Abstract
Background
Amniotic membrane tissue has been thought to potentiate healing in many soft tissue conditions. Specifically, recent studies have shown its therapeutic potential for treatment in the setting of spinal pathologies. The purpose of this study is to thoroughly review the existing scientific literature and evidence concerning the clinical use of amniotic membrane–derived biologic agents on postoperative outcomes following spinal surgery.
Methods
A systematic review was conducted following preferred reporting items for systematic reviews and meta-analyses guidelines using PubMed, Embase, and Cochrane databases up to December 2020 to identify animal and clinical studies examining the therapeutic potential for amniotic membrane tissue in the setting of spinal pathologies (including disc herniation, prevention of epidural fibrosis, and spinal fusion). Studies were broken down into 2 categories: experimental model type and the type of amnion product being analyzed.
Results
A total of 12 studies (4 clinical studies and 8 studies utilizing animal models) met inclusion criteria. Additionally, the major types of amnion product were divided into cryopreserved/freeze-dried amniotic membrane, human amniotic fluid, human amniotic membrane, cross-linked amniotic membrane, and amnion-derived epithelial cells. While heterogeneity of study design precludes definitive specific results reporting, most studies showed positive benefits on healing/outcomes with amniotic augmentation. Specifically, amnion products have shown promising effects in reducing epidural adhesions and scar tissue after spine surgery, improving spinal fusion rate and postoperative pain scores, and promoting better functional outcomes after spine surgery.
Conclusions
A review of the limited number of reported studies revealed a wide variety of amniotic membrane preparations, treatment regimens, and indications, which limit definitive conclusions. To date, while there is no definitive clinical proof that amniotic tissues enhance tissue repair or regeneration, the aggregate results demonstrate promising basic science and outcomes potential in spinal surgery. Further study is warranted to determine whether this application is appropriate in the clinical setting.
Clinical Relevance
This systematic review provides a summary of the existing literature regarding the use of amniotic membrane preparations, treatment regimens, and indications within spinal surgery. With the growing popularity and utilization of biologic agents such as amniotic membrane-derived products in orthopedic and neurologic surgery, this systematic review gives physicians a concise summary on the outcomes and indications associated with amniotic membrane products.
Level of Evidence
4.
Keywords: amniotic membrane, chorionic membrane, umbilical cord, epidural injection, microdiscectomy, intervertebral disc, disc herniation, epidural fibrosis, spinal dysraphism
Introduction
The use of biologic agents in orthopedic and spine surgery remains an area of continued growth and interest.1 Biologic agents such as platelet-derived growth factor, bone marrow aspirate (BMA) concentrate, platelet-rich plasma, demineralized bone matrix, and bone morphogenic proteins have all been used with varying success in an effort to reduce inflammation, stimulate angiogenesis, and ultimately induce healing after orthopedic and spine procedures.2–8 A common source for many of these agents is mesenchymal stem cells (MSC). Recently, the use of MSCs and their growth factors in orthopedics and spinal surgery has increased in popularity as MSC-derived products have become more widely available, in addition to promising research study results.9–19
MSCs and their associated growth factors can be isolated from a variety of tissue types including placental tissue, bone marrow, synovial tissue, and adipose tissue.20–24 However, placental tissue has gained favor as a source of abundant MSCs in addition to some of the other regenerative factors mentioned above.25 The human placenta is made up of several membranes and tissue that surround the developing fetus and provide sustenance and protection. The umbilical cord, amnion, and chorion are of particular importance in regard to their use as a potential source of MSCs and associated growth factors; however, the focus of the present analysis will be amniotic-derived cell-free products.
The amnion is a placental tissue that originates from trophoblasts and envelopes the developing fetus.26 The amnion has an epithelial cell layer and a mesenchymal cell layer that are both sources of MSCs. Amniotic epithelial cells are known to manufacture hepatocyte growth factor, epidermal growth factor, keratinocyte growth factor, and fibroblast growth factor, which are strong promoters of epithelization, tenocyte proliferation, and neural differentiation.1,27,28 They also inhibit the local immune response and possess the unique ability to differentiate into cells of all 3 germ lines.29,30 In clinical practice, it is the amnion-derived growth factors, such as those mentioned above, that are potentially beneficial in promoting healing and decreasing fibrosis. The vast majority of amnion products in the United States are “cell free,” meaning the MSCs themselves are excluded in favor of the growth factors they produce. Similarly, embryonic-derived mesenchymal stromal cells have the capacity to stimulate angiogenesis and suppress local innate and adaptive immune responses via the production of a variety of growth factors that are isolated for use in clinical applications.1,29 Notably, the unique qualities of amnion-derived epithelial and mesenchymal cells have exhibited strong osteogenic and chondrogenic differentiation, especially when compared with other sources of MSCs.31
Despite the emerging research and prevalent marketing/promotion of amnion-derived products in spine surgery, there exists a paucity in the literature to fully support their clinical use. As the use of amnion-derived products in spine surgery gains momentum, the outcomes of their use in animal and human models are of particular importance. Therefore, the purpose of this study is to thoroughly review the existing scientific literature and evidence concerning the clinical use of amniotic membrane–derived biologic agents on postoperative outcomes following spinal surgery.
Methods
This systematic review was conducted using preferred reporting items for systematic reviews and meta-analyses guidelines. Two independent reviewers conducted the initial literature search in December 2020 using PubMed, Embase, and Cochrane Central Register of Controlled Trials databases. A broad-based search was conducted to ensure no studies were missed using the following search terms: “‘amnion and spine’ or ‘amniotic and spine’.” All searches were conducted from database inception to the time of search (December 2020). Studies were included in the systematic review if they reported clinical, biological, biomechanical, patient-reported outcomes, or radiographic findings of human or animal studies examining the effect of amniotic membrane after spinal surgery. Only full-text manuscripts written in the English language were included, and no level of evidence restrictions were imposed. Technique articles, review articles, letters to the editor, and conference abstracts or studies not published in the English language were excluded.
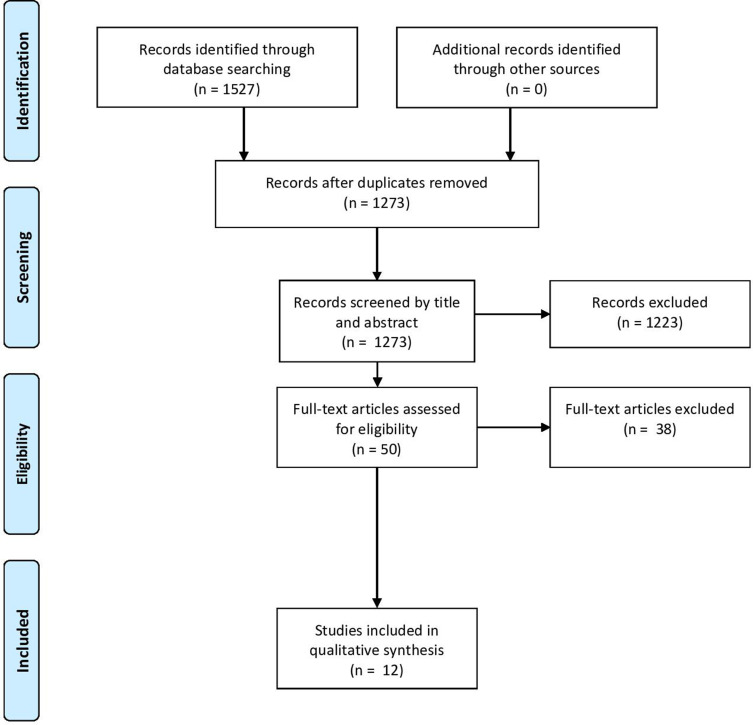
The search results were reviewed independently by 2 authors to select studies for inclusion in the review. After removal of duplicates, the initial keyword literature search produced a total of 1273 references. Fifty studies were identified for inclusion from the literature search based on appropriateness of title and abstract content and were related to the application of amniotic tissue for the treatment of disc pathology or prevention of scar tissue formation after spinal interventions. These 50 studies then underwent full-text review to confirm appropriateness for inclusion. The reference list and text of each latter manuscript were cross-referenced to identify any additional studies related to the study topic not previously found. Following full-text review and cross-referencing, 12 studies met all criteria for inclusion and were included in the review.32–43 After each step of the review process, any disagreement on inclusion of a study was resolved by discussion between the 2 reviewers. If consensus could not be reached, then inclusion was decided by a third reviewer. A flow diagram outlining the selection process is found in the Figure.
Figure.
Preferred reporting items for systematic reviews and meta-analyses flowchart outlining the literature review and selection process.
The 12 studies were subsequently divided based on study design to allow for simplified organization and improved comparison between similar studies. The 2 main categories were experimental model type and the type of amnion product being analyzed. Within the experimental model type category, subcategories were human models, rat models, sheep model, rabbit model, and dog model. Additionally, the major types of amnion product could be divided into cryopreserved/freeze-dried amniotic membrane (cAM/FAM), human amniotic fluid (HAF), human amniotic membrane (HAM), cross-linked amniotic membrane (CAM), and amnion-derived epithelial cells (AECs).
For all selected studies, the full text was accessed and thoroughly reviewed. The study design, experimental model, tissue type being experimented with, study objective, study methods, and main results for each article were all recorded and summarized in Table 1. The level of evidence was also collected for each article according to the Journal of Bone and Joint Surgery Levels of Evidence classification.44 Given the heterogeneity of the included studies, no calculable aggregate data or meta-analyses are presented in this review.
Table 1.
Summary of literature review for amniotic membrane–derived biologic agents on postoperative outcomes following spinal surgery.
| Study | Design | Tissue Type | Objective | Methods | Results |
| Animal Studies | |||||
| Bolat et al33 (2013) | Retrospective animal (rat) study | HAF | Evaluate effects of mitomycin-C, sodium hyaluronate, and amniotic fluid on prevention of spinal epidural fibrosis | A total of 4 groups (10 each): control, mitomycin-C, sodium hyaluronate, and amniotic fluid. L5 total laminectomy performed and assessed for epidural fibrosis 4-wk postoperative | Significant difference in amount of scar tissue (none) in experimental groups compared with control group |
| Choi et al34 (2011) | Experimental animal (rat) study | FAM | Evaluate effects of amniotic membrane on epidural adhesions after laminectomy | A total of 20 rats, 2 groups. Laminectomy with or without amniotic membrane. Assessment at 1-, 3-, and 8-wk postoperative | Significant decrease in amount and tenacity of scar tissue in amniotic membrane group |
| Cunningham et al35 (2019) | Experimental animal (sheep) study | Dual-layer, chorion-free amnion patch from HAM | Evaluate effect of dual-layer, chorion-free amnion path following lumbar laminectomy | A total of 12 sheep, 2 groups: control and amnion. Laminectomy performed with or without amnion, and half were evaluated at 4 wk, half at 10 wk | Significant decrease in amount of fibroblast infiltration and tissue tenacity with the use of amnion |
| Goldschlager et al36 (2011) | Experimental animal (sheep) study | AECs | Comparison of allogeneic mesenchymal precursor cells to AECs in promoting osteogenesis | A total of 29 sheep divided into 5 groups: (1) C3-C4 ACDF with autograft IC and IBC, (2) HA/TCP Mastergraft granules alone, (3) HA/TCP with 5 million MPCs, (4) HA/TCP with 5 million AECs, and (5) nonoperative | MPCs lead to significantly more fusion than any other group, and all AECs failed to have any fusion at all |
| Kara et al38 (2015) | Experimental animal (rat) study | HAF and HAM | Evaluate effectiveness of amniotic fluid and membrane on prevention of postlaminectomy spinal epidural fibrosis | A total of 27 rats underwent 2 nonconsecutive lumbar laminectomies were divided into either: (1) laminectomy alone, (2) laminectomy + AM, (3) laminectomy + AF. Sacrificed at 6 wk | No significant differences between groups in regard to epidural scar formation and mean fibroblast count |
| Luo et al39 (2019) | Experimental animal (rabbit) study | Amniotic suspension allograft containing particulated HAM and HAF | Evaluate whether amniotic suspension allograft increases intervertebral disc height and morphology after disc degeneration | A total of 12 rabbits underwent disc puncture and then 4 wk later were injected with either amniotic suspension allograft, sham control, or were left untreated. Assessed over 12 wk | At 12 wk, experimental group had significantly greater disc height, magnetic resonance imaging T2 relaxation times, and improved morphology compared with control and untreated groups |
| Oner et al40 (2015) | Experimental animal (rat) study | HAF | Assessment of 2 different bone grafts and amniotic fluid on vertebral fusion in rat model | A total of 48 rats were randomized into 1 of 4 groups: allograft group, allograft plus AF, DBM group, or DBM plus AF. Fusion of spine was assessed at 8 wk | Amniotic fluid significantly enhanced posterior spinal fusion when combined with allograft |
| Tao and Fan42 (2009) | Experimental animal (dog) study | FAM, CAM, and AFF | Evaluate whether AM can reduce epidural scar adhesion after laminectomy in canine model | A total of 24 dogs underwent laminectomy at L1, L3, L5, and L7 with FAM, CAM, AFF, and no treatment assigned randomly to each of the 4 sites. Animals were sacrificed at 1, 6, and 12 wk postoperative | CAM group had significantly lower amounts of epidural fibrosis compared with controls |
| Human Studies | |||||
| Anderson et al32 (2017) | Prospective, RCT | Cryopreserved amniotic membrane (cAM) | Compare pain, functional outcomes and recurrent herniation follow lumbar microdiscectomy w/ or w/o amniotic tissue graft | A total of 80 patients randomized to either amniotic tissue or no tissue following elective lumbar microdiscectomy | AM group had greater functional outcomes and no recurrent herniations at 2 years |
| Kamson and Smith37 (2020) | Prospective, RCT | Cryopreserved amniotic-derived products | Comparison of PROM after use of orthobiologic supplementation during endoscopic-assisted lumbar decompression surgery | A total of 269 patients randomized to receive either amniotic membrane, bone marrow aspiration, both, or none during lumbar decompression | Patients receiving either bone marrow aspirate or amniotic membrane had significantly decreased pain at all timepoints compared with control |
| Subach and Copay41 (2015) | Retrospective case series | Dehydrated human amnion/chorion membrane | Evaluation of AM on epidural scar formation after transforaminal lumbar interbody fusion | A total of 5 patients who had transforaminal lumbar interbody fusion with AM who subsequently underwent epidural re-exploration | Four of 5 cases had easily detachable tissue during epidural re-exploration |
| Walker et al43 (2018) | Retrospective case series | HAM | Evaluate HAM in the prevention of spinal retethering after detethering | A total of 14 patients received HAM after detethering. Followed for minimum of 6 mo | Only 1 patient required subsequent detethering |
Abbreviations: ACDF, anterior cervical discectomy and fusion; AECs, amnion-derived epithelial cells; AF, amniotic fluid; AFF, autologous-free fat; AM, amniotic membrane; CAM, cross-linked amniotic membrane; DBM, demineralized bone matrix; FAM, freeze-dried human amniotic membrane; HAF, human amniotic fluid; HA/TCP, hydroxyapatite-tricalcium phosphate; IBC, interbody cage; IC, iliac crest autograft; MPCs, mesenchymal precursor cells; RCT, randomized controlled trial.
Note: Boldface indicates the primary variables being measured or outcomes of interest in each selected study.
Results
A total of 12 studies published between 2009 and 2020 met all previously outlined inclusion and exclusion criteria and were included in the systematic review.32–43 Regarding levels of evidence, 2 studies were level I, 7 were level II, 1 was level III, and 2 were level IV. Four studies used human models,32,37,41,43 and 8 studies used animal models.33–36,38–40,42 Of the animal models, 4 used rat models,33,34,38,40 2 sheep models,35,36 1 dog model,42 and 1 rabbit model.39 Regarding amniotic product type, 5 studies utilized either cAM or FAM,32,34,37,41,42 4 utilized HAF,33,38–40 4 utilized HAM,35,38,39,43 1 utilized CAM,42 and 1 utilized AEC.36 Two studies analyzed both HAF and HAM in the same study, while another study analyzed both FAM and CAM. Study characteristics and major methodology and results are summarized in Table 1.
Animal Model
Bone Healing
Two studies assessed the effects of amnion products on bone healing.36,40 Goldschlager et al36 used AEC and mesenchymal precursor cells (MPCs), while Oner et al40 utilized HAF.
Goldschlager et al36 sought to compare the allogenic MPCs with AECs in promoting osteogenesis following anterior cervical discectomy. The investigation utilized 29 sheep divided into 5 groups receiving the following treatments: Fidji interbody cage (Abbott Spine, Bordeaux, France) packed with iliac crest autograph alone, hydroxyapatite-tricalcium phosphate (HA-TCP) Mastergraft granules (Medtronic, Fridley, Minnesota) alone, HA-TCP with 5 million MPCs, HA-TCP with 5 million AECs, and a group of age-matched nonoperative controls. The investigators found that there was significantly more fusion in the MPC group as compared with the 3 other experimental groups (P = 0.01). The MPC group found that 5 of the 6 sheep had continuous bony bridging at 3 months compared with 0 out of the 5 sheep in the AEC-treated group.36 Similarly, Oner et al40 reported rats receiving demineralized bone matrix combined with HAF had significantly better results in both radiologic and histologic evaluation of vertebral fusion results when compared with demineralized bone matrix alone following an L4-L6 spinal fusion 8 weeks after surgery (radiologic: P = 0.003; histologic: P < 0.001).
Inhibition of Scar Formation
A total of 5 studies assessed the formation of scar tissue following spinal procedures in animal models.33–35,38,42 Of these studies, 2 utilized HAF,33,38 2 utilized FAM,34,42 2 utilized HAM,35,38 and 1 utilized CAM.42
Bolat et al33 reported that all experimental groups (mitomycin-C, sodium hyaluronate, and HAF) had significantly less scar tissue compared with the control group 4 weeks after an L5 total laminectomy. Choi et al34 indicated that rats receiving FAM after laminectomy had significantly less scar tissue and a decrease in the tenacity of scar tissue when compared with the control group that did not receive FAM postlaminectomy (P < 0.05).
Cunningham et al35 evaluated the effects of dual-layer chorion-free amnion patch derived from HAM following lumbar laminectomy (at L3 and L5) in 12 sheep with 2 groups: control and amnion. The sheep served as their own control as the 2 laminectomy sites for each sheep were randomly assigned to the control or amnion group. The investigators found that at both 4 and 10 weeks postlaminectomy, there was a significant decrease in the amount of fibroblast infiltration (P < 0.05 for both 4 and 10 weeks). Additionally, at 10 weeks, tissue tenacity in the amnion-treated group was significantly less than the control (P < 0.05).35
Tao and Fan42 incorporated the use of 24 canine subjects that underwent laminectomy at L1, L3, L5, and L7. Experimental groups included: FAM, CAM, autologous-free fat, and a no treatment control. The study found that the CAM group experienced significantly lower scar burden (CAM vs autologous-free fat: P = 0.71; CAM vs control: P < 0.01) and epidural fibrosis and adhesion (CAM vs autologous-free fat: P = 0.36; CAM vs control: P < 0.01) when compared with the control group but not the autologous-free fat group. CAM was found to degrade more slowly when compared with FAM, which allowed an earlier infiltration by scar tissue. Additionally, the FAM, CAM, and no treatment groups all showed equivalent postlaminectomy bone growth.
In contrast, Kara et al38 found that rats undergoing laminectomy at 2 levels (L1 and L4) saw no significant difference between the HAF-treated rats, the HAM-treated rats, and the control group in terms of prevention of epidural scar tissue formation (HAF vs control: P = 0.718; HAM vs control: P = 0.400; HAF vs HAM: P = 0.140).
Disc Height
A single study by Luo et al39 utilized a rabbit experimental model to determine whether amniotic suspension allograft increases the intervertebral disc height and morphology after disc degeneration. Specifically, this study used amniotic suspension allograft derived from both particulated HAM and HAF. This analysis incorporated the use of 12 rabbits that underwent disc puncture and were then injected with amniotic suspension, sham control, or were untreated 4 weeks later. The rabbits were assessed over 12 weeks. Major findings showed that injection of amniotic suspension allograft derived from HAM and HAF had significant improvements in disc height and morphology when compared with the control and untreated groups (P = 0.043 for each).
Human Studies
Disc Herniation
Both Anderson et al32 and Kamson and Smith37 investigated the use of cAM/amniotic-derived products with similar outcomes. Specifically, Anderson et al found that when 80 patients were randomized to either a cAM group or control group following elective lumbar microdiscectomy, the cAM group experienced significantly greater functional outcomes and fewer recurrent herniations at 2 years postsurgery (P = 0.05 at 6 weeks and P = 0.02 at 24 months).32 Similarly, Kamson and Smith found that when 269 patients were randomized to receive either amniotic membrane, BMA, both, or no treatment during lumbar decompression, patients had significant decreases in pain. Patients receiving either BMA or amniotic membrane had significantly decreased mean visual analog scale measured back pain at 2 weeks (3.98 vs 5.01, P = 0.011), 2 months (3.22 vs 3.93, P = 0.04), 9 months (2.38 vs 4.11, P = 0.004), and 12 months (2.23 vs 3.58, P = 0.011).37 Moreover, mean visual analog scale measured leg pain had significant improvements for patients at 2 weeks (3.55 vs 4.77, P = 0.002), 6 months (2.34 vs 3.37, P = 0.026), and 9 months (2.18 vs 3.57, P = 0.01).37 There were no reportable complications noted intraoperatively in the 269 patients. Two patients experienced a reherniation (1 in the control group and 1 in the amnion group). The BMA only and both BMA and amniotic membrane groups experienced no reherniations.
Spinal Epidural Fibrosis/Scar Formation
Subach and Copay41 and Walker et al43 investigated the use of dehydrated human amnion/chorion membrane and HAM on the degree of fibrosis/scar tissue formation after spinal surgery, respectively. Subach and Copay utilized dehydrated human amnion/chorion membrane in 5 patients undergoing transforaminal lumbar interbody fusion and found that 4out of 5 cases had easily detachable fibrotic tissue during epidural re-exploration. Significant improvements in patient outcomes were also noted for back pain (P = 0.007), Oswestry Disability Index (P = 0.0032), and Medical Outcomes Study Questionnaire Short Form 36 (P = 0.0239).41 Additionally, Walker et al43 evaluated the effect of HAM in the prevention of spinal retethering after a detethering procedure in retrospective case series of 14 patients. The investigation found that only 1 patient experienced retethering after receiving a HAM graft in the prior detethering procedure suggesting that HAM grafts are a safe and potentially effective method of preventing microsurgical intradural adhesions.43
Discussion
As presented in this analysis, the few studies that have been conducted in animal models and human patients have shown promising effects in reducing epidural adhesions and scar tissue after spine surgery, improving spinal fusion rate and postoperative pain scores, and promoting better functional outcomes after spine surgery. Amniotic membrane tissue contains many factors that are theoretically optimal to support healing.1 It has historically been used for the treatment of burns and wounds.45 More recently, there is early evidence to support its role in the treatment of a number of musculoskeletal pathologies, including the spine.46 Notwithstanding, clinical research relating to amniotic membrane tissue in spinal surgery remains sparse. However, among the studies included in this analysis, very few complications have been reported suggesting the safety of amnion-derived products incorporated in spine procedures. Moreover, the existing evidence of amnion-derived biologic agents utilized in spine surgery shows promise in both animal and human models. Nevertheless, the current literature surrounding amniotic membrane products in spine surgery is inconclusive in its current state as this published research is mostly level IV evidence, heterogenous, consisting of many different treatment protocols, experimental subjects, and amnion-derived products.
Animal Studies
The evidence presented in this analysis is limited due to the nature of animal studies, which predominated the literature in this field. Eight of the 12 studies included in this analysis utilized animals as experimental models. The animals in these studies included dogs, rabbits, sheep, and rats.
Of these 8 animal studies, 6 showed promising results of amniotic membranes that may eventually translate to clinical practice. The encouraging outcomes included significant reductions in scar tissue and epidural fibrosis following spine surgery,33–35,42 greater disc height and improved morphology after disc degeneration,39 and enhanced posterior spinal fusion rates when combined with allograft.40 Studies by Goldschlager et al36 and Kara et al38 were the only animal studies that showed inconclusive results. Goldschlager et al found that AECs combined with HA/TCP failed to improve spinal fusion rates postlaminectomy.36 This is in contrast to the study by Oner et al that found improved spinal fusion rates when HAF was combined with bone allograft.40 Additionally, Kara et al discovered that HAF and HAM treatment groups showed no significant difference compared with the control in epidural scar formation and mean fibroblast count after undergoing lumbar laminectomy.38
While promising, results in the selected animal model studies came from a wide variety of amniotic products and experimental methodologies with some studies contradicting others, and it is essential to understand these results within their proper context. Animal models present researchers and scientists with a convenient and low-risk option in which to conduct preclinical studies; however, the translation to clinical trials and applicability often faces major barriers. Translation of medical treatments from animals to human subjects often disappoints for a variety of reasons including differing complexity, biology, and physiologic regulation between species.47 While experiments conducted on animal models is a necessity in order to discover effective treatments that can be used in humans, the jump to clinical practice is very wide and often unobtainable. Therefore, the findings from these studies should be interpreted with caution.
Amnion-Derived Products
Another shortfall of the applicability of the studies included in this analysis is the wide variety of amnion-derived products, preparations, dosages, and administration. Amnion products and preparations included cAM, FAM, dehydrated human amnion membrane, HAF, HAM, dual-layer chorion-free amnion patch from HAM, CAM, AECs, and amniotic suspension allograft containing particulated HAM and HAF. This equates to 9 different amnion-derived products or preparations of amnion distributed between 12 total studies. Therefore, drawing a sensible conclusion is made exceedingly difficult as no 2 studies are exactly alike in their most basic methodologies and treatments. Tables 2 and 3 summarize the variety of terms used throughout these heterogenous studies.
Table 2.
Summary of general stem cell terminology frequently used among selected articles.
| Term | Distinctions |
| Amniotic membrane epithelial cells | These are cells that can be derived from the inner lining of the placenta after birth. As such, there are fewer ethical concerns. |
| Mesenchymal stem cells | These are cells that can be derived from a variety of sources, adult, and embryo, including bone marrow, liver, kidney, muscle, adipose, connective tissue, placenta, and the umbilical cord. |
| Chorion cells | These calls can be derived from the placenta, specifically the yolk sac, which is the outermost fetal membrane surrounding the embryo. |
| Umbilical cord tissue | The umbilical cord contains large amounts of mesenchymal stem cells. These cells are distinct from the stem cells found in umbilical cord blood. |
| Umbilical cord blood | The stem cells are contained in the umbilical cord blood. However, it can be difficult to obtain a unit large enough to be used in an adult. |
Table 3.
Summary and unique distinctions of more specific amnion terminology used throughout the selected articles. Additional summary of spine surgery uses among the selected articles.
| Term | Distinctions | Potential Uses in Spine Surgery |
| HAF | Amniotic fluid surrounds the embryo contained in the amniotic sac and is highly proliferative. This fluid can be obtained through amniocentesis with little risk to the fetus and the mother. | Bolat et al33 used to examine the effects of HAF on spinal epidural fibrosis. Kara et al38 used to examine prevention of postlaminectomy spinal epidural fibrosis. Luo et al39 used to examine impact on intervertebral disc height and morphology after disc degeneration. Oner et al40 used to determine effect on vertebral fusion. Walker et al43 used to determine prevention of spinal retethering after detethering. |
| HAM | The amniotic membrane is the inner lining of the placenta. This membrane can be harvested after cesarean section. | Kara et al38 used to examine prevention of postlaminectomy spinal epidural fibrosis. Luo et al39 used to examine impact on intervertebral disc height and morphology after disc degeneration |
| Freeze-dried HAM | Freeze drying is more abrasive than drying alone, as the process requires drying and freezing, both of which impose stress on biomaterials. | Choi et al34 used this to evaluate the effects on epidural adhesions after laminectomy. Tao and Fan42 used to reduce epidural scar adhesion after laminectomy. |
| Cryopreserved HAM | Cryopreservation includes storing the specimen in liquid nitrogen. | Kamson and Smith37 used this to study PROM after endoscopic-assisted lumbar decompression. Anderson et al32 studied the use of this to compare functional outcomes and recurrent herniation after lumbar microdiscectomy. |
| Dehydrated HAM | Amniotic membranes undergo a process that desiccates and removes all water from the tissue. Dried samples can be stored at room temperature and typically have a much longer shelf life. | Subach and Copay41 used this to evaluate epidural scar formation after transforaminal lumbar interbody fusion. |
| Cross-linked amniotic membrane | Amniotic membrane can be cross-linked through exposure to chemicals and radiation to increase stability of the biomolecules within the membrane. | Tao and Fan42 used to reduce epidural scar adhesion after laminectomy. |
| Amnion-derived epithelial cells | These are cells that can be derived from the inner lining of the placenta after birth. As such, there are fewer ethical concerns. | Goldschlager et al36 used to observe effect on promoting osteogenesis. |
| Dual-layered, chorion-free, amnion patch | This is derived from HAM and consists of 2 layers of amniotic membrane stacked on top of each other. | Cunningham et al35 used to follow effect following lumbar laminectomy. |
Abbreviations: HAF, human amniotic fluid; HAM, human amniotic membrane; PROM, patient-reported outcome measures.
This inconsistency between studies and the amnion-derived products is only a small example of a much wider issue within the field of commercial tissue allografts. Tissue allografts are regulated by the Food and Drug Administration as a type of product known as “human cells, tissues, and cellular and tissue-based products” (HCT/Ps) as defined in Section 361 of the Public Health Service Act and Title 21 of the Code of Federal Regulations.48 Under the Section 361 classification, HCT/Ps, which includes placenta-derived products such as amniotic membrane, are required to meet only 4 criteria to uphold their classification as HCT/Ps. These criteria are (1) minimal manipulation, (2) homologous use, (3) not combined with drugs or devices, and (4) not reliant on cell metabolic activity as a primary function.25 HCT/Ps also require no premarket approval and have one of the fastest and most direct pathways to commercialization of all medical devices and pharmaceuticals.25 If a product does not meet the above requirements for classification as a HCT/P, they are required to be regulated by the Food and Drug Administration as biological drugs under Section 351 of the Public Health Service Act.25 This pathway to approval is very long and expensive, because it requires manufacturers to obtain a Biologics License Application and complete phase I to III clinical trials.25 Therefore, the vague regulation, minimal HCT/P criteria under Section 361, and lack of oversight in regard to tissue allografts such as amnion-derived products have led to a wide variety of commercial products that make comparisons exceedingly difficult. Table 4 contains a summary of 10 available amnion-derived products commonly used in orthopedic surgery.49 However, this list is likely not comprehensive as amnion-based products are being consistently released, discontinued, and rebranded under different names and formulation. Due to the small number of animal studies examined in this review, with variability of animal model, indications for use, preparation of amniotic tissue, dose, and administration, it is not possible to perform any comparative analysis. More research is needed to fully elucidate these differences.
Table 4.
Summary of 10 commonly utilized amnion-derived biologic agents used in orthopedics.
| Product (Company) | Composition | Growth Factors | Processing | Storage | Shelf Life | Available Configurations | Proposed Effects and Uses |
| Affinity (Organogenesis); Canton, MA, USA | Fresh amniotic membrane | NL | NL | Refrigerator | NL | Liquid |
|
| Allogen (ViVex); Atlanta, GA, USA | Frozen allograft derived from amniotic fluid | TIMP-2, HGF, IL-6, TIMP-1, IL-1ra, GRO-a, TGF-B1, TGF-B2, TIMP-4 | NL | Ambient conditions, refrigerator, or frozen | 2 y at −40°C for frozen configuration and 2 y at 2–30°C in liquid configuration |
|
|
| AlloWrap DS (Allosource); Centennial, CO, USA | Amniotic membrane | TGF-B1-B3, TGF-A, EGF, KGF, HGF, bFGF, IL-1, IL-2, IL-10, MMP-1–4 | NL | Ambient conditions | 2 y |
|
|
| Amniofix (MiMedx Group Inc); Marietta, GA, USA | Amniotic tissue membrane composite | EGF, KGF, HA, IL-6 | PURION process a | Ambient conditions | 5 y |
|
|
| Clarix Surgical Matrix (Amniox Medical Inc); Miami, FL, USA | Cryopreserved human amniotic membrane | EGF, KGF, HA, IL-6 | CRYOTEK process b | Refrigerator or freezer | 3 mo at 1–10°C; 1 y at −49 to 0°C; 2 y at −85 to −50°C |
|
|
| FloGraft (Applied Biologics); Scottsdale, AZ, USA | Amnion and amniotic fluid | NL | NL | Refrigerator or freezer | NL | Liquid |
|
| NuCel (Organogenesis); Canton, MA, USA | Amnion and amniotic fluid | NL | NL | NL | NL | Liquid |
|
| PalinGen Flow/Sports Flow (Amnio Technology); Phoenix, AZ, USA | Amnion and amniotic fluid components | FGF, EGF, PDGF A and B, VEGF, TGFβ | NL | Freezer | NL | Liquid |
|
| ReNu (Organogenesis); Canton, MA, USA | Cryopreserved amniotic suspension allograft | NL | NL | Freezer | NL | Liquid |
|
| ViaShield (Globus Medical); Audubon, PA, USA | Chorion-free, dual-layer amnion patch | NL | NL | NL | NL | Sheet/membrane | NL |
Abbreviations: bFGF, basic fibroblast growth factor; EGF, epidermal growth factor; GRO, growth regulated protein alpha; HA, hyaluronic acid; HGF, hepatocyte growth factor; HGF, hepatocyte growth factor; IL, interleukin; KGF, keratinocyte growth factor; MMP, matrix matalloproteinase; NL, not listed; PDGF A and B, platelet derived growth factor alpha and beta; TGF, transforming growth factor; TIMP-2, tissue inhibitor of metalloproteinases 2; VEGF, vascular endothelial growth factor.
The PURION process of cleaning, dehydration, and sterilization safely and carefully separates placental tissues, cleans and reassembles layers, and subsequently dehydrates the tissue to preserve the key elements associated with healing. The amniotic membrane scaffolding is protected while blood components are removed, so an intact extracellular matrix is left behind. No chemical cross-linking or decellularization occurs during the process.
The CRYOTEK process uses cryopreservation (deep freezing) to maintain the innate biological and structural integrity of the natural tissue while maintaining its natural hydrated state.
Human Studies
The 4 human studies in this analysis presented a promising outlook for the use of amnion in spine surgery. Results included improved functional outcomes and decreased risk of herniation following lumbar microdiscectomy,32 decreased pain following lumbar decompression,37 decreased epidural scar formation after transforaminal interbody fusion,41 and decreased retethering rates following an initial detethering procedure.43 These human studies involving amnion-derived cells in spine surgery currently represent a potential therapeutic advancement in spine surgery, but further clinical research in this field is needed to define the safety benefits, indications, and contraindications of amnion-derived cells in spine surgery.
Conclusion
A review of the limited number of reported studies revealed a wide variety of amniotic membrane preparations, treatment regimens, and indications, which limit definitive conclusions. To date, while there is no definitive clinical proof that amniotic tissues enhance tissue repair or regeneration, the aggregate results demonstrate promising basic science and outcomes potential in spinal surgery. Further study is warranted to determine whether this application is appropriate in the clinical setting.
References
- 1. Riboh JC, Saltzman BM, Yanke AB, Cole BJ. Human amniotic membrane-derived products in sports medicine: basic science, early results, and potential clinical applications. Am J Sports Med. 2016;44(9):2425–2434. 10.1177/0363546515612750 [DOI] [PubMed] [Google Scholar]
- 2. Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976). 2002;27(21):2396–2408. 10.1097/00007632-200211010-00015 [DOI] [PubMed] [Google Scholar]
- 3. Caplan AI, Correa D. Pdgf in bone formation and regeneration: new insights into a novel mechanism involving MscS. J Orthop Res. 2011;29(12):1795–1803. 10.1002/jor.21462 [DOI] [PubMed] [Google Scholar]
- 4. De Long WG, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649–658. 10.2106/JBJS.F.00465 [DOI] [PubMed] [Google Scholar]
- 5. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-Rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602–608. 10.5435/00124635-200910000-00002 [DOI] [PubMed] [Google Scholar]
- 6. Hollinger JO, Hart CE, Hirsch SN, Lynch S, Friedlaender GE. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90(Suppl 1):48–54. 10.2106/JBJS.G.01231 [DOI] [PubMed] [Google Scholar]
- 7. Michelson JD, Curl LA. Use of demineralized bone matrix in hindfoot arthrodesis. Clin Orthop Relat Res. 1996;(325):203–208. 10.1097/00003086-199604000-00024 [DOI] [PubMed] [Google Scholar]
- 8. Smith B, Goldstein T, Ekstein C. Biologic adjuvants and bone: current use in orthopedic surgery. Curr Rev Musculoskelet Med. 2015;8(2):193–199. 10.1007/s12178-015-9265-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marcucio RS, Nauth A, Giannoudis PV, et al. Stem cell therapies in orthopaedic trauma. J Orthop Trauma. 2015;29 Suppl 12:S24–S27. 10.1097/BOT.0000000000000459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peçanha R, Bagno L de LES, Ribeiro MB, et al. Adipose-derived stem-cell treatment of skeletal muscle injury. J Bone Joint Surg Am. 2012;94(7):609–617. 10.2106/JBJS.K.00351 [DOI] [PubMed] [Google Scholar]
- 11. Jäger M, Hernigou P, Zilkens C, et al. Cell therapy in bone healing disorders. Orthop Rev (Pavia). 2010;2(2):e20. 10.4081/or.2010.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fairbairn NG, Meppelink AM, Ng-Glazier J, Randolph MA, Winograd JM. Augmenting peripheral nerve regeneration using stem cells: a review of current opinion. World J Stem Cells. 2015;7(1):11–26. 10.4252/wjsc.v7.i1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crevensten G, Walsh AJL, Ananthakrishnan D, et al. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng. 2004;32(3):430–434. 10.1023/b:abme.0000017545.84833.7c [DOI] [PubMed] [Google Scholar]
- 14. Lee EH, Hui JHP. The potential of stem cells in orthopaedic surgery. J Bone Joint Surg Br. 2006;88(7):841–851. 10.1302/0301-620X.88B7.17305 [DOI] [PubMed] [Google Scholar]
- 15. Chen D, Zeng W, Fu Y, Gao M, Lv G. Bone marrow mesenchymal stem cells combined with minocycline improve spinal cord injury in a rat model. Int J Clin Exp Pathol. 2015;8(10):11957–11969. [PMC free article] [PubMed] [Google Scholar]
- 16. Berebichez-Fridman R, Gómez-García R, Granados-Montiel J, et al. The Holy Grail of orthopedic surgery: mesenchymal stem cells-their current uses and potential applications. Stem Cells Int. 2017;2017:2638305. 10.1155/2017/2638305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bemenderfer TB, Anderson RB, Odum SM, Davis WH. Effects of cryopreserved amniotic membrane-umbilical cord allograft on total ankle arthroplasty wound healing. J Foot Ankle Surg. 2019;58(1):97–102. 10.1053/j.jfas.2018.08.014 [DOI] [PubMed] [Google Scholar]
- 18. Sultan AA, Piuzzi NS, Mont MA. Nonoperative applications of placental tissue matrix in orthopaedic sports injuries: a review of literature. Clin J Sport Med. 2020;30(4):383–389. 10.1097/JSM.0000000000000684 [DOI] [PubMed] [Google Scholar]
- 19. Noback PC, Donnelley CA, Yeatts NC, et al. Utilization of orthobiologics by sports medicine physicians: a survey-based study. J Am Acad Orthop Surg Glob Res Rev. 2021;5(1):e20. 10.5435/JAAOSGlobal-D-20-00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Capelli C, Gotti E, Morigi M, et al. Minimally manipulated whole human umbilical cord is a rich source of clinical-grade human mesenchymal stromal cells expanded in human platelet lysate. Cytotherapy. 2011;13(7):786–801. 10.3109/14653249.2011.563294 [DOI] [PubMed] [Google Scholar]
- 21. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–347. 10.1002/jcp.21200 [DOI] [PubMed] [Google Scholar]
- 22. De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. [DOI] [PubMed] [Google Scholar]
- 23. Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22(5):649–658. 10.1634/stemcells.22-5-649 [DOI] [PubMed] [Google Scholar]
- 24. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. 10.1091/mbc.e02-02-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McIntyre JA, Jones IA, Danilkovich A, Vangsness CT. The placenta: applications in orthopaedic sports medicine. Am J Sports Med. 2018;46(1):234–247. 10.1177/0363546517697682 [DOI] [PubMed] [Google Scholar]
- 26. Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447–458. 10.1007/s00441-012-1424-6 [DOI] [PubMed] [Google Scholar]
- 27. Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20(3):173–177. 10.1076/0271-3683(200003)2031-9FT173 [DOI] [PubMed] [Google Scholar]
- 28. Barboni B, Russo V, Curini V, et al. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012;21(11):2377–2395. 10.3727/096368912X638892 [DOI] [PubMed] [Google Scholar]
- 29. Insausti CL, Blanquer M, García-Hernández AM, Castellanos G, Moraleda JM. Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning. 2014;7(1):53–63. 10.2147/SCCAA.S58696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Li C, Jiang X, et al. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol. 2004;32(7):657–664. 10.1016/j.exphem.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 31. Topoluk N, Hawkins R, Tokish J, Mercuri J. Amniotic mesenchymal stromal cells exhibit preferential osteogenic and chondrogenic differentiation and enhanced matrix production compared with adipose mesenchymal stromal cells. Am J Sports Med. 2017;45(11):2637–2646. 10.1177/0363546517706138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson DG, Popov V, Raines AL, O’Connell J. Cryopreserved amniotic membrane improves clinical outcomes following microdiscectomy. Clin Spine Surg. 2017;30(9):413–418. 10.1097/BSD.0000000000000544 [DOI] [PubMed] [Google Scholar]
- 33. Bolat E, Kocamaz E, Kulahcilar Z, et al. Investigation of efficacy of mitomycin-C, sodium hyaluronate and human amniotic fluid in preventing epidural fibrosis and adhesion using a rat laminectomy model. Asian Spine J. 2013;7(4):253–259. 10.4184/asj.2013.7.4.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi HJ, Kim KB, Kwon YM. Effect of amniotic membrane to reduce postlaminectomy epidural adhesion on a rat model. J Korean Neurosurg Soc. 2011;49(6):323–328. 10.3340/jkns.2011.49.6.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cunningham BW, Seiber B, Riggleman JR, Van Horn MR, Bhat A. An investigational study of a dual-layer, chorion-free amnion patch as a protective barrier following lumbar laminectomy in a sheep model. J Tissue Eng Regen Med. 2019;13(9):1664–1671. 10.1002/term.2920 [DOI] [PubMed] [Google Scholar]
- 36. Goldschlager T, Ghosh P, Zannettino A, et al. A comparison of mesenchymal precursor cells and amnion epithelial cells for enhancing cervical interbody fusion in an ovine model. Neurosurgery. 2011;68(4):1025–1034; . 10.1227/NEU.0b013e31820d5375 [DOI] [PubMed] [Google Scholar]
- 37. Kamson S, Smith D. Orthobiologic supplementation improves clinical outcomes following lumbar decompression surgery. J Clin Med Res. 2020;12(2):64–72. 10.14740/jocmr3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kara D, Senol N, Kapucuoglu FN, Tureyen K, Ismailoglu O. Effectiveness of human amniotic fluid and amniotic membrane in preventing spinal epidural fibrosis in an experimental rat model. J Neurol Sci. 2015;32(2):293–302. [Google Scholar]
- 39. Luo TD, Vines JB, Zabarsky ZK, et al. Evaluation of percutaneous intradiscal amniotic suspension allograft in a rabbit model of intervertebral disc degeneration. Spine (Phila Pa 1976). 2019;44(6):E329–E337. 10.1097/BRS.0000000000002851 [DOI] [PubMed] [Google Scholar]
- 40. Oner M, Dulgeroglu TC, Karaman I, Guney A, Kafadar IH, Erdem S. The effects of human amniotic fluid and different bone grafts on vertebral fusion in an experimental rat model. Curr Ther Res Clin Exp. 2015;77:35–39. 10.1016/j.curtheres.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Subach BR, Copay AG. The use of a dehydrated amnion/chorion membrane allograft in patients who subsequently undergo reexploration after posterior lumbar instrumentation. Adv Orthop. 2015;2015:501202. 10.1155/2015/501202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tao H, Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18(8):1202–1212. 10.1007/s00586-009-1013-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walker CT, Godzik J, Kakarla UK, Turner JD, Whiting AC, Nakaji P. Human amniotic membrane for the prevention of intradural spinal cord adhesions: retrospective review of its novel use in a case series of 14 patients. Neurosurgery. 2018;83(5):989–996. 10.1093/neuros/nyx608 [DOI] [PubMed] [Google Scholar]
- 44. Introducing Levels of Evidence to The Journal: JBJS. https://journals.lww.com/jbjsjournal/Fulltext/2003/01000/Introducing_Levels_of_Evidence_to_The_Journal.1.aspx. 10 March 2021.
- 45. Bose B. Burn wound dressing with human amniotic membrane. Ann R Coll Surg Engl. 1979;61(6):444–447. [PMC free article] [PubMed] [Google Scholar]
- 46. Shaw KA, Parada SA, Gloystein DM, Devine JG. The science and clinical applications of placental tissues in spine surgery. Global Spine J. 2018;8(6):629–637. 10.1177/2192568217747573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brubaker DK, Lauffenburger DA. Translating preclinical models to humans. Science. 2020;367(6479):742–743. 10.1126/science.aay8086 [DOI] [PubMed] [Google Scholar]
- 48. CFR - Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=1271. 2 March 2021.
- 49. Huddleston HP, Cohn MR, Haunschild ED, Wong SE, Farr J, Yanke AB. Amniotic product treatments: clinical and basic science evidence. Curr Rev Musculoskelet Med. 2020;13(2):148–154. 10.1007/s12178-020-09614-2 [DOI] [PMC free article] [PubMed] [Google Scholar]