Abstract
Biological pretreatment to the lignocellulosic waste prior to anaerobic digestion is a popular method to increase biogas production. However, the long time needed for the pretreatment is not suitable to the practical application. A fungus strain, which could produce many kinds of lignocellulosic enzymes including CMCase, FPase, xylanase and laccase, was isolated from the soil of Tibet in this study. The fungus was identified as Trametes sp. W-4 by morphological and molecular characterization. The optimum culture temperature was 30 °C and the optimum nitrogen source was peptone. Under the optimum fermentation condition, the activity of CMCase, FPase, xylanase and laccase could reach 2.73 U/mL, 0.41 U/mL, 0.29 U/mL, and 1.11 U/mL, respectively. The results of pretreatment of Trametes sp. W-4 on the mixtures of high land barley straw, cow manure and pig manure for enhancement of biogas production showed that a very short time pretreatment of 3 days could obtain the highest cumulative methane production of 111.51 mL/g-VS, which was 63.81% higher than that of the control group of 68.07 mL/g-VS. The finding indicated that Trametes sp. W-4 pretreatment could be a candidate for the improving of biogas production from lignocellulosic waste.
Keywords: Anaerobic digestion, Lignocellulosic biomass, Biological pretreatment, Biomethane, Strain identification
1. Introduction
Due to the abundance and easy accessibility of lignocellulosic material, converting lignocellulosic waste into biofuel increasingly attracts the worldwide interest in recent years as it might be one of the ways to response the energy crisis [1]. Among various biofuel producing ways, biomethane production through anaerobic digestion is a promising option as which not only could eliminate the harmful environmental effect of the waste but also could obtain fertilizer since the residue produced during anaerobic fermentation consists of a great amount of stabilized organic matter and many kinds of plant nutrients, like N, P, K, and micro-nutrients [2]. Generally, process of anaerobic digestion takes place through four successive stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis [3]. Among the four stages, stage of hydrolysis needs many kinds of enzymes to break down the substrate and if there is refractory organics in the feedstock, hydrolysis will be very slow, and this step will restrict the overall rate of anaerobic digestion [4].
Lignocellulosic material, which is mainly composed of cellulose, hemicellulose, and lignin, is such a recalcitrant compound during the anaerobic fermentation. Previous studies showed that pretreatment to lignocellulosic material prior to anaerobic digestion could increase the yield of biogas production since the pretreatment could change the chemical composition and structure of lignocellulose and make natural lignocellulose macromolecules susceptible to degrade [5]. Currently, pretreatment technologies can be roughly classified into physical, chemical, and biological method [6,7]. Among these pretreatment methods, biological pretreatment with advantages of environmentally benign, less energy consumption and fewer inhibiting compounds production attracts more and more attention [8]. Biological pretreatment usually can be divided into two categories: microbial pretreatment and enzyme pretreatment. The former primarily involves using single or multi-organisms to treat lignocellulose, while the latter has not been scaled-up into industrial level due to the high cost of commercial enzymes [9]. Even though microbial pretreatment has many advantages compared with other pretreatment methods, the long time pretreatment process is unfavorable to the practical application. Many reports in the literature showed that several weeks were commonly needed for the microbial pretreatment [[10], [11], [12]]. In order to short the pretreatment time and increase the performance, several strategies could be used, such as using mixed microorganism, combining with other pretreatment methods, or screening new strains with peculiar characterizations [13]. Recently, the method of combination biological pretreatment with other pretreatment has been studied by many researchers [[14], [15], [16], [17]]. Though they obtained desirable biogas production, the process was either energy excessive consumed or less environmentally friendly since the combination was mostly conducted with physical or chemical method. Since mixed culture could produce much more kinds of lignocellulosic enzymes compared with single culture, mixed culture pretreatment was considered more effectively [2]. However, the pretreatment time of mixed culture was still much longer than that of the physical or chemical pretreatment as far as the authors know. Most cases of mixed culture pretreatment in the literature also need several days or weeks, although many of them are shorter than the pure culture pretreatment. For examples, Ali et al. [12] pretreated creosote oil-treated pine sawdust using two constructed microbial consortia, significant reduction in lignocellulosic content and increasing in methane yields were obtained after 12 days of biological pretreatment. Another case comes from the results of Zhao et al. [18], when they studied the effects of the pretreatment via microbial consortium on the methane production from stover, they found that the total methane production was highest after 6 days of pretreatment.
The ultimate advantage of single culture pretreatment over mixed culture pretreatment would be due to the fact that the growth condition of single culture is easier to control and could form stable characterizations no matter how many generations have been produced. However, the microbial structure of mixed culture could be changed during the long time cultivation and thus decrease the ability of degradation [2]. Therefore, if the pretreatment time of single culture could be shortened, it would be significant to the biological pretreatment. Screening new strains with particular characterization such as high titer lignocellulosic enzymes production is one of the possible ways to the application of single culture pretreatment in the future. Recently, there are some researchers set focus on the isolation of lignocellulose–degradation microorganism for enhancing the biogas production [[19], [20], [21]]. However, most of the limited studies focused on the performance of the pretreatment and methane yield but not on the time of pretreatment. In nature environment, there are many kinds of microorganism that could degrade lignocellulosic material, such as bacteria, actinomycetes and fungi. Hence, peculiar lignocellulose–degradating strain could be possibly isolated from the nature environment. In this study, a novel fungus which could produce several kinds of lignocellulosic enzymes was isolated, and most importantly, 3 days of pretreatment by this strain could reach the highest biogas production. The pretreatment time by single strain that could obtain the highest performance of anaerobic digestion in this study is the shortest as far as the authors know. This strain could be a candidate for the biological pretreatment in practical application.
2. Materials and methods
2.1. Materials
Soil samples were obtained from Sangri County, Shannan Prefecture, Tibet province (92°28 '2.13 "E, 29°15' 18.27" N, altitude 3379 m). Highland barley straw and cattle/pig manures were collected from the campus of Tibet Agriculture & Animal Husbandry University with an altitude of 3000 m above mean sea level in Linzhi, China. Highland barley straw, cattle and pig manures were air-dried, ground into powder and sealed in laboratory for later use. Anaerobic inoculum was obtained from an anaerobic sequence batch reactor (about 30 L) which had been fed with cattle manure and operated under 30 °C temperature condition for a long time (over 2 years) in the laboratory. Characterizations of feedstocks and inoculum are shown in Table 1.
Table 1.
Characterizations of the feedstock and inoculum.
| Highland barley straw | Cow manure | Pig manure | Inoculum | |
|---|---|---|---|---|
| TS (%) | 91.4 ± 4.2 | 91.6 ± 3.7 | 93.4 ± 2.9 | 21.48 ± 1.1 |
| VS (%TS) | 96.41 ± 2.1 | 68.14 ± 2.4 | 57.80 ± 1.8 | 38.52 ± 0.9 |
| TOC (%TS) | 53.56 ± 2.1 | 37.86 ± 1.6 | 32.11 ± 1.7 | 21.4 ± 0.9 |
| pH | 5.60 ± 0.02 | 6.84 ± 0.02 | 6.32 ± 0.01 | 7.53 ± 0.03 |
| Cellulose (% TS) | 39.23 ± 2.31 | 9.04 ± 0.11 | 3.09 ± 0.08 | / |
| Hemicellulose (% TS) | 26.71 ± 2.94 | 7.18 ± 0.28 | 1.09 ± 0.03 | / |
| Lignin (% TS) | 13.11 ± 1.97 | 10.44 ± 1.31 | 0.46 ± 0.08 | / |
2.2. Strain isolation and determination
For the isolation of cellulase-producing microorganism, about 1 g soil was suspended in 100 ml sterile saline water (0.85% NaCl), agitated for 30 min under room temperature and then 0.1 ml suspension was spread over sodium carboxyl methyl cellulose (CMC-Na) agar plates, which contained 2 g/L peptone, 0.5 g/L MgSO4, 2 g/L KH2PO4, 1 g/L K2HPO4, 0.5 g/L NaCl, 2 g/L CMC-Na, and 15 g/L agar. The plates were incubated at 25 °C until sufficient growth appeared, then were stained with Gram’s Iodineto to visualize the halo around each colony [22]. Strains showed clear zones were picked and subcultured repeatedly to obtain pure cultures. The pure isolates were spot cultured on the CMC-Na agar medium plate, and were kept under the desired temperature conditions including 5 °C, 10 °C, 15 °C, 20 °C, 25 °C, 30 °C, and 35 °C to determine the temperature range of growth. After incubation for 6 days, the plates were taken out and the diameter of the colony was measured. Each plate was repeated three times and the average values were plotted. After the growth temperature test, the strain was cultured at the different nitrogen source condition of peptone, yeast extract, urea, NH4Cl and NaNO3 to determine the optimal nitrogen source for producing CMCase. The medium was the same as the screening medium except that no agar was added, the nitrogen source was equally added in weight, respectively. Then the strain was cultured at the optimal temperature and nitrogen source conditions for the later determination. 5 mL fermentation broth was taken out at the desired time to analyze the activities of CMCase, FPase, xylanase, and laccase.
Strain identification was based on morphological and molecular taxonomy. The former was performed according to the colony morphology, shape and size of conidiophores, metulae, phialides, and their texture and color as well, whereas the latter was performed by using Internal Transcribed Spacer (ITS) gene sequencing as reported by Li et al. [23]. The genomic DNA from the fungal isolate was extracted and amplified using the forward primer for ITS 1 (5′-TCCGT AGGTG AACCT TGCGG - 3′) and reverse primer for ITS 4 (5′ -TCCTC CGCTT ATTGA TATGC - 3′). The amplified gene product was purified using Polymerase Chain Reaction (PCR) purification kit (Qiagen) and followed by sequencing. The resulting sequences were submitted to the Basic Local Alignment Search Tool (BLAST) search program (http://www.ncbi.nlm.nih.gov/BLAST) of the National Center for Biotechnology Information (NCBI) to find similar sequences [24]. Several homologous sequences from BLAST results were aligned and used to make a phylogenetic tree by neighbor-joining method using MEGA 7.0 software.
2.3. Pretreatments and anaerobic digestion
The pretreatment experiments were conducted in beakers with effective volume of 1000 mL. Highland barley straw, cow dung, and pig manure were used as substrates and mixed according to 1:1:1 (dry weight ratio). Moisture content was adjusted to 70–80% by adding tap water [9]. The beakers containing the mixture of substrates were divided into two equal groups. One was subjected to sterilization in an autoclave for killing the indigenous microorganisms [10]. Then both the sterilization group and the unsterilization group were inoculated with the isolated fungus which had been spot cultured on the agar plates at 30 °C for 6 days in advance. Each beaker was inoculated with two discs of fungal mycelia which were cut along the edge of fungal colonies. After thoroughly mixed, the beaker was covered with gauze and placed in a incubator with consistent temperature of 30 °C. The beakers were shaken by hand for about 1 min once a day to increase the oxygen content and make substrate well-mixed. The pretreatment time was set as 3 d, 7 d, 14 d, respectively, and 0 d treatment group inoculated with heat-inactivated fungal mycelia was used as control. After pretreatment, the substrate was subjected to anaerobic digestion for biogas production.
Anaerobic digestion experiments were carried out in 500 mL serum bottles with working volume of 350 mL. The pretreated feedstock and anaerobic inoculum were well mixed with a dry weight ratio of 1:1, and then bottled. All bottles were purged with nitrogen gas for 3–5 min to make an anaerobic condition. Thereafter, these bottles were placed in a thermostat incubator at 30 °C and shaken for about 1 min by hand once a day to ensure that the substrates were fully mixed. The control bottles were a mixture of anaerobic inoculum and feedstock from the control pretreatment groups. The anaerobic digestion was terminated when no significant biogas production was observed. Before and after the pretreatment and anaerobic digestion, thoroughly mixed samples were taken out and stored at −20 °C for the later composition analysis. All experiments were done in duplicates.
2.4. Analytical methods
The activities of CMCase, FPase, and xylanase of the fermentation solution were measured using dinitrosalicylic acid (DNS) assay method [[25], [26], [27]]. 5 mL fermentation solution was centrifuged at 10,000 rotations per minute for 5 min, and the supernatant was used for the enzyme assay. One unit of CMCase or FPase or xylanase activity was defined as the amount of enzyme that released 1 μmol of reducing sugar (measured as glucose, xylose equivalent) from the substrate per min under assay conditions. Laccase activity was determined according to the method of Qiu er al [28]. One unit (U) of laccase activity was defined as the amount of enzyme required to catalyze the oxidation of 1 μmol substrate 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) per minute.
For the determination of total solids (TS) content, 5 g samples were dried in a drying oven at 105 °C to constant mass. The dry matter was then burned in a furnace at 550 °C for 2 h, and the loss of weight was considered as the volatile solids (VS) content [29]. The biogas was collected with an aluminum foil bag and monitored at a fixed time of each day. The volume was measured using water displacement method and corrected to the standard pressure and temperature condition (1 atm, 273 K) [30]. CH4 content was determined by a gas chromatograph (GC122, Shanghai, China) [31]. All biogas yield were expressed in N mL/g-VS initial. Lignin, cellulose, and hemicellulose were analyzed according to the method of Van Soest et al. [32]. Dry matter loss and the degradation of lignin, cellulose, and hemicellulose were calculated using the equations reported by Zhao et al. [33].
Statistical analyses were carried out by using SPSS version 17.0 (SPSS China Inc.).
3. Results and discussion
3.1. Isolation and identification of cellulolytic fungus
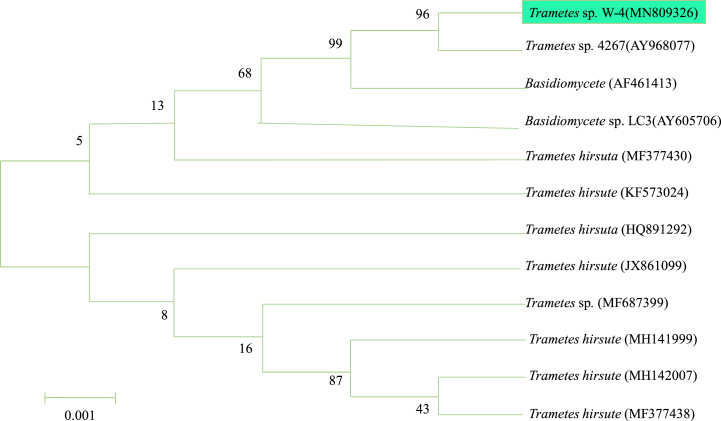
Soil samples obtained from Tibet were used for the isolation of cellulolytic microorganisms at 25 °C. More than 20 cellulolytic strains were obtained, among them, 5 strains with larger hydrolytic circle were preserved, and the others were discarded due to slow growth or very small hydrolytic circle. The strain with largest halo of cellulolytic activity was subjected to morphology and molecular identification. Colony morphology results showed that mycelium of the strain was white, and the colony on the agar plate was fluffy. Molecular identification showed that this strain was highly affiliated to Trametes sp. 4267 (Gen bank No. AY968077) by fungal 5S–18S sequences comparison with the data of NCBI (Fig. 1). The strain was thereafter named as Trametes sp. W-4. The ITS gene sequence of this strain was deposited in the GenBank nucleotide database with accession number of MN809326 (http://www.ncbi.nlm.nih.gov).
Fig. 1.
Phylogenetic dendrogram of Trametes sp. W-4 using neighbor-joining method. (Bootstrap values are shown at the nodes. The sequence accession numbers are given in parentheses following the species name. The scale bar represents the number of substitutions per site.)
3.2. Characterizations of Trametes sp. W-4
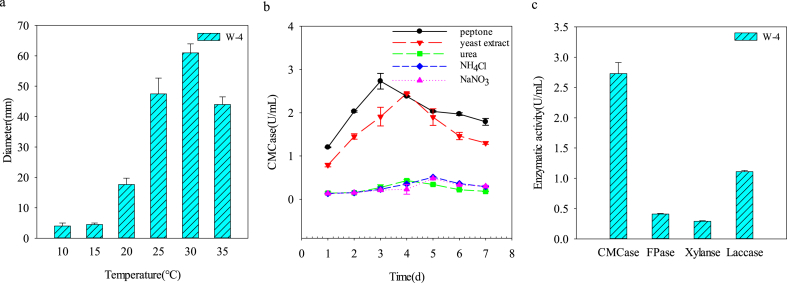
The effects of temperature on the growth of Trametes sp. W-4 are shown in Fig. 2a. The results showed that Trametes sp. W-4 had a very wide growth temperature range and the optimum temperature was 30 °C. When the strain was cultivated on the agar plate for 6 days at 30 °C, the diameter of the colony could reach 61 mm. Additionally, Trametes sp. W-4 could grow well even at very low temperature condition. At 10 °C temperature condition, the diameter of the colony on the agar plate could reach 4 mm after 6 days incubation. However, when the environment temperature dropped below 4 °C, Trametes sp. W-4 could not grow any more. According to the classification on organisms that inhabit environments by Morita [34], Trametes sp. W-4 is a kind of mesophilic organism and could grow under low temperature condition.
Fig. 2.
Growth characteristics and enzymatic activity of Trametes sp. W-4. (a. Diameter of strain colony on solid agar plate at different temperatures; b. Effect of nitrogen sources on CMCase activity of Trametes sp. W-4; c. Enzymatic activity of Trametes sp. W-4 under the optimum fermentation conditions: 30 °C, peptone as nitrogen source, cultured for 4 days in the liquid medium.)
Besides carbon source, nitrogen source is another important factor that could affect the strain growth and enzyme production [35]. Therefore, the influence of various nitrogen sources including yeast exact, peptone, urea, NH4Cl and NaNO3 on the CMCase activity of Trametes sp. W-4 during different growth phase was analyzed in this study. The results showed that the CMCase activity in peptone and yeast groups was significantly higher than that of the other groups, indicating that yeast exact and peptone are two more suitable nitrogen sources for the producing of lignocellulosic enzymes of Trametes sp. W-4. Additionally, CMCase activity with peptone as nitrogen source was significantly higher than that of yeast extract (Fig. 2b). The CMCase activity increased with the developing of cultivation, and reached peak of 2.73 U/mL at 3 days when using peptone as the nitrogen source. However, when using yeast as the nitrogen source, CMCase activity reached a lower peak (2.45 U/mL) in longer time (4 days). It seems obviously that peptone is the best nitrogen source for the CMCase production of Trametes sp. W-4. Besides CMCase, other lignocellulosic enzymes including FPase, xylanase, and laccase were also determined under the optimum nitrogen source condition. The results showed that the observed highest activities of FPase, xylanase and laccase of Trametes sp. W-4 were 0.41 U/mL, 0.29 U/mL, and 1.11 U/mL, respectively. Compared with the results in the literature, these four enzymes produced by Trametes sp. W-4 were at very high level [19] (Fig. 2c). Therefore, Trametes sp. W-4 could be used as a potential candidate for the treatment of lignocellulose waste for the further utilization.
3.3. Effect of Trametes sp. W-4 pretreatment on biogas production
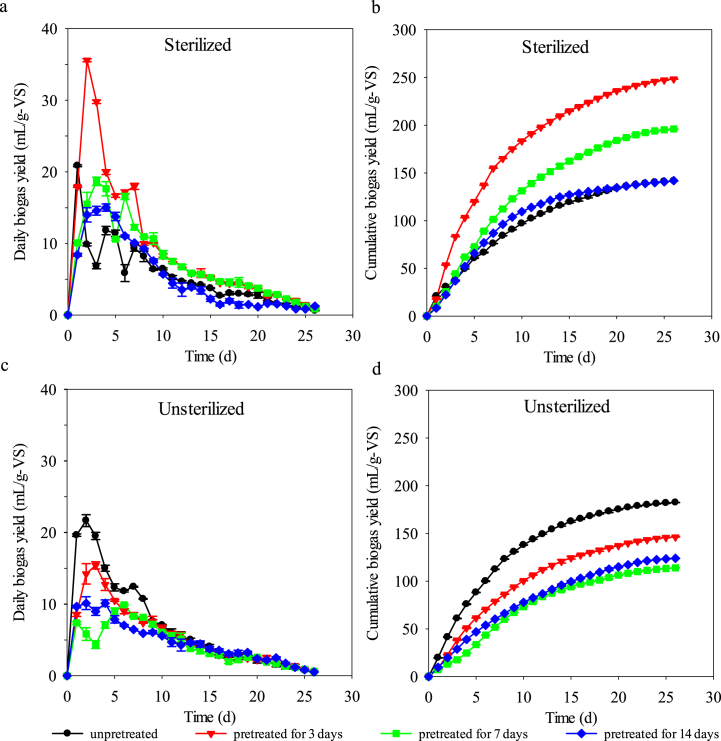
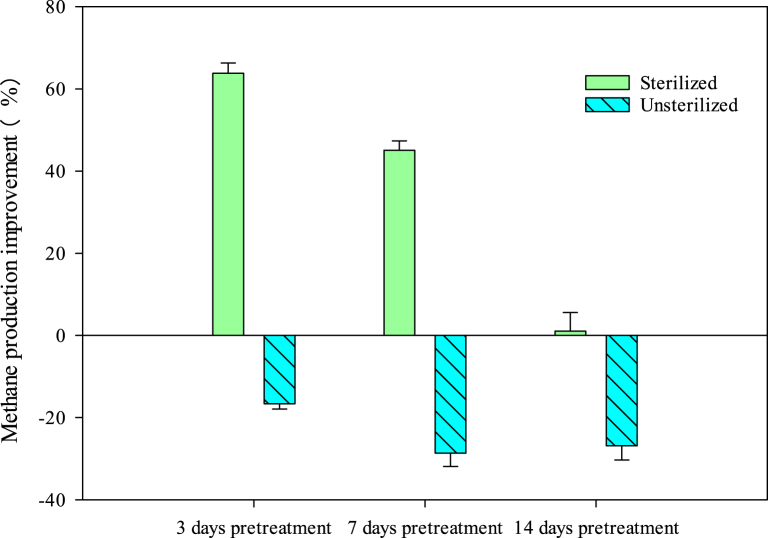
Daily biogas yield and cumulative biogas production from the pretreated feedstock by Trametes sp. W-4 are shown in Fig. 3. It could be easily observed that both sterilization and pretreatment by Trametes sp. W-4 could significantly affect the biogas production. When the feedstock was sterilized, the pretreatment by Trametes sp. W-4 could significantly increase the biogas production (Fig. 3 a and b), whereas under unsterilized condition the pretreatment was negative to the biogas production (Fig. 3 c and d). This result indicated that the native microrganism in the feedstock would consume the nutrition during the pretreatment stage and thus decrease the subsequent biogas production. Fig. 3 also shows that the pretreatment time was not as long as good. Under sterilized condition, daily biogas yield and cumulative biogas production decreased with the developing of pretreatment when the pretreatment time exceeded 3 days. The highest cumulative biogas production of 248.29 mL/g-VS and the highest daily biogas yield of 35.58 mL/g-VS was obtained from the treatment of 3 d group, then was the 7 d group of 196.01 mL/g-VS and 18.67 mL/g-VS, respectively, whereas the biogas production of 14 d treatment group was just comparable to the control group. Expectedly, the methane production was similar with biogas production. The highest methane production of 111.51 mL/g-VS was also obtained from the 3 d group, which was 63.81% higher than that of the control group of 68.07 mL/g-VS, then was the 7 d group (Fig. 4).
Fig. 3.
Daily biogas yield and cumulative biogas production under different pretreatment conditions.
Fig. 4.
Improvement rate of CH4 production from different pretreatments compared with unpretreated group.
The decreasing of biogas production under longer time pretreatment condition might be due to the fact that more nutrient consumed or more phenolic compounds produced with the continuing of pretreatment by Trametes sp. W-4 since this strain could produce high titer laccase (Fig. 2c) and thus inhibited the activity or growth of methanogens. This result is consistent with that in the literature, in which the pretreatment is considered as a kind of tradeoff between treatment time and “dry matter loss” [2]. However, the pretreatment time of 3 days was so short that it was able to comparable to the physical or chemical pretreatment. The so short pretreatment time might result from the characterizations of the enzymes produced by Trametes sp. W-4. As shown in the above results, Trametes sp. W-4 could produce versatile lignocellulosic enzymes including a very high titer laccase (Fig. 2c). The enhancing of biogas production by pretreatment does not need the thorough degradation of lignocellulosic material and excessive degradation of lignin might release a mount of lignin-derived phenolics that was harmful to the biogas production [36]. Partial degradation of the lignin may enoughly expose cellulose and hemicellulose for the later anaerobic digestion and thus result in the higher biogas production under shorter time pretreatment condition.
Compared with the biogas production in control groups between sterilization and unsterilization, it could be found that both daily biogas yield and cumulative biogas production of sterilized control group were lower than that of the unsterilized control group, respectively. Additionally, pretreatment to the unsterilized feedstock by Trametes sp. W-4 did not show positive effect on the biogas or methane production. It could be easily observed that the cumulative biogas production as well as the methane production of all the pretreatment groups were lower than that of the control group under the unsterilized condition (Fig. 3, Fig. 4). This result indicated that sterilization alone could negatively affect the biogas production although many researchers in the literature showed that sterilization had no influence on the anaerobic digestion or could enhance biogas production when combined with acid or alkaline pretreatment method [5]. The increased biogas production by sterilization combined with Trametes sp. W-4 pretreatment in this study might result from the degradation of Trametes sp. W-4 and the inhibition of sterilization to the nutrition consuming of the native microorganisms.
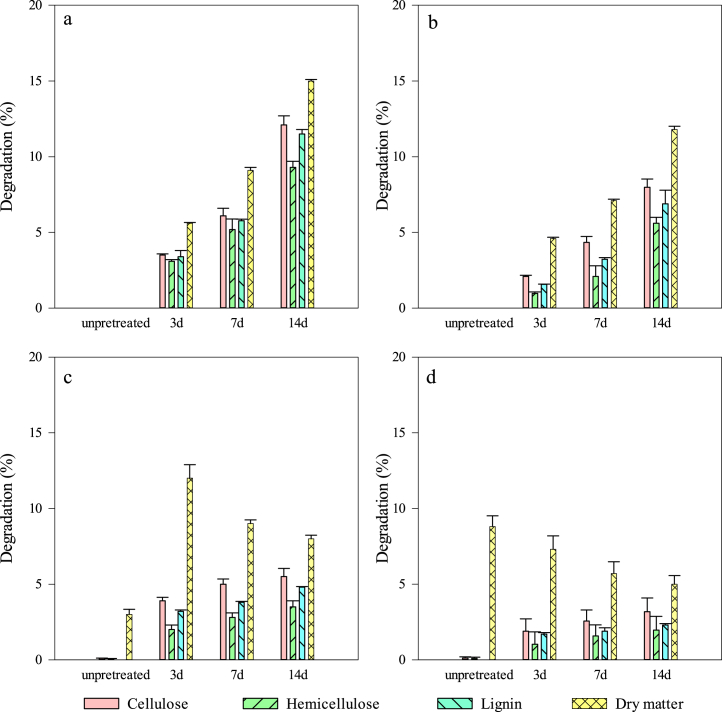
3.4. Effects of Trametes sp. W-4 pretreatment on the composition of feedstock
The degradation of lignocellulosic component and dry matter loss during the pretreatment by Trametes sp. W-4 and anaerobic digestion process are shown in Fig. 5. Trametes sp. W-4 could obviously decompose all of the components (i.e., cellulose, hemicellulose and lignin) of the feedstock during the pretreatment, and the degradation was increased with the prolonging of pretreatment time (Fig. 5 a and b). The dry matter loss and degradation under sterilized condition were higher than that of under unsterilized condition, indicating that the effect of indigenous microorgaisms in the feedstock on the pretreatment was not neglected. When Trametes sp. W-4 was inoculated into feedstock, the propagation of indigenous microorganisms might inhibit the growth of Trametes sp. W-4 and thus decrease the degradation of lignocellulosic content, as many researchers have confirmed that sterilization was necessary to the fungus pretreatment for increasing biogas production [19,37]. Although the degradation of lignocellulosic component was very low under unsterilized condition, the dry matter loss was still very high (Fig. 5b), which might result from the consuming of easily degradable materials like proteins and soluble sugars by indigenous microorganisms and thus leading to the lower biogas production in the latter anaerobic digestion stage (Fig. 3d).
Fig. 5.
Compositional changes in the feed stock during the pretreatment with Trametes sp. W-4 (a: sterilized; b: unsterilized) and the subsequent anaerobic digestion stage (c: sterilized; d: unsterilized) under different pretreatment time.
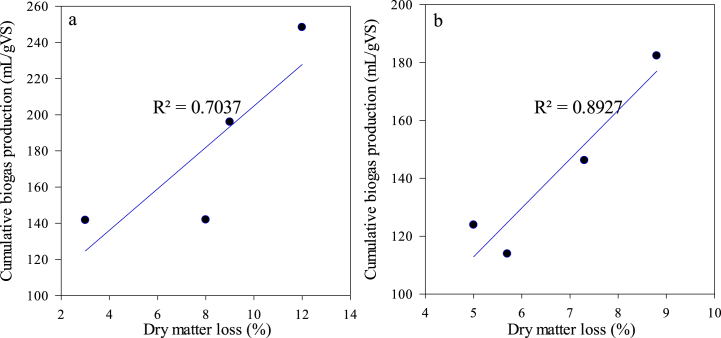
Compared with the degradation between pretreatment stage and anaerobic digestion stage, it could be found that cellulose, hemicellulose and lignin could be degradated continuously during the anaerobic digestion except that the degradation was very low (Fig. 5). This result might be related to the characterization of Trametes sp. W-4, as which is aerobic, could not grow during the anaerobic digestion stage. The degradation of lignocellulosic component under anaerobic condition might result from the function of lignocellulosic enzymes secreted during the pretreatment stage. However, the dry matter loss during the anaerobic digestion stage was significant different from that of the pretreatment stage. With the increasing of pretreatment time from 3d to 14d, the dry matter loss decreased during the anaerobic digestion stage (Fig. 5 c and d), and it could be found that the dry matter loss was consistent with the biogas production (Fig. 6 a and b). This result confirmed that long time pretreatment was not good for increasing the biogas production. It is worth noting that the degradation of cellulose, hemicellulose and lignin was almost not detectable in control groups, i.e. 0d treatment groups during both the pretreatment and the anaerobic digestion stages, indicating that there was no lignocellulosic enzymes activity in these groups.
Fig. 6.
Correlation between cumulative biogas production and dry matter loss. (a: sterilized; b: unsterilized).
4. Conclusions
A lignocellulosic enzymes-producing strain, named as Trametes sp. W-4, was obtained from the soil of Tibet. The optimum growth temperature of this strain was 30 °C and it could grow well even at very low temperature condition (10 °C). Trametes sp. W-4 could produce many kinds of lignocellulosic enzymes. Among various nitrogen sources, peptone is the best nitrogen source for the CMCase production of Trametes sp. W-4. Under the optimum fermentation conditions, the activity of CMCase, FPase, xylanase and laccase of this strain could reach 2.73 U/mL, 0.41 U/mL, 0.29 U/mL and 1.11 U/mL, respectively. The results of pretreatment of Trametes sp. W-4 on lignocellulosic materials for increasing biogas production showed that a very short time pretreatment of 3 days could obtain the highest cumulative methane production of 111.51 mL/g-VS, which was 63.81% higher than that of the control group of 68.07 mL/g-VS, while the longer time of pretreatment was not good to the biogas production due to more nutrient was consumed and more lignin was degradated. In addition, the pretreatment need the combination with sterilization, other wise, eighter of them could not obtain the highest biogas production.
Author contribution statement
Xiaoli Jin: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Suzhen Wei: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Dr. Suzhen wei was supported by Base and Talent Program in Tibet Autonomous Region [XZ202101JD0003F].
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Saravanan A., Senthil Kumar P., Jeevanantham S., Karishma S., Vo D.N. Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Bioresour. Technol. 2022;344 doi: 10.1016/j.biortech.2021.126203. [DOI] [PubMed] [Google Scholar]
- 2.Wei S. The application of biotechnology on the enhancing of biogas production from lignocellulosic waste. Appl. Microbiol. Biotechnol. 2016;100(23):9821–9836. doi: 10.1007/s00253-016-7926-5. [DOI] [PubMed] [Google Scholar]
- 3.Chiu S.L.H., Lo I.M.C. Reviewing the anaerobic digestion and co-digestion process of food waste from the perspectives on biogas production performance and environmental impacts. Environ. Sci. Pollut. Res. 2016;23(24):24435–24450. doi: 10.1007/s11356-016-7159-2. [DOI] [PubMed] [Google Scholar]
- 4.Ma S., Li Y., Li J., Yu X., Cui Z., Yuan X., Zhu W., Wang H. Features of single and combined technologies for lignocellulose pretreatment to enhance biomethane production. Renew. Sustain. Energy Rev. 2022;165 doi: 10.1016/j.rser.2022.112606. [DOI] [Google Scholar]
- 5.Poddar B.J., Nakhate S.P., Gupta R.K., Chavan A.R., Singh A.K., Khardenavis A.A., Purohit H.J. A comprehensive review on the pretreatment of lignocellulosic wastes for improved biogas production by anaerobic digestion. Int. J. Environ. Sci. Technol. 2022;19:3429–3456. doi: 10.1007/s13762-021-03248-8. [DOI] [Google Scholar]
- 6.Weide T., Baquero C.D., Schomaker M., Brügging E., Wetter C. Effects of enzyme addition on biogas and methane yields in the batch anaerobic digestion of agricultural waste (silage, straw, and animal manure) Biomass Bioenergy. 2020;132 doi: 10.1016/j.biombioe.2019.105442. [DOI] [Google Scholar]
- 7.Paul S., Dutta A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recycl. 2018;130:164–174. doi: 10.1016/j.resconrec.2017.12.005. [DOI] [Google Scholar]
- 8.Zhen G., Lu X., Kato H., Zhao Y., Li Y.-Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017;69:559–577. doi: 10.1016/j.rser.2016.11.187. [DOI] [Google Scholar]
- 9.Tisma M., Planinic M., Bucic-Kojic A., Panjicko M., Zupancic G.D., Zelic B. Corn silage fungal-based solid-state pretreatment for enhanced biogas production in anaerobic co-digestion with cow manure. Bioresour. Technol. 2018;253:220–226. doi: 10.1016/j.biortech.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J., Zheng Y., Li Y. Fungal pretreatment of yard trimmings for enhancement of methane yield from solid-state anaerobic digestion. Bioresour. Technol. 2014;156:176–181. doi: 10.1016/j.biortech.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Intasit R., Khunrae P., Meeinkuirt W., Soontorngun N. Fungal pretreatments of Napier grass and sugarcane leaves for high recovery of lignocellulosic enzymes and methane production. Ind. Crop. Prod. 2022;180 doi: 10.1016/j.indcrop.2022.114706. [DOI] [Google Scholar]
- 12.Ali S.S., Mustafa A.M., Kornaros M., Sun J., Khalil M., El-Shetehy M. Biodegradation of creosote-treated wood by two novel constructed microbial consortia for the enhancement of methane production. Bioresour. Technol. 2021;323 doi: 10.1016/j.biortech.2020.124544. [DOI] [PubMed] [Google Scholar]
- 13.Yadav M., Vivekanand V. Combined fungal and bacterial pretreatment of wheat and pearl millet straw for biogas production – a study from batch to continuous stirred tank reactors. Bioresour. Technol. 2021;321 doi: 10.1016/j.biortech.2020.124523. [DOI] [PubMed] [Google Scholar]
- 14.Guan R., Li X., Wachemo A.C., Yuan H., Liu Y., Zou D., Zuo X., Gu J. Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pretreatment. Sci. Total Environ. 2018;637–638:9–17. doi: 10.1016/j.scitotenv.2018.04.366. [DOI] [PubMed] [Google Scholar]
- 15.Bala R., Mondal M.K. Study of biological and thermo-chemical pretreatment of organic fraction of municipal solid waste for enhanced biogas yield. Environ. Sci. Pollut. Res. 2020;27(22):27293–27304. doi: 10.1007/s11356-019-05695-w. [DOI] [PubMed] [Google Scholar]
- 16.Narinthorn R., Choorit W., Chisti Y. Alkaline and fungal pretreatments for improving methane potential of Napier grass. Biomass Bioenergy. 2019;127 doi: 10.1016/j.biombioe.2019.105262. [DOI] [Google Scholar]
- 17.Fang Y., Si B., Qiu J., Wen Q., An M., Wang B., Jiang W. Bioconversion of bamboo shoot shell to methane assisted by microwave irradiation and fungus metabolism. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138268. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y., Xu C., Ai S., Wang H., Gao Y., Yan L., Mei Z., Wang W. Biological pretreatment enhances the activity of functional microorganisms and the ability of methanogenesis during anaerobic digestion. Bioresour. Technol. 2019;290 doi: 10.1016/j.biortech.2019.121660. [DOI] [PubMed] [Google Scholar]
- 19.Yadav M., Vivekanand V. Biological treatment of lignocellulosic biomass by Curvularia lunata for biogas production. Bioresour. Technol. 2020;306 doi: 10.1016/j.biortech.2020.123151. [DOI] [PubMed] [Google Scholar]
- 20.Noonari A.A., Mahar R.B., Sahito A.R., Brohi K.M. Effects of isolated fungal pretreatment on bio-methane production through the co-digestion of rice straw and buffalo dung. Energy. 2020;206 doi: 10.1016/j.energy.2020.118107. [DOI] [Google Scholar]
- 21.Dollhofer V., Dandikas V., Dorn-In S., Bauer C., Lebuhn M., Bauer J. Accelerated biogas production from lignocellulosic biomass after pre-treatment with Neocallimastix frontalis. Bioresour. Technol. 2018;264:219–227. doi: 10.1016/j.biortech.2018.05.068. [DOI] [PubMed] [Google Scholar]
- 22.Kasana R.C., Salwan R., Dhar H., Dutt S., Gulati A. A rapid and easy method for the detection of microbial cellulases on agar plates using gram's iodine. Curr. Microbiol. 2008;57(5):503–507. doi: 10.1007/s00284-008-9276-8. [DOI] [PubMed] [Google Scholar]
- 23.Li Q., Ng W.T., Wu J.C. Isolation, characterization and application of a cellulose-degrading strain Neurospora crassa S1 from oil palm empty fruit bunch. Microb. Cell Factories. 2014;13:8. doi: 10.1186/s12934-014-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 25.Wu H., Liu B., Ou X., Pan S., Shao Y., Huang F. Streptomyces thermoalkaliphilus sp. nov., an alkaline cellulase producing thermophilic actinomycete isolated from tropical rainforest soil. Antonie Leeuwenhoek. 2018;111(3):413–422. doi: 10.1007/s10482-017-0964-x. [DOI] [PubMed] [Google Scholar]
- 26.Adney B.J. 1996. Measurement of Cellulase Activities: Laboratory Analytical Procedure (LAP) [Google Scholar]
- 27.de Carvalho Silvello M.A., Martinez J., Goldbeck R. Low-frequency ultrasound with short application time improves cellulase activity and reducing sugars release. Appl. Biochem. Biotechnol. 2020;191(3):1042–1055. doi: 10.1007/s12010-019-03148-1. [DOI] [PubMed] [Google Scholar]
- 28.Qiu X., Wang S., Miao S., Suo H., Xu H., Hu Y. Co-immobilization of laccase and ABTS onto amino-functionalized ionic liquid-modified magnetic chitosan nanoparticles for pollutants removal. J. Hazard Mater. 2020;401 doi: 10.1016/j.jhazmat.2020.123353. [DOI] [PubMed] [Google Scholar]
- 29.Franco R.T., Buffière P., Bayard R. Co-ensiling of cattle manure before biogas production: effects of fermentation stimulants and inhibitors on biomass and methane preservation. Renew. Energy. 2018;121:315–323. doi: 10.1016/j.renene.2018.01.035. [DOI] [Google Scholar]
- 30.Pham C.H., Triolo J.M., Cu T.T., Pedersen L., Sommer S.G. Validation and recommendation of methods to measure biogas production potential of animal manure. Asian-Australas. J. Anim. Sci. 2013;26(6):864–873. doi: 10.5713/ajas.2012.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei S., Zhang H., Cai X., Xu J., Fang J., Liu H. Psychrophilic anaerobic co-digestion of highland barley straw with two animal manures at high altitude for enhancing biogas production. Energy Convers. Manag. 2014;88:40–48. doi: 10.1016/j.enconman.2014.08.018. [DOI] [Google Scholar]
- 32.Van-Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 33.Zhao J., Ge X., Vasco-Correa J., Li Y. Fungal pretreatment of unsterilized yard trimmings for enhanced methane production by solid-state anaerobic digestion. Bioresour. Technol. 2014;158C:248–252. doi: 10.1016/j.biortech.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Morita R.Y. Psychrophilic bacteria. Bacteriol. Rev. 1975;39:144–167. doi: 10.1128/br.39.2.144-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong X., Cai M. Science press; Beijing: 2001. Manual of Determinative Bacteriology. [Google Scholar]
- 36.Zanellati A., Spina F., Bonaterra M., Dinuccio E., Varese G.C., Scarpeci T.E. Screening and evaluation of phenols and furans degrading fungi for the biological pretreatment of lignocellulosic biomass. Int. Biodeterior. Biodegrad. 2021;161 doi: 10.1016/j.ibiod.2021.105246. [DOI] [Google Scholar]
- 37.Suksong W., Wongfaed N., Sangsri B., Kongjan P., Prasertsan P., Podmirseg S.M., Insam H., O-Thong S. Enhanced solid-state biomethanisation of oil palm empty fruit bunches following fungal pretreatment. Ind. Crop. Prod. 2020;145 doi: 10.1016/j.indcrop.2020.112099. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.