Abstract
The bones are a common site for metastasis arising from solid tumors such as breast and prostate cancer. Chemotherapy, including immunotherapy is rarely curative. Radiotherapy with pain palliation can temporize bone metastases but is generally considered a short-term solution and retreatment is difficult. Surgery is often necessary, yet recovery times might exceed life expectancy. Therefore, there is a need to develop new approaches to bone metastases that are effective but minimally invasive. Near-infrared photoimmunotherapy (NIR-PIT) uses antibodies labeled with IRDye700DX (IR700) which is activated by light, results in rapid cell membrane damage and immunogenic cell death. NIR-PIT using an anti-epidermal growth factor receptor (EGFR) antibody-IR700 conjugate in patients with recurrent head and neck cancer received qualified approval in Japan in 2020 and is now widely used there. However, no bone metastases have yet been treated. In this study, the efficacy of NIR-PIT for bone metastases was investigated using a bone metastases mouse model successfully established by caudal artery injection of a human triple-negative breast cancer cell line, MDAMB468-GFP/luc. The bone metastatic lesions were treated with NIR-PIT using the anti-EGFR antibody, panitumumb-IR700 conjugate. Bioluminescence imaging and histological evaluation showed that EGFR-targeting NIR-PIT has a therapeutic effect on bone metastatic lesions in mice. In addition, micro-CT showed that repeated NIR-PIT led to repair of metastasis-induced bone destruction and restored bone cortex continuity consistent with healing. These data suggest that NIR-PIT has potential for clinical application in the treatment of bone metastases.
Keywords: near-infrared, photoimmunotherapy, IR700, bone metastasis, breast cancer
Graphical abstract
Introduction
Bones are one of the most common sites of metastasis especially for cancers arising from the breast, prostate, kidney and lung and are often a strong negative prognostic factor. [1–4] According to one large study [5], the cumulative incidence of bone metastases in patients with solid tumors is 4.8% at one year, 6.9% at five years, and 8.4% at ten years, findings that have been verified in other large studies. [2] Once cancer metastasizes to bone, it is difficult to completely cure and metastases often cause pain, paralysis or fractures that severely impact the activities of daily living (ADL) and the quality of life (QOL) of patients.
Treatment methods for bone metastases include external beam radiation therapy, surgery, and medical therapy inclusive of chemotherapy, immunotherapy and pain medications. [6, 7] External beam radiation therapy, the current gold standard treatment, can provide short term pain relief and prophylaxis against fractures but recurrence is common. [8] Surgery for bone metastasis is limited to decompression surgery for spinal cord paralysis and fixation surgery for pathologic fractures but is invasive and often requires a long recovery period. A number of systemic therapies are used in bone metastases including chemotherapy, hormonal therapy, bone modifying agents (BMA) and radio-isotopes such as Sr-89 and Ra-223. Although effective in some cases, none of the above treatments is intended to be curative. Effective, minimally invasive therapies with few side effects are needed.
Near-infrared photoimmunotherapy (NIR-PIT) is a cancer treatment that uses antibodies labeled with NIR photon absorbing silicon phthalocyanine dye, IRDye700DX (IR700) which is injected approximately 24 hours prior to the application of NIR light from a laser. [9–11] Intravenously administered antibody-photon absorber conjugates (APCs) specifically bind to target cancer cells, and subsequent irradiation with NIR light (wavelength around 690nm) causes photochemical changes in the conjugate, changing it from hydrophilic to hydrophobic. Hydrophobic APCs aggregate on cell membranes and cause physical damage to the cell membrane. [12] In a wide variety of mouse subcutaneous tumor models, the therapeutic effects of NIR-PIT using various antibody-IR700 conjugates have been reported. [13–16] Phase 1/2a clinical trials using epidermal growth factor receptor (EGFR)-targeted NIR-PIT in patients with inoperable head and neck cancer have demonstrated clinically meaningful efficacy with a tolerable side effect profile. [17, 18] Based on the positive results of these clinical trials, the first EGFR-targeting NIR-PIT drug (Akalux®, Rakuten Medical Inc., San Diego, CA, USA) combined with a 690 nm laser system (BioBlade®, Rakuten Medical Inc.) received qualified approval from the Japanese Ministry of Health, Labor and Welfare in September 2020 for general clinical use. In addition, an FDA-designated fast-tracked global Phase 3 clinical trial is in progress (https://clinicaltrials.gov/ct2/show/NCT03769506).
It might be presumed that light-based therapies would be ineffective in bony lesions because of limited light penetration in bone. To test this, we investigated whether NIR-PIT is effective in treating bone metastases in a bone metastasis mouse model of the EGFR-positive MDAMB468-GFP/luc tumors (human triple-negative breast cancer cell line) using the caudal artery injection technique.
Materials and methods
Reagents
Water-soluble, silica-phthalocyanine-derivative, IRDye700DX NHS ester (IR700) was purchased from LI-COR Bioscience (Lincoln, NE). Panitumumab (Vectibix®), a fully human IgG2 monoclonal antibody against human epidermal growth factor receptor (EGFR), was purchased from Amgen (Thousand Oaks, CA). D-Luciferin was purchased from Gold Biotechnology (St. Louis, MO). All other chemicals were of reagent grade.
Antibody-IR700 Conjugation
Conjugation of IR700 to Panitumumab was performed as previously described. [19] Briefly, Panitumumab (1 mg, 6.8 nmol) was incubated with IR700 (34.2 nmol, 10 mmol/L in DMSO) in 0.1mol/L Na2HPO4 (pH 8.5) at room temperature for one hour. The reactants were purified using a Sephadex G 25 column (PD-10 column, GE Healthcare, Piscataway, NJ). IR700 conjugated with Panitumumab is abbreviated as Pan-IR700.
Cell culture
A human triple-negative breast cancer cell line, MDAMB468-GFP/luc [20], a human prostate cancer cell line, PC3-pip [21], and a human gastric cancer cell line N87-GFP [22], as NIR-PIT treatable tumors, and a murine preosteoblast cell line, MC3T3-E1 as a control, were used in this study. MDAMB468-GFP/luc, PC3-pip, and N87-GFP cells were cultured in an RPMI 1640 medium (GIBCO, Waltham, MA) supplemented with 10% FBS (GIBCO) and penicillin (100 units/mL)/streptomycin (100 μg/mL) (GIBCO) in a humidified incubator in an atmosphere of 95% air and 5% carbon dioxide at 37 degrees Celsius. MC3T3-E1 cells were maintained in ascorbic acid free α-minimum essential medium (αMEM, GIBCO) supplemented with 10% FBS and penicillin/streptomycin (maintenance medium).
Binding assay of Pan-IR700
Flowcytometric analysis was performed to evaluate the binding of Pan-IR700 to MDAMB468-GFP/luc cells or MC3T3-E1 cells. Half a million MDAMB468-GFP/luc cells or MC3T3-E1 cells were incubated with 1 μg of Pan-IR700 for 30 minutes on ice. After washing with PBS, cells were analyzed by FACSLyric (BD Biosciences, San Jose, CA) and FlowJo Software (Version 10.6.2; BD Biosciences). To confirm that Pan-IR700 bound to cells in an EGFR-specific manner, excess unconjugated panitumumab (10 μg) was added to block Pan-IR700.
Fluorescence microscopic studies
Morphological changes before and after NIR-PIT were observed using a fluorescence microscope (BX61; Olympus America Inc., Center Valley, PA, USA). Twenty thousand MDAMB468-GFP/luc cells with or without 2 × 104 MC3T3-E1 cells were seeded on a 6-well plate with a cover glass in, and cultured for 24 hours. The next day, Pan-IR700 was added to the culture medium at a final concentration of 10 μg/ml. After 3 hours of incubation at 37°C, the cover glass was picked up and washed with PBS. After setting the cover glass on the microscope, it was irradiated with a NIR-laser (690±5 nm, 150 mW/cm2) at a density of 16 J/cm2 using an ML7710 laser system (Modulight Inc, Tampere, Finland). Transmitted light differential interference contrast (DIC) images were acquired before and after NIR-PIT. A filter set consisting of an excitation bandpass filter from 590 to 650 nm and an emission bandpass filter from 665 to 740 nm was used to detect IR700 fluorescence.
In vitro NIR-PIT
The cytotoxic effects of NIR-PIT were determined by flow cytometric analysis as previously described. [23] In brief, 2×105 MDAMB468-GFP/luc cells or MC3T3-E1 cells were seeded into 12-well plates and cultured for 1 day. The medium was replaced with fresh culture medium containing 10 μg/mL of Pan-IR700 and incubated for 3 hours at 37°C. After washing with PBS, cells were irradiated with a NIR-laser (690±5 nm) at indicated energy density (ML7710, Modulight Inc, Tampere, Finland). Cells were detached 1 hour after irradiation, and propidium iodide (PI) was added. Samples were analyzed by FACSLyric and FlowJo Software. The cytotoxic effects of NIR-PIT were also examined by bioluminescence imaging. Ten million MDAMB468-GFP/luc were seeded in a 10 cm cell culture dish and cultured for 1 day. The medium was replaced with fresh culture medium containing 10 μg/mL of Pan-IR700 and incubated for 3 hours at 37°C. After washing with PBS, the dish was exposed to NIR-laser irradiation at the defined power density. After D-luciferin was added to the dish, bioluminescence images were acquired with Photon Imager (Biospace Lab, Nesles la Vallee, France). LDH cytotoxicity assay and CCK8 cell viability assay were performed using Cytotoxicity LDH Assay Kit-WST and Cell Counting Kit-8 (Dojindo Molecular Technologies, Rockville, MD). Twenty thousand MDAMB468-GFP/luc cells were seeded into 96-well plates and cultured for 1 day. Pan-IR700 was added to each well (final conc. 10 μg/mL), and cells were incubated for 3 hours at 37°C. After the medium change, cells were irradiated with NIR light at indicated energy density. The cells were further incubated for 1 hour at 37°C before LDH cytotoxicity, and CCK8 cell viability was measured. These assays were performed according to the manufacturer’s protocol.
Animal Models
All animal procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals (2010) by the National Research Council and approved by the National Cancer Institute Animal Care and Use Committee. 8-week-old female homozygote athymic nude mice were used (Charles River, NCI-Frederick, Frederick, MD). Mice were maintained under a 12h light-dark cycle. During experimental procedures, mice were anesthetized via inhalation of 2.5%–4.0% isoflurane or intraperitoneal injection of sodium pentobarbital (Nembutal Sodium Solution, Ovation Pharmaceuticals Inc., Deerfield, IL, USA). The bone metastasis model was established as previously reported. [24] In brief, 1×106 MDAMB468-GFP/luc, or PC3-pip, or N87-GFP cells were suspended in 100 μl PBS and injected rapidly via the caudal artery in the tail. Caudal artery injection of MDAMB468-GFP/luc cells results in bone metastases in the hind limbs (mainly in the distal femur and proximal tibia). The other two cell lines form multiple tumors not only in bone, but also in pelvic organs and soft tissues that shortened the survival of tumor-bearing mice. Four weeks later, bioluminescence imaging (BLI) was performed to confirm the formation of bone metastases. Mice with bone metastasis formation were used in subsequent experiments.
Bioluminescence imaging (BLI)
For acquisition of bioluminescence images, 200 μL of D-Luciferin diluted to 15 mg/ml was injected into the abdominal cavity of mice. Bioluminescence images were obtained with the Photon Imager 15 minutes after D-luciferin injection to estimate tumor burden. For quantifying the counts per minute of relative light units, regions of interest (ROIs) were manually drawn on the mouse hind limbs and analyzed with M3 Vision Software (Biospace Lab). To evaluate the therapeutic effect of NIR-PIT, the percentage decrease in luciferase activity was calculated using the following formula: [(relative light units after treatment) / (relative light units before treatment) × 100 (%)].
NIR-PIT for bone metastases
Tumor bearing mice were divided into three groups: No treatment group, APC i.v. group, and NIR-PIT group (n = 7–8, for each group). 100 μg of Pan-IR700 was administered via tail vein one day before NIR-PIT. The mouse knee area was irradiated with a NIR-laser (690±5 nm) at 30 J/cm2 energy density (ML7710).
Histological study
One day after NIR-PIT, mice were euthanized, and the bones of hind limbs were excised for histological studies. The resected bones (femur and tibia) were fixed with 10% neutral buffered formaldehyde, decalcified, and then embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (H&E). Microscopic pictures were obtained using an Olympus BX43 microscope (Olympus, Tokyo, Japan).
Micro Computed tomography (micro-CT)
Plain CT images of hind-limb bones were obtained with NanoSPECT/CT (BIOSCAN, Washington, D.C.). Mice were anesthetized with inhalation of 2.5% isoflurane. The following parameters were used for the acquisition of CT images: Frame Resolution Ultra Fine, Tube Voltage 55 kVp, Exposure Time 1000 msec, Number of Projections 360, and Pitch 1. Obtained data were reconstructed using InVivoScope version 1.43 software (BIOSCAN). The following parameters were used for CT reconstruction: Resolution Standard, Algorithm Exact Cone Beam FBP, FBP filter SheppLogan.
Statistical Analysis
All statistical analyses were performed with GraphPad Prism software version 8 (GraphPad Software, La Jolla, CA). Data were presented as mean ± standard error of mean (SEM) from a minimum of four experiments unless otherwise indicated. A one-way analysis of variance (ANOVA) followed by Tukey’s test was performed to compare cell viability. A two-way repeated measures ANOVA followed by Tukey’s test was used for the comparison of luciferase activities. Fisher’s exact t-test was used for the comparison of the analysis of necrotic foci formation. A p-value less than 0.05 was considered statistically significant.
Results
In vitro NIR-PIT using Pan-IR700 on MDAMB468-GFP/luc cells
First, we tested the specific binding of Pan-IR700 to MDAMB468-GFP/luc cells (Figure 1A). After incubating MDAMB468-GFP/luc cells with Pan-IR700, flow cytometry detected a high fluorescence signal in MDAMB468-GFP/luc cells. The signal was blocked by adding an excess amount of Panitumumab. These results showed Pan-IR700 specifically bound to MDAMB468-GFP/luc cells via hEGFR. Then, the cytotoxic effect of NIR-PIT using Pan-IR700 on MDAMB468-GFP/luc cells was examined in vitro. Incubation of MDAMB468-GFP/luc cells with Pan-IR700 followed by exposure to NIR light immediately induced cell swelling, bleb formation and rupture (Figure 1B). Quantitative assessment of the cytotoxic effect of NIR-PIT by flow cytometry with PI staining, bioluminescence imaging, WST-1 assay, and LDH cytotoxicity assay showed cell death increasing in a light-dose dependent manner (Figure 1C–F). Exposure to NIR light alone or APC alone failed to show cytotoxicity (Figure 1C).
Figure 1. In vitro efficacy of NIR-PIT using Pan-IR700 on MDAMB468-GFP/luc cells.
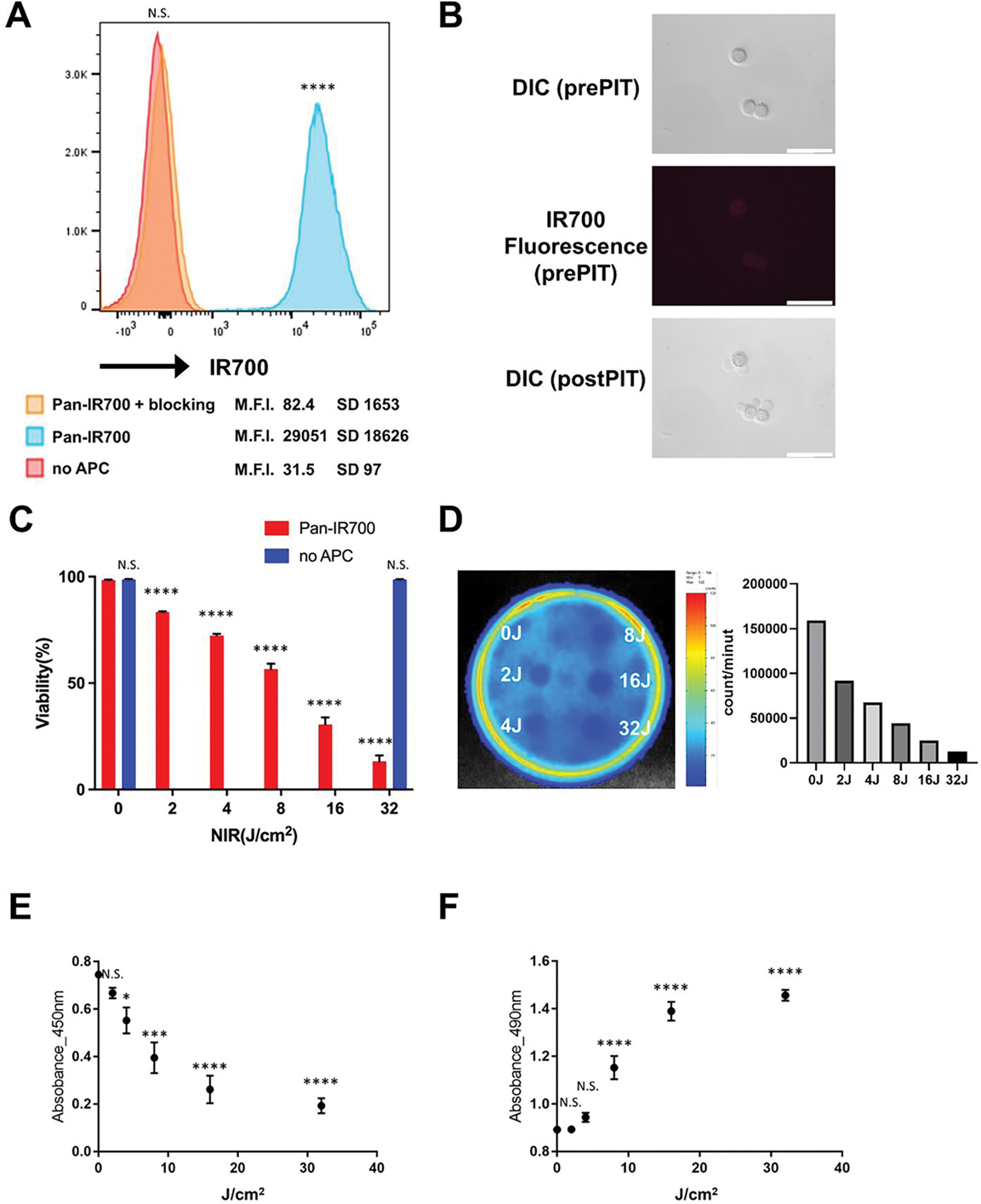
(A) Binding assay of Pan-IR700 to MDAMB468-GFP/luc cells using flow cytometry. Red, blue and orange histograms show unstained, Pan-IR700 staining without blocking and with blocking, respectively. (M.F.I., mean fluorescence intensity of 95,000 live cells) ****p <0.0001 vs unstained cells (B) Differential interference contrast (DIC) images and IR700 fluorescence images before and after NIR-PIT. White scale bar = 50 μm. (C) Light dose-dependent cell death in MDAMB468-GFP/luc cells induced by NIR-PIT using Pan-IR700. Cell viability was determined by flow cytometry with PI staining. Data are shown as mean ± SEM (n = 4; ****p < 0.0001 vs no light exposure group; N.S., not significant). (D) Dose-dependent cytotoxicity in MDAMB468-GFP/luc cells induced by NIR-PIT using Pan-IR700. Bioluminescence imaging showed decreased luciferase activities in a NIR-light dose-dependent manner. (E) Evaluation of cell viability by WST-1 assay. Data are shown as mean ± SEM (n = 4; ****p < 0.0001 vs no light exposure group; N.S., not significant) (F) Evaluation of cell viability by LDH cytotoxicity assay. Data are shown as mean ± SEM (n = 4; *p < 0.05, ***p < 0.001, ****p < 0.0001 vs no light exposure group; N.S., not significant)
Therapeutic efficacy of NIR-PIT in bone metastasis
Next, we investigated the therapeutic effect of NIR-PIT on bone metastases. For this experiment, mice was prepared by caudal artery injection of MDAMB468-GFP/luc cells. Figure 2A shows the treatment regimen and imaging protocol. After confirming bone metastasis formation in the mouse hindlimbs by bioluminescence imaging, the mice were divided into three groups: No treatment group, APC i.v. group and NIR-PIT group. One day after the intravenous injection of 100 μg of Pan-IR700, the hind limbs of mice in the NIR-PIT group were irradiated with NIR light (30J/cm2) (Figure 2B). The therapeutic effect was evaluated by the change in luciferase activity before and after treatment. Luciferase activity in the NIR-PIT group was significantly lower than those in the other two groups. On the other hand, administration of Pan-IR700 alone had no therapeutic effect (Figure 2C and 2D). One day after treatment, bone samples were collected for histological analysis. Formalin-fixed paraffin-embedded sections of mouse hindlimb bones were stained with hematoxylin and eosin (Figure 2E). H&E images of normal murine bone were obtained as controls in Figure 2E. While normal murine bone marrow was filled with hematopoietic cells, the bone marrow of the bone metastasis model mice was replaced by tumor cells. These pathological findings are consistent with what occurs in humans. The bone marrow of the no-treatment group and the APC i.v. only group was filled with viable tumor cells, whereas the tumor cells in the bone marrow of the NIR-PIT group were necrotic. These results demonstrated that NIR-PIT was effective in killing bone metastases.
Figure 2. Therapeutic effect of NIR-PIT for naturally grown bone metastasis.
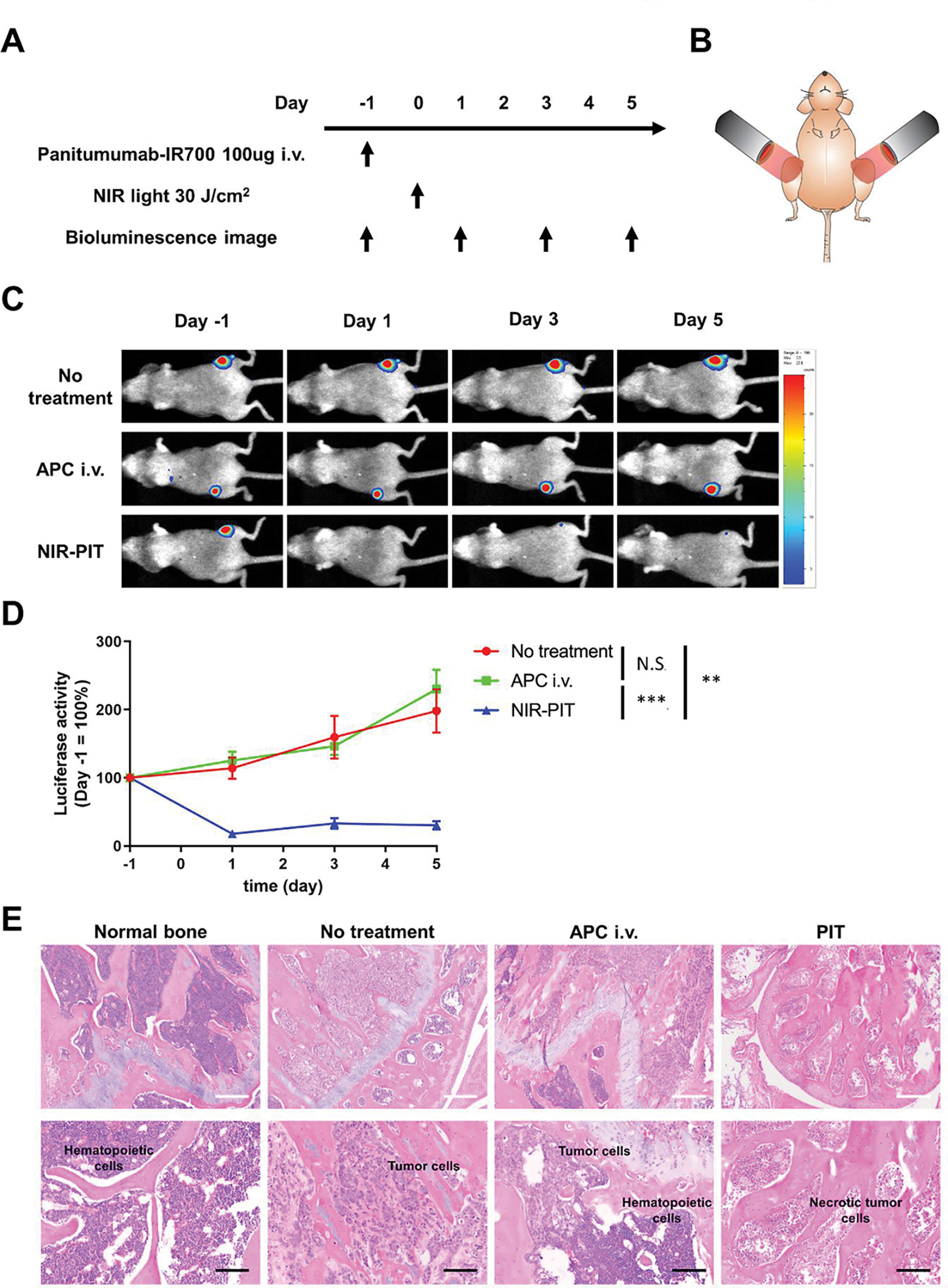
(A) Treatment protocol. Bioluminescence images were acquired at each time point as indicated. (B) Schema of NIR light exposure. NIR light was irradiated around the knee of the mouse. (C) Representative bioluminescence images of mice with bone metastases from each group. (D) Quantitative assessment of luciferase activity (values before treatment were set to 100%; n = 7–8 per group; two-way repeated measures ANOVA followed by Tukey’s multiple comparison test; **p < 0.01, ***p < 0.001; N.S., not significant). (E) Hematoxylin and eosin staining of mouse hind leg bone specimens excised 1 day after NIR-PIT (White scale bars, 100 μm; black scale bars, 50 μm). Necrotic foci which are shown in the image with NIR-PIT are present in all specimens of the treated group (n=3), yet are not shown in any control group (n=3). (p<0.05)
Therapeutic efficacy of repeated NIR-PIT for bone metastasis
The therapeutic effect of repeated NIR-PIT on bone metastases was also examined. Mice in the APC i.v. group were intravenously injected with Pan-IR700 once a week for 5 weeks. Mice in the repeated NIR-PIT group underwent NIR-PIT once a week for 5 weeks. Bioluminescence images were acquired once a week for 5 weeks. Micro CT was performed once every 2 weeks for 6 weeks (Figure 3A). Luciferase activities in the repeated NIR-PIT group were significantly lower than those in the other two groups. On the other hand, the administration of Pan-IR700 alone had no significant therapeutic effect (Figure 3B and 3C).
Figure 3. Therapeutic effect of repeated NIR-PIT for bone metastasis.
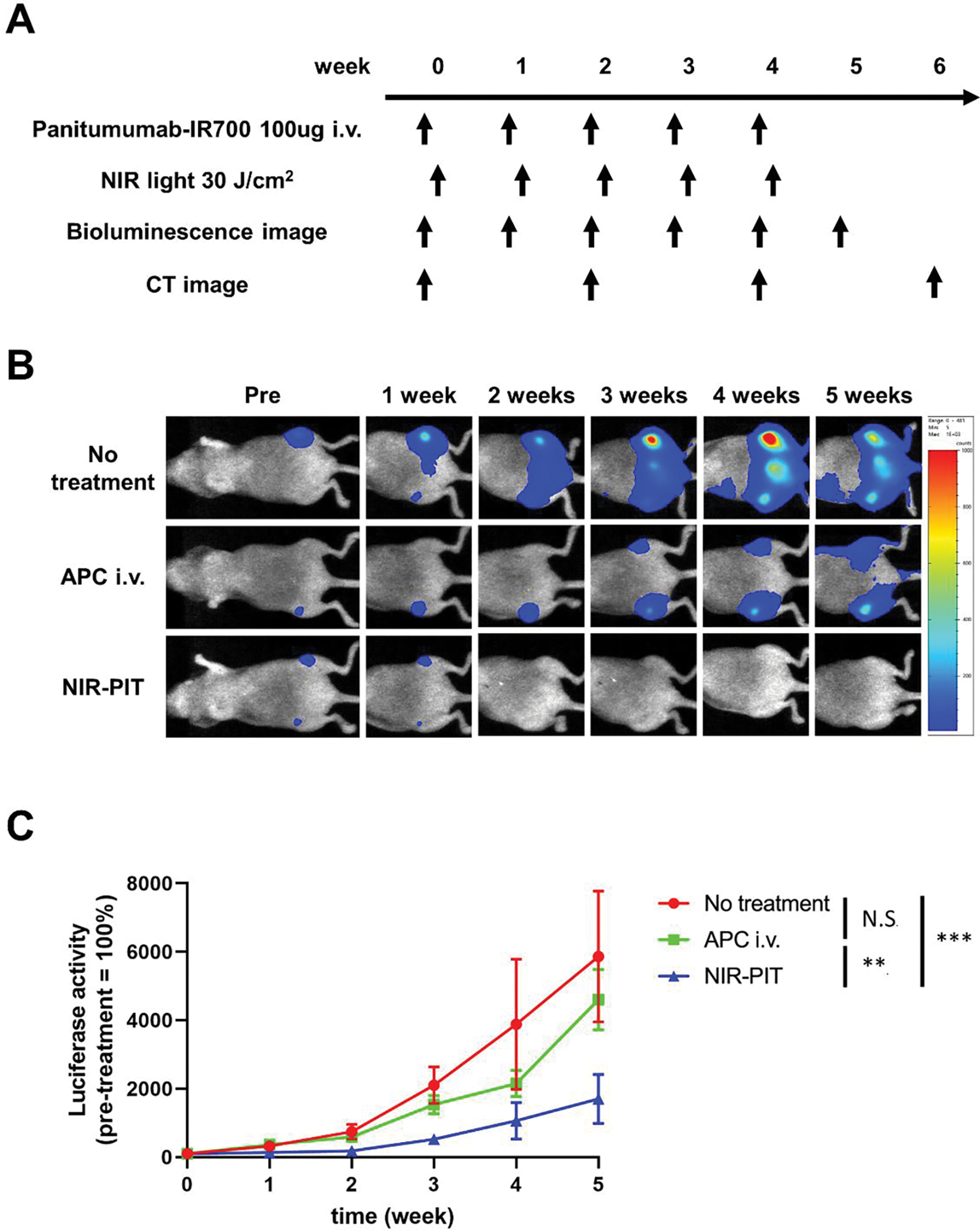
(A) Treatment protocol. Bioluminescence images were acquired at each time point as indicated. (B) Representative bioluminescence images of mice with bone metastases from each group. (C) Quantitative analysis of luciferase activity (values before treatment were set to 100%; n = 7–8 per group; two-way repeated measures ANOVA followed by Tukey’s multiple comparison test; **p < 0.01, ***p < 0.001; N.S., not significant).
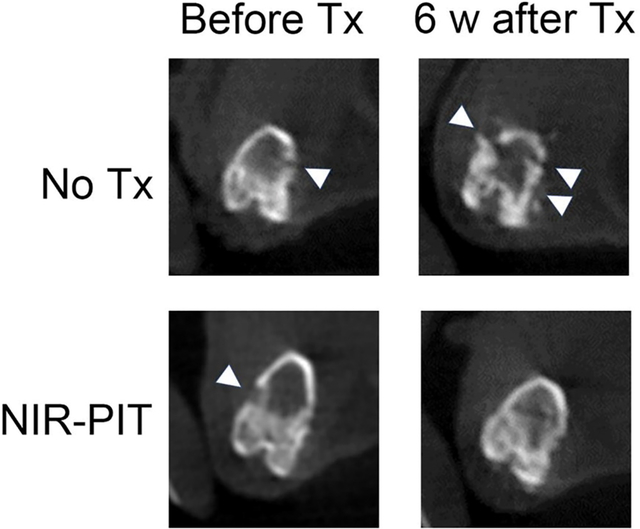
Next, morphological changes of hind-limb bones were evaluated using micro-CT (Figure 4). Normal bones maintained the continuity of the bone cortex, whereas metastatic bones showed destruction of the bone cortex. In the repeated NIR-PIT group, the destroyed bones were gradually repaired, and the continuity of the bone cortex was restored.
Figure 4. Micro-CT images before and after NIR-PIT.
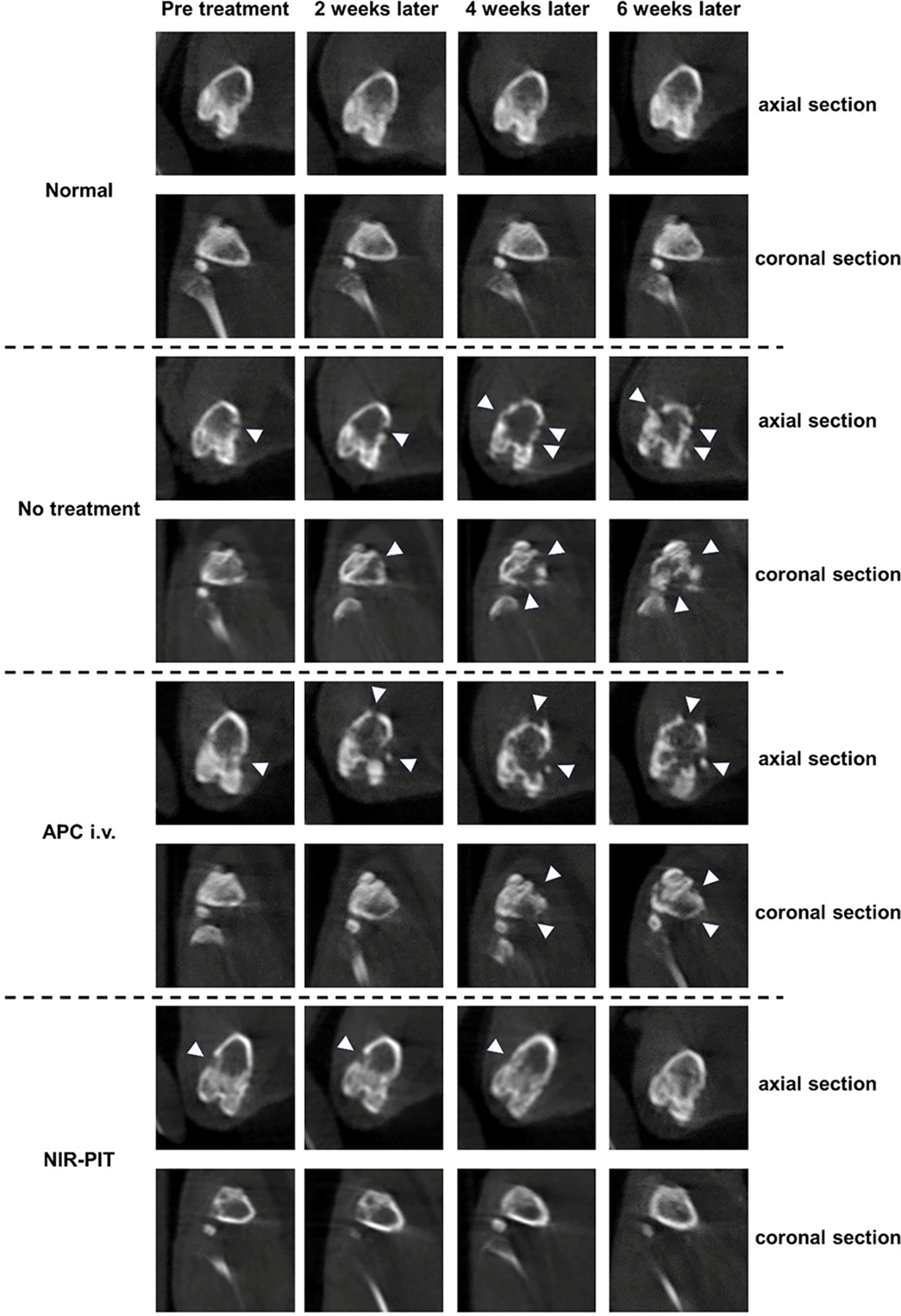
Plain computed tomography (CT) images of mouse hind-limb bones. CT scans were taken every 2 weeks. White arrowheads indicate destructed parts of bone cortex. (upper: axial section, lower: coronal section) Radiological changes of the bone that were separately evaluated by two board-certified radiologists (H.W and H.K) are consistent in all mice (n=3) in each group.
Discussion
NIR-PIT has proven to be an effective therapy in many soft tissue tumors both in mouse models and humans. However, there is doubt whether it will be useful in bony lesions given the limited transmittance of light in such tissues. Several previous studies have examined the transmittance of near-infrared light through various bone samples and shown that about 4.5% of light with a wavelength of around 690 nm, the excitation wavelength of IR700, was transmitted through a 5 mm thick bone fragment. This small amount of transmitted light appears to be sufficient to induce NIR-PIT and offers a potential application of NIR-PIT in treating bone tumors. [25] Therefore, in the present study, we performed NIR-PIT on bone metastatic lesions and found that NIR-PIT was quite effective in the treatment of bone metastases in this mouse model.
A variety of animal models have been used and each have strengths and limitations. Intraosseous, intracardiac, intravenous and orthotopic inoculations have been reported to generate animal models of bone metastasis. [26, 27] Although left ventricular intracardiac injection of cancer cells is a standard technique, this method is technically demanding and can also result in brain metastases. Brain metastases are life-shortening and often do not permit animals to be followed-up for sufficient periods of time. Long-term observation was necessary for this study; therefore, we employed a caudal artery injection method to establish bone metastases. [24] This method allows selective delivery of MDAMB468-GFP/luc cancer cells to the hindlimb bone marrow and can successfully mimic the early stage of bone metastasis. Other cell lines, such as PC3-pip and N87-GFP cells when injected in a similar manner caused pelvic and soft tissue masses that shortened overall survival.
Our study has several limitations. The first limitation is that the small size of mouse bones does not represent the human situation well. In this study, mice were irradiated with NIR light externally. Although NIR light was attenuated to some extent, the amount of light penetrating into the bone marrow space was sufficient for the treatment of the murine bone metastasis model. However, when considering a clinical application to humans, efficient NIR light delivery methods that allows light to reach deep into the body will be required. CT-guided placement of cylindrical light diffusers would allow NIR light delivery into deep bone lesions. The immune response generated in humans might generate abscopal effects in other lesions. There is precedence for inserting probes into bone metastases for therapeutic purposes. Radiofrequency ablation (RFA) and cryoablation for bone metastases require the insertion of similar probes to physically disrupt the metastasis. [28, 29] NIR-PIT will require similar intervention to place optical catheters. A second limitation is the use of an athymic mouse model, which does not recapitulate the intact tumor immune microenvironment. The interactions between host cells such as osteoblasts and osteoclasts and tumor cells are important for the development of bone metastasis occurring naturally. This is not possible because of the species mismatch between the human cells and the murine stroma. Thus, the immune response after immunogenic cell death of cancer cells induced by NIR-PIT could not be studied in this model. Additionally, we could successfully establish this EGFR-positive, bone metastasis model only with the MDAMB468-GFP/luc triple-negative breast cancer cells because other cell lines injected in a similar manner grew tumors in the soft tissue and pelvic organs shortening mouse survival. Nonetheless, we were gratified to see the successful flattening of the growth curves and repair of the bone cortex structure without the benefit of the immune response, which bodes well for clinical translation. Further study using syngeneic mouse cancer cell lines will be needed to explore the immune effects of NIR-PIT of bone metastasis.
In conclusion, this study demonstrated that NIR-PIT was effective for the treatment of bone metastases without affecting osteogenesis. Although many challenges remain to be solved for clinical applications, NIR-PIT could be a promising treatment option for bone metastases.
Highlights.
NIR-PIT targeting EGFR has been approved for clinical use u in Japan.
EGFR is expressing on 50–70% of triple negative breast cancer.
Bone metastasis model of EGFR-positive, triple negative breast cancer is established.
NIR-PIT successfully treated bone metastasis with repairing bone cortex structure.
NIR-PIT has a potential for clinical application to the treatment of bone metastases.
Funding Information
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research [grant numbers ZIA BC011513].
Footnotes
Conflict of Interest
The authors have no conflict of interest.
Disclosure:
- Approval of the research protocol by an Institutional Reviewer Board: N/A
- Informed Consent: N/A
- Registry and the Registration No. of the study/trial: N/A
- Animal Studies: All in vivo procedures were conducted in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996), US National Research Council, and approved by the local Animal Care and Use Committee (MIP-003; project number P214396).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Huang JF, Shen J, Li X, Rengan R, Silvestris N, Wang M, Derosa L, Zheng X, Belli A, Zhang XL, Li YM, Wu A, Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study, Ann Transl Med, 8 (2020) 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Svensson E, Christiansen CF, Ulrichsen SP, Rorth MR, Sorensen HT, Survival after bone metastasis by primary cancer type: a Danish population-based cohort study, BMJ Open, 7 (2017) e016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Silvestris N, Pantano F, Ibrahim T, Gamucci T, De Vita F, Di Palma T, Pedrazzoli P, Barni S, Bernardo A, Febbraro A, Satolli MA, Bertocchi P, Catalano V, Giommoni E, Comandone A, Maiello E, Riccardi F, Ferrara R, Trogu A, Berardi R, Leo S, Bertolini A, Angelini F, Cinieri S, Russo A, Pisconti S, Brunetti AE, Azzariti A, Santini D, Natural history of malignant bone disease in gastric cancer: final results of a multicenter bone metastasis survey, PLoS One, 8 (2013) e74402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim SU, Kim DY, Park JY, Ahn SH, Nah HJ, Chon CY, Han KH, Hepatocellular carcinoma presenting with bone metastasis: clinical characteristics and prognostic factors, J Cancer Res Clin Oncol, 134 (2008) 1377–1384. [DOI] [PubMed] [Google Scholar]
- [5].Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH, Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States, BMC Cancer, 18 (2018) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Coleman R, Hadji P, Body JJ, Santini D, Chow E, Terpos E, Oudard S, Bruland O, Flamen P, Kurth A, Van Poznak C, Aapro M, Jordan K, E.G.C.E.a. clinicalguidelines@esmo.org, Bone health in cancer: ESMO Clinical Practice Guidelines, Ann Oncol, 31 (2020) 1650–1663. [DOI] [PubMed] [Google Scholar]
- [7].Shibata H, Kato S, Sekine I, Abe K, Araki N, Iguchi H, Izumi T, Inaba Y, Osaka I, Kato S, Kawai A, Kinuya S, Kodaira M, Kobayashi E, Kobayashi T, Sato J, Shinohara N, Takahashi S, Takamatsu Y, Takayama K, Takayama K, Tateishi U, Nagakura H, Hosaka M, Morioka H, Moriya T, Yuasa T, Yurikusa T, Yomiya K, Yoshida M, Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology, ESMO Open, 1 (2016) e000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, Sharma M, Ripamonti CI, Committee EG, Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines, Ann Oncol, 29 (2018) iv166–iv191. [DOI] [PubMed] [Google Scholar]
- [9].Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H, Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules, Nat Med, 17 (2011) 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kobayashi H, Choyke PL, Near-Infrared Photoimmunotherapy of Cancer, Acc Chem Res, 52 (2019) 2332–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kobayashi H, Griffiths GL, Choyke PL, Near-Infrared Photoimmunotherapy: Photoactivatable Antibody-Drug Conjugates (ADCs), Bioconjug Chem, 31 (2020) 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sato K, Ando K, Okuyama S, Moriguchi S, Ogura T, Totoki S, Hanaoka H, Nagaya T, Kokawa R, Takakura H, Nishimura M, Hasegawa Y, Choyke PL, Ogawa M, Kobayashi H, Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy, ACS Cent Sci, 4 (2018) 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nagaya T, Nakamura Y, Okuyama S, Ogata F, Maruoka Y, Choyke PL, Kobayashi H, Near-Infrared Photoimmunotherapy Targeting Prostate Cancer with Prostate-Specific Membrane Antigen (PSMA) Antibody, Mol Cancer Res, 15 (2017) 1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lum YL, Luk JM, Staunton DE, Ng DKP, Fong WP, Cadherin-17 Targeted Near-Infrared Photoimmunotherapy for Treatment of Gastrointestinal Cancer, Mol Pharm, 17 (2020) 3941–3951. [DOI] [PubMed] [Google Scholar]
- [15].Kiss B, van den Berg NS, Ertsey R, McKenna K, Mach KE, Zhang CA, Volkmer JP, Weissman IL, Rosenthal EL, Liao JC, CD47-Targeted Near-Infrared Photoimmunotherapy for Human Bladder Cancer, Clin Cancer Res, 25 (2019) 3561–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Inagaki FF, Kato T, Furusawa A, Okada R, Wakiyama H, Furumoto H, Okuyama S, Choyke PL, Kobayashi H, Disialoganglioside GD2-Targeted Near-Infrared Photoimmunotherapy (NIR-PIT) in Tumors of Neuroectodermal Origin, Pharmaceutics, 14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cognetti DM, Johnson JM, Curry JM, Kochuparambil ST, McDonald D, Mott F, Fidler MJ, Stenson K, Vasan NR, Razaq MA, Campana J, Ha P, Mann G, Ishida K, Garcia-Guzman M, Biel M, Gillenwater AM, Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma, Head Neck, 43 (2021) 3875–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tahara M, Okano S, Enokida T, Ueda Y, Fujisawa T, Shinozaki T, Tomioka T, Okano W, Biel MA, Ishida K, Hayashi R, A phase I, single-center, open-label study of RM-1929 photoimmunotherapy in Japanese patients with recurrent head and neck squamous cell carcinoma, Int J Clin Oncol, 26 (2021) 1812–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fujimura D, Inagaki F, Okada R, Rosenberg A, Furusawa A, Choyke PL, Kobayashi H, Conjugation Ratio, Light Dose, and pH Affect the Stability of Panitumumab-IR700 for Near-Infrared Photoimmunotherapy, ACS Med Chem Lett, 11 (2020) 1598–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nagaya T, Sato K, Harada T, Nakamura Y, Choyke PL, Kobayashi H, Near Infrared Photoimmunotherapy Targeting EGFR Positive Triple Negative Breast Cancer: Optimizing the Conjugate-Light Regimen, PLoS One, 10 (2015) e0136829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Watanabe R, Hanaoka H, Sato K, Nagaya T, Harada T, Mitsunaga M, Kim I, Paik CH, Wu AM, Choyke PL, Kobayashi H, Photoimmunotherapy targeting prostate-specific membrane antigen: are antibody fragments as effective as antibodies?, J Nucl Med, 56 (2015) 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nagaya T, Nakamura Y, Sato K, Harada T, Choyke PL, Kobayashi H, Improved micro-distribution of antibody-photon absorber conjugates after initial near infrared photoimmunotherapy (NIR-PIT), J Control Release, 232 (2016) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Inagaki FF, Fujimura D, Furusawa A, Okada R, Wakiyama H, Kato T, Choyke PL, Kobayashi H, Fluorescence Imaging of Tumor-Accumulating Antibody-IR700 Conjugates Prior to Near-Infrared Photoimmunotherapy (NIR-PIT) Using a Commercially Available Camera Designed for Indocyanine Green, Mol Pharm, 18 (2021) 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kuchimaru T, Kataoka N, Nakagawa K, Isozaki T, Miyabara H, Minegishi M, Kadonosono T, Kizaka-Kondoh S, A reliable murine model of bone metastasis by injecting cancer cells through caudal arteries, Nat Commun, 9 (2018) 2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nakamura YA, Okuyama S, Furusawa A, Nagaya T, Fujimura D, Okada R, Maruoka Y, Eclarinal PC, Choyke PL, Kobayashi H, Near-infrared photoimmunotherapy through bone, Cancer Sci, 110 (2019) 3689–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Simmons JK, Hildreth BE 3rd, Supsavhad W, Elshafae SM, Hassan BB, Dirksen WP, Toribio RE, Rosol TJ, Animal Models of Bone Metastasis, Vet Pathol, 52 (2015) 827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dai J, Hensel J, Wang N, Kruithof-de Julio M, Shiozawa Y, Mouse models for studying prostate cancer bone metastasis, Bonekey Rep, 5 (2016) 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dupuy DE, Liu D, Hartfeil D, Hanna L, Blume JD, Ahrar K, Lopez R, Safran H, DiPetrillo T, Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial, Cancer, 116 (2010) 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tomasian A, Jennings JW, Hot and Cold Spine Tumor Ablations, Neuroimaging Clin N Am, 29 (2019) 529–538. [DOI] [PubMed] [Google Scholar]


