Summary
Direct electrical recordings from conventional boutons in the mammalian central nervous system have proven challenging due to their small size. Here, we provide a protocol for direct whole-cell patch-clamp recordings from small presynaptic boutons of primary dissociated cultured neurons of the rodent neocortex. We describe steps to prepare primary neocortical cultures and recording pipettes, followed by identifying boutons and establishing a whole-cell bouton recording. We then provide details on precise pipette capacitance compensation required for high-resolution current-clamp recordings from boutons.
For further details on the use and execution of this protocol, please refer to Ritzau-Jost et al.1
Subject areas: Biophysics, Cell Biology, Cell Culture, Neuroscience
Graphical abstract
Highlights
-
•
Identification and whole-cell recording from small boutons in mouse neuronal cultures
-
•
Optimization and compensation of pipette capacitance for high-resolution recordings
-
•
Adaptations of recording equipment for small bouton recordings
-
•
Applicable to small boutons of cultures and acute brain slices of other brain areas
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Direct electrical recordings from conventional boutons in the mammalian central nervous system have proven challenging due to their small size. Here, we provide a protocol for direct whole-cell patch-clamp recordings from small presynaptic boutons of primary dissociated cultured neurons of the rodent neocortex. We describe steps to prepare primary neocortical cultures and recording pipettes, followed by identifying boutons and establishing a whole-cell bouton recording. We then provide details on precise pipette capacitance compensation required for high-resolution current-clamp recordings from boutons.
Before you begin
The protocol below has been developed and optimized for bouton recordings in mouse primary neocortical neuron-glia co-cultures (referred to as neocortical cultures below).1,2 We first describe preparatory steps for bouton recordings, including the required equipment and solutions. Subsequently, we describe how low-capacitance quartz glass pipettes are manufactured, which are optimally suited for bouton recordings. We next outline strategies for bouton identification based on morphological and functional criteria. We then describe the procedure for whole-cell bouton recordings and provide advice on how to overcome the pitfalls most commonly encountered. Finally, we describe errors related to pipette capacitance and how to achieve optimal pipette capacitance compensation required for high-resolution bouton recordings. We have successfully used this protocol for bouton recordings in mouse primary neocortical cultures and rat acute neocortical brain slices1,2 and for dopaminergic axon recordings in rat acute striatal brain slices.3 Furthermore, we are currently adapting this protocol for bouton recordings in rat hippocampal cultures and human iPSC-derived neuronal cultures. Whole-cell recordings have previously also been performed from small boutons of cultured cerebellar and hippocampal neurons4,5,6,7 and acute cerebellar and hippocampal brain slices.6,8,9,10,11
Animal procedures provided in this protocol were in accordance with the European guidelines (EU Directive 2010/63/EU, Annex IV for animal experiments), German national guidelines (Tierschutzgesetz) and those of the regional Saxonian directorate (Landesdirektion; animal protocol: T41/16) as well as the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal procedures were approved in advance by the institutional Leipzig University Ethics Committees, the federal Saxonian Animal Welfare Committee as well as the V.A. Portland Health Care System Institutional Animal Care and Use Committee.
Institutional permissions
Laboratories wishing to follow this protocol first need to seek approval for the outlined animal procedures from their institutional and all relevant federal and / or national regulatory bodies.
Patch-clamp recording setup
For the general layout of a patch-clamp setup, we refer to excellent introductory literature (e.g., ref.12,13 or The Axon Guide by Molecular Devices, San Jose, CA, USA; https://www.moleculardevices.com/eN/Assets/user-guide/dd/cns/axon-guide-to-electrophysiology-and-biophysics-laboratory-techniques). In the following, we outline specifications of our setup facilitating small bouton recordings.
-
1.Microscope system:
- a.
- b.
-
2.
Electrophysiological recording equipment: We use a hybrid voltage-clamp/current-clamp amplifier with high precision in current-clamp recordings.1 Pipette capacitance is quantified in voltage-clamp mode and adopted for compensation in current-clamp mode (see Part IV). Therefore, the use of a hybrid voltage-clamp/current-clamp amplifier that provides the pipette capacitance value in voltage-clamp mode and allows for manual adjustment of pipette capacitance compensation in current-clamp mode is required.
-
3.
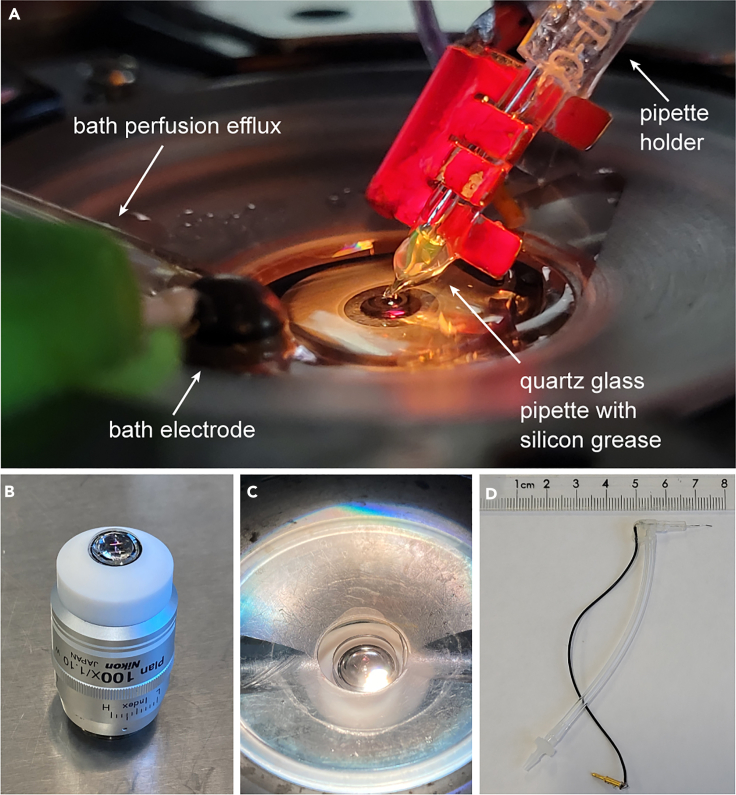
Pipette holder: We use custom-built pipette holders with short electrode wires and apply silicone grease to minimize holder capacitance (Figure 1D, see below).
-
4.Bath heating and perfusion
-
a.Establish bath perfusion, and perfusion heating if needed, 30 min before starting recordings to ensure constant bath temperature and solution flow.
-
b.We use a recirculating perfusion system with a gravity fed inlet and a peristaltic pump-driven outlet.
-
c.Most of our bouton recordings are performed at near-physiological temperature (34.5°–36°C) or at room temperature (20.0°–24.0°C).
-
d.We recommend exchanging the recirculating solution after 3–4 h of use with fresh solution in order to prevent osmolality increases due to evaporation. This is especially important when recording at near-physiological temperatures.
-
a.
-
5.
Micromanipulator: Smooth pipette movements are important for successful bouton recordings. Therefore, it is important to use the micromanipulator at a slow movement speed setting of ∼0.25–0.5 μm/s to not affect bouton membrane integrity while approaching with the pipette.
Figure 1.
Use of a high-resolution water-immersion objective on an inverted microscope
(A) Recording chamber configuration for bouton recordings. The red clamp attaches the pipette to the Kleindiek micromanipulator.
(B) Inverted water-immersion objective (100×, see key resources table) with Teflon sleeve to keep water in place between chamber and objective.
(C) Recording chamber with inverted water-immersion 100× objective.
(D) Custom-built pipette holder for Kleindiek micromanipulator with short electrode wire.
Preparation of neocortical neuron-glia co-cultures
Timing: 2–3 weeks before experiments, 1.5 h for culture preparation, both cortices from one mouse pup will be sufficient for 1–2 24-well plates
-
6.Coating of coverslips with Corning Matrigel Matrix.
-
a.Insert sterilized 13 mm coverslips into two 24-well plates by using, e.g., a fire-cleansed forceps or a suction-coupled glass Pasteur pipette.
-
b.Add 50 μL of freshly-thawed, diluted Matrigel (1:50 in cold MEM) onto the center of each coverslip. Gently spread Matrigel with the pipette tip and place 24-well plates into the incubator for ≥ 30 min without shaking off Matrigel.
-
a.
CRITICAL: Store Matrigel stock at −20°C to prevent preterm gelation. Thaw aliquots at 4°C, dilute in cold MEM immediately before use, and keep on ice while adding Matrigel to coverslips.
-
7.
For a step-by-step protocol of the dissection and dissociation procedure of the neocortex of mice, e.g., see ref.14. We plate 50.000 vital cells per coverslip, but the resulting culture density may vary with the use of different protocols and may have to be adjusted accordingly.
-
8.
Following plating, neuronal-glial co-cultures are grown in a MEM-based culture medium (for composition, see materials and equipment) at 37°C, 93% humidity, and room air plus 5% CO2.
Alternatives: Rat hippocampal neurons grown in Neurobasal™ A–based medium following, e.g., the protocol of ref.15). If other protocols for culture preparation and recordings are used, the solutions (see the following paragraph) may have to be adjusted to, e.g., differences in osmolality.
-
9.
Wrap 24-well plates with Parafilm M to maintain medium osmolality during culture maturation if incubator is frequently used.
-
10.
Use neuronal cultures for bouton recordings from 14 days in vitro (DIV) on.
Solution preparation (external recording solution, internal recording solution)
Timing: 2.5 h
-
11.External recording solution.
-
a.Add all components (see materials and equipment) to a beaker with ∼950 mL sterile double-distilled water (ddH2O) and stir until reagents are fully dissolved.
-
b.Titrate pH to 7.35 using 2 M NaOH.
-
c.Decant solution into a 1 l conical flask, thoroughly rinse beaker with ddH2O and use to make 1 l of solution. Osmolality of the final solution should be 305–310 mOsm.
-
d.Filter and store at 4°C until use. The solution can be kept for one week.
-
a.
-
12.Internal recording solution.
-
a.Add all components (see materials and equipment) to a beaker with ∼95 mL ddH2O and stir until reagents are fully dissolved.
-
b.Add ddH2O for a total volume of 100 mL and test osmolality (∼290 mOsm).
-
c.Titrate pH to 7.35 using 2 M KOH.
-
d.Sterile-filter solution (using, e.g., a syringe-mounted sterile 0.2 μm filter) and prepare 1 mL aliquots. Keep aliquots at −20°C and thaw individually before use.
-
e.Addition of a fluorescent dye (final concentration 20–50 μM of Atto 488-Carboxy) to the internal recording solution will facilitate confirmation of whole-cell recording configuration. To minimize final perturbation of concentration of other solution constituents start with 125% concentrated stock of pipette solution or use a 1000-fold concentrated stock dye solution.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Sodium chloride | Merck, Darmstadt, Germany | CAS# 7647-14-5 |
| Potassium chloride | Merck, Darmstadt, Germany | CAS# 7447-40-7 |
| HEPES | Merck, Darmstadt, Germany | CAS# 7365-45-9 |
| Glucose | Merck, Darmstadt, Germany | CAS# 50-99-7 |
| Magnesium chloride | Merck, Darmstadt, Germany | CAS# 7786-30-3 |
| Calcium chloride | Merck, Darmstadt, Germany | CAS# 10043-52-4 |
| Potassium gluconate | Merck, Darmstadt, Germany | CAS# 299-27-4 |
| Magnesium adenosine 5′-triphosphate | Merck, Darmstadt, Germany | CAS# 74804-12-9 |
| Sodium guanosine 5′-triphosphate | Merck, Darmstadt, Germany | CAS# 36051-31-7 |
| Potassium-HEPES | Merck, Darmstadt, Germany | CAS# 82207-62-3 |
| Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid | Merck, Darmstadt, Germany | CAS# 200-651-2 |
| Corning® Matrigel® Matrix | Corning, NY, USA | Cat# 354230 |
| Minimum Essential Medium (MEM) Eagle with Earle’s salts and L-glutamine | Thermo Fisher Scientific, Waltham, MA, USA | Cat# M4655 |
| Sodium bicarbonate | Merck, Darmstadt, Germany | CAS# 144-55-8 |
| Holo-Transferrin bovine | Merck, Darmstadt, Germany | CAS# 11096-37-0 |
| Insulin bovine | Merck, Darmstadt, Germany | Cat# I6634 |
| B-27™ supplement (50X) | Thermo Fisher Scientific, Waltham, MA, USA | Cat# 17504044 |
| FM®1-43 | Thermo Fisher Scientific, Waltham, MA, USA | Cat# T3163 |
| Atto 488-Carboxy | ATTO-TEC, Siegen, Germany | Cat# AD 488-21 |
| KORASILON® paste, medium viscosity | Kurt Obermeier GmbH, Bad-Berleburg, Germany | https://www.obermeier.de/en/products/silicones/ |
| Experimental models: Organisms/strains | ||
| C57BL/6N mice, postnatal day 0/1, both sexes | Charles River Laboratories, Wilmington, MA, USA; maintained at Animal Facility of the Medical Faculty, University of Leipzig |
RRID:IMSR_CRL:027 |
| Other | ||
| 24 well plates | Thermo Fisher Scientific, Waltham, MA, USA | Cat# 142475 |
| Parafilm M® | Merck, Darmstadt, Germany | Cat# P7793 |
| Electrode silver wire | Science Products, Hofheim, Germany | Cat# AG-8T |
| Nikon TI-inverted microscope; any equivalent high-end inverted microscope can be used | Nikon Instruments, Melville, NY, USA | RRID:SCR_021242 |
| Nikon CFI Plan Apochromat DM Lambda 100X Oil objective or Nikon CFI Plan 100XC W | Nikon Instruments, Melville, NY, USA | Cat# MRD31905 or Cat# MRL07920 |
| Nikon Digital Sight DS-2MBW CCD camera | Nikon Instruments, Melville, NY, USA | N/A |
| Multiclamp 700A patch-clamp amplifier | Molecular Devices, San Jose, CA, USA | RRID:SCR_021040 |
| MultiClamp 700A Commander | Molecular Devices, San Jose, CA, USA | https://support.moleculardevices.com/s/article/Axon-MultiClamp-700A-Commander-Download-page; version 1.3.0.05 |
| Kleindiek MM3A-LS micromanipulator | Kleindiek Nanotechnik, Reutlingen, Germany | https://www.nanotechnik.com/mm3a-ls.htmL |
| Ismatec ISM845 peristaltic perfusion pump | VWR International GmbH, Darmstadt, Germany | https://pr.vwr.com/store/ product/39213393/masterflex-ismatec-fixed-speed-rack-mount-minicartridge-peristaltic-pumps-avantor, Cat# MFLX78012-97-EU |
| TC-324C heater controller with SH-27B inline heater and TA-29 thermistor | Warner Instruments, Holliston, MA, USA | https://www.warneronline.com/single-channel-temperature-controller-tc-324c; Order# 64-2400, 64-0102, and 64-0107 |
| Quartz glass (without filament), 2 mm outer diameter, 1 mm inner diameter; HSQ 300 grade | Heraeus Quarzglas, Kleinostheim, Germany | N/A |
| DMZ Universal Electrode Puller modified according to Dudel et al. (2000) to operate with oxygen-hydrogen burner | Zeitz Instruments, Martinsried, Germany; ref.16 | https://www.zeitz-puller.com |
| Custom made electrode holder (Figure 1D) | N/A | N/A |
| Olympus SZ61 stereo microscope | Olympus, Tokyo, Japan | RRID:SCR_018950 |
| Olympus CX41 bench top microscope with Olympus LMPlanFL N 100X | Olympus, Tokyo, Japan | Cat# LMPLFLN100x |
Materials and equipment
Experimental model
We generate neocortical neuronal-glial co-cultures from C57BL/6N mice. Alternatively, cultures can be generated from, e.g., CD® (Sprague Dawley) IGS rats (RRID:RGD_734476) or human induced pluripotent stem cell cultures (hiPSC) of the Neurogenin-2-overexpression model (https://hpscreg.eu/cell-line/BIHi005-A-24).
External recording solution
| Reagent (MW = molecular weight in g/mol) | Final concentration | Amount |
|---|---|---|
| Sodium chloride (NaCl, MW = 58.3) | 150 mM | 8.74 g |
| Potassium chloride (KCl, MW = 74.6) | 4 mM | 298 mg |
| HEPES (MW = 238.3) | 10 mM | 2.383 g |
| Glucose (MW = 180.0) | 10 mM | 1.80 g |
| Magnesium chloride (MgCl2, MW = 95.2) | 1.1 mM | 105 mg |
| Calcium chloride (CaCl2, MW = 111.0) | 1.1 mM | 122 mg |
| ddH2O | Add for total volume of 1 L | |
| Total | N/A | 1 L |
External recording solution can be stored at 4°C for up to one week.
Internal recording solution
| Reagent (MW = molecular weight in g/mol) | Final concentration | Amount |
|---|---|---|
| Potassium gluconate (Kglu, MW = 234.3) | 150 mM | 3514 mg |
| Magnesium adenosine 5′-triphosphate (Mg-ATP, MW = 507.2) | 3 mM | 152 mg |
| Sodium guanosine 5′-triphosphate (Na-GTP, MW = 523.0) | 0.3 mM | 15.7 mg |
| Potassium-HEPES (K-HEPES, MW = 276.4) | 10 mM | 276 mg |
| NaCl (MW = 58.3) | 10 mM | 58.4 mg |
| Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA, MW = 380.4) | 0.05 mM | 1.9 mg |
| ddH2O | Add for total volume of 100 mL | |
| Total | N/A | 100 mL |
Osmolality 290 mOsm. Instead of solid EGTA, consider using, e.g., a 10 mM EGTA stock solution. Internal recording solution can be stored at −20°C for up to 2 months. Do not freeze and thaw repetitively. From 2 months after fabrication onwards, we noticed a steep decline in recording success.
Culture medium
| Reagent (MW = molecular weight in g/mol) | Final concentration | Amount |
|---|---|---|
| MEM with Earle’s salts and L-glutamine | N/A | 1 L |
| Glucose (MW = 180.0) | 27.8 mM | 5 g |
| Sodium bicarbonate (NaHCO3, MW = 81.01) | 2.5 mM | 200 mg |
| Holo-Transferrin bovine (MW ∼ 80.000) | 1.25 μM | 100 mg |
| Insulin bovine (MW ∼ 5700) | 4.4 μM | 25 mg |
| Fetal bovine serum | N/A | 50 mL |
| B-27™ supplement | N/A | 10 mL |
| Total | N/A | 1 L |
Fully dissolve all components in MEM medium. Sterile-filtered culture medium can be stored at 4°C for several weeks.
Alternative setup components
The components of the patch-clamp setup adjusted for bouton recordings are listed in the key resources table. The following alternatives to the listed components may be considered:
Multiclamp 700A patch-clamp amplifier: We tested the precision of different amplifiers in current-clamp mode.1 The Multiclamp 700A performed best and we assume similar precision for the Multiclamp 700B successor model (Molecular Devices, San Jose, CA, USA; RRID:SCR_018455).
MultiClamp 700A Commander: MultiClamp 700 amplifiers can also be run using the pCLAMP software suite (Molecular Devices, San Jose, CA, USA; RRID:SCR_011323).
Kleindiek MM3A-LS micromanipulator: Bouton recordings are possible with very stable micromanipulators of other manufacturers. For alternatives see, e.g., Sensapex (uMp series; https://www.sensapex.com/products/ump-micromanipulators/) or Luigs-Neumann (LN Mini 25; https://www.luigs-neumann.org/mini; ref.17).
Modified DMZ Universal Electrode Puller: Quartz glass capillaries can be pulled by laser-based pullers (e.g., P-2000, Sutter Instruments, Navato, CA, USA; https://www.sutter.com/MICROPIPETTE/p-2000.htmL), but may need additional coating to reduce pipette capacitance. Alternatively, borosilicate glass pipettes may be used (see Part IV).
Step-by-step method details
Preparation of quartz glass pipettes (part I)
Timing: 1 h
Part I describes the preparation of quartz glass pipettes for low-noise recordings18,19 from boutons using a puller designated for pulling thick-walled quartz glass pipettes using an oxygen-hydrogen burner.16 We recommend to fabricate all pipettes required for experimentation at once before starting recordings. Quartz glass pipettes optimized for bouton recordings have resistances of 7–12 MOhm when filled with potassium gluconate-based internal solution.
-
1.
Cut glass capillary into ∼5 cm lengths using a triangular file. Clean glass dust from the cut ends using compressed air spray or wipe with finger.
-
2.
Pull quartz glass pipettes from capillaries.
Note: Steady-state operation of the pipette puller is typically reached following a few consecutive rounds of pipette pulling.
-
3.
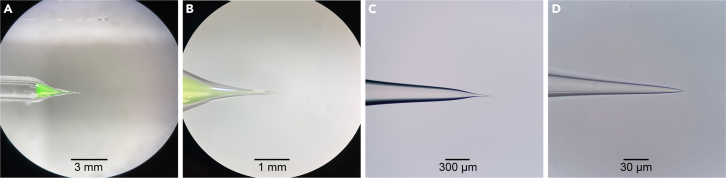
Inspect pipettes individually at low magnification (e.g., with a stereo microscope) and intermediate magnification (e.g., with a 10× objective) for taper shape (Figures 2A–2C), and at high magnification (e.g., using a 100× air objective with 3.4 mm working distance and 0.8 numerical aperture, Figure 2D; see key resources table) for pipette intactness and tip opening diameter.
Note: A steep tapering of the pipette shank and thick-walled pipette tips are preferred.
Pause Point: We typically record with newly fabricated pipettes and do not keep pipettes for more than 8 h. However, pulled pipettes can be stored for multiple days in a sealed container.
-
4.
Fill pipette tips with minimal internal recording solution (1–2 μL) using Microloader™ pipette tips (Eppendorf, Hamburg, Germany).
-
5.
Shorten pipettes to minimal length such that the electrode fits under the condenser (∼15 mm for the custom-built holder in Figure 1D).
-
6.
Clean cut ends of glass dust and mount on pipette holder (Figures 1A and 1D).
Optional: We have successfully re-used quartz glass pipettes upon cleaning following the protocol by ref.20 for bouton recordings.
Figure 2.
Quartz glass pipettes for bouton recordings
(A and B) Quartz glass pipette filled with fluorophore-containing internal recording solution at low and high stereoscopic magnification.
(C and D) Quartz glass pipette tip with 10× objective and 100× objective.
Identification of boutons (part II)
Timing: 5–15 min
Part II describes the identification of boutons for whole-cell recordings. Bouton identification is based on morphological and functional properties. For morphological identification, we use high magnification, high NA optics as described under Patch-clamp recording setup. For functional identification we use live-staining of active boutons and / or cell-attached recordings prior to establishing whole-cell mode.
-
7.Morphological identification of boutons in unstained cultures.
-
a.Insert coverslip into bath chamber and stabilize position with a metal grid if needed.Note: Each coverslip can be used for multiple cycles of bouton identification and recording attempts within ∼1h after insertion into external bath solution. If cells do not survive for at least 1h, see problem 1.
-
b.Boutons in primary dissociated cultures can be found as both, isolated boutons without an apparent postsynaptic target (labeled a in Figures 3A–3C) or boutons adjacent to neuronal cell bodies or dendrites (labeled b and c in Figures 3A–3C). If boutons cannot be identified visually in unstained cultures, see problem 2.Optional: Identification of boutons in unstained tissue can be facilitated by prior dye filling in a somatic whole-cell recording. Dye diffusion into the axon subsequently delineates boutons as repetitive axonal enlargements, e.g., as seen in Figure 4E of Ritzau-Jost et al. (2021). Bouton identification in acute brain slices requires prior neuronal dye filling1 or fluorophore-expressing transgenic animals3 due to the native tissue density precluding visual bouton identification.
-
a.
-
8.Live-staining of active boutons using FM®1-43 (Figure 3B; ref.21).
-
a.Prepare a high-K external solution (i.e., external recording solution with 40 mM NaCl replaced stoichiometrically by KCl).
-
b.Add FM®1-43 to 1 mL of high-K external solution in a petri dish (final FM®1-43 concentration 4 μM) and place in a dark box.
-
c.Insert a fresh coverslip into the petri dish with FM®1-43 high-K external solution in the dark box and incubate for 30–60 s.
-
d.Transfer the coverslip into another petri dish with regular external solution for washing and subsequently transfer into the recording chamber for imaging. If bouton live-staining does not work, see problem 3.
-
a.
-
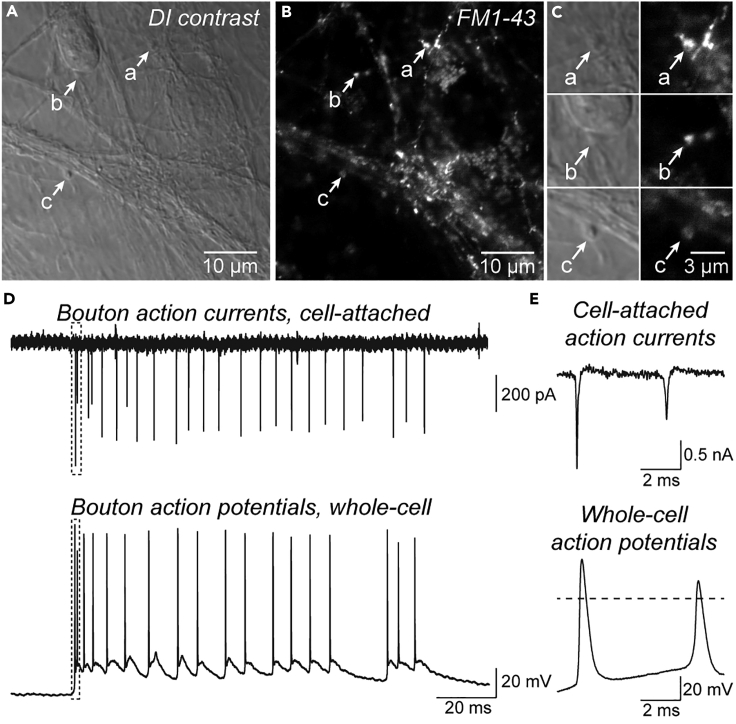
9.Cell-attached action potential recordings (Figures 3D and 3E).
-
a.In cell-attached mode, boutons can be identified by action currents resulting from spontaneous spiking.
-
a.
Note: Action currents are recorded in voltage-clamp on-cell mode at the zero-current potential22 and impose as biphasic, often asymmetric spikes. If differentiation of action currents from noise artifacts is difficult, see problem 4.
Figure 3.
Morphological and functional identification of boutons
(A) Example difference-interference contrast (DIC) image of a neocortical primary dissociated culture with isolated boutons (a) and boutons on neuronal somata or dendrites (b and c).
(B) Fluorescence image of the same area as in A stained for active synapses with FM®1-43.
(C) Magnification of DIC and fluorescence images of boutons (a–c).
(D) Spontaneous action currents (top) recorded from a bouton in cell-attached mode. Action potentials recorded from the same bouton after establishment of whole-cell mode are provided below (bottom).
(E) Initial spontaneous action currents recorded in cell-attached mode and action potentials after establishing whole-cell mode in the same bouton as in (D) (box). Note that the time points of action potentials differ in the upper and lower trace in panel D and E, representing similarly looking, but different bursts of spontaneous activity.
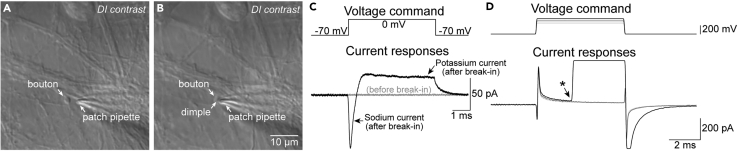
Figure 4.
Establishing whole-cell mode in bouton recordings
(A) DI contrast image of a patch pipette placed axially in close proximity to the targeted bouton.
(B) Configuration as in A after the pipette was placed on the bouton surface using axial advance of the pipette, creating a dimple on the bouton surface.
(C) Test pulse to 0 mV and evoked currents (leak-subtracted) in cell-attached mode (gray flat line) and after transition whole-cell mode (characterized by clearly discernable sodium and potassium currents).
(D) Voltage zap protocol for membrane breakdown in cell attached mode (currents not leak-subtracted). Membrane breakdown is indicated by a sudden deviation of the passive current transients (asterisk) leading to amplifier saturation (flat current signals following membrane breakdown during de- and repolarization).
Establishment of whole-cell bouton recordings (part III)
Timing: 10 min per attempt, recordings last for 5–15 min
Part III provides a step-by-step description of a whole-cell bouton recording. For the basic steps during conventional somatic patch-clamp recordings, we refer to detailed introductory literature elsewhere (e.g., ref.13,23,24). We recommend to first identify a bouton for recording and subsequently immerse the pipette into the bath. Throughout each attempt, a membrane test pulse is applied to monitor pipette or seal resistance.
-
10.
Set holding potential to nominal 0 mV (minus liquid junction potential)25 and monitor pipette resistance using a test pulse.
-
11.
Immerse pipette into bath solution with moderate positive pressure (20–40 mbar). Reduce pressure after crossing the bath surface to the minimum level required for solution efflux.
-
12.
Position the pipette tip in a focal plane just above the targeted bouton.
Note: Reducing pipette pressure will lower the risk of pipette block by particles within the internal solution. If very small tip pipettes and / or high fluorophore concentration in the internal solution are used, filter internal solution with 0.1 μm filter (Sartorius Minisart® PES 0.1) before pipette loading.
-
13.
Bring the pipette in proximity to the bouton (∼1–2 μm; Figure 4A).
-
14.
Set pipette current to zero.
-
15.
Set the pipette pressure to 5–15 mbar.
-
16.
Approach the bouton in the direction of the longitudinal pipette axis at minimum micromanipulator speed until you appear to touch the bouton (i.e., the bouton slightly moves). In some cases, a dimple is visible on the bouton surface (Figure 4B).
-
17.
Release positive pipette pressure slowly and do not apply suction. The recorded bouton will attach to the pipette tip and a gigaohm seal will form with according changes in the test pulse.
-
18.
Action currents can be recorded in the cell-attached configuration (see 9.). For difficulties to obtain cell-attached configuration, see problem 5.
-
19.
Gently retract the pipette along its longitudinal axis by ∼1 μm to decrease mechanical stress on the bouton membrane.
Note: This will also reduce the risk of sucking the bouton into the pipette while attempting to establish whole-cell mode.
-
20.
Determine pipette capacitance using a test pulse.
-
21.
Compensate for pipette capacitance using the built-in pipette capacitance compensation in cell-attached voltage-clamp mode.
Note: This capacitance is referred to as Cfast in the EPC10 amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany) or C1 and C2 in the Multiclamp 700A amplifier (Molecular Devices, San Jose, CA, USA). In the latter case, only C1 was used and C2 was set to zero.1
-
22.
Apply mild, as short as possible suction pulses to break the membrane at the pipette tip. In parallel, monitor membrane intactness using a test pulse.
Note: Because of the small capacitance of the bouton and higher series resistance compared to somatic recordings, typical changes in the response to small 5–10 mV test pulses are often not readily visible when establishing bouton whole-cell mode. We instead apply test pulse to 0 mV (duration 5 ms) to monitor active membrane conductance. In this case, upon membrane rupture a sodium current will be elicited that occurs with a delay of <300 μs relative to the test pulse onset followed by a slower and opposed potassium current (Figure 4C). Larger delays and large amplitudes of sodium currents (>500 pA) may indicate close proximity to a nearby soma.
Optional: Instead of pressure pulses, we successfully used the voltage “zap” protocol by ref.26 for membrane break down. Specifically, we use 250 mV depolarizations from resting membrane potential for 3 ms with 50 mV increments. Typically, membrane break down and thus transition to whole-cell mode occur at 350–400 mV zap amplitude (Figure 4D).
-
23.
Upon membrane rupture, whole-cell mode is established. Switch amplifier to whole-cell mode accordingly.
-
24.
If a dye is contained in the internal recording solution, the recorded bouton and adjacent axon can be identified in fluorescence imaging.
-
25.
Holding current at −80 mV should be between 0 and -15 pA. Holding currents exceeding -15 pA are a sign of a poor seal or an unhealthy bouton.
-
26.
Whole-cell recordings can be maintained for 10–15 min under optimal conditions of no mechanical and chemical stress.
CRITICAL: For stable whole-cell bouton recordings, use constant solution flow in the bath, minimize the pressure of the intracellular solution in the pipette (including adhesive forces), minimize phototoxicity during fluorescence imaging, and minimize the drift of the micromanipulator and the pipette in the pipette holder (see example of holders in Figures 1A and 1D for Kleindiek micromanipulators and ref.17 for headstage mounted micromanipulators). If whole-cell recordings cannot be maintained for 10–15 min, see problem 6.
Pipette capacitance compensation for current-clamp recordings from boutons (part IV)
Timing: 2 min for pipette filling and coating per attempt
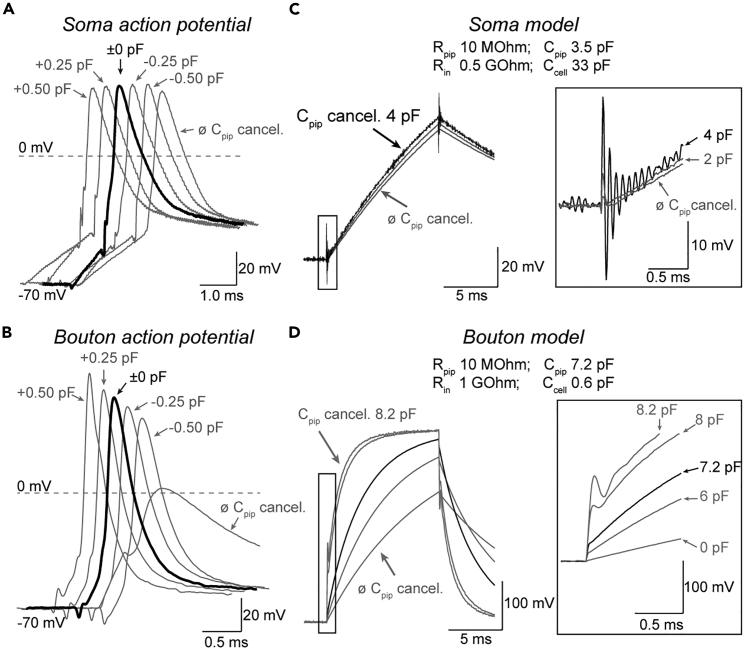
Current-clamp recordings rely on precise compensation of pipette capacitance including the capacitance of the pipette holder and the headstage of the amplifier.24,27,28 In contrast to somatic action potentials that are less sensitive to erroneously compensated pipette capacitance (Figure 5A), bouton action potentials are smaller and broader when recorded with insufficiently compensated pipette capacitance or larger and narrower with overcompensated pipette capacitance (Figure 5B).1,29 Therefore, part IV describes precise pipette capacitance compensation (27.) and minimization of total pipette capacitance (28.).
-
27.Capacitance compensation in voltage- and current-clamp mode deploys different circuits and amplifiers vary in their capacitance compensation precision.
-
a.In somatic current-clamp recordings, pipette capacitance compensation can be obtained by increasing the compensation until voltage oscillations occur upon current injection (Figure 5C).23 However, this method cannot be applied to bouton recordings due to their small membrane capacitance obscuring oscillation onset (Figure 5D). We therefore previously established a hybrid voltage-/current-clamp approach, in which the pipette capacitance is first determined in the voltage-clamp mode in the cell-attached configuration and this value is subsequently used for compensation in the whole-cell configuration after switching to current-clamp mode.1
- b.
-
c.We previously compared various amplifiers and found accurate capacitance compensation and hence small errors in recorded action potentials using the Multiclamp 700A amplifier (Molecular Devices, San Jose, CA, USA) in combination with the hybrid voltage-/current-clamp approach.
-
a.
CRITICAL: Accurate action potential recordings were only possible when total pipette capacitance was ≤ 5 pF (Figure 3 in Ritzau-Jost et al., 2021).1
-
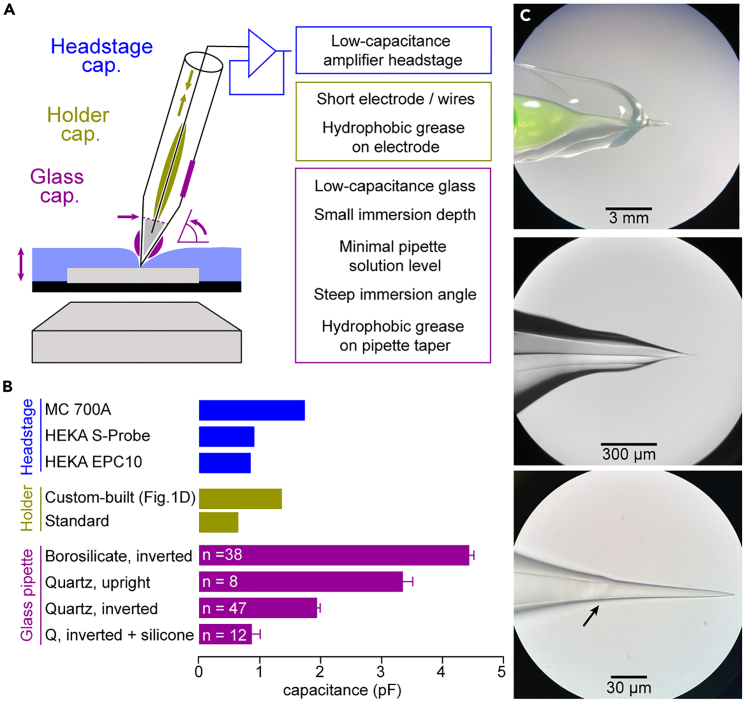
28.Three components mainly contribute to total pipette capacitance: Amplifier headstage input capacitances, pipette holder capacitance (including electrode wire), and the capacitance generated across the immersed pipette glass itself (Figure 6A).
-
a.Headstage input capacitance depends on the amplifier model.
-
b.Pipette holder capacitance is reduced by shortening connective and electrode wires and by applying hydrophobic silicone grease (e.g., KORASILON® paste, medium viscosity) on the electrode wire to minimize adhesion of internal solution (Figures 6B and 6C).
-
c.Pipette glass capacitance is reduced by low-capacitance glass types (e.g., quartz glass) and minimal pipette immersion using lowest possible bath solution levels on an inverted microscope.Note: The hydrophobicity of quartz glass additionally prevents adhesion of the extra- and intracellular solution along the glass and the silver wire. Adhesion of bath solution along the pipette taper can be further prevented by using a steep pipette immersion angle.
-
d.If recordings are performed on an upright microscope, consider lifting the objective from the bath solution after establishing the recording.
-
e.Application of hydrophobic silicone grease on the pipette taper and tip region under a binocular guidance further reduces pipette capacitance (Figures 6B and 6C).Optional: We previously also used borosilicate glass pipettes for bouton recordings. However, these have a higher capacitance than quartz glass pipettes (Figure 6B) and may therefore affect signals recorded from boutons (for a comparison of somatic and bouton action potentials recorded with quartz and borosilicate glass pipettes, see Figure S4E in Ritzau-Jost et al., 2021).1 Furthermore, borosilicate pipettes have higher resistances than quartz glass pipettes of similar tip opening diameter (Figure S2 in Ritzau-Jost et al., 2021).1 We therefore recommend to test the effect of different pipette types on bouton recordings prior to data interpretation. However, we have not systematically tested application of silicone grease or extensive coating to borosilicate glass pipettes. Therefore, careful capacitance minimization of borosilicate glass pipettes might allow current-clamp recordings of equal resolution as obtained with quartz glass pipettes.
-
a.
Figure 5.
Precise capacitance compensation is required for boutons current-clamp recordings
(A) Example soma action potentials recorded without compensation of pipette capacitance (ø Cap. comp), with compensation of the capacitance value determined in voltage-clamp mode (±0 pF), and with small undercompensation (-0.25 pF, -0.5 pF) and overcompensation (+0.25 pF, +0.5 pF) of pipette capacitance. Note the small sensitivity of somatic action potential shape to changes in pipette capacitance compensation.
(B) Example bouton action potentials recorded with varying pipette capacitance compensation as in (A). Note the strong effect of small deviations in capacitance compensation on the bouton action potential amplitude and duration.
(C) Increasing pipette capacitance compensation in current-clamp mode in a circuit model of a neuronal soma leads to voltage oscillations separable from slower voltage changes across the somatic capacitance. Box: Expansion of the initial current injection-evoked voltage change for different pipette capacitance compensations.
(D) Current injection-evoked voltage responses in a circuit model of a bouton for different pipette capacitance compensations. Box: Expansion of the initial current injection-evoked voltage changes. Panels (C and D) were modified from Ritzau-Jost et al. (2021).1
Figure 6.
Components and reduction of total pipette capacitance
(A) Scheme of the capacitance components adding up to total pipette capacitance (capacitances of the headstage in blue, pipette holder in yellow, and pipette glass in purple). Means to reduce pipette capacitance are illustrated and provided on the right.
(B) Capacitances for different glass types and treatments, pipette holders, and headstages. Color code as in (A). Glass pipette capacitances are represented as mean ± SEM.
(C) Example quartz glass pipette with KORASILON® silicone grease to reduce pipette capacitance at increasing magnification (from top to bottom). Arrow indicates edge of applied silicone grease.
Expected outcomes
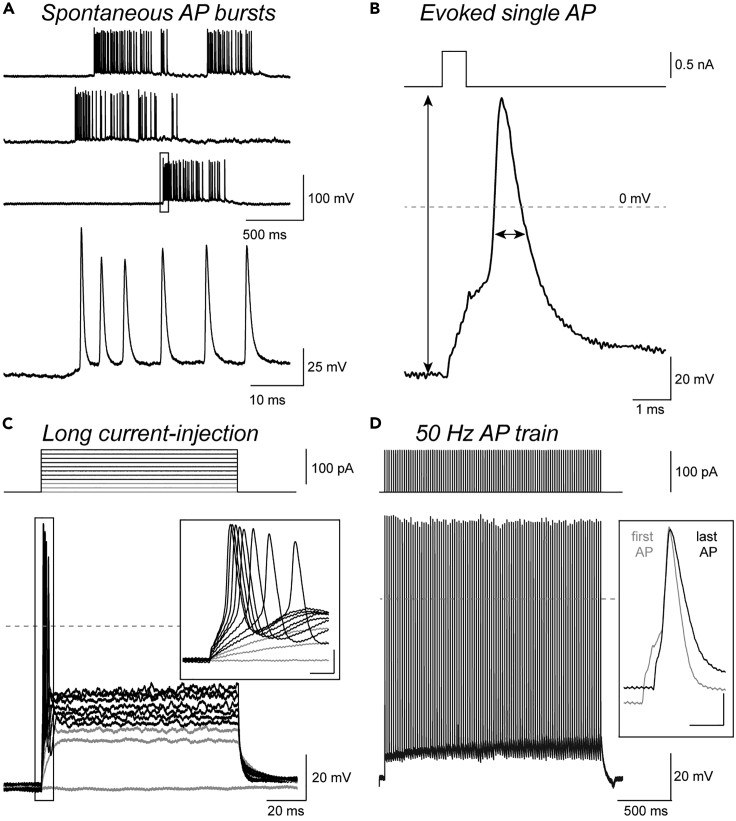
Boutons recorded in whole-cell mode typically have resting membrane potentials below -60 mV (liquid junction potential corrected). Spontaneous and evoked action potentials (Figures 7A and 7B) can be quantified. Action potentials in small boutons of dissociated primary cultures of the neocortex have large amplitudes of ∼120 mV (measured from resting to peak potential) and brief half-duration of ∼0.4 ms when recorded at physiological temperature. Thus, bouton action potentials are 5 mV larger and about half as narrow as somatic action potentials recorded under the same conditions. In contrast to neuronal somata, boutons characteristically fire single action potentials upon prolonged current-injections (Figure 7C). During repetitive action potential stimulation at a frequency of 50 Hz, bouton action potentials are broadened while amplitudes remain stable (Figure 7D) as previously described for hippocampal mossy fiber boutons.30
Figure 7.
Whole-cell current-clamp recordings of bouton action potentials
(A) Spontaneous bouton action potential bursts (top) and expansion of an initial burst phase (bottom).
(B) Bouton action potential evoked upon incremental 0.3 ms current injections. Action potential amplitude and half-duration are indicated by arrows.
(C) Bouton action potentials evoked by incremental 100 ms current injections. Note that boutons typically fire a single action potential in response to long current injections.
(D) Example train of bouton action potentials (top: 90 × 0.3 ms current injections at 50 Hz, bottom: evoked action potential train). Box: Overlay of the first and last train action potential indicating stable action potential amplitude and action potential broadening upon high-frequency stimulation. Panel (B) was modified from Ritzau-Jost et al. (2021).1
Limitations
The protocol was developed for current-clamp recordings at boutons with high temporal resolution. High pipette capacitance and different amplifiers may limit the resolution and result in erroneous bouton action potential shape. An important limitation of this technique is the exchange of the cytosol by the internal solution, which might alter cellular function. Finally, the duration of these recordings is usually limited to <15 min, which precludes continuous analysis of longer-lasting processes. Furthermore, the cell capacitance recorded in whole-cell mode likely reflects the capacitance of the recorded bouton and the adjacent axonal arbor. Thus, the estimation of the bouton surface area and the determination of membrane conductances are difficult (see also the discussion in ref.5. Additionally, due to the small size and fragile structure of boutons, pulling outside-out patches is more challenging during bouton recordings as compared to somatic recordings.
Troubleshooting
Problem 1
Cells die rapidly after insertion in the bath solution (step 7).
Potential solution
Adjust the osmolality and pH of the external solution as appropriate. Make sure to adjust the pH at the same temperature at which recordings are performed (pH of external recording solution changes between 23°C and 35°C by -0.2). Additionally, renew external solution after 3–4 h of perfusion in order to prevent increases in osmolality, especially when recording at physiological temperature. Lower bath perfusion flow to reduce mechanical stress of cells.
Problem 2
Boutons cannot be identified visually (step 7).
Potential solution
Bouton identification may be impeded by high culture density, therefore test a decrease in the number of plated cells during culture generation. Because we apply cells in the cover slip center while plating, culture density decreases towards the edge of the cover slip. Thus, alternatively move towards the cover slip edge or apply cells eccentrically on cover slip during culture generation.
If low culture density impedes bouton identification, increase the number of plated cells or move towards cover slip center.
Problem 3
Boutons are not stained using live-staining (step 8).
Potential solution
Increase the incubation time in high-K loading solution or the potassium concentration in order to enforce vesicle turn over. Alternatively, FM dyes can be incubated in the growth medium for, e.g., 1h prior to recordings. Alternatively, increase dye concentration in incubation solution. In order to maintain labeling following removal from loading solution and subsequent washing, consider blocking activity-induced destaining by using blockers in the external bath solution (1–2 mM kynurenic acid, or 20 μM 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo [f]quinoxaline-7-sulfonamide (NBQX), or 1 μM Tetrodotoxin depending on the type of experiment).
Problem 4
Differentiation of action currents from noise artifacts in cell-attached recordings (step 9).
Potential solution
Action currents show the following properties that may help in discriminating them from noise: Action currents often have asymmetrical shape with a sharp first deflection and a slower second deflection in the reverse direction (Figure 3E). The peak-to-peak duration between both deflections reflects the half-duration of the underlying action potential (∼0.4–1 ms).1 Action currents often occur in bursts and may be reduced within bursts.
Problem 5
Boutons swell or shrink rapidly when approached by pipette or adhere to the side of the electrode (step 18).
Potential solution
Reduce positive pipette pressure and thereby internal solution efflux from the pipette as mechanical damage of the bouton may occur (typically, low pressure values of <15 mbar are needed in close vicinity to the bouton). Adjust the osmolality gradient such that the osmolality of the internal solution is 5–10 mOsm smaller than the external solution. If boutons attach and adhere to the side of the electrode tip when releasing pressure for seal formation, make sure to approach the bouton with the pipette perpendicular to the bouton surface. Also, release positive pressure slowly and reduce the indentation of the bouton before releasing positive pressure.
Problem 6
Boutons swell or shrink after establishing whole-cell mode (step 26).
Potential solution
Adjust internal solution or external solution such that the internal solution osmolality is 5–10 mOsm below external solution osmolality. Make sure any negative or positive pressure is released from the pipette holder tubing after establishing whole-cell mode by ventilating the tubing. If the bouton is increasingly sucked into the pipette, apply minimal constant positive pressure. If internal recording solution contains fluorophores, minimize light intensity and record fluorescence images after finishing the recording on order to prevent phototoxicity.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Stefan Hallermann (hallermann@medizin.uni-leipzig.de).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate new datasets or code.
Acknowledgments
This work was supported by a European Research Council Consolidator Grant (ERC CoG 865634) to S.H., the German Research Foundation (HA6386/10-1 to S.H.), and by grants awarded by the U.S. Department of Veterans Affairs (BX002547) and NIGMS (GM134110) to S.M.S.
Author contributions
A.R-.J., S.M.S., and S.H. developed bouton recordings. A.R-.J., J.N., T.K., and T.T. performed bouton recordings. A.R-.J., S.M.S., and J.N. established the bouton live staining. M.K. and I.B. performed the electron microscopic analysis of patch-clamp pipettes. B.B. designed the electrical circuit of the bouton model. A.R-.J. and S.H. wrote the manuscript with input from all other authors.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Andreas Ritzau-Jost, Email: andreas.ritzau-jost@medizin.uni-leipzig.de.
Stefan Hallermann, Email: hallermann@medizin.uni-leipzig.de.
References
- 1.Ritzau-Jost A., Tsintsadze T., Krueger M., Ader J., Bechmann I., Eilers J., Barbour B., Smith S.M., Hallermann S. Large, stable spikes exhibit differential broadening in excitatory and inhibitory neocortical boutons. Cell Rep. 2021;34:108612. doi: 10.1016/j.celrep.2020.108612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steiger L., Tsintsadze T., Mattheisen G.B., Smith S.M. Somatic and terminal CB1 receptors are differentially coupled to voltage-gated sodium channels in neocortical neurons. bioRxiv. 2022 doi: 10.1101/2022.08.12.503665. Cell Report 2023 in press; Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C., Cai X., Ritzau-Jost A., Kramer P.F., Li Y., Khaliq Z.M., Hallermann S., Kaeser P.S. An action potential initiation mechanism in distal axons for the control of dopamine release. Science. 2022;375:1378–1385. doi: 10.1126/science.abn0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivekananda U., Novak P., Bello O.D., Korchev Y.E., Krishnakumar S.S., Volynski K.E., Kullmann D.M. Kv1.1 channelopathy abolishes presynaptic spike width modulation by subthreshold somatic depolarization. Proc. Natl. Acad. Sci. USA. 2017;114:2395–2400. doi: 10.1073/pnas.1608763114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novak P., Gorelik J., Vivekananda U., Shevchuk A.I., Ermolyuk Y.S., Bailey R.J., Bushby A.J., Moss G.W.J., Rusakov D.A., Klenerman D., et al. Nanoscale-targeted patch-clamp recordings of functional presynaptic ion channels. Neuron. 2013;79:1067–1077. doi: 10.1016/j.neuron.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawaguchi S.y., Sakaba T. Control of inhibitory synaptic outputs by low excitability of axon terminals revealed by direct recording. Neuron. 2015;85:1273–1288. doi: 10.1016/j.neuron.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi S.Y., Sakaba T. Fast Ca2+ buffer-dependent reliable but plastic transmission at small CNS synapses revealed by direct bouton recording. Cell Rep. 2017;21:3338–3345. doi: 10.1016/j.celrep.2017.11.072. [DOI] [PubMed] [Google Scholar]
- 8.Begum R., Bakiri Y., Volynski K.E., Kullmann D.M. Action potential broadening in a presynaptic channelopathy. Nat. Commun. 2016;7:12102. doi: 10.1038/ncomms12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Southan A.P., Robertson B. Patch-clamp recordings from cerebellar basket cell bodies and their presynaptic terminals reveal an asymmetric distribution of voltage-gated potassium channels. J. Neurosci. 1998;18:948–955. doi: 10.1523/jneurosci.18-03-00948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu H., Jonas P. A supercritical density of Na+ channels ensures fast signaling in GABAergic interneuron axons. Nat. Neurosci. 2014;17:686–693. doi: 10.1038/nn.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu H., Roth F.C., Vandael D., Jonas P. Complementary tuning of Na+ and K+ channel gating underlies fast and energy-efficient action potentials in GABAergic interneuron axons. Neuron. 2018;98:156–165.e6. doi: 10.1016/j.neuron.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penner R. In: Single-Channel Recordings. Sakmann B., Neher E., editors. 2009. A practical guide to patch clamping; pp. 9–17. [Google Scholar]
- 13.Numberger M., Draguhn A. Spektrum Akademischer Verlag; 1996. Patch-Clamp-Technik. [Google Scholar]
- 14.Beaudoin G.M.J., Lee S.H., Singh D., Yuan Y., Ng Y.G., Reichardt L.F., Arikkath J. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 2012;7:1741–1754. doi: 10.1038/nprot.2012.099. [DOI] [PubMed] [Google Scholar]
- 15.Bullmann T., Radivojevic M., Huber S.T., Deligkaris K., Hierlemann A., Frey U. Large-scale mapping of axonal arbors using high-density microelectrode arrays. Front. Cell. Neurosci. 2019;13:404. doi: 10.3389/fncel.2019.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudel J., Hallermann S., Heckmann M. Quartz glass pipette puller operating with a regulated oxy-hydrogen burner. Pflugers Arch. 2000;441:175–180. doi: 10.1007/s004240000407. [DOI] [PubMed] [Google Scholar]
- 17.Vandael D., Okamoto Y., Borges-Merjane C., Vargas-Barroso V., Suter B.A., Jonas P. Subcellular patch-clamp techniques for single-bouton stimulation and simultaneous pre- and postsynaptic recording at cortical synapses. Nat. Protoc. 2021;16:2947–2967. doi: 10.1038/s41596-021-00526-0. [DOI] [PubMed] [Google Scholar]
- 18.Levis R.A., Rae J.L. The use of quartz patch pipettes for low noise single channel recording. Biophys. J. 1993;65:1666–1677. doi: 10.1016/S0006-3495(93)81224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levis R.A., Rae J.L. Low-noise patch-clamp techniques. Methods Enzymol. 1998;293:218–266. doi: 10.1016/S0076-6879(98)93017-8. [DOI] [PubMed] [Google Scholar]
- 20.Kolb I., Stoy W.A., Rousseau E.B., Moody O.A., Jenkins A., Forest C.R. Cleaning patch-clamp pipettes for immediate reuse. Sci. Rep. 2016;6:35001. doi: 10.1038/srep35001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith S.M., Bergsman J.B., Harata N.C., Scheller R.H., Tsien R.W. Recordings from single neocortical nerve terminals reveal a nonselective cation channel activated by decreases in extracellular calcium. Neuron. 2004;41:243–256. doi: 10.1016/S0896-6273(03)00837-7. [DOI] [PubMed] [Google Scholar]
- 22.Perkins K.L. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J. Neurosci. Methods. 2006;154:1–18. doi: 10.1016/j.jneumeth.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marty A., Neher E. In: Single-Channel Recordings. Sakmann B., Neher E., editors. Springer; 2009. Tight-seal whole-cell recordings; pp. 31–52. [Google Scholar]
- 24.Barbour B. Electronics for electrophysiologists. 2018. http://129.199.30.71/IMG/pdf/electronics_for_electrophysiologists.pdf
- 25.Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-C. [DOI] [PubMed] [Google Scholar]
- 26.Kanda H., Ling J., Tonomura S., Noguchi K., Matalon S., Gu J.G. TREK-1 and TRAAK are principal K+ channels at the nodes of Ranvier for rapid action potential conduction on mammalian myelinated afferent nerves. Neuron. 2019;104:960–971.e7. doi: 10.1016/j.neuron.2019.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purves R.D. Academic Press; 1981. Microelectrode Methods for Intracellular Recording and Ionophoresis. [Google Scholar]
- 28.Brette R., Destexhe A. Handbook of Neural Activity Measurement. Cambridge University Press; 2012. Intracellular recording; pp. 44–91. [Google Scholar]
- 29.Oláh V.J., Tarcsay G., Brunner J. Small size of recorded neuronal structures confines the accuracy in direct axonal voltage measurements. eNeuro. 2021;8 doi: 10.1523/ENEURO.0059-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geiger J.R., Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/S0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate new datasets or code.