Abstract
Brunner's gland hyperplasia (BGH) or Brunneroma is an uncommon benign proliferative lesion of the small intestine. It is primarily found in the duodenal submucosa, and its main function is to create an alkaline-based mucus to protect the duodenum from stomach acid. BGH can manifest as hyperplasia or a polypoidal tumor and is often discovered incidentally during endoscopy or imaging. Less than 200 cases have been reported in the literature, and it rarely causes gastrointestinal bleeding. In this case report, we present a case of a 25-year-old male who presented with bloody stools and fainting due to severe anemia and underlying gastrointestinal pathology.
Keywords: tumor, gi oncology, young adult, gastrointestinal bleeding, brunner’s gland hyperplasia
Introduction
Although the small bowel makes up 75% of the digestive tract, tumors of this region are exceedingly uncommon (5%), with duodenal pathology being more prevalent than jejunal and ileal tumors [1]. It is extremely rare to get gastrointestinal bleeding due to Brunner's gland hyperplasia (BGH). It can manifest from hyperplasia to a polypoidal tumor characterized as a Brunner's gland adenoma or hamartoma, respectively [2]. Ten percent of all benign duodenal tumors are BGH, an uncommon benign proliferative lesion with an estimated prevalence of 0.008% [1]. The primary physiological purpose of Brunner's gland, which is situated in the duodenal submucosa, is to create an alkaline-based mucus to shield the duodenal mucosa from the acid released in the stomach [3]. Less than 200 cases have been reported, and it has seldom been discussed in the literature [4]. BGH is mainly discovered incidentally during endoscopy or imaging techniques. Thus, a duodenal polyp or mass must be histopathologically examined for a specific diagnosis and course of treatment [1-2]. We report a case of a young 25-year-old male who presented with the complaint of bloody stools and an episode of fainting secondary to severe anemia and underlying gastrointestinal pathology.
Case presentation
A 25-year-old male presented in the emergency department with a complaint of dizziness, an episode of fainting, and blood in his stool for the past one week. He mentioned the stools to be black initially, followed by fresh blood mixed with the stool. He had never had these symptoms before. He did not report any hematemesis or epigastric pain. He denied any use of medications or drugs. He was a nonsmoker and nonalcoholic. He had no past medical or surgical history. On examination, the patient looked pale with no abdominal pain. The digital rectal examination was positive for melena, but no fresh blood was seen. The laboratory investigations revealed his hemoglobin to be 6.3 (Table 1).
Table 1. Laboratory investigations at admission.
White Blood Cells (WBC), Red Blood Cells (RBC), Hemoglobin (Hgb), Hematocrit (Hct), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC), Red Cell Distribution Width-Coefficient of Variation (RDW-CV), Platelets (PLT), Mean Platelet Volume (MPV), Platelet Distribution Width (PDW), Absolute Neutrophil Count (ANC), Reticulocyte Number (Retic #), Reticulocyte Percentage (Retic %), Prothrombin Time (PT), International Normalized Ratio (INR), Aptt, Urea, Creatinine, Sodium, Potassium, Chloride, Bicarbonate, Bilirubin Total, Protein, Albumin, Alkaline Phosphatase (ALK Phos), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Troponin-T, High Sensitivity C-Reactive Protein (HS CRP).
| Parameters | Results | Reference Range |
| WBC | 11.3 | 4.0 – 10.0 x10^3/uL |
| RBC | 2.1 | 4.5 – 5.5 x10^6/uL |
| Hgb | 6.3 | 13.0 – 17.0 gm/dL |
| Hct | 18.6 | 40.0 – 50.0 % |
| MCV | 88.2 | 83.0 – 101.0 fL |
| MCH | 29.9 | 27.0 – 37.0 pg |
| MCHC | 33.9 | 31.5 – 34.5 gm/dL |
| RDW-CV | 13.9 | 11.6 – 14.0 % |
| Platelets | 342 | 150 – 450 x10^3/uL |
| MPV | 9.3 | 9.7 – 13.2 fL |
| PDW | 9.0 | 9.4 -10.6 fL |
| Absolute Neutrophil count Auto # (ANC) | 6.0 | 2.0 – 7.0 x10^3/uL |
| Lymphocytes Auto # | 4.4 | 1.0 – 3.0 x10^3/uL |
| Monocytes Auto# | 0.6 | 0.2 – 1.0 x10^3/uL |
| Eosinophil Auto# | 0.26 | 0.02 – 0.50 x10^3/uL |
| Basophils Auto# | 0.04 | 0.02 – 0.10 x10^3/uL |
| Neutrophil Auto % | 53.1 | 50 – 70 % |
| Lymphocyte Auto % | 38.8 | 20 – 40 % |
| Monocyte Auto % | 5.4 | 0.0 – 15.0 % |
| Eosinophil Auto % | 2.3 | 0.0 – 6.0 % |
| Basophil Auto % | 0.4 | 0.0 – 2.0 % |
| Retic # | 153.7 | 50.0 – 100.0 x10^3/uL |
| Retic % | 7.2 | 0.5 – 2.5 % |
| Prothrombin time | 13.7 | 9.4 – 12.5 seconds |
| INR | 1.2 | 1.0 – 1.2 |
| Apt* | 29.4 | 29.1 – 36.5 seconds |
| Urea | 3.8 | 2.5 – 7.8 mmol/L |
| Creatinine | 92 | 62 – 106 umol/L |
| Sodium | 141 | 133 – 146 mmol/L |
| Potassium | 3.7 | 3.5 – 5.3 mmol/L |
| Chloride | 1.7 | 95 – 108 mmol/L |
| Bicarbonate | 28 | 22 – 29 mmol/L |
| Bilirubin T | 6 | 0 – 21 umol/L |
| Total Protein | 63 | 60 – 80 gm/L |
| Albumin | 30 | 35 – 50 gm/L |
| ALK phosphate | 70 | 40 – 129 U/L |
| ALT | 20 | 0 – 41 U/L |
| AST | 20 | 0 – 40 U/L |
| Troponin-T HS | *8 | 3 – 15 ng/L |
| CRP | *3.3 | 0.0 – 5.0 mg/L |
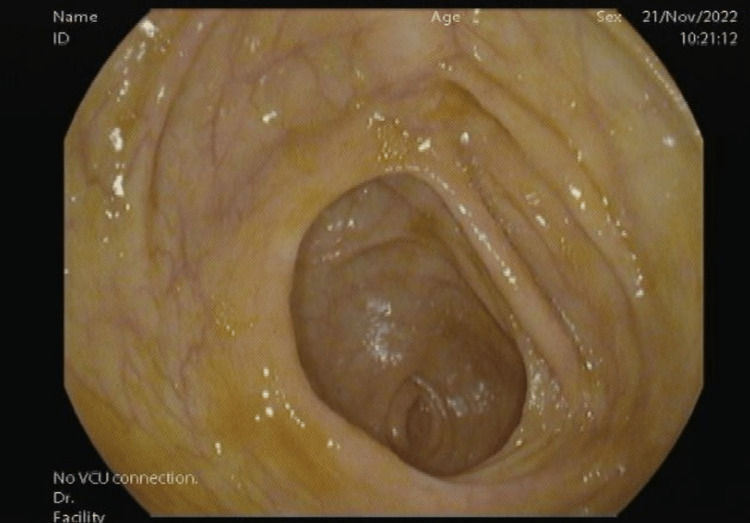
He required a blood transfusion of 1 unit. He was vitally stable with a blood pressure of 120/72, a heart rate of 81, a temperature of 37.5 °C, and a respiratory rate of 18. He was referred to Gastroenterology for colonoscopy and esophagogastroduodenoscopy. The colonoscopy was unremarkable for any lower gastrointestinal bleeding as the whole of the colonic mucosa was normal (Figure 1).
Figure 1. Normal colonic mucosa under colonoscopy; no evidence of bleeding.
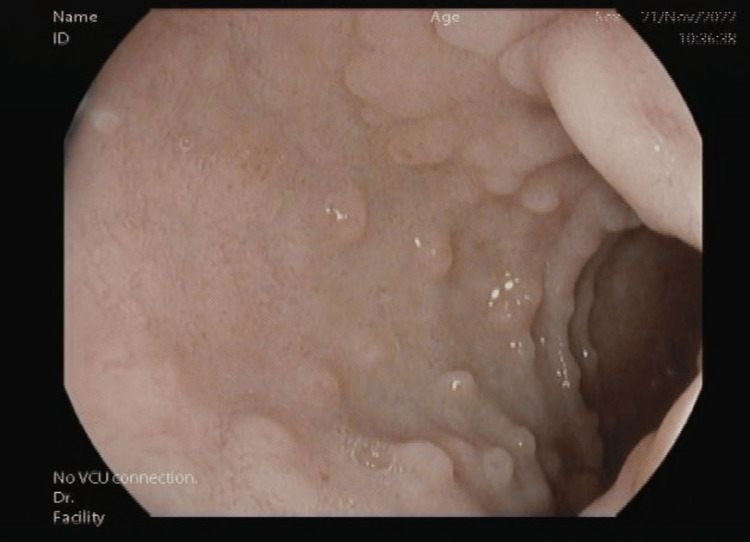
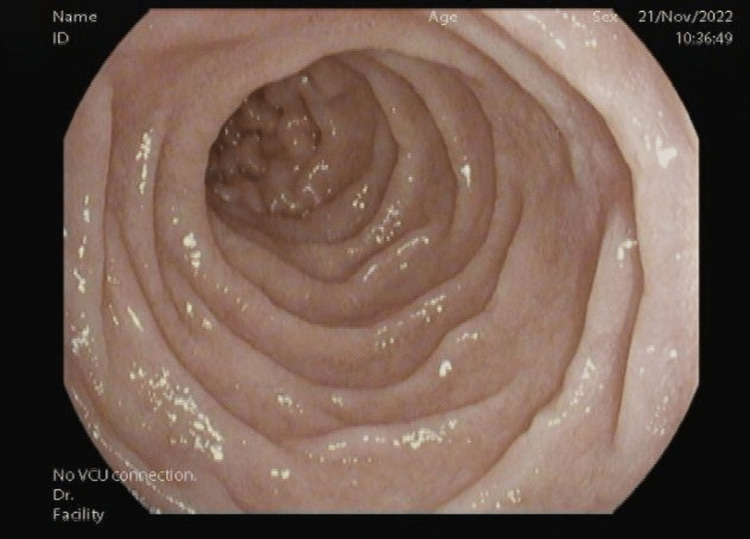
Esophagogastroduodenoscopy showed duodenal D1 nodularity, suspecting it to be BGH (Figure 2). Esophagogastroduodenoscopy was negative for any ulcers or lesions in the esophagus and parts of the stomach, such as the fundus, body, or antrum. The biopsies were taken for histopathological examination. D2 showed normal mucosa (Figure 3).
Figure 2. D1 mucosa showing nodularity with no signs of active bleeding.
Figure 3. D2 mucosa normal with no nodularity, mass, or bleeding.
The histopathology biopsy results of the nodules demonstrated small bowel mucosa with BGH, although negative for villous atrophy, crypt hyperplasia, dysplasia, or malignancy. He was therefore treated with supportive measures. Conservative management was opted for as the nodular lesions were multiple and less than 5 mm (2-3 mm). Esmoprazole, 40 mg BID, is administered IV as a 5-day inpatient. During the hospital course, he did not develop further bleeding episodes such as melena or hematemesis. Upon discharge, he has prescribed pantoprazole 40 mg for 3 months. The follow-up for 90 days was uneventful. His hemoglobin also improved.
Discussion
The American Institute of Radiologic Pathology refers to lesions that are less than 5 mm in size as BGH, while those that are more than 5mm as "Brunner's gland hamartoma" [1]. The literature review says that it’s quite uncommon to get upper gastrointestinal bleeding from isolated Brunner gland hyperplasia. According to histology, BGH is a single or multiple nodular lesion comprising enlarged Brunner's glands divided by fibrous septa. The isolated mass known as Brunner's gland hamartoma comprises a variety of Brunner's glands, ducts, smooth muscle, fibrous tissue, adipose tissue, lymphocytes, etc [1,2,5,6]. These tubulomucinous glands release an alkaline mucin that shields the mucosa of the duodenum from an acidic pH. These are mainly located in the proximal portion of the duodenum's mucosa and submucosa. BGH makes up 3.9%-10% of all benign duodenal polyps and masses. The patients are usually in their 5th or 6th decade with no gender predominance [1,2,6,7]. Though, this patient was relatively young.
The underlying etiology of BGH is unknown. Numerous articles and studies have hypothesized that BGH results from excessive acid secretion, Helicobacter pylori infection, or inflammation encouraging the production of alkaline secretions by the Brunner's gland cells [1-4]. The reduced pancreatic exocrine function may also be the cause. However, it is now believed that the primary component causing BGH is the exocrine modifying factor, which includes the hormone, vagus nerve, and intestinal mucous membrane factor [3,4].
The clinical presentation of Brunner Gland hyperplasia is usually asymptomatic and found incidentally in the elderly. The symptomatic presentation occurs in terms of gastrointestinal bleeding as hematemesis or melena. However, there have been about 15 cases reported for acute hemorrhage leading to hypovolemic shock [5-7].
If BGH is discovered incidentally, it is controversial to treat and requires further research [8]. There are no proper reviews on the medical management of BGH, particularly the smaller diffuse lesions (<5 mm). However, proton pump inhibitors are prescribed to reduce gastric acid secretion if found to be the etiology [3]. All polypoidal masses larger than 2 cm in size, whether symptomatic or not, require treatment. Endoscopic snare-polypectomy or surgical excision is the current treatment option [8].
Regarding our case, there wasn’t any clear evidence of a known underlying cause or previous clinical presentation related to BGH's diagnosis.
Conclusions
The conclusion that can be drawn from this case report is that BGH should be considered a potential cause of upper gastrointestinal bleeding in adolescents, even though it is a rare condition. This case report highlights the importance of considering rare causes of upper gastrointestinal bleeding in young adults, as it can lead to severe anemia and melena. Healthcare providers should be aware of the potential for rare causes of upper gastrointestinal bleeding in young adults and consider BGH a potential diagnosis. Further research is needed to better understand the prevalence and clinical presentation of BGH in adolescents.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Brunner’s gland hyperplasia: a massive duodenal lesion. Bhatti S, Alghamdi M, Omer E. Cureus. 2020;12:0. doi: 10.7759/cureus.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunner's gland hyperplasia: a rare cause of gastrointestinal bleeding. Kamani L, Raj R, Ali R, Siddiqi F. Clin Pract. 2020;10:1267. doi: 10.4081/cp.2020.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunner's gland hyperplasia: treatment of severe diffuse nodular hyperplasia mimicking a malignancy on pancreatic-duodenal area. Lee WC, Yang HW, Lee YJ, et al. J Korean Med Sci. 2008;23:540–543. doi: 10.3346/jkms.2008.23.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Case report of Brunner's gland hyperplasia: a rare "mimic" of malignant pathology. McCafferty J, Tokhi A, Krishnamoorthy S, Pande G. Int J Surg Case Rep. 2021;81:105827. doi: 10.1016/j.ijscr.2021.105827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Large Brunner’s gland hyperplasia with bleeding: a case report. Okutomi Y, Kato T, Aizawa H, et al. Case Rep Surg. 2021;2021:8861308. doi: 10.1155/2021/8861308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner's gland hyperplasia associated with lipomatous pseudohypertrophy of the pancreas presenting with gastrointestinal bleeding: a case report. Nguyen LC, Vu KT, Vo TT, et al. World J Clin Cases. 2021;9:9670–9679. doi: 10.12998/wjcc.v9.i31.9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner’s gland hamartoma of the duodenum: a literature review. Zhu M, Li H, Wu Y, et al. Adv Ther. 2021;38:2779–2794. doi: 10.1007/s12325-021-01750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massive upper gastrointestinal hemorrhage in Brunner’s gland hamartoma of duodenum. Dhali A, Ray S, Ghosh R, Sarkar A, Dhali GK. Cureus. 2021;13:0. doi: 10.7759/cureus.15875. [DOI] [PMC free article] [PubMed] [Google Scholar]