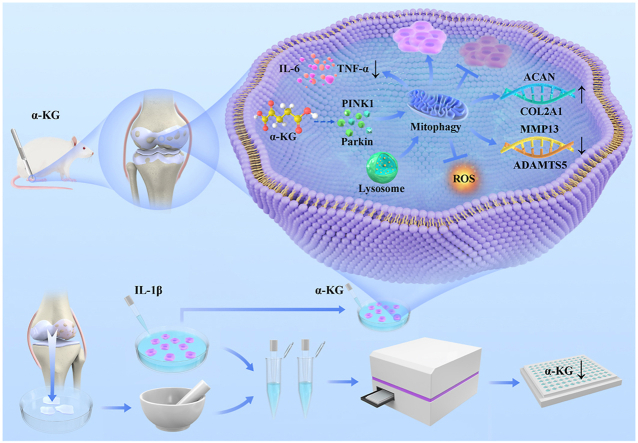
Abstract
Osteoarthritis (OA) is an age-related metabolic disease. Low-grade inflammation and oxidative stress are the last common pathway of OA. α-ketoglutarate (α-KG) is an essential physiological metabolite from the mitochondrial tricarboxylic acid (TCA) cycle, with multiple functions, including anti-inflammation and antioxidation, and exhibits decreased serum levels with age. Herein, we aimed to investigate the effect and mechanism of α-KG on OA. We first quantified the α-KG levels in human cartilage tissue and osteoarthritic chondrocytes induced by IL-1β. Besides, IL-1β-induced osteoarthritic chondrocytes were treated with different concentrations of α-KG. Chondrocyte proliferation and apoptosis, synthesis and degradation of extracellular matrix, and inflammation mediators were analyzed. RNA sequencing was used to explore the mechanism of α-KG, and mitophagy and oxidative stress levels were further detected. These results were verified in an anterior cruciate ligament transection (ACLT) induced age-related OA rat model. We found that α-KG content decreased by 31.32% in damaged medial cartilage than in normal lateral cartilage and by 36.85% in IL-1β-induced human osteoarthritic chondrocytes compared to control. α-KG supplementation reversed IL-1β-induced chondrocyte proliferation inhibition and apoptosis, increased the transcriptomic and proteinic expression of ACAN and COL2A1 in vivo and in vitro, but inhibited the expression of MMP13, ADAMTS5, IL-6, and TNF-α. In mechanism, α-KG promoted mitophagy and inhibited ROS generation, and these effects could be prevented by Mdivi-1 (a mitophagy inhibitor). Overall, α-KG content decreased in human OA cartilage and IL-1β-induced osteoarthritic chondrocytes. Moreover, α-KG supplementation could alleviate osteoarthritic phenotype by regulating mitophagy and oxidative stress, suggesting its potential as a therapeutic target to ameliorate OA.
Keywords: α-ketoglutarate, Osteoarthritis, Cartilage, Mitophagy, Oxidative stress
Graphical abstract
Highlights
-
•
The content of α-ketoglutarate is decreased in human osteoarthritis cartilage and IL-1β-induced chondrocytes.
-
•
α-ketoglutarate supplementation can alleviate osteoarthritic phenotype by regulating mitophagy and oxidative stress.
-
•
α-ketoglutarate could be a potential therapeutic target and medication for osteoarthritis.
1. Introduction
Osteoarthritis (OA) is a prevalent chronic and crippling disease characterized by cartilage destruction, osteophyte formation, and joint pain [1,2]. As of 2015, about 237 million people were suffering from OA worldwide, accounting for 3.3% of the total population [3,4]. By 2017, the prevalent cases of hip and knee OA reached 303.1 million [5], and OA became the fourth leading cause of years lived with disability and the leading source of societal cost in older adults globally [1]. Joint replacement is usually indicated for end-stage disease, while there is a lack of effective intervention for early-stage OA [6]. Therefore, there is an urgent need for early biomarkers to provide effective treatment for OA to reduce its burden on society and individuals.
It is widely thought that oxidative stress, chronic low-grade inflammation, decreased chondrocyte proliferation and extracellular matrix synthesis, and increased chondrocyte apoptosis and extracellular matrix degradation participate in the pathogenesis of OA [[7], [8], [9], [10]]. Current evidence suggests that mitochondria metabolism plays various regulatory roles in the pathogenesis of OA, including reactive oxygen species (ROS) production, bioenergetics metabolism, inflammatory responses, apoptosis, aging-related responses, and calcium metabolism [11]. Mitochondrial metabolism disorder is a major source of ROS [11], which increases gradually with OA progression [12]. The increased ROS levels lead to low-grade inflammation in cartilage and synovium, resulting in the secretion of extracellular matrix-degrading enzymes to increase cartilage degradation, and causing the downregulation of cartilage-specific anabolic genes (aggrecan (ACAN) and collagen type II alpha 1 (COL2A1)) gene expression, finally compromising chondrocyte function and joint structures [11,13]. Hence, targeting mitochondrial metabolism homeostasis and oxidative stress represents potential treatment strategies for patients with OA.
The tricarboxylic acid (TCA) cycle, also known as citric acid or Krebs cycle, occurs in the mitochondria in eukaryotes and regulates mitochondrial function and oxidative stress [[14], [15], [16], [17]]. α-ketoglutarate (α-KG) is a key intermediate metabolite in the TCA cycle, also known as 2-oxoglutarate [18], which contributes to a variety of metabolic processes, including the biogenesis of numerous amino acids, nucleotide, lipid, and carnitine biosynthesis, and as a cofactor in numerous dioxygenases [[19], [20], [21], [22]]. There is an increasing consensus that α-KG is involved in maintaining mitochondrial metabolism homeostasis [23], collagen synthesis [24], antioxidative defense [25], anti-inflammation [26], promoting cell proliferation [27], epigenetic modification [28], and tumor suppression [29]. Moreover, Tian et al. found that the circulatory α-KG concentration was gradually reduced with age [30], and α-KG supplementation decreased the levels of systemic inflammatory cytokines (such as interleukin (IL)-2, IL-6, and tumor necrosis factor-alpha (TNF-α)) to promote a longer, healthier life in aging mice model [26]. As an age-related and chronic inflammatory disease, whether OA can be alleviated by α-KG supplementation?
In the present study, we measured the α-KG levels in human OA cartilage tissue and osteoarthritic chondrocytes induced by IL-1β. The effects of α-KG on proliferation, apoptosis, extracellular matrix synthesis and degradation, and inflammation were assayed in IL-1β-induced OA-like chondrocytes. RNA sequencing was also performed to explore the mechanism. The rat OA model was finally established to confirm the therapeutic effect of α-KG and the underlying mechanisms. Overall, we sought to confirm the role and mechanism of α-KG in OA and lay the groundwork for future research on the early and effective treatment of this patient population.
2. Methodology
2.1. Materials and reagents
Dimethyl (DM)-α-KG (CAS.13192-04-6, No.1173917) was purchased from Haohong Scientific™ (Shanghai, China). Trizol® was purchased from Omega Bio-Tek™ (USA). Reverse transcription and Taq Pro Universal SYBR qPCR Master Mix kits were procured from Vazyme Biotech™ (Nanjing, China). COL2A1 (ab34712) and TNF-α (ab183218) antibodies were purchased from Abcam™ (Shanghai, China). Glyceraldehyde 3-phosphate dehydrogenase (GADPH) (AC001), matrix metallopeptidase 13 (MMP13) (A11148), IL-6 (A11115), and PTEN-induced putative kinase 1 (PINK1) (A7131) for western blot were procured from Abclonal™ (Wuhan, China). PINK1 (23274-1-AP) for immunohistochemistry and Parkin RBR E3 ubiquitin-protein ligase (Parkin) (14060-1-AP) were purchased from Proteintech™ (Wuhan, China). Oligonucleotide primers purchased from TIANYIHUIYUAN™ (Guangzhou, China). Isoflurane was obtained from Baxter Healthcare™ (Deerfield, IL, USA). DMEM/F-12 medium and fetal bovine serums (FBS) were obtained from Gibco™ (California, USA). Mdivi-1 (CAS. 338967-87-6, HY-15886) and Bafilomycin A1 (CAS. 88899-55-2, HY-100558) were purchased from MedChemExpress™ (Monmouth Junction, NJ, USA). ROS assay kits® (S0033S), Mito-Tracker Green® (C1048), and Lyso-Tracker Red® (C1046) were purchased from Beyotime™ (Shanghai, China). Recombinant Human IL-1β (200-01B-10UG) was obtained from PreproTech™ (Princeton, NJ, USA). The remaining materials and agents were of analytical grade.
2.2. Ethics statement
All OA patients provided consent for harvesting and using their tissues. All animal experiments were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (Revised 1996). Moreover, animal-based studies were performed in line with ethical guidelines/protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Jishou University Center for Animal Experiments. The study protocol was approved by the Ethics Committee (Approval no.202201).
2.3. α-KG content measurements
For the in vivo experiment, the cartilage tissue was collected from patients that underwent total knee replacement surgery. The damaged medial cartilage and normal lateral cartilage were separated from each sample. The tissues were transferred to an EP tube after being ground. For in vitro experiments, human primary chondrocytes were collected after treatment with 10 ng/ml IL-1β for 24 h. Then, the α-KG content was measured according to the protocol of α-KG assay kit® (G0861W, GraceBiotechnology™, Suzhou, China). The absorbance was detected at a wavelength of 450 nm by an enzyme-linked immunosorbent assay reader (TECAN™, Australia). A blank control was set to exclude the influence of other factors.
2.4. Cellular culturing
Primary human articular chondrocytes were extracted from normal lateral cartilage of patients that underwent total knee replacement surgery. Briefly [2], cartilage was exposed to type II collagenase (2 mg/mL, Invitrogen™, USA) at 37 °C for 8 h after shearing. Then, chondrocytes were harvested after centrifugation at 500g for 5 min and seeded with DMEM/F-12 medium and 10% FBS with 100 mg/ml streptomycin and 100 U/ml penicillin. In this study, Il-1β, which is commonly used to mimic in vitro pathological conditions, was employed as a positive control to induce the osteoarthritic phenotype of chondrocytes [31,32]. And human chondrocytes received 10 ng/ml IL-1β with or without different concentrations of α-KG for different times according to the experiment design. Three biological and two technical replicates were set for these assays.
2.5. Cell counting Kit-8 (CCK-8) & EdU assays
For the CCK-8 assay [33], chondrocytes were made into 1-5x10 [3]/L cell suspension and seeded in 96-well plates cultured with α-KG at the concentration of 0, 0.5, 1, 2, and 4 mM for 24, 48, and 72 h. Optical density was assayed using the CCK-8® assay kit (GK10001, GLPBIO™, Montclair, CA, USA) according to the manufacturer's protocol. Absorption levels were detected at 450 nm by an enzyme-linked immunosorbent assay reader (TECAN™, Australia). For the EdU assay, chondrocytes were cultured in 6-well plates with 10 ng/ml IL-1β and/or 2 mM α-KG and/or 50 μM Mdivi-1 for 24 h. Then, cells were treated with the EdU Kit® (MA0425, Meilunbio™, Dalian, China) according to the instructions. Briefly, after an equal volume of EdU (20 μM) at 37 °C was added to the 6-well-plates, samples were incubated for 2 h at 37 °C before fixed with 4% paraformaldehyde for 15 min. 0.5% Triton X-100 was added for 10 min at room temperature. After rinsing with PBS 3 times, cells were incubated in Click solution (including 430 μl Click Reaction Buffer, 20 μl CuSO4, 1 μl 555-Azide, and 50 μl Click-iT Additive Solution for one sample) for 30 min in darkness. Finally, samples were photographed under a fluorescence microscope (EVOS fl auto, ThermoFisher Scientific™, USA) after rinsing with PBS 3 times. ImageJ software (v.1.52q, NIH, Bethesda, MD, USA) was used for the quantitative analysis of 6 different visual fields for each sample (n = 3).
2.6. Flow cytometry
Chondrocytes were collected after treatment with 10 ng/ml IL-1β and/or 2 mM α-KG and/or 50 μM Mdivi-1. The Annexin V - FITC/PI double staining cell detection kit (BB-4101-50T, BestBio™, Shanghai, China) was used for cell apoptosis assays [33]. According to the manufacturer's instructions, the cells were washed twice with PBS by centrifugation (500 g, 5 min, 4 °C) and re-suspended with 400 μl 1X Annexin V binding solution. 5 μl Annexin V - FITC staining solution was added to the cell suspension and incubated for 15 min at 2–8 °C in darkness. 5 μl PI staining solution was then added to the cell suspension. The samples were analyzed immediately by flow cytometry (CytoFLEX, Beckman Coulter™, Brea, CA, USA).
2.7. Western blotting analysis
Briefly [34], cells were harvested and lysed in a RIPA lysis solution containing 1 mM PMSF on ice for 20 min. The supernatants were collected by 12000 g centrifugation at 4 °C for 10 min. The protein concentration was determined by BCA protein quantity kits (P0010S, Beyotime™, Shanghai, China). Equal amounts of supernatants (30 μg per lane) were resolved by 10% SDS-PAGE gel with 120 voltage and transferred to polyvinylidene difluoride membranes with 300 mA current. Then, the membranes were blocked in TBST solution supplemented with 5% non-fat milk for 1 h at room temperature. The blocked membranes were incubated overnight at 4 °C with primary antibodies for PINK1 (1:500 dilution), Parkin (1:1000 dilution), MMP13 (1:1000 dilution), IL-6 (1:500 dilution), TNF-α (1:500 dilution), and GAPDH (1:2000 dilution). After rinsing with TBST 3 times, membranes were incubated with HRP-linked secondary antibodies (1:5000 dilution) at room temperature for 1 h. Finally, immunoreactive bands were detected with ECL reagents after rinsing with TBST 3 times. Image J software was used for densitometric analysis.
2.8. Cellular immunofluorescence (IF) & ROS assay
For IF [2], after 24 h of treatment, the cells in confocal dishes were fixed in 4% formaldehyde for 20 min. 0.5% Triton X-100 was added to the dishes and washed with PBS three times. Then, the samples were blocked for 1 h with 3% BSA and incubated overnight with primary antibodies for COL2A1 (1:200 dilution). The cells were washed with TBST three times and incubated with Cy3-conjugated secondary antibody (1:2000 dilution) for 1 h at room temperature. Nuclei were counterstained with DAPI for 5 min at room temperature. The samples were rinsed with TBST three times in the dark and observed by a confocal microscope (Leica- LCS-SP8-STED®, Leica™, Germany). The intracellular ROS levels were measured by ROS assay kit® [35]. The cells were seeded in 6-well plates and treated with IL-1β or various concentrations of α-KG for 24h. DCFH-DA was diluted at 1:1000 with serum-free medium and added into the cells at 37 °C. Then, the samples were rinsed with a serum-free medium three times. The ROS level of samples was observed using a fluorescence microscope (EVOS fl auto, ThermoFisher Scientific™, USA). The average optical density (AOD) of 6 different fields for each sample was calculated by Image J software.
2.9. RNA extraction & real-time fluorescence quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from articular cartilage tissues/chondrocytes by TRIzol reagent [2]. Briefly, 1 ml TRIzol and 200 μl chloroform were added to the samples in 1.5 ml EP tubes. After fully mixed, the mixture was placed on ice for 10 min and centrifugated at 12000 g for 15 min. 400 μl upper liquid was transferred into a new 1.5 ml EP tube, and placed for 10 min at room temperature after the same amount of isopropanol was added and mixed. After centrifugation at 12000g for 10 min, RNA was washed with 1 ml 75% precooled ethanol twice and dissolved with free RNA enzyme water. The quality of the isolated RNA was determined by NanoDrop 2000 micronuclei acid analyzer (ThermoFisher Scientific™, USA). The A260/A280 ratio of all samples between 1.8 and 2.0 was considered as qualified samples. Then, 1 μg total RNA was synthesized into cDNA according to the kit protocol. cDNA was amplified with StepOnePlus™ Real-Time PCR System (AppliedBiosystems by ThermoFisher Scientific™, USA) using Taq Pro Universal SYBR qPCR Master Mix kits according to the manufacturer's protocol. The primer sequences are shown in Table S1. The real-time PCR conditions were as follows: 95 °C/30 s, 95 °C/10 s, and 62 °C/30 s (40 cycles). The relative mRNA expression was normalized against GAPDH using formula Y = 2-△△Ct.
2.10. RNA sequencing
The RNA of chondrocyte samples from the control group, IL-1β treated group, and 2 mM α-KG combined with IL-1β treated group was sequenced on an Illumina Novaseq platform [36], and 150 bp paired-end reads were generated. Raw reads were first processed through in-house Perl scripts. At the same time, Q20, Q30, and GC content of the clean data were calculated. All downstream analyses were based on clean data. The clean data with high quality were aligned with the reference genome using Hisat2 V2.0.5. Differential expression analysis was performed using the DESeq2 R package (1.20.0). Genes with an adjusted P ≤ 0.05 and absolute fold change of 2 were considered differentially expressed genes (DEGs). Gene Ontology (GO) and KEGG enrichment analysis of DEGs was conducted using the clusterProfiler R package.
2.11. Transmission electron microscopy (TEM)
Briefly [35], chondrocytes and one cubic millimeter of cartilage tissue from each collected specimen were fixed with 2.5% glutaraldehyde for 2 h and post-fixed in 1% osmium tetroxide for 1 h. After rinsing, dehydrating, and baking, the specimen was cut into ultrathin sections (60 nm) with LKB-Vultra microtome (Bromma™, Sweden), after which the sections were stained with uranyl acetate and lead citrate, and examined with a transmission electron microscope (H6000, Hitachi™, Tokyo, Japan).
2.12. Detection of mitochondrial and lysosomal colocalization
It is widely acknowledged that the colocalization of mitochondria and lysosomes indicates mitophagy. In experiment [35], confocal plates were used for cell culture. After human chondrocytes were treated with 10 ng/ml IL-1β and/or 2 mM α-KG and/or 50 μM Mdivi-1 for 24 h according to the experimental design. The culture medium was removed, and the samples were cultured at 37 °C for 15 min in DMEM/F-12 medium including Mito-Tracker Green (20 nM) and Lyso-Tracker Red (50 nM). Then, samples were stained with DAPI for 5–10 min followed by three rinses with PBS. Finally, the staining was photographed by a confocal microscope (LCS-SP8-STED®, Leica™, Germany). ImageJ software was used to measure the number of colocalized particles per cell, where Lyso-Tracker and Mito-Tracker were both positive. Six different visual fields were randomly selected from each group. The average of colocalized particles per cell in each visual field was counted.
2.13. Seahorse mito stress test
Seahorse mito stress test was performed according to the manufacturer's protocol. Briefly [37], chondrocytes (20000 cells/well) were plated in a Seahorse 24-well XF Cell Culture Microplate (100777-004, Agilent™, USA) and were treated with or without 10 ng/ml IL-1β and/or 2 mM α-KG for 24 h. Meanwhile, a sensor cartridge (102340-100, Seahorse XFe24 FluxPak, Agilent™, USA) was hydrated in Seahorse XF Calibrant (100840-000, Agilent™, USA) at 37 °C in a CO2-free incubator overnight. On the second day, the cell medium was exchanged for Seahorse assay medium (Seahorse XF DMEM (103680-100, Agilent™, USA) supplemented with 1 mM pyruvate (Agilent, 103578-100), 2 mM glutamine (103579-100, Agilent™, USA), and 10 mM glucose (103577-100, Agilent™, USA)) and incubated at 37 °C in a CO2-free incubator for 60 min. Oxygen consumption rate (OCR) was detected by the Seahorse XFe24 Extracellular Flux Analyzer (Agilent™, USA) with Seahorse XF Cell Mito Stress Test Kit (103015-100, Agilent™, USA) (15 μM oligomycin, 5 μM FCCP, and 5 μM rotenone/antimycin). Seahorse Wave Desktop v2.6 software was used to analyze the data.
2.14. Animals and treatments
Specific pathogen-free female Wistar rats (8 weeks old) (SCXK 2019-0004, weighing 200 ± 20 g) were obtained from Slyke Jingda Laboratory Animal™ (Hunan, China) and were raised under standard conditions (12 h light/dark cycle at 22–24 °C and 60% humidity with free access to food and water). Animal procedures and ethics were followed as described above. One week after adaptive feeding, anterior cruciate ligament transection (ACLT) was used to induce a normal mechanical loading-associated OA in the right knee [10]. The control group underwent a sham operation by opening the joint capsule and then suturing the incision. After the operation, free activity was allowed in the cage. After a week of operation, all animals were divided into the Sham group, Sham+α-KG group, ACLT group, and ACLT+α-KG group (n = 12). Consistent with the literature [26,35], rats in the Sham+α-KG group and ACLT+α-KG group were administrated with 2% α-KG in water for 8 weeks, while the remaining rats were given normal drinking water. Then, rats were anesthetized with 2% isoflurane and euthanized via cervical dislocation, followed by harvesting of right knee joints from the hind limbs. Some samples (n = 6/group) were fixed in 4% paraformaldehyde for histological analysis, and the remaining knee joint samples were stored at −80 °C until further analysis.
2.15. Safranin O-fast green staining & histological scoring
After fixing with 4% paraformaldehyde for 7 days, samples were decalcified in 20% EDTA (pH 7.4), which were replaced by the decalcification solution every three days until the tissue softened. The samples were then dehydrated before paraffin embedding. 5 μm-thick sagittal slices were prepared for Safranin O-fast green staining as described previously [2]. Briefly, after being deparaffinized to water (the specimens were sequentially placed in xylene I−xylene II−anhydrous ethanol I−anhydrous ethanol II−90% alcohol−85% alcohol−75% alcohol and then washed with water), sections were stained with Fast green (G1053-2, Servicebio™, Wuhan, China) for 5 min, followed by Safranin O (G1053-1, Servicebio™, Wuhan, China) for 5 s before being quickly dehydrated with absolute ethanol. Finally, after sealing with neutral gum, the sections were cleared with xylene for 5 min. AH-2® light microscope (Olympus™, Japan) was used to photograph. Post-staining images were used for histological scoring according to Osteoarthritis Research Society International (OARSI) scoring system by Pritzker [38]. Grade 1.0: intact chondrocytes; grade 1.5: cell death by apoptosis or necrosis; grade 2.0: the surface discontinuity consists of fibrillation; grade 2.5: abrasion of the surface with loss of a portion of the superficial cartilage zone; grade 3.0: simple fissures or clefts that penetrate the mid-zone; grade 3.5: extension of fissures to become branched or complex fissures; grade 4.0: loss of the superficial zone only (erosion); grade 4.5: absence of the mid-zone; grade 5.0 (denudation): presence of a bone surface which consists of intact calcified cartilage or sclerotic bone; grade 5.5: reparative fibrocartilaginous tissue or new bone formation; grade 6.0: deformation of the joint geometry at the joint margins; grade 6.5: both the joint margins and force bearing areas show deformation changes. OA staging was conducted based on the horizontal extent of the involved cartilage surface. Stage 1: less than 10% involvement; stage 2: 10–25% involvement; stage 3: 25–50% involvement; stage 4: more than 50%. . The final scores were calculated by two researchers independently.
2.16. Immunohistochemistry (IHC)
For IHC [2], after 5 μm-thick sagittal slices were obtained, the specimens were deparaffinized to water. Sodium citrate buffer was boiled for antigen retrieval. After blocking in BSA for 1 h, specimens were incubated with primary antibody (1:200 for COL2A1, 1:100 for MMP13, 1:1000 for PINK1, and 1:50 for Parkin) in a humidified chamber at 4 °C overnight. HRP-conjugated secondary antibody was used to detect the primary antibody for 30 min on the second day. Finally, the DAB substrate was used for coloration. Images were photographed by an AH-2® light microscope (Olympus™, Japan). The staining intensities were determined by AOD of 3 different visual fields for each sample calculated by ImageJ software (n = 6).
2.17. Statistical analysis
SPSS 20® (SPSS Science Inc™, USA) and Prism 7® (Graph Pad Software™, USA) software was employed for all analyses and graphical representations. Quantitative data were expressed by the mean ± standard deviation (S.D.), and qualitative data were presented as the median with an interquartile range. Paired Student's t-test analyzed the α-KG content in human cartilage, and comparisons between two groups were performed by two-tailed unpaired Student's t-test. One-way analysis of variance (ANOVA) was used for more than two groups, followed by post hoc multiple comparisons when homogeneity of variance and normal distribution were observed. For quantitative data with no homogeneity of variance or non-parametric distribution and qualitative data, the Kruskal-Wallis H test was used prior to pairwise comparison with the Nemenyi test. P < 0.05 was statistically significant.
3. Results
3.1. α-KG content in the human OA cartilage and IL-1β-treated primary chondrocytes and its effect on proliferation and apoptosis of chondrocytes
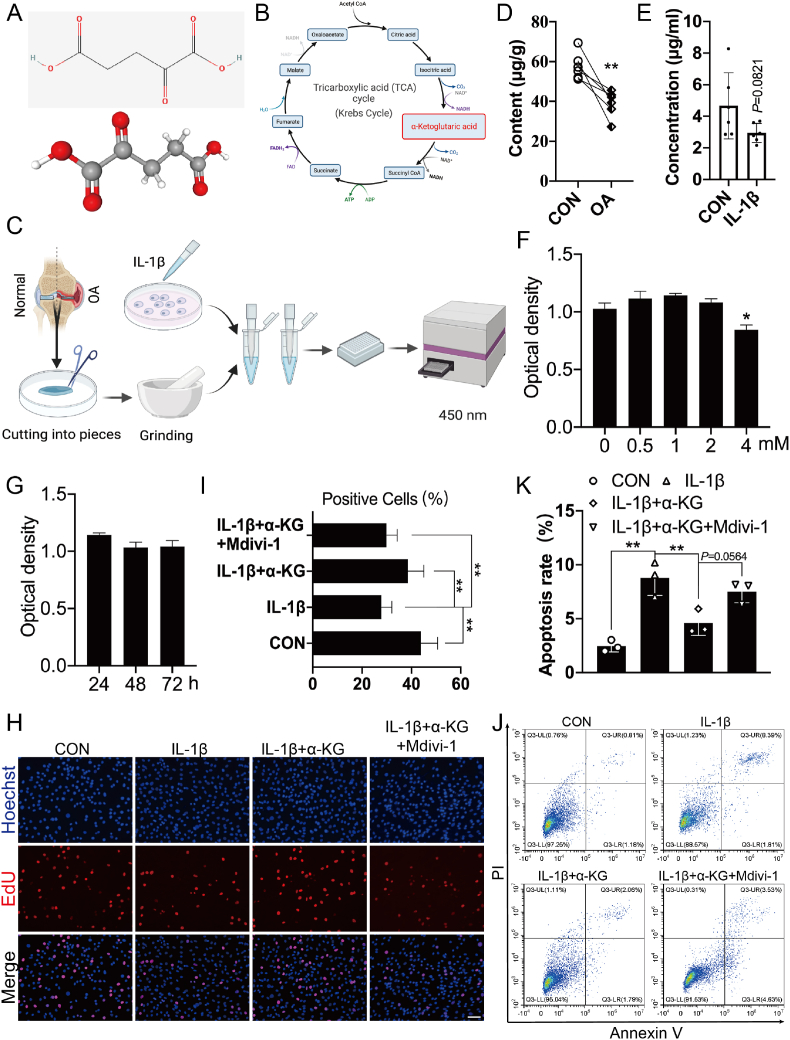
α-KG is one of the keto derivatives of glutaric acid (Fig. 1A), which comes from isocitric acid and generates succinyl CoA in TCA in physiology (Fig. 1B). Therefore, we first detected the content of α-KG in human cartilage tissue and chondrocytes induced by IL-1β (Fig. 1C). The results showed that α-KG content in the damaged medial cartilage decreased by 31.32% than in the normal lateral cartilage (P < 0.01, Fig. 1D), and by 36.85% in human chondrocytes after treatment with IL-1β for 24 h, although no significant difference was observed (P = 0.0821, Fig. 1E). Then, the influence of α-KG on primary human chondrocytes was assessed. The CCK-8 assay showed that chondrocyte viability was not significantly influenced when the α-KG concentration was less than 2 mM or when the duration was less than 72 h (P < 0.05, Fig. 1F and G). Therefore, we assessed the effect of treatment with 2 mM α-KG for 24 h on the proliferation and apoptosis of human chondrocytes induced by IL-1β. The EdU results showed that IL-1β inhibited the proliferation of primary human chondrocytes, but it could be reversed by α-KG (P < 0.01, Fig. 1H and I). Flow cytometry suggested that IL-1β increased the apoptosis of primary human chondrocytes, which could be also mitigated by α-KG to a certain extent (P < 0.01, Fig. 1J and K).
Fig. 1.
α-KG content and its effect on proliferation and apoptosis of chondrocytes induced by IL-1β. (A) The molecular structure of α-KG from PubChem database; (B) α-KG is a crucial metabolite in the tricarboxylic acid (TCA) cycle; (C) Schemes of detection of α-KG levels in the cartilage tissue and chondrocytes; The content of α-KG in the human cartilage tissue (D) and primary chondrocytes treated by IL-1β for 24 h (E), n = 6; (F–G) CCK-8 assay of chondrocyte activity treated with different concentrations of α-KG (for 24 h) and time (at 2 mM), n = 10. (H–I) EdU assay was applied to compare the cell proliferation ability, scale bar = 500 μm, n = 6. (J) Flow cytometry was applied to detect apoptosis of chondrocytes; (K) The statistical analysis of apoptosis rates in IL-1β-induced human chondrocytes after treatment with α-KG, n = 3. Values are expressed as the means ± S.D. *P < 0.05, **P < 0.01 vs. the corresponding control.
3.2. Effects of α-KG on the osteoarthritic phenotype of chondrocytes induced by IL-1β
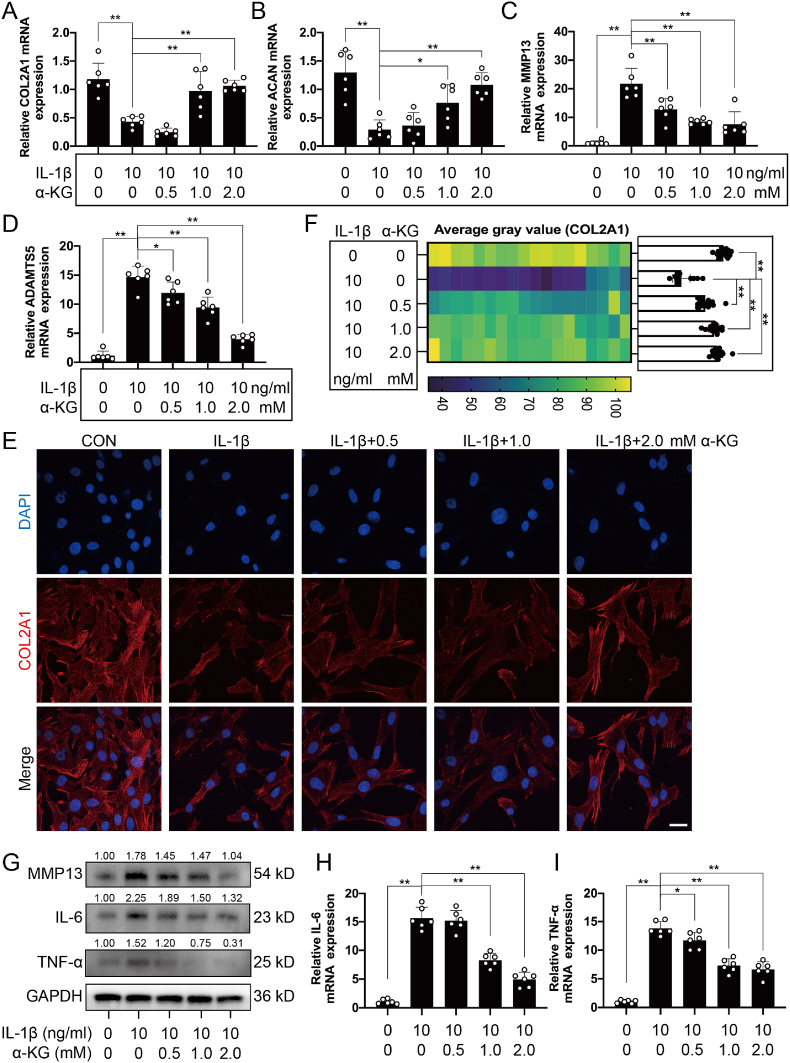
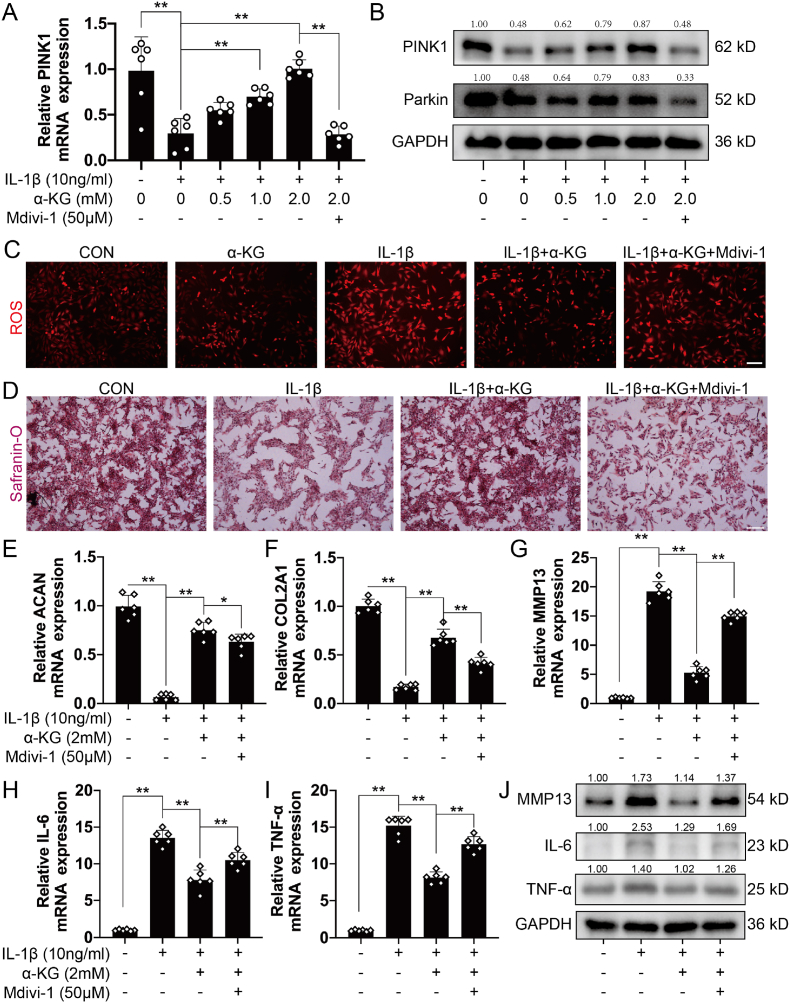
In addition to chondrocytes, the extracellular matrix (ECM) is another major component of cartilage tissue [32]. Therefore, RT-qPCR was used to detect the transcriptomic expression of genes related to matrix synthesis (ACAN and COL2A1) and degradation (MMP13 and ADAMTS5). The results demonstrated that α-KG promoted mRNA expression of ACAN and COL2A1 and reduced the expression of MMP13 and ADAMTS5 in a concentration-dependent manner in osteoarthritic chondrocytes induced by IL-1β (P < 0.05, P < 0.01, Fig. 2A–D). IF staining and western blot also showed that α-KG promoted protein expression of COL2A1 but inhibited MMP13 protein expression in a concentration-dependent manner in human chondrocytes induced by IL-1β (P < 0.01, Fig. 2E–G, S1A). We further assayed the effect of α-KG on inflammatory mediators. The RT-qPCR and western blot results showed that IL-1β increased the transcriptomic and protein expression levels of IL-6 and TNF-α, but α-KG decreased the transcriptomic and protein expression levels of IL-6 and TNF-α in a concentration-dependent manner in human chondrocytes induced by IL-1β (P < 0.05, P < 0.01, Fig. 2G–I, S1B, C).
Fig. 2.
The effect of α-KG on matrix synthesis, degradation and inflammation in human chondrocytes induced by IL-1β. (A–D) The mRNA expression of COL2A1, ACAN, MMP13, and ADAMTS5 were determined through RT-qPCR, n = 6; (E) The expression of COLA21 protein detected by IF assay, scale bar = 25 μm, n = 3; (F) Semi-quantitative IF assay of COLA21; (G) Western blot was used to determine protein expression of MMP13, IL-6, and TNF-α, n = 3; (H–I) RT-qPCR was used to determine the mRNA expression of IL-6 and TNF-α, n = 6. Values are expressed as the means ± S.D. *P < 0.05, **P < 0.01 vs. the corresponding control.
3.3. Influences of α-KG on mitophagy and ROS generation in osteoarthritic chondrocytes
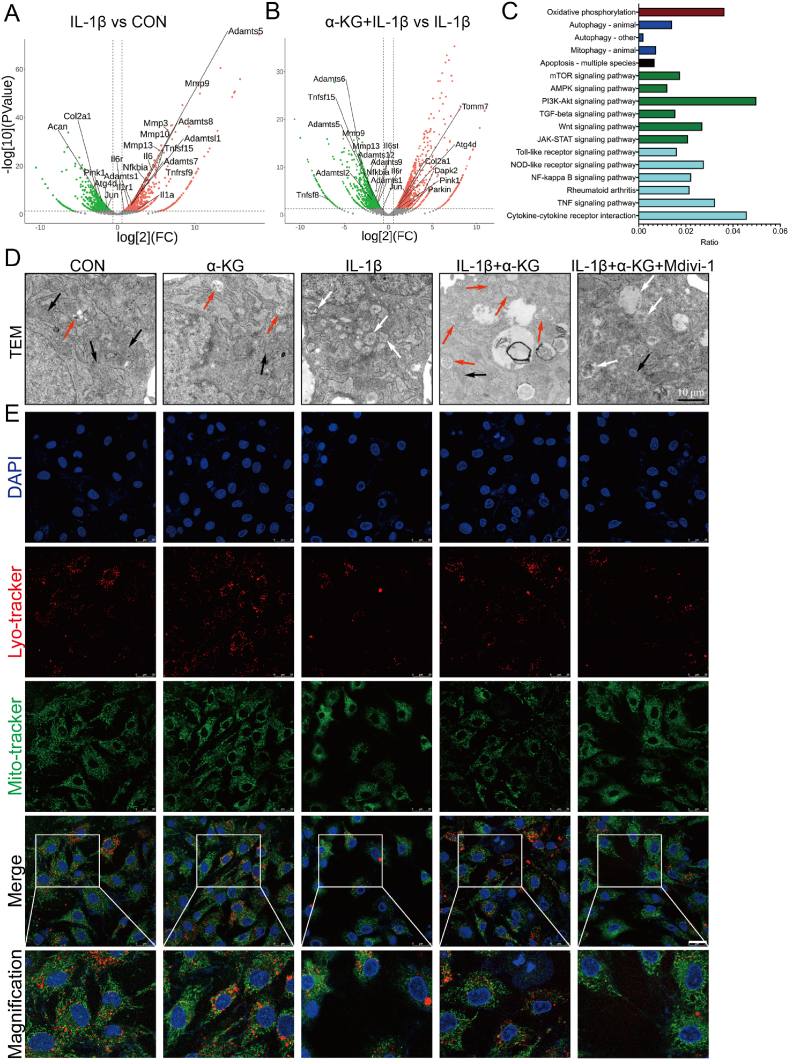
To explore the mechanism of α-KG in alleviating osteoarthritic phenotype, RNA sequencing was performed. The sequencing results suggested that compared with the control group, IL-1β treatment reduced the transcriptomic expression of genes related to matrix synthesis (ACAN and COL2A1) and upregulated the expression of genes related to matrix degradation (such as MMP3, MMP9, MMP10, MMP13, ADAMTS1, ADAMTSL1, ADAMTS5, ADAMTS7, and ADAMTS8) and inflammation mediators (such as IL-1α, IL-1R, IL-6, IL-6R, NFKBIA, TNFRSF9, and TNFRSF15) (Fig. 3A). However, compared with treatment IL-1β alone, α-KG combined with IL-1β upregulated genes related to matrix synthesis (COL2A1) and downregulated the expression for genes related to matrix degradation (such as MMP9, MMP13, ADAMTS1, ADAMTSL2, ADAMTS5, ADAMTS6, ADAMTS9, and ADAMTS12) and inflammation mediators (IL-6st, IL-6R, NFKBIA, TNFSF8, and TNFSF15) (Fig. 3B). Interestingly, changes in the expression of genes related to mitophagy were consistent with the expression of genes related to matrix synthesis, degradation, and inflammation mediators. IL-1β decreased the transcriptomic expression of PINK1, ATG4D, and JUN, while α-KG increased the expression of PINK1, Parkin, ATG4D, JUN, TOMM7, and DAPK2 (Fig. 3A and B). KEGG analysis also showed changes in pathways related to inflammation, proliferation, apoptosis, oxidative phosphorylation, and mitophagy (Fig. 3C).
Fig. 3.
The effect of α-KG on mitophagy of human chondrocytes induced by IL-1β. (A–B) Volcano plot showing changes in RNA expression; (C) KEGG analysis of RNA sequencing after α-KG treatment in IL-1β induced chondrocytes; (D) TEM was used to assay the mitophagy before and after α-KG treatment in IL-1β induced chondrocytes, and normal (black arrow), swelling (white arrow), and autophagic (red arrow) mitochondria were as indicated; (E) Colocalization of mitochondria and lysosomes in chondrocytes, scale bar = 25 μm, n = 3. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Therefore, TEM was further used to assay mitophagy. The results showed that the control mitochondria exhibited normal, regular and complete morphology, while swelling, vacuolization, a reduced density, and an incomplete structure were observed in IL-1β-induced chondrocytes (Fig. 3D). α-KG addition improved the mitochondrial structure and promoted mitophagy, thereby increasing the clearance of damaged mitochondria in IL-1β-induced chondrocytes (Fig. 3D). Mitochondrial and lysosomal colocalization by IF also showed α-KG increased their interaction in human chondrocytes induced by IL-1β (P < 0.05, P < 0.01, Fig. 3E, S2A). Bafilomycin A1, which prevents the late stage of autophagic flux by inhibiting lysosomal acidification [39], was further used to assay the mitophagy flux. The results showed that α-KG significantly increased mitophagy flux in IL-1β-induced osteoarthritic chondrocytes (P < 0.01, Figs. S2B and C). RT-qPCR and western blot also confirmed that α-KG upregulated the expression of genes related to mitophagy markers (PINK1 and Parkin) (P < 0.05, P < 0.01, Fig. 4A, B, S2D, E). Finally, mitochondrial function was assayed. The results showed α-KG treatment significantly improved the basal, maximal, ATP-linked mitochondrial respiration and spare respiratory capacity in IL-1β-induced chondrocytes (P < 0.05, P < 0.01, Figs. S3A–F). Meanwhile, ROS generation in human chondrocytes induced by IL-1β was also decreased by α-KG (P < 0.05, P < 0.01, Fig. 4C, S4A). These results suggested that α-KG increased mitophagy, improved mitochondrial function, and inhibited ROS generation in osteoarthritic chondrocytes.
Fig. 4.
The effect of Mdivi-1 on α-KG activity in human chondrocytes. (A) RT-qPCR was used to determine the mRNA expression of PINK1, n = 6; (B) Western blot was used to determine protein expression of PINK1 and Parkin, n = 3; (C) ROS levels in human chondrocytes, scale bar = 500 μm, n = 3; (D) Safranin O staining assayed the matrix content in chondrocytes, scale bar = 500 μm, n = 3; (E–I) The mRNA expression of ACAN, COL2A1, MMP13, IL-6, and TNF-α were determined through RT-qPCR, n = 6; (J) The protein expression of MMP13, IL-6, and TNF-α were determined by western blot, n = 3. Values are expressed as means ± S.D. *P < 0.05, **P < 0.01 vs. the corresponding control. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Influences of Mdivi-1 on α-KG activity in chondrocytes
It is well-established that Mdivi-1 is an inhibitor of mitophagy [40,41]. To further explore the role of mitophagy in mediating the alleviating effect of α-KG on the IL-1β-induced osteoarthritic phenotype of human chondrocytes, Mdivi-1 was used to treat IL-1β-induced chondrocytes combined with α-KG. TEM and IF results showed that treatment with α-KG and Mdivi-1 inhibited mitochondrial and lysosomal colocalization and mitochondrial morphology improvement, and increased ROS generation (P < 0.05, P < 0.01, Fig. 3, Fig. 4C, S2A, S4A). Combining α-KG and Mdivi-1 also decreased the transcriptomic and proteinic expression of PINK1 and Parkin (P < 0.05, P < 0.01, Fig. 3, Fig. 4A, B, S2D, E). These results indicated that Mdivi-1 reversed the effect of α-KG on mitophagy.
EdU and flow cytometry assays were then used to determine the proliferation and apoptosis of chondrocytes, which suggested that Mdivi-1 canceled the effect of α-KG on proliferation promotion and apoptosis inhibition (with an increased tendency) (P = 0.0564, P < 0.01, Fig. 1H–K). Safranin O staining exhibited enhanced staining following α-KG supplementation, which was suppressed by Mdivi-1 (P < 0.01, Fig. 4D, S4B). RT-qPCR showed that Mdivi-1 reversed the increased transcriptomic expression of genes related to matrix synthesis (ACAN and COL2A1) (P < 0.05, P < 0.01, Fig. 4E and F), the decreased expression of genes related to matrix degradation (MMP13 and ADAMTS5) (P < 0.05, P < 0.01, Fig. 4G, S4C), and the inflammatory mediators (IL-6 and TNF-α) (P < 0.01, Fig. 4H and I) induced by α-KG in IL-1β-treated human chondrocytes. Western blot and IF further confirmed that Mdivi-1 suppressed the increased protein expression of COL2A1 and the decreased expression of MMP13, IL-6, and TNF-α (P = 0.0559, P < 0.05, P < 0.01, Fig. 4J, S4D-H).
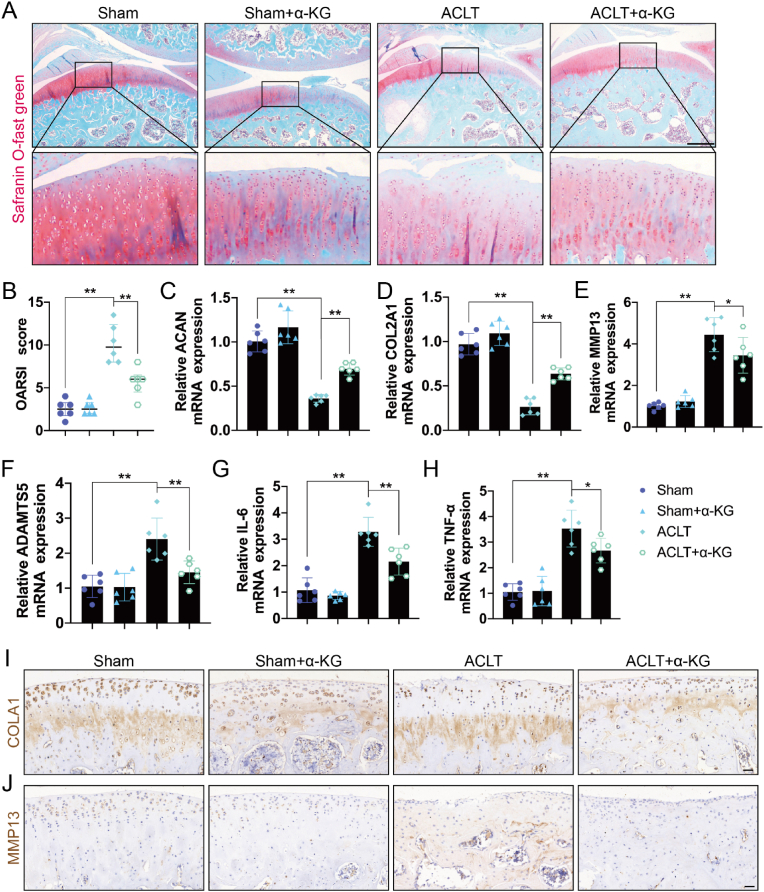
3.5. Effects of α-KG supplementation on OA phenotype in rats
To further evaluate the effect of α-KG, a rat OA model was established by ACLT. Safranin O-fast green staining revealed that ACLT caused loss of staining, interrupted or disappeared tidemark, cartilage fibrillation, cartilage fissures and erosion, thinner cartilage, and increased OARSI scores, which were restored by α-KG (P < 0.01, Fig. 5A and B). RT-qPCR showed that ACLT downregulated the mRNA expression levels of genes related to cartilage matrix synthesis (ACAN and COL2A1) and upregulated the mRNA expression levels of genes related to matrix degradation (MMP13 and ADAMTS5) and inflammation mediators (IL-6 and TNF-α) compared with the control group, but these were reversed in the ACLT+α-KG treatment group (P < 0.05, P < 0.01, Fig. 5C–H). IHC further demonstrated that α-KG reversed the decreased protein expression of COL2A1 and the increased MMP13 levels initially induced by ACLT (P < 0.01, Fig. 5I, J, S5A, B).
Fig. 5.
The effect of α-KG supplementation on ACLT-induced rat OA model. (A) Safranin O - Fast Green staining of knee cartilage after treatment with α-KG in ACLT induced rat model, scale bar = 1000 μm; (B) OARSI scores of safranin O - Fast Green staining of knee cartilage, and data are presented as median with interquartile range; (C–H) The mRNA expression of ACAN, COL2A1, MMP13, ADAMTS5, IL-6, and TNF-α were determined by RT-qPCR; (I–J) The protein expression of COL2A1 and MMP13 was detected by IHC, scar bar = 40 μm. Values are expressed as means ± S.D., n = 6. *P < 0.05, **P < 0.01 vs. the corresponding control. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
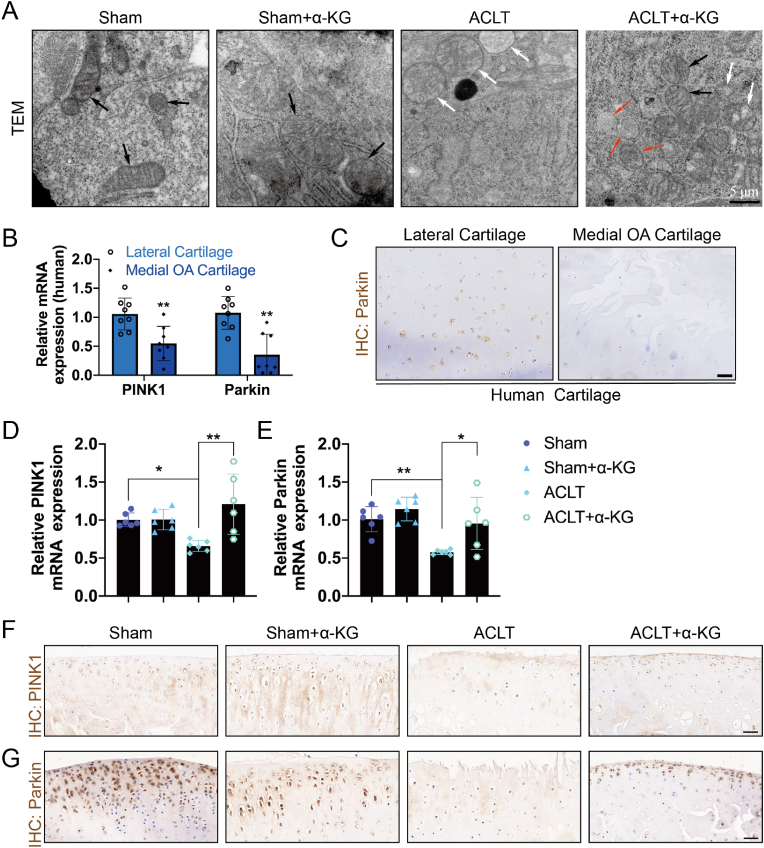
3.6. Influences of α-KG supplementation on mitophagy in cartilage tissue
Finally, mitophagy was assayed in cartilage tissue. TEM showed that ACLT caused swelling, vacuolization, and an incomplete structure of mitochondria, while mitochondria with normal regular and complete morphology were observed in the ACLT+α-KG group (Fig. 6A). RT-qPCR and IHC confirmed that transcriptomic expression of PINK1 and Parkin and protein levels of Parkin decreased in human OA cartilage tissue (P < 0.05, P < 0.01, Fig. 6B, C, S5C). Consistently, the transcriptomic and proteinic expression of PINK1 and Parkin was also decreased in ACLT-induced rat OA cartilage tissue (P < 0.05, P < 0.01, Fig. 6D–G, S5D-E). However, these were reversed by α-KG administration (P < 0.05, P < 0.01, Fig. 6D–G, S5D-E). These results indicated that mitophagy levels decreased in OA, and α-KG administration promoted mitophagy in rat cartilage tissue of the ACLT-induced rat OA model.
Fig. 6.
The mitophagy changes in human OA cartilage and ACLT-induced rat OA model treated with or without α-KG supplementation. (A) TEM was used to assay changes in the mitophagy before and after α-KG supplementation and normal (black arrow), swelling (white arrow), and autophagic (red arrow) mitochondria were as indicated, scale bar = 5 μm; (B) RT-qPCR was used to determine mRNA expression of PINK1 and Parkin in human cartilage, n = 8; (C) IHC was used to assay changes in protein expression of Parkin of human OA cartilage tissue, scale bar = 200 μm, n = 3; (D–E) RT-qPCR was used to determine mRNA expression of PINK1 and Parkin, n = 6; IHC was used to assay changes in protein expression of PINK1 (F) and Parkin (G) of ACLT-induced rat OA cartilage tissue before and after α-KG supplementation, scale bar = 40 μm, n = 6. Values are expressed as means ± S.D. *P < 0.05, **P < 0.01 vs. the corresponding control. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
OA is a common joint disease that predominantly affects the middle-aged and elderly. An increasing body of evidence suggests that OA is a metabolic disease [[42], [43], [44]]. Various changes in metabolites are involved in OA. Epidemiological and experimental studies have indicated that the higher prevalence of OA in women than men might be attributed to the decline of estrogen after menopause since estrogen can promote the production of proteoglycans by chondrocytes, and inhibit the production of dangerous mediators, such as ROS, inducible nitric oxide synthetase (iNOS) and nuclear factor-κB (NF-κB) [42,45]. In addition, with increasing age, chondrocyte proliferation decreased, and mitochondrial senescence and dysfunction emerged, thereby increasing ROS production and the levels of fatty acids, glucose, and inflammation mediators and, eventually, cartilage damage [46]. α-KG is an essential metabolite of the mitochondrial TCA cycle that can regulate mitochondrial metabolism, antioxidation, and anti-inflammation [[14], [15], [16], [17]], and its serum levels gradually decrease with age [30]. Accordingly, in the present study, we first quantified α-KG content in cartilage. The results showed that α-KG content in damaged medial cartilage was 31.32% lower than in normal lateral cartilage. Haseeb et al. found that IL-1β and TNF-α could alter the activity and expression of isocitrate dehydrogenases in human chondrocytes, which was related to decreased α-KG levels [47]. Therefore, we detected the concentration of α-KG in human OA-like chondrocytes induced by IL-1β. Consistent with the findings reported by Haseeb et al., α-KG was reduced by 36.85% in human OA-like chondrocytes induced by IL-1β, although no significant difference was observed (P = 0.0821). These results suggest that α-KG may be involved in the development of OA.
It is well-established that α-KG is a weak acid, which limits its permeability through the cell membrane [48]. Moreover, in rats, around 80% of dietary α-KG is rapidly removed from the bloodstream by oral administration [49]. The remaining α-KG enters the liver and kidney and is converted to proline, leucine, and other amino acids, which results in no significant change in the blood α-KG levels [50]. Therefore, DM-α-KG, a derivative of α-KG, was selected for in vivo and in vitro experiments in this study, which exhibits better absorption and readily diffuses into cells to be converted to α-KG absolutely by cellular esterases [51,52].
Articular cartilage is an avascular tissue consisting of chondrocytes and an extracellular matrix [42]. With the development and progression of OA, chondrocyte proliferation and extracellular matrix synthesis gradually decrease, while chondrocyte apoptosis and matrix degradation increase [53]. Previous studies showed that α-KG promoted the proliferation of Indian Capra hircus fibroblasts and chondrocytes in vitro [54]. In the present study, 2 mM α-KG could reverse IL-1β-induced proliferation inhibition and apoptosis in primary human chondrocytes. Furthermore, α-KG increased the mRNA and protein expression of genes related to matrix synthesis (ACAN and COL2A1) but inhibited the expression of genes related to matrix degradation (MMP13 and ADAMTS5) in a concentration-dependent manner in IL-1β-induced human OA-like chondrocytes. In a rat model of ACLT-induced OA, α-KG could also significantly improve cartilage quality, increase cartilage matrix synthesis (expression of ACAN and COL2A1), and inhibit its degradation (expression of MMP13 and ADAMTS5). In addition, chronic low-grade inflammation has been also documented as a typical and crucial pathology of OA [55]. Multiple inflammatory mediators, such as IL-6 and TNF-α, were increased in OA cartilage and IL-1β-induced OA-like chondrocytes [55]. In the present study, animal and cellular studies showed that α-KG reduced the expression of IL-6 and TNF-α, which were validated by RNA sequencing, consistent with the results found by Asadi Shahmirzadi et al., who reported that α-KG reduced multiple inflammatory mediators in aged mice, including IL-6 and TNF-α [26]. Overall, the present study found that α-KG supplementation promoted cartilage metabolic homeostasis and inhibited chronic low-grade inflammatory response, thereby alleviating OA.
Mitochondrial metabolic imbalance and dysfunction is a hallmark of OA [56]. Mitophagy maintains mitochondrial homeostasis and function via eliminating impaired organelles and unwanted proteins and reducing cellular stress caused by harmful stimuli [57]. Therefore, the basal level of mitophagy plays an important role in cartilage. Impaired mitophagy was implicated in various diseases, and promoting mitophagy yielded promising effects on these diseases, including cardiovascular disease [58], synapse defects [59], intervertebral disc degeneration (IVDD) and OA [60]. When mitochondria are damaged, PINK1 accumulates rapidly on mitochondrial membranes and recruits Parkin from the cytoplasm, thus inducing the degradation of damaged mitochondria [61]. RNA sequencing and experimental results in this study showed that α-KG changed the proliferation, apoptosis, inflammation and autophagy-related pathways in osteoarthritic chondrocytes induced by IL-1β. Moreover, RNA sequencing indicated that gene expression related to mitophagy exhibited changes consistent with genes related to chondrocyte matrix synthesis, degradation, and inflammation mediators. In osteoarthritic chondrocytes induced by IL-1β and ACLT-induced rat OA cartilage tissue, the expression of PINK1 and Parkin was decreased, and swelling, vacuolation, reduced density, and incomplete structure mitochondrial damage were observed. Moreover, α-KG supplementation reversed the above changes and promoted the mitophagy flux. Concurrently, mitochondrial function assay also showed α-KG significantly improved mitochondrial respiration damaged by IL-1β by increasing the basal, maximal, ATP-linked mitochondrial respiration and spare respiratory capacity, which is in line with previous findings that the activation of mitophagy can increase the mitochondrial health and respiratory function in OA [37,62]. These results suggest that α-KG increases the mitophagy of ACLT-induced OA rat cartilage tissue and osteoarthritic chondrocytes induced by IL-1β and improves the mitochondrial function.
Mitochondrial damage increases ROS production, which in turn further leads to mitochondrial damage and dysfunction, thus forming a “vicious circle” [35], which has been implicated in the development of OA [11]. Increased mitophagy is often accompanied by reduced oxidative stress [34,35]. Moreover, it has been reported that chronic low-grade inflammation and oxidative stress are the last common pathway of OA [11]. Based on the above findings, we further detected ROS levels. The results showed that IL-1β increased ROS production in human chondrocytes, while α-KG increased ROS scavenging, which is consistent with the findings of An et al. [35] and Faulkner et al. [63] that α-KG reduced ROS production in mouse myocardium induced by transverse aortic constriction (TAC) and muscle pericytes. Faulkner et al. further revealed that the beneficial effect of α-KG on ROS status was unlikely to be due to inducing antioxidant defense systems [63]. To further explore whether ROS changes are caused by mitophagy, IL-1β-induced osteoarthritic chondrocytes were co-treated with α-KG and Mdivi-1, a mitophagy inhibitor [40,41]. The results showed that Mdivi-1 eliminated the effects of α-KG on mitophagy and ROS production. Moreover, Mdivi-1 suppressed the effects of α-KG on anti-inflammation, antioxidation (ROS production), and maintenance of chondrocyte homeostasis (the chondrocyte proliferation and apoptosis and extracellular matrix synthesis and degradation). Taken together, the above findings indicate that α-KG alleviates OA by promoting mitophagy and inhibiting ROS production.
5. Conclusions
Overall, this study confirmed that the content of α-KG, a physiological metabolite from the mitochondrial TCA cycle, is decreased in human OA cartilage and IL-1β-induced osteoarthritic chondrocytes. Moreover, α-KG supplementation can promote chondrocyte proliferation and apoptosis inhibition and reverse the decreased synthesis and increased degradation of extracellular matrix and inflammatory reaction in vivo and in vitro, mediated by the ability of α-KG to promote mitophagy in chondrocytes and cartilage tissue and reduce oxidative stress. Besides, Mdivi-1, a mitophagy inhibitor, can suppress the effect of α-KG. In a nutshell, our findings provided hitherto undocumented evidence of the role of α-KG in maintaining cartilage homeostasis, suggesting it can be a potential therapeutic target and medication for OA.
Declaration of competing interest
All authors declare no conflicts of interest.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (No. 82260444), the Hunan Provincial Department of Education Project (NO.20A300), the Scientific Research Project of Hunan Health Commission (NO. B2019149 and C2019141), and the Natural Science Foundation of Jishou University (NO. JDlc1904 and NO. JDlc2001).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102663.
Contributor Information
Jun Zhao, Email: zhaojunhnjs@163.com.
Huan Geng, Email: gengh1989@163.com.
Chi Ma, Email: machiwhu@whu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2.Liu L., Li B., Li Q., et al. Transforming growth factor-β receptor 1: an intervention target for genetic poor cartilage quality induced by prenatal dexamethasone exposure. J. Adv. Res. 2022 doi: 10.1016/j.jare.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England) 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.March L., Smith E.U., Hoy D.G., et al. Burden of disability due to musculoskeletal (MSK) disorders. Best Pract. Res. Clin. Rheumatol. 2014;28(3):353–366. doi: 10.1016/j.berh.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Safiri S., Kolahi A.A., Smith E., et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020;79(6):819–828. doi: 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
- 6.Glyn-Jones S., Palmer A.J., Agricola R., et al. Osteoarthritis. Lancet (London, England) 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 7.Hui W., Young D.A., Rowan A.D., Xu X., Cawston T.E., Proctor C.J. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann. Rheum. Dis. 2016;75(2):449–458. doi: 10.1136/annrheumdis-2014-206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodgkinson T., Kelly D.C., Curtin C.M., O'Brien F.J. Mechanosignalling in cartilage: an emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol. 2022;18(2):67–84. doi: 10.1038/s41584-021-00724-w. [DOI] [PubMed] [Google Scholar]
- 9.Kawata M., Teramura T., Ordoukhanian P., et al. Krüppel-like factor-4 and Krüppel-like factor-2 are important regulators of joint tissue cells and protect against tissue destruction and inflammation in osteoarthritis. Ann. Rheum. Dis. 2022;81(8):1179–1188. doi: 10.1136/annrheumdis-2021-221867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang G., Chen S., Xie Z., et al. TGFβ attenuates cartilage extracellular matrix degradation via enhancing FBXO6-mediated MMP14 ubiquitination. Ann. Rheum. Dis. 2020;79(8):1111–1120. doi: 10.1136/annrheumdis-2019-216911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco F.J., Valdes A.M., Rego-Pérez I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat. Rev. Rheumatol. 2018;14(6):327–340. doi: 10.1038/s41584-018-0001-0. [DOI] [PubMed] [Google Scholar]
- 12.Scott J.L., Gabrielides C., Davidson R.K., et al. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis. 2010;69(8):1502–1510. doi: 10.1136/ard.2009.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi W., Fang F., Kong Y., et al. Dynamic hyaluronic acid hydrogel with covalent linked gelatin as an anti-oxidative bioink for cartilage tissue engineering. Biofabrication. 2021;14(1) doi: 10.1088/1758-5090/ac42de. [DOI] [PubMed] [Google Scholar]
- 14.Averina O.A., Permyakov O.A., Emelianova M.A., et al. Mitochondrial peptide Mtln contributes to oxidative metabolism in mice. Biochimie. 2022;204:136–139. doi: 10.1016/j.biochi.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Wei P., Bott A.J., Cluntun A.A., et al. Mitochondrial pyruvate supports lymphoma proliferation by fueling a glutamate pyruvate transaminase 2-dependent glutaminolysis pathway. Sci. Adv. 2022;8(39) doi: 10.1126/sciadv.abq0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhary V., Ah Kioon M.D., Hwang S.M., et al. Chronic activation of pDCs in autoimmunity is linked to dysregulated ER stress and metabolic responses. J. Exp. Med. 2022;219(11) doi: 10.1084/jem.20221085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L.Y., Lyu Y.Y., Zhang H.Y., et al. Nuclear receptor NR1D1 regulates abdominal aortic aneurysm development by targeting the mitochondrial tricarboxylic acid cycle enzyme aconitase-2. Circulation. 2022;146(21):1591–1609. doi: 10.1161/CIRCULATIONAHA.121.057623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan Y., Zhu C., Wang Y., et al. α-Ketoglutaric acid ameliorates hyperglycemia in diabetes by inhibiting hepatic gluconeogenesis via serpina1e signaling. Sci. Adv. 2022;8(18) doi: 10.1126/sciadv.abn2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L., Venneti S., Nagrath D. Glutaminolysis: a hallmark of cancer metabolism. Annu. Rev. Biomed. Eng. 2017;19:163–194. doi: 10.1146/annurev-bioeng-071516-044546. [DOI] [PubMed] [Google Scholar]
- 20.Chang L.C., Chiang S.K., Chen S.E., Hung M.C. Targeting 2-oxoglutarate dehydrogenase for cancer treatment. Am J Cancer Res. 2022;12(4):1436–1455. [PMC free article] [PubMed] [Google Scholar]
- 21.Bott M. Offering surprises: TCA cycle regulation in Corynebacterium glutamicum. Trends Microbiol. 2007;15(9):417–425. doi: 10.1016/j.tim.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B., Peng H., Zhou M., et al. Targeting BCAT1 combined with α-ketoglutarate triggers metabolic synthetic lethality in glioblastoma. Cancer Res. 2022;82(13):2388–2402. doi: 10.1158/0008-5472.CAN-21-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y., He P., Hou Y., Liu Z., Zhang X., Li N. Osmundacetone modulates mitochondrial metabolism in non-small cell lung cancer cells by hijacking the glutamine/glutamate/α-KG metabolic axis. Phytomedicine. 2022;100 doi: 10.1016/j.phymed.2022.154075. [DOI] [PubMed] [Google Scholar]
- 24.Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003;22(1):15–24. doi: 10.1016/s0945-053x(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 25.He L., Wu J., Tang W., et al. Prevention of oxidative stress by α-ketoglutarate via activation of CAR signaling and modulation of the expression of key antioxidant-associated targets in vivo and in vitro. J. Agric. Food Chem. 2018;66(43):11273–11283. doi: 10.1021/acs.jafc.8b04470. [DOI] [PubMed] [Google Scholar]
- 26.Asadi Shahmirzadi A., Edgar D., Liao C.Y., et al. Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metabol. 2020;32(3):447–456 e446. doi: 10.1016/j.cmet.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.TeSlaa T., Chaikovsky A.C., Lipchina I., et al. α-Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metabol. 2016;24(3):485–493. doi: 10.1016/j.cmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsukada Y., Fang J., Erdjument-Bromage H., et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439(7078):811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 29.Morris JPt, Yashinskie J.J., Koche R., et al. α-Ketoglutarate links p53 to cell fate during tumour suppression. Nature. 2019;573(7775):595–599. doi: 10.1038/s41586-019-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian Q., Zhao J., Yang Q., et al. Dietary alpha-ketoglutarate promotes beige adipogenesis and prevents obesity in middle-aged mice. Aging Cell. 2020;19(1) doi: 10.1111/acel.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.P., Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 32.Ohzono H., Hu Y., Nagira K., et al. Targeting FoxO transcription factors with HDAC inhibitors for the treatment of osteoarthritis. Ann. Rheum. Dis. 82(2) 2022:262–271. doi: 10.1136/ard-2021-221269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L., Geng H., Mei C., Chen L. Zoledronic acid enhanced the antitumor effect of cisplatin on orthotopic osteosarcoma by ROS-PI3K/AKT signaling and attenuated osteolysis. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/6661534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Q., Li S., Jiang N., et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019;26 doi: 10.1016/j.redox.2019.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An D., Zeng Q., Zhang P., et al. Alpha-ketoglutarate ameliorates pressure overload-induced chronic cardiac dysfunction in mice. Redox Biol. 2021;46 doi: 10.1016/j.redox.2021.102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orzechowska K., Kopij G., Paukszto L., et al. Chemerin effect on transcriptome of the porcine endometrium during implantation determined by RNA-sequencing. Biol. Reprod. 2022;107(2):557–573. doi: 10.1093/biolre/ioac063. [DOI] [PubMed] [Google Scholar]
- 37.D'Amico D., Olmer M., Fouassier A.M., et al. Urolithin A improves mitochondrial health, reduces cartilage degeneration, and alleviates pain in osteoarthritis. Aging Cell. 2022;21(8) doi: 10.1111/acel.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pritzker K.P., Gay S., Jimenez S.A., et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Huang T., Xu T., Wang Y., et al. Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy. 2021;17(11):3592–3606. doi: 10.1080/15548627.2021.1885203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizumura K., Cloonan S.M., Nakahira K., et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Invest. 2014;124(9):3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Y., Ge X., Chen Y., et al. Mycobacterium bovis induces mitophagy to suppress host xenophagy for its intracellular survival. Autophagy. 2022;18(6):1401–1415. doi: 10.1080/15548627.2021.1987671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bortoluzzi A., Furini F., Scirè C.A. Osteoarthritis and its management - epidemiology, nutritional aspects and environmental factors. Autoimmun. Rev. 2018;17(11):1097–1104. doi: 10.1016/j.autrev.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Courties A., Sellam J., Berenbaum F. Metabolic syndrome-associated osteoarthritis. Curr. Opin. Rheumatol. 2017;29(2):214–222. doi: 10.1097/BOR.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 44.Tomi A.L., Sellam J., Lacombe K., et al. Increased prevalence and severity of radiographic hand osteoarthritis in patients with HIV-1 infection associated with metabolic syndrome: data from the cross-sectional METAFIB-OA study. Ann. Rheum. Dis. 2016;75(12):2101–2107. doi: 10.1136/annrheumdis-2016-209262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roman-Blas J.A., Castañeda S., Largo R., Herrero-Beaumont G. Osteoarthritis associated with estrogen deficiency. Arthritis Res. Ther. 2009;11(5):241. doi: 10.1186/ar2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loeser R.F., Collins J.A., Diekman B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016;12(7):412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haseeb A., Makki M.S., Haqqi T.M. Modulation of ten-eleven translocation 1 (TET1), Isocitrate Dehydrogenase (IDH) expression, α-Ketoglutarate (α-KG), and DNA hydroxymethylation levels by interleukin-1β in primary human chondrocytes. J. Biol. Chem. 2014;289(10):6877–6885. doi: 10.1074/jbc.M113.512269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miedema H., Felle H., Prins H.B. Effect of high pH on the plasma membrane potential and conductance in Elodea densa. J. Membr. Biol. 1992;128(1):63–69. doi: 10.1007/BF00231871. [DOI] [PubMed] [Google Scholar]
- 49.Filip R., Pierzynowski S.G. The absorption, tissue distribution and excretion of enteraly administered alpha-ketoglutarate in rats. J. Anim. Physiol. Anim. Nutr. 2008;92(2):182–189. doi: 10.1111/j.1439-0396.2007.00725.x. [DOI] [PubMed] [Google Scholar]
- 50.Gyanwali B., Lim Z.X., Soh J., et al. Alpha-Ketoglutarate dietary supplementation to improve health in humans. Trends Endocrinol. Metabol. 2022;33(2):136–146. doi: 10.1016/j.tem.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Liu P.S., Wang H., Li X., et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017;18(9):985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 52.Mariño G., Pietrocola F., Kong Y., et al. Dimethyl α-ketoglutarate inhibits maladaptive autophagy in pressure overload-induced cardiomyopathy. Autophagy. 2014;10(5):930–932. doi: 10.4161/auto.28235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal P., Lee H.P., Smeriglio P., et al. A dysfunctional TRPV4-GSK3β pathway prevents osteoarthritic chondrocytes from sensing changes in extracellular matrix viscoelasticity. Nat Biomed Eng. 2021;5(12):1472–1484. doi: 10.1038/s41551-021-00691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh D., Vishnoi T., Kumar A. Effect of alpha-ketoglutarate on growth and metabolism of cells cultured on three-dimensional cryogel matrix. Int. J. Biol. Sci. 2013;9(5):521–530. doi: 10.7150/ijbs.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson W.H., Lepus C.M., Wang Q., et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016;12(10):580–592. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D'Amico D., Olmer M., Fouassier A.M., et al. Urolithin A improves mitochondrial health, reduces cartilage degeneration, and alleviates pain in osteoarthritis. Aging Cell. 2022;21(8) doi: 10.1111/acel.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu S., Zhang C., Ni L., et al. Stabilization of HIF-1α alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis. 2020;11(6):481. doi: 10.1038/s41419-020-2680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bravo-San Pedro J.M., Kroemer G., Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ. Res. 2017;120(11):1812–1824. doi: 10.1161/CIRCRESAHA.117.311082. [DOI] [PubMed] [Google Scholar]
- 59.Choi G.E., Lee H.J., Chae C.W., et al. BNIP3L/NIX-mediated mitophagy protects against glucocorticoid-induced synapse defects. Nat. Commun. 2021;12(1):487. doi: 10.1038/s41467-020-20679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun K., Jing X., Guo J., Yao X., Guo F. Mitophagy in degenerative joint diseases. Autophagy. 2021;17(9):2082–2092. doi: 10.1080/15548627.2020.1822097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narendra D.P., Jin S.M., Tanaka A., et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8(1) doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin Z., Chang B., Wei Y., et al. Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113092. [DOI] [PubMed] [Google Scholar]
- 63.Faulkner A., Tamiato A., Cathery W., et al. Dimethyl-2-oxoglutarate improves redox balance and mitochondrial function in muscle pericytes of individuals with diabetes mellitus. Diabetologia. 2020;63(10):2205–2217. doi: 10.1007/s00125-020-05230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.