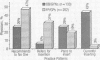Abstract
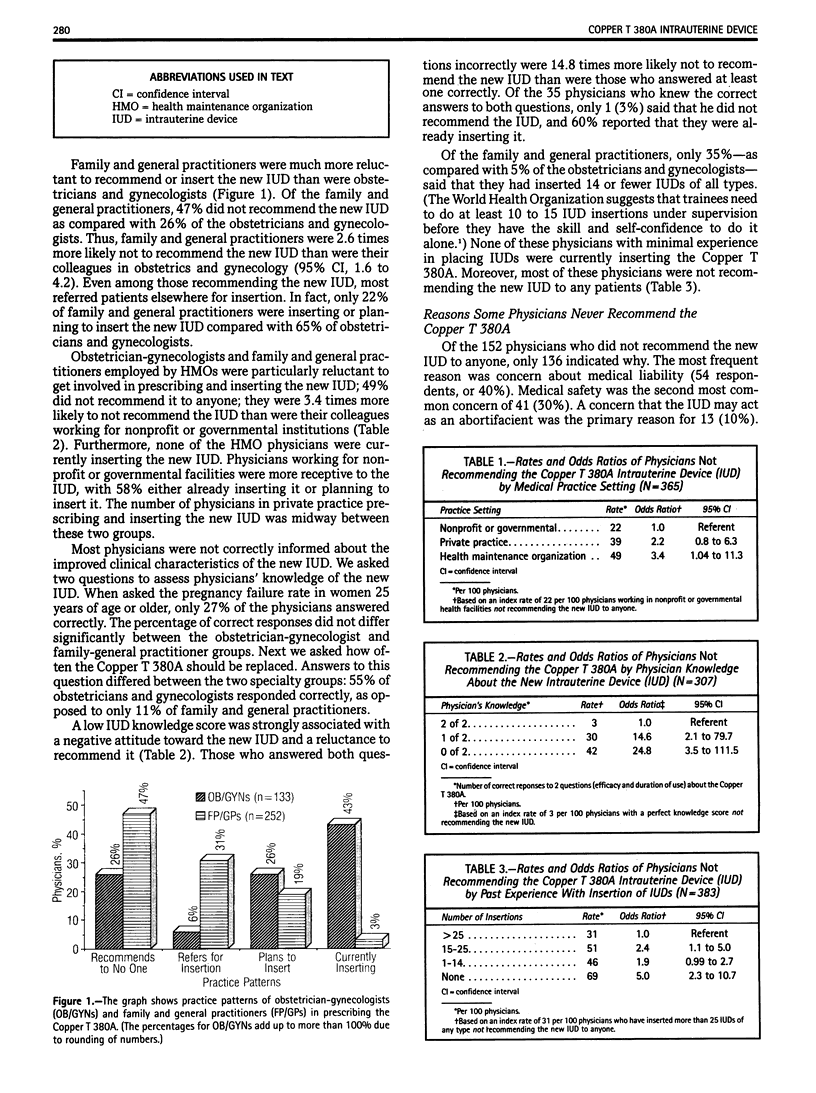
From a questionnaire sent to all obstetricians and gynecologists and all family and general practitioners in San Diego County, California, regarding the Copper T 380A intrauterine device, substantial barriers to prescribing it were identified. Of all physicians responding, 40% reported that they were not recommending the Copper T 380A to anyone, the single most common reason given being concern about medical liability. A lack of knowledge about the new device, a lack of intrauterine device insertion skills, and certain medical practice settings were also important barriers to prescribing it. The new intrauterine device is considered in the context of innovation-diffusion theory. Substantial amounts of education and training and improvement in the medical-legal climate are needed before current barriers to prescribing the new device are removed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burkman R. T. Association between intrauterine device and pelvic inflammatory disease. Obstet Gynecol. 1981 Mar;57(3):269–276. [PubMed] [Google Scholar]
- Cramer D. W., Schiff I., Schoenbaum S. C., Gibson M., Belisle S., Albrecht B., Stillman R. J., Berger M. J., Wilson E., Stadel B. V. Tubal infertility and the intrauterine device. N Engl J Med. 1985 Apr 11;312(15):941–947. doi: 10.1056/NEJM198504113121502. [DOI] [PubMed] [Google Scholar]
- Daling J. R., Weiss N. S., Metch B. J., Chow W. H., Soderstrom R. M., Moore D. E., Spadoni L. R., Stadel B. V. Primary tubal infertility in relation to the use of an intrauterine device. N Engl J Med. 1985 Apr 11;312(15):937–941. doi: 10.1056/NEJM198504113121501. [DOI] [PubMed] [Google Scholar]
- Forrest J. D. The end of IUD marketing in the United States: what does it mean for American women? Fam Plann Perspect. 1986 Mar-Apr;18(2):52–57. [PubMed] [Google Scholar]
- Grimes D. A. Intrauterine devices and pelvic inflammatory disease: recent developments. Contraception. 1987 Jul;36(1):97–109. doi: 10.1016/0010-7824(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Lee N. C., Rubin G. L., Borucki R. The intrauterine device and pelvic inflammatory disease revisited: new results from the Women's Health Study. Obstet Gynecol. 1988 Jul;72(1):1–6. [PubMed] [Google Scholar]
- Sivin I., Schmidt F. Effectiveness of IUDs: a review. Contraception. 1987 Jul;36(1):55–84. doi: 10.1016/0010-7824(87)90061-8. [DOI] [PubMed] [Google Scholar]
- Sivin I., Tatum H. J. Four years of experience with the TCu 380A intrauterine contraceptive device. Fertil Steril. 1981 Aug;36(2):159–163. [PubMed] [Google Scholar]