Abstract
BACKGROUND:
Substance use disorders (SUDs) are chronically recurring illnesses, where stress and drug cues significantly increase drug craving and risk of drug use recurrence. This study examined sex differences in functional magnetic resonance imaging (fMRI) brain responses to stress and drug cue exposure and assessed their prospective association with future drug use post-treatment.
METHODS:
Inpatient, treatment engaged men (N=46) and women (N=26) with SUDs, including alcohol, cocaine and/or cannabis use disorders, participated in an fMRI scan that assessed subjective (anxiety, drug craving), heart rate and neural responses to brief individualized script-driven imagery of stress, drug, and neutral-relaxing trials. Prospective follow-up interviews post-treatment assessed future drug use recurrence over 90 days.
RESULTS:
During fMRI, stress and drug versus neutral cue exposure led to increased anxiety, heart rate and craving responses (p’s<.004) in both men and women, but greater drug cue-induced anxiety (p<.017) and higher drug use days during follow-up (p<.006) in women relative to men. In whole brain analyses of stress and drug cues (p<.05 FWE corrected), and in whole brain correlation (p<.05, FWE corrected) with drug use days, significant sex differences revealed drug cue-related striatal hyperactivation (caudate, putamen) in men, but drug cue-related cortico-limbic (insula and dorsolateral prefrontal cortex) hypoactivation and stress-related hypoactivation in the ventromedial prefrontal cortex (VmPFC) in women; and these were significantly associated with higher future drug use days.
CONCLUSIONS:
Findings indicate sex-specific pathophysiology of SUD recurrence and support the need for differential treatment development for men and women with SUD to improve drug use outcomes.
Keywords: stress, drug cues, neuroimaging, recurrence of drug use, sex differences
Introduction
Approximately 37.9 million adults have a substance use disorder (SUD), characterized by repeated drug and/or alcohol misuse resulting in neural and behavioral negative adaptations (SAMHSA, 2021). Preclinical and clinical studies on sex differences in SUD suggest that women are more vulnerable to misuse of drugs than men and transition more quickly to addiction after drug initiation (Becker and Koob, 2016; Becker et al., 2017). For example, female rats self-administer more drug than male rats, (Carroll et al., 2002; Lynch and Carroll, 1999) and escalate their drug use more quickly than male rats (Becker et al., 2017; Roth and Carroll, 2004). Similarly, an early study reported that women who use cocaine had more struggles related to their cocaine use, more cocaine use days, and shorter abstinence periods than men (Kosten et al., 1993). Additionally, we previously reported that women relative to men with SUD reported higher childhood trauma scores than men, and that childhood emotional trauma was specifically predictive of SUD recurrence risk in women (Hyman et al., 2008). However, most functional imaging studies have not evaluated sex differences in SUD samples and its impact on recurrence of SUD risk (Lind et al., 2017).
Exposure to stress and adversity are key factors in the cycle of addiction. Stressful life events and childhood trauma as well as drug-related stimuli are associated with increases in craving and recurrence of SUD risk (Cooney et al., 2007; Sinha, 2011). Growing evidence indicates that drugs like cocaine, alcohol and cannabis activate peripheral stress arousal responses with increases in the hypothalamic-pituitary-adrenal (HPA) axis and autonomic arousal responses, that are altered and disrupted with chronic and binge use and misuse of these substances (Wemm and Sinha, 2019). Furthermore, individuals with SUD entering outpatient addiction treatment show disrupted peripheral stress responses and also report high levels of drug craving, both of which are associated with higher risk of future substance use (Sinha, 2011; Sinha et al., 2011; Sinha et al., 2006).
In addition, neuroimaging studies have examined the brain responses to provoked stress and drug-cue exposure using functional magnetic resonance imaging (fMRI). For example, a study conducted by Kosten and colleagues showed that return to cocaine use was associated with increased fMRI brain activation to cocaine cues in sensory, motor, and cognitive-emotional processing areas in cocaine-dependent patients (Kosten et al., 2006). Similarly, cocaine-cue activation in the right putamen and insula, as well as bilateral occipital regions was significantly associated with positive cocaine urine drug screen test (Prisciandaro et al., 2013). Studies from our laboratory have demonstrated that hypoactivity in the ventral medial prefrontal cortex (VmPFC) during stress and alcohol cue exposure was predictive of a reduced time to resumed substance use (Seo et al., 2013), and that alcohol craving, altered cortisol responses and altered VmPFC responses each predicted future recurrence of SUD risk (Blaine et al., 2017). However, direct examination of sex differences in neural responses to stress and drug cue exposure that are specifically associated with future recurrence of SUD and drug use has not been studied thus far.
It is important to examine sex differences in brain responses as sex differences in brain responses in healthy samples and in individuals with neuropsychiatric disorders including SUDs have been documented (Bangasser and Valentino, 2014; Becker et al., 2017; Kosten et al., 1993; Potenza et al., 2012; Seo et al., 2011). Research shows that healthy men and healthy women differ in brain activation in response to alcohol cues, and healthy women have a differential neural activation to stress and to alcohol cues relative to healthy men (Seo et al., 2011; Volkow et al., 2011). For example, Seo et. al., reported that healthy women showed greater alcohol-cue-related activity in the superior and middle frontal gyrus (SFG/MFG) than healthy men, whereas healthy men displayed greater cue-related activation in the ventral striatum and stress-related activations in the medial prefrontal cortex (mPFC), rostral anterior cingulate cortex (ACC), posterior insula, amygdala, and hippocampus than healthy women (Seo et al., 2011). In a recent study using a visual pictures block design procedure, Goldfarb et al. reported that healthy men have a greater stress response in the medial prefrontal cortex (PFC) regions of the dorsomedial prefrontal cortex, subgenual anterior cingulate cortex, and Brodmann area 11, whereas healthy women had a stronger response in the limbic/striatal regions, hippocampus, right insula/putamen, and pallidum (Goldfarb et al., 2019). Furthermore, we previously showed sex-specific differences in the corticostriatal-limbic responses to stress and/or drug cue exposure in men and women with cocaine use disorder (CUD), where women with CUD showed greater corticostriatal limbic reactivity to stress relative to healthy women, while men with CUD showed greater striatal reactivity to drug cues relative to healthy men (Potenza et al., 2012).
Based on this previous literature indicating sex differences in brain responses to stress in healthy men and women and in samples with SUD, the goal of this study was to examine sex differences in neural responses to stress and to drug cues relative to control neutral-relaxing cues in recently abstinent, treatment engaged women and men with SUD to specifically assess their association to future drug use post-treatment. Considering prior work, we hypothesized that men and women with SUD will show differential neural responses to stress and to drug cues, and with sex-specific distinct brain responses in the medial prefrontal, striatal and limbic regions, that will in turn predict future drug use and recurrence of SUD. Given previous sex differences in childhood trauma among SUD samples and their effects on recurrence of SUD risk (Hyman et al., 2008), we also assessed childhood trauma and accounted for its influence in fMRI analyses and in the association to future drug use and recurrence of SUD. To test these hypotheses, we studied 3–4 week abstinent, inpatient, treatment-engaged men and women, with current DSM-IVTR diagnoses of primary cocaine use disorder and/or alcohol use disorder and including those who also may have current cannabis use disorder. All subjects participated in an fMRI session where they were exposed to stress, neutral and drug-related cues using established personalized guided imagery procedures as in our previous work (see below). Patients were then followed prospectively over 90 days with in-person follow-up interviews at days 14, 30 and 90 to assess recurrence of drug use post-treatment (Figure 1).
Figure 1. Study Design.
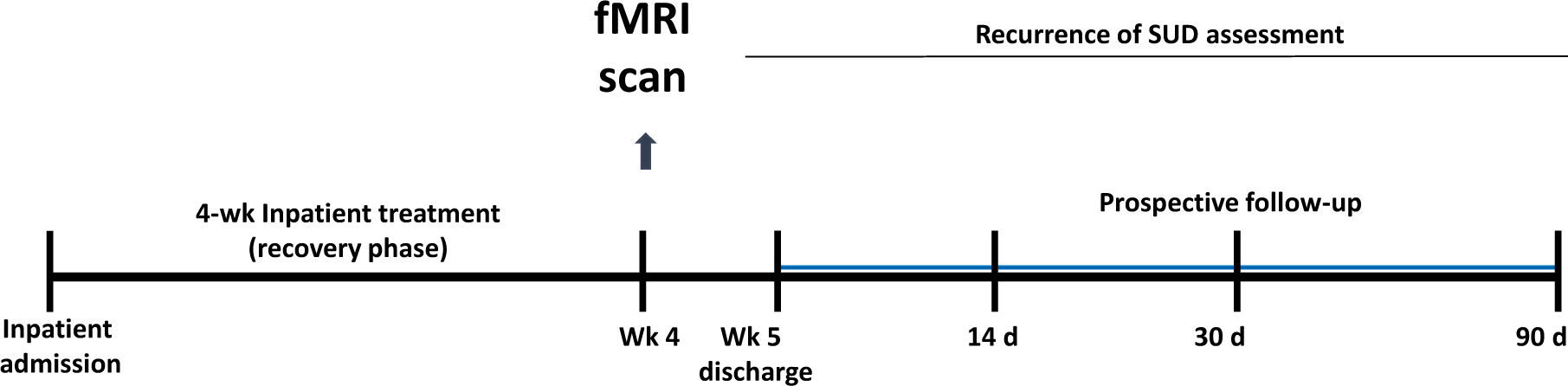
Seventy-two individuals with substance use disorder (SUD) residing in an inpatient treatment research facility for 4 weeks were assessed with functional magnetic resonance image (fMRI) testing in week 3/4. Participants also participated in prospective follow-up interviews at 14, 30, and 90 days following inpatient treatment discharge. Associations between neural responses and future drug use severity and drug use days were examined in a subset of 65 participants (42 men, 23 women, 90% of sample), who completed follow-up assessments to evaluate recurrence of SUD.
Methods
Participants
Seventy-two treatment seeking individuals (46 men and 26 women, aged 25 to 50 years), who met DSM-IVTR criteria for SUD with primary diagnoses of cocaine use disorder (CUD), and/or alcohol use disorder (AUD), and with or without cannabis use disorder were recruited from advertisements in local newspapers, on websites, and flyers, and addiction treatment centers in the greater New Haven, Connecticut, area (see Table 1). All participants were self-motivated for addiction treatment and for admission to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) to participate in inpatient treatment and research and were abstinent for approximately 3–4 weeks at the time of the fMRI scan. No study participation was in the context of an involuntary inpatient admission. Weekly substance use prior to inpatient admission was verified by a combination of self-report, breathalyzer, and urine toxicology assessments. Individuals with SUD specific to cocaine, alcohol, and cannabis (and nicotine) were admitted while those misusing opioids, sedatives, or methamphetamines were excluded. Those with other current Axis I psychiatric diagnoses and receiving psychoactive medications for those illnesses, and those with any history of psychosis were excluded. Individuals with secondary lifetime and/or current major depressive disorder, post-traumatic stress disorder (PTSD) or anxiety disorders were not excluded given the high comorbidity with SUD (Martins and Gorelick, 2011). Participants with any history of serious head trauma (defined by a blow or knock to the head with accompanying loss of consciousness > 30 minutes) or other neurological conditions were excluded. All participants gave both written and verbal informed consent. The study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Table 1:
Demographics and Clinical Characteristics of the Sample
| All SUD Participants n=72 | SUD Participants w/Follow-Up n=65 | |||
|---|---|---|---|---|
| Demographic Variables | Men=46 | Women=26 | Men=42 | Women=23 |
| No. Sex (%) | 46 (63.9) | 26 (36.1) | 42 (64.6) | 23 (35.4) |
| Age, mean (SD), years | 38.5 (6.4) | 34.7 (7.4) * | 39.3 (5.8) | 35.4 (6.2) * |
| Education, mean (SD), years | 12.5 (1.7) | 12.4 (1.3) | 12.5 (1.8) | 12.3 (1.3) |
| No. (%) Nicotine Smokers | 37 (80.4) | 22 (84.6) | 32 (76.2) | 20 (87.0) |
| No. (%) Minority Race | 17 (37.0) | 14 (53.8) | 14 (33.4) | 13 (56.5) |
| No. (%) African American | 16 (34.8) | 14 (53.8) | 13 (31.0) | 13 (56.5) |
| No. (%) Hispanic/Latino | 1 (2.2) | 0 (0.0) | 1 (2.4) | 0 (0.0) |
| Duration of substance abuse | ||||
| Any substance use, mean (SD), years | 19.4 (7.8) | 15.6 (7.2) * | 20.0 (7.4) | 16.0 (6.3) * |
| Alcohol use, mean (SD), years | 19.2 (8.1) | 13.0 (7.5) * | 20.4 (6.7) | 13.1 (6.9) * |
| Cocaine use, mean (SD), years | 9.4 (7.0) | 8.6 (6.6) | 10.0 (7.2) | 9.1 (6.7) |
| Marijuana use, mean (SD), years | 10.3 (7.3) | 11.5 (8.2) | 10.0 (7.3) | 11.8 (7.0) |
| Current Substance Use Disorder Diagnoses | * | |||
| No. (%) Alcohol | 22 (47.8) | 6 (23.1) | 22 (52.4) | 4 (17.4) |
| No. (%) Cocaine | 8 (17.4) | 9 (34.6) | 8 (19.0) | 9 (39.1) |
| No. (%) Marijuana | 0 | 0 | 0 | 0 |
| No. (%) Alcohol and Cocaine | 14 (30.4) | 6 (23.1) | 11 (26.2) | 6 (26.1) |
| No. (%) Alcohol and Marijuana | 0 | 0 | 0 | 0 |
| No. (%) Cocaine and Marijuana | 1 (2.2) | 4 (15.4) | 0 | 3 (13.0) |
| No. (%) Alcohol, Cocaine and Marijuana | 1 (2.2) | 1(3.8) | 1 (2.4) | 1 (4.3) |
| Lifetime Substance Use Disorder Diagnoses | ||||
| No. (%) Alcohol | 12 (26.1) | 3 (11.5) | 12 (28.6) | 1 (4.3) |
| No. (%) Cocaine | 5 (10.9) | 5 (19.2) | 5 (11.9) | 5 (21.7) |
| No. (%) Marijuana | 0 | 0 | 0 | 0 |
| No. (%) Alcohol and Cocaine | 15 (32.6) | 5 (19.2) | 12 (28.6) | 5 (21.7) |
| No. (%) Alcohol and Marijuana | 3 (6.5) | 2 (7.7) | 3 (7.1) | 2 (8.7) |
| No. (%) Cocaine and Marijuana | 1 (2.3) | 5 (19.2) | 1 (2.4) | 4 (17.4) |
| No. (%) Alcohol, Cocaine and Marijuana | 10 (21.7) | 6 (23.1) | 9 (21.4) | 6 (26.1) |
| Any psychiatric history No. (%) | 12 (26.1) | 11 (42.3) | 10 (23.8) | 10 (43.5) |
| Depression | ||||
| Lifetime | 6 (13.0) | 6 (23.1) | 5 (11.9) | 5 (21.7) |
| PTSD | ||||
| Lifetime | 5 (10.9) | 8 (30.8) * | 4 (9.5) | 8 (34.8) * |
| Anxiety disorder, excluding PTSD | ||||
| Lifetime | 5 (10.9) | 3 (11.5) | 4 (9.5) | 3 (13.0) |
| CTQ- Score, mean (SD) | 46.70 (18.6) | 53.08 (18.5) | 45.4 (18.1) | 55.0 (18.5) * |
| Mean (SD) Emotion | 11.0 (5.6) | 12.3 (5.9) | 10.6 (5.3) | 12.9 (6.0) |
| Mean (SD) Physical | 9.3 (4.6) | 9.5 (4.5) | 9.2 (4.6) | 9.8 (4.5) |
| Mean (SD) Sexual | 7.0 (4.6) | 9.9 (6.6) * | 7.0 (4.7) | 10.5 (6.8) * |
| Mean (SD) eNeglect | 11.3 (5.6) | 13.3 (5.2) | 10.9 (5.3) | 13.4 (5.3) |
| Mean (SD) pNeglect | 8.1 (4.0) | 8.0 (3.6) | 7.7 (3.4) | 8.4 (3.6) |
Independent t-Test and chi-square analyses were conducted to determine group differences. 90.3% of SUD participants provided follow-up interviews for drug use data.
Denotes p < .05.
Procedures
During week 1 of admission, participants provided demographic and medical history information, psychiatric history using the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1996), and childhood maltreatment history using the Childhood Trauma Questionnaire (CTQ) (Bernstein and Fink, 1998). Participants then completed an fMRI scan between weeks 3–4 of abstinence and inpatient stay. Participants were discharged from inpatient treatment and referred to outpatient aftercare. All participants were also scheduled for repeated face-to-face follow-up interviews at 14, 30, and 90 days after discharge, at which time drug use outcomes were evaluated using urine and breathalyzer samples and the Form 90 day substance use calendar (Miller and Del Boca, 1994).
Standard Behavioral Counseling
All participants admitted into the study participated in specialized substance abuse treatment that included weekly individual therapy provided by psychiatry residents and twice weekly standardized group drug counseling provided by an addiction specialist. The drug treatment was part of the inpatient program, initiated upon admission, and included additional group programming from 9:00 am to 3:30 pm on daily life skills and other structured activities, as described previously (Rando et al., 2013).
Individualized imagery method and script development
Prior to the fMRI session, individually-tailored, brief 2-minute guided imagery scripts were developed based on the description by participants via standardized, structured interviews using Scene Construction Questionnaires (adopted from (Miller et al., 1987; Sinha, 2009)) as described and validated in previous studies (Sinha, 2009). Two individualized scripts each for stress, drug-cue and neutral-relaxing conditions were developed. For stress scripts, participants described their recent experience of most distressing situations, and only those situations rated as 8 or above (on a 10-point Likert scale with 10=the most stressful) were selected for script development. Drug-cue scripts were developed from personal experiences of drug related situations that led to subsequent drug consumption (e.g., seeing others drinking alcohol, use cocaine and cannabis). Neutral scripts were developed based on commonly experienced, individual neutral-relaxing situations (e.g., laying on the beach and listening to the waves or sitting in a park and reading on a Sunday afternoon). Stress and neutral-relaxing scripts did not involve substance-related material. The script style, content format and length were standardized across conditions and subjects, while preserving individual stimulus and response content specific to the individual experience as previously described (Sinha, 2009). Each 2-minute script was audio-taped and presented in random order during the fMRI scanning session resulting in a total of 6 trials (2 stress, 2 drug cue and 2 neutral trials). These methods have been extensively used previously in human laboratory and functional neuroimaging studies that have validated neural stress and drug cue-related brain activation and related robust increases in drug craving as well as prospective prediction of future drug use outcomes (for e.g., Blaine et al., 2017; Potenza et al., 2012; Seo et al., 2013; Sinha et al., 2009; Sinha et al., 2011; Sinha et al., 2006).
fMRI acquisition and procedures
MRI data were collected using a 3T Siemens Trio MRI system equipped with a standard quadrature head coil, using T2*-sensitive gradient-recalled single shot echo planar pulse sequence (see supplemental materials for fMRI parameters). Six fMRI trials (two per condition) were acquired using a block design. The order of three script conditions were randomized and counterbalanced across subjects. Each script was presented only once for a subject and scripts in the same condition were not presented consecutively. Each trial lasted 5 min including a 1.5-min quiet baseline period followed by a 2.5-min imagery (2 min of read-imagery and 0.5 min of quiet-imagery) and a 1-min quiet recovery. During baseline, participants were instructed to stay still in the scanner without engaging in any mental activity. Before and after each trial, anxiety and craving ratings were elicited verbally for each using a 10-point Likert scale (0=not at all, 10=extremely high). Between fMRI runs and after each trial, participants were engaged in 2-min progressive relaxation to normalize any residual anxiety or craving from prior trials. This technique is mainly focused on relaxing physiological muscle tension and does not involve mental relaxation or imagery. After relaxation, anxiety and craving ratings returned to baseline, and there were no baseline differences in these ratings across trials.
Follow-up interviews and Future Drug Use Assessment
After discharge from inpatient treatment, all patients were set up for day 14, day 30 and day 90 follow-up interviews to assess drug use post-inpatient treatment. Follow-up assessment was successfully completed on 42 men and 23 women (N=65, 65/72, 90% follow-up rate), with prospective face-to-face follow-up interviews that included urine and breathalyzer samples and the Form 90 substance use calendar (SUC) (Miller and Del Boca, 1994) based on the timeline follow-back method (Sobell et al., 1996) at each follow-up timepoint and, where necessary, collateral informant report (i.e., family members or significant others) was also obtained as in previous work (Rando et al., 2011; Seo et al., 2013).
Estimation of Recurrence of Substance Use
The Kaplan-Meier (survival) estimator (Kaplan and Meier, 1958) was used to examine sex differences in recurrence of substance use assessed as time to first use of any drug (i.e., alcohol, cocaine, or cannabis), covarying for baseline duration of drug use, age and CTQ scores, and also assessing sex differences in fMRI whole-brain regression analyses on number of days to first use as a measure of time to resume drug use. In addition to examining resumption of substance use risk, we assessed number of days of any drug use in the 90-day follow-up period. The number of follow-up days used during the 90-day period was assessed as one measure of recurrence of SUD severity and included in whole brain regression analyses to assess sex differences in brain responses to stress and drug cues that are correlated with days of drug used.
FMRI Data Processing
fMRI data were converted from Digital Imaging and Communication in Medicine format to analyze format using XMedCon (Nolfe, 2003). To achieve steady-state equilibrium between radio-frequency pulsing and relaxation, the first ten images of each trial were discarded, leaving 180 measurements for analysis. Images were slice-time corrected using a custom-designed MATLAB program. Motion correction was implemented using Statistical Parametric Mapping (SPM5) for three translational and three rotational directions (Friston et al., 1996), removing trials with linear motion greater than 1.5 mm and a rotation exceeding 2 degrees The recovery period (1 min) was excluded from the data analysis to prevent carryover effects from the imagery period.
Individual-level analysis was conducted using a General Linear Model (GLM) on each voxel with the entire brain volume and a task-specific regressor for each of the 2 stress, 2 drug cue and 2 neutral trials where the 2.5 min imagery was assessed relative to each trial’s individual 1.5 min baseline, and using Yale BioImageSuite (http://www.bioimagesuite.org/(Duncan et al., 2004)). The trials per condition were then averaged together to result in individual level stress, drug cue and neutral cue provoked activation, relative to each trial baseline. To account for potential variability in baseline fMRI signal, drift correction was included in the GLM model; drift regressors were used to remove the mean time course, linear trend, quadratic trend, and cubic trend for each run. Each trial was normalized against the immediate baseline period preceding the script and then two trials of the same type were averaged. Functional images were spatially smoothed with a 6 mm Gaussian kernel, resulting in normalized beta-maps in the acquired space (3.44mmx3.44mmx4mm). To adjust for individual anatomical differences, three registrations were performed within the Yale BioImageSuite; a linear individual registration of raw functional image into 2D anatomical image, 2D to 3D (1×1×1 mm) linear registration, and a non-linear registration to reference 3D image, which is the Colin Brain(Holmes et al., 1998) in Montreal Neurological Institute (MNI) space. Then the output maps were converted to AFNI format for a group level analysis.
Statistical Analysis
Statistical analyses were performed in R v4.1.0. Linear mixed effects (LME) models with a random intercept were conducted to assess main effects and interactions for sex 2(between subjects) and condition 3(within subject: neutral, stress, drug cues) effects on anxiety, heart rate, and craving outcomes. T-tests and Chi-square analyses were used to compare the women vs men on demographic, clinical and follow-up number of substance use days used variables. Significant sex differences in any demographic variable like age was included as a covariate in all analyses. Additionally, as childhood trauma histories may vary by sex, CTQ scores were included as a covariate in all analyses. Pearson’s correlations were used to evaluate if years of alcohol use or a diagnosis of PTSD and current diagnosis were correlated with craving, anxiety, and HR responses, as well as post-hoc exploratory analyses of associations between subjective, physiological, and neural responses to stress and drug cues and in the association with future substance use. Figures were created with GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA).
Second level fMRI Group analysis was conducted with Analysis of Functional NeuroImages Software (AFNI;(Cox, 1996) http://afni.nimh.nih.gov), using LME models to assess sex differences in brain response to each condition (stress, drug and neutral cue imagery period relative to baseline for each trial) and their relationship to drug use outcomes. In addition, sex (men, women) was the between-subjects fixed-effect factor, condition was the within-subjects fixed-effect factor, subject was the random effect, and age and CTQ scores were included as covariates. We conducted LME regression models to assess sex differences in the association of brain responses to stress, drug, versus neutral cues and future drug use days and time to resumed substance use, with follow-up whole brain correlation analyses to assess source of significant associations in the LME regression models. The whole brain correlation assessed the correlation between brain responses to stress, drug, versus neutral cues and future drug use days and time to resume substance use for each sex separately. A FamilyWise Error rate (FWE) correction of alpha p<.05 was applied to correct for multiple comparisons using AFNI AlphaSim via Monte Carlo Simulation (Cox, 1996; Xiong et al., 1995) for all whole brain analyses and for the whole brain correlations.
Results
Demographic Characteristics
Demographic, substance use, CTQ, nicotine smoking status, and psychiatric history for all study participants and those with follow-up drug use data are provided in Table 1. Men with SUD were significantly older than women with SUD (p<.05), and as expected, men with SUD had significantly more years of any substance use (19.4 years) compared to women with SUD (15.6 years) (p<.05) but this was due to greater years of alcohol use in men relative to women (p<.05) but no sex differences in years of cocaine use or marijuana use. Men with SUD and women with SUD had similar CTQ total and subscale scores, but in the follow-up sample, women with SUD had significantly higher scores on the total CTQ than men with SUD (p<.05), stemming from higher scores of the sexual trauma subscale in the women compared to men (p<.05). There was also a significant sex difference in this SUD sample for the number of women vs men with lifetime PTSD (8/26 women, 5/46 men, p<.05) (see Table 1).
Behavioral and Physiological Responses during fMRI
We found a significant effect of condition for drug craving (F (2, 140)=45.76; p<.0001) stemming from greater craving during stress cue (p<.0001) and drug cue (p<.0001) trials compared to neutral-relaxing cue trials (Figure 2A). In addition, craving was greater in response to the drug cue compared to stress cue trials (p<.001). There was no sex main effect (F (1,70)=1.26; p<.266) or a condition by sex interaction (F(2, 140)=.95; p<.390) observed for drug craving.
Figure 2: Sex differences in subjective and physiologic responses during fMRI and follow-up days of substance use.
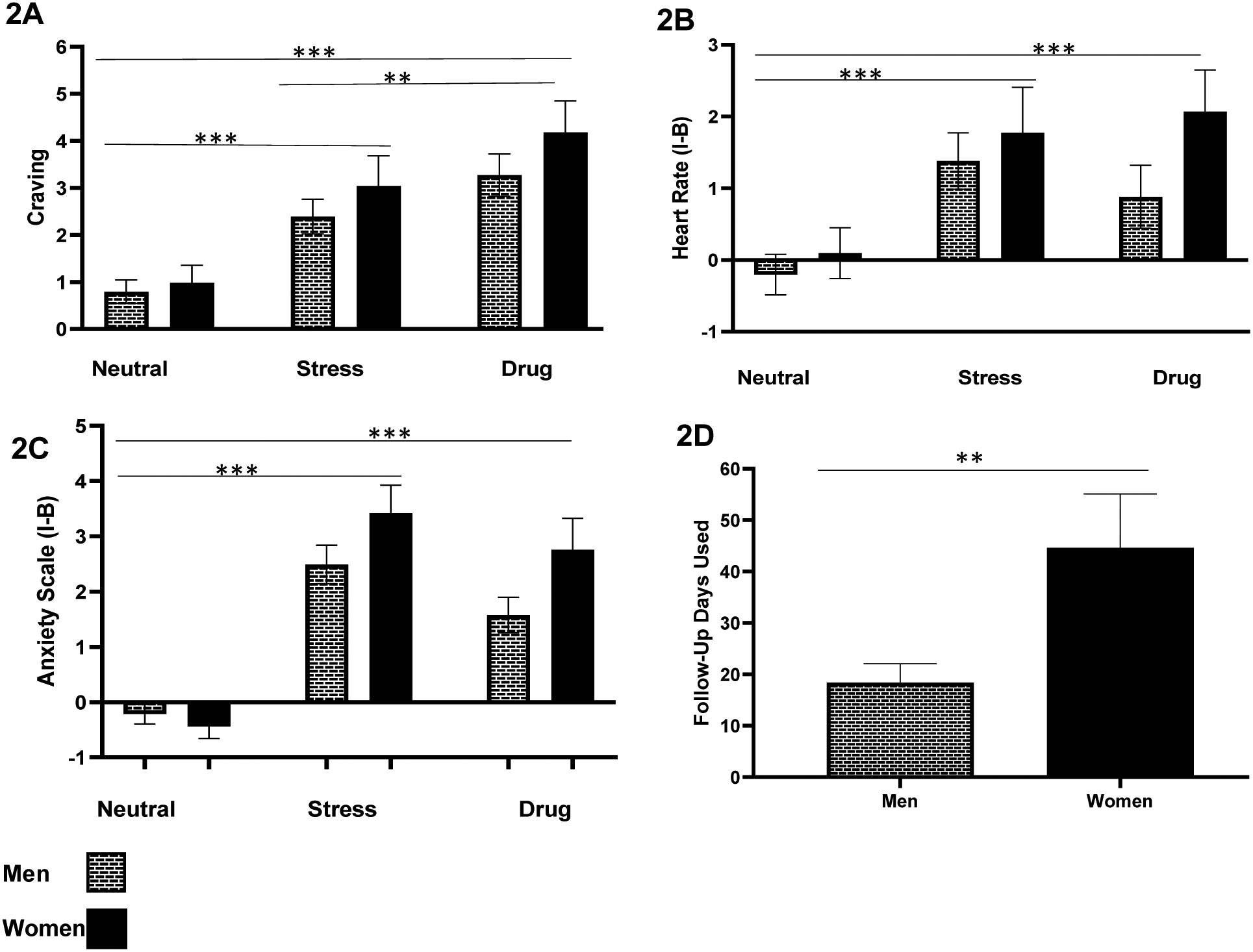
Mean craving, heart rate and anxiety ratings in response to neutral-relaxing (N), stress cue (S), and drug cue (D) imagery trials relative to baseline of each trial (imagery minus baseline for each trial, then averaged per condition) [I-B] and days of substance use in the follow up period in men and women with SUD. Drug craving (2A) was significantly higher in response to stress and drug cue compared to neutral imagery (S>N: p<.0001; D>N: p<.0001; D>S: p<.001) (for most used substance: alcohol, cocaine, or cannabis). Heart rate (2B) was significantly higher in response to stress and drug cue compared to neutral imagery trials (S>N: p<.0001; D>N: p<.0001; D/S: p<0.751). Anxiety (2C) was significantly higher in response to stress and drug cue imagery compared to neutral (S>N: p<.0001; D>N: p<.0001; and S>D; p<.014 (only in men). Also, Condition X Sex interaction for anxiety (F [2, 140] =3.1; p<.048) was observed stemming from women with SUD showing significantly higher anxiety in response to the drug cue compared to men with SUD (p<.017), and men showed higher anxiety in response to the stress compared to drug cue condition (p<.014), which was not seen in women (p<.20). (2D) Sex differences in the 90-day follow-up of mean days of use (and standard error) with women showing greater number of follow-up of days used than men (p<.006). Mean number of days used in women was 44.61 (±10.50) compared to 18.43 (±3.65) days used in men (Independent t-test t (27.4)=2.35, p<.006) (women, n=23; men, n=42). All data are displayed as mean ± S.E.M.
A main effect of condition for average heart rate responses (F(2,136) =15.7; p<.0001) was observed. A significantly higher heart rate response was observed during stress cue (p<.0001) and drug cue (p<.0001) trials compared to the neutral-relaxing cue trials (Figure 2B). There was no sex main effect (F(1,68)=1.3; p<.259) or a condition by sex effect (F(2,136)=1.04, p<.356) on heart rate. There were also no significant differences between stress and drug cue trials for heart rate responses (p<.751).
A significant effect of condition (F(2,140)=57.6; p< .0001) was observed for anxiety ratings which were significantly higher in the stress (p< .0001) and drug cue (p< .0001) trials compared to the neutral-relaxing cue trials (see Figure 2C). While there was not a statistically significant main effect of sex for anxiety ratings (F (1,70)=3.34; p<.07), a condition by sex interaction was observed (F(2,140)=3.1; p<.048) for anxiety ratings. This significant interaction stemmed from the following simple effects: women with SUD showing significantly higher anxiety in response to the drug cue compared to men with SUD (p<.017), with this effect at trend levels for the stress condition (p< 0.06), and men with SUD showed higher anxiety ratings in response to the stress cue compared to their anxiety responses during drug cues (p<.014), while no condition differences in anxiety were observed in women with SUD (p<.20).
Finally, we also assessed whether years of alcohol use or a diagnosis of PTSD was correlated with craving, anxiety and HR responses within each sex at a significance threshold of p<.01 selected to account for multiple correlations. No significant associations were found at this corrected threshold.
Prospective Drug Use Outcomes
Sixty-five of the 72 participants with SUD (90.3%) were successfully followed for assessment of drug use outcomes over 90 days post-treatment. Notably, women with SUD had significantly greater number of follow-up days of substance use compared to men with SUD [Women=44.6 days, Men=18.4 days; t(27.4) = −2.35, p<.006] (see Figure 2D). This was due to the fact that women with SUD had significantly more cocaine use days (Women=16.7 days and 9.6 days) in the 90-day follow-up compared to men with SUD (see Supplemental Table 1). While there was no statistically significant difference in men with SUD compared to women with SUD in time to return to substance use (p=.702), women with SUD resumed substance use earlier (median=14.0 days, SE=4.19) compared to men with SUD (median=21.0, SE=4.86) (see Supplemental Figure 1). Time to resume substance use and sex differences in number of follow-up days used were not associated with PTSD, specific SUD diagnoses, or greater years of alcohol use.
Sex Differences in Whole-Brain Neural Responses to Stress and Drug Cues
Voxel based-whole brain analyses were conducted to assess sex differences in men and women with SUD and in response to stress, drug cue and neutral-relaxing cue exposure (imagery-baseline period for each trial, per condition), controlling for age and CTQ scores (FWE whole brain corrected, p<.05). A significant sex main effect was observed which showed greater overall functional activation across all conditions in men with SUD relative to women with SUD in the thalamus, hypothalamus, hippocampus and right caudate (see Figure 3A, also Supplemental Table 2). Further, a significant sex X condition interaction was also observed, which arose from greater stress cue-induced activation in men with SUD compared to women with SUD in the caudate, thalamus, hypothalamus, right hippocampus, and left striatum (Figure 3B, also Supplemental Table 3). There were no sex differences, that survived whole brain correction, between men with SUD and women with SUD in the whole brain response to drug cues or to the neutral conditions.
Figure 3: Sex differences in neural fMRI whole brain responses to neutral, stress, and drug cue provocation (FWE, p<.05).
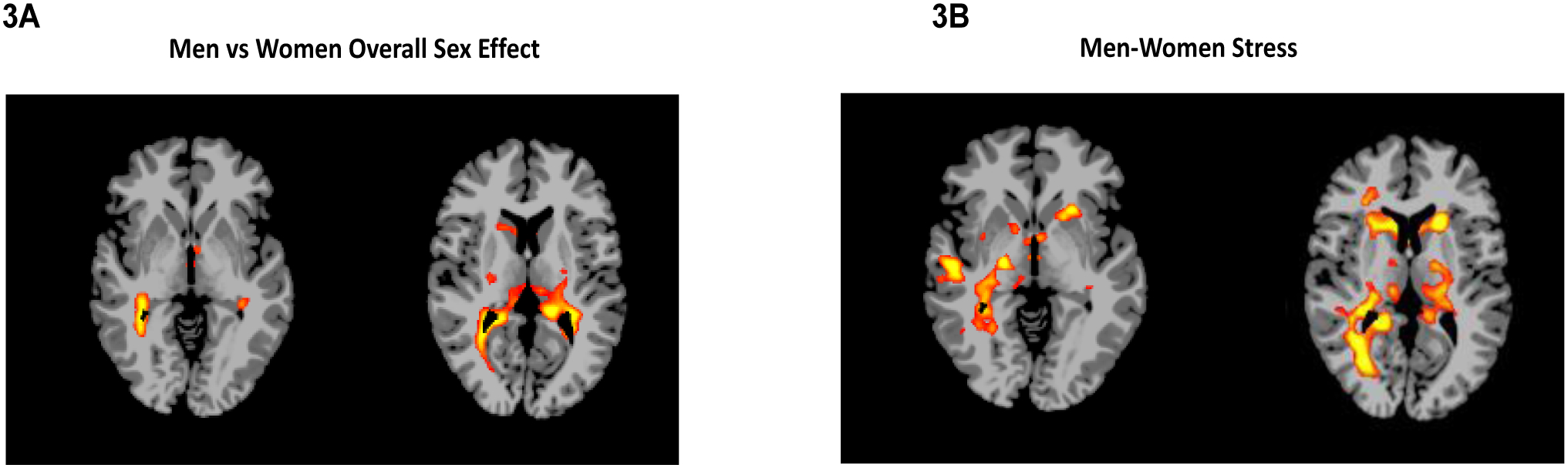
Whole-brain voxel-based analysis of sex, condition and sex x condition interaction (p<.05, whole-brain corrected). 3A: After controlling for CTQ and age, a sex main effect in whole brain response indicated overall higher activation in thalamus, hippocampus, hypothalamus, and right caudate in men compared to women. 3B: A significant sex x condition interaction (3B) indicated greater activation in men relative to women for the stress cue brain responses in the caudate, thalamus, hypothalamus, right hippocampus, and left striatum (FWE, p<.05), but no significant sex differences in the neural responses to drug cues and neutral provocation. (See Supplemental Table 2–3 for MNI coordinates).
Association of Brain Responses and Future Days of Substance Use
Results of the whole brain LME regression analyses assessing sex differences in neural responses to stress and to drug cues that predicted future drug use days yielded significant sex X follow-up days used and sex X follow-up days used X condition interaction effects (FWE whole brain corrected, p<.05), but no sex effects on the time to resume substance use outcome. Thus, follow-up whole brain correlation analyses were conducted separately for men and women to assess the source of the sex differences in the assocaition between brain responses to stress, drug cues and neutral conditions and with number of follow up days of drug use (FWE corrected at p<.05). These analyses revealed that drug cue-induced hyperactivation in the putamen and the caudate for men with SUD was significantly correlated with number of follow-up days of substance use (Figure 4A), but drug cue-induced hypoactivation in the left DLPFC and left insula for women with SUD was significantly correlated with future number of days used (Figure 4C). Moreover, stress cue-induced hypoactivation in the VmPFC was significantly associated with future days of substance use only for women with SUD (Figure 4B) (also see Supplementary Table 4). No significant correlations were found for brain responses and number of days to resumed substance use.
Figure 4: Sex differences in association between neural responses to drug and stress cue and future number of days of any substance use during follow-up.
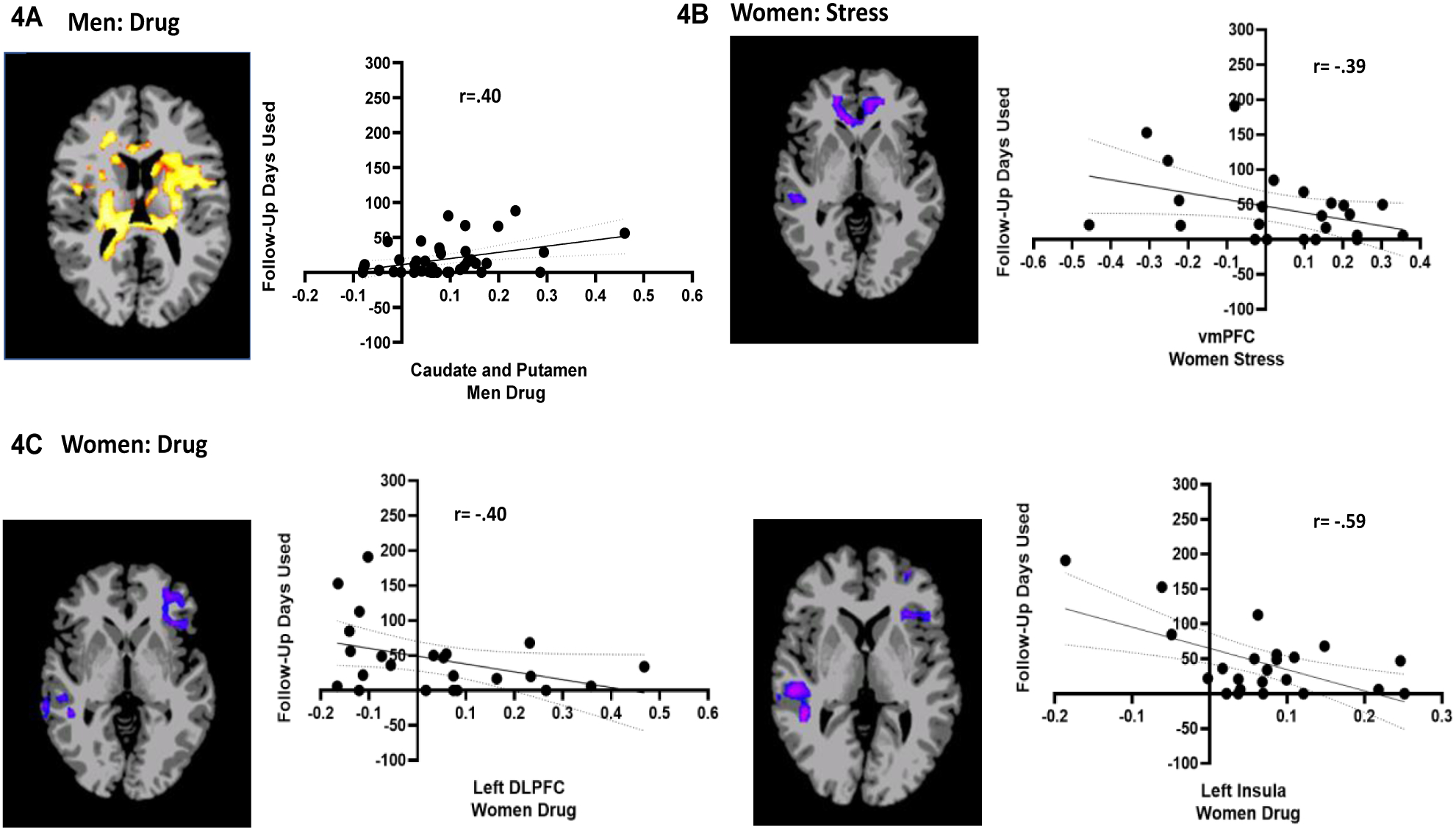
Whole-brain correlation analysis (FWE corrected p<.05). During the drug cue trials, putamen and caudate hyperactivity was significantly associated with higher follow-up days used in men (4A). During the stress cue trials, VmPFC hypoactivity (−0.57,45.19, −0.69) was significantly associated with higher follow-up days used in women (4B). However, hypoactivity during the drug cue trials in the left dLPFC (−39.61, 43.43, 7.24) and left insula was significantly associated with higher follow-up days used in women (4C). Note: Positive correlations shown in red/yellow; negative correlations shown in blue/purple. MNI coordinates for peak clusters for the VmPFC and DlPFC are provided above (also see Supplemental Tables 4–5). Also, all correlations remain significant even with removal of more extreme values.
Post-Hoc Exploratory Analyses of Associations between Subjective, Physiological and Neural Responses to Stress and Drug Cues and in the Assocaition with Future Substance Use.
As there were significant sex interactions in anxiety responses to stress and drug cues and also condition main effects showing stress and cue-induced craving and heart rate increases, we assessed whether these responses were associated with brain activations during stress and drug cue exposure. Pearson product-moment correlations were conducted between subjective and HR responses and regions of interest (ROIs) beta weights for regions identified in the whole brain analyses and shown in Supplementary Tables 2–4). We also set a more stringent significance threshold of p<.01 to adjust for the high number of correlation tests conducted. We found that there were no significant associations that met this threshold. In addition, years of alcohol use and PTSD diagnoses which were different between men and women were also assessed for association with activated brain ROIs during stress and cues and these did not yield significant results above the set corrected threshold.
Discussion
The current findings are the first to show significant sex differences in neural responses to stress and to drug cues in abstinent treatment engaged men and women with SUD. Notably, while there were no sex differences in craving and heart rate, there were significant sex differences in brain response and in subjective anxiety responses to stress and to drug cues. Furthermore, the clinical relevance of these sex differences in neural responses was reflected in sex-specific relationships between brain response to stress and to drug cues and the number of follow-up days of alcohol, cocaine, or marijuana use. Notably, women with SUD not only showed significantly higher number of future days of substance use overall during the prospective 90 day follow up period with significantly higher number of cocaine use days compared to men, but also displayed hypoactivity in the VmPFC during stress cue exposure and hypoactivity in the left DLPFC and left insula in response to drug cue exposure, which were each significantly correlated with higher days of substance use during 90-day follow-up. For men with SUD, hyperactive responses in striatal regions during drug cue exposure predicted higher days of substance use during follow-up.
Consistent with previous findings, we found that script-guided imagery effectively elicited increases in stress and cue reactivity in both men and women with SUD. Main effects of condition on drug craving, heart rate, and anxiety were observed, where stress and drug cue conditions were associated with higher subjective drug craving, heart rate, and anxiety as compared with the neutral-relaxing condition. As in previous research, we found no sex effect on provoked drug craving suggesting that both men and women with SUD have similar levels of stress- and drug cue- induced subjective craving, and this is consistent with our previous work in cocaine use disorder and alcohol use disorder (Potenza et al., 2012; Seo et al., 2011). While some previous work has shown greater levels of craving in women with opioid use disorder (OUD) relative to men with OUD (Back et al., 2011; Moran et al., 2018; Yu et al., 2007), other research reported no sex differences in craving levels (Herbeck et al., 2016; Kennedy et al., 2013). For example, Yu et al., reported that women had a greater response in heroin craving, systolic blood pressure, diastolic blood pressure, and decreased joy to imagery and/or paraphernalia cues compared to men (Yu et al., 2007). On the other hand, other research showed that sex - differences in response to craving was drug-specific. For instance, Moran and colleagues observed greater opioid craving in women in response to stress events whereas, men showed a greater cocaine craving in response to stress events (Moran et al., 2018). As our sample did not include those with opioid use disorder, it is possible that sex differences in subjective craving may be more specific to opioid use disorder and not different in co-occurrence of alcohol, cocaine, and cannabis use disorder. Greater study of sex differences in craving and the factors contributing to such differences is warranted in future research.
An overall sex by condition interaction for anxiety was also observed, where women with SUD had higher overall anxiety across conditions than men with SUD. This is in line with previous research that shows sex differences in anxiety levels during stress exposure in women versus men (al’Absi et al., 2021; Jastreboff et al., 2013; Sinha, 2008). Fox et al., reported that abstinent women with cocaine dependence experienced increased stress-induced anxiety and sadness compared with abstinent men with cocaine dependence, while men were emotionally and physiologically more reactive in the drug cue condition (Fox et al., 2008). Interestingly, in our sample women had significantly higher drug cue-induced anxiety relative to men (between sex group effect), whereas men with SUD had significantly higher anxiety during stress cue versus drug cue exposure (within subject effect). One explanation for this observation could be that men with SUD had significantly more years of substance use and years of alcohol use, suggesting perhaps that greater substance use severity may have made them more sensitive to the stress pathophysiology of addiction (Sinha, 2001. 2008). On the other hand, women showed equivalently increased anxiety ratings in response to stress and to drug cues, suggesting that negative affect and distress-related factors play a role in stress and drug cue reactivity for women, and that since women are known to have higher rates of internalizing mood and anxiety related illnesses, that may also contribute to this equivalent reactivity (Bangasser and Valentino, 2014; Chaplin et al., 2008; Hankin and Abela, 2005). Women may also have a greater sensitivity to negative drug effects and related greater dysregulation in the corticotrophin releasing factor (CRF)-HPA axis along with ANS in individuals with substance use disorder (Back et al., 2010; Sinha et al., 2009; Sinha et al., 2003; Seo et al., 2019), that in turn may contribute to higher drug cue-related anxiety equivalent to stress-related provoked anxiety levels.
Importantly, our findings show that women with SUD, in the early recovery period were found to resume substance use earlier (Supplemental Figure 1) and use drugs, specifically cocaine (Supplemental Table 1), on more days than men with SUD (Figure 2D) during the prospective 90-day follow-up period. This is in line with previous clinical observations that show women may experience greater withdrawal and protracted abstinence effects in early recovery that may increase recurrence of SUD and drug use risk (Becker and Chartoff, 2019; Becker et al., 2017; Becker et al., 2012). Furthermore, women with SUD had significantly higher childhood trauma scores specifically, sexual trauma compared to men with SUD (Table 1). Hyman et al. observed that greater severity of childhood emotional trauma, sexual trauma, and overall childhood trauma was associated with higher follow-up days of cocaine used and an increased risk of recurrence of SUD in women with cocaine dependence, and this association was not found in men (Hyman et al., 2008). Furthermore, higher childhood trauma levels have also been found to be associated with greater current days of substance use after inpatient treatment (Van Dam et al., 2014), and polysubstance use is associated with greater SUD severity and worse treatment outcomes (Crummy et al., 2020). Together, this previous work supports and explains our findings that women with higher levels of childhood trauma than men, combined with their polysubstance use history, may be at greater risk for recurrence of SUD and using on more days after resuming substance use, i.e., with greater severity, than men.
With regards to neuroimaging, overall functional hyperactivation in striatal and midbrain regions was found in men with SUD compared to women with SUD (Figure 3A). These results are consistent with our hypothesis that men and women have differential functional brain responses, and we found this to be specifically coming from differences in the stress condition (Figure 3B). These results are in line with the role of dopamine and the striatal reward pathways in the pathophysiology of substance use disorders(Volkow et al., 2007). Sex differences in the dopaminergic system, may contribute to these differences in men and women with SUD. Men have been found to have more D1 dopaminergic receptors in the striatum, while women had higher dopamine transporter mRNA density in the striatum (Becker, 1999). This suggest that women may experience greater sensitivity to drug effects and perhaps more craving and drug use associated with greater dopamine neurotransmitter turnover in response to increased dopamine transporter expression in the striatum. Moreover, females have more dopaminergic projections to the medial prefrontal cortex (mPFC) compared to males (Kritzer and Creutz, 2008), and it is known that the mPFC matures earlier in females compared to males (Perry et al., 2021). This is critical because the mPFC is involved in cognitive control, but it also is disrupted with early trauma, which may impact women with SUD more so than men with SUD due to their higher rates of early childhood trauma. Furthermore, ovarian hormones such as estrogen and progesterone are known to modulate chronic drug effects specifically during stress and in striatal regions, and such differences might explain the varying functional brain responses between sexes (Becker, 1999, 2016; Chase et al., 2011; Childress et al., 1999).
Human neuroimaging studies have demonstrated significant alterations in the mesocorticolimbic (PFC, insula, hippocampus, ACC, and amygdala) circuitry in individuals with SUD in response to drug cues compared to neutral cues (Chase et al., 2011; Childress et al., 1999; Schacht et al., 2013). In line with these studies, we found blunted responses to drug cues in the left DLPFC and left insula in women with SUD (Figure 4C) but heightened striatal responses to drug cues in men with SUD (Figure 4A) that each respectively predicted drug use in the subsequent 90-day follow-up. Research into the pathophysiology of addiction has shown dysregulation in executive control in both DLPFC and insula in individuals with SUD (Goldstein and Volkow, 2011; Naqvi and Bechara, 2010). Additionally, individuals with cocaine misuse and/or dependency showed increased functional connectivity between prefrontal cortex-striatum and decreased striatum-dorsal anterior cingulate connectivity during abstinence (Hu et al., 2015). While this previous research is consistent with our findings on the specific cortico-limbic striatal circuits involved in recurrence of SUD, the previous research included majority male samples and thus the sex-specific differential activation pattern and sex-specificity of regions associated with drug use has not been reported previously.
Impaired emotional regulation in individuals with SUD has been shown to be a predictor of abstinence and risk of resuming substance (Wilcox et al., 2016). Consistent with these observations women with SUD had blunted VmPFC during stress cue (Figure 4B). VmPFC plays a critical role in reward and decision-making(Hiser and Koenigs, 2018). Our previous work showed that craving, adrenal sensitivity, and VmPFC hypoactivity following exposure to stress-related and alcohol cue-related imagery predicted lower days of abstinence and more heavy drinking days (Blaine et al., 2020). This VmPFC dysregulation might explain why women with SUD in our study had more substance use days during the follow-up period. Further, women in our study also had higher sexual trauma and PTSD which may contribute to VmPFC dysregulation and subsequent SUD recurrence risk. For example, blunted VmPFC function to stress, threat, and trauma cues has been reported in PTSD and in risk for PTSD (Li & Sinha, 2008; Roekner et al., 2021), while greater cue-related striatal/putamen responses in men has also been associated with greater drug craving, and future drug and alcohol use as well as greater withdrawal severity (Martinez et al., 2007; Sinha et al., 2021b). Blunted responses in the VmPFC and rostral ACC in women points to greater obstacles in self-regulation and in control over emotions that may then be associated with increases in drug-seeking and more days of substance use in women. On the other hand, greater striatal and midbrain activation during drug cue and stress responses in men may signal greater compulsive drive for drug that in turn was associated with greater risk of follow-up use days.
Importantly, in this study we covaried for childhood trauma in all analysis, as women with SUD relative to men with SUD had higher CTQ scores in the follow-up subgroup and women with SUD also had higher rates of PTSD than men with SUD, and even after covarying for these differences, we found differences in sex differences in brain responses and drug use outcomes. This is notable as in previous work we have shown that higher CTQ scores were associated with greater number of substances use days in the follow up period in patients with SUD (Van Dam et al., 2014). Moreover, Poppa et al., observed that sexual trauma was associated with reduced orbitofrontal connectivity, in inpatient treatment-seeking SUD women (Poppa et al., 2019). This suggests that exposure to childhood trauma, and particularly sexual trauma, may not only increase vulnerability to greater substance use severity in women with SUD, but also contribute to the blunted VmPFC and rACC responses to stress, as reported in our current findings.
Clinical Implications
Our findings indicate that women with SUD compared to men with SUD show differential neural responses to stress and to drug cues in the association with subsequent drug use. Greater number of follow up drug use days was found in women compared to men. In addition, higher number of follow-up drug use days was associated with drug cue -related hyperactivation in the striatum in men and hypoactivation in left DLPFC and left insula in women. In addition, stress cu- induced VmPFC hypoactivation was associated with greater follow-up drug use days in women. These findings are clinically important because they suggest that men and women may likely benefit from differential sex-specific clinical interventions to improve treatment outcomes. Treatment interventions for women with SUD may benefit from a focus on stress regulation and coping and management skills whereas, men with SUD may benefit from a focus on control over compulsions and drug treatment programs and approved medications. Lastly, trauma-focused counseling to address enduring effects of childhood trauma as a part of ongoing intervention and therapy may improve SUD recurrence risk and lower number of drug use days in SUD women.
Limitations
Finally, it is important to note several limitations of the current study. Despite small sample sizes, we were able to assess sex differences in brain responses to stress and drug cues. However, the sample of women with SUD was low and the sample size for assessing recurrence of SUD outcomes was modest. Thus, the findings need replication in future studies that also have larger samples of men and women. These future studies may also be better powered to assess associations between anxiety, HR and craving and brain responses to stress and drug cues separately in men and women. Nonetheless, significant effects in post-treatment drug use days in women compared to men with higher days of substance use in the follow up period needs further attention in future studies. Moreover, men had more years of substance use than women, and these differences may have further contributed to the sex differences in neural effects. The majority of the sample was also using nicotine, and so co-morbid nicotine use may have also influenced the findings. Also, while significantly more women in our study had PTSD, sexual trauma, and overall childhood trauma compared to men, the numbers with PTSD were low, and thus the possible differential effects of PTSD, sexual trauma and CTQ on brain responses and recurrence of SUD needs further attention in future studies with larger samples. Finally, the current study included those with alcohol, cocaine, and/or cannabis polysubstance misuse, and so the findings may not be generalizable to those using opiates and methamphetamines. Despite these limitations, the current findings are the first to demonstrate sex differences in prefrontal and striatal-limbic brain responses to stress and to drug cues in a sample of men and women with substance use disorders. We found that women not only had higher days of substance use in the follow-up indicative of greater severity for recurrence of SUD, but also had blunted/decreased neural response to drug cues in the left DLPFC and left insula and blunted responses to stress in the VmPFC, whereas men had heightened/increased neural response to drug cues in the striatum, which were each in turn associated prospectively with future substance use days. These data suggest that behavioral and medication interventions that restore PFC/insula function in women and reduce striatal hyperresponsiveness in men would be of benefit to improve recurrence of SUD outcomes.
Supplementary Material
Highlights.
Treatment engaged men and women with substance use disorders (SUD) experience high craving, heart rate, and anxiety in response to stress and drug cue exposure.
Women with SUD show significantly higher provoked anxiety and greater number of subsequent follow-up days of substance use compared to men with SUD.
Men show greater activation in the striatum during stress compared to women, and greater activation in striatal regions during drug cues. This activation is associated with higher post-treatment future drug use days for men.
Women display hypoactivation in the ventromedial prefrontal cortex (VmPFC) to stress and in left dorsolateral prefrontal cortex (DLPFC) and left insula in response to drug cues, which predicted greater number of future drug use days post-treatment.
Findings suggest differential functional brain response patterns to stress and to drug cues predicts future substance use and supports the need for sex-specific treatment development for men and women with SUD.
Author Disclosures
This work was funded by grants R01-AA013892 and P50-DA016556 (PI: Rajita Sinha) from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and National Institute on Drug Abuse (NIDA). KS is supported by NIDA fellowship 5T32DA022975-12. The analyses and results described in this manuscript were presented in poster format at the 84th Annual Meeting for The College on Drug Dependence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Authors have no relevant conflicts of interest to disclose.
CRediT authorship contribution statement
KS was responsible for data analyses, contributed to interpretation of the results, and was a major contributor to manuscript preparation. CL was responsible for neuroimaging acquisition, processing and analysis. VM contributed to the manuscript preparation and made critical revisions of the manuscript for intellectual content. NF assisted with data analysis and contributed to manuscript preparation. RS conceptualized and designed the study, and was a major contributor to data analytic strategy, interpretation of study findings, manuscript preparation and made critical revisions of the manuscript. All authors approved the final version of the paper for submission.
References
- al’Absi M, Ginty AT, Lovallo WR, 2021. Neurobiological mechanisms of early life adversity, blunted stress reactivity and risk for addiction. Neuropharmacology 188, 108519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT, 2010. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend 106, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Lawson KM, Singleton LM, Brady KT, 2011. Characteristics and correlates of men and women with prescription opioid dependence. Addict Behav 36, 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ, 2014. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 35, 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, 1999. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 64, 803–812. [DOI] [PubMed] [Google Scholar]
- Becker JB, 2016. Sex differences in addiction. Dialogues Clin Neurosci 18, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Chartoff E, 2019. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44, 166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68, 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG, 2017. Sex differences, gender and addiction. J Neurosci Res 95, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C, 2012. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D, Fink L, 1998. Childhood Trauma Questionnaire: A retrospective self-report questionnaire and manual. Psychological Corp, San Antonio, TX. [Google Scholar]
- Blaine SK, Seo D, Sinha R, 2017. Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addict Biol 22, 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Wemm S, Fogelman N, Lacadie C, Seo D, Scheinost D, Sinha R, 2020. Association of Prefrontal-Striatal Functional Pathology With Alcohol Abstinence Days at Treatment Initiation and Heavy Drinking After Treatment Initiation. Am J Psychiatry 177, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK, 2002. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 161, 304–313. [DOI] [PubMed] [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, Sinha R, 2008. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcoholism, clinical and experimental research 32, 1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L, 2011. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry 70, 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP, 1999. Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinberg HR, Oncken CA, 2007. Alcohol and tobacco cessation in alcohol-dependent smokers: analysis of real-time reports. Psychol Addict Behav 21, 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, Staib LH, 2004. Geometric strategies for neuroanatomic analysis from MRI. Neuroimage 23 Suppl 1, S34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1996. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R, 2008. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology, 33, 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R, 1996. Movement-related effects in fMRI time-series. Magn Reson Med 35, 346–355. [DOI] [PubMed] [Google Scholar]
- Goldfarb EV, Seo D, Sinha R, 2019. Sex differences in neural stress responses and correlation with subjective stress and stress regulation. Neurobiol Stress 11, 100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12, 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abela JR, 2005. Development of psychopathology: A vulnerability-stress perspective. Sage Publications. [Google Scholar]
- Herbeck DM, Jeter KE, Cousins SJ, Abdelmaksoud R, Crevecoeur-MacPhail D, 2016. Gender differences in treatment and clinical characteristics among patients receiving extended release naltrexone. J Addict Dis 35, 305–314. [DOI] [PubMed] [Google Scholar]
- Hiser J, Koenigs M, 2018. The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biological psychiatry 83, 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC, 1998. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22, 324–333. [DOI] [PubMed] [Google Scholar]
- Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y, 2015. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry 72, 584–592. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R, 2008. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend 92, 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN, 2013. Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes Care 36, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EL, Meier P, 1958. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association 53, 457–481. [Google Scholar]
- Kennedy AP, Epstein DH, Phillips KA, Preston KL, 2013. Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend 132, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ, 1993. Gender differences in cocaine use and treatment response. J Subst Abuse Treat 10, 63–66. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE, 2006. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology 31, 644–650. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM, 2008. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci 28, 9525–9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind KE, Gutierrez EJ, Yamamoto DJ, Regner MF, McKee SA, Tanabe J, 2017. Sex disparities in substance abuse research: Evaluating 23 years of structural neuroimaging studies. Drug Alcohol Depend 173, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME, 1999. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144, 77–82. [DOI] [PubMed] [Google Scholar]
- Martins SS, Gorelick DA, 2011. Conditional substance abuse and dependence by diagnosis of mood or anxiety disorder or schizophrenia in the U.S. population. Drug Alcohol Depend 119, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Levin D, Kozak M, Cook E, McLean A, Lang P, 1987. Individual differences in imagery and the psychophysiology of emotion. Cognitive Emotion 1, 367–390. [Google Scholar]
- Miller WR, Del Boca FK, 1994. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl 12, 112–118. [DOI] [PubMed] [Google Scholar]
- Moran LM, Kowalczyk WJ, Phillips KA, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, Preston KL, 2018. Sex differences in daily life stress and craving in opioid-dependent patients. Am J Drug Alcohol Abuse 44, 512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A, 2010. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct 214, 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolfe E, 2003. XMedCon- An open-source medical image conversion toolkit. European journal of nuclear medicine 30 (Supp.2). [Google Scholar]
- Perry CJ, Campbell EJ, Drummond KD, Lum JS, Kim JH, 2021. Sex differences in the neurochemistry of frontal cortex: Impact of early life stress. J Neurochem 157, 963–981. [DOI] [PubMed] [Google Scholar]
- Poppa T, Droutman V, Amaro H, Black D, Arnaudova I, Monterosso J, 2019. Sexual trauma history is associated with reduced orbitofrontal network strength in substance-dependent women. Neuroimage Clin 24, 101973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R, 2012. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry 169, 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Myrick H, Henderson S, McRae-Clark AL, Brady KT, 2013. Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug Alcohol Depend 131, 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, Sinha R, 2011. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry 168, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando K, Tuit K, Hannestad J, Guarnaccia J, Sinha R, 2013. Sex differences in decreased limbic and cortical grey matter volume in cocaine dependence: a voxel-based morphometric study. Addict Biol 18, 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME, 2004. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav 78, 199–207. [DOI] [PubMed] [Google Scholar]
- SAMHSA, 2021. Substance Abuse and Mental Health Services Administration. (2021). Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- Schacht JP, Anton RF, Myrick H, 2013. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R, 2011. Sex differences in neural responses to stress and alcohol context cues. Human brain mapping 32, 1998–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R, 2013. Disrupted Ventromedial Prefrontal Function, Alcohol Craving, and Subsequent Relapse Risk. JAMA Psychiatry, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Rabinowitz AG, Douglas RJ, Sinha R, 2019. Limbic response to stress linking life trauma and hypothalamus-pituitary-adrenal axis function. Psychoneuroendocrinology 99, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2008. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141, 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2009. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol 14, 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2011. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep 13, 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM, 2009. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology 34, 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ, 2011. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of general psychiatry 68, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ, 2006. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of general psychiatry 63, 324–331. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ, 2003. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 170, 62–72. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB, 1996. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend 42, 49–54. [DOI] [PubMed] [Google Scholar]
- Van Dam NT, Rando K, Potenza MN, Tuit K, Sinha R, 2014. Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry 71, 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F, 2007. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol 64, 1575–1579. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Goldstein RZ, Alia-Klein N, Wong C, 2011. Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PLoS One 6, e16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemm SE, Sinha R, 2019. Drug-induced stress responses and addiction risk and relapse. Neurobiol Stress 10, 100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Pommy JM, Adinoff B, 2016. Neural Circuitry of Impaired Emotion Regulation in Substance Use Disorders. The American journal of psychiatry 173, 344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Gao J-H, Lancaster JL, Fox PT, 1995. Clustered pixels analysis for functional MRI activation studies of the human brain.
- Yu J, Zhang S, Epstein DH, Fang Y, Shi J, Qin H, Yao S, Le Foll B, Lu L, 2007. Gender and stimulus difference in cue-induced responses in abstinent heroin users. Pharmacol Biochem Behav 86, 485–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


