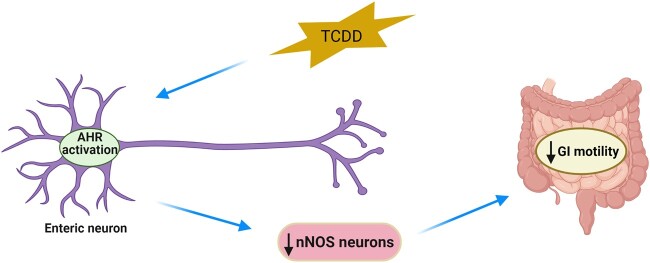
Abstract
Despite progress describing the effects of persistent organic pollutants (POPs) on the central nervous system, the effect of POPs on enteric nervous system (ENS) function remains underexplored. We studied the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a POP, and a potent aryl hydrocarbon receptor (AHR) ligand, on the ENS and intestinal motility in mice. C57Bl/6J mice treated with TCDD (2.4 µg/kg body weight) for 8 weeks (once per week) exhibited significant delay in intestinal motility as shown by reduced stool frequency, prolonged intestinal transit time, and a persistence of dye in the jejunum compared to control mice with maximal dye retention in the ileum. TCDD significantly increased Cyp1a1 expression, an AHR target gene, and reduced the total number of neurons and affected nitrergic neurons in cells isolated from WT mice, but not Ahr−/− mice. In immortalized fetal enteric neuronal cells, TCDD-induced nuclear translocation of AHR as well as increased Cyp1a1 expression. AHR activation did not affect neuronal proliferation. However, AHR activation resulted in enteric neuronal toxicity, specifically, nitrergic neurons. Our results demonstrate that TCDD adversely affects nitrergic neurons and thereby contributes to delayed intestinal motility. These findings suggest that AHR signaling in the ENS may play a role in modulating TCDD-induced gastrointestinal pathophysiology.
Keywords: TCDD, AHR, ENS, nNOS, intestinal transit
Graphical Abstract
Graphical Abstract.
Introduction
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a persistent organic pollutant (POP; Van den Berg et al., 2006) causes reproductive and developmental defects, tumorigenesis, liver fibrosis, immunological dysfunction, and neurotoxicity in animals (Duval et al., 2017; Fling et al., 2020; Gray et al., 1997a,b; Kim and Yang, 2005; Knerr and Schrenk, 2006; Marshall and Kerkvliet, 2010; Tomasini et al., 2012; Zhang et al., 2021). Legare et al., reported impaired brain function resulting from developmental exposure to TCDD (Legare et al., 2000). Peripheral neuropathy and progression of brain atrophy has been reported in Vietnam veterans exposed to Agent Orange/TCDD (Kim et al., 2003; Lee et al., 2022; Yi et al., 2014) raising public health concerns.
The biological and toxicological effects of TCDD are mediated through the activation of the aryl hydrocarbon receptor (AHR), a ligand-activated transcription factor that belongs to the bHLH-PAS family (Nebert, 2017). AHR plays a key role in the development and function of the nervous system. Studies have reported the temporal and spatial patterns of AHR expression in mouse brain (Kimura and Tohyama, 2017; Kimura et al., 2021), and its physiologic and toxicologic significance in the developing brain (Kakeyama and Tohyama, 2003). Research has shown that AHR activation in the brain regulates the expression of several genes that are involved in various processes such as neurogenesis (Latchney et al., 2013), neuronal migration (Kimura et al., 2017), proliferation (Latchney et al., 2011), differentiation (Jung et al., 2009), maturation (Collins et al., 2008), and survival (Juricek and Coumoul, 2018). However, dysregulation of these genes can impact normal development and function of the nervous system. In addition to being found in the mouse brain, AHR expression has also been studied in the developing and adult human and mouse enteric nervous system (ENS) using transcriptome and immunohistochemical analysis (Memic et al., 2018; Obata et al., 2020). These studies have found that AHR is mainly expressed in the colonic ENS, with a relatively weaker signal detected in the neurons of the distal ileum.
Intestinal dysmotility is a hallmark symptom of functional bowel disorders (FBDs), including functional constipation, irritable bowel disease, and functional bloating/distention. A recent multinational study investigating 22 functional gastrointestinal disorders (FGIDs) across 33 countries, found that more than 40% of the surveyed population suffer from FGIDs (Sperber et al., 2021). FBDs were the most common of all included FGIDs, affecting nearly 29% of participants and functional constipation was the most frequently reported FBD (Sperber et al., 2021). Chronic constipation is associated with colorectal cancer, Parkinson’s disease, multiple sclerosis, and mood disorders (Choung et al., 2016). Studies have not focused on the role of POPs in inducing dysmotility in these disease conditions including irritable bowel syndrome (IBS) associated with abdominal discomfort, diarrhea, and constipation. However, a genome-wide association study by Jankipersadsing et al. suggested a role for AHR in the biology of stool frequency, changes of which are one of the hallmarks of IBS (Jankipersadsing et al., 2017). The study was done with two population-based cohorts from the Netherlands (LifeLines-Deep, LLD) and Sweden (PopCol, PC) where the average number of bowel movements per day (BM/d) was extracted from daily records kept by both populations. The study highlighted plausible candidate genes and biological pathways. One of the top 10 loci from the meta-analysis data was the rs1979097 locus containing AHR. The effect of the assessed allele at each locus was positive indicating an effect on the average number of BM/d (increased number of stool passes).
Understanding the impact of AHR signaling in enteric neurons and how AHR activity induced via POPs modulates intestinal motility has not been studied. In this current study, we sought to examine the effects of TCDD on the ENS and intestinal motility in mice. To do this, we used both in vivo and in vitro models. To determine whether TCDD modulates gut motility, we assessed AHR activity in enteric neurons, and associated changes in intestinal motility by treating C57Bl/6J mice with TCDD. We also evaluated the expression of various neuronal subtypes in the myenteric plexus of control and TCDD-treated mice. Immorto fetal enteric neuronal (IM-FEN) cells were used to investigate the effects of TCDD on enteric neuronal cell proliferation and survival. Studies have reported both the relatively high doses (100–1000 nM) of TCDD exposure (Xu et al., 2013a,b) and much lower doses (0.1–10 nM) of TCDD (Latchney et al., 2011) in cultured neuronal cells causing cytotoxicity. In this study, we used 0.1 nM, 1 nM, and 10 nM TCDD doses based on our initial dose-dependent TCDD (10−4 nM to 10 nM) study assessing Cyp1a1 expression. To determine whether the effects of TCDD on enteric neurons observed is through AHR signaling, studies were carried out using primary enteric neuronal culture cells isolated from WT and Ahr−/− mice. Our findings provide new evidence that AHR activation by TCDD in the ENS contributes to delayed intestinal motility by adversely affecting nitrergic neurons.
Materials and methods
Mice
Nine- to 10-week-old male C57Bl/6J mice (Ahr+/+) (Strain No. 000664; Jackson Laboratory, Bar Harbor, ME) were housed (3 or 4 per cage) in temperature- and light-controlled rooms and given water and food ad libitum. Prior to treatment, mice were placed in individual cages and trained to eat dough pills (transgenic bacon-flavored dough diet from Bio-Serv, Flemington, NJ), prepared with a tablet mold, for 5 days. During the treatment, mice were placed in individual cages with a control or a dough pill with TCDD and monitored until the entire dough pill was eaten before placing them back into respective cages. Mice were divided into 2 groups, one for short term (ST) study (5 days), and the other for long-term (LT) study (8 weeks). In the ST study, mice received one dough pill with TCDD (2.4 µg/kg body weight) or a control pill on day 1 and were followed until day 5. During the LT study, mice received one dough pill with TCDD (2.4 µg/kg body weight) or a control pill once per week for 8 weeks. This dosage has been empirically tested on mice to induce dysmotility without affecting survival and effectively induce AHR activation in intestine and colon as assessed by AHR target gene, Cyp1a1 expression. Further, TCDD was provided through the diet as it represents a common exposure route. Tissues were collected immediately following sacrifice by CO2 asphyxiation and stored at −80°C until analysis. Congenic Ahr−/− mice on the C57Bl/6J background were obtained and bred as previously described (Lahoti et al., 2015). All animal studies were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University and were performed according to the university guidelines for the ethical treatment of animals.
Assessment of colonic emptying by stool frequency
Stool frequency (number of stool pellets extruded per mouse per hour) was measured in both ST and LT, vehicle and TCDD-treated mice as described previously (Li et al., 2006). Briefly, each mouse was placed in a clean cage without bedding material for 1 h. Fecal pellets collected immediately after expulsion were placed in sealed microcentrifuge tubes to avoid evaporation. Tubes were weighed to obtain the wet weight of the stool, which were then dried overnight at 65°C and reweighed to obtain the dry weight.
Total GI transit time
Mice treated with vehicle and TCDD for LT were gavaged with 300 µl of 6% (w/v) carmine red dye (Sigma-Aldrich, Burlington, MA) in 0.5% (w/v) methylcellulose (Sigma-Aldrich, Burlington, MA) and the time from gavage until the emergence of the first red-color pellet was used an index of total GI transit time (Li et al., 2011).
Intestinal transit assessment
Mice treated with vehicle or TCDD for LT were fasted for 6 h, followed by gavage with 70 kDa fluorescein isothiocyanate-dextran (FITC-Dextran) (Sigma-Aldrich, Burlington, MA). After 1 h mice were sacrificed, the small intestine was evenly cut into 8 segments and the colon was cut into 2 segments. The level of fluorescence in these segments including the cecum was determined as described (Woting and Blaut, 2018). Transit was analyzed using the intestinal geometric center of the distribution of FITC-dextran in the distal ileum and was calculated as described previously (Miller et al., 1981).
Whole mount tissue staining
Longitudinal muscle strips with intact myenteric ganglia (longitudinal muscle myenteric plexus, LMMP), from the proximal colon of the mice treated with vehicle and TCDD (LT), were used for nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase and acetylcholinesterase (ACh) staining and was performed as described previously (Anitha et al., 2006). Five randomly selected fields per mouse were evaluated from 3 mice per treatment group. LMMP from the distal colon were fixed in 4% paraformaldehyde and immunostaining protocol for TUJ1, nNOS, and ChAT was followed as previously published (Anitha et al., 2012). The number of neurons stained for a specific marker was determined per unit area. At least 10 random fields were scored in a blinded fashion.
Cell culture
Immorto fetal enteric neuronal cells (Anitha et al., 2008) were used for in vitro experiments. Cells were cultured in modified N2 medium (Heuckeroth et al., 1998) containing 10% fetal bovine serum (FBS), glial cell line-derived neurotrophic factor (GDNF) (10 ng/mL) (Shenandoah Biotechnology, Inc. Warminster, PA), and 20 U/mL recombinant mouse interferon-γ (MilliporeSigma, Burlington, MA) in a humidified tissue culture incubator containing 10% CO2 at the permissive temperature of 33°C for 24 to 48 h. At this temperature, cells proliferate until confluent monolayers are formed. Then the medium was changed to neurobasal-A medium (NBM) containing B-27 serum-free supplement (Thermo Fisher Scientific, Waltham, MA), 1 mmol/L glutamine, 1% FBS, and 10 ng/mL GDNF, and transferred to an atmosphere of 5% CO2 at 39°C. Cells were allowed to differentiate and then treated with various doses of TCDD (0.1, 1, and 10 nM) for 24 h.
Primary culture preparation
Primary culture of mouse ileum and colonic myenteric neurons was prepared using previously published protocols (Smith et al., 2013; Zhang and Hu, 2013) from 9-week-old C57Bl/6J (WT) and Ahr–/– mice. Myenteric neurons were seeded onto poly-D lysine and laminin-coated plates (Corning, NY) and cultured at 37°C with 5% CO2 in complete NBM prepared as described above. Half of the medium was replaced every 24 h, and after 5 days, the neurons were treated with 0.1 and 1 nM TCDD for 24 h.
BrdU cell proliferation assay
Immorto fetal enteric neuronal cells cultured at 33°C were treated with varying doses of TCDD (0.1, 1, and 10 nM) for 24 h. BrdU was added 3 h prior to the end of the TCDD treatment incubation period. Assay steps were followed according to the instructions provided by the manufacturer (BrdU Cell Proliferation Assay kit from MilliporeSigma, Burlington, MA).
LDH-Glo cytotoxicity assay
Immorto fetal enteric neuronal cells were cultured in 96-well white plates with clear bottoms at 39°C and treated with varying doses of TCDD (0.1, 1, and 10 nM) in complete NBM for 24 h. The LDH-Glo Cytotoxicity Assay kit from Promega (Madison, WI) was used to measure the percentage cytotoxicity according to the manufacturer’s instructions.
Caspase-Glo 3/7 assay
Immorto fetal enteric neuronal cells were cultured in 96-well white plates with clear bottoms at 39°C and treated with various doses of TCDD (0.1, 1, and 10 nM) in complete NBM for 24 h. Caspase 3/7 activity was measured using the Caspase-Glo 3/7 Assay kit from Promega (Madison, WI) following the manufacturer’s protocol. Luminometer readings were taken 1 h after adding the Caspase-Glo 3/7 Reagent.
Real-time PCR
Total RNA from vehicle and TCDD (0.1, 1, and 10 nM) treated IM-FEN cells, cultured primary myenteric neurons from the ileum and colon, and snap frozen sections of the ileum and colon LMMP was extracted using TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s specifications. Complementary DNA (cDNA) was synthesized from 1 µg of total RNA using the qScript cDNA SuperMix (Quantabio, Beverly, MA). Quantitative real-time PCR was performed with cDNA using PowerUp SYBR Green Master Mix (Applied Biosystems, Waltham, MA) on QuantStudio 3 Real-Time PCR System (Applied Biosystems, Waltham, MA) using primers listed in Table 1. Ct values obtained were normalized to GAPDH.
Table 1.
Primers used for real-time PCR
| Gene | Forward primer (5′→3′) | Reverse primer (5′→3′) |
|---|---|---|
| Cyp1a1 | CTC TTC CCT GGA TGC CTT GAA | GGA TGT GGC CCT TCT CAA ATG |
| Tubb3 | AACCATGGACAGTGTTCGGTCT | TATAGTGCCCTTTGGCCCAGTT |
| Nos1 | AGGAGGATGCTGGTGTGTTC | CTCAGATCTAAGGCGGTTGG |
| Chat | CCCTCCAGCTGGCTTACTAC | CAGGAGTGGCCGATCTGATG |
| Syp | TTGGCTTCGTGAAGGTGCTGCA | ACTCTCCGTCTTGTTGGCACAC |
| B2M | CATGGCTCGCTCGGTGAC | CAGTTCAGTATGTTCGGCTTCC |
| GAPDH | TTGTGATGGGTGTGAACCACGA | TCTTCTGGGTGGCAGTGATGG |
Western blotting
Cell lysates were obtained from IM-FEN cells treated with or without TCDD (0.1, 1, and 10 nM) for 24 h and 15 µg of protein/lane were loaded onto a 4–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA) and transferred to PVDF membrane. The membranes were probed for neuronal marker β-tubulin III (TUJ1), neuronal nitric oxide synthase (nNOS), and choline acetyltransferase (ChAT) using respective specific antibodies (Table 2). GAPDH was used as a loading control. A semiquantitative measurement of the band intensity was performed using the Scion Image computer software program (Scion Corporation, Frederick, MD) and expressed as a ratio of band intensity with respect to the loading control.
Table 2.
Antibodies used in western blot (WB) and Immunofluorescence (IF)
| Antibody/host | Company | Cat. No. | Dilution |
|---|---|---|---|
| AHR/Rabbit | Enzo Life Sciences (Farmingdale, New York) | BML-SA550-0100 | 1:1000 (WB), 1:200 (IF) |
| TUJ1/Mouse | abcam (Waltham, Massachusetts) | ab78078 | 1:1000 (WB), 1:500 (IF) |
| nNOS/Rabbit | abcam (Waltham, Massachusetts) | ab76067 | 1:2000 (WB), 1:500 (IF) |
| ChAT/Goat | MilliporeSigma (Burlington, Massachusetts) | AB144P | 1:400 (WB), 1:100 (IF) |
| Histone H3/Rabbit | Cell Signaling Technology (Danvers, Massachusetts) | 9715 | 1:2000 (WB) |
| GAPDH/Rabbit | Cell Signaling Technology (Danvers, Massachusetts) | 2118 | 1:2500 (WB) |
| HRP-conjugated anti-rabbit | Cell Signaling Technology (Danvers, Massachusetts) | 7074 | 1:2000-5000 (WB) |
| HRP-conjugated anti-mouse | Cell Signaling Technology (Danvers, Massachusetts) | 7076 | 1:2000 (WB) |
| HRP-conjugated anti-goat | R&D Systems (Minneapolis, Minnesota) | HAF017 | 1:800 (WB) |
| Donkey anti-Rabbit, Alexa Fluor 594 | Invitrogen (Waltham, Massachusetts) | A21207 | 1:200 (IF) |
| Donkey anti-Goat, Alexa Fluor 594 | Invitrogen (Waltham, Massachusetts) | A32758 | 1:200 (IF) |
| Donkey anti-Mouse, Alexa Fluor 488 | Invitrogen (Waltham, Massachusetts) | A21202 | 1:200 (IF) |
Nuclear and cytoplasm extraction
Nuclear and cytosolic extracts from IM-FEN cells treated with or without TCDD (0.1, 1, and 10 nM) for 30 min were prepared as previously described (Muku et al., 2017). Briefly, cell pellets were resuspended in MENG (25 mM MOPS, 1 mM EDTA, 0.02% sodium azide, 10% glycerol) including protease inhibitors and homogenized with a stainless-steel Dura-Grind Dounce homogenizer (Wheaton Instruments, Millville, NJ). Cell homogenates were centrifuged at 1000 × g for 20 min. The supernatant was then subjected to centrifugation at 42,000 × g for 30 min to generate cytosolic extracts. The nuclear pellet was washed 3 times with MENG, extracted with MENG + 500 mM NaCl and the extract collected after centrifugation at 42,000 × g for 30 min. The cytosolic and nuclear extracts were then analyzed for AHR by western blotting. The Histone H3 and GAPDH were used as nuclear and cytoplasmic loading controls, respectively.
Immunocytochemistry
Immorto fetal enteric neuronal cells were treated with vehicle, 1 nM TCDD with or without 10 µM CH-223191, a potent and specific antagonist of AHR for 24 h. Cells were pretreated with 10 µM CH-223191 for 1 h prior to the addition of TCDD. Cells were fixed using 4% paraformaldehyde (30 min) and permeabilized (10 min) with 0.3% Triton X-100. This was followed by blocking the cells with 3% normal donkey serum (1 h) and overnight incubation with different primary antibodies (AHR, TUJ1, and nNOS, Table 2). Secondary detection was performed in conjunction with Alexa Fluor 594 or 488. DAPI (AnaSpec, Fremont, CA) was used to counterstain the nucleus. As a negative control, the primary antibody was omitted in certain wells. At least 2 wells (200 cells/well) were scored in a blinded fashion for each condition in each of the experiments and the experiment was repeated 3 times. Images were captured on a Nikon ECLIPES 50i microscope with the NIS Elements imaging Software (Melville, NY).
Immunofluorescence staining of primary culture cells
Myenteric neurons from WT and Ahr–/– mice were treated with 1 nM TCDD for 24 h at 37°C. Cells were fixed with 4% paraformaldehyde for 20 min, blocked for 1 h in blocking and permeabilization solution containing 0.3% Triton X-100 and 5% BSA (MilliporeSigma, Burlington, MA) in PBS followed by 2 h incubation with primary antibodies (TUJ1, nNOS, and ChAT, Table 2) at room temperature. Secondary detection was performed in conjunction with Alexa Fluor 594 or 488. For quantitation, fluorescence intensity of the images was measured using the ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical analysis
Graph Pad Prism 9.1.1 version software (GraphPad, San Diego, CA) was used to analyze the data. Statistics was done with the student’s t test for 2 treatment groups, or with one-way analysis of variance (ANOVA) for multiple data sets, followed by Tukey’s multiple comparison test. Results are expressed as mean ± SEM. A p value of .05 or less was considered statistically significant.
Results
TCDD-treated mice exhibit delayed intestinal motility
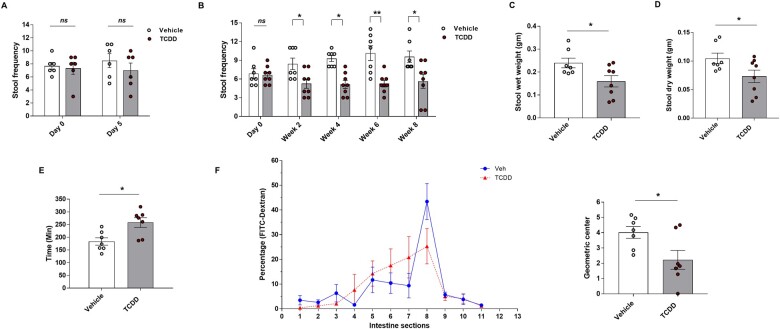
We investigated the physiological role of AHR in the ENS following exposure to TCDD. We used a mouse model to determine whether AHR signaling activated by TCDD in enteric neurons regulates gut motility. Ten-week-old C57Bl/6J mice were fed with control or TCDD pills (2.4 µg/kg body weight) for 5 days (ST) and 8 weeks (LT). We first tested the gut motility by stool frequency test where the frequency of bowel movements was measured as an average number of fecal pellets passed per hour in both ST and LT treatment groups. No significant reduction in the pellet frequency was observed in ST-treated mice (Fig. 1A). However, in LT-treated mice there was a moderate reduction in stool number at week 2 and a significant reduction by weeks 6 and 8 in TCDD-treated mice compared to control mice (Fig. 1B). Accordingly, the wet weight and the dry weight of stool pellets from week 8 were also significantly lower in TCDD-treated mice (p < .05; Fig. 1C and D). The total intestinal transit time (ITT) in LT-treated mice was measured by recording the time for expulsion of the first red stool pellet after gavage of the nonabsorbable red carmine dye. The total ITT was found to be significantly longer in TCDD-treated mice compared to control mice (Fig. 1E) (p < .05). Further, intestinal transit (measured as a relative distribution of FITC-Dextran fluorescence) was also found to be significantly delayed in LT TCDD-treated mice, as noted from a significantly reduced intestinal geometric center (p < .05; Fig. 1F). Control mice exhibited maximal FITC fluorescence retention within ileal segment, whereas TCDD-treated mice displayed a broader pattern of FITC fluorescence in jejunum and ileum. These results suggest that TCDD treatment resulted in delayed intestinal motility in mice.
Figure 1.
Delayed intestinal transit in C57Bl/6J mice treated with TCDD (2.4 µg/kg body weight). Ten-week-old C57Bl/6J mice were fed with transgenic dough pill with either vehicle for the control group or TCDD for the treatment group, for 5 days (ST) and 8 weeks (LT). GI motility was assessed by stool frequency (number of stool pellets per hour per mouse) test in both short-term and LT groups, total GI transit time (time for expulsion of the first red stool pellet after gavage of the red carmine dye) and 70-KDa FITC-Dextran distribution (assess the distribution of FITC-Dextran in the segments of the small intestine (1–8), cecum (9), and colon (10 and 11)), in LT group mice. A, Stool frequency in ST-treated mice. B–D, Stool frequency, wet weight, and dry weight of LT-treated mice. E, Whole gut transit by carmine red dye test on mice from LT study. F, FITC-Dextran distribution in LT group mice treated for 8 weeks, n = 7–8 mice. Results are mean ± SEM, *p < .05; **p < .01.
Delayed intestinal motility induced by TCDD is associated with decreased number of nNOS neurons in the colon
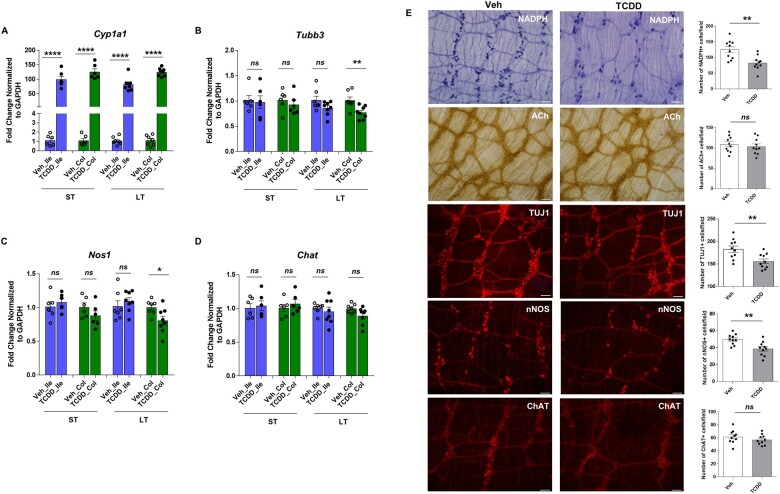
AHR signaling in the ileum and colon myenteric plexi of both ST- and LT-treated mice was examined by assessing Cyp1a1 expression by qPCR. TCDD-induced Cyp1a1 expression significantly in both ST and LT tissues compared to control mice (p < .0001; Fig. 2A). We next assessed the total number of neurons and the type of neurons affected by TCDD in both ST and LT colon and ileum myenteric plexi by qPCR. TCDD induced significant reduction in Tubb3 (gene symbol for TUJ1, representing total neurons, p < .01) and Nos1 (gene symbol for nNOS) gene expression (p < .05) in LT colonic myenteric plexi. There was no change in the Tubb3 and Nos1 expression in ST colon and Ileum, and LT ileum in control and TCDD-treated mice (Fig. 2B and C). Chat (gene symbol for ChAT) expression was not affected by TCDD in both ST and LT colon and ileum myenteric plexi (Fig. 2D). Consistent with this observation, we found a reduction in nitrergic neurons stained with NADPH diaphorase (proximal colon) or nNOS (distal colon) specific antibody, and the total number of neurons (neuronal marker, TUJ1) in TCDD-treated mice (LT) compared with control mice, with no difference in cholinergic neurons assessed by acetylcholine (ACh) or ChAT staining (Fig. 2E). Our in vivo data demonstrated a role for AHR signaling in modulating intestinal motility and influencing nNOS neurons in response to environmental toxicant TCDD.
Figure 2.
TCDD induces activation of AHR signaling in the intestine and colon of mice treated with TCDD and affects colonic nitrergic neurons. RNA was extracted from the myenteric plexi of ileum, and colon of ST and LT-treated mice and qPCR performed to assess the effect of TCDD on gene expression of (A) Cyp1a1, and (B–D) Tubb3, Nos1 and Chat. E, Representative photographs and histograms for proximal colon whole mount staining for NADPH diaphorase; ACh; and distal colon whole mount staining for TUJ1, nNOS, and ChAT from LT group mice treated for 8 weeks, are shown. The number of neurons stained for a specific marker was determined per unit area. At least 10 random fields were scored in a blinded fashion. Scale bar, 50 µm. n = 7–8 mice. Results are mean ± SEM, *p < .05; **p < .01; ****p < .0001.
AHR activation by TCDD leads to loss of nNOS neurons
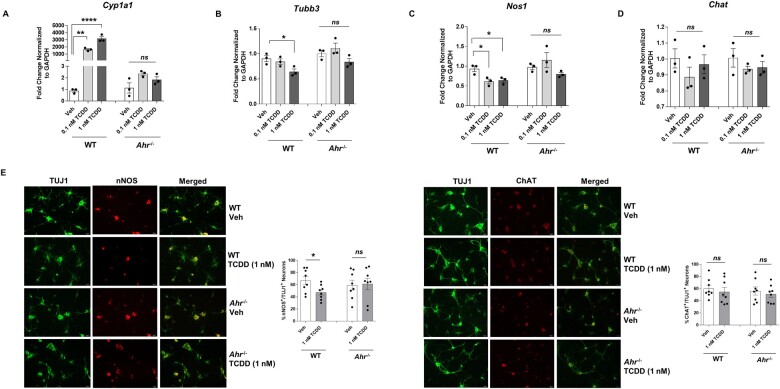
To verify whether reduction in nitrergic neurons is due to TCDD-induced AHR activation, we compared the effects of TCDD on enteric neuronal primary culture cells isolated from WT and Ahr−/− mice. Cells were treated with either vehicle or TCDD (0.1 and 1 nM) for 24 h. Lack of AHR activity in Ahr−/− mice was demonstrated by assessing Cyp1a1 expression by qPCR. TCDD stimulates Cyp1a1 expression in primary culture cells isolated from WT mice but not the cells isolated from Ahr−/− mice (Fig. 3A). We next assessed the effect of TCDD on enteric neuronal numbers and neuronal subtypes by qPCR. 1 nM TCDD significantly reduced the total number of neurons (Tubb3) and affected nitrergic neurons (Nos1) in cells isolated from WT mice (Fig. 3B and C), but not Ahr−/− mice. No change was noted in the expression of Chat in cells from both mice (Fig. 3D). qPCR data was validated by immunostaining of primary culture cells for nNOS and ChAT costained with neuronal marker TUJ1 (Fig. 3E). These results confirmed the involvement of AHR in reducing nitrergic neurons upon activation by TCDD.
Figure 3.
TCDD stimulates Cyp1a1 expression in primary enteric neuronal culture cells isolated from WT mice and affects enteric neurons but not the cells isolated from Ahr−/− mice. Enteric neuronal primary culture cells isolated from WT and Ahr−/− mice were treated with either vehicle or TCDD (0.1 and 1 nM) for 24 h. Gene expression of (A) Cyp1a1, and (B–D) Tubb3, Nos1, and Chat by qPCR. E, Representative photographs and histograms from the immunostaining of primary culture cells for nNOS and ChAT costained with neuronal marker TUJ1. Scale bar, 50 µm. n = 3. Results are mean ± SEM, *p < .05; **p < .01; ****p < .0001.
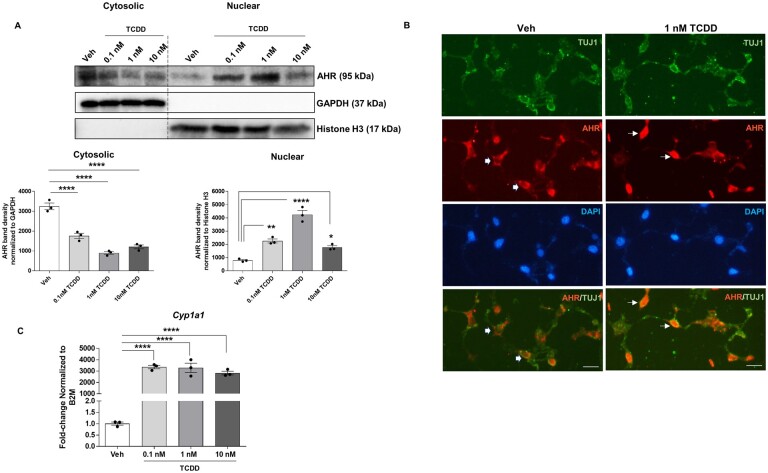
TCDD induces activation of AHR signaling in IM-FEN cells
We first examined the protein levels of AHR in subcellular fractions of IM-FEN cells treated with various doses of TCDD (0.1, 1, and 10 nM) for 30 min by immunoblot analyses. We observed that exposure of cells to TCDD induced a significant dose-response shift in AHR protein from the cytosolic to the nuclear compartment (Fig. 4A). The decreased level of nuclear AHR following 10 nM TCDD treatment vs 1 nM could be due to increased proteasomal degradation. We validated this observation by immunostaining the cells with AHR and neuronal marker TUJ1. Neurons treated with 1 nM TCDD exhibited more nuclear signal indicating AHR translocation into nucleus compared to vehicle (Fig. 4B). In addition, qPCR analysis revealed that the Cyp1a1 expression in cells treated with all three doses of TCDD was significantly increased (∼3000-fold, p < .0001) compared to vehicle-treated cells (Fig. 4C). These results indicate that IM-FEN cells express AHR and TCDD induces AHR activation in these cells.
Figure 4.
TCDD induces translocation of AHR to the nucleus and target gene Cyp1a1 expression in IM-FEN cells. A, Western blot for AHR protein expression in the cytoplasmic and nuclear fractions of cells treated with TCDD (0.1, 1, and 10 nM) for 30 min, showing its nuclear translocation upon activation by TCDD. B, Representative images of AHR (red), TUJ1 (green), and DAPI (blue) immunostaining of IM-FEN cells treated with 1 nM TCDD for 24 h. Thick arrows point to cytoplasmic and line arrow point to nuclear (arrow) signals. C, Cyp1a1 gene expression in cells treated with various doses of TCDD (0.1, 1, and 10 nM) for 24 h by qPCR. Scale bar, 50 µm. Similar results were obtained in 3 independent experiments. Results are mean ± SEM, *p < .05; **p < .01; ****p < .0001.
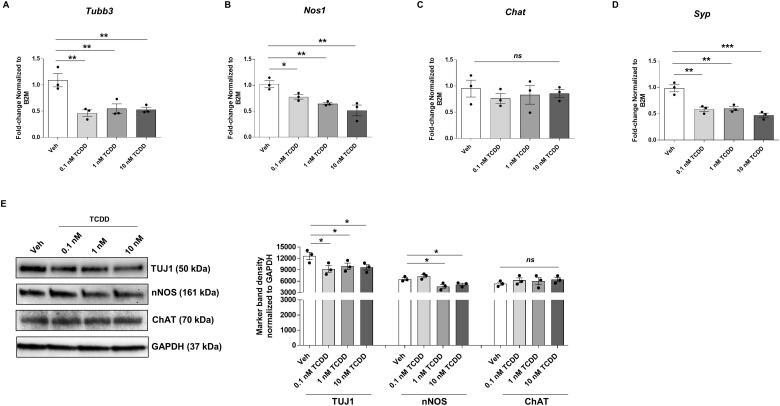
TCDD affects IM-FEN cells with reduced nNOS and synaptophysin expression
Next, we investigated the effect of TCDD on total number of neurons and subtypes by examining changes in neuronal marker TUJ1 and neuronal subtypes like nNOS and ChAT, and synaptic marker synaptophysin. TCDD treatment for 24 h caused a significant decrease in the expression of neuronal marker Tubb3 (p < .01; Fig. 5A) and a dose-dependent reduction in Nos1 and Syp (gene symbol for synaptophysin) RNA expression (Fig. 5B and D) while Chat expression was unchanged (Fig. 5C) compared to vehicle. This data was supported by western blot analysis with similar results (Fig. 5E), although 0.1 nM TCDD did not affect nNOS protein expression in these cells.
Figure 5.
TCDD affects IM-FEN cells and alters nNOS and synaptophysin expression. Cells were treated with vehicle or various doses of TCDD (0.1, 1, and 10 nM) for 24 h. qPCR was performed to assess the effect of TCDD on total number of neurons and subtypes by examining changes in neuronal marker beta Tubulin 3 (Tubb3), and neuronal subtypes like neuronal nitric oxide synthase (Nos1), Chat, and synaptic neuron marker synaptophysin (Syp). A–D, Gene expression of Tubb3, Nos1, Chat, and Syp by qPCR. E, Representative Western blot for TUJ1, nNOS, ChAT, and the graphical representation. Similar results were obtained in 3 independent experiments. Results are mean ± SEM, *p < .05; **p < .01; ***p < .001.
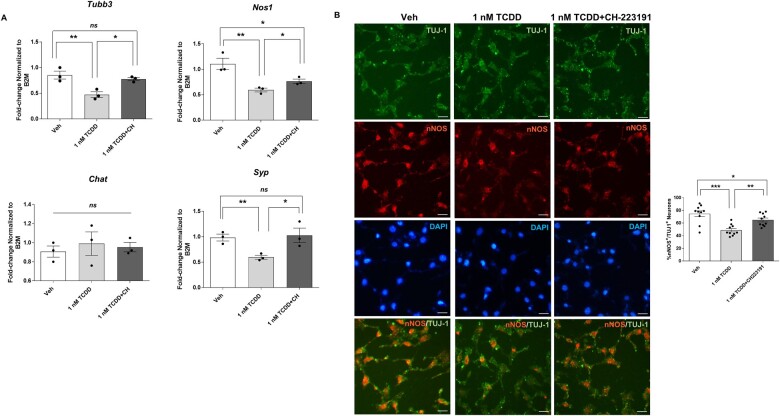
Reduced expression of neuronal markers in IM-FEN cells exposed to TCDD is AHR dependent
CH-223191 has been shown to preferentially inhibit AHR-TCDD binding and subsequent AHR-dependent gene expression (Zhao et al., 2010). To explore the involvement of AHR in TCDD-elicited suppression of neuronal marker expression, cells were pretreated with an AHR antagonist, CH-223191 (10 µM) for an hour and then treated with 1 nM TCDD for 24 h. qPCR was performed to assess the changes in the neuronal marker Tubb3, and neuronal subtypes like Nos1, Chat, and Syp (synaptic marker). TCDD reduced Tubb3, Nos1, and Syp expression significantly (p < .01) and had no effect on the expression of cholinergic neurons, Chat. CH-223191 reversed the inhibition of expression of Tubb3, Nos1, and Syp induced by TCDD (Fig. 6A) indicating that AHR was involved in the TCDD-induced effect. Similar results were obtained with immunocytochemistry of IM-FEN cells treated with 1 nM TCDD in the presence or absence of CH-223191 for nNOS. CH-223191 partially reversed the effect of TCDD on nNOS protein expression (Fig. 6B). These results confirm the involvement of AHR signaling in affecting neuronal subtypes and synaptic protein upon exposure to TCDD.
Figure 6.
Reduced expression of neuronal markers in IM-FEN cells exposed to TCDD is AHR dependent. Cells were pretreated with an AHR antagonist, CH-223191 (10 µM) for an hour and then treated with 1 nM TCDD for 24 h. A, Real-time PCR was done to assess the changes in Tubb3, Nos1, Chat, and Syp (synaptic marker). B, Representative images of neuronal marker TUJ-1 (green), nNOS (red), and DAPI (blue) immunostaining in IM-FEN cells treated with 1 nM TCDD in the presence or absence of 10 µM CH-223191. Data represent three independent experiments. Scale bar, 50 µm. Results are mean ± SEM, *p < .05; **p < .01; ***p < .001.
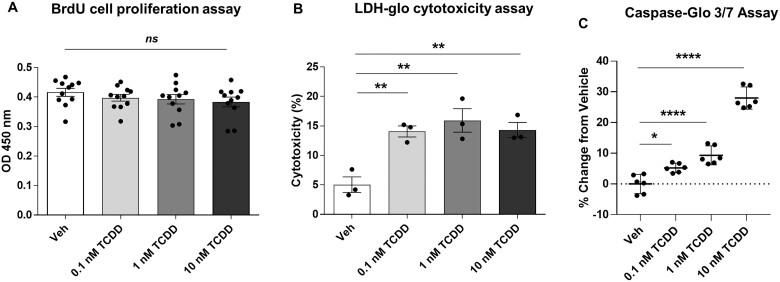
TCDD affects IM-FEN cell survival, but not proliferation by eliciting apoptosis in IM-FEN cells
Our in vivo findings demonstrated a role for TCDD-induced AHR signaling in modulating intestinal motility and influencing nNOS neurons. We sought to determine whether the reduction in neuronal marker expression is due to reduced cell proliferation or cell survival. We used IM-FEN cells to examine the effect of TCDD (0.1, 1, and 10 nM) on enteric neuronal cell proliferation and survival. We have previously shown that IM-FEN cells cultured at the permissive temperature, 33°C proliferate until confluent monolayers were formed and then differentiate at 39°C (Anitha et al., 2008). Cell proliferation was assessed by BrdU labeling as described in the Methods section. There was no significant change in the BrdU labeling among treated cells ruling out the role of TCDD on cell proliferation (Fig. 7A). Cytotoxicity in cells treated with TCDD for 24 h was assessed by the lactate dehydrogenase (LDH) release assay, a well-established assay for cell viability. All three doses of TCDD induced a statistically significant increase of LDH release in the culture medium of IM-FEN cells (p < .01; Fig. 7B) demonstrating the potential of TCDD to promote neuron cell death. Having observed the cytotoxic effect of TCDD, we next assessed the susceptibility of IM-FEN cells to apoptosis upon exposure to TCDD by looking at the activity of caspase 3/7, an important executioner of the apoptosis process. TCDD (1 and 10 nM) caused a significant increase (p < .0001; Fig. 7C) in caspase 3/7 activity compared to the vehicle-treated cells. These data suggest that TCDD can affect cell survival by inducing apoptosis in enteric neurons.
Figure 7.
TCDD affects IM-FEN cell survival, but not proliferation by eliciting apoptosis in IM-FEN cells. Cells were treated with vehicle or various doses of TCDD (0.1, 1, and 10 nM) for 24 h. A, BrdU labeling of IM-FEN cells grown at the permissive temperature (33°C) in medium containing interferon-γ. BrdU was added to the medium 3 h before fixation. The OD reading corresponds to the BrdU concentration in the cells. B, Cytotoxicity assessed by LDH release assay. C, Cell death by apoptosis assessed by measuring Caspase 3/7 activity 1 h after adding the Caspase-Glo 3/7 Reagent. Results are mean ± SEM, *p < .05; **p < .01; ****p < .0001.
Discussion
Our studies show delayed intestinal motility in LT TCDD-treated mice as demonstrated by reduced stool frequency, increased total GI transit time, and delayed intestinal transit compared to control mice. Our results from enteric neuronal primary cells, isolated from WT and Ahr−/− mice, confirmed the involvement of AHR in reducing the total number of neurons and affecting nitrergic neurons upon activation by TCDD. We also found that TCDD-induced cytotoxicity in IM-FEN cells by eliciting apoptosis. Taken together, our findings suggest AHR activation by TCDD in enteric neurons has an influential role in modulating the survival of nitrergic neurons and intestinal motility.
AHR is a pleiotropic signal transductor and is responsive to several exogenous and endogenous molecules including polycyclic aromatic hydrocarbons (PAHs). The major exposure route for humans to PAHs is dietary sources and up to 70% of PAH exposure for a nonsmoking person can be associated with their diet (Rengarajan et al., 2015). Epidemiological studies have indicated that the increasing cancer prevalence can be partly attributed to PAHs exposure (Harris et al., 2013; Rengarajan et al., 2015; Sampaio et al., 2021). A recent human study reported an association between PAH exposure and diarrhea in adults (Wu et al., 2021). Additionally, recent studies have shown that the dietary and microbial means can increase the amount of health-promoting AHR ligands to manage inflammatory bowel diseases and prevent colon cancer (Han et al., 2021).
In the 50 years since AHR was discovered as a cytosolic receptor and ligand-activated transcription factor for TCDD in mammalian tissues and cells, our knowledge of the physiologic role AHR plays in determining health and disease has burgeoned. Our findings build on previous studies that demonstrated the role of AHR in gut motility (Obata et al., 2020). The ENS is composed of neurons and glial cells organized into two intrinsic neural networks, the myenteric plexus and the submucosal plexus within the gut wall, regulating various processes such as blood flow, fluid exchange, and peristalsis (Furness, 2012). We found AHR activity in the intestine and colon as indicated by Cyp1a1 expression, although we did not look at AHR expression in these tissues. Obata et al. showed the Ahr deletion in enteric neurons, or neuron-specific constitutive overexpression of Cyp1a1 reduced peristaltic activity in the colon of mice (Obata et al., 2020) and obtained similar results in microbiota-depleted specific-pathogen-free mice. The authors attributed this delayed motility to microbiota- and ligand-dependent activation of AHR signaling in colonic neurons. Recent work has shown decreased motility in the small intestine of global Ahr−/− mice (Zhou et al., 2023) and the authors attributed this dysmotility to dietary, rather than microbial, metabolite-mediated AHR activation. Although the above studies appear to happen due to temporal and regional factors, in our current study, we noticed delayed motility in the small intestine, based on intestinal transit assessment using 70 kDa FITC-dextran study (1 h), as well as in the colon, based on the stool frequency test and total GI transit time using carmine red dye, in mice with LT TCDD treatment. These mice were on standard chow diet and treatment was for an extended period of 8 weeks during which we can expect ligand- and microbiota-dependent activation of AHR and signaling in intestine and colon. In addition, TCDD likely induced Cyp1a1 expression significantly in mouse ileum and colonic LMMP, enough to act as negative feedback regulator (Schiering et al., 2017) in enteric neurons which might phenocopy the effect of neuron-specific or global deletion of Ahr on intestinal motility, as observed by Obata et al. and Zhou et al. However, reduced expression of Tubb3 and Nos1 was evident only in colonic myenteric neurons of TCDD-treated mice. Intestinal motility is controlled by enteric nitrergic or inhibitory (relaxation) and cholinergic or excitatory (contraction) motor neurons that innervate the layers of smooth muscle. Loss of nNOS neurons can lead to altered coordination between cholinergic and nitrergic neurons thereby contributing to a hypercontractility, abnormal peristalsis, and subsequent constipation (Anitha et al., 2012; Brookes, 1993). Most of the effects of NO, the main enteric inhibitory neurotransmitter responsible for GI relaxation (Sanders and Ward, 2019) are mediated by NO-sensitive guanylate cyclase (NO-GC) and further transduced by cGMP-dependent mechanisms. Studies with knockout animal models have reported NO/cGMP as the main signaling pathway which influences GI motility (Dhaese et al., 2009; Groneberg et al., 2016). By regulating the expression of guanylate cyclases, AHR may indirectly influence the signaling pathways that are activated by NO. In Caenorhabditis elegans, Qin et al., have shown that the AHR ortholog, AHR-1 controls the expression of soluble guanylate cyclase genes in the URX neurons and regulates aggregation behavior (Qin et al., 2006). However, the role of AHR in the sensing of NO and the mechanisms by which AHR regulates the expression of guanylate cyclases is still not fully understood. We report that AHR activation by TCDD leads to loss of nNOS-expressing neurons which might result in reduced production of nitric oxide (NO) thereby affecting the cGMP/guanylyl cyclase (Gcy) signaling pathways. Further studies are warranted to investigate potential connections between AHR and NO/cGMP signaling pathways.
Our results from primary enteric neuronal culture cells isolated from the ileum and colons of mice treated with TCDD, suggest reduction in Tubb3 and Nos1 expression is due to AHR signaling induced by TCDD in the myenteric neurons. Although AHR acts as a regulator of peristatic activity under homeostatic conditions, its function in the ENS following exposure to environmental POPs has not been extensively studied. Fader et al., report an increase in whole gut transit time and intestinal permeability in TCDD-treated mice compared to control mice (Fader et al., 2017). They observed decreased gut motility in accordance with increased intestinal reabsorption and decreased fecal excretion of bile acids in TCDD-treated mice. To our knowledge, our study is the first linking AHR-mediated modulation of the ENS upon activation by TCDD and delayed intestinal motility in mice.
Sexual dimorphism in response to toxic substances may occur due to differences in physical, physiological, or behavioral characteristics, as well as the specific toxicant and dose. For instance, xenobiotics like TCDD can interact with AHR differently in male and female animals. A dose of TCDD that was lethal to wild-type male mice was not lethal to wild-type females or to Cyp1a1−/− males (Uno, 2004). Sex-related differences have been noticed in common functional gastroenterological disorders including IBS where women are more likely to have severe symptoms including constipation or bloating, and diarrhea than men (Narayanan et al., 2021). Mice studies have shown sexual dimorphism in gut motility (Soni et al., 2019). Including both male and female sexes in experiments could help better understand differences in drug responses between males and females, disease pathogeneses, and thus improve treatment strategies. Although our studies were done with male mice to avoid sex as a confounding factor, it is important for future studies to examine female mice.
The impact of TCDD on neurogenesis, survival, proliferation, and differentiation of neurons has been extensively studied in the CNS (Jung et al., 2009; Juricek and Coumoul, 2018; Latchney et al., 2011, 2013) but not in the ENS. We used various doses of TCDD (0.1, 1, and 10 nM) for in vitro study using IM-FEN cells. These cells express AHR and TCDD induces nuclear translocation of AHR with significant induction of Cyp1a1 expression, demonstrating their suitability for studying AHR signaling. Further, TCDD exposure affected IM-FEN cells as noted by reduced neuronal marker TUJ1, nNOS, and the synaptic marker synaptophysin. We report that TCDD affected the enteric neurons at a lower dose of 0.1 nM TCDD. CH-223191, an AHR antagonist reversed this effect indicating the involvement of AHR in TCDD-elicited suppression of nitrergic neuron expression and the synaptic marker showing its importance in the organization of the ENS during development and injury. When we investigated the cause of this reduced expression, it was evident that TCDD-induced cytotoxicity in these cells. Studies have shown TCDD induced activation of apoptotic signals in NGF-differentiated pheochromocytoma (dPC12) cells, mouse cerebellar granule cells, primary cultures of cerebral cortical neurons, and zebrafish larvae (Hill, 2003; Sánchez-Martín et al., 2010, 2011; Tomasini et al., 2012). We did not observe any effect of TCDD on IM-FEN cell proliferation as opposed to reduced proliferation seen in primary neural precursor cells isolated from the ventral forebrain of embryonic mice and SK-N-SH human neuronal cells (Jin et al., 2004; Latchney et al., 2011). Studies have shown synaptopathy, disruptions in synaptic structure and function, as the initial event preceding subsequent neurodegeneration diseases like Alzheimer’s and Parkinson’s disease (Bae and Kim, 2017). Synaptophysin immunohistochemistry has shown changed intestinal distributions of synaptophysin-positive synaptic vesicles in intestinal motility disorders (Dzienis-Koronkiewicz et al., 2005). We have previously shown cultured IM-FEN cells expressing synaptic protein synaptophysin (Anitha et al., 2008). Reduced expression of synaptophysin was observed with TCDD treatment suggesting TCDD effect may be associated with enteric neuropathies thereby contributing to the pathogenesis of intestinal motility disorders.
Taken together, our findings suggest that optimum AHR activation is needed for normal intestinal motility and physiology and excess activation leads to damage to neurons. Future studies will need to identify the potential target and effector genes of AHR activation in the ENS and examine the mechanism of AHR in regulating neuronal cell death. AHR is a novel target in neurotoxicity and neurodegeneration, thus pharmacological or dietary interventions that modulate AHR activity could offer a strategy for the management of intestinal motility disorders.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
A.D.P. was supported by the National Insitute of Environmental Health Sciences (NIEHS) grants (ES028288, S10OD021750), the PA Department of Health using Tobacco funds, and the USDA National Institute of Food and Federal Appropriations under Project PEN047702 and Accession number 1009993. G.H.P. was supported by NIEHS R35 ES028244. S.S. was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants (R01DK080684, R01DK044234) and VA Merit Review Awards (I01 BX000136).
Contributor Information
Anitha Vijay, Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, Pennsylvania 16802, USA.
Nina R Boyle, Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, Pennsylvania 16802, USA.
Supriya M Kumar, Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, Pennsylvania 16802, USA.
Gary H Perdew, Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, Pennsylvania 16802, USA.
Shanthi Srinivasan, Department of Digestive Diseases, Emory University School of Medicine, Atlanta, Georgia, USA; Atlanta VA Medical Center, Decatur, Georgia, USA.
Andrew D Patterson, Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, Pennsylvania 16802, USA.
References
- Anitha M., Joseph I., Ding X., Torre E. R., Sawchuk M. A., Mwangi S., Hochman S., Sitaraman S. V., Anania F., Srinivasan S. (2008). Characterization of fetal and postnatal enteric neuronal cell lines with improvement in intestinal neural function. Gastroenterology 134, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha M., Gondha C., Sutliff R., Parsadanian A., Mwangi S., Sitaraman S. V., Srinivasan S. (2006). GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J. Clin. Invest. 116, 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha M., Vijay-Kumar M., Sitaraman S. V., Gewirtz A. T., Srinivasan S. (2012). Gut microbial products regulate murine gastrointestinal motility via toll-like receptor 4 signaling. Gastroenterology 143, 1006–1016.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J. R., Kim S. H. (2017). Synapses in neurodegenerative diseases. BMB Rep. 50, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes S. J. (1993). Neuronal nitric oxide in the gut. J. Gastroenterol. Hepatol. 8, 590–603. [DOI] [PubMed] [Google Scholar]
- Choung R. S., Rey E., Richard Locke G. 3rd, Schleck C. D., Baum C., Zinsmeister A. R., Talley N. J. (2016). Chronic constipation and co‐morbidities: A prospective population‐based nested case‐control study. United European Gastroenterol. J. 4, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L. L., Williamson M. A., Thompson B. D., Dever D. P., Gasiewicz T. A., Opanashuk L. A. (2008). 2,3,7,8-Tetracholorodibenzo-p-dioxin exposure disrupts granule neuron precursor maturation in the developing mouse cerebellum. Toxicol. Sci. 103, 125–136. [DOI] [PubMed] [Google Scholar]
- Dhaese I., Vanneste G., Sips P., Buys E. S., Brouckaert P., Lefebvre R. A. (2009). Small intestinal motility in soluble guanylate cyclase α1 knockout mice: (Jejunal phenotyping of sGCα1 knockout mice). Naunyn. Schmiedebergs. Arch. Pharmacol. 379, 473–487. [DOI] [PubMed] [Google Scholar]
- Duval C., Teixeira-Clerc F., Leblanc A. F., Touch S., Emond C., Guerre-Millo M., Lotersztajn S., Barouki R., Aggerbeck M., Coumoul X. (2017). Chronic exposure to low doses of dioxin promotes liver fibrosis development in the C57BL/6J diet-induced obesity mouse model. Environ. Health Perspect. 125, 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzienis-Koronkiewicz E., Debek W., Chyczewski L. (2005). Use of synaptophysin immunohistochemistry in intestinal motility disorders. Eur. J. Pediatr. Surg. 15, 392–398. [DOI] [PubMed] [Google Scholar]
- Fader K. A., Nault R., Zhang C., Kumagai K., Harkema J. R., Zacharewski T. R. (2017). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-elicited effects on bile acid homeostasis: alterations in biosynthesis, enterohepatic circulation, and microbial metabolism. Sci. Rep. 7, 5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling R. R., Doskey C. M., Fader K. A., Nault R., Zacharewski T. R. (2020). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) dysregulates hepatic one carbon metabolism during the progression of steatosis to steatohepatitis with fibrosis in mice. Sci. Rep. 10, 14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B. (2012). The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Ostby J. S., Kelce W. R. (1997a). A dose–response analysis of the reproductive effects of a single gestational dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male Long Evans hooded rat offspring. Toxicol. Appl. Pharmacol. 146, 11–20. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Wolf C., Mann P., Ostby J. S. (1997b). In UteroExposure to low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin alters reproductive development of female Long Evans hooded rat offspring. Toxicol. Appl. Pharmacol. 146, 237–244. [DOI] [PubMed] [Google Scholar]
- Groneberg D., Voussen B., Friebe A. (2016). Integrative control of gastrointestinal motility by nitric oxide. Curr. Med. Chem. 23, 2715–2735. [DOI] [PubMed] [Google Scholar]
- Han H., Safe S., Jayaraman A., Chapkin R. S. (2021). Diet–host–microbiota interactions shape aryl hydrocarbon receptor ligand production to modulate intestinal homeostasis. Annu. Rev. Nutr. 41, 455–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. L., Banks L. D., Mantey J. A., Huderson A. C., Ramesh A. (2013). Bioaccessibility of polycyclic aromatic hydrocarbons: relevance to toxicity and carcinogenesis. Expert Opin. Drug Metab. Toxicol. 9, 1465–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuckeroth R. O., Lampe P. A., Johnson E. M., Milbrandt J. (1998). Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev. Biol. 200, 116–129. [DOI] [PubMed] [Google Scholar]
- Hill A., Howard C. V., Strahle U., Cossins A. (2003). Neurodevelopmental defects in zebrafish (Danio rerio) at environmentally relevant dioxin (TCDD) concentrations. Toxicol. Sci. 76, 392–399. [DOI] [PubMed] [Google Scholar]
- Jankipersadsing S. A., Hadizadeh F., Bonder M. J., Tigchelaar E. F., Deelen P., Fu J., Andreasson A., Agreus L., Walter S., Wijmenga C., et al. (2017). A GWAS meta-analysis suggests roles for xenobiotic metabolism and ion channel activity in the biology of stool frequency. Gut 66, 756–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. Q., Jung J. W., Lee Y. S., Kim J. A. (2004). 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits cell proliferation through arylhydrocarbon receptor-mediated G1 arrest in SK-N-SH human neuronal cells. Neurosci. Lett. 363, 69–72. [DOI] [PubMed] [Google Scholar]
- Jung J. E., Moon J. Y., Ghil S. H., Yoo B. S. (2009). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibits neurite outgrowth in differentiating human SH-SY5Y neuroblastoma cells. Toxicol. Lett. 188, 153–156. [DOI] [PubMed] [Google Scholar]
- Juricek L., Coumoul X. (2018). The aryl hydrocarbon receptor and the nervous system. Int. J. Mol. Sci. 19, 2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakeyama M., Tohyama C. (2003). Developmental neurotoxicity of dioxin and its related compounds. Ind. Health. 41, 215–230. [DOI] [PubMed] [Google Scholar]
- Kim J. S., Lim H. S., Cho S. I., Cheong H. K., Lim M. K. (2003). Impact of agent orange exposure among Korean Vietnam veterans. Ind. Health. 41, 149–157. [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Yang J. H. (2005). Neurotoxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in cerebellar granule cells. Exp. Mol. Med. 37, 58–64. [DOI] [PubMed] [Google Scholar]
- Kimura E., Kubo K. I., Endo T., Nakajima K., Kakeyama M., Tohyama C. (2017). Excessive activation of AhR signaling disrupts neuronal migration in the hippocampal CA1 region in the developing mouse. J. Toxicol. Sci. 42, 25–30. [DOI] [PubMed] [Google Scholar]
- Kimura E., Kohda M., Maekawa F., Fujii-Kuriyama Y., Tohyama C. (2021). Neurons expressing the aryl hydrocarbon receptor in the locus coeruleus and island of Calleja major are novel targets of dioxin in the mouse brain. Histochem. Cell Biol. 156, 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura E., Tohyama C. (2017). Embryonic and postnatal expression of aryl hydrocarbon receptor mRNA in mouse brain. Front. Neuroanat. 11, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerr S., Schrenk D. (2006). Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol. Nutr. Food Res. 50, 897–907. [DOI] [PubMed] [Google Scholar]
- Lahoti T. S., Boyer J. A., Kusnadi A., Muku G. E., Murray I. A., Perdew G. H. (2015). Aryl hydrocarbon receptor activation synergistically induces lipopolysaccharide-mediated expression of proinflammatory chemokine (c–c motif) ligand 20. Toxicol. Sci. 148, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchney S. E., Hein A. M., O'Banion M. K., DiCicco-Bloom E., Opanashuk L. (2013). Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. J. Neurochem. 125, 430–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchney S. E., Lioy D. T., Henry E. C., Gasiewicz T. A., Strathmann F. G., Mayer-Pröschel M., Opanashuk L. A. (2011). Neural precursor cell proliferation is disrupted through activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Stem Cells Dev. 20, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. A., Kyeong S., Kim D. H. (2022). Long-term effects of defoliant exposure on brain atrophy progression in humans. Neurotoxicology 92, 25–32. [DOI] [PubMed] [Google Scholar]
- Legare M. E., Hanneman W. H., Barhoumi R., Burghardt R. C., Tiffany-Castiglioni E. (2000). 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters hippocampal astroglia-neuronal gap junctional communication. Neurotoxicology 21, 1109–1116. [PubMed] [Google Scholar]
- Li Z., Chalazonitis A., Huang Y. Y., Mann J. J., Margolis K. G., Yang Q. M., Kim D. O., Côté F., Mallet J., Gershon M. D. (2011). Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 31, 8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. S., Schmauss C., Cuenca A., Ratcliffe E., Gershon M. D. (2006). Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: Analysis of dopamine receptor expression. J. Neurosci. 26, 2798–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N. B., Kerkvliet N. I. (2010). Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann. N. Y. Acad. Sci. 1183, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memic F., Knoflach V., Morarach K., Sadler R., Laranjeira C., Hjerling-Leffler J., Sundström E., Pachnis V., Marklund U. (2018). Transcription and signaling regulators in developing neuronal subtypes of mouse and human enteric nervous system. Gastroenterology 154, 624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. S., Galligan J. J., Burks T. F. (1981). Accurate measurement of intestinal transit in the rat. J. Pharmacol. Methods. 6, 211–217. [DOI] [PubMed] [Google Scholar]
- Muku G. E., Lahoti T. S., Murray I. A., Podolsky M. A., Smith K. J., Hubbard T. D., Kuzu G., Gowda K., Amin S. G., Perdew G. H. (2017). Ligand-mediated cytoplasmic retention of the Ah receptor inhibits macrophage-mediated acute inflammatory responses. Lab. Invest. 97, 1471–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S. P., Anderson B., Bharucha A. E. (2021). Sex- and gender-related differences in common functional gastroenterologic disorders. Mayo Clin. Proc. 96, 1071–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W. (2017). Aryl hydrocarbon receptor (AHR): “Pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog. Lipid Res. 67, 38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y., Castaño Á., Boeing S., Bon-Frauches A. C., Fung C., Fallesen T., de Agüero M. G., Yilmaz B., Lopes R., Huseynova A., et al. (2020). Neuronal programming by microbiota regulates intestinal physiology. Nature 578, 284–289. [DOI] [PubMed] [Google Scholar]
- Qin H., Zhai Z., Powell-Coffman J. A. (2006). The Caenorhabditis elegans AHR-1 transcription complex controls expression of soluble guanylate cyclase genes in the URX neurons and regulates aggregation behavior. Dev. Biol. 298, 606–615. [DOI] [PubMed] [Google Scholar]
- Rengarajan T., Rajendran P., Nandakumar N., Lokeshkumar B., Rajendran P., Nishigaki I. (2015). Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 5, 182–189. [Google Scholar]
- Sampaio G. R., Guizellini G. M., da Silva S. A., de Almeida A. P., Pinaffi-Langley A. C. C., Rogero M. M., de Camargo A. C., Torres E. A. F. S. (2021). Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 22, 6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Martín F. J., Fernández-Salguero P. M., Merino J. M. (2010). 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces apoptosis in neural growth factor (NGF)-differentiated pheochromocytoma PC12 cells. Neurotoxicology 31, 267–276. [DOI] [PubMed] [Google Scholar]
- Sánchez-Martín F. J., Fernández-Salguero P. M., Merino J. M. (2011). Aryl hydrocarbon receptor-dependent induction of apoptosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin in cerebellar granule cells from mouse: TCDD toxicity in cerebellar granule cells. J. Neurochem. 118, 153–162. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Ward S. M. (2019). Nitric oxide and its role as a non‐adrenergic, non‐cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br. J. Pharmacol. 176, 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering C., Wincent E., Metidji A., Iseppon A., Li Y., Potocnik A. J., Omenetti S., Henderson C. J., Wolf C. R., Nebert D. W., et al. (2017). Feedback control of AHR signalling regulates intestinal immunity. Nature 542, 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. H., Ngwainmbi J., Grider J. R., Dewey W. L., Akbarali H. I. (2013). An In-vitro Preparation of isolated enteric neurons and glia from the myenteric plexus of the adult mouse. JoVE (78), 50688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni K. G., Halder T., Conner M. E., Preidis G. A. (2019). Sexual dimorphism in upper gastrointestinal motility is dependent on duration of fast, time of day, age, and strain of mice. Neurogastroenterol. Motil. 31, e13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber A. D., Bangdiwala S. I., Drossman D. A., Ghoshal U. C., Simren M., Tack J., Whitehead W. E., Dumitrascu D. L., Fang X., Fukudo S., et al. (2021). Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology 160, 99–114.e3. [DOI] [PubMed] [Google Scholar]
- Tomasini M. C., Beggiato S., Ferraro L., Tanganelli S., Marani L., Lorenzini L., Antonelli T. (2012). Prenatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin produces alterations in cortical neuron development and a long-term dysfunction of glutamate transmission in rat cerebral cortex. Neurochem. Int. 61, 759–766. [DOI] [PubMed] [Google Scholar]
- Uno S., Dalton T. P., Sinclair P. R., Gorman N., Wang B., Smith A. G., Miller M. L., Shertzer H. G., Nebert D. W. (2004). Cyp1a1(−/−) male mice: Protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol. Appl. Pharmacol. 196, 410–421. [DOI] [PubMed] [Google Scholar]
- Van den Berg M., Birnbaum L. S., Denison M., De Vito M., Farland W., Feeley M., Fiedler H., Hakansson H., Hanberg A., Haws L., et al. (2006). The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 93, 223–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woting A., Blaut M. (2018). Small intestinal permeability and gut-transit time determined with low and high molecular weight fluorescein isothiocyanate-dextrans in C3H Mice. Nutrients 10, 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.-C., Fang W.-H., Wang C.-C., Lai C.-H., Chen W.-L. (2021). Association between polycyclic aromatic hydrocarbon exposure and diarrhea in adults. Atmosphere 12, 919. [Google Scholar]
- Xu G., Zhou Q., Wan C., Wang Y., Liu J., Li Y., Nie X., Cheng C., Chen G. (2013a). 2,3,7,8-TCDD induces neurotoxicity and neuronal apoptosis in the rat brain cortex and PC12 cell line through the down-regulation of the Wnt/β-catenin signaling pathway. Neurotoxicology 37, 63–73. [DOI] [PubMed] [Google Scholar]
- Xu G., Duan Z., Chen G., Nie X., Liu J., Zhang Y., Li Y., Wan C., Jiang J. (2013b). Role of mitogen-activated protein kinase cascades in 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced apoptosis in neuronal pheochromocytoma cells. Hum. Exp. Toxicol. 32, 1278–1291. [DOI] [PubMed] [Google Scholar]
- Yi S. W., Hong J. S., Ohrr H., Yi J. J. (2014). Agent Orange exposure and disease prevalence in Korean Vietnam veterans: The Korean veterans health study. Environ. Res. 133, 56–65. [DOI] [PubMed] [Google Scholar]
- Zhang T., Zhou X., Ren X., Zhang X., Wu J., Wang S., Wang Z. (2021). Animal toxicology studies on the male reproductive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin: Data analysis and health effects evaluation. Front. Endocrinol. 12, 696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Hu W. (2013). Mouse enteric neuronal cell culture. Methods Mol. Biol. 1078, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Degroot D. E., Hayashi A., He G., Denison M. S. (2010). CH223191 is a ligand-selective antagonist of the ah (dioxin) receptor. Toxicol. Sci. 117, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Chakraborty D., Murray I. A., Coslo D., Kehs Z., Vijay A., Ton C., Desai D., Amin S. G., Patterson A. D., et al. (2023). Aryl hydrocarbon receptor activation coordinates mouse small intestinal epithelial cell programming. Lab. Invest. 103, 100012. [DOI] [PubMed] [Google Scholar]