Abstract
Background
In a prior report, no patient with rodenticidal hepatotoxicity who met Kochi criteria (MELD score ≥36 or baseline INR ≥6 with hepatic encephalopathy) (PMID: 26310868) for urgent liver transplantation survived with medical management alone. Plasma exchange (PLEX) may improve survival in these patients.
Objectives
We describe our experience with low-volume PLEX (PLEX-LV) in treating rodenticide ingestion induced hepatotoxicity in children.
Methods
From prospectively collected database of rodenticidal hepatotoxicity patients managed as in-patient with department of Hepatology from December 2017 to August 2021, we retrospectively studied outcomes in children (≤18 years). Hepatotoxicity was categorized as acute liver injury (ALI, coagulopathy alone) or acute liver failure (ALF, coagulopathy and encephalopathy). Kochi criteria was used to assess need for urgent liver transplantation. The primary study outcome was one-month survival.
Results
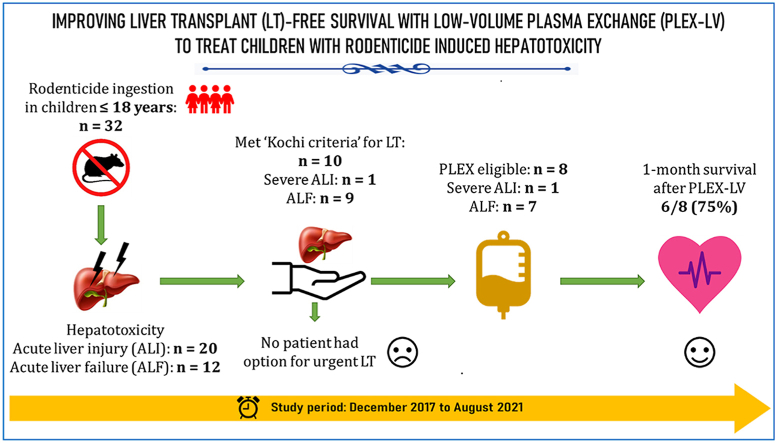
Of the 110 rodenticidal hepatotoxicity patients, 32 children (females: 56%; age: 16 [4.7–18] years; median, range) constituted the study patients. The study patients presented 4 (1–8) days after poison consumption (impulsive suicidal intent:31, accidental:1). Twenty children (62%) had ALI [MELD: 18 (8–36)] and 12 (38%) had ALF [MELD: 37 (24–45)].
All children received standard medical care, including N-acetyl cysteine; ALF patients also received anti-cerebral edema measures. None of the patient families opted for liver transplantation. Seventeen children (ALI: 6, ALF: 11) were treated with PLEX-LV (3 [1–5] sessions, volume of plasma exchanged per session: 26 [13–38] ml/kg body weight) and peri-procedure low dose prednisolone.
At 1 month, 28 of the 32 children (87.5%) were alive (4 ALF patients died). Of 10 children who met Kochi listing criteria for urgent liver transplantation, two children were ineligible for PLEX-LV (due to hemodynamic instability) and of the remaining 8 children treated by PLEX-LV, 6 (75%) survived.
Conclusions
PLEX-LV shows promise as an effective non-liver transplant treatment in children with rodenticidal hepatotoxicity.
Keywords: acute liver failure, Kochi criteria, rat killer, yellow phosphorus
Abbreviations: AKI, acute kidney injury; ALF, acute liver failure; ALI, acute liver injury; FFP, fresh frozen plasma; MELD, model for end-stage liver disease; PLEX-LV, low-volume plasma exchange
Graphical abstract
Rodenticide ingestion (mostly with suicidal intent) has become an important cause of hepatotoxicity in Tamil Nadu state in India.1 The acute hepatotoxicity in rodenticide poisoning is due to the yellow phosphorus or metal phosphides contained in it.2 The clinical severity depends on the amount of yellow phosphorus consumed and the delay in presentation to a medical facility.3 There is no antidote and urgent liver transplantation is recommended for patients with rodenticide induced acute liver failure (ALF).4,5 There is dearth of published literature guiding management of rodenticidal hepatotoxicity in children.6
Therapeutic plasma exchange (PLEX) improves survival in ALF patients of different etiologies.7, 8, 9 PLEX is postulated to benefit by removing inflammatory debris/macro-molecules in circulation in this clinical scenario. Raised plasma levels of one such inflammatory macro-molecule, von Willebrand factor (VWF), an endothelial activation marker, predicts poor outcome in patients with rodenticide hepatotoxicity.10 In a previous study, we described the potential of VWF reducing strategy in managing these patients.10 Our understanding of the role of PLEX in managing rodenticidal hepatotoxicity is evolving.
In this current study, we describe our experience with low volume PLEX (PLEX-LV) to treat children with rodenticidal hepatotoxicity.
Methods
Patient
A prospectively collected database of patients with rodenticidal hepatotoxicity managed as in-patient with department of Hepatology, from December 2017 to July 2021 was retrospectively analysed. Demography, laboratory parameters, treatment and one-month survival of all children ≤18 years old with rodenticidal hepatotoxicity were noted.
Liver Disease Severity Assessment
The patients were categorized based on severity of liver injury as—acute liver injury (ALI, abnormal liver function tests (LFT) with coagulopathy) and ALF (abnormal LFT with coagulopathy and encephalopathy). Model for end-stage liver disease (MELD) score11 and Sequential Organ Failure Assessment (SOFA) score12 were calculated at baseline. The highest MELD score during hospital stay was also recorded. Listing criteria (MELD score ≥36 or baseline INR ≥6 with hepatic encephalopathy)13 for urgent liver transplantation (Kochi criteria) in patients with rodenticide induced hepatotoxicity, was used to identify patients requiring urgent liver transplantation.
The markers used to assess the reticulo-endothelial activation were plasma VWF, serum ferritin and CD25 levels. The normal values are plasma VWF 50–150%, serum ferritin 15–140 ng/ml and serum soluble CD25 levels 1555–10,800 pg/ml.
Treatment Given
All patients received standard supportive management14 including parenteral N-acetyl cysteine. Anti-cerebral edema measures were given to patients with encephalopathy. Urgent liver transplantation was advised as first line treatment option in patients who met listing criteria for the same. Decision to initiate PLEX-LV was taken by the treating clinician on a case-to-case basis in patients with severe ALI or ALF. Hemodynamic instability and active sepsis were considered as contraindications for PLEX.15
Surveillance and Management of Infections
All patients were screened for sepsis (by clinical examination and baseline blood culture). Repeat blood cultures were taken if and when patient developed fever or had any clinical deterioration. All patients were given prophylactic intravenous antibiotics (cefoperazone-sulbactum). Presence of active sepsis was considered a contra-indication for PLEX-LV. If a patient developed bacteremia/clinical sepsis after starting PLEX-LV, further PLEX sessions were withheld and antibiotics were upgraded, after obtaining repeat blood culture specimen.
PLEX-LV Protocol16
PLEX-LV was done by either membranous or centrifugal technique via a central venous access. During each session of PLEX-LV, 50% of estimated plasma volume (calculated as per Kaplan formula17) was exchanged with an equal volume of synchronously transfused fresh frozen plasma (FFP). An initial target of three PLEX sessions (performed daily or on alternate days) was made. The need for further PLEX sessions was decided on a case-to-case basis by the treating clinician based on clinical progress. Most patients treated with PLEX-LV also received a small dose of steroid (body weight >50 kg: Tablet Prednisolone 10 mg once daily, 25–50 kg: 5 mg once daily and <25 kg: 2.5 mg once daily) peri-PLEX which was rapidly tapered afterwards. Low dose prednisolone was given to ameliorate the cytokine storm seen in ALF patients, while avoiding the risk of sepsis with higher doses of prednisolone.18 As increased gut permeability may contribute to ALF pathogenesis, we give oral zinc acetate for 2–4 weeks with an aim to reduce gut permeability.16 The dose of elemental zinc was 50 mg and 25 mg twice daily for body weight >25 kg and <25 kg, respectively. Calcium supplements were also given prior to and during each session of PLEX-LV.
Outcome
Primary outcome was survival at one month. This was documented from medical records and by telephonic contact in patients who did not come for follow up visit.
Statistical Analysis
The descriptive analysis of demography, laboratory data, treatment and outcome were presented as median and range. Comparison of variables among the groups was done by non-parametric tests. Statistical Package for the Social Sciences (SPSS) version 21.0, (IBM Bangalore, India) was used for the statistical analysis. P-value less than 0.05 was considered significant.
Results
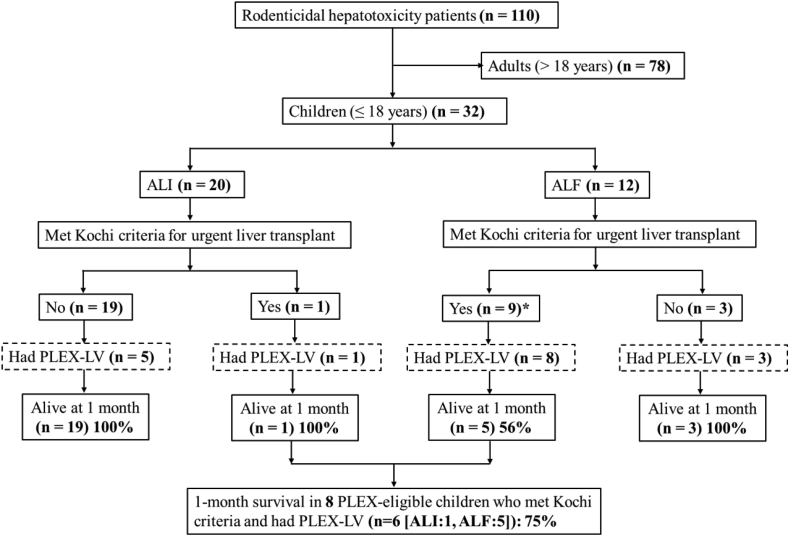
Figure 1 shows the flow diagram of patients with rodenticidal hepatotoxicity included in this study.
Figure 1.
Flow diagram of the patients with rodenticidal hepatotoxicity. ALI acute liver injury, ALF acute liver failure, PLEX-LV low-volume plasma exchange. ∗Two children were PLEX-ineligible, one of these children had PLEX-LV.
Demography and Baseline Characteristics
During the study period, 110 patients (78 adults; 32 children [29%]) with rodenticidal hepatotoxicity were managed in our department. Among the study cases (32 children), there were 18 (56%) females and the age was 16 (4.7–18; median, range) years. Two children were less than 10 years old (aged 4.7 and 7.4 years). The remaining 30 were aged ≥12 years.
Table 1 shows the baseline characteristics of children with rodenticide hepatotoxicity classified into 2 groups (ALI and ALF) based on the highest disease severity during the hospital stay. The highest MELD score in those with ALI and ALF were 26 (12–36) and 37 (25–50) respectively. The rodenticide consumption was with impulsive suicidal intent in 30 (93.8%), got poisoned in 1 (3.1%) and accidental in 1 (3.1%). Yellow phosphorus (3%) paste (Ratol®) was consumed by 28 children and the exact chemical was not known in the remaining 4 children. The parents of these 4 children were able to re-collect it as rat poison, these patients had elevated liver enzymes and prolonged INR after 4 days, typically seen in phosphorus poisoning. The interval from poison consumption to presentation to our institution was 4 (1–8) days.
Table 1.
Baseline Characteristics of Children with Rodenticidal Hepatotoxicity in ALI and ALF Groups.
| Parameters | ALI (n) = 20 | ALF (n) = 12 | P value |
|---|---|---|---|
| Age (years) | 16 (4.7–18) | 16.3 (7.4–18) | 0.741 |
| Females (%) | 10 (50%) | 8 (67%) | 0.355 |
| Interval from poison ingestion to hospital admission (days) | 3 (1–7) | 5 (1–8) | 0.207 |
| Quantity of poison consumed (mg) (n = 19) | 225 (112–450, n = 13) | 338 (112–450, n = 6) | 0.406 |
| Total bilirubin (mg/dl) | 2.7 (0.4–11.6) | 8.6 (2.4–19.9) | <0.001 |
| INR | 2.5 (1.1–10.0) | 8 (2.6–10.0) | <0.001 |
| Serum creatinine (mg/dL) | 0.59 (0.43–1.25) | 0.66 (0.42–3.07) | 0.509 |
| VWF antigen (U/dl) | 334 (109–649, n = 19) | 538 (271–685, n = 10) | 0.015 |
| Serum ferritin (ng/ml) | 1625 (161–21180, n = 15) | 1378 (12–34807, n = 11) | 0.872 |
| Serum soluble CD25 levels (pg/ml) | 2800 (1000–4750, n = 8) | 1800 (1300–6200, n = 5) | 0.944 |
| MELD score at admission | 18 (8–36) | 37 (24–45) | <0.001 |
| SOFA score at admission | 2 (0–4) | 6 (3–13) | <0.001 |
| Fulfilled Kochi criteria | 1 | 9 | <0.001 |
ALI, acute liver injury; ALF, acute liver failure; MELD, model for end-stage liver disease; SOFA, sequential organ failure assessment; VWF, von Willebrand factor; INR, International normalised ratio. All values are in number (percentage) and median (range).
The median length of hospital stay was 7.5 (5–16) days for children with ALI and 9 (3–26) days for those with ALF.
Treatment Details
All patient families opted against urgent liver transplantation. All children received standard management, including N-acetyl cysteine; ALF patients also received anti-cerebral edema measures. Ten patients with ALF and two with ALI were treated in intensive care unit (ICU) and the remaining 20 patients were treated in high dependency unit (HDU). Nine children (ALI: 7, ALF: 2) received FFP transfusions apart from FFPs transfused during PLEX-LV. Twenty-two children (ALI: 14, ALF: 8) were given oral zinc acetate. Fourteen children (ALI: 5, ALF: 9) received prednisolone.
PLEX-LV Details
Seventeen patients (ALI: 6, ALF: 11) had PLEX-LV (membranous 14, centrifugal 3). The median number of PLEX sessions was 3 (1–5) sessions. The volume of plasma exchanged at each session was 26 (13–38) ml/kg body weight. PLEX-LV was initiated within 2 (1–4) days of admission to our hospital.
Two children aged 4.7 and 7.4 years had transient hypotension within 10 min of starting PLEX and were managed with intravenous fluids and inotropes for 4 h. No other PLEX related complications were noted.
Extra-hepatic Complications
Of the 32 study children, 13 developed extrahepatic complications/needed mechanical ventilation (Table 2).
Table 2.
Extrahepatic Complications/Ventilation in 32patientswith Rodenticide Induced Hepatotoxicity.
| Type of complication | Incidence | No. of patients who were PLEX-eligible and had PLEX-LV | 1-month survival in PLEX-eligible patients who had PLEX-LV |
|---|---|---|---|
| Grade ≥2 hepatic encephalopathy | 12 (37.5%) | 9 | 8 (88.8%) |
| Acute kidney injury | 9 (28.1%) | 7 | 7 (100%) |
| Infection (bacteremia, fungemia) | 5 (15.6%) | 3 | 3 (100%) |
| Mechanical ventilation | 5 (15.6%) | 3 | 1 (33.3%) |
| Patients with >1 of above complications/mechanical ventilation | 10 (31.2%) | 9 | 7 (77.7%) |
PLEX-LV low-volume plasma exchange.
Hepatic Encephalopathy
Twelve children had hepatic encephalopathy of ≥ grade 2 (grade 2: 5, grade 3: 5, grade 4: 2). Two of them had focal seizures and computerized tomography brain showed cerebral edema. Eleven of them underwent PLEX-LV and 8 children survived. The two children with grade 4 encephalopathy, two with grade 3 and none with grade 2 encephalopathy died.
Mechanical Ventilation
Five children required mechanical ventilation. The indication for mechanical ventilation was low sensorium in 3 children and severe metabolic acidosis with hypotension in two. Four among the five ventilated children died. Three children had ventilator associated pneumonia and two of them died.
Acute Kidney Injury (AKI)
Nine children had AKI according to the Kidney Disease Improving Global Outcomes (KDIGO)19 criteria. Six children had stage 1 AKI, one had stage 2 AKI and two had stage 3 AKI. The 2 children with stage 3 AKI got Continuous Renal Replacement Therapy (CRRT) and both of them died (one of them also had PLEX). The remaining 7 children (stage 1 AKI [n = 6]; stage 2 AKI [n = 1] were treated with PLEX and survived.
Bacteremia and Other Infections
Four children developed bacteremia (E coli [n = 1], Stenotrophomonas [n = 1], Acinetobacter [n = 2]). Three of them developed bacteremia after PLEX-LV was started, which was detected in the subsequent culture. All three had completed the target PLEX-LV sessions (3, 3 and 5 sessions respectively) and were on steroids when bacteremia developed. One child (with Acinetobacter) was not treated with PLEX-LV. The antibiotics were upgraded to intravenous meropenem in three children and polymyxin in one child based on antibiotic susceptibility. Further PLEX sessions were not done and central venous access for PLEX was removed. All the 4 children survived.
One child had urinary tract infection (Pseudomonas) in addition to Acinetobacter bacteremia. He was treated with appropriate antibiotics and underwent PLEX-LV (along with steroids) and survived.
Another child in whom PLEX-LV was initiated, the baseline blood culture sample grew Candida tropicalis after his death.
Overall Survival at 1 Month in Children with Rodenticidal Hepatotoxicity
At one month, all 20 children with ALI (100%) and 8 children with ALF (66.6%) survived. The overall 1-month survival of children with rodenticidal hepatotoxicity (n = 32) was 87.5%. Survival was worse in those who needed mechanical ventilation (20%).
Patients Who Met Kochi Criteria and Were PLEX-Ineligible
Two children with ALF fulfilling Kochi criteria were ineligible for PLEX-LV: one child was treated with PLEX and the other was not. The child who was treated with PLEX, aged 15.7 years, had shock requiring 3 inotropes, and candidemia. He was treated with CRRT in addition to PLEX-LV. His baseline blood culture sample grew C. tropicalis after death. The other child aged 15 years with ALF also had shock requiring 3 inotropes and succumbed at 40 h after admission and PLEX could not be performed.
Survival in PLEX-eligible Children Who Met Kochi Criteria for Urgent Liver Transplantation
Among the 10 children who met Kochi criteria for liver transplant, 8 (ALI 1, ALF 7) were PLEX-eligible. Nine children (ALI 1, ALF 8) underwent PLEX-LV. One of the 9 patients was treated with PLEX despite having contra-indication (hemodynamic instability and candidemia [detected later]).
Of 8 PLEX-eligible patients who met Kochi criteria and underwent PLEX-LV [aged 16.3 (7.4–18) years, females (75%), MELD 37 (36–41), INR 9.5 (5.5–10), hepatic encephalopathy grade 2: 4, grade 3: 2], 6 patients (75%) survived (Figure 1).
Cause of Death
Four children died. Three were treated with PLEX-LV. Two (boys, aged 15.5 and 17 years) who had normal sensorium at admission, developed cerebral edema and died despite anti-cerebral edema treatment and 4 sessions of PLEX. One child (boy, aged 15.5 years) had candidemia, multiorgan failure and shock and died after one session of PLEX. Another child (girl, aged 15 years) had multiorgan failure and shock and died at 40 h after admission, she did not have PLEX-LV.
Rodenticidal Hepatotoxicity in Adults
During the same study period, 78 adult patients (>18 years) with rodenticidal hepatotoxicity were managed in our department. Thirty of them met Kochi criteria and 18 underwent PLEX-LV. The one-month survival in those who underwent PLEX-LV was 56% (n = 10).
Discussion
In this study, we present our experience with use of PLEX-LV to treat rodenticidal hepatotoxicity in children. Of 32 children with rodenticidal hepatotoxicity, one-month survival was 87.5%. We also demonstrate efficacy of PLEX-LV in children with ALI and ALF caused by phosphorus poisoning. 75% of children (6/8) who fulfilled Kochi criteria13 for urgent liver transplantation and were PLEX-eligible were rescued by PLEX-LV. In contrast, in rodenticidal hepatotoxicity patients who met Kochi criteria, Saraf et al. reported no survivors in patients treated medically and 85% survival in patients treated by urgent liver transplantation (none of the patients were treated with PLEX).13
This, to our knowledge, is the first report of PLEX-LV in children with rodenticidal hepatotoxicity. Large volume7, 8, 9 and standard volume7, 8, 9 PLEX improve liver-transplant free survival in adult patients with ALF of other etiologies.
Rodenticidal hepatoxicity is common in southern India, with most patients not having access to urgent liver transplantation.20 In a recent study from Tamil Nadu spanning a period of six months, most of the toxin induced liver injury was secondary to rodenticidal intake (685/702 patients). 159 (35%) of these patients succumbed to acute hepatotoxicity, and only one patient underwent liver transplantation.1 PLEX-LV provides a non-transplant therapeutic option in these patients.
The innate immune response (including reticuloendothelial activation) is more rapid than adaptive immune response and may be the dominant mode of immune mediated liver injury in ALF.21 Plasma VWF levels, serum ferritin and CD25 levels are clinical tools to assess innate immune activation (reticuloendothelial activation) in patients with liver diseases.22 In patients with rodenticidal hepatotoxicity, innate immune activation is seen23,24 and levels of reticuloendothelial activation markers in circulation correlate with the severity of liver disease.23, 24 PLEX may improve survival in ALF patients by attenuating innate immune activation and ameliorating multi-organ dysfunction.7 We have proposed that VWF reduction may be one of the mechanisms underlying the efficacy of PLEX-LV in liver failure syndromes.9,10,25
Among the 17 children who were treated with PLEX-LV in the present study, 15 were PLEX-eligible. Two children were PLEX-ineligible as per the TN-ISG (Tamil Nadu chapter of Indian Society of Gastroenterology) guidelines to manage rodenticide poisoning.15
In our study, patients who needed mechanical ventilation had poorer survival. Sedative overdose may occur inadvertently in patients with acute liver dysfunction, as most sedatives are metabolised in the liver. It is advisable to avoid sedative drugs in patients with rodenticidal hepatotoxicity, if possible. This may prevent drowsiness and need for mechanical ventilation and may improve survival.15,26
With an aggressive policy of surveillance for and management of infections, none of the 4 patients who developed bacteremia while on PLEX-LV died.
Preliminary observations in the current study suggest better survival in children with rodenticidal hepatotoxicity who were treated with PLEX-LV. Children fare better than adults in paracetamol overdose induced liver damage.27 The exact mechanisms why children with toxic hepatitis fare better than adults needs to be studied.
The study is limited by retrospective nature. We have not documented long term follow up in these children.
In conclusion, PLEX-LV is a life-saving therapeutic option in children with rodenticide induced acute liver injury and failure.
Credit authorship contribution statement
Conceptualized and designed the study, collected and analysed data, reviewed literature and prepared initial draft of the manuscript: LT, JC, AG, EJ, BC, KS, IA, SV, VGD, DD, JM, VB, KAB, APL, DDA, KPPA, CEE, UZ.
Data analysis and manuscript preparation: LT, AG, UZ, CEE, EE.
All authors critically revised and approved the final version of the manuscript.
All authors have agreed to be accountable for the accuracy and integrity of the work.
Conflicts of interest
The authors have none to declare.
Funding
Nil.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2022.10.013.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Govindarajan R., Ramamoorthy G., Shanmugam R.M., et al. Rodenticide ingestion is an important cause of acute hepatotoxicity in Tamil Nadu, southern India. Indian J Gastroenterol. 2021 Aug;40:373–379. doi: 10.1007/s12664-021-01178-4. [DOI] [PubMed] [Google Scholar]
- 2.Mohanka R., Rao P., Shah M., et al. Acute liver failure secondary to yellow phosphorus rodenticide poisoning: outcomes at a center with dedicated liver intensive care and transplant unit. J Clin Exp Hepatol. 2021 Jul 1;11:424–434. doi: 10.1016/j.jceh.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra A.K., Devakiruba N.S., Jasmine S., et al. Clinical spectrum of yellow phosphorous poisoning in a tertiary care centre in South India: a case series. Trop Doct. 2017 Jul 1;47:245–249. doi: 10.1177/0049475516668986. [DOI] [PubMed] [Google Scholar]
- 4.Ates M., Dirican A., Ozgor D., et al. Living donor liver transplantation for acute liver failure in pediatric patients caused by the ingestion of fireworks containing yellow phosphorus. Liver Transpl. 2011;17:1286–1291. doi: 10.1002/lt.22384. [DOI] [PubMed] [Google Scholar]
- 5.Reddy M.S., Rajakumar A., Mathew J.S., et al. Liver Transplantation Society of India guidelines for the management of acute liver injury secondary to yellow phosphorus–containing rodenticide poisoning using the modified Delphi technique of consensus development. J Clin Exp Hepatol. 2021 Jul 1;11:475–483. doi: 10.1016/j.jceh.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons B.J., Day L.M., Ozanne-Smith J., et al. Rodenticide poisoning among children. Aust N Z J Public Health. 1996 Oct;20:488–492. doi: 10.1111/j.1467-842x.1996.tb01627.x. [DOI] [PubMed] [Google Scholar]
- 7.Larsen F.S., Schmidt L.E., Bernsmeier C., et al. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol. 2016 Jan 1;64:69–78. doi: 10.1016/j.jhep.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Maiwall R., Bajpai M., Singh A., et al. Standard-volume plasma exchange improves outcomes in patients with acute liver failure: a randomized controlled trial. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2022 Apr;20:e831–e854. doi: 10.1016/j.cgh.2021.01.036. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S.E., Goel A., Zachariah U., et al. Low volume plasma exchange and low dose steroid improve survival in patients with alcohol-related acute on chronic liver failure and severe alcoholic hepatitis – preliminary experience. J Clin Exp Hepatol. 2022 Mar – Apr;12:372–378. doi: 10.1016/j.jceh.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sardar D., Mathews N., Mammen J., et al. Rodenticidal hepatotoxicity: raised plasma Von Willebrand factor levels predict in-hospital survival and preliminary report of the outcome of Von Willebrand factor reducing management protocol. Indian J Gastroenterol. 2019 Dec 1;38:527–533. doi: 10.1007/s12664-019-00989-w. [DOI] [PubMed] [Google Scholar]
- 11.Malinchoc M., Kamath P.S., Gordon F.D., et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 12.Vincent J.-L., Moreno R., Takala J., et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996 Jul 1;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 13.Saraf V., Pande S., Gopalakrishnan U., et al. Acute liver failure due to zinc phosphide containing rodenticide poisoning: clinical features and prognostic indicators of need for liver transplantation. Indian J Gastroenterol. 2015 Jul 1;34:325–329. doi: 10.1007/s12664-015-0583-2. [DOI] [PubMed] [Google Scholar]
- 14.Anand A.C., Nandi B., Acharya S.K., et al. Indian national association for the study of liver consensus statement on acute liver failure (Part-2): management of acute liver failure. J Clin Exp Hepatol. 2020;10:477–517. doi: 10.1016/j.jceh.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eapen C.E., Balasubramanian V., Ramamoorthy G., et al. Management of rodenticide poisoning: Tamil Nadu chapter of Indian Society of Gastroenterology guidelines. Gastroenterol Hepatol Endosc Pract. 2022;2:1–6. [Google Scholar]
- 16.Alexander V., Zachariah U., Goel A., et al. Low-volume plasma exchange and low-dose steroid to treat secondary hemophagocytic lymphohistiocytosis: a potential treatment for severe COVID-19? Curr Med Issues. 2020;18:77–82. [Google Scholar]
- 17.Kaplan A.A. A simple and accurate method for prescribing plasma exchange. ASAIO (Am Soc Artif Intern Organs) Trans. 1990 Sep;36:M597–M599. [PubMed] [Google Scholar]
- 18.Zachariah U., Kumar S.E., Alexander V., et al. Low-volume plasma exchange and low-dose steroid to treat severe liver injury. Gastroenterol Hepatol Endosc Pract. 2021;1:47–54. [Google Scholar]
- 19.Khwaja A. KDIGO Clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 20.Eapen C.E., Venkataraman J. Rodenticide (yellow phosphorus poison)-induced hepatotoxicity in India: constraints during management. J Clin Exp Hepatol. 2021 Jul 1;11:414–417. doi: 10.1016/j.jceh.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z., Han M., Chen T., et al. Acute liver failure: mechanisms of immune-mediated liver injury. Liver Int. 2010;30:782–794. doi: 10.1111/j.1478-3231.2010.02262.x. [DOI] [PubMed] [Google Scholar]
- 22.Goel R., Eapen C.E. Recognizing dysfunctional innate and adaptive immune responses contributing to liver damage in patients with cirrhosis. J Clin Exp Hepatol. 2022;12:993–1002. doi: 10.1016/j.jceh.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijayalekshmi B., Alexander V., Sharma A., et al. Reticuloendothelial activation correlates with disease severity in liver injury due to rodenticidal poisoning. Indian J Gastroenterol. February 2022;41:S89. [Google Scholar]
- 24.Vijayalekshmi B., Chaudary A., Prabhu S., et al. Peripheral blood monocyte phenotype in acute liver failure due to rat-killer poisoning. Indian J Gastroenterol. February 2022;41:S88. [Google Scholar]
- 25.Goel A., Nair S., Zachariah U., et al. Targeting raised von Willebrand factor levels in liver diseases: opening up newer therapeutic avenues. Eur Med J Hepatol. 2020 Apr 27;8 [Google Scholar]
- 26.Vadivukkarasi T.J., Kandasamy S., Abhilash K.P.P., et al. Safe sedation practices in acute liver failure in resource-constrained settings: a viewpoint. Gastroenterol Hepatol Endosc Pract. 2021 Jan 1;1:17. [Google Scholar]
- 27.Penna A., Buchanan N. Paracetamol poisoning in children and hepatotoxicity. Br J Clin Pharmacol. 1991 Aug;32:143–149. doi: 10.1111/j.1365-2125.1991.tb03873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.