Abstract
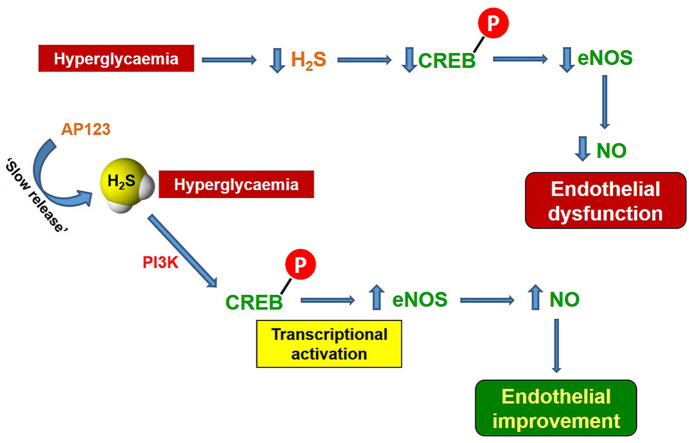
Diabetes is associated with severe vascular complications involving the impairment of endothelial nitric oxide synthase (eNOS) as well as cystathionine γ-lyase (CSE) activity. eNOS function is suppressed in hyperglycaemic conditions, resulting in reduced NO bioavailability, which is paralleled by reduced levels of hydrogen sulfide (H2S). Here we have addressed the molecular basis of the interplay between the eNOS and CSE pathways. We tested the impact of H2S replacement by using the mitochondrial-targeted H2S donor AP123 in isolated vessels and cultured endothelial cells in high glucose (HG) environment, at concentrations not causing any vasoactive effect per se. Aorta exposed to HG displayed a marked reduction of acetylcholine (Ach)-induced vasorelaxation that was restored by the addition of AP123 (10 nM). In HG condition, bovine aortic endothelial cells (BAEC) showed reduced NO levels, downregulation of eNOS expression, and suppression of CREB activation (p-CREB). Similar results were obtained by treating BAEC with propargylglycine (PAG), an inhibitor of CSE. AP123 treatment rescued eNOS expression, as well as NO levels, and restored p-CREB expression in both the HG environment and the presence of PAG. This effect was mediated by a PI3K-dependent activity since wortmannin (PI3K inhibitor) blunted the rescuing effects operated by the H2S donor. Experiments performed in the aorta of CSE−/− mice confirmed that reduced levels of H2S not only negatively affect the CREB pathway but also impair Ach-induced vasodilation, significantly ameliorated by AP123. We have demonstrated that the endothelial dysfunction due to HG involves H2S/PI3K/CREB/eNOS route, thus highlighting a novel aspect of the H2S/NO interplay in the vasoactive response.
Keywords: Endothelium, Hyperglycemia, Gasotransmitters, H2S donors, Vascular function
Graphical abstract
Highlights
-
•
Mitochondrial targeted H2S donor AP123 restores endothelial function through a mechanism involving PI3K/CREB/eNOS axis.
-
•
H2S availability is crucial for CREB/eNOS signaling in vitro as well as ex vivo.
-
•
Interplay between H2S and NO pathway plays a crucial role in the vasoactive function during hyperglycemia.
Abbreviations
- 3MST
3-mercaptopyruvate sulfurtransferase
- Ach
acethylcholine
- BAEC
bovine aortic edothelial cells
- CAT
cysteine aminotransferase
- Cav-1
caveolin 1
- CBS
cystathionine β-synthase
- CREB
cAMP response element binding protein
- pCREB
phosphorylated cAMP response element binding protein
- CSE
cystathionine γ-lyase
- H2S
hydrogen sulfide
- HG
high glucose
- Hsp90
heat shock protein 90
- KT
KT5720
- NG
normal glucose
- NO
nitric oxide
- eNOS
endothelial nitric oxide synthase
- NOx
nitrite and nitrate as nitric oxide species
- PAG
propargylglycine
- PE
phenilephrine
- PKA
proteina kinase A
- PI3K
phosphatidylinositol 3-kinase
- ROS
reactive oxigen species
- WM
wortmannin
- WT
wild type
1. Introduction
High concentrations of glucose in the bloodstream increase cell metabolism leading to the production of reactive oxygen species (ROS) within mitochondria that contributes to vascular damage [[1], [2], [3], [4]]. In physiological conditions, ROS are scavenged by several endogenous molecules with antioxidant activity [[5], [6], [7]]. The reduction of scavenger activity can lead to an uncontrolled increase of radicals that can affect vascular function [8,9]. This condition is typical of diabetes and includes coronary artery disease, stroke and peripheral vascular impairment, thus representing the major cause of morbidity and mortality in affected patients [10]. The vascular injury induced by hyperglycemia is also associated with alterations of endothelial molecular pathways controlling the vascular tone [[11], [12], [13]]. In fact, the therapeutic control of circulating glucose levels is of limited help in the improvement of cardiovascular complications in diabetic patients, where the hyperglycemic state has already triggered the vascular injury [[14], [15], [16], [17], [18]].
Hyperglycemia impairs the biosynthesis of endogenous gaseous vasodilators nitric oxide (NO) and hydrogen sulfide (H2S) and this contributes to endothelial dysfunction [[19], [20], [21]]. NO is physiologically synthesized by NO synthase, an enzyme expressed as three different isoforms: endothelial (eNOS), neuronal (nNOS) and inducible (iNOS). eNOS is the specific isoform expressed within the vasculature and it is activated by several means, including phosphorylation (p-eNOS) by Akt/PI3K or PKA, detachment of inhibitory protein caveolin-1 (Cav-1), binding of Ca2+-calmodulin complex or heat shock protein 90 (HSP90) [[22], [23], [24]]. Indeed, in diabetic vessels, the decreased availability of NO contributes to a reduced relaxation and this evidence is related to a decrease in eNOS expression and a lower level of p-eNOS, often associated with an overproduction of Cav-1 [21,[24], [25], [26]].
H2S is also recognized as a key vasodilator endogenously generated by three enzymes operating in the trans-sulfuration pathway, namely cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS) and cysteine aminotransferase/3-mercaptosulfurtransferase (CAT/3MST) [[27], [28], [29], [30]]. These enzymes are almost ubiquitously expressed within the human body; nonetheless, each of them shows peculiarity in specific tissues [31]. In particular, vasculature exhibits a differential distribution of CBS, 3MST and CSE, the latter being the major source of H2S within the vascular wall. Of note, similarly to NO, the decline of H2S concentrations occurring during the development of diabetes following CSE inhibition affects vascular function, suggesting a link between the two pathways in the control of vascular tone, which is improved by treatment with chronic H2S [20,[32], [33], [34], [35]].
The existence of an interplay between these gasotransmitters is therefore not novel as it has also been described in biological processes such as vasorelaxation, angiogenesis and inflammation or disease conditions (e.g. hypertension) [27,[36], [37], [38]]. Different experimental protocols have been utilized to study the interplay between NO and H2S but none focused specifically on this cross-talk in diabetes [[39], [40], [41]]. Indeed, the majority of the work in the field of NO–H2S cross-talk has been focused on the interplay between CBS and the NO pathway and used NO-donors [[42], [43], [44]]. In addition, in these studies, the interplay between H2S and the NO pathway has been assessed by using NaHS or other H2S donors at a concentration (10–100 μM) displaying per se a relaxant effect on the vessels [[45], [46], [47], [48], [49]]. Noteworthy, in many cases, the interplay between NO and H2S has been postulated based on the evaluation of single studies discussed in the review articles rather than designing a specific protocol aiming to dissect such interplay. To this extent, it might be intuitive that restoring physiological levels of H2S could represent a chance to recover NO-dependent endothelial function and could be used as a possible therapeutic approach to address vascular complications in diabetic patients, independently from glycaemia control. In this regard, the search for novel H2S-releasing compounds relies on the need to control the concentration and, possibly, localization of the H2S released, which has led to the discovery of AP123 [32]. AP123 is a slow donor that releases a very low amount of H2S within a specific timeframe. The release of H2S by AP123 was associated with the decreased hyperpolarization of the mitochondrial membrane and the suppression of the oxidant generation within mitochondria triggered by the HG environment. AP123 also increased the electron transport within the respiratory chain and improved the cellular metabolism, thus preserving endothelial cells from HG-induced injury. Taking advantage of the full characterization of the release kinetic of this donor, we aimed here to specifically investigate the interplay between the H2S and NO during hyperglycemia, by using AP123 at a concentration not exerting an intrinsic vascular effect.
2. Materials and methods
2.1. Isolated mouse aorta experiments
Animal care and experimental procedures in this study follow specific guidelines of the Italian and the European Council law for experiments involving animals. All procedures were approved by the local animal care office (Centro Servizi Veterinari, University of Naples, Federico II) and carried out following ARRIVE guidelines [50,51] and EU recommendations (Directive 2010/63/EU) for experimental design and analysis in pharmacology care. The procedure was authorized by Ministero della Salute (prot.n. 290-2018-PR). CD1 mice were used for ex vivo experiments, exposing the aorta to hyperglycemia. Experimental assessment of CSE−/− mice (in-house colony) aorta function was performed in comparison with age-matched male 8–12 weeks old C57Bl6 (Charles River, Lecco, Italy). Animals were kept at temperatures of 23 ± 2 °C, a humidity range of 40–70% and 12 h of light/dark cycles in pathogen-free cages (4 mice per cage) with free access to dry feed and water. Mice were anaesthetized with enflurane (5%) and then euthanized in CO2 chamber (70%). The thoracic aorta was rapidly harvested and adherent connective and fat tissue were removed. The aorta was then cut into rings of 1–1.5 mm in length and incubated in DMEM by using normoglycemic (NG, 25 mM) or hyperglycaemic (HG, 50 mM) conditions for 20 h with or without AP123 (1nM–100nM) in presence of wortmannin (WM, 100 nM), a PI3K inhibitor, or vehicle [52,53]. Following incubation, aorta rings were placed in organ baths (3.0 ml) filled with oxygenated (95% O2–5% CO2) Krebs’ solution (NaCl 118 mM, KCl 4.7 mM, MgCl2 1.2 mM, KH2PO4 1.2 mM, CaCl2 2.5 mM, NaHCO3 25 mM and glucose 10.1 mM) with ibuprofen (10 μM). The rings were connected to an isometric transducer (Fort 25, World Precision Instruments, 2Biological Instruments, Varese, Italy) associated with PowerLab 8/35 (World Precision Instruments, 2Biological Instruments, Varese, Italy). The optimal resting tension applied has been previously determined for each mouse strain. The rings were initially stretched until a resting tension of 1.5 g and then were allowed to equilibrate for at least 30 min; during this period the tension was adjusted, when necessary, to 1.5 g and the bath solution was periodically changed [29,54]. In each set of experiments, rings were firstly challenged with phenylephrine (PE, 1 μM) until the responses were reproducible. Cumulative concentration‐response curves to acetylcholine (Ach) (1 nM–30 μM) were performed on PE-precontracted rings to assess endothelial function. In another set of experiments, the direct vasodilatory effect of AP123 (1nM-30μM) was also tested on PE-precontracted rings.
In another set of experiments, aorta rings were collected from age-matched CSE−/− mice and used for vascular reactivity studies and western blot analysis, respectively. In particular, Ach-induced response was tested in aorta rings from CSE−/− mice incubated in DMEM by using normoglycemic (NG, 25 mM) conditions for 20 h with or without AP123 (10 nM).
2.2. Cell culture experiments
Bovine aortic endothelial cells (BAEC) were cultured in 10% fetal bovine serum (FBS) DMEM (without phenol red) and used at passages 5 to 10, without any differences in cell response. Following overnight starvation (serum-free medium), BAEC underwent normoglycemic (NG, 25 mM) or hyperglycaemic (HG, 50 mM) conditions for 3 h and then challenged with calcium ionophore A23187 (3 μM, 30 min, Sigma-Aldrich, Milan, Italy), as previously described [13,21] (supplemental method M1). Cells were grown until they reached 80% confluence and then plated for the experiments. AP123 (1nM–100nM) was administered at time 0 (t = 0, same time as hyperglycemia induction) or 1hr after hyperglycemia induction (t = 1).
All experiments using AP123 were performed by using the 1 h post-HG protocol. Cell supernatants and pellets were collected for nitrite/nitrate (NOx) and H2S levels determination and western blot analysis, respectively. In another set of experiments, BAEC were treated in a NG environment with CSE inhibitor propargylglycine (PAG, 10 mM, [55]) and, after 1 h, AP123 (10 nM) was added for 2 h in presence of PAG for a total length of 3 h experiment. Therefore, cell pellets were collected for western blot analysis. In a further series of experiments, BAEC cultured in HG conditions were also pre-incubated with PKA inhibitor KT5720 (1 μM) or PI3K inhibitor WM (100 nM) 30 min before the addition of AP123 (10 nM). Then, the supernatants and cell pellets were collected for NOx levels determination and western blot analysis, respectively. NaHS (10–100 nM) was used as a reference donor in comparison with AP123.
2.3. H2S determination
H2S levels were determined by a fluorometric assay in cell lysate and supernatant of BAEC that have undergone the different protocol treatments by using the specific fluorescent H2S probe SF7AM (Ex. 475 nm, Em. 500–550 nm, Tocris, UK). The volume of samples used was 50 μl for the supernatant, while cell lysate was used at a concentration of 0.5 mg/ml (50 μl). The samples, standards (Na2S) and blanks were pipetted into a black flat-bottomed 96-well plate by using RIPA solution as a dilution buffer. SF7AM was used at a final concentration of 10 μM and the total volume for each well was 200 μl. The plate was read at 37 °C throughout 90 min incubation period under orbital shaking conditions. Total H2S levels were quantified against a calibration curve obtained with Na2S (50nM-250μM). In another set of experiments, we measured live H2S production in cells. BAEC were incubated in NG or HG environment for 30 min and thereafter SF7AM (2.5 μM) was added for 30 min [56]. Following incubation, the medium was replaced to eliminate SF7AM excess and the cells were treated according to the different protocols described (AP123 10 nM or AP123 + PAG) for 2 h. Fluorescence readings were carried out straight after AP123 addition for 2 h at 30-min intervals (supplemental method M2).
2.4. NOx determination
NOx (nitrite/nitrate) levels quantification was carried out in supernatant (FBS-free DMEM without phenol red) of BAEC undergone different treatments or in plasma samples collected from CSE−/− mice and performed according to Misko et al. with modifications [13,26]. The samples were added with 0.49 M NH4Cl and 0.06 M Na2B4O7 (3:1) in presence of elementary cadmium to reduce nitrate to nitrite for 2 h at room temperature. After incubation, samples (160 μl) were centrifuged and added with a 10 μl solution of 2,3-diaminonaphtalene (DAN, 0.05 mg/ml) for 7 min in the dark and then 5 μl of NaOH 2.8 N were added in each sample to stop the reaction. Total nitrite levels were determined by fluorometric measurement of each sample (Ex. 365 nm, Em. 450 nm) against a calibration curve obtained with NaNO2 (50nM-2μM).
2.5. Western blot analysis
BAEC pellets or aorta segments were homogenized in modified RIPA buffer (Tris-HCl 50 mM, pH 7.4, Triton 1%, Na-deoxycholate 0.25%, NaCl 150 mM, EDTA 1 mM), added with protease inhibitor cocktail (Sigma-Aldrich). After centrifugation of homogenates at 6500 g for 10min, 60 μg of the denatured proteins were resolved on 10% SDS-PAGE gels and transferred to a polyvinylidene fluoride (PVDF) membrane. Unspecific binding on the membranes was minimized by using a blocking buffer solution (phosphate-buffered saline, PBS, containing 0.1% v/v Tween-20 and 3% non-fat dry milk, 1 h incubation). The incubation of membranes was carried out overnight at 4 °C with the following primary antibodies: mouse monoclonal anti-eNOS (1:1000, BD Biosciences, Milan, Italy), mouse monoclonal anti-cAMP response element-binding protein (CREB, 1:1000, Cell Signaling, Leiden, NL), mouse monoclonal anti-p-CREB (1:1000, Cell Signaling, Leiden, NL), rabbit polyclonal anti-GAPDH (1:1000, Merck, Milan, Italy) and mouse monoclonal anti-β-actin (1:1000, Merck, Milan, Italy). The filters were therefore washed in PBS containing 0.1% v/v Tween 20, before incubation for 2 h with anti-mouse horseradish peroxidase-conjugated secondary antibody. Membranes were washed and developed using Enhanced Chemiluminescence Substrate (ECL; Amersham Pharmacia Biotech, San Diego, CA, USA). Images for western blot have been obtained by using Chemidoc System (Bio-Rad, Milan, Italy). Band intensity has been analyzed by using ImageJ software and optical density (arbitrary units) has been reported.
2.6. Statistical analysis
All data were reported as mean ± SEM and the number of the replicated experiments for all data sets is n = 5 unless differently reported in figure legends. The relaxation data have been reported as % of relaxation calculated against the maximal contraction to phenylephrine‐induced tone. Statistical analysis has been performed by using one-way or two-way analysis of variance (ANOVA) where appropriate, followed by the Bonferroni or Dunnett post-hoc test, where applicable. Data were analyzed by using Prism Graphpad 8.0. Data sets were considered statistically significant when a value of p < 0.05 was reached.
3. Results
3.1. Effect of AP123 on Ach-induced vasorelaxation in HG environment
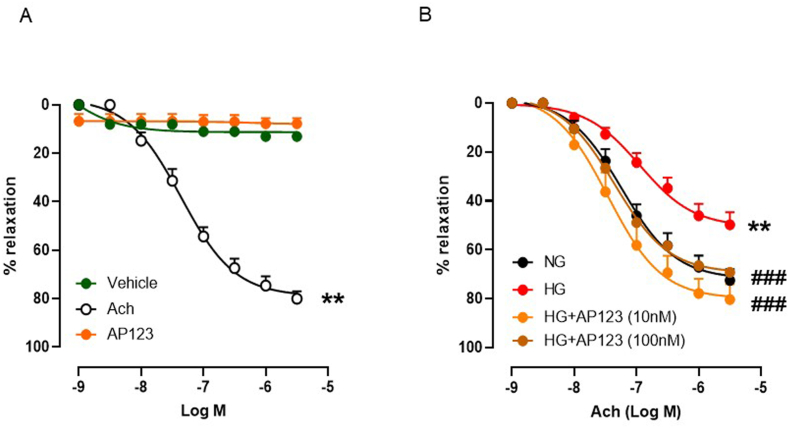
We first evaluated whether the H2S donor AP123 could exert a vasorelaxant effect per se in aorta rings precontracted with PE. The cumulative concentration-response curve (1nM-3μM) of AP123 did not show any vasoactivity in the range of the concentrations used (Fig. 1A). Therefore, we next evaluated the Ach-induced vasodilation in aortic rings incubated in a HG environment in presence of AP123 or vehicle (20 h). As expected, HG significantly reduced Ach-dependent vasorelaxation. Conversely, incubation with AP123 (10 nM and 100 nM) restored the normal vasodilatory activity induced by Ach (Fig. 1B). The concentration of 1 nM did not exert any appreciable effect (Supplemental Fig. S1).
Fig. 1.
Evaluation of endothelial function in mouse aorta rings incubated with AP123 during hyperglycemia. (A) Vasodilating effect exerted by Acetylcholine (Ach), AP123 and vehicle (DMSO) in aorta rings (n = 5) **p < 0.01 vs. vehicle and AP123. The Ach exerts a relaxant effect depending upon the concentrations used (1nM-3μM); conversely, neither AP123 nor DMSO triggers vasodilation when used within the same concentration range (1nM-3μM). (B) Ach-induced vasorelaxation in aorta rings incubated with AP123 (10–100 nM) exposed to HG or NG environment for 20 h **p < 0.01 vs. NG; ###p < 0.001 vs HG. Differences have been considered statistically significant when p was ≤0.05.
3.2. H2S and NO impairment in HG environment and rescuing effect by AP123
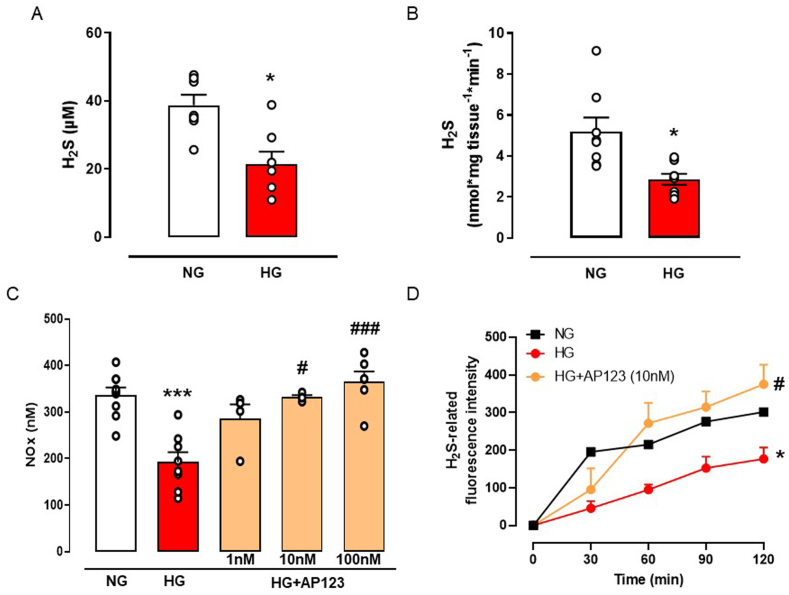
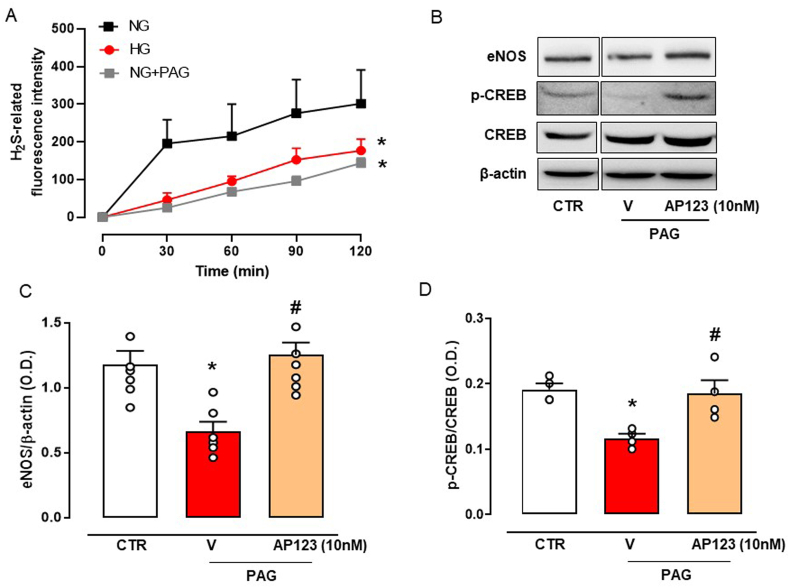
Next, we aimed to dissect the mechanism behind the beneficial effect of AP123 ex vivo in an in vitro model of hyperglycemia by using BAEC cells. We first assessed the impact of the HG environment on the physiological generation of H2S as well as NO. Cells undergoing HG treatment display significantly low levels of H2S measured in both cell supernatants (Fig. 2A) and total cell lysate (Fig. 2B). Similarly, NOx levels in the supernatant were also reduced by HG treatment, following previous findings (Fig. 2C) [26]. In a preliminary set of experiments, we chose the optimal treatment time (t = 0 or t = 1) to incubate AP123 in BAEC undergoing HG treatment [32]. We administered AP123 at the same time as HG exposure (t = 0) or 1 h later (t = 1). Preliminary results showed that incubation of AP123 1 h after the HG exposure was able to restore normal NOx generation, thus we used this setting for all following experiments (Supplemental Fig. S2). Therefore, we tested AP123 at different concentrations (1–100 nM) and we observed that it restored the NOx level as found in NG conditions (Fig. 2C). This effect was concentration-dependent and became significant at the concentration of 10 nM. Interestingly, this concentration of AP123 replenished the amount of H2S lost in the HG environment, as shown in the measurement of live H2S generation in BAEC cultured in HG environment (Fig. 2D).
Fig. 2.
Measurement of H2S and NO levels in BAEC during hyperglycemia. (A) The concentration of H2S was detected in the supernatant of BAEC exposed to the HG environment (3 h). *p < 0.05 vs NG. (B) Levels of H2S in the total cell lysate of BAEC exposed to HG environment. *p < 0.05 vs NG. (C) The concentration of total NO levels (expressed as total nitrate/nitrite, NOx) in BAEC exposed to HG environment and in presence of an increasing concentration of H2S donor AP123 (1–100 nM, 2 h). ***p < 0.001 vs NG; #p < 0.05, ###p < 0.001 * vs HG. (D) Live fluorometric quantification of H2S produced in BAEC exposed to NG, HG and HG with H2S donor AP123 (10 nM). *p < 0.05 vs NG; #p < 0.05 vs HG. The graphs show the mean values ± SEM for each group (n = 3–5). Differences have been considered statistically significant when p was ≤0.05.
3.3. Modulation of eNOS pathway by AP123 in HG environment
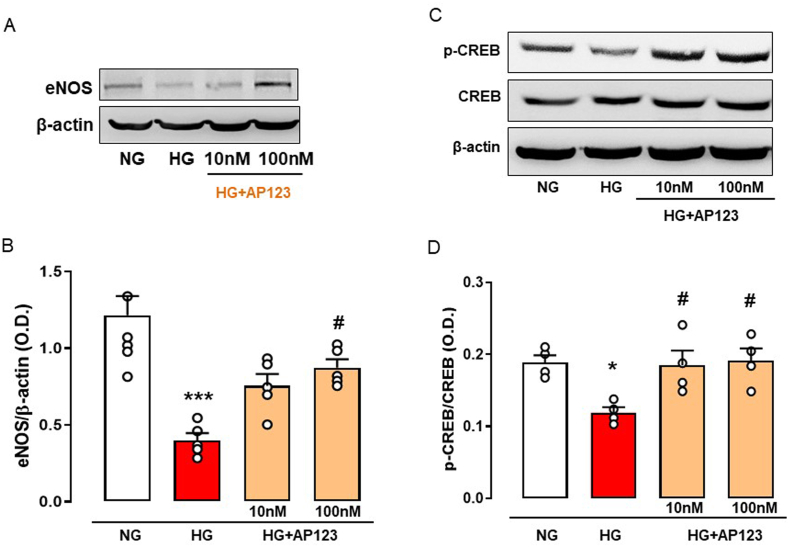
Next, following the quantification of NOx levels, we performed a western blot analysis to determine the influence of AP123 on eNOS expression in the HG environment. BAEC exposed to hyperglycemia showed a significant reduction in eNOS expression compared to the NG (Fig. 3A–B). The administration of AP123 to cells in the HG environment restored the physiological abundance of eNOS by significantly increasing its level. No effect was observed on eNOS levels when we used NaHS as a donor (Supplemental Fig. S3).
Fig. 3.
Protein expression levels of eNOS, p-CREB and CREB in BAEC treated with AP123 during hyperglycemia. (A) Western blot analysis showing cropped images of the expression of eNOS in BAEC treated or not with AP123 (10 nM and 100 nM, 2 h) exposed to HG environment (3 h). (B) Densitometric analysis of eNOS. (C) Western blot analysis showing cropped images of the expression and/or phosphorylation of CREB in BAEC treated or not with AP123 (10 nM and 100 nM, 2 h) exposed to HG environment. (D) Densitometric analysis of pCREB/CREB ratio. The graphs represent the mean ± SEM for each group (n = 4–5). The β-actin has been used as a housekeeping control protein. *p < 0.05 vs NG; ***p < 0.001 vs. NG; #p < 0.05 vs. HG. Differences have been considered statistically significant when p was ≤0.05.
HG exposure and AP123 treatment modified the expression of eNOS. Therefore, we hypothesized a transcriptional control operated by AP123 on eNOS gene activation and among the transcription factors regulating eNOS expression, we evaluated the cAMP response element-binding protein, also referred to as CREB [57,58], which is activated upon phosphorylation (p-CREB). BAEC cultured in the HG environment displays a reduction of p-CREB, with no changes in its constitutive form (Fig. 3C–D). Noteworthy, the treatment with AP123 (10 and 100 nM) increased CREB phosphorylation, thus re-establishing the levels observed in the control condition, e.g. NG experimental setting (Fig. 3C–D).
3.4. CREB activation and eNOS expression following AP123 treatment in H2S deficiency conditions
HG exposure is associated with a reduction of H2S biosynthesis in vivo [20] and this also applies to our experimental setting (see Fig. 2). Thus, we checked whether the low expression of p-CREB could be related to the low H2S levels. To test this hypothesis, we inhibited CSE activity by using PAG in BAEC exposed to the NG environment. This approach resulted in the reduction of the basal generation of H2S, thus mimicking the HG environment (Fig. 4A). PAG treatment, similarly to the HG setting, significantly downregulated eNOS expression and reduced activation of CREB, as indicated by lower levels of p-CREB expression (Fig. 4B–D). Interestingly, the administration of AP123 (10 nM) in PAG-treated BAEC restored the expression of both eNOS and p-CREB (Fig. 4B–D).
Fig. 4.
Measurement of H2S, eNOS and CREB protein expression levels in BAEC pre-treated with a CSE inhibitor in presence of AP123. (A) Fluorimetric quantification of live H2S concentration produced in BAEC pre-treated with the CSE inhibitor, PAG (10 mM, 15 min) and stimulated with AP123 (10 nM). (B) Western blot analysis showing cropped images of the expression of eNOS and pCREB/CREB in AP123-stimulated BAEC pre-treated or not with PAG. Densitometric analysis of (C) eNOS and (D) pCREB/CREB ratio. The graphs represent the mean ± SEM for each group (n = 3–6). CTR: untreated cells; V: cells treated with PAG only; AP123: cells treated with PAG + AP123 10 nM. The β-actin has been used as a housekeeping control protein. *p < 0.05 vs. NG or CTRL; #p < 0.05 vs. vehicle + PAG. Differences have been considered statistically significant when p was ≤0.05.
3.5. Role of endogenous H2S in CREB activation pathway
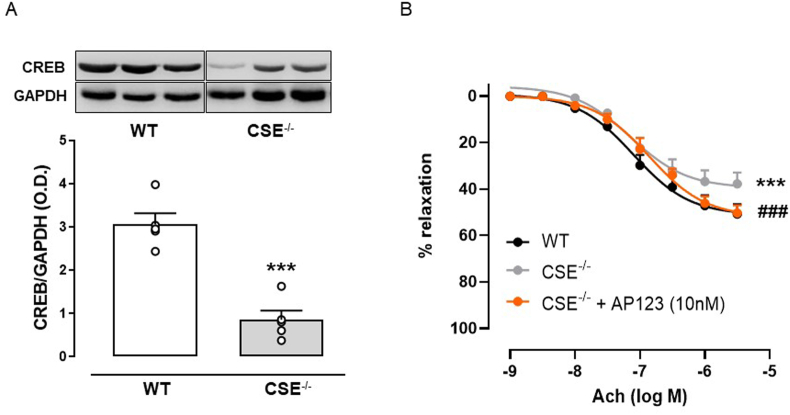
Endogenous modulation by H2S on the CREB pathway has also been investigated in mice lacking functional CSE (CSE−/− mice). Therefore, we checked the levels of CREB in the unstimulated aorta and we found that its expression was significantly downregulated in CSE−/− mice compared to wild type (WT) (Fig. 5A). We next evaluated the effect of Ach administration in the aorta from CSE−/− mice. Here, we observed that the relaxant effect induced by Ach was reduced by 20% in CSE−/− mice compared to wild type (WT) (Fig. 5B). We, therefore, tested the Ach-induced vasorelaxation in the aorta from CSE−/− mice following the incubation of AP123. The presence of AP123 significantly enhanced the Ach vasodilating effect in CSE−/− aorta, replicating the physiological vasodilation observed in WT mice.
Fig. 5.
Role of CSE-derived H2S in aorta from CSE−/−mice. (A) Western blot and densitometric analysis showing cropped images of CREB expression in the aorta harvested from CSE−/− and WT mice. (B) Ach-induced vasorelaxation in aorta rings harvested from CSE−/− and WT mice and Ach-induced vasorelaxation in aorta rings harvested from CSE−/− incubated with AP123 (20 h, 10 nM). The graphs represent the mean ± SEM for each group (n = 4–5). The GAPDH has been used as a housekeeping control protein ***p < 0.001 vs. WT. ###p < 0.001 vs CSE−/−. Differences have been considered statistically significant when p was ≤0.05.
3.6. Mechanism of CREB activation operated by AP123
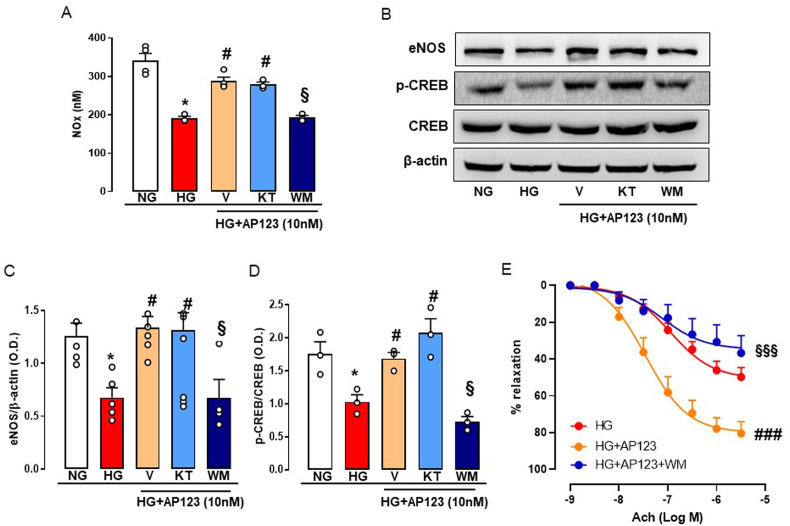
Next, we evaluated the possible involvement of two kinases (PKA and PI3K/Akt) pathways, known to be involved in NO and H2S signalling, in the activation of CREB following AP123 administration. Therefore, we quantified NOx levels in BAEC cultured in HG conditions and treated with AP123, in presence of KT5720 (KT) or wortmannin (WM), an inhibitor of PKA and PI3K, respectively. As already shown in Fig. 2C, the treatment with AP123 restored NOx levels in BAEC exposed to HG and this effect was abolished by WM, but not by KT (Fig. 6A). Next, we performed western blot analysis to establish whether the effect of WM and KT on changes in NOx levels induced by AP123 was also associated with a modulation of the eNOS pathway. Administration of KT did not modify the beneficial effect of AP123 on eNOS expression during hyperglycemia, while in presence of WM, AP123 was no longer able to restore eNOS expression in the HG environment (Fig. 6B–C). Next, we evaluated p-CREB expression in presence of KT and WM to investigate the upstream signalling. Similarly, p-CREB levels were restored by the treatment with AP123 in the HG environment and this effect was abrogated by WM, while no effect was observed following incubation with KT (Fig. 6B and D). Following the in vitro results, we tested whether the loss of effect observed when AP123 was used together with WM was also applied to our ex vivo setting. Therefore, we incubated the aorta rings pre-treated with WM (100 nM) in the HG environment in presence of AP123 (10 nM, 20 h). The beneficial effect on Ach-induced vasorelaxation in the HG environment was completely abolished by WM treatment (Fig. 6E). This effect was specific to AP123 since incubation with WM in the HG environment did not exert any effect on Ach-induced relaxation (Supplemental Fig. S4).
Fig. 6.
Effect of AP123 on BAEC pre-treated with either PKA or PI3K inhibitor in HG conditions and on isolated vessels. (A) The concentration of total NOx levels in AP123-treated (10 nM, 2 h) BAEC pre-incubated with PKA inhibitor, KT5720 (1 μM, 30 min), or PI3K inhibitor, WM (100 nM, 30 min) and exposed to HG environment (3 h). *p < 0.05 vs. NG; #p < 0.05 vs. HG; §p < 0.05 vs. HG + AP123 + WM (B) Western blot analysis showing cropped images of the expression and/or phosphorylation of eNOS and CREB in AP123-stimulated BAEC pre-treated or not with KT5720 or WM exposed to HG environment. Densitometric analysis of (C) eNOS and (D) pCREB/CREB ratio. *p < 0.05 vs. NG; #p < 0.05 vs. HG; §p < 0.05 vs. HG + AP123 + WM. The graphs represent the mean ± SEM for each group (n = 3–5). (E) Ach-induced vasorelaxation in aorta rings stimulated with AP123 (10 nM) pre-treated with PI3K inhibitor, WM (100 nM, 30 min), and exposed to HG environment for 20 h (n = 5). The β-actin has been used as a housekeeping control protein. ###p < 0.001 vs. HG + AP123; §§§p < 0.001 vs. HG + AP123 + WM. Differences have been considered statistically significant when p was ≤0.05.
4. Discussion
AP123 is a mitochondrial slow-release H2S donor fully characterized for what concerns the kinetic of H2S release [32]. In particular, a concentration of 100 μM of AP123 can release in a cell-free system ∼25 μM of H2S after 72 h, even though it does not cause any relaxant effect on mouse aorta precontracted with PE up to a dose of 3 μM. To understand the role played by H2S in HG conditions, we selected a concentration of 10 nM that: i) does not have any direct effect on the vessel, ii) is well within an achievable endogenous level of hydrogen sulfide. Aorta rings exposed to the HG environment lead to a marked inhibition of Ach-induced vasorelaxation, mainly due to eNOS/NO signaling impairment, as we have also previously shown [20,52,53]. Interestingly, the incubation of aorta rings with AP123 10 nM in the HG environment re-established the Ach-induced relaxations at the control level. Therefore, we have investigated if AP123 could positively modulate the eNOS pathway. In this regard, it has been shown that AP123 can protect b. End3 murine microvascular endothelial cells against oxidative stress associated with a hyperglycaemic environment [32]. To gain further insights into the mechanism triggered by AP123 in HG, we used an established in vitro cellular model of endothelial dysfunction induced by hyperglycemia. In this setting, BAEC were exposed to an HG environment leading to a marked reduction in H2S and NO. Following incubation with AP123, we observed a full restoration in NO levels, similar to those observed in normoglycemic conditions suggesting that AP123 does not act only by replenishing the hydrogen sulfide levels but also affects eNOS/NO signaling. Indeed, when we explored the expression of eNOS protein we found that AP123 positively modulated its levels, thereby contributing to restoring basal eNOS expression. Interestingly, at the same concentration of 10 nM, NaHS did not exert such a beneficial effect, thus indicating that the mode of H2S release might be crucial to exert a downstream molecular effect. In this context, a suboptimal mitochondrial activity is also associated with low levels of local H2S, due to defective enzymatic generation or altered translocation from cytosol to mitochondria, i.e. CSE, [59,60]. In addition, impaired mitochondrial function is associated with different diseases, including diabetes [[61], [62], [63], [64], [65]]. Of note, the chemical feature of AP123 being a mitochondrial-targeted H2S donor may explain this difference with NaHS, since it releases H2S in this specific sub-cellular compartment.
It is known that the cAMP response element-binding protein (CREB) is a transcriptional factor controlling, among other proteins, eNOS gene expression [57,58,66,67]. Thus, we wanted to test whether CREB could be involved in AP123 modulation of eNOS expression. In the cellular in vitro experiments, we found that HG exposure suppressed CREB activation, evaluated as changes in the phosphorylated form p-CREB. As expected, this effect was counteracted by incubation of cells with AP123 at the same concentration that operated the rescue of the Ach vasorelaxant effect. These data imply that AP123 modulation of eNOS expression involves the activation of CREB. To gain insights into the possible cross-talk with the H2S pathway, we evaluated whether the changes in the p-CREB/eNOS pathway were associated with the reduced levels of H2S observed in hyperglycemia.
CSE is the major enzyme involved in H2S production within the vessels. It is known from the literature that the inhibition of the endogenous CSE-derived H2S with PAG impairs Ach-induced vasorelaxation and mimics the HG effect [54]. When cells were incubated with PAG, i.e. the endogenous production was inhibited, there was a reduction of eNOS expression as well as of CREB activation. Thus, the endogenous basal production of CSE-derived H2S is involved in the regulation of CREB activation, which in turn affects eNOS expression levels. This hypothesis is confirmed by the finding that the incubation of BAEC with AP123 (10 nM) restores the p-CREB/eNOS axis in HG. All these data strongly imply that CREB activation requires H2S availability. A similar interplay has been identified in vivo in an animal model of cerebral ischemia-reperfusion injury, where the administration of H2S donors prevented cerebral damage by activating CREB signalling pathway [68]. Noteworthy, the action of AP123 is evident at nM concentration, in all the experimental settings used, and does not rely on direct H2S action on vessels. This evidence strongly suggests that AP123 release mimics the “physiological” role of CSE-derived hydrogen sulfide, impaired in a high glucose environment. To further address this hypothesis, we used aorta isolated from CSE−/− mice [65]. These mice display an impaired vascular function, a marked reduction of the endogenous hydrogen sulfide and, as also shown by others, isolated aorta rings displayed a reduced NO-dependent vasodilating response to Ach [[69], [70], [71]]. In addition, CSE−/− mice fed with a high-fat diet display a degenerative phenotype leading to leukocyte infiltration, atherosclerotic lesions and vascular stiffness [72,73]. In our setting, the shape of the Ach concentration-response curve displayed by CSE−/− mice overlaps that obtained in the experiments performed in an HG environment, or the presence of PAG, suggesting that the impairment of CSE activity/expression is involved in hyperglycemic-induced vascular dysfunction. In line with our results, the finding that aortas harvested from CSE−/− mice display reduced levels of CREB expression also reinforces our hypothesis. To further confirm our assumption, we incubated aorta rings harvested by CSE−/− mice with AP123 at 10 nM. AP123 restored the physiological vasodilatory effect induced by Ach, as can be seen by the overlapping of the concentration-response curve with that of wild-type mice. Thus, the impaired vascular reactivity of CSE−/− mice involves CREB-reduced expression and it can be reverted by the addition of the donor AP123. In summary, the absence of CSE impairs i) the vasodilatory response of aorta rings to Ach, ii) the level of circulating NO, iii) the basal expression level of CREB.
CREB is normally activated through a phosphorylation step operated by PKA, together with other kinases, including PI3K [74,75]. Thus, we have evaluated in our experimental setting whether PKA and/or PI3K could be involved in the molecular mechanism associated with eNOS activation by AP123 in HG-exposed endothelial cells. The pharmacological modulation study demonstrated that inhibition of PKA did not affect AP123 beneficial effect in the HG environment and CREB phosphorylation. Conversely, PI3K blockade blunted AP123 rescuing effect as well as CREB activation. To support this hypothesis, we performed a pharmacological modulation on isolated rings confirming that this molecular mechanism also drives the AP123 effect on vascular function. In isolated aorta rings the inhibition of PI3K with a specific inhibitor significantly abolished the effect of AP123, further indicating PI3K as a primary switch in the CREB activation process. Thus, AP123 rescuing action on the vasorelaxant effect of Ach relies on the PI3K/CREB/eNOS axis. This hypothesis of mechanism well fit with previous evidence showing that activation of PI3K/eNOS signaling by H2S directly modulated NO levels in BAEC [76] and exerts a protective effect in HG-induced injury in HUVEC cells [77]. Furthermore, the activation of this pathway has also been demonstrated to be beneficial in vivo, in a model of cardiac dysfunction associated with sepsis [78] as well as in neuronal hypoxia-ischemia injury or angiogenesis models [79,80].
Overall we demonstrate that the interplay between H2S and NO pathways plays a crucial role in the vasoactive response triggered by Ach in hyperglycemic conditions. This evidence sheds new light on the role of vascularly derived H2S, adding more knowledge to the complex mechanisms associated with the control of vessel functions.
5. Conclusions
Here we have demonstrated that mitochondrial-targeted H2S donor AP123 restore endothelial function through a mechanism involving PI3K and CREB activation in a model of hyperglycemia ex vivo and in vitro. Our findings show that the use of specific mitochondrial-targeted H2S donors may represent a feasible approach to address the endothelial dysfunction associated with HG. Indeed, the mechanism described operates at a local level within the vasculature re-establishing the physiological response to the endogenous agonist, i.e. Ach, independently from the control of blood glucose
Funding
This work was supported by the Ministry of Education, Universities and Research (MIUR) [“Progetti di Rilevante Interesse Nazionale (PRIN)”, grant number 2017NKB2N4_004; “Programma Operativo Nazionale (PON)”, grant number ARS01_01081].
Author contributions
Conceptualization, V.B., G.C. and M.W.; methodology, R.M., V.V., R.T., O.L.M., G.M.C., M.S.; methodology for animal care: S.C.; software, V.V.; validation, R.M., R.T. and O.L.M.; formal analysis, R.M. and V.V.; investigation, R.M., V.V., O.L.M.; data curation, R.M., O.L.M., F.C.; writing and original draft preparation, R.M., and V.B.; writing, reviewing and editing, V.B., E.M., R.d.E.d.V.B., G.T., M.B., R.S., M.W.; supervision, V.B. and R.d.E.d.V.B.; project administration, V.B.; funding acquisition, V.B. and G.T.; R.d.E.d.V.B. and V.B. have equally contributed as last authors to this work. All authors have read and agreed to the published version of the manuscript.
Ethical consideration
The study was conducted according to the ARRIVE guidelines and approved by the Institutional Review Board (Centro Servizi Veterinari, Università degli Studi di Napoli, Federico II) and by Ministero della Salute, n.24/2016 and n.718/2016 (prot.n. 290-2018-PR).
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We thank the personnel of Biotechnology Centre Animal Facility, Cardarelli Hospital, for hosting CSE−/− mice colony and for support and technical advices.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102657.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplemental figure S1. Evaluation of AP123 effect on Ach vasodilating properties in mouse aorta rings. Ach-induced vasorelaxation in aortic rings exposed to HG environment for 20 h in the absence or presence of AP123 (1 nM). **p < 0.01 vs. NG. Differences have been considered statistically significant when p was ≤0.05.
Supplemental figure S2. Measurement of NO in BAEC treated with AP123 and time-dependency. The concentration of total NOx levels in BAEC treated with AP123 (100 nM) at the same time (t = 0) or 1h (t = 1) following the exposure to the HG environment (3h) (n = 3). *p < 0.05 vs. NG; °p < 0.01 vs. HG. Differences have been considered statistically significant when p was ≤0.05.
Supplemental figure S3. Protein expression levels eNOS in BAEC treated with NaHS during hyperglycemia. (A) Western blot analysis of the expression of eNOS in BAEC treated with NaHS (10 nM and 100 nM, 2h) or vehicle and exposed to HG environment (3h). Densitometric analysis of (B) eNOS/β-actin ratio. The graphs represent the mean ± SEM for each group (n = 5). The β-actin has been used as a housekeeping control protein. Differences have been considered statistically significant when p was ≤0.05. ***p < 0.001 vs. NG.
References
- 1.Cheng Z., Kishore R. Potential role of hydrogen sulfide in diabetes-impaired angiogenesis and ischemic tissue repair. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101704. S2213-2317(20)30909-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki K., Olah G., Modis K., Coletta C., Kulp G., Gero D., Szoleczky P., Chang T., Zhou Z., Wu L., Wang R., Papapetropoulos A., Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. U. S. A. 2010;108:13829–13834. doi: 10.1073/pnas.1105121108. 1105121108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiorentino T.V., Prioletta A., Zuo P., Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharmaceut. Des. 2013;19:5695–5703. doi: 10.2174/1381612811319320005. CPD-EPUB-20130220-7 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Schulze P.C., Yoshioka J., Takahashi T., He Z., King G.L., Lee R.T. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J. Biol. Chem. 2004;279:30369–30374. doi: 10.1074/jbc.M400549200. M400549200 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Thews O., Lambert C., Kelleher D.K., Biesalski H.K., Vaupel P., Frank J. Impact of therapeutically induced reactive oxygen species and radical scavenging by alpha-tocopherol on tumor cell adhesion. Oncol. Rep. 2007;18:965–971. [PubMed] [Google Scholar]
- 6.Dickinson D.A., Forman H.J. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002;64:1019–1026. doi: 10.1016/s0006-2952(02)01172-3. S0006295202011723 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Baba S.P., Bhatnagar A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018;7:133–139. doi: 10.1016/j.cotox.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obrosova I.G., Minchenko A.G., Marinescu V., Fathallah L., Kennedy A., Stockert C.M., Frank R.N., Stevens M.J. Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia. 2001;44:1102–1110. doi: 10.1007/s001250100631. [DOI] [PubMed] [Google Scholar]
- 9.Inoguchi T., Sonta T., Tsubouchi H., Etoh T., Kakimoto M., Sonoda N., Sato N., Sekiguchi N., Kobayashi K., Sumimoto H., Utsumi H., Nawata H. Protein kinase c-dependent increase in reactive oxygen species (ros) production in vascular tissues of diabetes: role of vascular nad(p)h oxidase. J. Am. Soc. Nephrol. 2003;14:S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- 10.Thiruvoipati T., Kielhorn C.E., Armstrong E.J. Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J. Diabetes. 2015;6:961–969. doi: 10.4239/wjd.v6.i7.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Civelek M., Manduchi E., Riley R.J., Stoeckert C.J., Jr., Davies P.F. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ. Res. 2009;105:453–461. doi: 10.1161/CIRCRESAHA.109.203711. CIRCRESAHA.109.203711 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriya J., Ferrara N. Inhibiting the response to vegf in diabetes. Sci. Signal. 2014;7 doi: 10.1126/scisignal.2004996. pe1. 7/307/pe1 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Bucci M., Roviezzo F., Brancaleone V., Di Lorenzo A., Evangelista S., Gori M., Cirino G. Ace-inhibition ameliorates vascular reactivity and delays diabetes outcome in nod mice. Vasc. Pharmacol. 2008;49:84–90. doi: 10.1016/j.vph.2008.06.002. S1537-1891(08)00063-3 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Akbari C.M., Saouaf R., Barnhill D.F., Newman P.A., LoGerfo F.W., Veves A. Endothelium-dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. J. Vasc. Surg. 1998;28:687–694. doi: 10.1016/s0741-5214(98)70095-3. S0741521498002973 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Williams S.B., Goldfine A.B., Timimi F.K., Ting H.H., Roddy M.A., Simonson D.C., Creager M.A. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 16.Makimattila S., Virkamaki A., Groop P.H., Cockcroft J., Utriainen T., Fagerudd J., Yki-Jarvinen H. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94:1276–1282. doi: 10.1161/01.cir.94.6.1276. [DOI] [PubMed] [Google Scholar]
- 17.Mannucci E., Dicembrini I., Lauria A., Pozzilli P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care. 2013;36(Suppl 2):S259–S263. doi: 10.2337/dcS13-2018. 36/Supplement_2/S259 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giorgino F., Leonardini A., Laviola L. Cardiovascular disease and glycemic control in type 2 diabetes: now that the dust is settling from large clinical trials. Ann. N. Y. Acad. Sci. 2013;1281:36–50. doi: 10.1111/nyas.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Z., Shen X., Jiang X., Shan H., Cimini M., Fang P., Ji Y., Park J.Y., Drosatos K., Yang X., Kevil C.G., Kishore R., Wang H. Hyperhomocysteinemia potentiates diabetes-impaired edhf-induced vascular relaxation: role of insufficient hydrogen sulfide. Redox Biol. 2018;16:215–225. doi: 10.1016/j.redox.2018.02.006. S2213-2317(18)30028-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brancaleone V., Roviezzo F., Vellecco V., De Gruttola L., Bucci M., Cirino G. Biosynthesis of h2s is impaired in non-obese diabetic (nod) mice. Br. J. Pharmacol. 2008;155:673–680. doi: 10.1038/bjp.2008.296. bjp2008296 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vellecco V., Mitidieri E., Gargiulo A., Brancaleone V., Matassa D., Klein T., Esposito F., Cirino G., Bucci M. Vascular effects of linagliptin in non-obese diabetic mice are glucose-independent and involve positive modulation of the endothelial nitric oxide synthase (enos)/caveolin-1 (cav-1) pathway. Diabetes Obes. Metabol. 2016;18:1236–1243. doi: 10.1111/dom.12750. [DOI] [PubMed] [Google Scholar]
- 22.Fleming I. Molecular mechanisms underlying the activation of enos. Pflügers Archiv. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 23.Fulton D., Fontana J., Sowa G., Gratton J.P., Lin M., Li K.X., Michell B., Kemp B.E., Rodman D., Sessa W.C. Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in golgi and plasma membrane defines the existence of two pools of active enzyme. J. Biol. Chem. 2002;277:4277–4284. doi: 10.1074/jbc.M106302200. M106302200 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Du X.L., Edelstein D., Dimmeler S., Ju Q., Sui C., Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the akt site. J. Clin. Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noyman I., Marikovsky M., Sasson S., Stark A.H., Bernath K., Seger R., Madar Z. Hyperglycemia reduces nitric oxide synthase and glycogen synthase activity in endothelial cells. Nitric Oxide. 2002;7:187–193. doi: 10.1016/s1089-8603(02)00106-4. S1089860302001064 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Bucci M., Roviezzo F., Brancaleone V., Lin M.I., Di Lorenzo A., Cicala C., Pinto A., Sessa W.C., Farneti S., Fiorucci S., Cirino G. Diabetic mouse angiopathy is linked to progressive sympathetic receptor deletion coupled to an enhanced caveolin-1 expression. Arterioscler. Thromb. Vasc. Biol. 2004;24:721–726. doi: 10.1161/01.ATV.0000122362.44628.09. 01.ATV.0000122362.44628.09 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Hosoki R., Matsuki N., Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. S0006-291X(97)96878-9 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Shibuya N., Mikami Y., Kimura Y., Nagahara N., Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. mvp111 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Mitidieri E., Tramontano T., Gurgone D., Citi V., Calderone V., Brancaleone V., Katsouda A., Nagahara N., Papapetropoulos A., Cirino G., d'Emmanuele di Villa Bianca R., Sorrentino R. Mercaptopyruvate acts as endogenous vasodilator independently of 3-mercaptopyruvate sulfurtransferase activity. Nitric Oxide. 2018;75:53–59. doi: 10.1016/j.niox.2018.02.003. S1089-8603(17)30260-4 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Zhao W., Zhang J., Lu Y., Wang R. The vasorelaxant effect of h(2)s as a novel endogenous gaseous k(atp) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao X., Ding L., Xie Z.Z., Yang Y., Whiteman M., Moore P.K., Bian J.S. A review of hydrogen sulfide synthesis, metabolism, and measurement: is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxidants Redox Signal. 2019;31:1–38. doi: 10.1089/ars.2017.7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gero D., Torregrossa R., Perry A., Waters A., Le-Trionnaire S., Whatmore J.L., Wood M., Whiteman M. The novel mitochondria-targeted hydrogen sulfide (h2s) donors ap123 and ap39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 2016;113:186–198. doi: 10.1016/j.phrs.2016.08.019. S1043-6618(16)30631-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G.D., Mitchison T.J., Moskowitz M.A., Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1:112–119. doi: 10.1038/nchembio711. nchembio711 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Jain S.K., Bull R., Rains J.L., Bass P.F., Levine S.N., Reddy S., McVie R., Bocchini J.A. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxidants Redox Signal. 2010;12:1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng H.H., Yildiz G.S., Ku J.M., Miller A.A., Woodman O.L., Hart J.L. Chronic nahs treatment decreases oxidative stress and improves endothelial function in diabetic mice. Diabetes Vasc. Dis. Res. 2017;14:246–253. doi: 10.1177/1479164117692766. [DOI] [PubMed] [Google Scholar]
- 36.Coletta C., Papapetropoulos A., Erdelyi K., Olah G., Modis K., Panopoulos P., Asimakopoulou A., Gero D., Sharina I., Martin E., Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. 1202916109 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gheibi S., Jeddi S., Kashfi K., Ghasemi A. Regulation of vascular tone homeostasis by no and h2s: implications in hypertension. Biochem. Pharmacol. 2018;149:42–59. doi: 10.1016/j.bcp.2018.01.017. S0006-2952(18)30017-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo Faro M.L., Fox B., Whatmore J.L., Winyard P.G., Whiteman M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. 2014;41:38–47. doi: 10.1016/j.niox.2014.05.014. S1089-8603(14)00246-8 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Shirazi M.K., Azarnezhad A., Abazari M.F., Poorebrahim M., Ghoraeian P., Sanadgol N., Bokharaie H., Heydari S., Abbasi A., Kabiri S., Aleagha M.N., Enderami S.E., Dashtaki A.S., Askari H. The role of nitric oxide signaling in renoprotective effects of hydrogen sulfide against chronic kidney disease in rats: involvement of oxidative stress, autophagy and apoptosis. J. Cell. Physiol. 2019;234:11411–11423. doi: 10.1002/jcp.27797. [DOI] [PubMed] [Google Scholar]
- 40.Giuffre A., Vicente J.B. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/6290931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagpure B.V., Bian J.S. Interaction of hydrogen sulfide with nitric oxide in the cardiovascular system. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/6904327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kabil O., Weeks C.L., Carballal S., Gherasim C., Alvarez B., Spiro T.G., Banerjee R. Reversible heme-dependent regulation of human cystathionine beta-synthase by a flavoprotein oxidoreductase. Biochemistry. 2011;50:8261–8263. doi: 10.1021/bi201270q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C.N., Duan G.L., Liu Y.J., Yu Q., Tang X.L., Zhao W., Li X.H., Zhu X.Y., Ni X. Overproduction of nitric oxide by endothelial cells and macrophages contributes to mitochondrial oxidative stress in adrenocortical cells and adrenal insufficiency during endotoxemia. Free Radic. Biol. Med. 2015;83:31–40. doi: 10.1016/j.freeradbiomed.2015.02.024. S0891-5849(15)00090-8 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Monti M., Hyseni I., Pacini A., Monzani E., Casella L., Morbidelli L. Cross-talk between endogenous h(2)s and no accounts for vascular protective activity of the metal-nonoate zn(pipnono)cl. Biochem. Pharmacol. 2018;152:143–152. doi: 10.1016/j.bcp.2018.03.025. S0006-2952(18)30130-8 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Mitidieri E., Gurgone D., Caiazzo E., Tramontano T., Cicala C., Sorrentino R., d'Emmanuele di Villa Bianca R. L-cysteine/cystathionine-beta-synthase-induced relaxation in mouse aorta involves a l-serine/sphingosine-1-phosphate/no pathway. Br. J. Pharmacol. 2020;177:734–744. doi: 10.1111/bph.14654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yong Q.C., Lee S.W., Foo C.S., Neo K.L., Chen X., Bian J.S. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1330–H1340. doi: 10.1152/ajpheart.00244.2008. 00244.2008 [pii] [DOI] [PubMed] [Google Scholar]
- 47.Altaany Z., Yang G., Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J. Cell Mol. Med. 2013;17:879–888. doi: 10.1111/jcmm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King A.L., Polhemus D.J., Bhushan S., Otsuka H., Kondo K., Nicholson C.K., Bradley J.M., Islam K.N., Calvert J.W., Tao Y.X., Dugas T.R., Kelley E.E., Elrod J.W., Huang P.L., Wang R., Lefer D.J. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. U. S. A. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. 1321871111 [pii]. 201321871 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang B., Chen C.T., Chen C.S., Wang Y.M., Hsieh H.J., Wang D.L. Laminar shear flow increases hydrogen sulfide and activates a nitric oxide producing signaling cascade in endothelial cells. Biochem. Biophys. Res. Commun. 2015;464:1254–1259. doi: 10.1016/j.bbrc.2015.07.115. S0006-291X(15)30345-4 [pii] [DOI] [PubMed] [Google Scholar]
- 50.Kilkenny C., Browne W., Cuthill I.C., Emerson M., Altman D.G. Animal research: reporting in vivo experiments: the arrive guidelines. Br. J. Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. BPH872 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lilley E., Stanford S.C., Kendall D.E., Alexander S.P.H., Cirino G., Docherty J.R., George C.H., Insel P.A., Izzo A.A., Ji Y., Panettieri R.A., Sobey C.G., Stefanska B., Stephens G., Teixeira M., Ahluwalia A. Arrive 2.0 and the british journal of pharmacology: updated guidance for 2020. Br. J. Pharmacol. 2020;177:3611–3616. doi: 10.1111/bph.15178. BPH15178 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Venu V.K.P., Saifeddine M., Mihara K., Faiza M., Gorobets E., Flewelling A.J., Derksen D.J., Hirota S.A., Marei I., Al-Majid D., Motahhary M., Ding H., Triggle C.R., Hollenberg M.D. Metformin prevents hyperglycemia-associated, oxidative stress-induced vascular endothelial dysfunction: essential role for the orphan nuclear receptor human nuclear receptor 4a1 (nur77) Mol. Pharmacol. 2021;100:428–455. doi: 10.1124/molpharm.120.000148. molpharm.120.000148 [pii] [DOI] [PubMed] [Google Scholar]
- 53.Chandra S., Fulton D.J.R., Caldwell R.B., Caldwell R.W., Toque H.A. Hyperglycemia-impaired aortic vasorelaxation mediated through arginase elevation: role of stress kinase pathways. Eur. J. Pharmacol. 2019;844:26–37. doi: 10.1016/j.ejphar.2018.11.027. S0014-2999(18)30682-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brancaleone V., Esposito I., Gargiulo A., Vellecco V., Asimakopoulou A., Citi V., Calderone V., Gobbetti T., Perretti M., Papapetropoulos A., Bucci M., Cirino G. D-penicillamine modulates hydrogen sulfide (h2s) pathway through selective inhibition of cystathionine-gamma-lyase. Br. J. Pharmacol. 2016;173:1556–1565. doi: 10.1111/bph.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brancaleone V., Vellecco V., Matassa D.S., d'Emmanuele di Villa Bianca R., Sorrentino R., Ianaro A., Bucci M., Esposito F., Cirino G. Crucial role of androgen receptor in vascular h2s biosynthesis induced by testosterone. Br. J. Pharmacol. 2015;172:1505–1515. doi: 10.1111/bph.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin V.S., Lippert A.R., Chang C.J. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging h2o2-dependent h2s production. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7131–7135. doi: 10.1073/pnas.1302193110. 1302193110 [pii]. 201302193 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Min J., Jin Y.M., Moon J.S., Sung M.S., Jo S.A., Jo I. Hypoxia-induced endothelial no synthase gene transcriptional activation is mediated through the tax-responsive element in endothelial cells. Hypertension. 2006;47:1189–1196. doi: 10.1161/01.HYP.0000222892.37375.4d. 01.HYP.0000222892.37375.4d [pii] [DOI] [PubMed] [Google Scholar]
- 58.Lee S.Y., Min J. Regulation of no from endothelial cells by the decrease of cellular camp under arsenite exposure. J. Microbiol. Biotechnol. 2008;18:392–395. 7153 [pii] [PubMed] [Google Scholar]
- 59.Fu M., Zhang W., Wu L., Yang G., Li H., Wang R. Hydrogen sulfide (h2s) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2943–2948. doi: 10.1073/pnas.1115634109. 1115634109 [pii]. 201115634 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohta J., Ubuka T., Kodama H., Sugahara K., Yao K., Masuoka N., Kinuta M. Increase in cystathionine content in rat liver mitochondria after d,l-propargylglycine administration. Amino Acids. 1995;9:111–122. doi: 10.1007/BF00805832. [DOI] [PubMed] [Google Scholar]
- 61.Sergi D., Naumovski N., Heilbronn L.K., Abeywardena M., O'Callaghan N., Lionetti L., Luscombe-Marsh N. Mitochondrial (dys)function and insulin resistance: from pathophysiological molecular mechanisms to the impact of diet. Front. Physiol. 2019;10:532. doi: 10.3389/fphys.2019.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sivitz W.I., Yorek M.A. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxidants Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. 10.1089/ars.2009.2531 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paul B.D., Snyder S.H., Kashfi K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021;38 doi: 10.1016/j.redox.2020.101772. S2213-2317(20)30977-0 [pii]. 101772 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwak S.H., Park K.S., Lee K.U., Lee H.K. Mitochondrial metabolism and diabetes. J. Diabetes Investig. 2010;1:161–169. doi: 10.1111/j.2040-1124.2010.00047.x. JDI47 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cirino G., Szabo C., Papapetropoulos A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 2023;103:31–276. doi: 10.1152/physrev.00028.2021. [DOI] [PubMed] [Google Scholar]
- 66.Si H., Yu J., Jiang H., Lum H., Liu D. Phytoestrogen genistein up-regulates endothelial nitric oxide synthase expression via activation of camp response element-binding protein in human aortic endothelial cells. Endocrinology. 2012;153:3190–3198. doi: 10.1210/en.2012-1076. en.2012-1076 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niwano K., Arai M., Koitabashi N., Hara S., Watanabe A., Sekiguchi K., Tanaka T., Iso T., Kurabayashi M. Competitive binding of creb and atf2 to camp/atf responsive element regulates enos gene expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006;26:1036–1042. doi: 10.1161/01.ATV.0000215179.76144.39. 01.ATV.0000215179.76144.39 [pii] [DOI] [PubMed] [Google Scholar]
- 68.Dai H.B., Ji X., Zhu S.H., Hu Y.M., Zhang L.D., Miao X.L., Ma R.M., Duan M.L., Li W.Y. Hydrogen sulphide and mild hypothermia activate the creb signaling pathway and prevent ischemia-reperfusion injury. BMC Anesthesiol. 2015;15:119. doi: 10.1186/s12871-015-0097-6. 10.1186/s12871-015-0097-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitidieri E., Vellecco V., Brancaleone V., Vanacore D., Manzo O.L., Martin E., Sharina I., Krutsenko Y., Monti M.C., Morretta E., Papapetropoulos A., Caliendo G., Frecentese F., Cirino G., Sorrentino R., d'Emmanuele di Villa Bianca R., Bucci M. Involvement of 3',5'-cyclic inosine monophosphate in cystathionine gamma-lyase-dependent regulation of the vascular tone. Br. J. Pharmacol. 2021;178:3765–3782. doi: 10.1111/bph.15516. BPH15516 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., Snyder S.H., Wang R. H2s as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. 322/5901/587 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia H., Li Z., Sharp T.E., 3rd, Polhemus D.J., Carnal J., Moles K.H., Tao Y.X., Elrod J., Pfeilschifter J., Beck K.F., Lefer D.J. Endothelial cell cystathionine gamma-lyase expression level modulates exercise capacity, vascular function, and myocardial ischemia reperfusion injury. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017544. JAH35516 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mani S., Li H., Untereiner A., Wu L., Yang G., Austin R.C., Dickhout J.G., Lhotak S., Meng Q.H., Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. CIRCULATIONAHA.113.002208 [pii] [DOI] [PubMed] [Google Scholar]
- 73.Yuan S., Yurdagul A., Jr., Peretik J.M., Alfaidi M., Al Yafeai Z., Pardue S., Kevil C.G., Orr A.W. Cystathionine gamma-lyase modulates flow-dependent vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2018;38:2126–2136. doi: 10.1161/ATVBAHA.118.311402. ATVBAHA.118.311402 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng B., Li Y., Niu B., Wang X., Cheng Y., Zhou Z., You T., Liu Y., Wang H., Xu J. Involvement of pi3k/akt/foxo3a and pka/creb signaling pathways in the protective effect of fluoxetine against corticosterone-induced cytotoxicity in pc12 cells. J. Mol. Neurosci. 2016;59:567–578. doi: 10.1007/s12031-016-0779-7. 10.1007/s12031-016-0779-7 [pii] [DOI] [PubMed] [Google Scholar]
- 75.Steven A., Friedrich M., Jank P., Heimer N., Budczies J., Denkert C., Seliger B. What turns creb on? And off? And why does it matter? Cell. Mol. Life Sci. 2020;77:4049–4067. doi: 10.1007/s00018-020-03525-8. 10.1007/s00018-020-03525-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Predmore B.L., Julian D., Cardounel A.J. Hydrogen sulfide increases nitric oxide production from endothelial cells by an akt-dependent mechanism. Front. Physiol. 2011;2:104. doi: 10.3389/fphys.2011.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin F., Yang Y., Wei S., Huang X., Peng Z., Ke X., Zeng Z., Song Y. Hydrogen sulfide protects against high glucose-induced human umbilical vein endothelial cell injury through activating pi3k/akt/enos pathway. Drug Des. Dev. Ther. 2020;14:621–633. doi: 10.2147/DDDT.S242521. 242521 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J., Li J., Tian P., Guli B., Weng G., Li L., Cheng Q. H2s attenuates sepsis-induced cardiac dysfunction via a pi3k/akt-dependent mechanism. Exp. Ther. Med. 2019;17:4064–4072. doi: 10.3892/etm.2019.7440. ETM-0-0-7440 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li T., Li J., Zhao Y., Ke H., Wang S., Liu D., Wang Z. L-cysteine provides neuroprotection of hypoxia-ischemia injury in neonatal mice via a pi3k/akt-dependent mechanism. Drug Des. Dev. Ther. 2021;15:517–529. doi: 10.2147/DDDT.S293025. 293025 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang W., Liu C., Deng M., Wang F., Ren X., Fan Y., Du J., Wang Y. H2s promotes developmental brain angiogenesis via the nos/no pathway in zebrafish. Stroke Vasc. Neurol. 2020;6:244–251. doi: 10.1136/svn-2020-000584. svn-2020-000584 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.