Abstract
TFII-I is a transcription factor that shuttles between the cytoplasm and nucleus and is regulated by serine and tyrosine phosphorylation. Tyrosine phosphorylation of TFII-I can be regulated in a signal-dependent manner in various cell types. In B lymphocytes, Bruton's tyrosine kinase has been identified as a TFII-I tyrosine kinase. Here we report that JAK2 can phosphorylate and regulate TFII-I in nonlymphoid cells. The activity of TFII-I on the c-fos promoter in response to serum can be abolished by dominant negative JAK2 or the specific JAK2 kinase inhibitor AG490. Consistent with this, we have also found that JAK2 is activated by serum stimulation of fibroblasts. Tyrosine 248 of TFII-I is phosphorylated in vivo upon serum stimulation or JAK2 overexpression, and mutation of tyrosine 248 to phenylalanine inhibits the ability of JAK2 to phosphorylate TFII-I in vitro. Tyrosine 248 of TFII-I is required for its interaction with and phosphorylation by ERK and its in vivo activity on the c-fos promoter. These results indicate that the interaction between TFII-I and ERK, which is essential for its activity, can be regulated by JAK2 through phosphorylation of TFII-I at tyrosine 248. Thus, like the STAT factors, TFII-I is a direct substrate of JAK2 and a signal-dependent transcription factor that integrates signals from both tyrosine kinase and mitogen-activated protein kinase pathways to regulate transcription.
TFII-I (also known as SPIN and BAP-135) plays multiple roles in broad biological processes including transcription and signal transduction. Deletions of TFII-I are associated with Williams-Beuren syndrome, which is a human neurodevelopmental disease (33). It was initially described as an Inr-dependent transcription factor (22, 37–39) but has been also shown to bind E-box elements and activate transcription from sites upstream of initiator elements (36, 37). In addition, it can form complexes with the serum response factor (SRF) and STAT transcription factors (10, 15). We have previously found that TFII-I can bind to sites overlapping the serum response element (SRE) and c-sis–platelet-derived growth factor inducible factor element (SIE) of the c-fos promoter. We have also demonstrated that transcriptional enhancement of the c-fos promoter by TFII-I in vivo is dependent on the SIE and SRE and also on its own binding sites, which overlap with the c-fos SIE and SRE (15).
The c-fos promoter is the best-studied immediate-early gene promoter and is responsive to a variety of extracellular stimuli (5, 9, 18, 29). The mechanism of the responses to calcium, cyclic AMP, tyrosine kinases, and mitogen-activated protein MAP kinases have been well documented (4, 46). However, there is still a major pathway that regulates c-fos transcription which has not been fully characterized. Substantial evidence suggests that there is an alternative pathway leading to the activation of SRE which operates through SRF independently of TCF (7, 14, 34). This serum-responsive pathway is regulated by the activation of the small G protein RhoA (12). It has been recently suggested that the actin dynamics can regulate this pathway (41). The finding that SRF interacts with TFII-I suggests that TFII-I may play a role in the RhoA-SRF pathway.
A notable structural feature of TFII-I is its six helix-loop-helix repeats. It also contains a putative leucine zipper domain at the N-terminal region. TFII-I also has a D-box motif, which is a consensus MAP kinase binding domain (51, 52). We have shown that TFII-I binds the ERK1 MAP kinase through its D box in a signal transduction-dependent manner and that this interaction is required for the activity of TFII-I on the c-fos promoter (16). Furthermore, analysis of the primary sequence of TFII-I reveals two consensus tyrosine phosphorylation sites. Interestingly, it has been demonstrated that TFII-I (BAP135) can associate with the Bruton's tyrosine kinase (BTK) and that the tyrosine phosphorylation of TFII-I can be regulated by BTK in B cells (32, 53). Tyrosine phosphorylation of TFII-I is also required for its transcriptional initiation activity for the TATA-less Vβ1 promoter in T cells (31). In addition, we have previously shown that growth factor stimulation can enhance the tyrosine phosphorylation of TFII-I and potentiate its enhancement of the c-fos promoter in fibroblasts (15).
In an attempt to understand the regulation of TFII-I by tyrosine phosphorylation in nonlymphoid cells, we show here that TFII-I can be phosphorylated by JAK2 and that this phosphorylation is required for the activity of TFII-I. We also find for the first time that JAK2 is also a serum-responsive tyrosine kinase. Moreover, we find that JAK2 regulates the ability of TFII-I to interact with extracellular signal-regulated kinase (ERK) through tyrosine phosphorylation of TFII-I. These results suggest that TFII-I functions as a signaling molecule for the activation of the c-fos promoter by integrating signals from both JAK2 tyrosine kinase and the MAP kinase pathway.
MATERIALS AND METHODS
Plasmids and antibodies.
For transient overexpression of human TFII-I, the pEBG–TFII-I construct was used as previously described (15). The wild-type c-fos–luciferase construct and pRL-TK luciferase construct for reporter assays were previously described (15). Activated (E665K) or dominant negative (K788E) JAK2 expression plasmids were previously described (3, 20). V12-Ras, BXB-Raf, L63-RhoA, and HA-ERK1 expression plasmids were previously described (6, 8, 30, 35). The v-Src expression plasmid was a gift of Larry Feig. pCDNA3 empty vector was obtained from Invitrogen. Anti-glutathione S-transferase (GST) antibody was obtained from Sigma, and anti-HA antibody was obtained from Boehringer Mannheim. Anti-phospho-ERK (pERK) antibody was obtained from New England Biolabs. Anti-JAK2 antibody and antiphosphotyrosine antibody (4G10) were obtained from UBI. Anti-TFII-I antibody was previously described (16). To generate phosphospecific anti-pY248 antibody, a phosphopeptide with the sequence CVKEESEDPDpYYQYN was synthesized and coupled to keyhole limpet hemocyanin. The keyhole limpet hemocyanin-conjugated phosphopeptide was used to immunize rabbits. For the experiment in Fig. 8a, 1-μg aliquots of the indicated peptides were spotted onto nitrocellulose and air dried for dot blotting. Y462 and pY462 peptides were used as negative controls to confirm the specificity of the anti-pY248 antibody. The sequences for the peptides as follows: Y248, CVKEESEDPDYYQYN; pY248, CVKEESEDPDpYYQYN; Y462, CKFEAHPNDLYVEGL; and pY462, CKFEAHPNDLpYVEGL. Anti-pY248 primary antibody incubations for the experiments in fig. 8 were carried out at 1:1,000 dilutions of the antisera in the presence of 2 μg of the unphosphorylated cognate Y248 peptide per ml.
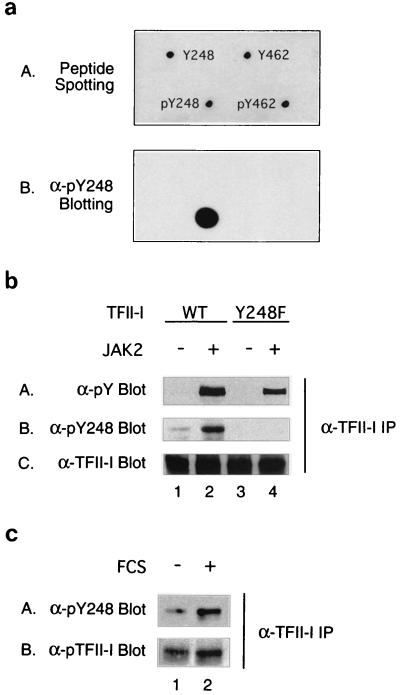
FIG. 8.
Tyrosine 248 of TFII-I is phosphorylated in vivo upon JAK2 overexpression or serum stimulation. (a) A 1-μg aliquot of each indicated peptide (sequences are described in Materials and Methods) was spotted onto a nitrocellulose membrane and air dried. The spotting positions for different peptides are indicated in panel A, and the dot blot result using anti-pY248 antibody is shown in panel B. (b) The wild-type–TFII-I (lane 1 and 2) or Y248F–TFII-I (lane 3 and 4) expression plasmid was transfected into COS-1 cells in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of activated JAK2 expression construct. The transfected cells were maintained in 2.5% FCS-containing medium for 48 h before harvest. Cell lysates were immunoprecipitated with anti-TFII-I antibody and fractionated by SDS-PAGE (10% polyacrylamide). Antiphosphotyrosine (panel A), anti-pY248 (panel B), or anti-TFII-I (panel C) antibody was used for Western blot analyses. (c) NIH 3T3 fibroblasts were serum starved for 36 h (lane 1) and stimulated with 15% FCS (lane 2) for 10 min. Total extracts were immunoprecipitated with anti-TFII-I antibody. The immunoprecipitates were fractionated by SDS-PAGE (10% polyacrylamide) and blotted with anti-pY248 antibody (panel A) or anti-TFII-I antibody (panel B).
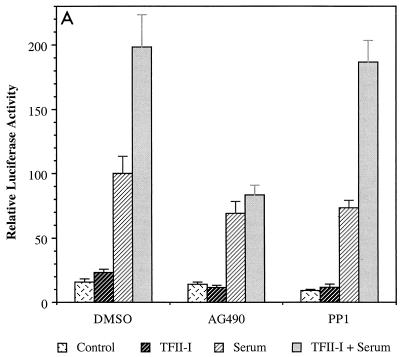
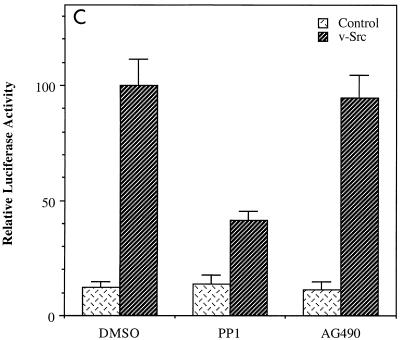
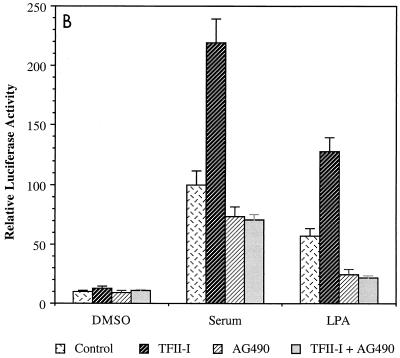
FIG. 1.
JAK2 inhibitor AG490 abolishes the activity of TFII-I on the c-fos promoter. (A) NIH 3T3 cells were transfected with the c-fos–luciferase reporter construct in the absence or presence of TFII-I and serum starved for 36 h in 0.5% CS following the transfection. The transfected cells were then incubated with 25 μM AG490 or 100 nM PP1 for 1 h prior to serum stimulation. Each inhibitor was also included during the 4-h FCS stimulation. Cell extracts were then processed for luciferase activity. (B) NIH 3T3 cells were transfected with the c-fos–luciferase reporter construct with or without TFII-I and serum starved for 40 h in 0.5% CS. Then the transfected cells were treated with 25 μM AG490 for 1 h prior to and during stimulation with 10% FCS or 10 μM LPA for 4 h as indicated. Cell extracts were then processed for luciferase activity. (C) The v-Src expression plasmid was transfected into NIH 3T3 cells with the c-fos–luciferase reporter construct, and the cells were maintained for 48 h in 0.5% CS following the transfection. AG490 (25 μM) or of PP1 (100 nM) was included in the culture for final 12 h before harvest as indicated. Cell extracts were then processed for luciferase activity. DMSO, dimethyl sulfoxide.
Cell culture, transfection and infection.
Murine NIH 3T3 fibroblasts were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% calf serum (CS). Rat-1 and COS-1 cells were cultured in DMEM with 10% fetal calf serum (FCS). For transient transfection, Fugene 6 (Boehringer Mannheim) was used. For reporter assays, 100 ng of reporter construct, 250 ng of pEBG-TFII-I (or pEBG) expression plasmid, and 25 ng of pRL-TK normalization plasmid were used per well of a 12-well plate. Twenty-five nanograms of V12-Ras, BXB-Raf, L63-RhoA, or v-Src construct and 100 ng of dominant negative JAK2 expression plasmid were included where indicated. The total amount of DNA per well was kept at 400 ng using pCDNA3 to adjust total DNA amounts where necessary. The dual luciferase assay was carried out as previously described (15). All transfection experiments were performed in duplicate, and the results were normalized to the expression of the Renilla luciferase transfection control. Relative luciferase activities presented are from a representative experiment, and similar results were obtained in all cases in at least three additional independent experiments. For inhibitor treatment, PP1 (19) and AG490 (26) were obtained from Calbiochem. Human recombinant epidermal growth factor EGF was purchased from R&D Systems. For Western blot analyses, COS-1 cells were maintained before harvest for 36 h in medium containing 10% FCS following transfection, except for the experiment in Fig. 8b where the cells were incubated in medium containing 2.5% FCS after transfection. Five micrograms of the indicated expression plasmids was used per 100-mm plate. The total amount of DNA was kept at 15 μg per 100-mm dish using pCDNA3 to adjust total DNA amounts where necessary. For the experiment in Fig. 4, Rat-1 cells were infected with a vector control adenovirus or SOCS-1-encoding adenovirus for 12 h (3,000 particles per cell) in the normal growth media and then serum starved for 36 h in medium with 0.5% CS. Stimulation was carried out with 10% FCS for 10 min prior to harvest. SOCS-1 adenovirus was a gift from Dan Frantz and Steve Shoelson.
FIG. 4.
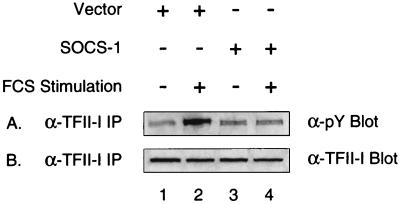
SOCS-1 inhibits tyrosine phosphorylation of TFII-I. Rat-1 cells were infected with empty vector (lanes 1 and 2) or SOCS-1-encoding adenovirus (lanes 3 and 4) for 12 h. Following the infection, the cells were maintained for 30 h in 0.5% FCS-containing DMEM (lanes 1 and 3) before a 10-min FCS stimulation was carried out (lanes 2 and 4). Cell extracts were then immunoprecipitated using anti-TFII-I antibody, and the immunoprecipitated proteins were fractionated by SDS-PAGE (10% polyacrylamide) and analyzed by antiphosphotyrosine (panel A) or anti-TFII-I (panel B) antibody Western blotting.
GST pulldown, immunoprecipitation, and Western blot analysis.
Cell lysates for the experiments in Fig. 3a, 4, 6, 8b, 8c, and 9 were prepared in buffer containing 20 mM Tris (pH 7.8), 200 mM KCl, 10% glycerol, 0.4% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride, and 1 μg each of leupeptin, antipain, pepstatin, and chymostatin per ml. The lysates were then centrifuged for 10 min at 10,000 × g at 4°C, and the supernatants were used for GST pulldown or immunoprecipitation assays as previously described (15, 16). Western blot analyses were carried out under standard conditions.
FIG. 3.
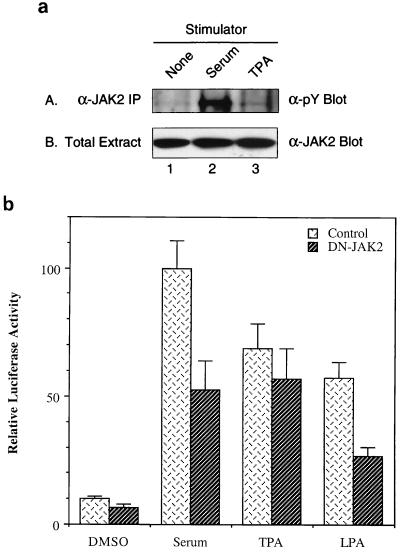
Serum stimulation enhances tyrosine phosphorylation of JAK2, and dominant negative JAK2 reduces serum induction of the c-fos promoter. (a) NIH 3T3 fibroblasts were serum starved for 36 h (lane 1) and stimulated with 15% FCS (lane 2) or 25 ng of TPA per ml (lane 3) for 10 min. Total cell extracts were immunoprecipitated with anti-JAK2 antibody. The immunoprecipitates (panel A) and total extracts (panel B) were fractionated by SDS-PAGE (8% polyacrylamide) and blotted with antiphosphotyrosine antibody (panel A) or anti-JAK2 antibody (panel B). (b) Dominant negative JAK2 expression plasmid was transfected into NIH 3T3 cells with the c-fos–luciferase reporter construct, and the cells were maintained for 36 h in 0.5% CS following the transfection. Then the transfected cells were stimulated with 10% FCS, 25 ng of TPA per ml, or 10 μM LPA for 4 h before harvest. Cell extracts were then processed for luciferase activity.
FIG. 6.
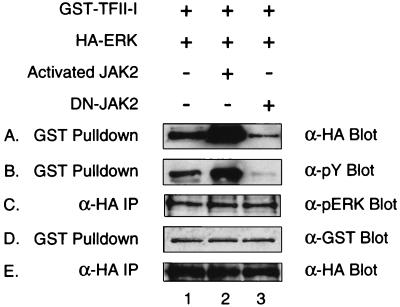
JAK2 can regulate the interaction between TFII-I and ERK. pEBG-TFII-I and HA-ERK1 expression plasmids were cotransfected into COS-1 cells in the presence of pCDNA3 (lane 1), activated JAK2 (lane 2), or dominant negative JAK2 (lane 3). Transfected COS-1 cells were lysed and subjected to a GST-pulldown assay. Glutathione-Sepharose bead-bound proteins (panels A, B, and D) and anti-HA antibody immunoprecipitates (panels C and E) were fractionated by SDS-PAGE (12% polyacrylamide) and analyzed by anti-HA (panels A and E), antiphosphotyrosine (panel B), anti-pERK (panel C), or anti-GST (panel D) antibody Western blotting.
FIG. 9.
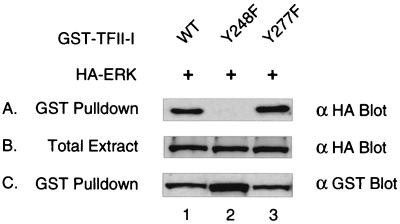
Tyrosine 248 of TFII-I is required for the ERK interaction. pEBG–TFII-I (wild type) (lane 1), pEBG–Y248F–TFII-I (lane 2), or pEBG–Y277F–TFII-I (lane 3) expression plasmids were cotransfected with the HA-ERK expression plasmid into COS-1 cells. Transfected COS-1 cells were lysed and subjected to a GST-pulldown assay. Glutathione-Sepharose bead-bound proteins (panels A and C) and total-cell extracts (panel B) were fractionated by SDS-PAGE (12% polyacrylamide) and blotted by anti-HA (panel A and B) or anti-GST (panel C) antibody.
In vitro mutagenesis and isolation of overexpressed TFII-I.
The Y248F-TFII-I and Y277F-TFII-I mutants were generated as previously described (16). The following complementary oligonucleotides were used to synthesize mutated DNA strands (mutated bases in boldface): Y248F (+), GGAAGAATCAGAAGATCCTGATTtTTATCAATATAACATTCAAGGAAGCC; Y248F (−), GGCTTCCTTGAATGTTATATTGATAAaAATCAGGATCTTCTGATTCTTCC; Y277F (+), CCAGCAGAAGATGATGATTtTTCTCCACCGTCTAAGAGACC; and Y277F (−), GGTCTCTTAGACGGTGGAGAAaAATCATCATCTTCTGCTGG. The wild-type or various mutant TFII-I proteins were purified from transfected COS-1 cells as previously described (15), and equal amount of each protein was used for in vitro kinase assays.
In vitro kinase assay.
HA-JAK2 (see Fig. 7) or HA-ERK (see Fig. 10) expression construct (10 μg per 100-mm plate) were transfected into COS-1 cells, and the cells were maintained for 36 h in medium containing 10% FCS and stimulated with 25 ng of EGF per ml for 10 min before harvest. Cell lysates were prepared in buffer containing 25 mM Tris (pH 7.5), 500 mM NaCl, 10 mM MgCl2, 5 mM MnCl2, 0.2 mM Na3VO4, 10% glycerol, 1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride, and 1 μg each of leupeptin, antipain, pepstatin, and chymostatin per ml. The lysates were then centrifuged for 10 min at 10,000 × g at 4°C, and the supernatants were used for anti-HA antibody immunoprecipitation. Immunoprecipitated JAK2- or ERK-containing beads were washed with kinase buffer (25 mM Tris [pH 7.5], 100 mM NaCl, 10 mM MgCl2, 5 mM MnCl2, 0.2 mM Na3VO4). The immune complex kinase assay was performed essentially as described previously (40). Briefly, immunoprecipitates were incubated for 30 min at room temperature with 20 μCi (6,000 Ci/mmol) of [γ-32P]ATP in either the presence or absence of substrate proteins. The labeled proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (8% polyacrylamide), fixed, dried and visualized by autoradiography. Each band was also quantitated by using Phosphorimager analysis for the reaction efficiency comparison.
FIG. 7.
JAK2 can phosphorylate TFII-I in vitro. (a) pEBG empty vector (lane 1) or HA-JAK2 expression plasmid (lane 2) was transfected into COS-1 cells. Following the transfection, the cells were maintained for 40 h in 10% FCS-containing DMEM before being subjected to EGF stimulation. Cell extracts were then immunoprecipitated to pull down JAK2 using anti-HA antibody, and an in vitro kinase assay was carried out in the presence of purified TFII-I protein and [γ-32P]ATP as described in Materials and Methods. The amounts of TFII-I and JAK2 proteins used in each reaction were compared by anti-TFII-I (panel B) or anti-HA (panel C) antibody Western blotting. (b) A JAK2 in vitro kinase assay was carried out with the wild-type–TFII-I (lane 2), Y248F–TFII-I (lane 3), or Y277F–TFII-I (lane 4) proteins as substrates. Only [γ-32P]ATP without substrate was added to the reaction mixture in lane 1 as a negative control. 32P incorporation of each band was quantitated using PhosphorImager analysis in panel B. A comparable amount of the purified TFII-I protein used in each reaction as substrate was shown by anti-GST antibody Western blotting in panel C.
FIG. 10.
Tyrosine 248 of TFII-I is required for its phosphorylation by ERK. HA-ERK expression plasmid was transfected into COS-1 cells. Following the transfection, the cells were maintained for 40 h in 10% FCS-containing DMEM before being subjected to a 10-min EGF stimulation. Cell extracts were then immunoprecipitated using anti-HA antibody, and an in vitro kinase assay was carried out in the presence of the wild-type–TFII-I (lane 2), Y248F–TFII-I (lane 3), or Y277F–TFII-I (lane 4) protein and [γ-32P]ATP as described in Materials and Methods. [γ-32P]ATP but no substrate was added to the reaction mixture in lane 1 as a negative control (panel A). 32P incorporation of each band was quantitated using a PhosphorImager (panel B). A comparable amount of the purified TFII-I protein was used in each reaction, as shown by anti-GST antibody Western blotting (panel C).
RESULTS
JAK2 is required for the activity of TFII-I.
We have previously shown that growth factor stimulation enhances the activity of TFII-I and its tyrosine phosphorylation (15). To attempt to identify which tyrosine kinases are required for the tyrosine phosphorylation of TFII-I in nonlymphoid cells, we initially examined the effect of various kinase inhibitors on the activity of TFII-I. JAK2 and Src tyrosine kinase inhibitors were selected for investigation since we have demonstrated that TFII-I is regulated by Ras (15) and both JAK2 and Src have been implicated in Ras-associated signal transduction pathways (27, 42, 44, 48, 50). Thus, the c-fos–luciferase reporter construct was transfected into NIH 3T3 fibroblasts in the absence or presence of TFII-I and the transfected cells were maintained in 0.5% calf serum for 36 h. Prior to serum stimulation, the cells were preincubated for 1 h with AG490 (26), a specific JAK2 inhibitor, or PP1 (19), a specific inhibitor of Src family kinases. The luciferase assay was carried out on cellular extracts after 4 h serum stimulation. As can be seen in Fig. 1A, the enhancement of the c-fos promoter by TFII-I in response to serum was abolished by the JAK2 inhibitor AG490. This result suggests that TFII-I functions in a manner that is dependent on the activity of the JAK2 tyrosine kinase. In the absence of TFII-I overexpression, AG490 diminished overall c-fos promoter induction in response to serum by 30%. The lack of complete inhibition of the c-fos promoter by AG490 suggests that other JAK2-independent pathways contribute to promoter induction by serum. This is to be expected given the multiplicity of pathways activated by serum stimulation that converge on the c-fos promoter (46). Lysophosphatidic acid (LPA) is believed to activate c-fos principally through the RhoA-SRF pathway (12). Interestingly, when AG490 was added to LPA-stimulated cells transfected with c-fos–luciferase, overall induction of the c-fos promoter was inhibited by more than 50% and the further activation of the c-fos promoter by TFII-I was completely abrogated, as shown in Fig. 1B. These results suggest that JAK2 plays a significant role in LPA induction of c-fos.
In contrast, PP1 did not have significant effects on the activity of TFII-I (Fig. 1A), indicating that Src kinase is not required for the activity of TFII-I in this assay. To rule out the possibility that PP1 was not active in the experiment, a similar c-fos reporter assay using the v-Src expression construct was carried out. The activation of the c-fos promoter by v-Src overexpression was efficiently diminished by PP1 treatment (Fig. 1C), indicating that PP1 was effective as an inhibitor in the reporter assays, but did not affect the activity of TFII-I on the c-fos promoter. AG490 had no significant effect on v-Src activation of c-fos, indicating that AG490 did not inhibit Src family tyrosine kinases at the concentration used in these experiments.
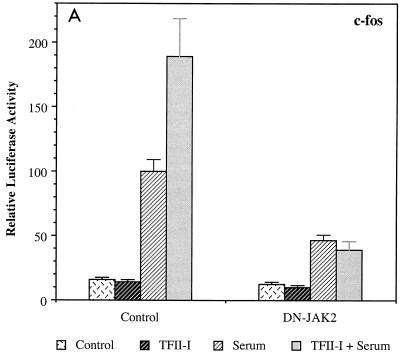
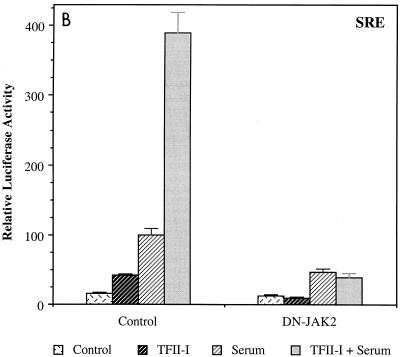
To further confirm the functional cooperation between TFII-I and JAK2, dominant negative JAK2 was transfected into NIH 3T3 fibroblasts with the c-fos–luciferase reporter in the absence or presence of TFII-I and luciferase activity was measured after serum stimulation. The results are shown in Fig. 2A. Dominant negative JAK2 eliminated the ability of TFII-I to enhance the c-fos promoter in response to serum stimulation and reduced the overall serum responsiveness of the promoter. Consistent with the results in Fig. 1, these results also indicate that the activity of TFII-I on the c-fos promoter requires JAK2. To determine whether dominant negative JAK2 affects the c-fos induction mainly through inactivation of the SIE via the JAK/STAT pathway, the effects of dominant negative JAK2 on a heterologous promoter driven by an isolated SRE lacking the STAT binding site SIE were examined (Fig. 2B). Very similar to the c-fos promoter, dominant negative JAK2 completely abolished enhancement of the SRE promoter by TFII-I and attenuated the overall promoter induction by serum. Interestingly, TFII-I cotransfection had a greater enhancement effect on the isolated SRE-driven promoter than on the full-length c-fos promoter. Thus, consistent with the results in Fig. 1, these results imply that the majority of the TFII-I/JAK2 activity on the c-fos promoter is mediated by SRF and the SRE.
FIG. 2.
Dominant negative JAK2 inhibits the transcriptional activity of TFII-I. (A and B) The dominant negative JAK2 expression plasmid was transfected into NIH 3T3 fibroblasts with the c-fos–luciferase (A) or an isolated SRE-luciferase (B) reporter construct in the absence or presence of TFII-I, and the cells were maintained for 40 h in 0.5% CS prior to a 4-h FCS stimulation. Cell extracts were then processed for luciferase activity. (C) The L63-RhoA expression plasmid was transfected into NIH 3T3 fibroblasts with the c-fos–luciferase reporter construct in the absence or presence of TFII-I, and the dominant negative JAK2 expression plasmid was also included where indicated. The transfected cells were maintained for 40 h in 0.5% CS before harvest. Cell extracts were then processed for luciferase activity.
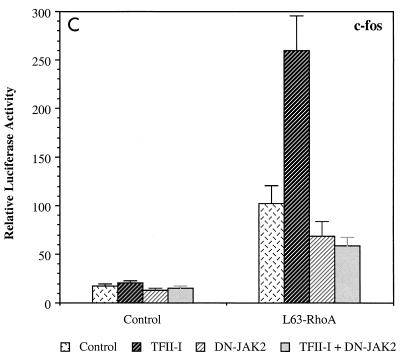
We have previously shown that RhoA can functionally cooperate with TFII-I for activation of the c-fos promoter (16), and RhoA has been shown to be the major upstream activator of the c-fos SRE in response to serum and LPA (12). To further understand the role of JAK2 in the activation of the c-fos SRE, we examined whether dominant negative JAK2 can affect RhoA induction of the c-fos promoter. The results are shown in Fig. 2C. TFII-I enhanced c-fos induction by activated L63-RhoA, and dominant negative JAK2 reduced it. Moreover, c-fos promoter enhancement by TFII-I in conjunction with L63-RhoA was completely eliminated by dominant negative JAK2 coexpression, suggesting that JAK2 is required for the functional cooperation between TFII-I and RhoA.
Serum stimulation activates JAK2.
JAK2 has been extensively investigated in various cytokine and growth factor signal transduction pathways (1, 49). However, it has not been demonstrated that JAK2 is activated by serum growth factors. Since JAK2 affects the activity of TFII-I on the c-fos promoter in response to serum, we examined whether JAK2 itself can be activated by serum stimulation. NIH 3T3 fibroblasts were serum starved for 36 h and stimulated with serum or tetradecanoyl phorbol acetate (TPA) for 10 min. Total-cell extracts were immunoprecipitated with anti-JAK2 antibody and blotted with antiphosphotyrosine antibody to determine JAK2 activation by different stimuli. As shown in panel A of Fig. 3a, tyrosine phosphorylation of JAK2 was increased on serum stimulation (lane 2) but not by TPA treatment (lane 3). The anti-JAK2 Western blot (panel B in Fig. 3a) shows that the overall protein level of JAK2 in the cells remained the same before and after stimulation. These results suggest that JAK2 becomes activated through tyrosine phosphorylation in response to serum but not to TPA. Consistent with this, Fig. 3b shows that dominant negative JAK2 inhibited c-fos induction by serum and LPA but not by TPA. This result, coupled with the inhibitory effects of AG490 (Fig. 1A and B) and SOCS-1 (see Fig. 4), indicates that JAK2 is active after serum stimulation. Thus, we conclude that serum growth factors lead to the activation of this kinase.
SOCS-1 inhibits serum-induced tyrosine phosphorylation of endogenous TFII-I in Rat-1 fibroblasts.
Since serum activates JAK2 and the in vivo function of TFII-I requires JAK2, we next examined whether the tyrosine phosphorylation of endogenous TFII-I can be affected by JAK2 inactivation. To do this, Rat-1 fibroblasts were infected with recombinant adenovirus overexpressing SOCS-1 (for “suppressor of cytokine signaling 1”). SOCS-1 has been well documented to be a potent JAK family kinase inhibitor (43). A vector adenovirus not expressing SOCS-1 was also used in the experiment as a negative control. After the infection, Rat-1 cells were serum starved for 36 h and stimulated with serum for 10 min before harvest. Total-cell extracts were immunoprecipitated with anti-TFII-I antibody and blotted with antiphosphotyrosine antibody. As shown in panel A of Fig. 4, tyrosine phosphorylation of TFII-I can be enhanced by serum stimulation in the control adenovirus-infected cells (lane 2) and this inducible tyrosine phosphorylation of TFII-I was absent in the SOCS-1-infected cells (lane 4). Panel B shows that the protein levels of TFII-I were comparable between the samples. Consistent with other data presented in this work, these results indicate that endogenous JAKs play an important role in the signal-dependent tyrosine phosphorylation of TFII-I.
TFII-I requires JAK2 for its activity independently of MAP kinase activation by JAK2.
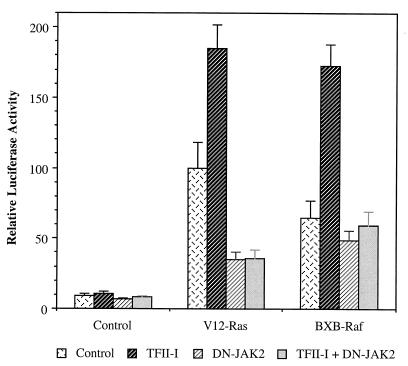
JAK2 is required for the activity of TFII-I (Fig. 1 and 2) and also has been implicated in the activation of Raf leading to the activation of ERK (42, 48, 50). We have previously demonstrated that TFII-I requires functional ERK for its activity (16). Therefore, to clearly understand the regulation of TFII-I by JAK2, we sought to determine whether JAK2 affects the activity of TFII-I through its affects on the MAP kinase cascade or through distinct mechanisms. To differentiate between these possibilities, the c-fos reporter assay was carried out after transfection with different combinations of V12-Ras, BXB-Raf, and dominant negative JAK2 in the absence or presence of TFII-I and the luciferase activity was measured. The results are shown in Fig. 5. The activation of the c-fos promoter by activated V12-Ras was diminished 65% by dominant negative JAK2, whereas the effects of activated BXB-Raf, which should be less dependent on JAK2, were only 25% inhibited. This is consistent with previous reports showing that JAK2 can participate in Raf activation by Ras (42, 48, 50). If the effects of JAK2 on TFII-I were primarily through its ability to activate ERK, then BXB-Raf should be able to overcome the inhibitory effect of dominant negative JAK2 on TFII-I, since its ability to activate ERK is Ras and JAK2 independent (11). However, c-fos promoter enhancement by TFII-I in the presence of either V12-Ras or BXB-Raf was completely abolished by dominant negative JAK2 coexpression, suggesting that TFII-I requires JAK2 for its activity independently of MAP kinase activation. These results indicate that JAK2 functions largely downstream of or in parallel to Ras but upstream of Raf to lead to c-fos promoter activation. This conclusion is further confirmed by the experiments below.
FIG. 5.
BXB-Raf fails to bypass the JAK2 requirement for the activity of TFII-I. pCDNA3 (Control), V12-Ras, or BXB-Raf expression plasmids were transfected into NIH 3T3 fibroblasts with the c-fos–luciferase reporter construct in the absence ( and
and  ) or presence (
) or presence ( and
and  ) of TFII-I. pCDNA3 (
) of TFII-I. pCDNA3 ( and
and  ) or dominant negative JAK2 (DN-JAK2) (
) or dominant negative JAK2 (DN-JAK2) ( and
and  ) plasmid was also included in the transfections where indicated. Following the transfection, the cells were maintained for 40 h in 0.5% CS before harvest. Cell extracts were then processed for luciferase activity.
) plasmid was also included in the transfections where indicated. Following the transfection, the cells were maintained for 40 h in 0.5% CS before harvest. Cell extracts were then processed for luciferase activity.
The interaction between TFII-I and ERK can be regulated by JAK2.
We have previously demonstrated that TFII-I interacts with the MAP kinase ERK in a signal transduction-dependent manner and that this binding is essential for the activity of TFII-I (16). To correlate the regulation of TFII-I by JAK2 with ERK interaction, we examined whether constitutively activated or dominant negative JAK2 can affect ERK binding to TFII-I. Thus, GST–TFII-I and HA-ERK expression plasmids were cotransfected into COS-1 cells in the presence of various JAK2 expression plasmids. Protein complexes were isolated by GST pulldown and analyzed by Western blot analyses. The interaction between TFII-I and ERK was detected by Western blot analyses using anti-HA antibody, and the tyrosine phosphorylation of TFII-I was determined by antiphosphotyrosine antibody blotting. The results are shown in Fig. 6. Coexpression of the activated JAK2 enhanced the interaction between TFII-I and ERK (lane 2), whereas the dominant negative JAK2 reduced it (lane 3), as can be seen in panel A. These results suggest that JAK2 can regulate the interaction between TFII-I and ERK. Panels D and E show that there was comparable expression of the transfected TFII-I and ERK between the samples. Interestingly, TFII-I tyrosine phosphorylation was affected by JAK2 (panel B) while phosphorylation state of ERK remained relatively unchanged (panel C). The activated JAK2 also increased the tyrosine phosphorylation of TFII-I (lane 2 of panel B), and the dominant negative JAK2 significantly decreased it (lane 3 of panel B), which correlates with the observed interaction with ERK. This result is of interest because JAK2 is required for the activity of TFII-I independently of MAP kinase activation, as indicated in Fig. 5. Therefore, it is likely that tyrosine phosphorylation of TFII-I by JAK2 regulates its interaction with ERK, which is in turn necessary for its enhancement of the c-fos promoter (16).
Mutation of tyrosine 248 diminishes JAK2 phosphorylation of TFII-I in vitro.
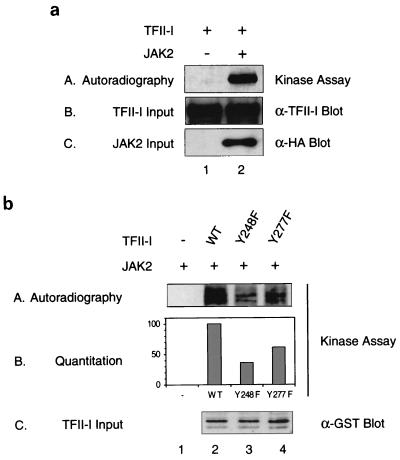
Since the regulation of TFII-I by JAK2 is likely to occur through its tyrosine phosphorylation, we attempted to determine whether TFII-I can be a direct substrate of JAK2. To do this, HA-tagged JAK2 was overexpressed in COS-1 cells and immunoprecipitated using anti-HA antibody and an in vitro kinase assay was carried out in the presence of [γ-32p]ATP as described in Materials and Methods. GST–TFII-I protein purified as previously described (15) was added to the reaction mixture as substrate. As can be seen in panel A of Fig. 7a, TFII-I was efficiently phosphorylated in vitro by the HA-JAK2 immunoprecipitate (lane 2). In the absence of HA-JAK2 transfection, no phosphorylation of the TFII-I substrate was observed (lane 1). This shows that JAK2 (or a tightly associated kinase) can phosphorylate TFII-I. The input amounts of TFII-I and JAK2 were shown in panel B and C.
Analysis of the TFII-I protein sequence reveals two consensus tyrosine phosphorylation sites (244 EDPDY 248, 273 EDDDY 277). To identify the JAK2 phosphorylation sites on TFII-I, two tyrosine point mutations of TFII-I were generated by changing tyrosines 248 or 277 of TFII-I to phenylalanines. These mutants are referred to below as Y248F–TFII-I or Y277F–TFII-I. The non-PCR-based QuickChange mutagenesis method (Stratagene) was used to avoid the introduction of other mutations into the protein, and each mutation was verified by sequencing. To determine whether the mutation of either tyrosine 248 or 277 alters the ability of JAK2 to phosphorylate TFII-I, purified Y248F–TFII-I or Y277F–TFII-I were used as substrates of the JAK2 in vitro kinase assay, as shown in Fig. 7b. Compared to the wild type (lane 2, panel A), the Y248F–TFII-I mutant exhibited greatly reduced phosphorylation by JAK2 (lane 3, panel A) and Y277F–TFII-I also showed a substantial decrease in its phosphorylation (lane 4, panel A). Both of these mutation failed to denature or significantly affect the folding of TFII-I as they both retained the ability to bind DNA in a sequence-specific manner (data not shown). Phosphorylation efficiency of different TFII-I mutants by JAK2 in comparison with the wild type was quantitated by measuring 32P incorporation of each band using PhosphorImager analysis (panel B). The amount of the purified TFII-I protein used in each reaction as substrate was determined by anti-GST antibody Western blotting and is shown in panel C. These results suggest that both tyrosine 248 and 277 of TFII-I can be phosphorylated by JAK2 but to different extents and that tyrosine 248 is a more significant in vitro substrate site than tyrosine 277.
Tyrosine 248 is phosphorylated in vivo in a signal-dependent manner.
To confirm the phosphorylation of Y248 in vivo, a phosphospecific antibody against the pY248 peptide (VKEESEDPDpYYQYN) was generated as described in Materials and Methods. To characterize the specificity of this antiserum a dot blot using anti-pY248 antibody was carried out against phospho-and nonphospho-Y248 peptides. Both phospho- and nonphospho-Y462 peptides were included in the experiment as unrelated peptide controls. The sequence of each peptide is described in Materials and Methods. Figure 8a shows that anti-pY248 antibody only recognizes the pY248 peptide and none of the other peptides spotted onto the nitrocellulose filter. These results indicate that this antiserum (α-pY248) specifically recognizes the phosphorylated form of the Y248 peptide and in addition does not simply recognize all phosphotyrosines, since the pY462 peptide is not recognized by this antibody.
To demonstrate that the anti-pY248 antibody recognizes in vivo phosphorylation of Y248 in the context of the native TFII-I protein, wild-type–TFII-I or Y248F–TFII-I expression plasmids were transfected into COS-1 cells in the absence or presence of activated JAK2. The transfected cells were grown in low-serum medium for 40 h, and the whole-cell lysates were immunoprecipitated with anti-TFII-I antibody. The isolated TFII-I was then analyzed by Western blot analyses using antiphosphotyrosine, anti-pY248, or anti-TFII-I antibody. The results are shown in Fig. 8b. Anti-pY248 antibody recognized pY248 in the context of the full length TFII-I protein (lane 2 of panel B), and mutation of this site abolished this recognition (lane 4 of panel B). Consistent with our in vitro results in Fig. 7b, cotransfection of JAK2 stimulated Y248 phosphorylation of TFII-I in vivo. From the results with the antiphosphotyrosine antibody (panel A), we found that mutation of Y248 diminished but did not abolish tyrosine phosphorylation of TFII-I, suggesting that there are additional sites of tyrosine phosphorylation as previously suggested (31, 32).
To further determine whether serum stimulates the phosphorylation of endogenous TFII-I on Y248, NIH 3T3 fibroblasts were serum starved for 36 h and stimulated with serum for 10 min. Total-cell extracts were immunoprecipitated with anti-TFII-I antibody and then blotted with the anti-pY248 antibody. As shown in panel A of Fig. 8c, Y248 phosphorylation of TFII-I was increased upon serum stimulation. The anti-TFII-I Western blot (panel B) shows that the overall protein level of TFII-I between samples was comparable. These results demonstrate that serum stimulation results in enhanced Y248 phosphorylation of endogenous TFII-I and that Y248 is indeed a signal-dependent in vivo phosphorylation site of TFII-I.
The interaction between TFII-I and ERK requires tyrosine 248.
We have found that JAK2 can regulate the interaction between TFII-I and ERK (Fig. 6) and can phosphorylate TFII-I in vitro in a Y248- and Y277-dependent manner (Fig. 7). Moreover, signal-dependent Y248 phosphorylation was observed in vivo (Fig. 8). To evaluate the role of these JAK2 phosphorylation sites of TFII-I in ERK binding in vivo, we examined whether these tyrosine mutants are capable of interacting with ERK. To compare ERK binding between the wild type and the mutants, GST-fused wild-type–TFII-I, Y248F–TFII-I, or Y277F–TFII-I expression plasmids were cotransfected with HA-ERK expression plasmid into COS-1 cells. Western blot analysis using anti-HA antibody was carried out following GST-pulldown to determine the interaction between the mutant TFII-I and ERK. As can be seen in Fig. 9, Y248F-TFII-I completely lost its ability to form a stable complex with ERK (lane 2, panel A) compared to the wild-type TFII-I (lane 1, panel A) whereas Y277–TFII-I retained its affinity for ERK (lane 3, panel A). Panel B shows that there was comparable expression of the transfected ERK between samples, and panel C confirms the expression of the different mutants of TFII-I. These results clearly indicate that Y248 of TFII-I is required for the interaction with ERK but that Y277 of TFII-I, which is a poorer phosphorylation site than Y248, is dispensable for ERK binding. Based on the results in Fig. 6 to 9, we conclude that tyrosine phosphorylation of TFII-I on Y248 by JAK2 is essential to enable TFII-I to interact with ERK.
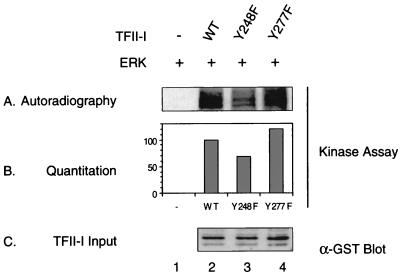
Phosphorylation of TFII-I by ERK is decreased by the tyrosine 248 mutation.
We have previously demonstrated that TFII-I must bind ERK and be phosphorylated by it in order to enhance c-fos promoter activation (16). Moreover, mutation of the ERK binding site on TFII-I severely impaired its ability to be phosphorylated by ERK. Since the tyrosine 248 mutation resulted in the loss of ERK binding (Fig. 9), we examined whether Y248F–TFII-I also impairs phosphorylation by ERK. An in vitro ERK kinase assay was carried out as previously described (16) with the wild-type–TFII-I, Y248F–TFII-I and Y277F–TFII-I as substrates. The results are shown in Fig. 10. The reduction of ERK interaction caused by tyrosine 248 mutation of TFII-I also impaired the phosphorylation of TFII-I by ERK substantially (lane 3), suggesting that this phosphorylation site of TFII-I modulates both its interaction with ERK and its ability to serve as an ERK substrate. Consistent with the results in Fig. 9, the phosphorylation of Y277F–TFII-I by ERK was unaffected (lane 4) since it retained ERK binding. Each reaction was quantitated as shown in panel B, and a control confirming comparable amounts of input TFII-I present in each sample is shown in panel C.
Tyrosine 248 is required for the activity of TFII-I for the c-fos promoter.
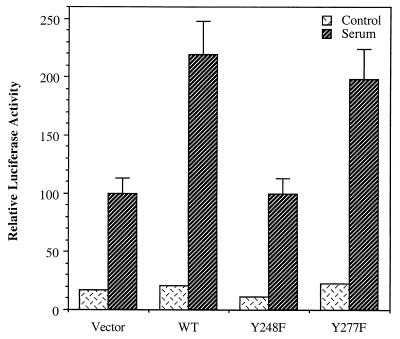
To assess the significance of Y248 of TFII-I on its function, we examined whether tyrosine 248 is required for the in vivo activity of TFII-I for the c-fos promoter in the reporter assay system. Y248F–TFII-I was transfected into NIH 3T3 fibroblasts with the c-fos–luciferase reporter construct, and luciferase activity was measured after serum stimulation. Empty vector, wild-type–TFII-I, and Y277F–TFII-I were also used in the same experiment as negative or positive controls. The results are shown in Fig. 11. The mutant Y248F–TFII-I, which is defective for ERK binding due to its loss of the JAK2 phosphorylation site, was not able to potentiate the c-fos promoter on serum stimulation, whereas the wild-type TFII-I enhanced the promoter response as expected. Y277F–TFII-I, which interacts normally with ERK (Fig. 9), showed the same level of enhancement activity for the c-fos promoter as did the wild type, as anticipated. Thus, it can be concluded that tyrosine 248, which is a JAK2 phosphorylation site of TFII-I and is also required for ERK interaction, is essential for the activity of TFII-I on the c-fos promoter enhancement in vivo.
FIG. 11.
TFII-I requires tyrosine 248 for its in vivo activity for the c-fos promoter. pEBG, pEBG–TFII-I (wild type), pEBG–Y248F–TFII-I, or pEBG–Y277F–TFII-I plasmids were transfected into NIH 3T3 fibroblasts with the c-fos–luciferase reporter construct. The cells were serum starved for 36 h in 0.5% CS and then stimulated for 4 h with 10% FCS. Cell extracts were then processed for luciferase activity.
DISCUSSION
Previously in experiments with B cells, TFII-I has been found to functionally and physically associate with the lymphoid cell-specific BTK and is a substrate of it (32, 53). Here we find that the activity of TFII-I on the c-fos promoter in fibroblasts is inhibited by dominant negative JAK2 and by the JAK2-specific inhibitor AG490. Consistent with this, we also show that activated or dominant negative JAK2 coexpression can affect tyrosine phosphorylation of transfected TFII-I in COS-1 cells and that SOCS-1 overexpression by adenovirus infection efficiently abolished serum-induced tyrosine phosphorylation of the endogenous TFII-I in Rat-1 fibroblasts. Similar to the STAT transcription factors, we demonstrate here that TFII-I can be a direct substrate of JAK2 and that this phosphorylation of TFII-I by JAK2 is significantly reduced by mutation of tyrosine 248 of TFII-I to phenylalanine. Interestingly, Y248F–TFII-I is not capable of interacting with ERK or of being phosphorylated by ERK and is no longer able to activate the c-fos promoter in vivo. Dominant negative JAK2 has a similar effect on TFII-I. These results suggest that tyrosine phosphorylation of TFII-I by JAK2 regulates its association with ERK and its activity on the c-fos promoter.
Potentially complicating the interpretation of these results is the observation that TFII-I is regulated by the Ras-ERK pathway (16) and that JAK2 has been found to be able to regulate the Raf-MAP kinase pathway in conjunction with Ras (42, 48, 50). Thus, it is necessary to distinguish the effects of JAK2 kinase on the activation of ERK from its affects on TFII-I. Several lines of evidence suggest that JAK2 phosphorylation of TFII-I is important independently of its effects on ERK. One of these is the finding that activated Raf, which bypasses JAK2 and Ras for ERK activation, fails to bypass the inhibitory effects of dominant negative JAK2 on TFII-I activity, as shown in Fig. 5. The other line of evidence is that mutation of the putative JAK2 phosphorylation site at Y248, which does not affect ERK activation per se, inhibits its association with ERK (Fig. 9), its ability to be phosphorylated by ERK (Fig. 10), and its in vivo activity on the c-fos promoter (Fig. 11). Thus, while it may indeed be the case that JAK2 may contribute to ERK activation, these data indicate that JAK2 also regulates TFII-I at a distinct level by modulating its functional association with ERK.
TFII-I binds to ERK though its MAP kinase binding motif (D box) like Elk-1 (16, 51, 52). Our findings here are the first example of regulated binding of MAP kinase to a D box by phosphorylation of the D-box-containing protein. Targeted interaction of ERK with its substrates such as Elk-1 has been shown to be regulated by the activation state of ERK and probably by its intracellular compartmentalization; i.e., the D-box protein Elk-1 resides in the nucleus prior to ERK activation and translocation to the nucleus. Our finding that mutation of a phosphorylation site of TFII-I outside of the D box disrupts the interaction with ERK is the first example of the regulation of ERK interaction with a substrate by modification of the substrate through tyrosine phosphorylation. The mechanism by which tyrosine phosphorylation of TFII-I may enhance ERK association is unclear. However, since the D box (amino acids 282 to 293) and tyrosine 248 of TFII-I are clearly separated in the primary sequence of TFII-I, it is likely that phosphorylation of Y248 leads to a conformational change of the molecule that makes the D box accessible to ERK.
Here we also show that JAK2 is activated by serum. It has been previously reported that JAK kinases can lead to ERK activation (48, 50). This suggests that JAK kinases may play an important role in the serum stimulation of ERK, which has been one of the major means of activating ERK experimentally. Moreover, the finding that JAK2 is serum regulated suggests that JAK2 may play a role in many serum-regulated signaling pathways. We are currently investigating these possibilities further.
Previously, the JAKs have been shown to be associated with various cytokine receptors and growth factor receptors for the activation (1, 47, 49). Serum is of course a complex mixture of factors, so JAK2 may be activated by any of a number of serum growth factors or cytokines present in serum. However, LPA is believed to be a major serum mitogen and functions through a seven-transmembrane receptor (28). Consistent with this, it has previously been shown that the seven-transmembrane receptor for angiotensin II can stimulate JAK2 phosphorylation (23). Thus, LPA may also activate JAKs through a heterotrimeric G protein.
LPA activates the c-fos SRE through a RhoA- and Ras-dependent mechanism (12). Since TFII-I binds to SRF and the SRE and is regulated by RhoA and Ras (15, 16), it is also likely to be a component the serum-regulated SRF pathway. The fact that dominant negative JAK2 inhibits serum stimulation of the c-fos promoter further suggests that JAK2 is an important serum-regulated tyrosine kinase and may also function in the LPA-RhoA-SRF pathway.
There is evidence that BTK is itself activated by JAK in lymphoid cells (45). This raises the question whether JAKs also participate in TFII-I regulation in lymphoid cells. In fact, both kinases are activated by IL-6 stimulation (24, 25). This could be the case since it appears that TFII-I is tyrosine phosphorylated on multiple sites. It is also possible that there may be other tyrosine kinases that phosphorylate TFII-I in nonlymphoid cells. Indeed, Src family kinases are known to be activated by serum (21). However, our observation that the Src family kinase inhibitor PP1 fails to affect TFII-I activity suggests that at least in fibroblasts the Src family kinases are not required for the regulation of TFII-I by the serum pathway. Nevertheless, it is likely that there are other tyrosine kinases that phosphorylate TFII-I, since we and others have found that TFII-I retains some tyrosine phosphorylation even in unstimulated cells (15, 31).
There are four known members of the JAK family (13), and a question that arises from our results is whether other JAK family members may also participate in TFII-I regulation. Here we have found that three different JAK inhibitors affect TFII-I: AG490, dominant negative JAK2, and SOCS-1. Together, these data suggest an important role for JAK2 in fibroblasts since AG490 and dominant negative JAK2 should primarily target JAK2 (2, 26). However, the JAKs in several cytokine receptors functions as heterodimers (13), and the same could be true here. Moreover, the expression of the various JAK family members varies among cell types. Therefore, it is possible that other JAK family members could substitute for JAK2 in cells where it is not expressed well (17). Further work is needed to determine whether other JAKs may regulate TFII-I.
In sum, we have found that JAK2 regulates TFII-I activity and its association with ERK. Future work will be directed toward identifying the role of phosphorylation in the cellular localization of TFII-I and its association with various upstream activators including SRF and STAT factors.
ACKNOWLEDGMENTS
We are grateful to Larry Feig, Alan D'Andrea, and Philip Tsichlis for providing some of the expression plasmids used in these experiments. We are especially grateful to Dan Frantz and Steve Shoelson for the gift of SOCS-1 adenovirus.
This work was supported by NIH grant R01-GM51551 to B.H.C.
REFERENCES
- 1.Argetsinger L, Campbell G S, Yang X, Witthuhn B A, Silvennoinen O, Ihle J N, Carter-Su C. Identification of JAK2 as a growth hormone receptor associated tyrosine kinase. Cell. 1993;74:237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- 2.Bright J J, Du C, Sriram S. Tyrphostin B42 inhibits IL-12-induced tyrosine phosphorylation and activation of Janus kinase-2 and prevents experimental allergic encephalomyelitis. J Immunol. 1999;162:6255–6262. [PubMed] [Google Scholar]
- 3.Chaturvedi P, Reddy M V, Reddy E P. Src kinases and not JAKs activate STATs during IL-3 induced myeloid cell proliferation. Oncogene. 1998;16:1749–1758. doi: 10.1038/sj.onc.1201972. [DOI] [PubMed] [Google Scholar]
- 4.Cochran B H. Regulation of immediate early gene expression. NIDA Res Monogr. 1993;125:3–24. [PubMed] [Google Scholar]
- 5.Cochran B H, Zullo J, Verma I M, Stiles C D. Expression of the c-fos gene and of a fos-related gene is stimulated by platelet-derived growth factor. Science. 1984;226:1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- 6.Feig L A, Pan B T, Roberts T M, Cooper G M. Isolation of ras GTP-binding mutants using an in situ colony-binding assay. Proc Natl Acad Sci USA. 1986;83:4607–4611. doi: 10.1073/pnas.83.13.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham R, Gilman M. Distinct protein targets for signals acting at the c-fos serum response element. Science. 1991;251:189–192. doi: 10.1126/science.1898992. [DOI] [PubMed] [Google Scholar]
- 8.Grammatikakis N, Lin J-H, Grammatikakis A, Tsichlis P N, Cochran B H. p50cdc37 acting in concert with Hsp90 is required for Raf-1 function. Mol Cell Biol. 1999;19:1661–1672. doi: 10.1128/mcb.19.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg M E, Ziff E B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984;311:433–442. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- 10.Grueneberg D A, Henry R W, Brauer A, Novina C D, Cheriyath V, Roy A L, Gilman M. A multifunctional DNA-binding protein that promotes the formation of serum response factor/homeodomain complexes: identity to TFII-I. Genes Dev. 1997;11:2482–2493. doi: 10.1101/gad.11.19.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidecker G, Huleihel M, Cleveland J L, Kolch W, Beck T W, Lloyd P, Pawson T, Rapp U R. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol Cell Biol. 1990;10:2503–2512. doi: 10.1128/mcb.10.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill C S, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 13.Ihle J N, Witthuhn B A, Quelle F W, Yamamoto K, Thierfelder W E, Kreider B, Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994;19:222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 14.Johansen F E, Prywes R. Two pathways for serum regulation of the c-fos serum response element require specific sequence elements and a minimal domain of serum response factor. Mol Cell Biol. 1994;14:5920–5928. doi: 10.1128/mcb.14.9.5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D-W, Cheriyath V, Roy A L, Cochran B H. TFII-I enhances activation of the c-fos promoter through interactions with upstream elements. Mol Cell Biol. 1998;18:3310–3320. doi: 10.1128/mcb.18.6.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D W, Cochran B H. Extracellular signal-regulated kinase binds to TFII-I and regulates its activation of the c-fos promoter. Mol Cell Biol. 2000;20:1140–1148. doi: 10.1128/mcb.20.4.1140-1148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotenko S V, Izotova L S, Pollack B P, Muthukumaran G, Paukku K, Silvennoinen O, Ihle J N, Pestka S. Other kinases can substitute for Jak2 in signal transduction by interferon-gamma. J Biol Chem. 1996;271:17174–17182. doi: 10.1074/jbc.271.29.17174. [DOI] [PubMed] [Google Scholar]
- 18.Kruijer W, Cooper J A, Hunter T, Verma I M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984;312:711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Bishop A, Witucki L, Kraybill B, Shimizu E, Tsien J, Ubersax J, Blethrow J, Morgan D O, Shokat K M. Structural basis for selective inhibition of src family kinases by PP1. Chem Biol. 1999;6:671–678. doi: 10.1016/s1074-5521(99)80118-5. [DOI] [PubMed] [Google Scholar]
- 20.Luo H, Rose P, Barber D, Hanratty W P, Lee S, Roberts T M, D'Andrea A D, Dearolf C R. Mutation in the Jak kinase JH2 domain hyperactivates Drosophila and mammalian Jak-Stat pathways. Mol Cell Biol. 1997;17:1562–1571. doi: 10.1128/mcb.17.3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luttrell L M, Hawes B E, van Biesen T, Luttrell D K, Lansing T J, Lefkowitz R J. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 22.Manzano-Winkler B, Novina C D, Roy A L. TFII-I is required for transcription of the naturally TATA-less but initiator-containing Vβ promoter. J Biol Chem. 1996;271:12076–12081. doi: 10.1074/jbc.271.20.12076. [DOI] [PubMed] [Google Scholar]
- 23.Marrero M B, Schieffer B, Paxton W G, Heerdt L, Berk B C, Delafontaine P, Bernstein K E. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–250. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda T, Fukada T, Takahashi-Tezuka M, Okuyama Y, Fujitani Y, Hanazono Y, Hirai H, Hirano T. Activation of Fes tyrosine kinase by gp130, an interleukin-6 family cytokine signal transducer, and their association. J Biol Chem. 1995;270:11037–11039. doi: 10.1074/jbc.270.19.11037. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda T, Takahashi-Tezuka M, Fukada T, Okuyama Y, Fujitani Y, Tsukada S, Mano H, Hirai H, Witte O N, Hirano T. Association and activation of Btk and Tec tyrosine kinases by gp130, a signal transducer of the interleukin-6 family of cytokines. Blood. 1995;85:627–633. [PubMed] [Google Scholar]
- 26.Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder J S, Freedman M, Cohen A, Gazit A, Levitzki A, Roifman C M. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 27.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 28.Moolenaar W H. Mitogenic action of lysophosphatidic acid. Adv Cancer Res. 1991;57:87–102. doi: 10.1016/s0065-230x(08)60996-3. [DOI] [PubMed] [Google Scholar]
- 29.Muller R, Bravo R, Burckhardt J, Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984;312:716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- 30.Nobes C D, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 31.Novina C D, Cheriyath V, Roy A L. Regulation of TFII-I activity by phosphorylation. J Biol Chem. 1998;273:33443–33448. doi: 10.1074/jbc.273.50.33443. [DOI] [PubMed] [Google Scholar]
- 32.Novina C D, Kumar S, Bajpai U, Cheriyath V, Zhang K, Pillai S, Wortis H H, Roy A L. Regulation of nuclear localization and transcriptional activity of TFII-I by Bruton's tyrosine kinase. Mol Cell Biol. 1999;19:5014–5024. doi: 10.1128/mcb.19.7.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez Jurado L A, Wang Y K, Peoples R, Coloma A, Cruces J, Francke U. A duplicated gene in the breakpoint regions of the 7q11.23 Williams-Beuren syndrome deletion encodes the initiator binding protein TFII-I and BAP-135, a phosphorylation target of BTK. Hum Mol Genet. 1998;7:325–334. doi: 10.1093/hmg/7.3.325. [DOI] [PubMed] [Google Scholar]
- 34.Rivera V M, Sheng M, Greenberg M E. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum induction. Genes Dev. 1990;4:255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- 35.Robbins D J, Cheng M, Zhen E, Vanderbilt C A, Feig L A, Cobb M H. Evidence for a Ras-dependent extracellular signal-regulated protein kinase (ERK) cascade. Proc Natl Acad Sci USA. 1992;89:6924–6928. doi: 10.1073/pnas.89.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy A L, Carruthers C, Gutjahr T, Roeder R G. Direct role for Myc in transcription initiation mediated by interactions with TFII-I. Nature. 1993;365:359–361. doi: 10.1038/365359a0. [DOI] [PubMed] [Google Scholar]
- 37.Roy A L, Du H, Gregor P D, Novina C D, Martinez E, Roeder R G. Cloning of an Inr- and E-box-binding protein, TFII-I, that interacts physically and functionally with USF1. EMBO J. 1997;16:7091–7104. doi: 10.1093/emboj/16.23.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy A L, Malik S, Meisterernst M, Roeder R G. An alternative pathway for transcription initiation involving TFII-I. Nature. 1993;365:355–359. doi: 10.1038/365355a0. [DOI] [PubMed] [Google Scholar]
- 39.Roy A L, Meisterernst M, Pognonec P, Roeder R G. Cooperative interaction of an initiator-binding transcription initiation factor and the helix-loop-helix activator USF. Nature. 1991;354:245–248. doi: 10.1038/354245a0. [DOI] [PubMed] [Google Scholar]
- 40.Simon A, Rai U, Fanburg B, Cochran B. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;44:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 41.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 42.Stancato L F, Sakatsume M, David M, Dent P, Dong F, Petricoin E F, Krolewski J J, Silvennoinen O, Saharinen P, Pierce J, Marshall C J, Sturgill T, Finbloom D S, Larner A C. Beta interferon and oncostatin M activate Raf-1 and mitogen-activated protein kinase through a JAK1-dependent pathway. Mol Cell Biol. 1997;17:3833–3840. doi: 10.1128/mcb.17.7.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, Hilton D J. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 44.Stokoe D, McCormick F. Activation of c-Raf-1 by Ras and Src through different mechanisms: activation in vivo and in vitro. EMBO J. 1997;16:2384–2396. doi: 10.1093/emboj/16.9.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi-Tezuka M, Hibi M, Fujitani Y, Fukada T, Yamaguchi T, Hirano T. Tec tyrosine kinase links the cytokine receptors to PI-3 kinase probably through JAK. Oncogene. 1997;14:2273–2282. doi: 10.1038/sj.onc.1201071. [DOI] [PubMed] [Google Scholar]
- 46.Treisman R. Journey to the surface of the cell: Fos regulation and the SRE. EMBO J. 1995;14:4905–4913. doi: 10.1002/j.1460-2075.1995.tb00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vignais M L, Sadowski H B, Watling D, Rogers N C, Gilman M. Platelet-derived growth factor induces phosphorylation of multiple JAK family kinases and STAT proteins. Mol Cell Biol. 1996;16:1759–1769. doi: 10.1128/mcb.16.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winston L A, Hunter T. JAK2, Ras, and Raf are required for activation of extracellular signal-regulated kinase/mitogen-activated protein kinase by growth hormone. J Biol Chem. 1995;270:30837–30840. doi: 10.1074/jbc.270.52.30837. [DOI] [PubMed] [Google Scholar]
- 49.Witthuhn B A, Quelle F W, Silvennoinen O, Yi T, Tang B, Miura O, Ihle J N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 50.Xia K, Mukhopadhyay N K, Inhorn R C, Barber D L, Rose P E, Lee R S, Narsimhan R P, D'Andrea A D, Griffin J D, Roberts T M. The cytokine-activated tyrosine kinase JAK2 activates Raf-1 in a p21ras-dependent manner. Proc Natl Acad Sci USA. 1996;93:11681–11686. doi: 10.1073/pnas.93.21.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang S H, Whitmarsh A J, Davis R J, Sharrocks A D. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. EMBO J. 1998;17:1740–1749. doi: 10.1093/emboj/17.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang S H, Yates P R, Whitmarsh A J, Davis R J, Sharrocks A D. The Elk-1 ETS-domain transcription factor contains a mitogen-activated protein kinase targeting motif. Mol Cell Biol. 1998;18:710–720. doi: 10.1128/mcb.18.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang W, Desiderio S. BAP-135, a target for Bruton's tyrosine kinase in response to B cell receptor engagement. Proc Natl Acad Sci USA. 1997;94:604–609. doi: 10.1073/pnas.94.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]