Abstract
Background
Shift work results in sleep‐wake disturbances, which cause sleepiness during night shifts and reduce sleep length and quality in daytime sleep after the night shift. In its serious form it is also called shift work sleep disorder. Various pharmacological products are used to ameliorate symptoms of sleepiness or poor sleep length and quality.
Objectives
To evaluate the effects of pharmacological interventions to reduce sleepiness or to improve alertness at work and decrease sleep disturbances whilst off work, or both, in workers undertaking shift work in their present job and to assess their cost‐effectiveness.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, PubMed and PsycINFO up to 20 September 2013 and ClinicalTrials.gov up to July 2013. We also screened reference lists of included trials and relevant reviews.
Selection criteria
We included all eligible randomised controlled trials (RCTs), including cross‐over RCTs, of pharmacological products among workers who were engaged in shift work (including night shifts) in their present jobs and who may or may not have had sleep problems. Primary outcomes were sleep length and sleep quality while off work, alertness and sleepiness, or fatigue at work.
Data collection and analysis
Two authors independently selected studies, extracted data and assessed risk of bias in included trials. We performed meta‐analyses where appropriate.
Main results
We included 15 randomised placebo‐controlled trials with 718 participants. Nine trials evaluated the effect of melatonin and two the effect of hypnotics for improving sleep problems. One trial assessed the effect of modafinil, two of armodafinil and one examined caffeine plus naps to decrease sleepiness or to increase alertness.
Melatonin (1 to 10 mg) after the night shift may increase sleep length during daytime sleep (mean difference (MD) 24 minutes, 95% confidence interval (CI) 9.8 to 38.9; seven trials, 263 participants, low quality evidence) and night‐time sleep (MD 17 minutes, 95% CI 3.71 to 30.22; three trials, 234 participants, low quality evidence) compared to placebo. We did not find a dose‐response effect. Melatonin may lead to similar sleep latency times as placebo (MD 0.37minutes, 95% CI ‐ 1.55 to 2.29; five trials, 74 participants, low quality evidence).
Hypnotic medication, zopiclone, did not result in significantly longer daytime sleep length compared to placebo in one low quality trial and we could not use the data from the study on lormetazepam.
Armodafinil taken before the night shift probably reduces sleepiness by one point on the Karolinska Sleepiness Scale (KSS) (MD ‐0.99, 95% CI ‐1.32 to ‐0.67; range 1 to 10; two trials, 572 participants, moderate quality evidence) and increases alertness by 50 ms in a simple reaction time test (MD ‐50.0, 95% CI ‐85.5 to ‐15.5) at three months' follow‐up in shift work sleep disorder patients. Modafinil probably has similar effects on sleepiness (KSS) (MD ‐0.90, 95% CI ‐1.45 to ‐0.35; one trial, 183 participants, moderate quality evidence) and alertness in the psychomotor vigilance test in the same patient group. Post‐marketing, severe skin reactions have been reported. Adverse effects reported by trial participants were headache, nausea and a rise in blood pressure. There were no trials in non‐patient shift workers.
Based on one trial, caffeine plus pre‐shift naps taken before the night shift decreased sleepiness (KSS) (MD ‐0.63, 95% CI ‐1.09 to ‐0.17).
We judged most trials to have a low risk of bias even though the randomisation method and allocation concealment were often not described.
Authors' conclusions
There is low quality evidence that melatonin improves sleep length after a night shift but not other sleep quality parameters. Both modafinil and armodafinil increase alertness and reduce sleepiness to some extent in employees who suffer from shift work sleep disorder but they are associated with adverse events. Caffeine plus naps reduces sleepiness during the night shift, but the quality of evidence is low. Based on one low quality trial, hypnotics did not improve sleep length and quality after a night shift.
We need more and better quality trials on the beneficial and adverse effects and costs of all pharmacological agents that induce sleep or promote alertness in shift workers both with and without a diagnosis of shift work sleep disorder. We also need systematic reviews of their adverse effects.
Keywords: Humans; Azabicyclo Compounds; Azabicyclo Compounds/therapeutic use; Benzhydryl Compounds; Benzhydryl Compounds/therapeutic use; Caffeine; Caffeine/therapeutic use; Hypnotics and Sedatives; Hypnotics and Sedatives/therapeutic use; Melatonin; Melatonin/therapeutic use; Modafinil; Piperazines; Piperazines/therapeutic use; Randomized Controlled Trials as Topic; Sleep; Sleep/drug effects; Sleep/physiology; Sleep Disorders, Circadian Rhythm; Sleep Disorders, Circadian Rhythm/drug therapy; Wakefulness; Wakefulness/drug effects; Wakefulness/physiology; Wakefulness‐Promoting Agents; Wakefulness‐Promoting Agents/therapeutic use
Plain language summary
Drugs for treating people with sleepiness during shift work and sleep problems after shift work
People who work shifts often report sleepiness at work and problems with sleep between work shifts. This is called shift work sleep disorder when the difficulties with sleep after the night shift and sleepiness during the night shift are persistent. We evaluated the effect of drugs, such as melatonin, to improve shift workers' sleep quality after night shift work. We also examined the effect of drugs, such as caffeine, to help shift workers stay awake. We also wanted to evaluate cost‐effectiveness but there were no studies.
Studies found
We performed a literature search up to 20 September 2013. We included 15 trials with 718 participants. Trials evaluated the effect of melatonin and hypnotics on sleep after the shift and the effect of modafinil, armodafinil and caffeine plus naps on sleepiness during the shift.
Effect on sleep length and quality
People who take melatonin may sleep for 24 minutes longer during the daytime after the night shift but there may be no effect on other sleep outcomes, such as time needed to fall asleep (low quality evidence). Side effects of melatonin use were rare.
For hypnotics (zopiclone), there is insufficient evidence to know whether or not they affect sleep length (very low quality evidence). We did not find reports on their side effects in shift workers.
Effect on alertness or sleepiness during the shift
People that take modafinil and armodafinil probably have a small reduction in sleepiness and an increase in alertness during the night shift, based on evidence at three months' follow‐up in people with shift work sleep disorder (moderate quality evidence). Headache and nausea were the most common side effects both in the short and long term follow‐up. However, serious skin disorders have been reported since these drugs have come on the market. We found no trials in shift workers without a diagnosis of shift work sleep disorder.
We found one trial which showed that people that took caffeine before the night shift in combination with a nap before the shift had increased alertness during the night shift.
What do we still need to find out?
The evidence was of low quality and mostly from small trials. Both sleep and alertness promoting agents have potentially serious adverse effects. Therefore, we need more trials to determine the beneficial and harmful effects of these drugs.
Summary of findings
Summary of findings for the main comparison. Melatonin versus placebo for sleep disturbances caused by shift work.
| Melatonin versus placebo for sleep disturbances caused by shift work | ||||||
| Patient or population: patients with sleep disturbances caused by shift work Settings: work setting Intervention: Melatonin versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Melatonin versus placebo | |||||
| Total sleep time, next day Diaries and actigraphy. Scale from: 120 to 600. | The mean total sleep time, next day in the control groups was 333 minutes | The mean total sleep time, next day in the intervention groups was 24.34 higher (9.82 to 38.86 higher) | ‐ | 263 (7 trials) | ⊕⊕⊝⊝ low1,2 | The scale of total sleeping time was estimated as ranging from two to 10 hours. |
| Total sleep time, next night Diaries and actigraphy. Scale from: 120 to 800. | The mean total sleep time, next night in the control groups was 384 minutes | The mean total sleep time, next night in the intervention groups was 16.97 higher (3.71 to 30.22 higher) | ‐ | 234 (3 trials) | ⊕⊕⊝⊝ low2 | Total sleep time estimated as ranging from two to 10 hours. |
| Sleep onset latency, next day Diaries and actigraphy. Scale from: 0 to 120. | The mean sleep onset latency, next day in the control groups was 12 minutes | The mean sleep onset latency, next day in the intervention groups was 0.15 lower (2.48 lower to 2.18 higher) | ‐ | 74 (5 trials) | ⊕⊕⊝⊝ low1,2 | Range of outcomes for sleep onset latency estimated as from 0 to two hours. |
| Sleepiness during the night shift KSS Scale from: 1 to 9. | The mean sleepiness during the night shift in the control groups was 4.3 score points | The mean sleepiness during the night shift in the intervention groups was 0.2 lower (0.91 lower to 0.51 higher) | ‐ | 34 (1 trial) | ⊕⊕⊝⊝ low3 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; Karolinska Sleepiness Scale (KSS). | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Two out of seven trials were at high risk of bias. The high risk trials contributed 25% in the meta‐analysis and the results changed after omission of these trials. 2 All trials are small with the sum of participants still being less than 400. 3 One small trial only.
Summary of findings 2. Armodafinil versus placebo for sleepiness caused by shift work.
| Armodafinil versus placebo for sleepiness caused by shift work | |||||
| Patient or population: patients with sleepiness caused by shift work Settings: work setting Intervention: Armodafinil versus placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Armodafinil versus placebo | ||||
|
Sleepiness during the night shift
KSS at final visit. Scale from: 1 to 9. Follow‐up: mean 3 months |
The mean sleepiness during the night shift in the control groups was 6 score points of KSS | The mean sleepiness during the night shift in the intervention groups was 0.99 lower (1.32 to 0.67 lower) | ‐ | 572 (2 trials) | ⊕⊕⊕⊝ moderate1 |
|
Sleep latency
MSLT. Scale from: 0 to 20 minutes. Follow‐up: mean 3 months |
The mean sleep latency in the control groups was 5.3 minutes on MSLT | The mean sleep latency in the intervention groups was 2.5 higher (1.36 to 3.64 higher) | ‐ | 216 (1 trial) | ⊕⊕⊝⊝ low2,3 |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; Karolinska Sleepiness Scale (KSS); Multiple sleep latency test (MSLT). | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 We included one trial at unclear risk of bias and one at low risk of bias. 2 This is a laboratory test and not a field test. 3 There was only one included trial.
Summary of findings 3. Modafinil versus placebo for sleepiness caused by shift work.
| Modafinil versus placebo for sleepiness caused by shift work | |||||
| Patient or population: patients with sleepiness caused by shift work Settings: work setting Intervention: Modafinil versus placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Modafinil versus placebo | ||||
| Sleepiness during the night shift KSS. Scale from: 1 to 9. Follow‐up: mean 3 months | The mean sleepiness during the night shift in the control groups was 6.7 score points on KSS | The mean sleepiness during the night shift in the intervention groups was 0.9 lower (1.45 to 0.35 lower) | ‐ | 183 (1 trial) | ⊕⊕⊕⊝ moderate1 |
|
Sleep latency
MSLT. Scale from: 0 to 20 minutes. Follow‐up: mean 3 months |
The mean sleep latency in the control groups was 2.37 minutes on MLST | The mean sleep latency in the intervention groups was 1.4 higher (1.27 to 1.53 higher) | ‐ | 153 (1 trial) | ⊕⊕⊝⊝ low1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; Karolinska Sleepiness Scale (KSS); Multiple sleep latency test (MSLT). | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 We included only one trial. 2 This is a laboratory test and not a field test.
Background
Description of the condition
Shift work is a common experience for many workers. At its simplest, shift work has been defined as "a way of organising daily working hours in which different people or teams work in succession to cover more than the usual 8‐hour day, up to and including the whole 24 hours" (Costa 2003). More precisely, in this systematic review of intervention trials, shift work is defined as any work schedule in which at least 25% of the work days involve the majority of working hours outside the time period between 08:00 and 17:00.
Many industries and services have long been required to develop in a society that functions at similar levels at all hours (Costa 2004). This means that an increasing proportion of workers spend at least some time in their working life performing shift work. For example, in a survey conducted in 2003, 14% of the Australian workforce performed shift work in the previous four weeks (ABS 2004) and between 8% and 24% of workers in the Organisation for Economic Co‐operation and Development (OECD) countries reported performing shift work in 2002 (OECD 2004).
In 2004 in the United States of America (USA), according to the Bureau of Labor Statistics (US BLS 2005), almost 15% of full‐time salaried workers usually worked on shifts including nights (16.7% men and 12.4% women). There were differences in the likelihood of working in shifts between different ethnic groups. The likelihood of undertaking shift work appears to decrease progressively with age. According to the Third European Survey of Working Conditions carried out in 2000 (Eurofound 2000), only 24% of the working population (27% employed and 8% self‐employed, both men and women) from the 15 member countries at the time were engaged in so‐called 'normal' or 'standard' day work; that is they started work sometime between the hours of 07:00 and 08:00, and ended work between the hours of 17:00 and 18:00, working from Monday to Friday (Costa 2004). This means that large numbers of workers are engaged in 'non‐standard' working hours, including shift and night work, part‐time work, weekend work, compressed work week, extended working hours, split shifts, on‐call work or flexible work hours.
Problems that result from the temporal misalignment of the habitual sleep‐wake cycle (i.e. having to stay awake during the time normally reserved for sleep and having to sleep during the normally active hours) are commonly referred to as sleep‐wake disturbances. Sleepiness during night shifts and sleep disturbances following them are the most frequently reported health problems in shift workers and they can jeopardise occupational health and safety by causing human errors and changes in basic biological and physiological functions (Driscoll 2007; Folkard 2003; Rogers 2001a; Rogers 2001b; Scott 2000). The International Agency for Research on Cancer has concluded that shift work that involves circadian disruption (i.e. having to adjust to working at night and sleeping during the day, and then having to readjust back) is probably carcinogenic to humans (Straif 2007). Prevalence rates of falling asleep at work and insomnia symptoms are about 30% higher, with an absolute difference of three percentage points (circa 10% versus 7%) for shift workers of 40 to 45 years of age compared to their day‐working counterparts (Ursin 2009). In addition, habitual sleep length is somewhat shorter for shift workers than for day workers (Åkerstedt 2008; Ursin 2009). Quality of sleep is also compromised due to misalignment between working hours and the human circadian system (Åkerstedt 1998; Drake 2004; Sallinen 2003). The occurrence of sleep‐wake disturbances also differs between various shifts within the group of shift workers. For example, the risk for severe sleepiness at work has been found to be between six to 14 times higher on night shifts and two times higher on early morning shifts compared to day shifts in a group of Finnish train drivers and railway traffic controllers (Härmä 2002). Also, the type of shift system plays a role in sleep‐wake disturbances. For example, ships' bridge officers who work two six‐hour watches per day report more frequent 'nodding off' on duty (7.3%) than their counterparts who have only two four‐hour watches per day (1.5%) (Härmä 2008).
International and national legislation regulates working time. In the EU, weekly working time should not exceed 48 hours and every 24‐hour period should contain a minimum daily rest period of 11 consecutive hours and every week should contain a rest period of 24 hours plus the 11 hours' daily rest. Moreover, normal hours of work for night workers should not exceed an average of eight hours in any 24‐hour period, and night workers whose work involves special hazards or heavy physical or mental strain should not work more than eight hours in any period of 24 hours during which they perform night work (Directive 2003/88/EC).
Description of the intervention
To counterbalance the negative effects of shift work on alertness and sleeping, there are several possibilities that can be classified as either work‐directed, such as changes in the shift system (see Erren 2013), or worker‐directed, such as pharmacological or non‐pharmacological interventions (see Herbst 2013). In this review, we investigated pharmacological interventions only. Pharmacological interventions may help shift workers by either reducing sleepiness and improving alertness during work‐shifts, or by reducing sleep disturbances while off work. We thus considered two categories of drugs in this review:
substances to help shift workers prevent drowsiness or improve alertness during shift work; or
substances to improve sleep quality or sleep length after a shift work period.
How the intervention might work
Ideally, these drugs or chemical substances would work by realigning the disruption of the circadian system. The outcome of the intervention might be better sleep or more sleep whilst off work, less sleepiness whilst at work, or both (Åkerstedt 1998). Melatonin comes closest to a substance that could potentially achieve this. In humans, melatonin is a hormone secreted by the pineal gland. The melatonin signal forms part of the system that regulates the sleep‐wake cycle by chemically causing drowsiness and lowering the body temperature. Exogenous melatonin appears to have some use against other circadian rhythm sleep disorders, such as jet lag (Herxheimer 2008) and the sleep‐related problems of people who work rotating or night shifts. Melatonin is available without prescription in the USA, Canada and also in many countries in Europe. Melatonin may be administered orally, as capsules, tablets or liquid, sublingually, or as transdermal patches.
Stimulants of the central nervous system, such as caffeine, have long been used by people to prevent drowsiness. Caffeine has been found to reduce the number of errors and improves cognitive performance of shift workers (Ker 2010). However, the authors found no difference in the effect upon injuries by trials comparing caffeine with other interventions among shift workers. More recently other stimulants, such as modafinil and armodafinil, have come on the market and especially target shift workers.
Lastly, hypnotic drugs are meant to induce sleep. Most of these drugs are known to have side effects, such as dependence and tolerance after long term use, and may cause drowsiness after the sleep period in short term usage.
Why it is important to do this review
A recent Cochrane Review (Ker 2010) examined the effect of caffeine on injuries and cognitive performance in shift workers. However, that review did not include sleep problems as an outcome. Herxheimer 2008 explored the effect of melatonin for people with jet lag. However, that review did not include shift workers, in whom one can expect the intervention to work similarly. As far as we know, there is no systematic review of pharmacological interventions for shift work‐related fatigue or sleeping problems.
Objectives
To evaluate the effects of pharmacological interventions to reduce sleepiness or to improve alertness at work and sleep disturbances whilst off work, or both, in workers undertaking shift work in their present job. In addition, we planned to assess the cost‐effectiveness of the interventions.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and cross‐over RCTs with a sufficiently long wash‐out period in between, as we expect the treatment to be symptomatic only.
Types of participants
We included all RCTs conducted with workers who undertake shift work (including night shifts) in their present jobs and who may or may not have sleep problems. We included only field trials in which real shift workers were studied whilst occupied in real work duties. In other words we excluded all trials that had used simulated shift work tasks because the results of these trials probably do not apply to shift workers in a real life working environment. There is already a review of melatonin for jet lag (Herxheimer 2008), therefore we excluded studies in which participants are airline staff, military personnel or similar for whom crossing time zones is frequent.
We also included participants with diagnosed shift work disorder or shift work sleep disorder (SWD). The second edition of the International Classification of Sleep Disorders list the following criteria for the diagnosis of this condition:
complaints of insomnia or excessive sleepiness associated with a work schedule that overlaps with usual time for sleep;
symptoms associated with work for at least a month;
evidence for sleep‐time misalignment present for seven days or more using a sleep log or actigraphic recording; and
the sleep disturbance cannot be explained by another disorder, medication or substance abuse.
However, the operationalisation of these criteria still leads to a widely varying number of people with SWD. Shift work sleep disorder can thus be seen as one end of the spectrum of sleep problems that shift workers can encounter (Wright 2013).
We excluded studies in which more than 10% of the participants had been diagnosed with one of the following conditions that would preclude them from shift work: type I diabetes, severe neurological disease with insomnia or sleeping problems other than caused by the shift work or severe psychiatric disease with insomnia.
Types of interventions
We included trials with any pharmacological intervention aimed at preventing or reducing sleepiness at work or sleep disturbances caused by shift work. We categorised interventions into preventive interventions that offer treatment to workers who are not actively seeking medical assistance for their sleep problems, and into treatment interventions that were given in response to a worker's request for help or that are based on a diagnosis of a shift work‐related sleep disorder.
We excluded interventions directed at treating individuals diagnosed with a specific disease other than SWD.
Examples of relevant pharmacological agents are caffeine and other stimulants such as modafinil and armodafinil to increase alertness during the shift and melatonin, melatonin agonists and hypnotic drugs to increase the quality and quantity of sleep following the shift. We included trials that compared pharmacological agents to placebo or to an alternative agent.
Types of outcome measures
Primary outcomes
Sleep length and sleep quality while off work;
Alertness and sleepiness, or fatigue, at work.
Two primary outcomes are justified as they both represent the same problem, namely sleep‐wake disturbance associated with shift work, i.e. sleep problems and fatigue.
We included trials that used the following types of outcome measures for sleep length and quality while the participants were off work:
Total sleep time (diary or actigraphy); or
Sleep quality as measured by sleep onset latency (diary, actigraphy), number of sleep time awakenings (diary) and subjective sleep quality (diary).
We included trials that used the following types of outcome measures for alertness or sleepiness or fatigue while the participants were working during the night shift (Cursio 2001):
Subjective measures of sleepiness: e.g. Karolinska Sleepiness Scale (KSS) (Åkerstedt 1990);
Objective sleep propensity measures: e.g. Multiple Sleep Latency Test (MSLT) (Carskadon 1979);
Objective performance decrease measures: psychomotor tasks (simple reaction time, psychomotor vigilance task).
Fatigue usually refers to exhaustion or tiredness due to long‐lasting exertion or mental concentration but it can also refer to a situation with insufficient sleep. Because there are some differences in the use of these terms in different countries (e.g. between Europe and Australia), we also included fatigue as an outcome measure when it was used as a measure of sleepiness at work.
Secondary outcomes
We considered the following as secondary outcomes:
Economic outcomes;
Resource use and associated costs of the intervention; and
Injuries and accidents and their risk at work and during the commute to and from work.
Adverse events
We planned to report adverse outcomes of interventions, such as side effects of chemical substances if trials reported them.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Occupational Health Group Specialized Register, CENTRAL, MEDLINE, EMBASE, PubMed and PsycINFO from inception up to 20 September 2013 and ClinicalTrials.gov up to July 2013. We employed a systematic search strategy designed primarily for PubMed, which we then adapted to EMBASE, PsycINFO and other relevant databases (see Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8). The strategy consisted of separate sets of search terms for:
various difficulties related to the onset or maintenance of sleep or symptoms of fatigue or sleepiness at work;
known pharmacological interventions for promoting either sleep or alertness;
known descriptors of shift work;
the revised Cochrane Collaboration strategy for finding RCTs;
the Cochrane Occupational Health Field most sensitive strategy for finding work‐related intervention trials.
We then combined: ((#1 OR #2) AND #3) AND (#4 OR #5).
In the search strategy, we had no language restrictions.
Searching other resources
We screened references from original articles and we also intended to check conference proceedings and abstracts but due to limited resources and limited expected yield we did not do this.
Data collection and analysis
Selection of studies
After using the search strategies, we developed a standardized selection form to make a first selection of the relevant trials, based on the following criteria: (1) the trial design was a RCT; (2) trial population consisted of shift work employees who participated in night shift work; and (3) trial outcomes were sleepiness or alertness at work, or sleep quality or length after a work shift.
We divided the identified references between all review authors and two review authors examined each reference. Each review author then independently assessed whether each trial in his or her share met the inclusion criteria or not. We resolved any disagreements by consensus. We sought to obtain further information from the trial authors when we thought a paper did not contain sufficient information to confirm eligibility. We intend to perform a new search for trials every two years and to update the review accordingly.
Data extraction and management
Two review authors independently extracted data from each of the included trials regarding: the country where the trial was conducted, the type of trial design used, characteristics of the trial participants (as per trial inclusion criteria) such as the occupation, type of work and branch of industry, and types of interventions and outcomes. We also extracted results data (means and standard deviation values for continuous outcomes and count data for dichotomous outcomes) for the purpose of meta‐analysis. We resolved disagreements by consensus.
Assessment of risk of bias in included studies
Two review authors independently assessed the quality, in terms of risk of bias, of the included trials. We used a consensus method of mutual agreement if disagreements occurred. If information was absent for evaluation of the methodological criteria, we contacted the trial authors to provide additional information. We evaluated trial validity according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) as implemented in RevMan 2011 to assess the risk of bias of the included RCTs and cluster‐RCTs. We independently assessed the risk of bias of each included trial based on the following six domains, applying a rating of low, high or unclear risk of bias:
Sequence generation
The method used to generate the allocation sequence ought to have been described in sufficient detail so that we could assess whether it should have produced comparable groups.
Allocation concealment
The method used to conceal the allocation sequence ought to have been described in sufficient detail so that we could assess whether intervention schedules could have been foreseen in advance of or during recruitment.
Blinding of outcome assessors, care providers and participants
Any measures used to blind outcome assessors, care providers and participants ought to have been described in sufficient detail so that we could assess the level of knowledge a given participant might have acquired regarding the intervention to which they had been assigned.
Incomplete outcome data
If included RCTs did not report intention‐to‐treat analyses, we sought to obtain missing data by contacting the trial authors. We extracted and report data on attrition and exclusions, as well as the numbers involved (compared with total randomised), reasons for attrition or exclusion where reported or obtained from investigators, and any re‐inclusions in analyses we performed (see also: 'Dealing with missing data' section below).
Selective outcome reporting
We assessed the possibility of selective outcome reporting by investigators by comparing the outcomes in the methods section to those reported. Of course selection is possible also without trial authors admitting having done it either in the methods or in the results section of their trial.
Other sources of bias
We assessed whether trials were apparently free of other problems that could put them at high risk of bias.
Measures of treatment effect
We reversed the scoring of scales if needed so that a high score denoted the same direction (good or bad) in all outcomes.
We plotted the results of each trial as point estimates, such as risk ratios (RR) for dichotomous outcomes, means and standard deviations (SD) for continuous outcomes or other data types as reported by the trial authors. When we could not plot the results, we described them in the 'Characteristics of included studies' table. We used standardised mean differences (SMD) for pooling outcome data from different instruments deemed similar enough for comparison.
Unit of analysis issues
If a trial compared several active interventions with no intervention then, for the purposes of meta‐analysis, we intended to divide the participants in the no‐intervention control group by the number of interventions as described in Higgins 2011 but we did not include any such trials. When a trial measured the same outcome with different measurement instruments such as diary and actigraphy we included only the actigraphy measurements in the same comparison. For trials that used clustered data, we intended to adjust for the clustering effect but due to a lack of data, this was not possible. For cross‐over trials, we intended to use the results from the paired statistical tests when available but the lack of appropriate data prevented this.
Dealing with missing data
We contacted trial authors to obtain data missing from their reports that we needed for meta‐analysis. Where statistics were missing, such as standard deviations or correlation coefficients and we could not obtain them from the trial authors, we calculated them from other available statistics, such as P values, according to the methods described in Higgins 2011 and available through the calculator in RevMan 2011.
Assessment of heterogeneity
First, we assessed clinical homogeneity based on the similarity of the intervention, control condition, outcome, population and follow‐up time. We determined similarity of interventions by our assessment of whether the interventions in question (e.g. melatonin at different doses) could reasonably be supposed to yield similar effects across different populations.
In addition, we tested for statistical heterogeneity by means of the Chi2 test as implemented in the forest plot in RevMan 2011. We used a significance level of P < 0.10 to indicate whether there was heterogeneity. Moreover, we quantified the degree of heterogeneity using the I2 statistic, where an I2 value of 25% to 50% indicates a low degree of heterogeneity, 50% to 75% a moderate degree of heterogeneity and over 75% a high degree of heterogeneity (Higgins 2003).
Assessment of reporting biases
To reduce the effect of reporting bias, we included trials and not publications in order to avoid the introduction of duplicated data (i.e. two articles could represent duplicate publications of the same study). Following the Cho 2000 statement on redundant publications, we attempted to detect duplicate trials and, where more articles reported on the same trial, we extracted data only once. To prevent location bias, we searched for trials across multiple databases. To prevent language bias, we did not exclude any articles based on language. Where sufficient data were available, we assessed publication bias using a funnel plot.
Data synthesis
We pooled data from trials that we judged to be clinically homogeneous and performed meta‐analyses. For continuous outcomes such as sleep length, we combined trials using mean differences. For outcomes measuring the same concept but measured on a different scale, we used standardised mean differences (SMDs).
Where trials were statistically heterogeneous according to RevMan 2011, we used a random‐effects model; otherwise we used a fixed‐effect model. We included a 95% confidence interval (CI) for all estimates.
We used the GRADE approach as described in Higgins 2011 and as implemented in GRADEpro 2008 to present the quality of evidence and 'Summary of findings' tables.
We based the downgrading of the quality of a body of evidence for a specific outcome on five factors: (1) limitations of trial; (2) indirectness of evidence; (3) inconsistency of results; (4) imprecision of results; and (5) publication bias.
For each comparison we started the quality of the body of evidence at high quality. If there were shortcomings for one or more of the factors mentioned above, we downgraded the quality by one or more levels. Thus we rated the evidence as either high, moderate, low or very low.
Subgroup analysis and investigation of heterogeneity
For trials that investigated melatonin in comparable populations and used the same outcome measures at the same follow‐up times, we conducted a subgroup analysis to compare trials conducted with various doses of the provided drugs, even though we did not mention this in the protocol. We also looked at participants drawn from different occupational settings or branches of industry (e.g. hospital staff).
Sensitivity analysis
We conducted sensitivity analyses to test the robustness of our meta‐analysis results by leaving out trials we judged to have a high risk of bias.
Results
Description of studies
Results of the search
In Figure 1 we present a PRISMA study flow diagram of included and excluded studies. After the initial search of electronic databases, we identified 4651 references. Based on screening of titles and abstracts we identified 207 eligible references for shift work interventions and retrieved the full text articles. Of these, 81 considered pharmacological interventions. We did not find any additional trials after checking the references of all articles retrieved as full text papers. After full text screening of these 81 articles, we included 15 RCTs in the review. These 15 trials consisted of nine trials on melatonin, two on armodafinil, two on hypnotics, and one each on modafinil and caffeine.
1.
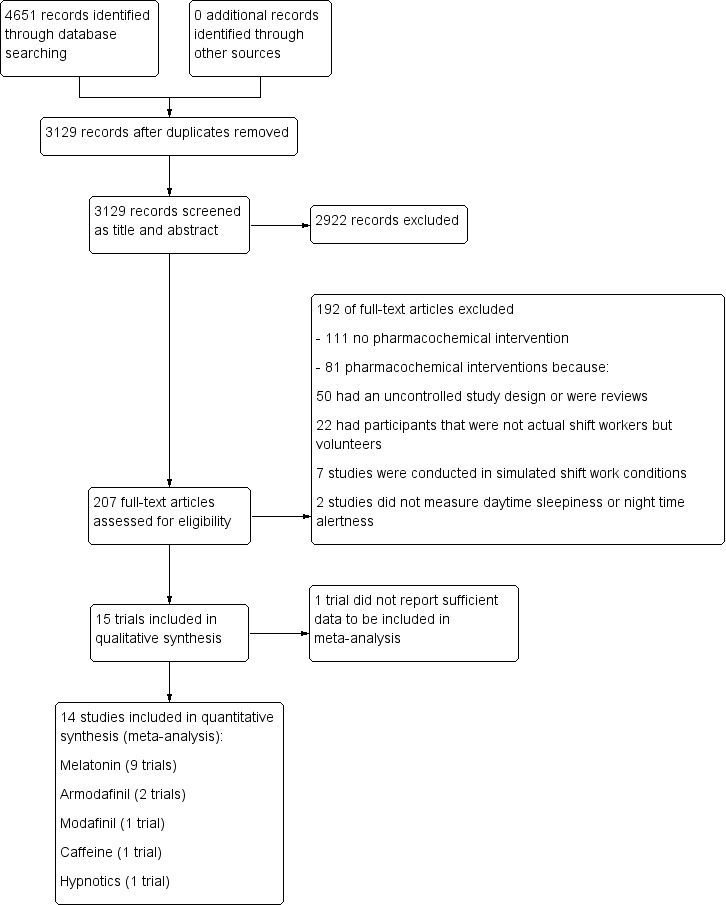
Study flow diagram.
Included studies
Melatonin
Characteristics of the trials and participants
We included nine RCTs that evaluated the effect of melatonin (Bjorvatn 2007; Cavallo 2005; Folkard 1993; James 1998; Jockovich 2000; Jorgensen 1998; Sadeghniiat‐Haghighi 2008; Wright 1998; Yoon 2002). Five trials were performed in the USA, one in the UK, one in Norway, one in Korea and one in Iran. Altogether the trials included 251 participants. The average age of the participants ranged between 24 and 42 years; the percentage of female participants ranged between 5% and 100%, typically varying by profession.
In seven RCTs, participants were hospital employees (Cavallo 2005; James 1998; Jockovich 2000; Jorgensen 1998; Sadeghniiat‐Haghighi 2008; Wright 1998; Yoon 2002), in one trial participants came from a police workforce (Folkard 1993) and in one trial they worked at an oil rig (Bjorvatn 2007). In seven trials, participants were volunteers with no reported sleeping problems (Cavallo 2005; Folkard 1993; James 1998; Jockovich 2000; Jorgensen 1998; Wright 1998; Yoon 2002) and in two trials participants were recruited if they had sleeping problems in association with shift work based on a preliminary questionnaire (Bjorvatn 2007; Sadeghniiat‐Haghighi 2008).
Melatonin administration
Seven trials administered melatonin in the morning after the night shift before the day time sleep period (Bjorvatn 2007; Cavallo 2005; Folkard 1993; James 1998; Jockovich 2000; Jorgensen 1998; Yoon 2002). The doses of melatonin varied between 1 mg (Jockovich 2000) to 10 mg (Jorgensen 1998), doses in all trials varied between 3 and 6 mg. Two trials administered melatonin in the evening after the night shift before going to sleep (Sadeghniiat‐Haghighi 2008; Wright 1998) using a dose of 5 mg melatonin.
Trial design and setting
Eight trials used a randomised double‐blind placebo‐controlled cross‐over design and randomised participants to the order of melatonin or placebo first. Participants acted as their own controls. Many trials included several consecutive days or nights in the measurement of the effects. Yoon 2002 reported using a repeated measures cross‐over design, with blinded allocation of the treatment and we included this trial in the data synthesis. This trial had three treatment arms, melatonin, placebo and a third arm with melatonin plus sunglasses to protect light exposure before day time sleep after the night shift (we excluded the third arm from our analyses as sunglasses did not enhance the effect of melatonin).
The wash‐out period between treatments (melatonin versus placebo) varied between three days (James 1998) and four weeks (Wright 1998). As the half‐life of exogenously administered melatonin is short, ranging between 10 and 60 minutes (Zawilska 2009), we considered the wash‐out period sufficient in all trials, meaning that all trial authors managed to prevent any carry‐over effect of melatonin.
Outcomes
All trials reported on the effect of orally administered melatonin on sleep length and quality after one or several consecutive night shifts. Five trials evaluated day time sleep after the night shift during a night shift period of several consecutive nights (Cavallo 2005; James 1998; Jockovich 2000; Jorgensen 1998; Yoon 2002). Two trials evaluated both day time sleep during the night shift and night time sleep after the night shift period (Bjorvatn 2007; Folkard 1993). Two trials evaluated night sleep after the night shift period (Sadeghniiat‐Haghighi 2008; Wright 1998). Several trials included several consecutive night shifts.
Seven trials recorded total sleep time by sleep diary for the daytime sleep after the night shift (Bjorvatn 2007; Cavallo 2005; Folkard 1993; James 1998; Jockovich 2000; Jorgensen 1998; Yoon 2002) and three trials recorded it for the night time sleep (Bjorvatn 2007; Sadeghniiat‐Haghighi 2008; Wright 1998). Five trials recorded sleep onset latency by diary for day sleep after the night shift (Bjorvatn 2007; Folkard 1993; James 1998; Jorgensen 1998; Yoon 2002) and three trials recorded it for the night time sleep (Bjorvatn 2007; Sadeghniiat‐Haghighi 2008; Wright 1998). It should be noted that sleep onset latency can be used in two different ways. During off‐shift rest periods, interventions aim to decrease sleep onset latency, i.e. to enable participants to fall asleep sooner. When used as a measure of alertness during shift work, the aim of interventions is to increase sleep onset latency, i.e. to enable participants to stay awake longer.
Bjorvatn 2007 used actigraphy for measurement of day time and night time total sleep time and sleep onset latency.
We did not find any trials that had used objective and more complicated measures of sleep length and quality, such as polysomnography (Krystal 2008).
Bjorvatn 2007 measured sleepiness during the night shift and during day time work after the night shift was measured with the KSS and Yoon 2002 measured alertness during the night shift by a Visual Analog Scale.
Armodafinil
Characteristics of the trials and participants
Two included RCTs (Czeisler 2009; Erman 2011) evaluated the effect of armodafinil on night shift alertness or sleepiness in shift workers who were excessively sleepy as a result of shift work sleep disorder (SWD). Both trials were clinical multicenter trials.
Czeisler 2009 rated participants (N = 245 treated) as moderately ill (N = 138; 56%) or markedly or severely ill (N = 104; 44%) in relation to shift work disorder symptoms. Most participants (N = 215; 87%) worked on permanent shift work and a minority engaged in rotating shift work (N = 33; 13%). The mean participant age was 38.9 in the intervention group and 40.3 years in the control group and the proportion of males was 66% and 64% in the two groups (armodafinil and placebo, respectively).
Erman 2011 rated participants (N = 383 randomised) as moderately ill (N = 207; 54%) or markedly or severely ill (N = 175; 46%). Most participants (N = 357; 93%) worked on permanent shift work and a minority in rotating shift work (N = 26; 7%). The mean ages of the participants were 36.1 to 36.7 years, and the proportion of males was 56%, in the two groups (armodafinil and placebo, respectively). Most participants were working in health care (25%). Other common occupations were protective services (15%) and transportation (10%).
Interventions
Both trials, Czeisler 2009 and Erman 2011, administered armodafinil (first night 50 mg, second and third nights 100 mg and from the fourth night 150 mg) or placebo in the evening before every night shift period. During the laboratory test night, Czeisler 2009 administered the dose at 10 PM. Armodafinil is licensed only in the US but not in Europe.
Trial design and setting
Czeisler 2009 was a multicenter clinical trial conducted in 42 sleep research centres in the US (37 centres) and Canada (five centres) between April and December 2004. This trial used a randomised double‐blind placebo‐controlled parallel‐group design. Participants were shift workers with moderate or severe SWD and excessive sleepiness due to that. The trial included a laboratory test night after three consecutive nights in shift work. Czeisler 2009 screened a total of 747 people and randomised 254, and 172 completed the trial.
Erman 2011 was a multicentre pharmacological phase IV clinical trial conducted in 45 sleep research clinics across the US between February and October 2010. This trial used a randomised double‐blind placebo‐controlled parallel‐group design. Participants were shift workers with moderate or severe SWD and excessive sleepiness due to that. Erman 2011 screened a total of 649 people and randomised 383, and 325 completed the trial.
Outcomes
Sleepiness during the night shift was measured by the KSS (Czeisler 2009; Erman 2011) and MSLT (Czeisler 2009) and alertness during the night shift by simple reaction time (Czeisler 2009). The open‐label extension of the trial of Czeisler 2009 also reported adverse events.
Modafinil
Characteristics of the trials and participants
We included one RCT in the analysis of the effect of modafinil on night shift alertness (Czeisler 2005). This was a multicentre clinical trial conducted in 28 sleep research centres in the USA between April 2001 and September 2002. It used a randomised double‐blind placebo‐controlled design. Participants were shift workers with moderate or severe sleepiness problems and shortened sleep latency and reduced sleep efficiency or both. The trial included a laboratory test night after three consecutive nights in shift work. Czeisler 2005 screened a total of 609 people and randomised 209, and 153 completed the trial.
Czeisler 2005 rated participants (N = 204 treated) as moderately ill (N = 102; 50%) or markedly or severely ill (N = 102; 50%). Most participants (N = 184; 90%) worked on permanent shift work and a minority in rotating shift work (N = 20; 10%). Participants' mean ages were 37.5 to 38.8 years, and the proportion of males was 60% to 62% in the two groups (modafinil and placebo, respectively).
Interventions
Czeisler 2005 administered modafinil (200 mg) or placebo in the evening before night shifts during a night shift period of three or more consecutive nights as the participants worked normally in their jobs. Modafinil is licensed both in the USA and Europe. The licence in Europe has been withdrawn for shift work sleep disorders as the European Medicines Agency (EMA) argued that the effects on both subjective and objective measures did not provide clear evidence of overall beneficial effect. At the same time, post‐authorisation surveillance of adverse effects revealed 21 cases of severe skin reactions, of which three were fatal. Due to this imbalance between benefits and harms, the EMA withdrew the licence for shift work sleep disorder in 2011 (EMA 2011).
Trial design and setting
Czeisler 2005 used a randomised placebo controlled cross‐over design. Randomisation was at the level of participants (modafinil or placebo). The trial included a laboratory test night after three or more consecutive nights in shift work both at baseline and during the trial. Participants were evaluated monthly during a three‐month period.
Outcomes
Czeisler 2005 measured sleepiness during the experimental night shift using the KSS and the MSLT and alertness during the night shift with the Psychomotor Vigilance Test, which used the number of lapses during the 20‐minute test session as an outcome.
Caffeine plus naps
Characteristics of the trials and participants
We included one RCT (Schweitzer 2006) in the analysis of the effect of caffeine on night shift alertness. The trial had two settings, one in the laboratory and the other in the field, but we only included the field trial which compared caffeine combined with naps to placebo. The trial was performed in the USA. The field trial participants (N = 53) were volunteer shift workers in the St. Louis and the San Diego area.
Interventions
Schweitzer 2006 administered caffeine (300 mg) in the evening before every night shift period. In the field trial, Schweitzer 2006 added evening naps (60 to 120 minutes) to the caffeine intervention for the two first evenings during a shift work period of four consecutive nights before the caffeine administration.
Trial design and setting
Schweitzer 2006 used a randomised placebo controlled cross‐over design. Randomisation was at the level of participants (caffeine plus naps or placebo first). Participants functioned as their own controls. Schweitzer 2006 offered naps before the night shift only before caffeine administration. Caffeine or placebo was administered before all four consecutive night shifts.
Outcomes
Schweitzer 2006 measured sleepiness during the night shift with the KSS. We calculated missing standard deviations based on the P value for the KSS outcome, assuming that the authors used a paired analysis. The trial authors also measured alertness during the night shift by the Psychomotor Vgilance Test, which used the frequency of lapses (reaction time greater than 500 msec) during the 15‐minute session as the outcome. However there were insufficient data to use this outcome in our analyses.
Hypnotics
Characteristics of the trials and participants
We included two RCTs (Sastre‐y‐Hernandez 1982; Quera‐Salva 2002) in the analysis of the effect of hypnotics (lorazepam and zopiclone respectively) on post‐shift sleep length. One trial was performed in France and one in Germany. Participants were nurses with sleeping problems (N = 60) and employees in two factories (N = 28) with sleeping problems related to shift work.
Interventions
Sastre‐y‐Hernandez 1982 administered lorazepam (1 mg) during seven consecutive days but it was unclear when night shifts took place during that time. Quera‐Salva 2002 administered zopiclone (7.5 mg) in the morning after every night shift period for three consecutive night shifts.
Trial design and setting
Sastre‐y‐Hernandez 1982 reported the trial as a double‐blind placebo controlled trial. We understood this to mean that it was a parallel‐group trial with individual participant randomisation. Sastre‐y‐Hernandez 1982 measured sleep quality immediately after getting up.
Quera‐Salva 2002 used a randomised placebo controlled parallel‐group design: Randomisation was at the level of participants (zopiclone or placebo). Quera‐Salva 2002 administered zopiclone or placebo after three consecutive night shifts and measured sleep length during the next day's sleep.
Outcomes
Sastre‐y‐Hernandez 1982 used daily self‐reports of length, quality and awakenings. Quera‐Salva 2002 measured sleep length after the night shift with actigraphy during the next day sleep period.
Excluded studies
Of the 207 full‐texts retrieved, we excluded 111 studies because they did not examine the effectiveness of a pharmacochemical intervention. We excluded the remaining 81 full‐text articles that did examine the effectiveness of a pharmacochemical intervention because they employed an uncontrolled study design or were reviews (N = 50), they had participants that were not actual shift workers but volunteers (N = 22), they were conducted in simulated shift work conditions (N = 7) or they did not report relevant sleep length and quality or sleepiness and alertness outcomes (N = 2). For a more detailed description, see the Characteristics of excluded studies table.
Risk of bias in included studies
We presented the details of the 'Risk of bias' assessment for each included trial in the 'Characteristics of included studies' table. We summarised the results in Figure 2 and Figure 3.
2.
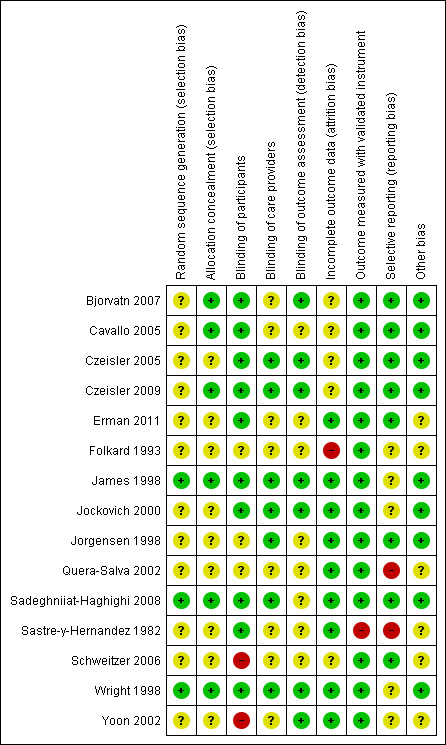
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
3.
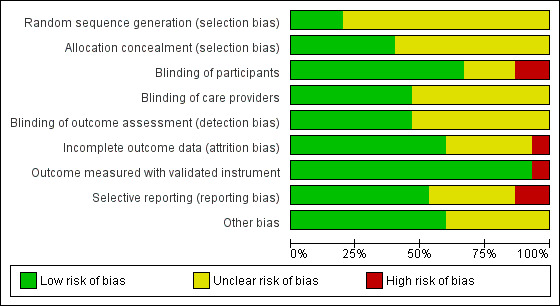
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
Of the 15 included RCTs, we judged ten trials to have a low risk of bias (Bjorvatn 2007; Cavallo 2005; Czeisler 2005; Czeisler 2009; Erman 2011; James 1998; Jockovich 2000; Jorgensen 1998; Sadeghniiat‐Haghighi 2008; Wright 1998). We judged the remaining five trials to have a high risk of bias due to high discontinuation (Folkard 1993;) missing baseline group comparability data (Quera‐Salva 2002), an unclear relation to shift work (Sastre‐y‐Hernandez 1982) or administering an unblinded intervention (Schweitzer 2006; Yoon 2002).
Allocation
Only three trials reported the details of sequence generation (James 1998; Sadeghniiat‐Haghighi 2008; Wright 1998).
Also allocation concealment was rarely reported and therefore we judged only six trials to have a low risk of bias regarding this factor (Bjorvatn 2007; Cavallo 2005; Czeisler 2009; James 1998; Sadeghniiat‐Haghighi 2008; Wright 1998).
Blinding
Many trials were reported as blinded or double blind but did not report details of who was blinded. In two trials, the intervention could not be blinded: in Schweitzer 2006 the trial arm included naps (with caffeine ‐ no naps with placebo) and in Yoon 2002 the third trial arm included sunglasses (with melatonin ‐ other arms melatonin and placebo without sunglasses.
Seven trials reported or we inferred blinding of outcome assessors (Bjorvatn 2007; Czeisler 2005; Czeisler 2009; James 1998; Jockovich 2000; Wright 1998; Yoon 2002). In these trials, the researchers responsible for collecting outcome data as well as the researchers responsible for analysing the data were blinded to treatment allocation.
Incomplete outcome data
All trials, except for three small ones (Quera‐Salva 2002; Wright 1998; Yoon 2002), reported drop‐outs during the intervention. The proportion of the participants who were followed‐up varied from a high proportion (James 1998 91%; Jorgensen 1998 90%; Sadeghniiat‐Haghighi 2008 88%; Erman 2011 84%; Jockovich 2000 79%; Czeisler 2005 75%; Schweitzer 2006 74%; Czeisler 2009 70%) to a much lower proportion (Cavallo 2005 62%; Bjorvatn 2007 45%; Folkard 1993 35%; Sastre‐y‐Hernandez 1982 6%).
The reasons for drop‐outs differed. In the three trials with the highest discontinuation rates, the employees in night work discontinued the participation during the intervention in an emergency hospital (Cavallo 2005), in a constabulary (Folkard 1993) and on an oil rig (Bjorvatn 2007) due to inconveniences of the trial protocol and changes in shift work schedules. We could not include these participants in the final analyses as there was no outcome data from those who discontinued.
Selective reporting
The reporting of outcomes was selective especially in trials where authors used many measures of night shift sleepiness or alertness, and where the results of most of the outcomes did not differ between interventions. This means that the numbers were omitted but results were expressed qualitatively for example stating that there was no significant difference between interventions.
Our primary outcomes, sleep length and quality of sleep during the day after the night shift (measured as e.g. total sleep time) and sleepiness or alertness during night shift (measured with e.g. KSS), were the most commonly reported by trial authors.
One trial (Quera‐Salva 2002) expressed the results as change scores, while others (e.g. Czeisler 2005; Czeisler 2009; Erman 2011; Schweitzer 2006) used graphs from which we had to estimate the numerical results. Sastre‐y‐Hernandez 1982 reported the results as categories that improved.
Other potential sources of bias
Baseline comparability
In trials with randomisation to two trial groups, three trials had good baseline comparability (Czeisler 2005; Czeisler 2009; Erman 2011). Quera‐Salva 2002 did not describe baseline data of two small (N = 14) groups and we considered this trial having a high risk of bias.
Co‐interventions
The duration of naps before the night shift and coffee and other stimulant consumption during the night shift (to reduce sleepiness in night shift) (Schweitzer 2006) and the duration of light exposure after the night shift (to reduce sleep disturbance during the day time sleep) (Yoon 2002) did not differ between trial arms.
In one trial, co‐interventions were a reason for discontinuation (Schweitzer 2006), where napping during the intervention (where napping was not scheduled) caused trial discontinuation.
Funding
Pharmaceutical companies funded five trials; three trials with stimulants (Czeisler 2005; Czeisler 2009; Erman 2011) which were clinical phase III and IV pharmacological multicentre trials (with strict protocols) and two trials with hypnotics (Sastre‐y‐Hernandez 1982; Quera‐Salva 2002).
Public funds supported two trials (Sadeghniiat‐Haghighi 2008; Schweitzer 2006). The other eight trials did not mention their funding sources.
Effects of interventions
See: Table 1; Table 2; Table 3
1. Melatonin taken after the night shift versus placebo
1.1 Sleep length and quality after the night shift
1.1.1 Total sleep time, next day
Six trials (Bjorvatn 2007; Cavallo 2005; Folkard 1993; James 1998; Jorgensen 1998; Yoon 2002) compared the effect of melatonin on sleep length the next day after the night shift using self‐report (diary). Self‐reported sleep length was longer after melatonin administration (MD 23.49 minutes, 95% CI 8.49 to 38.49; six trials, 225 participants, low quality evidence, Analysis 1.1)
1.1. Analysis.
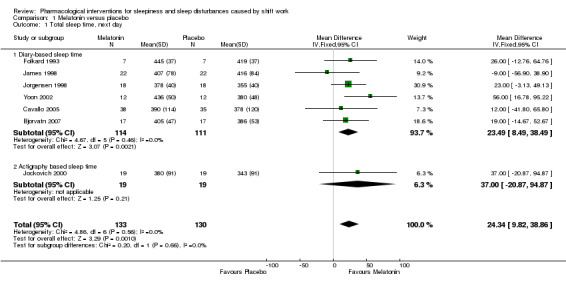
Comparison 1 Melatonin versus placebo, Outcome 1 Total sleep time, next day.
One trial (Jockovich 2000) compared the effect of melatonin on sleep length the next day after the night shift using actigraphy. The results of this trial showed that there is low quality evidence that sleep length is longer after melatonin administration (MD 37.00 minutes, 95% CI ‐20.87 to 94.87, one trial, 38 participants, Analysis 1.1) but the result was not statistically significant.
Overall, combining all seven trials, melatonin may increase sleep length on average by 24.34 minutes compared to placebo (95% CI 9.82 to 38.86; seven trials, 263 participants, low quality evidence, Analysis 1.1).
1.1.2 Total sleep time, next night
Two trials (Sadeghniiat‐Haghighi 2008; Wright 1998) compared the effect of melatonin on sleep length the next night after the night shift using self report (diary). The results of these trials showed that self‐reported sleep length is longer after melatonin administration (MD 19.05, 95% CI 4.47 to 33.63, low quality evidenceAnalysis 1.2).
1.2. Analysis.
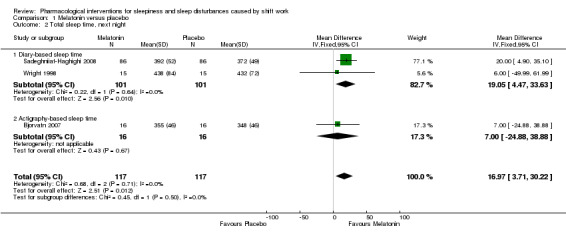
Comparison 1 Melatonin versus placebo, Outcome 2 Total sleep time, next night.
One trial (Bjorvatn 2007) compared the effect of melatonin on sleep length during the day after the night shift using actigraphy measurement. Sleep length may not be longer after melatonin administration (MD 7.00, 95% CI ‐24.88 to 38.88; one trial, 32 participants, low quality evidence, Analysis 1.2).
Combined in a meta‐analysis, the three trials showed that melatonin increased sleep length by 17 minutes compared to placebo (MD 95% CI 3.71 to 30.22; three trials, 234 participants, low quality evidence, Analysis 1.2).
1.1.3 Sleep onset latency, next day
Four trials (Folkard 1993; James 1998; Jockovich 2000; Yoon 2002) compared the effect of melatonin on next day sleep onset latency after the night shift using self‐reporting (diary). Self‐reported sleep onset latency may not differ after melatonin administration (MD 0.80 minutes, 95% CI ‐ 1.15 to 2.75; 5 trials, 59 participants, low quality evidence, Analysis 1.3).
1.3. Analysis.
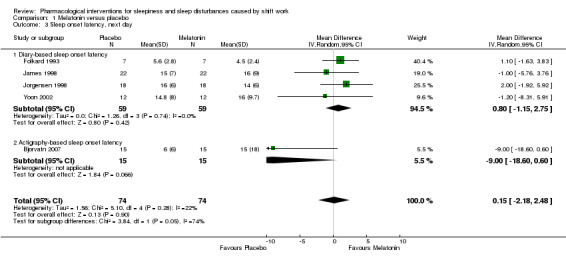
Comparison 1 Melatonin versus placebo, Outcome 3 Sleep onset latency, next day.
In one trial (Bjorvatn 2007) melatonin did not decrease the next day sleep onset latency after the night shift using actigraphy measurement (MD ‐9.0, 95% CI ‐18.60 to 0.60; one trial, 15 participants, Analysis 1.3)
Combined in a meta‐analysis, the five trials showed that melatonin may not reduce sleep onset latency compared to placebo (MD 0.15 minutes, 95% CI ‐2.18 to 2.48; five trials, 74 participants, low quality evidence, Analysis 1.3).
1.1.4 Sleep onset latency, next night
Two trials (Sadeghniiat‐Haghighi 2008; Wright 1998) compared the effect of melatonin on next night sleep onset latency after the night shift using self‐reporting (diary). These trial results were inconsistent with one trial showing a benefit of melatonin and one of placebo (Analysis 1.4).
1.4. Analysis.
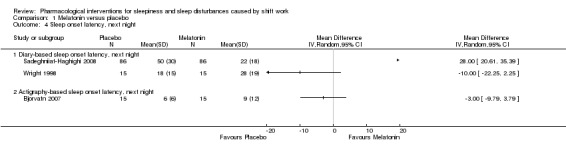
Comparison 1 Melatonin versus placebo, Outcome 4 Sleep onset latency, next night.
Bjorvatn 2007 showed that following melatonin administration sleep onset latency during the next night was not shorter compared to placebo when measured with actigraphy (MD ‐3.0, 95% CI ‐9.79 to 3.79; one trial, 15 participants, Analysis 1.4).
We could not combine the trials for meta‐analysis because the results were inconsistent.
1.1.5 Sleep quality, next day
Four trials measured subjective sleep quality with a visual analogue scale (Bjorvatn 2007; Cavallo 2005; Folkard 1993; Sadeghniiat‐Haghighi 2008). There was no significant difference between melatonin and placebo (SMD 0.08, 95% CI ‐0.15 to 0.31; four trials, 291 participants, low quality evidence, Analysis 1.5).
1.5. Analysis.
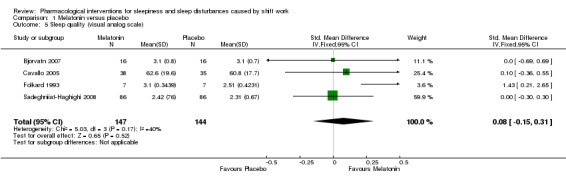
Comparison 1 Melatonin versus placebo, Outcome 5 Sleep quality (visual analog scale).
1.2 Sleepiness and alertness during the night shift
1.2.1 Alertness during night shift work after morning melatonin administration
One trial (Yoon 2002) compared the effect of melatonin on alertness during the night shift after morning melatonin administration using a visual analogue scale (scale ranged from 0 meaning most sleepy to 100 meaning fully alert). Alertness during the night shift was higher after melatonin administration compared to placebo (MD 8.70, 95% CI 1.49 to 15.91; one trial, 12 participants, Analysis 1.6).
1.6. Analysis.

Comparison 1 Melatonin versus placebo, Outcome 6 Alertness during the night shift work (VAS).
1.2.2 Sleepiness during the night shift after morning melatonin administration
Bjorvatn 2007 compared the effect of melatonin on sleepiness during the night shift after morning melatonin administration using the KSS (scale ranged from 1 meaning very alert to 9 meaning very sleepy). Sleepiness was similar after melatonin administration compared to placebo (MD ‐ 0.20, 95% CI ‐ 0.91 to 0.51; one trial, 17 participants, Analysis 1.7).
1.7. Analysis.

Comparison 1 Melatonin versus placebo, Outcome 7 Sleepiness during the night shift work (KSS).
1.2.3 Sleepiness during the day shift after evening melatonin administration
One included trial (Bjorvatn 2007) compared the effect of melatonin on sleepiness during the day shift after evening melatonin administration using the KSS. Sleepiness was similar after melatonin administration compared to placebo (MD ‐0.40, 95% CI ‐1.02 to 0.22; one trial, 17 participants, Analysis 1.8).
1.8. Analysis.

Comparison 1 Melatonin versus placebo, Outcome 8 Sleepiness during the day shift work (KSS).
1.3 Adverse effects
Six trials either passively reported (Bjorvatn 2007; James 1998; Jockovich 2000; Jorgensen 1998) or actively searched (Cavallo 2005; Wright 1998) for treatment side effects. Side effects did not differ between placebo and melatonin groups except for vivid dreams or nightmares during day time sleep (melatonin group, N = 3).
2. Armodafinil taken before the night shift versus placebo
2.1 Sleepiness and alertness during the night shift
Two included trials (Czeisler 2009; Erman 2011) compared the effect of armodafinil taken before the night shift on sleepiness during the night shift using five measurements with the KSS during the night shift. Based on these trials, armodafinil probably reduces night shift sleepiness (MD ‐0.89, 95% CI ‐1.37 to ‐0.40, two trials, 572 participants, moderate quality evidence, Analysis 2.1).
2.1. Analysis.
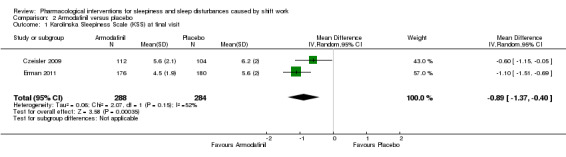
Comparison 2 Armodafinil versus placebo, Outcome 1 Karolinska Sleepiness Scale (KSS) at final visit.
One of these trials (Czeisler 2009) also compared the effect of armodafinil on how quickly participants fell asleep during the night shift in the laboratory using the MSLT. Armodafinil may increase sleep latency and thus reduce sleepiness (MD 2.50, 95% CI 1.36 to 3.64; one trial, 216 participants, low quality evidence, Analysis 2.2).
2.2. Analysis.

Comparison 2 Armodafinil versus placebo, Outcome 2 Multiple sleep latency test (MSLT).
The same trial (Czeisler 2009) also compared the effect of armodafinil on alertness during the night shift using simple reaction time as the outcome. Armodafinil administration reduces reaction time during the night shift and thus increases alertness (MD ‐50.00, 95% CI ‐84.40 ‐to ‐15.60, one trial, 112 participants, Analysis 2.3).
2.3. Analysis.

Comparison 2 Armodafinil versus placebo, Outcome 3 Mean simple reaction time.
2.2 Adverse effects
Adverse effects for armodafinil were few and mild. Headache was the most frequent adverse effect, reported by 12% in the armodafinil group and 10% in the placebo group. For nausea, these figures were 7% versus 3%, respectively. No single adverse event led to the withdrawal of more than one patient. However overall, severe adverse events were more frequent in the armodafinil group (Analysis 2.4). An open‐label extension of the Czeisler 2009 trial of armodafinil for shift work sleep disorder enrolled 113 of the patients of the trial Erman 2007. In this extended trial, 11% of participants withdrew because of adverse events. The rate of adverse events were similar to those reported in the original Czeisler 2009 trial. In addition, cardiovascular events and clinical relevant rises in blood pressure were reported in 6% and 18% of the participants.
2.4. Analysis.
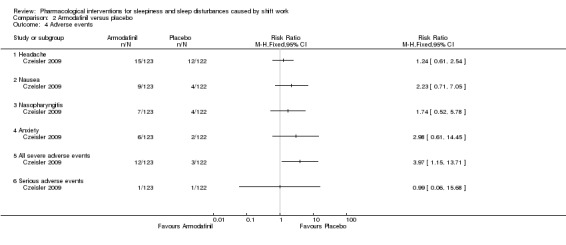
Comparison 2 Armodafinil versus placebo, Outcome 4 Adverse events.
3. Modafinil taken before the night shift versus placebo
3.1 Sleepiness and alertness during the night shift
One trial (Czeisler 2005) compared the effect of modafinil taken before the night shift on sleepiness during the night shift using the KSS during the night shift. Modafinil administration probably reduces night shift sleepiness (MD ‐0.90, 95% CI ‐1.45 to ‐0.35; one trial, 183 participants, moderate quality evidence, Analysis 3.1.
3.1. Analysis.

Comparison 3 Modafinil versus placebo, Outcome 1 Karolinska Sleepiness Scale (KSS).
Czeisler 2005 also compared the effect of modafinil on sleepiness during the night shift in the laboratory to placebo with the MSLT and found that modafinil may increase sleep latency (MD 1.40, 95% CI 1.27 to 1.53; one trial, 153 participants, low quality evidence, Analysis 3.2.
3.2. Analysis.

Comparison 3 Modafinil versus placebo, Outcome 2 Multiple sleep latency test (MSLT).
The same trial (Czeisler 2005) also compared the effect of modafinil on alertness during the experimental night shift after several night shifts using the psychomotor vigilance test (number of lapses during a 20‐minute test period) and found that modafinil administration increases night shift alertness and reduces sleepiness (MD ‐13.50, 95% CI ‐15.60 to ‐11.40, one trial, 69 participants, Analysis 3.3).
3.3. Analysis.

Comparison 3 Modafinil versus placebo, Outcome 3 Psychomotor Vigilance Test (number of lapses during 20 min test).
3.2 Adverse effects
The most commonly reported side effects from modafinil were headaches, nausea, and anxiety or nervousness, but they did not occur more frequently in the modafinil group except for insomnia (Analysis 3.4). Headache occurred in 26% of the modafinil group versus 19% in the placebo group (Erman 2007). For nausea it was 9% versus 3%, respectively. Day time sleep did not change as a result of modafinil treatment. Roth 2007, an open‐label trial extension trial, reported that the frequency of headache associated with modafinil was dose‐dependent. In post‐marketing surveillance, serious skin reactions were reported to the EMA and as a result the EMA decided to withdraw the licence for the indication shift work sleep disorder (EMA 2011). Despite a high suspicion that the drug could be abused as a mood‐enhancer or party drug, no such cases have been reported to our knowledge (Myrick 2004).
3.4. Analysis.
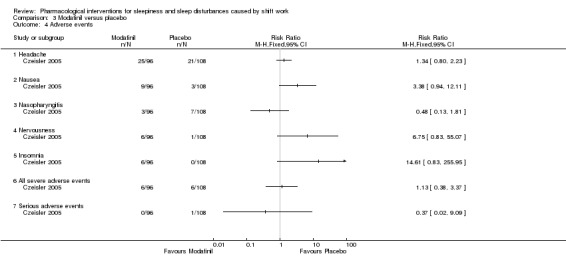
Comparison 3 Modafinil versus placebo, Outcome 4 Adverse events.
4. Caffeine plus naps versus placebo before the night shift
4.1 Sleepiness and alertness during the night shift
One trial (Schweitzer 2006) compared the effect of pre‐shift caffeine on sleepiness during several night shifts using the KSS in the laboratory and in a field trial. The field trial compared pre‐shift caffeine plus pre‐shift napping to placebo. There was low quality evidence that pre‐shift caffeine administration plus pre‐shift napping decreased sleepiness at the end of the night shift in a period of four night shifts compared to placebo (MD ‐0.63, 95% CI ‐1.09 to ‐0.17; one trial, 39 participants, Analysis 4.1).
4.1. Analysis.

Comparison 4 Caffeine plus naps versus placebo, Outcome 1 Karolinska sleepiness scale.
The trial authors also used the psychomotor vigilance test to compare pre‐shift caffeine plus pre‐shift napping to placebo in a field trial but there were insufficient data to analyse the results over the four nights.
4.2 Adverse effects
Adverse effects were not reported.
5. Hypnotics versus placebo
Zoplicone
One included trial (Quera‐Salva 2002) compared the effect of post‐shift zopiclone on sleep length using actigraphy measurement in shift workers with a sleeping problem. Zopiclone leads to a similar average total sleep length during the day after the night shift (after night shifts 1 and 2 during a night shift period) than placebo (MD 44.0, 95% CI ‐22.67 to 110.67; one trial, 28 participants, low quality evidence, Analysis 5.1).
5.1. Analysis.
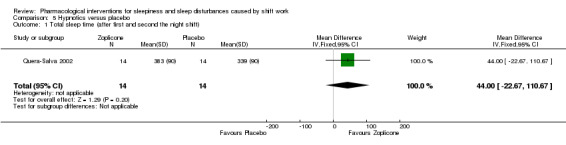
Comparison 5 Hypnotics versus placebo, Outcome 1 Total sleep time (after first and second the night shift).
Adverse effects of the medication were not reported.
Lormetazepam
Sastre‐y‐Hernandez 1982 did not report data that we could use in the meta‐analysis. The trial authors reported that in the lormetazepam group significantly more workers achieved a normal sleeping pattern (25/28 participants) than in the placebo group (18/28 participants). Both groups reported four adverse events and there was no difference in how well participants felt after waking up.
6. Sensitivity analysis
Melatonin versus placebo to improve day time sleep length and quality after the night shift
We judged seven melatonin trials to have a low risk of bias (Bjorvatn 2007; Cavallo 2005; James 1998; Jockovich 2000; Jorgensen 1998; Sadeghniiat‐Haghighi 2008; Wright 1998) and two to have a high risk of bias due to either a high discontinuation rate (Folkard 1993) or using an unblinded intervention (Yoon 2002). The Folkard 1993 trial also had very small standard deviations and therefore had a relatively large weight in the meta‐analysis.
When we excluded the two trials that had a high risk of bias from the primary outcome analysis, the effect of melatonin on sleep length the next day after the night shift became non‐significant. However, the combined effect with the one actigraphy trial (Jockovich 2000) in the meta‐analysis on the effect of melatonin on the same outcome was still significant.
Armodafinil versus placebo to reduce night shift sleepiness or alertness
We judged both trials to have a low risk of bias (Czeisler 2009; Erman 2011).
7. Subgroup analysis
We examined the effect of melatonin dose on next day sleep length using seven included trials. In four trials the melatonin dose was 5 to 10 mg (Folkard 1993; James 1998; Jorgensen 1998; Yoon 2002). In three trials (Bjorvatn 2007; Cavallo 2005; Jockovich 2000) the melatonin dose was 1 to 3 mg. There was no significant dose‐response effect of melatonin on next day sleep length (seven trials, 263 participants, Analysis 6.1).
6.1. Analysis.
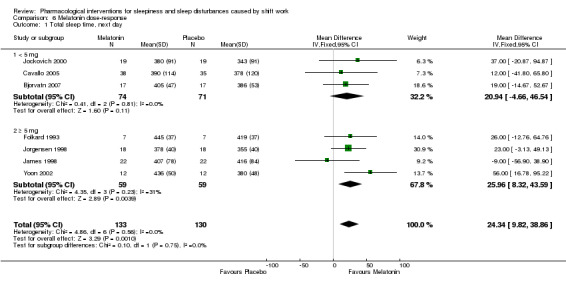
Comparison 6 Melatonin dose‐response, Outcome 1 Total sleep time, next day.
Seven trials included hospital employees (Cavallo 2005; James 1998; Jockovich 2000; Jorgensen 1998; Sadeghniiat‐Haghighi 2008; Wright 1998; Yoon 2002). The results did not differ when we excluded trials that included other participant populations.
8. Publication bias and quality of the evidence
Melatonin versus placebo was the only comparison where there was a sufficient number of trials to assess publication bias. The funnel plots did not indicate a considerable influence of publication bias (Figure 4). Therefore we did not downgrade the evidence provided in any of the comparisons for publication bias. Only two trials had a low risk of bias in the most important domains: randomisation, concealment and blinded outcome assessment. For the melatonin‐placebo comparison this meant that most trials had a high risk of bias and therefore we downgraded the evidence by two levels to low quality. For armodafinil and modafinil, we downgraded the quality of evidence by one level to moderate because of high risk of bias. For caffeine plus naps, we downgraded by two levels because of high risk of bias (one level) and small trial size (one level). For trials using hypnotics, we downgraded the evidence by two levels because of high risk of bias and small trial size. We did not downgrade the evidence because of indirectness or inconsistency of the results.
4.
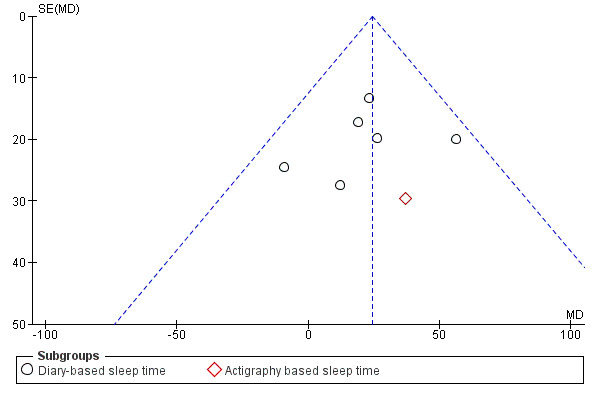
Funnel plot of comparison: 1 Melatonin versus placebo, outcome: 1.1 Total sleep time, next day.
9. Cost‐effectiveness and secondary outcomes
We did not find evidence on the cost‐effectiveness of the drugs in the included trials. No trials reported on the secondary outcomes of injuries or accidents.
Discussion
Summary of main results
Melatonin
The included trials provide low quality evidence that melatonin (from 1 mg to 10 mg) may increase sleep length compared to placebo after the night shift both during the next day sleep and during the next night sleep (low quality evidence,Table 1). The gain of extra sleep is around 25 minutes in day time sleep and around 15 minutes in night sleep after the night shift. Other sleep parameters did not change significantly. This result was based on the inclusion of two trials that we judged to have a high risk of bias. When we excluded these two trials from our analysis, the effect of melatonin on sleep length after the night shift was no longer significant. In subgroup analyses (examining the effect of a melatonin dose of 5 mg or more), higher dose did not increase the effect. The side effects of melatonin did not differ from placebo.
Armodafinil
Armodafinil (150 mg) is probably more effective than placebo in reducing sleepiness during the night shift in shift workers with a diagnosis of shift work sleep disorder based on two trials (moderate quality evidence, Table 2). Sleepiness on the KSS reduced from 6 (meaning some signs of sleepiness) to 5 (meaning neither alert nor sleepy), which is a 15% decrease compared to the control group score. Armodafinil was administered with gradually increasing dose (50 mg, 100mg, 150 mg) during the night shift period. Of the adverse effects, headache and nausea occurred most frequently in both groups and only slightly more often in the armodafinil‐treated group. Post‐marketing surveillance indicates that severe skin reactions are possible (EMA 2011).
Modafinil
Modafinil (200 mg) is probably more effective than placebo in reducing sleepiness during the night shift in shift workers with a shift work sleep disorder diagnosis, based on one trial (moderate quality evidence, Table 3). The decrease in sleepiness was similar to armodafinil, but the effect on sleepiness and alertness was most pronounced during the first hours of night shift and partly diminished towards the shift's end. Modafinil did not disturb sleep after or between night shifts. Of the adverse effects, headache and nausea occurred most frequently and were dose‐dependent. Other dose‐dependent side effects of modafinil were tachycardia and palpitation that probably resulted from adrenergic stimulation. Post‐marketing surveillance indicates that severe skin reactions are possible (EMA 2011).
Caffeine plus naps
Based on one trial, caffeine (300 mg, or 4 mg per kg) before the night shift combined with pre‐shift naps before the night shift may increase vigilance during the night shift (low quality evidence). In the trial, caffeine was administered in one dose before the night shift.
Hypnotics
Based on the currently available evidence, it is unclear whether or not the hypnotic drug zopiclone (7.5 mg) results in similar sleep length after the night shift during the two first post‐night shift day sleeps as placebo (very low quality evidence). The trial authors administered the drug before the planned day time sleep after the night shift. Adverse effects were not reported. For lormetazepam, we had insufficient information to draw conclusions.
Overall completeness and applicability of evidence
We searched for RCTs of pharmacological interventions conducted with shift workers in multiple databases and included trials regardless of language. We believe that we have located all available trials on shift workers.
Treatment side effects are a serious issue in interventions for sleepiness and sleep disturbances in shift workers. We only searched for RCTs and therefore we are unsure if we have captured all relevant studies on adverse effects. We discussed also those trials that reported on side effects, even if they did not meet our inclusion criteria for the primary outcome or trial design (such as Erman 2007; Roth 2007 and EU post marketing surveillance EMA 2011). Given the serious side effects of modafinil, a systematic review of the side effects is needed that also includes long‐term observational studies.
Given the extent of the sleep related problems, with about 25% of all workers engaged in shift work, the number of included trials is very small. There was only one trial on caffeine and three trials on other stimulants. In addition, caffeine was only studied in combination with naps and it is unclear if the sleepiness reducing effect is due to caffeine. We expected to find and include more trials on hypnotics as we imagined that this would be a frequently used treatment and because the addictive effects might be expected to be harmful (Keuroghlian 2012). Shy 2011 reports information on the prevalence of medication used to counterbalance the effects of shift work.
Also studies on recently introduced stimulating soft drinks, which can be considered psychostimulants for general use, were not identified in the search strategy.
The evidence presented in the included trials might not apply to all types of shift workers because trials used specific groups of shift workers. Participants in most melatonin trials were health care volunteers without severe shift work disorder symptoms. In the most recent trial (Bjorvatn 2007) the participants were employees at oil rigs who experienced sleeping problems. This is a very specific setting and the results might not apply to other occupational groups.
In the modafinil and armodafinil trials participants were shift workers in sleep research clinics in the USA and Canada who participated voluntarily and were screened for shift work sleep disorder i.e. sleeping problems and sleepiness in relation to shift work. The results of these trials apply most closely to those shift work employees who experience moderate or severe sleep and alertness problems in their work.
Participants in the caffeine trial were volunteer shift workers and thus may not be representative of the general shift work workforce. The same holds for the trial on hypnotics, in which participants were shift workers with sleeping problems.
We included only 'field studies' i.e. studies in the real work environments with participation of real shift work employees. Even though this led us to exclude some available studies, we believe that the results of laboratory studies are not easily transferable to the working situation and that we have included trials undertaken in the most relevant settings.
The included outcomes covered the three aspects of sleep that are disturbed most by shift work: sleep length and sleep quality when off work and alertness and sleepiness or fatigue while at work. Not all included trials reported on all specified outcomes. The majority of the included studies investigated melatonin. We did not include all assessments of sleep quality measured in individual trials because specific measures could not be combined.
Quality of the evidence
We judged most of the included trials to have a low risk of bias even though trials often did not describe the randomisation method and allocation concealment. Incomplete outcome data due to loss to follow‐up of participants presents a serious risk of bias. The common occurrence of this issue might be due to the difficulty of conducting trials in the field where shift workers have to take medication and fill in questionnaires in real life conditions. The more complicated the trial protocol (with many measurements e.g. during the night shift) and the longer the trial span, the more participants withdrew from the trials. To study the effect of an intervention across several night shifts in a row is conceptually difficult. The sleepiness problem is most prominent during the first two night shifts and starts to improve slightly during the consecutive nights due to a partial adjustment in day‐night rhythm in the case of slowly rotating shifts.
The central outcome in day time sleep analysis was total sleep time measured by sleep diary. Only two included trials used a more objective method, actigraphy. However, the diary and actigraphy measurement results were similar.
Trials measured night time sleepiness or alertness using many different methods. Outcome assessment has to consider natural fluctuation of alertness throughout the night shift by several measurement time‐points for both 'interventions' (active versus placebo). We combined the results of several nights to express the effect.
Most trials blinded participants and care providers but in many trials we judged the blinding of outcome assessment to introduce an unclear risk of bias. Two trials were unable to perform blinding because the protocol included co‐interventions (naps and sunglasses) that trial authors could not blindly introduce.
Potential biases in the review process
In this review, we did not restrict the inclusion of trials on the basis of the language(s) in which trials were published. Two trials included in the qualitative analysis were published in languages other than English (German and French).
We did not find or use overlapping trial results. So, the use of multiple publications from the same trial should not have been a problem.
Most melatonin trials used a cross‐over design. For testing the difference between intervention and control group, we used the unpaired t‐test as implemented in RevMan 2011 where actually a paired t‐test would be the appropriate way of testing the differences. This leads to CIs wider than they should really be and thus an underestimation of the intervention's effectiveness. We tried to use the paired t‐test to calculate the CIs but could only find enough data in one trial to do this. We therefore refrained from adjusting the results reported using RevMan 2011. In the same trials, there was another unit of analysis issue that may have caused bias in the opposite direction. The number of night shifts during which the outcome was measured in these trials varied from one to five. It was unclear from the trial reports whether trial authors reported these outcome measurements as the mean values of these measurements per individual participant or as the mean over all measurements, and we were unable to further clarify this with the trial authors. Analysis as the means over all outcome measurements, without taking the clustering per individual into account, would have artificially decreased the CIs. As these issues would be expected to bias the outcome measures in opposite directions, to some extent, they should cancel each other out. Therefore, we believe that the results remain reasonably valid.
We simplified outcome measures to the most common measurements. This disregarded many 'global assessment of effectiveness' type of outcomes. These were used only once in review articles. For example, we omitted sleep quality measurements from the analysis, even though we included it in our protocol, because the heterogeneity of the methods used in the potentially relevant trials was so extensive.
The timing of the intervention and outcome measurements varied. In melatonin trials next day sleep was measured most often, but three trials measured next night sleep because the night shift was for one night only. It is not clear what, if any, effect this may have had on the measured outcome.
The duration of treatment was from one to four nights, such as a series of consecutive night shifts. We did not assess the effects of the long‐time use of pharmacological aids in this review.
Agreements and disagreements with other studies or reviews
Ker 2010 concluded that caffeine may be an effective intervention for improving performance in shift workers but that there were no studies on the effect of caffeine on injuries. Morgenthaler 2007 reviewed countermeasures to shift work sleep disorder and recommended melatonin administration prior to day time sleep. Morgenthaler 2007 suggests using hypnotics to improve day‐time sleep but warns about the carryover of the sedative effect to the night‐time shift with potential adverse consequences for night‐time performance and safety. Morgenthaler 2007 recommended modafinil and caffeine as well‐tolerated stimulants that increase alertness.
Buscemi 2006 conducted a a review of melatonin use in sleep restriction caused by sleep disorders such as shift work and found that melatonin was not effective in reducing sleep onset latency. The authors observed no dose response. This is in accordance with the results of our meta‐analysis in this review where melatonin did not reduce sleep onset latency while total sleep time was somewhat increased (mean 24 minutes). The sleep time increase after night shift with melatonin represents an improvement of sleep length but we did not observe any effect on sleep quality. Neither was there any effect on sleepiness during the night shift. Sanchez‐Barcelo 2010 presented a non‐systematic overview of all potential uses of melatonin and concluded that for shift work related sleep problems it was difficult to draw conclusions due to the variability of the results.
Ballon 2006 and Kumar 2008 reported that modafinil reduces excessive sleepiness and illness severity in all three disorders for which it has been approved by the US Food and Drug Administration, i.e. narcolepsy, shift‐work sleep disorder and obstructive sleep apnoea with residual excessive sleepiness despite optimal use of continuous positive airway pressure (CPAP). However, it may interact with several pharmacological agents and its long term safety needs more investigation. However, the EMA approves the use of modafinil only in narcolepsy but not in obstructive sleep apnoea nor in shift‐work sleep disorder due to a suspected risk of cardiovascular problems, hypersensitivity and neuropsychiatric reactions (EMA 2011). The EMA argued that there were very limited benefits compared to potential serious harms. Basner 2005, in an editorial, argued along similar lines that the clinical relevance of the benefits are unclear.
In a review of the appropriate therapeutic selection of patients with shift work sleep disorder, Roth 2012 lists as 'guideline' (level 2 evidence) interventions timed melatonin administration and hypnotics for day‐time sleep, and alerting agents (modafinil, armodafinil and caffeine) for night‐time alertness for shift work employees with shift work disorder. The recommendations are partly supported by our findings. There are, however, few trials on hypnotics and caffeine.
There are two Cochrane protocols in process that concentrate on non‐pharmacological interventions (e.g. naps and bright light) (Herbst 2013) and shift schedule interventions (e.g. six or twelve hours, rotation models) (Erren 2013) to reduce sleepiness or improve alertness at work and reduce sleep disturbances whilst off work in shift workers.
Authors' conclusions
Implications for practice.
Melatonin may improve sleep length after a night shift but may not improve other sleep parameters (low quality evidence). Modafinil and armodafinil increase alertness and reduce sleepiness to some extent during the night shift in employees who suffer from shift work sleep disorder but both drugs have been associated with adverse events (moderate quality evidence) . Pre‐shift caffeine plus pre‐shift naps increases alertness during the night shift, but this is based on one small trial only. Based on the available evidence, it is unclear whether or not hypnotics improve sleep length and quality after a night shift (low quality evidence).
Implications for research.
Given the low quality evidence, additional placebo‐controlled trials of melatonin are needed. Trials should use objective electrophysiological monitoring of sleep length and quality.
More RCTs are needed of caffeine for shift workers under field conditions and they should also measure adverse effects. Long term adverse effects of modafinil and armodafinil should be studied in cohort and case‐control studies. These studies should also be summarised in a systematic review.
What's new
| Date | Event | Description |
|---|---|---|
| 20 February 2012 | Amended | A previous version of this protocol, now superseded by this one, was titled 'Interventions for sleepiness and sleep disturbances caused by shift work' (Liira 2010) and published by the Cochrane Collaboration Depression Anxiety and Neuroses Group. The systematic search strategy for the previous title yielded such a large amount of records that the authors decided to split the review into three. The new titles in addition to this one are "Person‐directed non‐pharmacological interventions for preventing and treating sleepiness and sleep disturbances caused by shift work" and "Adaptation of shift work schedules for preventing and treating sleepiness and sleep disturbances caused by shift work". All three will be hosted by the Cochrane Occupational Safety and Health Review Group as the titles fit better in their scope. |
Notes
This protocol is one of three which together supersede the protocol previously published by the Cochrane Collaboration Depression, Anxiety and Neurosis Group titled: "Interventions for sleepiness and sleep disturbances caused by shift work" (Liira 2010). The other two protocols are titled: "Person‐directed non‐pharmacological interventions for preventing and treating sleepiness and sleep disturbances caused by shift work" (Herbst 2013) and "Adaptation of shift work schedules for preventing and treating sleepiness and sleep disturbances caused by shift work" (Erren 2013).
Acknowledgements
We thank Mrs Merja Jauhiainen and Mrs Leena Isotalo for developing the search strategy, and Jane Dennis and the Cochrane Collaboration Depression, Anxiety and Neurosis Group for their support and advice on the previous version of the protocol entitled "Interventions for sleepiness and sleep disturbances caused by shift work" (Liira 2010). We also acknowledge Wim van Veelen, Ira Madan, Leena Isotalo, Kaisa Neuvonen, Sampsa Puttonen and Anneli Ojajärvi for their comments and Jani Ruotsalainen (JR) and Deirdre Walshe for copyediting the text.
Appendices
Appendix 1. MEDLINE search strategy
#1 "Sleep Disorders"[Mesh] OR "Chronobiology Disorders"[Mesh:NoExp] OR Sleep [Mesh] OR "Circadian Rhythm"[Mesh] OR "Psychomotor Performance"[Mesh] OR sleep*[tw] OR "circadian disruption"[tw] OR vigilance[tw] OR alertness[tw] OR wakefulness[tw] OR drowsiness[tw] OR fatigue[tw] OR insomnia[tw] OR hypersomnolence*[tw] OR dyssomnia*[tw] OR eveningness[tw] OR morningness[tw] OR "neurocognitive performance"[tw] OR "concentration difficulties"[tw] OR arousal[tw]
#2 "phase shift"[tw] OR "phase shifting"[tw] OR stimulant[tw] OR caffeine[tw] OR melatonin[tw] OR modafinil[tw] OR armodafinil[tw]
#3 #1 OR #2
#4 "night shift"[tw] OR "night shifts"[tw] OR "morning shift"[tw] OR "morning shifts"[tw] OR "evening shift"[tw] OR "evening shifts"[tw] OR "afternoon shift"[tw] OR "afternoon shifts"[tw] OR "shift work"[tw] OR shiftwork*[tw] OR "shift worker"[tw] OR "shift workers"[tw] OR "shift working"[tw] OR "work shift"[tw] OR "work shifts"[tw] OR "rotating shift"[tw] OR "shift combination"[tw] OR "shift combinations"[tw] OR "shift duration"[tw] OR "shift length"[tw] OR "shift system"[tw] OR "shift systems"[tw] OR "shift rotation"[tw] OR "clockwise rotation"[tw] OR "counter‐clockwise rotation"[tw] OR "shift roster"[tw] OR "shift rosters"[tw] OR "extended shifts"[tw] OR "extended work shifts"[tw] OR "night work"[tw] OR "evening work"[tw] OR "work schedule"[tw] OR "work schedules"[tw] OR "work hours"[tw] OR (("starting time"[tw] OR "early start"[tw]) AND (shift*[tw] OR Work[Mesh])) OR "irregular working hours"[tw] OR ("direction of rotation" [tw] AND shift*[tw])
#5 #3 AND #4
#6 ("randomised controlled trial" [pt] OR "controlled clinical trial" [pt] OR randomised [tiab] OR placebo [tiab] OR "clinical trials as topic" [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT (humans [mh] AND animals [mh]))
#7 (effect* [tw] OR controll*[tw] OR control[tw] OR controls*[tw] OR controli*[tw] OR controle*[tw] OR controla*[tw] OR evaluation* [tw] OR program* [tw]) AND (work[tw] OR works*[tw] OR work'*[tw] OR worka*[tw] OR worke*[tw] OR workg*[tw] OR worki*[tw] OR workl*[tw] OR workp*[tw] OR occupation* [tw] OR prevention* [tw] OR protect* [tw])
#8 "Costs and Cost Analysis"[Mesh] OR "economics"[MeSH Subheading] OR econom*[tiab] OR cost[tw] OR costs[tw]
#9 #5 AND (#6 OR #7 OR #8)
Appendix 2. PubMed search strategy and results
Updated strategy limited to RCTs, 9.9.2013/LI
| #7 | Search #5 AND #6 | 764 |
| #6 | Search (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab] NOT (animals [mh] NOT humans [mh])) | 2763494 |
| #5 | Search #3 AND #4 | 3995 |
| #4 | Search "night shift"[tw] OR "night shifts"[tw] OR "morning shift"[tw] OR "morning shifts"[tw] OR "evening shift"[tw] OR "evening shifts"[tw] OR "afternoon shift"[tw] OR "afternoon shifts"[tw] OR "shift work"[tw] OR shiftwork*[tw] OR "shift worker"[tw] OR "shift workers"[tw] OR "shift working"[tw] OR "work shift"[tw] OR "work shifts"[tw] OR "rotating shift"[tw] OR "shift combination"[tw] OR "shift combinations"[tw] OR "shift duration"[tw] OR "shift length"[tw] OR "shift system"[tw] OR "shift systems"[tw] OR "shift rotation"[tw] OR "clockwise rotation"[tw] OR "counter‐clockwise rotation"[tw] OR "shift roster"[tw] OR "shift rosters"[tw] OR "extended shifts"[tw] OR "extended work shifts"[tw] OR "night work"[tw] OR "evening work"[tw] OR "work schedule"[tw] OR "work schedules"[tw] OR "work hours"[tw] OR (("starting time"[tw] OR "early start"[tw]) AND (shift*[tw] OR Work[Mesh])) OR "irregular working hours"[tw] OR ("direction of rotation" [tw] AND shift*[tw]) | 10693 |
| #3 | Search #1 OR #2 | 491799 |
| #2 | Search stimulant*[tw] OR "Central Nervous System Stimulants"[Pharmacological Action] OR "Central Nervous System Stimulants"[Mesh:NoExp] OR "Hypnotics and Sedatives"[Mesh Terms] OR "modafinil"[Supplementary Concept] OR "modafinil"[All Fields] OR "armodafinil"[All Fields] OR "melatonin"[MeSH Terms] OR "melatonin"[All Fields] OR "caffeine"[MeSH Terms] OR "caffeine"[All Fields] | 142290 |
| #1 | Search "Sleep Disorders"[Mesh] OR "Chronobiology Disorders"[Mesh:NoExp] OR Sleep [Mesh] OR "Circadian Rhythm"[Mesh] OR "Psychomotor Performance"[Mesh] OR sleep*[tw] OR "circadian disruption"[tw] OR vigilance[tw] OR alertness[tw] OR wakefulness[tw] OR drowsiness[tw] OR fatigue[tw] OR insomnia[tw] OR hypersomnolence*[tw] OR dyssomnia*[tw] OR eveningness[tw] OR morningness[tw] OR "neurocognitive performance"[tw] OR "concentration difficulties"[tw] OR arousal[tw] | 368077 |
Appendix 3. EMBASE search strategy
#1 ('sleep disorder'/exp OR 'sleep'/exp)
#2 (sleep* OR 'circadian disruption' OR vigilance OR alertness/de OR alertness OR wakefulness/de OR wakefulness OR drowsiness/de OR drowsiness OR fatigue/de OR fatigue OR insomnia OR hypersomnolence* OR dyssomnia* OR eveningness OR morningness OR 'neurocognitive performance' OR 'concentration difficulties' OR arousal/de OR arousal)
#3 'phase shift' OR 'phase shifting' OR stimulant* OR 'caffeine'/de OR caffeine OR 'melatonin'/de OR melatonin OR 'modafinil'/de OR modafinil OR 'armodafinil'/de OR armodafinil
#4 'night shift' OR 'night shifts' OR 'morning shift' OR 'morning shifts' OR 'evening shift' OR 'evening shifts' OR 'afternoon shift' OR 'afternoon shifts' OR 'shift work' OR shiftwork* OR 'shift worker'/de OR 'shift worker' OR 'shift workers' OR 'shift working' OR 'work shift' OR 'work shifts' OR 'rotating shift' OR 'shift combination' OR 'shift combinations' OR 'shift duration' OR 'shift length' OR 'shift system' OR 'shift systems' OR 'shift rotation' OR 'clockwise rotation' OR 'counter‐clockwise rotation' OR 'shift roster' OR 'shift rosters' OR 'extended shifts' OR 'extended work shifts' OR 'night work'/de OR 'night work' OR 'evening work' OR 'work schedule'/de OR 'work
schedule' OR 'work schedules' OR 'work hours' OR ('starting time' OR 'early start' AND (shift* OR 'work'/de OR work)) OR 'irregular working hours' OR ('direction of rotation' AND shift*)
#5 #4 AND (#1 OR #2 OR #3)
#6 'randomised controlled trial'/exp OR 'randomised controlled trial' OR 'controlled clinical trial'/exp OR 'controlled clinical trial' OR randomised:ab,ti OR placebo:ab,ti OR 'clinical trials'/exp OR 'clinical trials' OR randomly:ab,ti OR trial:ti NOT ('animals'/exp OR 'animals' NOT ('humans'/exp OR 'humans')
#7 effect* OR control* OR evaluation* OR program* AND (work* OR occupation* OR prevention* OR protect*)
#8 econom* OR 'cost'/exp OR 'cost' OR costs
#9 #5 AND (#6 OR #7 OR #8) AND [embase]/lim
Appendix 4. EMBASE (embase.com) search strategy and results
Updated strategy limited to RCTs 10.9.2013/LI
#1 'sleep disorder'/exp OR 'sleep'/exp (213,714)
#2 sleep* OR 'circadian disruption' OR vigilance OR 'alertness'/de OR alertness OR 'wakefulness'/de OR wakefulness OR 'drowsiness'/de OR drowsiness OR 'fatigue'/de OR fatigue OR insomnia OR hypersomnolence* OR dyssomnia* OR eveningness OR morningness OR 'neurocognitive performance' OR 'concentration difficulties' OR 'arousal'/de OR arousal (424,774)
#3 stimulant* OR 'caffeine'/de OR caffeine OR 'melatonin'/de OR melatonin OR 'modafinil'/de OR modafinil OR 'armodafinil'/de OR armodafinil OR 'hypnotic sedative agent'/exp OR 'central stimulant agent'/exp (546,888)
#4 'night shift' OR 'night shifts' OR 'morning shift' OR 'morning shifts' OR 'evening shift' OR 'evening shifts' OR 'afternoon shift' OR 'afternoon shifts' OR 'shift work' OR shiftwork* OR 'shift worker'/de OR 'shift worker' OR 'shift workers' OR 'shift working' OR 'work shift' OR 'work shifts' OR 'rotating shift' OR 'shift combination' OR 'shift combinations' OR 'shift duration' OR 'shift length' OR 'shift system' OR 'shift systems' OR 'shift rotation' OR 'clockwise rotation' OR 'counter‐clockwise rotation' OR 'shift roster' OR 'shift rosters' OR 'extended shifts' OR 'extended work shifts' OR 'night work'/de OR 'night work' OR 'evening work' OR 'work schedule'/de OR 'work schedule' OR 'work schedules' OR 'work hours' OR ('starting time' OR 'early start' AND (shift* OR 'work'/de OR work)) OR 'irregular working hours' OR ('direction of rotation' AND shift*) (15,493)
#5 #4 AND (#1 OR #2 OR #3) (4,803)
#6 'randomized controlled trial(topic)'/exp OR 'randomized controlled trial' OR 'controlled clinical trial'/exp OR 'controlled clinical trial' OR 'clinical trials'/exp OR 'clinical trials' OR trial:ti OR random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR doubl* NEXT/1 blind* OR singl* NEXT/1 blind* OR assign* OR allocat* OR volunteer* (1,800,904)
#7 #5 AND #6 (673)
Search string #7
(('night shift' OR 'night shifts' OR 'morning shift' OR 'morning shifts' OR 'evening shift' OR 'evening shifts' OR 'afternoon shift' OR 'afternoon shifts' OR 'shift work' OR shiftwork* OR 'shift worker'/de OR 'shift worker' OR 'shift workers' OR 'shift working' OR 'work shift' OR 'work shifts' OR 'rotating shift' OR 'shift combination' OR 'shift combinations' OR 'shift duration' OR 'shift length' OR 'shift system' OR 'shift systems' OR 'shift rotation' OR 'clockwise rotation' OR 'counter‐clockwise rotation' OR 'shift roster' OR 'shift rosters' OR 'extended shifts' OR 'extended work shifts' OR 'night work'/de OR 'night work' OR 'evening work' OR 'work schedule'/de OR 'work schedule' OR 'work schedules' OR 'work hours' OR ('starting time' OR 'early start' AND (shift* OR 'work'/de OR work)) OR 'irregular working hours' OR ('direction of rotation' AND shift*)) AND (('sleep disorder'/exp OR 'sleep'/exp) OR (sleep* OR 'circadian disruption' OR vigilance OR 'alertness'/de OR alertness OR 'wakefulness'/de OR wakefulness OR 'drowsiness'/de OR drowsiness OR 'fatigue'/de OR fatigue OR insomnia OR hypersomnolence* OR dyssomnia* OR eveningness OR morningness OR 'neurocognitive performance' OR 'concentration difficulties' OR 'arousal'/de OR arousal) OR (stimulant* OR 'caffeine'/de OR caffeine OR 'melatonin'/de OR melatonin OR 'modafinil'/de OR modafinil OR 'armodafinil'/de OR armodafinil OR 'hypnotic sedative agent'/exp OR 'central stimulant agent'/exp))) AND ('randomized controlled trial(topic)'/exp OR 'randomized controlled trial' OR 'controlled clinical trial'/exp OR 'controlled clinical trial' OR 'clinical trials'/exp OR 'clinical trials' OR trial:ti OR random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR doubl* NEXT/1 blind* OR singl* NEXT/1 blind* OR assign* OR allocat* OR volunteer*)
Appendix 5. PsycINFO (Ovid) search strategy
#1 exp Sleep Disorders/ OR exp Sleep/ OR exp human biological rhythms/
#2 (sleep* or circadian disruption or vigilance or alertness or wakefulness or drowsiness or fatigue or insomnia or hypersomnolence* or dyssomnia* or eveningness or morningness or neurocognitive performance or concentration difficultie or arousal).mp. [mp=title, abstract, heading word, table of contents, key concepts]
#3 (phase shift or phase shifting or stimulant* or caffeine or melatonin or modafinil or armodafinil).mp. [mp=title, abstract, heading word, table of contents, key concepts]
#4 ('night shift' OR 'night shifts' OR 'morning shift' OR 'morning shifts' OR 'evening shift' OR 'evening shifts' OR 'afternoon shift' OR 'afternoon shifts' OR 'shift work' OR shiftwork* OR 'shift worker' OR 'shift workers' OR 'shift working' OR 'work shift' OR 'work shifts' OR 'rotating shift' OR 'shift combination' OR 'shift combinations' OR 'shift duration' OR 'shift length' OR 'shift system' OR 'shift systems' OR 'shift rotation' OR 'clockwise rotation' OR 'counter‐clockwise rotation' OR 'shift roster' OR 'shift rosters' OR 'extended shifts' OR 'extended work shifts' OR 'night work' OR 'evening work' OR 'work schedule' OR 'work schedules' OR 'work hours' OR (('starting time' OR 'early start') AND (shift* OR work)) OR 'irregular working hours' OR ('direction of rotation' AND shift*)).mp. [mp=title, abstract, heading word, table of contents, key concepts] OR exp workday shifts/
#5 #4 AND (#1 OR #2 OR #3)
#6 (('randomised controlled trial' or 'controlled clinical trial').mp. or randomized.ab,ti. or placebo.ab,ti. or 'clinical trials'.mp. or randomly.ab,ti. or trial.ti.) not (animals not humans).mp. [mp=title, abstract, heading word, table of contents, key concepts]
#7 ((effect* or control* or evaluation* or program*) and (work* or occupation* or prevention* or protect*)).mp.
[mp=title, abstract, heading word, table of contents, key concepts]
#8 (econom* or cost or costs).mp. [mp=title, abstract, heading word, table of contents, key concepts]
#9 #5 AND (#6 OR #7 OR #8)
Appendix 6. PsychINFO (ProQuest) search strategy and results
Updated strategy limited to RCTs 19.9.2013/LI
#1 SU.EXACT.EXPLODE("Sleep Disorders") (10309)
#2 SU.EXACT.EXPLODE("Sleep") (17950)
#3 AB,TI,SU(sleep* or "circadian disruption" or vigilance or alertness or wakefulness or drowsiness or fatigue or insomnia or hypersomnolence* or dyssomnia* or eveningness or morningness or "neurocognitive performance" or "concentration difficult*" or arousal) (101622)
#4 stimulant* or caffeine or melatonin or modafinil or armodafinil or hypnotic* (25468)
#5 SU.EXACT.EXPLODE("Hypnotic Drugs") OR SU.EXACT.EXPLODE("CNS Stimulating Drugs") (21752)
#6 1 or 2 or 3 or 4 or 5 (136265)
#7 "night shift*" or "morning shift*" or "evening shift*" or "afternoon shift*" or "shift work*" or shiftwork* or "'work shift*" or "rotating shift" or "shift combination*" or "shift duration*" or "shift length" or "shift system" or "shift systems" or "shift rotation" or "clockwise rotation" or "counter‐clockwise rotation" or "shift roster" or "shift rosters" or "extended shifts" or "extended work shifts" or "extended hour*" or "night work*" or "evening work*" or "work schedule*" or "work* hours" (4573)
#8 SU.EXACT.EXPLODE("Workday Shifts") OR SU.EXACT.EXPLODE("Work Scheduling") (2367)
#9 7 or 8 (5336)
#10 6 and 9 (1537)
#11 SU.EXACT("Treatment Effectiveness Evaluation") OR SU.EXACT.EXPLODE("Treatment Outcomes") OR SU.EXACT("Placebo") OR SU.EXACT("Followup Studies") OR placebo* OR random* OR "comparative stud*" OR clinical NEAR/3 trial* OR research NEAR/3 design OR evaluat* NEAR/3 stud* OR prospectiv* NEAR/3 stud* OR (singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*) (323081)
#12 10 and 11 (196)
Search string #12
((SU.EXACT.EXPLODE("Sleep Disorders") OR SU.EXACT.EXPLODE("Sleep") OR AB,TI,SU(sleep* OR "circadian disruption" OR vigilance OR alertness OR wakefulness OR drowsiness OR fatigue OR insomnia OR hypersomnolence* OR dyssomnia* OR eveningness OR morningness OR "neurocognitive performance" OR "concentration difficult*" OR arousal) OR (stimulant* OR caffeine OR melatonin OR modafinil OR armodafinil OR hypnotic*) OR (SU.EXACT.EXPLODE("Hypnotic Drugs") OR SU.EXACT.EXPLODE("CNS Stimulating Drugs"))) AND (("night shift*" OR "morning shift*" OR "evening shift*" OR "afternoon shift*" OR "shift work*" OR shiftwork* OR "'work shift*" OR "rotating shift" OR "shift combination*" OR "shift duration*" OR "shift length" OR "shift system" OR "shift systems" OR "shift rotation" OR "clockwise rotation" OR "counter‐clockwise rotation" OR "shift roster" OR "shift rosters" OR "extended shifts" OR "extended work shifts" OR "extended hour*" OR "night work*" OR "evening work*" OR "work schedule*" OR "work* hours") OR (SU.EXACT.EXPLODE("Workday Shifts") OR SU.EXACT.EXPLODE("Work Scheduling")))) AND (SU.EXACT("Treatment Effectiveness Evaluation") OR SU.EXACT.EXPLODE("Treatment Outcomes") OR SU.EXACT("Placebo") OR SU.EXACT("Followup Studies") OR placebo* OR random* OR "comparative stud*" OR clinical NEAR/3 trial* OR research NEAR/3 design OR evaluat* NEAR/3 stud* OR prospectiv* NEAR/3 stud* OR (singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*))
Appendix 7. CENTRAL search strategy and results
Cochrane Central Register of Controlled Trials (CENTRAL) 2013 Issue 8 of 12, part of The Cochrane Library. www.the cochranelibrary.com (accessed 19 September 2013)/LI
#1 MeSH descriptor: [Sleep Disorders] explode all trees (3970)
#2 MeSH descriptor: [Sleep] explode all trees (3790)
#3 MeSH descriptor: [Chronobiology Disorders] this term only (16)
#4 #1 or #2 or #3 (6461)
#5 sleep* or "circadian disruption" or vigilance or alertness or wakefulness or drowsiness or fatigue or insomnia or hypersomnolence* or dyssomnia* or eveningness or morningness or "neurocognitive performance":ti,ab,kw (Word variations have been searched) (23017)
#6 stimulant* or caffeine or melatonin or modafinil or armodafinil or hypnotic*:ti,ab,kw (Word variations have been searched) (9312)
#7 MeSH descriptor: [Central Nervous System Stimulants] explode all trees (1561)
#8 MeSH descriptor: [Hypnotics and Sedatives] explode all trees (2575)
#9 #4 or #5 or #6 or #7 or #8 (29681)
#10 "night shift" or "night shifts" or "morning shift" or "morning shifts" or "evening shift" or "evening shifts" or "afternoon shift" or "afternoon shifts" or "shift work" or shiftwork* or "shift worker" or "shift workers" or "shift working" or "work shift" or "work shifts" or "rotating shift" or "shift combination" or "shift combinations" or "shift duration" or "shift length" or "shift system" or "shift systems" or "shift rotation" or "clockwise rotation" or "counter‐clockwise rotation" or "shift roster" or "shift rosters" or "extended shifts" or "extended work shifts" or "night work" or "evening work" or "work schedule" or "work schedules" or "work hours" or "working hours" (Word variations have been searched) (605)
#11 #9 and #10 (227 CLib records, 187 CENTRAL trials)
Appendix 8. OSH Update databases (including NIOSH, CISDOC) search strategies and results
Updated strategy limited to RCTs 20.9.2013/LI
#1 GW{shift work*} OR GW{shiftwork*} (3489)
#2 GW{'night shift' OR 'night shifts' OR 'morning shift' OR 'morning shifts' OR 'evening shift' OR 'evening shifts' OR 'afternoon shift' OR 'afternoon shifts'} (949)
#3 GW{'work shift' OR 'work shifts' OR 'rotating shift' OR 'shift combination' OR 'shift combinations' OR 'shift duration' OR 'shift length' OR 'shift system' OR 'shift systems' OR 'shift rotation' OR 'clockwise rotation' OR 'counter‐clockwise rotation'} (2694)
#4 GW{'shift roster' OR 'shift rosters' OR 'extended shifts' OR 'extended work shifts' OR 'night work' OR 'evening work' OR 'work schedule' OR 'work schedules' OR 'work hours' OR 'working hours'} (3614)
#5 #1 OR #2 OR #3 OR #4 (7812)
#6 GW{sleep* OR 'circadian disruption' OR vigilance OR alertness OR wakefulness OR drowsiness OR fatigue OR insomnia OR hypersomnolence* OR dyssomnia* OR eveningness OR morningness OR 'neurocognitive performance' OR 'concentration difficulties' OR arousal} (14996)
#7 GW{stimulant* OR caffeine OR melatonin OR modafinil OR armodafinil OR hypnotic* OR pharmacol*} (15194)
#8 #5 AND (#6 OR #7) (2261)
#9 GW{random* OR factorial* OR crossover* OR 'cross over*' OR placebo* OR 'double blind*' OR 'single blind*' OR assign* OR allocat* OR volunteer* } (19177)
#10 #8 AND #9 (220)
Data and analyses
Comparison 1. Melatonin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total sleep time, next day | 7 | 263 | Mean Difference (IV, Fixed, 95% CI) | 24.34 [9.82, 38.86] |
| 1.1 Diary‐based sleep time | 6 | 225 | Mean Difference (IV, Fixed, 95% CI) | 23.49 [8.49, 38.49] |
| 1.2 Actigraphy based sleep time | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 37.0 [‐20.87, 94.87] |
| 2 Total sleep time, next night | 3 | 234 | Mean Difference (IV, Fixed, 95% CI) | 16.97 [3.71, 30.22] |
| 2.1 Diary‐based sleep time | 2 | 202 | Mean Difference (IV, Fixed, 95% CI) | 19.05 [4.47, 33.63] |
| 2.2 Actigraphy‐based sleep time | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [‐24.88, 38.88] |
| 3 Sleep onset latency, next day | 5 | 148 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐2.18, 2.48] |
| 3.1 Diary‐based sleep onset latency | 4 | 118 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐1.15, 2.75] |
| 3.2 Actigraphy‐based sleep onset latency | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐9.0 [‐18.60, 0.60] |
| 4 Sleep onset latency, next night | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Diary‐based sleep onset latency, next night | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Actigraphy‐based sleep onset latency, next night | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Sleep quality (visual analog scale) | 4 | 291 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.15, 0.31] |
| 6 Alertness during the night shift work (VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Sleepiness during the night shift work (KSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Sleepiness during the day shift work (KSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Armodafinil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Karolinska Sleepiness Scale (KSS) at final visit | 2 | 572 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.37, ‐0.40] |
| 2 Multiple sleep latency test (MSLT) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mean simple reaction time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Nasopharyngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Anxiety | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 All severe adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Modafinil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Karolinska Sleepiness Scale (KSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Multiple sleep latency test (MSLT) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Psychomotor Vigilance Test (number of lapses during 20 min test) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Nasopharyngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Nervousness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Insomnia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 All severe adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Caffeine plus naps versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Karolinska sleepiness scale | 1 | Mean Difference (Fixed, 95% CI) | ‐0.63 [‐1.09, ‐0.17] |
Comparison 5. Hypnotics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total sleep time (after first and second the night shift) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 44.0 [‐22.67, 110.67] |
Comparison 6. Melatonin dose‐response.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total sleep time, next day | 7 | 263 | Mean Difference (IV, Fixed, 95% CI) | 24.34 [9.82, 38.86] |
| 1.1 < 5 mg | 3 | 145 | Mean Difference (IV, Fixed, 95% CI) | 20.94 [‐4.66, 46.54] |
| 1.2 ≥ 5 mg | 4 | 118 | Mean Difference (IV, Fixed, 95% CI) | 25.96 [8.32, 43.59] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bjorvatn 2007.
| Methods | Trial design: Cross‐over RCT Country: Bergen, Norway Work settings: Oil rig on the North Sea Shift system: Two weeks on a 12‐hour shift, first week on night shift (18:30 to 06:30) and the second week on day shift (06:30 to 18:30). On the "swing" day workers ended night shift at 04:00 and started day work at 10:00. After three to four weeks off, the schedule was repeated. Randomisation procedure: One of the authors scheduled the exposure Recruitment: All people working night shift at oil rig (N = 109) Follow‐up: April 2002 to April 2003 Washout period: Three to four weeks off work |
|
| Participants | ||
| Interventions | Trial intervention: Melatonin 3 mg Comparison intervention: Placebo Treatment frequency and duration: Melatonin 3 mg for four days during night shifts and four days of the second week during the day period one hour before bedtime Control: Identical placebo Part of a three‐armed trial, other arm intervention bright light 10,000 lux for 30 minutes (Bjorvatn reported in another review) |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: Total sleep time (actigraphy, diary); sleep onset latency (actigraphy and diary); sleep quality (diary) Cognitive performance, sleepiness and fatigue at work: Sleepiness (KSS) |
|
| Notes | Ethics: The Regional National Committee for Research Ethics and the Norwegian Medicines Agency | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The workers were given melatonin and bright light in a randomised, double‐blind, placebo‐controlled crossover design", " the exposure was scheduled individually by one of the authors" (P. 205). |
| Allocation concealment (selection bias) | Low risk | "The code was broken after all of the data had been collected and entered into worksheets so that any bias would be reduced." (P. 205). |
| Blinding of participants All outcomes | Low risk | "The placebo and melatonin capsules were identical in size and colour, and the two treatments were administered blind, both for the participants and for everyone else involved in the study" (P. 205) (bright light arm of the trial was not blinded). |
| Blinding of care providers | Unclear risk | See above (exposure was scheduled by one of the authors). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised: Screened 109, included 38, followed‐up 17. Lost to follow‐up: Discontinued 22. |
| Outcome measured with validated instrument All outcomes | Low risk | Sleep length, sleep onset and quality while off work: Total sleep time (actigraphy, diary); sleep onset latency (actigraphy and diary); sleep quality (diary). Cognitive performance, sleepiness and fatigue at work: Sleepiness (KSS). |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting (discontinuation in the trial was a problem). |
| Other bias | Low risk | Balance in baseline characteristics: Cross‐over design, good balance in baseline characteristics. Balance in interventions: High proportion of discontinuation (> 50%) Funding public/private: No data. |
Cavallo 2005.
| Methods | Trial design: Double‐blind, randomised, placebo‐controlled cross‐over trial Country: Cincinnati, Ohio, USA Work settings: Pediatric residents in night work 11 to 12 nights over a two‐week period Randomisation procedure: Drug pharmacist used simple randomizations |
|
| Participants | Inclusion: Healthy second‐year paediatric residents working two night float rotations which is a total of 11 to 12 days when residents have the inverted activity‐sleep schedule distributed over a two‐week period. Number randomised: Screened, randomised, followed‐up 45 Lost to follow‐up: 28 completed two arms of the trial, 17 completed only one arm of the trial (7 melatonin, 10 placebo) (total of 73 trial periods, 35 melatonin, 38 placebo). Age mean: 28.6 ± 1.9 Sex: Intervention male 16/45 and control male 16/45 Exclusion: No alcohol or sedative during the trial period, infants or toddlers in household, chronic illness, depression. |
|
| Interventions | Trial intervention: Melatonin 3 mg in gelatin capsule Comparison intervention: Placebo Treatment providers: Hospital's investigational drug pharmacist Treatment frequency and duration: Participants took melatonin (3 mg) or a placebo before bedtime in the morning after night shift (not later than 13.00) |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: Sleep duration, sleep quality, number of awakenings (diary) Cognitive performance, sleepiness and fatigue at work: Conners Continuous performance test, Profile of Mood States (POMS). |
|
| Notes | Ethics: The Institutional Review Board approved the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The hospital’s investigational drug pharmacist used simple randomizations with no restriction and prepared gelatin capsules of melatonin and placebo that were identical in appearance. |
| Allocation concealment (selection bias) | Low risk | The pharmacist dispensed the capsules in a regular medication container for the first eight participants and in a container fitted with an automated medication monitoring event system (track cap) for all subsequent participants to monitor compliance with treatment. For a total of 676 treatment days with capsules dispensed in track caps, there were 661 track cap data points and 660 diary data points. |
| Blinding of participants All outcomes | Low risk | See above, estimated. |
| Blinding of care providers | Unclear risk | No data. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No data. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of these 45 residents, 28 participated in the trial during the two night float rotations, completing the two treatment arms of the cross‐over design. The other 17 residents completed only one treatment arm: seven were assigned to take melatonin and 10 were assigned to take placebo. |
| Outcome measured with validated instrument All outcomes | Low risk | Sleep length, sleep onset and quality while off work: Sleep duration, sleep quality, number of awakenings (diary). Cognitive performance, sleepiness and fatigue at work: Conners Continuous performance test, POMS. |
| Selective reporting (reporting bias) | Low risk | Not detected, (see attrition bias); individual observations summed. |
| Other bias | Low risk | Balance in baseline characteristics: Cross‐over design, good balance in baseline characteristics. Balance in interventions: Good balance in interventions, few withdrawals from the trial Funding public/private: No data. |
Czeisler 2005.
| Methods | Trial design: randomised, double‐blind, placebo controlled trial Country: USA, 28 centre trial Work settings: Shift workers with reported excessive sleepiness (shift work sleep disorder) symptoms Randomisation procedure: Random assignment (1:1), central randomisation process, stratified with the use of permuted blocks of two Recruitment: Advertising the trial, contact by phone Inclusion period: Follow‐up: December 2001 to September 2002; three months follow‐up for each participant |
|
| Participants | Inclusion: Adults between the ages of 18 and 60 years were eligible if they worked each month at least five night shifts for 12 hours or less, with six hours or more worked between 10 pm and 8 am and at least three shifts occurring consecutively and were diagnosed with shift‐work sleep disorder in accordance with criteria stipulated in the International Classification of Sleep Disorders. Number randomised: Screened 609, randomised 209, treated 204, followed‐up 153 Lost to follow‐up: Discontinued 51 (7 first month; 26 second month; 18 third month) Age mean: Intervention 37.5 ± 9.2 years control 38.8 ± 9.1 years Sex: Intervention male 60%, control male 62% Duration of SWD at baseline: > three months, excessive sleepiness syndrome Subjects were evaluated monthly during an overnight laboratory shift after having worked for three or more consecutive nights. |
|
| Interventions | Trial intervention: Randomly assigned to 200 mg modafinil 30 to 60 minutes before the night shift Comparison intervention: Placebo Treatment providers:Cephalon pharmaceutical company (Provigil) Treatment frequency and duration: 200 mg of modafinil taken 30 to 60 minutes before the start of each night shift; control: placebo |
|
| Outcomes | For the review: Sleepiness : KSS (range from 1 (very alert) to 9 (very sleepy)), MSLT (measured by polysomnography at two‐hour intervals, starting at 2 am) Alertness: Performance measure, a 20‐minute Psychomotor Vigilance Test was administered every two hours, starting at 1 am For the trial: There were two prespecified primary efficacy variables. The first was the rating on the Clinical Global Impression of Change test for sleepiness during the night shift, including the commute to and from work, at the final visit. Additional measures of sleepiness was KSS. The second prespecified primary efficacy variable was the change between baseline and the final visit (i.e. at the third month or at withdrawal from the trial) in overall mean sleep latency on the basis of results of the nighttime MSLT (P. 477). |
|
| Notes | Source of funding: Supported by Cephalon, Frazer, Pa. Ethics: Participants gave informed written consent. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization was stratified by centre, and within each centre, it was stratified by those patients who worked five to 10 nights per month and those patients who worked more than 10 night shifts per month. The randomisation was generated to enable the balanced treatment assignments for each of two strata (supplementary appendix, available at www.nejm.org). |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants All outcomes | Low risk | Patients were randomly assigned (in a 1:1 ratio) to receive 200 mg of modafinil (Provigil, Cephalon), formulated as 100 mg tablets, or an identical‐appearing placebo, taken 30 to 60 minutes before the start of each night shift. |
| Blinding of care providers | Low risk | Patients were randomly assigned (in a 1:1 ratio) to receive 200 mg of modafinil (Provigil, Cephalon), formulated as 100 mg tablets, or an identical‐appearing placebo, taken 30 to 60 minutes before the start of each night shift. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Supplementary appendix: outcome measures (MSLT, psychomotor vigilance test) were scored centrally without knowledge of treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 209 patients who were randomly assigned to receive the trial drug, 204 patients received the drug and 153 patients completed the trial. The participants were withdrawn from the follow‐up equally in both groups (modafinil 24, placebo 27) (p.479). The patients that completed the trial and those who did not had similar baseline values for the primary outcome variables (MSLT and Clinical Global Impression of Severity test) and similar results on polysomnography (P. 480). All analyses were performed on the observed data only except final visit analysis, where last‐observation‐carried‐forward methodology was used (Suppl. Appendix p.5). Final visit analysis included MSLT (placebo 96/108, modafinil 86/96), KSS (placebo 97/108, modafinil 86/96) and psychomotor vigilance test (placebo 66/108, modafinil 66/96). Total drop‐out rate in psychomotor vigilance test was about 30%; effect size among missing outcome is enough to induce clinically relevant bias in observed effect size (Higgins 2011 page 200). |
| Outcome measured with validated instrument All outcomes | Low risk | Sleepiness : KSS (range from 1 (very alert) to 9 (very sleepy)), MSLT (measured by polysomnography at two‐hour intervals, starting at 2 am) Alertness: Performance measure, a 20‐minute Psychomotor Vigilance Test was administered every two hours, starting at 1 am. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. |
| Other bias | Low risk | Balance at baseline: there were no significant differences in demographic variables, shift‐work type, sleepiness, performance, and results on polysomnography. |
Czeisler 2009.
| Methods | Trial design: This 12‐week, randomised, double‐blind, placebo‐controlled, parallel‐group, multicentre trial was conducted with a common protocol at 42 centres in the USA (37) and Canada (five) from 2 April to 23 December 2004, in compliance with the International Conference on Harmonization's Good Clinical Practice Consolidated Guidance. An independent ethics committee or institutional review board at each centre approved the protocol. Country: USA and Canada Randomisation procedure: The trial sponsor created and maintained the randomisation code, all clinical person, sponsors, investigators, patients remained blinded. A central interactive voice response system for the randomisation process ensured am overall balance among treatment groups within each country (p.959) Follow‐up: Three months |
|
| Participants | Inclusion: Men and women between the ages of 18 and 65 years who worked five or more night shifts per month (each shift ≤ 12 hours, with ≥ six hours worked between 10 pm and 8 am and with ≥ three shifts occurring on consecutive nights) and planned to maintain this schedule for the duration of the treatment were screened for inclusion. Only individuals who exhibited signs and symptoms of SWD of moderate or greater severity, as documented by a CGI‐S rating of 4 or higher for sleepiness on work nights, including the commute to and from work, were enrolled in the trial. Number randomised: Screened 747, randomised 254, treated 245, followed‐up 172 Lost to follow‐up: discontinued 68 (52 first month, 15 second month, 1 third month); discontinuation due to adverse effect: 7 armodafinil, 4 placebo Age mean: Intervention 38.9 ±10.8, control 40.3 ± 10.8 Sex: intervention male 54%, control male 52% Duration of SWD at baseline: duration of excessive sleepiness > 3 months Exclusion: Prescrition drugs, caffeine > 600 mg, substance abuse, medical condition. |
|
| Interventions | Trial intervention: Armodafinil 50 mg (first night) 100 mg (second and third night), 150 mg (fourth night and later) during a period of night shifts Comparison intervention: Placebo Treatment providers: Cephalon Inc (pharmaceutical company) Treatment frequency and duration: Trial participants were randomly assigned (1:1) to receive armodafinil, 150 mg (Cephalon Inc, Frazer, PA), formulated as 50 mg tablets or matching placebo 30 to 60 minutes before each night shift and no later than 11 pm. Patients received a dose of 50 mg on the first night, 100 mg on the second and third nights, and 150 mg on all subsequent nights. Patients took trial medication only on nights when they worked the night shift or attended the sleep laboratory. During laboratory night shifts, trial medication was administered at 10 pm (± 30 minutes). |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: Cognitive performance, sleepiness and fatigue at work: (tests during an overnight laboratory test after a night shift period). Sleep propensity during laboratory night shifts was evaluated electrophysiologically using 20‐minute MSLT19 sessions at midnight and at 2, 4, 6, and 8 am. Sleep latency was measured as the time from lights out to the first 30‐second epoch scored as sleep according to standard criteria. Patient‐estimated sleepiness was evaluated using the KSS. Patients completed the KSS before every MSLT session. The computerized Cognitive Drug Research (CDR) system was administered at 12:30, 2:30, 4:30, 6:30, and 8:30 am of each laboratory night shift. The CDR battery included tests of memory (e.g. numeric working memory test, word recognition test, immediate word recall test, delayed word recall test, and picture recognition test) and attention (e.g. simple reaction time test, choice reaction time test, and digit vigilance task). |
|
| Notes | Source of funding: Caphlaon Inc (pharmaceutical company) Ethics: An independent ethics committee or institutional review board at each centre approved the protocol. Trial Registration: clinical trials.gov identifier NCT00080288 (safety/efficacy). Supplemental Appendix: 8% of night workers and 5.6% of rotating shift workers met clinical criteria for shift work sleep disorder (sleepiness at night and insomnia) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial participants were randomly assigned (1:1) to receive armodafinil, 150 mg (Cephalon Inc, Frazer, PA), formulated as 50 mg tablets or matching placebo 30 to 60 minutes before each night shift and no later than 11 pm. The trial sponsor generated and maintained the randomisation code, and all clinical personnel from the sponsor, investigators and patients remained blinded to the identity of the trial drug for the duration of the trial. A central interactive voice response system for the randomisation process ensured an overall balance among treatment groups within each country (p.959). |
| Allocation concealment (selection bias) | Low risk | See above. |
| Blinding of participants All outcomes | Low risk | See above. |
| Blinding of care providers | Low risk | See above. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The mean (SD) number of nights that patients received trial medication was 42.4 (19.3) for the armodafinil group and 39.2 (18.2) for the placebo group. Sixty‐eight (28%) of 245 patients withdrew from the trial (30 in the armodafinil group and 38 in the placebo group). Reasons for discontinuing were adverse events (7 in the armodafinil group, 4 in the placebo group), consent withdrawn (3 in the armodafinil group, 16 in the placebo group), loss to follow‐up (3 in the armodafinil group, 5 in the placebo group), non‐adherence with trial procedures (6 in the armodafinil group, 2 in the placebo group), and other (11 in the armodafinil group, 11 in the placebo group) (p.961). The efficacy analysis included 216 (85%) of 254 patients. Patients were severely sleepy at baseline. The efficacy analysis used last‐observation‐carried‐forward method and used final visit (last recorded visit) in analysis. MSLT, KSS and mean simple reaction time tests had all 216/245 observations (placebo 104/122, armodafinil 112/123). |
| Outcome measured with validated instrument All outcomes | Low risk | Sleep propensity during laboratory night shifts was evaluated electrophysiologically using 20‐minute MSLT, 19 with sessions at midnight and at 2, 4, 6, and 8 am. Sleep latency was measured as the time from lights out to the first 30‐second epoch scored as sleep according to standard criteria. Patient‐estimated sleepiness was evaluated using the KSS. Patients completed the KSS before every MSLT session. Simple reaction time measurement included into computerized Cognitive Drug Research (CDR) test battery. |
| Selective reporting (reporting bias) | Low risk | Three outcomes was chosen for comparison (MSLT, KSS and simple reaction time); the number of subjects at 12 week were 87 armodafinil/83 placebo for MSLT, 95/90 for KSS and 88/84 for simple reaction time (originally 123 armodafinil, 122 placebo). |
| Other bias | Low risk | ‐ |
Erman 2011.
| Methods | Trial design: Randomized, double blind, placebo controlled, according to Guideline for Good Clinical Practice Country: USA Healthcare settings: 45 centres (insomnia research and treatment) Work settings: Shift workers 18 to 65 years, 25% health care sector, 15% protective services and 10% in transportation Randomisation procedure: Inclusion period: Feb to Oct 2010 Follow‐up: Six weeks |
|
| Participants | Inclusion: Regular shift workers (minimum five nights/month, minimum three consecutive nights) age 18 to 65 with excessive sleepiness associated with shift work sleep disorder (score > 4 in CGI‐C, < 7 in GAF and > 6 in average KSS scales) Number randomised: Screened 649, randomised 383, treated 371, followed‐up 313 Lost to follow‐up: Not started 12, discontinued 58 Age mean: Intervention 36.1, control 36.7 Sex: Intervention male 53%, control male 56% Exclusion: Obstructive sleep apnoea, medical or psychiatric disorder causing sleepiness; use of modafinil, caffeine > 600 mg/day, melatonin, amphetamine, sedating antidepressants or other medication causing sleepiness. |
|
| Interventions | Trial intervention: armodafinil 150 mg; 50 mg at first evening, 100 mg at second and third evening and 150 mg from fourth evening onwards. Comparison intervention: Placebo Treatment providers: Cephalon Inc pharmaceutical company Treatment frequency and duration: Once 30 to 60 minutes before night shift, latest at 11 o'clock; six weeks; four visits and one telephone call during the trial period. |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: Cognitive performance, sleepiness and fatigue at work: (during the last night of a shift period) Clinical global impression of Change ‐ Severity of illness (CGI‐C), Global assessment of Functioning (GAF), KSS in baseline and in weeks three and six. |
|
| Notes | Source of funding: Trial was sponsored by Cephalon (producer of armodafinil) Ethics: According to Quideliness of Good Clinical Practice Trial Registration: clinical trials.gov identifier NCT01080807 (tolerability) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Phase IV clinical trial, randomisation by protocol; trial according to Guideline for Good Clinical Practice; Trial register: clinical trials.gov identifier NCT01080807 Allocation: Randomized Endpoint classification: Safety/efficacy trial Intervention model: Parallel assignment Masking: Double blind (subject, caregiver, investigator, outcomes assessor) Primary Purpose: Treatment At the baseline visit, patients who continued to meet eligibility criteria were randomly assigned (1:1) to receive either 150 mg of armodafinil or matching placebo treatment only on nights worked for six weeks. Trial drug was taken once nightly, 30 to 60 minutes prior to the start of the night shift, on nights worked. Armodafinil treatment was titrated as follows (only on nights worked): the first dose was 50 mg (one tablet), the second and third doses were 100 mg (two tablets), and the fourth and subsequent doses were 150 mg (three tablets). Placebo tablets matching armodafinil tablets were administered on the same schedule. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants All outcomes | Low risk | No description. |
| Blinding of care providers | Unclear risk | No description. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withrawal less than 20% during the trial (88% completed in placebo group, 82% in armodafinil group; 9/184 adverse effects in armodafinil group). The efficacy analysis included 256 to 259 (90%) of 283 patients. The efficacy analysis used last‐observation‐carried‐forward method and used final visit (last recorded visit) in analysis. KSS had 256/283 observations (placebo 180/190, armodafinil 176/193) and Global Assessment of Function. |
| Outcome measured with validated instrument All outcomes | Low risk | KSS (secondary efficacy variable)(patient assessed) Clinical global impression of Change ‐ Seveity of illness (CGI‐C)(secondary efficacy variable)(investigator assessed) Global Assessment of Functioning (GAF) (primary efficacy variable)(investigator assessed). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as planned. |
| Other bias | Unclear risk | ‐ |
Folkard 1993.
| Methods | Trial design: Double‐blind randomised cross‐over design Country: Surrey, England Work settings: Surrey Constabulary One complete cycle 28 day system operated by Surrey Constabulary comprise of four rest days, seven nights (22:00 to 06:00), two rest days, seven late shifts (14:00 to 22:00), one rest day and seven early shifts (6:00 to 14:00). For treatment legs the measures were taken for two blocks of 14 days, prior of two successive 28 days cycles of the shift system. |
|
| Participants | Inclusion: Number randomised: Screened, randomised, followed‐up 17 volunteer police officers in Surrey Constabulary, all healthy and free from medication, six subject completed all three phases of the trial, seven completed both placebo and melatonin legs, eight completed the baseline recording; the remainder (10) failed to provide sufficiently complete records or their pattern of shift deviated grossly from that scheduled. Lost to follow‐up: Full data were obtained from 7/17 participants (ten lost to follow ‐up) Age mean: Intervention control 29 ± 7 Sex: Intervention male 15/17 control male 15/17 |
|
| Interventions | Trial intervention: Melatonin 5 mg after six successive night shifts and prior to four normally timed night sleep Comparison intervention:placebo Treatment frequency and duration: once 30 to 60 minutes after night shift, latest at 11 o'clock; Follow‐up: six weeks; four visits and one telephone call during the trial period. |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: daily sleep diaries (sleep onset, sleep offset, sleep duration, sleep latency, rated sleep quality). Cognitive performance, sleepiness and fatigue at work: mood checklist, work load ratings, rating of alertness, letter search task for memory. |
|
| Notes | Ethics: University of Surrey Ethical Committee | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The design was double blind and order of treatments was randomised (P. 316), six subjects completed all three phases of the trial. |
| Allocation concealment (selection bias) | Unclear risk | No written information. |
| Blinding of participants All outcomes | Unclear risk | Melatonin (5 mg) in gelatin and placebo (lactose in gelatin) capsules (p.316). |
| Blinding of care providers | Unclear risk | No written information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Results are based on N = 7 for placebo and melatonin. Analyses are based on the six recorded day sleeps. The remainder (10) failed to provide sufficiently complete records or their pattern of shift deviated grossly from that scheduled. |
| Outcome measured with validated instrument All outcomes | Low risk | Sleep length, sleep onset and quality while off work: daily sleep diaries (sleep onset, sleep offset, sleep duration, sleep latency, rated sleep quality). Cognitive performance, sleepiness and fatigue at work: mood checklist, work load ratings, rating of alertness, letter search task for memory. |
| Selective reporting (reporting bias) | Unclear risk | No data, high attrition bias. |
| Other bias | Unclear risk | Balance in baseline characteristics: cross‐over design balanced baseline characteristics. Balance in interventions: Discontinuation of trial severely imbalanced trial groups Funding public/private: No data. |
James 1998.
| Methods | Trial design: Double blind single dose randomised cross‐over trial Country: Michigan, USA Randomisation procedure: Trough computer randomisation schedule; medication was identified only by sequential number; identification key was kept by a hospital pharmacist and seen by investigators only at the termination of the trial |
|
| Participants | Inclusion: Trial included 24 adult volunteers. The volunteers were pre‐hospital personnel (EMTs or paramedics) working rotating night shifts with the Kent County EMS system. Number randomised: 24 volunteers initially enrolled in the trial (1 excluded due tranquillizer use, 1 pregnant) Followed‐up: 22 volunteers Lost to follow‐up: 2 volunteers Age: mean 29 ± 8 (SD) Sex: intervention female/male 5/17 Exclusion: 1 tranquillizer use, 1 pregnant |
|
| Interventions | Trial intervention: Melatonin 6 mg once after three to six consecutive night shifts Comparison intervention: Placebo Treatment frequency and duration: Melatonin 6 mg one capsule orally 30 minutes before each consecutive day sleep, latest at 11 o'clock; total of four treatment cycles (2 melatonin, 2 placebo) six weeks; four visits and one telephone call during the trial period. |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: Sleep latency (time between bedtime and sleep onset), reported awakenings during sleep, sleep efficiency (total time asleep as a percentage of total time in bed), total sleep time and duration of daytime naps. Sleep quality was measured using a linear VAS, ranging from worst (score = 0) to best (score = 10). Cognitive performance, sleepiness and fatigue at work: The participant's mood during each post‐treatment night was measured using three bipolar VASs: alert to tired, calm to tense, and cheerful to depressed. Job performance on night shifts was measured using three monopolar VASs "demands on your time" (time pressure), "mental effort or concentration" (mental load), and "stress or emotional involvement". Linear analog scores were measured to the nearest millimetre (P. 368). |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After giving informed consent, volunteers were randomly assigned to take either a 6 mg melatonin capsule (Vitamin Research Products Inc., Carson City, Nevada) or placebo. Randomization was accomplished using a computer randomisation schedule" (P. 367). |
| Allocation concealment (selection bias) | Low risk | See above and below. |
| Blinding of participants All outcomes | Low risk | Trial medications were packaged in blister packs and identified only by a sequential number for computer coding purposes. The identification key was kept by a hospital pharmacist and seen by investigators only at the termination of the trial (P. 367). |
| Blinding of care providers | Low risk | See above. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 24 volunteers were initially enrolled in the trial. One participant was excluded because of prescription tranquillizer use. Another paramedic became pregnant and dropped out of the trial after completing one treatment cycle (melatonin). |
| Outcome measured with validated instrument All outcomes | Low risk | Sleep length and quality: Total sleep time; Sleep onset latency.Sleep quality was measured using a linear VAS, ranging from worst (score = 0) to best (score = 10). Cognitive performance, sleepiness and fatigue at work: The participant's mood during each posttreatment night was measured using three bipolar VASs: alert to tired, calm to tense, and cheerful to depressed. Job performance on night shifts was measured using three monopolar VASs "demands on your time" (time pressure), "mental effort or concentration" (mental load), and "stress or emotional involvement. '' Linear analog scores were measured to the nearest millimetre (P 368). |
| Selective reporting (reporting bias) | Unclear risk | Outcomes only partially reported. |
| Other bias | Low risk | Balance in baseline characteristics: Cross‐over design balances baseline characteristics Balance in interventions: Low discontinuation Funding public/private: No data |
Jockovich 2000.
| Methods | Trial design: Randomized, double‐blind, placebo‐controlled, cross‐over design Country: Jacksonville, Florida, USA Work settings: University of Florida emergency medicine (EM) residency in Jacksonville Randomisation procedure: Hospital pharmacy department prepared the test medicines, both investigators and the subjects were blinded to the process |
|
| Participants | Inclusion: Volunteer residents working in night shift in emergency medicine unit; minimum two series of at least three consecutive night shifts Number randomised: Screened, randomised, followed‐up 19 Lost to follow‐up: 0 Age mean: 28.2 years Sex: Intervention male 15/19 control male 75% Exclusion: Heavy alcohol use, heavy caffeine use, opioid or benzodiazepine use, restless legs |
|
| Interventions | Trial intervention: Melatonin 1 mg 30 to 60 minutes prior to anticipated daytime sleep session beginning after the first night shift Comparison intervention: Placebo |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: Wrist Actigraphs were used to obtain an objective measure of daytime sleep for each of the daytime sleep sessions throughout both strings of night shifts, and it calculates total sleep time, time in bed, sleep efficiency (total sleep time minutes/time in bed), and sleep onset latency. Cognitive performance, sleepiness and fatigue at work: The POMS and the Stanford Sleepiness Scale (SSS) were used to evaluate melatonin’s effect on sleepiness and mood. |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Subjects were randomly assigned to receive either melatonin (1 mg) or placebo, 30 to 60 minutes prior to their anticipated daytime sleep session. A cross‐over design was used, with subjects serving as their own controls on subsequent night shifts. |
| Allocation concealment (selection bias) | Unclear risk | No data. |
| Blinding of participants All outcomes | Low risk | The hospital pharmacy department prepared 1 mg melatonin and placebo caplets for use in the trial. The investigators and subjects were blinded regarding the true contents of the caplets. |
| Blinding of care providers | Low risk | See above. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The subjects reported 100% compliance with drug therapy, with the exception of one dose of melatonin, which was taken 15 minutes prior to the anticipated sleep session instead of the suggested 30 to 60 minutes. Three subjects failed to complete the SSS, POMS, or both for one night shift. One subject was awoken multiple times during a daytime sleep session secondary to the phone. Additionally, actigraphical data were incomplete for three subjects because of equipment failure or noncompliance. |
| Outcome measured with validated instrument All outcomes | Low risk |
Sleep length and quality: Total sleep time; Sleep onset latency. Wrist Actigraphs were used to obtain an objective measure of daytime sleep for each of the daytime sleep sessions throughout both strings of night shifts, and it calculates total sleep time, time in bed, sleep efficiency (total sleep time minutes/time in bed), and sleep latency. Cognitive performance, sleepiness and fatigue at work: POMS and the SSS were used to evaluate melatonin’s effect on EP sleepiness and mood. |
| Selective reporting (reporting bias) | Unclear risk | Most outcomes reported |
| Other bias | Low risk | Balance in baseline characteristics: Cross‐over design used, good balance Balance in interventions: Low drop‐out rate Funding public/private: No data |
Jorgensen 1998.
| Methods | Trial design: Double‐blind, placebo‐controlled cross‐over trial Country: Maryland, Baltimore, USA Healthcare settings: Emergency medicine department in veteran hospital Work settings: Emergency medicine residents and attending physicians working two to five consecutive eight or 12 hours night shifts (7 pm or 11 pm to 7 am) Randomisation procedure: Randomization procedure was design to ensure that even number of the subjects took placebo/melatonin first ‐ each subject was tested during two strings of nights of equal duration separated by a minimum washout period of five days (randomisation was made by unblinded third party) Recruitment: Emergency medicine residents and attending physicians |
|
| Participants | Inclusion: Emergency medicine residents and attending physicians, N = 18 Number randomised: 20 ‐ two excluded (18/20) Screened, randomised, followed‐up : 18/20 Lost to follow‐up: discontinued: no data Age mean: Intervention 32 years, median 31 years Sex: Intervention male 89 % Exclusion: Two excluded (alcohol, sedative use during the test period) |
|
| Interventions | Trial intervention: sublingual melatonin 10 mg in the morning (starting at day 2) during one string of nights, following after the wash‐out period other string of nights. Comparison intervention: placebo in a comparable manner; placebo and melatonin as often as first treatment. |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: Day sleep period (starting from day 2): Total sleep time; sleep onset latency: impression of day time seep (VAS). Cognitive performance, sleepiness and fatigue at work: During night shift SSS; impression of night time sleep (VAS). |
|
| Notes | Ethics: Protocol was approved by institutional review board. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure was designed to ensure that roughly even number of the subjects took placebo/melatonin first. Each subject was tested during two strings of nights of equal duration separated by a minimum washout period of five days (randomisation was made by unblinded third party). Unblinded third party assembled an equal number of "melatonin first" and "placebo first" packets (P.700). |
| Allocation concealment (selection bias) | Unclear risk | See above. |
| Blinding of participants All outcomes | Unclear risk | See above. |
| Blinding of care providers | Low risk | Blinded investigators used one bundle (of medication) at a time in the trial (P. 700). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low discontinuation rate. |
| Outcome measured with validated instrument All outcomes | Low risk | Sleep length, sleep onset and quality while off work: Total sleep time (diary/questionnaire), gestalt impression of dug effect day sleep/night alertness. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | Balance in baseline characteristics: Cross‐over design, good balance Balance in interventions: Low drop‐out rate Funding public/private: No data |
Quera‐Salva 2002.
| Methods | Trial design: Randomised double‐blind trial Country: France Work settings: Car manufacturing company and a paper mill employees Randomisation procedure: Random allocation to zopiclone (14) and placebo (14) treatment during the day after the night shift for three consecutive night shift periods (no description of randomisation procedure) Recruitment: Employess with sleeping problems in two work places |
|
| Participants | Inclusion: Employees with sleeping problems in shift work Number randomised: Screened 29, randomised 28, followed‐up 28 Lost to follow‐up: Discontinued 1 Age mean: 41 ± 7 years (both intervention and control group) Sex: Intervention:all male, control: all male Exclusion: Severe chronic illness |
|
| Interventions | Trial intervention: Zopiclone 7.5 mg Comparison intervention: Placebo Treatment frequency and duration: After three consecutive night shifts before sleeping period of the next day. |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: Sleep diary, wrist actigraphy Cognitive performance, sleepiness and fatigue at work: None |
|
| Notes | Source of funding: Probably Aventis pharmaceuticals. Ethics: Protocol accepted by the committee of biomedical research in hospital Amroise Paré. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Double blind allocation to treatment/placebo. |
| Allocation concealment (selection bias) | Unclear risk | No written data. |
| Blinding of participants All outcomes | Unclear risk | Double‐blind allocation of treatment. |
| Blinding of care providers | Unclear risk | See above. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Acticraphs used for sleep time detection. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up. |
| Outcome measured with validated instrument All outcomes | Low risk | Sleep length measured by actigraphy. |
| Selective reporting (reporting bias) | High risk | Non‐exact data of outcomes provided (change scores of outcome parameters; total sleeping time expressed in hours). |
| Other bias | Unclear risk | Balance in baseline characteristics: No information on baseline characteristics of participants, randomisation to two groups Balance in interventions: Low drop‐out rate Funding public/private: Aventis pharmaceutical company (?) |
Sadeghniiat‐Haghighi 2008.
| Methods | Trial design: Double‐blind, placebo‐controlled randomised cross‐over trial Country: Baharloo Hospital, Tehran, Iran Work settings: Hospital nurses working in night shift Randomisation procedure: Randomised to one of the two sequence (either placebo or melatonin first) Follow‐up: Over the following night sleep |
|
| Participants | Inclusion: Healthy, non‐smoking, non‐pregnant shift work nurses, who reported sleeping problems in the questionnaire (score of inclusion?) Number randomised: Screened : 118, randomised 98, followed‐up 86 Lost to follow‐up: Discontinued: 12 Age mean: 30 years Sex: Male 20% Duration of SWD at baseline: No data, all reported sleeping problems Exclusion: Pregnancy, smoking, no sleep problem in the preliminary questionnaire (20), not following the protocol (12) |
|
| Interventions | Trial intervention: Melatonin 5 mg Comparison intervention: placebo Treatment frequency and duration: Melatonin 5 mg/placebo, about 30 minutes before intended night‐time sleep, after the night shift, four days wash‐out period |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: Total sleep time; Sleep onset latency (diary) Cognitive performance, sleepiness and fatigue at work: None |
|
| Notes | Ethics: Ethical Committee of the Tehran University approved the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to one of two sequences: placebo followed by melatonin or melatonin followed by placebo. Randomization list was completed by using random number generator (.page 2/5). |
| Allocation concealment (selection bias) | Low risk | The treatment and placebo phases consisted of taking identical looking melatonin/placebo on the same schedule (page 2/5). |
| Blinding of participants All outcomes | Low risk | See above. |
| Blinding of care providers | Low risk | See above. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few drop‐outs. |
| Outcome measured with validated instrument All outcomes | Low risk | Sleep length and quality: sleep length, sleep onset latency, number of awakenings, difficulty falling asleep or staying asleep, problems waking up, sleep quality. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Balance in baseline characteristics: Cross‐over design, good balance Balance in interventions: Low drop‐out rate Funding public/private: Supported by Tehran University of medical Science |
Sastre‐y‐Hernandez 1982.
| Methods | RCT double‐blind placebo controlled | |
| Participants | Nurses (N = 60) with sleep problems (sleep latency > 30 mins, sleep length < 6 hours, two awakenings per night) | |
| Interventions | Lormetazepam 1 mg before going to bed during seven days Placebo in similar looking capsules |
|
| Outcomes | Self report or diary of sleep length, awakenings, sleep quality, global impression of change | |
| Notes | Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants All outcomes | Low risk | Double blind placebo controlled, similar capsules. |
| Blinding of care providers | Unclear risk | Double blind placebo controlled, similar capsules. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double blind placebo controlled, similar capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fifty‐six out of 60 cases analysed. |
| Outcome measured with validated instrument All outcomes | High risk | No reference to validation trial. |
| Selective reporting (reporting bias) | High risk | No raw outcomes reported, only outcomes in certain categories of improvement. |
| Other bias | Unclear risk | Authors employed by pharmaceutical company. |
Schweitzer 2006.
| Methods | Trial design: Field trial: Cross‐over design for four consecutive night shifts (caffeine 300 mg and naps in two evenings; placebo without naps) Country: USA Work settings: Voluntary participants (laboratory trial); voluntary shift workers (field trial) Randomisation procedure: Randomization into four test groups (laboratory trial); cross‐over trial; randomisation of the sequence (field trial) Recruitment: By media advertising |
|
| Participants | Inclusion: Recruitment by media (participants get paid) volunteers with no sleepiness problems, field trial; minimum five night shifts/month and minimum three consecutive nights with shift length 7 to 10 hours and minimum five hours between 10pm and 8am. Number randomised: Field trial: 53 met the inclusion criteria and were randomised; 39 complemented both arms of the trial. Field trial: 14 lost for follow‐up (6 did not complete the trial, 8 excluded due to protocol violations) Field trial 28 men, 11 women mean age 33.5, range 22 to 55 years Exclusion: Severe medical condition, use of medication, alcohol or caffeine (more than 400 mg/day), anxiety or depression disorders, excessive sleepiness |
|
| Interventions | Trial intervention: Caffeine 300 mg 30 minutes prior to night shift and two hour naps before shift start Comparison intervention: placebo Treatment frequency and duration: Four consecutive night in night shift work, separation of conditions from three to 24 days between schedules |
|
| Outcomes | Cognitive performance, sleepiness and fatigue at work: KSS, SSS, psychomotor vigilance task but insufficient data. | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Field trial: Individuals were randomly assigned to condition order in a counterbalanced manner; cross‐over design was used to compare two conditions; no description of the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants All outcomes | High risk | Interventions were different between two arms of trial; evening naps were introduced during the two first nights in caffeine group but not in placebo group (intervention not blinded). |
| Blinding of care providers | Unclear risk | Interventions were different between two arms of trial; evening naps were introduced during the two first nights in caffeine group (intervention not blinded). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In field trial, drop‐out rate was 26% (mostly due to protocol violations; napping during no‐nap period). |
| Outcome measured with validated instrument All outcomes | Low risk |
Sleep length and quality: Sleep length after night shift (not relevant in field trial where naps were taken before night shift) Cognitive performance, sleepiness and fatigue at work: KSS, SSS, psychomotor vigilance task, maintenance of wakefulness test |
| Selective reporting (reporting bias) | Low risk | All main outcomes reported; KSS and PVG presented. |
| Other bias | Unclear risk | Balance in baseline characteristics: Cross‐over design, good balance in baseline Balance in interventions: Drop‐out rate 26% Funding public/private: No industry funding, participants were paid |
Wright 1998.
| Methods | Trial design: Randomized placebo controlled double blind cross‐over trial Work settings: Dept Emergency Medicine, Vanderbilt University, Nashville, TN, USA Randomisation procedure: By pharmacist (cross‐over melatonin and placebo), computer generated random numbers. All personnel involved remained unaware of the treatment assignment Recruitment: All emergency medicine faculty MDs in night work |
|
| Participants | Inclusion: Emergency department faculty members working in night shift Number randomised: 20 eligible, 3 not in night work, 1 pregnant, 1 lactating, followed‐up: 15 Lost to follow‐up: None Age mean: 38.6 years Sex: Intervention and control, male 12/15 Exclusion: Not in night work, pregnant or lactating, melatonin use 30 days before, any sedative use. |
|
| Interventions | Trial intervention: After two night shifts subjects took melatonin 5 mg/placebo 30 min before going to bed in the evening of (post shift) day 1,2 and 3. Comparison intervention: Placebo Treatment providers: Blinded Treatment frequency and duration: Three days for each treatment |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: Total sleep time, sleep onset latency, awakenings (diary or questionnaire). Cognitive performance, sleepiness and fatigue at work: KSS (results not reported). |
|
| Notes | Intervention directed to normal night sleep after the a shift work (differs from day time sleep interventions). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by pharmacist (cross‐over melatonin and placebo), computer generated random numbers (P. 335). |
| Allocation concealment (selection bias) | Low risk | Computer generated random numbers. All personnel involved remained unaware of the treatment assignment. |
| Blinding of participants All outcomes | Low risk | All personnel involved remained unaware of the treatment assignment until completion of the trial. No interim analysis was conducted (P. 335). |
| Blinding of care providers | Low risk | See above. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low drop‐out. |
| Outcome measured with validated instrument All outcomes | Low risk |
Sleep length, sleep onset and quality while off work: Total sleep time, sleep onset latency. Cognitive performance, sleepiness and fatigue at work: KSS (results not reported). |
| Selective reporting (reporting bias) | Unclear risk | Sleepiness data missing (reported no difference between arms). |
| Other bias | Low risk | Balance in baseline characteristics: Cross‐over design, good balance Balance in interventions: Low drop‐out rate Funding public/private: No data |
Yoon 2002.
| Methods | Trial design: Repeated measure cross‐over design Country: Korea Work settings: Young‐In Mental Hospital Randomisation procedure: No data Recruitment: No data |
|
| Participants | Inclusion: Twelve female nurses, 23 to 27 years, working in night shift schedules for 0.5 to 3 yrs, counter‐clockwise shift rotation (night‐evening‐morning) Number randomised: 12 Lost to follow‐up: 0 Age: 23 to 27 years Sex: Intervention male control male Exclusion: Suspected depression (score above 16 in Beck depression inventory; definite morning and evening types |
|
| Interventions | Trial intervention: Three arms: placebo, melatonin 6 mg, or melatonin 6 mg plus sunglasses use in the mornings of days 2 to 4 Comparison intervention: Placebo Treatment frequency and duration: Melatonin 6 mg at 9:00 am at day 2 and 3 during four days of intervention during the shift work; sunlight exposure for 30 minutes was allowed for days 2 to 4; minimum five days wash‐out period between trial exposures |
|
| Outcomes |
Sleep length, sleep onset and quality while off work: Sleep latency, sleep period time, total sleep time, sleep efficiency, sleep‐onset time, sleep‐offset time (diary). Cognitive performance, sleepiness and fatigue at work: nocturnal alertness ‐ visual analog scale 24:00 ‐ 6:00 at two hour intervals (0‐100). |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | To control the sequence effect, two subjects were allocated to each of the six possible sequences; cross‐over design balanced the trial. |
| Allocation concealment (selection bias) | Unclear risk | Cross‐over design balances allocation (allocation concealment only possible between placebo and melatonin). |
| Blinding of participants All outcomes | High risk | Three arms: placebo, melatonin, melatonin with sunglasses; blinding is not possible in all arms (only between melatonin versus placebo). |
| Blinding of care providers | Unclear risk | See above. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low drop‐out rate. |
| Outcome measured with validated instrument All outcomes | Low risk | Sleep length, sleep onset and quality while off work: sleep latency, total sleep time (diary). Cognitive performance, sleepiness and fatigue at work: nocturnal alertness ‐ visual analog scale 24:00 to 6:00 at two hour intervals (0‐100). |
| Selective reporting (reporting bias) | Unclear risk | See above. Blinding was not possible, reporting may still be valid. |
| Other bias | Unclear risk | Balance in baseline characteristics: cross‐over design, good balance Balance in interventions: no withdrawals, unblinded design Funding: no data |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Babkoff 2002 | Participants were not actual shift workers. Caffeine and bright light in simulated shift work with volunteers. |
| Beaumont 2001 | Participants were not actual shift workers. Slow release caffeine in continuous wakefulness (64 hours) in volunteers. |
| Beaumont 2005 | Participants were not actual shift workers. Slow release caffeine after continuous wakefulness period in volunteers. |
| Bonnet 1994 | Participants were not actual shift workers. Naps and caffeine in experimental study with volunteers. |
| Caldwell 2003 | Study conducted in simulated shift work conditions. Temazepam in simulated night shift in air force personnel. |
| Carrier 2009 | Participants were not actual shift workers. Caffeine in laboratory night shift with volunteers. |
| Cohen 2010 | Participants were not actual shift workers. Ramelteon, a melatonin agonist, in simulated night shift study with volunteers. |
| Crowley 2003 | Participants were not actual shift workers. Melatonin in simulated night shift with volunteers. |
| Dagan 2006 | Participants were not actual shift workers. Caffeine and modafinil in simulated night shift with volunteers. |
| Dawson 1995 | Participants were not actual shift workers. Night shift bright light and day time melatonin in simulated night shift with volunteers. |
| Erman 2007 | Study did not measure daytime sleepiness or night time alertness. RCT on modafinil in multicentre study; outcomes were patient functioning, quality of life (no sleepiness, alertness outcomes). |
| Giggli 1993 | Study did not measure daytime sleepiness or night time alertness. Double‐blind cross‐over study on the safety of use (non alertness disturbing effect) of hypnotic brotizolam in shift work caused sleep problems (no daytime sleep related or night time alertness related outcomes). |
| Grady 2010 | Study conducted in simulated shift work conditions. Modafinil in laboratory study. |
| Hart 2005 | Study conducted in simulated shift work conditions. Methamphetamin and zolpidem in simulated night shift. |
| Hart 2006 | Study conducted in simulated shift work conditions. Modafinil in simulated night shift. |
| Jay 2006 | Participants were not actual shift workers. Energy drinks in volunteers. |
| Kantelip 1994 | Participants were not actual shift workers. Lormetazepam in medical students. |
| McLellan 2004 | Participants were not actual shift workers. Caffeine in simulated night shift in volunteers. |
| McLellan 2005 | Study conducted in simulated shift work conditions. Caffeine in experimental military trial with soldiers. |
| Muehlbach 1995 | Study conducted in simulated shift work conditions. Caffeine in simulated night shift work. |
| Nickelsen 2002 | Participants were not actual shift workers. Melatonin agonist LY156735 in simulated night shift with volunteers. |
| Porcu 1997 | Participants were not actual shift workers. Temazepam 1 mg for day time sleep in simulated study with volunteers. |
| Schweitzer 1992 | Participants were not actual shift workers. Caffeine in simulated night shift study with volunteers. |
| Sharkey 2001 | Study conducted in simulated shift work conditions. Melatonin in simulated night shift work. |
| Smith 1993 | Participants were not actual shift workers. Caffeine in simulated shift work with volunteers. |
| Smith 2005 | Participants were not actual shift workers. Melatonin in simulated night shift with volunteers. |
| Walsh 1990 | Participants were not actual shift workers. Caffeine in simulated night shift in volunteers. |
| Walsh 1991 | Participants were not actual shift workers. Triazolam in simulated night shifts in volunteers. |
| Walsh 2004 | Participants were not actual shift workers. Modafinil in simulated night shift in volunteers. |
| Wesensten 2007 | Participants were not actual shift workers. Ampakine (CX717) stimulant in simulated night shift in volunteers. |
| Wright 1997 | Participants were not actual shift workers. Bright light and caffeine in 45 hours sleep deprivation in volunteers. |
Characteristics of studies awaiting assessment [ordered by study ID]
Gill 2006.
| Methods | RCT |
| Participants | 25 emergency department resident and attending physicians |
| Interventions | Modafinil (200 mg) versus placebo "Due to the need for a washout period for the outcome analysis (two cognitive tests), the trial consisted of two sessions that took place at least seven weeks apart. During the trial sessions, the participants were required to work a previously scheduled overnight ED shift. Participants were asked to refrain from the caffeine equivalent of more than three cups of coffee within the 24 hours preceding the night shift. Following the night shift, participants were asked to refrain from napping before attending a scheduled didactic session. Participants were asked to take the pill in the bottles marked "session #1" and "session #2" consecutively for each session, to take the pill between 6:30 AM and 7:30 AM, and to only take it after patient care activities had been completed. They then proceeded to attend didactic sessions that began at 8:00 or 8:30 AM and lasted until 10:00 AM, 11:30 AM, or 1 PM , depending on the day of the session. The participants then underwent cognitive testing immediately following didactic sessions." |
| Outcomes | 10 cm Visual Analog Scale with items: 1) difficulty attending lecture after taking the pill, 2) difficulty falling asleep after testing, and 3) difficulty driving home. Scale ranged from 0 = "not very difficult, to 10 = "very difficult" "The questionnaire also contained a list of potential symptoms that the participants may have experienced and a line for them to list "other" symptoms. They were asked to circle any symptoms they experienced or elaborate under "other". Coding task of the Wechsler Adult Intelligence Scale AX version of the Continuous Performance Task |
| Notes | It is unclear if sleepiness or alertness during a classroom didactic session is the same as sleepiness or alertness during normal work duties. The authors also state having excluded participants who were unable to: "limit caffeine intake to a daily maximum of the equivalent of three cups of coffee for 24 hours before and during session 1 and session 2 simulated night shifts..." |
Walsh 1984.
| Methods | Cross‐over trial but unclear if the order of conditions was randomised |
| Participants | Ten rotating night shift workers |
| Interventions | Triazolam (0.5 mg) versus placebo |
| Outcomes | Total sleep time, sleep efficiency, adaptation to daytime sleep |
| Notes |
Wesnes 2005.
| Methods | Unclear |
| Participants | People with chronic shift work disorder |
| Interventions | Armodafinil (150 mg and 250 mg) |
| Outcomes | Memory function |
| Notes | Preliminary data provided in abstract; trial design (night shift) and detailed data missing |
Wright 2010.
| Methods | RCT |
| Participants | Shift workers tested in laboratory after three nights of shift work |
| Interventions | Armodafinil 150 mg, modafinil 200 mg versus placebo |
| Outcomes | MSLT and KSS |
| Notes | According to the authors armodafinil was twice as effective than modafinil and both were significantly more effective than placebo (MSLT). The publication is an abstract but we need the whole article to evaluate eligibility. |
Differences between protocol and review
We intended to check conference proceedings and abstracts but due to limited resources and limited expected yield we did not do this. We omitted sleep quality measurements from our analyses because the heterogeneity of the methods used in the potentially relevant trials was so extensive. We also conducted a subgroup analysis to compare trials conducted with various doses of the provided drugs, even though in the protocol we did not outline doing so.
Contributions of authors
Jos Verbeek (JV) and Jani Ruotsalainen (JR) conceived the protocol. JR, JV and Juha Liira (JL) wrote the first and the second draft. Tim Driscoll (TM), Naomi Rogers (NR), Mikael Sallinen (MS) and Giovanni Costa (GC) commented on both drafts.
JL wrote the systematic review. JV, JN, TD, MS and GC included eligible trials, and JV helped when authors could not agree. JL and JR did the 'Risk of bias' assessment and JV helped when these authors could not agree. JL, JR and JV extracted data from the trials.
JL and JV conducted the data synthesis. TD, NR, MS and GC, JR and JV commented on the systematic review text.
All authors were involved in designing the search strategy and choosing the comparison groups for data analyses. Leena Isotalo (LI) designed and implemented the systematic search strategy.
Sources of support
Internal sources
-
Finnish Institute of Occupational Health, Finland.
Salary for Juha Liira, Jani Ruotsalainen, Mikael Sallinen, Leena Isotalo and Jos Verbeek
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Bjorvatn 2007 {published data only}
- Bjørvatn B, Stangenes K, Oyane N, Forberg K, Lowden A, Holsten F, et al. Randomized placebo‐controlled field study of the effects of bright light and melatonin in adaptation to night work. Scandinavian Journal of Work, Environment and Health 2007;33(3):204‐14. [DOI] [PubMed] [Google Scholar]
Cavallo 2005 {published data only}
- Cavallo A, Ris MD, Succop P, Jaskiewicz J. Melatonin treatment of pediatric residents for adaptation to night shift work. Ambulatory Pediatrics 2005;5(3):172‐7. [DOI] [PubMed] [Google Scholar]
Czeisler 2005 {published data only}
- Czeisler CA, Walsh JK, Roth T, Hughes RJ, Wright KP, Kingsbury L, et al. Modafinil for excessive sleepiness associated with shift‐work sleep disorder. New England Journal of Medicine 2005;353(4):476‐86. [DOI] [PubMed] [Google Scholar]
Czeisler 2009 {published data only}
- Black JE, Hull SG, Tiller J, Yang R, Harsh JR. The long‐term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open‐label extension study. Journal of Clinical Sleep Medicine 2010;6(5):458‐66. [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Walsh JK, Wesnes KA, Arora S, Roth T. Armodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled study. Mayo Clinic Proceedings 2009;84(11):958‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erman 2011 {published data only}
- Erman MK, Seiden DJ, Yang R, Dammerman R. Efficacy and tolerability of armodafinil: effect on clinical condition late in the shift and overall functioning of patients with excessive sleepiness associated with shift work disorder. Journal of Occupational and Environmental Medicine/American College of Occupational and Environmental Medicine 2011;53(12):1460‐5. [DOI] [PubMed] [Google Scholar]
Folkard 1993 {published data only}
- Folkard S, Arendt J, Clark M. Can melatonin improve shift workers' tolerance of the night shift? Some preliminary findings. Chronobiology International 1993;10(5):315‐20. [DOI] [PubMed] [Google Scholar]
James 1998 {published data only}
- James M, Tremea MO, Jones JS, Krohmer JR. Can melatonin improve adaptation to night shift?. American Journal of Emergency Medicine 1998;16(4):367‐70. [DOI] [PubMed] [Google Scholar]
Jockovich 2000 {published data only}
- Jockovich M, Cosentino D, Cosentino L, Wears RL, Seaberg DC. Effect of exogenous melatonin on mood and sleep efficiency in emergency medicine residents working night shifts. Academic Emergency Medicine 2000;7(8):955‐8. [DOI] [PubMed] [Google Scholar]
Jorgensen 1998 {published data only}
- Jorgensen, K‐M. Witting MD. Does exogenous melatonin improve day sleep or night alertness in emergency physicians working night shifts?. Annals of Emergency Medicine 1998;31(6):699‐704. [DOI] [PubMed] [Google Scholar]
Quera‐Salva 2002 {published data only}
- Quera‐Salva MA, Philip P, Taillard J, Letrequeser R, Allain H, Garcia‐Acosta S, et al. Improvement of insomnia in shift workers treated with zopiclone: A study in the real work environment. Revue Neurologique 2002;158(11):1102‐6. [PubMed] [Google Scholar]
Sadeghniiat‐Haghighi 2008 {published data only}
- Sadeghniiat‐Haghighi K, Aminian O, Pouryaghoub G, Yazdi Z. Efficacy and hypnotic effects of melatonin in shift‐work nurses: double‐blind, placebo‐controlled crossover trial. Journal of Circadian Rhythms 2008;6(10):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sastre‐y‐Hernandez 1982 {published data only}
- Sastre‐y‐Hernández M, Vass K, Fichte K, Paschelke G. Drug therapy of sleep disorders in shift workers [Probleme der medikamentosen Behandlung von Schlafstorungen bei Schichtarbeitern]. Medizinische Klinik (Praxis‐Ausg.) 1982;77(8):52‐5. [PubMed] [Google Scholar]
Schweitzer 2006 {published data only}
- Schweitzer PK, Randazzo AC, Stone K, Erman M, Walsh JK. Laboratory and field studies of naps and caffeine as practical countermeasures for sleep‐wake problems associated with night work. Sleep 2006;29(1):39‐50. [DOI] [PubMed] [Google Scholar]
Wright 1998 {published data only}
- Wright SW, Lawrence LM, Wrenn KD, Haynes ML, Welch LW, Schlack HM. Randomized clinical trial of melatonin after night‐shift work: efficacy and neuropsychologic effects. Annals of Emergency Medicine 1998;32(3 Pt 1):334‐40. [DOI] [PubMed] [Google Scholar]
Yoon 2002 {published data only}
- Yoon IY, Song BG. Role of morning melatonin administration and attenuation of sunlight exposure in improving adaptation of night‐shift workers. Chronobiology International 2002;19(5):903‐13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Babkoff 2002 {published data only}
- Babkoff H, French J, Whitmore J, Sutherlin R. Single‐dose bright light and/or caffeine effect on nocturnal performance. Aviation, Space and Environmental Medicine 2002;73(4):341‐50. [PubMed] [Google Scholar]
Beaumont 2001 {published data only}
- Beaumont M, Batejat D, Pierard C, Coste O, Doireau P, Beers P, et al. Slow release caffeine and prolonged (64‐h) continuous wakefulness: effects on vigilance and cognitive performance. Journal of Sleep Research 2001;10(4):265‐76. [DOI] [PubMed] [Google Scholar]
Beaumont 2005 {published data only}
- Beaumont M, Batéjat D, Coste O, Doireau P, Chauffard F, Enslen M, et al. Recovery after prolonged sleep deprivation: residual effects of slow‐release caffeine on recovery sleep, sleepiness and cognitive functions. Neuropsychobiology 2005;51(1):16‐27. [DOI] [PubMed] [Google Scholar]
Bonnet 1994 {published data only}
- Bonnet MH, Arand DL. The use of prophylactic naps and caffeine to maintain performance during a continuous operation. Ergonomics 1994;37(6):1009‐20. [DOI] [PubMed] [Google Scholar]
Caldwell 2003 {published data only}
- Caldwell JL, Prazinko BF, Rowe T, Norman D, Hall KK, Caldwell JA. Improving daytime sleep with temazepam as a countermeasure for shift lag. Aviation, Space and Environmental Medicine 2011;74(2):153‐63. [PubMed] [Google Scholar]
Carrier 2009 {published data only}
- Carrier J, Paquet J, Fernandez‐Bolanos M, Girouard L, Roy J, Selmaoui B, et al. Effects of caffeine on daytime recovery sleep: A double challenge to the sleep‐wake cycle in aging. Sleep Medicine 2009;10(9):1016‐24. [DOI] [PubMed] [Google Scholar]
Cohen 2010 {published data only}
- Cohen DA, Wang W, Klerman EB, Rajaratnam SM. Ramelteon prior to a short evening nap impairs neurobehavioral performance for up to 12 hours after awakening. Journal of Clinical Sleep Medicine 2010;6(6):565‐71. [PMC free article] [PubMed] [Google Scholar]
Crowley 2003 {published data only}
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. Journal of Biological Rhythms 2003;18(6):513‐23. [DOI] [PubMed] [Google Scholar]
Dagan 2006 {published data only}
- Dagan Y, Doljansky JT. Cognitive performance during sustained wakefulness: A low dose of caffeine is equally effective as modafinil in alleviating the nocturnal decline. Chronobiology International 2006;23(5):973‐83. [DOI] [PubMed] [Google Scholar]
Dawson 1995 {published data only}
- Dawson D, Encel N, Lushington K. Improving adaptation to simulated night shift: timed exposure to bright light versus daytime melatonin administration. Sleep 1995;18(1):11‐21. [DOI] [PubMed] [Google Scholar]
Erman 2007 {published data only}
- Erman MK, Rosenberg R, Modafinil Shift Work Sleep Disorder Study Group. Modafinil for excessive sleepiness associated with chronic shift work sleep disorder: effects on patient functioning and health‐related quality of life. Primary Care Companion to the Journal of Clinical Psychiatry 2007;9(3):188‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giggli 1993 {published data only}
- Gigli GL, Maschio MCE, Diomedi M, Moroni M, Dell'orso S, Placidi F, et al. Brotizolam and cognitive performance: A double‐blind, crossover study versus placebo in a population of shift workers. Current Therapeutic Research 1993;53(2):129‐36. [Google Scholar]
Grady 2010 {published data only}
- Grady S, Aeschbach D, Wright KP Jr, Czeisler CA. Effect of modafinil on impairments in neurobehavioral performance and learning associated with extended wakefulness and circadian misalignment. Neuropsychopharmacology 2010;35(9):1910‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hart 2005 {published data only}
- Hart CL, Haney M, Nasser J, Foltin RW. Combined effects of methamphetamine and zolpidem on performance and mood during simulated night shift work. Pharmacology, Biochemistry and Behavior 2005;81(3):559‐68. [DOI] [PubMed] [Google Scholar]
Hart 2006 {published data only}
- Hart CL, Haney M, Vosburg SK, Comer SD, Gunderson E, Foltin RW. Modafinil attenuates disruptions in cognitive performance during simulated night‐shift work. Neuropsychopharmacology 2006;31(7):1526‐36. [DOI] [PubMed] [Google Scholar]
Jay 2006 {published data only}
- Jay SN, Petrilli RM, Ferguson SA, Dawson D, Lamond N. The suitability of a caffeinated energy drink for night‐shift workers. Physiology & Behavior 2006;87(5):925‐31. [DOI] [PubMed] [Google Scholar]
Kantelip 1994 {published data only}
- Kantelip JP, Patay M, Levy P, Mougin F, Didier JM. Effect of night medical guard on following day vigilance. Influence of hypnotic medication on recovery night and on the vigilance during the following day [Effet d'une nuit de garde medicale sur la vigilance du lendemain. Influence d'un hypnotique sur la nuit de recuperation et la vigilance dans la journee qui suit]. Therapie 1994;49(2):107‐12. [PubMed] [Google Scholar]
McLellan 2004 {published data only}
- McLellan TM, Bell DG, Kamimori GH. Caffeine improves physical performance during 24 h of active wakefulness. Aviation Space and Environmental Medicine 2004;75(8):666‐72. [PubMed] [Google Scholar]
McLellan 2005 {published data only}
- McLellan TM, Kamimori GH, Voss DM, Bell DG, Cole KG, Johnson D. Caffeine maintains vigilance and improves run times during night operations for special forces. Aviation Space and Environmental Medicine 2005;76(7):647‐54. [PubMed] [Google Scholar]
Muehlbach 1995 {published data only}
- Muehlbach MJ, Walsh JK. The effects of caffeine on simulated night‐shift work and subsequent daytime sleep. Sleep 1995;18(1):22‐9. [DOI] [PubMed] [Google Scholar]
Nickelsen 2002 {published data only}
- Nickelsen T, Samel A, Vejvoda M, Wenzel J, Smith B, Gerzer R. Chronobiotic effects of the melatonin agonist LY 156735 following a simulated 9H time shift: Results of a placebo‐controlled trial. Chronobiology International 2002;19(5):915‐36. [DOI] [PubMed] [Google Scholar]
Porcu 1997 {published data only}
- Porcù S, Bellatreccia A, Ferrara M, Casagrande M. Acutely shifting the sleep‐wake cycle: nighttime sleepiness after diurnal administration of temazepam or placebo. Aviation, Space and Environmental Medicine 1997;68(8):688‐94. [PubMed] [Google Scholar]
Schweitzer 1992 {published data only}
- Schweitzer PK, Muehlbach MJ, Walsh JK. Countermeasures for night work performance deficits: The effect of napping or caffeine on continuous performance at night. Work and Stress 1992;6(4):355‐65. [Google Scholar]
Sharkey 2001 {published data only}
- Sharkey KM, Fogg LF, Eastman CI. Effects of melatonin administration on daytime sleep after simulated night shift work. Journal of Sleep Research 2001;10(3):181‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1993 {published data only}
- Smith AP, Brockman P, Flynn R, Maben A, Thomas M. Investigation of the effects of coffee on alertness and performance during the day and night. Neuropsychobiology 1993;27(4):217‐23. [DOI] [PubMed] [Google Scholar]
Smith 2005 {published data only}
- Smith MR, Lee C, Crowley SJ, Fogg LF, Eastman CI. Morning melatonin has limited benefit as a soporific for daytime sleep after night work. Chronobiology International 2005;22(5):873‐88. [DOI] [PubMed] [Google Scholar]
Walsh 1990 {published data only}
- Walsh JK, Muehlbach MJ, Humm TM, Dickens QS, Sugerman JL, Schweitzer PK. Effect of caffeine on physiological sleep tendency and ability to sustain wakefulness at night. Psychopharmacology (Berl) 1990;101(2):271‐3. [DOI] [PubMed] [Google Scholar]
Walsh 1991 {published data only}
- Walsh JK, Schweitzer PK, Anch AM, Muehlbach MJ, Jenkins NA, Dickins QS. Sleepiness/alertness on a simulated night shift following sleep at home with triazolam. Sleep 1991;14(2):140‐6. [PubMed] [Google Scholar]
Walsh 2004 {published data only}
- Walsh JK, Randazzo AC, Stone KL, Schweitzer PK. Modafinil improves alertness, vigilance, and executive function during simulated night shifts. Sleep 2004;27(3):434‐9. [DOI] [PubMed] [Google Scholar]
Wesensten 2007 {published data only}
- Wesensten NJ, Reichardt RM, Balkin TJ. Ampakine (CX717) effects on performance and alertness during simulated night shift work. Aviation, Space and Environmental Medicine 2007;78(10):937‐43. [DOI] [PubMed] [Google Scholar]
Wright 1997 {published data only}
- Wright KP Jr, Badia P, Myers BL, Plenzler SC. Combination of bright light and caffeine as a countermeasure for impaired alertness and performance during extended sleep deprivation. Journal of Sleep Research 1997;6(1):26‐35. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gill 2006 {published data only}
- Gill M, Haerich P, Westcott K, Godenick KL, Tucker JA. Cognitive performance following modafinil versus placebo in sleep‐deprived emergency physicians: a double‐blind randomized crossover study. Academic Emergency Medicine 2006;13(2):158‐65. [DOI] [PubMed] [Google Scholar]
Walsh 1984 {published data only}
- Walsh JK, Muehlbach MJ, Schweitzer PK. Acute administration of triazolam for the daytime sleep of rotating shift workers. Sleep 1984;7(3):223‐9. [DOI] [PubMed] [Google Scholar]
Wesnes 2005 {published data only}
- Wesnes KA, Niebler GE. Cognitive effects of armodafinil in patients with excessive sleepiness and obstructive sleep apnea/hypopnea, narcolepsy or shift work sleep disorder. Sleep Medicine 2005;6(S2):S211. [Google Scholar]
Wright 2010 {published data only}
- Wright KP, Wyatt JK, Dammerman R, Bogan R. Pharmacotherapy for wakefulness in patients with excessive sleepiness associated with shift work disorder: Evaluation of armodafinil and modafinil. Sleep 2010;33:A188. [Google Scholar]
Additional references
ABS 2004
- Australian Bureau of Statistics. Working Arrangements, Australia, Nov 2003. http://www.abs.gov.au/ausstats/abs@.nsf/ProductsbyReleaseDate/7FBCC870780B923FCA2572E9001830F8?OpenDocument (accessed 22 July 2009).
Ballon 2006
- Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. Journal of Clinical Psychiatry 2006;67(4):554‐66. [DOI] [PubMed] [Google Scholar]
Basner 2005
- Basner RC. Shift‐work sleep disorder ‐‐ the glass is more than half empty. New England Journal of Medicine 2005;353(5):519‐21. [DOI] [PubMed] [Google Scholar]
Buscemi 2006
- Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta‐analysis. BMJ (Clinical research ed.) 2006;332(7538):385‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carskadon 1979
- Carskadon MA, Dement WC. Effects of total sleep loss on sleep tendency. Perceptual and Motor Skills 1979;48(2):495‐506. [DOI] [PubMed] [Google Scholar]
Cho 2000
- Cho BK, Rosenfeldt F, Turina MI, Karp RB, Ferguson TB, Bodnar E, et al. Joint statement on redundant (duplicate) publication by the editors of the undersigned cardiothoracic journals. Annals of Thoracic Surgery 2000;69(2):663. [DOI] [PubMed] [Google Scholar]
Costa 2003
- Costa G. Shift work and occupational medicine: an overview. Occupational Medicine 2003;53(2):83‐8. [DOI] [PubMed] [Google Scholar]
Costa 2004
- Costa G, Åkerstedt T, Nachreiner F, Baltieri F, Carvalhais J, Folkard S, et al. Flexible working hours, health, and well‐being in Europe: some considerations from a SALTSA project. Chronobiology International 2004;21(6):831‐44. [DOI] [PubMed] [Google Scholar]
Cursio 2001
- Cursio G, Casagrande M, Bertini M. Sleepiness: evaluating and quantifying methods. International Journal of Psychophysiology 2001;41(3):251‐63. [DOI] [PubMed] [Google Scholar]
Directive 2003/88/EC
- Directive 2003/88/EC of the European Parliament and of the Council of 4 November 2003 concerning certain aspects of the organisation of working time. http://eur‐lex.europa.eu/legal‐content/EN/ALL/?uri=CELEX:32003L0088.
Drake 2004
- Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep 2004;27(8):1453‐62. [DOI] [PubMed] [Google Scholar]
Driscoll 2007
- Driscoll TR, Grunstein RR, Rogers NL. A systematic review of the neurobehavioural and physiological effects of shiftwork systems. Sleep Medicine Reviews 2007;11(3):179‐94. [DOI] [PubMed] [Google Scholar]
EMA 2011
- European Medicines Agency. Assessment report for modafinil containing medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Modafinil_31/WC500105597.pdf 2011; Vol. EMA/4038/2011.
Erren 2013
- Erren TC, Herbst C, Koch MS, Fritschi L, Foster RG, Driscoll TR, et al. Adaptation of shift work schedules for preventing and treating sleepiness and sleep disturbances caused by shift work. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010639] [DOI] [Google Scholar]
Eurofound 2000
- Eurofound. Third European Survey on Working Conditions 2000. http://www.eurofound.europa.eu/publications/htmlfiles/ef0121.htm (accessed 31 July 2009).
Folkard 2003
- Folkard S, Tucker P. Shift work, safety and productivity. Occupational Medicine 2003;53(2):95‐101. [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. GRADE working group, 2008.
Herbst 2013
- Herbst C, Erren TC, Sallinen M, Fritschi L, Costa G, Driscoll TR, et al. Person‐directed non‐pharmacological interventions for preventing and treating sleepiness and sleep disturbances caused by shift work. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010641] [DOI] [Google Scholar]
Herxheimer 2008
- Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD001520] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Härmä 2002
- Härmä M, Sallinen M, Ranta R, Mutanen P, Müller K. The effect of an irregular shift system on sleepiness at work in train drivers and railway traffic controllers. Journal of Sleep Research 2002;11(2):141‐51. [DOI] [PubMed] [Google Scholar]
Härmä 2008
- Härmä M, Partinen M, Repo R, Sorsa M, Siivonen P. Effects of 6/6 and 4/8 watch systems on sleepiness among bridge officers. Chronobiology International 2008;25(2):413‐23. [DOI] [PubMed] [Google Scholar]
Ker 2010
- Ker K, Edwards PJ, Felix LM, Blackhall K, Roberts I. Caffeine for the prevention of injuries and errors in shift workers. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD008508] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keuroghlian 2012
- Keuroghlian AS, Barry AS, Weiss RD. Circadian dysregulation, zolpidem dependence, and withdrawal seizure in a resident physician performing shift work. American Journal on Addictions/American Academy of Psychiatrists in Alcoholism and Addictions 2012;21(6):576‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krystal 2008
- Krystal AD, Edinger JD. Measuring sleep quality. Sleep Medicine 2008;9(Suppl 1):S10‐7. [DOI] [PubMed] [Google Scholar]
Kumar 2008
- Kumar R. Approved and investigational uses of modafinil : an evidence‐based review. Drugs 2008;68(13):1803‐39. [DOI] [PubMed] [Google Scholar]
Liira 2010
- Liira J, Ruotsalainen JH, Driscoll TR, Rogers NL, Costa G, Sallinen M, et al. Interventions for sleepiness and sleep disturbances caused by shift work. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD008590] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgenthaler 2007
- Morgenthaler TI, Lee‐Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep 2007;30(11):1445‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myrick 2004
- Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical, and post‐marketing surveillance‐‐a review of abuse liability issues. Annals of Clinical Psychiatry 2004;16(2):101‐9. [DOI] [PubMed] [Google Scholar]
OECD 2004
- OECD. Employment Outlook 2004. www.oecd.org/document/62/0,3343,en_2649_33927_31935102_1_1_1_1,00.html (accessed 22 July 2009).
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rogers 2001a
- Rogers NL, Dinges DF. Shiftwork, circadian disruption and consequences. Trends in Evidence Based Psychiatry 2001;6(7):58‐64. [Google Scholar]
Rogers 2001b
- Rogers NL, Szuba MP, Staab JP, Evans DL, Dinges DF. Neuroimmunologic aspects of sleep and sleep loss. Seminars in Clinical Neuropsychiatry 2001;6(4):295‐307. [DOI] [PubMed] [Google Scholar]
Roth 2007
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. Journal of Clinical Sleep Medicine 2007;3(6):595‐602. [PMC free article] [PubMed] [Google Scholar]
Roth 2012
- Roth T. Appropriate therapeutic selection for patients with shift work disorder. Sleep Medicine 2012;13(4):335‐41. [DOI] [PubMed] [Google Scholar]
Sallinen 2003
- Sallinen M, Härma M, Mutanen P, Ranta R, Virkkala J, Müller K. Sleep‐wake rhythm in an irregular shift system. Journal of Sleep Research 2003;12(2):103‐12. [DOI] [PubMed] [Google Scholar]
Sanchez‐Barcelo 2010
- Sánchez‐Barceló EJ, Mediavilla MD, Tan DX, Reiter RJ. Clinical uses of melatonin: evaluation of human trials. Current Medicinal Chemistry 2010;17(19):2070‐95. [DOI] [PubMed] [Google Scholar]
Scott 2000
- Scott AJ. Shift work and health. Primary Care 2000;27(4):1057‐79. [DOI] [PubMed] [Google Scholar]
Shy 2011
- Shy BD, Portelli I, Nelson LS. Emergency medicine residents' use of psychostimulants and sedatives to aid in shift work. American Journal of Emergency Medicine 2011;29(9):1034‐6. [DOI] [PubMed] [Google Scholar]
Straif 2007
- Straif K, Baan R, Grosse Y, Secretan B, Ghissassi FN, Bouvard V, et al. Carcinogenicity of shift‐work, painting, and fire‐fighting. Lancet Oncology 2007;8(12):1065‐6. [DOI] [PubMed] [Google Scholar]
Ursin 2009
- Ursin R, Baste V, Moen BE. Sleep duration and sleep‐related problems in different occupations in the Hordaland Health Study. Scandinavian Journal of Work, Environment and Health 2009;35(3):193‐202. [DOI] [PubMed] [Google Scholar]
US BLS 2005
- US Bureau of Labor Statistics. Workers in flexible and shift schedules in May 2004. www.bls.gov/cps/ (accessed 22 July 2009).
Wright 2013
- Wright KP Jr, Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD). Sleep Medicine Reviews 2013;17(1):41‐54. [DOI] [PubMed] [Google Scholar]
Zawilska 2009
- Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacological Reports 2009;61(3):383‐410. [DOI] [PubMed] [Google Scholar]
Åkerstedt 1990
- Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. International Journal of Neuroscience 1990;52(1‐2):29‐37. [DOI] [PubMed] [Google Scholar]
Åkerstedt 1998
- Åkerstedt T. Is there an optimal sleep‐wake pattern in shift work?. Scandinavian Journal of Work, Environment and Health 1998;24(Suppl 3):18‐27. [PubMed] [Google Scholar]
Åkerstedt 2008
- Åkerstedt T, Ingre M, Broman JE, Kecklund G. Disturbed sleep in shift workers, day workers, and insomniacs. Chronobiology International 2008;25(2):333‐48. [DOI] [PubMed] [Google Scholar]


