Abstract
Objectives
Psoas muscle parameters have been proposed as a simple and quick method for sarcopenia assessment. The aim of this study was to assess sarcopenia in cirrhotics by psoas muscle on computed tomography and its impact on mortality.
Methods
One hundred and fifty patients (75 cirrhotics, 75 subjects) were assessed for psoas muscle on CT scan. Psoas muscle index (PMI) was calculated as ‘total psoas muscle area/(height of subject)2’. Cut off values for sarcopenia diagnosis were derived from local subjects (n = 75) who did not have cirrhosis/other causes of sarcopenia.
Results
Sarcopenia assessed by PMI was seen in 36% (n = 27) of the cirrhotics. Sarcopenia was significantly higher in patients having Child-Pugh C. Ascites, hepatic encephalopathy (HE) and gastro-intestinal bleed were seen in 48%, 18.7% and 24%, respectively. Sarcopenia was significantly associated with ascites and HE (P < 0.05). Out of the 75 cases, 53 cases completed the follow-up period of 1 year. Among the 20 cases who had sarcopenia, 35% (n = 7) succumbed to liver-related illness during 1 year follow-up, and out of the 33 cases without sarcopenia, only 6% (n = 2) died. The association of sarcopenia and 1 year mortality was statistically significant (P = 0.01).
Conclusions
The PMI, a simple method for sarcopenia assessment detected sarcopenia in 36% of cirrhotics. Patients with sarcopenia had a significantly higher 1 year mortality rate and appropriate prognostication of such patients is needed.
Keywords: sarcopenia, chronic liver disease, psoas muscle index, L3SMI, CT scan
Abbreviations: CLD, Chronic Liver Disease; CT, Computed Tomography; HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; HE, Hepatic Encephalopathy; HG, Hand Grip; GI, Gastro-Intestinal; MAC, Mid-Arm Circumference; MAMC, Mid-Arm Muscle Circumference; MELD, Model for End Stage Liver Disease; NASH, Non-Alcoholic Steato-Hepatitis; PBC, Primary Biliary Cholangitis; PMI, Psoas Muscle Index; PMTH, Psoas Muscle Thickness by Height of subject; SMI, Skeletal Muscle Index; TST, Tricep Skin fold Thickness
Sarcopenia is defined as generalised reduction in muscle mass and function which can be primary (due to ageing) or secondary (due to acute or chronic illness including chronic liver disease).1 The reported pooled prevalence of sarcopenia in patients with cirrhosis is 37.5% and has been shown to be an independent predictor of mortality.2 Additionally, patients having malnutrition and/or sarcopenia have higher rate of complications like longer hospital stay, ascites, renal impairment and increased mortality during hospitalisation.3,4
Multiple modalities have been used for the assessment of sarcopenia of which non-invasive imaging with computed tomography (CT) has been regarded as the most precise.5 Among CT-based measurements, skeletal muscle index (SMI) measured at the level of the third lumbar vertebrate (L3) has been shown to be one of the most is a reliable and valid measures and has been recommended by various society guidelines.6,7
L-3 SMI is measured as the cross-sectional muscle area of the skeletal muscles at level L3 divided by the square of height of subject. Although L-3 SMI is shown to be highly accurate, the derivation of the index requires dedicated specialized software. Whereas, SMI provides a composite muscle evaluation at the L3 level, alternative simpler CT-based indices based on single muscle measurements like psoas muscle thickness (PMT), psoas muscle index (PMI), and psoas muscle cross-sectional area (PMA) have been used for sarcopenia assessment.8 These measurements are easy and rapid and do not need the use of dedicated specialized softwares and have been shown to be reliable predictors of mortality in cirrhosis.8,9 However, overall, the literature about the use of psoas muscle-based indices are limited and some studies have questioned the robustness of these indices in patients with cirrhosis.10
In this background, we undertook this study to assess the sarcopenia in cirrhotics quantified by psoas muscle on CT scan and assess its impact on mortality and morbidity.
Materials and methods
This was an observational prospective study over a period of 18 months carried out at a tertiary care centre. One hundred and fifty patients (75 cases with cirrhosis and 75 subjects) undergoing CT scan were included in the study. The other inclusion criteria were as those with age >18 years and <70 years, patients diagnosed/known to have cirrhosis of liver, patients who underwent CT scan of abdomen for any indication. The combination of clinical, laboratory, endoscopic and radiological features were used for the diagnosis of liver. These included features like ascites (high serum ascites albumin gradient), nodular liver at imaging, grade IV liver fibrosis at elastography, oesophageal varices and other such features.
Patients diagnosed to have malignancy, tuberculosis, prolonged illness/hospital stay more than 1 month, HIV positive status, on steroid treatment and pregnant females were excluded.
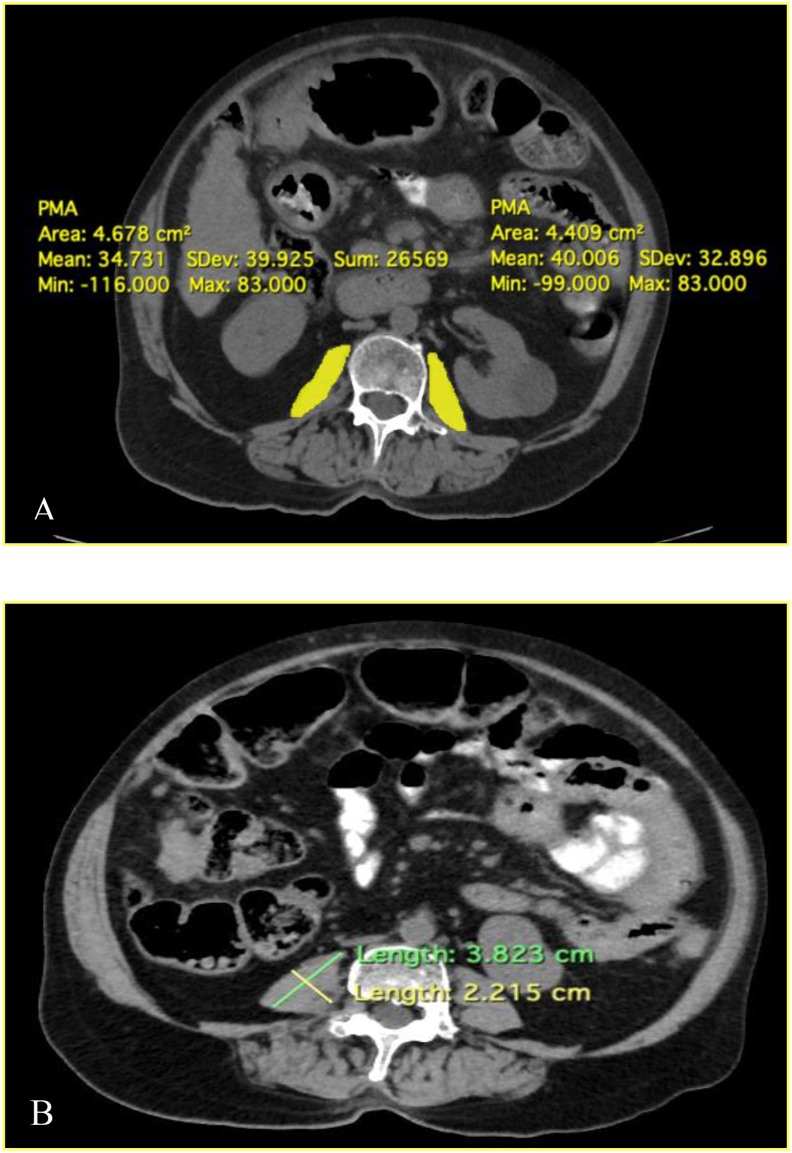
CT scans was performed as dictated from the medical condition of the patients or as part of pre-liver transplant evaluation. Data were collected and transverse CT sections were be analysed at the third lumbar vertebrae level (L3). Total psoas muscle area was measured including the right and left psoas muscle (Figure 1A). All CT images were analysed by two dedicated radiologists. Out of the 75 patients with cirrhosis , 53 patients completed a follow-up period of 1 year and were assessed for the mortality rate.
Figure 1.
(A) Psoas muscle area—yellow colour delineates the right and left psoas muscle with the respective areas (B) Psoas muscle thickness—green line indicates longest diameter of the psoas muscle and the yellow line indicates the psoas muscle thickness (largest diameter perpendicular to the longest diameter).
Cross-sectional psoas muscle area was normalized for stature by calculation of L3 PMI i.e. PMI (mm/m2) = Total psoas muscle area (mm)/(Height of subject)2 (m). The PMT was measured as the largest diameter perpendicular to the longest diameter of the psoas muscle on axial CT image at level of L4 vertebrae (Figure 1B). Psoas muscle thickness by height of subject (PMTH) was calculated by measuring the thickness of psoas muscle at level of fourth lumbar vertebrae divided by height of the patient as: PMTH (mm/m) = Psoas muscle thickness (mm)/Height of subject (m).11
In the previous studies, one of the main limiting factors was that local cut off values were not used for the diagnosis of sarcopenia and hence overestimating the prevalence. This is a major concern with studies from our region as most of the published cut off values are from the western literature. In this study, the cut off values for the diagnosis of sarcopenia was derived from a local control group (n = 75) who did not have cirrhosis/other obvious causes of sarcopenia. The control group were subjects who presented with a short illness (<7 days) like acute appendicitis, nephrolithiasis, acute cholecystitis in whom CT scan was indicated as per the clinical condition. The cut off values derived from the control group are shown in Table 1. The lower bound (mean - 2 SD) was taken as the cut off value for diagnosing sarcopenia.12
Table 1.
Mean, SD, Upper and Lower Bound of PMI and PMTH of Subjects (n = 75).
| PMTH (mm) |
PMI (mm2/m) |
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Mean | 21.97 | 18.48 | 753.95 | 544.70 |
| Standard deviation | 2.71 | 1.95 | 164.10 | 90.27 |
| Upper bound | 27.40 | 22.40 | 1082.15 | 725.27 |
| Lower bound (Cut off) | 16.53 | 14.57 | 425.72 | 364.14 |
PMI, Psoas Muscle Index; PMTH, Psoas Muscle Thickness/Height of subject.
Statistical Analysis
Qualitative data were represented in the form of frequency and percentage. Among qualitative data, Nominal data included group category (Case and Control), gender of the subjects, cirrhosis aetiology, ascites, PMTH L4-based sarcopenia, PMI-based Sarcopenia, muscle weakness and muscle mass. Association between qualitative variables was assessed by Chi-square test, with continuity correction for all 2 × 2 tables and by Fisher's Exact test for all 2 × 2 tables where Chi–Square test was not valid due to small counts. Fisher's Exact test was applied for all 2 × 2 tables where P-value of continuity correction was not valid due to small counts, in-spite of pooling of data. Quantitative data were represented using Mean ± SD and Median and IQR (Interquartile range).
Correlation between data was done by using spearman's rank correlation, as the data failed ‘Shapiro-wilk test normality’ test or was Ordinal data. Cohen's kappa coefficient (κ) was used to measures inter-test agreement for Qualitative data. Appropriate statistical software, including but not restricted to MS Excel, PSPP version 1.0.1 was used for statistical analysis. An alpha value (P-value) of ≤0.05 was used as the cut off for statistical significance. Results were graphically represented where deemed necessary.
Results
The cirrhosis group patients ranged in age from 31 to 70 with a mean (±s.d) of 52.17 (±7.86) years. The males were in slightly higher proportion (54.7%) than females. Child-Pugh B was seen in 41.3% of patients while Child-Pugh A and C were 33.3% and 25.3%, respectively. The mean MELD (±s.d) score was 13.12 (±4.87) and the median MELD score was 12. The patient with MELD score 9 or less were 28% whereas those with score between 10 and 19 were 61.3%. Patients with MELD score more than 20 were 10.7%. Among the different aetiologies of cirrhosis, non-alcoholic steato-hepatitis was the most common aetiology seen in 42.7% of the cases. The other aetiologies of cirrhosis included ethanol (16%), HBV (10.7%), HCV (10.7%), cryptogenic (9.3%) and autoimmune (6.7%). The baseline demographic features and aetiological diagnosis have been included in Table 2.
Table 2.
Baseline Demographic Features and Etiological Diagnosis.
| Variables | Mean value ± SD |
|---|---|
| Age (years) | 52.17 ± 7.86 |
| Sex (n) | |
| Male | 41 |
| Female | 34 |
| Height (cm) | 159.36 ± 9.56 |
| BMI (kg/m2) | 27.46 ± 5.32 |
| Aetiology (n) | |
| NASH | 32 |
| Alcohol | 12 |
| HBV | 8 |
| HCV | 8 |
| Cryptogenic | 7 |
| Miscellaneous | 8 |
| CHILD (n) | |
| A | 25 |
| B | 31 |
| C | 19 |
| MELD score | 13.12 ± 4.87 |
| Albumin (g/dL) | 3.14 ± 0.5 |
| Vitamin D (ng/dL) | 18.32 ± 6.68 |
HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; MELD, Model for End Stage Liver Disease; NASH, Non-Alcoholic Steato-Hepatitis.
The mean value of PMI was 753.95 mm2/m and 544.70 mm2/m in male and female subjects (control group), respectively. The mean value of PMTH was 21.97 mm and 18.48 mm in male and female subjects (control group), respectively. The mean value of PMI was 549.73 mm2/m and 457.88 mm2/m in male and female patients, respectively. The mean value of PMTH was 17.78 mm and 16 mm in male and female patients, respectively.
Sarcopenia assessed by PMI was seen in 36% of the total cirrhosis patients while 64% patients had no sarcopenia by the PMI assessment. Somewhat similar results were seen for sarcopenia assessment by the PMTH assessment. 34.7% of patients having cirrhosis had sarcopenia by PMTH assessment while 65.3% has no sarcopenia. The Kappa measure of agreement for the sarcopenia assessment by PMI and PMTH was 0.737 suggestive of good strength of agreement between the two methods. In five cases, sarcopenia was diagnosed by PMI method but not by PMTH method. In other four cases, sarcopenia was detected by PMTH method but not by PMI method.
Out of the patients who had ascites (36 patients), 61.1% (22 patients) of patients had sarcopenia while 38.9% (14 patients) of patients had no sarcopenia as noted by PMI. The P value for Chi-square test after continuity correction was significant (<0.05) suggesting significant association between ascites and PMI sarcopenia. There was also significant association between grade of ascites and PMI sarcopenia. Table 3 shows the association of grade of ascites and PMI sarcopenia. Out of the patients who had gastro-intestinal bleed (18 patients), 27.8% (5 patients) of patients had PMI sarcopenia and 72.2% (13 patients) of patients had no PMI sarcopenia. The P value for Chi-square test after continuity correction was not significant (P value - 0.581). Out of the patients who had HE (14 patients), 85.7% (12 patients) of patients had PMI sarcopenia and 14.3% (2 patients) of patients had no PMI sarcopenia. The P value for Chi-square test after continuity correction was significant (<0.05) suggesting the association between HE and PMI sarcopenia could be established.
Table 3.
Association of Grade of Ascites and PMI Sarcopenia.
| Grade of Ascites | PMI sarcopenia |
Total | ||
|---|---|---|---|---|
| Yes | No | |||
| Large | No. | 2 | 1 | 3 |
| Moderate | No. | 9 | 7 | 16 |
| Mild | No. | 11 | 6 | 17 |
| No ascites | No. | 5 | 34 | 39 |
| Total | No. | 27 | 48 | 75 |
| % | 36.0% | 64.0% | 100.0% | |
| Chi–Square tests | Value | Df | P-value | Association is- |
| Pearson Chi–square $ | 19.247 | 3 | 0.00024 | Significant |
| Pearson Chi–square ˆ | 16.909 | 1 | 3.92E-05 | Significant |
$ 2 cells (25.0%) have expected count less than 5. ˆ Row data pooled and Chi-Square test reapplied with Continuity Correction.
PMI - Psoas Muscle Index Sarcopenia
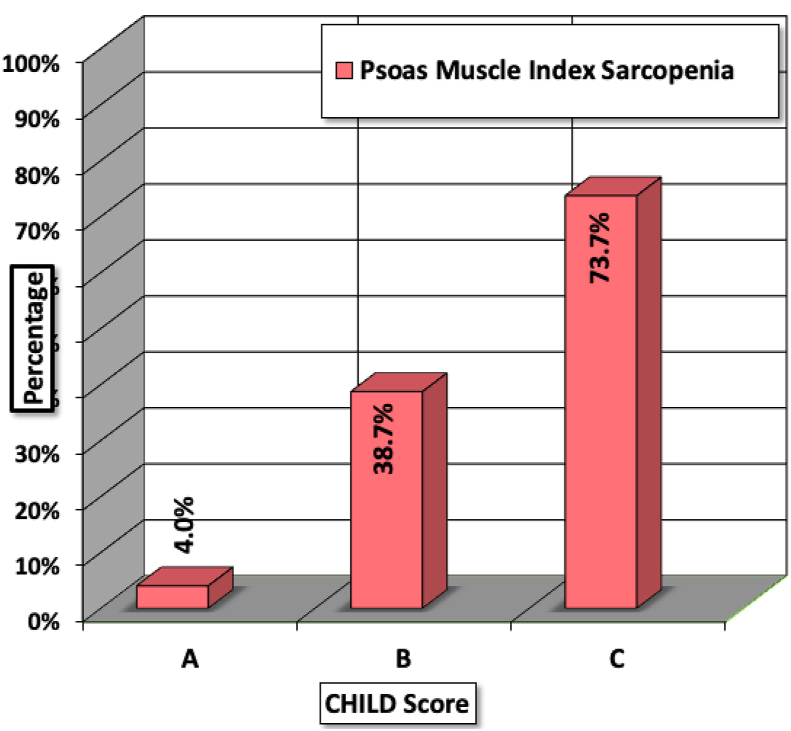
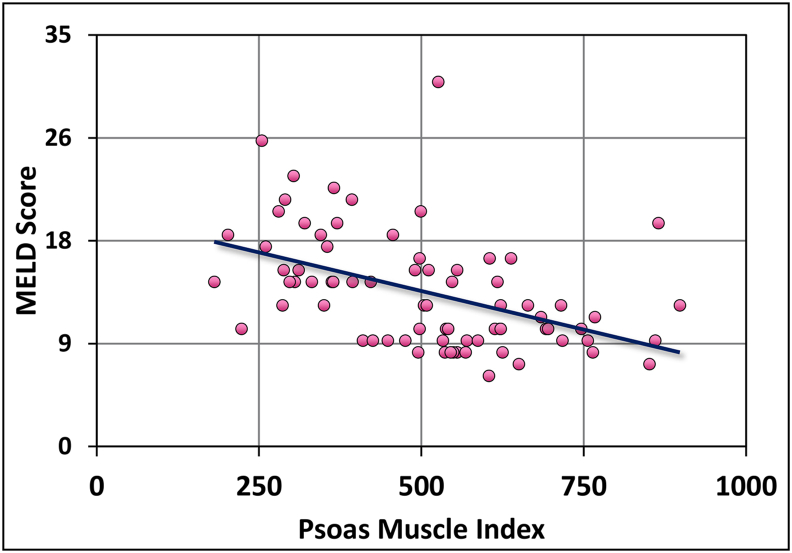
Out of the cirrhosis patients who had Child-Pugh A, only 4% had PMI sarcopenia while 73.7% patients with Child-Pugh C had PMI sarcopenia. The P value for Chi-square test after continuity correction was significant (<0.05). Hence, there was significant association between Child-Pugh class and PMI sarcopenia. Figure 2 shows the bar diagram depicting the association of Child-Pugh class and PMI sarcopenia. The Spearman's rho correlation coefficient for model for end-stage liver disease (MELD) score and PMI sarcopenia was −0.5169. The negative correlation suggested that, as the MELD score increased the PMI values reduced and vice-versa. The P value was significant (<0.05). Figure 3 shows the scatter graph between MELD score and PMI sarcopenia. The association between cirrhosis aetiology and PMI sarcopenia was not statistically significant (P value - 0.764).
Figure 2.
Association between Child-Pugh class and PMI sarcopenia.PMI, Psoas muscle index.
Figure 3.
Association of MELD Score and PMI sarcopenia. PMI, Psoas muscle index.
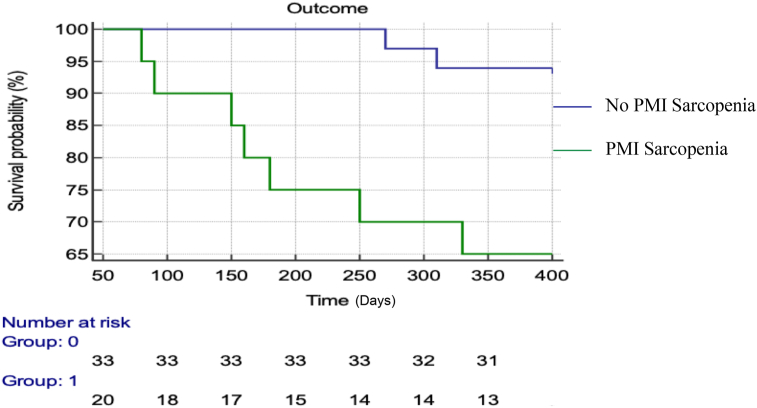
Out of the 75 patients having cirrhosis, 53 patients completed the follow-up period of 1 year. Among the 20 patients with sarcopenia, 35% (n = 7) of patients succumbed to liver related illness at 1 year follow-up. On the other hand, out of the 33 patients without sarcopenia, 6% (n = 2) died during follow-up at 1 year. The association of 1 year mortality and sarcopenia was statistically significant (P value = 0.01). Figure 4 shows the Kaplan–Meier curve depicting the mortality on 1 year follow-up among patients with and without sarcopenia. The hazard ratio for mortality in patients with sarcopenia was 7.5 (1.8–30.1).
Figure 4.
Shows the Kaplan–Meier curve depicting the mortality on 1 year follow-up among patients with and without sarcopenia. PMI, Psoas Muscle Index.
Discussion
Quantification of the skeletal muscle mass requires cross-sectional imaging like CT scan.13 CT image analysis at the L3 vertebra is now accepted as a standard method to quantify sarcopenia. This analysis involves the psoas, para spinal and abdominal wall muscles which are less likely to be affected by activity and water retention.
Recent studies have increasingly used psoas muscle parameters measured on CT to diagnose sarcopenia and prognosticate the patients.8,11,14 The psoas muscle has also been shown to be a simple predictor of survival for hepatocellular cancer patients treated with sorafenib.15 The psoas muscle parameters have also correlated well with L3 SMI values in a recent study with significant positive co-relations (r = 0.56, P < 0.01).14 However, another recent study although showing a robust co-relation (r > 7, P < 0.01) has questioned the robustness of psoas muscle indices and has suggested SMI as a better predictor of mortality especially in the male subgroup of cirrhosis.10
The cut off values for the diagnosis of psoas muscle sarcopenia was based on the local control group. The western cut off values for the diagnosis of sarcopenia were not used as they would over-estimate the prevalence of sarcopenia.16 This is due to the ethnicity differences among the Asian and European populations. However, studies from other Asian countries like Korea gave similar cut off values were found in our study (Table 4).12 The study by Sidhu et al. assessed Indian patients without cirrhosis (n = 3087) and found to have the mean SMI on CT scan to be 41.25 ± 4.42 in females and 44.33 ± 6.56 in males. These values are much lesser than the values reported for western population.17
Table 4.
Cut off Values of PMI in the Present Study and the Study by Kim JSet al.
| Present study (Subjects) |
Kim JS et al.33 |
|||
|---|---|---|---|---|
| PMI (mm2/m) |
PMI (mm2/m) |
|||
| Male | Female | Male | Female | |
| Lower bound (Cut off) | 425.72 | 364.14 | 465.7 | 287.6 |
PMI - Psoas Muscle Index.
In the study by Kim Ty et al., it was seen that the mortality risk was higher in cirrhotic patients with PMTH ≤14 mm/m as than those with PMTH >14 mm/m on follow-up.11 As shown in this Korean study, our study also found the values of psoas muscle was lower in female than male. In the current study, the cut off value derived from the control group for the diagnosis of sarcopenia was 16.5 mm/m and 14.5 mm/m for males and females, respectively. This difference persists even after adjustment for body weight and height and may be due to the sex hormones and difference in amount of physical activity.18, 19, 20
The prevalence of sarcopenia in the present study among the patients having cirrhosis was 36%. The previously published studies on sarcopenia from India have shown the prevalence from 12.8% to 47.8%.16,21 Benjamin J et al., in their published study in 2017 found the prevalence of sarcopenia in 12.8% of patients with alcoholic liver cirrhosis.16 Later on in 2020, another study published from the same centre by Kumar V et al., stated the prevalence of sarcopenia in pre-liver transplant patients to be 47.8%.21 This difference of prevalence may be due to the higher MELD score in the pre-liver transplant group of patients in the later study.
A few studies have noted that patients with alcoholic cirrhosis have greater prevalence of malnutrition than other aetiologies.22, 23, 24 In the study by Tai ML et al., it was seen that patients with alcoholic cirrhosis had a higher proportion of malnutrition than other aetiologies of cirrhosis, although it was not statistically significant.23 In the present study, the association of aetiology of cirrhosis and PMI sarcopenia was not significant. This is in agreement with the available literature that the aetiology of cirrhosis does not seem to have a major impact on the nutrition status.
MELD and Child-Pugh scores are commonly used to assess the severity of cirrhosis. Malnutrition and sarcopenia appear to have a higher prevalence among the patients who have higher severity of cirrhosis. In a Canadian study by Tandon P et al., it was seen that the prevalence of sarcopenia increased form Child-Pugh A to Child-Pugh C.25 Sarcopenia was present in 10%, 34% and 54% of patients with Child-Pugh A, Child-Pugh B and Child-Pugh C, respectively. In a recent study by Hiraoka A et al., it was seen that sarcopenia was present in 4%, 5% and 17% of patients with chronic hepatitis, Child-Pugh A cirrhotics and Child-Pugh B/C cirrhotics, respectively.26 In the current study, the prevalence of sarcopenia was 73.7% in Child-Pugh C cirrhotics, while only 4% of the Child-Pugh A cirrhotics had sarcopenia. Hence, the results of the present study are in accordance with the existing literature that the prevalence of sarcopenia is higher in CHILD C cirrhotic patients. The recent EASL clinical practice guidelines on nutrition in cirrhosis recommends that patients with advanced liver disease and Child-Pugh C class should directly go for a detailed nutritional assessment and a CT scan should be considered to measure the L3 muscle area in such patients.1
The different psoas muscle parameters have been described in literature for assessing sarcopenia.8,10,11,27 PMI and PMTH have shown to predict survival in cirrhosis patients.11,27 Golse N et al., measured different muscle indexes and concluded that PMA offered better accuracy than L3SMI and PMA/BSA, and the same accuracy as PMI for predicting 1 year survival post-liver transplant.27 In the present study, we found that the sarcopenia assessment by PMTH and PMI had a good agreement (kappa value - 0.737) and the association was statistically significant. The benefits of using psoas muscle for sarcopenia assessment include simplicity, no need for dedicated software, easy identification on CT scan, deep muscle (may not be affected by ascites compared to other parietal muscles) and prognostic value in patients having cirrhosis (independent of the MELD scores).
In two recent studies, it was seen that the prevalence of ascites differs with prevalence of frailty.28,29 In one study, ascites was seen in 28% of the patients having cirrhosis where frailty was seen in 18%.30 In the other study, ascites was seen in 52% of the cirrhosis patients and the prevalence of frailty was upto 43%.29 In the present study, ascites was seen in 61.1% of patients with sarcopenia compared to 38.9% of patients without sarcopenia. In the study by Hanai et al., the prevalence of HE was higher in patients with sarcopenia than in those without sarcopenia.31 In the present study, HE was present in 85.7% of patients with sarcopenia and in 14.3% of patients with no sarcopenia. The association of HE and sarcopenia was found to be statistically significant. Mortality risk was higher in cirrhotic patients with sarcopenia (PMTH ≤14 mm/m) than those without sarcopenia (PMTH >14 mm/m) on follow-up.11 In the current study, 35% of patients with sarcopenia died on follow-up due to liver-related illness compared to 6% of patients without sarcopenia. The difference was found to be statistically significant.
The Indian National Asssociation for the Study of the Liver (INASL) consensus on nutrition in cirrhotic patients recommends that the diagnosis of sarcopenia should be an integral part of the pre-transplant evaluation, as sarcopenia will have an adverse effect on the post-transplant outcome. Also, the sarcopenia is amenable to correction during the waiting time for transplantation. The consensus also recommends that cirrhotic patients who are planned for non-transplant surgery should be nutritionally rehabilitated when the surgery is elective.32
There were few limitations of this study. The results could be improved and made more precise by increasing sample size. Only 53 out of 75 patients completed the follow-up period of 1 year. Evaluation of the L3 SMI and its correlation with psoas muscle parameters would further establish the role of psoas muscle.
The psoas muscle is a simple and reliable method for sarcopenia assessment on CT scan. Measuring the psoas muscle does not require any special software. The PMI or PMTH method correlate well with each other and either of it can be used for sarcopenia assessment. Sarcopenia assessed by PMI was seen in 36% of the cirrhotics in the present study. Sarcopenia was significantly higher in patients with Child-Pugh C. Patients with large ascites and/or HE are more likely to have sarcopenia as compared to the patients without ascites and/or HE. Patients with sarcopenia have a higher 1 year mortality rate and appropriate prognostication of such patients should be done.
Credit authorship contribution statement
Gajanan Rodge: Data curation, Writing- Original draft preparation, Investigation, Software.
Usha Goenka: Visualization, Investigation, Supervision, Software.
Surabhi Jajodia: Visualization, Reviewing and Editing.
Rachit Agarwal: Supervision, Reviewing and Editing.
Shivaraj Afzalpurkar: Reviewing and Editing.
Akash Roy: Reviewing and Editing.
Mahesh Kumar Goenka: Conceptualization, Methodology, Supervision, Software.
ConflictS of interest
The authors have none to declare.
Funding
None.
References
- 1.European Association for the Study of the Liver EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tantai X., Liu Y., Yeo Y.H., et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;76:588–599. doi: 10.1016/j.jhep.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Sam J., Nguyen G.C. Protein-calorie malnutrition as a prognostic indicator of mortality among patients hospitalized with cirrhosis and portal hypertension. Liver Int. 2009;29:1396–1402. doi: 10.1111/j.1478-3231.2009.02077.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1478-3231.2009.02077.x [DOI] [PubMed] [Google Scholar]
- 4.Bernal W., Martin-Mateos R., Lipcsey M., et al. Aerobic capacity during cardiopulmonary exercise testing and survival with and without liver transplantation for patients with chronic liver disease. Liver Transplant. 2014;20:54–62. doi: 10.1002/lt.23766. https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/lt.23766 [DOI] [PubMed] [Google Scholar]
- 5.Buchard B., Boirie Y., Cassagnes L., Lamblin G., Coilly A., Abergel A. Assessment of malnutrition, sarcopenia and frailty in patients with cirrhosis: which tools should we use in clinical practice? Nutrients. 2020;12:186. doi: 10.3390/nu12010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey E.J., Lai J.C., Wang C.W., et al. Fitness, Life Enhancement, and Exercise in Liver Transplantation Consortium. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transplant. 2017;23:625–633. doi: 10.1002/lt.24750. https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/lt.24750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishikawa H., Shiraki M., Hiramatsu A., Moriya K., Hino K., Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease: recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951–963. doi: 10.1111/hepr.12774. https://onlinelibrary.wiley.com/doi/10.1111/hepr.12774 [DOI] [PubMed] [Google Scholar]
- 8.Durand F., Buyse S., Francoz C., et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151–1157. doi: 10.1016/j.jhep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Hou L., Deng Y., Wu H., et al. Low psoas muscle index associates with long-term mortality in cirrhosis: construction of a nomogram. Ann Transl Med. 2020;8 doi: 10.21037/atm.2020.02.49. https://atm.amegroups.com/article/view/38266/html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebadi M., Wang C.W., Lai J.C., et al. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopeni. 2018;9:1053–1062. doi: 10.1002/jcsm.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim T.Y., Kim M.Y., Sohn J.H., et al. Sarcopenia as a useful predictor for long-term mortality in cirrhotic patients with ascites. J Kor Med Sci. 2014;29:1253–1259. doi: 10.3346/jkms.2014.29.9.1253. https://jkms.org/DOIx.php?id=10.3346/jkms.2014.29.9.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J.S., Kim W.Y., Park H.K., Kim M.C., Jung W., Ko B.S. Simple age specific cutoff value for sarcopenia evaluated by computed tomography. Ann Nutr Metab. 2017;71:157–163. doi: 10.1159/000480407. [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu D.H., Kim M.Y., Seo Y.S., et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol. 2018;24:319–331. doi: 10.3350/cmh.2017.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashima M., Miyaaki H., Honda T., et al. Significance of psoas muscle thickness as an indicator of muscle atrophy in patients with hepatocellular carcinoma treated with sorafenib. Mol Clin Oncol. 2017;7:449–453. doi: 10.3892/mco.2017.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamin J., Shasthry V., Kaal C.R., et al. Characterization of body composition and definition of sarcopenia in patients with alcoholic cirrhosis: a computed tomography based study. Liver Int. 2017 Nov;37:1668–1674. doi: 10.1111/liv.13509. [DOI] [PubMed] [Google Scholar]
- 17.Sidhu S., Saggar K., Goyal O., Kishore H., Sidhu S.S. Normative values of sarcopenia in the Indian population. Indian J Gastroenterol. 2018;37 doi: 10.1007/s12664-018-0911-4. A1–A137. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner R.N., Waters D.L., Gallagher D., Morley J.E., Garry P.J. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–136. doi: 10.1016/S0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 19.Beneke R., Neuerburg J., Bohndorf K. Muscle cross-section measurement by magnetic resonance imaging. Eur J Appl Physiol Occup Physiol. 1991;63:424–429. doi: 10.1007/BF00868073. [DOI] [PubMed] [Google Scholar]
- 20.Bevier W.C., Wiswell R.A., Pyka G., Kozak K.C., Newhall K.M., Marcus R. Relationship of body composition, muscle strength, and aerobic capacity to bone mineral density in older men and women. J Bone Miner Res. 1989;4:421–432. doi: 10.1002/jbmr.5650040318. [DOI] [PubMed] [Google Scholar]
- 21.Janssen I., Heymsfield S.B., Wang Z.M., Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 22.Kumar V., Benjamin J., Shasthry V., et al. Sarcopenia in cirrhosis: fallout on liver transplantation. J Clin Exp Hepatol. 2020;10:467–476. doi: 10.1016/j.jceh.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao V.M., Ziegler T.R. Nutrition support in end-stage liver disease. Crit Care Nurs Clin. 2010;22:369-380. doi: 10.1016/j.ccell.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Tai M.L., Goh K.L., Mohd-Taib S.H., Rampal S., Mahadeva S. Anthropometric, biochemical and clinical assessment of malnutrition in Malaysian patients with advanced cirrhosis. Nutr J. 2010;9:27. doi: 10.1186/1475-2891-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roongpisuthipong C., Sobhonslidsuk A., Nantiruj K., Songchitsomboon S. Nutritional assessment in various stages of liver cirrhosis. Nutrition. 2001;17:761-765. doi: 10.1016/S0899-9007(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 26.Tandon P., Ney M., Irwin I., et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transplant. 2012;18:1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 27.Hiraoka A., Michitaka K., Ueki H., et al. Sarcopenia and two types of presarcopenia in Japanese patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2016;28:940-947. doi: 10.1097/MEG.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 28.Golse N., Bucur P.O., Ciacio O., et al. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transplant. 2017;23:143–154. doi: 10.1002/lt.24671. [DOI] [PubMed] [Google Scholar]
- 29.Tandon P., Tangri N., Thomas L., et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol. 2016;111:1759–1767. doi: 10.1038/ajg.2016.303. [DOI] [PubMed] [Google Scholar]
- 30.Cron D.C., Friedman J.F., Winder G.S., et al. Depression and frailty in patients with end-stage liver disease referred for transplant evaluation. Am J Transplant. 2016;16:1805–1811. doi: 10.1111/ajt.13639. [DOI] [PubMed] [Google Scholar]
- 31.Hanai T., Shiraki M., Watanabe S., et al. Sarcopenia predicts minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatol Res. 2017;47:1359–1367. doi: 10.1111/hepr.12873. [DOI] [PubMed] [Google Scholar]
- 32.Puri P., Dhiman R.K., Taneja S., et al. Nutrition in chronic liver disease: consensus statement of the Indian national association for study of the liver. J Clin Exp Hepatol. 2021;11:97–143. doi: 10.1016/j.jceh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]