ, Salinas R, Harding FV. Bilateral kinematic and kinetic analysis of the squat exercise after anterior cruciate ligament reconstruction. Arch. Phys. Med. Rehab. 2003;84:1211–1216. doi: 10.1016/S0003-9993(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 26.Slater LV, Hart JM, Kelly AR, Kuenze CM. Progressive changes in walking kinematics and kinetics after anterior cruciate ligament injury and reconstruction: a review and meta-analysis. J. Athl. Train. 2017;52:847–860. doi: 10.4085/1062-6050-52.6.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart HF, et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br. J. Sports Med. 2016;50:597–612. doi: 10.1136/bjsports-2015-094797. [DOI] [PubMed] [Google Scholar]
- 28.Della Villa, F., et al. Basketball Sports Medicine and Science (eds Laver, L. et al.) p. 723-736 (Springer Berlin Heidelberg, Berlin, Heidelberg, 2020).
- 29.Perry, A. et al. Acl rehabilitation: How can we lessen injury rates? Oper. Tech. Sports Med.30, 150892 (2022).
- 30.Rose MJ, Costello KE, Eigenbrot S, Torabian K, Kumar D. Inertial measurement units and application for remote health care in hip and knee osteoarthritis: Narrative review. JMIR Rehabil. Assist. Technol. 2022;9:e33521. doi: 10.2196/33521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Sullivan JW, et al. Accuracy of smartphone camera applications for detecting atrial fibrillation: a systematic review and meta-analysis. JAMA Netw. Open. 2020;3:e202064–e202064. doi: 10.1001/jamanetworkopen.2020.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warmerdam E, et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol. 2020;19:462–470. doi: 10.1016/S1474-4422(19)30397-7. [DOI] [PubMed] [Google Scholar]
- 33.Di Paolo S, et al. Rehabilitation and return to sport assessment after anterior cruciate ligament injury: quantifying joint kinematics during complex high-speed tasks through wearable sensors. Sensors. 2021;21:2331. doi: 10.3390/s21072331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tipton CC, Telfer S, Cherones A, Gee AO, Kweon CY. The use of microsoft kinect ™ for assessing readiness of return to sport and injury risk exercises: a validation study. Int. J. Sports Phys. Ther. 2019;14:724–730. doi: 10.26603/ijspt20190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guiotto A, et al. Reliability and repeatability of ACL Quick Check®: a methodology for on field lower limb joint kinematics and kinetics assessment in sport applications. Sensors. 2021;22:259. doi: 10.3390/s22010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Small SR, et al. Current clinical utilisation of wearable motion sensors for the assessment of outcome following knee arthroplasty: a scoping review. BMJ Open. 2019;9:e033832. doi: 10.1136/bmjopen-2019-033832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seshadri DR, et al. Wearable sensors for monitoring the internal and external workload of the athlete. NPJ Digit. Med. 2019;2:1–18. doi: 10.1038/s41746-019-0149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prill R, Walter M, Kr´olikowska A, Becker R. A systematic review of diagnostic accuracy and clinical applications of wearable movement sensors for knee joint rehabilitation. Sensors. 2021;21:8221. doi: 10.3390/s21248221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marques JB, et al. The use of wearable technology as an assessment tool to identify between-limb differences during functional tasks following acl reconstruction. a scoping review. Phys. Ther. Sport. 2022;55:1–11. doi: 10.1016/j.ptsp.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Peebles AT, Miller TK, Queen RM. Landing biomechanics deficits in anterior cruciate ligament reconstruction patients can be assessed in a non-laboratory setting. J. Orthop. Res. 2022;40:150–158. doi: 10.1002/jor.25039. [DOI] [PubMed] [Google Scholar]
- 41.Malik OA, Arosha Senanayake SMN, Zaheer D. A multisensor integration-based complementary tool for monitoring recovery progress of anterior cruciate ligament-reconstructed subjects. IEEE/ASME Trans. Mechatron. 2015;20:2328–2339. doi: 10.1109/TMECH.2014.2376199. [DOI] [Google Scholar]
- 42.Arosha Senanayake SMN, Ahmed Malik O, Mohammad Iskandar P, Zaheer D. Assessing post-anterior cruciate ligament reconstruction ambulation using wireless wearable integrated sensors. J. Med. Eng. Technol. 2013;37:498–510. doi: 10.3109/03091902.2013.837529. [DOI] [PubMed] [Google Scholar]
- 43.Malik OA, Arosha Senanayake S, Zaheer D. An intelligent recovery progress evaluation system for ACL reconstructed subjects using integrated 3-D kinematics and EMG features. IEEE J. Biomed. Health Inf. 2015;19:453–463. doi: 10.1109/JBHI.2014.2320408. [DOI] [PubMed] [Google Scholar]
- 44.Taborri J, et al. A machine-learning approach to measure the anterior cruciate ligament injury risk in female basketball players. Sensors. 2021;21:3141. doi: 10.3390/s21093141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molinaro L, et al. Sensor-based indices for the prediction and monitoring of anterior cruciate ligament injury: reliability analysis and a case study in basketball. Sensors. 2021;21:5341. doi: 10.3390/s21165341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurchiek RD, et al. Open-source remote gait analysis: a postsurgery patient monitoring application. Scientific Reports. 2019;9:17966. doi: 10.1038/s41598-019-54399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kordatos G, Stavrakis M. Design and evaluation of a wearable system to increase adherence to rehabilitation programmes in acute cruciate ligament (CL) rupture. Multimed. Tools Appl. 2020;79:33549–33574. doi: 10.1007/s11042-019-08502-3. [DOI] [Google Scholar]
- 48.Riskowski JL, Mikesky AE, Bahamonde RE, Burr DB. Design and validation of a knee brace with feedback to reduce the rate of loading. J. Biomech. Eng. 2009;131:084503. doi: 10.1115/1.3148858. [DOI] [PubMed] [Google Scholar]
- 49.Riskowski JL. Gait and neuromuscular adaptations after using a feedback-based gait monitoring knee brace. Gait Posture. 2010;32:242–247. doi: 10.1016/j.gaitpost.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Dan MJ, et al. Wearable inertial sensors and pressure MAT detect risk factors associated with ACL graft failure that are not possible with traditional return to sport assessments. BMJ Open Sport Exerc. Med. 2019;5:e000557. doi: 10.1136/bmjsem-2019-000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dowling AV, Favre J, Andriacchi TP. A wearable system to assess risk for anterior cruciate ligament injury during jump landing: measurements of temporal events, jump height, and sagittal plane kinematics. J. Biomech. Eng. 2011;133:071008. doi: 10.1115/1.4004413. [DOI] [PubMed] [Google Scholar]
- 52.Hurwitz D, Ryals A, Case J, Block J, Andriacchi T. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J. Orthop. Res. 2002;20:101–107. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 53.Sharma L, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41:1233–1240. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 54.Blackburn JT, Padua DA. Influence of trunk flexion on hip and knee joint kinematics during a controlled drop landing. Clin. Biomech. 2008;23:313–319. doi: 10.1016/j.clinbiomech.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Morgan AM, O’Connor KM. Evaluation of an accelerometer to assess knee mechanics during a drop landing. J. Biomech. 2019;86:125–131. doi: 10.1016/j.jbiomech.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 56.Pratt K, Sigward S. Inertial sensor angular velocities reflect dynamic knee loading during single limb loading in individuals following anterior cruciate ligament reconstruction. Sensors. 2018;18:3460. doi: 10.3390/s18103460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sigward SM, Chan M-SM, Lin PE. Characterizing knee loading asymmetry in individuals following anterior cruciate ligament reconstruction using inertial sensors. Gait Posture. 2016;49:114–119. doi: 10.1016/j.gaitpost.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dowling AV, Favre J, Andriacchi TP. Characterization of thigh and shank segment angular velocity during jump landing tasks commonly used to evaluate risk for ACL injury. J. Biomech. Eng. 2012;134:091006. doi: 10.1115/1.4007178. [DOI] [PubMed] [Google Scholar]
- 59.Dowling AV, Favre J, Andriacchi TP. Inertial sensor-based feedback can reduce key risk metrics for anterior cruciate ligament injury during jump landings. Am. Jo. Sports Med. 2012;40:1075–1083. doi: 10.1177/0363546512437529. [DOI] [PubMed] [Google Scholar]
- 60.Dallinga J, et al. Innovative Video Feedback on Jump Landing Improves Landing Technique in Males. Int. J. Sports Med. 2016;38:150–158. doi: 10.1055/s-0042-106298. [DOI] [PubMed] [Google Scholar]
- 61.Nyman E, Armstrong CW. Real-time feedback during drop landing training improves subsequent frontal and sagittal plane knee kinematics. Clin. Biomech. 2015;30:988–994. doi: 10.1016/j.clinbiomech.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 62.Munro BJ, Campbell TE, Wallace GG, Steele JR. The intelligent knee sleeve: a wearable biofeedback device. Sens. Actuat. B: Chem. 2008;131:541–547. doi: 10.1016/j.snb.2007.12.041. [DOI] [Google Scholar]
- 63.Islam R, et al. A nonproprietary movement analysis system (MoJoXlab) based on wearable inertial measurement units applicable to healthy participants and those with anterior cruciate ligament reconstruction across a range of complex tasks: validation study. JMIR mHealth uHealth. 2020;8:e17872. doi: 10.2196/17872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jarchi D, et al. Gait analysis from a single ear-worn sensor: reliability and clinical evaluation for orthopaedic patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2016;24:882–892. doi: 10.1109/TNSRE.2015.2477720. [DOI] [PubMed] [Google Scholar]
- 65.Chia L, et al. Evaluating the validity and reliability of inertial measurement units for determining knee and trunk kinematics during athletic landing and cutting movements. J. Electromyogr. Kinesiol. 2021;60:102589. doi: 10.1016/j.jelekin.2021.102589. [DOI] [PubMed] [Google Scholar]
- 66.Fan B, Xia H, Xu J, Li Q, Shull PB. IMU-based knee flexion, abduction and internal rotation estimation during drop landing and cutting tasks. J. Biomech. 2021;124:110549. doi: 10.1016/j.jbiomech.2021.110549. [DOI] [PubMed] [Google Scholar]
- 67.Weinhandl JT, Armstrong BS, Kusik TP, Barrows RT, O’Connor KM. Validation of a single camera three-dimensional motion tracking system. J. Biomech. 2010;43:1437–1440. doi: 10.1016/j.jbiomech.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vervaat W, Bogen B, Moe-Nilssen R. Within-day test-retest reliability of an accelerometer-based method for registration of step time symmetry during stair descent after ACL reconstruction and in healthy subjects. Physiother. Theory Pract. 2022;38:226–234. doi: 10.1080/09593985.2020.1723150. [DOI] [PubMed] [Google Scholar]
- 69.Ahmadian N, Nazarahari M, Whittaker JL, Rouhani H. Quantification of triple single-leg hop test temporospatial parameters: a validated method using body-worn sensors for functional evaluation after knee injury. Sensors. 2020;20:3464. doi: 10.3390/s20123464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oh J, et al. Estimation of ground reaction forces during stair climbing in patients with ACL reconstruction using a depth sensor-driven musculoskeletal model. Gait Posture. 2021;84:232–237. doi: 10.1016/j.gaitpost.2020.12.025. [DOI] [PubMed] [Google Scholar]
- 71.Chaaban CR, et al. Combining inertial sensors and machine learning to predict vgrf and knee biomechanics during a double limb jump landing task. Sensors. 2021;21:4383. doi: 10.3390/s21134383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cao, Z., Simon, T., Wei, S.-E. & Sheikh, Y. Realtime multi-person 2d pose estimation using part affinity fields. Paper presented at the IEEE conference on computer vision and pattern recognition, P. 7291–7299 (IEEE, 2017).
- 73.Cao Z, Hidalgo G, Simon T, Wei S-E, Sheikh Y. Openpose: Realtime multi-person 2d pose estimation using part affinity fields. IEEE Trans. Pattern Anal. Mach. Intell. 2021;43:172–186. doi: 10.1109/TPAMI.2019.2929257. [DOI] [PubMed] [Google Scholar]
- 74.H´ebert-Losier K, Hanzl´ıkov´a I, Zheng C, Streeter L, Mayo M. The ‘DEEP’ Landing Error Scoring System. Appl. Sci. 2020;10:892. doi: 10.3390/app10030892. [DOI] [Google Scholar]
- 75.Kawaguchi K, et al. Sex-based differences in the drop vertical jump as revealed by video motion capture analysis using artificial intelligence. Orthop. J. Sports Med. 2021;9:23259671211048188. doi: 10.1177/23259671211048188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kianifar R, Lee A, Raina S, Kuli´c D. Automated assessment of dynamic knee valgus and risk of knee injury during the single leg squat. IEEE J. Transl. Eng. Health Med. 2017;5:1–13. doi: 10.1109/JTEHM.2017.2736559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sell TC, Akins JS, Opp AR, Lephart SM. Relationship between tibial acceleration and proximal anterior tibia shear force across increasing jump distance. J. Appl. Biomech. 2014;30:75–81. doi: 10.1123/jab.2012-0186. [DOI] [PubMed] [Google Scholar]
- 78.Pratt KA, Sigward SM. Detection of knee power deficits following anterior cruciate ligament reconstruction using wearable sensors. J. Orthop. Sports Phys. Ther. 2018;48:895–902. doi: 10.2519/jospt.2018.7995. [DOI] [PubMed] [Google Scholar]
- 79.Christanell F, Hoser C, Huber R, Fink C, Luomajoki H. The influence of electromyographic biofeedback therapy on knee extension following anterior cruciate ligament reconstruction: a randomized controlled trial. Sports Med. Arthrosc. Rehab. Ther. Technol. 2012;4:41. doi: 10.1186/1758-2555-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chan M-S, Sigward SM. Individuals following anterior cruciate ligament reconstruction practice underloading strategies during daily activity. J. Orthop. Res. 2022;40:565–572. doi: 10.1002/jor.25070. [DOI] [PubMed] [Google Scholar]
- 81.Kuenze C, Pfeiffer K, Pfeiffer M, Driban JB, Pietrosimone B. Feasibility of a wearable-based physical activity goal-setting intervention among individuals with anterior cruciate ligament reconstruction. J. Athl. Train. 2021;56:555–564. doi: 10.4085/1062-6050-203-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beynnon BD, et al. The effects of level of competition, sport, and sex on the incidence of first-time noncontact anterior cruciate ligament injury. Am. J. Sports Med. 2014;42:1806–1812. doi: 10.1177/0363546514540862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Draper V. Electromyographic biofeedback and recovery of quadriceps femoris muscle function following anterior cruciate ligament reconstruction. Phys. Ther. 1990;70:11–17. doi: 10.1093/ptj/70.1.11. [DOI] [PubMed] [Google Scholar]
- 84.Havens KL, Cohen SC, Pratt KA, Sigward SM. Accelerations from wearable accelerometers reflect knee loading during running after anterior cruciate ligament reconstruction. Clin. Biomech. 2018;58:57–61. doi: 10.1016/j.clinbiomech.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Fischer F, et al. Isokinetic extension strength is associated with single-leg vertical jump height. Orthop. J. Sports Med. 2017;5:2325967117736766. doi: 10.1177/2325967117736766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Di Paolo S, Zaffagnini S, Pizza N, Grassi A, Bragonzoni L. Poor motor coordination elicits altered lower limb biomechanics in young football (soccer) players: implications for injury prevention through wearable sensors. Sensors. 2021;21:4371. doi: 10.3390/s21134371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldsack JC, et al. Verification, analytical validation, and clinical validation (v3): the foundation of determining fit-for-purpose for biometric monitoring technologies (biomets) NPJ Digit. Med. 2020;3:1–15. doi: 10.1038/s41746-020-0260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mauntel TC, et al. Automated quantification of the landing error scoring system with a markerless motion-capture system. J. Athl. Train. 2017;52:1002–1009. doi: 10.4085/1062-6050-52.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olivares-Jabalera J, et al. Is there association between cutting and jump-landing movement quality in semi-professional football players? implications for acl injury risk screening. Phys. Ther. Sport. 2022;56:15–23. doi: 10.1016/j.ptsp.2022.05.015. [DOI] [PubMed] [Google Scholar]
- 90.Boswell MA, et al. A neural network to predict the knee adduction moment in patients with osteoarthritis using anatomical landmarks obtainable from 2d video analysis. Osteoarthritis Cartilage. 2021;29:346–356. doi: 10.1016/j.joca.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uhlrich, S. D. et al. Opencap: 3d human movement dynamics from smartphone videos. Preprint at https://www.biorxiv.org/content/10.1101/2022.07.07.499061v1 (2022). [DOI] [PMC free article] [PubMed]
- 92.Myer GD, Ford KR, Paterno MV, Nick TG, Hewett TE. The effects of generalized joint laxity on risk of anterior cruciate ligament injury in young female athletes. Am. J. Sports Med. 2008;36:1073–1080. doi: 10.1177/0363546507313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sugimoto D, Myer GD, Barber Foss KD, Hewett TE. Dosage effects of neuromuscular training intervention to reduce anterior cruciate ligament injuries in female athletes: Meta- and sub-group analyses. Sports Med. 2014;44:551. doi: 10.1007/s40279-013-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sugimoto D, et al. Critical components of neuromuscular training to reduce acl injury risk in female athletes: meta-regression analysis. Br. J. Sports Med. 2016;50:1259. doi: 10.1136/bjsports-2015-095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Myklebust G, Skjølberg A, Bahr R. Acl injury incidence in female handball 10 years after the norwegian acl prevention study: important lessons learned. Br. J. Sports Med. 2013;47:476–479. doi: 10.1136/bjsports-2012-091862. [DOI] [PubMed] [Google Scholar]
- 96.McClincy M, et al. Perspectives on the gamification of an interactive health technology for postoperative rehabilitation of pediatric anterior cruciate ligament reconstruction: User-centered design approach. JMIR Serious Games. 2021;9:e27195. doi: 10.2196/27195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wiehr, F., Vujic, M., Kru¨ger, A. & Daiber, F. The jungle warm-up run: augmenting athletes with coach-guided dynamic game elements. Paper presented at the Augmented Humans International Conference, 1–12 (2020).
- 98.Theodoropoulos JS, Bettle J, Kosy JD. The use of gps and inertial devices for player monitoring in team sports: a review of current and future applications. Orthop. Rev. 2020;12:7863. doi: 10.4081/or.2020.7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harris JD, et al. Return to sport after acl reconstruction. Orthopedics. 2014;37:e103–e108. doi: 10.3928/01477447-20140124-10. [DOI] [PubMed] [Google Scholar]
- 100.Buckthorpe M. Recommendations for movement re-training after acl reconstruction. Sports Med. 2021;51:1601–1618. doi: 10.1007/s40279-021-01454-5. [DOI] [PubMed] [Google Scholar]
- 101.Thome´e R, et al. Muscle strength and hop performance criteria prior to return to sports after acl reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2011;19:1798. doi: 10.1007/s00167-011-1669-8. [DOI] [PubMed] [Google Scholar]
- 102.Gokeler A, Welling W, Zaffagnini S, Seil R, Padua D. Development of a test battery to enhance safe return to sports after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2017;25:192–199. doi: 10.1007/s00167-016-4246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davies WT, Myer GD, Read PJ. Is it time we better understood the tests we are using for return to sport decision making following acl reconstruction? a critical review of the hop tests. Sports Med. 2020;50:485–495. doi: 10.1007/s40279-019-01221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Undheim MB, et al. Isokinetic muscle strength and readiness to return to sport following anterior cruciate ligament reconstruction: is there an association? a systematic review and a protocol recommendation. Br. J. Sports Med. 2015;49:1305–1310. doi: 10.1136/bjsports-2014-093962. [DOI] [PubMed] [Google Scholar]
- 105.Hughes G, Musco P, Caine S, Howe L. Lower limb asymmetry after anterior cruciate ligament reconstruction in adolescent athletes: a systematic review and meta-analysis. J. Athl. Train. 2020;55:811–825. doi: 10.4085/1062-6050-0244-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Losciale JM, et al. Hop testing lacks strong association with key outcome variables after primary anterior cruciate ligament reconstruction: a systematic review. Am. J. Sports Med. 2020;48:511–522. doi: 10.1177/0363546519838794. [DOI] [PubMed] [Google Scholar]
- 107.Kotsifaki A, et al. Single leg hop for distance symmetry masks lower limb biomechanics: time to discuss hop distance as decision criterion for return to sport after acl reconstruction? Br. J. Sports Med. 2022;56:249–256. doi: 10.1136/bjsports-2020-103677. [DOI] [PubMed] [Google Scholar]
- 108.Read P, Mc Auliffe S, Wilson MG, Myer GD. Better reporting standards are needed to enhance the quality of hop testing in the setting of acl return to sport decisions: a narrative review. Br. J. Sports Med. 2021;55:23–29. doi: 10.1136/bjsports-2019-101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Mille P, Osmak J. Performance: bridging the gap after acl surgery. Curr. Rev. Musculoskelet. Med. 2017;10:297–306. doi: 10.1007/s12178-017-9419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Halilaj E, et al. Machine learning in human movement biomechanics: Best practices, common pitfalls, and new opportunities. J. Biomech. 2018;81:1–11. doi: 10.1016/j.jbiomech.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dorschky E, et al. Cnn-based estimation of sagittal plane walking and running biomechanics from measured and simulated inertial sensor data. Front. Bioeng. Biotechnol. 2020;8:604. doi: 10.3389/fbioe.2020.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnson WR, et al. Multidimensional ground reaction forces and moments from wearable sensor accelerations via deep learning. IEEE Trans. Biomed. Eng. 2020;68:289–297. doi: 10.1109/TBME.2020.3006158. [DOI] [PubMed] [Google Scholar]
- 113.Tan T, Wang D, Shull PB, Halilaj E. IMU and smartphone camera fusion for knee adduction and knee flexion moment estimation during walking. IEEE Transactions on Industrial Informatics. 2023;19:1445–1455. doi: 10.1109/TII.2022.3189648. [DOI] [Google Scholar]
- 114.Mahmood, N., Ghorbani, N., Troje, N. F., Pons-Moll, G. & Black, M. J. AMASS: Archive of motion capture as surface shapes. Paper presented at the International Conference on Computer Vision, p. 5442–5451 (2019).
- 115.Taneja K, et al. A feature-encoded physics-informed parameter identification neural network for musculo-skeletal systems. J. Biomech. Eng. 2022;144:121006. doi: 10.1115/1.4055238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bartsoen L, et al. Bayesian parameter estimation of ligament properties based on tibio-femoral kinematics during squatting. Mech. Syst. Signal Process. 2023;182:109525. doi: 10.1016/j.ymssp.2022.109525. [DOI] [Google Scholar]
- 117.Tian, Y., Zhang, H., Liu, Y. & Wang, L. Recovering 3d human mesh from monocular images: A survey. Preprint at https://arxiv.org/abs/2203.01923v2 (2022). [DOI] [PubMed]
- 118.Hu P, Ho ES-L, Munteanu A. 3dbodynet: fast reconstruction of 3d animatable human body shape from a single commodity depth camera. IEEE Trans. Multimedia. 2021;24:2139–2149. doi: 10.1109/TMM.2021.3076340. [DOI] [Google Scholar]
- 119.Loper M, Mahmood N, Romero J, Pons-Moll G, Black MJ. Smpl: a skinned multi-person linear model. ACM Trans. Graph. 2015;34:1–16. doi: 10.1145/2816795.2818013. [DOI] [Google Scholar]
- 120.Xu, H. et al. Ghum & ghuml: Generative 3d human shape and articulated pose models. Paper presented at the IEEE/CVF Conference on Computer Vision and Pattern Recognition. p. 6184-6193 (2020).
- 121.Scalona E, et al. Inter-laboratory and inter-operator reproducibility in gait analysis measurements in pediatric subjects. Int. Biomech. 2019;6:19–33. doi: 10.1080/23335432.2019.1621205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Perazzi, F. et al. A benchmark dataset and evaluation methodology for video object segmentation. Paper presented at the IEEE conference on computer vision and pattern recognition. p. 724-732 (2016).
- 123.Hu B, Rouse E, Hargrove L. Benchmark datasets for bilateral lowerlimb neuromechanical signals from wearable sensors during unassisted locomotion in able-bodied individuals. Front. Robot. AI. 2018;5:14. doi: 10.3389/frobt.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Camargo J, Ramanathan A, Flanagan W, Young A. A comprehensive, open-source dataset of lower limb biomechanics in multiple conditions of stairs, ramps, and level-ground ambulation and transitions. J. Biomech. 2021;119:110320. doi: 10.1016/j.jbiomech.2021.110320. [DOI] [PubMed] [Google Scholar]
- 125.Embry, K., Villarreal, D., Macaluso, R. & Gregg, R. The effect of walking incline and speed on human leg kinematics, kinetics, and emg. IEEE Dataport10, https://ieee-dataport.org/open-access/effect-walking-incline-and-speed-human-leg-kinematics-kinetics-and-emg (2018).
- 126.Wilkinson MD, et al. The fair guiding principles for scientific data management and stewardship. Scientific data. 2016;3:1–9. doi: 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stratford PW, Riddle DL. When minimal detectable change exceeds a diagnostic test–based threshold change value for an outcome measure: Resolving the conflict. Phys. Ther. 2012;92:1338–1347. doi: 10.2522/ptj.20120002. [DOI] [PubMed] [Google Scholar]
- 128.Richardson WS, et al. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12–A13. doi: 10.7326/ACPJC-1995-123-3-A12. [DOI] [PubMed] [Google Scholar]
- 129.Page MJ, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 2021;10:1–11. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (axis) BMJ Open. 2016;6:e011458. doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dar G, Yehiel A, Cale’ Benzoor M. Concurrent criterion validity of a novel portable motion analysis system for assessing the landing error scoring system (LESS) test. Sports Biomech. 2019;18:426–436. doi: 10.1080/14763141.2017.1412495. [DOI] [PubMed] [Google Scholar]
- 132.Eltoukhy M, et al. Concurrent validity of depth-sensing cameras for noncontact ACL injury screening during side-cut maneuvers in adolescent athletes: a preliminary study. J. Appl. Biomech. 2019;35:2–10. doi: 10.1123/jab.2018-0105. [DOI] [PubMed] [Google Scholar]
- 133.Gray AD, et al. Development and validation of a portable and inexpensive tool to measure the drop vertical jump using the microsoft kinect V2. Sports Health. 2017;9:537–544. doi: 10.1177/1941738117726323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Uhl´ar A, et al. Kinect azure-based accurate measurement of dynamic valgus position of the knee-a corrigible predisposing factor of osteoarthritis. Appl. Sci. 2021;11:5536. doi: 10.3390/app11125536. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new or unpublished data is included within the study.
All code relating to summary figure development is available on request to T.T.