Abstract
Epithelial-mesenchymal transition (EMT) is a biological process that transforms epithelial cells into a mesenchymal phenotype, conferring cell migration and invasion capabilities. EMT is involved in the progression and metastasis of colorectal cancer (CRC). Recently, emerging evidence has shown dysregulation of non-coding RNA (ncRNA) was linked to EMT. ncRNAs, including long non-coding RNA (lncRNA), regulate the transcription of downstream target genes (mRNA) through interaction with microRNAs (miRNAs); these are termed competitive endogenous RNA (ceRNA) networks. CeRNA dysregulation-induced EMT, which is linked to the progression and prognosis of CRC, has attracted wide attention. However, understanding the role of the regulation of the ceRNA network in the EMT of CRC remains limited. We discuss the molecular functions of lncRNA, the ceRNA networks related to miRNAs and mRNAs in EMT, as well as EMT transcription factors, such as the zinc finger E-box binding homeobox 1/2 (ZEB1/2), SNAIL, SLUG, and TWIST1/2. In addition, miRNAs and lncRNAs that directly target genes, thereby initiating different signaling pathways to promote EMT in CRC, were summarized. Clarifying the role of these molecules in EMT is critical for understanding molecular mechanisms and exploring the potential therapeutic targets of CRC.
Keywords: Colorectal cancer, Epithelial-mesenchymal transition, lncRNA, Signaling pathway
1. Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors globally [1]. In 2020, there were approximately 1.9 million new cases of CRC and 935,000 related deaths worldwide [2]. The poor prognosis of CRC is attributed to the lack of early diagnosis and effective treatment and to the involvement of unexplored molecular mechanisms [3,4]. In recent years, with the development of high-throughput sequencing technology, dysregulation of non-coding RNAs (ncRNAs) has been found in various phases of cancer progression [4,5]. NcRNAs include long non-coding RNA (lncRNA) —transcripts with a sequence >200 nucleotides without an open reading frame — and circular RNA (circRNA) with a covalently closed loop structure formed by reverse shearing [6]. NcRNAs play an important role in the regulation of miRNA-mediated expression. MiRNAs bind to partially complementary sequences of target gene transcription products, which are known as response elements (MREs). Endogenous RNA with the same MREs can competitively bind to miRNA, which is called competitive endogenous RNA (ceRNA) [7,8]. LncRNAs, cirRNAs, and pseudogenes can compete with miRNA for the same miRNA response elements and reduce miRNA inhibition, increasing the expression of miRNA target genes (mRNA) [7,[9], [10], [11]]. CeRNA is a novel regulatory mechanism in cancer, which is attracting widespread attention and is involved in many biological processes, including epithelial-mesenchymal transition (EMT), proliferation, apoptosis, metastasis, and chemoresistance [12,13]. EMT is a biological process that transforms epithelial cells into a mesenchymal phenotype, conferring cell migration and invasion capabilities. The adhesion of E-cadherin is decreased, and the proliferation, invasion, and metastasis of CRC cells are promoted. The main mechanism is the expression of EMT-related transcription factors and the increased expression of marker proteins E-cadherin and N-cadherin. The complex formed by E-cadherin and β-catenin plays an important role in maintaining cell adhesion and epithelial morphology. When EMT occurs, the stability of the E-cadherin/β-catenin complex decreases [14]. Recent emerging evidence has shown that dysregulation of ceRNA is related to EMT. The ceRNA regulatory network deepens our understanding of the mechanisms of oncogenesis, which is necessary for diagnosis, prognosis markers, new therapeutic drug discovery, and prediction of response to therapy.
In this review, based on the currently available knowledge from the clear and complete experimental evidence (through a literature search in the PubMed database), we discussed the molecular functions of lncRNA and the ceRNA network-related miRNAs and mRNAs involved in the EMT of CRC cells. The review summarizes information on the competitive combination of miRNA and lncRNA directly target genes, thereby initiating different signaling pathways to promote EMT in CRC [[15], [16], [17], [18], [19]]. In addition, the regulation of EMT-associated transcription factors (EMT-TCFs)- zinc finger E-box binding homeobox 1/2 (ZEB1/2), and TWIST1/2 by the ceRNA network axis is discussed [16,[20], [21], [22], [23], [24]].
2. Signaling pathways mediated by the ceRNA network implicated in the EMT of CRC
2.1. Wnt/β-catenin signaling pathway
Studies have shown that Wnt signaling pathway activation is associated with cytokines and TCFs that induce EMT in CRC [[25], [26], [27], [28]]. Following activation, Wnt binds to its membrane receptor FZD and inhibits the degradation of an Axin/APC/GSK3 and -catenin complex, resulting in β-catenin accumulation in the nucleus. When β-Catenin accumulates stably in the cytosol, it entered the nucleus and bound to the LEF/TCF transcription factor family, initiating transcription of downstream target genes [25,[29], [30], [31], [32]]. Through the ceRNA network, LncRNAs can activate the Wnt/β-catenin signaling pathway and promote the EMT of CRC cells by sponging miRNA as well as targeting mRNA (Fig. 1).
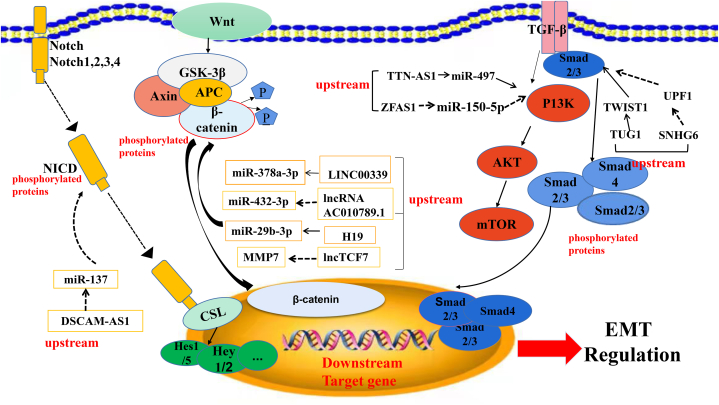
Fig. 1.
Signal pathways related to epithelial–mesenchymal transition (EMT) in colorectal cancer (CRC), mainly include Wnt/β-catenin, transforming growth factor-β (TGF-β), and Notch pathways. These three pathways play a central role in inducing EMT in CRC. Solid line: promoting, Dotted line: inhibiting.
Firstly, long intergenic non-coding RNA 339/miR-378a-3p/mediator complex subunit 19 (LINC00339/miR-378a-3p/MED19) activates the Wnt/β-catenin pathway to induce the EMT and promote the metastasis and invasion of CRC [33]. Upregulation of LINC00339 contributes to cell viability and motility in vitro and tumorigenesis in vivo, as well as reduces the expression of epithelial-cadherin (E-cadherin) and promotes that of vimentin (VIM) and neural-cadherin (N-cadherin). Upregulated expression of LINC00339 in CRC cells (HT-29, HCT-116, LoVo, W480) and tissues has been associated with TNM staging and Duke's staging, and arrested cell cycle transition from G0/G1 to the S phase. The direct interaction between LINC00339, miR-378a-3p, and MED19 mRNA was verified using the dual-luciferase reporter gene and RNA pull-down assays. LINC00339 acts as an oncogenic factor via upregulation of MED19 through competitive binding to miR-340–5p, as well as β-catenin, c-Myc, and cyclin D1 (CCND1). Notably, miR-378a-3p partially reverses these effects [33].
Secondly, AC010789.1, significantly higher in CRC tissues versus normal tissues, plays an important role in β-catenin activation. A tumor xenograft experiment in nude mice demonstrated that silencing AC010789.1 inhibited tumor proliferation and metastasis in vivo while increasing the expression of epithelial marker E-cadherin (epithelial marker) and decreasing that of mesenchymal maker VIM. AC010789.1 could directly interact with miR-432–3p to target ZEB1 as shown using LncBase Predicted v.2 data, plasmid transfection, and gain- or loss-of-function assays [34]. Furthermore, AC010789.1 knockdown reduced the levels of Wnt-responsive genes and β-catenin nuclear signaling, which could be partially restored by co-transfection with a miR-432–3p inhibitor. These findings suggest that silencing of AC010789.1 inhibited CRC progression via miR-432-3p-mediated ZEB1 downregulation and suppression of the Wnt/β-catenin signaling pathway [34].
Thirdly, Ding et al. reported that the H19/miR-29 b-3p/progranulin (PGRN) axis modulated the EMT of CRC cells through Wnt/β-catenin signaling. It has been shown that lncRNA H19 is upregulated in CRC tissues [35]. However, upregulated H19 was negatively correlated with miR-29 b-3p expression. High expression of H19 and low expression of miR-29 b-3p were observed in CRC patients with poor differentiation, T3+T4 stage, and M1 distant metastasis [35]. The relationship between H19 and miR-29 b-3p, miR-29 b-3p, and PGRN, as well as the activation of downstream Wnt signaling have been confirmed using the dual-luciferase reporter gene assay. Moreover, transfection with si-H19, a miR-29 b-3p mimic, or si-PGRN increased E-cadherin levels while decreasing those of mesenchymal markers SNAIL and VIM [35].
Furthermore, the lncRNA transcription factor 7 (lncTCF7) was found to be upregulated in CRC tissues and was correlated with the tumor size, lymph node metastasis, and TNM stage [36]. The overexpression of lncTCF7 stimulated CRC cell migration and invasion in vitro. LncTCF7 induced EMT by activating the Wnt signaling pathway and inhibiting EMT, inhibited the expression of the epithelial marker E-cadherin and increasing three classic mesenchymal markers (N-cadherin, VIM, and SLUG). It also increased the expression of c-Myc, CCND1, and MMP7 in CRC [36]. In summary, the regulation of the ceRNA network, including LINC00339/miR-378a-3p/MED19, AC010789.1/miR-432–3p/ZEB1, H19/miR-29 b-3p/PGRN, lncTCF7/c-Myc, CCND1, and MMP7 was critical in the Wnt-catenin mediated EMT of CRC.
2.2. The transforming growth factor-β (TGF-β) and phosphatidylinositol 3 kinase/protein kinase B/mechanistic target of rapamycin (PI3K/Akt/mTOR) signaling pathway
The TGF-β/Smad pathway is highly activated in the EMT of CRC [37]. Activated TGF-β phosphorylates downstream proteins Smad2 and Smad3 [38]. Further, the phosphorylated Smad2/Smad3/Smad4 complex enters the nucleus and regulates the EMT of CRC cells at the transcriptional level [39,40]. Emerging evidence has shown that multiple lncRNA-mediated ceRNA networks exert their biological functions by regulating the TGF-β/Smad signaling pathway (Fig. 1) [40,41]. Furthermore, small nucleolar RNA host gene 6 (SNHG6) was found to be highly expressed in CRC patients with poor survival [41]. SNHG6 targeted up-frameshift protein 1 (UPF1) to activate the TGF-β/Smad pathway directly or indirectly through an unknown miRNA [41]. A negative correlation between UPF1 with SNHG6 in CRC tissues has been reported whereby the knockdown of SNHG6 could upregulate the expression of UPF1 in RKO cells, as well as decrease that of Smad7, a regulator of downstream proteins in TGF-β pathway [41]. A previous study showed that miR-101–3p could directly bind to SNHG6 and ZEB1, favoring EMT and cancer progression [42,43]. Knockdown of SNHG6 increased E-cadherin expression while decreasing ZEB1, N-cadherin, VIM, SLUG, and SNAIL expression, thus inhibited the EMT of CRC. These results were consistent with previous studies [[41], [42], [43], [44]]. Shen et al. reported that TGF-β upregulated the expression of taurine upregulated 1 (TUG1), and TUG1 knockdown inhibited the EMT of CRC cells. TUG1 is highly expressed in CRC tissues and upregulated after treatment with TGF-β. In addition, it has been revealed that TWIST1 is a downstream target of TUG1. Knockdown of TWIST1 inhibited the EMT processes in CRC cells, as demonstrated by the reduced expression of VIM and increased levels of E-cadherin [45]. In short, the TGF-β/TUG1/TWIST1 signal pathway plays an important role in inducing EMT and promoting the metastasis of CRC. Hence, it could be used as a potential therapeutic target for the treatment of this disease [45].
Non-Smad signaling pathways are also mediated by TGF-β, including P13 K/Akt, and studies have demonstrated that multiple lncRNA-mediated ceRNA networks can activate P13 K/Akt/mTOR signaling to trigger EMT (Fig. 1) [46,47]. Titin-antisense RNA1 (TTN-AS1), as a ceRNA, can activate PI3K/Akt/mTOR signaling through direct binding to miR-497 [48]. TTN-AS1 was highly expressed in CRC tissues, which was associated with lymph node metastasis and an advanced TNM stage [48]. In SW620 cells, Knockdown of TTN-AS1 significantly increased E-cadherin levels while decreasing N-cadherin, VIM, phosphorylated-PI3K (p-PI3K), p-Akt, and p-mTOR levels [48]. Further, lncRNA ZNFX1 antisense RNA1 (ZFAS1) is upregulated in CRC cells in comparison to the corresponding normal colonic epithelial cells [49,50]. On chromosome 20q13, ZFAS1 is overexpressed and has been associated with tumor EMT, as well as growth and metastasis of various types of cancer [51,52]. TNM stage, pT stage, distant metastasis, and poor overall survival were all associated with rising ZFAS1 levels in clinical CRC specimens [49]. Chen et al. reported that SP1-induced ZFAS1 upregulated the expression of vascular endothelial growth factor A (VEGFA) through competitive binding with miR-150–5p [49]. The expression of ZFAS1 was significantly negatively correlated with that of miR-150–5p in CRC tissues. MiR-150–5p targeted both ZFAS1 and VEGFA. ZFAS1 knockdown inhibited the EMT process in CRC by inactivating the VEGFA/VEGFR and downstream Akt/mTOR signaling pathways [49] (Fig. 1). Thus, the SNHG6/UPF1/ZEB1 axis and TUG1/TGF-β/TWIST1/EMT axis are related to the TGF-β/Smad pathway [41,45]. TTN-AS1/miR-497 and ZFAS1/miR-150–5p/VEGFA/VEGFR2 activate PI3K/Akt/mTOR signaling pathway [48,49,53]. The TGF-β/Smad pathway and PI3K/Akt/mTOR have a mutual connection and synergistic effects. However, the role of ceRNA in the TGF-β/Smad, and the PI3K/Akt/mTOR, signal pathways, and EMT induction of CRC has not been thoroughly investigated.
2.3. Notch signaling pathway
The Notch signaling pathway has been implicated in multiple biological processes in the progression of cancer, including EMT. Notch signaling inhibits E-cadherin expression mainly by inducing transcription factors of SNAIL/SLUG. In addition, EMT-related TCFs had the ability to encourage Notch signaling (Fig. 1) [54,55]. Xu et al. reported in Down syndrome, the cell adhesion molecule-antisense RNA-1 (DSCAM-AS1)/miR-137/Notch-1 axis promoted the proliferation and migration of CRC cells. DSCAM-AS1 is highly expressed in CRC tissues and positively correlates with metastasis and the advanced stage of CRC [56]. Downregulation of DSCAM-AS1 could alleviate the EMT process in CRC cell lines. DSCAM-AS1 acted as a ceRNA to bind and inhibit miR-137. Experiments using the dual-luciferase reporter gene and RNA pull-down assays showed that miR-137 can bind to the 3′-untranslated region (3′-UTR) of Notch-1, consequently decreasing its expression. Overexpression of Notch-1 or inhibition of miR-137 can restore DSCAM-AS1 silencing-mediated inhibition of EMT (Fig. 1) [56].
Overall, DSCAM-AS1 acts as a ceRNA to bind and inhibit miR-137. MiR-137 can bind to the 3′-UTR of Notch-1 and increase its expression, thereby modulating EMT and the Notch signaling pathway (Fig. 1) [56]. The LINC00339/miR-378a-3p/MED19 axis [33], AC010789.1/miRNA-432–3p/ZEB1 axis [34], and lncTCF7 activate the Wnt/β-catenin pathway to promote EMT, metastasis, and invasion in CRC. Upregulation of these three lncRNAs increase the protein levels of β-catenin, c-Myc, and CCND1 (Fig. 1) [36]. The SNHG6/UPF1/ZEB1 axis and TUG1/TGF-β/TWIST1/EMT axis are related to the TGF-β/Smad pathway [41,45]. The TTN-AS1 axis could directly interact with miR-497 and blocks the activation of PI3K/Akt/mTOR signaling, and ZFAS1 by inhibiting miR-150-5p-mediated VEGFA/VEGFR2/Akt/mTOR signaling. Consequently, contributing to CRC progression and EMT (Fig. 1).
3. miRNA and lncRNA as ceRNAs directly regulate the expression of ZEB and TWIST proteins
In the ceRNA network, the most important relationship is that between lncRNA and EMT-TCFs. These inducible TCFs may serve as direct targets of miRNA, which is regulated by lncRNA and participates in the EMT of CRC (Fig. 2).
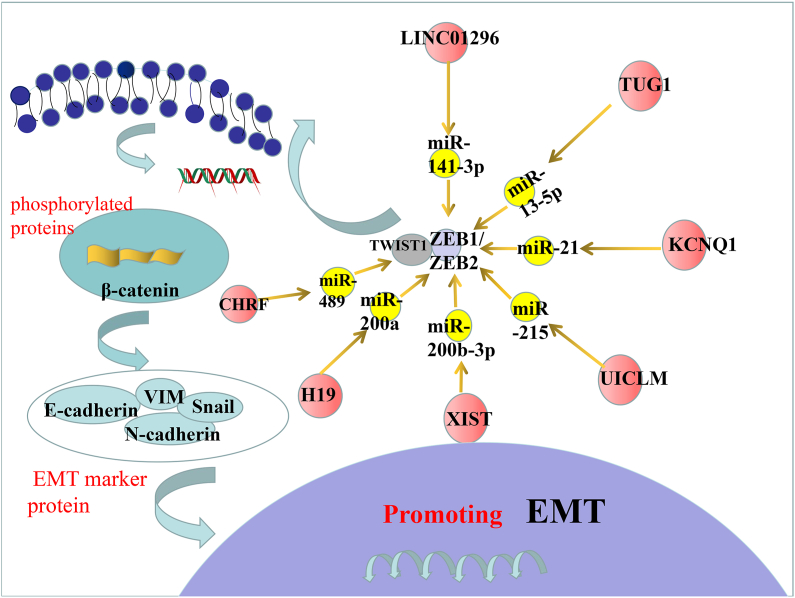
Fig. 2.
The ZEB1/2 and TWIST, as well as β-catenin pathway in promoting EMT. middle: the axis associated with ZEB1/2 and TWIST. Left:β-catenin promote the EMT.
The loss of E-cadherin expression and upregulation of the EMT-TCFs are the main causes of the EMT [16,57]. ZEB1/2, is the most extensively researched TCFs in the ceRNA network, inhibits the expression of E-cadherin in CRC. The TWIST family of proteins is a member of the basic helix-loop-helix (bHLH) family in TCFs, which can reduce the expression of E-cadherin and increase the mesenchymal phenotype protein [58,59]. Through ceRNA networks, these TCFs can regulate the expression and participate in the EMT process, which is associated with cell migration and invasion as well as poor survival. In this section, we discussed all miRNAs and lncRNAs that target E-cadherin, as well as ZEB and TWIST proteins [14,[58], [59], [60], [61]]. We illustrateds the regulatory axes of miRNA, lncRNA, and ceRNA (as direct regulators of ZEB and TWIST proteins) in the EMT (Fig. 2) .
3.1. LINC01296/miR-141–3p/ZEB1-ZEB2 axis
Sun et al. [61] found that LINC01296 was highly expressed in CRC cell lines (SW480 and HCT116) and clinical samples. LINC01296 overexpression promoted cell migration, invasion, and EMT, which is consistent with other cancers such as hepatocellular carcinoma [62], pancreatic ductal adenocarcinoma [63], and ovarian cancer [64]. The Cancer Genome Atlas database revealed that the miR-141–3p, which is significantly negatively correlated with LINC01296, could be the target of LINC01296 [61]. Through sponging of miR-141–3p, LINC01296 positively regulated the expression of EMT-related molecules (e.g., ZEB1 and ZEB2) and promoted CRC metastasis by activating the EMT [61]. The ZEB1 and ZEB2 proteins are involved in different biological events and can stimulate EMT. In conclusion, the LINC01296/miR-141–3p/ZEB1-ZEB2 axis is important in the promotion of EMT in CRC [61].
3.2. TUG1/miR-13–5p/ZEB2 axis
TUG1 is a 7.1 kb long non-coding RNA (lncRNA) that is found on human chromosome 22q12.2 [65]. The mouse retinal photoreceptors were the place TUG1 was first identified [66]. In addition to acting as a tumor promoter in CRC, it has been known to participate in and regulate the biological processes of many human diseases [65]. TUG1 expression was significantly higher in CRC tissues and regulated the expression of miR-138–5p and upregulated that of ZEB2 in LoVo cells, as well as promoted the occurrence and metastasis of CRC [67]. Downregulation of TUG1 decreased the expression of β-catenin, c-Myc, CCND1, VIM, SNAIL, and N-cadherin, while increased the expression of BCL-2 associated X (Bax), caspase-3 (CASP3), E-cadherin, and zona occludens 1 (ZO-1) [67]. MiR-144–3p bound to the 3′-UTR of ZEB1 and ZEB2 and inhibited their expression level, thereby impairing the EMT in CRC [68]. The inhibition of EMT caused by the upregulation of TUG1 could be reversed by downregulating of ZEB2 or overexpressing of miR-144–3p [67]. These findings indicated that the downregulation of TUG1 significantly inhibited ZEB2 expression and hindered the EMT in CRC. In addition, it has been confirmed that TUG1 inhibited the EMT in other malignant tumors (e.g., bladder cancer) by regulating the expression of ZEB2 [69]. Therefore, the role of the lncRNA TUG1/miR-138–5p/ZEB2 axis in EMT could offer a new direction for research and may provide a new therapeutic target in patients with CRC [67].
3.3. KCNQ1 overlapping transcript 1 (KCNQ1OT1)/miR-217/ZEB1 axis
KCNQ1 paired strand/antisense transcript 1 (KCNQ1OT1), also known as KCNQ1 overlapping transcript 1, is a 91-kb unspliced lncRNA on chromosome 11p15.5. KCNQ1OT1 regulates epigenetic modifications and is associated with the pathogenicity of various human diseases [70]. In CRC tissues and cell lines (DLD-1 and SW480), KCNQ1OT1 is significantly upregulated [71]. KCNQ1OT1 knockdown inhibits CRC cells proliferation and alters EMT-labeled mRNA and protein levels (i.e., increased E-cadherin, and decreased β-catenin, N-cadherin, and VIM) [71]. Simultaneously, silencing of KCNQ1OT1 significantly reduced the ZEB1 expression levels. The binding site of KCNQ1OT1 is found in miR-217 [71], Furthermore, ZEB1 may be a target of miR-217. The expression of ZEB1 is positively correlated with KCNQ1OT1, but negatively correlates with miR −217. Bioinformatics, qRT-PCR, western blotting, gain- and loss-of-function, and luciferase reporter gene analyses all confirmed the direct interaction between miR-217, KCNQ1OT1, and ZEB1 [71]. The facilitating effects of the KCNQ1OT1/miR-217/ZEB1 axis has been shown to promote EMT and cell migration in CRC [71].
3.4. Upregulation of the CRC liver metastasis (UICLM)/miR-215/ZEB2 axis
According to Chen et al. [72], the UICLM/miR-215/ZEB2 axis facilitated EMT in CRC. The lncRNA UICLM was found to be significantly upregulated in tumor tissue with liver metastases and it was linked to tumor size and clinical stage IV [72]. UICLM Knockdown reduced the migration and EMT of SW620 and DLD-1 cells. These effects were demonstrated by lower levels of mesenchymal markers (N-cadherin, VIM, SLUG) and higher levels of epithelial markers (E-cadherin, α-catenin, β-catenin) [72]. UICLM acts as a ceRNA to sponge miR-215, reduce mRNA and protein levels of ZEB2 in CRC cells, regulate the EMT, to ultimately influence cell invasion and metastasis in CRC [72].
3.5. LncRNA X inactive specific transcript (XIST)/miR-200 b-3p/ZEB1 axis
The lncRNA XIST can directly bind miR-200 b-3p to its target ZEB1. LncRNA XIST is upregulated in CRC cell lines compared with the normal colon epithelial cell line CCD-112CoN and high expression of the lncRNA XIST is positively correlated with distant metastasis, mainly in stages III– of the TNM staging [73]. Knockdown of lncRNA XIST inhibited cell proliferation and EMT. Immunofluorescence experiments confirmed that the knockdown of lncRNA XIST in SW620 and HCT116 cells increased E-cadherin expression while decreasing N-cadherin [73]. The ectopic expression of miR-200 b-3p and knockdown of lncRNA XIST significantly reduced protein levels of ZEB1 [73]. In contrast, inhibiting miR-200 b-3p increased the protein levels of ZEB1 in CRC cells. Moreover, the tumor suppressive effect of lncRNA XIST knockdown in cancer cells could be reversed by the re-expression of ZEB1. Ectopic expression of ZEB1 or inhibition of miR-200 b-3p can restore ZEB1 mRNA levels, which are decreased by lncRNA XIST knockdown [73].
3.6. LncRNA cardiac hypertrophy-related factor (CHRF)/miR-489/TWIST1 axis
Tao et al. [74] reported that the CHRF/miR-489/TWIST signal transduction axis was crucial to the EMT of CRC. High expression of TWIST1, a member of the TWIST family, induced EMT by upregulating proteins with a mesenchymal phenotype [74]. Luciferase activity analysis revealed that TWIST1 is a direct downstream target of miR-489 in CRC. In addition, a negative correlation between miR-489 and TWIST1 mRNA expression was observed in CRC tissues. The miR-489 was inhibited during the carcinogenesis of CRC [74]. Overexpression of miR-489 led to the upregulation of the epithelial marker E-cadherin, downregulation of VIM, and morphological changes in epithelial-like cells. Therefore, miR-489 may inhibit CRC cell metastasis and EMT by targeting TWIST1 [74]. The levels of lncRNA cardiac hypertrophy-related factor (lncRNA CHRF) were significantly higher in CRC tissues [74]. Notably, lncRNA CHRF reversely regulated the expression of miR-489 and promoted that of TWIST1, as well as the progression of EMT in CRC cells. In summary, the down-regulation of miR-489 induced by lncRNA CHRF may regulate EMT by targeting TWIST1, thereby promoting the migration and invasion of CRC cells [74].
3.7. The H19/miR-200a/ZEB1-ZEB2 axis
A study demonstrated that H19 competitively bound to miR-200a and inhibited β-catenin, which is involved in the pathogenesis of CRC [75]. H19 is highly expressed in CRC cell lines (HCT116 and SW480 cells) and in tissues. H19 knockdown inhibited the proliferation of the two aforementioned cell lines. Using online tools to screen potential miRNAs that bind to H19, researchers discovered that miR-200a may interact with H19, while β-catenin was identified as a candidate target gene for miR-200a [75]. Verification studies using gain- or loss-of-function assays and luciferase reporter gene detection revealed that miR-200a overexpression significantly reduced β-catenin expression levels, whereas overexpression of H19 exerted the opposite effect [75]. Another study reported that overexpression of H19 inhibited miR-200a function and miR-200a target genes ZEB1 and ZEB2, promoting EMT progression in CRC [76].
4. Conclusion
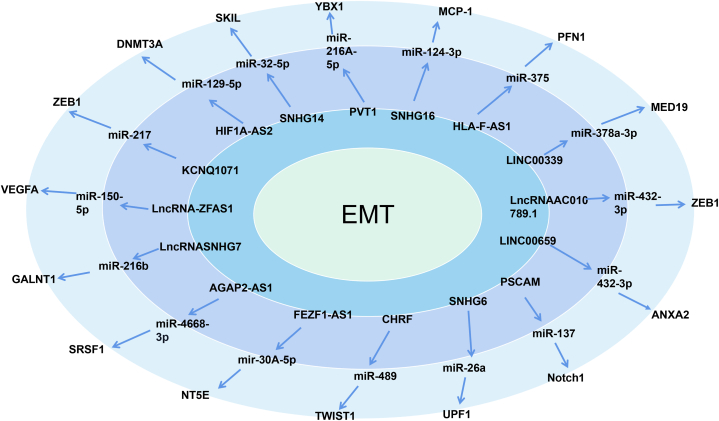
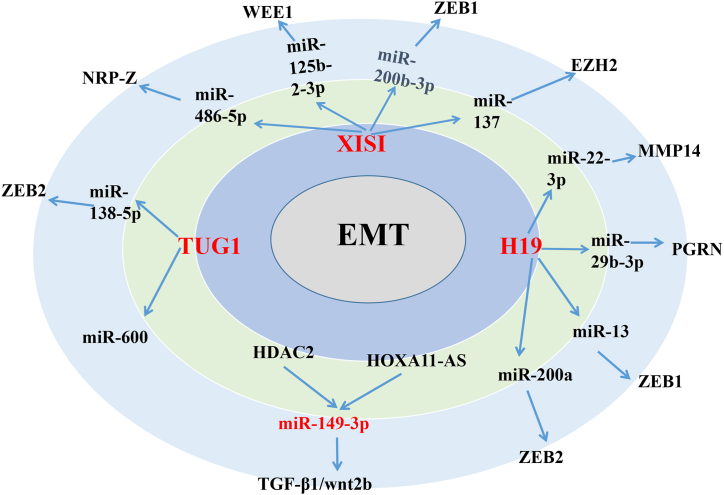
In this review, we summarized the currently available literature regarding the ceRNA network in CRC. Based on the available data, we constructed a ceRNA model consisting of 21 lncRNAs, 25 miRNAs, and 27 mRNAs, which are involved in EMT (Fig. 3). Fig. 3 illustrates that, in the process of inducing EMT, most lncRNA-miRNAs and their protein targets have a one-to-one regulatory relationship, such as PVT1, KCNQ1071, lncRNA-ZFAS1, FEZF1 antisense RNA 1 (FEZF1-AS1), SNHG6, HLA-F-AS1, LINC00339, LINC00659, and SNHG16, However, three lncRNAs (i.e., H19, lncRNA XIST, and TUG1) share multiple miRNA binding sites, which regulate the expression of multiple proteins (Fig. 4). Several studies have found that H19 can competitively bind to multiple miRNAs (e.g., miR-22–3p, miR-29 b-3p, miR-138, and miR-200a), regulate the expression of multiple mRNAs, (e.g., matrix metallopeptidase 14, PGRN, ZEB1, and ZEB2), and induce the EMT in CRC [35,[76], [77], [78]]. Previously reported lncRNA XIST-mediate ceRNA networks that promote EMT in CRC cells are lncRNA XIST/miR-125b-2-3p/WEE1 [78], XIST/miR-486–5p/Neuropilin 2 (XIST/miR-486–5p/NRP2) [79], lncRNA XIST/miR-137/enhancer of zeste homolog 2 (XIST/miR-137/EZH2) [80], and lncRNA XIST/miR-200 b-3p/ZEB1 [73]. In addition, TUG1 can competitively bind with miR-600 and miR-138–5p to regulate the expression of KIAA1199 and ZEB2, thereby promoting the occurrence of EMT in CRC [67,81]. We hypothesized that several lncRNAs with multiple binding sites may be associated with RNA-induced silencing complexes and contain various additional miRNA binding sites. In addition, they are highly expressed in CRC tissues and cells with high metastatic potential and are closely related to the metastasis of CRC. These studies strongly confirmed the key role of these three lncRNAs in regulating the EMT of CRC cells. Targeting these three lncRNAs may exert an important effect on the treatment of patients with CRC. Therefore, further investigation and research in this field are warranted.
Fig. 3.
The competing endogenous RNA (ceRNA) network is composed of long non-coding RNA (lncRNA), microRNA (miRNA), mRNA that regulate epithelial transition (EMT) (adapted from evidence in the literature). From the inner circle to the outer circle: innermost circle, EMT; second circle, lncRNA; third circle, miRNA; and fourth circle, mRNA. The red mark indicates that lncRNA can regulate multiple miRNAs/multiple mRNAs.
Fig. 4.
Multi-targets among ceRNA network in EMT of CRC. H19, lncRNA XIST, and TUG1 share multiple miRNA binding sites, which regulate the expression of multiple proteins. The HDAC2, HOXA11-AS acts on the same target and regulates the same downstream target genes, promoting the occurrence of EMT in CRC.
5. Future perspectives
The regulation of ceRNA in EMT is closely related to the progression and prognosis of CRC. However, a full understanding of the regulation of the ceRNA network in CRC remains limited. Determination of the contribution of ceRNAs in the development of CRC is critical for identifying new therapeutic targets and diagnostic markers for CRC. Additional research and an in-depth understanding of the EMT process are necessary.
Ethics approval and consent to participate
Not applicable.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Ph.D Xiaoling Gao was supported by National Natural Science Foundation of China [grant no.81860372]; Innovative Research Group Project of the National Natural Science Foundation of China [82260421].
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chan S.C.H., Liang J.Q. Advances in tests for colorectal cancer screening and diagnosis. Expert Rev. Mol. Diagn. 2022;22(4):449–460. doi: 10.1080/14737159.2022.2065197. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Dariya B., Aliya S., Merchant N., Alam A., Nagaraju G.P. Colorectal cancer biology, diagnosis, and therapeutic approaches. Crit. Rev. Oncog. 2020;25(2):71–94. doi: 10.1615/CritRevOncog.2020035067. [DOI] [PubMed] [Google Scholar]
- 4.Ogunwobi O.O., Mahmood F., Akingboye A. Biomarkers in colorectal cancer: current research and future Prospects. Int. J. Mol. Sci. 2020;21(15) doi: 10.3390/ijms21155311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkle M., El-Daly S.M., Fabbri M., Calin G.A. Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov. 2021;20(8):629–651. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q., Feng Z., Shi S., Zhang Y., Ren S. Comprehensive analysis of lncRNA-associated ceRNA network reveals the novel potential of lncRNA, miRNA and mRNA biomarkers in human rectosigmoid junction cancer. Oncol. Lett. 2021;21(2):144. doi: 10.3892/ol.2020.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin W., Liu H., Tang Y., Wei Y., Wei W., Zhang L., et al. The development and controversy of competitive endogenous RNA hypothesis in non-coding genes. Mol. Cell. Biochem. 2021;476(1):109–123. doi: 10.1007/s11010-020-03889-2. [DOI] [PubMed] [Google Scholar]
- 9.May J.M., Bylicky M., Chopra S., Coleman C.N., Aryankalayil M.J. Long and short non-coding RNA and radiation response: a review. Transl. Res. 2021;233:162–179. doi: 10.1016/j.trsl.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Liu X., Lin C., Jia X., Zhu H., Song J., et al. Noncoding RNAs regulate alternative splicing in Cancer. J. Exp. Clin. Cancer Res. 2021;40(1):11. doi: 10.1186/s13046-020-01798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020;21(8):475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- 12.Si L., Yang Z., Ding L., Zhang D. Regulatory effects of lncRNAs and miRNAs on the crosstalk between autophagy and EMT in cancer: a new era for cancer treatment. J. Cancer Res. Clin. Oncol. 2022;148(3):547–564. doi: 10.1007/s00432-021-03892-0. [DOI] [PubMed] [Google Scholar]
- 13.Hussen B.M., Shoorei H., Mohaqiq M., Dinger M.E., Hidayat H.J., Taheri M., et al. The impact of non-coding RNAs in the epithelial to mesenchymal transition. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.665199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chattopadhyay I., Ambati R., Gundamaraju R. Exploring the crosstalk between inflammation and epithelial-mesenchymal transition in cancer. Mediat. Inflamm. 2021 doi: 10.1155/2021/9918379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L., Cho K.B., Li Y., Tao G., Xie Z., Guo B. Long noncoding RNA (lncRNA)-Mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int. J. Mol. Sci. 2019;20(22) doi: 10.3390/ijms20225758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landeros N., Santoro P.M., Carrasco-Avino G., Corvalan A.H. Competing endogenous RNA networks in the epithelial to mesenchymal transition in diffuse-type of gastric cancer. Cancers. 2020;12(10) doi: 10.3390/cancers12102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C., Hu C., Li J., Jiang L., Zhao C. Identification of epithelial-mesenchymal transition-related lncRNAs that associated with the prognosis and immune microenvironment in colorectal cancer. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.633951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajamäki K., Taira A., Katainen R., Välimäki N., Kuosmanen A., Plaketti R.M., et al. Genetic and epigenetic characteristics of inflammatory bowel disease-associated colorectal cancer. Gastroenterology. 2021;161(2):592–607. doi: 10.1053/j.gastro.2021.04.042. [DOI] [PubMed] [Google Scholar]
- 19.Usman S., Waseem N.H., Nguyen T.K.N., Mohsin S., Jamal A., Teh M.T., et al. Vimentin is at the heart of epithelial mesenchymal transition (EMT) mediated metastasis. Cancers. 2021;13(19) doi: 10.3390/cancers13194985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frey P., Devisme A., Schrempp M., Andrieux G., Boerries M., Hecht A. Canonical BMP signaling executes epithelial-mesenchymal transition downstream of SNAIL1. Cancers. 2020;12(4) doi: 10.3390/cancers12041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vedagiri D., Gupta D., Mishra A., Krishna G., Bhaskar M., Sah V., et al. Retinoic acid-inducible gene I-like receptors activate snail to limit RNA viral infections. J. Virol. 2021;95(21) doi: 10.1128/jvi.01216-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y., Feng M., Bai L., Liao W., Zhou K., Zhang M., et al. Comprehensive analysis of EMT-related genes and lncRNAs in the prognosis, immunity, and drug treatment of colorectal cancer. J. Transl. Med. 2021;19(1):391. doi: 10.1186/s12967-021-03065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo J., Ha J., Kang E., Cho S. The role of epithelial-mesenchymal transition-regulating transcription factors in anti-cancer drug resistance. Arch Pharm. Res. (Seoul) 2021;44(3):281–292. doi: 10.1007/s12272-021-01321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng X., Dai F., Feng L., Zou H., Feng L., Xu M. Communication between epithelial-mesenchymal Plasticity and cancer stem cells: new insights into cancer progression. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.617597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheriyamundath S., Ben-Ze'ev A. Wnt/β-Catenin target genes in colon cancer metastasis: the special case of L1CAM. Cancers. 2020;12(11) doi: 10.3390/cancers12113444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji Y., Lv J., Sun D., Huang Y. Therapeutic strategies targeting Wnt/β-catenin signaling for colorectal cancer (Review) Int. J. Mol. Med. 2022;49(1) doi: 10.3892/ijmm.2021.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong Z.A., Michalski M.N., Stevens P.D., Sall E.A., Williams B.O. Regulation of Wnt receptor activity: implications for therapeutic development in colon cancer. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caspi M., Wittenstein A., Kazelnik M., Shor-Nareznoy Y., Rosin-Arbesfeld R. Therapeutic targeting of the oncogenic Wnt signaling pathway for treating colorectal cancer and other colonic disorders. Adv. Drug Deliv. Rev. 2021;169:118–136. doi: 10.1016/j.addr.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Qi J., Yu Y., Akilli Öztürk Ö., Holland J.D., Besser D., Fritzmann J., et al. New Wnt/β-catenin target genes promote experimental metastasis and migration of colorectal cancer cells through different signals. Gut. 2016;65(10):1690–1701. doi: 10.1136/gutjnl-2014-307900. [DOI] [PubMed] [Google Scholar]
- 30.Vu T., Datta P.K. Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers. 2017;9(12) doi: 10.3390/cancers9120171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J., Liu D., Sun X., Yang K., Yao J., Cheng C., et al. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/β-catenin signaling via transactivation of GSK-3β and Axin2 expression. Cell Death Dis. 2019;10(1):26. doi: 10.1038/s41419-018-1263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tewari D., Bawari S., Sharma S., DeLiberto L.K., Bishayee A. Targeting the crosstalk between canonical Wnt/β-catenin and inflammatory signaling cascades: a novel strategy for cancer prevention and therapy. Pharmacol. Ther. 2021;227 doi: 10.1016/j.pharmthera.2021.107876. [DOI] [PubMed] [Google Scholar]
- 33.Ye H., Li W., Wu K., Liu Y., Lv Y., Zhu Y., et al. The SP1-induced long noncoding RNA, LINC00339, promotes tumorigenesis in colorectal cancer via the miR-378a-3p/MED19 Axis. OncoTargets Ther. 2020;13:11711–11724. doi: 10.2147/ott.s277254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duan W., Kong X., Li J., Li P., Zhao Y., Liu T., et al. LncRNA AC010789.1 promotes colorectal cancer progression by targeting MicroRNA-432-3p/ZEB1 Axis and the wnt/β-catenin signaling pathway. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.565355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding D., Li C., Zhao T., Li D., Yang L., Zhang B. LncRNA H19/miR-29b-3p/PGRN Axis promoted epithelial-mesenchymal transition of colorectal cancer cells by acting on Wnt signaling. Mol. Cell. 2018;41(5):423–435. doi: 10.14348/molcells.2018.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li T., Zhu J., Wang X., Chen G., Sun L., Zuo S., et al. Long non-coding RNA lncTCF7 activates the Wnt/β-catenin pathway to promote metastasis and invasion in colorectal cancer. Oncol. Lett. 2017;14(6):7384–7390. doi: 10.3892/ol.2017.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunen D., Willems S.M., Kellner U., Midgley R., Simon I., Bernards R. TGF-β: an emerging player in drug resistance. Cell Cycle. 2013;12(18):2960–2968. doi: 10.4161/cc.26034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai J., Xia L., Li J., Ni S., Song H., Wu X. Tumor-associated macrophages derived TGF-β‒induced epithelial to mesenchymal transition in colorectal cancer cells through smad2,3-4/snail signaling pathway. Cancer Res. Treat. 2019;51(1):252–266. doi: 10.4143/crt.2017.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou B., Li W., Xia P., Zhao F., Liu Z., Zeng Q., et al. LHPP suppresses colorectal cancer cell migration and invasion in vitro and in vivo by inhibiting Smad3 phosphorylation in the TGF-β pathway. Cell Death Dis. 2021;7(1):273. doi: 10.1038/s41420-021-00657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malki A., ElRuz R.A., Gupta I., Allouch A., Vranic S., Al Moustafa A.E. Molecular mechanisms of colon cancer progression and metastasis: recent insights and advancements. Int. J. Mol. Sci. 2020;22(1) doi: 10.3390/ijms22010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X., Lai Q., He J., Li Q., Ding J., Lan Z., et al. LncRNA SNHG6 promotes proliferation, invasion and migration in colorectal cancer cells by activating TGF-β/Smad signaling pathway via targeting UPF1 and inducing EMT via regulation of ZEB1. Int. J. Med. Sci. 2019;16(1):51–59. doi: 10.7150/ijms.27359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan K., Tian J., Shi W., Xia H., Zhu Y. LncRNA SNHG6 is associated with poor prognosis of gastric cancer and promotes cell proliferation and EMT through epigenetically silencing p27 and sponging miR-101-3p. Cell. Physiol. Biochem. 2017;42(3):999–1012. doi: 10.1159/000478682. [DOI] [PubMed] [Google Scholar]
- 43.Chang L., Yuan Y., Li C., Guo T., Qi H., Xiao Y., et al. Upregulation of SNHG6 regulates ZEB1 expression by competitively binding miR-101-3p and interacting with UPF1 in hepatocellular carcinoma. Cancer Lett. 2016;383(2):183–194. doi: 10.1016/j.canlet.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 44.Chen B., Wang H., Li D., Lin X., Ma Z., Zeng Y. Up-frameshift protein 1 promotes tumor progression by regulating apoptosis and epithelial-mesenchymal transition of colorectal cancer. Technol. Cancer Res. Treat. 2021;20 doi: 10.1177/15330338211064438. 15330338211064438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen X., Hu X., Mao J., Wu Y., Liu H., Shen J., et al. The long noncoding RNA TUG1 is required for TGF-β/TWIST1/EMT-mediated metastasis in colorectal cancer cells. Cell Death Dis. 2020;11(1):65. doi: 10.1038/s41419-020-2254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang W.J., Luo C., Huang C., Pu F.Q., Zhu J.F., Zhu Z.M. PI3K/Akt/GSK-3β signal pathway is involved in P2X7 receptor-induced proliferation and EMT of colorectal cancer cells. Eur. J. Pharmacol. 2021;899 doi: 10.1016/j.ejphar.2021.174041. [DOI] [PubMed] [Google Scholar]
- 47.Alizadeh A., Jebelli A., Baradaran B., Amini M., Oroojalian F., Hashemzaei M., et al. Crosstalk between long non-coding RNA DLX6-AS1, microRNAs and signaling pathways: a pivotal molecular mechanism in human cancers. Gene. 2021;769 doi: 10.1016/j.gene.2020.145224. [DOI] [PubMed] [Google Scholar]
- 48.Cui Z., Han B., Wang X., Li Z., Wang J., Lv Y. Long non-coding RNA TTN-AS1 promotes the proliferation and invasion of colorectal cancer cells by activating miR-497-mediated PI3K/Akt/mTOR signaling. OncoTargets Ther. 2019;12:11531–11539. doi: 10.2147/ott.s229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X., Zeng K., Xu M., Hu X., Liu X., Xu T., et al. SP1-induced lncRNA-ZFAS1 contributes to colorectal cancer progression via the miR-150-5p/VEGFA axis. Cell Death Dis. 2018;9(10):982. doi: 10.1038/s41419-018-0962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang C., Zan J., Yue B., Liu C., He C., Yan D. Long non-coding ribonucleic acid zinc finger antisense 1 promotes the progression of colonic cancer by modulating ZEB1 expression. J. Gastroenterol. Hepatol. 2017;32(6):1204–1211. doi: 10.1111/jgh.13646. [DOI] [PubMed] [Google Scholar]
- 51.Zhou H., Wang F., Chen H., Tan Q., Qiu S., Chen S., et al. Increased expression of long-noncoding RNA ZFAS1 is associated with epithelial-mesenchymal transition of gastric cancer. Aging (Albany NY) 2016;8(9):2023–2038. doi: 10.18632/aging.101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y., Gao X., Li P., Song L., Shi L. LncRNA ZFAS1, as a poor prognostic indicator, promotes cell proliferation and epithelial-mesenchymal transition in endometrial carcinoma. Pers. Med. 2021;18(1):43–53. doi: 10.2217/pme-2020-0014. [DOI] [PubMed] [Google Scholar]
- 53.Chen X., Xu X., Pan B., Zeng K., Xu M., Liu X., et al. miR-150-5p suppresses tumor progression by targeting VEGFA in colorectal cancer. Aging (Albany NY) 2018;10(11):3421–3437. doi: 10.18632/aging.101656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Francesco E.M., Maggiolini M., Musti A.M. Crosstalk between Notch, HIF-1α and GPER in breast cancer EMT. Int. J. Mol. Sci. 2018;19(7) doi: 10.3390/ijms19072011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Misiorek J.O., Przybyszewska-Podstawka A., Kałafut J., Paziewska B., Rolle K., Rivero-Müller A., et al. Context matters: NOTCH signatures and pathway in cancer progression and metastasis. Cells. 2021;10(1) doi: 10.3390/cells10010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J., Wu G., Zhao Y., Han Y., Zhang S., Li C., et al. Long noncoding RNA DSCAM-AS1 facilitates colorectal cancer cell proliferation and migration via miR-137/notch1 Axis. J. Cancer. 2020;11(22):6623–6632. doi: 10.7150/jca.46562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashrafizadeh M., Ang H.L., Moghadam E.R., Mohammadi S., Zarrin V., Hushmandi K., et al. MicroRNAs and their influence on the ZEB family: mechanistic aspects and therapeutic applications in cancer therapy. Biomolecules. 2020;10(7) doi: 10.3390/biom10071040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romero S., Musleh M., Bustamante M., Stambuk J., Pisano R., Lanzarini E., et al. Polymorphisms in TWIST1 and ZEB1 are associated with prognosis of gastric cancer patients. Anticancer Res. 2018;38(7):3871–3877. doi: 10.21873/anticanres.12671. [DOI] [PubMed] [Google Scholar]
- 59.Kang E., Seo J., Yoon H., Cho S. The Post-translational regulation of epithelial-mesenchymal transition-inducing transcription factors in cancer metastasis. Int. J. Mol. Sci. 2021;22(7) doi: 10.3390/ijms22073591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Expósito-Villén A., Ea A., Franco D. Functional role of non-coding RNAs during epithelial-to-mesenchymal transition. Noncoding RNA. 2018;4(2) doi: 10.3390/ncrna4020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Z., Shao B., Liu Z., Dang Q., Guo Y., Chen C., et al. LINC01296/miR-141-3p/ZEB1-ZEB2 axis promotes tumor metastasis via enhancing epithelial-mesenchymal transition process. J. Cancer. 2021;12(9):2723–2734. doi: 10.7150/jca.55626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wan Y., Li M., Huang P. LINC01296 promotes proliferation, migration, and invasion of HCC cells by targeting miR-122-5P and modulating EMT activity. OncoTargets Ther. 2019;12:2193–2203. doi: 10.2147/ott.s197338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan Q., Zhang Y., Feng L., Jiang Y. Upregulated long noncoding RNA LINC01296 indicates a dismal prognosis for pancreatic ductal adenocarcinoma and promotes cell metastatic properties by affecting EMT. J. Cell. Biochem. 2019;120(1):552–561. doi: 10.1002/jcb.27411. [DOI] [PubMed] [Google Scholar]
- 64.Xu H., Zheng J.F., Hou C.Z., Li Y., Liu P.S. Up-regulation of long intergenic noncoding RNA 01296 in ovarian cancer impacts invasion, apoptosis and cell cycle distribution via regulating EMT. Cell. Signal. 2019;62 doi: 10.1016/j.cellsig.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 65.Li Z., Shen J., Chan M., Wu W. TUG1: a pivotal oncogenic long non-coding RNA of human cancers. Cell Prolif. 2016;49(4):471–475. doi: 10.1111/cpr.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young T.L., Matsuda T., Cepko C.L. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr. Biol.: CB. 2005;15(6):501–512. doi: 10.1016/j.cub.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 67.Yan Z., Bi M., Zhang Q., Song Y., Hong S. LncRNA TUG1 promotes the progression of colorectal cancer via the miR-138-5p/ZEB2 axis. Biosci. Rep. 2020;40(6) doi: 10.1042/bsr20201025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li T., Tang C., Huang Z., Yang L., Dai H., Tang B., et al. miR-144-3p inhibited the growth, metastasis and epithelial-mesenchymal transition of colorectal adenocarcinoma by targeting ZEB1/2. Aging (Albany NY) 2021;13(13):17349–17369. doi: 10.18632/aging.203225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Q., Liu H., Cheng H., Li Y., Li X., Zhu C. Downregulation of long noncoding RNA TUG1 inhibits proliferation and induces apoptosis through the TUG1/miR-142/ZEB2 axis in bladder cancer cells. OncoTargets Ther. 2017;10:2461–2471. doi: 10.2147/ott.s124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cagle P., Qi Q., Niture S., Kumar D. KCNQ1OT1: an oncogenic long noncoding RNA. Biomolecules. 2021;11(11) doi: 10.3390/biom11111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bian Y., Gao G., Zhang Q., Qian H., Yu L., Yao N., et al. KCNQ1OT1/miR-217/ZEB1 feedback loop facilitates cell migration and epithelial-mesenchymal transition in colorectal cancer. Cancer Biol. Ther. 2019;20(6):886–896. doi: 10.1080/15384047.2019.1579959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen D.L., Lu Y.X., Zhang J.X., Wei X.L., Wang F., Zeng Z.L., et al. Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression. Theranostics. 2017;7(19):4836–4849. doi: 10.7150/thno.20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen D.L., Chen L.Z., Lu Y.X., Zhang D.S., Zeng Z.L., Pan Z.Z., et al. Long noncoding RNA XIST expedites metastasis and modulates epithelial-mesenchymal transition in colorectal cancer. Cell Death Dis. 2017;8(8) doi: 10.1038/cddis.2017.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tao Y., Han T., Zhang T., Ma C., Sun C. LncRNA CHRF-induced miR-489 loss promotes metastasis of colorectal cancer via TWIST1/EMT signaling pathway. Oncotarget. 2017;8(22):36410–36422. doi: 10.18632/oncotarget.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang W., Ning N., Jin X. The lncRNA H19 promotes cell proliferation by competitively binding to miR-200a and derepressing β-catenin expression in colorectal cancer. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/2767484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang W.C., Fu W.M., Wong C.W., Wang Y., Wang W.M., Hu G.X., et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6(26):22513–22525. doi: 10.18632/oncotarget.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu X.T., Xing W., Zhao R.S., Tan Y., Wu X.F., Ao L.Q., et al. HDAC2 inhibits EMT-mediated cancer metastasis by downregulating the long noncoding RNA H19 in colorectal cancer. J. Exp. Clin. Cancer Res. 2020;39(1):270. doi: 10.1186/s13046-020-01783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng Z.L., Lu J.H., Wang Y., Sheng H., Wang Y.N., Chen Z.H., et al. The lncRNA XIST/miR-125b-2-3p axis modulates cell proliferation and chemotherapeutic sensitivity via targeting Wee1 in colorectal cancer. Cancer Med. 2021;10(7):2423–2441. doi: 10.1002/cam4.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu A., Liu L., Lu H. LncRNA XIST facilitates proliferation and epithelial-mesenchymal transition of colorectal cancer cells through targeting miR-486-5p and promoting neuropilin-2. J. Cell. Physiol. 2019;234(8):13747–13761. doi: 10.1002/jcp.28054. [DOI] [PubMed] [Google Scholar]
- 80.Liu X., Cui L., Hua D. Long noncoding RNA XIST regulates miR-137-EZH2 Axis to promote tumor metastasis in colorectal cancer. Oncol. Res. 2018;27(1):99–106. doi: 10.3727/096504018x15195193936573. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Sun J., Hu J., Wang G., Yang Z., Zhao C., Zhang X., et al. LncRNA TUG1 promoted KIAA1199 expression via miR-600 to accelerate cell metastasis and epithelial-mesenchymal transition in colorectal cancer. J. Exp. Clin. Cancer Res. 2018;37(1):106. doi: 10.1186/s13046-018-0771-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.