Abstract
The recent availability of susceptibility testing for Helicobacter pylori infections in the United Sates has resulted in paradigm shifts in the diagnosis, therapy, and follow-up of H. pylori infections. Here, we reviewed the English literature concerning changes in H. pylori diagnosis and therapy with an emphasis on the last 3 years. We focus on the new methods that offer rapid and convenient susceptibility testing using either invasive (endoscopic) or noninvasive (stool) methods of obtaining test material. We also discuss the implications of this availability on therapy and follow-up after therapy. The approach to therapy was categorized into four groups: (1) therapies that can be used empirically, (2) therapies that should be restricted to those that are susceptibility-based, (3) potentially effective therapies that have yet to be optimized for local use, and (4), therapies that contain unneeded antibiotics that should not be prescribed. The most convenient and efficient method of susceptibility testing is by using reflexive stool testing in which if the sample is positive, it is automatically also used for determination of susceptibility. Reflexive testing can also be done via reflexive ordering (e.g., for all positive urea breath tests). The post therapy test-of-cure has emerged as a critical component of therapy as it not only provides feedback regarding treatment success but when combined with susceptibility testing also provide evidence regarding the cause of failure (e.g., poor adherence versus emergence of resistance during therapy. Susceptibility testing has made even the most current H. pylori guidelines for diagnosis and therapy generally obsolete. Clarithromycin, metronidazole, and levofloxacin triple therapies should only be administered as susceptibility-based therapy. Regimens containing unneeded antibiotics should not be given. We provide recommendations regarding the details and indications for all current therapies.
Keywords: Helicobacter pylori, heteroresistance, molecular stool testing, optimization, paradigm shift, susceptibility testing, test-of-cure, treatment, treatment failure, treatment guidelines
Introduction
Helicobacter pylori infections are etiologically related to peptic ulcer, atrophic gastritis, and gastric cancer. Since H. pylori’s discovery in the 1980’s, antimicrobial therapy for H. pylori infections has primarily been empiric with potentially effective therapies being identified by trial and error; cure rates were generally low1 (Figures 1 and 2). It soon became apparent that finding an ideal therapy was going to be difficult as resistance to the commonly used antibiotics developed rapidly and exerted a detrimental effect on cure rates. Antibiotic regimens approved by the US Food and Drug Administration (FDA) also often had relatively low cure rates as the focus was on healing of peptic ulcers rather than on cure of the infection.2 Although H. pylori was declared a carcinogen by the World Health Organization in 19943 the clinical focus remained on peptic ulcer which, at that time, was a major health problem and, in the United States, gastric cancer was becoming increasingly rare. The concept that cure of the infection would also prevent development of atrophic gastritis, which had long been recognized a precursor lesion for gastric cancer,4 was not immediately recognized.
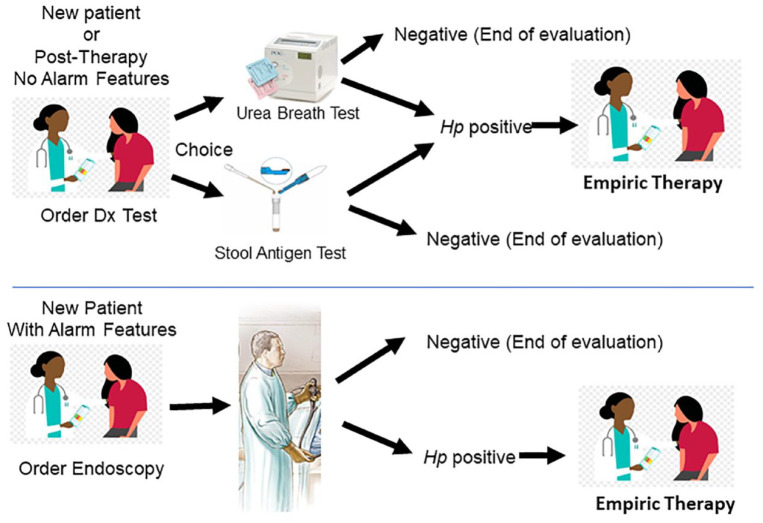
Figure 1.
An illustration of the current and now superseded approach to diagnosis and empiric therapy of Helicobacter pylori for patients with and without alarm features.
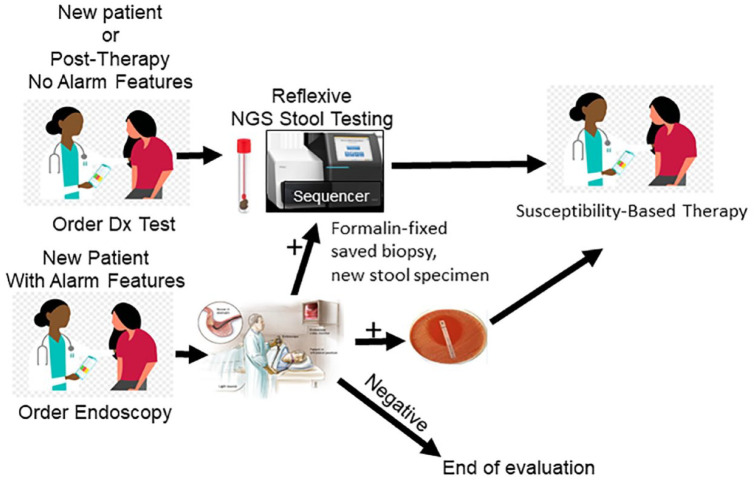
Figure 2.
An illustration of the use of next generation sequencing for Helicobacter pylori susceptibility using stool, fresh or formalin fixed biopsies, or bacteria from culture plates. Results include amoxicillin, metronidazole, clarithromycin, rifabutin, tetracycline, and levofloxacin.
Treatment of H. pylori changed the management of peptic ulcer from ‘once an ulcer, always an ulcer’ to a one-off condition as the focus switched from simply ulcer healing to eradication of the infection in order to cure a disease and to prevent and eliminate gastric cancer. The fact that the infection was chronic, largely inaccessible in the stomach, and its primary clinical manifestations were gastric diseases, resulted in Gastroenterology rather than Infectious Diseases taking the lead and effectively ‘owing’ the infectious disease. Hundreds, if not thousands, of comparative treatment trials were subsequently done in which differences in outcome rather than cure rates were the major outcome variable.2,5 Despite using therapies containing up to five antibiotics, resistance rates have continued to rise and cure rates decline.6 Clinical treatment guidelines and recommendations remained focused on empiric therapy despite that it is an infectious disease for which effective therapy relies on patient-specific susceptibility data as well as the implementation of the principles of antimicrobial stewardship.2,7 The European H. pylori study group even established a registry to collect data about what clinicians did unobtrusively (while not interfering in their management practices).8 Their initial results confirmed what should have been expected (i.e., cure rates were low, and the management practices were ‘heterogeneous, suboptimal and discrepant with current recommendations’).8 Probably the most important outcome of the registry was not recognized by the authors at the time of the initial report. That outcome was the insight that the registry participants actually had ready access to the practice- and patient-specific susceptibility data available in the data in the form of the post treatment test-of-cure results.9 Use of that data would likely have changed the outcome. An accompanying commentary noted that H. pylori treatment prescribed empirically without knowledge of its local effectiveness or susceptibility patterns was destined to fail and was contrary to the principles of antimicrobial stewardship which requires empiric therapies to not only be highly effective, but also to employ strategies designed to ensure that high effectiveness remains sustainable.9 The study design precluded the inclusion of a control group which in retrospect significantly limited the usefulness of the data obtained. For example, without a control group it is impossible to distinguish between natural history from being the result of participation in the registry as a cause of any changes in practice that occurred. Finally, with this type of data there is often a tendency to ‘salami-slice’. The original publication has tables of what appears to be comprehensive treatment data for all sites, and it is unclear if, or how many, of the subsequent region- or group-specific data publications were primarily derived from the initially published database. It is also not clear whether similar data could not have been obtained by analysis of insurance claims. The overall poor cure rates and the continuing high use of clarithromycin suggests that a better use of the funds needed to support the registry might have been to better educate the clinicians, for example in the use of test-of-cure as a surrogate method of providing immediate local feedback regarding success or after failure of each treatment or, to have designed a comparison of how to best offer susceptibility testing.
Patient-specific susceptibility testing for Helicobacter pylori has recently become universally available in the United States such that the experiment of observing how these tests integrated into practice is now actually underway (Table 1).10
Table 1.
Where to obtain Helicobacter pylori susceptibility testing in the United States.
| Test | Laboratory | Web address |
|---|---|---|
| Culture | ARUP Laboratories | https://ltd.aruplab.com/Tests/Pub/2006686 |
| Culture | Mayo Clinical Laboratories | https://www.mayocliniclabs.com/test-catalog/Overview/62769 |
| Culture | QUEST | https://testdirectory.questdiagnostics.com/test/test-detail/8395/helicobacter-pylori-culture?cc=MASTER |
| Culture | LabCorp | https://www.labcorp.com/tests/180885/i-helicobacter-pylori-i-culture |
| Culture | Microbiology Specialists Inc. | https://microbiologyspecialists.com/helicobacter-pylori-testing/ |
| Reflex stool by polymerase chain reaction | Mayo Clinical Laboratories | https://www.mayocliniclabs.com/test-catalog/Overview/607594 |
| Next generation sequencing | American Molecular Laboratories | http://amlaboratories.com/testing-services/helicobacter-pylori-detection-antibiotic-resistant-analysis/ |
| Reflex stool y next generation sequencing |
American Molecular Laboratories |
http://amlaboratories.com/testing-services/helicobacter-pylori-detection-antibiotic-resistant-analysis/ |
Source: From Graham and Moss’s10 study, with permission.
The widespread availability of testing presents new possibilities and expectations. For example, the field has progressed from obtaining a simple ‘infected or not infected’ result to the ability to select a therapy with a high likelihood of success based on antimicrobial susceptibility or test-of-cure results. Post therapy, not only can we identify treatment failure, but we can judge the reason for failure based on whether the infection remains susceptible to the drugs used or whether it has become resistant. How to use this information is discussed in detail below. First, we will consider how to identify the most efficient and cost-effective use of susceptibility testing in the diagnosis, treatment, and follow-up of patients with H. pylori infections.
Whom to test?
As the importance of H. pylori as a cause of human disease has increasingly been recognized, treatment recommendations have evolved from (a) curing those with peptic ulcer to (b) curing all H. pylori infections identified unless there are compelling reasons not to (e.g., advanced age, etc.)11,12 and to (c) proactively searching for and eradicating H. pylori with the goal of eliminating gastric cancer.13,14 The solution to the ‘whom to test’ question is predicated on the presumption that all those infected can be cured.11,12,15 The recommendations for whom to test have greatly expanded and now include all first-degree relatives of those with H. pylori infections, those with active or a history of peptic ulcer disease or gastric cancer and all those living in the same household as a patient discovered to have an H. pylori infection11,14,15 (Table 2).
Table 2.
Recommendations for Helicobacter pylori testing for individuals and populations.
| Recommendations | Agreement | Evidence level |
|---|---|---|
| Houston Consensus Conference Recommendations | ||
| When to test a specific individual?* | ||
| 1. With suspected H. pylori infection (e.g., active Duodenal Ulcer) | 100% | High |
| 2. With current or past gastric or duodenal ulcers | 100% | High |
| 3. With uninvestigated dyspepsia | 100% | High |
| 4. With gastric mucosa-associated lymphoid tissue lymphoma | 100% | Moderate |
| 5. Family members residing in same household of patients with proven active H. pylori infections | 91% | Moderate |
| 6. Family history of peptic ulcer disease | 91% | Moderate |
| 7. With family history of gastric cancer | 100% | Moderate |
| 8. First-generation immigrants from high prevalence areas | 82% | High |
| 9. High risk groups (e.g., in the United States: Latino and African American racial or other ethnic groups) | 91% | Low |
| Taipei Global Consensus Recommendations | ||
| Which specific populations to screen? | ||
| 1. Populations with high incidence of gastric cancer | 84% | Low |
| 2. Young adults in high incidence populations before the development of atrophic gastritis and intestinal metaplasia | 84% | Low |
| 3. Young adults in high incidence populations to reduce the transmission to their children | 92% | Low |
| 4. Populations with high incidence being integrated or included into the national healthcare priorities | 92% | Low |
Extension of treatment recommendations to family members and those with H. pylori-related diseases is based on the high frequency of transmission within families. China, a country with one of the highest rates of gastric cancer, has also recently introduced family screening as part of their efforts to eliminate H. pylori and gastric cancer.16
Which tests for the diagnosis of H. pylori
Treatment of H. pylori requires proof of an active infection and therefore excludes serology which cannot distinguish between an active infection and a serologic scar of past infections. Current tests for active infection include urea breath tests, stool antigen tests, histology with (immune)-staining for the bacteria, culture, and molecular tests to identify H. pylori DNA in biologic specimens such as stools or gastric biopsies.17 The availability of susceptibility testing resulted in the initial test needing to address (a) whether the patient is infected and (b) if so, what is the antibiotic susceptibility pattern? Until recently, obtaining susceptibility testing required both endoscopies to obtain gastric mucosa biopsies and a laboratory willing to provide culture and susceptibility testing. While gastroenterologists were ready and willing to do the required endoscopy and collect the specimens, there were few laboratories that offered culture and susceptibility testing. In the last year this has changed in the United States as most major diagnostic laboratories now offer H. pylori culture and susceptibility testing (Table 1).10,17 Although the ready availability of culture and susceptibility testing has provided a partial solution it did not alter the requirement for endoscopy. Obtaining and processing gastric biopsies is both time consuming and expensive; whenever specimens for culture must be shipped to distant locations, positive results are obtained in far less than 100% of cases.17 However, practical, rapid, less expensive, and noninvasive alternative methods are now commercially available.10 These new methods are based on the same principle as the stool antigen test (i.e., H. pylori reside in the stomach and are shed in the stool thus providing a ready source of H. pylori antigens and DNA). Molecular testing of stools using the polymerase chain reaction (PCR) can also detect genetic changes in H. pylori DNA that correlate with resistance.10,17 While PCR-based testing for clarithromycin and levofloxacin in stools or gastric biopsy specimens has long been available to research laboratories, only recently have kits for clarithromycin susceptibility testing of stools become commercially available.17,18 PCR testing for clarithromycin is now commercially offered in the United States by the Mayo Clinical Laboratory17 and kits for PCR-based testing for clarithromycin are available and have been approved for clinical use by European regulatory agencies.14,17,18
Molecular susceptibility testing based on next-generation sequencing (NGS) is also now available for the six commonly used antibiotics: amoxicillin, metronidazole, clarithromycin, tetracycline, rifabutin, and tetracycline.10 NGS-based susceptibility testing can be done using fresh, frozen or formalin-fixed gastric biopsies or from stool. Results are rapid and are obtained in nearly 100% of cases.10,19–21
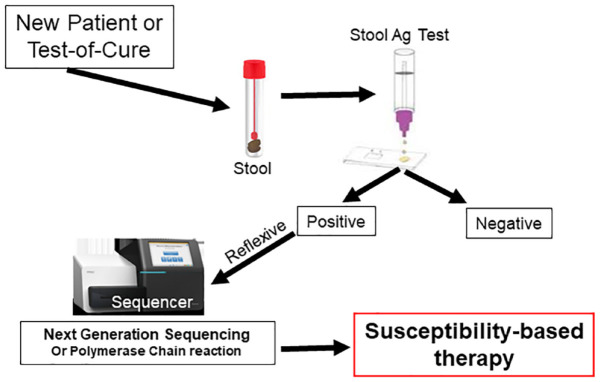
Clinically, and practically, molecular testing of stools is the most efficient and practical approach to H. pylori susceptibility testing, especially when preformed as a reflexive stool test (Figure 3). Reflexive refers to the method by which stool samples are handled and reported. First, the sample is tested for H. pylori using a stool antigen or stool PCR test. Negative tests are not processed further but are reported and are billed only for the diagnostic test. Samples that test positive reflexively (automatically) undergo molecular susceptibility testing, the susceptibility pattern is reported, and they are billed for molecular susceptibility testing. As noted above, such testing is now commercially available using NGS (American Molecular Laboratories) for the six commonly used antibiotics and a PCR-based reflexive test limited to clarithromycin (Mayo Clinical Laboratories).10 It is hoped that commercial stool PCR-based testing kits for fluoroquinolones will also become available for reflexive testing. The fact that government-approved commercial tests for PCR stool susceptibility tests for clarithromycin resistance are approved in Europe and theoretically such testing should be widely available17,18 in that most, if not all, European hospital laboratories already utilize PCR testing for COVID testing.17,18 It behooves European clinicians to request that service from their providers.
Figure 3.
Illustration of the steps in reflexive stool testing in which positive samples are automatically sent for next generation sequencing to provide noninvasive susceptibility testing.
Most recent treatment guidelines have become obsolete
This paradigm shift in the diagnosis and treatment of H. pylori was in part stimulated by the worldwide increase in antimicrobial resistance and the associated marked fall in cure rates with empiric therapy. The availability of susceptibility testing has made many of the current treatment guidelines obsolete and has shown that they also promoted antimicrobial misuse. Overall, the history and outcome of H. pylori treatment guideline development in the United States and Europe is not something to be especially proud of.18,22–26 The majority of guidelines have been based on retrospective analyses of trials that individually achieved poor cure rates. They also tended to focus on recommendations involving clarithromycin and to give lip service to the problems of increasing resistance and the role of recommended regimens as a major cause of antibiotic misuse. Over the years, the European guidelines have encouraged a prominent role for clarithromycin that persisted long after clarithromycin was proven to be no longer effective when given empirically.27,28 Finally, since the introduction of the proprietary version of bismuth quadruple therapy packaged for 10-day therapy (Pylera) and despite the rapid increase in metronidazole resistance, the European guidelines have favored 10 rather than 14 days as the ideal duration of therapy.
Bismuth triple therapy was the first highly effective therapy.29 It became a quadruple therapy by the addition of a proton pump inhibitor (PPI) to overcome a decline in effectiveness experienced because of increasing metronidazole resistance.30 It provides the best results in the presence of metronidazole when given for 14 days (discussed in studies5,26,31–33). In the presence of metronidazole-susceptible infections, therapy of 5–7 days is typically sufficient; we recommend 7 days. Pylera was marketed for only 10 days initially to differentiate it from other commercially available bismuth quadruple therapies and Pylera is highly effective for metronidazole susceptible infection.5 For populations with a relatively low prevalence of metronidazole resistance 10 and 14 day therapies will generally be very similar. However, if the target population is likely to contain metronidazole-resistant infections, a 7–10-day duration will generally be inferior to 14-day therapy.26,32 Valid comparative trials must state the prevalence of resistance. Currently it is generally best to assume a high prevalence of metronidazole and prescribe 14-day therapy. The higher the cure rate the better as each 1% decrease in cure rate results in 10,000 patients per million treatments requiring retreatment.
Many guidelines are based on meta-analyses of studies which focus on differences between treatments rather than actual cure rates and often include studies with unacceptably poor treatment outcomes which contain strawman comparisons with clinically unacceptable cure rates among the comparators.34 Guidelines fundamentally based on clinically unacceptable cure rates cannot be expected to provide clinically useful recommendations.
Many recent guidelines also recommend regimens associated with at least one unnecessary antibiotic (most often clarithromycin or metronidazole). Most contain four-drug combinations containing clarithromycin and metronidazole such as concomitant, sequential, or hybrid therapy. These regimens have resulted in the administration of many tens of thousands of kilograms of unneeded antibiotics per year and is likely a significant contributor to the global problem of antibiotic resistance.5,35,36 Vonoprazan clarithromycin triple therapy also contains unnecessary clarithromycin and should be avoided when possible.37 Clinically useful and unbiased guidelines can be recognized by their focus on (a) how to reliably obtain high cure rates; (b) a strong admonition not to use clarithromycin or levofloxacin unless the resistance rates are proven to be low locally and their local effectiveness is regularly confirmed; (c) an admonition against the misuse of antibiotics, especially against use of the clarithromycin-containing regimens described above and; and (d) a recommendation for 14-day bismuth quadruple therapy in the presence of metronidazole resistance unless local head-to-head comparisons confirm that a shorter duration produces high and clinically equivalent results.
The recent availability of susceptibility testing in the United States and Europe (culture, next generation sequencing, and stool PCR for clarithromycin in the United States and culture and stool PCR for clarithromycin in Europe)10,17 has resulted in the majority of published treatment guidelines, including the 2022 Maastricht H. pylori treatment guidelines becoming largely obsolete.
Which therapy?
The choice of antimicrobial therapy depends upon whether the infection is life threatening. Ideally, antimicrobial therapy given for a life-threatening condition should be given immediately and be the regimen most likely to succeed. Treatment and susceptibility testing are done simultaneously, and the antibiotic is changed based on the results of the susceptibility tests. For immediately non-life-threatening conditions therapy should be both susceptibility-based and infection-specific. However, local experience should identify one or more regimens that reliably yield high cure rates successfully without susceptibility testing (Table 3).
Table 3.
Recommended Helicobacter pylori therapies for the United States.
| Empiric therapies | |
|---|---|
| Bismuth quadruple therapy Bismuth subsalicylate q.i.d. 14 days |
Bismuth (e.g., PeptoBismol®) 2 tablets or 2 capsules q.i.d.
30 min before meals, tetracycline HCl 500 mg and metronidazole
500 mg 30 min after meals q.i.d. plus a PPI, 30 min b.i.d. before meals and bedtime (see PPI below) |
| Bismuth quadruple therapy Bismuth subsalicylate b.i.d. 14 days |
Bismuth (e.g., PeptoBismol) 2 tablets or 2 capsules q.i.d, 30 min before meals, tetracycline HCl 500 mg b.i.d. and metronidazole 500 mg, 30 min after meals q.i.d. plus a PPI, b.i.d. 30 min before morning and evening meals (see PPI below) |
| Bismuth quadruple therapy Pylera® formulation (bismuth citrate). 14 days |
Give combination tablets with means plus a PPI, q.i.d. 30 min before meals and bedtime (see PPI below) (see text for specific details). 14-day therapy recommended with metronidazole resistance likely. |
| Rifabutin triple therapy. 14 days | Rifabutin 150 mg b.i.d., amoxicillin 1-g t.i.d. plus 40 mg of esomeprazole or rabeprazole 30 min before meals b.i.d. (see PPI below) (see text for specific details) |
| Talicia® formulation of rifabutin triple therapy. 14 days | As directed by package insert |
|
Therapies only effective as susceptibility-based
therapy
Do not use empirically unless proven to cure > 90% locally | |
| Clarithromycin triple therapy. 14 days |
Clarithromycin 500 mg b.i.d., amoxicillin 1-g b.i.d. 30 min before meals (see PPI below) |
| Metronidazole triple therapy. 14 days |
Metronidazole 500 mg b.i.d., amoxicillin 1-g b.i.d., 30 min before meals (see PPI below) |
| Levofloxacin triple therapy. 14 days* |
Levofloxacin 500 mg in a.m., amoxicillin 1-g b.i.d., 30 min before meals (see PPI below) |
| PPI dose should at a minimum be 40 mg of omeprazole or equivalent b.i.d. (e.g., 45 mg lansoprazole, 20 mg of rabeprazole or esomeprazole). If cost is equivalent, we recommend 40 mg of rabeprazole or esomeprazole b.i.d. | |
| Potentially effective therapies that remain to be optimized before effective local use | |
| PPI or P-CAB-amoxicillin dual therapies | In western societies dual therapies are generally ineffective and remain to be optimized before they can be recommended |
|
Therapies that contain unneeded antibiotics
and should not be used
All include at least one antibiotic that offers no therapeutic benefit and only serves to increase global antimicrobial resistance: concomitant, hybrid, reverse hybrid, sequential therapies, vonoprazan clarithromycin triple therapy. | |
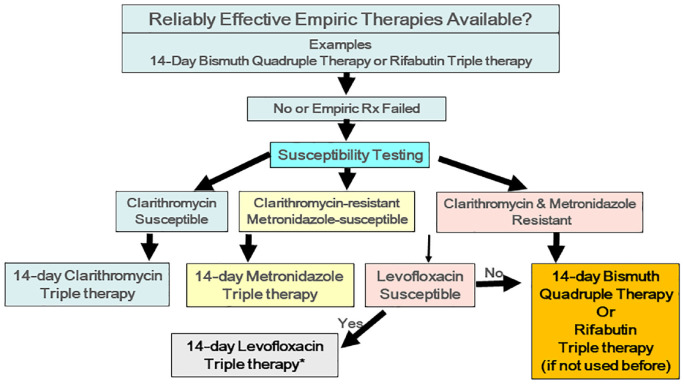
Table 3 and Figure 4 illustrate one approach to H. pylori therapy that utilizes either an empiric or susceptibility-based therapy.14,19 In most areas, the list of potential empiric therapies will include a version of bismuth quadruple therapy consisting of a PPI, bismuth, tetracycline-HCl, and high dose (1500–1600 mg) metronidazole all given for 14 days.26,32,33 Where available, furazolidone may substitute for metronidazole.39,40 In the United States, the second option is currently rifabutin triple therapy consisting of high dose PPI, rifabutin, and amoxicillin with the caveat that the dosages and duration of that regimen generally have not been optimized to reliably achieve high (i.e., >90%) cure rates locally.31 We anticipate that eventually high dose PPI (40 mg of esomeprazole or rabeprazole b.i.d.) or a potassium competitive acid blocker (P-CAB) (e.g., vonoprazan) plus amoxicillin dual therapy will have been be optimized and join this list, possibly as first choice in western countries.41 Failure to optimize a regimen before marketing has remained the Achille’s heel of FDA or European-approved H. pylori therapies.
Figure 4.
Flow diagram of a stepwise approach to Helicobacter pylori therapy that starts with empiric therapy using a proven locally highly effective regimen. If not, one goes immediately to susceptibility-based therapy.
The decision to employ an empiric therapy depends on the availability of therapies proven to reliably provide high cure rates locally. Because of the high prevalence of resistance, therapies containing clarithromycin or levofloxacin should no longer be used empirically and empiric metronidazole use should be restricted to bismuth quadruple therapy. As discussed below, treatment outcome must always be confirmed by test-of-cure to provide feedback regarding continued use of that regimen as an empiric therapy (Figure 5).34
Figure 5.
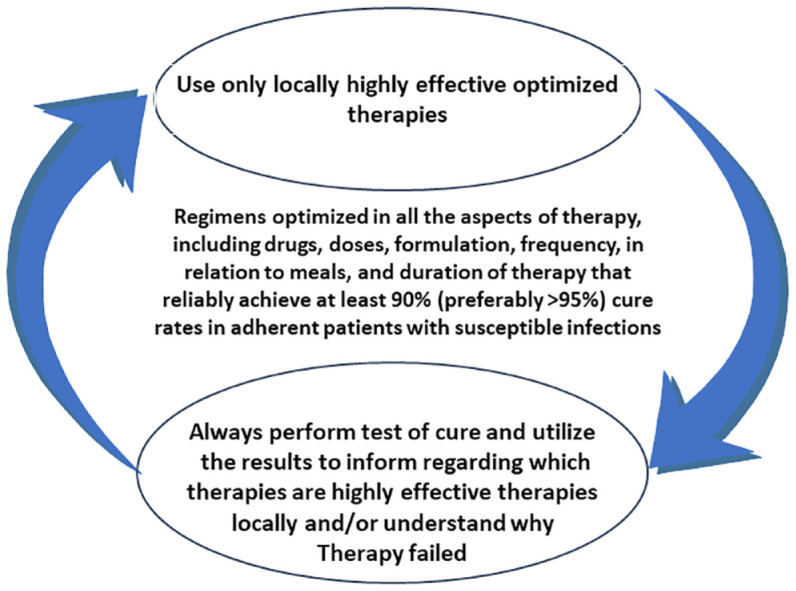
Illustration of the importance of the post treatment test-of-cure as feedback to provide the clinician with updated information regarding the local effectiveness of locally optimized therapies.
Source: From Graham’s34 study, with permission.
Is there still a role for therapies containing clarithromycin, levofloxacin, or metronidazole?
Worldwide, resistance to these antimicrobials has resulted in low overall cure rates such that they should no longer be used empirically. It an attempt to salvage clarithromycin-containing therapies, the Maastricht Guidelines suggested the use of an arbitrary level of resistance (e.g., 15%) as a cutoff for use. The evidence was graded as very low and importantly few, if any, clinicians had a method for determining the percent of local resistance other than whether the therapy was successful. Clinically 65 and 90% cure rates are likely difficult to distinguish in any clinical practice.23 Experience has shown that clarithromycin resistance has continued to increase and the rare regions where clarithromycin was previously effective (e.g., Thailand and Southeast Asia) are generally experiencing unacceptably high rates of resistance. However, while these three antimicrobials should no longer be prescribed empirically, they can still be used for susceptibility-based therapy provided that one uses locally optimized regimens and confirms treatment all results using test-of-cure.
The is no longer any role for concomitant, sequential, and hybrid therapies?
Antimicrobial therapies for H. pylori can be categorized into one of three categories: (a) can often be successfully given empirically, (b) should not be used because they each contain at least one unnecessary antibiotic, and (c) those that should only be used in susceptibility-based therapies (Table 3).
These complex regimens were introduced as empiric therapies to empirically overcome clarithromycin resistance. Basically, the concept was to add another antibiotic, generally metronidazole so that treatment failure required dual metronidazole and clarithromycin resistance. Sequential therapy proved effective in regions with high clarithromycin resistance but low metronidazole resistance. However, when given to patients with both clarithromycin and metronidazole resistance the regimen failed.6 Alternate formulations include concomitant therapy which provided all four drugs simultaneously and hybrid therapy which used a PPI plus amoxicillin followed by all four drugs (concomitant therapy). All three regimens were most effective when given for 14 days and all had their efficacy undermined by the presence of dual clarithromycin and metronidazole resistance. Sequential therapy has long been considered obsolete. Over time, the cure rates with concomitant therapy have also declined as dual clarithromycin and metronidazole resistance rates have increased. Critically, it was soon recognized that all of these multidrug therapies violated the principles of antimicrobial stewardship and, as discussed above, even when successful, all patients receive at least one unnecessary antibiotic.2 It now appears likely that these therapies are a major iatrogenic cause of increasing global antimicrobial resistance and are responsible for tens of thousands of kilograms of unnecessary antibiotics being prescribed annually.37 This is especially critical for clarithromycin which is listed by the World Health Organization as a ‘necessary drug’.42
Dose and type of antisecretory agent for H. pylori therapy
As noted previously, the first highly successful therapy was a triple therapy consisting of bismuth, tetracycline, and metronidazole. It used acid-independent antibiotics and needed no antisecretory drug.30 The rapid increase in metronidazole resistance reduced its effectiveness which was restored by the addition of a PPI which produced bismuth quadruple therapy.43 Clarithromycin, fluoroquinolones, and amoxicillin are acid-dependent antibiotics and thus require concomitant antisecretory drugs to achieve high treatment success.44 They are all also susceptible to emergence of resistance during therapy. Emergence of resistance can be markedly reduced by the addition of a low dose of amoxicillin.5 H. pylori therapy is also duration-dependent with 14-day therapy generally being the most effective. Part of this may be due to the fact that PPIs require 3 or 4 days to achieve full effectiveness. Drugs that achieve full effect more rapidly, such as P-CABs, may be fully effective with a duration shorter than 14 days. Amoxicillin is likely the most acid-dependent of the antibiotics. Amoxicillin is a penicillin and acts by inhibiting production of the bacterial cell thus requiring active bacterial replication to be effective. The effectiveness is therefore dependent on the effectiveness of the antisecretory drug to produce a milieu that encourages the organism to divide and thus become susceptible to the antibiotic.45,46 H. pylori replication occurs when the local pH is between 6 and 8.47–49 Achieving this pH at the site where the organisms reside requires almost complete suppression of acid secretion and thus the effectiveness requires high level acid suppression using either a PPI or P-CAB. Amoxicillin has a dual role. When used in combination therapy with clarithromycin, metronidazole, or levofloxacin) one role is to prevent the emergence of resistance (heteroresistance) to the antibiotic.5 High-level acid inhibition is not needed such that therapy with H2-receptor antagonists, PPIs or P-CABs is effective. When used as dual therapy (e.g., amoxicillin and antisecretory drug) in which all the antimicrobial effect resides in the amoxicillin treatment success requires high level acid suppression such as obtained with a high dose of a PPI (e.g., 40 mg of esomeprazole or rabeprazole b.i.d. or use of a P-CAB).50,51 In Japan, vonoprazan 20 mg b.i.d. is approximately equivalent to the high dose PPI therapy52 and they are approximately equally effective for dual therapy).53,54 In western countries a reliably highly effective dual therapy (antisecretory drug plus amoxicillin) protocol has not yet been identified.41 The outcome of dual therapy can often be improved by giving the amoxicillin every 6 or 8 h44 but in areas where PPIs provide highly effective acid suppression b.i.d. therapy appears adequate.55–57 Dual amoxicillin-antisecretory therapy remains to be optimized in terms of drugs, doses, duration of therapy, and use of adjuvants.41 Preliminary data suggests that the effectiveness of vonoprazan in raising and maintaining the intragastric pH can be enhanced by the addition of an H2-receptor antagonist.58 We await the results of the studies needed to optimize vonoprazan-containing regimens in order to achieve reliably effective dual therapy. The US/European trials of vonoprazan-clarithromycin triple therapy and vonoprazan-amoxicillin dual therapy failed to achieve acceptable cure rates even when given only to susceptible infections.41 Importantly, and surprisingly, the PPI triple therapy also failed with clarithromycin susceptible infections. These unexpected and unprecedented results suggest that there was possibly an as yet unidentified flaw in the experiment or study design.
Test-of-cure and investigating the cause of treatment failures
The test-of-cure is as important as the initial choice of therapy. The test-of-cure provides a feedback loop that confirms that hypotheses and thoughts underpinning the initial choice of therapy such as the presumed local pattern of resistance. It also confirms that the doses and duration of therapy were appropriate for the population being treated and provides confirmation about whether the therapy in use should be revised or discarded (Figure 6). It has become increasingly clear that the test-of-cure data provides critical information to allow clinicians to self-test their judgment based on the outcome of each decision. It is also clear test-of-cure data are a critical resource that should be shared within the community and disseminated to all practitioners to inform about the current status of therapy with any regimen.9
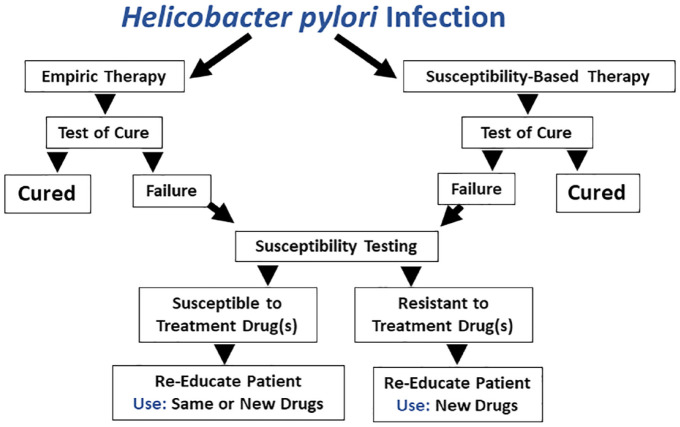
Figure 6.
Schematic of the entire course of therapy starting with either empiric or susceptibility-based therapy through one treatment failure.
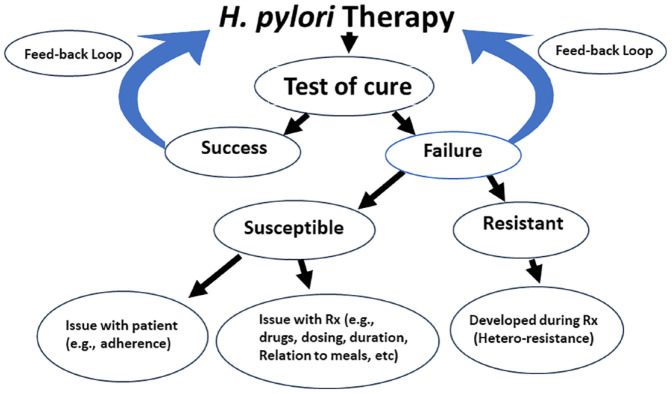
Understanding why treatment failed has proven difficult. The advent of NGS stool-based susceptibility testing not only greatly simplified pretreatment susceptibility testing but also has made it possible to better understand the cause(s) of treatment failure. Failures following use of an optimized therapy treatment generally involve a problem with the patient or a problem with the therapy (Figure 7). Ideally, the most efficient approach would be for universal use of reflexive stool testing for both diagnosis and test-of-cure. This is both a cost-effective and informative method as it determines the presence or absence of an active H. pylori infection. For those with active infection it automatically provides for susceptibility-based therapy. When reflexive stool testing is used for test-of-cure, it provides information regarding whether the infection was cured or in case of treatment failure, it allows one to distinguish whether failure was due to emergence of resistance, and finally provides susceptibility data for planning the next therapy (Figure 5). Treatment failure without developing resistance points to issues related to adherence, or an inactive drug. In contrast, post treatment resistance implies that resistance emerged during therapy5 which is most often due to the presence of small and unrecognized subpopulation of resistant organism being present (i.e., hetero-resistance).5
Figure 7.
An algorithm emphasizing the information obtainable from always utilizing the post treatment test-of-cure to both provide information regarding effectiveness of the therapy used and also to provide information regarding the likely cause of treatment failure.
Cost-effectiveness of H. pylori susceptibility testing
The recent change in the category of H. pylori infections from a Gastroenterology problem to an Infectious Disease problem changed the nature of whether to focus on susceptibility such that the clinician’s question changed from ‘whether susceptibility testing?’ to ‘what do these tests cost?’. The cost issue encompasses not only costs associated with susceptibility testing and includes costs of not employing testing which is a key element of antimicrobial stewardship and the underpinning of infectious disease therapy. Above, we have discussed both how susceptibility testing is used in the management of infectious diseases and the role of empiric therapy. We show that susceptibility testing is not a knee jerk-option but rather, its use depends both on the clinical situation and the local experience with antimicrobial therapy of H. pylori infections. Examination of the costs before widespread availability of susceptibility testing shows that the Gastroenterology approach was associated with many, often hidden, costs. For example, currently, the average cure rate is about 70% meaning that 30% of patients require one or more retreatments and all the costs associated with retreatment (e.g., doctor visits, costs of drugs used in the initial and subsequent treatments, cost of side effects, diagnostic tests, time off from work, travel time, office visits, etc.). In addition, the drugs used in the unsuccessful treatment add to the global problems of antimicrobial resistance and antibiotic misuse. One of the common responses to treatment failure has been to repeat the same therapy. Current guidelines recommend susceptibility-based therapy as an option or to use therapies such as concomitant therapy which contain unnecessary antibiotics. For those with infections that are still susceptible to the antibiotics used are likely to have been poorly adherent to the protocol. Without susceptibility testing this underlying problem would likely remain unrecognized.
The availability of noninvasive susceptibility testing also largely eliminates the need for endoscopy and all its associated costs when used for obtaining specimens for culture or other types of susceptibility testing requiring gastric tissue. The real question is not what testing costs (although that is important), but rather what the costs of not using susceptibility-based therapy are.
Summary
The paradigm shifts in management of H. pylori infections associated with the widespread availability and use of susceptibility testing has resulted in fundamental changes in the diagnosis and treatment of the disease. Even if the only change available was the availability of stool clarithromycin susceptibility testing, current guidelines would still be largely rendered obsolete. Empiric therapy is still a possibility for initial treatment. The most important requirement is for every treatment to undergo post treatment confirmation of cure and to offer susceptibility testing for every treatment failure (Figure 7). Achieving and maintaining high cure rates likely also requires that the pre- and post-treatment testing data be retained and compiled, preferably locally or regionally as it would be difficult for a busy practitioner to remember accurate details regarding his/her practice statistics; thus cure rates could fall significantly and not be recognized.
Acknowledgments
None.
Footnotes
ORCID iD: David Y. Graham  https://orcid.org/0000-0002-6908-8317
https://orcid.org/0000-0002-6908-8317
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): David Y. Graham: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr David Y. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center.
Dr David Y. Graham is a consultant for RedHill Biopharma and Phathom Pharmaceuticals regarding novel Helicobacter pylori therapies and has received research support for culture of H. pylori. He is also a consultant with Janssen Research & Development regarding potential gastrointestinal effects of drugs under development and has collaborated on research projects with American Molecular regarding molecular diagnostics for H. pylori.
Availability of data and materials: Not applicable.
References
- 1. Borsch GM, Graham DY. Helicobacter pylori. In: Collen MJ, Benjamin SB. (eds) Pharmacology of peptic ulcer disease, handbook of experimental pharmacology. Vol 99. Berlin: Springer-Verlag, 1991, pp.107–148. [Google Scholar]
- 2. Graham DY. Transitioning of Helicobacter pylori therapy from trial and error to Antimicrobial Stewardship. Antibiotics (Basel) 2020; 9: 20201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anonymous. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum 1994; 61: 1–241. [PMC free article] [PubMed] [Google Scholar]
- 4. Hurst AF. Schorstein lecture on the precursors of carcinoma of the stomach. Lancet 1929: 214: 1023–1028. [Google Scholar]
- 5. Graham DY, Liou JM. Primer for development of guidelines for Helicobacter pylori therapy using antimicrobial stewardship. Clin Gastroenterol Hepatol 2022; 20: 973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graham DY. Illusions regarding Helicobacter pylori clinical trials and treatment guidelines. Gut 2017; 66: 2043–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Core elements of antibiotic stewardship. Centers for disease control and prevention. Epub ahead of print 8/15/2019. [Google Scholar]
- 8. Nyssen OP, Bordin D, Tepes B, et al. European Registry on Helicobacter pylori management (Hp-EuReg): patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2021; 70: 40–54. [DOI] [PubMed] [Google Scholar]
- 9. Graham DY, El-Serag HB. European registry on Helicobacter pylori management shows that gastroenterology has largely failed in its efforts to guide practitioners. Gut 2021; 70: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graham DY, Moss SF. Antimicrobial susceptibility testing for Helicobacter pylori is now widely available: when, how, why. Am J Gastroenterol 2022; 117: 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. el-Serag HB, Kao JY, Kanwal F, et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol 2018; 16: 992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64: 1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 14. Lee YC, Dore MP, Graham DY. Diagnosis and treatment of Helicobacter pylori Infection. Annu Rev Med 2022; 73: 183–195. [DOI] [PubMed] [Google Scholar]
- 15. Liou JM, Malfertheiner P, Lee YC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut 2020; 69: 2093–2112. [DOI] [PubMed] [Google Scholar]
- 16. Ding S-Z, Du Y-Q, Lu H, et al. Chinese consensus report on family-based Helicobacter pylori infection control and management (2021 Edition). Gut 2022; 71: 238–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dore MP, Graham DY. Modern approach to the diagnosis of Helicobacter pylori infection. Aliment Pharmacol Ther 2022; 55: S14–S21. [DOI] [PubMed] [Google Scholar]
- 18. Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut 2022; 71:1724–1762. [Google Scholar]
- 19. Moss SF, Dang LP, Chua D, et al. Comparable results of Helicobacter pylori antibiotic resistance testing of stools vs gastric biopsies using next-generation sequencing. Gastroenterology 2022; 162: 2095–2097 [DOI] [PubMed] [Google Scholar]
- 20. Hulten KG, Genta RM, Kalfus IN, et al. Comparison of culture with antibiogram to next-generation sequencing using bacterial isolates and formalin-fixed, paraffin-embedded gastric biopsies. Gastroenterology 2021; 161: 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Graham DY. Molecular-based Helicobacter pylori susceptibility testing is almost ready for prime time. Gastroenterology 2021; 160: 1936–1937. [DOI] [PubMed] [Google Scholar]
- 22. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am J Gastroenterol 2017; 112: 212–239. [DOI] [PubMed] [Google Scholar]
- 23. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]
- 24. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection–the Maastricht IV/ Florence Consensus Report. Gut 2012; 61: 646–664. [DOI] [PubMed] [Google Scholar]
- 25. Altintas E, Sezgin O, Ulu O, et al. Maastricht II treatment scheme and efficacy of different proton pump inhibitors in eradicating Helicobacter pylori. World J Gastroenterol 2004; 10: 1656–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: the good, the bad, and the ugly. Gastroenterol Clin North Am 2015; 44: 537–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janssen MJ, Van Oijen AH, Verbeek AL, et al. A systematic comparison of triple therapies for treatment of Helicobacter pylori infection with proton pump inhibitor/ ranitidine bismuth citrate plus clarithromycin and either amoxicillin or a nitroimidazole. Aliment Pharmacol Ther 2001; 15: 613–624. [DOI] [PubMed] [Google Scholar]
- 28. Laheij RJ, Rossum LG, Jansen JB, et al. Evaluation of treatment regimens to cure Helicobacter pylori infection- a meta-analysis. Aliment Pharmacol Ther 1999; 13: 857–864. [DOI] [PubMed] [Google Scholar]
- 29. Borody TJ, Cole P, Noonan S, et al. Recurrence of duodenal ulcer and Campylobacter pylori infection after eradication. Med J Aust 1989; 151: 431–435. [DOI] [PubMed] [Google Scholar]
- 30. George LL, Borody TJ, Andrews P, et al. Cure of duodenal ulcer after eradication of Helicobacter pylori. Med J Aust 1990; 153: 145–149. [DOI] [PubMed] [Google Scholar]
- 31. Shiotani A, Roy P, Lu H, et al. Helicobacter pylori diagnosis and therapy in the era of antimicrobial stewardship. Therap Adv Gastroenterol 2021; 14: 17562848211064080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Graham DY, Dore MP, Lu H. Understanding treatment guidelines with bismuth and non-bismuth quadruple Helicobacter pylori eradication therapies. Expert Rev Anti Infect Ther 2018; 16: 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016; 65: 870–878. [DOI] [PubMed] [Google Scholar]
- 34. Graham DY, Hernaez R, Rokkas T. Cross-roads for meta-analysis and network meta-analysis of H. pylori therapy. Gut 2022; 71: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dang BN, Graham DY. It is time to rethink H. pylori therapy. J Gastrointestin Liver Dis 2017; 26: 115–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dang BN, Graham DY. Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat Rev Gastroenterol Hepatol 2017; 7: 383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Graham DY, Lu H, Shiotani A. Vonoprazan-containing Helicobacter pylori triple therapies contribution to global antimicrobial resistance. J Gastroenterol Hepatol 2021; 36: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 38. Keller A. Fluoroquinolones, 2020, https://www.consumernotice.org/drugs-and-devices/fluoroquinolones/.
- 39. Mohammadi M, Attaran B, Malekzadeh R, et al. Furazolidone, an underutilized drug for H. pylori eradication: lessons from Iran. Dig Dis Sci 2017; 62: 1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Graham DY, Lu H. Furazolidone in Helicobacter pylori therapy: misunderstood and often unfairly maligned drug told in a story of French bread. Saudi J Gastroenterol 2012; 18: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chey WD, Mégraud F, Laine L, et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the US and Europe: randomized clinical trial. Gastroenterology 2022; 163: 608–619. [DOI] [PubMed] [Google Scholar]
- 42. WHO Expert Committee on Drug Dependence, World Health Organization. The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, 2013 (including the 18th WHO Model List of Essential Medicines and the 4th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization, 2014. [Google Scholar]
- 43. Borody TJ, Andrews P, Shortis NP, et al. Optimal H. pylori (HP) therapy - a combination of omeprazole and triple therapy (TT). Gastroenterology 1994; 106: A55. [Google Scholar]
- 44. Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori Infection. Gastroenterol Clin North Am 2010; 39: 465-480. DOI: S0889-8553(10)00049-X [pii];10.1016/j.gtc.2010.08.007 [doi]. [DOI] [PubMed] [Google Scholar]
- 45. Sachs G, Meyer-Rosberg K, Scott DR, et al. Acid secretion and Helicobacter pylori. Digestion 1997; 58: 8–13. [DOI] [PubMed] [Google Scholar]
- 46. Scott D, Weeks D, Melchers K, et al. The life and death of Helicobacter pylori. Gut 1998; 43: S56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marcus EA, Inatomi N, Nagami GT, et al. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther 2012; 36: 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sachs G, Wen Y, Scott DR. Gastric infection by Helicobacter pylori. Curr Gastroenterol Rep 2009; 11: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sachs G, Meyer-Rosberg K, Scott DR, et al. Acid, protons and Helicobacter pylori. Yale J Biol Med 1996; 69: 301–316. [PMC free article] [PubMed] [Google Scholar]
- 50. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol 2018; 6: 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Graham DY, Lu H, Dore MP. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter 2019; 24: e12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects–a randomised open-label cross-over study. Aliment Pharmacol Ther 2015; 42: 719–730. [DOI] [PubMed] [Google Scholar]
- 53. Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut 2020; 69: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Furuta T, Yamade M, Kagami T, et al. Dual therapy with vonoprazan and amoxicillin is as effective as triple therapy with vonoprazan, amoxicillin and clarithromycin for eradication of Helicobacter pylori. Digestion 2020; 1–1: 743–751. [DOI] [PubMed] [Google Scholar]
- 55. Hou X, Meng F, Wang J, et al. Vonoprazan non-inferior to lansoprazole in treating duodenal ulcer and eradicating Helicobacter pylori in Asian patients. J Gastroenterol Hepatol 2022; 37: 1275–1283. [DOI] [PubMed] [Google Scholar]
- 56. Xiao Y, Zhang S, Dai N, et al. Phase III, randomised, double-blind, multicentre study to evaluate the efficacy and safety of vonoprazan compared with lansoprazole in Asian patients with erosive oesophagitis. Gut 2020; 69: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tack J, Vladimirov B, Horny I, et al. Randomized clinical trial: a double-blind, proof-of-concept, phase 2 study evaluating the efficacy and safety of vonoprazan 20 or 40 mg versus esomeprazole 40 mg in patients with symptomatic gastro-esophageal reflux disease and partial response to a healing dose of a proton-pump inhibitor. Neurogastroenterol Motil 2023; 35: e14468. [DOI] [PubMed] [Google Scholar]
- 58. Suzuki T, Kagami T, Uotani T, et al. Comparison of effect of an increased dosage of vonoprazan versus vonoprazan plus lafutidine on gastric acid inhibition and serum gastrin. Eur J Clin Pharmacol 2018; 74: 45–52. [DOI] [PubMed] [Google Scholar]