Highlights
-
•
Phenolic profiles of proso millet were modified substantially through germination.
-
•
Feruloylquinic acid and N,N′-bis-(p-coumaroyl) putrescine were firstly identified.
-
•
Germination significantly improved antioxidant activity of proso millet.
-
•
Germinated proso millets could serve as healthy ingredients in various foods.
Keywords: Proso millet, Germination process, UPLC-ESI-MS/MS, Polyphenols, Antioxidant capacity
Abstract
Changes in phenolic profiles and antioxidant activity of three varieties of proso millet during germination were investigated. Total phenolic content (TPC) and total flavonoid content (TFC) increased significantly with prolongation in germination period. After germination for 6 days, TPC of the free and bound fractions increased 6.30–8.66-fold and 77.65–116.18%, respectively. The free and bound phenolic compounds identified by UPLC-MS/MS, displayed significant variations. Feruloylquinic acid and N,N′-bis-(p-coumaroyl)-putrescine biosynthesized during germination, are reported for the first time in proso millets. Other phenolics including trans- and cis-ferulic, trans-p-coumaric, vanillic acid and ferulic acid dimers (DFAs) were increased significantly along with a new DFA (8,5′-DFA) seemingly produced during germination. The germinated proso milllets displayed superior antioxidant activity than the corresponding ungerminated samples indicating that germination could be one applicable method for improving phenolic profiles and antioxidant capacity of proso millets. Thus germinated proso millet could be exploited as a functional ingredient in several products.
Introduction
Proso millet (Panicum miliaceum L.) likely originated from the Loess Plateau area in China and it is generally considered as one of the major cereal crops in the arid and semi-arid regions of China (Hunt et al., 2018). Proso millet shows a high nutritional value providing significant amounts of nutrients including carbohydrates, proteins, vitamins and minerals along with some other phytochemical components such as flavonoids, phenolic acid derivatives and alkaloids (Shen et al., 2018) which exhibit various physiological functional properties including antioxidant, hypoglycemic, hypolipidemic, inhibition of cancer cell proliferation, prevention of liver damage (Kumari et al., 2017, Park et al., 2008, Zhang et al., 2014).
Proso millet has been reported to have good potential on prevention of cardiovascular disease, type 2 diabetes, cancer and obesity (Andersson et al., 2014, Saleh et al., 2013). The health functions of proso millets are largely attributed to their different sorts of antioxidant phytochemicals, particularly phenolic compounds. However, the use of proso millets in the food system is limited partly due to the presence of some anti-nutritional factors, such as phytic acid and protease inhibitors.
Some applicable food processing technologies, such as germination, cooking and fermentation, are known to reduce these undesirable components, thus improving their acceptability and nutritional quality (Pradeep & Sreerama, 2015). Germination allows grains not only to soften texture and facilitate processing, but also to improve palatability, flavor, and safety (Aparicio-Garcia et al., 2021, Arouna et al., 2020). Moreover, germination is considered as an important processing method for low-cost and sustainable improvement in nutritional quality and health benefits of grains. It has been reported that proper germination treatment could reduce or eliminate anti-nutritional factors in grains (Wang et al., 2015), improve the utilization of nutrients such as minerals and vitamins (Cho and Chung, 2020, Kapravelou et al., 2020), and also increase the bioavailability and digestibility of polyphenols in grains (Gong, Chen, Li, Sun, & Liu, 2018). As a part of the life cycle of grain seeds, germination has a very complex mechanism and process, which activates the metabolic activity of grains following soaking. Subsequently, kernel modification and enzyme synthesis take place which could result in improvement of intrinsic phytochemicals and antioxidant capacity during the germination process (Xu et al., 2009).
Phenolic profiles and antioxidant activity of whole grains are likely modified significantly via the germination process. Until now, limited research has been carried out on the effects of germination on phenolic components and antioxidant activity of proso millet. Therefore, the main purpose of this study were (1) to investigate dynamic changes in the free and bound phenolic contents and their antioxidant activities, and (2) to characterize the phenolic profiles and the dynamic changes of the individual phenolic contents in free and bound forms at different germination stages. The findings will contribute to the exploration and utilization of germinated proso millet as a healthy food material.
Materials and methods
Materials
Reagents and standards
Folin-Ciocalteu reagent, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2′-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), were acquired from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). HPLC and MS grade methanol, and formic acid were purchased from Thermo Fisher Scientific Reagent Co., Ltd. (Waltham, Massachusetts, USA). The polyphenol standards, including caffeic acid, chlorogenic acid, protocatechualdehyde, p-hydroxybenzaldehyde, p-hydroxybenzoic acid, p-coumaric acid, protocatechuic acid, ferulic acid, vanillic acid and rutin, were acquired from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). All the other regular chemicals, including sodium carbonate (Na2CO3), ferric trichloride (FeCl3), sodium hydroxide (NaOH), aluminum trichloride (AlCl3), sodium nitrite (NaNO2) ethyl acetate and N-hexane, were acquired from Tianjin Deen Chemical Reagent Co., Ltd. (Tianjin, China).
Proso millets
The proso millet samples were harvested from Shaanxi and Gansu provinces in China and classified into three different categories based on the growing location and grain varietal characteristics (YL-B: from Yulin, Shaanxi Province, with black seed coat; QY-R: from Qingyang, Gansu Province, with red seed coat; DX-Y: from Dingxi, Gansu Province, with yellow seed coat).
Germination procedure
The raw, ungerminated proso millet samples were recorded as G0. The proso millet grains were washed and soaked in distilled water for 12 h in the dark. Excess water was drained and some of the samples were taken out as soaked samples and recorded as S1. The other samples were evenly spread in a self-made germination plate and germinated at constant temperature inside a humidity incubator (160HL, Jinyi Instrument, Jiangsu, China) at 25 °C and 90% relative humidity for 6 days. Samples were collected every 2 days, and recorded as G2, G4 and G6, respectively. All samples were freeze-dried immediately after collecting, pulverized with a grinder, and the powder samples were sieved through mesh 40. The ground samples were stored at −80 °C until extraction and analysis.
Methods
Extraction of phenolic compounds
The free and bound phenolic fractions were obtained using our reported method (Xiang, Apea-Bah, Ndolo, Katundu, & Beta, 2019). The ground proso millet sample was defatted for 15 min with N-hexane (1:10 w/v), and the process was repeated. Then, the defatted sample was mixed and extracted with 80% methanol solution for 1 h under dark. The extraction process was repeated twice, and supernatant was collected and combined to obtain the free phenolic extract.
The residue after extracting free phenolics was air dried at room temperature and then digested with NaOH solution (2 mol L-1). After hydrolyzing for 2 h, the resulting slurry was adjusted to pH 1.5–2.0 with HCl solution (6 mol L-1), centrifuged to obtain the hydrolysate, and then extracted three times with ethyl acetate (1:1 v/v) to get the bound phenolic extract. All the resultant liquid extracts were evaporated to dryness with rotary evaporator, and then re-dissolved in 50% methanol solution to obtain the phenolic fractions for the subsequent analysis.
Total phenolic content
The total phenolic content (TPC) of the sample was determined by using the colorimetric method, according to Xiang, Zhang, Apea-Bah, and Beta (2019). Firstly, 20 μL of the properly diluted extract of sample was withdrawn and mixed well with 40 μL of Folin-Ciocalteu working reagent in a microplate (BIOLAND Biotechnology Co., Ltd. Hangzhou, China). The mixture was kept under dark for 5 min at room temperature. Then 160 μL of saturated Na2CO3 solution was added to each microplate well and the reaction allowed to proceed in darkness at 25 °C for 1.5 h. The absorbance at wavelength of 750 nm was read on a microplate reader (BIO-RAD Instruments Inc., USA). Ferulic acid was used as the standard, and the TPC of the sample was calculated and expressed as milligrams of ferulic acid equivalents per kilogram (mg FAE/kg) of sample.
Total flavonoid content
The total flavonoid content (TFC) of sample was quantified with AlCl3 colorimetric method (Xiang, Yang, Beta, Liu, & Yang, 2019). Firstly, 0.5 mL of the phenolic extract and 1.0 mL of distilled water were added in a 5 mL plastic tube, followed by mixing with 150 μL of 10% AlCl3 solution and 75 μL of 5% NaNO2 solution. The mixture was then kept for 1 min followed by adding 0.5 mL of 4% NaOH solution and a certain amount of distilled water to make the total volume of the mixture to 2.5 mL before incubating at 25 °C. The absorbance was read at 510 nm on the microplate reader after incubation for 15 min. Rutin was used as the standard, and the TFC was calculated and expressed as milligram of rutin equivalents (RE) per kilogram (mg RE/kg) of sample.
ABTS assay
The measurement of ABTS·+ scavenging capacity was performed according to our reported method (Xiang, Li, Ndolo, & Beta, 2019). In brief, the stock solution of ABTS radical cations was diluted with ethanol to get the dilute solution of ABTS radical cations as the working solution with an absorbance of about 0.90 at 750 nm. Subsequently, 190 μL of the freshly prepared working ABTS·+ solution and 10 μL of the properly diluted phenolic extract of sample were mixed thoroughly. Absorbance of the mixture was read at 750 nm on the microplate reader after the reaction at 25 °C for 30 min. Trolox was used as the standard and the ABTS radical cations scavenging capacity of the extract was calculated and expressed as micromoles of Trolox equivalents per gram (μmol TE/g) of sample.
DPPH assay
The DPPH radical scavenging capacity of the sample was determined by the previously reported procedure (Xiang, Apea-Bah, et al., 2019). Briefly, 10 μL of the properly diluted extract of sample and 190 μL of 60 μmol L-1 DPPH radical solution were added into a microplate. The mixture was reacted for 30 min at 25 °C. Subsequently, the absorbance was read at 515 nm on the microplate reader. Trolox was used as the standard. DPPH radical scavenging capacity of the extract was calculated and expressed μmol TE/g of sample.
FRAP assay
The FRAP assay was applied to measure the reducing capacity of the phenolic extract according to our previously published method (Zhang et al., 2021). Firstly, 10 μL of each phenolic extract of proso millet sample was combined with 300 μL ferric-TPTZ solution, which was composed of 300 mmol L-1 acetate buffer, 10 mmol L-1 TPTZ in 40 mmol L-1 hydrochloric acid solution, and 20 mmol L-1 FeCl3 solution, in the volume ratio of 10:1:1. After the mixture reacted at 25 °C for 2 h, the absorbance was measured at 600 nm. The FRAP of the phenolic extract was quantified as μmol TE/g of sample.
Quantification of the individual polyphenols and UPLC-ESI-QqQ-MS/MS conditions
An UPLC (Waters UPLC H-Class) coupled to a QqQ-MS (Waters Xevo TQ- S/micro) was applied for the qualitative and quantitative analysis of phenolic compounds. A C18 column (100 × 3 mm, Thermo Fisher Scientific, Waltham, USA) was used, and the column temperature was set at 25 °C. The binary mobile phase composed of pure water containing 0.1% formic acid and methanol containing 0.1% formic acid was applied and flow rate was set at 0.4 mL/min. A 25 min linear gradient was programmed as follows: 0–3.81 min, 9–14% B; 3.81–4.85 min, 14–15% B; 4.85–5.89 min, 15% B; 5.89–8.32 min, 15–17% B; 8.32–9.71 min,17–19% B; 9.71–10.40 min, 19% B; 10.40–12.48 min, 19–26% B; 12.48–13.17 min, 26–28% B; 13.17–14.21 min, 28–35% B; 14.21–15.95 min, 35–40% B; 15.95–16.64 min, 40–48% B; 16.64–18.37 min, 48–53% B; 18.37–22.53 min, 53–70% B; 22.53–22.88 min, 70–9% B; 22.88–25.00 min, 9% B, according to Zheng, Yuan, et al. (2022). Ten μL phenolic extract was injected, and the phenolic components was identified by comparing retention times (RTs) with authentic standards or putatively identified by comparing UV spectra and MS/MS information available in related literatures. The QqQ-MS was calibrated and run in negative mode through the mass range of 50–1000 with the resolution of 5. Full mass spectra were recorded by using a cone voltage of 20 V and a capillary voltage of 1 kV. The flow rates of desolvation gas (N2) and cone gas (He) were set at 1000 and 50 L/h, respectively. The collision energies of 20 V, the desolvation gas temperature of 500 °C, and the ion source temperature of 150 °C were set separately to gain the MS2 spectra of polyphenol compounds.
The phenolics were quantified by using external calibration curves following the method of Zheng, Ding et al. (2022). The contents of individual phenolic compounds were calculated from their peak areas at wavelengths of 320 nm for hydroxycinnamic acid derivatives (chlorogenic acid, caffeic acid, p-coumaric acid and ferulic acid), and 280 nm for hydroxybenzonic acid derivatives (vanillic acid, p-hydroxybenzoic acid, p-hydroxybenzaldehyde and protocatechualdehyde). Where standard was unavailable, the phenolic derivative was quantified as equivalent of an analogue or the corresponding aglycone. The content of each phenolic compound was presented as milligrams per kilogram (mg/kg) of sample.
Data processing and statistical analysis
Three parallel experiments were carried out, and all the data were processed by IBM SPSS Statistics (Version 26, IBM Corp., Armonk, NY, USA). The data were presented as mean ± standard deviation (SD). Differences of mean values were carried out by using ANOVA followed by Tukey’s HSD test, and p < 0.05 were considered statistically significant.
Results and discussion
Changes of TPC during germination process
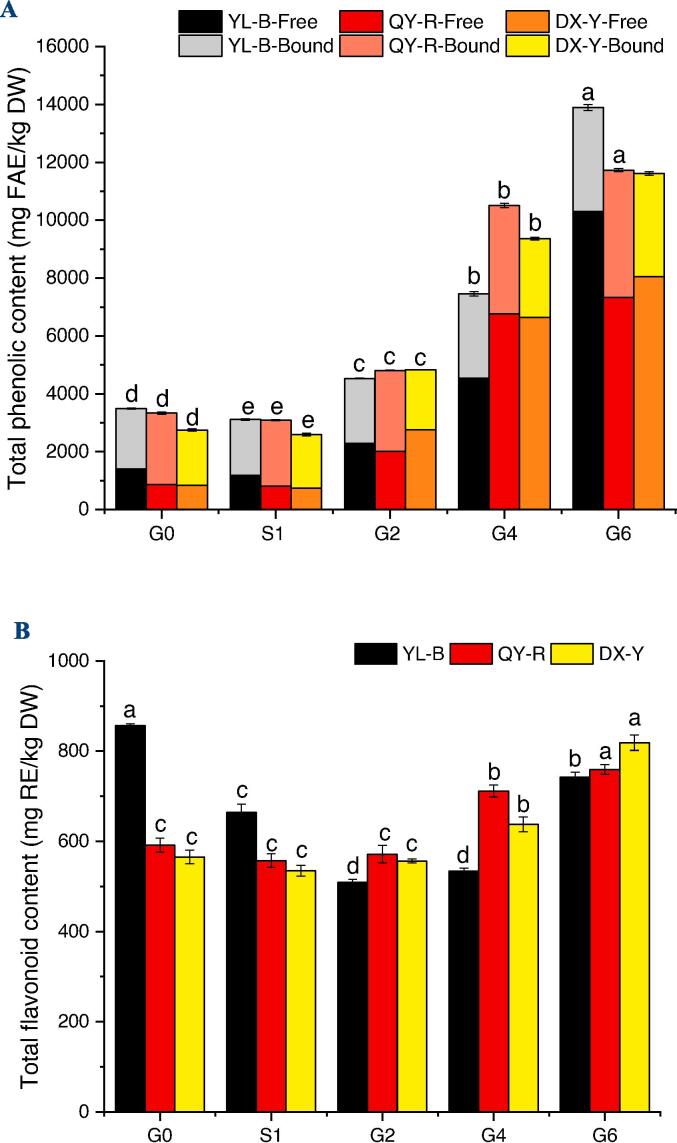
The changes of the TPCs of the free and bound fractions from the three proso millet cultivars (YL-B, QY-R and DX-Y) during germination are shown in Fig. 1(A). Compared with raw grains, both the free and bound phenolics decreased significantly (p < 0.05) after soaking. That was probably caused by the loss of water-soluble phenolic compounds during the soaking stage. Subsequently, the TPCs of the free soluble and bound insoluble fractions from the proso millets significantly increased (p < 0.05) in a time-dependent manner during the germination process (G2-G6).
Fig. 1.
Changes in total phenolic content (A) and total flavonoid content (B) of proso millets during the germination process. G0 stands for raw, ungerminated proso millet, S1 means soaked proso millets, and G2, G4 and G6 imply proso millet germinated for 2, 4 and 6 days. Different lowercase letters (a-e) in the same bar chart indicate significant differences (p < 0.05).
For the free phenolic extracts, the TPC of YL-B from stage G0 to G2 and from G2 to G4 increased by 62.76% and 97.75%, respectively, and then significantly increased by 126.81% from stage G4 to G6. The increasing trends in the TPCs of QY-R and DX-Y were similar to YL-B. However, the increases in TPC of QY-R and DX-Y were higher in the early stages of germination. From stage G0 to G2, the TPCs of QY-R and DX-Y increased by 134.06% and 231.66%, respectively. Moreover, from G2 to G4, TPCs increased by 236.62% and 140.52%, respectively. After germination for 6 days, the free TPCs of the three proso millet cultivars (YL-B, QY-R and DX-Y) improved 6.30, 7.55 and 8.66-fold, respectively, when comparing with the corresponding ungerminated seeds. The free phenoilcs from the black proso millet (YL-B) showed the highest TPC value of 10299 mg FAE/kg DW after 6 days of germination. The contribution of the free phenolics to the sum of phenolics was thereby increased from 40.40% to 74.10%. The TPCs of the red variety (QY-R) and the yellow proso millet (DX-Y) also increased from 858 to 7334 mg FAE/kg DW and 833 to 8054 mg FAE/kg DW, respectively. The contributions of the free phenolics were increased from 25.74% to 62.52% and 30.33% to 69.35%, respectively.
As for the bound phenolic fractions, the TPCs of YL-B, QY-R and DX-Y showed a continuously increasing trend during the whole germination process. The bound TPCs of the three proso millets, YL-B, QY-R and DX-Y, increased by 116.18%, 77.65% and 85.97%, respectively. The red variety (QY-R) displayed the highest bound TPC at the G6 stage, reaching 4396.74 mg FAE/kg DW. It had been reported that some pathways involving synthesis of phenolics, together with key enzymes, are activated by germination (Lemmens et al., 2019). Phenylalanine pathway was believed to be the most important and common metabolic pathway for synthesis of phenolics, and phenylalanine ammonia lyase (PAL), the major rate-limiting enzyme for synthesis of plant polyphenols, was generally activated by germination. The phenolics in beans, cereals, pseudocereals and other seeds were reported to be accumulated by germination treatment (Aloo et al., 2021, Lemmens et al., 2019).
However, the increases of bound TPC of the germinated proso millets were much lower than the free TPC. This may be attributed to the conversion from bound phenolics to free form under the action of hydrolases, which changes the cell wall, and then continuously modifies and releases some phenolic acids (Sharma et al., 2016, Ti et al., 2014). Other similar effects of germination on total phenolic content have been observed in brown rice (Ti et al., 2014) and corn (Xiang, Guo, Liu, Li, Hu, & Brennan, 2017).
Changes of TFC during germination
Changes of the total flavonoid contents at different germination stages are presented in Fig. 1 (B). For QY-R and DX-Y, the TFCs increased from 591 to 759 mg RE/kg DW and 565 to 819 mg RE/kg DW, respectively, after 6 days of germination. The TFCs were not significantly different (p greater than 0.05) after soaking and first germination stage but increased at the later stages reaching the highest levels at G6 with increases of 28.41% and 44.86%, respectively, when compared with the ungerminated sample. However, the TFC of YL-B decreased from stages G0 to G4, and then significantly (p < 0.05) increased at G6 even though it was still 13.38% lower than the ungerminated sample.
It has been reported that polyphenol oxidase and peroxidase are activated in the early stage of germination, which may lead to the decrease of TFC (Xu, Rao, & Chen, 2020). With prolonged germination times, the increase in TFC may be attributed to the activation of various flavonoid biosynthetic enzymes in germinated proso millets (Pradeep & Sreerama, 2015).
Modification of phenolic profiles by germination
Some representative polyphenol profiles of the free and bound extracts from the germinated proso millets are provided in Fig. S1. The retention times (RT) in the UPLC chromatograms, the corresponding identification results and changes in phenolic compositions during germination are listed in Table 1.
Table 1.
Individual phenolic compounds identified by UPLC-MS/MS in the free and bound extracts from proso millets during germination.
| No. | Retention time |
[M−H]− (m/z) |
Compounds identified | *G0 | G2 | G4 | G6 | |
|---|---|---|---|---|---|---|---|---|
| Free | 1 | 4.810 | 353 | Neochlorogenic acid | + | + | – | – |
| 2 | 5.340 | 137 | Protocatechualdehyde a | + | + | – | – | |
| 3 | 6.610 | 137 | p-Hydroxybenzoic acid a | + | + | – | – | |
| 4 | 6.611 | 162 | Cinnamic acid derivative | + | – | – | – | |
| 5 | 8.140 | 121 | p-Hydroxybenzaldehyde a | + | + | + | + | |
| 6 | 8.294 | 353 | Chlorogenic acid a | + | – | – | – | |
| 7 | 8.531 | 367 | Feruloylquinic acid | – | – | + | + | |
| 8 | 10.146 | 353 | Cryptochlorogenic acid | + | – | – | – | |
| 9 | 11.710 | 379 | N,N′-bis-(p-coumaroyl) putrescine | – | – | + | + | |
| 10 | 13.649 | 323 | Hydroxycinnamic acid derivatives | – | – | + | + | |
| 11 | 14.240 | 163 | trans-p-Coumaric acid a | + | + | + | + | |
| 12 | 15.131 | 468 | N′, N″-dicaffeoylspermidine | + | + | + | + | |
| 13 | 15.650 | 193 | trans-Ferulic acid a | + | + | + | + | |
| 14 | 16.182 | 482 | N′-caffeoyl-N″-feruloylspermidine | + | + | + | + | |
| 15 | 18.070 | 321 | N-(p-coumaroyl) serotonin | + | + | + | + | |
| 16 | 18.270 | 351 | N-feruloylserotonin | + | + | + | + | |
| 17 | 18.650 | 301 | Homoeriodictyol | + | + | + | + | |
| Bound | 1 | 5.521 | 137 | Protocatechualdehyde a | + | + | + | + |
| 2 | 6.682 | 137 | p-Hydroxybenzoic acid a | + | + | + | + | |
| 3 | 6.621 | 162 | Cinnamic acid derivative | + | – | – | – | |
| 4 | 8.081 | 121 | p-Hydroxybenzaldehyde a | + | + | + | + | |
| 5 | 8.843 | 167 | Vanillic acid a | + | + | + | + | |
| 6 | 14.050 | 163 | trans-p-Coumaric acid a | + | + | + | + | |
| 7 | 15.170 | 163 | cis-p-Coumaric acid a | + | + | + | + | |
| 8 | 15.552 | 193 | trans-Ferulic acid a | + | + | + | + | |
| 9 | 15.841 | 385 | 8,8′-aryltetralin-DFA | + | + | + | + | |
| 10 | 16.460 | 193 | cis-Ferulic acid | + | + | + | + | |
| 11 | 16.823 | 385 | 8,8′-DFA | + | + | + | + | |
| 12 | 18.245 | 387 | Ferulic truxilic acid | + | + | – | – | |
| 13 | 18.643 | 577 | TFA | + | + | – | – | |
| 14 | 18.650 | 385 | 8-5′-DFA | – | – | + | + | |
| 15 | 19.192 | 385 | Trans-trans-8-O-4′–DFA | + | + | + | + | |
| 16 | 19.521 | 385 | 5-5′-DFA | + | + | + | + | |
| 17 | 19.733 | 385 | 8-5′-DFA benzofuran | – | – | + | + | |
| 18 | 20.481 | 385 | Trans-cis-8-O-4′–DFA | – | – | + | + | |
*G0 stands for raw, ungerminated proso millet and G2, G4 and G6 imply proso millet germinated for 2, 4 and 6 days.
DFA -diferulic acid. TFA -triferulic acid.
+, detected; –, not detected.
Identification of the compound was confirmed using authentic standards. All other compounds were tentatively identified by comparing their mass spectral characteristics with those reported in literatures.
Free phenolic compounds
The free phenolic profiles of proso millets were greatly modified by the germination process. Six polyphenols were confirmed using authentic standards. The putative identifications of the other eight phenolic derivatives were based on UPLC-ESI-MS/SM analysis using retention times, elution orders, UV and MS/MS spectra, as well as literature comparisons. They were identified as neochlorogenic acid, protocatechualdehyde, p-hydroxybenzoic acid, cinnamic acid derivative, p-hydroxybenzaldehyde, chlorogenic acid, cryptochlorogenic acid, trans-p-coumaric acid, N′, N″-dicaffeoylspermidine, trans-ferulic acid, N′-caffeoyl-N″-feruloylspermidine, N-(p-coumaroyl) serotonin, N-feruloylserotonin, homoeriodictyol, respectively (Li et al., 2021, Yuan et al., 2022). Compared with ungerminated samples (G0), chlorogenic acid, cryptochlorogenic acid and a cinnamic acid derivative were not detected at stage G2, which may have been metabolized at the initial stage of germination. As germination progressed, neochlorogenic acid, protocatechualdehyde and p-hydroxybenzoic acid disappeared (at stage G4) while three new phenolic compounds were detected which showed deprotonated molecular ions [M−H]− at m/z 367, 379, 323, respectively.
Compound 7 showed a [M−H]− ion at m/z 367, MS/MS spectrum displayed a main fragment at m/z 193 (100%) and two relatively minor fragment ions at m/z 134 (10%) and 149 (10%). The MS/MS and UV spectrum are shown in Fig. S2. Comparing the MS/MS information along with UV spectrum of the reference (Asamenew et al., 2019, Kiselova-Kaneva et al., 2022), compound 7 was putatively assigned as feruloylquinic acid. This seems to be the first detection of feruloylquinic acid in germinated proso millet.
Compound 9 was the largest peak, indicating predominance in phenolic composition in the germinated proso millets. The MS/MS and UV spectrum are shown in Fig. S2 (C and D). This compound had a hydroxycinnamic acid moiety, responsible for the typical UV absorption characteristics at 300–325 nm. The MS/MS showed the fragmentation pattern that is consistent with p-coumaroyl-feruloylputrescine, which was identified by Moreau, Nunez, and Singh (2001), and compound 9 displayed a deprotonated molecular ion [M−H]− at m/z 379, which was 30 amu lower than that of p-coumaroyl-feruloylputrescine. In addition, the fragmentation exhibited a characteristic fragment of p-coumaric acid at m/z 119 (25%) suggesting the substitution of feruloyl by coumaroyl in p-coumaroyl-feruloylputrescine. Therefore, compound 9 was putatively identified as N, N′-bis-(p-coumaroyl)-putrescine, which had been isolated from corn bran by Choi et al. (2007). To our knowledge, this is the first report on N, N′-bis-(p-coumaroyl)-putrescine existing in proso millets.
Compound 10 was also classified as a hydroxycinnamic acid derivative, the MS2 information and UV spectrum were presented in Fig. S2 (E and F). Peak 10 displayed a [M−H]− ion at m/z 323 and its MS2 fragmentations produced a main fragment at m/z 146 (100%) and two relatively minor fragment ions at m/z 179 (35%) and 107 (15%), respectively. This peak was probably a caffeic acid derivative.
Bound phenolic compounds
From our previous report (Yuan et al., 2022), the bound phenolics of proso millets were mainly composed of seventeen phenolic acid derivatives, including protocatechualdehyde, p-hydroxybenzoic acid, cinnamic acid derivative, p-hydroxybenzaldehyde, vanillic acid, cis-p-coumaric acid, trans-p-coumaric acid, trans-ferulic acid, cis-ferulic acid, ferulic truxilic acid, ferulic acid dehydrotrimers (TFAs), as well as four ferulic acid dimers (DFAs). In this study, eighteen phenolic derivatives were confirmed or putatively identified in the bound extract of the germinated proso millets. Results from UPLC-ESI-MS/MS analysis showed that the ferulic truxilic acid and TFAs gradually disappeared during germination whereas compounds 14, 17 and 18 were newly detected with [M−H]− at m/z 385, indicating new DFAs formed.
In total, seven DFAs were detected in bound form in the germinated proso millets, and the detailed MS/MS data and UV spectra are shown in Fig. S3. Six DFAs including 8-8′-aryltetralin, 8-8′, 5-5′, 8-5′ benzofuran and 8-5′-DFA had previously been detected in white asparagus cell walls (Jaramillo, Rodríguez, Jiménez, Guillén, Fernández-Bolaños, & Heredia, 2007) and some millets (Chandrasekara & Shahidi, 2011). Trans-trans-8-O-4′-DFA could be identified by the UV spectrum of the standard and its characteristic MS2 fragment ion at m/z 193. The MS2 fragmentations at m/z 134, 149, and 178 were produced by losses of –CH3 plus –COOH, –COOH, and –CH3 from the characteristic fragment at m/z 193, respectively (Ostrowski, Swarcewicz, Nolka, & Stobiecki, 2016; Xiang, Zhang, et al., 2019). Trans-cis-8-O-4′ DFA, the isomer of trans–trans-8-O-4′ DFA, presented the same characteristic fragment patterns in MS2 spectrum, but showed a higher retention time in the UPLC chromatogram (Gong et al., 2017; Xiang, Zhang, et al., 2019). Therefore, compounds 9, 11, 14, 15, 16, 17 and 18 were referred to as 8-8′-aryltetralin, 8-8′, trans–trans-8-O-4′, 5-5′, 8-5′, 8-5′ benzofuran and trans–cis-8-O-4′-DFA, respectively. Among them, trans–trans-8-O-4′, 8-5′ benzofuran and trans–cis-8-O-4′-DFA were accumulated, and 8,5′-DFA was produced by germination process.
Contents of the individual phenolic compounds
The dynamic changes of the main phenolic compounds in the free and bound phenolic fractions from the three germinated proso millets (YL-B, QY-R, DX-Y) are shown in Table 2, Table 3, Table 4, respectively. The results revealed similar trends in release and biosynthesis of polyphenols during germination.
Table 2.
Contents of individual phenolic compounds (mg/kg DW a) in black proso millet (YL-B) b at different germination stages.
| Phenolic compounds | *G0 | S1 | G2 | G4 | G6 | |
|---|---|---|---|---|---|---|
| Free | Hydroxybenzoic acids | |||||
| Protocatechualdehyde | 41.30 ± 1.12a | 35.53 ± 1.09b | 21.9 ± 0.38c | n.d. | n.d. | |
| p-Hydroxybenzoic acid | 42.74 ± 0.91a | 8.59 ± 0.49b | 4.31 ± 0.22c | n.d. | n.d. | |
| p-Hydroxybenzaldehyde | 37.20 ± 0.99a | 5.68 ± 0.75bc | 3.87 ± 0.13cd | 2.25 ± 0.34 d | 5.87 ± 0.82b | |
| Hydroxycinnamic acids | ||||||
| trans-p-Coumaric acid | 37.07 ± 1.17a | 5.66 ± 0.09bc | 3.32 ± 0.25d | 4.29 ± 0.06 cd | 5.93 ± 0.53b | |
| trans-Ferulic acid | 6.67 ± 0.29c | 5.63 ± 0.13c | 8.72 ± 0.13c | 28.70 ± 2.39b | 39.79 ± 2.39a | |
| Feruloylquinic acid | n.d. | n.d. | n.d. | 13.83 ± 0.85b | 66.91 ± 5.39a | |
| N,N′-bis-(p-coumaroyl)- putrescine | n.d. | n.d. | n.d. | 12.56 ± 1.06b | 50.32 ± 1.60a | |
| Hydroxycinnamic acid derivatives | n.d. | n.d. | n.d. | 16.64 ± 2.64b | 62.18 ± 4.78a | |
| Hydroxycinnamic acid spermidines | ||||||
| N′,N″-dicaffeoylspermidine | 3.79 ± 0.06a | 2.56 ± 0.11b | 0.64 ± 0.02 e | 1.06 ± 0.04 d | 1.52 ± 0.10c | |
| N′-caffeoyl-N″-feruloyl spermidine |
8.94 ± 0.14bc | 8.14 ± 0.14c | 4.56 ± 0.14 d | 11.31 ± 1.20b | 22.73 ± 2.13a | |
| Hydroxycinnamic acid serotonins | ||||||
| N-(p-coumaroyl)serotonin | 43.29 ± 1.24a | 22.14 ± 1.27b | 13.73 ± 0.13c | 6.11 ± 0.25d | 9.54 ± 0.73e | |
| N-feruloylserotonin | 15.41 ± 0.13c | 15.23 ± 0.15c | 10.19 ± 0.16d | 21.36 ± 1.44b | 29.31 ± 1.56a | |
| Bound | Hydroxybenzoic acids | |||||
| Protocatechualdehyde | 31.86 ± 0.92a | 8.78 ± 1.06b | 3.74 ± 0.12 d | 3.76 ± 0.10 d | 6.01 ± 0.18c | |
| p-Hydroxybenzoic acid | 69.93 ± 1.50a | 13.56 ± 0.77b | 1.43 ± 0.12c | 1.64 ± 0.09c | 1.95 ± 0.07c | |
| p-Hydroxybenzaldehyde | 142.91 ± 3.22a | 110.53 ± 1.24b | 109.51 ± 1.42b | 27.22 ± 1.22 d | 45.65 ± 1.12c | |
| Vanillic acid | 80.50 ± 1.18d | 75.18 ± 1.06e | 110.40 ± 1.38c | 166.74 ± 1.50b | 219.65 ± 2.87a | |
| Hydroxycinnamic acids | ||||||
| trans-p-Coumaric acid | 397.65 ± 1.55 d | 320.67 ± 1.58 e | 421.39 ± 2.05c | 453.17 ± 2.91b | 464.81 ± 3.51a | |
| cis-p-Coumaric acid | 33.22 ± 0.99a | 27.10 ± 0.70b | 22.50 ± 1.11c | 7.50 ± 0.50e | 10.72 ± 1.03d | |
| trans-Ferulic acid | 353.21 ± 2.73e | 381.54 ± 1.76d | 462.34 ± 2.23c | 2599.90 ± 6.90b | 4253.10 ± 4.62a | |
| 8,8′-aryltetralin-DFA | 9.54 ± 0.29c | 10.78 ± 0.84c | 11.33 ± 0.79c | 16.15 ± 0.67b | 29.60 ± 0.80a | |
| cis-Ferulic acid | 53.18 ± 0.91c | 43.20 ± 1.03 d | 57.25 ± 1.00c | 180.76 ± 1.62b | 343.68 ± 2.76a | |
| 8,8′-DFA | 12.12 ± 0.51c | 12.54 ± 0.60c | 13.09 ± 0.19c | 37.45 ± 0.79b | 70.48 ± 1.91a | |
| 8,5′-DFA | n.d. | n.d. | 35.37 ± 1.44c | 112.93 ± 2.70b | 245.86 ± 2.69a | |
| Trans-trans-8-O-4′–DFA | 31.43 ± 1.23c | 34.69 ± 1.28c | 35.38 ± 1.34c | 93.81 ± 1.71b | 211.22 ± 2.40a | |
| 5-5′-DFA | 19.19 ± 0.82d | 24.46 ± 1.27cd | 30.29 ± 1.83c | 98.59 ± 3.22b | 234.85 ± 4.30a | |
| 8-5′-DFA benzofuran | 11.92 ± 0.16b | 9.17 ± 0.06c | 9.32 ± 1.12c | 9.37 ± 0.17c | 16.62 ± 0.84a | |
| Trans-cis-8-O-4′–DFA | 9.72 ± 0.17c | 6.13 ± 0.08d | 9.69 ± 0.18c | 18.49 ± 0.64b | 36.90 ± 0.79a | |
*G0 stands for raw, ungerminated proso millet, S1 means soaked proso millets, and G2, G4 and G6 imply proso millet germinated for 2, 4 and 6 days.
n.d., not detected.
DW, dry weight of sample.
Results are expressed as mean ± SD. Values with no letters in common are significantly different (p < 0.05).
Table 3.
Contents of individual phenolic compounds (mg/kg DWa) in red proso millet (QY-R)b at different germination stages.
| Phenolic compounds | *G0 | S1 | G2 | G4 | G6 | |
|---|---|---|---|---|---|---|
| Free | Hydroxybenzoic acids | |||||
| Protocatechualdehyde | 55.55 ± 2.18a | 31.74 ± 1.47b | 25.02 ± 1.78c | n.d. | n.d. | |
| p-Hydroxybenzoic acid | 37.55 ± 1.02a | 3.51 ± 0.56b | 1.55 ± 0.40c | n.d. | n.d. | |
| p-Hydroxybenzaldehyde | 34.59 ± 1.35a | 1.48 ± 0.13c | 2.09 ± 0.07bc | 2.30 ± 0.07bc | 3.54 ± 0.31b | |
| Hydroxycinnamic acids | ||||||
| trans-p-Coumaric acid | 41.20 ± 1.14a | 7.01 ± 0.77b | 6.26 ± 0.27b | 2.23 ± 0.11c | 2.83 ± 0.08c | |
| trans-Ferulic acid | 4.86 ± 0.30bc | 3.70 ± 0.47c | 5.94 ± 0.07b | 22.20 ± 0.77a | 23.33 ± 0.91a | |
| Feruloylquinic acid | n.d. | n.d. | n.d. | 21.84 ± 0.90b | 25.22 ± 0.90a | |
| N,N′-bis-(p-coumaroyl)- putrescine | n.d. | n.d. | n.d. | 6.68 ± 0.12b | 12.30 ± 0.68a | |
| Hydroxycinnamic acid derivatives | n.d. | n.d. | n.d. | 13.72 ± 0.35b | 15.58 ± 0.49a | |
| Hydroxycinnamic acid spermidines | ||||||
| N′,N″-dicaffeoylspermidine | 3.44 ± 0.11a | 0.78 ± 0.13c | 0.77 ± 0.12c | 0.72 ± 0.05c | 1.13 ± 0.08b | |
| N′-caffeoyl-N″-feruloylspermidine | 23.54 ± 0.77a | 20.54 ± 0.73b | 9.69 ± 0.32e | 14.19 ± 1.00d | 17.61 ± 0.68c | |
| Hydroxycinnamic acid serotonins | ||||||
| N-(p-coumaroyl)serotonin | 34.16 ± 5.10a | 5.56 ± 0.18b | 5.25 ± 0.10b | 2.68 ± 0.04b | 2.70 ± 0.03b | |
| N-feruloylserotonin | 5.10 ± 0.13b | 3.63 ± 0.04c | 2.68 ± 0.05d | 5.39 ± 0.51b | 7.05 ± 0.44a | |
| Bound | Hydroxybenzoic acids | |||||
| Protocatechualdehyde | 35.32 ± 1.28a | 1.63 ± 0.29 d | 3.22 ± 0.27cd | 4.15 ± 0.12bc | 4.86 ± 0.12b | |
| p-Hydroxybenzoic acid | 72.90 ± 2.75a | 11.83 ± 1.14b | 7.75 ± 1.09c | 0.27 ± 0.04d | 0.20 ± 0.04d | |
| p-Hydroxybenzaldehyde | 115.75 ± 2.34a | 103.52 ± 4.56b | 87.23 ± 1.68c | 25.61 ± 1.40d | 2.84 ± 0.24e | |
| Vanillic acid | 134.74 ± 2.72d | 125.19 ± 1.83e | 180.21 ± 1.44c | 277.94 ± 2.39b | 284.24 ± 2.03a | |
| Hydroxycinnamic acids | ||||||
| trans-p-Coumaric acid | 546.40 ± 2.85d | 559.20 ± 3.68cd | 573.42 ± 3.28bc | 579.23 ± 9.79b | 646.48 ± 4.01a | |
| cis-p-Coumaric acid | 25.24 ± 1.10c | 25.13 ± 0.99c | 25.88 ± 1.16bc | 28.23 ± 1.13b | 32.41 ± 1.08a | |
| trans-Ferulic acid | 538.49 ± 1.78d | 541.95 ± 3.84d | 705.56 ± 3.86c | 2795.28 ± 3.84b | 3180.94 ± 5.38a | |
| 8,8′-aryltetralin-DFA | 10.88 ± 0.36bc | 9.49 ± 0.56c | 10.51 ± 0.40bc | 12.40 ± 0.85b | 20.65 ± 1.45a | |
| cis-Ferulic acid | 66.50 ± 1.02c | 61.79 ± 1.77c | 63.28 ± 1.82c | 371.09 ± 2.73b | 509.52 ± 2.90a | |
| 8,8′-DFA | 13.48 ± 0.17d | 13.17 ± 0.15d | 17.81 ± 0.16c | 36.34 ± 1.19b | 53.31 ± 1.35a | |
| 8,5′-DFA | n.d. | n.d. | 44.57 ± 1.30c | 150.52 ± 2.08b | 209.34 ± 1.52a | |
| Trans-trans-8-O-4′–DFA | 29.90 ± 0.59d | 30.25 ± 0.69d | 46.59 ± 1.14c | 123.31 ± 2.04b | 169.79 ± 2.63a | |
| 5-5′-DFA | 22.13 ± 0.67d | 22.82 ± 0.96d | 33.70 ± 1.07c | 131.74 ± 1.80b | 179.58 ± 2.77a | |
| 8-5′-DFAbenzofuran | 8.10 ± 0.23d | 6.55 ± 0.06e | 11.49 ± 0.23c | 12.92 ± 0.23b | 13.83 ± 0.16a | |
| Trans-cis-8-O-4′–DFA | 5.39 ± 0.09c | 5.99 ± 0.10c | 6.98 ± 0.08c | 24.44 ± 1.07b | 29.85 ± 1.09a | |
*G0 stands for raw, ungerminated proso millet, S1 means soaked proso millets, and G2, G4 and G6 imply proso millet germinated for 2, 4 and 6 days.
n.d., not detected.
DW, dry weight of sample.
Results are expressed as mean ± SD. Values with no letters in common are significantly different (p < 0.05).
Table 4.
Contents of individual phenolic compounds (mg/kg DW a) in yellow proso millet (DX-Y) b at different germination stages.
| Phenolic compounds | *G0 | S1 | G2 | G4 | G6 | |
|---|---|---|---|---|---|---|
| Free | Hydroxybenzoic acids | |||||
| Protocatechualdehyde | 56.09 ± 1.17a | 42.46 ± 0.98b | 31.46 ± 1.25c | n.d. | n.d. | |
| p-Hydroxybenzoic acid | 35.43 ± 1.14a | 5.06 ± 0.07b | 1.74 ± 0.17c | n.d. | n.d. | |
| p-Hydroxybenzaldehyde | 37.20 ± 0.67a | 7.63 ± 0.22b | 6.20 ± 0.17c | 1.86 ± 0.18d | 0.60 ± 0.10e | |
| Hydroxycinnamic acids | ||||||
| trans-p-Coumaric acid | 40.31 ± 1.19a | 7.22 ± 0.12b | 6.82 ± 0.17b | 2.33 ± 0.11c | 2.66 ± 0.11c | |
| trans-Ferulic acid | 3.18 ± 0.16d | 3.44 ± 0.10d | 9.30 ± 0.35c | 21.53 ± 0.76b | 21.63 ± 0.65a | |
| Feruloylquinic acid | n.d. | n.d. | n.d. | 21.46 ± 1.24b | 24.07 ± 1.07a | |
| N,N′-bis-(p-coumaroyl)- putrescine | n.d. | n.d. | n.d. | 15.49 ± 0.57b | 26.35 ± 0.58a | |
| Hydroxycinnamic acid derivatives | n.d. | n.d. | n.d. | 15.29 ± 0.53b | 34.52 ± 1.23a | |
| Hydroxycinnamic acid spermidines | ||||||
| N′,N″-dicaffeoylspermidine | 0.93 ± 0.06a | 0.33 ± 0.03c | 0.19 ± 0.04d | 0.65 ± 0.05b | 0.72 ± 0.04b | |
| N′-caffeoyl-N″-feruloylspermidine | 3.72 ± 0.09e | 4.16 ± 0.06 d | 5.85 ± 0.16c | 9.19 ± 0.07b | 11.10 ± 0.09a | |
| Hydroxycinnamic acid serotonins | ||||||
| N-(p-coumaroyl)serotonin | 24.85 ± 0.07a | 4.95 ± 0.06b | 5.16 ± 0.10b | 2.09 ± 0.11d | 2.49 ± 0.16c | |
| N-feruloylserotonin | 5.25 ± 0.09b | 2.44 ± 0.12 d | 2.95 ± 0.07c | 5.49 ± 0.17b | 6.10 ± 0.11a | |
| Bound | Hydroxybenzoic acids | |||||
| Protocatechualdehyde | 37.04 ± 1.71a | 3.10 ± 0.08c | 5.34 ± 0.22b | 5.35 ± 0.12b | 6.72 ± 0.07b | |
| p-Hydroxybenzoic acid | 92.40 ± 1.30a | 35.49 ± 1.12b | 25.31 ± 0.70c | 0.89 ± 0.09d | 0.90 ± 0.03d | |
| p-Hydroxybenzaldehyde | 153.56 ± 1.61a | 131.48 ± 1.83b | 124.11 ± 1.42c | 30.55 ± 0.74e | 40.15 ± 1.72d | |
| Vanillic acid | 80.03 ± 1.58c | 43.39 ± 1.50e | 56.35 ± 1.06d | 84.47 ± 1.04b | 97.56 ± 0.97a | |
| Hydroxycinnamic acids | ||||||
| trans-p-Coumaric acid | 403.12 ± 2.30e | 452.50 ± 2.28d | 472.37 ± 2.34c | 524.08 ± 3.08b | 572.61 ± 2.48a | |
| cis-p-Coumaric acid | 70.62 ± 1.73a | 59.51 ± 1.19b | 55.57 ± 1.19c | 30.45 ± 0.79e | 38.31 ± 1.04d | |
| trans-Ferulic acid | 297.97 ± 1.33e | 328.65 ± 2.32d | 413.36 ± 2.30c | 2794.80 ± 5.75b | 2890.73 ± 4.47a | |
| 8,8′-aryltetralin-DFA | 8.50 ± 0.17 d | 8.77 ± 0.10d | 9.69 ± 0.17c | 19.13 ± 0.08b | 21.14 ± 0.08a | |
| cis-Ferulic acid | 78.55 ± 1.41c | 72.26 ± 1.33 d | 78.52 ± 1.24c | 400.48 ± 1.58b | 434.02 ± 1.80a | |
| 8,8′-DFA | 9.93 ± 0.16c | 10.82 ± 0.45bc | 12.02 ± 0.17b | 41.25 ± 0.81a | 42.17 ± 1.18a | |
| 8,5′-DFA | n.d. | n.d. | 22.52 ± 0.61c | 137.44 ± 1.27b | 154.55 ± 1.45a | |
| Trans-trans-8-O-4′–DFA | 17.05 ± 0.83c | 18.45 ± 0.79c | 19.55 ± 1.07c | 124.07 ± 1.32b | 142.53 ± 1.05a | |
| 5-5′-DFA | 12.15 ± 0.54c | 13.12 ± 0.45c | 13.64 ± 0.64c | 141.87 ± 2.54b | 168.59 ± 3.69a | |
| 8-5′-DFAbenzofuran | 13.39 ± 0.53b | 8.34 ± 0.23d | 10.58 ± 0.62c | 13.88 ± 0.64b | 25.34 ± 1.39a | |
| Trans-cis-8-O-4′–DFA | 9.40 ± 0.42c | 8.01 ± 0.46c | 8.86 ± 0.23c | 29.51 ± 1.45b | 33.35 ± 1.45a | |
*G0 stands for raw, ungerminated proso millet, S1 means soaked proso millets, and G2, G4 and G6 imply proso millet germinated for 2, 4 and 6 days.
n.d., not detected.
DW, dry weight of sample.
Results are expressed as mean ± SD. Values with no letters in common are significantly different (p < 0.05).
The levels of trans-ferulic acid and cis-ferulic acid increased significantly (p < 0.05) during germination process. For the free extracts, the levels of trans-ferulic acid in YL-B, QY-R, and DX-Y were improved for 4.97, 3.80, and 5.80 times, respectively, after 6 days of germination process. Moreover, trans-ferulic acid levels in bound form were much higher than those in free form, and the bound trans-ferulic acid in YL-B, QY-R, and DX-Y at stage G6 increased by 11.04, 4.91, and 8.70 times, respectively. The germinated black proso millet from Yulin (YL-B) displayed the highest bound trans-ferulic acid of 4253 mg/kg DW (at G6). However, cis-ferulic acid was only detected in bound form, and cis-ferulic acid in QY-R reached the highest value of 509 mg/kg DW after 6 days of germination process.
The trans-p-coumaric acid was the predominant phenolic compounds in bound form, contributing to more than 90% of the total phenolics in the germinated proso millets. The levels of bound trans-p-coumaric acid showed significant (p < 0.05) increase in all the three proso millets (YL-B, QY-R and DX-Y) during the germination process, while trans-p-coumaric acid in free form was significantly reduced to trace amount.
Vanillic acid was detected only in bound form. The levels of vanillic acid in YL-B, QY-R and DX-Y showed an increasing trend during the germination process, and increased by 172.86%, 110.95% and 21.90%, respectively at G6. The germinated QY-R presented the highest vanillic acid content of 284.24 mg/kg DW.
Protocatechualdehyde existed in both free and bound forms in proso millets. The three cultivars (YL-B, QY-R, DX-Y) presented similar changes during germination. The free protocatechualdehyde decreased significantly to undetectable levels, while the bound protocatechualdehyde showed a significant (p < 0.05) decrease at first from G0 to G2 and then increased from stage G2 to G6.
Both of the free and bound p-hydroxybenzoic acid decreased significantly during germination. The free p-hydroxybenzoic acid in the three proso millets decreased to undetectable levels while the bound form was also reduced to trace amounts with the germination process.
Feruloylquinic acid and N, N′-bis-(p-coumaroyl) putrescine were detected for the first time at stage G4 and increased significantly (p < 0.05) from the stage G4 to G6. YL-B showed higher increase in feruloylquinic acid and N, N'-bis-(p-coumaroyl) putrescine than the other two proso millets, reaching 66.91 mg/kg DW and 56.91 mg/kg DW, respectively. N, N′-bis-(p-coumaroyl) putrescine was a new compound and abundantly synthesized phenolic derivative during germination. Dong et al. (2015) reported that putrescine conjugates in rice mainly accumulated in the roots. At the later germination stage of proso millets, the root growth may be the reason for the production and significant increase of the hydroxycinnamic acid putrescine conjugate. It was reported that putrescine combined with antioxidant molecules, for example hydroxycinnamic acid, to make plants more resistant to oxidative damage (Dong et al., 2015).
The contents of N′, N″-dicaffeoylspermidine in YL-B, QY-R and DX-Y decreased first and then increased at later germination stages. Overall, germination caused the loss of this hydroxycinnamic acid spermidine by 59.89%, 67.15% and 22.58%, respectively in the three grains. N′-caffeoyl-N″-feruloylspermidine in YL-B and QY-R showed a similar decreasing trend with N′, N″-dicaffeoylspermidine during the germination process. However, DX-Y showed a significant increase (p < 0.05) in N′-caffeoyl-N″-feruloylspermidine during the germination process of 198.39% and reaching 11 mg/kg DW after 6 days of germination.
N-(p-coumaroyl) serotonin was reduced significantly (p < 0.05) by the germination process. Compared with the raw sample, N-(p-coumaroyl) serotonin in the germinated YL-B, QY-R and DX-Y decreased by 77.96%, 92.10%, and 89.98% at G6 respectively. However, N-feruloylserotonin, the other hydroxycinnamic acid serotonin conjugate, significantly (p < 0.05) decreased at first and then significantly increased (p < 0.05) from the stage G2 to G6. YL-B presented the highest N-feruloylserotonin at G6 of 29.31 mg/kg DW, which was improved by 90.20% compared to the raw, ungerminated sample.
Antioxidant properties
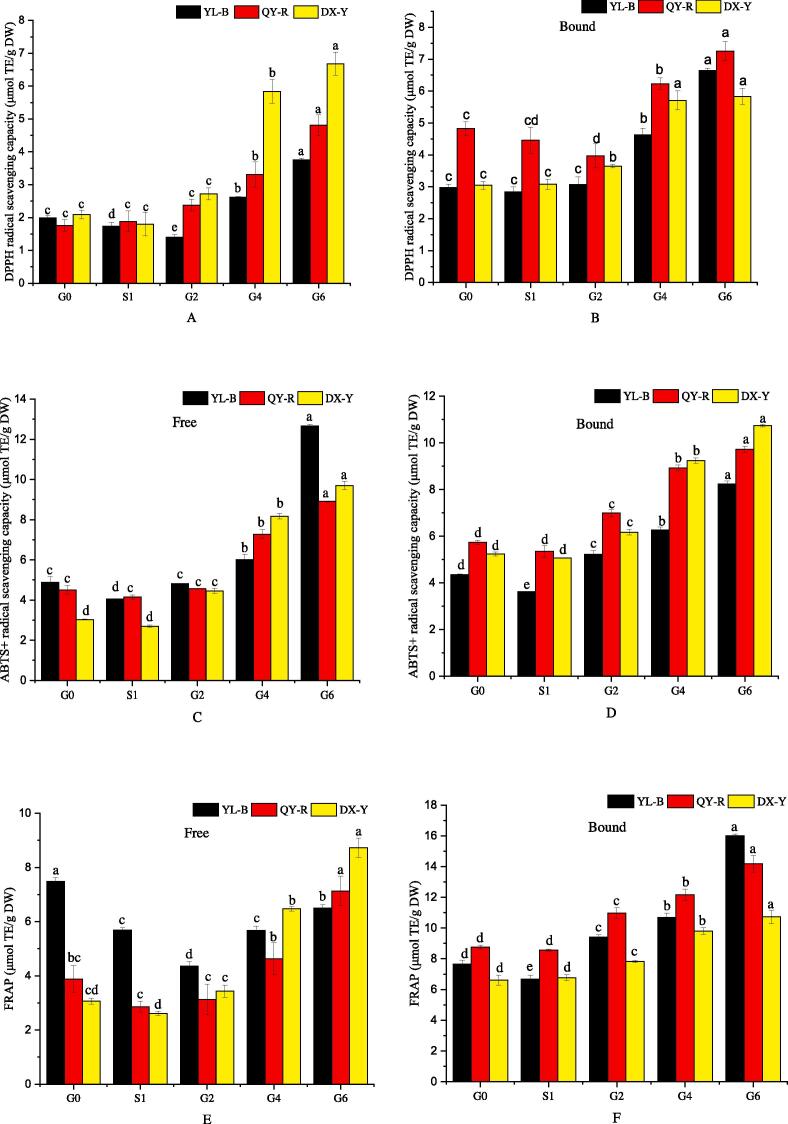
The changes of antioxidant activity of proso millets during the germination are shown in Fig. 2. As seen in Fig. 2(A) and (B), the DPPH radical scavenging activity of the free fractions from YL-B decreased first by 29.69% during G0-G2, then significantly increased during G2-G6, and increased by 89.43% at G6 compared to the ungerminated sample. However, the DPPH radical scavenging activity of the free fractions from QY-R and DX-Y presented continuous increase for the whole germination process (G0-G6), increasing by 172.54% and 218.88%, respectively. The free phenolics of DX-Y sprout exhibited the highest DPPH radical scavenging activity of 6.68 μmol TE/g among the three proso millet cultivars.
Fig. 2.
Changes in antioxidant activity of proso millets during germination. A: DPPH radical scavenging capacity of free phenolics, B: DPPH radical scavenging capacity of bound phenolics, C: ABTS+ radical scavenging capacity of free phenolics, D: ABTS+ radical scavenging capacity of bound phenolics, E: FRAP of free phenolics, F: FRAP of bound phenolics. G0 stands for raw, ungerminated proso millet, S1 means soaked proso millets, and G2, G4 and G6 imply proso millet germinated for 2, 4 and 6 days. Different lowercase letters (a-e) in the same bar chart indicate significant differences (p < 0.05).
Concerning the bound phenolics of the germinated proso millets, the DPPH radical scavenging activity of YL-B and DX-Y significantly increased continuously (p < 0.05) during the germination process, reaching 123.18% and 91.15% at G6, respectively. On the contrary, the DPPH radical scavenging activity of QY-R first decreased by 17.89% and then increased significantly (p < 0.05) during the subsequent stages. The bound phenolics of QY-R sprouts displayed the highest DPPH radical scavenging activity of 7.25 μmol TE/g among the three proso millets.
As seen in Fig. 2(C) and (D), for both free and bound fractions, the ABTS·+ scavenging capacities were significantly (p < 0.05) improved by germinating the three proso millet cultivars. The ABTS·+ radical scavenging activities of the free phenolics of YL-B, QY-R and DX-Y increased by 159.44%, 97.63% and 220.05%, respectively whereas those of bound phenolics increased by 89.82%, 69.32% and 105.12%, respectively.
As seen in Fig. 2(E) and (F), for the free phenolics, the ferric reducing antioxidant power (FRAP) of YL-B significantly (p < 0.05) decreased by 41.7% at first (G0-G2), and then increased to 6.50 μmol TE/g after germination which was still significantly (p < 0.05) lower than in ungerminated grains. However, the FRAP was significantly (p < 0.05) increased in QY-R and DX-Y by 83.95% and 184.62%, respectively as a result of germination. The free phenolics of DX-Y displayed the highest FRAP of 8.73 μmol TE/g. The FRAP significantly (p < 0.05) increased continually for YL-B, QY-R and DX-Y during germination by 109.09%, 61.96% and 62.00%, respectively for the bound phenolics.
Overall, the germination of proso millet grains could significantly improve their antioxidant activity. Similar results have been reported on red cabbage seed (Aloo, Ofosu, Daliri, & Oh, 2021), brown rice (Ti et al., 2014) and buckwheat (Xu et al., 2020) following germination. The improvement in antioxidant activity is likely attributable to the complex biochemical metabolism of phenolic biosynthesis and conversion during the process of germination, which resulted in the generation of antioxidant phytochemicals, in particular, phenolic derivatives.
Conclusion
With the prolonged germination time, the total phenolic and total flavonoid contents and the antioxidant activity were significantly increased when compared to the ungerminated proso millets. However, the three proso millets displayed significant differences at different germination stages. The free phenolic profiles were significantly modified by germination, with feruloylquinic acid and N,N′-bis-(p-coumaroyl)-putrescine being produced in abundance and becoming the predominant phenolic compound. Some intrinsic phenolic compounds, such as p-hydroxybenzoic acid, protocatechualdehyde, decreased significantly and even disappeared after germination. The bound phenolics showed significant improvements as the predominant compounds in proso millets including trans-p-coumaric acid, cis-ferulic acid and trans-ferulic acid. In addition, the levels of ferulic acid dimers were significantly increased with a new ferulic acid dimer (8,5′-DFA) being formed through the process of germination. These newly biosynthesized phenolic derivatives likely enhanced the antioxidant activity of germinated proso millets. The findings demonstrate that germinated proso millets containing more phenolic derivatives has the potential to be exploited as a functional food.
CRediT authorship contribution statement
Jinle Xiang: Conceptualization, Supervision, Writing – original draft. Yuan Yuan: Methodology. Lin Du: Visualization, Investigation. Youyang Zhang: Software. Chunqiu Li: Validation. Trust Beta: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the Project for Introduction of Senior Foreign Experts in Henan Province of China (Grant No. HNGD2022056) and the Internationalization for Senior Ranking Personnel in Henan Province of China (Grant No. 21010157).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100628.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
UPLC profiles of free and bound phenolics of proso millets germinated for 6 days. Detection was at 320 nm for free (A) and bound (B) phenolics.
MS/MS spectrum (A) and UV spectrum (B) of feruloylquinic acid, MS/MS spectrum (C) and UV spectrum (D) of N,N′-bis-(p-coumaroyl) putrescine, MS/MS spectrum (E) and UV spectrum (F) of hydroxycinnamic acid derivatives.
MS/MS (A) and UV spectra (A′) of 8,8′-aryltetralin-DFA, MS/MS (B) and UV spectra (B′) of 8,8′-DFA, MS/MS (C) and UV spectra (C′) of 8-5′-DFA, MS/MS (D) and UV spectra (D′) of trans–trans-8-O-4′–DFA, MS/MS (E) and UV spectra (E′) of 5-5′-DFA, MS/MS (F) and UV spectra (F′) of 8-5′-DFA benzofuran, MS/MS (G) and UV spectra (G′) of trans–cis-8-O-4′–DFA.
Data availability
Data will be made available on request.
References
- Aloo S.-O., Ofosu F.-K., Oh D.-H. Effect of germination on alfalfa and buckwheat: phytochemical profiling by UHPLC-ESI-QTOF-MS/MS, bioactive compounds, and in-vitro studies of their diabetes and obesity-related functions. Antioxidants (Basel) 2021;10(10):1613. doi: 10.3390/antiox10101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloo S.O., Ofosu F.K., Daliri E.B., Oh D.H. UHPLC-ESI-QTOF-MS/MS metabolite profiling of the antioxidant and antidiabetic activities of red cabbage and broccoli seeds and sprouts. Antioxidants (Basel) 2021;10(6) doi: 10.3390/antiox10060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A.A.M., Dimberg L., Åman P., Landberg R. Recent findings on certain bioactive components in whole grain wheat and rye. Journal of Cereal Science. 2014;59(3):294–311. doi: 10.1016/j.jcs.2014.01.003. [DOI] [Google Scholar]
- Aparicio-Garcia N., Martinez-Villaluenga C., Frias J., Penas E. Sprouted oat as a potential gluten-free ingredient with enhanced nutritional and bioactive properties. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.127972. [DOI] [PubMed] [Google Scholar]
- Arouna N., Gabriele M., Pucci L. The impact of germination on sorghum nutraceutical properties. Foods. 2020;9(9) doi: 10.3390/foods9091218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asamenew G., Kim H.W., Lee M.K., Lee S.H., Lee S., Cha Y.S.…Kim J.B. Comprehensive characterization of hydroxycinnamoyl derivatives in green and roasted coffee beans: A new group of methyl hydroxycinnamoyl quinate. Food Chemistry X. 2019;2 doi: 10.1016/j.fochx.2019.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekara A., Shahidi F. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. Journal of Functional Foods. 2011;3(3):144–158. doi: 10.1016/j.jff.2011.03.007. [DOI] [Google Scholar]
- Cho E.E., Chung N.-J. Enhancement of amino acid contents by germination in a Korean weedy rice germplasm. Journal of Crop Science and Biotechnology. 2020;23(4):375–384. doi: 10.1007/s12892-020-00046-5. [DOI] [Google Scholar]
- Choi S.W., Lee S.K., Kim E.O., Oh J.H., Yoon K.S., Parris N.…Moreau R.A. Antioxidant and antimelanogenic activities of polyamine conjugates from corn bran and related hydroxycinnamic acids. Journal of Agricultural and Food Chemistry. 2007;55(10):3920–3925. doi: 10.1021/jf0635154. [DOI] [PubMed] [Google Scholar]
- Dong X., Gao Y., Chen W., Wang W., Gong L., Xianqing L., Luo J. Spatiotemporal distribution of phenolamides and the genetics of natural variation of hydroxycinnamoyl spermidine in rice. Molecular Plant. 2015;8(1):111–121. doi: 10.1093/mp/ssu101. [DOI] [PubMed] [Google Scholar]
- Gong E.S., Luo S.J., Li T., Liu C.M., Zhang G.W., Chen J.…Liu R.H. Phytochemical profiles and antioxidant activity of processed brown rice products. Food Chemistry. 2017;232:67–78. doi: 10.1016/j.foodchem.2017.03.148. [DOI] [PubMed] [Google Scholar]
- Gong K., Chen L., Li X., Sun L., Liu K. Effects of germination combined with extrusion on the nutritional composition, functional properties and polyphenol profile and related in vitro hypoglycemic effect of whole grain corn. Journal of Cereal Science. 2018;83:1–8. doi: 10.1016/j.jcs.2018.07.002. [DOI] [Google Scholar]
- Hunt H.V., Rudzinski A., Jiang H., Wang R., Thomas M.G., Jones M.K. Genetic evidence for a western Chinese origin of broomcorn millet (Panicum miliaceum) Holocene. 2018;28(12):1968–1978. doi: 10.1177/0959683618798116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo S., Rodríguez R., Jiménez A., Guillén R., Fernández-Bolaños J., Heredia A. Effects of storage conditions on the accumulation of ferulic acid derivatives in white asparagus cell walls. Journal of the Science of Food and Agriculture. 2007;87(2):286–296. doi: 10.1002/jsfa.2718. [DOI] [Google Scholar]
- Kapravelou G., Martínez R., Perazzoli G., Sánchez González C., Llopis J., Cantarero S.…Porres J.M. Germination improves the polyphenolic profile and functional value of mung bean (Vigna radiata L.) Antioxidants (Basel) 2020;9(8):746. doi: 10.3390/antiox9080746. https://www.mdpi.com/2076-3921/9/8/746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselova-Kaneva Y., Galunska B., Nikolova M., Dincheva I., Badjakov I. High resolution LC-MS/MS characterization of polyphenolic composition and evaluation of antioxidant activity of Sambucus ebulus fruit tea traditionally used in Bulgaria as a functional food. Food Chemistry. 2022;367 doi: 10.1016/j.foodchem.2021.130759. [DOI] [PubMed] [Google Scholar]
- Kumari D., Madhujith T., Chandrasekara A. Comparison of phenolic content and antioxidant activities of millet varieties grown in different locations in Sri Lanka. Food Science & Nutrition. 2017;5(3):474–485. doi: 10.1002/fsn3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens E., Moroni A.V., Pagand J., Heirbaut P., Ritala A., Karlen Y.…Delcour J.A. Impact of cereal seed sprouting on its nutritional and technological properties: A critical review. Comprehensive Reviews in Food Science and Food Safety. 2019;18(1):305–328. doi: 10.1111/1541-4337.12414. [DOI] [PubMed] [Google Scholar]
- Li W., Wen L., Chen Z., Zhang Z., Pang X., Deng Z.…Guo Y. Study on metabolic variation in whole grains of four proso millet varieties reveals metabolites important for antioxidant properties and quality traits. Food Chemistry. 2021;357 doi: 10.1016/j.foodchem.2021.129791. [DOI] [PubMed] [Google Scholar]
- Moreau R.A., Nunez A., Singh V. Diferuloylputrescine and p-coumaroyl-feruloylputrescine, abundant polyamine conjugates in lipid extracts of maize kernels. Lipids. 2001;36(8):839–844. doi: 10.1007/s11745-001-0793-6. [DOI] [PubMed] [Google Scholar]
- Ostrowski W., Swarcewicz B., Nolka M., Stobiecki M. Differentiation of phenylpropanoid acids cyclobutane- and dehydrodimers isomers in barley leaf cell walls with LC/MS/MS system. International Journal of Mass Spectrometry. 2016;407:77–85. doi: 10.1016/j.ijms.2016.07.003. [DOI] [Google Scholar]
- Park K.O., Ito Y., Nagasawa T., Choi M.R., Nishizawa N. Effects of dietary Korean proso-millet protein on plasma adiponectin, HDL cholesterol, insulin levels, and gene expression in obese type 2 diabetic mice. Bioscience, Biotechnology, and Biochemistry. 2008;72(11):2918–2925. doi: 10.1271/bbb.80395. [DOI] [PubMed] [Google Scholar]
- Pradeep P.M., Sreerama Y.N. Impact of processing on the phenolic profiles of small millets: Evaluation of their antioxidant and enzyme inhibitory properties associated with hyperglycemia. Food Chemistry. 2015;169:455–463. doi: 10.1016/j.foodchem.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Saleh A.S.M., Zhang Q., Chen J., Shen Q. Millet grains: Nutritional quality, processing, and potential health benefits. Comprehensive Reviews in Food Science and Food Safety. 2013;12(3):281–295. doi: 10.1111/1541-4337.12012. [DOI] [Google Scholar]
- Sharma S., Saxena D.C., Riar C.S. Analysing the effect of germination on phenolics, dietary fibres, minerals and γ-amino butyric acid contents of barnyard millet (Echinochloa frumentaceae) Food Bioscience. 2016;13:60–68. doi: 10.1016/j.fbio.2015.12.007. [DOI] [Google Scholar]
- Shen R., Ma Y., Jiang L., Dong J., Zhu Y., Ren G. Chemical composition, antioxidant, and antiproliferative activities of nine Chinese proso millet varieties. Food and Agricultural Immunology. 2018;29(1):625–637. doi: 10.1080/09540105.2018.1428283. [DOI] [Google Scholar]
- Ti H., Zhang R., Zhang M., Li Q., Wei Z., Zhang Y., Tang X., Deng Y., Liu L., Ma Y. Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chemistry. 2014;161:337–344. doi: 10.1016/j.foodchem.2014.04.024. [DOI] [PubMed] [Google Scholar]
- Wang X., Yang R., Jin X., Zhou Y., Han Y., Gu Z. Distribution of phytic acid and associated catabolic enzymes in soybean sprouts and indoleacetic acid promotion of Zn, Fe, and Ca bioavailability. Food Science and Biotechnology. 2015;24(6):2161–2167. doi: 10.1007/s10068-015-0288-4. [DOI] [Google Scholar]
- Xiang J., Apea-Bah F.B., Ndolo V.U., Katundu M.C., Beta T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chemistry. 2019;275:361–368. doi: 10.1016/j.foodchem.2018.09.120. [DOI] [PubMed] [Google Scholar]
- Xiang J., Li W., Ndolo V.U., Beta T. A comparative study of the phenolic compounds and in vitro antioxidant capacity of finger millets from different growing regions in Malawi. Journal of Cereal Science. 2019;87:143–149. doi: 10.1016/j.jcs.2019.03.016. [DOI] [Google Scholar]
- Xiang J., Yang C., Beta T., Liu S., Yang R. Phenolic profile and antioxidant activity of the edible tree peony flower and underlying mechanisms of preventive effect on H2O2-induced oxidative damage in Caco-2 cells. Foods. 2019;8(10) doi: 10.3390/foods8100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J., Zhang M., Apea-Bah F.B., Beta T. Hydroxycinnamic acid amide (HCAA) derivatives, flavonoid C-glycosides, phenolic acids and antioxidant properties of foxtail millet. Food Chemistry. 2019;295:214–223. doi: 10.1016/j.foodchem.2019.05.058. [DOI] [PubMed] [Google Scholar]
- Xiang N., Guo X., Liu F., Li Q., Hu J., Brennan C.S. Effect of light- and dark-germination on the phenolic biosynthesis, phytochemical profiles, and antioxidant activities in sweet corn (Zea mays L.) sprouts. International Journal of Molecular Sciences. 2017;18(6) doi: 10.3390/ijms18061246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.G., Tian C.R., Hu Q.P., Luo J.Y., Wang X.D., Tian X.D. Dynamic Changes in phenolic compounds and antioxidant activity in oats (Avena nudaL.) during steeping and germination. Journal of Agricultural and Food Chemistry. 2009;57(21):10392–10398. doi: 10.1021/jf902778j. [DOI] [PubMed] [Google Scholar]
- Xu M., Rao J., Chen B. Phenolic compounds in germinated cereal and pulse seeds: Classification, transformation, and metabolic process. Critical Reviews in Food Science and Nutrition. 2020;60(5):740–759. doi: 10.1080/10408398.2018.1550051. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Xiang J., Zheng B., Sun J., Luo D., Li P., Fan J. Diversity of phenolics including hydroxycinnamic acid amide derivatives, phenolic acids contribute to antioxidant properties of proso millet. Lwt. 2022;154 doi: 10.1016/j.lwt.2021.112611. [DOI] [Google Scholar]
- Zhang L., Liu R., Niu W. Phytochemical and antiproliferative activity of proso millet. PloS One. 2014;9(8):e104058. doi: 10.1371/journal.pone.0104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Xu Y., Xiang J., Zheng B., Yuan Y., Luo D., Fan J. Comparative evaluation on phenolic profiles, antioxidant properties and α-glucosidase inhibitory effects of different milling fractions of foxtail millet. Journal of Cereal Science. 2021;99 doi: 10.1016/j.jcs.2021.103217. [DOI] [Google Scholar]
- Zheng B., Ding Y., Johnson J.B., Xiang J., Li Z., Zhang Y., Luo D. Enrichment and bioactivities of polyphenols of crude extract by deep eutectic solvent extraction from foxtail millet bran. International Journal of Food Science & Technology. 2022 doi: 10.1111/ijfs.16155. [DOI] [Google Scholar]
- Zheng B., Yuan Y., Xiang J., Jin W., Johnson J.B., Li Z.…Luo D. Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: Optimization, comparison and bioactivities. Lwt. 2022;154 doi: 10.1016/j.lwt.2021.112740. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
UPLC profiles of free and bound phenolics of proso millets germinated for 6 days. Detection was at 320 nm for free (A) and bound (B) phenolics.
MS/MS spectrum (A) and UV spectrum (B) of feruloylquinic acid, MS/MS spectrum (C) and UV spectrum (D) of N,N′-bis-(p-coumaroyl) putrescine, MS/MS spectrum (E) and UV spectrum (F) of hydroxycinnamic acid derivatives.
MS/MS (A) and UV spectra (A′) of 8,8′-aryltetralin-DFA, MS/MS (B) and UV spectra (B′) of 8,8′-DFA, MS/MS (C) and UV spectra (C′) of 8-5′-DFA, MS/MS (D) and UV spectra (D′) of trans–trans-8-O-4′–DFA, MS/MS (E) and UV spectra (E′) of 5-5′-DFA, MS/MS (F) and UV spectra (F′) of 8-5′-DFA benzofuran, MS/MS (G) and UV spectra (G′) of trans–cis-8-O-4′–DFA.
Data Availability Statement
Data will be made available on request.