Abstract
Ureaplasma urealyticum and Ureaplasma parvum are important causes of septic arthritis in patients with hypogammaglobulinemia. The diagnosis can be challenging, leading to prolonged illness and increased morbidity, and mortality. This is driven by the complex growth media requirements of Ureaplasma species and the difficulty in identifying the organisms on routine culture media. Herein, we present a case of native joint polyarticular septic arthritis and vertebral infection secondary to disseminated U. urealyticum in a patient maintained on rituximab. The diagnosis was established through a positive species-specific U. urealyticum polymerase chain reaction (PCR) after a meticulous workup including synovial fluid biopsy, cultures and broad-range bacterial PCR returned negative. Septic arthritis caused by Ureaplasma species should be considered in the differential diagnosis especially in immunocompromised patients with hypogammaglobulinemia, even if the initial microbiological workup is non-revealing. Delayed diagnosis and treatment are associated with increased morbidity.
Keywords: Ureaplasma urealyticum, Polyarticular septic arthritis, Discitis, Osteomyelitis, Polymerase-chain reaction, PCR
Introduction
Ureaplasmas are small, free-living organisms that colonize the genitourinary tract of healthy humans at varying frequencies [1], [2]. The genus Ureaplasma comprises two species, Ureaplasma urealyticum and Ureaplasma parvum which are implicated in a number of urogenital infections such as epididymitis, chorioamnionitis and nongonococcal urethritis [2], [3]. In immunocompromised patients such as those with hypogammaglobulinemia [4], [5], [6], hematologic malignancy [7], and solid organ transplant recipients [8], these organisms can lead to extragenital and disseminated infections. Septic arthritis caused by Ureaplasma species has been well described in patients with X-linked agammaglobulinemia (XLA) and common variable immunodeficiency (CVI) [4], [5], [6], [9]. This clinical presentation has been increasingly recognized in patients receiving immunosuppressive therapy, especially rituximab, which leads to prolonged B-cell depletion and secondary hypogammaglobulinemia [10]. Herein, we present a case of polyarticular septic arthritis and discitis/osteomyelitis secondary to disseminated U. urealyticum in a patient maintained on rituximab. The challenges in diagnosis are illustrated by the extensive negative initial microbiological workup, including broad-range bacterial polymerase chain reactions (PCR) on multiple tissue samples.
Case presentation
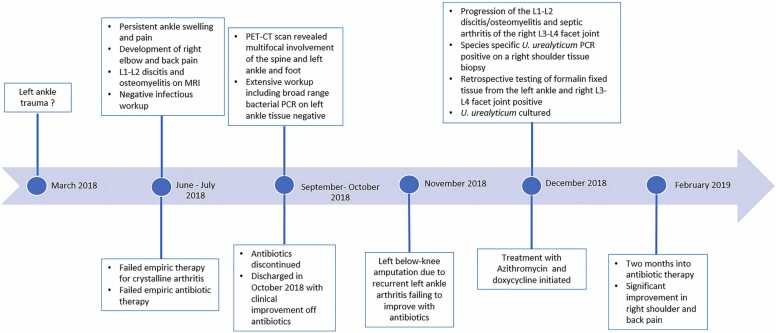
The patient was a 54-year-old man who had developed progressive joint pains for several months. He had a history of multiple sclerosis and was treated with rituximab infusions every 6 months but was otherwise healthy. He was sexually active with one female partner and had no known history of sexually transmitted infections. The patient’s timeline is shown in Fig. 1. His symptoms began in early 2018 with pain and swelling in the left ankle. During initial evaluations, crystalline arthritis was suspected; however, empiric treatment with non-steroidal anti-inflammatory medications, colchicine, and intra-articular steroid injections were ineffective and symptoms progressed to involve the right elbow. Aspiration of the elbow joint revealed >100,000 cells per microliter with neutrophilic predominance. Crystals were absent on microscopic examination and cultures were negative. There was no documentation of blood cultures obtained at the time. He underwent arthroscopic irrigation and debridement of the right elbow at an external facility. Given his immunocompromised state, he was prescribed a course of antibiotics including trimethoprim-sulfamethoxazole, levofloxacin, and vancomycin to empirically target Staphylococcus aureus, including methicillin-resistant strains, and Pseudomonas spp. The right elbow symptoms abated, however the left ankle pain persisted despite antibiotic therapy, and this was followed by the development of severe back pain in July 2018. Laboratory evaluation revealed an erythrocyte sedimentation rate (ESR) of 96 mm/ 1 h (reference range: 0–22 mm/ 1 h), C-reactive protein (CRP) of 171 mg/L (reference range: ≤8.0 mg/L) and MRI showed L1-L2 discitis and osteomyelitis (Fig. 2). Percutaneous biopsies of the L1 vertebral body and L1-L2 disc space revealed inflammation; however, bacterial cultures were negative. Antibiotics were modified to ertapenem and daptomycin, but without improvement.
Fig. 1.
Patient’s timeline.
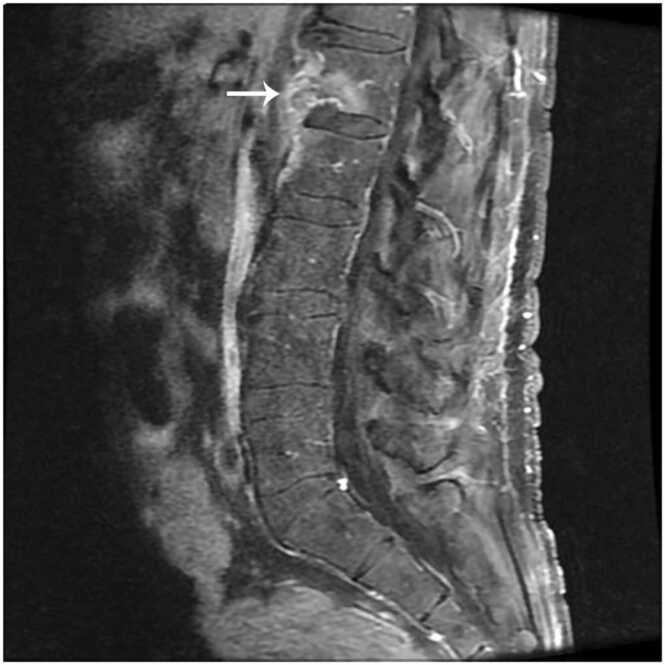
Fig. 2.
Gadolinium enhanced, T1 weighted MRI of the lumbar spine showing abnormal enhancement of the L1-L2 endplates and paraspinal soft tissues.
In September 2018, he presented to our institution for a second opinion. On physical examination, the left ankle was warm, swollen, erythematous and tender to the touch. He also experienced pain on active and passive range of motion. The right elbow examination was not concerning for active infection or arthritis, and he did not have point tenderness on palpation of the spine. Antibiotics were held on admission given the lack of improvement despite several months of continuous therapy. Several attempts at aspiration of the left ankle yielded minimal fluid for analysis. These were prioritized for bacterial, fungal, and mycobacterial cultures, which were negative. Given the multifocal involvement, a PET-CT scan was obtained which revealed L1-L2 discitis/osteomyelitis with progressive osseous destruction and early paraspinal extension, in addition to multifocal FDG activity within the joints of the left ankle and foot (Fig. 3). There were no findings concerning for infective endocarditis on transesophageal echocardiography. Open biopsy of the left ankle synovium demonstrated acute inflammation, while percutaneous L1 vertebral body and L1-L2 disk space biopsies showed benign bone and focal fibrosis. Bacterial, fungal, and mycobacterial cultures were negative. Tissue blocks were reviewed, and no organisms were seen on special stains. Broad range bacterial PCR from the ankle was positive for Streptococcus mitis group from one of two specimens, but this was not considered significant. An infectious workup for Whipple’s disease, endemic mycoses, Cryptococcus spp., Coxiella burnetii, Brucella spp., Sporothrix spp., Lyme disease, Parvovirus B19, human immunodeficiency virus (HIV), Neisseria gonorrhea, hepatitis B and C, and syphilis were negative. Concomitantly, evaluation for non-infectious causes including inflammatory arthritis (particularly spondyloarthritis and SAPHO syndrome) and crystalline arthritis was negative. The patient reported subjective improvement and requested discharge after a one-month hospitalization. He was discharged without anti-infective therapy but with plans for close outpatient follow-up.
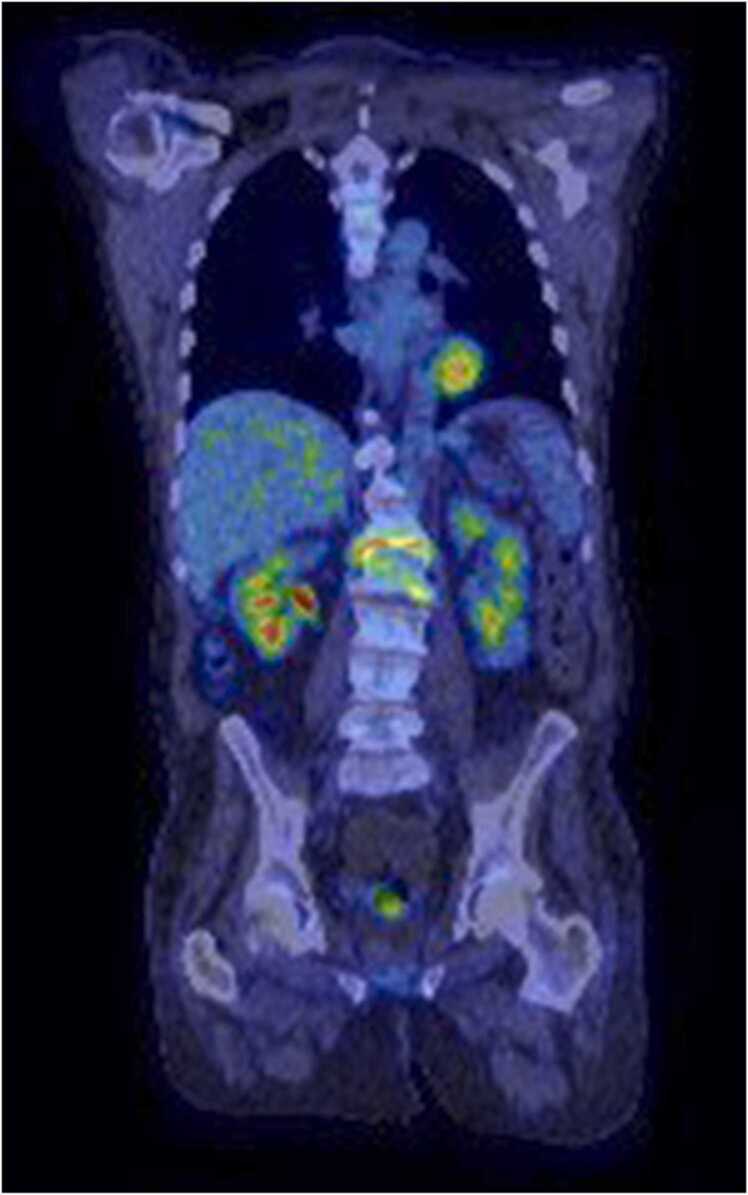
Fig. 3.
PET-CT scan showing L1-L2 discitis/osteomyelitis with progressive osseous destruction.
Following discharge, his left ankle arthritis worsened, and he was hospitalized in other institutions and underwent left below-knee amputation. Symptoms spread to other areas. The patient was readmitted to our facility in December 2018 with bilateral shoulder and chest pain. MRI of the spine demonstrated progression of the L1-L2 discitis/osteomyelitis and septic arthritis of the right L3-L4 facet joint. Laboratory studies showed absence of CD19/20 cells and hypogammaglobulinemia with an IgG level of 417 mg/dL (reference range: 767 – 1590 mg/dL). In the setting of hypogammaglobulinemia, infections related to mycoplasmas were reconsidered. A right shoulder tissue biopsy was sent for species-specific Ureaplasma spp. and Mycoplasma spp. PCR and returned positive for U. urealyticum. Retrospective testing of formalin-fixed tissue from the left ankle and right L3-L4 facet joint confirmed the diagnosis of septic arthritis due to disseminated U. urealyticum infection. The organism was successfully cultured in 10B broth at a reference laboratory, and antimicrobial susceptibility testing performed via microbroth dilution [11] demonstrated susceptibility to tetracycline, erythromycin, and levofloxacin. Treatment with doxycycline 100 mg orally twice daily and azithromycin 500 mg orally daily was initiated and continued for 4 months with complete resolution of symptoms.
Discussion
Previous studies have demonstrated that mycoplasmas and ureaplasmas can remain viable inside neutrophils in the absence of antibodies [12], and patients with hypogammaglobulinemia are susceptible to mucosal surface colonization by these organisms, likely due to the absence of protective mucosal antibodies [4], [6]. This combination of factors is hypothesized to facilitate the dissemination of these organisms inside neutrophils to areas of active neutrophil recruitment, such as traumatic joints [4]. Rarely, Ureaplasma species can cause septic arthritis in immunocompetent patients, however, the infection seems to predominantly affect older patients with prosthetic joints [9], [13], [14], [15].
Early in the disease course, patients typically present with monoarthritis involving the shoulder, elbow, knee, or hip, which may be misdiagnosed as inflammatory arthritis, leading to delays in adequate treatment and subsequent dissemination and polyarticular joint involvement [5]. Our patient initially presented with septic arthritis of the left ankle, which later progressed to polyarticular septic arthritis and discitis/osteomyelitis. These organisms require specialized media for cultivation, and cultures are performed in a few reference laboratories in the United States [11]. Nucleic acid amplification assays such as 16S rRNA polymerase chain reaction (PCR), microbial cell-free DNA [9], and species-specific PCR have become more widely available and are replacing cultures for clinical diagnosis. Susceptibility testing however requires isolation of the organism in culture media. The diagnosis in our patient was challenging as initial broad range bacterial PCR obtained on multiple left ankle tissue samples returned negative.
Ureaplasmas lack a peptidoglycan cell wall and do not synthesize folic acid, rendering them resistant to agents acting on these targets such as β-lactams, trimethoprim, and sulfonamides [16], which are commonly used to treat septic arthritis. This explains the lack of improvement in our patient despite prolonged empiric courses of vancomycin, trimethoprim-sulfamethoxazole, daptomycin and ertapenem. Treatment entails the use of quinolones, tetracyclines, or macrolides as these organisms are usually susceptible to these classes of antibiotics [16]. The duration of treatment is variable and is guided by the clinical syndrome, extent of infection and response to therapy. In instances where antimicrobial susceptibilities are not available, empiric treatment with two potentially active antibiotics may be considered since clinical failures with monotherapy have been reported [5], [10], [16].
In conclusion, Ureaplasma spp. are uncommon, but important causes of septic arthritis in immunocompromised patients with hypogammaglobulinemia. The infection can manifest with mono-, oligo-, or polyarticular involvement and should be highly suspected when bacterial cultures return negative and patients fail to improve on empiric antibiotics. Nucleic acid amplification assays are the mainstay of diagnosis for this infection; however, false-negative results may be encountered. Empiric therapy containing a macrolide, quinolone, tetracycline, or a combination of these antibiotics may be considered in patients with typical host factors and a consistent clinical presentation, as delays in diagnosis and treatment are associated with high morbidity.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Said El Zein: Writing, Tom Garvey: Writing, Shreyasee Amin: Writing, Aaron Tande: Conceptualization, reviewing draft.
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.McCormack W.M., Rosner B., Alpert S., Evrard J.R., Crockett V.A., Zinner S.H. Vaginal colonization with Mycoplasma hominis and Ureaplasma urealyticum. Sex Transm Dis. 1986:13. doi: 10.1097/00007435-198604000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Sweeney E.L., Dando S.J., Kallapur S.G., Knox C.L. The human ureaplasma species as causative agents of chorioamnionitis. Clin Microbiol Rev. 2017;30:349–379. doi: 10.1128/CMR.00091-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeton M.L., Payne M.S., Jones L. The role of Ureaplasma spp. in the development of Nongonococcal urethritis and infertility among men. Clin Microbiol Rev. 2019:32. doi: 10.1128/CMR.00137-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom K.A., Chung D., Cunningham-Rundles C. Osteoarticular infectious complications in patients with primary immunodeficiencies. Curr Opin Rheuma. 2008;20:480–485. doi: 10.1097/BOR.0b013e3282fd6e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franz A., Webster A.D., Furr P.M., Taylor-Robinson D. Mycoplasmal arthritis in patients with primary immunoglobulin deficiency: clinical features and outcome in 18 patients. Br J Rheuma. 1997;36:661–668. doi: 10.1093/rheumatology/36.6.661. [DOI] [PubMed] [Google Scholar]
- 6.Furr P.M., Taylor-Robinson D., Webster A.D. Mycoplasmas and ureaplasmas in patients with hypogammaglobulinaemia and their role in arthritis: microbiological observations over twenty years. Ann Rheum Dis. 1994;53:183–187. doi: 10.1136/ard.53.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balsat M., Galicier L., Wargnier A., Pereyre S., Itzykson R., Zouakh M., et al. Diagnosis of ureaplasma urealyticum septic polyarthritis by PCR assay and electrospray ionization mass spectrometry in a patient with acute lymphoblastic leukemia. J Clin Microbiol. 2014;52:3456–3458. doi: 10.1128/JCM.00963-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon C.A., Corcorran M.A., Shaw K.W., Montenovo M., Sibulesky L., Reyes J.D., et al. Hyperammonemia syndrome due to Ureaplasma infection after liver-kidney transplant. Transpl Infect Dis. 2020;22 doi: 10.1111/tid.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asif A.A., Roy M., Ahmad S. Rare case of Ureaplasma parvum septic arthritis in an immunocompetent patient. BMJ Case Rep. 2020:13. doi: 10.1136/bcr-2020-236396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jhaveri V.V., Lasalvia M.T. Invasive ureaplasma infection in patients receiving rituximab and other humoral immunodeficiencies—a case report and review of the literature. Open Forum Infect Dis. 2019:6. doi: 10.1093/ofid/ofz399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2011. Methods for antimicrobial susceptibility testing for human mycoplasmas; approved guideline. CLSI document M43-A. [PubMed] [Google Scholar]
- 12.Webster A.D., Furr P.M., Hughes-Jones N.C., Gorick B.D., Taylor-Robinson D. Critical dependence on antibody for defence against mycoplasmas. Clin Exp Immunol. 1988;71:383–387. [PMC free article] [PubMed] [Google Scholar]
- 13.Rouard C., Pereyre S., Abgrall S., Guillet-Caruba C., Diviné P., Bourgeois-Nicolaos N., et al. Early prosthetic joint infection due to Ureaplasma urealyticum: Benefit of 16S rRNA gene sequence analysis for diagnosis. J Microbiol Immunol Infect. 2019;52:167–169. doi: 10.1016/j.jmii.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Farrell J.J., Larson J.A., Akeson J.W., Lowery K.S., Rounds M.A., Sampath R., et al. Ureaplasma parvum prosthetic joint infection detected by PCR. J Clin Microbiol. 2014;52:2248–2250. doi: 10.1128/JCM.00432-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sköldenberg O.G., Rysinska A.D., Neander G., Muren O.H., Ahl T.E. Ureaplasma urealyticum infection in total hip arthroplasty leading to revision. J Arthroplast. 2010;25 doi: 10.1016/j.arth.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Fernández J., Karau M.J., Cunningham S.A., Greenwood-Quaintance K.E., Patel R. Antimicrobial susceptibility and clonality of clinical ureaplasma isolates in the United States. Antimicrob Agents Chemother. 2016;60:4793–4798. doi: 10.1128/AAC.00671-16. [DOI] [PMC free article] [PubMed] [Google Scholar]