Abstract
The efficacy of redosing the recombinant adeno-associated virus (rAAV) vector rAAV2.5T to ferret lung is limited by AAV neutralizing antibody (NAb) responses. While immunosuppression strategies have allowed for systemic rAAV repeat dosing, their utility for rAAV lung-directed gene therapy is largely unexplored. To this end, we evaluated two immunosuppression (IS) strategies to improve repeat dosing of rAAV2.5T to ferret lungs: (1) a combination of three IS drugs (Tri-IS) with broad coverage against cellular and humoral responses (methylprednisolone [MP], azathioprine, and cyclosporine) and (2) MP alone, which is typically used in systemic rAAV applications. Repeat dosing utilized AAV2.5T-SP183-fCFTRΔR (recombinant ferret CFTR transgene), followed 28 days later by AAV2.5T-SP183-gLuc (for quantification of transgene expression). Both the Tri-IS and MP strategies significantly improved transgene expression following repeat dosing and reduced AAV2.5T NAb responses in the bronchioalveolar lavage fluid (BALF) and plasma, while AAV2.5T binding antibody subtypes and cellular immune responses by ELISpot were largely unchanged by IS. One exception was the reduction in plasma AAV2.5T binding immunoglobulin G (IgG) in both IS groups. Only the Tri-IS strategy significantly suppressed splenocyte expression of IFNA (interferon α [IFN-α]) and IL4. Our studies suggest that IS strategies may be useful in clinical application of rAAV targeting lung genetic diseases such as cystic fibrosis.
Keywords: cystic fibrosis transmembrane conductance regulator, CFTR, recombinant adeno-associated virus, rAAV, ferret, gene therapy, immune response, immunosuppression, neutralizing antibody, cytotoxic T lymphocytes, AAV readministration
Graphical abstract
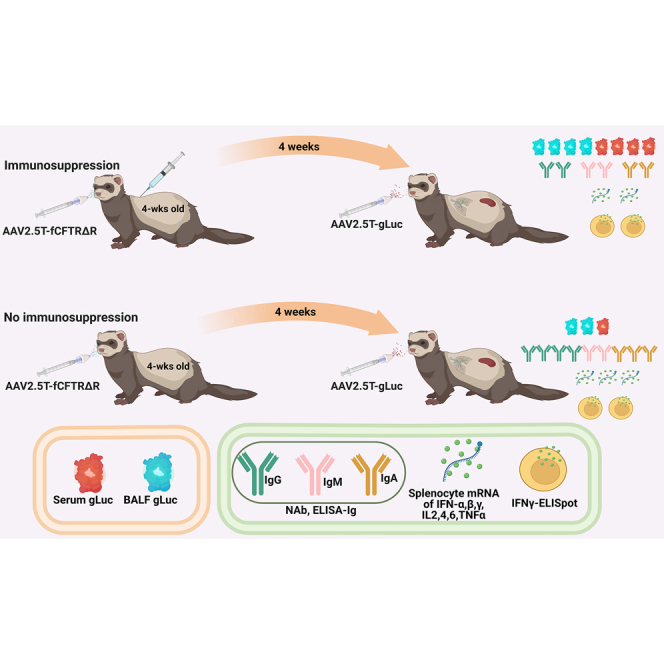
John Engelhardt and colleagues developed an immunosuppressive strategy to mitigate neutralizing antibody responses to an AAV2.5T (adeno-associated viral vector) and improve transgene expression following repeat administration to the ferret lung for gene therapy of cystic fibrosis.
Introduction
Cystic fibrosis (CF) affects at least 70,000 people worldwide according to the patient registry of the CF Foundation. CF is an autosomal recessive genetic disorder caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, which encodes a chloride and bicarbonate channel expressed in epithelial cells.1 More than 2,000 distinct CFTR variants have been identified,2 among which approximately 300 mutations cause CF.3 CFTR interacts with other ion channels, such as the epithelial sodium channel (ENaC), to regulate fluid movement across epithelia and thus can have cell-intrinsic functions based on the composition of other channels co-expressed with CFTR.4 Lung disease is the primary cause of morbidity and mortality in patients with CF, where the loss of CFTR-mediated chloride and bicarbonate transport leads to impaired mucus clearance and chronic bacterial infection.
Recently, the FDA approved the triple combination of CFTR modulators (elexacaftor/tezacaftor/ivacaftor) as an effective treatment for ∼90% of patients with CF who have at least one copy of the F508del CFTR mutation. However, development of other therapeutic strategies is required to treat the remaining patients with CF who have drug-refractory missense mutations, who produce little to no CFTR protein, or who cannot tolerate treatment with CFTR modulators.5 To this end, CFTR gene replacement strategies are being pursued to treat patients with CF in a mutation-agnostic fashion.6
A recombinant adeno-associated virus (rAAV) type 2 vector (rAAV2) was used in the first rAAV clinical trials in patients with CF.7,8 While these trials failed to meet efficacy endpoints due to the lack of CFTR mRNA expression, they did demonstrate that rAAV delivered to the human lung was safe.7 Other advantages of rAAV for pulmonary administration include its low immunogenicity, low risk of integration, and episomal persistence of vector genomes in transduced cells.9,10,11
Reasons for the lack of efficacy in the rAAV2 CF clinical trial were later discovered to involve both the biology of rAA2 transduction in airway epithelia and vector design.4 First, apical transduction of human airway epithelium (HAE) with rAAV2 encounters a proteasome-dependent post-entry block in transport of the virus to the nucleus.12,13 Second, the small vector package capacity of rAAV necessitated the use of the weak intrinsic promoter activity of the inverted terminal repeat (ITR) sequence for expression of the full-length CFTR cDNA. To solve these obstacles, a human airway tropic AAV capsid variant (AAV2.5T) was identified by directed evolution of a library of AAV2 and AAV5 shuffled and mutated capsids after multiple rounds of apical transduction in HAE.14 We have previously demonstrated that rAAV2.5T can transduce polarized ferret airway epithelium grown at an air-liquid interphase (ALI) and ferret lung in vivo.15 Other advancements in vector design involved generating a smaller CFTR minigene (CFTRΔR)16 and a strong short synthetic promoter/enhancer (SP183) element that is efficiently expressed in human and ferret airway epithelial cells.17 These last two improvements enabled packaging of the SP183-fCFTRΔR transgene cassette in the absence of vector genome truncations.17 These advances in vector design led to the recombinant virus (rAAV2.5T-SP183-fCFTRΔR) used in this study.17
Cells accessible to a gene transfer vector from the airway luminal surface primarily include ciliated cells and secretory cells. Ciliated cells are terminally differentiated, while secretory cells have the capacity to divide. The durability of an episomal persistent viral vector, such as rAAV, is dependent on the lifespan of transduced cells. While the lifespan of human airway cell types remains unclear, the average half-life of a mouse ciliated cell is ∼6 months in the trachea and ∼17 months in the intralobar airways.18 Because of the relatively slow turnover rate of airway epithelia, functional complementation in transduced cells will likely persist for a finite period. However, the rate of cellular turnover in the terminally diseased human CF lung is predicted to be 75-fold higher than healthy lungs, based on the number of dividing cells,19 suggesting that repeat dosing of rAAV will be required. Despite the low immunogenicity compared with other viral vectors, rAAV vectors still induce host immunity, including innate and adaptive immune responses.4,20,21 Thus, the extent of the immune response will also impact durability of rAAV-mediated therapy and the dosing interval required to maintain a therapeutic effect.
Our previous study demonstrated that the repeat dosing of rAAV2.5T to juvenile ferret lungs at a monthly interval resulted in reduced transgene expression from the second dose of rAAV2.5T and in significant increases in the level of neutralizing antibodies (NAbs) in plasma and bronchoalveolar lavage fluid (BALF) compared with single-vector administration to age-matched naive ferrets.15 Immune responses elicited by rAAV administration can include innate, adaptive humoral (B cell), and/or cellular (T cell) responses, which can be significantly elevated by repeated exposure to the homologous capsid.22 Pharmacologic immunosuppression can dampen immune responses observed following repeat dosing of rAAV. Several immunosuppression strategies have been explored in animal models to improve the efficacy of rAAV repeat dosing and/or the durability of transgene expression. For example, sustained expression of dystrophin was achieved in striated muscles of rAAV-injected dystrophic dogs treated with a short course of the cyclosporine and mycophenolate mofetil immunosuppressants.23,24 Similarly, treatment with dexamethasone prior to rAAV9 dosing augmented factor IX expression from the liver of C57BL/6 mice.25
Several clinical trials have reported that the combined use of corticosteroids with rAAV delivery can improve transgene expression and control inflammatory responses. During the in-life phase of the hemophilia B gene therapy with rAAV encoding factor IX–R338L, two subjects that developed elevated liver enzymes were administrated prednisone, following which factor IX coagulant activity was sustained.26 In another AAV gene therapy clinical trial for Leber’s hereditary optic neuropathy, corticosteroids were used to manage the ocular inflammation after intravitreal administration of rAAV2/2-ND4.27
The triple combination of methylprednisolone (MP), azathioprine, and cyclosporine has been successfully used in patients undergoing orthotopic lung transplantation to inhibit the adaptive T cell and B cell immune responses to the donor lung. Our group has successfully used this triple immunosuppression (Tri-IS) regimen in ferret lung transplants to slow rejection.28 In this present study, we sought to compare the efficacy of Tri-IS and MP to enhance rAAV2.5T repeat administration to ferret lungs. Tri-IS was used to achieve maximal IS, and MP was evaluated as a practical option for use in CF subjects. In our study, we first utilized an AAV2.5T vector (AAV2.5T-SP183-fCFTRΔR) to selectively induce a humoral response to the AAV2.5T capsid. For the vector redosing, we used the AAV2.5T-SP183-gLuc vector that allowed for accurate quantitation of Gaussia luciferase expression soon after AAV delivery, thus limiting the impact of immune responses against Gaussia luciferase (gLuc). For maximal effect, the immunosuppressive drugs were administered prior to and immediately following the first dose of AAV2.5T, and treatment was maintained for 30 days prior to AAV2.5T redosing 4 weeks after the first dose. The level and duration of expression was compared with repeat-dose animals that were not immunosuppressed.
Results
IS significantly improves transgene expression following repeat dosing of rAAV2.5T
We have previously shown that repeat rAAV2.5T dosing at a 4-week interval to lungs of juvenile ferrets generates a local and systemic NAb response that limits the effectiveness of the second vector administration.15 In this previous work, the first vector dose was AAV2.5T-SP183-fCFTRΔR, and the second vector dose was AAV2.5T-SP183-gLuc, which expressed a secreted gLuc reporter and provided temporal and quantitative assessment of second vector transgene expression. As expected, these studies demonstrated that NAbs originating from AAV2.5T-SP183-fCFTRΔR exposure reduced the efficiency of AAV2.5T-SP183-gLuc transduction in the lung compared with naive controls receiving only AAV2.5T-SP183-gLuc. Thus, we hypothesized that transduction efficiency following vector readministration may be improved if the immune responses to the first vector administration were dampened by IS.
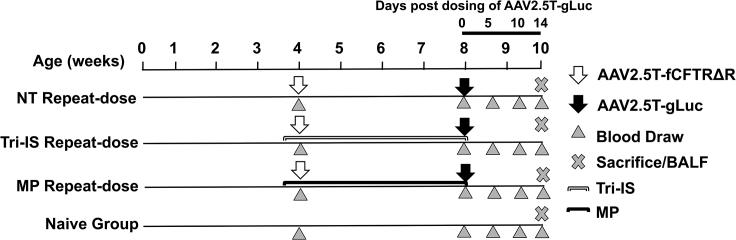
We have previously demonstrated that a combination of cyclosporine, azathioprine, and MP effectively inhibits immune rejection of ferret orthotopic lung transplants28; this IS regimen is also the standard of care for patients that have had an orthotopic lung transplant.29,30 For this rAAV2.5T readministration study, we utilized the three immunosuppressive drug combination (Tri-IS group) treatment and compared its outcomes with both that of MP alone treatment (MP group) and no IS (NT group). Ferrets received the immunosuppressants starting 2 days before administration of the first vector (AAV2.5T-SP183-fCFTRΔR) and were maintained on immunosuppressants for a total of 30 days until the administration of the second vector (AAV2.5T-SP183-gLuc). As a control for gene expression, age-matched ferrets in the NT group were administered the two doses of vector without IS (Figure 1). The activity of gLuc in plasma was monitored following the administration of the second vector (0, 5, 10, and 14 days; Figure 2B). BALF was collected at the time of tissue harvest (14 days following AAV2.5T-SP183-gLuc administration).
Figure 1.
Study design for immunosuppression and repeat dosing of rAAV2.5T to ferret lung
1 × 1013 DRP/kg body weight rAAV2.5T-fCFTRΔR was administrated intratracheally to 4-week-old ferrets, and 4 weeks later, 1 × 1013 DRP/kg rAAV2.5T-gLuc was readministered intratracheally to the lung. The MP or Tri-IS drugs were given daily starting 2 days before the first vector dose and then daily until the day of the second vector dose, for a total duration of 30 days of immunosuppression. Plasma and bronchioalveolar lavage fluid (BALF) was harvested at the indicated time points. n = 15 animals in each group. MP, methylprednisolone alone treatment group; Tri-IS, triple immunosuppression (cyclosporine, MP, and azathioprine) treatment group; NT, no immunosuppression group. The naive group did not receive virus or immunosuppression.
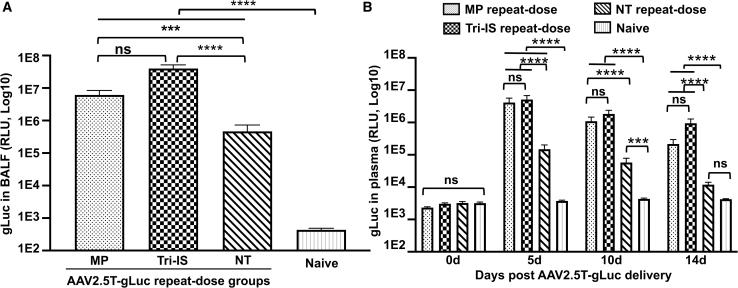
Figure 2.
Efficiency of rAAV2.5T redosing to ferret lung
Secreted gLuc expression in (A) BALF and (B) plasma following intratracheal delivery of rAAV2.5T-fCFTRΔR to 4-week-old ferrets, followed by rAAV2.5T-gLuc 4 weeks later. BALF was harvested 14 days after rAAV2.5T-gLuc delivery, and plasma was harvested at the indicated time points. Results are presented as the mean ± SEM. Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test using log2-transformed data, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. ns, not significant. n = 15 animals per group.
Results from this study found that gLuc activity in the BALF of ferrets in either the MP or Tri-IS groups was significantly higher (13.2-fold, p < 0.001, and 85.6-fold, p < 0.0001), respectively, when compared with the NT group (Figure 2A). We observed no difference in plasma gLuc activity between MP and Tri-IS groups at any time point following the AAV2.5T repeat dosing, although both groups were significantly greater than the NT group (p < 0.0001). The plasma gLuc activity declined between 5 and 14 days in all groups. At the peak of plasma gLuc expression (5 days post-second vector dosing), the gLuc activities in MP and Tri-IS groups were 27.7- and 34.5-fold higher than that in the NT group (Figure 2B).
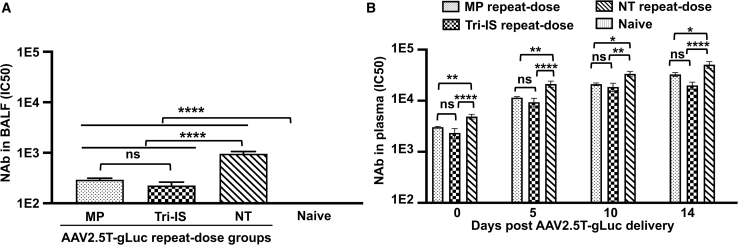
IS inhibits NAb responses against rAAV2.5T in the BALF and plasma
NAb titers in the BALF were significantly lower in the immunosuppressed groups, with the NT group having 4.2- and 3.3-fold higher (p < 0.0001) NAb titers than those of the Tri-IS and MP groups, respectively (Figure 3A). NAb levels in the plasma of ferrets just prior to (day 30) the administration of the AAV2.5T-SP183-gLuc vector revealed NAbs in all groups transduced with AAV2.5T-SP183-fCFTRΔR. Notably, the baseline (day 30) NAb titer in animals of the NT group was significantly higher than that seen in the plasma of animals from both the Tri-IS (2.1-fold, p < 0.0001) and MP (1.6-fold, p < 0.01) groups (Figure 3B), indicating that both immunosuppressive regimens suppressed the production of NAbs elicited from the first vector dose with AAV2.5T-SP183-fCFTRΔR. Although plasma NAb levels from the animals of all treatment groups increased with time in a similar pattern, the levels of NAb in the immunosuppressed groups were significantly lower than that in the NT group at 5, 10, and 14 days after the rAAV2.5T-SP183-gLuc dosing (Figure 3B). These findings demonstrate that IS can effectively inhibit the local lung immune responses that limit second vector administration.
Figure 3.
Level of AAV2.5T-specific neutralizing antibody (NAb) in BALF and plasma following AAV2.5T redosing
NAb titers in the (A) BALF and (B) plasma following intratracheal delivery of rAAV2.5T-fCFTRΔR at 4 weeks of age, followed by rAAV2.5T-gLuc 4 weeks later. Results showed as mean ± SEM. The average NAb titer of the naive group was below the limits of detection in both BALF and plasma. Statistical significance was analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test using log2(Y+1)-transformed data, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. ns, not significant. n = 15 animals in each group.
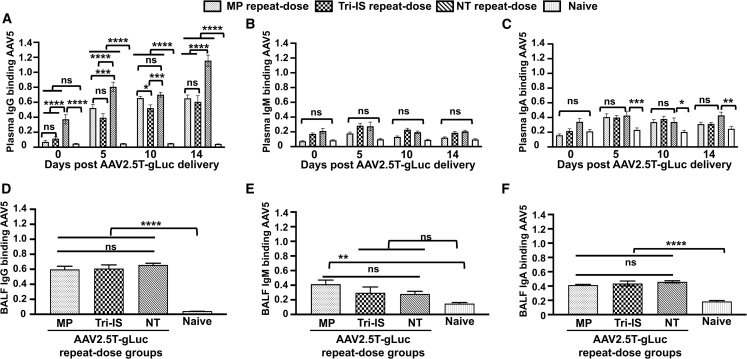
Mapping the AAV2.5T capsid-binding immunoglobulin isotype in the plasma and BALF following IS
We profiled the immunoglobulin (Ig) isotypes that bound to the AAV2.5T capsid following vector redosing in the presence or absence of IS using previously described methods.15 IgG accounts for ∼70%–75% of the total Ig in plasma. Thus, it was not surprising that IgG was the dominant capsid-binding Ig isotype in the plasma of all treatment groups, with the exception of the naive group (Figure 4C). Compared with the NT group, the Tri-IS and MP treatments significantly suppressed (p < 0.0001) capsid-binding IgG in the plasma to background levels following the first dose of AAV2.5T (Figure 4A; day 0 prior to vector redosing). AAV2.5T vector redosing led to increases in plasma capsid-binding IgG in all treatment groups, with animals from the Tri-IS and MP groups having significantly lower titers than the NT group; this difference reached the maximum at 14 days post-vector redosing and was no different compared with the immunosuppressed treatment group (Figure 4A). By contrast, plasma IgM and IgA capsid-binding titers were low and not significantly different between the NT and immunosuppressed treatment groups prior to, or following, vector redosing (Figures 4B and 4C). Similarly, BALF capsid-binding IgG, IgM, and IgA titers were also not different between the NT and immunosuppressed treatment groups at 14 days following vector redosing (Figures 4B–4F). Thus, the dominant effect of IS on the antibody isotype profile was a reduction in plasma capsid-binding IgG generated after the first dose of rAAV2.5T.
Figure 4.
AAV2.5T-binding immunoglobulin (Ig) isotype in plasma and BALF
(A–C) Plasma was harvested at the indicated time points post-rAAV2.5T-gLuc redosing for analysis of AAV2.5T-binding (A) IgG (diluted 4,000-fold for the 0-, 5-, and 10-day samples and 16,000-fold for the 14-day samples), (B) IgM (diluted to 1:2,000), and (C) IgA (diluted to 1:20). (D–F) Neat undiluted BALF was used for analysis of AAV2.5T-binding (D) IgG, (E) IgM, and (F) IgA. Results are presented as the mean ± SEM. Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. Statistical significance is noted as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. ns, not significant. n = 15 animals in each group.
Mapping the splenocyte cytokine transcriptional profiles and the impact of IS following redosing with rAAV2.5T
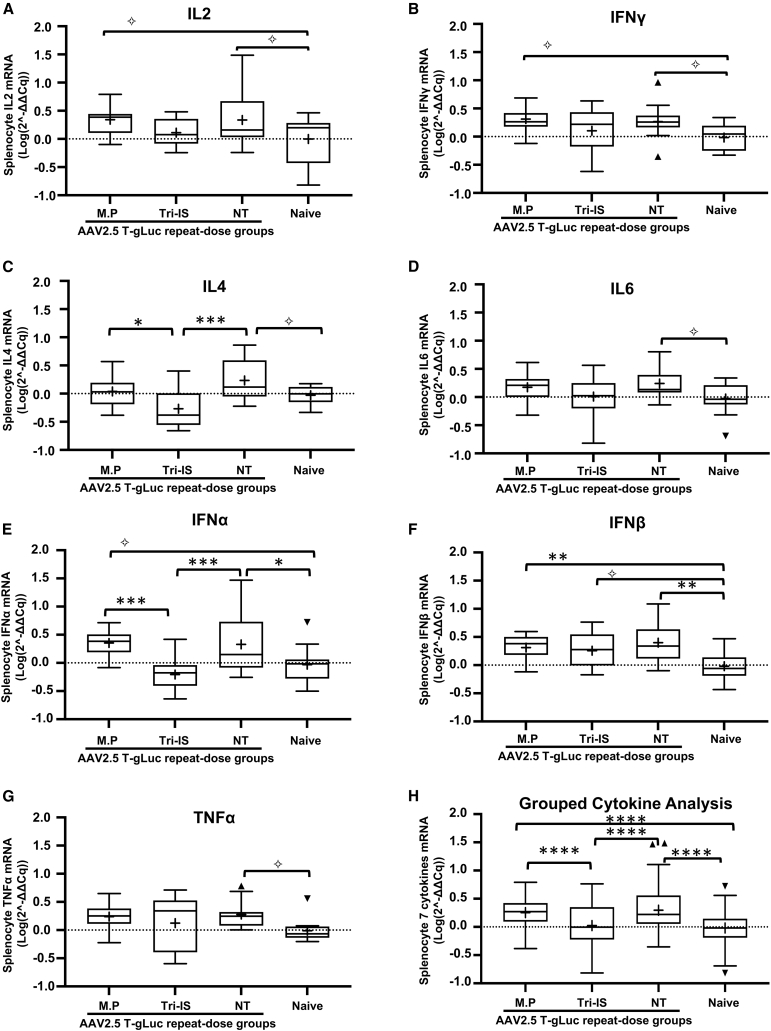
rAAV vectors can activate innate and adaptive immune responses facilitated by the paracrine cytokine signaling.31 Briefly, in response to pathogens, naive CD4 T cells differentiate into effector Th1 and Th2 cells, which secrete cytokines that activate and resolve immune responses. Interleukin-2 (IL-2) and interferon γ (IFN-γ) belong to Th1-type cytokines and are involved in cell-mediated pro-inflammatory responses to viruses and other pathogens.32,33,34 By contrast, IL-4 and IL-6 are able to promote Th2 cell differentiation, which mediates B cell activation, antibody production, and Th1 response regulation.35,36 Type I IFNs including IFN-α and IFN-β play important roles in resolving viral infection and induce an anti-virus state in both infected and bystander cells.37 Tumor necrosis factor α (TNF-α) is a pivotal pro-inflammatory cytokine involved in many aspects of viral immune responses and is induced by macrophages, activated T cells, natural killer cells, and mast cells.38 Here, we quantitated the levels of all these cytokine mRNAs in splenocytes isolated from rAAV2.5T-infected ferrets to index pathways inhibited by IS (Figure 5). mRNAs were quantified by qRT-PCR, and we used ACTB (β-actin) mRNA levels for normalization. Of the seven splenocyte cytokines evaluated, Tri-IS significantly reduced IL-4 (Figure 5C) and IFNA (IFN-α) (Figure 5E) relative to the NT group. Notably, when compared with the NT group, MP treatment had no significant effect on any of the splenocyte cytokine transcripts (Figure 5). As expected with the robust IS provided by Tri-IS, a combined analysis by mixed-effects modeling demonstrated that the Tri-IS group had significantly (p < 0.0001) lower cytokines levels compared with MP and NT groups (Figure 5H).
Figure 5.
Splenocytes cytokine mRNA levels following repeat dosing of rAAV2.5T to the ferret lung
(A–G) Cytokine mRNAs were quantified by reverse transcriptase TaqMan qPCR using β-actin as housekeeping gene for normalization. Individual cytokines are shown in (A)–(G), and (H) represents a combined analysis of all cytokines. The 2(−ΔΔCq) value was transformed to log(n,10) value before doing statistical analysis. The statistical analyses were conducted using R (v.4.0.4). The random intercept mixed-effects model was fit using R package lmer4 (v.1.1.27.1). Tukey’s method was used for post hoc pairwise comparison with p values calculated with ANOVA for (A)–(G) and mixed-effects model for (H). The mean value is indicated as a plus symbol (+). The outlier is indicated as solid triangles. Statistical significance is noted as ✧p < 0.1, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. ns, not significant. n = 12–15 ferrets/group.
IS exerts no significant effect on the T cell response as measured by IFN-γ ELISpot
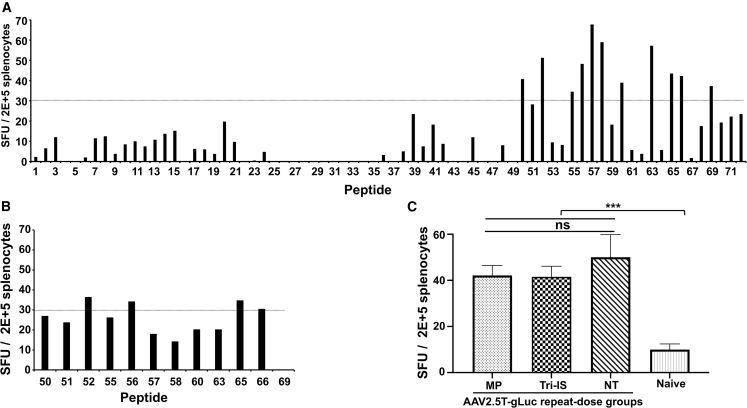
The observation that IFNG (IFN-γ) was significantly (p = 0.0106) upregulated in splenocytes in the NT vs. naive groups (Figure 5B) suggested the potential for AAV2.5T antigen-specific T cell responses following redosing. To this end, we developed an IFN-γ ELISpot assay to measure the AAV2.5T-specific T cell responses. We first synthesized 72 15-mer peptides with a 5-amino acid overlap that spans the entire VP1 sequence of the AAV2.5T capsid. Each peptide was individually screened by ELISpot using splenocytes harvested from three ferrets that were administered two doses of rAAV2.5T to the lung intratracheally (i.t.) and a third dose of AAV2.5T that was dosed half via i.t. and half intramuscularly (i.m.) (Figure 6A). We chose four positive peptides (#52, #56, #65, and #66), which consistently gave >30 spot-forming units (SFU)/2 × 105 splenocytes (Figure 6B), as stimuli in experimental ELISpot assays. We observed a significant increase in SFUs in ferrets of the NT group (44.9 ± 9 SFUs, n = 13 ferrets) vs. the splenocytes from naive ferrets (10 ± 2.4 SFUs, n = 15 ferrets) (Figure 6C; p < 0.001). Likewise, the immune responses observed in the ferrets of both the MP and Tri-IS redosing treatment groups were significantly greater (p < 0.001) compared with the naive splenocytes, but neither the MP nor the Tri-IS groups were significantly different than the NT group (Figure 6C). These results suggest that the immunosuppressive strategies used here had a limited effect on suppressing the cellular immune responses to rAAV2.5T. Given the similar SFU between all vector treated groups with differing transduction efficiencies, the cellular responses may not limit the efficacy of rAAV2.5T redosing in the ferret model.
Figure 6.
AAV2.5T redosing elicits AAV2.5T-specific T cell activation
(A) T cell responses against specific peptides of the AAV2.5T capsid. Seventy-two 15-mer peptides with a 5-mer overlapping sequence were synthesized. As a positive control, splenocytes were harvested from three ferrets that were dosed three times with rAAV2.5T using intratracheal and intramuscular delivery (see materials and methods for details). Three naive ferrets served as negative controls. Each peptide (1 μg/mL) was used as stimulus to induce 2 × 105 splenocytes. The total number of SFUs (spot forming units) was calculated as SFUs in the presence of peptides minus the SFUs with media alone. The positive stimulation control was PMA (10 ng/mL) + ionomycin (0.2 μg/mL), and media only was used as a negative control. (B) Secondary screen of reactive peptides identified in the primary peptide library screen in (A). Peptides that resulted in SFUs >30 in (A) were used in this secondary screen. (C) Peptides 52 (GMTNNLQGSNTYALE), 56 (TSESETQPVNRVAYN), 65 (NTPVPGNITSFSDVP), and 66 (FSDVPVSSFITQYST) were pooled for use in the ELISpot-based analysis of rAAV2.5T repeat-dosed ferrets and naive controls. Data are presented as the mean ± SEM. Statistical significance was analyzed by one-way ANOVA followed by the Tukey’s multiple comparison test with ∗∗∗p < 0.001. ns, not significant.
Discussion
Ferrets and humans have a high level of similarity in airway cytoarchitecture, and thus ferrets have been used to model human respiratory virus infections and genetically inherited pulmonary diseases.39,40,41 For example, submucosal glands in the cartilaginous airways play important roles in lung innate immunity through the secretion of anti-microbial and anti-viral proteins, and the distribution of these structures is conserved between ferret and human but not mice. We have developed CFTR-knockout and CFTR-G551D ferrets that develop a human-like CF disease phenotypes in the lung, pancreas, gallbladder, liver, and intestine.40,41
While gene therapy studies in CF ferrets will ultimately be needed to validate the efficacy of gene therapy approaches, several unsolved questions remain prior to embarking on studies in CF ferrets. For example, any gene replacement approach will likely require repeated administration due to increased cellular turnover in the inflamed and infected CF lung. Thus, in the context of rAAV applications, knowledge of the immune responses that limit efficacy following repeated administration is needed in wild-type animals prior to tackling potentially altered rAAV immunology in the diseased CF lung. Similarly, the efficiency of airway transduction with rAAV vectors in wild-type ferret lungs may be reduced in CF animals due to mucus accumulation in the airways. Lastly, immunosuppressive strategies to mitigate humoral and cellular responses that limit readministration and durability of transgene expression come with potential complications when used in the setting of chronic bacterial infection. To understand how the environment of the CF lung impacts efficacy of rAAV-based therapies, baseline studies in wild-type animals are needed. Thus, this study provides baseline data on immunosuppressive strategies that improve the efficacy of rAAV repeat dosing in the ferret lung.
Management of the humoral and cellular immune responses to AAV vector-based gene therapeutics is paramount to clinical translation of gene-based molecular therapies. Indeed, FDA-approved gene-based treatments, including AAV gene therapeutics for vision loss, caused by biallelic RPE65 mutation-associated retinal dystrophy,42,43 and spinal muscular atrophy (SMA) type I,44,45 incorporate corticosteroids to mitigate the inflammatory consequences of rAAV application in humans. rAAV-mediated gene therapies for CF lung disease have to contend with a diseased lung with significant preexisting inflammation coupled with a higher rate of turnover of airway epithelial cells due to established disease.46 Given that the rAAV genome persists as a circular episome11,12,47 and is diluted with cell division or turnover, it is likely that repeat administration of an rAAV vector therapeutic will be required, especially during the initial phases of treatment and until disease is stabilized.
Different mitigation strategies to inhibit the host immune responses in order to maximize the efficiency of rAAV repeat dosing have been developed and tested in animal models.48,49 In the present study, we sought to evaluate two immunosuppressive regimens (MP and Tri-IS) to improve the efficacy of repeat administration of rAAV2.5T to ferret lungs. Overall, our studies demonstrated that both MP and Tri-IS were efficacious in improving transgene expression following rAAV2.5T redosing. This improvement appears to be largely due to the blunting of the humoral NAb response following the first vector administration, with baseline plasma NAb and capsid-binding antibody titers prior to the vector redosing being reduced in the immunosuppressed vs. naive groups. These reductions in plasma NAb and capsid-binding antibody titers were also sustained for the 14-day period following redosing when IS was stopped, at which time NAb titers in the BALF were also significantly lower in the MP and Tri-IS groups.
While preexisting humoral immunity to the AAV capsid is known to be a major barrier to redosing, the activation of cytotoxic T lymphocyte (CTL) responses to the AAV capsid can also lead to the destruction of transduced cells.21 Glucocorticoids, such as MP, are strong immunosuppressive and anti-inflammatory agents that act broadly on the endocrine and immune system.50 Cyclosporine A and its derivates prevent T cell activation via the formation of a tripartite complex that includes cyclophilin A, CsA, and calcineurin, which inhibits NFAT translocation to the nucleus in T cells.51 Azathioprine, which belongs to the thiopurine family of drugs, inhibits purine synthesis and slows cell proliferation within the immune system.52 We expected that the combination of all three of these immunosuppressive drugs (Tri-IS) would be more effective than MP alone if CTL involvement limited transgene expression following AAV2.5T redosing. Notably, we observed no difference between the Tri-IS and NT groups when comparing ELISpot splenocyte IFN-γ responses with AAV2.5T capsid peptide stimulation. This finding was consistent with the lack of change in splenocyte IFNG mRNA levels between Tri-IS and NT groups. Although all rAAV redosing groups presented with elevated AAV2.5T-reactive splenocytes relative to naive animals, the fold change was small (∼5-fold). In previously published studies, similar results were reported with AAV-treated macaques, where oral prednisone had no significant effect on splenocyte-derived T cell responses as measured by IFN-γ-based ELISpot assays.53 Thus, we interpret the similar action of MP and Tri-IS in improving the efficacy of rAAV2.5T redosing in the ferret to be consistent with lack of significant T cell responses against the rAAV2.5T capsid. One potential caveat is that lung-resident T cells within the lymph nodes may have different levels of T cell reactivity between the redosing groups. While the difference in gLuc activity in the redosing MP and Tri-IS groups did not reach significance, gLuc expression trended lower in the MP group, which may be due to greater resident T cell suppression by Tri-IS.
Cytokines play an important role in coordinating immune responses and their resolution. Our analysis of the splenocyte Th1, Th2, and pro-inflammatory cytokines demonstrated that Tri-IS blunted the IL-2 (Th1), IL-4 (Th2), and TNF-α (pro-inflammatory) responses to AAV2.5T redosing compared with the MP group. Furthermore, the combined analysis of all seven cytokines demonstrated significant suppression in the Tri-IS groups compared with the MP and NT groups. Although Tri-IS led to greater IS, this level of suppression did not significantly enhance transgene expression following redosing when compared with the treatment with MP alone. Thus, in the context of CF, the MP immunosuppressive regimen appears to be best suited for enhancing the efficacy of AAV2.5T redosing. Additional studies evaluating the duration of IS required to improve redosing of AAV2.5T in airway are still needed.
Limitations of our study include the likelihood of a humoral and/or a cellular response against gLuc, which was used for quantification of the effectiveness of AAV2.5T redosing. For example, the positive yet low-level T cell responses in all redosing groups may be the result of cellular immune responses against gLuc. While the study impact of cellular and/or humoral immunity against gLuc remains unclear, future redosing studies will need to be performed using barcoded AAV2.5T-SP183-fCFTRΔR vectors, in which synonymous mutations are introduced into the CFTR coding sequences of the two vectors evaluated. Thus, the capsid is the only foreign protein. These changes will enable the discrimination of endogenous CFTR mRNA from both of the sequentially delivered vector-derived CFTR transcripts. The current study has established many of the parameters needed for a more in-depth analysis of the immunologic responses to AAV2.5T readministration.
Since most patients with CF have inflamed airways in the presence and even in the absence of infection,54 the use of IS drugs in these patients will need to be carefully considered. While controversial, some reports have suggested that prednisone can slow lung disease progression in patients with CF,55 and ongoing clinical studies in patients with CF using a combination of antibiotics and prednisone may provide better clarity on the parameters of steroid use in this patient population.56 Other relevant issues include a greater than 30% prevalence of anti-AAV5 NAbs detected in large international cohorts of healthy donors and patients with hemophilia A or B.57,58
In our studies, the combination of IS with rAAV2.5T administration to the ferret lung improves the efficiency of redosing by significantly blunting the AAV2.5T NAb responses. While our study was designed to exaggerate rAAV2.5T-specific immune responses (i.e., a short interval between the first and the second doses of AAV2.5T) and to evaluate the strength of two immunosuppressive strategies (MP vs. Tri-IS), several important questions remain prior to clinical application. These include the length of IS required for improved efficacy of redosing and the durability of the NAb responses in the lung. The latter of these questions may be impacted by the interval of AAV2.5T redosing required for the sustained clinical benefit in the CF lung. Our study provides a context for understanding the impact of humoral and cellular immune responses in clinical applications of rAAV2.5T to the CF lung.
Materials and methods
Production of rAAV2.5T virus vector
AAV2.5T vectors expressing ferret CFTRΔR (AAV2.5T-SP183-fCFTRΔR), firefly luciferase (AAV2.5T-SP183-fLuc), and gLuc (AAV2.5T-SP183-gLuc) were prepared as previously described.15 Briefly, HEK293 cells were transfected with the AAV capsid, pAV2.5Trepcap, adenovirus helper pAd4.1, and the AAV pro-viral vector with AAV2 ITR sequences plasmids. rAAV vectors released from the transfected HEK293 cells were purified on an iodixanol cushion by ultracentrifuge followed by two cesium chloride (CsCl)-density gradients. Titers were determined as DNase-I-resistant particles (DRPs) by TaqMan qPCR with primers and probes specific to each transgene. The purity of each vector stock was confirmed by SDS-PAGE after silver staining.
Animal dosing
Four-week-old juvenile wild-type ferrets (n = 15 per experimental group) were anesthetized with a mixture of ketamine and xylazine injected subcutaneously and were i.t. administered AAV2.5T-SP183-fCFTRΔR at a dose of 1 × 1013 DRP/kg body weight (BW). Four weeks later, anesthetized ferrets were administered 1 × 1013 DRP/kg BW AAV2.5T-SP183-gLuc. The ferrets were dosed using a microsprayer aerosolizer (PennCentury, model IA-1B). The AAV2.5T vector inoculum contained doxorubicin at a concentration of 200 μM, and the administered volume was normalized to ferret BW at 2 mL/kg BW.
Daily administration of immunosuppressants started 2 days before the first vector dose and continued until the dosing day of the second vector dose (30 days of total dosing). Specifically, the cyclosporine (1 mg/kg BW, Novartis) and MP (2 mg/kg BW, Pfizer) were administered intraperitoneally, and azathioprine (2 mg/kg BW, Wedgewood Pharmacy) was given orally. The antibiotic Baytril (10 mg/kg BW, Bayer) was injected daily subcutaneously to prevent bacterial infection in the immunosuppressed ferrets.
Measurement of gLuc activity in plasma and BALF
On days 0, 5, 10, and 14 after the delivery of the AAV2.5T-SP183-gLuc vector, blood was drawn from the jugular vein of anesthetized ferrets and collected into heparinized tubes for plasma isolation. The experiments were terminated at 14 days post-second vector administration. Ferrets were euthanized with Euthasol (Virbac AH), and BALF was collected by lavage of the trachea/lung cassette using 5 mL PBS per 300 g BW. The gLuc activities in plasma (5 μL) or BALF (5 μL) were immediately measured after sample collection using a Renilla Luciferase Assay Kit (Promega).
Titration of AAV2.5T NAb and capsid-binding antibody levels in plasma and BALF
The level of NAbs in the plasma and BALF was quantified using a microneutralization assay based on the transduction of A549 cells using AAV2.5T-SP183-fLuc incubated with serially diluted plasma or BALF as previously described.15,59 fLuc activity in cell lysates was measured using a Firefly Luciferase Assay Kit (Promega). The titers of AAV2.5T NAbs in each plasma or BALF sample were calculated as the IC50 using Prism software (GraphPad). The levels of total capsid-binding IgG, IgM, and IgA in the plasma and BALF were determined by ELISA using rAAV5 as the capture antigen and previously described methods.15 rAAV5 was used since the VP2 and the VP3 capsid proteins of AAV2.5T are derived from AAV5 with a single A581T mutation. We have previously shown that the use of rAAV5 or rAAV2.5T vectors as the capture antigen gives similar quantification of anti-AAV2.5T capsid-binding antibodies by ELISA.15
Quantitative analysis of cytokines by TaqMan PCR
To investigate the immune responses following rAAV2.5T delivery to the lung, the mRNA expression of pro-inflammatory cytokines IL-2, IL-4, IL6, IFN-α, IFN-β, IFN-γ, and TNF-α was evaluated in splenocytes using TaqMan RT-PCR. β-Actin mRNA was used as an internal control for transcript normalization. Details on the primers and probes used for TaqMan RT-PCR are provided in Table S1.
IFNγ-ELISpot analysis of ferret T cell responses
The T cell responses to the rAAV2.5T capsid were measured using a ferret IFNγ-ELISpot assay (Mabtech, Cincinnati, OH, USA). For AAV2.5T epitope mapping, we used a library of 72 15-mer synthetic peptides with 5-mer overlapping sequences that spanned the entire amino acid sequence of the VP1 of the AAV2.5T capsid protein.
Splenocytes from positive control (AAV2.5T-SP183-fCFTRΔR-administered) ferrets (n = 3) were used to screen for immunoreactive capsid peptides within the AAV2.5T capsid. In brief, the ferrets were administered three doses of AAV2.5T-SP183-fCFTRΔR at 2-week intervals between dosing and beginning at 1 month of age. The first and second doses of vector (1 × 1013 DRP/kg BW) were i.t. administered. The third dose of vector was delivered both i.t. and i.m. (5 × 1012 DRP/kg BW for each), since i.m. delivery of an antigen can induce a high level of cellular immune response. Two weeks after the last dose of vector, splenocytes were isolated and cryopreserved in CryoStor CS10 media (StemCell Technologies). Three age-matched naive ferrets were used to harvest naive control splenocytes.
For the IFN-γ ELISpot assay, splenocytes were thawed, washed, and counted. Phorbol 12-myristate 13-acetate (PMA; 10 ng/mL, Sigma) and ionomycin (0.2 mg/mL, Sigma) were used as a positive control for splenocyte responses, and media alone (i.e., no stimulation) was used as a negative control. Controls and peptide antigens (1 μg/mL) were added to each well (n = 3) containing splenocytes (2 × 105/well). The number of SFUs per well was determined using an Immunospot plate reader (Astor, Mabtech), and results were expressed as SFU/2 × 105 cells after subtracting the SFU from the media-alone negative control. Of the 72 capsid peptides screened, the 4 peptides with the highest SFUs (peptides 52, 56, 65, and 66) were pooled to stimulate ferret splenocytes in the subsequent rAAV2.5T dosing study that evaluated the impact of IS.
Statistical analysis
The experimental data are presented as mean ± standard error of the mean (SEM), and Prism 9 (GraphPad Software) was used for the data analyses. The statistical significance between multiple groups was determined using one-way analysis of variance (ANOVA) followed by Tukey’s test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). In Figure 2, the data were log2 transformed before ANOVA and the Tukey test because of the large range in values. In Figure 3, log2(values +1)-transformed data were used before ANOVA and the Tukey test because certain samples returned an IC50 of zero because of the lack of a clear dose response. In Figure 5, the 2(−ΔΔCq) value was transformed to log(n,10) prior to statistical analysis with R (v.4.0.4). The random intercept mixed-effects model was fit using R package lmer4 (v.1.1.27.1).
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL152960, DK054759 and 75N92019C00010 to J.F.E.) and a sponsored research agreement (SRA) from Spirovant Sciences, Inc. All animal experimentation was performed according to protocols approved by the institutional animal care and use committees of the University of Iowa.
Author contributions
Conceptualization, Y.T., Z.Y., and J.F.E.; investigation, Y.T., S.F., E.D.H., P.W., W.Y.F., J.L., and F.Y.; methodology, Y.T. and Z.Y.; validation, Y.T., S.F., E.D.H., Z.F., P.W., W.Y.F., J.L., and F.Y.; formal analysis, Y.T., K.W., and J.F.E.; writing – original draft, Y.T.; writing – review & editing, K.J.E., M.P.L., R.K., K.W., Z.Y., and J.F.E.; project administration, K.J.E., M.P.L., and R.K.; supervision, J.F.E.; funding acquisition, J.F.E.
Declaration of interests
This work was funded by an SRA from Spirovant Sciences, Inc.; Z.Y. and J.F.E. are paid consultants for Spirovant Sciences, Inc.; and K.J.E., M.P.L., and R.K. are employees of Spirovant Sciences, Inc.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2023.02.015.
Contributor Information
Ziying Yan, Email: ziying-yan@uiowa.edu.
John F. Engelhardt, Email: john-engelhardt@uiowa.edu.
Supplemental information
References
- 1.Riordan J.R., Rommens J.M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J.L., et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Bareil C., Bergougnoux A. CFTR gene variants, epidemiology and molecular pathology. Arch. Pediatr. 2020;27(Suppl 1):eS8–eS12. doi: 10.1016/S0929-693X(20)30044-0. [DOI] [PubMed] [Google Scholar]
- 3.Pereira S.V.N., Ribeiro J.D., Ribeiro A.F., Bertuzzo C.S., Marson F.A.L. Novel, rare and common pathogenic variants in the CFTR gene screened by high-throughput sequencing technology and predicted by in silico tools. Sci. Rep. 2019;9:6234. doi: 10.1038/s41598-019-42404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Y., Yan Z., Engelhardt J.F. Viral vectors, animal models, and cellular targets for gene therapy of cystic fibrosis lung disease. Hum. Gene Ther. 2020;31:524–537. doi: 10.1089/hum.2020.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooney A.L., McCray P.B., Jr., Sinn P.L. Cystic fibrosis gene therapy: looking back, looking forward. Genes. 2018;9:538. doi: 10.3390/genes9110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi S.H., Engelhardt J.F. Gene therapy for cystic fibrosis: lessons learned and paths forward. Mol. Ther. 2021;29:428–430. doi: 10.1016/j.ymthe.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loring H.S., ElMallah M.K., Flotte T.R. Development of rAAV2-CFTR: history of the first rAAV vector product to be used in humans. Hum. Gene Ther. Methods. 2016;27:49–58. doi: 10.1089/hgtb.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flotte T., Carter B., Conrad C., Guggino W., Reynolds T., Rosenstein B., Taylor G., Walden S., Wetzel R. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum. Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- 9.Kearns W.G., Afione S.A., Fulmer S.B., Pang M.C., Erikson D., Egan M., Landrum M.J., Flotte T.R., Cutting G.R. Recombinant adeno-associated virus (AAV-CFTR) vectors do not integrate in a site-specific fashion in an immortalized epithelial cell line. Gene Ther. 1996;3:748–755. [PubMed] [Google Scholar]
- 10.Penaud-Budloo M., Le Guiner C., Nowrouzi A., Toromanoff A., Chérel Y., Chenuaud P., Schmidt M., von Kalle C., Rolling F., Moullier P., et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol. 2008;82:7875–7885. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan D., Sharma P., Yang J., Yue Y., Dudus L., Zhang Y., Fisher K.J., Engelhardt J.F. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J. Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan D., Yue Y., Yan Z., Yang J., Engelhardt J.F. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Z., Zak R., Luxton G.W.G., Ritchie T.C., Bantel-Schaal U., Engelhardt J.F. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J. Virol. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Excoffon K.J.D.A., Koerber J.T., Dickey D.D., Murtha M., Keshavjee S., Kaspar B.K., Zabner J., Schaffer D.V. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc. Natl. Acad. Sci. USA. 2009;106:3865–3870. doi: 10.1073/pnas.0813365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Y., Yan Z., Lin S., Huntemann E.D., Feng Z., Park S.Y., Sun X., Yuen E., Engelhardt J.F. Repeat dosing of AAV2.5T to ferret lungs elicits an antibody response that diminishes transduction in an age-dependent manner. Mol. Ther. Methods Clin. Dev. 2020;19:186–200. doi: 10.1016/j.omtm.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostedgaard L.S., Rokhlina T., Karp P.H., Lashmit P., Afione S., Schmidt M., Zabner J., Stinski M.F., Chiorini J.A., Welsh M.J. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc. Natl. Acad. Sci. USA. 2005;102:2952–2957. doi: 10.1073/pnas.0409845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan Z., Sun X., Feng Z., Li G., Fisher J.T., Stewart Z.A., Engelhardt J.F. Optimization of recombinant adeno-associated virus-mediated expression for large transgenes, using a synthetic promoter and tandem array enhancers. Hum. Gene Ther. 2015;26:334–346. doi: 10.1089/hum.2015.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawlins E.L., Hogan B.L.M. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leigh M.W., Kylander J.E., Yankaskas J.R., Boucher R.C. Cell proliferation in bronchial epithelium and submucosal glands of cystic fibrosis patients. Am. J. Respir. Cell Mol. Biol. 1995;12:605–612. doi: 10.1165/ajrcmb.12.6.7766425. [DOI] [PubMed] [Google Scholar]
- 20.Ronzitti G., Gross D.A., Mingozzi F. Human immune responses to adeno-associated virus (AAV) vectors. Front. Immunol. 2020;11:670. doi: 10.3389/fimmu.2020.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber T. Anti-AAV antibodies in AAV gene therapy: current challenges and possible solutions. Front. Immunol. 2021;12:658399. doi: 10.3389/fimmu.2021.658399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin J.H., Yue Y., Srivastava A., Smith B., Lai Y., Duan D. A simplified immune suppression scheme leads to persistent micro-dystrophin expression in Duchenne muscular dystrophy dogs. Hum. Gene Ther. 2012;23:202–209. doi: 10.1089/hum.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z., Kuhr C.S., Allen J.M., Blankinship M., Gregorevic P., Chamberlain J.S., Tapscott S.J., Storb R. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol. Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- 25.Chai Z., Zhang X., Dobbins A.L., Samulski R.J., Merricks E.P., Nichols T.C., Li C. Dexamethasone transiently enhances transgene expression in the liver when administered at late-phase post long-term adeno-associated virus transduction. Hum. Gene Ther. 2022;33:119–130. doi: 10.1089/hum.2021.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J., et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouquet C., Vignal Clermont C., Galy A., Fitoussi S., Blouin L., Munk M.R., Valero S., Meunier S., Katz B., Sahel J.A., Thomasson N. Immune response and intraocular inflammation in patients with leber hereditary optic neuropathy treated with intravitreal injection of recombinant adeno-associated virus 2 carrying the ND4 gene: a secondary analysis of a phase 1/2 clinical trial. JAMA Ophthalmol. 2019;137:399–406. doi: 10.1001/jamaophthalmol.2018.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sui H., Olivier A.K., Klesney-Tait J.A., Brooks L., Tyler S.R., Sun X., Skopec A., Kline J., Sanchez P.G., Meyerholz D.K., et al. Ferret lung transplant: an orthotopic model of obliterative bronchiolitis. Am. J. Transplant. 2013;13:467–473. doi: 10.1111/j.1600-6143.2012.04337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott J.K., Girgis R.E. Individualizing immunosuppression in lung transplantation. Glob Cardiol Sci Pract. 2018;2018:5. doi: 10.21542/gcsp.2018.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandrashekaran S., Crow Pharm S.A., Shah S.Z., Arendt Pharm C.J., Kennedy C.C. Immunosuppression for lung transplantation: current and future. Curr. Transplant. Rep. 2018;5:212–219. doi: 10.1007/s40472-018-0199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martino A.T., Markusic D.M. Immune response mechanisms against AAV vectors in animal models. Mol. Ther. Methods Clin. Dev. 2020;17:198–208. doi: 10.1016/j.omtm.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalia V., Sarkar S. Regulation of effector and memory CD8 T cell differentiation by IL-2-A balancing act. Front. Immunol. 2018;9:2987. doi: 10.3389/fimmu.2018.02987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Souza W.N., Schluns K.S., Masopust D., Lefrançois L. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J. Immunol. 2002;168:5566–5572. doi: 10.4049/jimmunol.168.11.5566. [DOI] [PubMed] [Google Scholar]
- 34.Kang S., Brown H.M., Hwang S. Direct antiviral mechanisms of interferon-gamma. Immune Netw. 2018;18:e33. doi: 10.4110/in.2018.18.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Junttila I.S. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 2018;9:888. doi: 10.3389/fimmu.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diehl S., Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002;39:531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 37.McNab F., Mayer-Barber K., Sher A., Wack A., O'Garra A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantaert T., Baeten D., Tak P.P., van Baarsen L.G.M. Type I IFN and TNFalpha cross-regulation in immune-mediated inflammatory disease: basic concepts and clinical relevance. Arthritis Res. Ther. 2010;12:219. doi: 10.1186/ar3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He N., Liu X., Vegter A.R., Evans T.I.A., Gray J.S., Guo J., Moll S.R., Guo L.J., Luo M., Ma N., et al. Ferret models of alpha-1 antitrypsin deficiency develop lung and liver disease. JCI Insight. 2022;7:e143004. doi: 10.1172/jci.insight.143004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun X., Yi Y., Yan Z., Rosen B.H., Liang B., Winter M.C., Evans T.I.A., Rotti P.G., Yang Y., Gray J.S., et al. In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci. Transl. Med. 2019;11:eaau7531. doi: 10.1126/scitranslmed.aau7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X., Olivier A.K., Liang B., Yi Y., Sui H., Evans T.I.A., Zhang Y., Zhou W., Tyler S.R., Fisher J.T., et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am. J. Respir. Cell Mol. Biol. 2014;50:502–512. doi: 10.1165/rcmb.2013-0261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.FDA FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-novel-gene-therapy-treat-patients-rare-form-inherited-vision-loss
- 43.Russell S., Bennett J., Wellman J.A., Chung D.C., Yu Z.-F., Tillman A., Wittes J., Pappas J., Elci O., McCague S., et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65 -mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.FDA ZOLGENSMA. 2019. https://www.fda.gov/media/126109/download
- 45.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K., et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 46.Voynow J.A., Fischer B.M., Roberts B.C., Proia A.D. Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am. J. Respir. Crit. Care Med. 2005;172:1013–1018. doi: 10.1164/rccm.200410-1398OC. [DOI] [PubMed] [Google Scholar]
- 47.Yang J., Zhou W., Zhang Y., Zidon T., Ritchie T., Engelhardt J.F. Concatamerization of adeno-associated virus circular genomes occurs through intermolecular recombination. J. Virol. 1999;73:9468–9477. doi: 10.1128/jvi.73.11.9468-9477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L., Warzecha C.C., Kistner A., Chichester J.A., Bell P., Buza E.L., He Z., Pampena M.B., Couthouis J., Sethi S., et al. Prednisolone reduces the interferon response to AAV in cynomolgus macaques and may increase liver gene expression. Mol. Ther. Methods Clin. Dev. 2022;24:292–305. doi: 10.1016/j.omtm.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velazquez V.M., Meadows A.S., Pineda R.J., Camboni M., McCarty D.M., Fu H. Effective depletion of pre-existing anti-AAV antibodies requires broad immune targeting. Mol. Ther. Methods Clin. Dev. 2017;4:159–168. doi: 10.1016/j.omtm.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strehl C., Ehlers L., Gaber T., Buttgereit F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front. Immunol. 2019;10:1744. doi: 10.3389/fimmu.2019.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuda S., Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 52.McWilliam M., Khan U. Azathioprine and the neurologist. Pract. Neurol. 2020;20:69–74. doi: 10.1136/practneurol-2018-002161. [DOI] [PubMed] [Google Scholar]
- 53.Cramer M.L., Shao G., Rodino-Klapac L.R., Chicoine L.G., Martin P.T. Induction of T-cell infiltration and programmed death ligand 2 expression by adeno-associated virus in rhesus macaque skeletal muscle and modulation by prednisone. Hum. Gene Ther. 2017;28:493–509. doi: 10.1089/hum.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosen B.H., Evans T.I.A., Moll S.R., Gray J.S., Liang B., Sun X., Zhang Y., Jensen-Cody C.W., Swatek A.M., Zhou W., et al. Infection is not required for mucoinflammatory lung disease in CFTR-knockout ferrets. Am. J. Respir. Crit. Care Med. 2018;197:1308–1318. doi: 10.1164/rccm.201708-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng K., Ashby D., Smyth R.L. Oral steroids for long-term use in cystic fibrosis. Cochrane Database Syst. Rev. 2015;2015:CD000407. doi: 10.1002/14651858.CD000407.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waters V., Mathews A. Prednisone in cystic fibrosis pulmonary exacerbations (PIPE, NCT03070522) 2022. https://clinicaltrials.gov/ct2/show/NCT03070522
- 57.Klamroth R., Hayes G., Andreeva T., Gregg K., Suzuki T., Mitha I.H., Hardesty B., Shima M., Pollock T., Slev P., et al. Global seroprevalence of pre-existing immunity against AAV5 and other AAV serotypes in people with hemophilia A. Hum. Gene Ther. 2022;33:432–441. doi: 10.1089/hum.2021.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kruzik A., Fetahagic D., Hartlieb B., Dorn S., Koppensteiner H., Horling F.M., Scheiflinger F., Reipert B.M., de la Rosa M. Prevalence of anti-adeno-associated virus immune responses in international cohorts of healthy donors. Mol. Ther. Methods Clin. Dev. 2019;14:126–133. doi: 10.1016/j.omtm.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu P., Lu J., Zhang X., Mei M., Feng L., Peng D., Hou J., Kang S.M., Liu X., Tang Y. Single dose of consensus hemagglutinin-based virus-like particles vaccine protects chickens against divergent H5 subtype influenza viruses. Front. Immunol. 2017;8:1649. doi: 10.3389/fimmu.2017.01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.