Abstract
Scalable cognitive paradigms that provide metrics such as the Computerized Cognitive Composite (C3) may be sensitive enough to relate to Alzheimer's disease biomarkers in the preclinical clinically unimpaired (CU) stage. We examined CU older adults (n = 3287) who completed alternate versions of the C3 approximately 51 days apart. A subset of CU with abnormal amyloid also completed tau positron emission tomography (PET) imaging. C3 initial performance and practice effects were examined in relation to amyloid status and continuous regional tau burden. Initial C3 performance was associated with amyloid status across all participants, and with tau burden in the medial temporal lobe and early cortical regions in CU with abnormal amyloid. Short‐term practice effects were associated with reduced tau in these regions in CU with abnormal amyloid, but were not associated with amyloid status. Thus, computerized cognitive testing repeated over a short follow‐up period provides additional insights into early Alzheimer's disease processes.
Keywords: amyloid, computerized assessments, practice effects, preclinical Alzheimer's disease, tau
1. BACKGROUND
A major barrier for Alzheimer's disease (AD) prevention strategies is the identification of sensitive cognitive tests that change during the course of preclinical AD and can be administered in a large‐scale multi‐site setting. Computerized assessments, 1 providing metrics such as the Computerized Cognitive Composite (C3), 2 , 3 , 4 are one promising approach as they require relatively low burden and cost, and are correlated with gold‐standard paper‐and‐pencil measures of early cognitive decline such as the Preclinical Alzheimer's Cognitive Composite (PACC). 4 Practice effects, or expected improved performance on repeated testing reflecting learning, 5 , 6 can easily be quantified from repeat testing over short intervals on computerized assessments. Diminished practice effects have been associated with future cognitive decline, 7 , 8 , 9 amyloid status, 10 , 11 and neurodegeneration biomarkers including CSF amyloid and tau 12 across the AD spectrum. 13 Importantly, practice effects can be due to conceptual‐ and form‐related familiarity as individuals gain experience with both general task procedures and specific test stimuli if the same test versions are used, or conceptual‐related familiarity alone if alternate stimuli are used. How performance on computerized metrics like the C3, both from single and repeat assessments, relate to both early amyloid and tau burden in a large multi‐site cohort of preclinical AD individuals has not yet been examined.
The C3 consists of three memory‐based measures targeting discrimination, associative memory, and visual memory that are sensitive to early decline in AD and change over time. 2 , 3 , 4 Cross‐sectional C3 performance distinguishes between impaired and clinically unimpaired (CU) individuals, 2 , 3 , 14 as well as between CU individuals with abnormal (A+) and normal (A−) amyloid. 4 , 15 A+ CU show significantly worse cross‐sectional C3 performance than A−, but practice effects are comparable between A‐ and A+ CU. 4 Only one study to date has examined initial C3 performance and practice effects in relation to tau. This study of 81 A− and 33 A+ CU found no association between C3 initial performance and tau. However, lower C3 practice effects over 3 months derived from a mixture of same (capturing both conceptual‐ and form‐related familiarity) and alternate (capturing conceptual‐related familiarity alone) test versions were associated with higher amyloid burden and tau deposition in entorhinal cortex and inferior temporal (IT) lobe. 16
While the literature on how C3 performance, specifically, is impacted by AD biomarkers is small, reduced practice effects on other neuropsychological tests have been shown to reflect underlying AD neuropathology (for review, see 13 ). For example, domain‐specific practice effects on the Repeatable Battery for the Assessment of Neuropsychological Status memory subscales only were identified in CU older adults and were reduced in the A+ group. 10 Reduced practice effects have even been identified in A− CU older adults who are APOE e4 carriers, highlighting the utility of practice effects for early detection. 17
The goal of the present study was to extend our understanding of cross‐sectional performance and practice effects on the C3 in a large‐scale multi‐site study of preclinical AD. We leveraged a sample of 4141 CU individuals with amyloid positron emission tomography (PET) and two C3s derived from alternate test versions completed approximately 51 days apart, including a subset of 343 A+ CU with tau PET imaging. 18 , 19 We hypothesized that initial C3 performance would relate to global amyloid status, and that practice effects would provide an additional unique signal of tau in the medial temporal lobe (MTL) and select early cortical regions.
RESEARCH IN CONTEXT
Systemic Review: The authors reviewed the literature on practice effects and computerized cognitive assessments with particular attention to the Computerized Cognitive Composite (C3), a metric derived from three computerized memory‐focused tests. Crucially, no previous studies have examined how performance on computerized metrics like the C3, both from single and repeat assessments, relate to both early amyloid and tau burden in a large multi‐site cohort of preclinical Alzheimer's disease.
Interpretation: In clinically unimpaired older adults, initial C3 performance was significantly associated with both amyloid and tau, whereas short‐term practice effects demonstrated a unique additional signal reflecting tau burden in the medial temporal lobe and early cortical regions in amyloid positive individuals.
Future Directions: Future work is needed to determine if practice effects on computerized assessments predict future cognitive decline and how practice effects obtained from same versus alternate test versions affect this predictive power.
2. METHODS
2.1. Participants
The Anti‐Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) study (https://a4study.org) is a secondary prevention trial in preclinical AD that screened participants 65–85 years old who were CU (Mini Mental State Examination score of 25–30, global Clinical Dementia Rating score of 0, Logical Memory Delayed Recall IIa score of 6–18). 18 All participants provided written informed consent prior to participation and Institutional Review Board approval was obtained at each study site. Participants completed the C3 at initial Visit 1 and repeat Visit 3 (median = 51, interquartile range = 38–70 days apart). Eligibility based on unimpaired cognitive status and lack of significant co‐morbid medical conditions was assessed during Visit 1. Those meeting Visit 1 eligibility underwent an amyloid PET scan with18‐F‐florbetapir to determine Aβ status at Visit 2. Of the 4486 participants who completed amyloid PET, 99 participants with Japanese as their primary language did not complete the C3 due to concerns about test validity, leaving 4387 remaining participants. After exclusion related to missingness and score validity (Figure 1A), 4287 participants were included in analyses focused on initial C3 performance and 4141 participants were included in analyses focused on demographics, practice effects, and amyloid status. A smaller subset of 446 participants completed tau PET imaging with18‐F‐flortaucipir. We focused our analyses examining C3 relationships with regional tau on A+ individuals given that most participants with tau PET data were A+ (only 55/446 scanned with tau PET were A− 20 ). Based on the intersection of available tau PET data with valid C3 data, 354 A+ participants were included in analyses examining initial C3 performance and 343 A+ participants were included in practice effect analyses in relation to tau.
FIGURE 1.
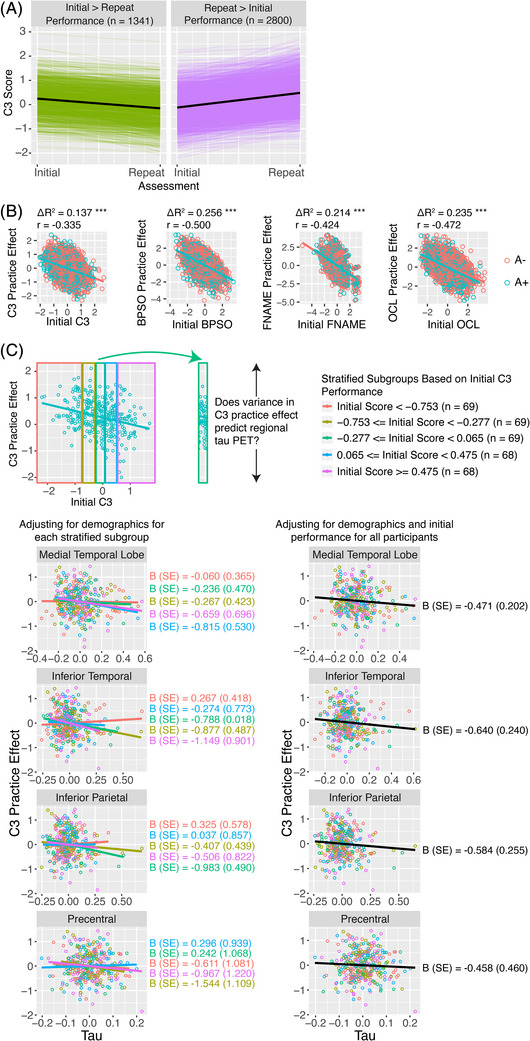
C3 practice effects. (A) C3 score changes from initial to repeat assessments. Colored lines depict scores for an individual subject. Black lines indicate the mean score change for those who declined from initial to repeat assessments (n = 1341) and for those who improved from initial to repeat assessments (n = 2800). (B) Relation between initial performance and practice effects after controlling for age, sex, education, race, and ethnicity. (C) Association between tau and C3 practice effects. Participants were first stratified into 5 quartiles based on initial C3 performance. By examining stratified groups (i.e., restricting the range of baseline values), we removed the negative association between initial performance and practice effects. Associations between tau and C3 practice effects for each stratified group after adjusting for demographic variables were examined (left column). Despite the reduced power in the stratified groups, the beta weights are similar to those calculated from all participants after adjusting for demographics and initial performance (right column). A−, normal amyloid; A+, abnormal amyloid; BPSO, behavioral pattern separation task‐object; C3, computerized cognitive composite; FNAME, Face Name Associative Memory Examination; OCL, One Card Learning.
2.2. Amyloid status and tau PET imaging
Amyloid status was determined using a hybrid quantitative/qualitative method used by A4. 2 Flortaucipir data (5‐min frames) and T1‐weighted MRI were downloaded from LONI and processed as recently described. 20 Tau SUVRs in bilateral MTL (average of bilateral entorhinal cortex and amygdala regions), IT, inferior parietal (IP), and precentral regions were calculated using cerebellar cortex as the reference region.
2.3. Computerized cognitive testing
The C3 combines three memory‐based scores: (1) Behavioral Pattern Separation Task‐Object (BPSO) lure discrimination index, (2) Face Name Associative Memory Exam (FNAME) accuracy on the face‐name matching task, and (3) One Card Learning (OCL) accuracy from the CogState Brief Battery (Table S1). A composite score was created in part to maximize signal to noise ratio in a population with expected subtle cognitive deficits. 4
In BPSO, participants are shown the same image interspersed with novel and similar images, and participants must categorize each image as old, similar, or new. 21 A higher lure discrimination index (a d'prime metric computed using “similar” responses / similar items—“similar” responses / new items) indicates an improved ability to correctly identify lures, which has been linked to pattern separation mechanisms in the hippocampus. 22 In FNAME, participants are shown 12 face‐name pairs and after a delay, participants complete a first letter name recall task, a face‐name matching task, and a face recognition task. Accuracy from the face‐name matching task has previously been linked to amyloid burden in normal aging cohorts. 23 In OCL, participants learn a series of playing cards by indicating “yes” or “no” as to whether the card was previously presented. 24 Poorer performance on this task has been related to higher levels of CSF phosphorylated‐tau‐181/AB‐42 in late middle‐aged participants. 25 Alternate versions of C3 memory subtests were used at the initial and repeat assessments.
2.4. Statistical analyses
Because we were interested in change in scores over time, z‐scores for the BPSO, FNAME, and OCL tasks at the initial visit and at Visit 3 were internally normed using the means and standard deviations from all valid data at the initial assessment (n = 4387). The C3 was created from mean z‐scores if at least two of the three C3 tasks were completed. Practice effects were defined as change (i.e., subtraction) in z‐scores from initial to repeat assessments. All analyses were repeated with practice effects defined as reliable change indices using the Jacobson and Traux (1991) 26 formula and all results remained consistent.
A series of linear regressions were used to examine the impact of demographic factors on initial C3 performance and C3 practice effects. Given the short time between the initial and repeat administration of the C3, and to understand potential regression to the mean effects, we additionally assessed the relationship between initial performance and practice effects. We then examined biomarker (i.e., amyloid status, MTL tau burden) relationships with initial C3 performance, C3 practice effects, and C3 practice effects accounting for initial performance; separate models were used for amyloid status and MTL tau. Follow‐up analyses using the individual BPSO, FNAME, and OCL scores were also conducted to determine if any one subtest was driving effects. MTL tau models were repeated using IT, IP, and precentral regions to explore C3 relationships with tau outside of the MTL. Additional details are provided in Methods S1.
3. RESULTS
3.1. Demographics
Participant demographics are listed in Table 1 and the CONSORT diagram is shown in Figure S1. Initial C3 performance and practice effects were associated with several demographic variables. Specifically, worse initial C3 performance was associated with older age and lower education, and worse performance was observed in males, non‐White, and Hispanic/Latino participants (Table 2A). Diminished C3 practice effects were also associated with older age, younger education, and non‐White and Hispanic/Latino status, but only after adjusting for initial performance (Tables 2B,C). Similar patterns were observed for individual BPSO, FNAME, and OCL tests.
TABLE 1.
Demographic and clinical information for participants included in initial performance analyses as well as analyses focused on practice effects.
| Initial performance subset (n = 4287) | Practice effect subset (n = 4141) | A+ initial performance subset with tau data (n = 354) | A+ practice effect subset with tau data (n = 343) | |
|---|---|---|---|---|
| Age, mean (SD) | 71.25 (4.648) | 71.23 (4.643) | 71.93 (4.706) | 71.95 (4.721) |
| Sex, n (%) | ||||
| Male | 1715 (40%) | 1663 (40%) | 142 (40%) | 137 (40%) |
| Female | 2572 (60%) | 2478 (60%) | 212 (60%) | 206 (60%) |
| Education, mean (SD) | 16.61 (2.844) | 16.61 (2.853) | 16.23 (2.841) | 16.23 (2.841) |
| Race, n (%) | ||||
| White | 4003 (93%) | 3869 (94%) | 338 (95%) | 327 (95%) |
| Asian | 68 (2%) | 65 (2%) | 2 (1%) | 2 (1%) |
| Black | 155 (4%) | 148 (4%) | 9 (3%) | 9 (3%) |
| Other | 61 (1%) | 59 (1%) | 5 (1%) | 5 (1%) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 136 (3%) | 132 (3%) | 9 (3%) | 8 (2%) |
| Not Hispanic or Latino | 4117 (96%) | 3976 (96%) | 338 (95%) | 329 (96%) |
| Unknown or Not Reported | 34 (1%) | 33 (1%) | 7 (2%) | 6 (2%) |
| APOE, n (%) | ||||
| E2/E2 | 25 (1%) | 24 (1%) | 1 (0%) | 1 (0%) |
| E2/E3 | 427 (10%) | 414 (10%) | 15 (4%) | 15 (4%) |
| E2/E4 | 110 (3%) | 105 (3%) | 13 (4%) | 12 (3%) |
| E3/E3 | 2296 (54%) | 2223 (54%) | 129 (36%) | 126 (37%) |
| E3/E4 | 1252 (29%) | 1209 (29%) | 168 (47%) | 163 (48%) |
| E4/E4 | 137 (3%) | 134 (3%) | 24 (7%) | 24 (7%) |
| Unknown | 40 (0.9%) | 32 (0.8%) | 4 (1.1%) | 2 (0.6%) |
| Amyloid Status, n (%) | ||||
| A− | 3017 (70%) | 2906 (70%) | 0 (0%) | 0 (0%) |
| A+ | 1270 (30%) | 1235 (30%) | 354 (100%) | 343 (100%) |
| PACC, mean (SD) | 0.008 (2.530) | −0.003 (2.530) | −0.591 (2.785) | −0.576 (2.771) |
| GDS, mean (SD) | 1.005 (1.445) | 0.998 (1.429) | 0.983 (1.275) | 0.980 (1.271) |
| STAI, mean (SD) | 9.917 (3.123) | 9.887 (3.101) | 10.150 (3.056) | 10.130 (3.062) |
Abbreviations: A−, normal amyloid; A+, abnormal amyloid; C3, computerized cognitive composite; GDS, Geriatric Depression Scale; PACC, Preclinical Alzheimer's Cognitive Composite; STAI, State‐Trait Anxiety Inventory.
TABLE 2.
Beta weights (standard errors) and p‐values for demographic variables predicting (A) initial performance and (B) practice effects, as well as (C) the association between demographic variables and initial performance in predicting practice effects.
| C3 | BPSO | FNAME | OCL | |
|---|---|---|---|---|
| A. Initial performance | ||||
| Age | −0.034 (0.002); p < 0.001 | −0.031 (0.003); p < 0.001 | −0.040 (0.003 ); p < 0.001 | −0.032 (0.003); p < 0.001 |
| Sex (male vs. female) | −0.099 (0.020); p < 0.001 | −0.027 (0.031); p = 0.387 | −0.289 (0.031); p < 0.001 | 0.015 (0.031); p = 0.632 |
| Education | 0.027 (0.003); p < 0.001 | 0.017 (0.005); p = 0.001 | 0.028 (0.005); p < 0.001 | 0.038 (0.005); p < 0.001 |
| Race (Black vs. White) | −0.400 (0.052); p < 0.001 | −0.465 (0.081); p < 0.001 | −0.398 (0.079); p < 0.001 | −0.317 (0.080); p < 0.001 |
| Race (Asian vs. White) | −0.176 (0.077); p = 0.023 | −0.306 (0.121); p = 0.012 | −0.360 (0.119); p = 0.002 | 0.132 (0.120); p = 0.271 |
| Race (Other vs. White) | −0.229 (0.083); p = 0.006 | −0.326 (0.130); p = 0.012 | 0.013 (0.130); p = 0.919 | −0.352 (0.130); p = 0.007 |
| Ethnicity (Hispanic/Latino vs. not Hispanic/Latino | −0.341 (0.056); p < 0.001 | −0.351 (0.089); p < 0.001 | −0.289 (0.087); p < 0.001 | −0.344 (0.088); p < 0.001 |
| Ethnicity (Unknown/Not eported vs. not Hispanic/Latino) | 0.059 (0.109); p = 0.590 | −0.030 (0.169); p = 0.857 | 0.085 (0.166); p = 0.610 | 0.119 (0.169); p = 0.479 |
| B. Practice effects | ||||
| Intercept | 0.281 (0.013); p < 0.001 | 0.420 (0.022); p < 0.001 | 0.299 (0.025); p < 0.001 | 0.123 (0.019); p < 0.001 |
| Age | −0.008 (0.002); p < 0.001 | 0.000 (0.003); p = 0.901 | −0.021 (0.004); p < 0.001 | −0.006 (0.003); p = 0.043 |
| Sex (male vs. female) | −0.000 (0.200); p = 0.991 | 0.028 (0.033); p = 0.394 | −0.039 (0.039); p = 0.316 | −0.015 (0.030); p = 0.614 |
| Education | 0.001 (0.003); p = 0.714 | 0.002 (0.006); p = 0.679 | 0.008 (0.007); p = 0.227 | −0.008 (0.005); p = 0.131 |
| Race (Black vs. White) | 0.011 (0.518); p = 0.826 | 0.322 (0.087); p < 0.001 | −0.225 (0.100); p = 0.025 | −0.099 (0.078); p = 0.201 |
| Race (Asian vs. White) | −0.122 (0.773); p = 0.114 | −0.287 (0.130); p = 0.027 | 0.048 (0.152); p = 0.753 | −0.075 (0.116); p = 0.519 |
| Race (other vs. White) | 0.044 (0.083); p = 0.592 | 0.043 (0.139); p = 0.755 | −0.012 (0.164); p = 0.940 | 0.107 (0.125); p = 0.390 |
| Ethnicity (Hispanic/Latino vs. not Hispanic/Latino | −0.024 (0.056); p = 0.668 | −0.032 (0.094); p = 0.737 | 0.006 (0.110); p = 0.957 | −0.010 (0.084); p = 0.909 |
| Ethnicity (unknown/not reported vs. not Hispanic/Latino) | −0.053 (0.108); p = 0.621 | −0.107 (0.180); p = 0.551 | 0.053 (0.208); p = 0.797 | −0.107 (0.162); p = 0.508 |
| C. Practice effects controlling for initial performance | ||||
| Intercept | 0.305 (0.012); p < 0.001 | 0.446 (0.019); p < 0.001 | 0.381 (0.022); p < 0.001 | 0.132 (0.017); p < 0.001 |
| Age | −0.021 (0.002); p < 0.001 | −0.016 (0.003); p < 0.001 | −0.043 (0.004); p < 0.001 | −0.020 (0.003); p < 0.001 |
| Sex (male vs. female) | −0.035 (0.019); p = 0.059 | 0.015 (0.029); p = 0.611 | −0.195 (0.035); p < 0.001 | 0.022 (0.026); p = 0.398 |
| Education | 0.011 (0.003); p < 0.001 | 0.011 (0.005); p = 0.030 | 0.023 (0.006); p < 0.001 | 0.010 (0.005); p = 0.033 |
| Race (Black vs. White) | −0.138 (0.048); p = 0.004 | 0.078 (0.075); p = 0.300 | −0.469 (0.089); p < 0.001 | −0.247 (0.068); p < 0.001 |
| Race (Asian vs. White) | −0.184 (0.072); p = 0.010 | −0.441 (0.112); p < 0.001 | −0.148 (0.135); p = 0.274 | −0.014 (0.101); p = 0.889 |
| Race (other vs. White) | −0.042 (0.077); p = 0.581 | −0.112 (0.120); p = 0.350 | −0.003 (0.146); p = 0.985 | −0.073 (0.109); p = 0.502 |
| Ethnicity (Hispanic/Latino vs. not Hispanic/Latino | −0.144 (0.052); p = 0.006 | −0.214 (0.081); p = 0.008 | −0.160 (0.098); p = 0.102 | −0.173 (0.074); p = 0.018 |
| Ethnicity (unknown/not reported vs. not Hispanic/Latino) | −0.037 (0.100); p = 0.710 | −0.144 (0.155); p = 0.352 | 0.100 (0.184); p = 0.586 | −0.058 (0.141); p = 0.682 |
| Initial performance | −0.365 (0.014); p < 0.001 | −0.532 (0.014); p < 0.001 | −0.575 (0.017); p < 0.001 | −0.458 (0.013); p < 0.001 |
Abbreviations: BPSO, behavioral pattern separation task‐object; C3, computerized cognitive composite; FNAME, Face Name Associative Memory Examination; OCL, One Card Learning.
3.2. Regression to the mean and practice effects
The overall magnitude of practice effects at the group level was positive and significantly different than zero (indicated by the model intercept terms in Table 2B,C), showing that on average, participants improved on the C3 from their initial to repeat assessments. Direct examination of C3 scores indicated a significant positive practice effect, t(4140) = −29.036, p < 0.001, from the initial (mean C3 = −0.002) to repeat (mean C3 = 0.277) assessment with 2800/4141 (68%) of participants showing improved performance (Figure 1A). C3 initial performance was also negatively associated with practice effects (Figure 1B) suggesting regression to the mean; initial performance was not at ceiling for any C3 component. Both practice effects and regression to the mean effects were present for the C3 and the individual components (Table 2C).
3.3. Initial C3 performance and biomarkers
Worse initial C3 performance was associated with amyloid positivity and greater tau burden in the MTL, IT, and IP (Table 3). Amyloid explained 0.2% of the variance in C3 performance across the full sample of A− and A+, whereas MTL, IT, and IP tau explained 1%–3% of the variance within the A+ group (Figure 2). For individual components, BPSO performance was significantly related to both amyloid positivity and greater MTL tau (marginally associated with IT tau), FNAME was associated with MTL tau, and OCL was associated with amyloid positivity and IP tau. Associations with precentral tau were not significant with the exception of an association with FNAME.
TABLE 3.
Beta weights (Standard Errors) and p‐values for amyloid status and tau predicting initial computerized cognitive composite performance, computerized cognitive composite practice effects, and computerized cognitive composite practice effects controlling for initial performance. All models additionally control for age, sex, education, race, and ethnicity.
| C3 | BPSO | FNAME | OCL | ||
|---|---|---|---|---|---|
| Initial performance | |||||
| Amyloid status (A+ vs. A−) | n = 4287 | −0.075 (0.021); p < 0.001 | −0.081 (0.033); p = 0.015 | −0.027 (0.033); p = 0.405 | −0.113 (0.033); p < 0.001 |
| MTL tau in A+ only | n = 354 | −0.728 (0.220); p = 0.001 | −0.725 (0.337); p = 0.032 | −0.791 (0.346); p = 0.023 | −0.587 (0.346); p = 0.090 |
| IT tau in A+ only | n = 354 | −0.782 (0.264); p = 0.003 | −0.742 (0.399); p = 0.064 | −0.652 (0.415); p = 0.117 | −0.862 (0.038); p = 0.038 |
| IP tau in A+ only | n = 354 | −0.721 (0.281); p = 0.011 | −0.592 (0.423); p = 0.163 | −0.744 (0.444); p = 0.095 | −0.747 (0.440); p = 0.091 |
| Precentral tau in A+ only | n = 354 | 0.835 (0.510); p = 0.102 | 0.407 (0.766); p = 0.596 | 1.623 (0.795); p = 0.042 | 0.630 (0.797); p = 0.430 |
| Practice effects | |||||
| Amyloid status (A+ vs. A−) | n = 4141 | 0.009 (0.021); p = 0.687 | 0.007 (0.035); p = 0.839 | −0.007 (0.041); p = 0.856 | 0.017 (0.032); p = 0.597 |
| MTL tau in A+ only | n = 343 | −0.228 (0.212); p = 0.283 | −0.433 (0.375); p = 0.249 | −0.551 (0.441); p = 0.212 | 0.288 (0.321); p = 0.370 |
| IT tau in A+ only | n = 343 | −0.378 (0.252); p = 0.135 | −0.603 (0.444); p = 0.175 | −0.761 (0.526); p = 0.149 | 0.217 (0.384); p = 0.572 |
| IP tau in A+ only | n = 343 | −0.347 (0.268); p = 0.198 | −0.436 (0.470); p = 0.355 | −0.761 (0.564); p = 0.235 | −0.010 (0.408); p = 0.981 |
| Precentral tau in A+ only | n = 343 | −0.716 (0.484); p = 0.140 | 0.803 (0.849); p = 0.345 | −2.760 (1.002); p = 0.006 | −0.123 (0.737); p = 0.867 |
| Practice effects controlling for initial performance | |||||
| Amyloid status (A+ vs. A−) | n = 4141 | −0.018 (0.020); p = 0.348 | −0.033 (0.030); p = 0.282 | −0.019 (0.036); p = 0.599 | −0.038 (0.028); p = 0.174 |
| MTL tau in A+ only | n = 343 | −0.471 (0.202); p = 0.020 | −0.811 (0.335); p = 0.016 | −1.074 (0.381); p = 0.005 | 0.021 (0.281); p = 0.941 |
| IT tau in A+ only | n = 343 | −0.640 (0.240); p = 0.008 | −0.988 (0.395); p = 0.013 | −1.187 (0.454); p = 0.009 | −0.178 (0.337); p = 0.598 |
| IP tau in A+ only | n = 343 | −0.584 (0.255); p = 0.023 | −0.739 (0.419); p = 0.079 | −1.155 (0.487); p = 0.018 | −0.353 (0.357); p = 0.324 |
| Precentral tau in A+ only | n = 343 | −0.458 (0.460); p = 0.320 | −1.009 (0.757); p = 0.183 | −1.745 (0.877); p = 0.047 | 0.164 (0.643); p = 0.799 |
Note: Amyloid status and tau were examined using separate linear models.
Abbreviations: A−, normal amyloid; A+, abnormal amyloid; BPSO, behavioral pattern separation task‐object; C3, computerized cognitive composite; FNAME, Face Name Associative Memory Examination; IP, inferior parietal; IT, inferior temporal; MTL, medial temporal lobe; OCL, One Card Learning.
FIGURE 2.
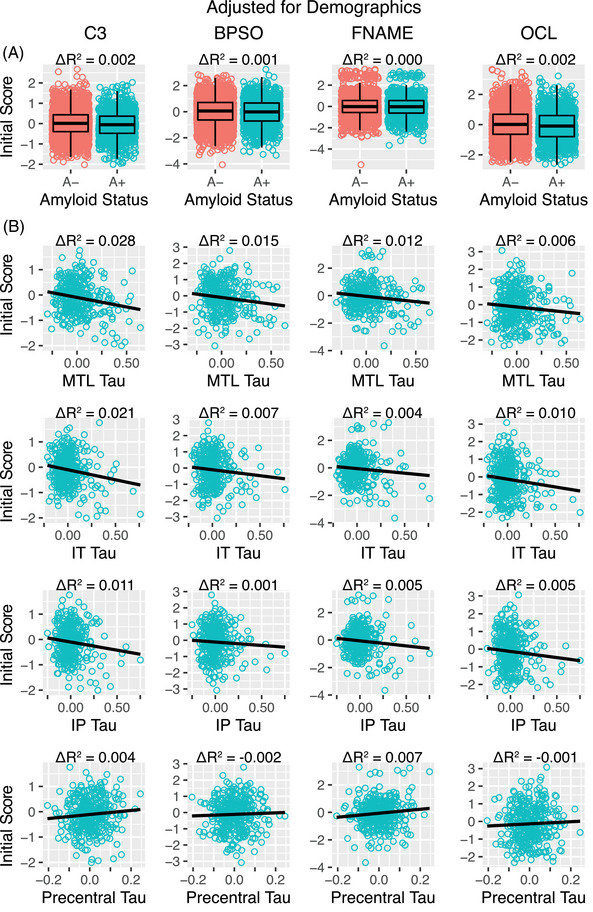
Initial C3 score performance and its association with amyloid and tau. (A) Initial C3 performance was higher in amyloid negative (A−, pink) than amyloid positive (A+, green) individuals. (B) Greater tau burden was associated with worse initial C3 performance in A+ individuals. C3 scores shown here were adjusted for age, sex, education, race, and ethnicity for visualization purposes. BPSO, behavioral pattern separation task‐object; C3, computerized cognitive composite; FNAME, Face Name Associative Memory Examination; OCL, One Card Learning.
3.4. C3 practice effects and biomarkers
Reduced MTL, IT, and IP tau were significantly associated with stronger practice effects using the C3 and individual components of BPSO and FNAME when controlling for initial performance (Table 3; Figure 3). However, C3 practice effects were not associated with either amyloid or tau when initial performance was not considered (Table 3; Figure S2). This pattern demonstrates that for a given initial C3 performance level, the variance in practice effects is associated with regional tau, an effect that would be undetected without controlling for initial performance (presumably because of the strong regression to the mean effect, Figure 1B). To further confirm this finding, we divided participants into five equally sized quantiles based on initial C3 performance to reduce the range of initial C3 performance values (Methods S2). Doing so removed any significant association between initial performance and practice effects, and confirmed that variance in practice effects continued to be related to regional tau (Figure 1C). This analysis provides further evidence that variability in task performance is associated with elevated tau for a given level of initial C3 performance. Practice effects on the C3 or individual components were not related to amyloid status, with and without controlling for initial performance (Table 3).
FIGURE 3.
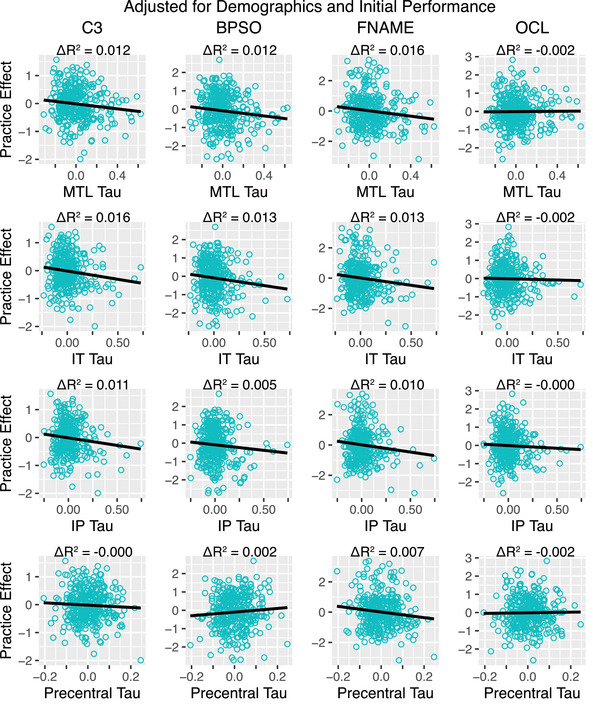
Relation between tau and C3 practice effects within A+ individuals after adjusting for demographics and initial performance. Practice effects and tau SUVRs shown here were adjusted for age, sex, education, race, ethnicity, and initial C3 performance for visualization purposes. BPSO, behavioral pattern separation task‐object; C3, computerized cognitive composite; FNAME, Face Name Associative Memory Examination; OCL, One Card Learning.
4. DISCUSSION
In a large sample of CU participants screened for a preclinical AD prevention trial, computerized cognitive testing performance using the C3 was related to amyloid, MTL tau, and early cortical tau burden. Initial C3 performance was significantly associated with both amyloid and tau, whereas short‐term practice effects demonstrated a unique additional signal reflecting tau burden in the MTL and early cortical regions. The consistent relationships between C3 performance and AD biomarkers suggest that computerized assessments repeated over a short timescale could potentially be a cost‐effective and sensitive measure that reflects biomarker evidence of AD, though effect sizes and demographic biases must be considered.
Our study demonstrates that initial and repeated C3 assessments are associated with amyloid and tau biomarkers in preclinical AD. In a previously published study examining the feasibility and validity of C3 in A4, initial performance on the C3 as well as BPSO and OCL tests significantly differed between A+ and A− CU groups, and participants showed significant practice effects (using alternate versions) that did not differ in strength between amyloid groups. 4 We replicate and extend these findings to show that amyloid explains 0.2% of the initial performance variance across A− and A+, whereas MTL, IT, and IP tau explains 1%–3% of the initial variance in C3 performance within A+. Although 1%–3% explained variance is relatively low, effects in preclinical AD are typically small and even genetic risk factors including APOE only explain 8%–10% of the variation in cognitive decline in this early AD stage. 27 Interestingly, we also show a C3 practice effect of approximately ¼ of a standard deviation, a magnitude that is consistent with other cognitive tests, patient samples, retest intervals, and demographic variables. 5 , 6 , 28
The lack of amyloid association with practice effects contributes to the larger mixed literature that has shown both diminished practice effects (predominantly examined using the same test versions) associated with higher levels of amyloid 10 , 29 , 30 , 31 as well as no significant relationship. 4 , 31 , 32 , 33 Importantly, with our additional assessment of tau, we show that although practice effects are not related to amyloid status, diminished practice effects are significantly related to tau burden in the MTL, IT, and IP among A+ CUs when initial performance is considered. This is consistent with a previous study showing that diminished practice effects on FNAME is associated with increased tau in entorhinal cortex and amygdala in 33 A+ CU, and partially consistent with examinations of 81 A– and 33 A+ CU showing that C3 practice effects were associated with both global amyloid and tau in the entorhinal cortex and IT. 16 Critically, the use of alternate versions in A4 may have weakened the strength of practice effects, which could in turn be diminishing relations with biomarkers. Indeed, reduced practice effects have been associated with greater amyloid and tau burden on the FNAME when the same test stimuli was used, but these biomarker relationships were not present when alternate test versions were used. 34 Thus, our findings may be underestimating practice effect associations with biomarkers. Nevertheless, our study demonstrates that in a large cohort of preclinical AD individuals, repeated computerized assessment over a short timescale of approximately 2 months yields additional unique information about tau burden.
Initial C3 performance was strongly related to all demographic variables. Older, lower educated, male, non‐White, and Hispanic/Latino participants performed worse on the C3 as well as its subcomponents. Black participants performed almost half a point worse than White participants on the C3, Asian and Other races performed around 0.2 points worse than White participants, and Hispanic/Latino participants performed around 0.3 points worse than non‐Hispanic/Latino participants; these race and ethnicity effects were generally present on the individual BPSO, FNAME, and OCL tests. Notably, these race and ethnicity effects were present despite efforts to design and use more culturally neutral tests. Given that it is well known that recognition of own‐race faces is better than other‐race faces 35 and the majority of the participants were White, 30% of faces presented in the FNAME task were non‐White. Additionally, the use of cards in the OCL task minimizes reliance on language. 24 , 36 Further research is needed to understand the cause of lower scores in underrepresented populations so that appropriate test adjustments can be made. Refinement of tasks to minimize the effects of culture, race, and ethnicity; adjustment for demographic variables; and calculation of demographically adjusted norms will be critical for widespread valid implementation of new computerized assessments.
There has been suggestion that practice effects may be a unique cognitive variable that is less influenced by demographic variables since an individual's performance is compared with their own prior performance. 37 In line with this suggestion, our results show that practice effects are not consistently related to any demographic variables when initial performance is not considered. However, our results also demonstrate that when initial performances are factored into practice effects, there are consistent relationships with demographic variables, similar to those seen with initial performance. This discrepancy is likely due to the strong relationship between initial performance and practice effects reflecting a regression to the mean effect. With additional assessments, such as what is done in burst designs for digital cognitive assessments that involve high frequency testing over a short time span, 38 scores are expected to eventually converge at the mean, presumably reflecting a more accurate and robust quantification of an individual's ability. Taken together, our results suggest that regression to the mean effects can obscure other important relationships, highlighting the need to factor in initial performance.
Our results also show that practice effects provide a reliable and unique signal of tau that is not captured by initial cognitive performance. We demonstrated that diminished C3 practice effects were consistently related to increased tau in the MTL, IT, and IP within A+ individuals; there was no association with amyloid status. Significant tau accumulation within and beyond the MTL is common in the preclinical AD stage. 20 The MTL is crucial for encoding new information into long‐term memory, 39 a necessary process for improved performance on repeated task exposure, and tau deposition in this region is associated with poorer memory performance even among CU. 40 , 41 In addition to the initial manifestation of tau in the MTL, IT and IP also show early tau accumulation 42 and are regions with relatively high rates of annual tau accumulation among A+ CU and A+ mild cognitive impairment. 43 The association between practice effects and tau burden was also observed when examining practice effects on individual tests of BPSO and FNAME, but not OCL. While both BPSO 44 , 45 , 46 and FNAME 47 , 48 , 49 , 50 were developed on the basis of fMRI paradigms shown to robustly activate the MTL and cortical regions important for memory, OCL is a more continuous working memory‐type measure that was not specifically designed to be sensitive in AD. Thus, OCL may be less reliant on the MTL in comparison to BPSO and FNAME. Notably, across the C3 and its individual tests, practice effects were not significantly related to amyloid status or tau when initial effects were not considered. Thus, consideration of the regression to the mean effect has important implications for uncovering relationships between practice effects and tau burden.
Several limitations are important to consider. First, our study consisted of predominantly White, highly educated individuals who self‐selected into the study. The generalizability of findings is to be determined and this will be especially important to examine in the context of digital literacy, especially as technology familiarity was not assessed. Second, the current C3 subcomponents focus on recognition instead of recall, and retrieval‐based measures may yield even stronger relationships with AD biomarkers. Third, we are unable to comment on all types of practice effects because alternate test versions were used. Although present, the practice effects detected here with alternate test versions may be weaker than what has been shown in other studies that used the same test stimuli. Weaker practice effects may have allowed for an especially strong regression to the mean effect and may partially explain the lack of association between practice effects and tau burden when baseline performance was not included. Thus, same versus alternate test versions have important implications for detecting biomarker presence 34 and use of the same test stimuli could more strongly capture biomarker associations in preclinical AD. Fourth, whether baseline performance should be included when assessing change is a topic of statistical debate. 51 , 52 Finally, other factors such as medication changes can affect neuropsychological performance but were outside the scope of the current study.
The present study has important implications for clinical trials. Our results show that a single administration of the C3 relates to both amyloid status and tau burden, and that repeated administration over a short timescale provides an additional independent association with tau burden within preclinical AD individuals. Thus, frequently administered computerized cognitive assessments yield unique variables, such as practice effects, that are sensitive enough to relate to AD biomarkers. Although the effect sizes were relatively small and demographic biases must be considered, computerized assessments and quantification of practice effects for clinical trials have the potential to reduce site burden and cost 8 as well as increase accessibility given its potential for remote assessment. Additionally, our findings highlight that practice effects on cognitive tests are present even when alternate test versions are used. Together with other studies that have demonstrated similar effects, 11 our results challenge the notion that alternate test forms eliminate practice effects.
CONFLICT OF INTEREST STATEMENT
Elizabeth C. Mormino is a paid consultant for Roche, Genentech, and Eli Lilly. Kathleen L. Poston received consulting fees from CuraSen Therapeutics Inc and serves on the Scientific Advisory Board for Amprion and Curasen Therapeutics Inc. Kathryn V. Papp is a paid consultant for Prothena and CogState. Keith A. Johnson is a paid consultant for Merck and Novartis. Reisa A. Sperling is a paid consultant for from AC Immune, Acumen, Alnylam, Cytox, Genentech, Janssen, JOMDD, Nervgen, Neuraly, Neurocentria, Oligomerix, Prothena, Renew, Shionogi, Vigil Neuroscience, Ionis, and Vaxxinity. Dorene M. Rentz is a paid consultant for Biogen Idec, Novartis, and the Dana Foundation. All other authors have no disclosures. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants provided written informed consent prior to participation and Institutional Review Board approval was obtained at each study site.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The A4 Study is a secondary prevention trial in preclinical AD, aiming to slow cognitive decline associated with brain amyloid accumulation in clinically normal older individuals. The A4 Study is funded by a public‐private‐philanthropic partnership, including funding from the National Institutes of Health‐National Institute on Aging, Eli Lilly and Company, Alzheimer's Association, Accelerating Medicines Partnership, GHR Foundation, an anonymous foundation and additional private donors, with in‐kind support from Avid and Cogstate. The companion observational Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) Study is funded by the Alzheimer's Association and GHR Foundation. The A4 and LEARN Studies are led by Dr. Reisa Sperling at Brigham and Women's Hospital, Harvard Medical School and Dr. Paul Aisen at the Alzheimer's Therapeutic Research Institute (ATRI), University of Southern California. The A4 and LEARN Studies are coordinated by ATRI at the University of Southern California, and the data are made available through the Laboratory for Neuro Imaging at the University of Southern California. The participants screening for the A4 Study provided permission to share their de‐identified data in order to advance the quest to find a successful treatment for AD. We acknowledge the dedication of all the participants, the site personnel, and all of the partnership team members who continue to make the A4 and LEARN Studies possible. The complete A4 Study Team list is available on: a4study.org/a4‐study‐team. This study was supported by grants K99AG071837 (Dr. Young), P30AG066515 (Drs. Mormino, Poston), R01NS115114 (Drs. Mormino, Poston), K01AG051718 (Dr. Mormino), P01AG036694 (Drs. Johnson, Sperling), and P50AG005134 (Drs. Johnson, Sperling) from the National Institute of Health; and AARFD‐21‐849349 (Dr. Young) from the Alzheimer's Association.
Young CB, Mormino EC, Poston KL, et al. Computerized cognitive practice effects in relation to amyloid and tau in preclinical Alzheimer's disease: Results from a multi‐site cohort. Alzheimer's Dement. 2023;15:e12414. 10.1002/dad2.12414
REFERENCES
- 1. Öhman F, Hassenstab J, Berron D, Schöll M, Papp KV. Current advances in digital cognitive assessment for preclinical Alzheimer's disease. Alzheimers Dement Diagn Assess Dis Monit. 2021;13:e12217. doi: 10.1002/dad2.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rentz DM, Dekhtyar M, Sherman J, et al. The feasibility of at‐home iPad cognitive testing for use in clinical trials. J Prev Alzheimers Dis. 2016;3:8‐12. doi: 10.14283/jpad.2015.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buckley RF, Sparks KP, Papp KV, et al. Computerized cognitive testing for use in clinical trials: a comparison of the NIH toolbox and CogState C3 batteries. J Prev Alzheimers Dis. 2017;4:3‐11. doi: 10.14283/jpad.2017.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papp KV, Rentz DM, Maruff P, et al. The computerized cognitive composite (C3) in A4, an Alzheimer's disease secondary prevention trial. J Prev Alzheimers Dis. 2021;8:59‐67. doi: 10.14283/jpad.2020.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldberg TE, Harvey PD, Wesnes KA, Snyder PJ, Schneider LS. Practice effects due to serial cognitive assessment: implications for preclinical Alzheimer's disease randomized controlled trials. Alzheimers Dement Amst Neth. 2015;1:103‐111. doi: 10.1016/j.dadm.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hausknecht JP, Halpert JA, Di Paolo NT, Moriarty Gerrard MO. Retesting in selection: a meta‐analysis of coaching and practice effects for tests of cognitive ability. J Appl Psychol. 2007;92:373‐385. doi: 10.1037/0021-9010.92.2.373 [DOI] [PubMed] [Google Scholar]
- 7. Duff K, Lyketsos CG, Beglinger LJ, et al. Practice effects predict cognitive outcome in amnestic mild cognitive impairment. Am J Geriatr Psychiatry. 2011;19:932‐939. doi: 10.1097/JGP.0b013e318209dd3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanderson‐Cimino M, Elman JA, Tu XM, et al. Cognitive practice effects delay diagnosis of MCI: implications for clinical trials. Alzheimers Dement Transl Res Clin Interv. 2022;8:e12228. doi: 10.1002/trc2.12228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hassenstab J, Ruvolo D, Jasielec M, Xiong C, Grant E, Morris JC. Absence of practice effects in preclinical Alzheimer's disease. Neuropsychology. 2015;29:940‐948. doi: 10.1037/neu0000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng B, Udeh‐Momoh C, Watermeyer T, et al. Practice effect of repeated cognitive tests among older adults: associations with brain amyloid pathology and other influencing factors. Front Aging Neurosci. 2022;14:909614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aschenbrenner AJ, Hassenstab J, Wang G, et al. Avoid or embrace? practice effects in Alzheimer's disease prevention trials. Front Aging Neurosci. 2022;14:883131. doi: 10.3389/fnagi.2022.883131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oltra‐Cucarella J, Sánchez‐SanSegundo M, Ferrer‐Cascales R. Predicting Alzheimer's disease with practice effects, APOE genotype and brain metabolism. Neurobiol Aging. 2022;112:111‐121. doi: 10.1016/j.neurobiolaging.2021.12.011 [DOI] [PubMed] [Google Scholar]
- 13. Jutten RJ, Grandoit E, Foldi NS, et al. Lower practice effects as a marker of cognitive performance and dementia risk: a literature review. Alzheimers Dement Amst Neth. 2020;12:e12055. doi: 10.1002/dad2.12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maruff P, Lim YY, Darby D, et al. Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer's disease. BMC Psychol. 2013;1:30. doi: 10.1186/2050-7283-1-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sato K, Ihara R, Suzuki K, et al. Predicting amyloid risk by machine learning algorithms based on the A4 screen data: application to the Japanese Trial‐Ready Cohort Study. Alzheimers Dement Transl Res Clin Interv. 2021;7:e12135. doi: 10.1002/trc2.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jutten RJ, Rentz DM, Fu JF, et al. Monthly at‐home computerized cognitive testing to detect diminished practice effects in preclinical Alzheimer's disease. Front Aging Neurosci. 2022;13:800126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lim YY, Baker JE, Mills A, et al. Learning deficit in cognitively normal APOE ε4 carriers with LOW β‐amyloid. Alzheimers Dement Diagn Assess Dis Monit. 2021;13:e12136. doi: 10.1002/dad2.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sperling RA, Donohue MC, Raman R, et al. Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol. 2020;77:735‐745. doi: 10.1001/jamaneurol.2020.0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Young CB, Winer JR, Younes K, et al. Divergent cortical tau positron emission tomography patterns among patients with preclinical Alzheimer disease. JAMA Neurol. 2022;79:592‐603. doi: 10.1001/jamaneurol.2022.0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442‐2449. doi: 10.1016/j.neuropsychologia.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirwan CB, Stark CEL. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn Mem Cold Spring Harb N. 2007;14:625‐633. doi: 10.1101/lm.663507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rentz DM, Amariglio RE, Becker JA, et al. Face‐name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49:2776‐2783. doi: 10.1016/j.neuropsychologia.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fredrickson J, Maruff P, Woodward M, et al. Evaluation of the usability of a brief computerized cognitive screening test in older people for epidemiological studies. Neuroepidemiology. 2010;34:65‐75. doi: 10.1159/000264823 [DOI] [PubMed] [Google Scholar]
- 25. Racine AM, Clark LR, Berman SE, et al. Associations between performance on an abbreviated cogstate battery, other measures of cognitive function, and biomarkers in people at risk for Alzheimer's disease. J Alzheimers Dis JAD. 2016;54:1395‐1408. doi: 10.3233/JAD-160528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12‐19. doi: 10.1037//0022-006x.59.1.12 [DOI] [PubMed] [Google Scholar]
- 27. Ge T, Sabuncu MR, Smoller JW, Sperling RA, Mormino EC. Dissociable influences of APOE ε4 and polygenic risk of AD dementia on amyloid and cognition. Neurology. 2018;90:e1605‐e1612. doi: 10.1212/WNL.0000000000005415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calamia M, Markon K, Tranel D. Scoring higher the second time around: meta‐analyses of practice effects in neuropsychological assessment. Clin Neuropsychol. 2012;26:543‐570. doi: 10.1080/13854046.2012.680913 [DOI] [PubMed] [Google Scholar]
- 29. Duff K, Foster NL, Hoffman JM. Practice effects and amyloid deposition: preliminary data on a method for enriching samples in clinical trials. Alzheimer Dis Assoc Disord. 2014;28:247‐252. doi: 10.1097/WAD.0000000000000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duff K, Hammers DB, Dalley BCA, et al. Short‐term practice effects and amyloid deposition: providing information above and beyond baseline cognition. J Prev Alzheimers Dis. 2017;4:87‐92. doi: 10.14283/jpad.2017.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ihara R, Iwata A, Suzuki K, et al. Clinical and cognitive characteristics of preclinical Alzheimer's disease in the Japanese Alzheimer's Disease Neuroimaging Initiative Cohort. Alzheimers Dement N Y. 2018;4:645‐651. doi: 10.1016/j.trci.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson RS, Capuano AW, Yu L, et al. Neurodegenerative disease and cognitive retest learning. Neurobiol Aging. 2018;66:122‐130. doi: 10.1016/j.neurobiolaging.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Machulda MM, Hagen CE, Wiste HJ, et al. Practice effects and longitudinal cognitive change in clinically normal older adults differ by Alzheimer imaging biomarker status. Clin Neuropsychol. 2017;31:99‐117. doi: 10.1080/13854046.2016.1241303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samaroo A, Amariglio RE, Burnham S, et al. Diminished Learning Over Repeated Exposures (LORE) in preclinical Alzheimer's disease. Alzheimers Dement Diagn Assess Dis Monit. 2020;12:e12132. doi: 10.1002/dad2.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanaka JW, Kiefer M, Bukach CM. A holistic account of the own‐race effect in face recognition: evidence from a cross‐cultural study. Cognition. 2004;93:B1‐B9. doi: 10.1016/j.cognition.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 36. Maruff P, Thomas E, Cysique L, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol. 2009;24:165‐178. doi: 10.1093/arclin/acp010 [DOI] [PubMed] [Google Scholar]
- 37. Duff K, Callister C, Dennett K, Tometich D. Practice effects: a unique cognitive variable. Clin Neuropsychol. 2012;26:1117‐1127. doi: 10.1080/13854046.2012.722685 [DOI] [PubMed] [Google Scholar]
- 38. Brewster PWH, Rush J, Ozen L, Vendittelli R, Hofer SM. Feasibility and psychometric integrity of mobile phone‐based intensive measurement of cognition in older adults. Exp Aging Res. 2021;47:303‐321. doi: 10.1080/0361073X.2021.1894072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87‐96. doi: 10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- 40. Lowe VJ, Bruinsma TJ, Wiste HJ, et al. Cross‐sectional associations of tau‐PET signal with cognition in cognitively unimpaired adults. Neurology. 2019;93:e29‐e39. doi: 10.1212/WNL.0000000000007728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maass A, Lockhart SN, Harrison TM, et al. Entorhinal tau pathology, episodic memory decline, and neurodegeneration in aging. J Neurosci. 2018;38:530‐543. doi: 10.1523/JNEUROSCI.2028-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110‐119. doi: 10.1002/ana.24546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Young CB, Landau SM, Harrison TM, Poston KL, Mormino EC. Influence of common reference regions on regional tau patterns in cross‐sectional and longitudinal [18F]‐AV‐1451 PET data. NeuroImage. 2021;243:118553. doi: 10.1016/j.neuroimage.2021.118553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stark SM, Yassa MA, Stark CEL. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem Cold Spring Harb N. 2010;17:284‐288. doi: 10.1101/lm.1768110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age‐related memory deficits linked to circuit‐specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011;108:8873‐8878. doi: 10.1073/pnas.1101567108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL. High‐resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. NeuroImage. 2010;51:1242‐1252. doi: 10.1016/j.neuroimage.2010.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miller SL, Celone K, DePeau K, et al. Age‐related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105:2181‐2186. doi: 10.1073/pnas.0706818105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Brien JL, O'Keefe KM, LaViolette PS, et al. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74:1969‐1976. doi: 10.1212/WNL.0b013e3181e3966e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Celone KA, Calhoun VD, Dickerson BC, et al. Alterations in memory networks in mild cognitive impairment and alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222‐10231. doi: 10.1523/JNEUROSCI.2250-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sperling R, Chua E, Cocchiarella A, et al. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. NeuroImage. 2003;20:1400‐1410. doi: 10.1016/S1053-8119(03)00391-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001;1:6. doi: 10.1186/1471-2288-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? an example with education and cognitive change. Am J Epidemiol. 2005;162:267‐278. doi: 10.1093/aje/kwi187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


