Abstract
Background
Nintedanib slows lung function decline for patients with non-idiopathic pulmonary fibrosis progressive pulmonary fibrosis (PPF) in clinical trials, but the real-world safety and efficacy are not known.
Methods
In this retrospective cohort study, standardised data were collected from patients in whom nintedanib was initiated for PPF between 2019 and 2020 through an early-access programme across eight centres in the United Kingdom. Rate of lung function change in the 12 months pre- and post-nintedanib initiation was the primary analysis. Symptoms, drug safety, tolerability and stratification by interstitial lung disease subtype and computed tomography pattern were secondary analyses.
Results
126 patients were included; 67 (53%) females; mean±sd age 60±13 years. At initiation of nintedanib, mean forced vital capacity (FVC) was 1.87 L (58% predicted) and diffusing capacity of the lung for carbon monoxide (DLCO) was 32.7% predicted. 68% of patients were prescribed prednisolone (median dose 10 mg) and 69% were prescribed a steroid-sparing agent. In the 12 months after nintedanib initiation, lung function decline was significantly lower than in the preceding 12 months: FVC −88.8 mL versus −239.9 mL (p=0.004), and absolute decline in DLCO −2.1% versus −6.1% (p=0.004). Response to nintedanib was consistent in sensitivity and secondary analyses. 89 (71%) out of 126 patients reported side-effects, but 86 (80%) of the surviving 108 patients were still taking nintedanib at 12 months with patients reporting a reduced perception of symptom decline. There were no serious adverse events.
Conclusion
In PPF, the real-world efficacy of nintedanib replicated that of clinical trials, significantly attenuating lung function decline. Despite the severity of disease, nintedanib was safe and well tolerated in this real-world multicentre study.
Short abstract
In a real-world multicentre observational study of patients with non-IPF progressive pulmonary fibrosis, nintedanib was safe, efficacious and well tolerated, reducing annual lung function decline with a similar magnitude of effect as in clinical trials https://bit.ly/3ElA10n
Background
Progressive pulmonary fibrosis (PPF) occurs across a range of interstitial lung diseases (ILDs) including fibrotic hypersensitivity pneumonitis (fHP) and connective tissue disease-associated (CTD)-ILD [1–4]. The natural history of PPF mirrors idiopathic pulmonary fibrosis (IPF) [5, 6], ultimately leading to respiratory failure and early mortality [4]. It is estimated that 18–40% of patients with ILD develop PPF despite conventional therapy [6–9]. The PROGRESS study [6] reported substantial progression of disease in patients with PPF despite 91.4% of patients receiving immunosuppressive therapy, highlighting the urgent need for effective treatment.
Nintedanib, a tyrosine kinase inhibitor [10], slows the annual rate of lung function decline in patients with IPF [11] and most recently in patients with progressive non-IPF fibrosing lung diseases [12, 13].
The INBUILD study and subsequent subanalyses confirm the antifibrotic efficacy of nintedanib independent of ILD diagnosis, once progression despite optimal management has occurred [12, 14, 15]. This study has shown that participants who experience PPF despite conventional management over the preceding 24 months demonstrate subsequent disease progression with the same magnitude of decline as untreated IPF [11, 16, 17].
While there is randomised controlled trial evidence supporting the use of nintedanib for non-IPF PPF [12, 13], the extent to which the benefits observed in trials translates into real-world efficacy, safety and tolerability for patients is unknown.
Nintedanib was offered for the treatment of patients in the United Kingdom (UK) with a diagnosis of non-IPF PPF in a national early-access programme between May 2019 and August 2020. The aim of this multicentre study was to assess the impact of nintedanib on the clinical course of patients with PPF in a real-world setting.
Methods
Patient population
The study was granted ethical approval (Integrated Research Application System identifier 292810, research ethics committee reference 21/LO/0091) and was registered locally at each participating centre. Applications for access to nintedanib were submitted by specialist ILD physicians for eligible patients (aged ≥18 years) meeting the following INBUILD criteria for PPF [12] over the preceding 24 months: 1) relative decline in forced vital capacity (FVC) ≥10%; 2) relative decline in FVC of 5–10% with worsening respiratory symptoms or increased extent of fibrosis on high-resolution computed tomography (HRCT) scan; and 3) worsening respiratory symptoms and increased extent of fibrosis on HRCT.
All applications were centrally reviewed and only approved for patients meeting these criteria. A case report form (CRF) collected retrospective and prospective data for each patient (supplementary material). Lung function data were obtained annually. Other data to suggest clinical worsening, including increasing oxygen requirements, were collected as a descriptor within the CRF, but were not used to inform eligibility for nintedanib. Participating centres were responsible for monitoring blood tests and documenting adverse events. Data were collected as per clinical practice from electronic patient records by each participating centre and anonymised prior to collation.
Outcomes and analyses
The primary outcome measure was the difference in FVC change (mL) in the 12±6 months post-nintedanib initiation relative to the change in the 12±6 months pre-initiation of nintedanib. Secondary outcomes were change in the percentage predicted diffusing capacity of the lungs for carbon monoxide (ppDLCO) and percentage predicted transfer coefficient of the lung for carbon monoxide (ppKCO) post-initiation relative to pre-initiation. All reported lung function variables were pre-bronchodilator measurements.
Secondary analyses assessed differences in lung function change in individual patient groups (sex, diagnosis and radiological pattern on computed tomography (CT)), based on the difference between lung function changes in the preceding and subsequent 12 months from nintedanib initiation.
Change in symptoms of shortness of breath, exercise tolerance and cough at initiation of nintedanib and at 12 months’ follow-up were recorded as worsened, no change or improved based on clinical review of patient records, and included as an additional secondary outcome. Adverse effects including 12-month mortality and side-effects as well as compliance data were collected to ascertain the overall tolerability and safety of nintedanib in this population.
Statistical methods
Multilevel models for repeat measures were employed to account for all lung function tests recorded across the cohort; restricted linear splines were used to compare rate of change in the year pre- and post-nintedanib with a knot at initiation.
In secondary analyses of between-group differences, multilevel models were adjusted for the a priori covariates age, sex, diagnostic group (fHP, CTD-ILD, other), radiological pattern (usual interstitial pneumonia (UIP) pattern of fibrosis on HRCT) and early cessation of nintedanib treatment (prior to 12 months).
Sensitivity analyses were performed using percentage predicted FVC (ppFVC), timing of ppFVC from nintedanib initiation as a continuous variable instead of discrete, and three single imputation strategies for missing ppFVC data assumed not missing at random: median imputation at time point, a carried-forward or -backward rule and a 10% relative decline imputation. Sensitivity analyses were also performed in a restricted cohort of patients with complete lung function at all three time points, including paired t-test of pre- and post- changes in lung function, as well as unpaired tests of differences in change between sex, radiological pattern and diagnostic group.
To compare symptom changes at initiation of nintedanib and at follow-up after 12 months of treatment, dichotomised categories of “worse/no change” or “improved” were included in McNemar's tests on discordant pairs. Relationships between early cessation of nintedanib treatment and patient characteristics were tested using Fisher's exact tests and Wilcoxon rank-sum tests. Similar tests were performed to assess for bias in patients with complete lung function. All analyses were performed in Stata SE16; p<0.05 was considered significant for all outcomes.
Results
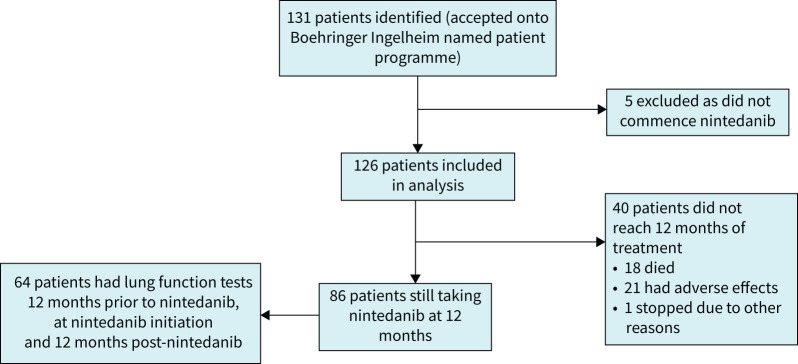
Data were collected from 126 eligible patients who received nintedanib across eight ILD centres in the UK (figure 1, supplementary table S1). There were 67 (53%) females; mean±sd age was 60±13 years (table 1). At initiation of nintedanib, mean±sd FVC was 1.86±0.70 L (57.62±18.56% pred) and mean±sd ppDLCO was 32.66±11.04%. Five patients who were approved for nintedanib declined to commence therapy.
FIGURE 1.
Consolidated Standards of Reporting Trials flow diagram of study population demonstrating the number of eligible participants, those initiated on nintedanib, those reaching primary analysis criteria of sequential lung function and adherence to the compassionate access programme.
TABLE 1.
Baseline characteristics and lung function at initiation of nintedanib
| Patients | 126 |
| Age (years) | 60±13 |
| Baseline lung function | |
| FVC (L) (n=103) | 1.86±0.70 |
| FVC (% pred) (n=102) | 57.62±18.56 |
| DLCO (% pred) (n=79) | 32.66±11.04 |
| KCO (% pred) (n=74) | 65.85±18.97 |
| Gender | |
| Male | 59 (47) |
| Female | 67 (53) |
| ILD diagnosis | |
| fHP | 44 (34.9) |
| CTD-ILD | 44 (34.9) |
| Unclassifiable IIP | 9 (7.1) |
| Fibrotic NSIP | 8 (6.3) |
| Fibrotic OP | 4 (3.2) |
| PPFE | 3 (2.4) |
| Asbestosis | 3 (2.4) |
| Smoking-related ILD | 3 (2.4) |
| Familial PF | 1 (0.8) |
| Fibrotic sarcoid | 1 (0.8) |
| DIP | 1 (0.8) |
| HIV-associated ILD | 1 (0.8) |
| Other | 4 (3.2) |
| Smoking status | |
| Current smoker | 2 (1.6) |
| Ex-smoker | 46 (36.5) |
| Never-smoker | 63 (50) |
| No data | 15 (11.9) |
| Hospital | |
| Guy's and St Thomas' NHS Foundation trust | 10 (7.9) |
| Manchester University NHS Foundation Trust | 13 (10.3) |
| North Bristol NHS Trust | 3 (2.4) |
| Oxford University Hospital | 16 (12.7) |
| Royal Papworth Hospital | 15 (11.9) |
| Royal Brompton Hospital | 61 (48.4) |
| Royal Devon and Exeter Hospital | 4 (3.2) |
| University Hospital Birmingham | 4 (3.2) |
| MRC dyspnoea score | |
| 1 | 4 (3) |
| 2 | 18 (14) |
| 3 | 26 (21) |
| 4 | 27 (21) |
| 5 | 16 (13) |
| No data | 35 (28) |
| Home oxygen (ambulatory/LTOT) | |
| Yes | 79 (62.7) |
| No | 45 (35.7) |
| Unknown | 2 (1.6) |
| Fibrotic pattern on CT | |
| UIP | 30 (24) |
| Non-UIP | 95 (75) |
| No data | 1 (0.8) |
| Indication for starting nintedanib | |
| Progressive symptoms | 111 (88) |
| Lung function decline | 100 (79) |
| CT progression | 85 (67) |
| Concurrent immunosuppression | |
| Yes | 113 (90) |
| No | 13 (10) |
| Prednisolone | 86 (68) |
| Mycophenolate mofetil | 70 (56) |
| Azathioprine | 6 (5) |
| Hydroxychloroquine | 13 (10) |
| Leflunomide | 3 (2) |
| Rituximab | 9 (7) |
| Tacrolimus | 1 (0.8) |
| Methotrexate | 3 (2) |
| Change in immunosuppression following nintedanib initiation | |
| Unchanged | 76 (60) |
| Increased | 29 (23) |
| Decreased | 13 (10) |
| Other | 8 (6) |
Data are presented as n, mean±sd or n (%). FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; KCO: transfer coefficient of the lung for carbon monoxide; ILD: interstitial lung disease; fHP: fibrotic hypersensitivity pneumonitis; CTD-ILD: connective tissue disease-associated ILD; IIP: idiopathic interstitial pneumonia; NSIP: nonspecific interstitial pneumonia; OP: organising pneumonia; PPFE: pleuroparenchymal fibroelastosis; PF: pulmonary fibrosis; DIP: desquamative interstitial pneumonia; NHS: National Health Service; MRC: Medical Research Council; LTOT: long-term oxygen therapy; CT: computed tomography; UIP: usual interstitial pneumonia.
The most frequent diagnoses were fHP (n=44, 35%) and CTD-ILD (n=44, 35%). For the purposes of the secondary analyses, the remainder of patients were categorised as “other” diagnoses and these included unclassifiable idiopathic interstitial pneumonia (n=9, 7%), fibrotic nonspecific interstitial pneumonia (NSIP) (n=8, 6%) and fibrotic organising pneumonia (n=4, 3%) (table 1). The CTD-ILD group comprised 24 patients with systemic sclerosis-associated ILD (SSc-ILD), seven patients with rheumatoid arthritis-associated ILD and 13 patients with other connective tissue diseases.
The radiological pattern of fibrosis was definite UIP in 30 (24%) out of 126 patients. Among those with non-UIP fibrosis, the CT was most commonly reported as fHP (n=35) and fibrotic NSIP (n=33).
At the time of nintedanib initiation, the most prescribed immunosuppressive drugs were prednisolone and mycophenolate mofetil (MMF) in 86 (68%) and 70 (56%) out of 126 patients, respectively. The median dose of prednisolone was 10 mg (interquartile range (IQR) 9–10 mg) and the median dose of MMF was 2 g (IQR 1.5–2 g) per day. In total, 87 (69%) patients were prescribed a steroid-sparing agent and 79 (63%) were prescribed oxygen. At the time of data collection, 44 (35%) patients had been or were currently being assessed for lung transplantation. Six patients were active on the lung transplant list; an additional 19 patients were undergoing assessment; and 19 patients had previously been assessed and declined for transplantation.
Primary lung function analysis
In 123 people with lung function data available, the data were obtained a median of 346.5 days (IQR 281–429 days) prior to initiation, at nintedanib initiation and 340 days (IQR 267–396 days) post-initiation. Incorporating all lung-function time-point data available, mean FVC was 2.10 L (95% CI 1.97–2.22 L) pre-initiation, 1.86 L (95% CI 1.73–2.00 L) at drug initiation and 1.77 L (95% CI 1.63–1.90 L) post-initiation. The mean ppDLCO was 38.8% (95% CI 36.4–41.2%) pre-initiation, 32.7% (95% CI 28.9–35.4%) at initiation of nintedanib and 32.4% (95% CI 29.5–35.4%) post-initiation.
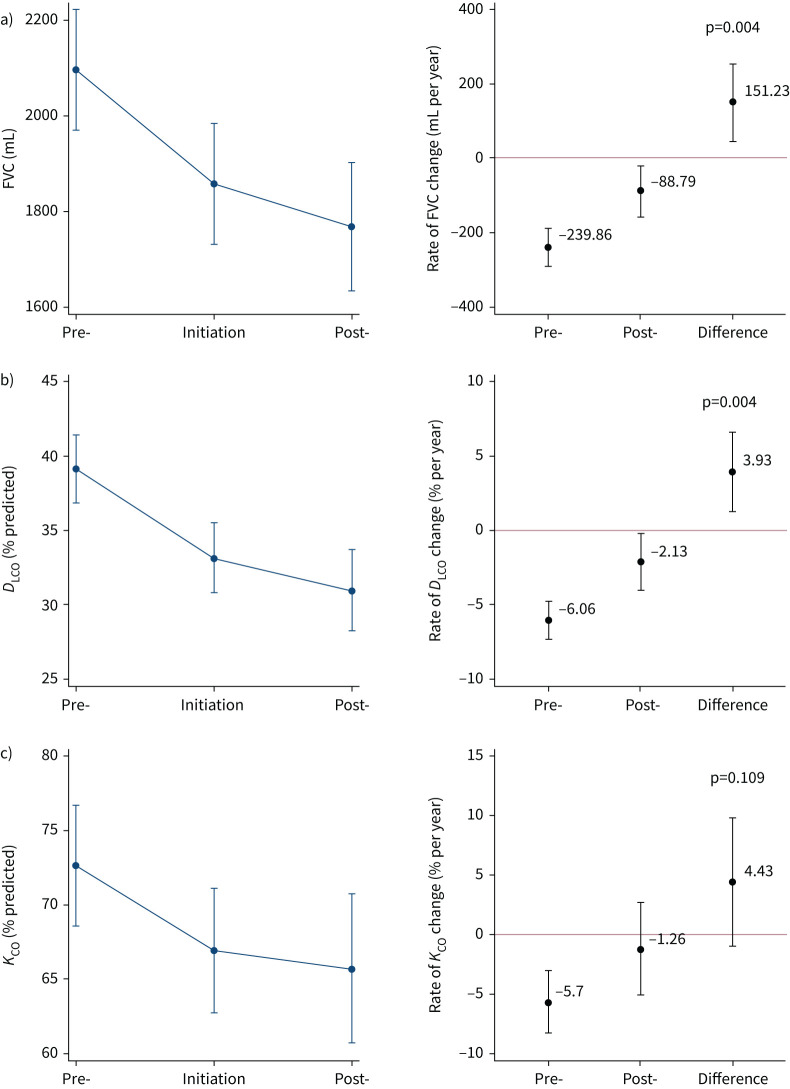
In 123 people with an FVC recorded, mean FVC change was −239.9 mL (95% CI −292.7– −187.0 mL) in the year (±6 months) pre-initiation and −88.8 mL(95% CI −156.2– −21.3 mL) in the year (±6 months) post-initiation with a significant attenuation of 151.2 mL (95% CI 47.5–255.0 mL; p=0.004) (figure 2a, supplementary table S2).
FIGURE 2.
Difference in lung function change in the 12 months pre- and post-initiation of nintedanib (n=123). Mean lung function a) forced vital capacity (FVC) (n=123), b) diffusing capacity of the lung for carbon monoxide (DLCO) (n=114) and c) transfer coefficient of the lung for carbon monoxide (KCO) (n=108) values at pre-initiation, initiation and post-initiation, and the difference in rate of lung function change pre- and post-initiation. Estimates plotted with 95% confidence intervals from multilevel model.
In 114 people with ppDLCO recorded, mean ppDLCO change was −6.1% (95% CI −7.4– −4.8%) pre-initiation and −2.1% (95% CI −4.1– −0.2%) post-initiation, with significant attenuation between rates of 3.93% (95% CI 1.3–6.6%; p=0.004) (figure 2b, supplementary table S2). In 108 people with ppKCO recorded, there was a mean ppKCO change of −5.7% (95% CI −8.4– −3.0%) pre-initiation with a post-initiation change of −1.3% (95% CI −5.2–+2.7%) and a nonsignificant reduction in rate of change of 4.43% (95% CI −1.0–9.9%; p=0.109) (figure 2c, supplementary table S2).
Results of the sensitivity analyses were similar to the overall analysis (supplementary table S3, supplementary figure S1). Patients experienced a mean ppFVC change of −6.6% per year (95% CI −8.5– −4.8% per year) pre-initiation and −3.0% per year (95% CI −5.2– −0.7% per year) post-initiation, with an attenuation of 3.7% per year (95% CI 0.3–7.0% per year; p=0.032). 64 (51%) patients out of 126 had complete lung function at all three time points; patients with incomplete lung function (n=62) were older (mean age 62.7 versus 57.8 years; p=0.034) and had more severe Medical Research Council (MRC) dyspnoea scores (p=0.002), but there was no difference in gender, baseline FVC or ppDLCO, diagnosis or CT pattern. Strategies for handling missing lung function suggested a similar attenuation of decline post-nintedanib.
Secondary lung function analyses
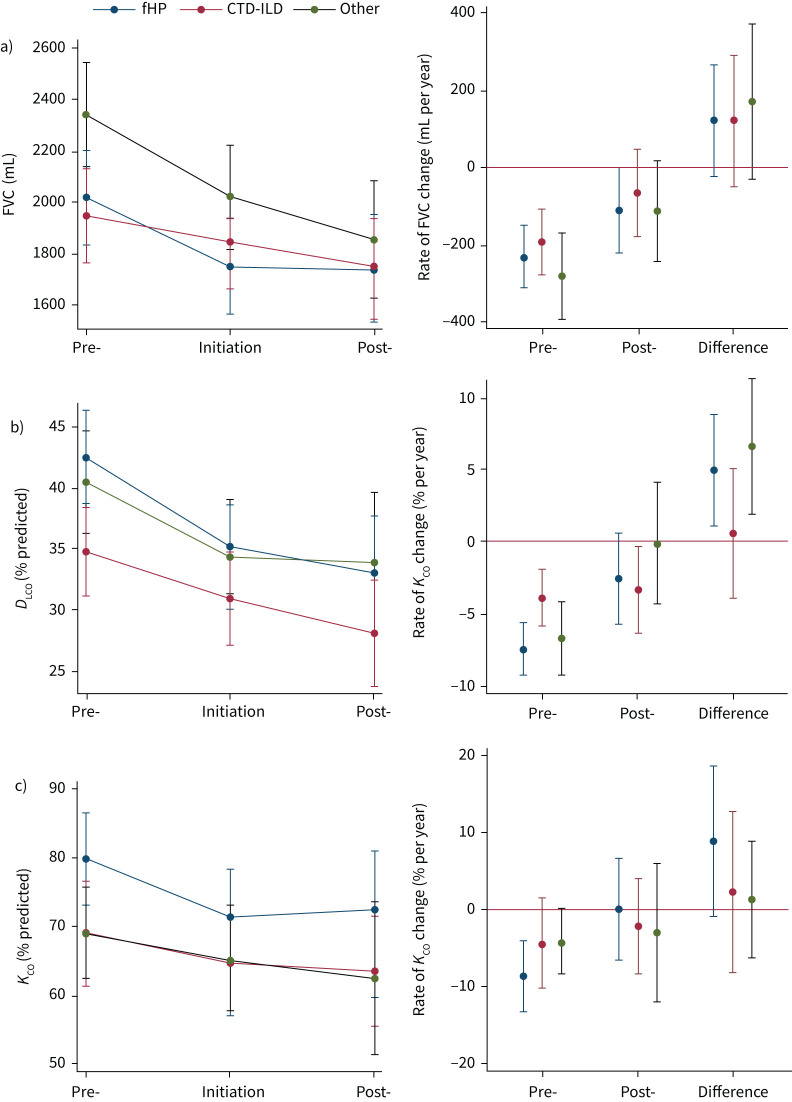
Regardless of diagnosis, patients experienced a similar reduced rate of decline in FVC with nintedanib; however, statistical significance was not reached (figure 3, supplementary table S2). The difference between the 12-month post-nintedanib and pre-nintedanib rate of FVC change was +125.4 mL per year (95% CI −18.4–+269.1 mL per year; p=0.087) in patients with fHP; +122.5 mL per year (95% CI −47.7–+292.7 mL per year; p=0.158) in patients with CTD-ILD; and +172.7 mL per year (95% CI −25.8–+371.2 mL per year; p=0.088) for the “other” diagnosis group. A significant attenuation in the rate of ppDLCO change was observed in individuals with fHP (+5.0% per year, 95% CI +1.1–+8.9% per year; p=0.013) and the “other” diagnosis grouping (+6.6% per year, 95% CI +1.9–+11.4% per year; p=0.006), but not in those with CTD-ILD (+0.6% per year, 95% CI −3.9–+5.1; p=0.803). No significant difference in ppKCO change was observed across diagnoses.
FIGURE 3.
Difference in lung function change between 12 months pre- and post-nintedanib according to diagnostic group (n=123). Adjusted estimates of mean lung function a) forced vital capacity (FVC) (n=123), b) diffusing capacity of the lung for carbon monoxide (DLCO) (n=113) and c) transfer coefficient of the lung for carbon monoxide (KCO) (n=107) values at pre-initiation, initiation and post-initiation, and the difference in rate of lung function change pre- and post-initiation, according to diagnostic group. Estimates plotted with 95% confidence intervals. fHP: fibrotic hypersensitivity pneumonitis; CTD-ILD: connective tissue disease-associated interstitial lung disease.
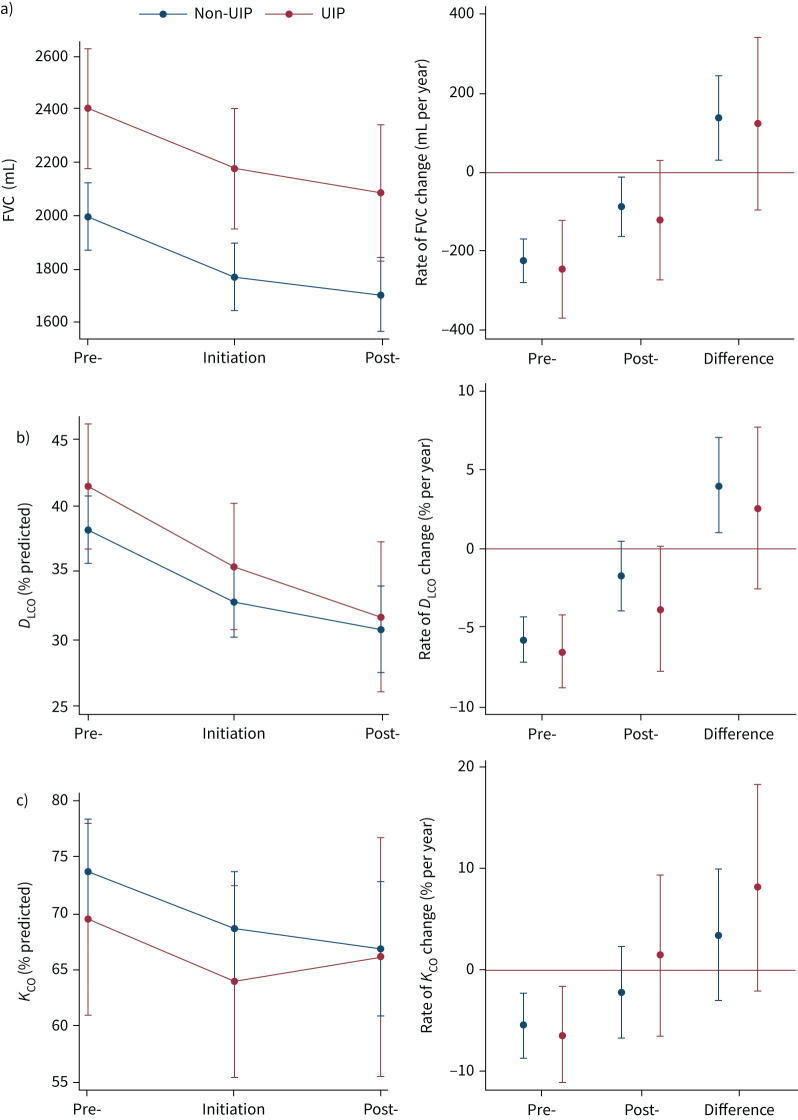
There was a reduced rate of FVC and ppDLCO decline in those with a non-UIP pattern and similar in UIP, although this did not reach significance (figure 4, supplementary table S2). There was a significant FVC decline in males, but not females, and a significant ppDLCO decline in females, but not males; estimates were in the same direction for both sexes (supplementary figure S2, supplementary table S2).
FIGURE 4.
Difference in lung function change between 12 months pre- and post-nintedanib according to computed tomography (CT) pattern (n=123). Adjusted estimates of mean lung function a) forced vital capacity (FVC) (n=123), b) diffusing capacity of the lung for carbon monoxide (DLCO) (n=113) and c) transfer coefficient of the lung for carbon monoxide (KCO) (n=107) values at pre-initiation, initiation and post-initiation, and the difference in rate of lung function change pre- and post-initiation, according to CT pattern. Estimates plotted with 95% confidence intervals. UIP: usual interstitial pneumonia.
All secondary subgroup analyses were comparable in restricted analysis of the difference in change (Δ) in FVC across subgroups (supplementary figure S3).
Symptoms, safety and tolerability
Patient symptoms
Of those surviving to follow-up (108 out of 126), the proportion of patients who reported an improvement in symptoms increased after nintedanib treatment. Improvements in shortness of breath were reported in 25.9% (28 out of 108) of people after 12 months of nintedanib compared to 12.0% (13 out of 108) in the 12 months prior to drug initiation (relative difference 15.8%, 95% CI 7.5–24.0%; p=0.0007). Reported exercise tolerance was similarly improved in 29.6% (32 out of 108) after nintedanib compared to 13.0% (14 out of 108) at initiation (relative difference 19.1%, 95% CI 10.0–28.3%; p=0.0003). No change was observed in reported cough symptoms (relative difference 3.0%, 95% CI −11.1–17.1%; p=0.839).
Drug side-effects and tolerability
Across the entire cohort (n=126), at least one side-effect was experienced by 89 (71%) out of 126 patients (table 2). Diarrhoea was the most common side-effect (n=49, 39%). Hepatotoxicity was reported in eight (6%) patients and was not more commonly reported in patients on steroid-sparing agents (p=0.702). No serious adverse events associated with nintedanib treatment were recorded. 18 (14%) patients died during the study period, for which complications or progression of ILD accounted for eight.
TABLE 2.
Tolerability, safety and survival related to nintedanib
| Patients | 126 |
| Adverse effects | |
| Yes | 89 (71) |
| Diarrhoea | 49 (39) |
| Nausea and vomiting | 31 (25) |
| Weight loss | 31 (25) |
| Abdominal pain | 22 (17) |
| Appetite loss | 11 (9) |
| Hepatotoxicity | 8 (6) |
| Headache | 8 (6) |
| Constipation | 3 (2) |
| Lethargy | 3 (2) |
| Other | 2 (2) |
| None | 37 (29) |
| Nintedanib dose reduction? | |
| Yes | 37 (29) |
| Surviving patients who completed ≥6 months of nintedanib | |
| Yes | 102 (81) |
| No | 24 (19) |
| Surviving patients on nintedanib at 12 months | |
| Yes | 86 (80) |
| No | 22 (20) |
| Survival at 12 months | |
| Alive | 108 (86) |
| Dead | 18 (14) |
Data are presented as n or n (%).
The dose of nintedanib was reduced in 37 (29%) out of 126 patients, most commonly due to diarrhoea (23 (62%) out of 37). Patients whose nintedanib dose was reduced experienced a similar benefit in lung function stabilisation (supplementary figure S4, supplementary table S4). 50 (40%) patients underwent a change in immunosuppressive treatment in the year following nintedanib initiation, but this was not associated with a difference in rate of lung function change (supplementary table S5).
Drug discontinuation
Nintedanib was discontinued before 1 year of treatment in 40 (32%) out of 126 patients due to death (n=18), side-effects (n=21) or other reasons (n=1). Patients who stopped nintedanib before 12 months of treatment were nonsignificantly older (mean difference 4.9 years; 95% CI −0.02–9.87 years; p=0.051). No difference was observed in FVC or DLCO at treatment initiation in those who discontinued nintedanib, but these patients had a lower KCO (mean difference −11.70%, 95% CI −21.28– −2.12%; p=0.017). Patients with higher MRC scores were less likely to continue treatment for 12 months; 19 (66%) of the 29 patients who survived but stopped treatment before 12 months had an MRC score of 4 or 5 at drug initiation compared to 24 (39%) out of 62 patients who continued treatment to 12 months (p=0.027).
Discussion
This study has demonstrated that for patients with non-IPF PPF in a real-world setting, the decline of lung function is significantly attenuated following the introduction of nintedanib. Although it is challenging to compare real-world data directly with data acquired from highly protocolised clinical trials, patients experienced a mean FVC decline of −240 mL in the 12 months prior to nintedanib treatment, as compared with −89 mL in the 12 months after treatment initiation (difference of 151 mL per year) mirroring the results of the INBUILD trial (FVC decline of −187 mL per year with placebo versus −80 mL per year with nintedanib) [12]. Furthermore, there was parallel attenuation in ppDLCO decline associated with nintedanib, an effect which, historically, has been challenging to observe in multicentre clinical trials due to interlaboratory variability. The early-access nature of this study meant that the patients had more advanced disease at drug initiation compared to those recruited to INBUILD (mean FVC 1.86 L versus 2.34 L and DLCO 32.7% versus 44.4%). Despite this, the observation that nintedanib is effective in slowing the rate of lung function decline is highly reassuring. Previous real-world studies have shown that nintedanib slows the progression of IPF [18–20]; however, this is the first real-world study assessing its efficacy in non-IPF PPF.
The attenuating effect was observed across diagnostic subgroups, HRCT patterns and sex to similar extents. Nintedanib was safe and well tolerated when prescribed alongside a broad range of immunosuppressive regimes, some of which were restricted in previous clinical trials. This supports data from subgroup analyses of the SENSCIS study that showed no adverse interaction between nintedanib and mycophenolate mofetil [21]. Most patients were prescribed corticosteroids and at least one steroid-sparing agent with infrequent reports of hepatoxicity or debilitating gastrointestinal side-effects. Although 71% of patients reported at least one side-effect, most commonly diarrhoea, 80% of surviving patients were still taking nintedanib at 12 months. Dose reductions were required in 29% of patients, but did not appear to reduce the efficacy of nintedanib. 17% of patients discontinued nintedanib before 12 months due to side-effects; these patients had greater baseline dyspnoea and a lower ppKCO, that may reflect additional comorbidity.
In this prevalent PPF population with severe disease at initiation, nintedanib was potentially associated with a reduced perception of symptomatic decline with a greater proportion of patients reporting improvements in shortness of breath and exercise tolerance following nintedanib initiation than before treatment. This finding is hypothesis-generating and may be explained by the placebo effect of a new therapy or indeed the concomitant introduction of palliative treatments. Although data regarding pulmonary rehabilitation were not available, the study period coincided with the height of the coronavirus disease 2019 (COVID-19) pandemic when services were highly restricted, making it less likely that widespread uptake of rehabilitation could explain these results. These observations have implications for patient selection, as advanced age, frailty and disease severity should not necessarily preclude antifibrotic treatment. However, the lack of patient-reported impact on cough does highlight an unmet need.
Strengths and limitations
Eight specialist ILD centres contributed to the study with only 10 (8%) out of 126 patients not meeting newly published official American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thorax Association clinical practice guidelines for patients with PPF [3], supporting the generalisability of the results. The breakdown of ILD diagnoses in this study paralleled INBUILD; the most common diagnoses were fHP and CTD-ILD [12, 14]. There were more patients with SSc-ILD (24%) than there were in INBUILD (<7%) [12], but the proportion was similar to the PROGRESS study [6] and thus may be a more accurate reflection of a real-world population. The INBUILD cohort had a higher proportion of UIP scans incorporating both definite and probable UIP [3], whereas in our study only definite UIP was classified as such. Almost all patients who were eligible received treatment with only a small number (n=5) electing not to start nintedanib; this means that selection bias is unlikely to have skewed the results.
As this was a real-world study of patients treated with nintedanib through an early-access programme, data were acquired through routine clinical care as opposed to a protocolised clinical study, which resulted in missing data, particularly of lung function records. Study recruitment and follow-up were conducted between May 2019 and August 2021, therefore the COVID-19 pandemic further compounded the issue as recommended shielding guidance and repositioning of health resource impacted lung function visits, hospitalisations and acute exacerbations. Thus, comparisons drawn between lung function decline pre- and post-treatment exposure may be limited by the time and progression course. Patients not providing lung function data at the final time point could overestimate the benefit of the drug due to survivorship bias.
To ensure that all patients entered onto the early-access programme were represented in primary analyses, multilevel models for repeat measures were used. Sensitivity analysis restricted solely to those patients who provided lung function data at three time points demonstrated a similar magnitude of effect with nintedanib. Routine clinical lung function is frequently missing not at random, due to the effort required in severe disease. Further sensitivity analyses were performed with alternative single imputation strategies for data missing not at random [22], and continued to support an attenuation in lung function decline.
The secondary analysis was limited by small numbers in subgroups, restricting power to detect between-group differences including potential confounding effects of immunosuppression. Patient-reported symptom scores were evaluated subjectively from clinic letters and not prospectively assessed using validated questionnaires. While intolerable side-effects due to nintedanib were not reported, improvement in symptoms are subject to bias and require independent validation. UIP and non-UIP HRCT patterns were reported by ILD teams based on radiology reports and not centrally evaluated; however, all centres are specialist ILD units and local interpretation is considered acceptable for many drug clinical trials.
Conclusion
In this first, antifibrotic-treated, real-world multicentre study of patients with non-IPF PPF, we have demonstrated that nintedanib was safe, well tolerated and associated with an attenuation of lung function decline despite the severity of ILD in this cohort. Nintedanib did not appear to have excess deleterious effects when taken in combination with other immunosuppressants, supporting its use for the pharmacological treatment of non-IPF ILD where progression has occurred despite optimal management.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00423-2022.supplement (107KB, pdf)
Supplementary figure 1 00423-2022.figureS1 (36.4KB, jpg)
Supplementary figure 2 00423-2022.figureS2 (66.1KB, jpg)
Supplementary figure 3 00423-2022.figureS3 (103.8KB, jpg)
Supplementary figure 4 00423-2022.figureS4 (42.7KB, jpg)
Supplementary figure 5 00423-2022.figureS5 (72.7KB, jpg)
Form 00423-2022.form (227.7KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: P.M. George conceived the study, coordinated data collection, analysed the data and drafted the manuscript. L. Raman coordinated data collection, analysed the data and wrote the first draft of the manuscript. I. Stewart provided statistical oversight, analysed the data and drafted the manuscript. P.M. George, L. Raman and I. Stewart had direct access to the data. All authors were involved in data collection, critical appraisal and approved the final version of the manuscript.
Conflict of interest: I. Stewart receives funding from the Rayne Foundation. F. Chua reports personal fees from Boehringer Ingelheim. N. Chaudhuri reports personal fees from Boehringer Ingelheim, Redex, Novartis, Liminal Biosciences, Vicor Pharma, Bridge Biotherapeutics and Roche. M. Gibbons reports personal fees for advisory boards and support for attending conferences from Boehringer Ingelheim. C. Hogben has received personal fees from Boehringer Ingelheim. V. Kouranos reports personal fees from Boehringer Ingelheim and Novartis. S. Mulholland reports personal fees from Boehringer Ingelheim. M. Naqvi reports honoraria from Boehringer Ingelheim, Astra Zeneca and Roche, and grant support paid to their institution from an NHSX digital award. E.A. Renzoni reports fees paid to their institution from Boehringer Ingelheim, Novartis and Roche, and research grants paid to their institution from Boehringer Ingelheim. M. Thillai reports personal fees from Boehringer Ingelheim, research grants paid to their institution from Boehringer Ingelheim and support for conference attendance from Boehringer Ingelheim. A.U. Wells is president elect of WASOG, and reports personal fees from Boehringer Ingelheim and Roche, and support for conference from Boehringer Ingelheim. P.M. George reports personal fees from Boehringer Ingelheim, Roche, Teva, Cipla and Brainomix, research grants paid to their institution from Boehringer Ingelheim, and support for conference attendance from Boehringer Ingelheim and Roche. No other authors report any conflicts of interest.
References
- 1.George PM, Spagnolo P, Kreuter M, et al. . Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med 2020; 8: 925–934. doi: 10.1016/S2213-2600(20)30355-6 [DOI] [PubMed] [Google Scholar]
- 2.Simpson T, Barratt SL, Beirne P, et al. . The burden of progressive fibrotic interstitial lung disease across the UK. Eur Respir J 2021; 58: 2100221. doi: 10.1183/13993003.00221-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Remy-Jardin M, Richeldi L, et al. . Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022; 205: e18–e47. doi: 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med 2020; 383: 958–968. doi: 10.1056/NEJMra2005230 [DOI] [PubMed] [Google Scholar]
- 5.Brown KK, Martinez FJ, Walsh SLF, et al. . The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J 2020; 55: 2000085. doi: 10.1183/13993003.00085-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasser M, Larrieu S, Si-Mohamed S, et al. . Progressive fibrosing interstitial lung disease: a clinical cohort (the PROGRESS study). Eur Respir J 2021; 57: 2002718. doi: 10.1183/13993003.02718-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson A, Hartmann N, Patnaik P, et al. . Estimation of the prevalence of progressive fibrosing interstitial lung diseases: systematic literature review and data from a physician survey. Adv Ther 2021; 38: 854–867. doi: 10.1007/s12325-020-01578-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson AL, Patnaik P, Hartmann N, et al. . Prevalence and incidence of chronic fibrosing interstitial lung diseases with a progressive phenotype in the United States estimated in a large claims database analysis. Adv Ther 2021; 38: 4100–4114. doi: 10.1007/s12325-021-01786-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijsenbeek M, Kreuter M, Olson A, et al. . Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin 2019; 35: 2015–2024. doi: 10.1080/03007995.2019.1647040 [DOI] [PubMed] [Google Scholar]
- 10.Wollin L, Wex E, Pautsch A, et al. . Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J 2015; 45: 1434–1445. doi: 10.1183/09031936.00174914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richeldi L, du Bois RM, Raghu G, et al. . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. doi: 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 12.Flaherty KR, Wells AU, Cottin V, et al. . Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019; 381: 1718–1727. doi: 10.1056/NEJMoa1908681 [DOI] [PubMed] [Google Scholar]
- 13.Distler O, Highland KB, Gahlemann M, et al. . Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019; 380: 2518–2528. doi: 10.1056/NEJMoa1903076 [DOI] [PubMed] [Google Scholar]
- 14.Wells AU, Flaherty KR, Brown KK, et al. . Nintedanib in patients with progressive fibrosing interstitial lung diseases – subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 2020; 8: 453–460. doi: 10.1016/S2213-2600(20)30036-9 [DOI] [PubMed] [Google Scholar]
- 15.Matteson EL, Kelly C, Distler JHW, et al. . Nintedanib in patients with autoimmune disease-related progressive fibrosing interstitial lung diseases: subgroup analysis of the INBUILD trial. Arthritis Rheumatol 2022; 74: 1039–1047. doi: 10.1002/art.42075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King TE Jr, Bradford WZ, Castro-Bernardini S, et al. . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. doi: 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 17.Noble PW, Albera C, Bradford WZ, et al. . Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769. doi: 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 18.Brunnemer E, Wälscher J, Tenenbaum S, et al. . Real-world experience with nintedanib in patients with idiopathic pulmonary fibrosis. Respiration 2018; 95: 301–309. doi: 10.1159/000485933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright WA, Crowley LE, Parekh D, et al. . Real-world retrospective observational study exploring the effectiveness and safety of antifibrotics in idiopathic pulmonary fibrosis. BMJ Open Respir Res 2021; 8: e000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harari S, Caminati A, Poletti V, et al. . A real-life multicenter national study on nintedanib in severe idiopathic pulmonary fibrosis. Respiration 2018; 95: 433–440. doi: 10.1159/000487711 [DOI] [PubMed] [Google Scholar]
- 21.Highland KB, Distler O, Kuwana M, et al. . Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med 2021; 9: 96–106. doi: 10.1016/S2213-2600(20)30330-1 [DOI] [PubMed] [Google Scholar]
- 22.Jakobsen JC, Gluud C, Wetterslev J, et al. . When and how should multiple imputation be used for handling missing data in randomised clinical trials – a practical guide with flowcharts. BMC Med Res Methodol 2017; 17: 162. doi: 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00423-2022.supplement (107KB, pdf)
Supplementary figure 1 00423-2022.figureS1 (36.4KB, jpg)
Supplementary figure 2 00423-2022.figureS2 (66.1KB, jpg)
Supplementary figure 3 00423-2022.figureS3 (103.8KB, jpg)
Supplementary figure 4 00423-2022.figureS4 (42.7KB, jpg)
Supplementary figure 5 00423-2022.figureS5 (72.7KB, jpg)
Form 00423-2022.form (227.7KB, pdf)