Abstract
BACKGROUND
The role of aspirin in reducing lipoprotein(a)-mediated atherothrombotic events in primary prevention is not established.
OBJECTIVES
This study sought to assess whether low-dose aspirin benefits individuals with elevated plasma lipoprotein(a)-associated genotypes in the setting of primary prevention.
METHODS
The study analyzed 12,815 genotyped individuals ≥70 years of age of European ancestry and without prior cardiovascular disease events enrolled in the ASPREE (ASPirin in Reducing Events in the Elderly) randomized controlled trial of 100 mg/d aspirin. We defined lipoprotein(a)-associated genotypes using rs3798220-C carrier status and quintiles of a lipoprotein(a) genomic risk score (LPA-GRS). We tested for interaction between genotypes and aspirin allocation in Cox proportional hazards models for incidence of major adverse cardiovascular events (MACE) and clinically significant bleeding. We also examined associations in the aspirin and placebo arms of the trial separately.
RESULTS
During a median 4.7 years (IQR: 3.6-5.7 years) of follow-up, 435 MACE occurred, with an interaction observed between rs3798220-C and aspirin allocation (P = 0.049). rs3798220-C carrier status was associated with increased MACE risk in the placebo group (HR: 1.90; 95% CI: 1.11-3.24) but not in the aspirin group (HR: 0.54; 95% CI: 0.17-1.70). High LPA-GRS (vs Low) was associated with increased MACE risk in the placebo group (HR: 1.70; 95% CI: 1.14-2.55), with risk attenuated in the aspirin group (HR: 1.41; 95% CI: 0.90-2.23), but the interaction was not statistically significant. In all participants, aspirin reduced MACE by 1.7 events per 1,000 person-years and increased clinically significant bleeding by 1.7 events per 1,000 person-years. However, in the rs3798220-C and high LPA-GRS subgroups, aspirin reduced MACE by 11.4 and 3.3 events per 1,000 person-years respectively, without significantly increased bleeding risk.
CONCLUSIONS
Aspirin may benefit older individuals with elevated Lipoprotein(a) genotypes in primary prevention.
Keywords: aspirin, cardiovascular disease, genetics, lipoprotein(a), primary prevention
Lipoprotein(a) [Lp(a)] is a highly prevalent, independent, and likely causal risk factor for atherosclerotic cardiovascular disease (CVD).1,2 Elevated plasma Lp(a) levels (>30 mg/dL or >75 nmol/L) confer up to 4-fold increased risk of CVD3 with an estimated 20% to 30% of the general population affected (>100 million people in the United States, and ~1.4 billion globally).4-7 Despite the high burden and prevalence of elevated plasma Lp(a), there are currently no approved pharmacological therapies for targeted treatment. Although promising candidates are in development for the secondary prevention of Lp(a)-mediated CVD,2,8 it will be many years before these candidates are assessed for primary prevention.
Aspirin is one of the most widely used therapies for reducing risk in individuals with a prior history of atherothrombotic CVD events (secondary prevention).9 The high homology of apolipoprotein(a) and plasminogen, along with in vitro data suggesting that Lp(a) prevents plasminogen activation to plasmin, has given rise to the hypothesis that elevated Lp(a) levels may have antifibrinolytic effects and tilt the balance to prothrombosis.10-12 Lp(a) may also induce platelet aggregation by activating platelet PAR-1 (protease-activated receptor-1) and CD36 receptors via its apolipoprotein(a) and oxidized phospholipid components.11,13 Furthermore, the antifibrinolytic properties of Lp(a) may imply a lower propensity to bleeding. Given the relationship between Lp(a), thrombosis, fibrinolysis, and platelet function,14 it can be proposed that aspirin may benefit individuals with elevated Lp(a) in the setting of primary prevention of CVD.
Plasma Lp(a) levels are largely genetically determined and heritable through genetic variants in the LPA gene.15,16 Therefore, genotypes associated with elevated plasma Lp(a) levels can be used as a proxy to estimate elevated Lp(a). A previous analysis of the Women’s Health Study (WHS)17 demonstrated that carrier status for the rs3789220-C LPA gene variant (present in approximately 3.5% of Europeans) was associated with 8-fold higher plasma Lp(a) compared with noncarriers and with increased CVD risk (consistent with other studies).18,19 The WHS analysis suggested that healthy women carrying the rs3789220-C variant benefited from 100 mg aspirin every other day compared with noncarrier women. However, the analysis of aspirin interaction was limited to only low-risk, younger women and a single LPA gene variant. Since then, a 43-variant lipoprotein(a) genetic risk score (LPA-GRS) has been derived, which explained approximately 60% of the variation in directly measured plasma Lp(a) levels and demonstrated robust performance for CVD risk prediction.15
The relationship between Lp(a) genotypes and plasma levels is not absolute, as Lp(a) genotypes may have the potential to confer risk independently of directly measured plasma Lp(a) levels. Here, using Lp(a) genotype, rather than phenotype, as a predictor, we hypothesized that low-dose aspirin may specifically benefit older individuals with elevated Lp(a)-associated genotypes in the ASPREE (ASPirin in Reducing Events in the Elderly) trial, a randomized, placebo-controlled trial of 100 mg aspirin once daily in healthy older people with no history of CVD. The overall results of the ASPREE trial failed to demonstrate a significant reduction in CVD events with aspirin, but this was associated with a significant increase in major bleeding.20,21 We sought to examine whether a subgroup of ASPREE participants benefited, specifically those who carry genotypes associated with elevated Lp(a) levels [rs3798220-C carrier status and a high burden of polygenic Lp(a) risk]. We examined whether individuals enrolled into the ASPREE trial carrying these genotypes benefitted specifically from aspirin (Central Illustration).
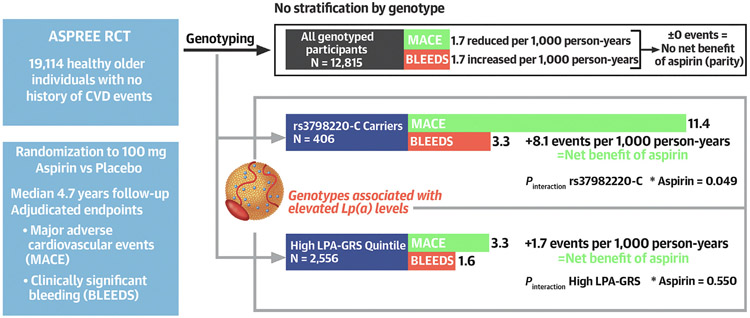
CENTRAL ILLUSTRATION. Aspirin, Lipoprotein(a) Genotypes, and Primary Prevention of Cardiovascular Disease Events.
Our study provides evidence that aspirin specifically benefits older individuals with elevated lipoprotein(a) [Lp(a)] genotypes in the setting of primary prevention of cardiovascular disease (CVD). In 12,815 genotyped participants in the ASPREE (ASPirin in Reducing Events in the Elderly) trial, aspirin reduced major adverse cardiovascular events (MACE) by 1.7 events per 1,000 person-years and increased clinically significant bleeding (BLEEDS) by 1.7 events per 1,000 person-years (indicating no net benefit of aspirin). However, in the rs3798220-C carrier group and the highest quintile of a lipoprotein(a) genomic risk score (LPA-GRS) distribution, aspirin reduced MACE by 11.4 and 3.3 events, respectively, without significantly increased bleeding risk, indicating a shift toward the net benefit of aspirin.
METHODS
STUDY SAMPLE.
The study included genotyped participants from the ASPREE trial conducted in Australia and the United States (design and results reported previously).20-22 The ASPREE trial was a randomized, double-blind, placebo-controlled trial investigating the effect of daily 100 mg aspirin on a primary endpoint of disability-free survival over a median follow-up of 4.7 years (IQR: 3.6-5.7 years). The study enrolled 19,114 individuals ≥70 years of age (≥65 years of age for U.S. minorities) with no prior history of CVD events (exclusion criteria included previous diagnosis of myocardial infarction, heart failure, angina pectoris, stroke, atrial fibrillation, or systolic blood pressure ≥180 mm Hg). All participants provided written informed consent. The study was approved by the Alfred Hospital Research Ethics Committee (390/15) and registered on ClinicalTrials.gov (NCT01038583).
ENDPOINTS.
We considered the endpoints of major adverse cardiovascular events (MACE), a composite of fatal coronary heart disease (excluding death from heart failure), nonfatal myocardial infarction, or fatal or nonfatal ischemic stroke, and clinically significant bleeding (CSB) events, which included major hemorrhage and intracranial bleeding. All components of these endpoints were adjudicated by expert committees masked to randomization using processes described previously.20
GENOTYPING.
DNA samples provided by 14,052 ASPREE Biobank participants were genotyped using the Axiom 2.0 Precision Medicine Diversity Research Array (Thermo Fisher Scientific). Variant calling used a custom pipeline aligned to human reference genome GRCh38. For the present genetic analyses, we included participants of European descent who were ≥70 years of age at enrollment (N = 12,815). Genetic ancestry was defined using principal component analysis with the 1000 Genomes Project reference population.23 ASPREE participants who did not overlap the non-Finnish European cluster were excluded (Supplemental Figure 1). Imputation was performed using the Haplotype Reference Consortium panel.24 Postimputation quality control removed variants with low imputation quality scores (r2 < 0.30). Heterozygote and homozygote rs3798220-C carriers were considered as a single group (carriers) and analyzed using an additive model. We calculated the LPA-GRS using Plink v1.9, following published protocols15 and using variants listed in Supplemental Table 1.
STATISTICAL ANALYSIS.
For rs3798220-C carriers vs noncarriers, we estimated the cumulative incidence of MACE and CSB in the aspirin and placebo groups. We used Cox proportional hazards regression to model relationships between carriers and noncarriers with incidence of MACE and CSB events, adjusting for age at randomization, sex, smoking, alcohol consumption, body mass index, previous regular aspirin use, hypertension, diabetes, chronic kidney disease, and nonsteroidal anti-inflammatory drug use at baseline. We tested for interaction between aspirin allocation and rs3798220-C carrier status in the overall genotyped cohort. We then estimated the separate associations in the placebo and aspirin groups (regardless of statistical significance or otherwise of the interaction).
For the LPA-GRS analysis, we estimated cumulative incidence of MACE and CSB events between participants in the highest vs lowest GRS quintiles and used Cox proportional hazards regression to model relationships with incidence of MACE and CSB events, adjusting for age, sex, and 3 principal components (principal component analysis) of genetic ancestry (consistent with previous studies).15 We tested for interaction between aspirin allocation and LPA-GRS quintile (a 4 degree-of-freedom interaction test), reporting the result of the interaction between the highest and lowest quintile and between aspirin and placebo allocation
Finally, we compared the numbers of MACE and CSB events per 1,000 person-years in the aspirin and placebo groups in the whole cohort, then stratified by genotypic subgroups (in rs3798220-C carriers, and in individuals in the highest LPA-GRS quintile). To quantify the overall benefit vs harm of aspirin, we compared the reduction in MACE in the aspirin group per 1,000 person-years, and the corresponding increase in CSB events.
RESULTS
The baseline characteristics of the genotyped participants are shown in Table 1. The median age was 75.1 years, 54.9% were women, 3.1% were current smokers, and 9.3% had diabetes. Some differences in baseline characteristics were observed between genotyped and nongenotyped participants in the ASPREE trial, including sex, alcohol use, diabetes, and chronic kidney disease (Supplemental Table 2). Baseline characteristics between the high vs low LPA-GRS quintiles differed only for sex and low-density lipoprotein cholesterol (Supplemental Table 3). During follow-up, incident MACE occurred in 435 (3.4%) of 12,815 genotyped participants (Figure 1).
TABLE 1.
Baseline Characteristics of the Study Population, Stratified by Treatment Group
| Total (N = 12,815) |
Treatment Group |
||
|---|---|---|---|
| Placebo (n = 6,439) |
Aspirin (n = 6,376) |
||
| Age, y | 75.1 ± 4.2 | 75.0 ± 4.2 | 75.1 ± 4.2 |
| Sex | |||
| Male | 5,779 (45.1) | 2,912 (45.2) | 2,867 (45.0) |
| Female | 7,036 (54.9) | 3,527 (54.8) | 3,509 (55.0) |
| BMI, kg/m2 | 27.97 ± 4.55 | 27.97 ± 4.51 | 27.98 ± 4.59 |
| Smoking | |||
| Never/former | 12,422 (96.9) | 6,237 (96.9) | 6,185 (97.0) |
| Current | 393 (3.1) | 202 (3.1) | 191 (3.0) |
| Alcohol use | |||
| Never | 1,985 (15.5) | 1,011 (15.7) | 974 (15.3) |
| Former | 617 (4.8) | 314 (4.9) | 303 (4.8) |
| Current | 10,213 (79.7) | 5,114 (79.4) | 5,099 (80.0) |
| Diabetes | |||
| No | 11,624 (90.7) | 5,828 (90.5) | 5,796 (90.9) |
| Yes | 1,191 (9.3) | 611 (9.5) | 580 (9.1) |
| HDL cholesterol, mg/dL | 61.3 ± 17.7 | 61.2 ± 17.7 | 61.4 ± 17.7 |
| LDL cholesterol, mg/dL | 119.0 ± 33.7 | 119.0 ± 33.3 | 119.0 ± 34.0 |
| Total cholesterol, mg/dL | 204.0 ± 37.7 | 204.0 ± 37.4 | 204.0 ± 37.9 |
| SBP, mm Hg | 139.0 ± 16.3 | 140.0 ± 16.3 | 139.0 ± 16.3 |
| DBP, mm Hg | 77.2 ± 10.0 | 77.0 ± 9.90 | 77.3 ± 10.0 |
| Chronic kidney disease | |||
| No | 9,757 (74.3) | 4,902 (74.4) | 4,855 (74.2) |
| Yes | 3,058 (25.7) | 1,537 (25.6) | 1,521 (25.8) |
| Baseline NSAID | |||
| No | 11,261 (87.9) | 5,669 (88.0) | 5,592 (87.3) |
| Yes | 1,554 (12.1) | 770 (12.0) | 784 (12.3) |
| Previous regular aspirin use | |||
| Yes | 1,135 (8.0) | 559 (8.6) | 576 (9.0) |
Values are mean ± SD or n (%). Participants were enrolled in the ASPREE trial, were ≥70 years of age, and were without a prior history or current diagnosis of atherothrombotic cardiovascular disease, a diagnosis of dementia, significant physical disability, or life-threating cancer diagnoses.
ASPREE = ASPirin in Reducing Events in the Elderly; BMI = body mass index; DBP = diastolic blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein; NSAID = nonsteroidal anti-inflammatory drug; SBP = systolic blood pressure.
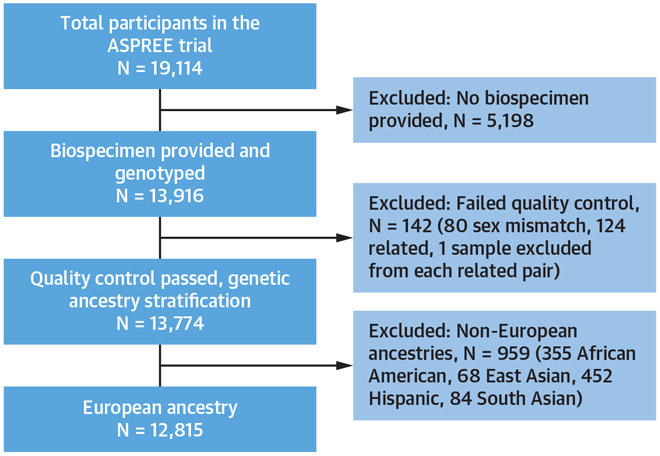
FIGURE 1. Study Flow Chart Indicating the Selection of the Final Data Set.
The ASPREE (ASPirin in Reducing Events in the Elderly) trial enrolled 19,114 individuals ≥70 years (≥65 years for U.S. minorities) with no prior history of cardiovascular disease events. DNA samples were provided by 14,052 ASPREE Biobank participants at enrollment. Genotyped samples were excluded for failed quality control (n = 142) and ancestry stratification (n = 959). The final data set (n = 12,815) included only genotyped participants with European descent ≥70 years of age.
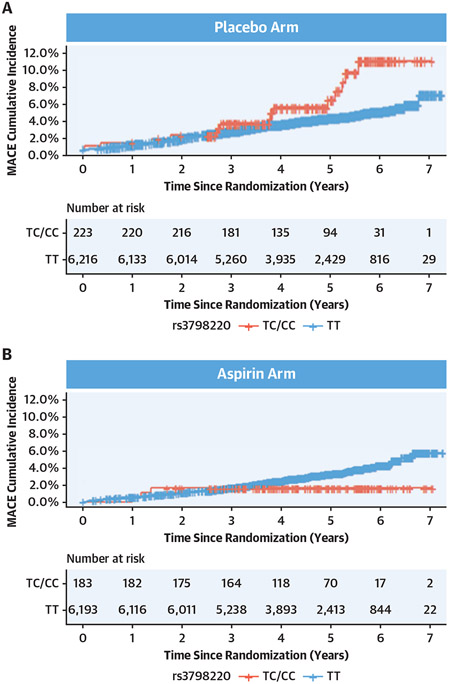
Among the 12,815 participants, 406 (3.2%) were carriers of the rs3798220-C allele (404 heterozygotes, 2 homozygotes, considered as a single carrier group). A higher cumulative incidence of MACE was observed in rs3798220-C carriers in the placebo arm of the trial (Figure 2A). However, this risk was attenuated in the aspirin arm, with a lower cumulative incidence of MACE observed in rs3798220-C carriers than in non-carriers (Figure 2B).
FIGURE 2. Cumulative Incidence of MACE Stratified by rs3798220-C Carrier Status.
Among 12,815 genotyped participants, 406 (3.2%) were carriers of the rs3798220-C allele (404 heterozygotes, 2 homozygotes, considered as a single carrier group). For rs3798220-C carriers (red line) vs noncarriers (blue line), we estimated the cumulative incidence of major adverse cardiovascular events (MACE) in the aspirin and placebo groups. (A) A higher cumulative incidence of MACE was observed in rs3798220-C carriers in the placebo arm of the trial. (B) However, this risk was attenuated in the aspirin arm, with a lower cumulative incidence of MACE observed in rs3798220-C carriers than noncarriers.
In the placebo arm of the trial, rs3798220-C carrier status was associated with increased MACE risk (HR: 1.90; 95% CI: 1.11-3.24; P = 0.018) (Table 2). However, in the aspirin arm, MACE risk was attenuated, with no statistically significant association observed between rs3798220-C carriers vs noncarriers for MACE risk (HR: 0.54; 95% CI: 0.17-1.70; P = 0.294). This interaction between rs3798220-C carrier status and aspirin allocation was significant in the overall cohort (P = 0.049) (Table 2).
TABLE 2.
Association of rs3798220-C Carrier Status With MACE and Major Bleeding Risk in the ASPREE Trial
| All Participants (N = 12,815) |
Placebo Arm (n = 6,439) |
Aspirin Arm (n = 6,376) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Events | HR (95% CI) | P Value | Events | HR (95% CI) | P Value | Events | HR (95% CI) | P Value | |
| MACE | |||||||||
| rs3798220-C carriers | 435 (3.4) | 1.28 (0.80-2.06) | 0.29 | 243 (3.7) | 1.90 (1.11-3.24) | 0.018 | 192 (3.0) | 0.54 (0.17-1.71) | 0.29 |
| Pinteraction rs3798220-C * aspirin | 0.049 | ||||||||
| CSB | |||||||||
| rs3798220-C carriers | 395 (3.1) | 1.62 (0.47-5.60) | 0.508 | 169 (2.6) | 0.72 (0.27-1.92) | 0.508 | 226 (3.5) | 1.14 (0.54-2.41) | 0.736 |
| Pinteraction rs3798220-C * aspirin | 0.447 | ||||||||
Values are n (%), unless otherwise indicated.
ASPREE = ASPirin in Reducing Events in the Elderly; CSB = clinically significant bleeding; MACE = major adverse cardiovascular events.
rs3798220-C carrier status was not significantly associated with increased risk of CSB events, neither in the aspirin group nor in the placebo group (Table 2, Figure 3). No interaction between rs3798220-C carrier status and aspirin allocation was observed in the overall cohort for CSB (P = 0.447) (Figure 3).
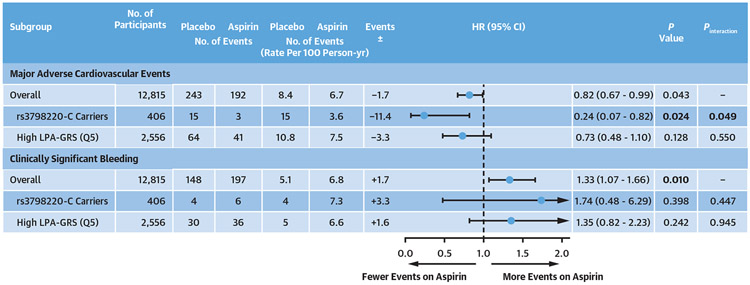
FIGURE 3. Subgroup Analyses of MACE and Bleeding Events in the ASPREE Trial.
Among 12,815 genotyped participants of the ASPREE (ASPirin in Reducing Events in the Elderly) trial, we used Cox proportional hazards regression to model relationships between genotype groups of interest [rs3798220-C carriers and noncarriers, and high vs low quintile of a lipoprotein(a) genomic risk score (LPA-GRS)] with incidence of major adverse cardiovascular events (MACE) and clinically significant bleeding events, adjusting for covariates. We tested for interaction between aspirin allocation and rs3798220-C carrier status, and high vs low LPA-GRS quintile (quintile 5 [Q5]), in the overall genotyped cohort. Interaction between rs3798220-C carrier status and aspirin allocation was significant for MACE (P = 0.049) but not for bleeding in the overall cohort. No interaction between the highest vs lowest LPA-GRS quintile and aspirin allocation was observed for MACE (P = 0.55) or clinically significant bleeding (P = 0.94).
The LPA-GRS as a continuous variable was associated with increased MACE risk in all participants (HR per SD of the GRS: 1.14; 95% CI: 1.05-1.24; P = 0.001).
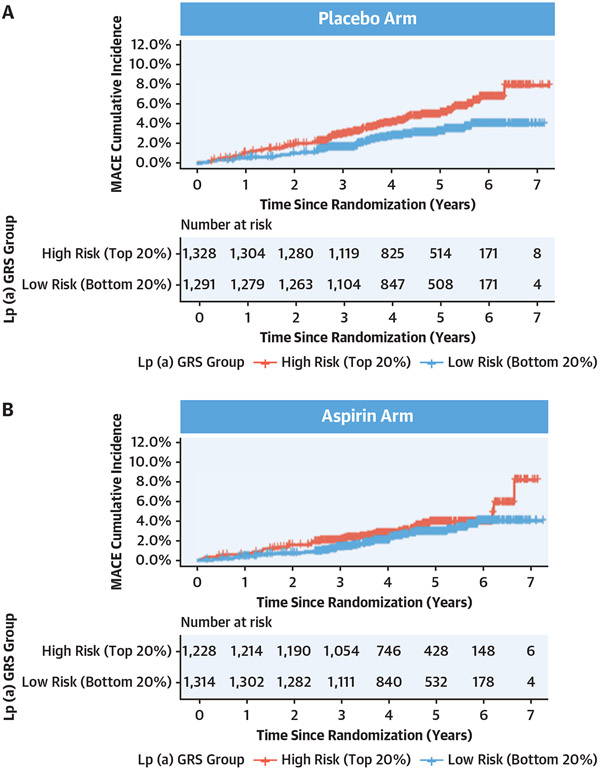
In the placebo arm, a higher cumulative incidence of MACE was observed in the high vs low LPA-GRS quintile (Figure 4A). However, in the aspirin arm, a lower cumulative incidence of MACE was observed in the highest vs lowest LPA-GRS quintile (Figure 4B).
FIGURE 4. Cumulative Incidence of MACE in Highest vs Lowest LPA-GRS Quintiles.
We calculated an LPA-GRS using variants listed in Supplemental Table 1. We estimated the cumulative incidence of MACE between participants in the highest (red line) vs lowest (blue line) quintiles of the LPA-GRS distribution. (A) In the placebo arm, a higher cumulative incidence of MACE was observed in the high vs low LPA-GRS quintile. (B) However, in the aspirin arm, a lower cumulative incidence of MACE was observed in the highest vs lowest LPA-GRS quintile. Lp(a) = lipoprotein(a); other abbreviations as in Figure 3.
In the placebo arm, high LPA-GRS quintile (vs low LPA-GRS quintile) was associated with increased MACE risk (HR: 1.70; 95% CI: 1.14-2.55; P = 0.008) (Table 3). However, this risk was attenuated in the aspirin arm, with no statistically significant association observed between the high LPA-GRS quintile and MACE risk (HR: 1.41; 95% CI: 0.90-2.23; P = 0.130) (Figure 4). No interaction between the highest LPA-GRS quintile and aspirin allocation was observed for MACE (P = 0.55) or CSB (P = 0.94).
TABLE 3.
Association of a LPA-GRS With MACE Risk in the ASPREE Trial
| MACE | Placebo Arm (n = 6,439) | Aspirin Arm (n = 6,376) | ||||
|---|---|---|---|---|---|---|
| Events/participants | 243 (3.8) | 192 (3.0) | ||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Low LPA-GRS (Q1, 0%-20%) | Reference | Reference | ||||
| Medium LPA-GRS (Q2-Q4, 21%-80%) | 1.27 | 0.88-1.81 | 0.19 | 1.23 | 0.84-1.81 | 0.27 |
| High LPA-GRS (Q5, 81%-100%) | 1.70 | 1.14-2.55 | 0.008 | 1.41 | 0.90-2.23 | 0.13 |
| Pinteraction high LPA-GRS * aspirin | 0.5487 | |||||
Values are n (%) unless otherwise indicated. Bold indicates a statistically significance association detected (P < 0.05).
LPA-GRS = Lipoprotein(a) genomic risk score; Q = quintile; other abbreviations as in Table 2.
Randomization to aspirin resulted in a higher incidence of major bleeding events in all ASPREE genotyped participants (HR: 1.33; 95% CI: 1.07-1.66; P = 0.010) (Figure 3). However, there was no statistically significant increase in bleeding risk on aspirin in either rs7398220-C carriers (P = 0.398) or the highest LPA-GRS quintile (P = 0.242) (Figure 3).
Furthermore, there was no difference in the cumulative incidence of CSB events observed in the highest vs lowest LPA-GRS quintiles when analyzing placebo and aspirin groups separately (Supplemental Figure 2).
In all genotyped participants, aspirin reduced MACE by 1.7 events per 1,000 person-years and increased CSB events by 1.7 events per 1,000 person-years (net difference = 0 events) (Figure 3). Described in simple terms, this suggested parity between overall benefit vs harm in all genotyped participants of the ASPREE trial when considered independently of genotype (assuming equal weighting of the benefit-to-harm ratio for the MACE-to-CSB events ratio).
However, in the rs3798220-C subgroup, aspirin reduced MACE by 11.4 events per 1,000 person-years (a >6-fold higher magnitude of CVD risk reduction than in the overall cohort) with a bleeding risk of 3.3 events per 1,000 person-years (Figure 3). Hence in rs3798220-C carriers, aspirin appeared to have a net benefit of 8.1 events per 1,000 person-years.
In the highest LPA-GRS quintile, aspirin reduced MACE by 3.3 events per 1,000 person-years (approximately 2-fold higher magnitude of risk-reduction compared with the overall cohort), with an increase in bleeding risk of 1.6 events per 1,000 person-years (almost identical bleeding risk to the overall cohort) (Figure 3). This shifted the benefit vs harm balance in the highest LPA-GRS quintile to a net benefit of 1.7 events per 1,000 person-years.
DISCUSSION
In this analysis of the ASPREE trial, we found evidence suggesting that older individuals with genotypes associated with elevated Lp(a) levels may benefit from low-dose aspirin for the primary prevention of CVD events. We identified elevated Lp(a)-associated genotypes using 2 methods: rs3798220-C carrier status and high LPA-GRS. Using both methods, we observed fewer CVD events in participants carrying these genotypes who were randomized to aspirin, including a significant interaction between rs3798220-C carrier status and aspirin allocation. Further, our study suggests that the reduction in CVD events with aspirin in older individuals with these Lp(a) genotypes may outweigh the associated bleeding risk, which was higher in all ASPREE participants on aspirin, before stratifying by genotype. By contrast, the reduction in CVD events was observed in a genotype-specific manner, specifically in individuals with elevated Lp(a)-associated genotypes.
Our results provide new evidence to support the potential use of aspirin to target individuals with elevated Lp(a) for the primary prevention of CVD events. However, our results would be strengthened by the use of directly measured plasma Lp(a)levels, in addition to Lp(a) genotypes. This could help answer the question of whether Lp(a) genotypes may confer risk independently of plasma levels. Genetic risk scores tend to identify individuals with elevated Lp(a) but are not fully predictive of plasma Lp(a) levels, and at most account for 60% of their variation.15 Plasma Lp(a) would directly interact with the coagulation system and platelets and summates all known genetic and nongenetic influences on Lp(a) levels; therefore, it would be expected to be informative in all subjects.
Nonetheless, given the lack of any currently approved therapies for targeting elevated Lp(a), our findings may have widespread clinical implications, adding evidence to the rationale that aspirin may be a viable option for reducing Lp(a)-mediated CVD risk. The role of aspirin in primary prevention of CVD remains widely debated. Identification of population subgroups who may benefit from aspirin (in which the net benefit-harm balance shifts favorably) may enable a more guided and precise use of aspirin in primary prevention. Our epidemiological study follows evidence from in vitro25 and in vivo26 studies suggesting that aspirin can lower serum Lp(a) levels. Our analysis provides some of the first randomized controlled trial evidence to support such a rationale, from a large primary prevention placebo-controlled study of aspirin involving both men and women.
Previously, the relationship between aspirin and Lp(a)-mediated CVD risk has been reported in the WHS. The WHS compared aspirin 100 mg every other day with placebo for primary CVD prevention in initially healthy women ≥45 years of age who were followed for 10 years.17 In the WHS, women with elevated plasma Lp(a) (>44.0 mg/dL) were 1.47 times more likely to develop CVD events than women with low Lp(a) (<3.4 mg/dL). Analysis of the role of genotypes focused on a single variant relevant to only 3.5% of Europeans. Women carrying the LPA rs3789220-C variant, associated with highly elevated Lp(a) levels (heterozygous ~80 mg/dL, homozygous ~154 mg/dL, normal <30 mg/dL),16-18,27 had a ~2-fold higher risk of events than noncarrier women. In addition, the reduction of CVD risk among rs3789220-C carriers in the WHS was observed only in those randomized to aspirin (~ 2-fold risk reduction) and not in those randomized to placebo. No higher risk of bleeding was observed in women with elevated Lp(a), perhaps owing to the prothrombotic properties of Lp(a).28 These results, in the absence of any other randomized controlled trial evidence or approved therapy for treating Lp(a)-associated risk, have been used by some physicians as justification for prescribing aspirin in patients with elevated Lp(a). These findings from the WHS have long required validation.
In the present study of the ASPREE trial population, our results were consistent with the WHS analysis, despite randomizing older individuals (both men and women) ≥70 years of age (≥65 years of age for U.S. minorities) with no prior history of CVD events at baseline. Our validation of the findings of the WHS included the association between rs3789220-C carrier status and increased CVD risk, and an apparent benefit of aspirin specific to rs3798220-C carriers, vs bleeding risk. Although the preponderance of data suggests rs3798220-C is a tagging SNV for small isoforms, ex vivo studies suggest that the isoleucine to methionine substitution of this SNV may be more antifibrinolytic than wild-type apolipoprotein(a). For example, rs3798220-C is associated with decreased coagulation time, increased fibrin clot lysis time, and increased fibrin fiber width in plasma, while having no effect on fiber density as compared with wild-type apolipoprotein(a).29
Our validation of the WHS rs3789220-C result provides evidence that a very high-risk subgroup of individuals with highly elevated Lp(a) (~3.5% of Europeans carrying the rs3789220-C allele) may benefit from low-dose aspirin for the primary prevention of CVD events. Further, the benefits in this subgroup specifically may outweigh any bleeding risk (with the acknowledged caveat of low numbers of bleeding events in our analysis). This was not the case in the overall ASPREE trial population, in which the benefit-to-harm equation was balanced or suggested overall net harm, with a lower magnitude of CVD risk reduction on aspirin and a higher relative bleeding risk overall.20,21
However, rs3798220-C carriers comprise only a small portion of all individuals with elevated Lp(a) in the general population. It is estimated that 20% to 30% of the general population have elevated plasma Lp(a) (>30 mg/dL).4-7 Although the identification of a very high-risk rs3798220-C carrier subgroup may represent an opportunity to target aspirin therapy, further efforts are needed to identify and characterize others with elevated Lp(a). To that end, we used a recently derived 43-variant LPA-GRS15 to stratify all genotyped ASPREE participants. The LPA-GRS explains ~60% of the variation in directly measured plasma Lp(a) levels and has the potential advantage of being able to identify a larger group of individuals with genetically elevated Lp(a) compared with the rs3789220-C variant alone. We used the GRS to identify the highest 20% (quintile) of the distribution with genetically elevated Lp(a). We observed that ASPREE participants with in the highest LPA-GRS quintile had increased MACE risk in the placebo group only and that this risk was attenuated and not statistically significant in those in the aspirin arm of the trial. We also found that the overall balance between benefit and harm in individuals in the ASPREE trial with a high LPA-GRS shifted toward more a net benefit than harm (more MACE reduced than CSB events induced) compared with in the overall cohort.
No significant interaction was observed in the LPA-GRS analysis. Therefore, it is not clear to what extent the LPA-GRS results add further evidence to suggest that individuals with elevated Lp(a), beyond rs3798220-C carriers, may be more likely to benefit from aspirin. If the benefit of aspirin extends beyond very high-risk rs3798220-C carriers alone, to the broader 20% to 30% of individuals with elevated Lp(a) the potential utility of aspirin for the primary prevention of CVD events would increase substantially.
STUDY STRENGTHS AND LIMITATIONS.
Strengths of our study include placebo-controlled randomization to aspirin to minimize selection bias; a very well-characterized clinical trial population, who had no prior history of diagnosed CVD events; the large sample size of genotyped participants; rigorously adjudicated endpoints, including both CVD and bleeding events; and the biological plausibility for the results, given that Lp(a) has specific interactions with hemostasis (thrombosis or fibrinolysis and platelet function). The ASPREE cohort included surviving healthy older adults with low rates of diabetes and overall low CVD risk. Therefore, it is possible that the benefits of low-dose aspirin observed in the ASPREE population [for those in the ASPREE trial with elevated Lp(a) genotypes] may underestimate the true potential benefit of aspirin in the general population, and in younger higher-risk subgroups, with the same genotypes.
Limitations include the use of genetically predicted Lp(a) rather than directly measured levels. Genetic prediction will never be as accurate as directly measured plasma Lp(a).17 However, given the availability of genetic data from the ASPREE trial, Lp(a) genotypes have acted as a powerful surrogate in lieu of directly measured plasma Lp(a) levels. The prevalence and biological effects of rs3798220-C can vary by genetic ancestry, limiting the generalizability of our results beyond individuals of European descent. For example, rs3798220-C is associated with differential isoforms size and Lp(a) levels across ancestries and is not present in individuals from Africa. By contrast, rs3798220-C is present in >40% of Hispanic individuals, in which it is associated with large isoform size and low Lp(a) levels.30 Further limitations include the limited power for subgroup analysis of bleeding events, owing to low event numbers. Our results pertaining to genotype-specific bleeding risks on aspirin therefore require validation.
The ASPREE cohort comprised healthy, older individuals at baseline (including only 3% current smokers and 9% with diabetes). Elevated Lp(a) in these individuals did not manifest in premature CVD events before 70 years of age, making the ASPREE trial a highly ascertained healthy older population. The findings of our study, therefore, cannot necessarily be generalized to the broader general population including younger individuals and require validation in higher-risk, younger cohorts. However, as mentioned previously, in younger, higher-risk population-based cohorts, the effects of aspirin in reducing Lp(a)-mediated CVD risk may actually be stronger than those observed in the ASPREE trial, given that the ASPREE trial population was depleted for susceptible individuals at high CVD risk. Analyses of other aspirin randomized controlled trial populations with younger participants may derive more accurate estimates regarding the magnitude of risk reduction achievable by aspirin.
Our study included both statin users and nonusers in the analysis. Statins can raise plasma Lp(a) levels, and evidence suggests that Lp(a) levels are predictive of risk in both statin-treated and non-statin-treated patients.6 Consideration of statin users and nonusers together reflects clinical practice and is particularly relevant for clinicians interested in whether aspirin provides benefit beyond statins. Finally, our analysis of the net effects of aspirin (reduction in MACE vs harm from bleeding) considered MACE-to-CSB events with equal 1:1 weighting. Additionally, while this allowed a quantification of benefits vs harm, it over-simplifies a clinical situation in which various other factors need to be considered.
CONCLUSIONS
Our study provides evidence that aspirin may specifically benefit older individuals with genotypes associated with elevated plasma Lp(a) in the setting of high-risk primary prevention of CVD events and that overall benefit may outweigh harm related to major bleeding. Aspirin is a widely available, well-tolerated, and low-cost therapeutic option and could play an important role in reducing Lp(a)-mediated CVD risk. In clinical practice, the benefits of aspirin must be balanced with bleeding risks, which increase with age and other factors. Evidence from other randomized controlled trials, and future analysis of directly measured Lp(a) levels in the ASPREE trial, are required to further delineate aspirin’s role in targeting Lp(a)-mediated CVD risk.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
In a large cohort of genotyped patients without prior cardiovascular events randomized to aspirin or placebo, those at elevated Lp(a) genomic risk and aspirin allocation had a lower risk of MACE than those patients on placebo.
TRANSLATIONAL OUTLOOK:
Additional trials are needed to confirm the efficacy of aspirin for cardiovascular risk reduction in people with elevated plasma Lp(a) levels in the primary prevention settings.
ACKNOWLEDGMENTS
The authors thank the ASPREE trial staff and participants, and the general practitioners and staff of the medical clinics who cared for the participants.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
The ASPREE Biobank is supported by a Flagship cluster grant (including the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne), grants U01AG029824 and U19AGO62682 from the National Institute on Aging and the National Cancer Institute at the National Institutes of Health, by grants 334047 and 1127060 from the National Health and Medical Research Council of Australia, and Monash University and the Victorian Cancer Agency. Dr Lacaze is supported by a National Heart Foundation Future Leader Fellowship (102604). Dr McNeil is supported by a National Health and Medical Research Council Leadership Fellowship (IG1173690). Dr Bhatia is partially supported by National Institutes of Health grant 5T32HL079891, as part of the University of California San Diego Integrated Cardiovascular Epidemiology Fellowship. Dr Natarajan is supported by grants from the National Heart, Lung, and Blood Institute/National Institutes of Health (R01HL142711, R01HL127564). Bayer AG provided low-dose aspirin and placebo tablets for the clinical trial but had no other relationship with the work. Dr Riaz is a paid employee of Regeneron Genetics Center, a Subsidiary of Regeneron Pharmaceuticals Inc. Dr Natarajan has received investigator-initiated grants from Amgen, Apple, AstraZeneca, Boston Scientific, and Novartis; has received personal fees from Apple, AstraZeneca, Blackstone Life Sciences, Foresite Labs, Novartis, and Roche/Genentech; is a co-founder of TenSixteen Bio; is a shareholder of geneXwell and TenSixteen Bio; and has a spouse who is an employee of Vertex, all unrelated to the present work. Dr Nicholls has received research support from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Eli Lilly, Esperion, Resverlogix, Novartis, InfraReDx, and Sanofi-Regeneron; and has served as a consultant for Amgen, Akcea, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi-Regeneron, and Novo Nordisk. Dr Tonkin has received research support from Bayer for materials in ASPREE; and has received honoraria for Advisory Board participation or lectures from Amgen, AstraZeneca, Boehringer Ingelheim, and Pfizer. Dr Tsimikas is a co-inventor and has received royalties from patents owned by the University of California-San Diego; is a co-founder of and has an equity interest in Oxitope, LLC and its affiliates (Kleanthi Diagnostics, LLC and Covicept Therapeutics, Inc); and has a dual appointment at the University of California San Diego and Ionis Pharmaceuticals; although these relationships have been identified for conflict-of-interest management based on the overall scope of the project, the research findings included in this particular publication may not necessarily relate to the interests of the previous companies; the terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict-of-interest policies. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CSB
clinically significant bleeding
- CVD
cardiovascular disease
- Lp(a)
lipoprotein(a)
- LPA-GRS
lipoprotein (a) genomic risk score
- MACE
major adverse cardiovascular events
- SNV
single-nucleotide variation
- WHS
Women’s Health Study
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For supplemental figures and tables, please see the online version of this paper.
REFERENCES
- 1.Tsimikas S. Lp (a) as a new target for reduction of risk of cardiovascular disease and emergence of novel therapies to lower Lp (a). Curr Opin Endocrinol Diabetes Obes. 2016;23:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382:244–255. [DOI] [PubMed] [Google Scholar]
- 3.Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36:2239–2245. [DOI] [PubMed] [Google Scholar]
- 6.Willeit P, Ridker PM, Nestel PJ, et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet. 2018;392:1311–1320. [DOI] [PubMed] [Google Scholar]
- 7.Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI Working Group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szarek M, Bittner VA, Aylward P, et al. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. Eur Heart J. 2020;41:4245–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennekens CH, Dyken ML, Fuster V. Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1997;96:2751–2753. [DOI] [PubMed] [Google Scholar]
- 10.Boffa MB, Koschinsky ML. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res. 2016;57:745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rand ML, Sangrar W, Hancock MA, et al. Apolipoprotein(a) enhances platelet responses to the thrombin receptor-activating peptide SFLLRN. Arterioscler Thromb Vasc Biol. 1998;18:1393–1399. [DOI] [PubMed] [Google Scholar]
- 12.Martinez C, Rivera J, Loyau S, et al. Binding of recombinant apolipoprotein(a) to human platelets and effect on platelet aggregation. Thromb Haemost. 2001;85:686–693. [PubMed] [Google Scholar]
- 13.Podrez EA, Byzova TV, Febbraio M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding WY, Protty MB, Davies IG, Lip GYH. Relationship between lipoproteins, thrombosis, and atrial fibrillation. Cardiovasc Res. 2022;118:716–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinder M, Uddin MM, Finneran P, Aragam KG, Natarajan P. Clinical utility of lipoprotein(a) and LPA genetic risk score in risk prediction of incident atherosclerotic cardiovascular disease. JAMA Cardiol. 2020;6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. [DOI] [PubMed] [Google Scholar]
- 17.DI Chasman, Shiffman D, Zee RY, et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 2009;203:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luke MM, Kane JP, Liu DM, et al. A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arterioscler Thromb Vasc Biol. 2007;27:2030–2036. [DOI] [PubMed] [Google Scholar]
- 19.Shiffman D, O’Meara ES, Bare LA, et al. Association of gene variants with incident myocardial infarction in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2008;28:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med. 2018;379:1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das S, Forer L, Schonherr S, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azuma H, Yamaguchi H, Mima N, et al. An in vitro system for identifying agents capable of changing serum lipoprotein(a) concentration by regulating the transcriptional activity of the apolipoprotein(a) gene promoter. Biochem Biophys Res Commun. 1996;227:570–575. [DOI] [PubMed] [Google Scholar]
- 26.Akaike M, Azuma H, Kagawa A, et al. Effect of aspirin treatment on serum concentrations of lipoprotein(a) in patients with atherosclerotic diseases. Clin Chem. 2002;48:1454–1459. [PubMed] [Google Scholar]
- 27.Arai K, Luke MM, Koschinsky ML, et al. The I4399M variant of apolipoprotein(a) is associated with increased oxidized phospholipids on apolipoprotein B-100 particles. Atherosclerosis. 2010;209:498–503. [DOI] [PubMed] [Google Scholar]
- 28.Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and Low Risk of major bleeding in brain and airways in the general population: a Mendelian randomization study. Clin Chem. 2017;63:1714–1723. [DOI] [PubMed] [Google Scholar]
- 29.Scipione CA, McAiney JT, Simard DJ, et al. Characterization of the I4399M variant of apolipoprotein(a):implications for altered prothrombotic properties of lipoprotein (a). J Thromb Haemost. 2017;15:1834–1844. [DOI] [PubMed] [Google Scholar]
- 30.Lee SR, Prasad A, Choi YS, et al. LPA gene, ethnicity, and cardiovascular events. Circulation. 2017;135:251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.