Abstract
Chronic hypoxia induces smooth muscle cell proliferation and vessel wall remodeling in the vasculature of the lung. One well-characterized component of the hypoxic response is transcriptional activation of genes encoding vascular smooth muscle cell (VSMC) mitogens. We report here that chronic hypoxia can also prolong the growth of human VSMC by inducing telomerase activity and telomere stabilization. We demonstrate that hypoxia induced phosphorylation of the telomerase catalytic component (TERT) and sustained high levels of TERT protein expression in VSMC compared to normoxia. Furthermore, inhibition of telomerase shortened cell life span in hypoxic cultures, whereas constitutive expression of TERT extended the life span of cells under normoxic conditions. Our data indicate that hypoxic induction of telomerase activity could be involved in long-term growth of VSMC and may thus contribute to human vascular disorders.
Hypoxia is an important regulator of physiologic processes, including erythropoiesis, angiogenesis, and glycolysis (6). In the vasculature, chronic hypoxia has been shown to cause proliferation of vascular smooth muscle cells (VSMC), leading to vessel wall remodeling, a key pathophysiologic component of pulmonary hypertension (11). The mechanisms by which hypoxia regulates VSMC growth include direct cell cycle-specific effects, as well as indirect effects, via the regulation of VSMC mitogen production by endothelial cells (10). Hypoxia triggers a cellular adaptive response that is primarily mediated by the transcription factor hypoxia-inducible factor 1 (HIF-1) (20). Expression of HIF-1 target genes serves to maintain cellular homeostasis. Transcriptional activation of hypoxia-responsive genes represents one major component of the vascular cell hypoxic response; however, the mechanisms regulating long-term VSMC proliferation in the vessel wall under chronic hypoxia remain to be elucidated.
Telomere integrity is essential for chromosome stability (5) and therefore plays a crucial role in long-term cell proliferation. Telomere length is maintained by telomerase, a ribonucleoprotein that uses its associated RNA moiety as a template to add telomeric repeats onto chromosome ends. High levels of telomerase activity are readily detected in cancer cells but not in most normal somatic cells, indicating that telomerase function is required for tumor growth (9). Recent studies indicate that telomerase activity is also present in highly proliferative somatic cell types, such as activated lymphocytes (7). Moreover, in later generations of mice lacking telomerase RNA, there is decreased cell proliferation in highly proliferative organs (12), suggesting that telomerase activity may also be required for proliferation and long-term viability in these cells.
The mammalian telomerase RNA component (TERC) has been identified, and its expression was shown to correlate with cell proliferation as well as with telomerase activity in cancer cells (3). Recently, two protein components have been isolated, the mammalian homologue of the Tetrahymena RNA binding protein p80 (TEP1) (19) and the mammalian homologue of the yeast EST2 protein (TERT) (16). TERT mRNA levels were reported to correlate with telomerase activity and to be implicated in the regulation of telomerase activity in cancer cells (16). Furthermore, telomerase activity in telomerase-negative cells can be restored by ectopic expression of TERT, suggesting that in certain cases, TERT is the only limiting factor for telomerase activation (1).
We report here that primary VSMC express high levels of telomerase activity when exposed to hypoxia and demonstrate a causal role of telomerase activation in long-term growth of VSMC under chronic hypoxia. Hypoxia significantly induced the phosphorylation of TERT protein, resulting in increased telomerase activity and prolongation of cell life span. TERT protein levels remained elevated under hypoxia compared to those found under normoxia for significantly longer population doublings (PD). Moreover, constitutive overexpression of TERT prolonged VSMC life span under normoxia. These findings suggest that hypoxia, a well-known regulator of vascular tone and wall structure, not only regulates gene expression but also regulates cell growth and survival via modulating telomerase activation in VSMC.
MATERIALS AND METHODS
Cell culture.
Primary cultures of rat aortic VSMC were grown in Dulbecco's modified Eagle's medium (DMEM; GIBCO, Grand Island, N.Y.) with 10% fetal calf serum and were used between passages 5 and 10. Primary cultures of human aortic VSMC were purchased from BioWhittaker (Walkersville, Md.) and were cultured according to the manufacturer's instructions. A7r5 rat fetal VSMC were obtained from the American Type Culture Collection and were cultured in DMEM with 10% fetal calf serum. Media were changed every 3 days. Exposure of cells to hypoxia (1% oxygen) was performed as described previously (17). Human VSMC were cultured under normoxia (21% oxygen) and hypoxia, and the media and gas were exchanged every 3 to 4 days. Whenever the culture became nearly confluent, the cells were trypsinized, counted using trypan blue exclusion, and subcultured at a density of 3,500 cells/cm2. The cultures were terminated and regarded as senescent when the cell population did not increase in 2 weeks. The senescent phenotype was confirmed by β-galactosidase activity assay as previously described (22). PD were calculated as follows: PD = log (number of cells obtained/initial number of cells)/log 2. For inhibition of telomerase, the cells were cultured in the presence of 3,3′-diethyloxadicarbocyanine (Calbiochem, San Diego, Calif.) or the hexameric telomere-mimic TTAGGG phosphorothioate oligonucleotides (Calbiochem).
Preparation of cell extracts.
Cytoplasmic S-100 extracts were prepared as described previously except for the use of 0.5% 3-[-(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) in place of n-nonanoyl-N-methylglucamide (21). Cell nuclei were isolated by centrifugation (3,000 × g) after homogenization of the cells in hypotonic buffer (10 mM HEPES [pH 8], 10 mM KCl, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 1 μg of leupeptin/μl, 1 μg of aprotinin/μl, and 0.2% Nonidet P-40). The nuclear pellets were resuspended in high-salt buffer (20 mM HEPES [pH 8], 400 mM NaCl, 1 mM EGTA, 1 mM dithiothreitol, 0.5 mM PMSF, 1 μg of leupeptin/μl, 1 μg of aprotinin/μl, 10% glycerol) and were rocked for 30 min at 4°C, and nuclear extracts were obtained by centrifugation (15,000 × g).
Telomerase activity measurement.
Telomerase activity was assayed by two methods: stretch PCR (21) and modified telomeric repeat amplification protocol (TRAP; Oncor, Gaithersburg, Md.) assays. Briefly, in the stretch PCR assay, 20 μl of a 2× reaction mix (100 mM Tris-HCl [pH 8.5], 1 mM dATP, 1 mM TTP, 1 mM dGTP, 10 mM β-mercaptoethanol, 2 mM EGTA, 0.1 mg of bovine serum albumin/ml, 2 mM spermidine, 0.2 mM spermine, 20 pmol of forward primer 5′-GTA AAA CGA CGG CCA GTT TGG GGT TGG GGT TGG GGT TG-3′) was added to 20 μl of S-100 extracts, followed by incubation at 30°C for 60 min. The reaction was terminated by heat inactivation (90°C, 1 min), and the mixture was treated with 0.15 mg of proteinase K/ml at 37°C for 15 min. DNA products were isolated by phenol-chloroform extraction and were then ethanol precipitated with ammonium acetate. The precipitated DNA was dissolved in 39 μl of PCR buffer (20 mM Tris-HCl [pH 8.3], 75 mM KCl, 1.5 mM MgCl2, 0.005% Tween 20) with 5 pmol of reverse primer 5′-CAG GAA ACA GCT ATG ACC CCT AAC CCT AAC CCT AAC CCT-3′. Eleven microliters of a Taq mix (10 μl of PCR buffer, 0.3 μl of 5-U/μl Taq polymerase [Boehringer Mannheim]), 0.2 μl of dNTP mix (10 mM concentrations of dATP, TTP, and dGTP and 1 mM dCTP), and 0.5 μl (3,000 Ci) of [α-32P]dCTP/mmol were added to the mixture, which was preheated at 95°C, and then a PCR (93°C, 1 min; 68°C, 1 min; 72°C, 2 min for 25 cycles) was performed. PCR products were analyzed on 7 M urea–7% polyacrylamide sequencing gel. The TRAP assay was performed according to the manufacturer's instructions. Telomerase activity was visualized by the characteristic 6-bp ladder and was represented as the intensity of the entire ladder. We performed pilot experiments using serially diluted samples and determined the linear range of the assay. All analyses for telomerase activity were carried out within this range. The specificity of telomerase products was determined by their sensitivity to preincubation with RNase for each sample. Telomerase products were quantified using a PhosphorImager analyzer (Molecular Dynamics, Sunnywale, Calif.).
Introduction of TERT-FLAG in A7r5 cells.
The expression vector, pcDNA3 TERT-FLAG, was the kind gift of F. Ishikawa (Department of Life Science, Tokyo Institute of Technology, Tokyo, Japan). Transfection of A7r5 cells was performed by Fugene (Boehringer Mannheim, Indianapolis, Ind.) according to the manufacturer's instructions. Transfected cells were grown in the presence of 1 mg of G418/ml, and approximately 30 G418-resistant cell lines were isolated. All clones were maintained in 250 μg of G418/ml.
Constitutive expression of TERT in human VSMC.
A cDNA encoding human TERT, obtained by PCR cloning using pcDNA3 TERT-FLAG as a template, was cloned into a pLNCX retroviral vector (Clontech, Palo Alto, Calif.). Retroviral stocks were generated by transient transfection of the packaging cell line (PT67; Clontech) and were stored at −80°C until use. Human VSMC (passages 5 to 8) were plated at 5 × 105 per 100-mm-diameter dish 24 h before infections. For infections, the culture medium was replaced with retroviral stocks supplemented with 8 μg of Polybrene (Sigma)/ml, and the cells were incubated at 37°C for 18 h. Forty-eight hours after infections, the infected-cell populations were selected by culture in 1 mg of G418/ml for 7 days. Cells infected with empty vector (pLNCX) were used as the control.
Southern blot analysis.
Genomic DNA extracted by standard methods was digested with HinfI and RsaI and was resolved in 0.5% agarose gels. The blot was then hybridized with 32P-labeled (CCCTAA)3 at 42oC overnight to detect telomeric DNA, washed under highly stringent conditions with 0.1× SSC–0.1% sodium dodecyl sulfate (SDS) (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and analyzed with a PhosphorImager (Molecular Dynamics). The mean length of telomere restriction fragments was calculated by converting the intensity of signals to molecular size based on DNA molecular size markers using the formula L = (ODI)/(ODI /LI), where ODI is the integrated signal at position I and LI is the length of the DNA fragment in position I.
Western blot analysis.
Whole-cell lysates or nuclear extracts were resolved by SDS–6% polyacrylamide gel electrophoresis (PAGE). Proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, Mass.) and incubated with anti-TERT antibody (Calbiochem) or anti-FLAG antibody M2 (Sigma) followed by an anti-rabbit immunoglobulin G–horseradish peroxidase antibody or anti-mouse immunoglobulin G–horseradish peroxidase antibody (Jackson, West Grove, Pa.). Specific proteins were detected using enhanced chemiluminescence (Amersham, Piscataway, N.J.).
In vitro kinase reaction.
S-100 extracts (10 μg) in kinase buffer (50 mM HEPES [pH 7.4], 10 mM MgCl2) were incubated at 30°C in the presence or absence of 10 mM ATP for a time as indicated. In some reactions, the protein kinase inhibitors herbimycin A, tyrphostin A25, H7, myristoylated protein kinase A (PKA) inhibitory peptide 14-22, myristoylated PKC inhibitory peptide 651-658, PKG inhibitory peptide 29-35, and PD98059 (Calbiochem) were also added to the reaction mixture. The reactions were carried out in 10 μl, and half of each mixture was analyzed for telomerase activity.
In vitro labeling of TERT.
For detection of phosphorylated TERT in vitro, S-100 extracts (100 μg) in 50 μl of the reaction mixture (50 mM HEPES [pH 7.4], 10 mM MgCl2, 1 mM ATP, and 1 μCi of [γ-32P]ATP/μl) were incubated for the indicated time intervals. After the reaction was terminated by EDTA, the reaction mixtures were diluted by NP-40 buffer (10 mM Tris-HCl [pH 8], 150 mM NaCl, 1% Nonidet P-40, 0.5 mM PMSF, 1 μg of leupeptin/μl, 1 μg of aprotinin/μl, 50 mM sodium fluoride, 0.2 mM sodium vanadate, 10 U of RNase inhibitor/ml) and were precleared by incubation with 50 μl of protein G-Sepharose (Pierce, Rockford, Ill.). Anti-FLAG antibody was added to precleared samples and incubated for 2 h. Protein G-Sepharose was then added to the samples, which were incubated for an additional 2 h. The immunoprecipitates were washed four times with NP-40 buffer and resuspended in SDS sample buffer, and SDS–6% PAGE analysis was performed.
Orthophosphate labeling.
To detect phosphorylated TERT, cultures were preincubated in labeling medium (phosphate-free DMEM) for 60 min. Then cells were incubated in the labeling medium containing 0.5 mCi of [32P]orthophosphate/ml for 6 h under normoxia (21%) or hypoxia (1%) in the presence or absence of H7. After incubation, whole-cell extracts were prepared and subjected to immunoprecipitation with anti-FLAG antibody as described above, except that radioimmunoprecipitation assay buffer (10 mM Tris-HCl [pH 8], 140 mM NaCl, 5 mM EDTA, 0.025% NaN3, 1% Triton X-100, 1% deoxycholate, 0.1% SDS, 50 mM sodium fluoride, 0.2 mM sodium vanadate, 0.5 mM PMSF, 1 mg of leupeptin/ml, and 1 mg of aprotinin/ml) was used instead of NP-40 buffer. The immunoprecipitates were then resolved by SDS–6% PAGE and transferred onto a PVDF membrane followed by autoradiography and immunoblotting.
RESULTS
Chronic hypoxia extends replicative life span of human VSMC.
We examined the effects of chronic hypoxia on long-term growth and survival of VSMC in culture. Human primary VSMC were cultured under normoxia (21% oxygen) or hypoxia (1% oxygen), and the medium and gas were exchanged every 3 to 4 days. Whenever the culture became nearly confluent, the cells were trypsinized, counted, and subcultivated. As shown in Fig. 1A, chronic hypoxia significantly extended the life span of human VSMC compared to that of normoxic cultures. Similar results were obtained with VSMC from four different donors (two representative results are shown in Fig. 1A, donors 1 and 2). Under hypoxic conditions, VSMC continued to proliferate and maintained the morphology of early passage cells (Fig. 1B, upper panel), whereas normoxic cultures adopted a flat, enlarged shape and ceased proliferation at subconfluent densities, thus manifesting characteristics of senescence (Fig. 1B, lower panel). The cytochemical senescent phenotype was also examined by use of senescence-associated β-galactosidase (SA β-Gal) activity, which has been shown to be a marker of senescence in vascular cells (22) as well as in fibroblasts. Almost all normoxic cultures revealed SA β-Gal activity, while only a few cells were stained in hypoxic cultures at multiple comparable PD (Fig. 1C; data not shown).
FIG. 1.
Hypoxia prolongs the life span of human VSMC. (A) VSMC life span under normoxia and hypoxia. Primary cultures of human VSMC were passaged under normoxia (21%) or hypoxia (1%) for the indicated number of days, and PD since the onset of the experiment were calculated as described in Materials and Methods. Results with cells derived from two different donors (donors 1 and 2) are shown. Similar results were obtained with cells from four different donors. (B) VSMC morphology at late passages. Cultures propagated under hypoxia retained a morphology characteristic of early passage VSMC (PD 38), whereas normoxic cultures adopted a flat, enlarged shape (PD 33). Both cultures shown here were derived from the same donor. Magnification, ×40. (C) SA β-Gal activity. SA β-Gal staining was performed on normoxic cultures at PD 33. Magnification, ×40.
Hypoxia induces telomerase activity.
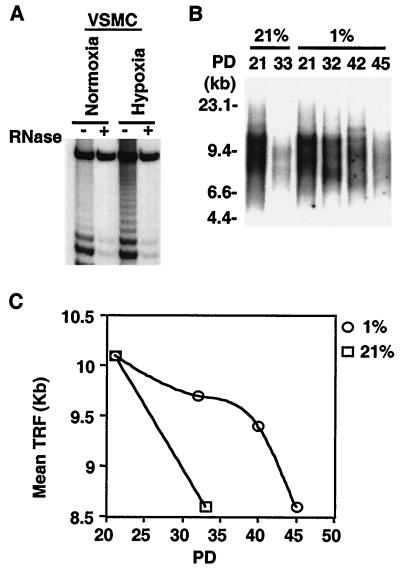
To ascertain whether proliferating VSMC express telomerase activity, cell extracts prepared from subconfluent, primary, cultured rat, and cultured human VSMC were assayed using either the stretch PCR or the TRAP technique. Significant telomerase activity was detected in VSMC cultured under standard conditions (Fig. 2A, normoxia). The level of telomerase activity decreased as cultures approached confluency and was greatly reduced upon serum deprivation of confluent cells, suggesting a correlation of active telomerase with VSMC proliferation (results not shown). VSMC telomerase activity was significantly induced by hypoxia in cultured VSMC as early as 6 h after exposure of cultures to 1% O2 (Fig. 2A, hypoxia).
FIG. 2.
Hypoxia induces telomerase activity. (A) Induction of telomerase activity by hypoxia in VSMC. Subconfluent human VSMC were exposed to normoxia (21%) or hypoxia (1%), and S-100 extracts (10 μg) were analyzed for telomerase activity by the TRAP assay as described in Materials and Methods. Telomerase activity is represented by the characteristic 6-bp ladder and is proportional to the intensity of the entire ladder. The specificity of the TRAP assay is validated by sensitivity to preincubation of the extracts with RNase A. A fourfold induction of telomerase activity was detected at 6 h of hypoxic exposure. Similar results were observed in three independent experiments using either stretch PCR or the TRAP assay. −, absence of RNase; +, presence of RNase. (B) TRF analysis. Genomic DNA (2 μg), isolated from VSMC passaged under normoxia (21%) or hypoxia (1%) for the indicated PD, was digested with HinfI and RsaI and resolved in 0.5% agarose gels. The resultant Southern blot was probed with 32P-labeled (CCCTAA)3 as described in Materials and Methods. (C) Quantitation of mean TRF length in normoxic (21%) and hypoxic (1%) cultures. Length is given in kilobases. Similar results were observed in cultures of VSMC from three different donors.
To ascertain whether telomerase induction by hypoxia affects telomere length, we assayed for terminal restriction fragment (TRF) length by Southern blotting. In contrast to progressive telomere shortening observed in normoxic cultures, telomere length was preserved in hypoxic cultures (Fig. 2B and C), consistent with sustained telomerase activation under hypoxia. Similar preservation of telomere length and of telomerase activity was observed in hypoxic cultures derived from three donors (data not shown).
Inhibition of telomerase activity shortens cell life span of hypoxic cultures.
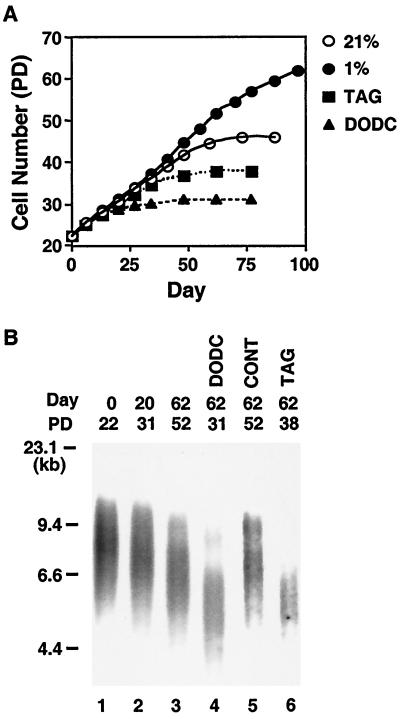
To determine whether activation of telomerase was responsible for prolonging VSMC life span in hypoxic cultures, we inhibited telomerase activity using the telomerase inhibitors 3,3′-diethyloxadicarbocyanine (DODC) and telomere-mimic oligonucleotides (TAG). DODC has previously been shown to bind selectively to single DNA strands rich in guanine, resulting in telomerase inhibition (4). TAG are hexameric, telomere-mimic phosphorothioate oligonucleotides (TTAGGG) that have been shown to suppress telomerase activity (15, 23). Both agents effectively suppressed telomerase activity in human VSMC in the range of 0.1 to 1 μM (data not shown) and significantly shortened the cell life span of hypoxic cultures (Fig. 3A, compare 1% with TAG or DODC). Hypoxic cultures treated with TAG or DODC exhibited a significantly accelerated rate of telomere shortening compared to untreated controls at the corresponding time point (day 62) (Fig. 3B, lanes 3, 4, and 6) or number of PD (Fig. 3B, PD 31, lanes 2 and 4). Control phosphorothioate oligonucleotides (TGTGAG) did not affect telomere length (Fig. 3B, CONT). There was no significant cell death in the cultures treated with control, DODC, or TAG at the concentrations used; VSMC underwent growth arrest and manifested typical senescent morphology, and the expression levels of the three telomerase components were unaffected (data not shown). Thus, our data indicate that the extension of VSMC life span conferred by hypoxia requires elevated telomerase activity and can be reversed by accelerated rates of telomere shortening.
FIG. 3.
Effects of telomerase inhibition on cell life span under hypoxia. (A) Telomerase inhibitors prevent the hypoxia-induced prolongation of VSMC life span. Human VSMC were passaged under normoxia (21%) or hypoxia in the absence of telomerase inhibitors (1%) or hypoxia in the presence of DODC (0.1 μM) or of TAG (1 μM). PD values were calculated as described in Materials and Methods. (B) Progressive telomere shortening by telomerase inhibition. Genomic DNA (2 μg), which was extracted from VSMC passaged under hypoxia in the absence of telomerase inhibitors (lanes 1 to 3) or in the presence of DODC (lane 4), of control oligonucleotides (lane 5), or of telomere-mimic oligonucleotides (TAG, lane 6) for the indicated number of days, was analyzed for TRF length by Southern blotting as shown in Fig. 2. Length is given in kilobases. The number of PD since the onset of the experiment for each culture is also indicated.
Constitutive expression of TERT prolongs the growth of human VSMC.
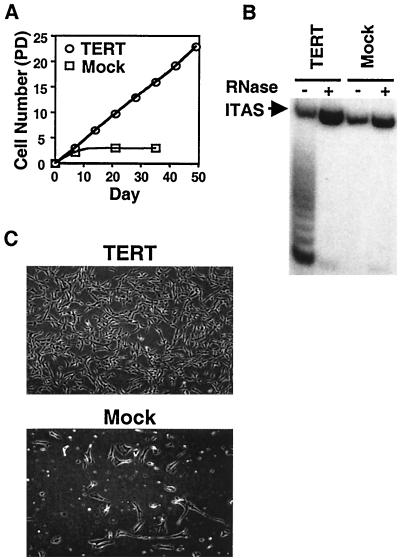
To prove a causal role for telomerase expression in long-term VSMC growth, we constitutively overexpressed TERT in human VSMC. Primary VSMC cultures were infected with a retroviral vector expressing the TERT open reading frame, and several clones were isolated. After cloning, the replicative life span of TERT-transfected clones was examined and compared with that of mock-transfected clones. The time point when transfected clones were propagated to confluency in a 100-mm-diameter dish was designated day 0 (approximately 20 to 30 PD after infection). Mock-transfected clones ceased proliferating at 3 to 5 PD after cloning, whereas TERT-overexpressing lines showed a longer life span and are still proliferating to date (Fig. 4A). TERT lines revealed high levels of telomerase activity, whereas no telomerase activity was detected in mock-transfected cells after cloning (Fig. 4B). Consistent with high levels of telomerase activity in TERT lines, telomere length was maintained after extensive passaging (data not shown). The morphology of TERT lines after extensive passage remained similar to that of early passage cell populations (Fig. 4C, upper photograph), while mock-transfected clones revealed the typical morphology of senescence after cloning (Fig. 4C, lower photograph). Similar observations were obtained from all four lines that we examined and from pooled populations of TERT-overexpressing VSMC. Furthermore, TERT lines expressed markers of VSMC, such as smooth muscle light-chain kinase, and retained normal growth control in response to serum deprivation and high cell density (data not shown). Thus, constitutive expression of TERT greatly extended the life span of human VSMC but did not appear to induce a transformed phenotype.
FIG. 4.
Overexpression of TERT extends the life span of human VSMC. (A) Replicative life span of TERT-overexpressing VSMC lines. Human VSMC were infected with a retroviral vector encoding TERT or the empty vector (Mock), and infected clones were isolated and expanded. The time point when isolated lines had been propagated to near confluency in a 100-mm-diameter dish was designated day 0 (approximately 20 to 30 PD after infection). Cultures were subsequently passaged under normoxia for the indicated time, and PD were calculated as for Fig. 1. Similar results were obtained from four independently isolated lines as well as from pooled populations. (B) Telomerase activity in TERT-infected VSMC lines. S-100 extracts (0.1 μg) were prepared from TERT- or mock-infected clones at day 0 and were analyzed by TRAP assay as described for panel A. −, absence of RNase; +, presence of RNase; ITAS, internal telomerase assay standard. (C) Morphology of TERT-overexpressing lines. After extensive passage, TERT-expressing lines manifested a morphology similar to that of early passage VSMC (upper photograph), while mock-infected lines revealed the typical morphology of senescence (lower photograph).
Protein phosphorylation of TERT contributes to telomerase activation.
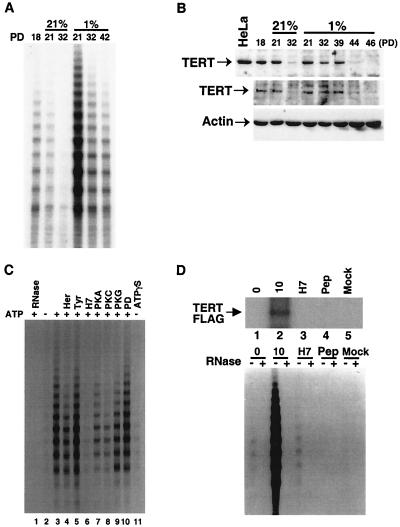
Telomerase activity was significantly higher in VSMC cultured under hypoxia than under normoxia at any PD value and decreased in hypoxia only at very high PD values (Fig. 5A). We noted that levels of the telomerase RNA component and TEP1 were not altered in our long-term hypoxic cell culture model as determined by reverse transcriptase PCR (data not shown). By contrast, sustained high expression of TERT protein was observed in hypoxic cultures for a significantly longer period and higher PD than in normoxic cultures (Fig. 5B, upper panel for nuclear extracts and middle panel for whole-cell extracts). TERT protein was significantly reduced earlier in normoxic cells (Fig. 5B, PD 32) and considerably later in hypoxic cells (Fig. 5B, PD 44 and 46). However, there was no strict quantitative relationship between telomerase activity and TERT expression levels. For example, although telomerase activity in hypoxic cultures was 10 times as high as that in normoxic cultures at PD 21 (Fig. 5A), TERT protein levels were similar at PD 21 under normoxia or hypoxia (Fig. 5B). In addition, short-term hypoxic exposure did not affect TERT protein levels, whereas telomerase activity was induced by four- to fivefold (Fig. 2A and data not shown). Therefore, in addition to TERT expression levels, these findings imply the existence of additional posttranslational mechanisms for the regulation of telomerase activity.
FIG. 5.
Protein phosphorylation contributes to telomerase activation. (A) Telomerase activity in normoxic and hypoxic cultures. S-100 extracts (10 μg) from cell populations of donor 1 under normoxia (21%) and hypoxia (1%) at the indicated PD were assayed for telomerase activity using the stretch PCR assay as described in Materials and Methods. Similar results were obtained in cultures from three different donors. (B) TERT levels under normoxia and hypoxia. Nuclear extracts (15 μg, upper panel) or whole-cell lysates (50 μg, middle panel), extracted from normoxic (21%) or hypoxic cultures (1%) at the indicated PD, were analyzed by Western blotting using an anti-TERT antibody. Nuclear extracts (20 μg) from HeLa cells were used as a positive control. The same blot was reprobed with anti-β-actin antibody to verify equal loading among lanes (lower panel). Similar results were obtained in VSMC from three different donors. (C) Effects of protein kinase inhibitors on in vitro telomerase activity. S-100 extracts (10 μg) prepared from serum-deprived rat VSMC were incubated at 30°C for 10 min in the presence (+) or absence (−) of 10 μM ATP, as indicated. Lanes 4 to 10 included the following protein kinase inhibitors: herbimycin A (10 μM) (Her); tyrphostin A25 (100 μM) (Tyr); H7 (50 μM); PKA inhibitory peptide (1 μM); PKC inhibitory peptide (50 μM); PKG inhibitory peptide (100 μM); and PD98059 (50 μM) (PD). Lane 11 included 10 μM ATPγS. After incubation, reaction mixtures were assayed for telomerase activity using the stretch PCR assay. Results shown are representative of three similar experiments. Similar results were obtained in human VSMC. (D) Phosphorylation of TERT in vitro. S-100 extracts (100 μg) prepared from TERT-FLAG cells (lanes 1 to 4) and mock-transfected cells (lane 5) that had been deprived of serum for 3 days were supplemented with 1 mM ATP and 1 μCi of [γ32P]ATP/μl and were immunoprecipitated with anti-FLAG antibody immediately (lane 1) or after 10 h of incubation (lanes 2 to 5). H7 (10 mM) was present in lane 3, and 20 μg of FLAG-competing peptide (Pep) was present in lane 4. Immunoprecipitates were then resolved by SDS–6% PAGE, and phosphorylated TERT-FLAG was detected by autoradiography (upper gel). One-tenth of each immunoprecipitate was also directly assayed for telomerase activity using the stretch PCR assay (lower gel). −, absence of RNase; +, presence of RNase. 0, 0 h; 10, 10 h.
Recent reports have implied sensitivity of telomerase activity to protein phosphorylation (13). We hypothesized that the activation of telomerase by hypoxia may be mediated by such a mechanism. To test this hypothesis, we first examined the effects of ATP on telomerase activity in vitro. Cell extracts prepared from rat VSMC that had been deprived of serum for 7 days (a situation that greatly suppresses telomerase activity) were incubated with ATP in the presence of 10 mM MgCl2 and were then assayed for telomerase activity. As shown in Fig. 5C, telomerase activity was dramatically stimulated by 10 min of incubation with ATP prior to the assay (Fig. 5C, lanes 2 and 3). adenosine-5′-γ-thiotriphosphate (ATPγS) did not induce telomerase activity (Fig. 5C, lane 11), indicating that ATP hydrolysis is required for this activation. The kinase inhibitors H7 (Fig. 5C, lane 6), PKC (Fig. 5C, lane 8), and to a lesser degree, PKA inhibitory peptide (Fig. 5C, lane 7) prevented the stimulation of telomerase activity, whereas the tyrosine kinase inhibitors herbimycin A (Fig. 5C, lane 4) and tyrphostin (Fig. 5C, lane 5) or the MAP kinase kinase (MEK) inhibitor PD98059 (Fig. 5C, lane 10) had no significant effect. Similar results were observed in human VSMC (data not shown). These findings suggest that protein phosphorylation, in particular H7-sensitive kinase(s), mediates the stimulation of telomerase activity by ATP in vitro.
More recently, phosphorylation of TERT has been shown to regulate telomerase activity in cancer cells (14). Thus, we speculated that phosphorylation of the TERT component may be responsible for the activation of telomerase in VSMC. Rat fetal VSMC (A7r5 line) were transfected with TERT-FLAG expression vector, and lines which constitutively express TERT-FLAG protein were established. Expression of TERT-FLAG protein was determined by Western blotting using anti-FLAG antibody (data not shown). To determine whether protein phosphorylation of TERT contributed to telomerase activation, we prepared cell extracts from TERT-FLAG cells deprived of serum for 3 days to suppress telomerase activity and created an in vitro kinase reaction in the presence of 1 mM ATP and 1 μCi of [γ-32P]ATP/μl. In vitro kinase reaction mixtures were then immunoprecipitated with anti-FLAG antibody. One-tenth of the immunoprecipitates was also assayed for telomerase activity. TERT-FLAG protein was strongly phosphorylated 10 min after incubation with ATP and was associated with a stimulation of telomerase activity (Fig. 5D, lane 2). Preincubation with FLAG peptide completely abolished detection of TERT-FLAG as well as abolishing telomerase activity in the immunoprecipitate (Fig. 5D, lane 4). In addition, no TERT-specific band was detected in the reaction mixtures of mock-transfected cells (Fig. 5D, lane 5), suggesting that the anti-FLAG antibody specifically immunoprecipitated the telomerase complex. H7 inhibited both telomerase activity and the phosphorylation of TERT-FLAG protein (Fig. 5D, lane 3), indicating that phosphorylation of TERT is involved in stimulating telomerase activity.
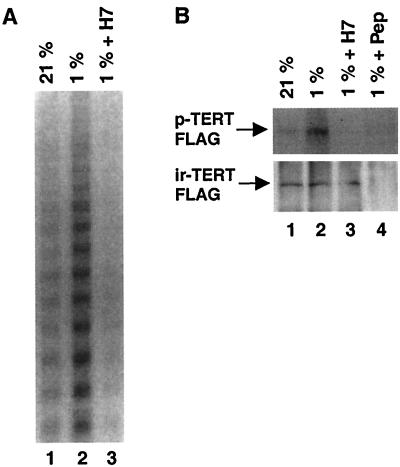
We next sought to determine whether TERT protein is phosphorylated in cells in response to hypoxia and whether phosphorylation was required for telomerase activation. VSMC were treated with H7 and exposed to hypoxia for 6 h. Treatment with H7 prevented the hypoxic induction of telomerase activity in both rat and human VSMC cultures (Fig. 6A). We therefore examined phosphorylated TERT levels in normoxic and hypoxic cultures in the absence or presence of H7. VSMC transfected with TERT-FLAG expression vector were labeled with [32P]orthophosphate for 6 h under normoxia or hypoxia, and TERT-FLAG protein was immunoprecipitated with anti-FLAG antibody. Phosphorylated TERT was detected in both normoxic and hypoxic cultures; however, phosphorylation was remarkably enhanced by hypoxia (Fig. 6B, upper panel, lanes 1 and 2). Western blotting confirmed comparable levels of immunoreactive TERT in the immunoprecipitates (Fig. 6B, lower panel). Furthermore, treatment of hypoxic cultures with H7 reduced levels of phosphorylated TERT (Fig. 6B, lane 3), suggesting that protein phoshorylation of TERT modulates telomerase activity under hypoxia.
FIG. 6.
Phosphorylation of TERT protein in VSMC by hypoxia. (A) Effect of protein kinase inhibitors on telomerase activity in cultured cells in vivo under hypoxia. Human VSMC were cultured under normoxia (left lane) or hypoxia for 6 h in the presence (right lane) or absence (middle lane) of H7 (50 μM). After hypoxic exposure, S-100 extracts were prepared and incubated at 30°C for 10 min in the presence of 10 μM ATP. Reaction mixtures were assayed for telomerase activity using the stretch PCR assay. Results shown are representative of three independent experiments and were similar between human and rat VSMC. (B) Phosphorylation of TERT in vivo. VSMC transfected with TERT FLAG expression vector were labeled with [32P]orthophosphate for 6 h under normoxia (lane 1) or hypoxia in the absence (lanes 1, 2, and 4) or the presence (lane 3) of H7 (10 μM). TERT FLAG protein was immunoprecipitated with anti-FLAG antibody in the absence (lanes 1 to 3) or the presence (lane 4) of FLAG-competing peptide. The immunoprecipitates were then subjected to SDS–6% PAGE analysis and transferred onto a PVDF membrane followed by autoradiography to detect phosphorylated TERT FLAG (p-TERT FLAG) and by immunoblotting with anti-FLAG antibody to detect immunoreactive TERT FLAG (ir-TERT FLAG).
DISCUSSION
Hypoxia regulates multiple physiologic and pathological processes in the body. Many cardiopulmonary disorders are associated with hypoxia and result in increased morbidity and mortality. Hypoxia has profound effects on blood vessel tone and wall structure by regulating gene expression and cell-cell interaction in vascular cells (10). In the lung vasculature, chronic hypoxia leads to smooth muscle cell hyperplasia with medial hypertrophy of the pulmonary arterioles and the clinical picture of pulmonary hypertension. These VSMC proliferative changes have been attributed to the increased growth factors released by endothelial cells in response to hypoxia. It has previously been reported that VSMC have a hypoxic response independent of endothelial cells. In VSMC, hypoxia stimulates the transcription of heme oxygenase-1, the principal enzyme responsible for carbon monoxide generation (18), as well as stimulating the expression of E2F-1, a key cell cycle-specific transcription factor (17). Thus, independently of endothelial cells, hypoxia modulates VSMC gene expression and cell cycle progression. In the present report, we have demonstrated a causal role of telomerase activation and telomere function for the long-term growth and viability of VSMC under conditions of hypoxia. First, we detected relatively high levels of telomerase activity in VSMC that are not usually observed in normal somatic cells. Second, chronic hypoxia extended the growth and life span of human VSMC and was associated with telomere stabilization as well as higher levels of telomerase activity. Third, the life span of hypoxic human VSMC was effectively shortened by inhibition of telomerase activity, while it was extended in normoxic cells by constitutive expression of TERT. Combined, these data support a physiologic role for telomerase activity in regulating VSMC proliferation under hypoxic conditions.
Multiple adaptive responses to hypoxia involve transcriptional activation of vascular cell gene expression. In the case of telomerase, additional mechanisms control its activity in response to hypoxia. We propose here mechanisms whereby telomerase is activated by hypoxia via phosphorylation of TERT protein at least partly by a PKC-dependent pathway. Consistent with this, the potent mitogenic response to hypoxia has been reported to be partially mediated by PKC activation (2). We observed that high levels of TERT protein were sustained in long-term hypoxic cultures, while short-term hypoxia did not change expression levels of TERT. The half-life of telomerase has been shown to be relatively long (24 h) (8). Thus, it is assumed that constitutive activation of telomerase by hypoxia is regulated mainly by protein phosphorylation of TERT or potentially other, additional posttranslational mechanisms.
Telomerase activity appeared to be related to cell proliferation in VSMC; however, whether telomerase activity is necessary for short-term proliferation of VSMC remains unclear. Recent studies using mice null for the telomerase RNA component indicated that telomerase deficiency per se did not affect short-term cell proliferation in embryonic fibroblasts but did reduce long-term cell viability in proliferative organs. Thus, it is likely that mitogenic stimuli, for instance hypoxia, activate both telomerase and the cell cycle machinery, allowing VSMC to continuously proliferate with minimal telomere shortening. Consistent with this, hypoxic exposure was shown to promote VSMC proliferation by increasing the expression of the cell cycle transcription factor E2F-1 (17). Enhanced cell cycle progression combined with telomere stabilization may be important in highly proliferative pathological conditions in the vasculature that are characterized by sustained and long-standing vessel wall remodeling.
In summary, we have shown a critical role for telomerase activity in long-term growth and viability of VSMC in culture in response to hypoxia. Hypoxia induced telomerase activity in VSMC by stimulating TERT protein phosphorylation, resulting in telomere stabilization and the maintenance of high expression levels of TERT. Telomerase activation under chronic hypoxia may result in enhanced and sustained VSMC proliferation and vessel wall remodeling, findings characteristic of proliferative vascular disorders, such as pulmonary hypertension. Further investigations on telomerase and telomere function on the growth and long-term survival of VSMC would provide new insights into the pathophysiology and treatment of human vasculopathies.
ACKNOWLEDGMENTS
This work was supported by the American Heart Association and by National Institutes of Health grants RO1 HL55454 and SCOR 1P50 HL56398.
We thank F. Ishikawa for pcDNA3 TERT-FLAG and L. Lynch for expert technical assistance. We also thank J. Johnson for her expert assistance in the preparation of the manuscript.
REFERENCES
- 1.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C-P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 2.Dempsey E C, McMurtry I F, O'Brien R F. Protein kinase C activation allows pulmonary artery smooth muscle cells to proliferate to hypoxia. Am J Physiol. 1991;260:L136–L145. doi: 10.1152/ajplung.1991.260.2.L136. [DOI] [PubMed] [Google Scholar]
- 3.Feng J, Funk W D, Wang S-S, Weinrich S L, Avilion A A, Chiu C-P, Adams R R, Chang E, Allsopp R C, Yu J, Le S, West M D, Harley C B, Andrews W H, Greider C W, Villeponteau B. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 4.Fu W, Begley J G, Killen M W, Mattson M P. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999;274:7264–7271. doi: 10.1074/jbc.274.11.7264. [DOI] [PubMed] [Google Scholar]
- 5.Greider C W. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 6.Guillemin K, Krasnow M A. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 7.Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek M A, Shay J W, Ishioka S, Yamakido M. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995;155:3711–3715. [PubMed] [Google Scholar]
- 8.Holt S E, Wright W E, Shay J W. Regulation of telomerase activity in immortal cell lines. Mol Cell Biol. 1996;16:2932–2939. doi: 10.1128/mcb.16.6.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L C, Coviello G M, Wright W E, Weinrich S L, Shay J W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 10.Kourembanas S, Morita T, Christou H, Liu Y, Koike H, Brodsky D, Arthur D, Mitsialis S A. Hypoxic responses of vascular cells. Chest. 1998;114:25S–28S. doi: 10.1378/chest.114.1_supplement.25s-a. [DOI] [PubMed] [Google Scholar]
- 11.Kourembanas S, Morita T, Liu Y, Christou H. Mechanisms by which oxygen regulates gene expression and cell-cell interaction in the vasculature. Kidney Int. 1997;51:438–443. doi: 10.1038/ki.1997.58. [DOI] [PubMed] [Google Scholar]
- 12.Lee H-W, Blasco M A, Gottlieb G J, Horner J W I, Greider C W, DePinho R A. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Zhao L L, Funder J W, Liu J-P. Protein phosphatase 2A inhibits nuclear telomerase activity in human breast cancer cells. J Biol Chem. 1997;272:16729–16732. doi: 10.1074/jbc.272.27.16729. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Zhao L, Zhiyong Y, Funder J W, Liu J-P. Telomerase is controlled by protein kinase Cα in human breast cancer cells. J Biol Chem. 1998;273:33436–33442. doi: 10.1074/jbc.273.50.33436. [DOI] [PubMed] [Google Scholar]
- 15.Mata J E, Joshi S S, Palen B, Pirruccello S J, Jackson J D, Elias N, Page T J, Medlin K L, Iversen P L. A hexameric phosphorothioate oligonucleotide telomerase inhibitor arrests growth of Burkitt's lymphoma cells in vitro and in vivo. Toxicol Appl Pharmacol. 1997;144:189–197. doi: 10.1006/taap.1997.8103. [DOI] [PubMed] [Google Scholar]
- 16.Meyerson M, Counter C M, Eaton E N, Ellisen L W, Steiner P, Caddle S D, Ziaugra L, Beijersbergen R L, Davidoff M J, Liu Q, Aacchetti S, Haber D A, Weinberg R A. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 17.Morita T, Mitsialis S A, Koike H, Liu Y, Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J Biol Chem. 1997;272:32804–32809. doi: 10.1074/jbc.272.52.32804. [DOI] [PubMed] [Google Scholar]
- 18.Morita T, Perrella M A, Lee M-E, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA. 1995;92:1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama J-I, Saito M, Nakamura H, Matsuura A, Ishikawa F. TLP1: a gene encoding a protein component of mammalian telomerase is a novel member of WD repeats family. Cell. 1997;88:875–884. doi: 10.1016/s0092-8674(00)81933-9. [DOI] [PubMed] [Google Scholar]
- 20.Semenza G L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 21.Tatematsu K-I, Nakayama J-I, Danbara M, Shionoya S, Sato H, Omine M, Ishikawa F. A novel quantitative “stretch PCR assay” that detects a dramatic increase in telomerase activity during the progression of myeloid leukemias. Oncogene. 1996;13:2265–2274. [PubMed] [Google Scholar]
- 22.van der Loo B, Fenton M J, Erusalimsky J D. Cytochemical detection of a senescence-associated β-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp Cell Res. 1998;241:309–315. doi: 10.1006/excr.1998.4035. [DOI] [PubMed] [Google Scholar]
- 23.Zahler A M, Williamson J R, Cech T R, Prescott D M. Inhibition of telomerase by G-quartet DNA structures. Nature. 1991;350:718–720. doi: 10.1038/350718a0. [DOI] [PubMed] [Google Scholar]