Abstract
Significance:
Although corona virus disease 2019 (COVID-19) has now gradually been categorized as an endemic, the long-term effect of COVID-19 in causing multiorgan disorders, including a perturbed cardiovascular system, is beginning to gain attention. Nonetheless, the underlying mechanism triggering post-COVID-19 cardiovascular dysfunction remains enigmatic. Are cardiac mitochondria the key to mediating cardiac dysfunction post-severe acute respiratory syndrome coronavirus 2 (post-SARS-CoV-2) infection?
Recent Advances:
Cardiovascular complications post-SARS-CoV-2 infection include myocarditis, myocardial injury, microvascular injury, pericarditis, acute coronary syndrome, and arrhythmias (fast or slow). Different types of myocardial damage or reduced heart function can occur after a lung infection or lung injury. Myocardial/coronary injury or decreased cardiac function is directly associated with increased mortality after hospital discharge in patients with COVID-19. The incidence of adverse cardiovascular events increases even in recovered COVID-19 patients. Disrupted cardiac mitochondria postinfection have been postulated to lead to cardiovascular dysfunction in the COVID-19 patients. Further studies are crucial to unravel the association between SARS-CoV-2 infection, mitochondrial dysfunction, and ensuing cardiovascular disorders (CVD).
Critical Issues:
The relationship between COVID-19 and myocardial injury or cardiovascular dysfunction has not been elucidated. In particular, the role of the cardiac mitochondria in this association remains to be determined.
Future Directions:
Elucidating the cause of cardiac mitochondrial dysfunction post-SARS-CoV-2 infection may allow a deeper understanding of long COVID-19 and resulting CVD, thus providing a potential therapeutic target. Antioxid. Redox Signal. 38, 599–618.
Keywords: long COVID-19, SARS-CoV-2, cardiovascular disorders, cardiac mitochondria, inflammation
Introduction
Since the emergence of the corona virus disease 2019 (COVID-19) pandemic, the number of confirmed cases and deaths are on the rise globally according to the World Health Organization (WHO) (Dong et al, 2020). COVID-19 is a complex lung disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), accompanied by severe lung infection and multiple organ damage (Wu et al, 2020a). The virion structure of coronaviruses is composed of a nucleocapsid (N) containing single-stranded genomic RNA and phosphorylated nucleocapsid (N) protein.
Interspersed between the S proteins are the membrane (M) and envelope (E) proteins (Chan et al, 2020; Li et al, 2020). An investigation into the genomic sequence and protein structure of SARS-CoV-2 revealed a 79.6% genomic sequence identity to SARS-CoV (Wu et al, 2020a; Zhou et al, 2020b) and 51.8% identity to Middle East respiratory syndrome corona virus (MERS-CoV) (Lu et al, 2020; Ren et al, 2020), with similar biological features, including cellular entry via binding of the viral S protein to angiotensin-converting enzyme 2 (ACE2) on the surface of the host cell and mainly spreading through the respiratory tract (Hoffmann et al, 2020).
COVID-19 first appeared as a respiratory infection. In severe cases, it can lead to viral pneumonia and acute respiratory distress syndrome. Acute lung injury may also accompany, and uncontrolled SARS-CoV-2 infection can trigger an excessive inflammatory response, whereby an overload of pro-inflammatory cytokines such as interleukin (IL)-1β and IL-6 are produced. Progressive severity of COVID-19 is assessed via the presence of inflammatory markers mediated by neutrophils and macrophages, which then induce upregulated byproducts of the coagulation cascade (Garcia-Olivé et al, 2020; Tay et al, 2020).
Clinical studies have further found that COVID-19 can have direct and indirect risk of damage to myocardial or coronary microvasculature while also been capable of producing inflammatory cytokines, inflammatory storm, and lung injury/pulmonary circulation damage associated with COVID-19 (Khan et al, 2020). Elderly patients, obese patients, patients with diabetic cardiomyopathy, cancer, and other more severe diseases are at higher risk for these events (Zhao et al, 2020). In this regard, older age and heart failure after lung infection are among the important risk factors for all-cause mortality. Patients >60 years old and diagnosed with COVID-19 infection had more systemic symptoms, as well as more severe pneumonia, especially those with high blood pressure, coronary heart disease, and diabetes (Zhao et al, 2020).
Early clinical data have alluded to a bidirectional interaction between COVID-19 and the heart with an increase in susceptibility to cardiovascular disorders (CVD) (Guo et al, 2020a; Shi et al, 2020a; Shi et al, 2020b) in adults with subsequent heightened mortality rate. This striking observation has been demonstrated, particularly in COVID-19 patients with a history of CVD (30%–60%) (Ramachandran et al, 2022). COVID-19 has also been demonstrated to cause defects in the coagulation cascade culminating in thromboembolism in many patients (Bikdeli et al, 2020; Connors and Levy, 2020).
In children, extensive hemophagocytosis, consistent with multisystem inflammatory injury and Kawasaki disease were also detected with elevated ILs (primarily IL-6) (Del Valle et al, 2020; Leppkes et al, 2020). Although these COVID-19-inflammatory markers and ACE2 interaction have been proposed to predispose to CVD in the COVID-19 patients, the exact mechanism underlying this association is unknown (Riphagen et al, 2020), whereas adverse cardiovascular outcomes in COVID-19 patients remain neglected.
So, Why Does COVID-19 Predisposes to Cardiac Injury?
Potential causes to COVID-19-mediated CVD include elevated cardiac stress due to respiratory failure/hypoxia/hypoxemia caused by severe pulmonary inflammatory injury or acute lung injury, indirect injury of myocardium, or multiple organs caused by systemic inflammatory response, all of the aforementioned pathological mechanisms can affect the myocardium or microvessels, resulting in myocardial direct injury. This is the main cause of myocardial infection or cardiac dysfunction caused by SARS-CoV-2. Inflammatory infiltration is mainly caused by macrophages and CD4+ T cells (Xiaohong et al, 2020; Xu et al, 2020).
This mechanism have been detected in the necrotic regions of the heart amounting to myocarditis (Fung et al, 2016; Thomas Aretz, 1987). The ACE2 enzyme is mainly involved in the regulation of blood pressure levels, and is expressed in cells of various organs such as the heart, lung, and kidney (Bhatti et al, 2020; Ma et al, 2021). SARS-CoV-2 has also been found to infect and induce cytotoxicity in the cardiomyocytes (Bojkova et al, 2020b; Kwon et al, 2020; Lindner et al, 2020; Marchiano et al, 2021; Navaratnarajah et al, 2021; Wong et al, 2020) and patients (Escher et al, 2020; Lindner et al, 2020; Tavazzi et al, 2020; Tian et al, 2020), mirroring the detection of viral genome alongside decreased levels of ACE2 and increased hypertrophy in the myocardium (Abbasi, 2022; Escher et al, 2020; Oudit et al, 2009).
Yet, upregulated expression of ACE2 in certain cardiac cells such as the pericytes promotes dysfunction while disrupting coronary microcirculation (Chen et al, 2020). What is the mechanism by which cardiomyocytes are damaged by COVID-19, and which type of cardiac cells are more susceptible to the virus? In this regard, a deeper understanding of the mechanisms underlying COVID-19-infection mediated myocardial injury is needed to formulate a therapeutic strategy to prevent long-term adverse cardiovascular events in the COVID-19 patients.
What Is the Effect of SARS-CoV-2 Infection to the Heart?
The risk of cardiovascular disease in patients with COVID-19 is related to underlying diseases, including cerebrovascular disease, myocardial injury, coronary microvascular injury, septic cardiomyopathy, inflammatory heart disease, ischemic heart disease, and hypertrophic cardiomyopathy and other cardiac dysfunctions. The increase in susceptibility to CVD is also prevalent in COVID-19 patients without any prior history of CVD as well as those who had mild symptoms of COVID-19. The intensity of this susceptibility to CVD increases proportionately across the spectrum from the nonhospitalized cohort to those admitted to intensive care (Lindner et al, 2020).
Increased thrombotic and inflammatory inclination precipitate in acute coronary syndromes and acute myocardial infarction (AMI). The AMI incidence ratio within 7 days of infection is between 2.8 and 10.1, highlighting the association between viral infection and AMI, with AMI contributing to mortality as observed in the SARS epidemic (Peiris et al, 2003).
With regard to cardiac function, current data are inconclusive regarding whether ejection fraction (EF) is still preserved after COVID-19-induced myocarditis. Normal heart function has been observed in patients with uncomplicated lymphocytic myocarditis with most patients leaning toward preserved EF with concomitant elevated cardiac troponin I (cTnI) and brain-type natriuretic peptide (BNP) (Ammirati et al, 2018; Hu et al, 2021; Inciardi et al, 2020). Both hypo- and hypertension as well as arrhythmias are also prevalent in the COVID-19 patients particularly the critically ill patients. Palpitations have been detected in ∼7% of the patients, whereas a higher proportion (∼18%) develop arrhythmias, including tachycardia, bradycardia, and cardiac arrest (Coromilas et al, 2021). Whether these blood pressure abnormalities are the cause or end-effect of the infection or related to potential derangements in ACE2 expression remains unknown.
Although COVID-19 has been associated with ensuing cardiovascular dysfunction, most of the investigation into adverse cardiovascular outcomes have been carried out in hospitalized patients with a short duration of follow-up and a narrow selection of cardiovascular outcomes (Ayoubkhani et al, 2021; Daugherty et al, 2021; Huang et al, 2021). In general, an elevation of cardiac damage biomarkers such as cTnI (Ruan et al, 2020) and BNP (Arentz et al, 2020) have been detected in COVID-19 patients in association with a worse prognosis (Shi et al, 2020a). Previously, in a Wuhan cohort, myocardial damage and heart failure were found to contribute to 40% of deaths in the COVID-19 patients, irrespective of whether they had respiratory failure (Ruan et al, 2020).
There is also a significant risk of mortality associated with myocardial damage and cardiac dysfunction even when adjusted against other factors such as age and other comorbidities such as the presence of diabetes mellitus, or inflammatory cardiomyopathy (Shi et al, 2020a). In another single-center retrospective observational study of 257 hospitalized patients with COVID-19, ∼22% of the patients succumbed to in-hospital mortality or discharge to hospice. Patients admitted to hospital with COVID-19 had significantly higher cTnI within 24 hours (≥0.012 ng/mL) and higher in-hospital mortality (52% vs. 10%, p < 0.0001). Interestingly, circulating mitochondrial DNA (mtDNA) can also fully reflect the diagnostic and prognostic value of cTnI to a certain extent in prospectively collected cell-free plasma samples of patients hospitalized for COVID-19 (Scozzi et al, 2021).
Elevated circulating mtDNA levels were also determined to be an independent risk factor for intensive care unit (ICU) admission, renal replacement therapy, or eventual mortality. Circulating mtDNA levels also had a similar area under the curve when compared against other emerging or clinically established measures of inflammation such as IL-6, C5b-9, and neutrophil-to-lymphocyte ratio. The observations from this particular study was reflected in two mtDNA genes, namely mitochondrial-encoded gene cytochrome B (mtCYTB) and mtCOX3 (Scozzi et al, 2021). The discovery of mtDNA in the plasma of COVID-19 patients leads to the question as to whether cardiac mitochondria mediating COVID-19-related cardiac injury?
So, How Would the SARS-CoV-2 Infection Mediate Mitochondrial Dysfunction?
Starting from the entry of the virus into the cell …
The spike glycoprotein of SARS-CoV-2 is used on the host transmembrane serine protease 2 (TMPRSS2) (Hoffmann et al, 2020) as well as ACE2 host receptor (Cao et al, 2020b) (Fig. 1). The former can make spinous process protein enter host cells through endocytosis, whereas the latter will cut spinous process protein at s1/s2 and S2′ sites through host enzymes (Hoffmann et al, 2020; Ou et al, 2020). Androgen-induced TMPRSS2 is currently thought to affect mitochondrial function mainly by acting on estrogen-related receptor alpha, mainly because estrogen-related receptor alpha can regulate mitochondrial function transcription and energy homeostasis, which indirectly regulates intracellular homeostasis (Hoffmann et al, 2020; Singh et al, 2020; Xu et al, 2018).
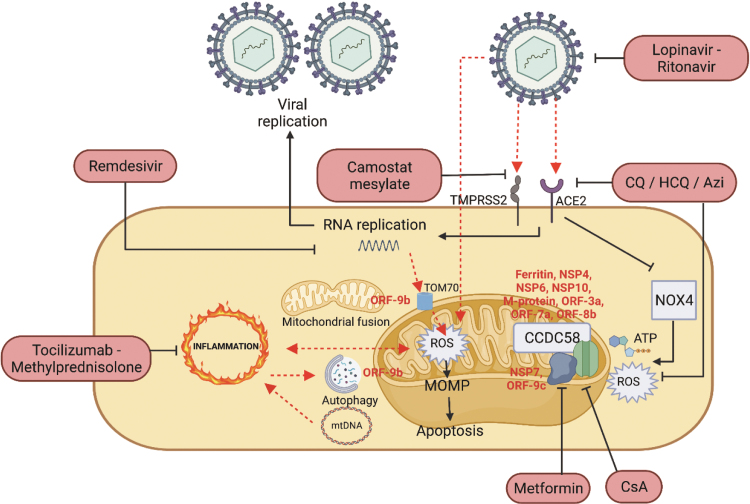
FIG. 1.
Interaction between SARS-CoV-2 infection and the resulting inflammation and mitochondrial dysfunction. SARS-CoV-2 infects the cell via the ACE2 and TMPRSS2 structure. Once inside the cell, viral replication is induced, leading to various events such as the interaction with the mitochondrial TOM70 to mediate inflammation signaling via upregulation of mitochondrial ROS. The mitochondrial ROS produced will also predispose to mitochondrial outer membrane permeabilization, release of mtDNA and apoptosis. The released mtDNA will also further trigger inflammation. In addition to viral replication, the different viral proteins will also induce mitochondrial dysfunction via targeting mitochondrial dynamics in the form of morphology shifting and perturbation to autophagy, as well as disrupting the mitochondrial respiratory supercomplex and permeability transition pore. To prevent the deleterious effects of long COVID-19, different pharmacological compounds (as indicated in pink capsules) are being used to target the different facets of SARS-CoV-2 infection. ACE2, angiotensin-converting enzyme 2; COVID-19, corona virus disease 2019; mtDNA, mitochondrial DNA; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane serine protease 2.
Whether the priming of the virus S protein by TMPRSS2 modifies its function and availability and its subsequent effects on the mitochondria remains to be investigated. ACE2 exerts effects on mitochondrial functions whereby the adenosine triphosphate (ATP) and reactive oxygen species (ROS) production is directly correlated with ACE2 levels. A depressed or disturbed host ACE2 by the SARS-CoV-2 virus modifies NADPH oxidase 4 (NOX4) in the mitochondria thereby affecting mitochondrial ROS (Singh et al, 2020), which can be utilized for destroying pathogens or induce apoptosis in the infected cell.
The use of ACE2 receptors for cell entry by SARS-CoV-2 intensifies the problem of low availability of this enzyme in the aged or diabetic subjects thus weakening the anti-inflammatory ability of ACE2 while contributing inflammatory reaction and complications of diabetes (Obukhov et al, 2020). In the comorbid settings of aging plus diabetes, the aging-associated reduction of mitochondrial function and obesity-associated meta-inflammation increases severity of COVID-19.
Mitochondria are the energy metabolism centers of cardiomyocytes, which can generate ATP required through the process of oxidative phosphorylation to meet the higher energy demands of the myocardium. The heart has important physiological activities, such as myocardial contraction and maintenance of cellular homeostasis, which require ATP produced by mitochondria. Therefore, optimal mitochondrial structure and function are the premise and basis for the normal physiological activities of the myocardium and the normal blood ejection function of the heart (Chang et al, 2021).
Upon entry to the cell, the virus triggers an inflammatory response with cytokines such as tumor necrosis factor-α (TNF-α), interferon (IFN)-γ, and IL-10 initiating an upregulation of ROS production (Saleh et al, 2020). As shown in Figure 2, a vicious cycle of ROS-induced ROS increase in the mitochondria then ensues with stimulation of additional proinflammatory cytokine production (Li et al, 2013), resulting in mitochondrial membrane permeabilization and apoptosis. When dysfunctional or damaged mitochondria release their contents into the cardiomyocyte cytoplasm, it further leads to the production of inflammatory cytokines and further mediates the activation of apoptotic pathways (Saleh et al, 2020).
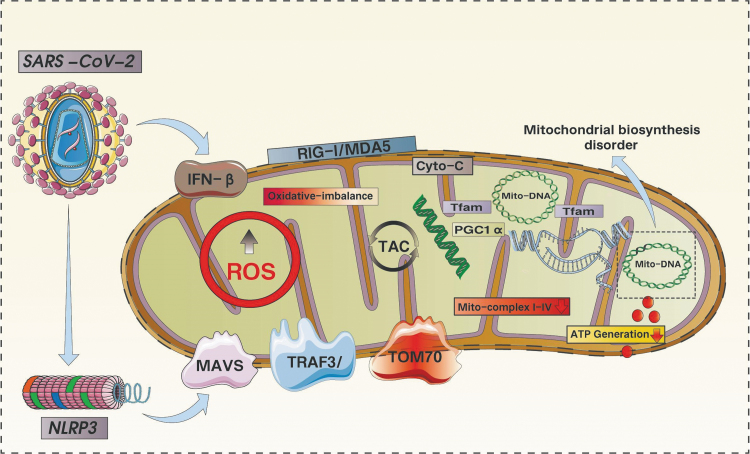
FIG. 2.
SARS-CoV-2-mediated ROS burst-induced mitochondrial oxidative stress damages good inflammatory responses in a vicious cycle in mitochondria. SARS-CoV-2 attack can lead to the activation of NLRP3 inflammasome and the excessive production of ROS. Mitochondria are the main site of ROS generation and the main target of ROS attack. Dysfunction, the level of oxidative phosphorylation was significantly reduced. At the same time, the reduced levels of PGC1a and Tfam also led to mtDNA dysfunction, severe mitochondrial biosynthesis was blocked, and the level of ATP synthesis was significantly reduced. Activation of the NLRP3 inflammasome also leads to dysfunction of MAVS/TRAF3 and TOM70, which ultimately leads to damage to the inner and outer mitochondrial membranes, further mediating the release of Cyto-C into the cytoplasm and activating apoptosis via the caspase pathway. ATP, adenosine triphosphate; MAVS, mitochondria antiviral signaling.
High levels of mtDNA and fragments from the mtCYTB are found in the cytoplasm, according to new research (Scozzi et al, 2021). The findings aim to better predict adverse COVID-19 outcomes. The study found that the level of mtCYTB is closely related to the level of plasma SC5b-9. Plasma SC5b-9 is a marker of complement activation, and the appearance of the marker indicates the formation of membrane attack complex (Scozzi et al, 2021). Intramitochondrial proteins and mtDNA released in the cytoplasmic space can be recognized by intracellular immune receptors, leading to neutrophil recruitment and cytokine production by monocytes, thereby affecting the homeostatic balance of the immune system (Jang et al, 2018).
To evade the host cell defense mechanism, it has been postulated that the virus hides inside mitochondria-derived double membrane vesicles (Jang et al, 2018)—a theory based on the HIV mechanism (Somasundaran et al, 1994) as well as the computational analysis of the 5′ and 3′ untranslated regions of SARS-CoV-2 primarily for host mitochondrial localization (Wu et al, 2020b). SARS-CoV-2 is able to replicate in these specialized double-membrane vesicles (Wolff et al, 2020), thereby promoting viral replication and viral replication-transcription complex (RTC) critical for nonstructural protein synthesis (NSPS) (Yan et al, 2020). The assembly process of RTC is critical for SARS-CoV infection and has a similar function for SARS-CoV-2 (Sakai et al, 2017). Therefore, SARS-CoV-2 can have important effects on mitochondrial function via a mediation of viral attack and inflammatory response, and promoting the occurrence of hijacking mechanisms, especially the inhibition of mitochondrial biogenesis (Singh et al, 2020).
A more definitive link between the virus and the human mitochondria lies in “SARS-CoV-2-human protein-protein” interaction map from recent purified mass spectrometry analysis (Gordon et al, 2020), whereby it can be inferred that SARS-CoV-2 protein and human mitochondrial protein can produce a certain level of interaction mechanism. Human mitochondrial proteins can overlap with available information about SARS-CoV under different circumstances. Interestingly, COVID-19 can significantly inhibit the transcription levels of mtDNA-encoded genes and increase the transcription levels of nuclear-encoded mitochondrial respiratory complexes, mitochondrial permeability transition pore (mPTP), and mitotic phagocytosis-related genes. Alongside this finding was the increased detection of the cardiac injury markers such as troponin-C and IL-6 in the plasma.
Among the proteins localized to the organelles, the nuclear-encoded mitochondrial protein, CCDC58, which also happens to localize to the mitochondria, has been found to be elevated in COVID-19 peripheral blood mononuclear cells (PBMCs), alongside significant upregulation of mPTP complex components SPG7 and cyclophilin D was seen in Caco-2 cells infected with SARS-CoV-2 (Bojkova et al, 2020a; Shanmughapriya et al, 2015).
The role of CCDC58 in modulating calcium sequestration by the mitochondria is exemplified by experiments knocking down CCDC58 leading to depressed capability of the mitochondria to sequester calcium, even in the presence of upregulated SARS-CoV-2 M protein. SPG7, PPIF, and CCDC58 are able to generate an interaction mechanism, and the mPTP complex itself can also bind to some SARS-CoV-2 viral proteins (ORF3a, ORF9b, and nonstructural protein [NSP] 6), thus suggesting that SARS-CoV-2 viral proteins may be involved in SARS-CoV-2-induced mitochondrial dysfunction and cell death (Ramachandran et al, 2022).
Different neutral proteins also promoted suppression of mitochondrial energy metabolism levels (mitochondrial basal or maximal respiration) and reduced mitochondrial capacity to regulate calcium homeostasis—an effect reversed by administration of cyclosporin A (CsA). In brief, the observations from the study point toward a potential role of the SARS-CoV-2 proteins in sensitizing the mPTP to open thus triggering subsequent cell death (Ramachandran et al, 2022).
Based on the results obtained from the overall transcriptomic profiling of SARS-CoV-2 infection in PBMCs of healthy volunteers and patients with COVID-19-infected disease, the transcriptional levels of mitochondrial ion channels, mitophagy, and other pathways such as oxidative phosphorylation, ATP biosynthesis were found to be significantly downregulated, whereas the transcriptional levels of apoptosis regulators of the mitochondrial pathway were significantly increased. Yet, the ATP energy required for T cell regulation and initial activation of neutrophils comes from mitochondria, so once the mitochondrial oxidative phosphorylation and the tricarboxylic acid cycle are affected, it will lead to mitochondrial dysfunction and dysregulation of intracellular homeostasis. As shown in Figure 3, during antigen presentation and processing, both ATP and mitochondrial Ca2+ are also needed while ROS acts to activate inflammatory proteins (Angajala et al, 2018).
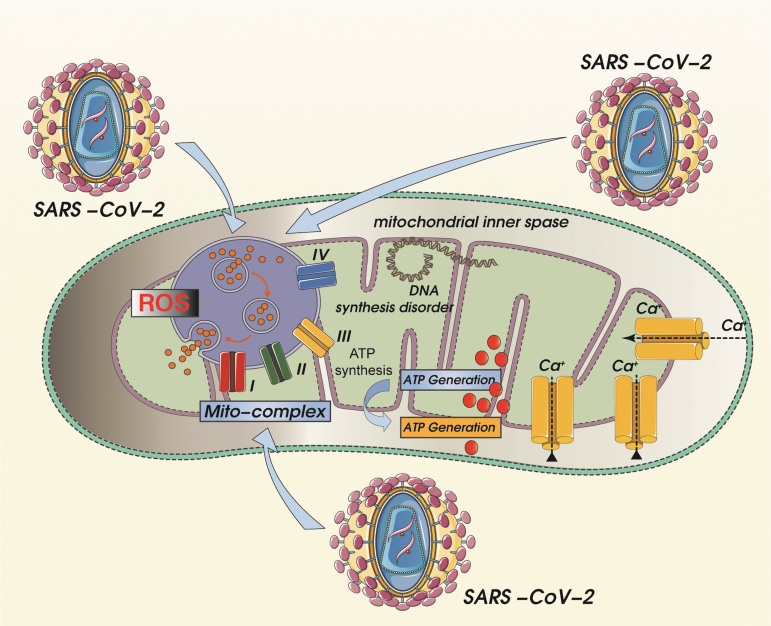
FIG. 3.
SARS-CoV-2-induced mitochondrial respiratory chain dysfunction and mitochondrial calcium overload with concomitant mtDNA synthesis dysfunction. Mitochondrial oxidative stress damage caused by SARS-CoV-2-mediated mitochondrial ROS overproduction can further lead to mitochondrial respiratory chain dysfunction, and induction of calcium overload can cause oxidative phosphorylation dysfunction in mitochondria. Accompanied by the reduction of mitochondrial membrane potential, mitochondrial ATP synthesis disorder will eventually lead to and promote irreversible cell damage. The initial calpain activation further leads to influx of extracellular Ca2+ through calcium channels, followed by transfer to the cell membrane, activating the apoptotic pathway.
In addition to viral replication, some SARS-CoV-2 proteins are able to localize to the outer or inner mitochondrial membrane (IMM), as well as the endoplasmic reticulum (ER), to further affect cellular functions (Ramachandran et al, 2022). Some transcripts involved in calcium transport, such as STIM1 and L-type calcium channels, were significantly upregulated in PBMCs from patients with COVID-19. In human induced pluripotent stem cells (hiPSC)-coiled membranes (CMs) expressing SARS-CoV-2 proteins, the baseline of cellular calcium is severely disrupted, whereas the L-type calcium channel activity was almost completely blocked. In this regard, the viral proteins have been found to localize to the mitochondria, bind and associate with the mPTP complex, alter mitochondrial dynamics and function, impair Ca2+ signaling and inhibit voltage dependent cation channel (VDCC) channel activity, ultimately leads to dysregulated calcium homeostasis and severe mitochondrial dysfunction.
The single-stranded positive-sense genomic RNA from SARS-CoV-2 has also been postulated to localize to the mitochondria, thus potentially mediating an impairment of mitochondrial function (Wu et al, 2020b). In addition, transcriptomic profiles associated with oxidative phosphorylation regulatory mechanisms were also detected in peripheral mononuclear leukocytes and bronchoalveolar lavage fluid of patients with COVID-19 (Gardinassi et al, 2020). Yet all of these data were based on computational analysis and prediction, whereby further laboratory investigation will be required to verify the significance of these findings. Indeed, subsequent studies carried out as described hereunder further corroborated the relevance of the transcriptional and translational aspects of SARS-CoV-2 in mediating mitochondrial dysfunction.
Both SARS-CoV-1 and SARS-CoV-2 contain open reading frames (ORF-9b, ORF-7a, and ORF-8b) that are localized to mitochondria and capable of interacting with mitochondrial function-regulating signals. ORF3a, apart from mediating mitochondrial apoptosis (Padhan et al, 2008), also targets the mitochondrial deubiquitinase Ubiquitin-specific protease 30 (USP30), thereby altering mitochondrial homeostasis/mitochondrial quality control (including mitochondrial biogenesis, mitochondrial dynamics, and mitophagy) (Pasquier and Robichon, 2021). Apart from the genetic components that exert an effect on mitochondrial function, the protein components of the SARS-CoV-2 also affect mitochondrial function and fate.
Among the 28 gene products encoded by SARS-CoV-2, viral structural protein membrane (M) and NSP6, NSP10, ORF3a, and ORF9c proteins are mainly localized in mitochondria or ER. The reorganization of M protein in hiPSC-CMs can inhibit the release and regulation function of CaV1.2 channel current. Ectopic expression of M protein in human cells can further induce mitochondrial swelling, possibly by interacting with mPTP components SPG7 and CCDC58 to further enhance pore opening and lead to dysregulation of mitochondrial homeostasis (Ramachandran et al, 2022). Some of the SARS-CoV-2 proteins that interact with the mitochondria, including NSP4 (with the translocase of the inner membrane) (Gordon et al, 2020).
Critically ill COVID-19 patients also have a high level of ferritin (Alroomi et al, 2021; Dimopoulos et al, 2021; Lino et al, 2021; Papamanoli et al, 2021), which when excessive promotes oxidative stress and impairs mitochondrial oxygen consumption. Iron metabolization is impaired in the dysfunctional mitochondria, causing an iron buildup and ferroptosis. In addition, the oxidative stress caused by ferritin overload also affects COVID-19 patients with diabetes as glucose tolerance is impaired (Tummalacharla et al, 2022).
How Is Inflammation Involved in the Relationship Between SARS-CoV-2 Infection, and the Mitochondria?
IFN-I induction is a crucial step in the immunological defense against viral infection (Stetson and Medzhitov, 2006). Intracellular antiviral response mechanisms are primarily initiated by activation of RIG-I-like receptors (e.g., RIG-I/MDA5) that trigger signaling complexes on the mitochondrial outer membrane, including adaptor protein-mitochondria antiviral signaling (MAVS) proteins and TRAF3/TRAF6/TOM70 upon detection of viral RNA (Liu et al, 2010).
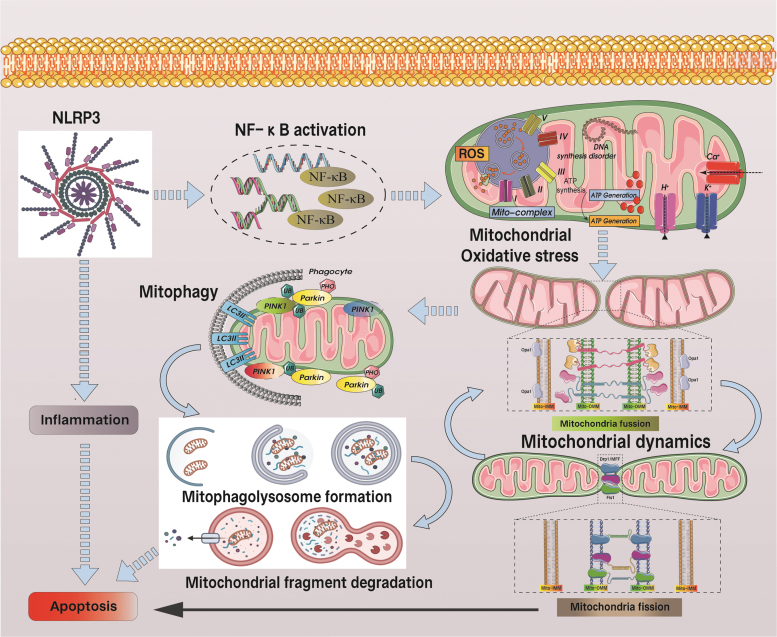
Although SARS-CoV-2 triggers only a very low level of IFN-1 (Blanco-Melo et al, 2020), ORF3b disrupts antiviral defense by inhibiting type I IFN induction via interacting with the mitochondrial virus detection protein RIG-1 (Freundt et al, 2009)—a similar interaction also detected with SARS-CoV-2 ORF-9b within the nucleocapsid (N) gene, although via an interaction with TOM70 (Shi et al, 2019), which is an intracellular multiprotein complex responsible for regulating caspase-1 activation and processing of the pro-inflammatory cytokine IL-1β as well as triggering pyroptotic inflammatory cell death (Takahashi, 2019). As shown in Figure 4, an impaired clearance of dysfunctional mitochondria via autophagy inhibition and defective mitochondrial fission causes an escalation of ROS production to activate the NLRP3 inflammasome, eventually accelerating cardiac failure (Li et al, 2022; Park et al, 2015).
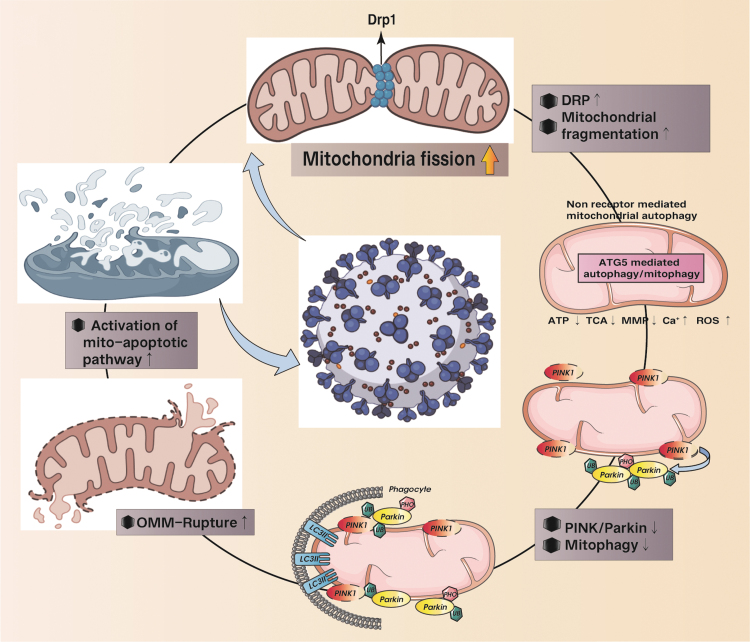
FIG. 4.
SARS-CoV-2-mediated mitochondrial hyperfission and the regulatory mechanism of autophagy/mitochondrial autophagy in the apoptosis process of mitochondrial pathway. SARS-CoV-2-mediated excessive mitochondrial fission leads to increased levels of mitochondrial fragmentation. Damaged or fragmented mitochondria/organelles are cleared by ATG-mediated autophagy and PINK/Parkin-mediated mitophagy. However, in the state of autophagy or mitophagy deficiency, damaged or fragmented mitochondria cannot be removed in time. Mitochondrial dysfunction or rupture of the outer membrane leads to the activation of the mitochondrial pathway to apoptosis. This is the main mechanism of ROS-mediated mitochondrial damage and dysregulation of cellular homeostasis under the action of SARS-CoV-2.
ORF9b triggers MAV degradation and further limits host cell IFN-producing responses (Shi et al, 2014). The ORF9c of SARS-CoV-2 can also interact with the production of negative regulators of MAVS signaling (NLRX1, NDFIP2), and the interaction mechanism of NSP13 with the MAVS effector TBK1 can also regulate the activation of MAVS, thereby affecting mitochondrial function (Gordon et al, 2020). Other studies have found that prohibitin (PHB1/2) can also react with SARS coronavirus NSP2, thereby affecting the activation of intracellular signals and leading to dysfunction of mitochondrial biogenesis (Cornillez-Ty et al, 2009; Yoshinaka et al, 2019). The cycle of SARS-CoV-2 entry into the cell, triggering inflammation, mitochondrial dysfunction, and excessive ROS production impairs mtDNA, releases mtDNA into the cytosol after cellular stress and damage and disrupts mitochondrial dynamics thereby preventing proper mitochondrial division and biosynthesis (as shown in Fig. 5).
FIG. 5.
NLRP3-mediated inflammatory response and mitochondrial ROS-mediated mitochondrial oxidative stress have effects on mitochondrial dynamics and mitophagy. NLRP3 promotes the activation of NF-KB while stimulating the overproduction of mitochondrial ROS, which mediates decreased levels of mitochondrial respiratory chain complexes and underproduction of ATP, as well as mitochondrial calcium overload and mitochondrial dysfunction. Under the influence of mitochondrial dysfunction, the level of OPA1-mediated mitochondrial fusion is deficient and the level of Drp1-mediated mitochondrial fission is elevated. Under persistent inflammation and ROS attack, mitophagy levels were also affected. Long-term mitochondrial dysfunction can lead to apoptosis. Drp1, dynamin-related protein 1; OPA1, optic atrophy 1.
The severity of COVID-19 also depends on the level of oxidative stress, presence of systemic hyperinflammation, and coagulopathy, whereby excessive oxidative stress in COVID-19 patients as evidenced by increased mitochondrial superoxide and lipid peroxidation causes local and systemic tissue damage thereby triggering inflammation and contributing to the clinical phenotypes of COVID-19 (Beltrán-García et al, 2020). In an in vitro model of monocytes cocultured with VERO E6-media containing SARS-CoV-2, an increase in lipid peroxidation-dependent inflammasome-derived IL-1β secretion was observed (Lage et al, 2022). Importantly, the elevated oxidative stress and inflammasome activity persisted after short-term patient recovery, thereby rendering the possibility of mitigating oxidative stress and inflammation as potential therapeutic targets to prevent long-term adverse outcomes of COVID-19 (Lage et al, 2022).
What Is the Association Between Mitochondrial Damage with SARS-CoV-2 Symptoms?
Elevated respiration as a result of diminished oxygen availability in the blood is detected and induced by the carotid bodies. The impaired constriction of the pulmonary artery and carotid body due to the infection-mediated disruption of mitochondrial complex I subunit NDUFS2 has been implicated in the occurrence of hypoxemia in patients infected with SARS-CoV-2 (Archer et al, 2020; Dunham-Snary et al, 2019). Carotid bodies also express ACE2 and may be directly infected by SARS-CoV-2 (Li and Schultz, 2006). Several subunits of Complex I can interact with the SARS-CoV-2 proteins NSP7 and ORF9c.
The mitochondria of monocytes from COVID-19 patients also have impaired bioenergetics with a reduced basal and maximal respiration, a defective oxidative and glycolytic metabolism alongside a reduced proton leak (Gibellini et al, 2020). The mitochondria were characterized by varying sizes with a high degree of swelling, although the mitochondrial membranes were intact.
Although cTnI is directly related to myocardial injury, cTnI is also a major structural protein closely related to the maintenance of myocardial contractile/diastolic function. The level of cTnI may serve as a key biomarker for myocardial injury caused by COVID-19 in the future, and is directly related to survival and prognosis (Al Abbasi et al, 2020).
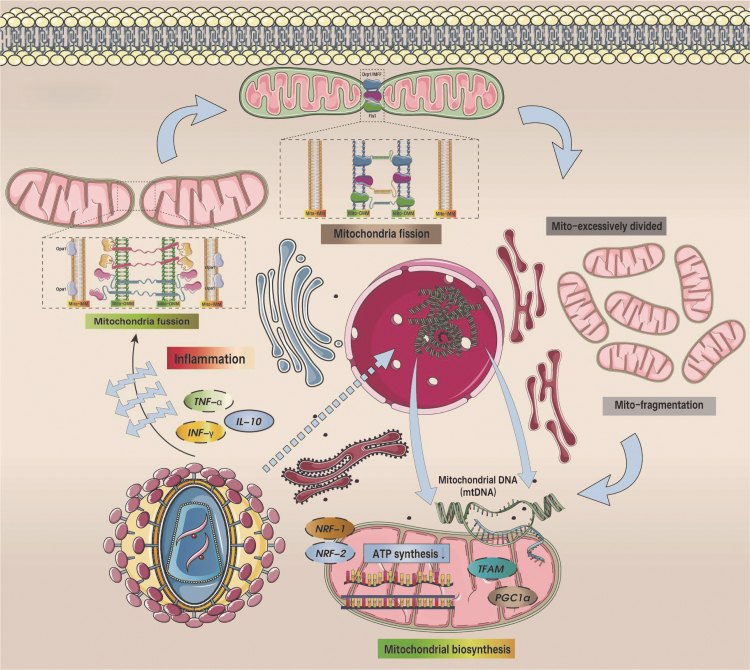
Changes to Mitochondrial Dynamics Post-SARS-CoV-2 Infection
To survive and replicate, viruses have been known to manipulate mitochondrial fission and fusion (Holder and Reddy, 2021). As shown in Figure 6, mitochondria are dynamic in the sense that the structure can be altered via fusion and fission processes (Chan, 2006a). Fusion of the outer mitochondrial membrane (OMM) and IMM is critical for mitochondrial dynamics and is mediated by the prefusion proteins mitofusin 1 and 2 (Mfn1 and Mfn2) and optic atrophy 1 (OPA1), respectively (Chan, 2006a; Chen and Chan, 2004). The normal fusion mechanism of mitochondria can further promote mtDNA synthesis, maintain mitochondrial protein homeostasis, and normal mitochondrial energy metabolism (Chan, 2006b).
FIG. 6.
SARS-CoV-2-mediated inflammatory response leads to dysregulated mitochondrial homeostasis and mitochondrial/endoplasmic reticulum dysfunction. Under the influence of SARS-CoV-2, TNF-α/IFN-γ was excessively activated, the expression level of IL-10 was inhibited, the levels of OPA1 and Mfn1/Mfn2 decreased under the mediation of inflammatory response, and mitochondrial fusion was inhibited. In contrast, Drp1/Mff/Fis1-mediated mitochondrial fission was overactivated. Mitochondrial function is closely related to mitochondrial morphology. Under normal circumstances, mitochondria are in a dynamic balance between fusion and fission processes. If mitochondrial fusion and/or fission are abnormal, mitochondrial function may be abnormal. Therefore, the disruption of mitochondrial dynamic balance will lead to mitochondrial dysfunction. During SARS-CoV-2 infection. NRF-1/-2 and TFAM/PGC1α were inhibited, and mtDNA damage was aggravated. Notably, SARS-CoV-2 also causes dysfunction of mitochondrial-ER contact points (MAMs), which further affects ER function and mediates ER stress. ER, endoplasmic reticulum; IFN, interferon; IL, interleukin; MAM, mitochondria-associated membrane; Mff, mitochondrial fission factor; Mfn, mitofusin; TNF-α, tumor necrosis factor-α.
Mitochondrial fission is mainly completed by OMM fission mediated by cytoplasmic GTPase-dynamin-related protein 1 (Drp1) and mitochondrial fission factor (Mff), and the interaction mechanism between Fis1 and Drp1 also plays a role in regulating mitochondrial fission (Smirnova et al, 1998; Liu and Chan, 2015; Otera et al, 2010; Zhou et al, 2017) and MiD49/51 (Losón et al, 2014; Otera et al, 2016; Palmer et al, 2013; Palmer et al, 2011). Under normal conditions, mitochondrial fission kinesins are capable of facilitating mitochondrial division and distribution during cell cycle as well as removal of the damaged mitochondria via mitophagy.
Enhanced mitochondrial fission via upregulation of Drp1 or degradation/inhibition of MAVS has been utilized in viral systems to upregulate mitophagy and modify occurrence of apoptosis (Khan et al, 2015). In certain viruses such as the hepatitis C and B viruses, mitochondrial fission inhibits apoptosis via upregulating mitophagy so as to allow the virus to survive longer and further replicate (Kim et al, 2014; Kim et al, 2013a). However, studies have found that SARS-CoV infection causes mitochondria to elongate in length. Changes in the length of mitochondria are mainly related to the formation of CMs (Barbier et al, 2017; Chatel-Chaix et al, 2016; Shi et al, 2014). How is this mitochondrial elongation associated with viral replication?
As shown in Figure 6, structural abnormalities or dysfunctions of mitochondria-associated membranes (MAMs)—contact sites between the ER and mitochondria—are thought to mediate an important bridge between mitochondrial length elongation and viral replication/attack. The contact between the ER and mitochondria determines the replication, division, and distribution of mitochondria, and the contact area between the ER and mitochondria is the starting area of these mitochondrial divisions. Contact between these two organelles allows mtDNA to replicate and divide.
This DNA splitting is then coupled with the splitting of the mitochondria themselves and the distribution of daughter mtDNA around the cell. MAMs can transfer a large number of molecules between the ER and mitochondria, and promote the activation and function of certain pattern recognition receptors (PRRs), including MAVS. Dysfunction of MAMs caused by COVID-19 attack may affect ion channel dysfunction between mitochondria and ER, ultimately leading to dysregulated mitochondrial homeostasis and ER stress, activating apoptotic pathways (Barazzuol et al, 2020; Friedman et al, 2011; Lewis et al, 2016; Vazquez and Horner, 2015).
CM formation after viral infection affects the location of mitochondrial contact site regions in the ER (Chatel-Chaix et al, 2016). SARS-CoV-2 NSP4 is required for SARS-CoV CM formation. SARS-CoV-2 NSP4 interacts with the mitochondrial import machinery (translocase of inner mitochondrial membrane [TIM]) complex (Gordon et al, 2020). Dysfunction of MAM can disrupt homeostatis in the intracellular environment, including autophagy or mitophagy, resulting in the failure of timely clearance of damaged or fragmented organelles/metabolites, thereby inducing further mitochondrial oxidative stress or dysfunction of energy metabolism (Gomez-Suaga et al, 2017; Zhou et al, 2021). ORF9b of SARS-CoV promotes dysregulated mitochondrial dynamics via hyperactivation of Mff Drp1 protein and upregulation of Mff/Fis1 (as shown in Fig. 7).
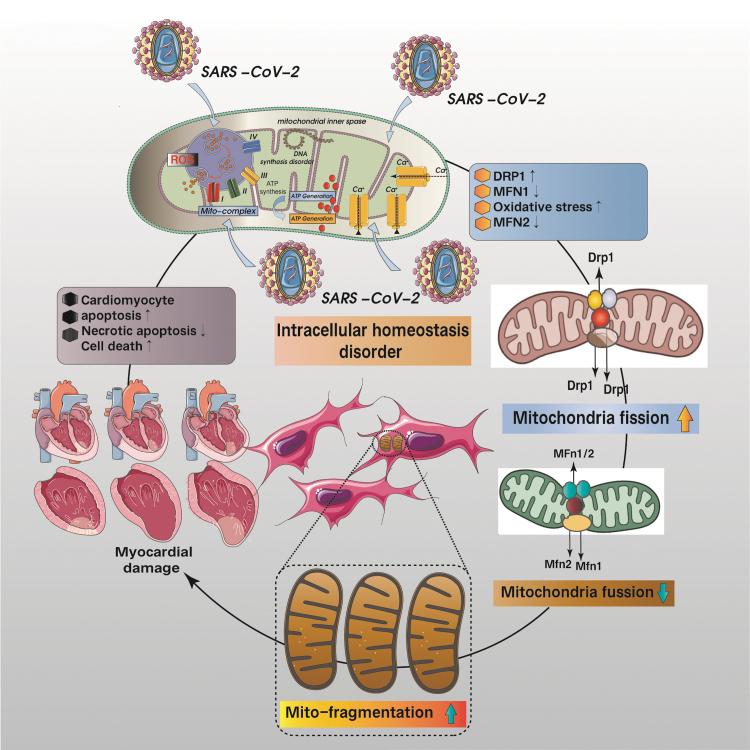
FIG. 7.
Cardiomyocyte homeostasis and myocardial injury due to mitochondrial dysfunction. SARS-CoV-2 attack results in mitochondrial calcium overload, mitochondrial oxidative stress damage, excessive mitochondrial fission, and increased levels of mitochondrial fragmentation, which ultimately lead to dysregulation of cardiomyocyte homeostasis, apoptosis/necroptosis, and cellular death, which is also the main cause of myocardial damage and nonregeneration.
Although proteosomal-degradation of Drp1 has been associated with SARS-CoV-1 ORF-9b, it is likely that the ORF-9b will perform the same function as well given the similarities in the genome (Shi et al, 2014). The ORF9b localizes on the mitochondrial membrane and modulates MAVS via interaction with TOM70 (Gordon et al, 2020). Although Mfn2-mediated mitochondrial fusion inhibits MAVS thereby repressing the IFN response, apoptosis can still be induced via other factors (ORF-6/-7a) (Schaecher et al, 2007; Ye et al, 2008).
Lower levels of mitochondrial basal/maximal and reserve respiration could be detected in HEK293 cells expressing ORF3a (Ramachandran et al, 2022). In this regard, M protein can participate in the protein interaction mechanism of mitochondrial energy metabolism, thereby affecting mitochondrial protein homeostasis (Gordon et al, 2020), whereas the M protein from SARS-CoV induces apoptosis (Chan et al, 2007). Whether the M-protein-mediated apoptosis is associated with the function of the nucleocapsid and ORF3a as in the case of SARS-CoV (Padhan et al, 2008; Zhang et al, 2007) remains to be determined.
Changes to Autophagy Post-SARS-CoV-2 Infection
The severity of SARS-CoV-2 has also been correlated to interference with platelet count and coagulation (Tang et al, 2020; Terpos et al, 2020). As shown in Figure 7, the increased coagulation and decreased platelet promote stroke formation alongside a potential impaired mitophagy machinery, which predisposes to apoptosis and thrombus formation (Lee et al, 2016). This phenomenon may be especially relevant to the diabetic patients whose mitochondria are susceptible to oxidative stress yet failed to be removed via mitophagy (Lee et al, 2016). Hyperinflammation and iron buildup in the COVID-19 patients further exacerbates platelet disruption (Saleh et al, 2020).
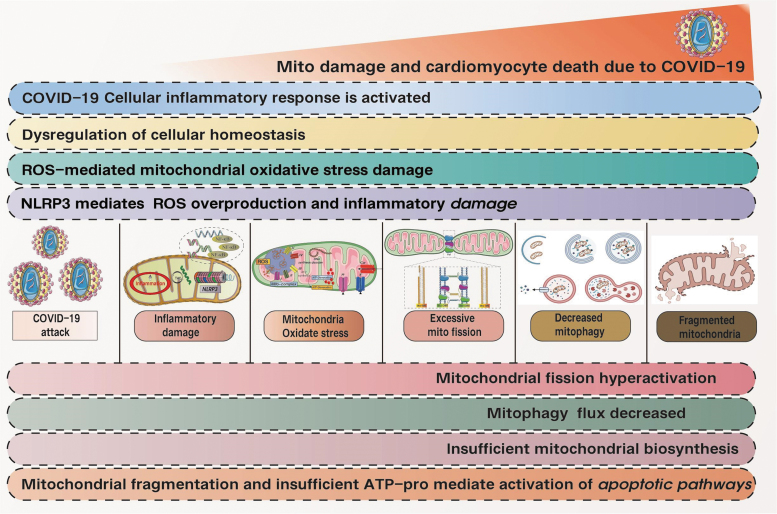
When the comorbidity of aging is present, the severity in COVID-19-infected aged patients is more pronounced when compared with a younger patient. This is expected as the mitochondria in the aged cell will have a less efficient respiratory and ATP production machinery. The lack or impairment of mitophagy in the aging cell (García-Prat et al, 2016) also leads to accumulation of defective mitochondria, release of damaged mitochondrial components or mtDNA into the cytosol and trigger of unregulated inflammasome activity (Shah, 2020), further putting the aged patients at risk of hyperinflammation and increased mortality (as shown in Fig. 8).
FIG. 8.
Pathological mechanism of COVID-19-mediated cardiomyocyte programmed death. The onset of COVID-19 leads to cellular inflammatory damage and dysregulation of cellular homeostasis. Mitochondrial ROS can lead to aggravated mitochondrial oxidative stress damage. It is also accompanied by excessive mitochondrial fission and insufficient mitophagy, impaired levels of autophagic flux, and increased fragmented mitochondria. Insufficient production or supply of mitochondrial ATP eventually leads to cardiomyocytes senescence or death. Lack or impairment of mitotic phagocytosis in senescent cells also leads to the accumulation of damaged mitochondria, thus triggering an unregulated inflammatory response, which further puts older patients at risk for excessive inflammation and increases mortality.
ORF-9b of SARS-CoV localizes to mitochondria and mediates changes in mitochondrial length by triggering ubiquitination and proteasomal degradation of Drp1—a stark difference compared with the Sendai virus that causes mitochondrial fragmentation and aggregation (Castanier et al, 2010) as well as the hepatitis C virus that promotes mitochondrial perinuclear clustering, Parkin translocation to mitochondria, and mitophagy (Kim et al, 2013b). In addition, after ORF-9b usurped PCBP2 and HECT domain E3 ligase AIP4, it could target the mitochondria-associated adaptor molecule MAVS signalosome to mediate the degradation of MAVS and the loss of TRAF3 and TRAF6, thereby suppressing host cell IFN response.
It is also worth noting that transient ORF-9b expression can also lead to strong induction of autophagy in HEK293 and A549 cells, although questions remain as to whether the observation only focused on ATG5-mediated autophagosome formation rather than the complete autophagic flux and how to reconcile the increased mitochondrial elongation with induction of autophagy (Shi et al, 2014). Based on findings in SARS-CoV-2-infected IFN-deficient VeroFM monkey kidney cells and IFN-active human lung Calu-3 cells, autophagy activation was observed to be inhibited. Its regulatory mechanism is mainly through the activation of autophagy inhibitory genes (AKT1, SKP2) and the inhibition of the expression levels of autophagy initiation proteins (AMPK, TSC2, ULK1), the reduction of phagocytic cell formation (BECN1, VPS34, ATG14), and autophagy.
Levels of lysosomal fusions (BECN1, ATG14 oligomers) decreased. The change in these proteins also mirrors the accumulation of LC3B-II and P62, and suggested changes in autophagic flux. The findings were studied in in vivo experimental models and in lung samples from patients with COVID-19. Exogenous administration of the polyamines spermidine and spermine, AKT1 inhibitor MK-2206 can further inhibit the in vitro reproduction of SARS-CoV-2 and further activate autophagy. This is more consistent with previous findings that the damage mechanism of SARS-CoV-2 is closely related to autophagy (Gassen et al, 2021).
Interestingly, SARS-CoV-2 infection in cardiomyocytes caused an increase in the number of autophagosomes and autophagolysosomes within 24 hours, which may imply that viral infection exacerbates increased organelle damage and fragmentation. This leads to an increase in the level of autophagic flux. However, the effect of autophagic flux corresponding to the proportion of dysfunctional mitochondria remains to be further verified. An increase in the proportion of damaged or mitochondrial fragmentation may further affect the homeostatic mechanism of cardiomyocytes (Ramachandran et al, 2022). An optimal level of mitophagy is crucial to preserve the quality and quantity of mitochondria in the cardiac myocytes, without which the cells fail to adapt to the stress from the increase in damaged mitochondria thus culminating in death. As COVID-19 patients experience fatigue and muscle weakness, the perturbation to mitochondrial dynamics may explain this shortness of energy.
What Are the Candidate Anti-COVID-19 Therapeutics That Also Target the Mitochondria?
To date, there has been no specific antiviral drugs that are clinically approved to target the disease, only to relieve symptoms. Several antiviral and antiparasitic drugs have been repositioned to relieve the symptoms of SARS-CoV-2 infection, particularly the pneumonia-associated symptoms of some of the COVID-19 patients (Aguiar et al, 2018; Colson et al, 2020; Guo et al, 2020b; Wang et al, 2020). The mechanistic targets of these pharmacological agents can be further subdivided into the different components of the virus itself and its resulting pathophysiological processes. Most of the drugs used to alleviate COVID-19 is based on the genetic makeup and function of SARS-CoV-2, specifically targeting proteases, ACE2, hemagglutinin esterase (HE), E proteins, and RNA-dependent polymerase actions (Kandimalla et al, 2020).
Targeting ACE2 and TMPRSS2
Chloroquine—hydroxychloroquine
Previously used for treating malaria, the quinine analogue, chloroquine (CQ) and hydroxychloroquine (HCQ), a derivative of CQ, have been used to combat COVID-19. CQ was found to interfere with terminal glycosylation of ACE2 and further inhibit the pH-dependent step of viral replication (Devaux et al, 2020; Vincent et al, 2005). It was further found in in vitro and clinical studies that HCQ, which has antiviral effects, can effectively limit SARS-CoV-2 infection, or attack and reduce the damage of autoantibodies to cells (Liu et al, 2020; Yao et al, 2020).
Although HCQ is currently only approved by the FDA for the prevention and treatment of malaria and autoimmune diseases (such as rheumatoid arthritis and systemic lupus erythematosus), it may be utilized to target COVID-19 in the future (Rainsford et al, 2015). Although the primary effect of HCQ in tackling COVID-19 lies in preventing viral entry by inhibiting interaction of the ACE2 receptor with the spike glycoprotein-ganglioside, HCQ also exerts its multitherapeutic effects by altering endosomal and lysosomal activities, attenuating the glycosylation process of host receptors, inhibiting proteolytic processes and inflammatory cytokine production.
It can further affect lysosomal activity and regulate autophagy and endocytic pathways. HCQ can further alter intracellular pH and is able to modulate lysosomal activity of antigen presenting cells, ultimately affecting cathepsin, MAP kinase, and autophagosome function, resulting in direct structural damage to the SARS-CoV-2 spike protein (Devaux et al, 2020; Zhou et al, 2020a). Further evidence shows that CQ and HCQ also induce alkalinization of autophagolysosomes and lead to impaired cell fusion and viral detachment. This process may directly affect the replication and attack of the virus.
The combined application of CQ and HCQ has been shown to directly affect the outcome of the disease and inhibit the occurrence and development of COVID-19 (Liu et al, 2020) by modulating the immune response via regulating the activation of autophagy, regulating TLR signaling, inhibiting the overexpression of TNF-α and IL-6, IL-17, and IL-22, which has far-reaching significance (Colson et al, 2020; Wang et al, 2020). However, it should be noted that different doses of HCQ and CQ may cause retinopathy and QTc prolongation, and may be accompanied by fatal arrhythmias (Kalil, 2020). A systematic review and meta-regression analysis were performed on studies of multiple clinical studies covering 5652 patients. Results showed that treatment of CQ or HCQ-treated COVID-19 patients was associated with an increased risk of drug-induced QT prolongation.
There is also an increased risk of ventricular tachycardia or cardiac arrest (Tleyjeh et al, 2021). Moreover, the application of these two drugs can also induce an increase in cardiotoxicity by inhibiting the activity of lysosomal enzymes in cardiomyocytes, thereby leading to autophagy/mitochondrial dysfunction in cardiomyocytes, leading to the risk of severe myocardial injury (Frustaci et al, 2012). These concerns have to take into account the risk of biventricular concentric hypertrophy and diastolic dysfunction. And at the ultrastructural level, the toxicity associated with HCQ is closely related to the intracellular accumulation of myelin and the formation of curvilinear bodies (Chatre et al, 2018).
Cardiac toxicity, in particular conduction disorders, from these drugs is more prevalent in women after chronic administration (median of 7 years) with a high cumulative dose (Chatre et al, 2018). HCQ can also block the inflow of potassium current IK1, and to a certain extent block the rapidly activated delayed potassium current IKr, resulting in prolongation of action potential. Studies have found that HCQ can also prolong spontaneous action potential firing by inhibiting multiple cardiac channels. However, this process increases the likelihood of ventricular ectopic and fatal ventricular arrhythmias. This is also an outcome that should be considered before clinical application.
Combining HCQ and azithromycin has also achieved positive benefits against COVID-19 (Gautret et al, 2020). In an unrandomized clinical trial of 36 patients, the combination of HCQ along with azithromycin reduced SARS-CoV-2 viral load (Gautret et al, 2020). Nonetheless, azithromycin has also been considered to be one of the main factors leading to a prolonged QT interval and a higher risk of sudden cardiac death in women and in the elderly via calcium overloading in the cardiomyocytes through a chronic usage-mediated increase in peak and late cardiac sodium current (Yang et al, 2017). In light of these observations, the Food and Drug Administration has cautioned against treatment of COVID-19 patients with CQ or HCQ in nonexperimental settings due to the risk of sudden cardiac death and severe arrhythmia (Oren et al, 2020).
Metformin
It is imperative to note that due to the localization of ACE2 in multiple cell types, blocking this receptor may inadvertently cause other side effects particularly in patients with comorbidities such as diabetes, renal failure, and CVD. Conversely, the ACE2 levels can be improved via the usage of ACE-inhibitors and angiotensin II-blockers in the diabetic patients (Alghatrif et al, 2020), which has also been demonstrated to protect against severe COVID-19—a benefit also seen in those who take the drug Metformin (Scheen, 2020). Metformin has also been known to inhibit mitochondrial complex I and associated excessive accumulation of ROS, thereby producing protective benefits such as reversing the aging- and diabetes-mediated inflammatory phenotype of T cells by activates autophagy/mitochondrial autophagy and promotes mitochondrial biogenesis (Bharath et al, 2020) as well as reducing IL-6 release (Soberanes et al, 2019).
In the COVID-19 patients, metformin has been found to inhibit viral infection via AMPK increase thereby mediating a Ser680 phosphorylation of the ACE2 receptor, thus impairing ACE2—viral spike protein binding. Viral replication is inhibited by metformin via it interacts by targeting vacuolar ATPase (V-ATPase) and endoplasmic Na+/H+ exchanger (ENHE), thereby increasing cellular and endoplasmic pH and inhibiting endocytic cycling and virion assembly (Scheen, 2020; Sharma et al, 2020). Metformin was also able to inhibit the activation of electron transport chain and mTORC1 signaling pathways, which further modulated viral protein-host protein (NDUF-NSP7 and LARP/FKBP7-N/ORF8) interactions. Thus, host-dependent viral replication is inhibited, viral protein synthesis is inhibited, and the level of viral particle release is reduced (Gordon et al, 2020).
Metformin also regulates the immune response by modulating the ACE2/AngII/AT1R axis and inhibiting NF-κB signaling, suppressing the expression level of inflammatory cytokines, the activation of macrophages can also be inhibited (Sharma et al, 2020). In brief, metformin can play a good therapeutic role in blood sugar control, immune regulation, redox response regulation, and anti-inflammatory. It has a strong advantage in the treatment of cardiovascular disease, especially diabetic cardiomyopathy. It can be used to prevent myocardial injury or endothelial dysfunction and provide vascular protection, thereby reducing microvascular complications and thrombotic events during hospitalization for SARS-CoV-2 infection and protecting the myocardium.
Camostat mesylate
Apart from targeting ACE2, camostat mesylate is primarily a serine protease inhibitor for the treatment of postoperative reflux esophagitis. Its regulatory mechanism mainly targets TMPRSS2, -13, and -11D/E/F (Kawase et al, 2012; Shirato et al, 2013). A twice daily administration of camostat mesylate reduced lethality by 60% in mice infected by administering 10,000 pfu of SARS-CoV virus via intranasal instillation (Zhou et al, 2015). Camostat mesylate also reduced SARS-CoV-2 spike protein-driven cellular entry in a human lung cell line and primary human lung epithelial cells (Hoffmann et al, 2020). However, the results of a double-blind randomized placebo-controlled multicenter trial study showed that camostat mesylate was not an effective drug for the treatment of patients hospitalized with COVID-19 (Gunst et al, 2021). This may be related to the metabolic pathway of the drug into the human body and the complex characteristics of the targeting mechanism, but it is worth further research on the pharmacological mechanism.
Targeting inflammation
Tocilizumab—methylprednisolone
Although the effectiveness in tackling COVID-19 is not pronounced, immunosuppresive drugs such as tocilizumab (TCZ) and sarilumab can target regulation of IL-6 activity and can also be used to suppress pathological immune responses (NCT04306705, NCT04322773).
Stemming from a previous single-center retrospective study, TCZ as a monoclonal antibody against IL-6 has now been used in various clinical trials to repress the inflammatory CRP and IL-6 in COVID-19 patients. In the previous study, TCZ was used in combination with methylprednisolone (MP) and also administered BID or more in COVID-19 patients with comorbidities such as diabetes mellitus, hypertension, and stroke. Although therapeutic effects of TCZ was observed from this study, those severely ill COVID-19 patients who only received a single dose had adverse outcomes (Luo et al, 2020). TCZ administration was found to produce certain cardiotoxicity and further induce mitochondrial oxidative stress and dysregulation of mitochondrial homeostasis, mediating increased levels of cardiomyocyte apoptosis. At the same time lead to the occurrence of cardiovascular adverse events (including hypertension, hypercholesterolemia, and myocardial infarction) (Gabay et al, 2016; Hermine et al, 2021).
Targeting the mitochondria
Cyclosporin A
Experimental data show that mPTP blocker and immunosuppressant cyclosporine A can also have a certain therapeutic effect on COVD-19, mirroring the effect of CCDC58 knockdown and may provide additional treatment options for COVID-19 patients in the future (Ramachandran et al, 2022). Although only tested in hiPSC-CMs, CsA can enhance mitochondrial ability to regulate calcium homeostasis, regulate Ca2+ influx and release, and regulate mitochondrial biogenesis. The exacerbation of cardiomyocytes autophagy and cardiac cell death after SARS-CoV-2 infection was suppressed by CsA treatment (Ramachandran et al, 2022).
Targeting viral replication
Remdesivir
Previously effective in combating Ebola, remdesivir (formerly GS-5734) is a broad-spectrum antiviral drug (nucleotide analog) that mainly acts on RdRp to inhibit RNA replication (Agostini et al, 2018; Brown et al, 2019). With a half-life of 35 h, pharmacokinetics and drug safety of remdesivir has been validated in clinical trials (single and multiple-dose phase I) (Humeniuk et al, 2020; Ram et al, 2020). Some COVID-19 patients administered with remdesivir have been found to accompanied by severe sinus bradycardia, unstable blood pressure, abnormal T waves, atrial fibrillation, and other symptoms. In addition, after the application of remdesivir, some cardiac arrest and conduction block phenomena have also occurred clinically. The patients experiencing adverse cardiovascular outcomes have been speculated to have structural cardiac problems.
Yet, remdesivir itself binds to human mitochondrial RNA polymerase (Sanchez-Codez et al, 2021) thus causing cardiotoxicity and risk of QT prolongation with decreased spontaneous beat rate and changes in sodium ion peak amplitude may be directly related to the duration of treatment (Choi et al, 2020). Using hiPSC-CMs, the remdesivir-induced cardiotoxicity occurs with 50% cytotoxic concentration (CC50) close to its estimated peak plasma concentration (Cmax 9 μM) (Choi et al, 2020). Evaluation of therapeutic concentrations of RDV and its active metabolites on isolated mitochondria showed that they did not directly affect mitochondrial energy metabolism and mitochondrial respiratory function (Bjork and Wallace, 2021; Fišar et al, 2021).
However, another in vitro study found in a hypoxic-induced hiPSC-CMs model that remdesivir could induce mitochondrial fission to a certain extent, leading to dysregulation of redox balance and inhibiting mitochondrial respiration levels under hypoxia (Kwok et al, 2021). Perturbations to electrophysiological properties and sarcomere disturbances/abnormalities were also observed, with some harmful effects and side effects persisting even after drug treatment is discontinued, ultimately leading to decreased levels of cellular activity and increased numbers of cells dying. Notably, remdesivir-induced cardiotoxicity could be ameliorated by modulating mitochondrial dynamics, inhibiting Drp1-mediated mitochondrial pathological polyfission. This suggests that the combination of remdesivir and mitochondrial fission inhibitors or derivative drugs may have a protective effect against myocardial injury caused by COVID-19 in the future, which is worthy of further pharmacological research in in vivo models (Kwok et al, 2021).
Lopinavir–ritonavir (LPV/r)
Similarly, the study found that two anti-HIV drugs, lopinavir and ritonavir, can be used to treat SARS-CoV-1 and MERS-CoV infections—have also been combined as an anti-COVID-19 drug whereby the β-coronavirus viral load was significantly reduced (Lim et al, 2020). Yet, when moved into a clinical trial on hospitalized adult patients with severe COVID-19 treated with lopinavir–ritonavir, the combination showed no benefit (Cao et al, 2020a). In total, 50% of COVID-19 patients show side effects associated with the combination of drugs.
In a report from a single pharmacovigilance center, male and multimorbid COVID-19 patients >75 years of age received a combination therapy of LPV/r and HCQ, significant prolongation of QTc time occurred during or within 7 days of drug treatment (Istampoulouoglou et al, 2021) due to LPV/r's potency to block voltage-gated potassium channels (Fresse et al, 2021). In a prospective study of critically ill elderly 41 COVID-19 patients receiving LPV/r for 10 days, 9 (22%) patients experienced bradycardia with the majority being sinus bradycardia, with the hypothesis that the COVID-19-mediated inflammatory damage increases risk of bradycardia in elderly patients (Beyls et al, 2020).
Although yet to be investigated in the human patients, chronic (8 weeks) LPV/r treatment increased levels of myocardial oxidative stress while also inhibiting the function of the ubiquitin-proteasome system (UPS), with an elevation of serum low-density lipoprotein-cholesterol, calcineurin, and connexin 43 expression thereby attenuating cardiac function in Wistar rats (Reyskens et al, 2013). In the human glioblastoma U-87 MG cell line, co-treatment with lopinavir and ritonavir (25 and 50 μM) promoted the excessive production and accumulation of ROS significantly increased, resulting in severe structural abnormalities and dysfunction of mitochondria, and activated the caspase pathway and induced apoptosis in a caspase pathway-independent manner (Gratton et al, 2018).
Summary
Mitochondrial dysfunction in the form of metabolic abnormality, energy deficit, excess ROS production, dysregulated Ca2+ homeostasis, imbalance dynamics, impaired autophagy, ER stress, and culmination of cellular death, constitutes an important axis in the complicated etiology of CVD.
Currently, most drugs for COVID-19 are targeted against respiratory disorders. Despite the presence of repurposed drugs that also confer protection for other organs, most of these are still being tested in preclinical studies. Symptomatic treatment remains the optimal intervention measure and although not directly targeted against myocardial injury, the ability to promptly and effectively inhibit inflammation not only improves patient outcomes but also prevents the onset of long-term adverse outcome. To preserve optimal cardiac function, interventions to conserve mitochondrial function and myocardial energy metabolism may prove to be a potential useful therapeutic.
As we now shift toward a postpandemic world, long COVID-19 is now the emerging concern whereby the association between COVID-19 infection and triggering of myocardial disorders remain to be unraveled, including whether viral infection would induce cardiac mitochondrial dysfunction thus predisposing to myocardial disorders? What exactly is the underlying mechanism to viral infection-induced cardiac mitochondrial dysfunction? Whether intact mitochondria would blunt the response to infection and thus avoid myocardial injury? and Could modulation of cardiac mitochondrial dynamics prevent viral-infection-induced myocardial injury and reduce infection-related mortality?
Detection of a high level of mitochondrial and cardiac damage biomarkers at the time of admission can function to risk-stratify the COVID-19 patients and allows for more advanced cardiac-targeted therapy and maintenance therapy for patients at higher risk of myocardial injury or cardiac function impairment. More notably, the maintenance of normal or relatively low levels of these biomarkers of myocardial injury allows physicians to identify patients with lower in-hospital mortality earlier and to expedite discharge, thereby promoting rational allocation of resources.
Abbreviations Used
- ACE2
angiotensin-converting enzyme 2
- AMI
acute myocardial infarction
- ATP
adenosine triphosphate
- BNP
brain-type natriuretic peptide
- CMs
coiled membranes
- COVID-19
corona virus disease 2019
- CQ
chloroquine
- CsA
cyclosporin A
- cTnI
cardiac troponin I
- CUHK
Chinese University of Hong Kong
- CVD
cardiovascular disorders
- Drp1
dynamin-related protein 1
- E
envelope
- EF
ejection fraction
- ER
endoplasmic reticulum
- HCQ
hydroxychloroquine
- IFN
interferon
- IL
interleukin
- IMM
inner mitochondrial membrane
- M
membrane
- MAMs
mitochondria-associated membranes
- MAVS
mitochondria antiviral signaling
- MERS-CoV
Middle East respiratory syndrome corona virus
- Mff
mitochondrial fission factor
- Mfn
mitofusin
- mPTP
mitochondrial permeability transition pore
- mtCYTB
mitochondrial-encoded gene cytochrome B
- mtDNA
mitochondrial DNA
- N
nucleocapsid
- NSP
nonstructural protein
- OMM
outer mitochondrial membrane
- OPA1
optic atrophy 1
- ORF
open reading frame
- PBMCs
peripheral blood mononuclear cells
- ROS
reactive oxygen species
- RTC
replication-transcription complex
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- TCZ
tocilizumab
- TMPRSS2
transmembrane serine protease 2
- TNF-α
tumor necrosis factor-α
Authors' Contributions
Conceptualization by S.-G.O. and S.-B.O. Writing by X.C., N.I.I., S.-G.O., and S.-B.O. Figure preparation by X.C. Information curation by A.R., D.X., and R.W.Y.C. Expert opinion (COVID-19) by R.W.Y.C. Reviewing and editing by S.-G.O. and S.-B.O.
Author Disclosure Statement
No conflict of interest was declared.
Funding Information
S.-G.O. is funded by the National Institutes of Health R00 HL130416 and R01 HL148756. S.-B.O. is funded by an Early Career Scheme (ECS) Award (CUHK 24110822) from the Research Grants Council HKSAR, a Direct Grant for Research 2020/21 (2020.035), a Project Impact Enhancement Fund (PIEF; Phase 2-COVID; PIEF/Ph2/COVID/08) from the Faculty of Medicine, The Chinese University of Hong Kong (CUHK), the Improvement on Competitiveness in Hiring New Faculties Funding Scheme from CUHK, and the Lui Che Woo Institute of Innovative Medicine.
References
- Abbasi J. The COVID heart-one year after SARS-CoV-2 infection, patients have an array of increased cardiovascular risks. JAMA 2022;327(12):1113–1114; doi: 10.1001/JAMA.2022.2411 [DOI] [PubMed] [Google Scholar]
- Agostini ML, Andres EL, Sims AC, et al. . Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 2018;9(2):e00221-18; doi: 10.1128/MBIO.00221-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar ACC, Murce E, Cortopassi WA, et al. . Chloroquine analogs as antimalarial candidates with potent in vitro and in vivo activity. Int J Parasitol Drugs Drug Resist 2018;8(3):459–464; doi: 10.1016/J.IJPDDR.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Abbasi B, Torres P, Ramos-Tuarez F, et al. . Cardiac troponin-I and COVID-19: A prognostic tool for in-hospital mortality. Cardiol Res 2020;11(6):398–404; doi: 10.14740/CR1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghatrif M, Cingolani O, Lakatta EG. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: Insights from cardiovascular aging science. JAMA Cardiol 2020;5(7):747–748; doi: 10.1001/JAMACARDIO.2020.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alroomi M, Rajan R, Omar AA, et al. . Ferritin level: A predictor of severity and mortality in hospitalized COVID-19 patients. Immun Inflamm Dis 2021;9(4):1648–1655; doi: 10.1002/IID3.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammirati E, Cipriani M, Moro C, et al. . Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: Multicenter Lombardy registry. Circulation 2018;138(11):1088–1099; doi: 10.1161/CIRCULATIONAHA.118.035319 [DOI] [PubMed] [Google Scholar]
- Angajala A, Lim S, Phillips JB, et al. . Diverse roles of mitochondria in immune responses: Novel insights into immuno-metabolism. Front Immunol 2018;9:1605; doi: 10.3389/FIMMU.2018.01605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, Sharp WW, Weir EK. Differentiating COVID-19 pneumonia from acute respiratory distress syndrome and high altitude pulmonary edema: Therapeutic implications. Circulation 2020;142(2):101–104; doi: 10.1161/CIRCULATIONAHA.120.047915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentz M, Yim E, Klaff L, et al. . Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020;323(16):1612–1614; doi: 10.1001/JAMA.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoubkhani D, Khunti K, Nafilyan V, et al. . Post-covid syndrome in individuals admitted to hospital with covid-19: Retrospective cohort study. BMJ 2021;372:n693; doi: 10.1136/BMJ.N693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzuol L, Giamogante F, Brini M, et al. . PINK1/Parkin mediated mitophagy, Ca2+ signalling, and ER-mitochondria contacts in Parkinson's disease. Int J Mol Sci 2020;21(5):1772; doi: 10.3390/IJMS21051772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier V, Lang D, Valois S, et al. . Dengue virus induces mitochondrial elongation through impairment of Drp1-triggered mitochondrial fission. Virology 2017;500:149–160; doi: 10.1016/J.VIROL.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-García J, Osca-Verdegal R, Pallardó FV, et al. . Oxidative stress and inflammation in COVID-19-associated sepsis: The potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants (Basel) 2020;9(10):1–20; doi: 10.3390/ANTIOX9100936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyls C, Martin N, Hermida A, et al. . Lopinavir-ritonavir treatment for COVID-19 infection in intensive care unit: Risk of bradycardia. Circ Arrhythm Electrophysiol 2020;13(8):862–865; doi: 10.1161/CIRCEP.120.008798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharath LP, Agrawal M, McCambridge G, et al. . Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab 2020;32(1):44.e6–55.e6; doi: 10.1016/J.CMET.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti JS, Bhatti GK, Khullar N, et al. . Therapeutic strategies in the development of anti-viral drugs and vaccines against SARS-CoV-2 infection. Mol Neurobiol 2020;57(11):4856–4877; doi: 10.1007/S12035-020-02074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B, Madhavan MV, Jimenez D, et al. . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 2020;75(23):2950–2973; doi: 10.1016/J.JACC.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JA, Wallace KB. Remdesivir; molecular and functional measures of mitochondrial safety. Toxicol Appl Pharmacol 2021;433:115783; doi: 10.1016/J.TAAP.2021.115783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. . Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020;181(5):1036.e9–1045.e9; doi: 10.1016/J.CELL.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojkova D, Klann K, Koch B, et al. . Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 2020a;583(7816):469–472; doi: 10.1038/S41586-020-2332-7 [DOI] [PubMed] [Google Scholar]
- Bojkova D, Wagner JUG, Shumliakivska M, et al. . SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res 2020b;116(14):2207–2215; doi: 10.1093/CVR/CVAA267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Won JJ, Graham RL, et al. . Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res 2019;169:104541; doi: 10.1016/J.ANTIVIRAL.2019.104541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Wang Y, Wen D, et al. . A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020a;382(19):1787–1799; doi: 10.1056/NEJMOA2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Li L, Feng Z, et al. . Comparative genetic analysis of the novel coronavirus (2019-NCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov 2020b;6(1):11; doi: 10.1038/S41421-020-0147-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanier C, Garcin D, Vazquez A, et al. . Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep 2010;11(2):133–138; doi: 10.1038/EMBOR.2009.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CM, Ma CW, Chan WY, et al. . The SARS-coronavirus membrane protein induces apoptosis through modulating the Akt survival pathway. Arch Biochem Biophys 2007;459(2):197–207; doi: 10.1016/J.ABB.2007.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: Dynamic organelles in disease, aging, and development. Cell 2006a;125(7):1241–1252; doi: 10.1016/j.cell.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 2006b;22:79–99; doi: 10.1146/annurev.cellbio.22.010305.104638 [DOI] [PubMed] [Google Scholar]
- Chan JFW, Kok KH, Zhu Z, et al. . Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 2020;9(1):221–236; doi: 10.1080/22221751.2020.1719902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Lochner A, Wang HH, et al. . Coronary microvascular injury in myocardial infarction: Perception and knowledge for mitochondrial quality control. Theranostics 2021;11(14):6766–6785; doi: 10.7150/THNO.60143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatel-Chaix L, Cortese M, Romero-Brey I, et al. . Dengue virus perturbs mitochondrial morphodynamics to dampen innate immune responses. Cell Host Microbe 2016;20(3):342–356; doi: 10.1016/J.CHOM.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatre C, Roubille F, Vernhet H, et al. . Cardiac complications attributed to chloroquine and hydroxychloroquine: A systematic review of the literature. Drug Saf 2018;41(10):919–931; doi: 10.1007/S40264-018-0689-4 [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Mitochondrial dynamics in mammals. Curr Top Dev Biol 2004;59:119–144; doi: 10.1016/S0070-2153(04)59005-1 [DOI] [PubMed] [Google Scholar]
- Chen L, Li X, Chen M, et al. . The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 2020;116(6):1097–1100; doi: 10.1093/CVR/CVAA078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Shin JS, Park SJ, et al. . Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antiviral Res 2020;184:104955; doi: 10.1016/J.ANTIVIRAL.2020.104955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P, Rolain JM, Lagier JC, et al. . Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents 2020;55(4):105932; doi: 10.1016/J.IJANTIMICAG.2020.105932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost 2020;18(7):1559–1561; doi: 10.1111/JTH.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornillez-Ty CT, Liao L, Yates JR, et al. . Severe acute respiratory syndrome coronavirus nonstructural protein 2 interacts with a host protein complex involved in mitochondrial biogenesis and intracellular signaling. J Virol 2009;83(19):10314–10318; doi: 10.1128/JVI.00842-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coromilas EJ, Kochav S, Goldenthal I, et al. . Worldwide survey of COVID-19-associated arrhythmias. Circ Arrhythm Electrophysiol 2021;14(3):285–295; doi: 10.1161/CIRCEP.120.009458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty SE, Guo Y, Heath K, et al. . Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2021;373:n1098; doi: 10.1136/BMJ.N1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle DM, Kim-Schulze S, Huang HH, et al. . An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020;26(10):1636–1643; doi: 10.1038/S41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux CA, Rolain JM, Colson P, et al. . New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int J Antimicrob Agents 2020;55(5):105938; doi: 10.1016/J.IJANTIMICAG.2020.105938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G, Sakelliou A, Flevari A, et al. . Ferritin levels in critically ill patients with COVID-19: A marker of outcome? Pneumon 2021;34(2):1–5; doi: 10.18332/PNE/135958 [DOI] [Google Scholar]
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20(5):533–534; doi: 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Snary KJ, Wu D, Potus F, et al. . Ndufs2, a core subunit of mitochondrial complex I, is essential for acute oxygen-sensing and hypoxic pulmonary vasoconstriction. Circ Res 2019;124(12):1727–1746; doi: 10.1161/CIRCRESAHA.118.314284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher F, Pietsch H, Aleshcheva G, et al. . Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Hear Fail 2020;7(5):2440–2447; doi: 10.1002/EHF2.12805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fišar Z, Ľupták M, Hroudová J. Little in vitro effect of remdesivir on mitochondrial respiration and monoamine oxidase activity in isolated mitochondria. Toxicol Lett 2021;350:143–151; doi: 10.1016/J.TOXLET.2021.07.015 [DOI] [PubMed] [Google Scholar]
- Fresse A, Viard D, Romani S, et al. . Spontaneous reported cardiotoxicity induced by lopinavir/ritonavir in COVID-19. An alleged past-resolved problem. Int J Cardiol 2021;324:255–260; doi: 10.1016/J.IJCARD.2020.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundt EC, Yu L, Park E, et al. . Molecular determinants for subcellular localization of the severe acute respiratory syndrome coronavirus open reading frame 3b protein. J Virol 2009;83(13):6631–6640; doi: 10.1128/JVI.00367-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, et al. . ER tubules mark sites of mitochondrial division. Science 2011;334(6054):358–362; doi: 10.1126/science.1207385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci A, Morgante E, Antuzzi D, et al. . Inhibition of cardiomyocyte lysosomal activity in hydroxychloroquine cardiomyopathy. Int J Cardiol 2012;157(1):117–119; doi: 10.1016/J.IJCARD.2012.03.112 [DOI] [PubMed] [Google Scholar]
- Fung G, Luo H, Qiu Y, et al. . Myocarditis. Circ Res 2016;118(3):496–514; doi: 10.1161/CIRCRESAHA.115.306573 [DOI] [PubMed] [Google Scholar]
- Gabay C, Mcinnes IB, Kavanaugh A, et al. . Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis 2016;75(10):1806–1812; doi: 10.1136/ANNRHEUMDIS-2015-207872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Olivé I, Sintes H, Radua J, et al. . D-dimer in patients infected with COVID-19 and suspected pulmonary embolism. Respir Med 2020;169:106023; doi: 10.1016/J.RMED.2020.106023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Prat L, Martínez-Vicente M, Perdiguero E, et al. . Autophagy maintains stemness by preventing senescence. Nature 2016;529(7584):37–42; doi: 10.1038/NATURE16187 [DOI] [PubMed] [Google Scholar]
- Gardinassi LG, Souza COS, Sales-Campos H, et al. . Immune and metabolic signatures of COVID-19 revealed by transcriptomics data reuse. Front Immunol 2020;11:1636; doi: 10.3389/FIMMU.2020.01636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen NC, Papies J, Bajaj T, et al. . SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals. Nat Commun 2021;12(1):3818; doi: 10.1038/S41467-021-24007-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P, Lagier JC, Parola P, et al. . Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020;56(1):105949; doi: 10.1016/J.IJANTIMICAG.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibellini L, Biasi S De, Paolini A, et al. . Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol Med 2020;12(12):e13001; doi: 10.15252/EMMM.202013001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Suaga P, Paillusson S, Stoica R, et al. . The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Curr Biol 2017;27(3):371–385; doi: 10.1016/J.CUB.2016.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DE, Jang GM, Bouhaddou M, et al. . A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020;583(7816):459–468; doi: 10.1038/S41586-020-2286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton R, Tricarico PM, Guimaraes RL, et al. . Lopinavir/ritonavir treatment induces oxidative stress and caspase-independent apoptosis in human glioblastoma U-87MG cell line. Curr HIV Res 2018;16(2):106–112; doi: 10.2174/1570162X16666180528100922 [DOI] [PubMed] [Google Scholar]
- Gunst JD, Staerke NB, Pahus MH, et al. . Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19—A double-blind randomized controlled trial. EClinicalMedicine 2021;35:100849; doi: 10.1016/J.ECLINM.2021.100849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Fan Y, Chen M, et al. . Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020a;5(7):811–818; doi: 10.1001/JAMACARDIO.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YR, Cao QD, Hong ZS, et al. . The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil Med Res 2020b;7(1):11; doi: 10.1186/S40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermine O, Mariette X, Tharaux PL, et al. . Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: A randomized clinical trial. JAMA Intern Med 2021;181(1):32–40; doi: 10.1001/JAMAINTERNMED.2020.6820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. . SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181(2):271.e8–280.e8; doi: 10.1016/J.CELL.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder K, Reddy PH. The COVID-19 effect on the immune system and mitochondrial dynamics in diabetes, obesity, and dementia. Neuroscientist 2021;27(4):331–339; doi: 10.1177/1073858420960443 [DOI] [PubMed] [Google Scholar]
- Hu H, Ma F, Wei X, et al. . Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J 2021;42(2):206; doi: 10.1093/EURHEARTJ/EHAA190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Huang L, Wang Y, et al. . 6-Month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021;397(10270):220–232; doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeniuk R, Mathias A, Cao H, et al. . Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci 2020;13(5):896–906; doi: 10.1111/CTS.12840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciardi RM, Lupi L, Zaccone G, et al. . Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5(7):819–824; doi: 10.1001/JAMACARDIO.2020.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istampoulouoglou I, Zimmermanns B, Grandinetti T, et al. . Cardiovascular adverse effects of lopinavir/ritonavir and hydroxychloroquine in COVID-19 patients: Cases from a single pharmacovigilance centre. Glob Cardiol Sci Pract 2021;2021(2):e202111; doi: 10.21542/GCSP.2021.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JY, Blum A, Liu J, et al. . The role of mitochondria in aging. J Clin Invest 2018;128(9):3662–3670; doi: 10.1172/JCI120842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil AC. Treating COVID-19-off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA 2020;323(19):1897–1898; doi: 10.1001/JAMA.2020.4742 [DOI] [PubMed] [Google Scholar]
- Kandimalla R, John A, Abburi C, et al. . Current status of multiple drug molecules, and vaccines: An update in SARS-CoV-2 therapeutics. Mol Neurobiol 2020;57(10):4106–4116; doi: 10.1007/S12035-020-02022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase M, Shirato K, van der Hoek L, et al. . Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol 2012;86(12):6537–6545; doi: 10.1128/JVI.00094-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IH, Savarimuthu S, Leung MST, et al. . The need to manage the risk of thromboembolism in COVID-19 patients. J Vasc Surg 2020;72(3):799–804; doi: 10.1016/J.JVS.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Syed GH, Kim SJ, et al. . Mitochondrial dynamics and viral infections: A close nexus. Biochim Biophys Acta 2015;1853(10 Pt B):2822–2833; doi: 10.1016/J.BBAMCR.2014.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-J, Khan M, Quan J, et al. . Hepatitis B virus disrupts mitochondrial dynamics: Induces fission and mitophagy to attenuate apoptosis. PLoS Pathog 2013a;9(12):e1003722; doi: 10.1371/journal.ppat.1003722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-J, Syed GH, Khan M, et al. . Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc Natl Acad Sci U S A 2014;111(17):6413–6418; doi: 10.1073/pnas.1321114111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Syed GH, Siddiqui A. Hepatitis C virus induces the mitochondrial translocation of Parkin and subsequent mitophagy. PLoS Pathog 2013b;9(3):e1003285; doi: 10.1371/JOURNAL.PPAT.1003285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok M, Lee C, Li HS, et al. . Remdesivir induces persistent mitochondrial and structural damage in human induced pluripotent stem cell derived cardiomyocytes. Cardiovasc Res 2021;118(12):2652–2664; doi: 10.1093/CVR/CVAB311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Nukala SB, Srivastava S, et al. . Detection of viral RNA fragments in human IPSC cardiomyocytes following treatment with extracellular vesicles from SARS-CoV-2 coding sequence overexpressing lung epithelial cells. Stem Cell Res Ther 2020;11(1):514; doi: 10.1186/S13287-020-02033-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage SL, Amaral EP, Hilligan KL, et al. . Persistent oxidative stress and inflammasome activation in CD14 high CD16—Monocytes from COVID-19 patients. Front Immunol 2022;12:799558; doi: 10.3389/FIMMU.2021.799558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Du J, Stitham J, et al. . Inducing mitophagy in diabetic platelets protects against severe oxidative stress. EMBO Mol Med 2016;8(7):779–795; doi: 10.15252/EMMM.201506046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppkes M, Knopf J, Naschberger E, et al. . Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 2020;58:102925; doi: 10.1016/J.EBIOM.2020.102925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SC, Uchiyama LF, Nunnari J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science 2016;353(6296):aaf5549; doi: 10.1126/SCIENCE.AAF5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Fan Y, Lai Y, et al. . Coronavirus infections and immune responses. J Med Virol 2020;92(4):424–432; doi: 10.1002/JMV.25685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Cui Y-J, Liu Y, et al. . ATP6AP2 knockdown in cardiomyocyte deteriorates heart function via compromising autophagic flux and NLRP3 inflammasome activation. Cell Death Discov 2022;8(1):161; doi: 10.1038/S41420-022-00967-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Fang P, Mai J, et al. . Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol 2013;6(1):19; doi: 10.1186/1756-8722-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YL, Schultz HD. Enhanced sensitivity of Kv channels to hypoxia in the rabbit carotid body in heart failure: Role of angiotensin II. J Physiol 2006;575(Pt 1):215–227; doi: 10.1113/JPHYSIOL.2006.110700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Jeon S, Shin HY, et al. . Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: The application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci 2020;35(6):e79; doi: 10.3346/JKMS.2020.35.E79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner D, Fitzek A, Bräuninger H, et al. . Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol 2020;5(11):1281–1285; doi: 10.1001/JAMACARDIO.2020.3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino K, Guimarães GMC, Alves LS, et al. . Serum ferritin at admission in hospitalized COVID-19 patients as a predictor of mortality. Brazilian J Infect Dis 2021;25(2):101569; doi: 10.1016/J.BJID.2021.101569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Cao R, Xu M, et al. . Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020;6(1):16; doi: 10.1038/S41421-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Chan DC. The mitochondrial fission receptor Mff selectively recruits oligomerized Drp1. Mol Biol Cell 2015;26(24):4466–4477; doi: 10.1091/mbc.E15-08-0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Wei B, Shi HX, et al. . Tom70 mediates activation of interferon regulatory factor 3 on mitochondria. Cell Res 2010;20(9):994–1011; doi: 10.1038/CR.2010.103 [DOI] [PubMed] [Google Scholar]
- Losón OC, Liu R, Rome ME, et al. . The mitochondrial fission receptor MiD51 requires ADP as a cofactor. Structure 2014;22(3):367–377; doi: 10.1016/j.str.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Zhao X, Li J, et al. . Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020;395(10224):565–574; doi: 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Liu Y, Qiu L, et al. . Tocilizumab treatment in COVID-19: A single center experience. J Med Virol 2020;92(7):814–818; doi: 10.1002/JMV.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Xu Y, Su Y, et al. . Single-cell transcriptome analysis decipher new potential regulation mechanism of ACE2 and NPs signaling among heart failure patients infected with SARS-CoV-2. Front Cardiovasc Med 2021;8:628885; doi: 10.3389/FCVM.2021.628885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiano S, Hsiang TY, Khanna A, et al. . SARS-CoV-2 infects human pluripotent stem cell-derived cardiomyocytes, impairing electrical and mechanical function. Stem Cell Reports 2021;16(3):478–492; doi: 10.1016/J.STEMCR.2021.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnarajah CK, Pease DR, Halfmann PJ, et al. . Highly efficient SARS-CoV-2 infection of human cardiomyocytes: Spike protein-mediated cell fusion and its inhibition. J Virol 2021;95(24):e0136821; doi: 10.1128/JVI.01368-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obukhov AG, Stevens BR, Prasad R, et al. . SARS-CoV-2 infections and ACE2: Clinical outcomes linked with increased morbidity and mortality in individuals with diabetes. Diabetes 2020;69(9):1875–1886; doi: 10.2337/DBI20-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren O, Yang EH, Gluckman TJ, et al. . Use of chloroquine and hydroxychloroquine in COVID-19 and cardiovascular implications: Understanding safety discrepancies to improve interpretation and design of clinical trials. Circ Arrhythm Electrophysiol 2020;13(6):586–590; doi: 10.1161/CIRCEP.120.008688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Miyata N, Kuge O, et al. . Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. J Cell Biol 2016;212(5):531–544; doi: 10.1083/jcb.201508099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Wang C, Cleland MM, et al. . Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol 2010;191(6):1141–1158; doi: 10.1083/jcb.201007152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X, Liu Y, Lei X, et al. . Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020;11(1):1620; doi: 10.1038/S41467-020-15562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit GY, Kassiri Z, Jiang C, et al. . SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 2009;39(7):618–625; doi: 10.1111/J.1365-2362.2009.02153.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhan K, Minakshi R, Towheed MAB, et al. . Severe acute respiratory syndrome coronavirus 3a protein activates the mitochondrial death pathway through P38 MAP kinase activation. J Gen Virol 2008;89(Pt 8):1960–1969; doi: 10.1099/VIR.0.83665-0 [DOI] [PubMed] [Google Scholar]
- Palmer CS, Elgass KD, Parton RG, et al. . Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission. J Biol Chem 2013;288(38):27584–27593; doi: 10.1074/jbc.M113.479873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CS, Osellame LD, Laine D, et al. . MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep 2011;12(6):565–573; doi: 10.1038/embor.2011.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamanoli A, Kalogeropoulos AP, Hotelling J, et al. . Association of serum ferritin levels and methylprednisolone treatment with outcomes in nonintubated patients with severe COVID-19 pneumonia. JAMA Netw Open 2021;4(10):e2127172; doi: 10.1001/JAMANETWORKOPEN.2021.27172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Won JH, Hwang I, et al. . Defective mitochondrial fission augments NLRP3 inflammasome activation. Sci Rep 2015;5:15489; doi: 10.1038/SREP15489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier C, Robichon A. Computational search of hybrid human/SARS-CoV-2 DsRNA reveals unique viral sequences that diverge from those of other coronavirus strains. Heliyon 2021;7(6):e07284; doi: 10.1016/J.HELIYON.2021.E07284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JSM, Chu CM, Cheng VCC, et al. . Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003;361(9371):1767–1772; doi: 10.1016/S0140-6736(03)13412-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainsford KD, Parke AL, Clifford-Rashotte M, et al. . Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015;23(5):231–269; doi: 10.1007/S10787-015-0239-Y [DOI] [PubMed] [Google Scholar]
- Ram AH, Badgujar KC, Zanznay R, et al. . Remdesivir for COVID-19: A review of pharmacology, mechanism of action, in-vitro activity and clinical use based on available case studies. J Drug Deliv Ther 2020;10(4-s):264–270; doi: 10.22270/JDDT.V10I4-S.4313 [DOI] [Google Scholar]
- Ramachandran K, Maity S, Muthukumar AR, et al. . SARS-CoV-2 infection enhances mitochondrial PTP complex activity to perturb cardiac energetics. iScience 2022;25(1):103722; doi: 10.1016/J.ISCI.2021.103722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren LL, Wang YM, Wu ZQ, et al. . Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. Chin Med J (Engl) 2020;133(9):1015–1024; doi: 10.1097/CM9.0000000000000722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyskens KMSE, Fisher TL, Schisler JC, et al. . Cardio-metabolic effects of HIV protease inhibitors (lopinavir/ritonavir). PLoS One 2013;8(9):e73347; doi: 10.1371/JOURNAL.PONE.0073347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riphagen S, Gomez X, Gonzalez-Martinez C, et al. . Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395(10237):1607–1608; doi: 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Yang K, Wang W, et al. . Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46(5):846–848; doi: 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y, Kawachi K, Terada Y, et al. . Two-amino acids change in the Nsp4 of SARS coronavirus abolishes viral replication. Virology 2017;510:165–174; doi: 10.1016/J.VIROL.2017.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh J, Peyssonnaux C, Singh KK, et al. . Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion 2020;54:1–7; doi: 10.1016/J.MITO.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Codez MI, Rodriguez-Gonzalez M, Gutierrez-Rosa I. Severe sinus bradycardia associated with remdesivir in a child with severe SARS-CoV-2 infection. Eur J Pediatr 2021;180(5):1627; doi: 10.1007/S00431-021-03940-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaecher SR, Touchette E, Schriewer J, et al. . Severe acute respiratory syndrome coronavirus gene 7 products contribute to virus-induced apoptosis. J Virol 2007;81(20):11054–11068; doi: 10.1128/JVI.01266-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen AJ. Metformin and COVID-19: From cellular mechanisms to reduced mortality. Diabetes Metab 2020;46(6):423–426; doi: 10.1016/J.DIABET.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scozzi D, Cano M, Ma L, et al. . Circulating mitochondrial DNA is an early indicator of severe illness and mortality from COVID-19. JCI Insight 2021;6(4):e143299; doi: 10.1172/JCI.INSIGHT.143299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. Novel coronavirus-induced NLRP3 inflammasome activation: A potential drug target in the treatment of COVID-19. Front Immunol 2020;11:1021; doi: 10.3389/FIMMU.2020.01021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmughapriya S, Rajan S, Hoffman NE, et al. . SPG7 is an essential and conserved component of the mitochondrial permeability transition pore. Mol Cell 2015;60(1):47–62; doi: 10.1016/J.MOLCEL.2015.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Ray A, Sadasivam B. Metformin in COVID-19: A possible role beyond diabetes. Diabetes Res Clin Pract 2020;164:108183; doi: 10.1016/J.DIABRES.2020.108183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CS, Nabar NR, Huang NN, et al. . SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov 2019;5(1):101; doi: 10.1038/S41420-019-0181-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C-S, Qi H-Y, Boularan C, et al. . SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J Immunol 2014;193(6):3080–3089; doi: 10.4049/JIMMUNOL.1303196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Qin M, Shen B, et al. . Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020a;5(7):802–810; doi: 10.1001/JAMACARDIO.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Shi S, Shi S, et al. . Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020b;41(22):2070–2079; doi: 10.1093/EURHEARTJ/EHAA408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K, Kawase M, Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol 2013;87(23):12552–12561; doi: 10.1128/JVI.01890-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Chaubey G, Chen JY, et al. . Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am J Physiol Cell Physiol 2020;319(2):C258–C267; doi: 10.1152/AJPCELL.00224.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Shurland DL, Ryazantsev SN, et al. . A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 1998;143(2):351–358; doi: 10.1083/jcb.143.2.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberanes S, Misharin AV, Jairaman A, et al. . Metformin targets mitochondrial electron transport to reduce air-pollution-induced thrombosis. Cell Metab 2019;29(2):335.e5–347.e5; doi: 10.1016/J.CMET.2018.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaran M, Zapp ML, Beattie LK, et al. . Localization of HIV RNA in mitochondria of infected cells: Potential role in cytopathogenicity. J Cell Biol 1994;126(6):1353–1360; doi: 10.1083/JCB.126.6.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity 2006;25(3):373–381; doi: 10.1016/J.IMMUNI.2006.08.007 [DOI] [PubMed] [Google Scholar]
- Takahashi M. Role of NLRP3 inflammasome in cardiac inflammation and remodeling after myocardial infarction. Biol Pharm Bull 2019;42(4):518–523; doi: 10.1248/BPB.B18-00369 [DOI] [PubMed] [Google Scholar]
- Tang N, Li D, Wang X, et al. . Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18(4):844–847; doi: 10.1111/JTH.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazzi G, Pellegrini C, Maurelli M, et al. . Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22(5):911–915; doi: 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay MZ, Poh CM, Rénia L, et al. . The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol 2020;20(6):363–374; doi: 10.1038/S41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. . Hematological findings and complications of COVID-19. Am J Hematol 2020;95(7):834–847; doi: 10.1002/AJH.25829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Aretz H. Myocarditis: The Dallas criteria. Hum Pathol 1987;18(6):619–624; doi: 10.1016/S0046-8177(87)80363-5 [DOI] [PubMed] [Google Scholar]
- Tian S, Xiong Y, Liu H, et al. . Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol 2020;33(6):1007–1014; doi: 10.1038/S41379-020-0536-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tleyjeh IM, Kashour Z, AlDosary O, et al. . Cardiac toxicity of chloroquine or hydroxychloroquine in patients with COVID-19: A systematic review and meta-regression analysis. Mayo Clin Proc Innov Qual Outcomes 2021;5(1):137–150; doi: 10.1016/J.MAYOCPIQO.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummalacharla SC, Pavuluri P, Maram SR, et al. . Serum activities of ferritin among controlled and uncontrolled type 2 diabetes mellitus patients. Cureus 2022;14(5):e25155; doi: 10.7759/CUREUS.25155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez C, Horner SM. MAVS coordination of antiviral innate immunity. J Virol 2015;89(14):6974–6977; doi: 10.1128/JVI.01918-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent MJ, Bergeron E, Benjannet S, et al. . Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005;2:69; doi: 10.1186/1743-422X-2-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Cao R, Zhang L, et al. . Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-NCoV) in vitro. Cell Res 2020;30(3):269–271; doi: 10.1038/S41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff G, Melia CE, Snijder EJ, et al. . Double-membrane vesicles as platforms for viral replication. Trends Microbiol 2020;28(12):1022–1033; doi: 10.1016/J.TIM.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CK, Luk HKH, Lai WH, et al. . Human-induced pluripotent stem cell-derived cardiomyocytes platform to study SARS-CoV-2 related myocardial injury. Circ J 2020;84(11):2027–2031; doi: 10.1253/CIRCJ.CJ-20-0881 [DOI] [PubMed] [Google Scholar]
- Wu F, Zhao S, Yu B, et al. . A new coronavirus associated with human respiratory disease in China. Nature 2020a;579(7798):265–269; doi: 10.1038/S41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KE, Fazal FM, Parker KR, et al. . RNA-GPS predicts SARS-CoV-2 RNA residency to host mitochondria and nucleolus. Cell Syst 2020b;11(1):102.e3–108.e3; doi: 10.1016/J.CELS.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaohong Y, Tingyuan L, Zhicheng H, et al. . A pathological report of three COVID-19 cases by minimal invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi 2020;49(5):411–417; doi: 10.3760/CMA.J.CN112151-20200312-00193 [DOI] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang Y, et al. . Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8(4):420–422; doi: 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wang Y, Xiao ZG, et al. . Nuclear receptor ERRα and transcription factor ERG form a reciprocal loop in the regulation of TMPRSS2:ERG fusion gene in prostate cancer. Oncogene 2018;37(48):6259–6274; doi: 10.1038/S41388-018-0409-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Zhang Y, Ge J, et al. . Architecture of a SARS-CoV-2 mini replication and transcription complex. Nat Commun 2020;11(1):5874; doi: 10.1038/S41467-020-19770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Prinsen JK, Bersell KR, et al. . Azithromycin causes a novel proarrhythmic syndrome. Circ Arrhythm Electrophysiol 2017;10(4):e003560; doi: 10.1161/CIRCEP.115.003560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Ye F, Zhang M, et al. . In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020;71(15):732–739; doi: 10.1093/CID/CIAA237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Wong CK, Li P, et al. . A SARS-CoV protein, ORF-6, induces caspase-3 mediated, ER stress and JNK-dependent apoptosis. Biochim Biophys Acta 2008;1780(12):1383–1387; doi: 10.1016/J.BBAGEN.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka T, Kosako H, Yoshizumi T, et al. . Structural basis of mitochondrial scaffolds by prohibitin complexes: insight into a role of the coiled-coil region. iScience 2019;19:1065–1078; doi: 10.1016/J.ISCI.2019.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wei L, Jiang D, et al. . SARS-CoV nucleocapsid protein induced apoptosis of COS-1 mediated by the mitochondrial pathway. Artif Cells Blood Substit Immobil Biotechnol 2007;35(2):237–253; doi: 10.1080/10731190601188422 [DOI] [PubMed] [Google Scholar]
- Zhao M, Wang M, Zhang J, et al. . Comparison of clinical characteristics and outcomes of patients with coronavirus disease 2019 at different ages. Aging (Albany NY) 2020;12(11):10070–10086; doi: 10.18632/AGING.103298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Dai SM, Tong Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother 2020a;75(7):1667–1670; doi: 10.1093/JAC/DKAA114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Hu S, Jin Q, et al. . Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of MROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved MPTP opening. J Am Heart Assoc 2017;6(3):e005328; doi: 10.1161/JAHA.116.005328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ren J, Toan S, et al. . Role of mitochondrial quality surveillance in myocardial infarction: From bench to bedside. Ageing Res Rev 2021;66:101250; doi: 10.1016/J.ARR.2020.101250 [DOI] [PubMed] [Google Scholar]
- Zhou P, Yang X-L, Wang X-G, et al. . A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020b;579(7798):270–273; doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Vedantham P, Lu K, et al. . Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res 2015;116:76–84; doi: 10.1016/J.ANTIVIRAL.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]