Abstract
Immunoassays are a powerful tool for sensitive and quantitative analysis of a wide range of biomolecular analytes in the clinic and in research laboratories. However, enzyme-linked immunosorbent assay (ELISA)—the gold-standard assay—requires significant user intervention, time, and clinical resources, making its deployment at the point-of-care (POC) impractical. Researchers have made great strides toward democratizing access to clinical quality immunoassays at the POC and at an affordable price. In this review, we first summarize the commercially available options that offer high performance, albeit at high cost. Next, we describe strategies for the development of frugal POC assays that repurpose consumer electronics and smartphones for the quantitative detection of analytes. Finally, we discuss innovative assay formats that enable highly sensitive analysis in the field with simple instrumentation.
Keywords: diagnostics, immunoassays, affordable, frugal detectors, smartphone
INTRODUCTION
Immunoassays—bioanalytical techniques to detect specific analytes—are ubiquitous in clinical and basic research laboratories and have revolutionized how we diagnose patients, monitor health, and study disease. Immunoassays use capture reagents—typically antibodies or antigens—to detect and quantify an analyte of interest from an unknown sample based on highly sensitive and specific binding interactions between the analyte and the capture reagent. While traditional laboratory-based assays, such as the enzyme-linked immunosorbent assay (ELISA), are the workhorses of clinical laboratories, they have several shortcomings that limit their overall utility. Notably, ELISA requires multiple timed incubation steps, trained personnel, and expensive equipment, and it can incur a long delay from sample to result (1). To democratize access to high-performance clinical tests that do not need to be carried out in a centralized facility, researchers have worked on developing point-of-care (POC) immunoassays that are easy to operate without sacrificing performance.
The lateral flow immunoassay (LFIA) is possibly the most widely used and successful POC test (2). LFIAs are paper-based platforms that enable qualitative detection—in the absence of a dedicated reader—of specific antigens, antibodies, or even nucleic acids (3). To operate an LFIA, a liquid sample—normally serum, plasma, whole blood, saliva, urine, or sweat—containing an analyte of interest is added to the LFIA on the adsorbent sample pad. The sample migrates via capillary action across a conjugate release pad that contains biomolecules specific to the analyte of interest that are functionalized with a transduction element. The analyte-biomolecule complex then migrates across a porous membrane—typically nitrocellulose—to a test line containing another biorecognition element specific to the analyte that captures the complex. In the presence of the analyte of interest, a line will appear that can be assessed by the naked eye for a qualitative “yes” or “no” readout. LFIAs also contain a control line to ensure that the liquid properly travels across the strip. Recent advancements in LFIA technology have been described elsewhere (4-6), but despite these improvements, LFIAs are often not sensitive enough to detect many biomarkers in their clinically relevant range of concentration, are difficult to multiplex and are qualitative, and their visual readout can be somewhat subjective (7).
Advances in protein engineering, signal transduction, surface chemistry, microfluidics, and microfabrication have led to the development of new POC tests that rival their clinical laboratory counterparts. Furthermore, parallel advances in detectors that leverage the imaging and computational power of smartphones, three-dimensional (3D) printing, and optics now enable the rapid prototyping of new portable detectors that interface with smartphones or that are stand-alone customized devices. Their size, cost, performance, and internet connectivity promise increased accessibility at the POC in hospitals and clinics, at home, and in global health settings (8). In this article, we first discuss state-of-the-art commercial systems that often use proprietary devices for assay readout. Next, we highlight how smartphones have been integrated into POC tests to improve their performance and accessibility. We also discuss other methods for frugal POC testing based on repurposed devices. Finally, we discuss promising technologies that we believe may enable robust and decentralized testing at the POC.
COMMERCIAL IMMUNOASSAYS AND DETECTORS
POC diagnostics are a US$34 billion industry that is expected to grow to $81 billion by 2028 (9). While glucose monitoring has dominated POC testing, disease diagnostics and screening recently surpassed glucose monitoring as the fastest growing sector (9), greatly accelerated by the demand for rapid diagnostic technologies for cardiac disease, cancer—and as highlighted by the ongoing COVID-19 pandemic—infectious disease (9). Although this review largely focuses on innovative academic research in POC immunoassays, there are a growing number of commercially available devices already in use at home and in the clinic that we briefly summarize in this section.
The digital pregnancy test is perhaps the most ubiquitous commercial POC immunoassay analyzer. First introduced by Clearblue in 2003 (10), this test integrated an objective digital readout into a traditional disposable LFIA pregnancy test. Rather than the user interpreting potentially ambiguous weak intensity bands, the built-in reflectance-based optical reader in the device converts the colorimetric readout into a digital signal that is reported as a binary positive/negative result to the user via its integrated microcontroller and digital display. The test has similar sensitivity compared to traditional analog LFIAs but demonstrates superior correlation between the interpreted and expected result for both trained technicians and end-user consumers. It also scored highest in user certainty of results when compared against nondigital versions of the test (11, 12). Lumos Diagnostics has developed a single-use disposable digital LFIA reader that can report quantitative results for any assay to a user’s smartphone via Bluetooth communication (13). Taking a cue from Clearblue, other companies have developed numerous analyzers to read and interpret LFIAs to eliminate subjectivity in the assay readout (14-16). Many of these devices share common features, including compatibility with third-party LFIA cartridges; qualitative, semiquantitative, or quantitative read-outs; visual, reflective, or fluorescent imaging; a wide field of view for multiplex assays; and some degree of image processing. Although most of these readers are designed for the benchtop, some companies are developing hand-held versions that can provide results in real time to the user. Examples include the Lumos Multi-use Disposable Reader (13), NOWDiagnostics ADEXUSDx Analyzer (17), and Detekt’s RDS-2500 (18).
Clinically approved POC immunoassays are dominated by benchtop analyzers that use proprietary cartridges: Siemens’ Stratus CS Acute Care and DCA Vantage, Quidel’s Sofia and Triage systems, Mitsubishi’s Pathfast, the BD Veritor, and Philips’ Minicare. These platforms can provide results in real time, but they are typically not portable and require some amount of clinical infrastructure to operate. They have demonstrated equivalent clinical sensitivities and specificities to their centralized counterparts. However, the availability of tests for many analytes of interest is limited, making it unlikely that a clinic can rely on a single platform for all tests (19-23). Furthermore, the costs of these devices are still prohibitive for many POC applications.
The Abbott i-STAT is the most advanced POC platform of its kind that is routinely used in the clinic. It is handheld and fully portable and can detect 28 different analytes using 16 unique cartridges through electrical and electrochemical transduction schemes. The proprietary cartridges are single use and house fluidic elements, reagents, and sensors specific for the test(s) of interest. Several tests, including for cardiac (cardiac troponin I, B-type natriuretic peptide, creatine kinase myocardial band), traumatic brain injury (glial fibrillary acidic protein and ubiquitin carboxy-terminal hydrolase L1), and pregnancy (β-human chorionic gonadotropin) biomarkers all utilize affinity immunoassays (24). The platform has demonstrated strong correlation to benchtop analyzers, giving confidence in its use in a clinical setting (25). The user only needs to add 2–3 drops of blood into the cartridge before inserting it into the instrument, and the i-STAT generates a quantitative result in ~5 minutes.
While these commercially available platforms offer quantitative and sensitive performance, they are often expensive and not broadly accessible outside a clinical setting. Most require some clinical infrastructure for their operation and are more useful to decrease the time-to-result for clinicians than to increase accessibility of the tests. The exceptions are the digital pregnancy tests that do not require any support infrastructure for use, though after ~20 years since their introduction, they have remained mostly unchanged. Researchers continue to strive to develop broadly accessible POC platforms that offer the same clinical performance as these commercially available devices at a lower cost and that can operate with minimal clinical infrastructure. The next section discusses how smartphones have been used toward meeting this goal.
SMARTPHONE-ENABLED IMMUNOASSAYS
Smartphones are an appealing platform for POC diagnostics because of their ubiquity. Researchers can also profitably leverage several features of smartphones for the development of POC detectors. First, modern smartphones have high-performance complementary metal oxide semiconductor cameras that can image and interpret optical immunoassays with high sensitivity. Second, smartphone processors can quickly process image data and minimize sample processing time. Third, smartphone connectivity using Wi-Fi, Bluetooth, and cellular data allows easy of transmission of data collected at the POC data to the clinic. Fourth, they are portable because they are small and battery operated. The extensive scope of smartphones in clinical diagnostics and mobile health has been reviewed elsewhere (26-28), so we will focus our discussion here on a few recent and representative innovations.
Adapter-Less Smartphone Detectors
The simplest smartphone detectors use an alignment jig to align a smartphone camera to photograph LFIAs (Figure 1a). For example, Zangheri et al. (28a) fabricated a chemiluminescent LFIA for cortisol that is inserted into a jig and scanned by a smartphone. This system had a detection range of 0.3–60 ng/mL for cortisol in patient saliva, showed good agreement with commercial ELISA kits, and only took 30 min for readout. Roda et al. (29) used a similar platform for the semiquantitative detection of immunoglobulin A (IgA) antibodies against SARS-CoV-2 and showed that this test could detect even the early stages of COVID-19 infection.
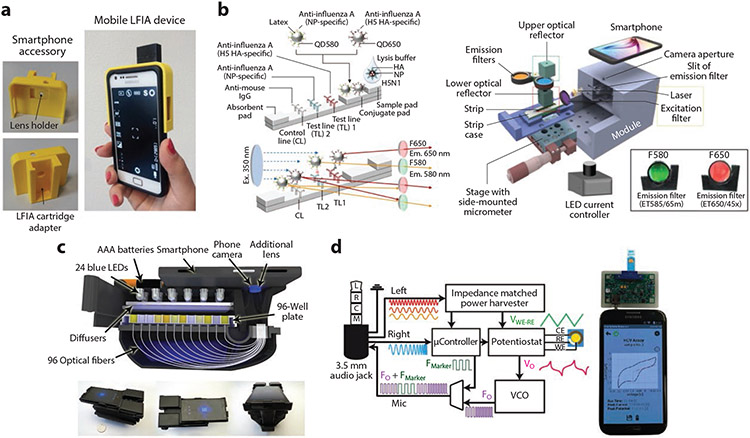
Figure 1.
(a) A 3D-printed LFIA-to-smartphone attachment for colorimetric LFIA photography. Panel adapted with permission from Reference 28a; copyright 2015 Elsevier. (b) Multiplexed fluorescence smartphone-based device for dual detection of influenza A subtypes H5 and NP. Panel adapted with permission from Reference 31 (CC BY-NC 4.0). (c) Smartphone-based ELISA colorimetric reader capable of 96 concurrent colorimetric readouts from a standard microtiter plate. Panel adapted with permission from Reference 32; copyright 2015 American Chemical Society. (d) Block diagram of a potentiostat smartphone attachment connected via the audio port, used for detection of hepatitis C antibodies. Panel adapted with permission from Reference 35; copyright 2016 Elsevier. Abbreviations: 3D, three-dimensional; C, common; CE, counter electrode; ELISA, enzyme-linked immunosorbent assay; em., emission; ex., excitation; F, frequency; HA, hemagglutinin; HCV, hepatitis C virus; IgG, immunoglobulin G; L, left; LED, light-emitting diode; LFIA, lateral flow immunoassay; M, microphone; NP, nucleoprotein; R, right; RE, reference electrode; VCO, voltage-controlled oscillator; WE, working electrode.
Fluorometric Smartphone Detectors
Fluorometric detection methods perform better than colorimetric methods because fluorescence methods are more sensitive and produce wider dynamic ranges than colorimetric methods. Furthermore, distinct fluorescent tags with narrow emission spectra that do not spectrally overlap allow multiplexed sensing, which is more difficult with colorimetric methods because of their much wider absorbance spectra. However, the main challenge with smartphone-based fluorescence detectors is their lower signal-to-noise ratios compared to larger desktop fluorescence detectors due to their smaller pixel size and the need to operate at ambient temperature, which introduces greater thermal noise compared to the cooled detectors that can be used in desktop detectors. There are several approaches to get around these problems and improve smartphone-based detector signal-to-noise performance. Ratiometric fluorescence detection—one such approach—measures the ratio between two probe intensities for self-calibration, which reduces the impact of environmental noise. Quantum dots, which are brighter than organic small-molecule fluorophores, are alternative fluorescent tags to improve signal intensity. Finally, clever optical design can further enhance performance by minimizing environmental noise and maximizing assay light collection.
Wang and colleagues (30) developed an LFIA system for POC testing of acute myocardial infarction using ratiometric fluorescence measurement. Their LFIA incorporated a mixture of composite nanostructures conjugated to capture and probe antibodies that produce two distinct emission bands. These bands are filtered by a long-pass (>500 nm) optical filter and imaged by the smartphone camera. The camera’s internal Bayer filter separates the colors into RGB values, and the relative hue ratio between the emission bands is correlated with the concentration of the analyte of interest (30). POC multiplex fluorescence detection was also demonstrated by Yeo et al. (31). Their system used a dual quantum dot influenza A subtyping LFIA inserted into a 3D-printed housing with an ultraviolet (UV) excitation laser and two toggleable optical filters tuned to the individual quantum dot emission bands. The device also incorporated custom software for image processing/readout and had step-by-step instructions programmed on the smartphone touchscreen. When tested on 14 H5N1-positive patients, the system yielded a receiver-operator curve with an area under the curve of 0.96 for influenza A and 0.89 for influenza H5 (Figure 1b). Berg et al. (32) developed a high-throughput smartphone microplate reader for POC ELISA quantification, which uses an optical fiber bundle to couple individual wells of a microplate sample to the smartphone camera lens. This allowed a readout of 96 wells simultaneously, and the results could then be transmitted via custom software to a data server (Figure 1c).
Smartphone-Integrated Electrochemical Biosensing
Electrochemical methods for immunoassay readout are a developing field in POC diagnostics (33, 34). Electrochemical immunoassays are attractive as they can enable real-time test readout. Smartphones can be modified to power electrochemical sensing circuits, process data, provide rapid readout, and transmit these results wirelessly. Custom interfaces have been developed between smartphones and electrochemical sensors, such as through the smartphone audio port or by near-field communication (NFC). Aronoff-Spencer et al. (35) developed an electrochemical immunoassay for the hepatitis C antibody, which was read using a custom potentiostat attachment connected to smartphone through the smartphone’s audio port (Figure 1d). The audio port provided channels that powered the electrochemical attachment, sent commands to the attachment’s microcontroller, and received data back from the attachment. This system exhibited a limit-of-detection (LOD) of 2 nM with a dynamic range of 4–250 nM against mouse anti-HCV antibody. Cheng et al. (36) developed a flexible immunosensor patch that used a smartphone’s NFC module to detect cortisol. The patch was designed with a cortisol electrode functionalized with anticortisol Abs on a polyethylene glycol (PEG)-coated surface above a layer of gold nanoparticles, an NFC chip for power and data transmission, a microcontroller for coordinating differential pulse voltammetry, and a potentiostat for current readout. By using NFC, users can simply hover a smartphone over the patch to read their cortisol levels without further intervention. This sensor had an LOD of ~7 nanomolar, which is several orders of magnitude worse than commercial ELISA kits that have an LOD of a ~0.3 picomolar. Further, correlation between both methods was poor, though trends were observed. Nevertheless, this system is a proof-of-concept of the utility of the NFC module for untethered personal diagnostics.
Limitations of Smartphone-Based Detection
There are several limitations to using smartphones as a POC detector. All the approaches described in this section are at a proof-of-concept stage and were designed to work with specific smartphones; none have been converted to products capable of widespread adoption. Validation studies were small and further clinical validation with larger patient populations is needed. Smartphone connectivity was rarely utilized to its full potential in these studies and they do not adequately address how to securely handle patient data at the POC. Transduction-specific problems exist as well. Smartphone color cameras have a lower quantum efficiency than monochrome camera sensors for optical detection. The Bayer color filters in smartphone cameras reduce light transmittance up to 66%, which dims the image, causing a loss of analytical sensitivity (37). Electrochemical methods are early in development, and have issues of stability, storage, and running minimally treated/diluted samples. Despite these limitations, these publications present valuable insights into smartphone-based technologies that may be useful outside the scope of POC diagnostics. Further development is needed, ideally in collaboration with smartphone manufacturers, to realize a future where smartphones become a commonplace diagnostic tool. In the meanwhile, alternative transduction methods for POC sensing are under development and are discussed in the next section.
FRUGAL DETECTORS AND SCANNERS
Although most researchers rely on commercially available or custom-built detectors to quantify and analyze their POC tests, others have cleverly opted for the more unconventional approach of repurposing consumer electronics and sensors to transduce and report the results of their tests. These do-it-yourself frugal detectors are typically easily accessible, are inexpensive, and require minimal to no modification. This eliminates the need for independent development of a stand-alone detector while maintaining many of the benefits that more specialized commercial detectors/readers offer. In this section, we discuss some specific examples of how this strategy has been adapted to carry out immunoassays at the POC.
Flatbed Scanners
An early example of using consumer electronics for affordable detection of immunoassays was the use of a flatbed document scanner as an optical transducer. Flatbed scanners offer a wide field of view (~600 mm2), modest depth of view (depending on the model), and reasonable spatial resolution (~10 μm), and they can scan in full color. Göröcs & Ozcan (38) wrote an early review of these technologies highlighting their use for immunoassays and other biomedical applications. Typically, these scanners are used as a more affordable version of the LFIA readers discussed earlier to increase sensitivity, enable quantification of the LFIA, and improve objectivity by removing human error in interpretation of LFIAs (39, 40). Göröcs et al. (41) added an array of oblique angled green light-emitting deodes (LEDs) and an emission filter to enable fluorescent imaging with a flatbed scanner. Aygun et al. (42) converted a flatbed scanner into a interferometric reflectance imaging sensor for DNA and protein microarrays by simply adding a small wedge to create an oblique angle in the scan head, allowing the sensor to collect reflected light from the microarray (Figure 2a).
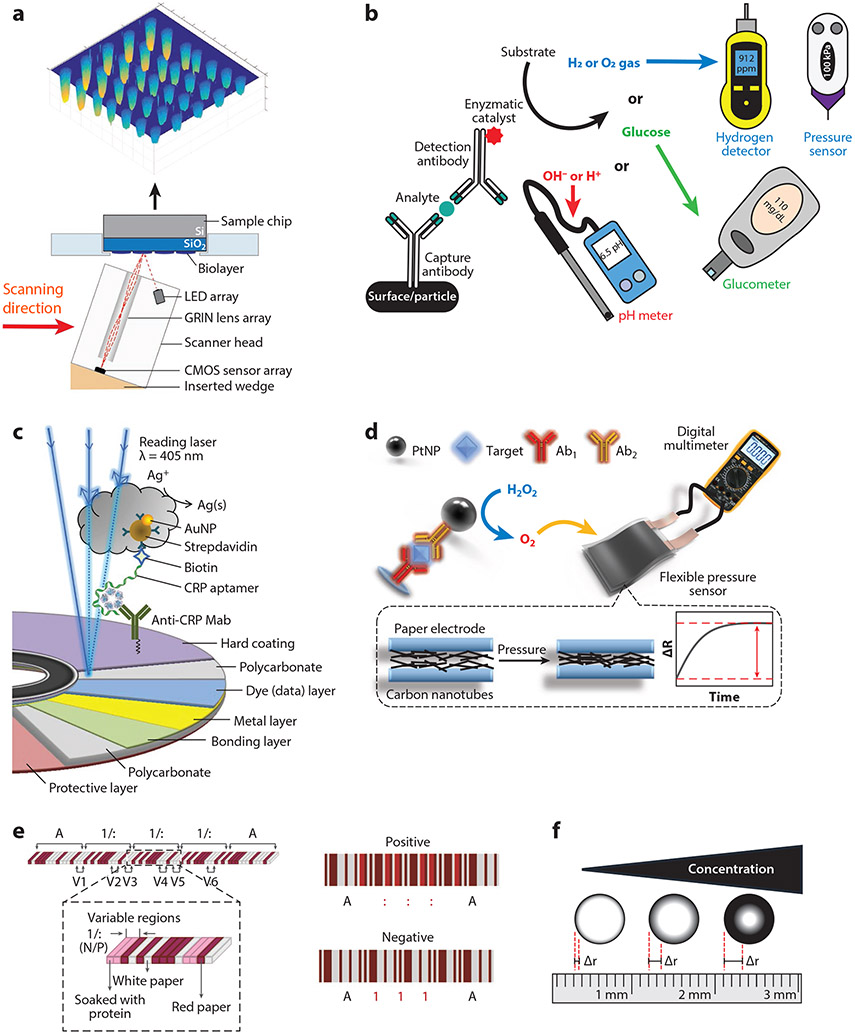
Figure 2.
Representative schematics that describe various frugal detection platforms built from repurposed consumer electronics. (a) A flatbed scanner modified with a 3D-printed wedge that converts the scanner into an IRIS. Panel adapted with permission from Reference 42; copyright 2017 American Chemical Society. (b) Simplified schematic of the enzymatic process flow utilized for several platforms. These strategies typically use an enzymatic labeled antibody to convert a substrate into a product and byproduct. Either the product itself is measured (e.g., by a glucometer) or the by-product is measured by a hydrogen detector, pressure sensor, or pH meter. (c) Blu-ray disc drive and immunoassay-modified disc is used to detect CRP antigen by using silver staining amplification to create read errors in the disc. The density of the errors correlates with analyte concentration. Panel adapted with permission from Reference 56; copyright 2020 American Chemical Society. (d) A sandwich immunoassay using a PtNP-conjugated antibody that enzymatically catalyzes the decomposition of hydrogen peroxide into H2O and O2. The resulting increase of pressure compresses a carbon nanotube situated between two electrodes, modulating the measured resistance when read by a multimeter. Panel adapted with permission from Reference 57; copyright 2019 American Chemical Society. (e) An LFIA design for multiplexed analyte detection that is read by a standard barcode scanner. Dyed, protein modified, and unmodified chromatography paper is stacked and skived to create a barcode. Individual bars corresponding to the protein modified layers change color depending on the presence or absence of a specific analyte of interest, changing how the barcode scanner interprets the test. Panel adapted with permission from Reference 62 (CC BY-NC 4.0). (f) A paper-based immunoassay that can be visually quantified with a ruler by evaluating the difference in diameter of the resulting coffee ring transport of a reporter dye. Abbreviations: 3D, three-dimensional; Ab, antibody; AuNP, gold nanoparticle; CMOS, complementary metal-oxide-semiconductor; CRP, C-reactive protein; dAb, detection antibody; GRIN, ; gradient index; IRIS, interferometric reflectance imaging sensor; LED, light-emitting diode; LFIA, lateral flow immunoassay; mAb, monoclonal antibody; PtNP, platinum nanoparticle.
Personal Glucose Monitors
Another strategy is to repurpose existing medical biosensors for new applications outside the scope of their originally designed purpose. Repurposing personal glucose monitors—which are affordable and widely available at most pharmacies and drug stores—is the most notable example of this strategy. Modern personal glucose monitors electrochemically measure the conversion of a redox probe (such as ferro/ferricyanide) catalyzed by the oxidation of glucose by an enzyme (glucose oxidase, glucose dehydrogenase, etc.) that generate electrons that can be measured as a current in an electrochemical cell. Immunoassays can be carried out using personal glucose monitors by introducing an antibody conjugated to an enzyme (i.e., invertase, glucoamylase that convert sugars such as sucrose into glucose) (Figure 2b). The antibody-enzyme conjugate is integrated into a sandwich or competition immunoassay. This strategy has the added benefit of amplifying the electrochemical signal through the enzymatic turnover of the enzyme that generates a micromolar concentration of glucose from a picomolar concentration of captured target. The glucose then reacts with the reagents preloaded onto a commercial glucose test strip, generating a response on the reader that is directly correlated to the concentration of the analyte (43, 44).
Recent work has focused on the use of nanoparticles to increase the density of the enzymes per biorecognition interaction. This has demonstrated high-sensitivity detection of hepatitis B surface antigens with an LOD of 0.4 ng/mL using an Al2O3 nanocarrier (45) and of prostate-specific antigen with an LOD of 0.1 pg/mL using rolling circle amplification to facilitate the hybridization of DNA-conjugated invertase onto gold nanoparticles, increasing the enzyme density per immunoreaction (46). Another strategy is to encapsulate glucose directly into a nanocarrier and use that as the labeled reagent, bypassing the need for an enzyme that catalyzes the production of glucose. Alshawawreh et al. (47) used glucose encapsulated liposomes with conjugated detection antibodies to detect procalcitonin with an LOD of 0.15 nM in whole blood.
pH Meters
pH meters have been converted into detectors for immunoassays (Figure 2b). The working principle is to leverage the modulation of H+ ions from an enzymatic reaction as the detection signal. The influx of H+ modulates the pH of the bulk solution, which can be measured quantitatively with a digital pH meter, or even semiquantitatively with pH test strips (48). Most examples utilize glucose oxidase (GOx)-labeled antibodies conjugated to microparticles (49, 50) or encapsulated within hollow microspheres or liposomes (51). Sun et al. (52) hypothesized that the conjugation of enzymes to nanomaterials may decrease catalytic activity of the enzyme, and they hence synthesized a cross-linked urease nanoparticle made entirely of the enzyme. This probe was conjugated directly to a detection antibody with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride/ n-hydroxysuccinimide (EDC/NHS) chemistry. They demonstrated a highly sensitive LOD of 5.2 pg/mL for detection of lipocalin-2 with no cross-reactivity with other common serum biomarkers such as neuron-specific enolase, alpha-fetoprotein, carcinoembryonic antigen, calcitonin, prostate-specific antigen, and human immunoglobulin using this approach (52).
Disc Drives
At their peak in 2012–2016, blu-ray disc players could be found in ~73% of US homes (53). The platform built upon the previously successful and technologically similar DVD and CD technologies. The large R&D investment into the development of disc technology provided an extremely high-performance optical transducer at an affordable price that has been repurposed for signal transduction in biosensors (54). Zhang et al. (55) used an unmodified blu-ray disc drive and off-the-shelf blu-ray discs retrofitted with polydimethylsiloxane (PDMS) microchannels as an immunosensor. The PDMS microchannels allowed for fluid control during surface functionalization and sample addition, as well as multiplexing of the assay (56). The disc surface was modified with NaOH to create carboxylic acid groups enabling conjugation of capture antibody by EDC/NHS chemistry. Gold nanoparticle–conjugated detection antibodies were used as the label to facilitate a silver staining amplification step in a competition format. The addition of Ag+ to the assay allowed the gold nanoparticles to reduce the Ag+ into Ag/Ag2O clusters at the assay location and interrupt the ability of the disc drive to read the data on the disc. Using a third party disc quality software program, Zhang et al. quantified the distribution of error codes and observed that they directly mapped to physical locations on the disc and that the density of those errors directly correlated with the analyte concentration (Figure 2c). With this method, they detected food toxin aflatoxin B1 in corn samples with an LOD of 0.27 ng/mL and a dynamic range of 0.5–100 ng/mL.
Multimeters
Tang and coworkers (57-59) have repurposed off-the-shelf digital multimeters for immunoassays. Their platform consists of a reaction chamber where a sandwich immunoreaction takes place that uses platinum nanoparticle (PtNP)-conjugated detection antibodies. When hydrogen peroxide is added to the reaction chamber, the PtNPs catalyze its decomposition, generating oxygen gas. The gas then enters a pressure-sensitive chamber that transduces the amount of oxygen produced—which is directly related to amount of antigen binding—into electrical resistance that can be measured by the multimeter (Figure 2b). The pressure sensor consists of two flexible electrodes with a pressure-sensitive dielectric between them that modulates the effective resistance between the electrodes. They used carbon nanotubes that contract (57), conductive polypyrrole/PDMS films in a sawtooth pattern that increase surface contact area (58), and 3D polypyrrole foams that condense as pressure-sensitive transducers (59). All three transducers demonstrated sensitive detection of carcinoembryonic antigen, with LODs ranging from 0.08 to 0.17 ng/mL.
Gas and pressure sensors
Rather than converting gas pressure into electrical resistance change like Tang and coworkers, other researchers have directly used commercial pressure sensors to measure pressure changes as a function of analyte binding (Figure 2b). Zhu et al. (60) used the same PtNP-conjugated detection antibody strategy in a sandwich assay to convert H2O2 into H2O and O2 gas, and the production of O2 was measured with a pressure sensor. They used this format to detect prostate-specific antigen with an LOD of 1.42 picomolar in human serum. Bu et al. (61) used gas production to transduce the affinity capture of Escherichia coli O157:H7 using a hydrogen gas sensor. In this scheme, polyclonal antibodies against the E. coli were immobilized on magnetic beads and used to capture E. coli cells. A synthetic hybrid nanocomposite containing an antimicrobial peptide and PtNPs served as the detection reagent. The peptide specifically binds to the E. coli while the PtNPs act as a catalyst for the hydrolysis of ammonia borane—generating hydrogen gas that is measured directly with a hydrogen meter (Figure 2b). This format could detect E. coli 0157:H7 with an LOD of 10 cfu/mL. The sensor is somewhat expensive (~US$800), but it highlights the ability to repurpose commercially available instruments for highly sensitive and quantitative measurements that can be carried out in the field (60, 61).
Barcode scanner
Yang et al. (62, 63) used a barcode scanner used in grocery store checkouts as a detector of multiplexed immunoassays that they named paper-based barcode chips. A barcode scanner works by converting a series of parallel lines of varied width and spacing into a string of alpha-numeric characters. In this study, sheets of chromatography paper were either dyed red, immobilized with a capture antibody, or left unmodified. These sheets were then stacked and glued on top of each other in a specific order corresponding to the standardized Codabar code. The stack was then skived into thin slices such that the resulting barcode was viewable from above. The assay is operated like a standard LFIA. After the assay is run, the red and white layers will remain unchanged, but the protein immobilized layers can change color from white to red depending on the presence of a target analyte. When the barcode scanner attempts to read the code, the interpreted alpha-numeric string will depend on the color of the immunoassay bar and adjacent static bars. In this way multiple assays can be strung together sequentially to create an easily readable multiplexed test (Figure 2e). They demonstrated comparable sensitivities to commercially available LFIAs but with the advantage that this assay is less subjective than the visual readout of an LFIA and is easy to use
Ruler
Zhang et al. (64) created a paper-based ELISA platform that can be quantified visually with only a standard ruler. In this study, human IgG was adsorbed onto starch-iodide paper. GOx-conjugated antihuman IgG antibody was incubated on the paper. After washing the paper, glucose was added, and the bound GOx catalyzed the oxidation of glucose to produce H2O2 that oxidized the iodide in the paper, creating an intense blue triiodide anion complex. As the substrate dried, the test leveraged evaporation-driven flow or coffee-ring transport of the complex to the test’s edge. Zhang et al. found that the difference in radius of the inner and outer rings were linearly correlated to the logarithmically transformed concentration of human IgG (64) (Figure 2f). This is a creative example of a POC test that enables quantitation of a visual colorimetric assay at little additional cost.
Though these frugal devices are attractive for their potential ease of adoption and low-cost quantitation attributes, most of the examples discussed in this section are at a proof-of-concept stage. There are also several practical concerns that need to be addressed to make these devices ready for implementation at the POC, such as their reproducibility, shelf-life, and resilience to environmental conditions that may fluctuate at the POC. However, these technologies present intriguing alternatives to the custom detector approach to quantitative immunoassays that currently dominate the diagnostics landscape. They also highlight the notion that innovations in assay design rather than device or detector design can transform an assay into a POC test. In the next section, we further explore this concept of assay-level innovations toward enabling POC immunoassays.
PROMISING TECHNOLOGIES
In this section, we highlight several POC platforms that we believe are promising technologies for robust and decentralized testing. Rather than collating an exhaustive list of POC tests, we limit our discussion to those that we believe have distinct benefits in terms of ease-of-use, ease-of-readout, multiplexing capacity, and analytical sensitivity.
Instrument-Free Immunoassays
As an alternative to LFIAs, Qin and coworkers (65) developed a volumetric bar-chart chip (V-Chip) to detect protein biomarkers visually without an external device for read out. The V-Chip uses a sandwich ELISA format where silica nanoparticles are functionalized with detection antibodies and conjugated to catalase. In the presence of a biomarker of interest, a sandwich complex is formed and hydrogen peroxide—introduced into the system via a sliding mechanism—reacts with the catalase to release oxygen which then displaces a red dye. The distance the dye travels is directly proportional to the biomarker concentration (Figure 3). In their initial study, Song et al. (65) used the V-Chip for quantitative and multiplexed detection of human chorionic gonadotropin carcinoembryonic antigen, estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2. However, the analytical sensitivity of the initial design of the V-Chip was worse than clinical instruments. Subsequently, Li et al. (66) modified the assay to include a real-time internal control by introducing a competition scheme between the sample of interest and a loading control of known concentration. Additional advancements include the use of more sensitive detection reagents, including horseradish peroxidase and platinum nanoparticle probes (17, 18, 67, 68), digital readout for improved precision (68), automated fluid handling (67), and on-chip blood separation (18). These advancements improved the LOD to the low picomolar regime for some V-Chip assays. The modular nature of the platform also enables detection of small molecules by a competition assay format (18). Collectively, the V-Chip is one of the most promising platforms for instrument-free POC testing, as it is low cost, rapid, and quantitative, and can be multiplexed. However, the V-Chip still requires multiple timed incubation steps, reagents must be stored at 4°C prior to testing, its sensitivity is insufficient for some biomarkers, and the assay readout is temperature-dependent.
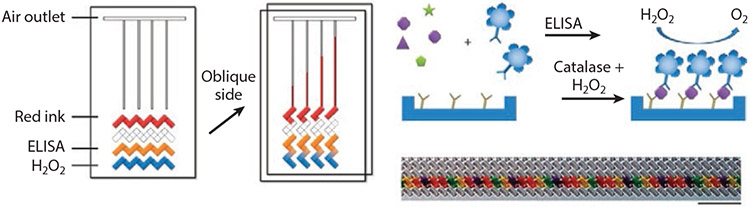
Figure 3.
Volumetric bar-chart chip (V-Chip) preloaded with red dye, antibodies for enzyme-linked immunosorbent assay (ELISA), and H2O2. Oblique sliding of the top plate forms isolated the channels, allowing the reaction between catalase and H2O2 to proceed that generates oxygen that displaces dye through the channel to a distance proportional to the concentration of the biomarker of interest. The V-Chip can be multiplexed by loading up to 50 different antibodies. Figure adapted with permission from Reference 65; copyright 2012 Nature Publishing Group.
Bioluminescence Homogeneous Immunoassays
Homogeneous immunoassays are another promising approach for POC testing, as they can be performed directly in solution and do not require multiple incubation or washing steps. In particular, bioluminescence-based sensors using either bioluminescence resonance energy transfer (BRET) or split luciferase strategies have been successfully used for the detection of small molecules, antigens, and antibodies. While other homogenous immunoassays using surface-enhanced Raman scattering nanotags (69), fluorescence (70-72), or inductively coupled plasma mass spectrometry (73) have been developed, we focus on bioluminescence-based assays in this section, as they do not require an external excitation source and are therefore more amenable to POC testing.
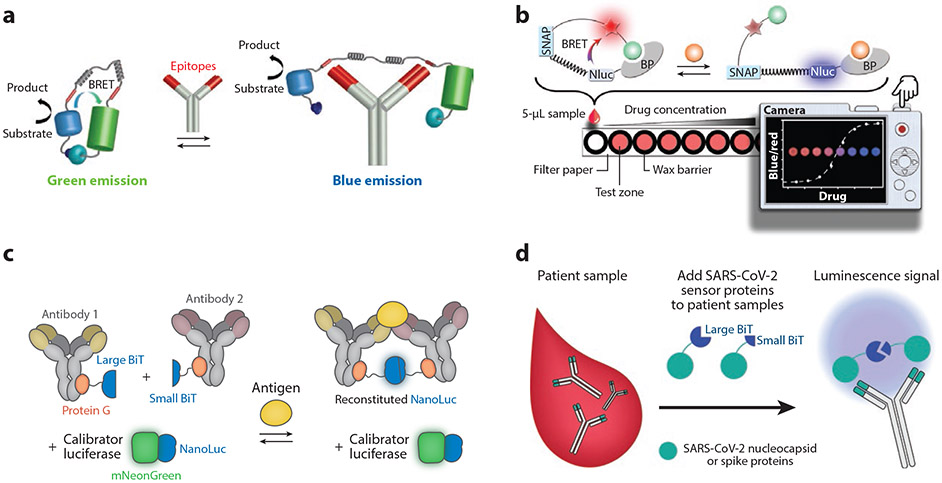
Arts et al. (74) developed LUMABS (luminescent antibody sensor), which consists of a blue-emitting luciferase—NanoLuc—fused to mNeonGreen via a semiflexible peptide linker with peptide epitopes for the antibody of interest flanking each linker (Figure 4a). In the absence of the antibody of interest, NanoLuc and mNeonGreen remain in close proximity, enabling efficient BRET, which leads to green emission. When the antibody of interest binds to each peptide epitope, the BRET efficiency decreases such that the emission signal changes from green to blue, and this shift in the fluorescence emission wavelength is a function of the antibody concentration. LUMABS was used with a smartphone camera and a customized Android app to detect antibody biomarkers of HIV and dengue virus type 1 directly from undiluted plasma with a pM LOD. LUMABS assays have also been developed to detect therapeutic monoclonal antibodies from plasma for drug monitoring (75). Subsequently, Tenda et al. (76) transformed LUMABS into a fully integrated sample-in-signal-out microfluidic paper-based analytical device, and they demonstrated multiplexed detection of anti-HIV1p17, anti-HA, and anti-dengue antibodies by a spatially discrete LUMABS assay that was imaged by a digital camera.
Figure 4.
Bioluminescence immunoassays. (a) Luminescent antibody sensor (LUMABS) assay. In the absence of the antibody of interest, NanoLuc and mNeonGreen are in close proximity, enabling efficient bioluminescence resonance energy transfer (BRET). Bivalent binding of the antibody of interest to the epitopes (red) in the semiflexible linker pulls the BRET partners apart, changing the color of emission from green to blue. Panel adapted with permission from Reference 74; copyright 2016 American Chemical Society. (b) A luciferase-based indicators of drugs (LUCID) sensor, composed of a SNAP-tag, NanoLuc (Nluc), and a binding protein (BP). The SNAP-tag is labeled with a molecule containing a fluorophore (red star) and a ligand (green ball) that can bind to the BP. The analyte of interest displaces the ligand, changing emission from red to blue, which is imaged by a digital camera. Panel adapted with permission from Reference 77; copyright 2017 John Wiley and Sons. (c) The ratiometric plug-and-play immunodiagnostics (RAPPID) platform. A pair of antibodies for an antigen of interest are functionalized with a split version of NanoLuc (large BiT and small BiT) via protein G–based photoconjugation. Antigen binding brings the antibody pair into proximity, resulting in the reconstitution of NanoLuc and emission of blue light after addition of the substrate. Panel adapted with permission from Reference 82 (CC BY-NC 4.0). (d) Split luciferase (spLUC) assay for anti-SARS-CoV-2 antibody detection. Antibodies are incubated with SARS-CoV-2 spike or nucleocapsid proteins fused to large BiT and small BiT. Approximately half of the antibodies will bind to the large BiT fusion with one arm and the small BiT fusion with the other, reconstituting active NanoLuc, which generates luminescence.
Similarly, Johnsson and coworkers (77) have developed LUCID (luciferase-based indicators of drugs) sensors that consist of NanoLuc fused to an antigen-binding fragment of an antibody, and a SNAP-tag containing a fluorophore for BRET linked with the analyte of interest (Figure 4b). The LUCID assay has been implemented in a competition format to detect methotrexate, theophylline, and quinine. Because these analytes are small molecules, they only have a single antibody-binding epitope and are hence not amenable to detection by a sandwich assay but can be detected by a competition assay. Johnsson and coworkers fabricated LUCID assays using lyophilized reagents on a paper-based device that was imaged with a low-cost digital camera for quantification of drug levels in serum and blood. Shortly thereafter, Merkx and coworkers (78) modified the LUMABS platform by introducing para-azidophenylalanine as a bio-orthogonal chemical handle to conjugate synthetic epitopes via strain-promoted azide–alkyne click chemistry. This allowed them to perform a similar competition assay as a LUCID to detect creatinine, a small molecule, with an LOD of 200 μM from diluted plasma.
Ni et al. (79) developed a modified LUMABS platform based on competitive intramolecular complementation of split NanoLuc (NB-LUMABS). The NB-LUMABS consists of a large fragment of NanoLuc fused to two small fragments—one of which is conjugated with a red fluorophore—enabling efficient BRET in the absence of the antibody of interest. Binding of the antibody of interest to the epitopes in the semiflexible linker disrupts the interaction with the small fragment of NanoLuc conjugated to the red fluorophore, changing the fluorescence emission from red to blue. They used NB-LUMABS to detect anti-HIV1-p17 and cetuximab antibodies in undiluted plasma using a digital camera. Importantly, the authors demonstrated that the dynamic range of the system can be adjusted by tuning the affinity between the large and small fragments of NanoLuc (80).
Hall et al. (81) developed a shelf-stable bioluminescence-based immunoassay where NanoLuc was split into three fragments: a large 18-kDa polypeptide and two smaller peptide chains. When all three fragments are in close proximity, they assemble to reconstitute active luciferase that catalyzes the production of bioluminescence from a substrate. To develop an immunoassay, they attached each peptide fragment to a capture and detection antibody for interleukin-6 (IL-6). In the presence of IL-6, a sandwich complex is formed, bringing the peptide chains into close proximity, and the reconstituted luciferase then catalyzes the generation of luminescence upon the addition of furimazine substrate. They also showed that all assay components could be lyophilized and reconstituted without drastically impacting the performance of the sensor, suggesting its potential utility for POC applications. Ni et al. (82) demonstrated a similar platform, named the ratiometric plug-and-play immunodiagnostics (RAPPID) platform, based on the complementation of two fragments of NanoLuc that are attached to two antibodies that have different binding epitopes for the same analyte (Figure 4c). This system was used to detect C-reactive protein, therapeutic monoclonal antibodies, and SARS-CoV-2 antigen and antibodies. A key innovation of this platform is the inclusion of an internal calibrator luciferase with a spectrally distinct emission profile that ensures that the ratiometric output signal is stable over time and less susceptible to experimental variation.
Staglijar and coworkers (83) developed a similar platform, called serological assay based on split tripart nanoluciferase (SATiN), to detect anti-SARS-CoV-2 antibodies against the spike protein. Here, NanoLuc is split into three fragments: two short peptides (β9 and β10 each containing 11 amino acids) and a 16-kDa fragment (Δ11S) (84). The spike protein was conjugated to the β10 peptide fragment, and protein G was conjugated to the β9 peptide. In the presence of antispike protein antibody and the Δ11S fragment, the activity of NanoLuc is regained, which generates a luminescence signal. The luminescence signal of the SATiN assay was highly correlated with a conventional ELISA; however, samples needed to be diluted to avoid saturation of protein G by nonspecific immunoglobulins. The same strategy was used by Kim et al. (85) to detect anti-tumor necrosis factor (TNF) therapeutic antibodies from serum, except that they used protein A to bind to the antibody of interest. Elledge et al. (86) designed a similar assay where NanoLuc was split into two fragments, each of which were fused to a viral SARS-CoV-2 antigen. In the presence of anti-SARS-CoV-2 antibodies—which are bivalent—the fragments are brought into proximity, activating NanoLuc and generating luminescence (Figure 4d). This assay had an overall specificity of 100% (for spike protein) and 99% (for nucleocapsid protein) and a sensitivity of 89% (for spike protein) and 98% (for nucleocapsid protein). Importantly, they demonstrated that the protein components can be lyophilized for ambient temperature storage, and the test can be carried out in 30 min or less, can be read with a battery-supported portable luminometer, and is compatible with whole blood and saliva. Collectively, bioluminescence-based immunoassay platforms are promising for POC testing; however, multiplexing remains challenging due to a limited number of orthogonal readouts. This limitation is significant because detecting multiple biomarkers simultaneously is important for diagnosing and monitoring many diseases (87-89).
Electrochemical Immunoassays
Electrochemical immunoassays are attractive for POC tests due to the simplicity, low-cost, and ability to miniaturize a potentiostat for detection. There are numerous ways to transduce the signal in these assays: DC or AC transduction, faradaic or nonfaradaic, or labeled or label-free, but the general working principle is the same for all configurations where immunoreaction binding events modulate the output impedance, current, or potential at an electrode interface that can be directly correlated to the concentration of an analyte. Because of the simplicity of the device and noninvasive nature of the measurement when the analyte is sampled from sweat or wound exudate, these assays can be designed as wearables (90), allowing for the exciting possibility of monitoring analyte levels continuously to provide dynamic real-time data to the user (91, 92). As other reviews have covered these affinity electrochemical immunosensors in detail (90, 93-96), in this section, we highlight several recent advancements toward improved POC electrochemical immunoassays.
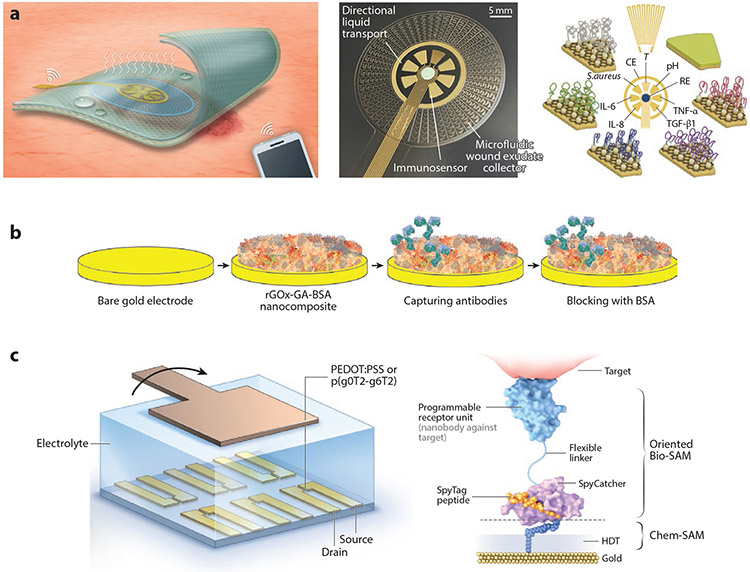
Gao et al. (97) demonstrated pseudocontinuous monitoring with their VeCare platform, a sensor-integrated bandage to monitor wound healing. The multiplexed sensor reports levels of inflammatory markers [tumor necrosis factor alpha (TNF-α), IL-6, IL-8), growth factors [transforming growth factor beta 1 (TGF-β1)], infection (Staphylococcus aureus), and physiological status (temperature and pH) from wound exudate (Figure 5a). The sensor can be seamlessly integrated into wound care procedures, giving the user updated levels of the biomarker and physiological parameters as often as the bandage is changed. The analyzer itself is wearable and can be interfaced directly with each new bandage that reports data to the user’s phone via Bluetooth. Gao et al. demonstrated good correlation between biomarker levels and wound size and healing time and successfully diagnosed infection of the wound and the effectiveness of antiseptic and antibiotic treatment.
Figure 5.
(a) Multiplexed electrochemical biosensor that directly interfaces with patient bandage for quasi-continuous monitoring of biomarkers, infection, and physiological status of the wound exudate. Panel adapted with permission from Reference 97 (CC BY-NC 4.0). (b) Schematic of a conductive nonfouling surfaces for improved electron transfer while preventing nonspecific binding an electrochemical sensor. Panel adapted with permission from Reference 99; copyright 2021 John Wiley and Sons. (c) Nanobody-conjugated organic electrochemical transistor for single-molecule quantification of SARS-CoV-2 and MERS-CoV spike antigens. Panel adapted with permission from Reference 102; copyright 2021 Nature Publishing Group. Abbreviations: BSA, bovine serum albumin; CE, counter electrode; GA, glutaraldehyde; HDT, 1,6-hexanedithiol; IL, interleukin; PEDOT:PSS, poly(3,4-ethylenedioxythiophene) polystyrene sulfonate; RE, reference electrode; rGOx, reduced graphene oxide nanoflake; SAM, self-assembled monolayer; T, temperature; TGF-β1, transforming growth factor-beta 1; TNF-α, tumor necrosis factor-alpha.
Guo et al. (102) engineered a nanobody-functionalized organic electrochemical transistor that allows single-molecule quantification of SARS-CoV-2 and MERS-CoV spike antigen from only 5 μL of unprocessed human saliva, serum, and nasopharyngeal swab in 15 min. The nanobody was specifically engineered with a flexible linker that allows it to bind with high density and controlled orientation, improving the biorecognition layer of the sensor (Figure 5c). This platform uses an organic electrochemical transistor that is specially designed to operate in accumulation mode versus the more common depletion mode. This improves device stability, requires less power to operate, and makes it compatible with portable integrated electronic readers. The authors attribute their sensors’ superior performance to the combination of these innovations.
D4 Immunoassays
Array-based systems are popular for clinical diagnostics, especially for multiplexed applications. The evolution has been spurred by development in microcontact or noncontact printing to fabricate the high-density arrays of biomolecules. Because these fabrication techniques have been reviewed elsewhere (103, 104), in this section, we highlight our group’s contribution to microarrays for POC diagnostics.
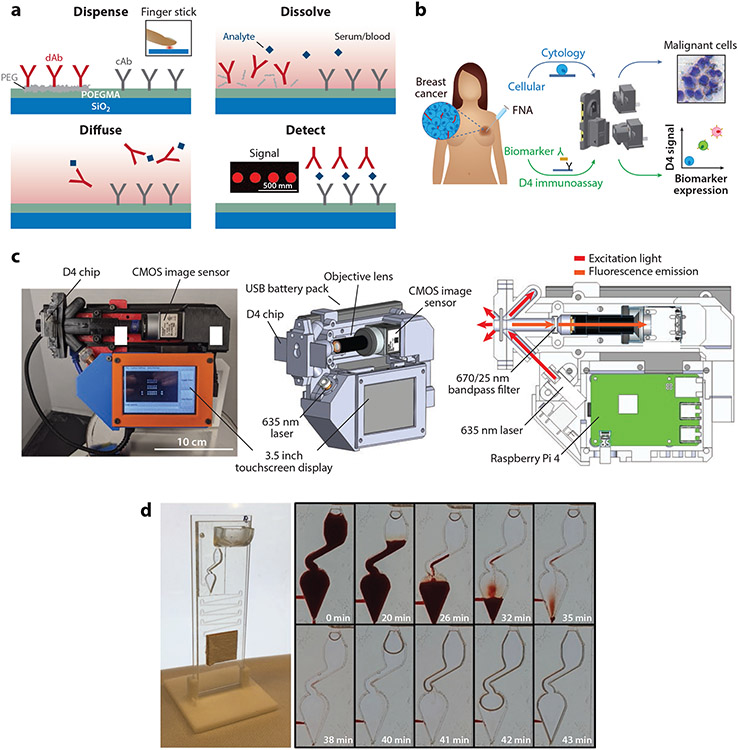
Our group has developed a microarray-based immunoassay, termed the D4, to quantify protein biomarkers directly from undiluted whole blood or serum (105). The D4 is built upon a nonfouling polymer brush composed of poly(oligo(ethylene glycol) methyl ether methacrylate) (POEGMA), which eliminates nearly all nonspecific protein adsorption and cellular adhesion (106-110), leading to high signal-to-noise ratios. The POEGMA brush contains all biomolecules necessary to complete the assay, such that when a liquid sample is dispensed onto the surface, the fluorescently labeled detection antibody dissolves from the brush, diffuses in solution, and forms a sandwich complex with the capture antibody and analyte of interest. The resulting sandwich can then be imaged with a fluorescence detector, and the fluorescence intensity scales with analyte concentration (Figure 6a).
Figure 6.
D4 immunoassays. (a) Schematic of the D4 immunoassay. A POEGMA brush is grown on a glass slide, followed by deposition of capture antibodies into the brush and fluorescently labeled detection antibodies on an excipient pad by inkjet printing. When a sample is dispensed onto the assay, the detection antibodies-fluorophore conjugates are liberated into solution due to dissolution of the underlying excipient pad, and the analyte and detection antibody-fluorophore conjugate diffuse across the brush. This leads to the formation of a sandwich complex at the spots where the capture antibody is printed. The fluorescence intensity of the complex is measured by a hand-held fluorescence detector, the D4Scope. Adapted with permission from Reference 105; copyright 2017 PNAS. (b) Schematic of the EpiView-D4 platform for detection of breast cancer biomarkers and imaging of fine needle aspirates from tumors. Panel adapted with permission from Reference 111 (CC BY-NC 4.0). (c) D4Scope image (left), 3D model of the D4Scope with key components labeled (middle), and optical illumination scheme (right). Panel adapted with permission from Reference 112 (CC BY-NC 4.0). (d) Microfluidic D4 cassette processing whole blood samples, with time lapse shown. Panel adapted with permission from Reference 115 (CC BY-NC 4.0). Abbreviations: cAb, capture antibody; CMOS, complementary metal-oxide-semiconductor; dAb, detection antibody; FNA, fine needle aspiration; PEG, polyethylene glycol; POEGMA, poly(oligo(ethylene glycol) methyl ether methacrylate).
In our initial study, we developed a D4 immunoassay to detect leptin—a biomarker of malnutrition—and established concordance with traditional laboratory-based ELISA. We obtained a LOD of roughly 40 pg/mL directly from undiluted serum or blood. We also demonstrated that D4 assays can be imaged with a smartphone-based fluorescence microscope; however, the LOD of the assay was approximately 20-fold higher compared to a traditional benchtop scanner (105). More recently, we developed a D4 assay to measure human epidermal growth receptor 2 directly from breast cancer fine needle aspirates resuspended in a lysis buffer (111). In this study, upgrades were made to the smartphone-based scanner—that had notably improved illumination and increased noise rejection efficiency—which improved the sensitivity approximately fivefold relative to the previous iteration. This new device, termed the EpiView-D4, could also be used as a brightfield microscope by attaching a different adapter, which we used to image stained cytology slides to identify malignant cells (Figure 6b).
Rather than using a smartphone scanner, we have recently built a stand-alone device, termed the D4Scope, which uses off-the-shelf components to image D4 assays with high sensitivity (Figure 6c). The D4Scope uses a coherent 638-nm red laser to excite fluorescently labeled detection reagents and captures their emission, after it is sent through a bandpass filter, using a complementary metal-oxide semiconductor sensor. We first used the D4Scope in conjunction with the D4 immunoassay to detect Ebola virus secreted glycoprotein, a novel biomarker of Ebola infection (112). We found that the D4Scope had an LOD of 0.10 ng/mL directly from undiluted pooled human serum, which is comparable to the LOD obtained on a traditional benchtop scanner (0.07 ng/mL). In an experiment with nonhuman primates where Ebola virus wash injected intramuscularly, the D4 assay was more sensitive than existing LFIAs and detected secreted glycoprotein one day earlier than polymerase chain reaction [PCR (113, 114)]. More recently, we have developed a serological D4 assay to detect antibodies against SARS-CoV-2 spike and nucleocapsid proteins (115). To design the assay, we used a double-antigen bridging format (DA-D4) that detects total antibody concentration, independent of isotype. We also incorporated the DA-D4 chip onto a newly developed passive capillary, a semigravity-driven microfluidic cassette, to completely automate the testing process. This automated D4-DA assay was highly sensitive and specific for COVID-19 two weeks after symptom onset and was able to differentiate between SAR-CoV-2 and other seasonal coronaviruses. Further, we showed that the assay could be performed directly from undiluted whole blood (Figure 6d).
The attractive features of the D4 POC assay include the ability to easily multiplex the assay by printing spatially discrete capture probes and detection reagents with a single fluorophore, which greatly simplifies assay readout. Further, the passive microfluidic cassette does not require any pumps or actuators and therefore is operated similarly to an LFIA, yet retains the sensitivity of traditional ELISA. However, unlike some digital ELISA platforms, discussed next, we have not yet been able to detect proteins in the low fg/mL or attomolar range, which limits our ability to detect protein analytes at very low concentrations in bodily fluids.
Digital Microfluidic Immunoassays
Digital microfluidics, a liquid-handling technology in which discrete droplets are controlled to implement a series of operations, has become increasingly popular for automating immunoassays. As digital microfluidic systems have been reviewed extensively elsewhere (116), in this section, we only discuss several innovative immunoassays that use them for POC applications.
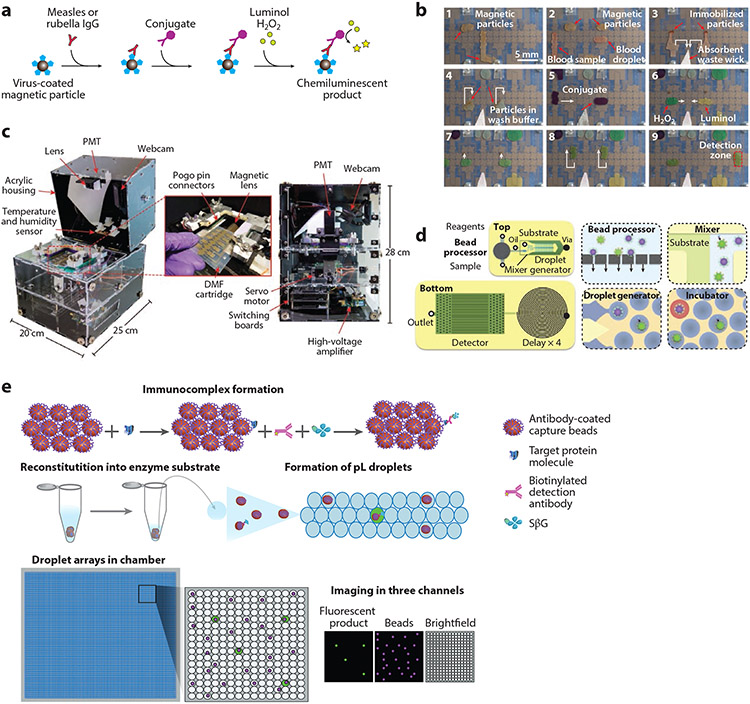
Ng et al. (117) developed a digital microfluidic immunoassay to detect antibodies against measles and rubella from whole blood at the POC. Their system uses magnetic particles coated with measles or rubella viral antigens (Figure 7a) and an automated liquid-handling system (Figure 7b) to perform the sequential steps of ELISA. This generates a chemiluminescence signal that is read using an instrument developed in-house, termed the Measles-Rubella Box (MR Box). The MR Box developed for POC testing (118, 119) combines all the electronic, mechanical, and software components in a single unit that weighs 4 kg and costs approximately US$2,500 (Figure 7c). In a field trial at the Kakuma refugee camp in Kenya, the MR Box had an overall agreement of 86% and 84% with laboratory-based tests for measles and rubella, respectively, thus highlighting the potential utility of the device at the POC.
Figure 7.
Digital microfluidic immunoassays. (a) Paramagnetic beads coated with measles or rubella virus antigens are incubated with sample. Anti-measles or antirubella antibodies bind to the beads, are washed, and are then labeled with antihuman immunoglobulin G conjugated to horseradish peroxidase. The beads are washed again and then incubated with luminol and H2O2, resulting in a chemiluminescent product. (b) Nine sequential steps of the DMF ELISA process. (c) Portable measles-rubella box for assay readout, highlighting device design and components. Panels a–c adapted with permission from Reference 117 (CC BY-NC 4.0). (d) Schematic of the μMD chip, showing all four modules of the μMD. Panel adapted with permission from Reference 120; copyright 2019 PNAS. (e) ddELISA schematic. First, antibody-coated paramagnetic beads are added to a sample, followed by incubation with biotinylated detection antibodies and SβG. Most beads will have zero immunocomplexes and a small fraction will have one. Beads are then reconstituted in enzyme substrate solution, partitioned into picoliter droplets, and then loaded into a chamber to form droplet arrays, which are imaged using three different channels. Panel adapted with permission from Reference 122; copyright 2020 AAAS. Abbreviations: ddELISA, droplet digital ELISA; DMF, digital microfluidic; ELISA, enzyme-linked immunosorbent assay; μMD, microdroplet megascale detector; PMT, photomultiplier tube; SβG, streptavidin-β-galactosidase.
Yelleswarapu et al. (120) developed an optofluidic platform, the microdroplet Megascale Detector (μMD), for multiplexed digital ELISA (dELISA) using multiple color-coded microbeads. In μMD, microbeads functionalized with antibodies capture a protein of interest from serum and are subsequently sandwiched by a detection antibody coupled to horseradish peroxidase. Individual beads are then encapsulated into water-in-oil droplets, traverse a microfluidic channel where enzymatic amplification occurs, and are finally read by a smartphone–based detector, where the droplets’ fluorescence is detected using time domain–encoded optofluidics (Figure 7d). Using this platform, they obtained an LOD of 3.7 fg/mL for granulocyte-macrophage colony-stimulating factor and 8.0 fg/mL for IL-6 in diluted fetal bovine serum, respectively. In comparison, Quanterix’s Simoa HD-1 Analyzer (121)—the gold standard commercial implementation of dELISA—has an LOD of 1.9 fg/mL. While the prototype instrumentation costs $500 and the disposables cost $5—costs that are reasonable though on the upper end for POC applications—this assay still requires a 3-h incubation that occurs off-chip with some manual intervention that limits its utility at the POC.
Similarly, Cohen et al. (122) developed a droplet digital ELISA (ddELISA) with attomolar sensitivity for protein targets. Paramagnetic beads coated with capture antibodies are added to a serum sample along with biotinylated detection antibodies and streptavidin-β-galactosidase. The magnetic beads are washed and loaded into picoliter-scale droplets to form droplet arrays containing one or zero beads. The signal output—the average number of enzymes per bead—is directly related to protein concentration (Figure 7e). The LOD of ddELISA was 30 attomolar for interferon gamma (IFN-γ) and was 20 attomolar for IL-2, which is at least tenfold more sensitive than Simoa. Although the ddELISA platform is extremely sensitive, multiple user intervention steps and the necessity for refrigeration limit its utility for POC testing in its current format. These platforms would benefit from further automation and integration into a microfluidic system that automates all steps of the assay, as has been done for microfluidic ELISAs (123).
OUTLOOK AND CONCLUSION
In this review, we have discussed innovative strategies and technologies used for POC immunoassays while minimizing cost and enhancing accessibility. The high cost and limited accessibility of commercially available platforms have motivated development of broadly accessible POC platforms that do not sacrifice performance relative to the analyzers used in clinical laboratories. Researchers have taken different approaches toward this goal. While some have modified existing platforms to be compatible with consumer electronics, such as smartphones, multimeters, pH meters, hydrogen meters, and glucometers, other researchers have developed readouts—signals—that are inherently easier to transduce in the field without the need for sophisticated equipment. These promising technologies include instrument-free methods, bioluminescence homogeneous immunoassays, electrochemical immunoassays, D4 microarrays, and digital microfluidics. These developments have the potential to democratize access to affordable clinical-quality immunoassays at the POC, but they also highlight the gap that remains to be filled before this goal can be fully realized. This is because many of these technologies are at a proof-of-concept stage and have not been validated in clinical studies. However, as the demand for POC immunoassays continues to increase, we are hopeful that some of these platforms will bridge this gap and find use at the POC.
ACKNOWLEDGEMENTS
This work was supported by the National Cancer Institute through grant UH3-CA211232; and through the National Institute of Health though grants R01-CA248491, R01-AI150888, and R01-AI159992
Footnotes
DISCLOSURE STATEMENT
Immucor Inc. has acquired the rights to the D4 assay on POEGMA brushes for in vitro diagnostics from Sentilus Inc. (cofounded by Ashutosh Chilkoti) featured in references 105, 107, 109-112, and 115. Jason Liu and Ashutosh Chilkoti are inventors on patent (No.PCT/US2020/031210) featured in reference 112 and 115. All authors are inventors on patent (No. PCT/US2021/046833) featured in reference 115.
LITERATURE CITED
- 1.Crowther JR. 2000. The ELISA Guidebook. Totowa, NJ: Humana Press. 2nd ed. [Google Scholar]
- 2.Sher M, Zhuang R, Demirci U, Asghar W. 2017. Paper-based analytical devices for clinical diagnosis: recent advances in the fabrication techniques and sensing mechanisms. Expert Rev. Mol. Diagn 17:351–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li F, You M, Li S, Hu J, Liu C, et al. 2020. Paper-based point-of-care immunoassays: recent advances and emerging trends. Biotechnol. Adv 39:107442. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Zhan L, Qin Z, Sackrison J, Bischof JC. 2021. Ultrasensitive and highly specific lateral flow assays for point-of-care diagnosis. ACS Nano 15:3593–611 [DOI] [PubMed] [Google Scholar]
- 5.Hu J, Wang S, Wang L, Li F, Pingguan-Murphy B, et al. 2014. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron 54:585–97 [DOI] [PubMed] [Google Scholar]
- 6.Mahmoudi T, de la Guardia M, Shirdel B, Mokhtarzadeh A, Baradaran B. 2019. Recent advancements in structural improvements of lateral flow assays towards point-of-care testing. Trends Anal. Chem 116:13–30 [Google Scholar]
- 7.O’Farrell B 2008. Evolution in lateral flow–based immunoassay systems. In Lateral Flow Immunoassay, ed. Wong RC, Tse HY, pp. 1–33. Totowa, NJ: Humana Press [Google Scholar]
- 8.Bosch I, de Puig H, Hiley M, Carre-Camps M, Perdomo-Celis F, et al. 2017. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci. Transl. Med 9. 10.1126/scitranslmed.aan1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insights FB. 2021. Point of care (POC) Diagnostics Market Size, Share & COVID-19 Impact Analysis, By Product (Blood Glucose Monitoring, Infectious Diseases, Cardiometabolic Diseases, Pregnancy & Infertility Testing, Hematology Testing, and Others), By End User (Hospital Bedside, Physician’s Office Lab, Urgent Care & Retail Clinics, and Homecare/Self-Testing) and Regional Forecast, 2021–2028, Fortune Business Insights. https://www.fortunebusinessinsights.com/industry-reports/point-of-care-diagnostics-market-101072 [Google Scholar]
- 10.Office of NIH History and Stetten Museum. The Thin Blue Line: The History of the Pregnancy Test. National Institutes of Health.https://history.nih.gov/display/history/Pregnancy+Test+Timeline [Google Scholar]
- 11.Johnson S, Cushion M, Bond S, Godbert S, Pike J. 2015. Comparison of analytical sensitivity and women’s interpretation of home pregnancy tests. Clin. Chem. Lab. Med 53:391–402 [DOI] [PubMed] [Google Scholar]
- 12.Tomlinson C, Marshall J, Ellis JE. 2008. Comparison of accuracy and certainty of results of six home pregnancy tests available over-the-counter. Curr. Med. Res. Opin 24:1645–49 [DOI] [PubMed] [Google Scholar]
- 13.Diagnostics Lumos. 2021. Lumos Diagnostics Point-of-Care Readers. https://lumosdiagnostics.com/solutions/readers/ [Google Scholar]
- 14.Urusov AE, Zherdev AV, Dzantiev BB. 2019. Towards lateral flow quantitative assays: detection approaches. Biosensors 9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faulstich K, Gruler R, Eberhard M, Lentzsch D, Haberstroh K. 2009. Handheld and portable reader devices for lateral flow immunoassays. In Lateral Flow Immunoassay, ed. Wong R, Tse H, pp. 1–27. Totowa, NJ: Humana Press [Google Scholar]
- 16.Pezzuto F, Scarano A, Marini C, Rossi G, Stocchi R, et al. 2019. Assessing the reliability of commercially available point of care in various clinical fields. Open Public Health J. 12:342–68 [Google Scholar]
- 17.Li Y, Xuan J, Song Y, Qi W, He B, et al. 2016. Nanoporous glass integrated in volumetric bar-chart chip for point-of-care diagnostics of non-small cell lung cancer. ACS Nano 10:1640–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Uddayasankar U, He B, Wang P, Qin L. 2017. Fast, sensitive, and quantitative point-of-care platform for the assessment of drugs of abuse in urine, serum, and whole blood. Anal. Chem 89:8273–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn J, Obuekwe J, Baun T, Rogers J, Patel T, Snow L. 2014. Prompt detection of influenza A and B viruses using the BD Veritor™ System Flu A+B, Quidel® Sofia® Influenza A+B FIA, and Alere BinaxNOW® Influenza A&B compared to real-time reverse transcription-polymerase chain reaction (RT-PCR). Diagnost. Microbiol. Infect. Dis 79:10–13 [DOI] [PubMed] [Google Scholar]
- 20.Beck ET, Paar W, Fojut L, Serwe J, Jahnke RR. 2021. Comparison of the Quidel Sofia SARS FIA Test to the Hologic Aptima SARS-CoV-2 TMA Test for Diagnosis of COVID-19 in Symptomatic Outpatients. J. Clin. Microbiol 59. 10.1128/JCM.02727-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reenen AV, Berger M, Moreau E, Bekx E, Bruinink T, et al. 2019. Analytical performance of a single epitope B-type natriuretic peptide sandwich immunoassay on the Minicare platform for point-of-care diagnostics. Pract. Lab. Med 15:e00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venge P, van Lippen L, Blaschke S, Christ M, Geier F, et al. 2017. Equal clinical performance of a novel point-of-care cardiac troponin I (cTnI) assay with a commonly used high-sensitivity cTnI assay. Clin. Chim. Acta 469:119–25 [DOI] [PubMed] [Google Scholar]
- 23.Christenson RH, Jacobs E, Uettwiller-Geiger D, Estey MP, Lewandrowski K, et al. 2017. Comparison of 13 commercially available cardiac troponin assays in a multicenter North American study. J. Appl. Lab. Med 1:544–61 [DOI] [PubMed] [Google Scholar]
- 24.Martin CL. 2010. i-STAT—Combining chemistry and haematology in PoCT. Clin. Biochem. Rev 31:81–84 [PMC free article] [PubMed] [Google Scholar]
- 25.Korley FK, Datwyler SA, Jain S, Sun X, Beligere G, et al. 2021. Comparison of GFAP and UCH-L1 measurements from two prototype assays: the Abbott i-STAT and ARCHITECT assays. Neurotrauma Rep. 2:193–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez-Neuta I, Neumann F, Brightmeyer J, Ba Tis T, Madaboosi N, et al. 2019. Smartphone-based clinical diagnostics: towards democratization of evidence-based health care. J. Intern. Med 285:19–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinhubl SR, Muse ED, Topol EJ. 2015. The emerging field of mobile health. Sci. Transl. Med 7:283rv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merazzo KJ, Totoricaguena-Gorrino J, Fernandez-Martin E, Del Campo FJ, Baldrich E. 2021. Smartphone-enabled personalized diagnostics: current status and future prospects. Diagnostics 11:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Zangheri M, Cevenini L, Anfossi L, Baggiani C, Simoni P, et al. 2015. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens. Bioelectron 64:63–68 [DOI] [PubMed] [Google Scholar]
- 29.Roda A, Cavalera S, Di Nardo F, Calabria D, Rosati S, et al. 2021. Dual lateral flow optical/chemiluminescence immunosensors for the rapid detection of salivary and serum IgA in patients with COVID-19 disease. Biosens. Bioelectron 172:112765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Jiang C, Jin J, Huang L, Yu W, et al. 2021. Ratiometric fluorescent lateral flow immunoassay for point-of-care testing of acute myocardial infarction. Angew. Chem. Int. Ed 60:13042–49 [DOI] [PubMed] [Google Scholar]
- 31.Yeo SJ, Kang H, Dao TD, Cuc BT, Nguyen ATV, et al. 2018. Development of a smartphone-based rapid dual fluorescent diagnostic system for the simultaneous detection of influenza A and H5 subtype in avian influenza A-infected patients. Theranostics 8:6132–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg B, Cortazar B, Tseng D, Ozkan H, Feng S, et al. 2015. Cellphone-based hand-held microplate reader for point-of-care testing of enzyme-linked immunosorbent assays. ACS Nano 9:7857–66 [DOI] [PubMed] [Google Scholar]
- 33.Sun AC, Hall DA. 2019. Point-of-care smartphone-based electrochemical biosensing. Electroanalysis 31:2–16 [Google Scholar]
- 34.Islam T, Hasan MM, Awal A, Nurunnabi M, Ahammad AJS. 2020. Metal nanoparticles for electrochemical sensing: progress and challenges in the clinical transition of point-of-care testing. Molecules 25:5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aronoff-Spencer E, Venkatesh AG, Sun A, Brickner H, Looney D, Hall DA. 2016. Detection of Hepatitis C core antibody by dual-affinity yeast chimera and smartphone-based electrochemical sensing. Biosens. Bioelectron 86:690–96 [DOI] [PubMed] [Google Scholar]
- 36.Cheng C, Li X, Xu G, Lu Y, Low SS, et al. 2021. Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat cortisol via near field communication. Biosens. Bioelectron 172:112782. [DOI] [PubMed] [Google Scholar]
- 37.Aidukas T, Eckert R, Harvey AR, Waller L, Konda PC. 2019. Low-cost, sub-micron resolution, wide-field computational microscopy using opensource hardware. Sci. Rep 9:7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Göröcs Z, Ozcan A. 2014. Biomedical imaging and sensing using flatbed scanners. Lab Chip 14:3248–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanjay ST, Dou M, Sun J, Li X. 2016. A paper/polymer hybrid microfluidic microplate for rapid quantitative detection of multiple disease biomarkers. Sci. Rep 6:30474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gogalic S, Sauer U, Doppler S, Preininger C. 2018. Investigating colorimetric protein array assay schemes for detection of recurrence of bladder cancer. Biosensors 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Göröcs Z, Ling Y, Yu MD, Karahalios D, Mogharabi K, et al. 2013. Giga-pixel fluorescent imaging over an ultra-large field-of-view using a flatbed scanner. Lab Chip 13:4460–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aygun U, Avci O, Seymour E, Urey H, Ünlü MS, Ozkumur AY. 2017. Label-free and high-throughput detection of biomolecular interactions using a flatbed scanner biosensor. ACS Sens. 2:1424–29 [DOI] [PubMed] [Google Scholar]
- 43.Lan T, Zhang J, Lu Y. 2016. Transforming the blood glucose meter into a general healthcare meter for in vitro diagnostics in mobile health. Biotechnol. Adv 34:331–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lisi F, Peterson JR, Gooding JJ. 2020. The application of personal glucose meters as universal point-of-care diagnostic tools. Biosens. Bioelectron 148:111835. [DOI] [PubMed] [Google Scholar]
- 45.Taebi S, Keyhanfar M, Noorbakhsh A. 2018. A novel method for sensitive, low-cost and portable detection of hepatitis B surface antigen using a personal glucose meter. J. Immunol. Methods 458:26–32 [DOI] [PubMed] [Google Scholar]
- 46.Sun F, Sun X, Jia Y, Hu Z, Xu S, et al. 2019. Ultrasensitive detection of prostate specific antigen using a personal glucose meter based on DNA-mediated immunoreaction. Analyst 144:6019–24 [DOI] [PubMed] [Google Scholar]
- 47.Alshawawreh FA, Lisi F, Ariotti N, Bakthavathsalam P, Benedetti T, et al. 2019. The use of a personal glucose meter for detecting procalcitonin through glucose encapsulated within liposomes. Analyst 144:6225–30 [DOI] [PubMed] [Google Scholar]
- 48.Kwon D, Joo J, Lee S, Jeon S. 2013. Facile and sensitive method for detecting cardiac markers using ubiquitous pH meters. Anal. Chem 85:12134–37 [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Yang J, Nie J, Yang J, Gao D, et al. 2016. Enhanced ELISA using a handheld pH meter and enzyme-coated microparticles for the portable, sensitive detection of proteins. Chem. Commun 52:3474–77 [DOI] [PubMed] [Google Scholar]
- 50.Li B, Ge L, Lyu P, Chen M, Zhang X, et al. 2021. Handheld pH meter-assisted immunoassay for C-reactive protein using glucose oxidase-conjugated dendrimer loaded with platinum nanozymes. Mikrochim. Acta 188:14–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang Y, Su Z, Zhang J, Cai M, Wu L. 2018. A novel electrochemical immunoassay for carcinoembryonic antigen based on glucose oxidase-encapsulated nanogold hollow spheres with a pH meter readout. Analyst 143:5271–77 [DOI] [PubMed] [Google Scholar]
- 52.Sun A-L, Qi Q-A, Zhi L-J. 2020. Cross-linkage urease nanoparticles: a high-efficiency signal-generation tag for portable pH meter-based electrochemical immunoassay of lipocalin-2 protein diagnostics. Microchim. Acta 187:485. [DOI] [PubMed] [Google Scholar]
- 53.Katsingris P. 2018. THE NIELSEN TOTAL AUDIENCE REPORT: Q1 2018. The Nielsen Company. https://www.nielsen.com/us/en/insights/report/2018/q1-2018-total-audience-report/# [Google Scholar]
- 54.Hwu EE-T, Boisen A. 2018. Hacking CD/DVD/blu-ray for biosensing. ACS Sens. 3:1222–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Wang H, Zhang X, Li X, Yu H-Z. 2020. Indirect competitive immunoassay on a blu-ray disc for digitized quantitation of food toxins. ACS Sens. 5:1239–45 [DOI] [PubMed] [Google Scholar]
- 56.Weng S, Li X, Niu M, Ge B, Yu H-Z. 2016. Blu-ray technology-based quantitative assays for cardiac markers: from disc activation to multiplex detection. Anal. Chem 88:6889–96 [DOI] [PubMed] [Google Scholar]
- 57.Yu Z, Tang Y, Cai G, Ren R, Tang D. 2019. Paper electrode-based flexible pressure sensor for point-of-care immunoassay with digital multimeter. Anal. Chem 91:1222–26 [DOI] [PubMed] [Google Scholar]
- 58.Yu Z, Cai G, Tong P, Tang D. 2019. Saw-toothed microstructure-based flexible pressure sensor as the signal readout for point-of-care immunoassay. ACS Sens. 4:2272–76 [DOI] [PubMed] [Google Scholar]
- 59.Yu Z, Cai G, Liu X, Tang D. 2020. Platinum nanozyme-triggered pressure-based immunoassay using a three-dimensional polypyrrole foam-based flexible pressure sensor. ACS Appl. Mater. Interfaces 12:40133–40 [DOI] [PubMed] [Google Scholar]
- 60.Zhu L, Lv Z, Yin Z, Li M, Tang D. 2021. Digital multimeter-based point-of-care immunoassay of prostate- specific antigen coupling with a flexible photosensitive pressure sensor. Sens. Actuators B Chem 343:130121 [Google Scholar]
- 61.Bu S-J, Wang K-Y, Bai H-S, Leng Y, Ju C-J, et al. 2019. Immunoassay for pathogenic bacteria using platinum nanoparticles and a hand-held hydrogen detector as transducer. Application to the detection of Escherichia coli O157:H7. Microchim. Acta 186:296. [DOI] [PubMed] [Google Scholar]
- 62.Yang M, Zhang W, Yang J, Hu B, Cao F, et al. 2017. Skiving stacked sheets of paper into test paper for rapid and multiplexed assay. Sci. Adv 3:eaao4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Sun J, Zou Y, Chen W, Zhang W, et al. 2015. Barcoded microchips for biomolecular assays. Anal. Chem 87:900–6 [DOI] [PubMed] [Google Scholar]
- 64.Zhang D, Gao B, Chen Y, Liu H. 2018. Converting colour to length based on the coffee-ring effect for quantitative immunoassays using a ruler as readout. Lab Chip 18:271–75 [DOI] [PubMed] [Google Scholar]
- 65.Song Y, Zhang Y, Bernard PE, Reuben JM, Ueno NT, et al. 2012. Multiplexed volumetric bar-chart chip for point-of-care diagnostics. Nat. Commun 3:1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y, Xuan J, Xia T, Han X, Song Y, et al. 2015. Competitive volumetric bar-chart chip with real-time internal control for point-of-care diagnostics. Anal. Chem 87:3771–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song Y, Wang Y, Qi W, Li Y, Xuan J, et al. 2016. Integrative volumetric bar-chart chip for rapid and quantitative point-of-care detection of myocardial infarction biomarkers. Lab Chip 16:2955–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Xuan J, Song Y, Wang P, Qin L. 2015. A microfluidic platform with digital readout and ultra-low detection limit for quantitative point-of-care diagnostics. Lab Chip 15:3300–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sebba D, Lastovich AG, Kuroda M, Fallows E, Johnson J, et al. 2018. A point-of-care diagnostic for differentiating Ebola from endemic febrile diseases. Sci. Transl. Med 10:eaat0944. [DOI] [PubMed] [Google Scholar]
- 70.Chen L, Zhang X, Zhou G, Xiang X, Ji X, et al. 2012. Simultaneous determination of human Enterovirus 71 and Coxsackievirus B3 by dual-color quantum dots and homogeneous immunoassay. Anal. Chem 84:3200–7 [DOI] [PubMed] [Google Scholar]
- 71.Akama K, Noji H. 2020. Multiplexed homogeneous digital immunoassay based on single-particle motion analysis. Lab Chip 20:2113–21 [DOI] [PubMed] [Google Scholar]
- 72.Takkinen K, Zvirbliene A. 2019. Recent advances in homogenous immunoassays based on resonance energy transfer. Curr. Opin. Biotechnol 55:16–22 [DOI] [PubMed] [Google Scholar]
- 73.Huang Z, Li Z, Jiang M, Liu R, Lv Y. 2020. Homogeneous multiplex immunoassay for one-step pancreatic cancer biomarker evaluation. Anal. Chem 92:16105–12 [DOI] [PubMed] [Google Scholar]
- 74.Arts R, den Hartog I, Zijlema SE, Thijssen V, van der Beelen SH, Merkx M. 2016. Detection of antibodies in blood plasma using bioluminescent sensor proteins and a smartphone. Anal. Chem 88:4525–32 [DOI] [PubMed] [Google Scholar]
- 75.van Rosmalen M, Ni Y, Vervoort DFM, Arts R, Ludwig SKJ, Merkx M. 2018. Dual-color bioluminescent sensor proteins for therapeutic drug monitoring of antitumor antibodies. Anal. Chem 90:3592–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tenda K, van Gerven B, Arts R, Hiruta Y, Merkx M, Citterio D. 2018. Paper-based antibody detection devices using bioluminescent BRET-switching sensor proteins. Angew. Chem. Int. Ed 57:15369–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xue L, Yu Q, Griss R, Schena A, Johnsson K. 2017. Bioluminescent antibodies for point-of-care diagnostics. Angew. Chem. Int. Ed 56:7112–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arts R, Ludwig SKJ, van Gerven BCB, Estirado EM, Milroy LG, Merkx M. 2017. Semisynthetic bioluminescent sensor proteins for direct detection of antibodies and small molecules in solution. ACS Sens. 2:1730–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ni Y, Arts R, Merkx M. 2019. Ratiometric bioluminescent sensor proteins based on intramolecular split luciferase complementation. ACS Sens. 4:20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dixon AS, Schwinn MK, Hall MP, Zimmerman K, Otto P, et al. 2016. NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chem. Biol 11:400–8 [DOI] [PubMed] [Google Scholar]
- 81.Hall MP, Kincaid VA, Jost EA, Smith TP, Hurst R, et al. 2021. Toward a point-of-need bioluminescence-based immunoassay utilizing a complete shelf-stable reagent. Anal. Chem 93:5177–84 [DOI] [PubMed] [Google Scholar]
- 82.Ni Y, Rosier B, van Aalen EA, Hanckmann ETL, Biewenga L, et al. 2021. A plug-and-play platform of ratiometric bioluminescent sensors for homogeneous immunoassays. Nat. Commun 12:4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yao Z, Drecun L, Aboualizadeh F, Kim SJ, Li Z, et al. 2021. A homogeneous split-luciferase assay for rapid and sensitive detection of anti-SARS CoV-2 antibodies. Nat. Commun 12:1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dixon AS, Kim SJ, Baumgartner BK, Krippner S, Owen SC. 2017. A tri-part protein complementation system using antibody-small peptide fusions enables homogeneous immunoassays. Sci. Rep 7:8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim SJ, Dixon AS, Adamovich PC, Robinson PD, Owen SC. 2021. Homogeneous immunoassay using a tri-part split-luciferase for rapid quantification of anti-TNF therapeutic antibodies. ACS Sens. 6:1807–14 [DOI] [PubMed] [Google Scholar]
- 86.Elledge SK, Zhou XX, Byrnes JR, Martinko AJ, Lui I, et al. 2021. Engineering luminescent biosensors for point-of-care SARS-CoV-2 antibody detection. Nat. Biotechnol 39:928–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borrebaeck CA. 2017. Precision diagnostics: moving towards protein biomarker signatures of clinical utility in cancer. Nat. Rev. Cancer 17:199–204 [DOI] [PubMed] [Google Scholar]
- 88.Dincer C, Bruch R, Kling A, Dittrich PS, Urban GA. 2017. Multiplexed point-of-care testing —xPOCT. Trends Biotechnol. 35:728–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yager P, Domingo GJ, Gerdes J. 2008. Point-of-care diagnostics for global health. Annu. Rev. Biomed. Eng 10:107–44 [DOI] [PubMed] [Google Scholar]
- 90.Takaloo S, Zand MM. 2021. Wearable electrochemical flexible biosensors: with the focus on affinity biosensors. Sens. Bio-Sens. Res 32:100403 [Google Scholar]
- 91.Upasham S, Prasad S. 2020. SLOCK (sensor for circadian clock): passive sweat-based chronobiology tracker. Lab Chip 20:1947–60 [DOI] [PubMed] [Google Scholar]
- 92.Kinnamon D, Ghanta R, Lin K-C, Muthukumar S, Prasad S. 2017. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci. Rep 7:13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grieshaber D, MacKenzie R, Vörös J, Reimhult E. 2008. Electrochemical biosensors— sensor principles and architectures. Sensors 8:1400–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mahshid SS, Flynn SE, Mahshid S. 2021. The potential application of electrochemical biosensors in the COVID-19 pandemic: a perspective on the rapid diagnostics of SARS-CoV-2. Biosens. Bioelectron 176:112905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wehmeyer KR, White RJ, Kissinger PT, Heineman WR. 2021. Electrochemical affinity assays/sensors: brief history and current status. Annu. Rev. Anal. Chem 14:109–31 [DOI] [PubMed] [Google Scholar]
- 96.Mathew M, Radhakrishnan S, Vaidyanathan A, Chakraborty B, Rout CS. 2021. Flexible and wearable electrochemical biosensors based on two-dimensional materials: recent developments. Anal. Bioanal. Chem 413:727–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao Y, Nguyen DT, Yeo T, Lim SB, Tan WX, et al. 2021. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv 7:eabg9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cho I-H, Lee J, Kim J, Kang MS, Paik JK, et al. 2018. Current technologies of electrochemical immunosensors: perspective on signal amplification. Sensors 18:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zupančič U, Jolly P, Estrela P, Moschou D, Ingber DE. 2021. Graphene enabled low-noise surface chemistry for multiplexed sepsis biomarker detection in whole blood. Adv. Funct. Mater 31:2010638 [Google Scholar]
- 100.Sabaté del Río J, Henry OYF, Jolly P, Ingber DE. 2019. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol 14:1143–49 [DOI] [PubMed] [Google Scholar]
- 101.Timilsina SS, Durr N, Yafia M, Sallum H, Jolly P, Ingber DE. 2021. Rapid antifouling nanocomposite coating enables highly sensitive multiplexed electrochemical detection of myocardial infarction and concussion markers. medRxiv 2021.06.13.21258856 [Google Scholar]
- 102.Guo K, Wustoni S, Koklu A, Díaz-Galicia E, Moser M, et al. 2021. Rapid single-molecule detection of COVID-19 and MERS antigens via nanobody-functionalized organic electrochemical transistors. Nat. Biomed. Eng 5:666–77 [DOI] [PubMed] [Google Scholar]
- 103.Romanov V, Davidoff SN, Miles AR, Grainger DW, Gale BK, Brooks BD. 2014. A critical comparison of protein microarray fabrication technologies. Analyst 139:1303–26 [DOI] [PubMed] [Google Scholar]
- 104.Barbulovic-Nad I, Lucente M, Sun Y, Zhang M, Wheeler AR, Bussmann M. 2006. Bio-microarray fabrication techniques—a review. Crit. Rev. Biotechnol 26:237–59 [DOI] [PubMed] [Google Scholar]
- 105.Joh DY, Hucknall AM, Wei Q, Mason KA, Lund ML, et al. 2017. Inkjet-printed point-of-care immunoassay on a nanoscale polymer brush enables subpicomolar detection of analytes in blood. PNAS 114:E7054–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heggestad JT, Fontes CM, Joh DY, Hucknall AM, Chilkoti A. 2020. In pursuit of zero 2.0: recent developments in nonfouling polymer brushes for immunoassays. Adv. Mater 32:e1903285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma H, Li D, Sheng X, Zhao B, Chilkoti A. 2006. Protein-resistant polymer coatings on silicon oxide by surface-initiated atom transfer radical polymerization. Langmuir 22:3751–56 [DOI] [PubMed] [Google Scholar]
- 108.Hucknall A, Rangarajan S, Chilkoti A. 2009. In pursuit of zero: polymer brushes that resist the adsorption of proteins. Adv. Mater 21:2441–46 [Google Scholar]
- 109.Hucknall A, Kim D-H, Rangarajan S, Hill RT, Reichert WM, Chilkoti A. 2009. Simple fabrication of antibody microarrays on nonfouling polymer brushes with femtomolar sensitivity for protein analytes in serum and blood. Adv. Mater 21:1968–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma H, Hyun J, Stiller P, Chilkoti A. 2004. “Non-fouling” oligo(ethylene glycol)-functionalized polymer brushes synthesized by surface-initiated atom transfer radical polymerization. Adv. Mater 16:338–41 [Google Scholar]
- 111.Joh DY, Heggestad JT, Zhang S, Anderson GR, Bhattacharyya J, et al. 2021. Cellphone enabled point-of-care assessment of breast tumor cytology and molecular HER2 expression from fine-needle aspirates. NPJ Breast Cancer 7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fontes CM, Lipes BD, Liu J, Agans KN, Yan A, et al. 2021. Ultrasensitive point-of-care immunoassay for secreted glycoprotein detects Ebola infection earlier than PCR. Sci. Transl. Med 13:eabd9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cross RW, Boisen ML, Millett MM, Nelson DS, Oottamasathien D, et al. 2016. Analytical validation of the ReEBOV antigen rapid test for point-of-care diagnosis of Ebola virus infection. J. Infect. Dis 214:S210–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boisen ML, Cross RW, Hartnett JN, Goba A, Momoh M, et al. 2016. Field validation of the ReEBOV antigen rapid test for point-of-care diagnosis of Ebola virus infection. J. Infect. Dis 214:S203–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heggestad JT, Kinnamon DS, Olson LB, Liu J, Kelly G, et al. 2021. Multiplexed, quantitative serological profiling of COVID-19 from blood by a point-of-care test. Sci. Adv 7:eabg4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Samiei E, Tabrizian M, Hoorfar M. 2016. A review of digital microfluidics as portable platforms for lab-on a-chip applications. Lab Chip 16:2376–96 [DOI] [PubMed] [Google Scholar]
- 117.Ng AHC, Fobel R, Fobel C, Lamanna J, Rackus DG, et al. 2018. A digital microfluidic system for serological immunoassays in remote settings. Sci. Transl. Med 10:eaar6076. [DOI] [PubMed] [Google Scholar]
- 118.Ng AH, Lee M, Choi K, Fischer AT, Robinson JM, Wheeler AR. 2015. Digital microfluidic platform for the detection of rubella infection and immunity: a proof of concept. Clin. Chem 61:420–29 [DOI] [PubMed] [Google Scholar]
- 119.Choi K, Ng AH, Fobel R, Chang-Yen DA, Yarnell LE, et al. 2013. Automated digital microfluidic platform for magnetic-particle-based immunoassays with optimization by design of experiments. Anal. Chem 85:9638–46 [DOI] [PubMed] [Google Scholar]
- 120.Yelleswarapu V, Buser JR, Haber M, Baron J, Inapuri E, Issadore D. 2019. Mobile platform for rapid sub-picogram-per-milliliter, multiplexed, digital droplet detection of proteins. PNAS 116:4489–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, et al. 2016. The Simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J. Lab. Autom 21:533–47 [DOI] [PubMed] [Google Scholar]
- 122.Cohen L, Cui N, Cai Y, Garden PM, Li X, et al. 2020. Single molecule protein detection with attomolar sensitivity using droplet digital enzyme-linked immunosorbent assay. ACS Nano 14:9491–501 [DOI] [PubMed] [Google Scholar]
- 123.Laksanasopin T, Guo TW, Nayak S, Sridhara AA, Xie S, et al. 2015. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci. Transl. Med 7:273re1. [DOI] [PubMed] [Google Scholar]