Abstract
Electronic cigarette use has especially risen among adolescents and young adults. The aim of this study was to investigate fasting blood glucose and lipid profiles in chronic combustible cigarette and electronic cigarette users. We evaluated participants aged 21 to 45 (n = 525, mean age 31 ± 7 years, 45% women) without established cardiovascular disease or risk factors who were combustible cigarette users (n = 290), electronic cigarette users (n = 131; 65 sole users and 66 dual users), or never users (n = 104). In the first wave of enrollment (2014–2017), electronic cigarette users reported their products as first, second and third generation devices (e-cig users) and were all largely current (i.e., dual) or former (sole) combustible cigarette users, whereas in the second wave of enrollment (2019–2020), electronic cigarette users all reported pod-based device use (pod users) and included more sole users who were never smokers. In multivariable-adjusted analyses comparing to never users, both sole e-cig users and combustible cigarette users had higher glucose and triglycerides and lower high-density lipoprotein (HDL) cholesterol levels. Dual e-cig users showed higher triglycerides and very-low-density lipoprotein cholesterol, and lower HDL cholesterol compared to never users. In contrast, pod users (both sole and dual) had lipid profiles and glucose levels similar to never users. Overall, users of early generation electronic cigarettes display adverse metabolic profiles. In contrast, pod-based electronic cigarette users have similar lipid profiles to never users. Future studies are needed to understand the cumulative effects of electronic cigarette use on cardiometabolic health.
Keywords: e-cigarettes, electronic cigarettes, fasting glucose, lipids, smoking, vaping
Introduction
Use of combustible cigarettes remains a leading risk factor for atherosclerotic cardiovascular disease (CVD).1 Altered cardiometabolic health can be characterized by adverse lipid profiles and higher glucose levels, which are key mediators of the atherosclerotic process.2, 3 Prior evidence suggests that combustible cigarette smoking has an adverse influence on lipid profiles.4–6 The contributing elements in combustible cigarette smoke that alter lipid metabolism have not been well-defined and could include reactive aldehydes, metals or nicotine.7
Since their introduction more than a decade ago, electronic cigarettes are increasingly popular. Many adults have switched from combustible to electronic cigarettes due to the potential of a lower harm profile because of less exposure to harmful constituents; however, information on the cardiovascular and metabolic health effects remain insufficient.8–10 In addition, the emergence of pod-based electronic cigarettes has corresponded with a drastic rise of use among adolescents and young adults, especially among never users of combustible tobacco products.11–14 It is not known whether electronic cigarettes, which contain some overlapping harmful constituents (i.e., nicotine and acrolein), but lack others present in combustible cigarettes (i.e., tar), would affect lipid and glucose levels.15 The probable effects of electronic cigarettes can only be inferred by the limited data available on nicotine and smokeless tobacco products.9 In addition, e-liquid formulations include propylene glycol, glycerin, and flavorings in addition to nicotine, which necessitates the study of electronic cigarettes as an independent entity.7
In the present study, we sought to characterize cardiometabolic parameters of electronic cigarette users compared to never users and combustible cigarette users in two enrollment periods of the Cardiovascular Injury due to Tobacco Use (CITU) study, an ongoing observational study conducted as a joint effort between Boston University School of Medicine and the University of Louisville School of Medicine.16
Methods
Study visits were scheduled after an 8-hour fast from food and beverages apart from water, and a 6-hour fast from tobacco product use. All participants gave written informed consent as approved by the Boston University Medical Center and the University of Louisville Institutional Review Boards.
Study sample
As previously described,16 the CITU study enrolled participants between the ages of 21 and 45 years without established cardiovascular disease (CVD) or CVD risk factors (dyslipidemia, hypertension, diabetes) in two enrollment periods between July 2014 and November 2017 and between April 2019 and March 2020.
We classified participants in the first enrollment period as one of the following:
Never users: no current use of cigarettes or other tobacco products, smoked fewer than 100 cigarettes in their lifetime, and a urinary cotinine level below 10 ng/mL;
Combustible cigarette users: current use of cigarettes for at least 5 days per week, cumulative consumption of at least 100 cigarettes in their lifetime, and no current use of electronic cigarettes;
Sole electronic cigarette users: current use of electronic cigarettes for at least 5 days per week, and no use of cigarettes for at least 3 months;
Dual electronic cigarette users: current use of both traditional and electronic cigarettes for at least 5 days per week, and a lifetime consumption of at least 100 cigarettes.
In the second enrollment period, we recruited participants using the same overall eligibility criteria between April 2019 and March 2020. Owing to temporal trends in the types of electronic cigarette products used in the US, electronic cigarette users in the first enrollment period all used first to third generation products whereas all electronic cigarette users in the second enrollment period reported use of pod-based devices. To distinguish between the earlier generation and pod-based products, we designate ‘e-cig users’ to represent first to third generation electronic cigarette users, and ‘pod users’ to represent pod-based electronic cigarettes. We further subdivided each of the electronic cigarette user groups into dual use (ongoing combustible cigarette use) or sole use (never combustible cigarette use or at least 3 months since quitting combustible cigarette use). Participants were excluded from the study if they were pregnant, had clinically evident CVD, diabetes mellitus, systolic blood pressure > 159 mmHg or diastolic blood pressure > 99 mmHg, clinically active cancer, liver disease, rheumatologic disease, inflammatory bowel disease, or thyroid disease.
Measurements of glucose and lipid profiles
Blood glucose and lipids were determined by the autoanalyzer Synchron Systems (Beckman Coulter, Brea, CA, USA) at the University of Louisville. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula, and very-low-density lipoprotein (VLDL) cholesterol estimates were obtained by dividing the triglyceride values by 5. If the triglyceride value exceeded 300 mg/dL, LDL and VLDL cholesterol were not calculated.
Analysis
All analyses were performed using STATA/SE, release 16.0 (StataCorp LLC, College Station, TX, USA). For clinical characteristics and urinary cotinine levels, one-way analysis of variance (ANOVA) and chi-squared testing were performed for continuous and nominal variables across all six tobacco use groups (never users, combustible cigarette users, sole e-cig users, dual e-cig users, sole pod users, and dual pod users). The distributions of triglycerides and VLDL cholesterol were skewed; hence, these values were log-transformed for linear regression analyses. Given the differences in rates of former smoking and in the product characteristics of earlier generation and pod-based electronic cigarettes, we elected to perform analyses of these two groups separately.
One-way ANOVA was performed to compare fasting glucose and lipid measures across the four tobacco use groups separately for the e-cig users. One-way ANOVA was performed to compare fasting glucose and lipid measures between the pod-based users (sole and dual) and never users. Multivariable regression models (adjusted for age, race, sex, and study site) were conducted comparing fasting glucose and lipid measures in sole and dual e-cig users and combustible cigarette users to never users (reference group). A separate multivariable model adjusting for the same covariates was conducted to compare sole e-cig users to combustible cigarette users. In separate models, we compared metabolic measures in pod users (sole and dual) to never users. We considered a two-sided p-value of less than 0.05 as statistically significant.
Results
Clinical characteristics
The CITU cohort included 530 participants in the use groups defined above with available fasting glucose and lipid profiles. Of these, five observations were excluded due to sample mishandling. Clinical characteristics of the cohort are shown in Table I.
Table I.
Clinical characteristics of the CITU cohort
| Never users | Combustible cigarette users | E-cig users |
Pod users |
p-value | |||
|---|---|---|---|---|---|---|---|
| Sole users | Dual users | Sole users | Dual users | ||||
|
n = 104 |
n = 290 |
n = 42 |
n = 47 |
n = 23 |
n = 19 |
||
| Age, years | 29 ± 6 | 33 ± 7 | 28 ± 6 | 33 ±7 | 26 ±7 | 24 ± 5 | 0.0000 |
| Female sex, n (%) | 53 (51 %) | 126 (44%) | 11 (26%) | 22 (47%) | 13 (57%) | 10 (52%) | 0.092 |
| Race (%) | 0.000 | ||||||
| American Indian | 0% | 1% | 2% | 0% | 0% | 0% | |
| Asian | 12% | 2% | 7% | 0% | 22% | 16% | |
| Black/African American | 38% | 52% | 10% | 48% | 17% | 16% | |
| White/Caucasian | 40% | 39% | 67% | 46% | 44% | 63% | |
| Other | 10% | 6% | 14% | 6% | 17% | 5% | |
| Ethnicity (%) | 0.790 | ||||||
| Hispanic | 8% | 8% | 10% | 9% | 17% | 11% | |
| Non-Hispanic | 92% | 92% | 90% | 91% | 83% | 89% | |
| Urinary cotinine (mg/dL) | 3 ± 3 | 927 ± 900 | 686 ± 799 | 851 ± 726 | 970 ± 970 | 508 ± 440 | 0.0000 |
Results expressed as mean ± SD or percentage as appropriate.
CITU, Cardiovascular Injury due to Tobacco Use (CITU) study.
Electronic cigarette product characteristics
The majority of e-cig users reported second and third generation products with few first generation products (online supplementary material: Table SI). Two sole and five dual e-cig users reported using e-liquids without nicotine. A total of 80% of the sole e-cig users were former combustible cigarette users who had switched to electronic cigarettes at least 3 months prior to study enrollment. Among pod users, 52% of sole users and 42% of dual users reported using JUUL-brand products. The other pod-based products used were BLU, Aspire, SMOK, Vuse, Ooze, Relx, and Suorin. All pod-based electronic cigarette users reported using e-liquids with nicotine. Among sole pod users, 48% were former combustible cigarette users who had switched to electronic cigarette use at least 6 months prior to study enrollment and 52% had never used combustible cigarettes.
Metabolic parameters
In unadjusted analyses of the e-cig users, there were differences in glucose, triglycerides, and HDL and VLDL cholesterol levels across the tobacco product use groups (Figure 1). No differences were noted in total and LDL cholesterols across the groups. No differences were noted in any of the measures comparing pod users to never users (online supplementary material: Table S2).
Figure I.
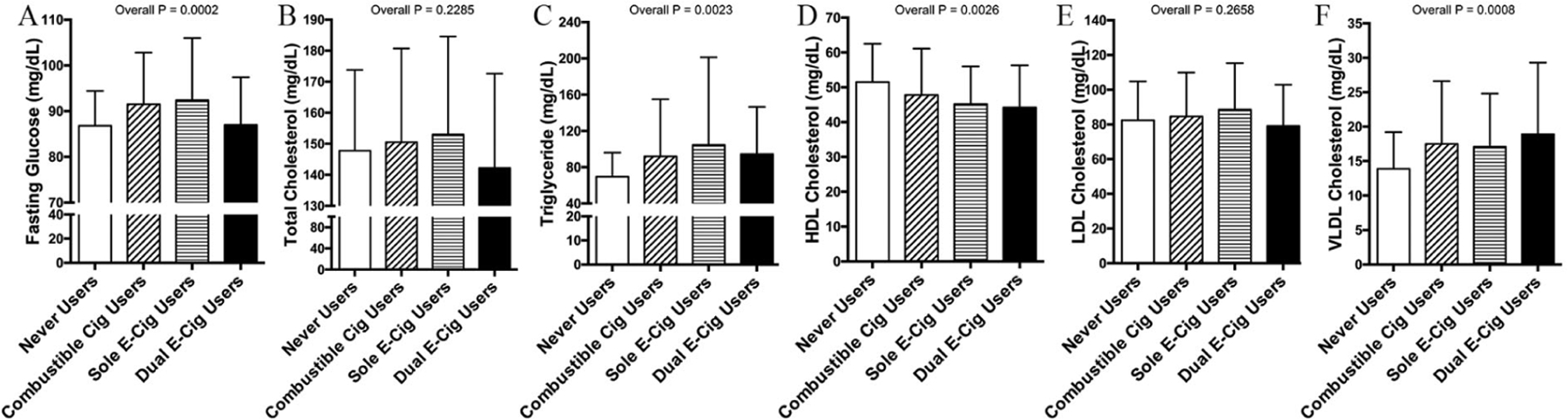
Electronic cigarette use alters fasting glucose and lipid profiles. Across tobacco user groups, there were differences in fasting glucose (A), triglycerides (C), HDL cholesterol (D), and VLDL cholesterol (F), but no differences in total (B) and LDL cholesterol (E) across the groups.
Results plotted are mean and SD.
Cig, cigarette; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
In multivariable regression models adjusted for age, race, sex, and study site, use of e-cigs alone or in conjunction with combustible cigarettes was associated with higher triglycerides and lower HDL cholesterol (Table 2). Sole e-cig use was also associated with higher fasting glucose. Dual e-cig use was also associated with higher VLDL cholesterol. Linear regression models were also constructed to compare combustible cigarette use to sole e-cig use (Table 3) and demonstrated no differences in the cardiometabolic parameters between the groups.
Table 2.
Multivariable-adjusted associations between tobacco product use and metabolic measures.
| Never users | Combustible cigarette users |
Sole e-cig users |
Dual e-cig users |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | ||
| Fasting glucose | Ref | 4.5 | 2.0, 7.1 | 0.001 | 5.1 | 1.2, 9.0 | 0.010 | 0.0 | −3.7, 3.8 | 0.995 |
| Total cholesterol | Ref | 0.8 | −6.2, 7.7 | 0.831 | 5.5 | −5.2, 16.1 | 0.315 | −8.0 | −18.3, 2.4 | 0.132 |
| Ln triglyceride | Ref | 0.2 | 0.1, 0.3 | 0.003 | 0.2 | 0.1, 0.3 | 0.032 | 0.2 | 0.0, 0.4 | 0.017 |
| HDL cholesterol | Ref | −3.6 | −6.5, −0.6 | 0.019 | −5.0 | −9.2, −0.0 | 0.049 | −7.2 | −1 1.6, −2.7 | 0.002 |
| LDL cholesterol | Ref | 1.0 | −4.8, 6.8 | 0.739 | 5.0 | −2.6, 15.5 | 0.162 | −4.9 | −13.6, 3.8 | 0.267 |
| Ln VLDL cholesterol | Ref | 0.2 | 0.1, 0.3 | 0.002 | 0.1 | −0.0, 0.4 | 0.143 | 0.2 | 0.1,04 | 0.006 |
Triglycerides and VLDL cholesterol were log-transformed.
Models adjusted for age, race, sex, and study site.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein; Ln, natural log.
Table 3.
Multivariable-adjusted associations between combustible cigarette users and sole e-cig users.
| Combustible cigarette users | Sole e-cig users |
|||
|---|---|---|---|---|
| β | 95% Cl | p-value | ||
| Fasting glucose | Ref | −0.4 | −4.3, 3.6 | 0.854 |
| Total cholesterol | Ref | 3.5 | −6.9, 13.9 | 0.508 |
| Ln triglyceride | Ref | 0.0 | −0.2, 0.2 | 0.949 |
| HDL cholesterol | Ref | 0.5 | −3.9, 5.0 | 0.815 |
| LDL cholesterol | Ref | 3.0 | −5.9, 1 1.9 | 0.513 |
| Ln VLDL cholesterol | Ref | −0.1 | −0.2, 0.1 | 0.505 |
Triglycerides and VLDL cholesterol were log-transformed.
Models adjusted for age, race, sex, and study site.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein; Ln, natural log.
For the pod users, we performed multivariable regression models comparing sole and dual pod users to never users (Table 4). Overall, pod users had similar lipid and glucose levels to never users. In sensitivity analyses that included just the pod users who had never used combustible cigarettes, the overall findings were similar.
Table 4.
Multivariable-adjusted associations between pod use and metabolic measures.
| Never users | Sole pod users |
Dual pod users |
||||||
|---|---|---|---|---|---|---|---|---|
| β | 95% Cl | p-value | β | 95% Cl | p-value | |||
| Fasting glucose | Ref | 1.6 | −2.2, 5.4 | 0.403 | 2.4 | −1.8, 6.6 | 0.265 | |
| Total cholesterol | Ref | 1.0 | −1 1.4, 13.3 | 0.878 | 0.5 | −13.3, 14.2 | 0.944 | |
| Ln triglyceride | Ref | 0.1 | −0.1, 0.3 | 0.215 | −0.1 | −0.3, 0.1 | 0.429 | |
| HDL cholesterol | Ref | −1.2 | −6.8, 4.4 | 0.674 | 2.3 | −3.9, 8.5 | 0.468 | |
| LDL cholesterol | Ref | 0.8 | −9.6, 1 1.2 | 0.886 | −1.3 | −12.7, 10.1 | 0.818 | |
| Ln VLDL cholesterol | Ref | 0.0 | −0.1, 0.2 | 0.589 | −0.1 1 | −0.3,0. | 0.442 | |
Discussion
The relative health effects of electronic cigarettes and combustible cigarettes continue to be critical questions given the number of adults switching between tobacco products as a potential health-protective strategy. In the present study, we evaluated important cardiometabolic health measures in a cross-sectional analysis of a cohort of tobacco product users and non-users. Our findings indicate that sole e-cig use was associated with higher fasting glucose and triglycerides and lower HDL cholesterol, all consistent with an adverse cardiometabolic profile. Similarly, dual use of combustible and electronic cigarettes was associated with higher triglycerides and VLDL cholesterol as well as decreased HDL cholesterol. The lipid profiles and glucose were similar when comparing sole e-cig use with combustible cigarette use. Interestingly, in a smaller group of pod users, we did not observe any differences in cardiometabolic parameters, suggesting that more prolonged tobacco product use, former combustible product use, or specific electronic cigarette components may be important contributors to the adverse metabolic profiles. Overall, our findings suggest that there may be residual effects of combustible cigarette use in earlier generation electronic cigarette users that were not observed in a smaller group of pod-based electronic cigarette users.
Prior studies have demonstrated the association of cigarette use with impaired glucose metabolism17 and atherogenic lipid profiles.6,18,19 The intensity of cigarette use measured as the number of cigarettes smoked daily and the number of pack years both relate to more adverse glucose and cholesterol levels. These associations have largely been unexplored for electronic cigarettes. A recent study exploring glucose tolerance in mouse models and a cross-sectional national cohort found no associations of electronic cigarette use to altered glucose or insulin resistance.20 However, the study was limited in the number of sole electronic cigarette and dual users when compared to non-users and cigarette users. Another study evaluating the lipidome signatures in chronic combustible cigarette users and electronic cigarette users failed to see differences when compared to the control group, which would suggest that neither alters lipid composition, but noted distinct sex-specific alterations in lipid species.21 In contrast, analyses of data from the Korean general population revealed electronic cigarette use to be associated with increased risk for high triglycerides, low HDL cholesterol, and metabolic syndrome.22
Here, the electronic cigarette users in the earlier enrollment period were comprised of users of earlier generation products with consistent use of electronic cigarette products for at least 3 months. We noted adverse lipid compositions in e-cig users using comparable approaches as has been previously reported in combustible cigarette users. Although the mechanisms by which smoking alters lipid parameters are not well understood, it has been linked to catecholamine release causing a rise in circulating free fatty acids and thereby altering cholesterol levels.23,24 Furthermore, these shifts have been attributed to nicotine, the addictive chemical that is present in tobacco products. Given the presence of nicotine in electronic cigarettes, it is possible that nicotine may be implicated in altering lipid metabolism.25 These differences may also be residual findings related to prolonged combustible cigarette use prior to switching to e-cig use.
We additionally analyzed lipid profiles of pod users. In both sole and dual users of pod-based devices, we did not observe unfavorable parameters when compared to never users of tobacco products. This could have been due to distinct product characteristics between early generation products and pod-based electronic cigarettes including differences in voltage, flavorings, and nicotine content. Pod-based electronic cigarettes contain nicotine salts, which may have differential health effects and generally have a fixed proportion of vehicle components, including polyethylene glycol and vegetable glycerol. Several types of early generation electronic cigarettes permit users to create their own e-liquids, which creates the possibility of complex mixtures. There is growing evidence elucidating the cardiovascular effects of specific electronic cigarette constituents on cardiovascular health components. Interestingly, in an animal model of electronic cigarette use, the vehicle chemicals appeared to be an important inducer of alterations in lipid metabolism,26 so it remains possible that the vehicle concentrations in the e-cig group but not the pod group are associated with lipid levels. Further studies with animal models would help explain the lipid-related toxicity of specific electronic cigarette constituents. The shifting demographics of electronic cigarette use may also underlie the observed differences in e-cig as compared to pod users. A greater proportion of e-cig users were former smokers whereas many pod users had never used any combustible products. In addition, pod-based users tended to be younger, thus the cumulative exposure to all tobacco products may be lower.
Study limitations
Our study is not without limitations. As with any cross-sectional study design, we were unable to assess a temporal relationship between exposure and outcome, limiting causal inference. In addition, the observational design of the study raises the possibility of residual confounding that would be addressed by a randomized study design. To mitigate the impact of confounding, we limited our cohort to individuals without established cardiovascular risk factors and all those of a young age and performed adjusted analyses. However, it remains possible that individuals who select to use electronic cigarettes have additional characteristics that are associated with lipid levels. Furthermore, to allow for any residual effects of cigarette use to subside, we required a minimum of 3 months of current electronic cigarette use alone for the sole electronic cigarette user group, as some lipid values, such as HDL cholesterol, can begin to change within weeks of smoking cessation. However, it is possible that longer electronic cigarette use is necessary to accrue significant improvements in fasting glucose and lipid profiles. It is also possible that tobacco-naive individuals who begin to use electronic cigarettes may have different metabolic parameters compared to former smokers who switch, as is suggested by our analysis of the pod-based users. Given the shorter current recruitment period to date of the pod users, there were fewer pod than e-cig users; thus, as indicated by the confidence intervals in the multivariable models, it remains possible that a difference would be observed with a larger sample size. Finally, electronic cigarette brands represent a diverse class of tobacco products with a wide range of operating conditions, use patterns, product characteristics, and e-liquid constituents, which often include varying levels of nicotine, several flavors, and different ratios of the vehicles – glycerin and propylene glycol. It may be helpful in future studies to include controlled interventional studies.
Conclusion
The present study provides insights into the associations of novel tobacco product use with known markers of cardiovascular health. Importantly, when compared to never users, we found use of there earlier generation of electronic cigarettes with and without combustible cigarette use to be associated with lower levels of HDL cholesterol and higher triglycerides. Metabolic measures were similar in sole e-cig users compared to combustible cigarette users, suggesting that electronic cigarettes may have a residual impact on cardiometabolic parameters. Our study suggests that fasting glucose and lipid measures, important preclinical risk factors for impaired metabolic regulation and atherosclerosis, are altered in individuals who use earlier generations of electronic cigarettes alone or in conjunction with combustible cigarettes. Although we found pod-based electronic cigarette users to have similar measures to never users, the long-term effects of consistent usage of electronic cigarettes have not been evaluated. Therefore, our findings further support the urgency in studying the longitudinal effects of electronic cigarette use in individuals who switch from combustible cigarettes, and especially in those who are never smokers.
Supplementary Material
Funding
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Numbers 5P50HL120163 and U54HL120163. Dr Hamburg is supported by a grant from the American Heart Association 20YVNR35500014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Heart Association.
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material
The supplementary material is available online with the article.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 update: A report from the American Heart Association. Circulation 2017; 135: el46–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson PWF. High-density lipoprotein, low-density lipoprotein and coronary artery disease. Am J Cardiol 1990; 66: 7A–10A. [DOI] [PubMed] [Google Scholar]
- 3.Marz W, Kleber ME, Schamagl H, et al. HDL cholesterol: Reappraisal of its clinical relevance. Clin Res Cardiol 2017; 106: 663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: An analysis of published data. Br Med J 1989; 298: 784–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan XJ, Jiao GP, Ren YJ, et al. Relationship between smoking and dyslipidemia in western Chinese elderly males. J Clin Lab Anal 2008; 22: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RB, Ducatman A. Associations between smoking and lipid/lipoprotein concentrations among US adults aged ≥20 years. J Circ Biomark 2018; 7: 1849454418779310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strongin RM. E-cigarette chemistry and analytical detection. Annu Rev Anal Chem 2019; 12: 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatnagar A, Whitsel LP, Ribisl KM, et al. Electronic cigarettes: A policy statement from the American Heart Association. Circulation 2014; 130: 1418–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol 2017; 14: 447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macdonald A, Middlekauff HR. Electronic cigarettes and cardiovascular health: What do we know so far? Vase Health Risk Manag 2019; 15: 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandi P, Cahn Z, Coding Sauer A, et al. Trends in e-cigarette use by age group and combustible cigarette smoking histories, U.S. adults, 2014–2018. Am JPrev Med 2021; 60: 151–158. [DOI] [PubMed] [Google Scholar]
- 12.Barrington-Trimis JL, Leventhal AM. Adolescents’ use of “pod mod” e-cigarettes — urgent concerns. N Engl J Med 2018:379: 1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public health consequences of e-cigarettes Eaton DL, Kwan LY, Stratton K (eds). Washington, DC: National Academies Press; (US: ), 2018. [PubMed] [Google Scholar]
- 14.Vallone DM, Cuccia AF, Briggs J, et al. Electronic cigarette and JUUL use among adolescents and young adults. JAMA Pediatr 2020; 174: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benowitz NL. Cigarette smoking and cardiovascular disease: Pathophysiology and implications for treatment. Prog Cardiovasc Dis 2003; 46: 91–111. [DOI] [PubMed] [Google Scholar]
- 16.Keith RJ, Fetterman JL, Riggs DW, et al. Protocol to assess the impact of tobacco-induced volatile organic compounds on cardiovascular risk in a cross-sectional cohort: Cardiovascular Injury due to Tobacco Use study. BMJ Open 2018; 8: e019850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakanishi N, Nakamura K, Matsuo Y, et al. Cigarette smoking and risk for impaired fasting glucose and type 2 diabetes in middle-aged Japanese men. Ann Intern Med 2000; 133: 183–191. [DOI] [PubMed] [Google Scholar]
- 18.Garrison RJ, Kannel WB, Feinleib M, et al. Cigarette smoking and HDL cholesterol: The Framingham offspring study. Atherosclerosis 1978; 30: 17–25. [DOI] [PubMed] [Google Scholar]
- 19.Selya AS, Hesse ND. Time to first cigarette and serum cholesterol levels. Soc Sc; Med 2017; 174: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orimoloye OA, Uddin SMI, Chen LC, et al. Electronic cigarettes and insulin resistance in animals and humans: Results of a controlled animal study and the National Health and Nutrition Examination Survey (NHANES 2013–2016). PLoS One 2019; 14: e0226744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Middlekauff HR, William KJ, Su B, et al. Changes in lipid composition associated with electronic cigarette use. J Transl Med 2020; 18: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim T, Choi H, Kang J, et al. Association between electronic cigarette use and metabolic syndrome in the Korean general population: A nationwide population-based study. PLoS One 2020; 15: e0237983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell SC, Moffatt RJ, Stamford BA. Smoking and smoking cessation—The relationship between cardiovascular disease and lipoprotein metabolism: A review. Atherosclerosis 2008; 201: 225–235. [DOI] [PubMed] [Google Scholar]
- 24.Gepner AD, Piper ME, Johnson HM, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: Outcomes from a randomized clinical trial. Am Heart J 2011; 161: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc Med 2016; 26: 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madison MC, Landers CT, Gu BH, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest 2019; 129: 4290–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


