Abstract
Background:
Platelet-rich plasma (PRP) and hyaluronic acid (HA) are non-surgical treatments for osteoarthritis (OA), but the comparison of their efficiency is still inconclusive.
Objectives:
The objectives of this study were to compare the efficacy of PRP and HA in the treatment of OA by meta-analysis and to explore the effects of different injection times and leukocyte concentration on the efficacy of PRP.
Design:
Meta-analysis and subgroup analysis were conducted. The data were analyzed by Review Manager v5.4.1.
Data sources and methods:
Articles were retrieved and screened from PubMed, the Cochrane Library, Web of Science, and Embase. The outcome included the total Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), the visual analog scale (VAS), adverse events (AEs), the International Knee Documentation Committee (IKDC), and the satisfaction rate.
Results:
A total of 30 articles involving 2733 patients were included. The total WOMAC score and IKDC score of the PRP group were better than those of the HA group at the last follow-up time, while there was no significant difference in AEs, satisfaction rate, and VAS between the two groups. In our subgroup analysis, there was no significant difference between single-injection PRP and triple-injection PRP. Leukocyte-poor PRP (LP-PRP) was better than leukocyte-rich PRP (LR-PRP) in IKDC, but there was no significant difference between them in the other scores.
Conclusions:
In the treatment of OA, compared with HA, PRP performed better in the improvement of the patient’s function. There was no significant difference in VAS and AEs between the two groups, and the safety was comparable. LP-PRP looked to be superior to LR-PRP in functional recovery, but there appeared to be no significant difference in pain relief between them. There was no significant difference between single PRP and triple PRP in the subgroup analysis.
Keywords: hyaluronic acid, intra-articular injection, meta-analysis, osteoarthritis, platelet-rich plasma
Introduction
Osteoarthritis (OA) is a degenerative joint disease that affects the entire joint1,2 and is characterized by localized articular cartilage loss, the formation of osteophytes, subchondral bone alterations, and synovial hyperplasia.2 However, the pathogenesis of OA is not yet fully understood.3 OA is the most common arthritis. With the extension of global life expectancy, the prevalence of OA is also increasing.4 Meanwhile, OA has a high disability rate. According to the statistics of the study published in Lancet,5 the total burden and age-standardized disability-adjusted-life year rate of OA significantly increased from 1990 to 2015. Another study showed that OA might also lead to a decline in people’s mental health.6 What’s worse, not only patients may have to cost a lot, but the consumption of medical resources may also be huge.6
However, the non-surgical treatments for OA now mainly include nonsteroidal anti-inflammatory drugs (NSAIDs), intra-articular injections of platelet-rich plasma (PRP), hyaluronic acid (HA), steroids, and mesenchymal stem cells (MSCs).7 Among them, HA is a high molecular weight biological polysaccharide, which can play a role in chondroprotection both in vivo and in vitro.8 In addition, the concentration and molecular weight of HA decrease with the development of OA.9 Now HA is widely used in the clinical practice of OA.10 However, its efficacy remains controversial.11 As for PRP, an autologous blood product with a high platelet concentration after centrifugation and concentration12 is increasingly used in musculoskeletal disease. Its low adverse events (AEs) rate makes it widely applied in clinical practice.13 PRP mainly contains concentrated platelets, optional leukocytes, and fibrin.14 Activated platelets via exocytosis release cytokines, transforming growth factors, and other substances which play roles in tissue repair.14 It is generally believed that PRP plays a role in the treatment of OA in three ways: inhibiting inflammatory reaction, regulating immunity, and regulating cell metabolism through growth factors.14 And it has different classifications depending on its composition and other influencing factors. For example, it can be classified into four types according to leukocytes and fibrin content.15 In addition, according to the coding classification system proposed in a literature,16 we can know the concentration and the concentration ratio of platelet, the purity of PRP, leukocyte concentration, whether PRP activation is endogeneous or whether PRP is activated prior to injection, and whether Ca2+ is added at the time of activation from the code. It is a promising method for the treatment of OA, and clinical data show that PRP is safe.17 However, there is no consensus on the best regimen for the content of PRP injections.18
At present, there are 37 meta-analyses comparing the efficacy of PRP and HA in the treatment of OA, most of which are knee OA or hip OA. The result in the scores of the total Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), visual analog scale (VAS), and International Knee Documentation Committee (IKDC) obtained from their research are inconsistent. The selection of PRP and HA for the treatment of OA is currently an inconclusive issue. In 2021, a randomized controlled trial (RCT)19 comparing PRP and HA indicated that the efficacy of HA was better than PRP, in contrast to the results of many previous studies. In addition, Belk et al.20 in 2022 found that PRP and HA have similar beneficial short-term clinical outcomes in the treatment of hip OA. The possible reason for the different results between different RCTs may be due to the different preparation methods, application protocols of their PRP, and their different PRP leukocyte concentrations.21,22 Some studies have found that leukocytes may have an impact on the treatment of OA.23,24 In addition, there are studies that have found that the number of PRP injections has an effect on the treatment of OA.25,26
Therefore, the comparison of efficacy between PRP and HA is still unclear. What’s more, many high-quality RCTs in recent years were not included in the previous meta-analysis, and the level of literature evidence included in the previous meta-analysis was different. At the same time, the contribution of leukocyte concentration in PRP and the number of PRP injections to the treatment of OA is controversial.20,27–29
Therefore, the purpose of this study is to compare the efficacy of PRP and HA in OA patients through meta-analysis. At the same time, subgroup analysis was carried out regarding the number of PRP injections, the leukocyte concentration in PRP, the final follow-up time, the injection site, and Kellgren–Lawrence (K-L) grade. Subgroup analysis can provide a reference for the standardization of PRP preparations in the future. Our hypothesis was that PRP would have better efficacy than HA, and leukocyte-poor PRP (LP-PRP) would have better efficacy than leukocyte-rich PRP (LR-PRP).
Method
Search strategy
PubMed, the Cochrane Library, Web of Science, and Embase were searched. The keywords were as follows: (osteoarthritis) AND (PRP OR platelet rich plasma OR platelet-derived growth factors OR PRGF OR PLG) AND (HA OR sodium hyaluronate OR Hylan G-F 20 OR hyaluronic acid) AND (randomized controlled trial). Duplicates were removed. The publication time of found studies was limited from 1 January 2000, to 28 March 2022.
Eligibility
The inclusion criteria were as follows: (1) Only articles with evidence level I were eligible for this study; (2) only articles including the PRP injection group and HA injection group were included. The exclusion criteria were as follows: (1) all studies not written in English were excluded; (2) studies on temporomandibular OA were excluded; (3) studies with only abstract among the retrieved articles were excluded; (4) protocols were excluded.
Study selection
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Two authors independently searched the databases and reviewed the retrieved articles. When in doubt, they further reviewed the full text of the article. When the two authors failed to reach a consensus, the article was handed over to the third author to evaluate whether to include it. The reviewers then established a list of studies that met all the inclusion criteria and integrated them. Duplicates were removed.
Data extraction
The two authors extracted data independently, followed by a joint review to produce accurate and consistent data. Differences were resolved through consultation with the senior author. The extracted data included sample size, study design, interventions, age, body mass index (BMI), gender, the location of OA, K-L grade of OA, follow-up time, and results especially scoring data. All scoring data adopted the data of the final follow-up time. Yaradilmis et al.30 contained two PRP groups, where the two sets of data were combined within the group and separated by type within the subgroup; and Trueba Vasavilbaso et al.31 contained three HA groups, which the three HA sets of data were combined. Although the RCT of Gormeli et al.26 also included two PRP groups, we only included the group with the same number of injections as HA, that is, the PRP group with three injections. In addition, the RCT of Raeissadat et al.32 included the PRP group and the PRGF group. We combined the data of the two groups in group analysis, but only the data of the PRGF group was used in subgroup analysis. The numerical rating scale (NRS) pain score in RCTs33,34 was converted to the VAS score.
Statistical analysis
A quantitative synthesis of the included results was made by using Review Manager v5.4.1. Two authors synthesized the results by random (I2 more than 50%) or fixed model (I2 less than 50%), and the results were presented in the form of a forest plot. In discrete data processing, if the number of events in both groups is 0, they will be artificially assigned a value of 1. Continuous data in each specific study was expressed as mean and standard deviation. If the continuous data in the article is expressed in other forms, such as quartile, it was first converted into mean and standard deviation form. If the data are presented in the form of a figure, we used Image J software to get the mean and standard deviation for the figure. WOMAC score of a RCT35 applied a different full score from the other papers, so we have standardized it.
Subgroup analysis
We used the software Review Manager v5.4.1 to continue the subgroup analysis on the scoring results for VAS, WOMAC, IKDC, AEs, and satisfaction rate. In the first group, we grouped the PRPs by single injection and triple injections and performed subgroup analysis on them. In the second group, we classified them into LP-PRP and LR-PRP according to the leukocytes they contained, and then further subgroup analysis was performed on them. The third and fourth subgroup analyses were grouped according to OA location and whether the last follow-up time was greater than 12 months. We also divided RCTs into two subgroups based on K-L grade. RCTs were divided into two subgroups: RCTs with K-L grade 0 or 1 and RCTs without K-L grade 0 or 1.
Outcomes
The primary outcome of this meta-analysis was the VAS score. Secondary outcomes included total WOMAC score, IKDC, AEs, and satisfaction rate. The VAS mainly evaluates pain,36–38 the IKDC mainly evaluates function,39 and the WOMAC evaluates symptoms and physical functional disability in patients as a whole in terms of pain, stiffness, and joint function.40
Bias assessment
The assessment of included studies was carried out by two reviewers independently. Three criteria were used: low risk, unclear risk, and high risk to assess the selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias via the software Review Manager v5.4.1. If all of the items were assessed to be ‘low risk’, the study was assessed to have a low risk of bias. If one or two items were assessed as ‘unknown risk’ or ‘high risk’, we assessed the study to have a moderate risk of bias. Or else we assessed there to be a high risk of bias in the study.41 Any disagreement would be settled by discussion. In addition, we conducted a funnel analysis of WOMAC scores, VAS scores, IKDC scores, and AEs by using the software Review Manager v5.4.1.
Results
Characteristics of included studies and patients
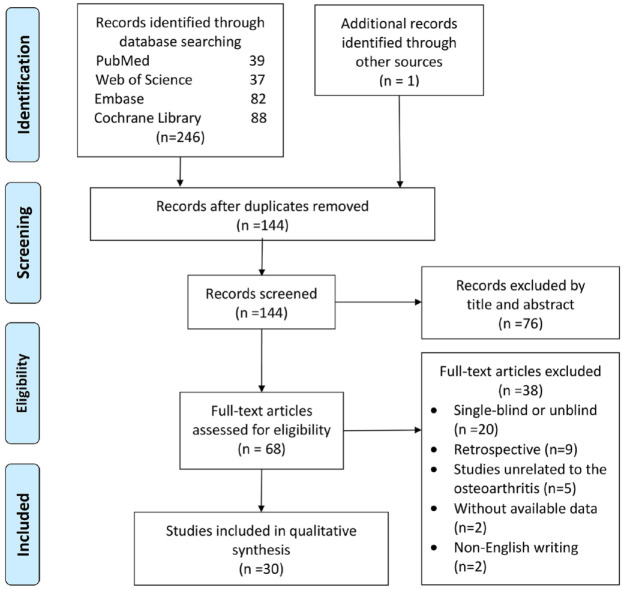
A total of 30 articles26,30–35,42–64 with an evidence level I were included out of the 144 records retrieved (Figure 1). This meta-analysis included 2733 patients (Table 1).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Table 1.
Characteristics of the included studies.
| Study | Country | No. of patients (PRP versus HA) |
The patients selection criteria | Number of injections (PRP versus HA) |
The injection technique used | Presence of leukocytes (PRP group) |
Age (mean ± SD), year | BMI, kg/m2 | OA grade (K-L) | Final Follow-up (months) | Questionnaires used | Results (preference for PRP or HA) | Level of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdelsabor Sabaah et al.42 | Egypt | 15 15 |
Thumb carpometacarpal OA | 1 1 |
Blind | NR | NR NR |
NR NR |
NR | 3 | VAS, AUSCAN | Preferred HA | 1 |
| Bansal et al.43 | India | 64 68 |
Symptomatic primary knee OA | 1 1 |
Blind | LP-PRP | 64.4 65.8 |
NR NR |
1–3 | 12 | WOMAC, IKDC | Preferred PRP | 1 |
| Cole et al.44 | USA | 49 50 |
Symptomatic unilateral knee OA | 3 3 |
Ultrasound guidance | LP-PRP | 55.9 ± 10.4 56.8 ± 10.5 |
27.4 ± 3.9 29.0 ± 6.4 |
1–4 | 13 | WOMAC pain, IKDC, VAS, Lysholm knee score | Preferred PRP | 1 |
| Di Martino45 2018 | Italy | 85 82 |
Chronic symptomatic degenerative knee changes and OA | 3 3 |
NR | LR-PRP | 52.7 ± 13.2 57.5 ± 11.7 |
27.2 ± 7.6 26.8 ± 4.3 |
0–3 | 64.3 ± 7.8 | IKDC, EQ-VAS, Tegner scores | No preference | 1 |
| Di Sante et al.46 | Italy | 21 22 |
Monolateral severe hip OA | 3 3 |
Ultrasound guidance | LP-PRP | 71.4 ± 6.0 73.6 ± 7.9 |
NR NR |
2–3 | 4 | WOMAC pain, WOMAC joint stiffness, WOMAC disability, VAS | Preferred HA for the long term | 1 |
| Dulic et al.47 | Serbia | 34 30 |
Knee pain and OA | 3 3 |
NR | LR-PRP | 58.8 ± 11.2 59.4 ± 14.0 |
28.47 ± 4.54 29.98 ± 5.24 |
2–4 | 12 | VAS, WOMAC, KOOS, IKDC | No preference | 1 |
| Duymus et al.48 | Turkey | 33 34 |
Mild–moderate and moderate knee OA | 2 1 |
Blind | NR | 60.4 ± 5.1 60.3 ± 9.1 |
27.6 ± 4.6 28.4 ± 3.6 |
2–3 | 12 | WOMAC, VAS | Preferred PRP | 1 |
| Filardo et al.49 | Italy | 54 55 |
Chronic pain or swelling of the knee and degenerative changes of the joint | 3 3 |
NR | LR-PRP | 55.0 58.0 |
27 26 |
0–3 | 12 | IKDC, EQ-VAS, Tegner score, KOOS | Preferred PRP in low grade degeneration | 1 |
| Filardo et al.50 | Italy | 94 89 |
Chronic pain or swelling of knee and degenerative changes | 3 3 |
NR | LR-PRP | 53.3 ± 13.2 57.6 ± 11.8 |
26.6 ± 4.0 26.9 ± 4.4 |
0–3 | 12 | IKDC, KOOS, EQ-VAS, Tegner score | No preference | 1 |
| Görmeli et al.26 | Turkey | 46 39 |
Knee OA | 3 3 |
Blind | NR | 53.7 ± 13.1 53.5 ± 14.0 |
28.541 ± 4.5664 29.7 ± 3.7 |
1–4 | 6 | EQ-VAS, IKDC | Preferred PRP for the early OA | 1 |
| Huang et al.51 | China | 40 40 |
Symptomatic knee OA | 3 3 |
Blind | LP-PRP | 54.5 ± 1.2 54.8 ± 1.1 |
25.23 ± 4.15 24.51 ± 3.09 |
1–2 | 12 | WOMAC, VAS | Preferred HA for the long term | 1 |
| Kirschner et al.33 | USA | 34 36 |
Chronic glenohumeral OA | 1 1 |
Ultrasound guidance | LP-PRP | 69.1 ± 11.5 68.4 ± 11.9 |
26.8 ± 5.2 27.9 ± 5.4 |
NR | 12 | SPADI, ASES, NRS | No preference | 1 |
| Kraeutler et al.35 | USA | 18 13 |
Hip OA | 3 3 |
Blind | LP-PRP | 53.3 ± 8.4 53.6 ± 7.6 |
23.7 ± 2.1 23.5 ± 2.0 |
2–3 | 24 | WOMAC | Preferred PRP | 1 |
| Lana et al.52 | Brazil | 36 36 |
Mild to moderate knee OA | 3 3 |
Ultrasound guidance | LR-PRP | 60.9 ± 7.0 60.0 ± 6.6 |
27.42 ± 6.89 28.24 ± 8.77 |
1–3 | 12 | WOMAC, VAS | Preferred PRP | 1 |
| Lin et al.53 | China | 31 29 |
Knee OA | 3 3 |
Blind | LP-PRP | 61.2 ± 13.1 62.5 ± 9.9 |
23.98 ± 2.62 26.26 ± 2.99 |
NR | 12 | WOMAC, IKDC | Preferred PRP | 1 |
| Lisi et al.54 | Italy | 30 28 |
Knee OA | 3 3 |
Ultrasound guidance | NR | 53.5 ± 15.1 57.1 ± 10.0 |
NR NR |
2–3 | 12 | WOMAC, Lysholm, Tegner, AKSS, Lequesne, VAS | Preferred PRP | 1 |
| Montañez-Heredia et al.55 | Spain | 27 26 |
Knee OA | 3 3 |
Blind | LP-PRP | 66.3 ± 8.3 61.5 ± 8.6 |
29.0 ± 5.5 30.4 ± 4.9 |
1–3 | 6 | VAS, KOOS, EUROQOL | Preferred PRP for lower OA grades | 1 |
| Papalia et al.56 | Italy | 23 24 |
End career athletes with degenerative cartilage lesions of the knee | 3 3 |
Blind | NR | NR NR |
NR NR |
1–2 | 12 | IKDC, KOOS, VAS | Preferred HA | 1 |
| Park et al.57 | Korea | 55 55 |
Symptomatic knee OA | 1 1 |
Blind | LR-PRP | 60.6 ± 8.2 62.3 ± 9.6 |
25.5 ± 2.2 25.9 ± 2.8 |
1–3 | 6 | IKDC, the Patient Global Assessment score, VAS, WOMAC, Samsung Medical Center patellofemoral score | Preferred PRP | 1 |
| Paterson et al.58 | Australia | 10 9 |
Knee OA | 3 3 |
Ultrasound guidance | LR-PRP | 49.9 ± 13.7 52.7 ± 10.3 |
27.92 ± 11.94 30.87 ± 5.64 |
2–3 | 3 | VAS, KOOS, KQoL | No preference | 1 |
| Raeissadat et al.32 | Iran | 103 49 |
Mild to moderate knee OA | 2, 2 3 |
Blind | NR, LP-PRP | 56.08 ± 6.1 57.91 ± 6.7 |
27.45 ± 2.35 27.46 ± 2.2 |
2–3 | 12 | VAS, WOMAC, Lequesne | Preferred PRP | 1 |
| Sánchez et al.59 | Spain | 79 74 |
Symptomatic knee OA | 3 3 |
NR | LP-PRP | 60.5 ± 7.9 58.9 ± 8.2 |
27.9 ± 2.9 28.2 ± 2.7 |
NR | 6 | WOMAC, OMERACT-OARSI | Preferred PRP | 1 |
| Sdeek et al.60 | Egypt | 95 94 |
Symptomatic knee OA | 3 3 |
NR | LP-PRP | 60.2 59.5 |
27.9 27.1 |
2–3 | 36 | IKDC, VAS, WOMAC | Preferred PRP | 1 |
| Spaková et al.34 | Slovakia | 60 60 |
Knee OA | 3 3 |
Blind | LR-PRP | 52.8 ± 12.4 53.2 ± 14.5 |
NR NR |
1–3 | 6 | WOMAC, NRS | Preferred PRP | 1 |
| Trueba Vasavilbaso et al.31 | Mexico | 10 30 |
Meniscal pathology and OA + Indication for knee arthroscopic debridement | 1 5, 4, 3 |
Blind | NR | 60.3 ± 9.5 64.8 ± 10.4 |
NR NR |
1–2 | 18 | WOMAC | Preferred HA | 1 |
| Vaquerizo et al.61 | Spain | 48 42 |
Symptomatic knee OA | 3 1 |
Blind | LP-PRP | 62.4 ± 6.6 64.8 ± 7.7 |
30.7 ± 3.6 31.0 ± 4.6 |
2–4 | 12 | WOMAC, Lequesne, OMERACT-OARSI | Preferred PRP | 1 |
| Villanova-Lopez et al.62 | Spain | 34 34 |
Hip OA | 1 1 |
Ultrasound-guided | LP-PRP | 61.2 ± 9.7 61.1 ± 12.3 |
28.6 ± 4.2 28.4 ± 4.5 |
1–4 | 12 | VAS, HHS, WOMAC | No preference | 1 |
| Yaradilmis et al.30 | Turkey | 60 30 |
Moderate knee OA | 3, 3 3 |
Blind | LP-PRP, LR-PRP | 59.6 ± 7.0 63.0 ± 9.2 |
31.9 ± 5.2712 32.4 ± 4.2 |
2–3 | 12 | VAS, WOMAC, Likert score | Preferred PRP | 1 |
| Yu et al.63 | China | 104 88 |
Knee OA | 6 5 |
NR | NR | 46.2 ± 8.6 51.5 ± 9.3 |
NR NR |
NR | 12 | WOMAC | Preferred PRP | 1 |
| Zhang et al.64 | China | 30 30 |
Refractory knee OA + infection | 4 4 |
Ultrasound guidance | NR | 65.4 ± 5.8 66.2 ± 4.9 |
NR NR |
2–3 | 3 | VAS, Lysholm knee scale | Preferred PRP | 1 |
AKSS, American Knee Society Score; ASES, American Shoulder and Elbow Surgeons score; AUSCAN, The Australian Canadian Osteoarthritis Hand Index; BMI, body mass index; EQ-VAS, EuroQol visual analog scale; EUROQOL, European Quality of Life scale; HA, hyaluronic acid; HHS, Harris hip score; IKDC, International Knee Documentation Committee subjective knee evaluation; K-L, Kellgren–Lawrence; KOOS, Knee Injury and Osteoarthritis Outcome Score; KQoL, Knee Quality of Life scale; LP-PRP, leukocyte-rich PRP; NR, not reported; NRS, numerical rating scale; OA, osteoarthritis; OMERACT-OARSI, Outcome Measures in Rheumatology Clinical Trials-Osteoarthritis Research Society and Health Assessment Questionnaire; PRGF, plasma rich in growth factors; PRP, platelet-rich plasma; SD, standard deviation; SPADI, Shoulder Pain and Disability Index; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
The upper part is PRP group and the lower part is HA group; only one decimal place is reserved for age.
Total WOMAC score
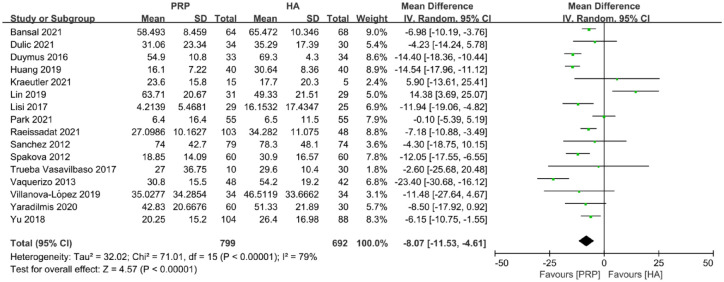
The total WOMAC score included a total of 16 articles and 1491 joints, of which 799 joints received PRP and 692 joints received HA. The total WOMAC score at the final follow-up time showed that the improvement of PRP was greater than that of the HA group [MD: −8.07; 95% confidence interval (CI): −11.53 to −4.61; p < 0.00001] (Figure 2).
Figure 2.
Forest plot showing mean difference in total WOMAC score between PRP and HA autografts.
HA, hyaluronic acid; PRP, platelet-rich plasma; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
VAS score
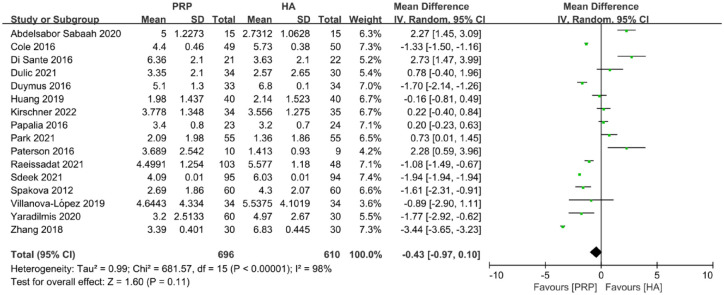
A total of 16 trials, including 1306 joints, provided useful data on VAS score. The data showed that there was no statistically significant difference between PRP and HA at the last follow-up time (MD: −0.43; CI: −0.97 to 0.10; p = 0.11) (Figure 3).
Figure 3.
Forest plot showing mean difference in VAS score between PRP and HA autografts.
HA, hyaluronic acid; PRP, platelet-rich plasma; VAS, visual analog scale.
Adverse events
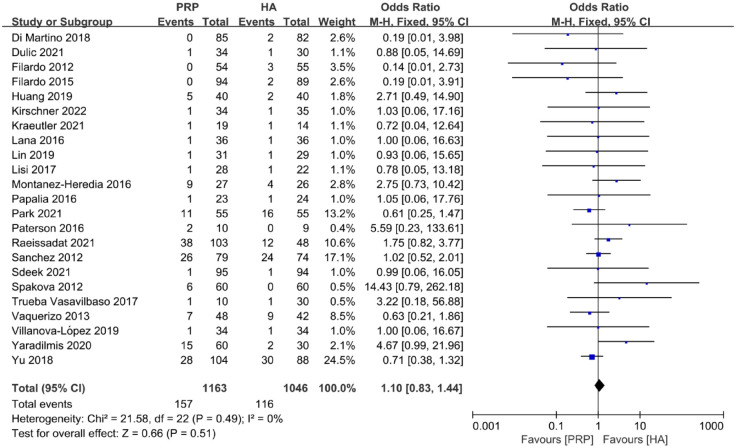
A total of 23 articles were included in the AEs evaluation, of which 1163 joints were in the PRP group and 1046 joints were in the HA group. At the final follow-up time, there was no significant difference between the PRP group and the HA group [odds ratio (OR): 1.10; 95% CI: 0.83 to 1.44; p = 0.51] (Figure 4). The AEs included pain, numbness, and swelling at the injection site and so on.51,59
Figure 4.
Forest plot show odds ratio in AEs between PRP and HA autografts.
AEs, adverse events; HA, hyaluronic acid; PRP, platelet-rich plasma.
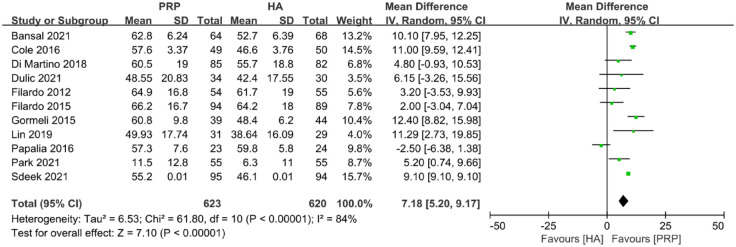
IKDC
Eleven studies were included in the IKDC analysis. In all, 623 joints were in the PRP group and 620 joints were in the HA group. PRP was better than HA at the final follow-up time (MD: 7.18; 95% CI: 5.20 to 9.17; p < 0.00001) (Figure 5).
Figure 5.
Forest plot showing mean difference in IKDC between PRP and HA autografts.
HA, hyaluronic acid; IKDC, International Knee Documentation Committee; PRP, platelet-rich plasma.
Satisfaction rate
In the assessment of satisfaction rate, including four studies, there was no significant difference between the PRP group and the HA group (OR: 1.30; 95% CI: 0.81 to 2.10; p = 0.28) (Supplementary Figure 1).
Subgroup analysis of injections and leukocyte concentration
A subgroup analysis was performed for the experiments with single-injection PRP and triple-injection PRP, and we found single-injection PRP significantly performed better than HA only in IKDC, while triple-injection PRP performed significantly better than HA in total WOMAC, IKDC. Nevertheless, there was no significant difference between single-injection PRP and triple-injection PRP in all subgroup analysis scores (Supplementary Figure 2–6). As for LP-PRP and LR-PRP, there was no significant difference between either of them and HA in terms of VAS, AEs, and satisfaction rate. LP-PRP and LR-PRP were both better than HA in IKDC score. There was no significant difference between LP-PRP and LR-PRP in total WOMAC, VAS, AEs, and the satisfaction rate, but LP-PRP was superior to LR-PRP in IKDC score (Supplementary Figure 7–11). We summarized the subgroup analysis regarding the number of injections and leukocyte concentrations in Supplementary Figure 12.
Subgroup analysis of final follow-up time and location of OA
A subgroup analysis of the final follow-up time and the location of the disease was conducted. There were no significant differences in total WOMAC, VAS, and AEs between HA and PRP in the short term (the final follow-up time < 12 months), while PRP showed better performance than HA in total WOMAC, VAS, and IKDC in the long term (the final follow-up time ⩾ 12 months). However, there were no significant differences between short-term follow-up results and long-term follow-up results when PRP was injected for OA (Supplementary Figure 13–16). As for the location of OA, PRP for knee OA was better than HA in total WOMAC and VAS scores, while there were no significant differences between PRP for non-knee OA and HA in total WOMAC, VAS, and AEs. However, PRP for knee OA showed better efficiency than PRP for non-knee OA in VAS (Supplementary Figure 17–19). We summarized the subgroup analysis regarding the final follow-up time and the location of OA in Supplementary Figure 20.
Subgroup analysis of Kellgren–Lawrence grade
A subgroup analysis of K-L grade was conducted. RCTs were divided into two subgroups based on whether the K-L grade of the patients included in the RCTs contained grade 0 or 1. There were no significant differences between the two subgroups in total WOMAC, VAS, AEs, IKDC, and satisfaction rate. In both subgroups, PRP performed better than HA in total WOMAC and IKDC, and there were no significant differences in VAS, AEs, and satisfaction rate scores between the PRP and the HA (Supplementary Figure 21–25). We summarized the subgroup analysis regarding the K-L grade in Supplementary Figure 26.
Bias assessment
Supplementary Figure 27 depicts the risk of bias assessment for randomized controlled trials. Four studies were rated as having a low risk of bias, 18 research as having a moderate risk of bias, and the remaining 8 studies were evaluated as having a high risk of bias. The most common reason for the research being unclear was that they did not disclose whether participants and outcome assessors were blinded and the specific method of randomization. Besides, among the 30 included studies, 23 provided data for the funnel plot of AEs, 16 for the funnel plot of the total WOMAC, 16 for the funnel plot of VAS, and 11 for the funnel plot of IKDC. The funnel plots of total WOMAC, VAS, AEs, and IKDC were symmetrical on the left and right sides, which showed there is no publication bias (Supplementary Figure 28–31).
Discussion
From comparing the different scoring results between PRP and HA at the final follow-up time, our study found that there was no significant difference between PRP and HA in VAS score, AEs, and satisfaction rate, which suggested that there may be no significant difference in safety and pain relief between PRP and HA in the treatment of OA. It was also found that the PRP group had a better effect in the total WOMAC score and IKDC, which suggested that PRP may have a better functional relief effect than HA. Besides, because of concerns about heterogeneity due to factors such as PRP formulation and the number of injections, subgroup analyses were performed according to injection times, leukocyte concentration in PRP, final follow-up time, location, and K-L grade of OA. It was found that there was no significant difference between single-injection PRP and triple-injection PRP, and there was no significant difference between LP-PRP and LR-PRP in total WOMAC, VAS, AEs, and satisfaction rate, but LP-PRP showed better improvement than LR-PRP in IKDC. It seemed that LP-PRP appeared to be more effective in functional relief compared with LR-PRP. Moreover, the differences in different scores between groups and subgroups were compared, and it was found that no matter whether single-injection PRP or triple-injection PRP and whether LP-PRP or LR-PRP, there was no significant difference between PRP and HA in VAS and AEs, which may indicate that the effect of different leukocyte content and injection times on pain relief and safety may not be as good as that on the functional repair. In addition, there was no significant difference between PRP for the short term and HA in total WOMAC and VAS. And PRP for the long term showed better efficiency than HA in total WOMAC, VAS, and IKDC. This may suggest that PRP has better long-term effects, but there were no significant differences between subgroups, so more studies are needed to be conducted. It also found that PRP may be more effective for pain relief in knee OA than it is for pain relief in non-knee OA.
There have been a number of studies28,29,65–68 lately comparing the efficacy and safety of PRP and HA for knee OA or hip OA, while our meta-analysis was conducted on OA including both knee OA and hip OA, as well as other OA meeting the criteria. What’s more, our study included some new RCTs.32,35,42,43,47,57,62 In addition, we conducted subgroup analyses for PRP type, number of injections, and other factors. Gong et al.65 indicated that the PRP group had a better effect than HA in WOMAC score, but there was no significant difference in IKDC, Tegner, EQ-VAS, and AEs. And our study showed PRP had a better performance compared with HA in total WOMAC score and IKDC score, while there was no significant difference between PRP and HA in VAS score at the final follow-up time. Tan et al.29 showed that PRP had a better total WOMAC, VAS, and IKDC than HA at 12 months. They also did subgroup analysis according to times and types of PRP (fresh and frozen), but found no meaningful result. Tang et al.28 found in the subgroup with injection ⩾ 2 and LP-PRP, PRP was significantly better than HA in WOMAC at 12 months. However, our subgroup analysis showed that LP-PRP had a better effect than the HA in IKDC, but there was no significant difference between single-injection PRP and triple-injection PRP. The reason for the difference between the above meta-analysis and our study may be that more studies were included in our study. What’s more, level II RCTs were excluded from our study, so the quality of the included studies was relatively high. In addition, the funnel plots of AEs, IKDC, VAS, and total WOMAC were made, which showed little publication bias.
Chouhan et al.25 showed that there was no significant difference between single-injection PRP and multiple-injection PRP in the short term (3 months), while the anti-inflammatory effect of the multiple PRP group was longer than that of the single PRP group and disease control group in the long term (6 months). But in the long run, the impact tends to weaken. It suggests that the anti-inflammatory effects of single-injection PRP and multiple-injection PRP were similar in the short term, and multiple injections of PRP may have a chondroprotective effect, but the effect is not long term. Chou et al.69 for the treatment of mild to moderate knee OA showed that the effect of triple injections of PRP was better than that of single and twice injections, which was consistent with the conclusion of the long-term result of Chouhan et al.25 The two studies hint to us that the difference in follow-up time of included studies may also be a reason for the inconsistency between our study and the above meta-analysis and the possibility that the chondroprotective effect of triple injections of PRP gradually deteriorates with time. Therefore, a subgroup analysis of the final follow-up time was also conducted, and it was found that there was no significant difference between PRP and HA in the short term in total WOMAC and VAS scores, but the long-term effect of PRP was better than HA in total WOMAC, VAS, and IKDC. However, there were no significant differences in PRP between the short-term group and the long-term group, which was probably because the other variables were not kept the same. This subgroup analysis may indicate that PRP has a better long-term effect than HA, and one of the factors influencing the scores in our injection subgroups may be the different final follow-up times.
B-lymphocytes, one type of leukocyte, contain interleukin 1 (IL-1),70 which can stimulate the expression of matrix metalloproteinases, promote chondrocyte apoptosis, and lead to cartilage degradation. In addition, IL-1 can also reduce the production of specific macromolecules, such as type II collagen in chondrocytes.71 Although transforming growth factor β1 (TGF-β1) contained in PRP can stimulate chondrocytes to produce cartilage extracellular matrix and interfere with IL-induced degradation by inducing the synthesis of IL-1 receptor antagonists71 (Supplementary Figure 32), experiments showed that the expression of TGF-β1 receptors decreased significantly in the late stage of OA,72 which makes TGF-β1 could no longer counteract the harmful effects of IL-1, and the interfere can be overwhelmed by excess leukocytes.70 Therefore, in the long term, this may lead to the long-term effect of leukocytes in the treatment of OA not being as good as that in the short term. This may be why more and more research results suggest the use of LP-PRP in the treatment of OA. This analysis is consistent with the result that the effect of LP-PRP in IKDC score in our subgroup analysis is better than that of LR-PRP. It may indicate that the reason why there was no significance in other scores between LP-PRP and PR-PRP may be the difference in the final follow-up time. In the short term, LR-PRP may have a better anti-inflammatory effect. However, in the long term, some cytokines produced by leukocytes may promote the apoptosis of chondrocytes and other cells, which aggravates the disease and leads to the final negative impact being greater than the positive impact.
Riboh et al.27 found LP-PRP was more effective than LR-PRP in function scores, while Abbas et al.73 suggested that LP-PRP and LR-PRP may have no significant difference in clinical practice. Besides, the double-blind randomized trial of Di Martino et al.74 also found no significant difference in clinical outcomes between LR-PRP and LR-PRP. However, our subgroup analysis showed that LP-PRP was better than LR-PRP in functional recovery. The reason for the inconsistent results may be the non-standardization of PRP preparation process and different follow-up times. In addition to leukocytes, there are other factors in PRP that may have an impact. Although the design of Di Martino74 tried to minimize the interference of other factors in PRP, the preparation process may still cause differences in other components. Therefore, more research is still needed to compare the differences between LP-PRP and LR-PRP and to try to standardize the preparation process of PRP.75
According to the results of subgroup analysis, LP-PRP seems to have a better effect on OA than HA. However, due to the inclusion criteria for high-quality literature, the quantity is insufficient to compare other scores, such as the short Form-36 score, and the PRP preparation process and other variables in subgroup analysis are not unique. Therefore, more studies are still needed to compare the effects of injection times, leukocyte concentration, and other factors on the therapeutic effect of PRP, and the results and suggestions are only used as a preliminary reference.
Limitations
The limitations of this meta-analysis study included that only the scores of the last follow-up time were compared without assessment of different time stages. The final follow-up time resulted in a bias. In the meantime, we did not analyze the BMI of patients in RCTs and types of HA, which may also have contributed to the bias. In addition, most of the OA studies included were knee OA studies. The number of included studies with other OA is small. Finally, the number of studies included in the satisfaction rate is too small to provide sufficient evidence to compare the efficacy of the PRP group and the HA group. More research is still needed to supplement.
Conclusion
In the treatment of OA, compared with HA, PRP performed better in the improvement of the patient’s function. There was no significant difference in VAS and AEs between the two groups, and the safety was comparable. LP-PRP looked to be superior to LR-PRP in functional recovery, but there appeared to be no significant difference in pain relief between them. There was no significant difference between single PRP and triple PRP in our subgroup analysis.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X231157043 for Comparison of clinical efficiency between intra-articular injection of platelet-rich plasma and hyaluronic acid for osteoarthritis: a meta-analysis of randomized controlled trials by Lili Chen, Shirong Jin, Yunheng Yao, Sixian He and Jinshen He in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors thank Figdraw (www.figdraw.com) for providing the original materials and drawing platform and Dr Matt Gong from University of Pittsburgh for the language editing.
Footnotes
ORCID iDs: Lili Chen  https://orcid.org/0000-0002-8572-9663
https://orcid.org/0000-0002-8572-9663
Shirong Jin  https://orcid.org/0000-0002-1862-4677
https://orcid.org/0000-0002-1862-4677
Yunheng Yao  https://orcid.org/0000-0002-7279-6306
https://orcid.org/0000-0002-7279-6306
Sixian He  https://orcid.org/0000-0002-7543-6613
https://orcid.org/0000-0002-7543-6613
Jinshen He  https://orcid.org/0000-0003-0277-3819
https://orcid.org/0000-0003-0277-3819
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lili Chen, Department of Orthopaedic Surgery, Third Xiangya Hospital of Central South University, Changsha, China.
Shirong Jin, Department of Orthopaedic Surgery, Third Xiangya Hospital of Central South University, Changsha, China.
Yunheng Yao, Department of Orthopaedic Surgery, Third Xiangya Hospital of Central South University, Changsha, China.
Sixian He, Department of Orthopaedic Surgery, Third Xiangya Hospital of Central South University, Changsha, China.
Jinshen He, Department of Orthopaedic Surgery, Third Xiangya Hospital of Central South University, Changsha 410013, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Lili Chen: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Shirong Jin: Conceptualization; Data curation; Validation; Writing – review & editing.
Yunheng Yao: Conceptualization; Data curation; Software; Validation; Writing – review & editing.
Sixian He: Conceptualization; Data curation; Validation; Writing – review & editing.
Jinshen He: Conceptualization; Data curation; Funding acquisition; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Education Reform Foundation of Central South University (No. 2021JY188), National Natural Science Foundation of China (No. 81802208), and Natural Science Foundation of Hunan Province (No. 2021JJ40922) funded this study.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: All the data are obtained from the articles which are selected from PubMed, the Cochrane Library, Web of Science, and EMBASE databases.
Systematic review registration number: CRD42021267342 (Website: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=267342)
References
- 1. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet 2015; 386: 376–387. [DOI] [PubMed] [Google Scholar]
- 2. Chen D, Shen J, Zhao W, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res 2017; 5: 16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Molnar V, Matišić V, Kodvanj I, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci 2021; 22: 9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sacitharan PK. Ageing and osteoarthritis. Subcell Biochem 2019; 91: 123–159. [DOI] [PubMed] [Google Scholar]
- 5. GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 and diseases injuries healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1603–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol 2018; 30: 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am 2020; 104: 293–311. [DOI] [PubMed] [Google Scholar]
- 8. Pereira H, Sousa DA, Cunha A, et al. Hyaluronic acid. Adv Exp Med Biol 2018; 1059: 137–153. [DOI] [PubMed] [Google Scholar]
- 9. Gupta RC, Lall R, Srivastava A, et al. Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front Vet Sci 2019; 6: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gigante A, Callegari L. The role of intra-articular hyaluronan (Sinovial) in the treatment of osteoarthritis. Rheumatol Int 2011; 31: 427–444. [DOI] [PubMed] [Google Scholar]
- 11. Billesberger LM, Fisher KM, Qadri YJ, et al. Procedural treatments for knee osteoarthritis: a review of current injectable therapies. Pain Res Manag 2020; 2020: 3873098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shahid M, Kundra R. Platelet-rich plasma (PRP) for knee disorders. EFORT Open Rev 2017; 2: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patricios J, Harmon KG, Drezner J. PRP use in sport and exercise medicine: be wary of science becoming the sham. Br J Sports Med 2022; 56: 66–67. [DOI] [PubMed] [Google Scholar]
- 14. Andia I, Atilano L, Maffulli N. Moving toward targeting the right phenotype with the right platelet-rich plasma (PRP) formulation for knee osteoarthritis. Ther Adv Musculoskelet Dis 2021; 13: 1759720X211004336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol 2009; 27: 158–167. [DOI] [PubMed] [Google Scholar]
- 16. Kon E, Di Matteo B, Delgado D, et al. Platelet-rich plasma for the treatment of knee osteoarthritis: an expert opinion and proposal for a novel classification and coding system. Expert Opin Biol Ther 2020; 20: 1447–1460. [DOI] [PubMed] [Google Scholar]
- 17. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med 2018; 11: 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci 2020; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bennell KL, Bayram C, Harrison C, et al. Trends in management of hip and knee osteoarthritis in general practice in Australia over an 11-year window: a nationwide cross-sectional survey. Lancet Reg Health West Pac 2021; 12: 100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of level I and II randomized controlled trials. Arthroscopy 2022; 38: 2035–2046. [DOI] [PubMed] [Google Scholar]
- 21. Cao Y, Zhu X, Zhou R, et al. A narrative review of the research progress and clinical application of platelet-rich plasma. Ann Palliat Med 2021; 10: 4823–4829. [DOI] [PubMed] [Google Scholar]
- 22. Andia I, Abate M. Platelet-rich plasma in the treatment of skeletal muscle injuries. Expert Opin Biol Ther 2015; 15: 987–999. [DOI] [PubMed] [Google Scholar]
- 23. Hsueh MF, Zhang X, Wellman SS, et al. Synergistic roles of macrophages and neutrophils in osteoarthritis progression. Arthritis Rheumatol 2021; 73: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loukov D, Karampatos S, Maly MR, et al. Monocyte activation is elevated in women with knee-osteoarthritis and associated with inflammation, BMI and pain. Osteoarthritis Cartilage 2018; 26: 255–263. [DOI] [PubMed] [Google Scholar]
- 25. Chouhan DK, Dhillon MS, Patel S, et al. Multiple platelet-rich plasma injections versus single platelet-rich plasma injection in early osteoarthritis of the knee: an experimental study in a guinea pig model of early knee osteoarthritis. Am J Sports Med 2019; 47: 2300–2307. [DOI] [PubMed] [Google Scholar]
- 26. Görmeli G, Görmeli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc 2017; 25: 958–965. [DOI] [PubMed] [Google Scholar]
- 27. Riboh JC, Saltzman BM, Yanke AB, et al. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med 2016; 44: 792–800. [DOI] [PubMed] [Google Scholar]
- 28. Tang JZ, Nie MJ, Zhao JZ, et al. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. J Orthop Surg Res 2020; 15: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tan J, Chen H, Zhao L, et al. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis of 26 randomized controlled trials. Arthroscopy 2021; 37: 309–325. [DOI] [PubMed] [Google Scholar]
- 30. Yaradilmis YU, Demirkale I, Safa Tagral A, et al. Comparison of two platelet rich plasma formulations with viscosupplementation in treatment of moderate grade gonarthrosis: a prospective randomized controlled study. J Orthop 2020; 20: 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trueba Vasavilbaso C, Rosas Bello CD, Medina López E, et al. Benefits of different postoperative treatments in patients undergoing knee arthroscopic debridement. Open Access Rheumatol 2017; 9: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raeissadat SA, Ghazi Hosseini P, Bahrami MH, et al. The comparison effects of intra-articular injection of platelet rich plasma (PRP), plasma rich in growth factor (PRGF), hyaluronic acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Musculoskelet Disord 2021; 22: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirschner JS, Cheng J, Creighton A, et al. Efficacy of ultrasound-guided glenohumeral joint injections of leukocyte-poor platelet-rich plasma versus hyaluronic acid in the treatment of glenohumeral osteoarthritis: a randomized, double-blind controlled trial. Clin J Sport Med 2022; 32: 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spaková T, Rosocha J, Lacko M, et al. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil 2012; 91: 411–417. [DOI] [PubMed] [Google Scholar]
- 35. Kraeutler MJ, Houck DA, Garabekyan T, et al. Comparing intra-articular injections of leukocyte-poor platelet-rich plasma versus low-molecular weight hyaluronic acid for the treatment of symptomatic osteoarthritis of the hip: a double-blind, randomized pilot study. Orthop J Sports Med 2021; 9: 2325967120969210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med 1988; 18: 1007–1019. [DOI] [PubMed] [Google Scholar]
- 37. Heller GZ, Manuguerra M, Chow R. How to analyze the visual analogue scale: myths, truths and clinical relevance. Scand J Pain 2016; 13: 67–75. [DOI] [PubMed] [Google Scholar]
- 38. da Costa BR, Saadat P, Basciani R, et al. Visual Analogue Scale has higher assay sensitivity than WOMAC pain in detecting between-group differences in treatment effects: a meta-epidemiological study. Osteoarthritis Cartilage 2021; 29: 304–312. [DOI] [PubMed] [Google Scholar]
- 39. Collins NJ, Misra D, Felson DT, et al. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res 2011; 63(Suppl. 11): S208–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salaffi F, Leardini G, Canesi B, et al. Reliability and validity of the Western Ontario and McMaster Universities (WOMAC) osteoarthritis index in Italian patients with osteoarthritis of the knee. Osteoarthritis Cartilage 2003; 11: 551–560. [DOI] [PubMed] [Google Scholar]
- 41. Robinson DM, Eng C, Makovitch S, et al. Non-operative orthobiologic use for rotator cuff disorders and glenohumeral osteoarthritis: a systematic review. J Back Musculoskelet Rehabil 2021; 34: 17–32. [DOI] [PubMed] [Google Scholar]
- 42. Abdelsabor Sabaah HM, El Fattah RA, Al Zifzaf D, et al. A comparative study for different types of thumb base osteoarthritis injections: a randomized controlled interventional study. Ortop Traumatol Rehabil 2020; 22: 447–454. [DOI] [PubMed] [Google Scholar]
- 43. Bansal H, Leon J, Pont JL, et al. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: correct dose critical for long term clinical efficacy. Sci Rep 2021; 11: 3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med 2017; 45: 339–346. [DOI] [PubMed] [Google Scholar]
- 45. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med 2019; 47: 347–354. [DOI] [PubMed] [Google Scholar]
- 46. Di Sante L, Villani C, Santilli V, et al. Intra-articular hyaluronic acid vs platelet-rich plasma in the treatment of hip osteoarthritis. Med Ultrason 2016; 18: 463–468. [DOI] [PubMed] [Google Scholar]
- 47. Dulic O, Rasovic P, Lalic I, et al. Bone marrow aspirate concentrate versus platelet rich plasma or hyaluronic acid for the treatment of knee osteoarthritis. Medicina 2021; 57: 1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Duymus TM, Mutlu S, Dernek B, et al. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc 2017; 25: 485–492. [DOI] [PubMed] [Google Scholar]
- 49. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med 2015; 43: 1575–1582. [DOI] [PubMed] [Google Scholar]
- 50. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord 2012; 13: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang Y, Liu X, Xu X, et al. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis: a prospective randomized controlled study. Orthopade 2019; 48: 239–247. [DOI] [PubMed] [Google Scholar]
- 52. Lana JF, Weglein A, Sampson SE, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med 2016; 12: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin KY, Yang CC, Hsu CJ, et al. Intra-articular injection of platelet-rich plasma is superior to hyaluronic acid or saline solution in the treatment of mild to moderate knee osteoarthritis: a randomized, double-blind, triple-parallel, placebo-controlled clinical trial. Arthroscopy 2019; 35: 106–117. [DOI] [PubMed] [Google Scholar]
- 54. Lisi C, Perotti C, Scudeller L, et al. Treatment of knee osteoarthritis: platelet-derived growth factors vs. Clin Rehabil 2018; 32: 330–339. [DOI] [PubMed] [Google Scholar]
- 55. Montañez-Heredia E, Irízar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritic knee pain: a randomized clinical trial in the context of the Spanish National Health Care System. Int J Mol Sci 2016; 17: 1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Papalia R, Zampogna B, Russo F, et al. Comparing hybrid hyaluronic acid with PRP in end career athletes with degenerative cartilage lesions of the knee. J Biol Regul Homeost Agents 2016; 30(Suppl. 1): 17–23. [PubMed] [Google Scholar]
- 57. Park YB, Kim JH, Ha CW, et al. Clinical efficacy of platelet-rich plasma injection and its association with growth factors in the treatment of mild to moderate knee osteoarthritis: a randomized double-blind controlled clinical trial as compared with hyaluronic acid. Am J Sports Med 2021; 49: 487–496. [DOI] [PubMed] [Google Scholar]
- 58. Paterson KL, Nicholls M, Bennell KL, et al. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Musculoskelet Disord 2016; 17: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sánchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy 2012; 28: 1070–1078. [DOI] [PubMed] [Google Scholar]
- 60. Sdeek M, Sabry D, El-Sdeek H, et al. Intra-articular injection of platelet rich plasma versus hyaluronic acid for moderate knee osteoarthritis. A prospective, double-blind randomized controlled trial on 189 patients with follow-up for three years. Acta Orthop Belg 2021; 87: 729–734. [DOI] [PubMed] [Google Scholar]
- 61. Vaquerizo V, Plasencia MÁ, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy 2013; 29: 1635–1643. [DOI] [PubMed] [Google Scholar]
- 62. Villanova-López MM, Núñez-Núñez M, Fernández-Prieto D, et al. Randomized, double-blind, controlled trial, phase III, to evaluate the use of platelet-rich plasma versus hyaluronic acid in hip coxarthrosis. Rev Esp Cir Ortop Traumatol 2020; 64: 134–142. [DOI] [PubMed] [Google Scholar]
- 63. Yu W, Xu P, Huang G, et al. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp Ther Med 2018; 16: 2119–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang YF. Platelet-rich plasma therapy in refractory knee osteoarthritis combined with infection. Int J Clin Exp Med 2018; 11: 4801–4807. [Google Scholar]
- 65. Gong H, Li K, Xie R, et al. Clinical therapy of platelet-rich plasma vs hyaluronic acid injections in patients with knee osteoarthritis: a systematic review and meta-analysis of randomized double-blind controlled trials. Medicine 2021; 100: e25168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhao D, Pan JK, Yang WY, et al. Intra-articular injections of platelet-rich plasma, adipose mesenchymal stem cells, and bone marrow mesenchymal stem cells associated with better outcomes than hyaluronic acid and saline in knee osteoarthritis: a systematic review and network meta-analysis. Arthroscopy 2021; 37: 2298–2314. [DOI] [PubMed] [Google Scholar]
- 67. Migliorini F, Driessen A, Quack V, et al. Comparison between intra-articular infiltrations of placebo, steroids, hyaluronic and PRP for knee osteoarthritis: a Bayesian network meta-analysis. Arch Orthop Trauma Surg 2021; 141: 1473–1490. [DOI] [PubMed] [Google Scholar]
- 68. Medina-Porqueres I, Ortega-Castillo M, Muriel-Garcia A. Effectiveness of platelet-rich plasma in the management of hip osteoarthritis: a systematic review and meta-analysis. Clin Rheumatol 2021; 40: 53–64. [DOI] [PubMed] [Google Scholar]
- 69. Chou SH, Shih CL. Efficacy of different platelet-rich plasma injections in the treatment of mild-moderate knee osteoarthritis: a systematic review and meta-analysis. Int J Clin Pract 2021; 75: e14068. [DOI] [PubMed] [Google Scholar]
- 70. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med 2011; 39: 2135–2140. [DOI] [PubMed] [Google Scholar]
- 71. Pujol JP, Chadjichristos C, Legendre F, et al. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect Tissue Res 2008; 49: 293–297. [DOI] [PubMed] [Google Scholar]
- 72. Boumediene K, Conrozier T, Mathieu P, et al. Decrease of cartilage transforming growth factor-beta receptor II expression in the rabbit experimental osteoarthritis – potential role in cartilage breakdown. Osteoarthritis Cartilage 1998; 6: 146–149. [DOI] [PubMed] [Google Scholar]
- 73. Abbas A, Du JT, Dhotar HS. The effect of leukocyte concentration on platelet-rich plasma injections for knee osteoarthritis: a network meta-analysis. J Bone Joint Surg Am 2022; 104: 559–570. [DOI] [PubMed] [Google Scholar]
- 74. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med 2022; 50: 609–617. [DOI] [PubMed] [Google Scholar]
- 75. Singh H, Knapik DM, Polce EM, et al. Relative efficacy of intra-articular injections in the treatment of knee osteoarthritis: a systematic review and network meta-analysis. Am J Sports Med 2021; 50: 3140–3148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X231157043 for Comparison of clinical efficiency between intra-articular injection of platelet-rich plasma and hyaluronic acid for osteoarthritis: a meta-analysis of randomized controlled trials by Lili Chen, Shirong Jin, Yunheng Yao, Sixian He and Jinshen He in Therapeutic Advances in Musculoskeletal Disease