Abstract
How rare protein-disrupting risk variants implicated in autism spectrum disorders (ASDs) interact or functionally converge is unknown. Pintacuda et al.1 perform proteomics in induced human neurons and identify more than 1,000 interactions, 90% of which were not previously reported, emphasizing the importance of cell-type- and isoform-specific protein interactions in ASD.
How rare protein-disrupting risk variants implicated in autism spectrum disorders (ASDs) interact or functionally converge is unknown. Pintacuda et al.1 perform proteomics in induced human neurons and identify more than 1,000 interactions, 90% of which were not previously reported, emphasizing the importance of cell-type- and isoform-specific protein interactions in ASD.
Main text
Neuropsychiatric disease research is currently guided by the promise that understanding genetic risk factors should lead us toward a mechanistic understanding of these disorders. In autism spectrum disorder (ASD), hundreds of risk genes have been identified and implicate pathways related to synaptic signaling, Wnt signaling, mTOR pathways, and chromatin remodeling.2, Single-cell transcriptomics has enabled identification of the cell types, developmental time points, and cellular processes where risk genes might converge. The expression of known ASD risk genes is concentrated in, but not exclusive to, excitatory neurons and the timing of expression peaks during fetal brain development.3,4 While genomics and transcriptomics have led to understanding the underlying biology of genetic risk for ASD in general, with exceptions,5,6,7 few studies have tackled proteomics in a neuronal cell framework.
Here, Pintacuda et al.1 create human neuronal protein-protein interaction (PPI) networks for a subset of ASD risk genes. By experimentally probing ASD-related PPIs in human stem-cell-derived neurons, the authors generated an unprecedented resource. Indeed, ∼90% of the >1,000 identified interactions are novel, potentially because most previous protein interaction studies were performed in non-neural cell lines or tissues. This work, along with data probing the PPI of ASD risk genes in mouse cortical neurons,6 demonstrates that most neurally relevant PPIs may be unknown. The authors uncover genes within the network that have not been implicated in disease and identify novel interactions of specific isoforms related to relevant disease pathways. This speaks to the enormous importance of interrogating the cell-type-specific protein interactomes of genes implicated in neuropsychiatric disorders to inform upon their molecular mechanisms.
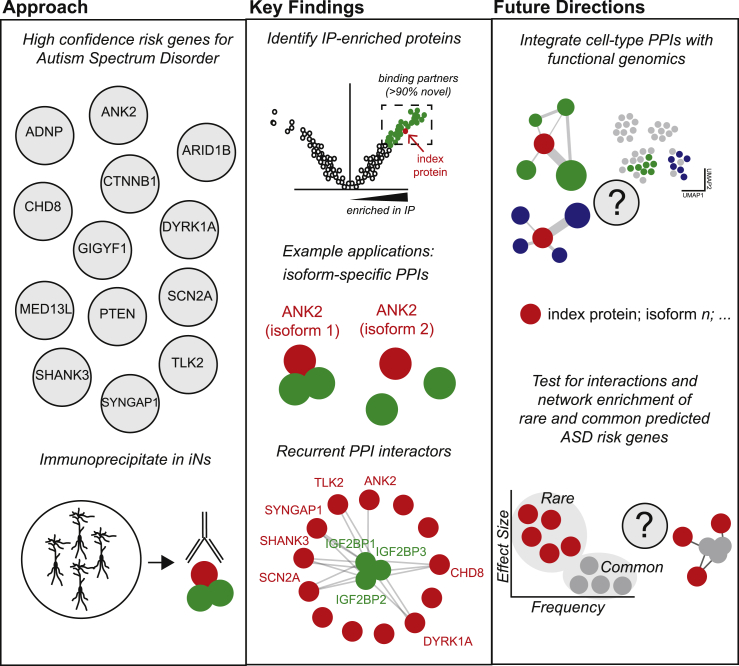
The PPI network was established from 13 of the highest-confidence risk genes associated with ASD8 (Figure 1). PPIs in stem-cell-derived neurogenin-2 induced excitatory neurons (iNs) were established through immunoprecipitation (IP) of these index proteins followed by mass spectrometry and quantified via liquid chromatography and tandem mass spectrometry (LC-MS/MS). The quality of each IP-MS experiment was assessed by enrichment of the index protein (Figure 1), demonstrating greater than 80% replication, as well as validation through western blotting. Between 3 (PTEN) and 604 (DYRK1A) interactors were identified per index protein, with little overlap between interacting proteins. In an independent experiment in postmortem human cerebral cortex, a more heterogeneous material, the authors observed moderate replication of protein interactors (∼40%), which may reflect either cell-type specificity, developmental differences, or technical effects. Nevertheless, the set of overlapping interactors between induced neurons in vitro and human brain are of high confidence.
Figure 1.
Summary of approach, key findings, and potential future directions in Pintacuda et al.
The PPI network established in this paper is derived from genes associated with ASD in the largest study of rare variation to date.8 PPIs in stem-cell-derived neurogenin-2 induced excitatory neurons (iNs) were then established through immunoprecipitation (IP) experiments. Key findings include neuron- and isoform-specific and recurrent protein interactions. Future directions may involve integrating cell-type-specific proteomics with other functional genomic modalities and data.
The authors highlight that these PPI networks can be used to nominate novel ASD risk genes and convergent pathways. They produce a “social Manhattan” plot to illuminate potential candidate ASD risk genes that have fallen below statistical significance in past studies but participate in PPIs with significant risk genes. They go on to prioritize highly interactive proteins to uncover central players within the ASD interactome.
The majority of interactors identified were specific to one index protein. But, insulin-like growth factor 2 mRNA-binding proteins (IGF2BP1-3), which together form a m6A-reader complex, were highly interconnected, each interacting with at least 5 index proteins, suggesting that these proteins may be major mediators in convergent biological pathways for ASD risk (Figure 1). Confirming this will require an expanded set of risk gene PPIs.
The authors also leverage published bulk RNA sequencing (RNA-seq) data to demonstrate co-expression patterns supportive of their identified PPIs. Interestingly, their PPI networks overlap with genes differentially expressed in layer II/III cortical glutamatergic neurons in ASD.9 This complements transcriptomic studies identifying enrichment of ASD risk gene expression in developing layer II/III projection neurons that underlie interhemispheric and cortical-cortical connectivity.3 These PPI data support the convergence in ASD on this class of neurons.
In addition, the authors focus on the integral membrane protein Ankyrin2 (ANK2), which exhibits multiple isoforms, including a neuron-specific transcript that retains a giant exon (exon 37). Using a CRISPR-Cas9 editing strategy, the authors generate a modified line incapable of generating giant ANK2 (ANK2 KO) while preserving other isoforms. While iNs were not viable in the ANK2 KO line, proteomic analysis of neural progenitor cells (NPCs) revealed numerous disease-relevant interactors that required the giant exon for interaction (Figure 1). The giant exon harbors many patient mutations, so these neuron-specific interactions may be an essential mechanism by which ANK2-related mutations increase risk for ASD.
This exciting work provides a rationale and framework for expanded PPI studies with ASD risk genes while highlighting many questions to explore with existing functional genomic data (Figure 1). Do rare de novo and common inherited variants inhabit the same PPI networks, similar to rare de novo and transmitted variants? Do co-expression modules identified in single-cell neuronal RNA-seq datasets overlap with the PPI networks identified? If so, single-cell RNA co-expression modules might provide insight in circumstances where IP-competent antibodies are not available or when interrogating PPI networks within less-accessible cell types and tissues. While it is not clear what mechanisms drive the observed differences in cell-type-specific PPI networks, existing RNA-seq data might provide insight on the role of cell-type-specific isoform expression. Indeed, transcriptomic work suggests that cell-type-enriched protein isoforms comprise substantial disease-relevant signals.10 The results here show that cell-type-specific transcriptomic interrogation of disease should be complemented by further probing of cell-type-specific PPIs. Pintacuda and colleagues not only present a valuable resource for the ASD field but also motivate us to consider cell-type-specific proteomics while investigating one of the pressing questions in ASD genetics: how do risk genes interact and functionally converge to impact cellular phenotypes?
Acknowledgments
We thank members of the Geschwind lab for helpful discussions and acknowledge funding from NIH grants to D.H.G. (NIMH: U01 MH115746, R01 MH110927, R01 MH100027) and K.W.E. (NIEHS: K00 ES032608), as well as funding from the Simons Foundation.
Declaration of interests
The authors declare no competing interests.
References
- 1.Pintacuda G., Hsu Y.-H.H., Tsafou K., Li K.W., Martin J.M., Riseman J., Biagini J.C., Ching J.K.T., Mena D., Gonzalez-Lozano M.A., et al. Protein interaction studies in human induced neurons indicate convergent biology underlying autism spectrum disorders. Cell Genom. 2023;3 doi: 10.1016/j.xgen.2022.100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de la Torre-Ubieta L., Won H., Stein J.L., Geschwind D.H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 2016;22:345–361. doi: 10.1038/nm.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parikshak N.N., Luo R., Zhang A., Won H., Lowe J.K., Chandran V., Horvath S., Geschwind D.H. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155:1008–1021. doi: 10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polioudakis D., de la Torre-Ubieta L., Langerman J., Elkins A.G., Shi X., Stein J.L., Vuong C.K., Nichterwitz S., Gevorgian M., Opland C.K., et al. A single-cell transcriptomic atlas of human neocortical development during mid-gestation. Neuron. 2019;103:785–801.e8. doi: 10.1016/j.neuron.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corominas R., Yang X., Lin G.N., Kang S., Shen Y., Ghamsari L., Broly M., Rodriguez M., Tam S., Trigg S.A., et al. Protein interaction network of alternatively spliced isoforms from brain links genetic risk factors for autism. Nat. Commun. 2014;5:3650. doi: 10.1038/ncomms4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murtaza N., Cheng A.A., Brown C.O., Meka D.P., Hong S., Uy J.A., El-Hajjar J., Pipko N., Unda B.K., Schwanke B., et al. Neuron-specific protein network mapping of autism risk genes identifies shared biological mechanisms and disease-relevant pathologies. Cell Rep. 2022;41 doi: 10.1016/j.celrep.2022.111678. [DOI] [PubMed] [Google Scholar]
- 7.Sakai Y., Shaw C.A., Dawson B.C., Dugas D.V., Al-Mohtaseb Z., Hill D.E., Zoghbi H.Y. Protein interactome reveals converging molecular pathways among autism disorders. Sci. Transl. Med. 2011;3:86ra49. doi: 10.1126/scitranslmed.3002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satterstrom F.K., Kosmicki J.A., Wang J., Breen M.S., De Rubeis S., An J.Y., Peng M., Collins R., Grove J., Klei L., et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180:568–584.e23. doi: 10.1016/j.cell.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velmeshev D., Schirmer L., Jung D., Haeussler M., Perez Y., Mayer S., Bhaduri A., Goyal N., Rowitch D.H., Kriegstein A.R. Single-cell genomics identifies cell type-specific molecular changes in autism. Science. 2019;364:685–689. doi: 10.1126/science.aav8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandal M.J., Zhang P., Hadjimichael E., Walker R.L., Chen C., Liu S., Won H., van Bakel H., Varghese M., Wang Y., et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362 doi: 10.1126/science.aat8127. [DOI] [PMC free article] [PubMed] [Google Scholar]