Abstract
Introduction:
Widespread white matter abnormalities and alterations in glutamate levels have been reported in patients with schizophrenia. We hypothesized that alterations in white matter integrity and glutamate levels in individuals at clinical high risk (CHR) for psychosis are associated with the subsequent development of psychosis.
Methods:
Participants included 33 antipsychotic naïve CHR (Female 7/Male 26, Age 19.55 (4.14) years) and 38 healthy controls (Female 10/Male 28, Age 20.92 (3.37) years). Whole brain diffusion tensor imaging for fractional anisotropy (FA) and right frontal white matter proton magnetic resonance spectroscopy for glutamate levels were acquired. CHR participants were clinically followed for 2 years to determine conversion to psychosis.
Results:
CHR participants that transitioned to psychosis (N = 7, 21%)were characterized by significantly lower FA values in the posterior thalamic radiation compared to those who did not transition and healthy controls. In the CHR group that transitioned to psychosis only, positive exploratory correlations between glutamate levels and FA values of the posterior thalamic radiation and the retrolenticular part of the internal capsule and a negative correlation between glutamate levels and the cingulum FA values were found.
Conclusion:
The results of the present study highlight that alterations in white matter structure and glutamate are related with the conversion to psychosis.
Keywords: Clinical high risk, Conversion to psychosis, Diffusion tensor imaging, Glutamate, Magnetic resonance spectroscopy
1. Introduction
Psychosis is a clinical feature of severe psychiatric illnesses, including schizophrenia and bipolar depression, that may be characterized by poor response to antipsychotic medications and increased functional disability following the onset of illness. Early identification of subjects at clinical high risk (CHR) for psychosis may provide early intervention opportunities to either prevent or reduce the impact of the disorder (Yung and Nelson, 2011). Despite several efforts to identify the CHR subjects who will progress to psychosis (Fusar-Poli and Schultze-Lutter, 2016), there are still no specific biomarkers to identify those who will develop a psychotic disorder (Lieberman et al., 2019).
The disconnection hypothesis of psychotic disorders proposes that alterations in brain connectivity lead to altered communication between regions (Friston, 1998) that could be mediated by white matter anatomical and neurotransmitter abnormalities (Skudlarski et al., 2010). Diffusion tensor imaging (DTI) is a magnetic resonance technique that provides white matter microstructure information, with fractional anisotropy (FA) being a commonly used index. Previous DTI studies have reported widespread white matter abnormalities in patients with schizophrenia (Chiappelli et al., 2015; Kochunov et al., 2014; Kochunov et al., 2017; Vitolo et al., 2017), including studies in drug-naïve first-episode psychosis patients (Alvarado-Alanis et al., 2015; Cheung et al., 2008; Ebdrup et al., 2016; Perez-Iglesias et al., 2010; Serpa et al., 2017). With respect to CHR, cross-sectional studies have found augmented (Schmidt et al., 2015), diminished (Epstein et al., 2014; Karlsgodt et al., 2009; Katagiri et al., 2015) or no differences (Peters et al., 2008) in FA values compared with healthy controls. Prospective DTI studies that assessed conversion to psychosis have shown mixed results, with decreased FA values in a region lateral to the right putamen and left superior temporal lobe (Bloemen et al., 2010), higher FA values in the genu and body of corpus callosum (Saito et al., 2017), and no FA differences in CHR individuals that converted to psychosis compared to those CHR who did not convert (Carletti et al., 2012; Peters et al., 2010; von Hohenberg et al., 2014). The reasons for these discrepancies are unclear, but could be explained by previous use of antipsychotics (Ebdrup et al., 2016; Szeszko et al., 2014) or different imaging protocols (Kelly et al., 2018).
Diversely, the glutamatergic hypothesis of schizophrenia was originally proposed due to observation that phencyclidine, a glutamate antagonist, induced positive and negative symptoms similar to those observed in schizophrenia patients (Javitt and Zukin, 1991). These observations led to the hypothesis that clinical phenomena observed in schizophrenia is associated with hypofunction of the N-methyl-d-aspartate (NMDA) receptor that leads to an increase in glutamate release (Olney and Farber, 1995). Several molecular abnormalities of the glutamate synapse have been described since, giving the notion of schizophrenia as a disorder of plasticity (initially reviewed in McCullumsmith et al., 2004), and positioning glutamate a prominent role in the pathophysiology of the disease (Krystal et al., 2005; Moghaddam and Javitt, 2012). Proton magnetic resonance spectroscopy (1H MRS) is an in vivo non-invasive technique that allows the measurement of glutamate levels in the brain.
Cross-sectional 1H MRS studies in CHR individuals have found increased (de la Fuente-Sandoval et al., 2011; de la Fuente-Sandoval et al., 2015; Grent-'t-Jong et al., 2018; Stone et al., 2009), decreased (Shakory et al., 2018; Stone et al., 2009) or unchanged (Liemburg et al., 2016; Modinos et al., 2018) levels of glutamate compared with healthy control participants. Similar to DTI studies, inconsistencies could be due to heterogeneity of the samples, difference in methods, and exposure to antipsychotic medication. Importantly, two prospective studies have shown that increased hippocampal (Bossong et al., 2019) and striatal (de la Fuente-Sandoval et al., 2013) glutamate levels were associated with the subsequent conversion to syndromal psychosis.
There is also a relationship between white matter microstructure and glutamate neurotransmission, which may be altered in the CHR. Neuregulin-1 (NRG-1) is a susceptibility gene associated with schizophrenia (Harrison and Law, 2006; Stefansson et al., 2002) that participates in neurodevelopment, myelination and glutamatergic transmission, among other functions (Mostaid et al., 2017). Also, oligodendrocytes, the glia cells that produce myelin sheaths in the central nervous system, express functional N-Methyl-d-Aspartate (NMDA) receptors that mediate myelin disruption in a high glutamatergic environment (Paoletti and Neyton, 2007). Finally, a recent study found that a variant of the excitatory amino acid transporter 2 (EAAT2), responsible for >90% of glutamate reuptake, is related to white matter integrity in schizophrenia patients (Mazza et al., 2019).
The aim of the present study was to explore the relationship between white matter microstructure alterations, frontal white matter glutamate levels, clinical symptoms and the subsequent development of psychosis in CHR individuals. Based on the results of prior 1H MRS prospective studies in CHR individuals (Bossong et al., 2019; de la Fuente-Sandoval et al., 2013), we hypothesized that frontal glutamate levels would be higher in the CHR individuals that transitioned to psychosis (CHR-T) compared to both CHR individuals that did not transition to psychosis (CHR-—N) and control subjects. Finally, due to the heterogeneity of the results of DTI prospective studies in the CHR population, we proposed an exploratory study of both the white matter microstructure alterations, along their relationships with glutamate levels.
2. Materials and methods
2.1. Participants
The study was approved by the Ethics and Scientific committees of the Instituto Nacional de Neurología y Neurocirugía in Mexico City (INNN). All participants were included in the study after an informed written procedure, which was obtained from both parents for participants under 18 years old. Thirty-three individuals meeting CHR criteria, as defined by the Structured Interview for Prodromal Syndromes (SIPS) (Miller et al., 2003), were recruited from the outpatient services at the INNN. All CHR subjects were antipsychotic naïve at the time of enrollment. Exclusion criteria included a concomitant medical or neurological illness, current substance abuse or history of substance dependence (excluding nicotine), a high risk for suicide, and psychomotor agitation. Thirty-eight age and sex-matched healthy control subjects were also recruited; control subjects with a present psychiatric disorder or a family history of psychosis were excluded. All participants were screened for drugs of abuse, including cannabis, cocaine, heroin, opioids, and benzodiazepines at the time of inclusion and 1 h prior to the imaging procedures.
2.2. Clinical assessments
CHR individuals and controls were assessed by three certified psychiatrists (PL-O, FR-M and CdlF-S) using the SIPS interview. Patients were clinically followed up for at least two years, using the Scale of Prodromal Symptoms (SOPS) criteria (Miller et al., 2003) to determine conversion to psychosis.
2.3. Neuroimaging procedures
2.3 1. Image acquisition
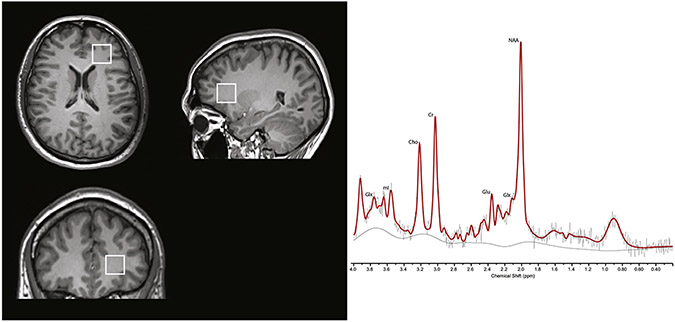
Magnetic Resonance Imaging studies were conducted at the INNN on a 3T scanner (Signa Excite HDxt; GE Healthcare, Wauwatosa, WI), using an eight-channel phased array receive-only head coil and a body-transmit coil (In vivo, Orlando, FL, USA). First, a high-resolution T1-weighted spoiled gradient recalled echo imaging series (TE = 5 ms, TR = 12 ms, inversion time = 450 ms, flip angle = 20°, FOV = 25.6 cm, 256 × 256 matrix, 186 slices, slice thickness = 1 mm), oriented above and parallel to the anterior commissure–posterior commissure line, was acquired. For the DTI acquisition, an image covering the whole brain was obtained using a diffusion-weighted single-shot echo planar image sequence (DW-EPI) in axial orientation (FOV = 256 mm, 60 slices, 128 × 128 matrix; ASSET factor = 2, TR = 12,000 ms, TE = 70 ms, b-value = 700 s/mm2; slice thickness = 2.6 mm, slice gap = 0 mm, with 35 non-collinear diffusion directions) and 1 non-diffusion-weighted image were acquired. For the 1H MRS acquisition, the T1-weighted image was reformatted to sagittal and coronal views for proper 1H MRS voxel placement. The 1H MRS spectra were obtained using point-resolved spectroscopy (PRESS, TE = 35 ms, TR = 2000 ms; spectral width = 5000 Hz; 4096 data points used; and 128 water-suppressed averages and 16 water-unsuppressed averages) in an 8 mL (2 × 2 × 2 cm) voxel centered on the right frontal lobe and aimed to include the maximum amount of white matter, encompassing the anterior corona radiata, the inferior longitudinal fasciculus, anterior thalamic radiation, inferior fronto-occipital fasciculus and uncinate fasciculus (a representative voxel and spectra is presented in Fig. 1).
Fig. 1.
Voxel placement in right frontal white matter and representative spectra from a healthy control. Glu, glutamate; Glx, glutamate + glutamine; NAA N-acetylaspartate + N-acetylaspartylglutamate; Cho, glycerophosphocholine + phosphocholine; mI, myo-inositol; Cr, creatine + phosphocreatine.
2.3.2. DTi image analysis
DTI analysis was performed by one of the investigators (PK) blinded to diagnosis. The DTI data were assembled by the ENIGMA DTI analysis pipeline (Jahanshad et al., 2013) and are available online http://enigma.ini.usc.edu/protocols/dti-protocols/). All data included in the analysis passed the ENIGMA DTI quality assurance or quality control procedures. DTI data for each subject was corrected for eddy current and motion artifacts using eddy tool distributed as a part of FMRIB Software Library (FSL) package (Smith et al., 2006), followed by the fitting of diffusion tensor and calculation of FA images (Kochunov et al., 2011). FA images from all subjects were aligned to the ENIGMA-DTI target brain using the motion correction method FSL's fast nonlinear image registration (Jenkinson et al., 2002) to create a minimal deformation target established on the images from the subjects in the study (Jahanshad et al., 2013). Next, FSL's Tract-based spatial statistics (TBSS) (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS) was used for processed data and an individual FA values projection was made on the hand-segment ENIGMA-DTI skeleton mask (Smith et al., 2006). After extracting the skeleton mask of white matter and the projection of individual FA values, ENIGMA tract-wise regions of interest were obtained from the Johns Hopkins University white matter parcellation atlas (Mori et al., 2008) and were transferred to extract the mean FA across the full skeleton and the average FA values for 24 white matter tracts. The whole-brain FA was obtained by the average of all white matter skeleton values (http://enigma.ini.usc.edu/ongoing/dti-working-group/). Mean values for each tract were calculated by the average of the tract region of interest in both hemispheres. Subsequently, the voxelwise FA values along the ENIGMA skeleton mask were analyzed.
2.3.3. 1H MRS analysis
All water-suppressed spectra were analyzed using LCModel version 6.3-0E (Provencher, 2001) by one of the investigators (GG-C) blinded to diagnosis. Spectra were referenced to the unsuppressed water signal, allowing for glutamate quantification, expressed in institutional units. A simulated basis set of metabolites, consisting of L-alanine, aspartate, creatine, creatine methylene group, γ-aminobutyric acid, glucose, glutamate, glutamine, glutathione, glycerophosphocholine, guanidinoacetate, L-lactate, myo-inositol, N-acetylaspartate, N-acetylaspartylglutamate, phosphocholine, phosphocreatine, scyllo-inositol, and taurine, along with lipids (Lip), and macromolecules (MM) (Lip09, Lip13a, Lip13b, Lip20, MM09, MM12, MM14, MM17 and MM20) was used for analysis. This basis set, included in LCModel, had the same sequence parameters used in our study. T1-weighted MRI scans used for voxel localization and segmentation. Spectroscopic voxels were segmented into gray matter, white matter and cerebrospinal fluid using Statistical Parametric Mapping 8 (SPM8, Wellcome Department of Imaging Neurosciences, University College London, UK). Glutamate metabolite levels were then corrected for the proportion of gray, white and cerebrospinal fluid tissue proportions according to methods described by Gasparovic et al. (2006). Glutamate fits with a %SD Cramér–Rao lower bound exceeding 20% spectra with a full-width at half maximum (FWHM) exceeding 12 Hz as reported by LCModel were considered poor quality and were excluded from further analyses.
2.4. Statistical analysis
Demographic, clinical characteristics, glutamate levels, and DTI FA were compared between controls, CHR-N and CHR-T groups with analysis of variance (ANOVA); post-hoc comparisons were carried out using Bonferroni's correction. Frequency data were analyzed using χ2 test and comparisons between transition and non-transition groups were analyzed using two-sample t-tests. Statistical comparisons were conducted at a p < 0.05 significance level.
Exploratory analyses of associations between glutamate levels, DTI FA values and clinical symptoms were explored using Pearson's product-moment correlation (r), or Spearman r for nonnormally distributed data. Multiple comparisons were controlled using the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995).
3. Results
Seven (21%) out of the 33 CHR participants converted to psychosis during the clinical follow up. Clinical and sociodemographic baseline data for the three groups, CHR-T, CHR-N and healthy controls, are presented in Table 1. Education level was different between groups (F[2, 68] = 9.37, p < 0.001) and post-hoc tests revealed that the control group had higher educational level compared to both CHR-T (t = 3.58, df = 40, p = 0.001) and CHR-N (t = 3.23, df = 59, p = 0.002) groups, with no significant differences between CHR-T and CHR-N groups (t = 1.46, df = 31, p = 0.18). There were no differences in any SIPS symptoms subscale between the CHR-T and CHR-N groups. All 3 groups were similar in age, sex, cannabis, and tobacco use.
Table 1.
Demographic and clinical characteristics of the sample.
| Mean (±SD) |
Statistics | |||
|---|---|---|---|---|
| CHR-T | CHR-N | Healthy controls | ||
| Age, years | 20.43 (5.99) | 19.31 (3.61) | 20.92 (3.37) | F[2, 68] = 1.43, p = 0.25 |
| Sex (male/female) | 5/2 | 21/5 | 28/10 | χ2 = 0.51, p = 0.77 |
| Education, years | 9.7 (3.3) | 11.7 (2.68) | 14 (2.81) | F[2, 68] = 9.37, p < 0.001 |
| Tobacco (ever used) | 2 | 6 | 5 | χ2 = 1.56, p = 0.46 |
| Cannabis (ever used) | 0 | 2 | 0 | χ2 = 3.56, p = 0.17 |
| SIPS positive symptoms | 12.29 (3.68) | 11.04 (4.51) | t = −0.76, p = 0.47 | |
| SIPS negative symptoms | 20.14 (3.81) | 16.46 (5.64) | t = −2.03, p = 0.06 | |
| SIPS disorganization symptoms | 10.29 (3.20) | 9.35 (3.89) | t = −0.66, p = 0.52 | |
| SIPS general symptoms | 9.43 (3.26) | 9.08 (4.03) | t = −0.24. p = 0.81 | |
CHR-T, Clinical high risk that transitioned to psychosis; CHR-—N, Clinical high risk that did not transition to psychosis; SIPS, Structured Interview for Prodromal Syndromes.
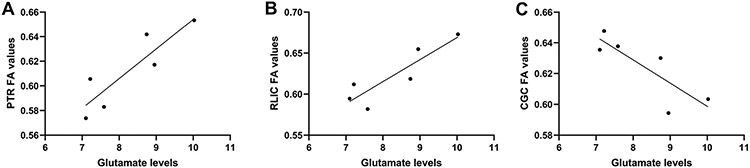
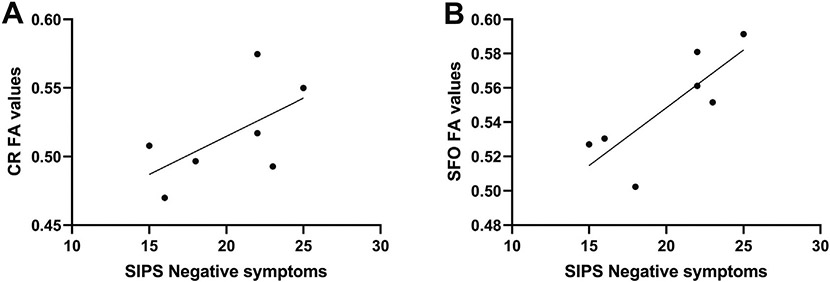
One 1H MRS spectra from a control subject and one from the CHR-T group, along with the DTI data of one individual from the CHR-N group were rejected due to poor quality. The analyses of the 24 tracts included (Supplementary Table 1), revealed significant differences in the FA values of the posterior thalamic radiation (F[2, 67] = 3.44, p = 0.04; η = 0.31); Bonferroni post hoc tests revealed that CHR-T subjects had lower FA values compared to both CHR-N (p = 0.01; Cohen's d = 0.34) and control groups (p = 0.02; Cohen's d = 0.33), with no statistical differences between CHR-N and controls (p = 0.88; Cohen's d = 0.01). The exploratory relationships between glutamate levels and white matter tracts (using Pearson's product-moment correlation) revealed significant correlations between glutamate levels and the posterior thalamic radiation (r = 0.88, p = 0.02), the retrolenticular part of internal capsule (r = 0.89, p = 0.02), and the cingulum (r = −0.83, p = 0.04) of the CHR-T group (Fig. 2). However, none of these correlations remained significant after correcting for multiple comparisons. All significant and non-significant exploratory correlations between glutamate levels and white matter tracts of the CHR-T group are presented in Supplementary Table 2. No correlations were seen between any of the studied tracts and glutamate levels in neither the CHR-N or control groups. Finally, exploratory correlations between the SIPS negative symptom subscale and the anterior corona radiata (r = 0.79, p = 0.03) and the superior fronto-occipital fasciculus (r = 0.81, p = 0.03) FA values were seen only in the CHR-T group (Fig. 3). These correlations did not survive the correction for multiple comparisons.
Fig. 2.
The relationship between A) the posterior thalamic radiation fractional anisotropy (FA) values and frontal white matter glutamate levels, B) retrolenticular part of the internal capsule FA values and glutamate levels, and C) cingulum FA values and glutamate levels. PTR, posterior thalamic radiation; RLIC, retrolenticular part of the internal capsule; CGC, cingulum.
Fig. 3.
The relationship between SIPS negative symptoms subscale and fractional anisotropy values of A) the anterior corona radiata, and B) the superior fronto-occipital fasciculus. CR, anterior corona radiata; SFO, superior fronto-occipital fasciculus; FA, fractional anisotropy.
There were no significant correlations between glutamate levels and the SIPS scores in any of the clinical groups. There were also no significant differences in glutamate levels, voxel tissue composition or spectral quality values among the 3 groups (Supplementary Table 3).
4. Discussion
In the present study, and contrary to our initial hypothesis, frontal glutamate levels of CHR participants that transitioned to psychosis did not differ from CHR individuals that did not transitioned nor healthy controls. However, CHR individuals that later transitioned to psychosis showed significantly lower FA values in the posterior thalamic radiation in comparison to both CHR that did not transition and healthy control groups. Moreover, we found several significant exploratory correlations only in the CHR-T group that included frontal glutamate levels and FA levels in the posterior thalamic radiation and the retrolenticular part of internal capsule, and a negative relationship with frontal glutamate levels and cingulum FA values, and positive relationships with SIPS negative symptoms subscale and FA values of the anterior corona radiata and the superior fronto-occipital fasciculus.
Our results somewhat differ with other studies that have found abnormalities in FA values of CHR individuals who later converted to psychosis. Bloemen et al. (Bloemen et al., 2010) found lower FA values of converters in contrast to healthy controls in bilateral medial frontal lobes, and lower FA values of CHR-T in comparison to CHR-N lateral to the right putamen and left superior temporal lobe. Another study found lower FA in the corpus callosum of CHR-T with regard to healthy controls (Rigucci et al., 2016). Finally, the study by Saito et al. (Saito et al., 2017) found higher FA values in the genu and body of corpus callosum in CHR-T in comparison to CHR-N individuals. Several reasons could explain the differences between studies. First, these studies included CHR individuals that were treated with antipsychotic medication. As previously reported, antipsychotic medication may affect FA values in several white matter tracts (Meng et al., 2019; Szeszko et al., 2014). Second, the studies used different analysis methodology to evaluate FA indices; some studies suggest that substantial heterogeneity arises by using different imaging protocols and scanner differences (Kelly et al., 2018). Third, other sociodemographic variables, such as age, sex, substance use, symptom duration and severity may significantly contribute to FA heterogeneity (Kelly et al., 2018; Samartzis et al., 2014).
Several DTI studies, however, agree with our FA results. White matter disturbances of the posterior thalamic radiation, specifically lower FA, have been described in early phases of the illness (Domen et al., 2017; Perez-Iglesias et al., 2010; Zhang et al., 2019) and also in chronic schizophrenia patients (Kelly et al., 2018).
The thalamus represents a central hub for cognitive, sensory and motor functions, and has been associated with the pathophysiology of psychosis. Postmortem studies have shown a reduced number of neurons in the mediodorsal nucleus and the pulvinar (Byne et al., 2002). Moreover, neurochemical abnormalities, including dopaminergic, GABAergic and glutamatergic systems, have been described in the thalamus of patients with schizophrenia (Clinton and Meador-Woodruff, 2004). More recently, neuroimaging studies have found smaller thalamic volumes, lower levels of glutamate and N-acetylaspartate, and thalamic connectivity abnormalities in different phases of the illness, including the CHR (Steullet, 2019). Specifically, the posterior thalamic radiation connects the thalamus with the occipital and posterior temporo-parietal cortices, and its implicated in the processing of visual attention and visual learning (Bundesen et al., 2005). Animal models have shown that perinatal hypoxia causes an abnormal neurodevelopment, involving oligodendrocytes malfunction as well as impaired GABAergic and glutamatergic pathways, with a subsequent aberrant thalamo-cortical connection (Dean et al., 2013). Diminished FA in the posterior thalamic radiation has been consistently observed in pre-term born adults (Ball et al., 2013), which correlates with lower visual short-term memory capacity (Menegaux et al., 2017). Finally, a recent study (De Herdt et al., 2013) found that CHR-T performed significantly worst in working memory and visual learning domains when compared with CHR-N subjects. Further studies could confirm the role of the thalamo-cortical pathways, along with its clinical manifestations, as predictors for transition to psychosis in at risk populations.
We did not find differences in right frontal lobe glutamate levels among the three groups (CHR-T, CHR-—N, and controls). This finding differs to other prospective studies that have found higher striatal (de la Fuente-Sandoval et al., 2013) and hippocampal (Bossong et al., 2019) glutamate levels in CHR individuals that later converted to psychosis. One reason for this discrepancy could be the studied region. While a previous spectroscopic imaging study found augmented white matter glutamate levels in the bilateral medial prefrontal region of stable patients with schizophrenia (Bustillo et al., 2017), a more recent study found no differences in the same region (Bustillo et al., 2019); moreover, two 1H MRS studies in medicated patients, performed in the centrum semiovale (Rowland et al., 2013) and in the white matter of the forceps minor area of the left hemisphere (Chiappelli et al., 2015), also found no differences in glutamate levels in patients with schizophrenia. Finally, recent meta-analyses have not found differences in glutamate levels in the frontal lobe of patients with schizophrenia (Iwata et al., 2018; Merritt et al., 2016).
Interestingly, we found a negative correlation between right frontal lobe glutamate levels and FA values of the cingulum. Some studies support the negative relationship between glutamate and FA levels. Oligodendrocytes express NMDA receptors that provoke excitotoxicity in glutamate rich environments and diminish the myelin production (Paoletti and Neyton, 2007). Also, a recent study found that a variant for the EEAT2 that reflects lower glutamate uptake activity its related with lower FA values in patients with schizophrenia (Mazza et al., 2019).
We also found positive correlations between glutamate levels and the posterior thalamic radiation and the retrolenticular part of internal capsule which may be explained by linking two mechanisms. First, white matter development is still an active process during adolescence and early adulthood, and peaks of maturation vary among tracts (Kochunov and Hong, 2014; Peters et al., 2012). Second, NMDA receptor-mediated myelination by oligodendrocytes has a different response to glutamate depending on the expression of NRG-1; when NRG-1 is expressed, myelination is augmented and accelerated in the presence of glutamate (Lundgaard et al., 2013). Since NRG-1 is involved in axon guiding and myelination (McIntosh et al., 2008; Mei and Xiong, 2008), glutamate could exert different effects depending upon the maturation stage and NRG-1 expression.
Finally, we found positive exploratory correlations between the SIPS negative symptoms subscale and FA values of the anterior corona radiata and the superior fronto-occipital fasciculus in the CHR-T group. These correlations did not survive correction for multiple comparisons but provide preliminary evidence of potential correlations between FA values and clinical symptoms that may survive multiple comparison corrections in larger studies.
Some limitations in the present study should be considered: First, the small sample (n = 7, 21%) of individuals that converted to psychosis limits the interpretation of the results. Second, cognitive tests were not included in this study; therefore, the relationship between white matter alterations, glutamate levels and cognition could not be explored. Third, our 1H MRS measurement was limited to the right frontal lobe white matter and it might not reflect the glutamatergic function of other white matter regions involved in the pathophysiology of psychosis. Finally, 1H MRS is unable to measure direct glutamate neurotransmission and is unable to differentiate vesicular and metabolic pools of glutamate.
Despite these limitations, the present study provides preliminary evidence of white matter alterations in antipsychotic-naïve individuals that were initially at risk and that later developed psychosis, contributes to a growing body of literature, and emphasizes the need for multi-site studies targeting white matter structure, function and chemistry in this population.
Supplementary Material
Acknowledgements
This project was supported by Consejo Nacional de Ciencia y Tecnología, Mexico, (CONACyT) Grant Nos. 182279 and 261895 to Camilo de la Fuente-Sandoval, and CONACyT's Sistema Nacional de Investigadores to Pablo León-Ortiz and Camilo de la Fuente-Sandoval.
Role of the funding source
Consejo Nacional de Ciencia y Tecnología and Sistema Nacional de Investigadores had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Declaration of competing interest
Pablo León-Ortiz has served as a speaker for Shire Pharmaceuticals, Francisco Reyes-Madrigal has served as a speaker for Janssen (Johnson & Johnson) and AstraZeneca, R. Mora-Durán has served as a speaker for Schwabe Pharmaceuticals, Laura M. Rowland has served as a consultant for Otsuka America Pharmaceutical Inc., and Camilo de la Fuente-Sandoval has served as a consultant for Janssen (Johnson & Johnson). The rest of the authors report no biomedical financial interests or potential conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.schres.2020.06.006.
References
- Alvarado-Alanis P, Leon-Ortiz P, Reyes-Madrigal F, Favila R, Rodriguez-Mayoral O, Nicolini H, Azcarraga M, Graff-Guerrero A, Rowland LM, de la Fuente-Sandoval C, 2015. Abnormal white matter integrity in antipsychotic-naive first-episode psychosis patients assessed by a DTI principal component analysis. Schizophr. Res 162 (1–3), 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Boardman JP, Aljabar P, Pandit A, Arichi T, Merchant N, Rueckert D, Edwards AD, Counsell SJ, 2013. The influence of preterm birth on the developing thalamocortical connectome. Cortex 49 (6), 1711–1721. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol 57 (1), 289–300. [Google Scholar]
- Bloemen OJ, de Koning MB, Schmitz N, Nieman DH, Becker HE, de Haan L, Dingemans P, Linszen DH, van Amelsvoort TA, 2010. White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol. Med 40 (8), 1297–1304. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Antoniades M, Azis M, Samson C, Quinn B, Bonoldi I, Modinos G, Perez J, Howes OD, Stone JM, Allen P, McGuire P, 2019. Association of hippocampal glutamate levels with adverse outcomes in individuals at clinical high risk for psychosis. JAMA Psychiatry 76 (2), 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundesen C, Habekost T, Kyllingsbaek S, 2005. A neural theory of visual attention: bridging cognition and neurophysiology. Psychol. Rev 112 (2), 291–328. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Jones T, Chen H, Lemke N, Abbott C, Qualls C, Stromberg S, Canive J, Gasparovic C, 2017. Glutamatergic and neuronal dysfunction in gray and white matter: a spectroscopic imaging study in a large schizophrenia sample. Schizophr. Bull 43 (3), 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustillo JR, Jones T, Qualls C, Chavez L, Lin D, Lenroot RK, Gasparovic C, 2019. Proton magnetic resonance spectroscopic imaging of gray and white matter in bipolar-I and schizophrenia. J. Affect. Disord 246, 745–753. [DOI] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L, 2002. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am. J. Psychiatry 159 (1), 59–65. [DOI] [PubMed] [Google Scholar]
- Carletti F, Woolley JB, Bhattacharyya S, Perez-Iglesias R, Fusar Poli P, Valmaggia L, Broome MR, Bramon E, Johns L, Giampietro V, Williams SC, Barker GJ, McGuire PK, 2012. Alterations in white matter evident before the onset of psychosis. Schizophr. Bull 38 (6), 1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE, 2008. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol. Med 38 (6), 877–885. [DOI] [PubMed] [Google Scholar]
- Chiappelli J, Hong LE, Wijtenburg SA, Du X, Gaston F, Kochunov P, Rowland LM, 2015. Alterations in frontal white matter neurochemistry and microstructure in schizophrenia: implications for neuroinflammation. Transl. Psychiatry 5, e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Meador-Woodruff JH, 2004. Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr. Res 69 (2–3), 237–253. [DOI] [PubMed] [Google Scholar]
- De Herdt A, Wampers M, Vancampfort D, De Hert M, Vanhees L, Demunter H, Van Bouwel L, Brunner E, Probst M, 2013. Neurocognition in clinical high risk young adults who did or did not convert to a first schizophrenic psychosis: a meta-analysis. Schizophr. Res 149 (1–3), 48–55. [DOI] [PubMed] [Google Scholar]
- Dean JM, McClendon E, Hansen K, Azimi-Zonooz A, Chen K, Riddle A, Gong X, Sharifnia E, Hagen M, Ahmad T, Leigland LA, Hohimer AR, Kroenke CD, Back SA, 2013. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci. Transl. Med 5 (168), 168ra167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domen P, Peeters S, Michielse S, Gronenschild E, Viechtbauer W, Roebroeck A, Os JV, Marcelis M, for Genetic R, Outcome of P, 2017. Differential time course of microstructural white matter in patients with psychotic disorder and individuals at risk: a 3-year follow-up study. Schizophr. Bull 43 (1), 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebdrup BH, Raghava JM, Nielsen MO, Rostrup E, Glenthoj B, 2016. Frontal fasciculi and psychotic symptoms in antipsychotic-naive patients with schizophrenia before and after 6 weeks of selective dopamine D2/3 receptor blockade. J. Psychiatry Neurosci 41 (2), 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein KA, Cullen KR, Mueller BA, Robinson P, Lee S, Kumra S, 2014. White matter abnormalities and cognitive impairment in early-onset schizophrenia-spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry 53 (3), 362–372 e361-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, 1998. The disconnection hypothesis. Schizophr. Res 30 (2), 115–125. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J, Graff-Guerrero A, 2011. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology 36 (9), 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Favila R, Stephano S, Graff-Guerrero A, 2013. Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. Int. J. Neuropsychopharmacol 16 (2), 471–475. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Solis-Vivanco R, Favila R, Graff-Guerrero A, Shungu DC, 2015. Cortico-striatal GABAergic and glutamatergic dysregulations in subjects at ultra-high risk for psychosis investigated with proton magnetic resonance spectroscopy. Int. J.Neuropsychopharmacol 19 (3), pyv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Schultze-Lutter F, 2016. Predicting the onset of psychosis in patients at clinical high risk: practical guide to probabilistic prognostic reasoning. Evid Based Ment Health 19 (1), 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA, 2006. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med 55 (6), 1219–1226. [DOI] [PubMed] [Google Scholar]
- Grent-'t-Jong T, Gross J, Goense J, Wibral M, Gajwani R, Gumley AI, Lawrie SM, Schwannauer M, Schultze-Lutter F, Navarro Schroder T, Koethe D, Leweke FM, Singer W, Uhlhaas PJ, 2018. Resting-state gamma-band power alterations in schizophrenia reveal E/I-balance abnormalities across illness-stages. Elife 7, e37799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ, 2006. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol. Psychiatry 60 (2), 132–140. [DOI] [PubMed] [Google Scholar]
- von Hohenberg CC, Pasternak O, Kubicki M, Ballinger T, Vu MA, Swisher T, Green K, Giwerc M, Dahlben B, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, Woodberry KA, Thermenos HW, Mulert C, McCarley RW, Seidman LJ, Shenton ME, 2014. White matter microstructure in individuals at clinical high risk of psychosis: a whole-brain diffusion tensor imaging study. Schizophr. Bull 40 (4), 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Nakajima S, Plitman E, Mihashi Y, Caravaggio F, Chung JK, Kim J, Gerretsen P, Mimura M, Remington G, Graff-Guerrero A, 2018. Neurometabolite levels in antipsychotic-naive/free patients with schizophrenia: a systematic review and meta-analysis of (1)H-MRS studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 86, 340–352. [DOI] [PubMed] [Google Scholar]
- Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, Blangero J, Brouwer RM, Curran JE, de Zubicaray GI, Duggirala R, Fox PT, Hong LE, Landman BA, Martin NG, McMahon KL, Medland SE, Mitchell BD, Olvera RL, Peterson CP, Starr JM, Sussmann JE, Toga AW, Wardlaw JM, Wright MJ, Hulshoff Pol HE, Bastin ME, McIntosh AM, Deary IJ, Thompson PM, Glahn DC, 2013. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage 81, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR, 1991. Recent advances in the phencyclidine model of schizophrenia. Am.J. Psychiatry 148 (10), 1301–1308. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17 (2), 825–841. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD, 2009. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol. Psychiatry 66 (6), 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri N, Pantelis C, Nemoto T, Zalesky A, Hori M, Shimoji K, Saito J, Ito S, Dwyer DB, Fukunaga I, Morita K, Tsujino N, Yamaguchi T, Shiraga N, Aoki S, Mizuno M, 2015. A longitudinal study investigating sub-threshold symptoms and white matter changes in individuals with an 'at risk mental state' (ARMS). Schizophr. Res 162 (1–3), 7–13. [DOI] [PubMed] [Google Scholar]
- Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, Andreassen OA, Arango C, Banaj N, Bouix S, Bousman CA, Brouwer RM, Bruggemann J, Bustillo J, Cahn W, Calhoun V, Cannon D, Carr V, Catts S, Chen J, Chen JX, Chen X, Chiapponi C, Cho KK, Ciullo V, Corvin AS, Crespo-Facorro B, Cropley V, De Rossi P, Diaz-Caneja CM, Dickie EW, Ehrlich S, Fan FM, Faskowitz J, Fatouros-Bergman H, Flyckt L, Ford JM, Fouche JP, Fukunaga M, Gill M, Glahn DC, Gollub R, Goudzwaard ED, Guo H, Gur RE, Gur RC, Gurholt TP, Hashimoto R, Hatton SN, Henskens FA, Hibar DP, Hickie IB, Hong LE, Horacek J, Howells FM, Hulshoff Pol HE, Hyde CL, Isaev D, Jablensky A, Jansen PR, Janssen J, Jonsson EG, Jung LA, Kahn RS, Kikinis Z, Liu K, Klauser P, Knochel C, Kubicki M, Lagopoulos J, Langen C, Lawrie S, Lenroot RK, Lim KO, Lopez-Jaramillo C, Lyall A, Magnotta V, Mandl RCW, Mathalon DH, McCarley RW, McCarthy-Jones S, McDonald C, McEwen S, McIntosh A, Melicher T, Mesholam-Gately RI, Michie PT, Mowry B, Mueller BA, Newell DT, O'Donnell P, Oertel-Knochel V, Oestreich L, Paciga SA, Pantelis C, Pasternak O, Pearlson G, Pellicano GR, Pereira A, Pineda Zapata J, Piras F, Potkin SG, Preda A, Rasser PE, Roalf DR, Roiz R, Roos A, Rotenberg D, Satterthwaite TD, Savadjiev P, Schall U, Scott RJ, Seal ML, Seidman LJ, Shannon Weickert C, Whelan CD, Shenton ME, Kwon JS, Spalletta G, Spaniel F, Sprooten E, Stablein M, Stein DJ, Sundram S, Tan Y, Tan S, Tang S, Temmingh HS, Westlye LT, Tonnesen S, Tordesillas-Gutierrez D, Doan NT, Vaidya J, van Haren NEM, Vargas CD, Vecchio D, Velakoulis D, Voineskos A, Voyvodic JQ, Wang Z, Wan P, Wei D, Weickert TW, Whalley H, White T, Whitford TJ, Wojcik JD, Xiang H, Xie Z, Yamamori H, Yang F, Yao N, Zhang F, Zhao J, van Erp TGM, Turner J, Thompson PM, Donohoe G, 2018. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol. Psychiatry 23 (5), 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Hong LE, 2014. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr. Bull 40 (4), 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Lancaster J, Thompson PM, Kochunov V, Rogers B, Fox P, Blangero J, Williamson DE, 2011. Fractional anisotropy of cerebral white matter and thickness of cortical gray matter across the lifespan. Neuroimage 58 (1), 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Chiappelli J, Wright SN, Rowland LM, Patel B, Wijtenburg SA, Nugent K, McMahon RP, Carpenter WT, Muellerklein F, Sampath H, Hong LE, 2014. Multimodal white matter imaging to investigate reduced fractional anisotropy and its age-related decline in schizophrenia. Psychiatry Res. 223 (2), 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Coyle TR, Rowland LM, Jahanshad N, Thompson PM, Kelly S, Du X, Sampath H, Bruce H, Chiappelli J, Ryan M, Fisseha F, Savransky A, Adhikari B, Chen S, Paciga SA, Whelan CD, Xie Z, Hyde CL, Chen X, Schubert CR, O'Donnell P, Hong LE, 2017. Association of white matter with core cognitive deficits in patients with schizophrenia. JAMA Psychiatry 74 (9), 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Perry EB Jr., Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D'Souza DC, 2005. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch. Gen. Psychiatry 62 (9), 985–994. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Small SA, Girgis RR, 2019. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am. J. Psychiatry 176 (10), 794–810. [DOI] [PubMed] [Google Scholar]
- Liemburg E, Sibeijn-Kuiper A, Bais L, Pijnenborg G, Knegtering H, van der Velde J, Opmeer E, de Vos A, Dlabac-De Lange J, Wunderink L, Aleman A, 2016. Prefrontal NAA and Glx levels in different stages of psychotic disorders: a 3T 1H-MRS study. Sci. Rep 6, 21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HO, Franklin RJ, Charles F-C, Attwell D, Karadottir RT, 2013. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11 (12), e1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza E, Spangaro M, Poletti S, Cavallaro R, Benedetti F, 2019. Genetic variability of glutamate reuptake: effect on white matter integrity and working memory in schizophrenia. Schizophr. Res 208, 457–459. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Clinton SM, Meador-Woodruff JH, 2004. Schizophrenia as a disorder of neuroplasticity. Int. Rev. Neurobiol 59, 19–45. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Moorhead TW, Job D, Lymer GK, Munoz Maniega S, McKirdy J, Sussmann JE, Baig BJ, Bastin ME, Porteous D, Evans KL, Johnstone EC, Lawrie SM, Hall J, 2008. The effects of a neuregulin 1 variant on white matter density and integrity. Mol. Psychiatry 13 (11), 1054–1059. [DOI] [PubMed] [Google Scholar]
- Mei L, Xiong WC, 2008. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci 9 (6), 437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegaux A, Meng C, Neitzel J, Bauml JG, Muller HJ, Bartmann P, Wolke D, Wohlschlager AM, Finke K, Sorg C, 2017. Impaired visual short-term memory capacity is distinctively associated with structural connectivity of the posterior thalamic radiation and the splenium of the corpus callosum in preterm-born adults. Neuroimage 150, 68–76. [DOI] [PubMed] [Google Scholar]
- Meng L, Li K, Li W, Xiao Y, Lui S, Sweeney JA, Gong Q, 2019. Widespread white-matter microstructure integrity reduction in first-episode schizophrenia patients after acute antipsychotic treatment. Schizophr. Res 204, 238–244. [DOI] [PubMed] [Google Scholar]
- Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK, 2016. Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry 73 (7), 665–674. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW, 2003. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull 29 (4), 703–715. [DOI] [PubMed] [Google Scholar]
- Modinos G, Simsek F, Horder J, Bossong M, Bonoldi I, Azis M, Perez J, Broome M, Lythgoe DJ, Stone JM, Howes OD, Murphy DG, Grace AA, Allen P, McGuire P, 2018. Cortical GABA in subjects at ultra-high risk of psychosis: relationship to negative prodromal symptoms. Int. J. Neuropsychopharmacol 21 (2), 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D, 2012. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37 (1), 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J, 2008. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40 (2), 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostaid MS, Mancuso SG, Liu C, Sundram S, Pantelis C, Everall IP, Bousman CA, 2017. Meta-analysis reveals associations between genetic variation in the 5′ and 3′ regions of Neuregulin-1 and schizophrenia. Transl. Psychiatry 7 (1), e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Farber NB, 1995. Glutamate receptor dysfunction and schizophrenia. Arch Gen. Psychiatry 52 (12), 998–1007. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J, 2007. NMDA receptor subunits: function and pharmacology. Curr Opin. Pharmacol 7 (1), 39–47. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, Barker GJ, McGuire PK, Roiz-Santianez R, Mata I, de Lucas EM, Quintana F, Vazquez-Barquero JL, Crespo-Facorro B, 2010. White matter defects in first episode psychosis patients: a voxelwise analysis of diffusion tensor imaging. Neuroimage 49 (1), 199–204. [DOI] [PubMed] [Google Scholar]
- Peters BD, de Haan L, Dekker N, Blaas J, Becker HE, Dingemans PM, Akkerman EM, Majoie CB, van Amelsvoort T, den Heeten GJ, Linszen DH, 2008. White matter fibertracking in first-episode schizophrenia schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology 58 (1), 19–28. [DOI] [PubMed] [Google Scholar]
- Peters BD, Dingemans PM, Dekker N, Blaas J, Akkerman E, van Amelsvoort TA, Majoie CB, den Heeten GJ, Linszen DH, de Haan L, 2010. White matter connectivity and psychosis in ultra-high-risk subjects: a diffusion tensor fiber tracking study. Psychiatry Res. 181 (1), 44–50. [DOI] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, Brauer J, Asato MR, Khong PL, James AC, Gallego JA, Malhotra AK, 2012. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr. Bull 38 (6), 1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW, 2001. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 14 (4), 260–264. [DOI] [PubMed] [Google Scholar]
- Rigucci S, Santi G, Corigliano V, Imola A, Rossi-Espagnet C, Mancinelli I, De Pisa E, Manfredi G, Bozzao A, Carducci F, Girardi P, Comparelli A, 2016. White matter microstructure in ultra-high risk and first episode schizophrenia: a prospective study. Psychiatry Res. Neuroimaging 247, 42–48. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, Holcomb HH, Barker PB, 2013. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr. Bull 39 (5), 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito J, Hori M, Nemoto T, Katagiri N, Shimoji K, Ito S, Tsujino N, Yamaguchi T, Shiraga N, Aoki S, Mizuno M, 2017. Longitudinal study examining abnormal white matter integrity using a tract-specific analysis in individuals with a high risk for psychosis. Psychiatry Clin. Neurosci 71 (8), 530–541. [DOI] [PubMed] [Google Scholar]
- Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M, 2014. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J. Neuroimaging 24 (2), 101–110. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Lenz C, Smieskova R, Harrisberger F, Walter A, Riecher-Rossler A, Simon A, Lang UE, McGuire P, Fusar-Poli P, Borgwardt SJ, 2015. Brain diffusion changes in emerging psychosis and the impact of state-dependent psychopathology. Neurosignals 23 (1), 71–83. [DOI] [PubMed] [Google Scholar]
- Serpa MH, Doshi J, Erus G, Chaim-Avancini TM, Cavallet M, van de Bilt MT, Sallet PC, Gattaz WF, Davatzikos C, Busatto GF, Zanetti MV, 2017. State-dependent microstructural white matter changes in drug-naive patients with first-episode psychosis. Psychol. Med 47 (15), 2613–2627. [DOI] [PubMed] [Google Scholar]
- Shakory S, Watts JJ, Hafizi S, Da Silva T, Khan S, Kiang M, Bagby RM, Chavez S, Mizrahi R, 2018. Hippocampal glutamate metabolites and glial activation in clinical high risk and first episode psychosis. Neuropsychopharmacology 43 (11) 2249–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska AA, Pearlson G, 2010. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol. Psychiatry 68 (1), 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE, 2006. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data Neuroimage 31 (4), 1487–1505. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K, 2002. Neuregulin 1 and susceptibility to schizophrenia Am. J. Hum. Genet 71 (4), 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, 2019. Thalamus-related anomalies as candidate mechanism-based biomarkers for psychosis. Schizophr. Res 10.1016/j.schres.2019.05.027. [DOI] [PubMed] [Google Scholar]
- Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, O'Gorman RL, Barker GJ, McGuire PK, Oasis, 2009. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol. Psychiatry 66 (6), 533–539. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ikuta T, Peters BD, Gallego JA, Kane J, Malhotra AK, 2014. White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacology 39 (6), 1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo E, Tatu MK, Pignolo C, Cauda F, Costa T, Ando A, Zennaro A, 2017. White matter and schizophrenia: a meta-analysis of voxel-based morphometry and diffusion tensor imaging studies. Psychiatry Res. Neuroimaging 270, 8–21. [DOI] [PubMed] [Google Scholar]
- Yung AR, Nelson B, 2011. Young people at ultra high risk for psychosis: a research update. Early Interv Psychiatry 5 (Suppl. 1), 52–57. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yang M, Du X, Liao W, Chen D, Fan F, Xiu M, Jia Q, Ning Y, Huang X, Wu F, Soares JC, Cao B, Wang L, Chen H, 2019. Glucose disturbances, cognitive deficits and white matter abnormalities in first-episode drug-naive schizophrenia. Mol. Psychiatry 10.1038/s41380-019-0478-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.