The endoplasmic reticulum (ER) is an expansive membrane network shown to be required for many essential cellular functions that include protein translation, lipid synthesis, vesicle trafficking, and in more recent years, the biogenesis and maintenance of membrane bound organelles through membrane contact sites. A recent study from the lab of Voeltz1 now demonstrates that the ER also forms contacts with a different type of cellular compartment: membraneless organelles (Figure 1A). Membraneless organelles (or “condensates”) are liquid-like assemblies of molecules that form by a process called liquid–liquid phase separation. Animal cells contain many types of membraneless organelles that play key biological roles. This study focused on Processing-bodies (P-bodies) and stress granules, assemblies of untranslated mRNA and associated proteins that form when translation is limited.2 The finding that contact between the ER and these membraneless assemblies is integral to their regulation is an exciting leap forward in our overall understanding of cellular organization and function.
Figure 1.
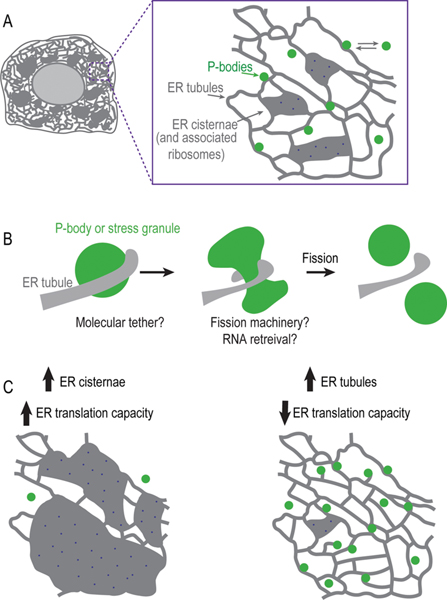
Regulation and tethering of membraneless organelles by the ER. (A) Schematic of the ER network in animal cells. Magnified view of membraneless assemblies (P-bodies) dynamically associated with ER tubules. (B) Schematic of an ER tubule wrapping around a membraneless organelle to define the site of fission. (C) Schematic summary of how the structure and/or protein translation capacity of the ER regulates the biogenesis of P-bodies and stress granules.
To determine if P-bodies and ER tubules form contact sites at molecular distances typical for membrane contact sites (10–30 nm), Voeltz and colleagues1 used live-imaging of dimerization-dependent fluorescent proteins (ddFP). ddFPs are a pair of nonfluorescent monomers that emit fluorescence upon dimerization with the key advantage that their binding is reversible. The fusion of each nonfluorescent monomer to markers for the ER and P-body revealed that contact sites between P-bodies and the ER are at a molecular distance and can be stable (>2 min) or short-lived (lasting <2 min). The variable duration of ER–P-body contact suggested to the authors that their tethering may depend on the composition of P-bodies. Consistent with this, the overexpression of a P-body component to change P-body composition increased the population of P-bodies that formed stable contacts with the ER. The composition of other types of membraneless organelles (e.g., P-granules) as well as in vitro liquid droplets has been shown to be heterogeneous, exhibiting distinct phases of liquid or partially gel-like characteristics.3,4 Future work is required to determine how the composition of P-bodies affects their interaction with the ER and the functional meaning behind the observed variation in the duration of contact.
The fact that some P-bodies are stably tethered to the ER raises the possibility that contact sites serve a role similar to membrane contact sites in which ER tubules define the site and aid in the division of membrane bound organelles (e.g., mitochondria). Indeed, the authors found that the fission of P-bodies and stress granules, a process for which there is very little known, coincides with ER tubules (Figure 1B). ER contact sites may recruit molecular machinery that pinches off membraneless organelles or may promote fission through destabilization of their liquid-like properties, leading to their spontaneous disassembly. It is known that, upon reinitiation of protein translation, stress granules disassemble because of the rapid retrieval of the mRNAs that maintain their liquid-like properties.4 The ER is a major site of protein translation and so ER contact sites may facilitate the disassembly of P-bodies and stress granules by the retrieval of mRNAs to ER-bound ribosomes. Interestingly, P-bodies prefer to associate with the curved edges of the ER tubules, which are less likely to be associated with ribosomes, suggesting that contact sites likely serve functions beyond mRNA retrieval.
To test the relationship between the translation capacity of the ER and the biogenesis of P-bodies and stress granules, the authors used molecular and genetic tools to change the shape of the ER. Five-fold more ribosomes are associated with ER sheets than ER tubules and so the authors induced ER sheets to confine mRNAs to the ER-associated ribosomes. A mostly sheet-like ER network decreased the number of P-bodies, while a shift to a more tubular ER network increased the P-body number (Figure 1C). Pharmacological inhibition of global translation increased the number of P-bodies even when the ER was more sheet-like, suggesting that the translation capacity of the ER, rather than ER shape, determines P-body number. To more directly test if the translation capacity of the ER drives P-body biogenesis, the authors induced ER stress at time scales previously shown to selectively increase translation of ER-resident proteins that mitigate ER stress (such as BiP). Short-term ER stress decreased P-body number, suggesting that mRNAs encoding for ER proteins are involved in the formation and/or stability of P-bodies under these conditions. A future direction will be to identify and manipulate the short-term stress-induced mRNAs bound by ER-associated ribosomes to directly determine their effect on P-body biogenesis.
A key step toward a mechanistic understanding of the role of ER contact sites in the biogenesis and fission of P-bodies or stress granules will be to identify the molecular tether. Stress granules and lysosomes are tethered by the protein annexin A11.5 Annexin A11 possesses a low-complexity domain that phase partitions into liquid droplets to associate with stress granules and a folded domain that binds to lipid headgroups on the surface of the lysosome. A similar combination of domains within an ER-associated protein could serve as a tether between membrane-less assemblies and the ER.
The findings by Voeltz and colleagues1 unveil many open questions about the function of ER contact sites with membraneless organelles and their role in overall cellular physiology. It will be exciting to know if the association of the ER with membraneless organelles is more broadly applicable to other liquid-like compartments such as P-granules that specify the germline during early development. In addition, the ER may function to regulate RNA granules important for neuronal function, which may explain why mutations in genes that encode for ER shaping proteins give rise to neuronal disorders. The regulation and function of ER–membraneless organelle contact may be purely biophysical, raising the possibility that the tethering of these compartments depends not only on the composition of the membraneless organelle but also on the composition of protein and lipid domains at the surface of the ER. The ER forms contacts with nearly every compartment in the cell, providing a way to integrate and coordinate different biochemical processes, an open area of research that will keep laboratories busy for many years ahead.
ACKNOWLEDGMENTS
We thank Sarah Barger and Michael Mauro for helpful comments on the manuscript.
Funding
Funding was provided by a NSF CAREER grant (1846010) and a NIH grant (R01GM131004) to S.B. and a Yale endowed Anderson Postdoctoral Fellowship to S.L.
Footnotes
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.biochem.0c00232
Contributor Information
Shirin Bahmanyar, Department of Molecular Cellular and Developmental Biology, Yale University, New Haven, Connecticut 06520, United States.
Shoken Lee, Department of Molecular Cellular and Developmental Biology, Yale University, New Haven, Connecticut 06520, United States.
REFERENCES
- (1).Lee JE, Cathey PI, Wu H, Parker R, and Voeltz GK (2020) Endoplasmic reticulum contact sites regulate the dynamics of membraneless organelles. Science 367, No. eaay7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Decker CJ, and Parker R. (2012) P-Bodies and Stress Granules: Possible Roles in the Control of Translation and mRNA Degradation. Cold Spring Harbor Perspect. Biol 4, a012286–a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wheeler J, Matheny T, Jain S, Abrisch R, and Parker R. (2016) Distinct stages in stress granule assembly and disassembly. eLife 5, No. e18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Putnam A, Cassani M, Smith J, and Seydoux G. (2019) A gel phase promotes condensation of liquid P granules in Caenorhabditis elegans embryos. Nat. Struct. Mol. Biol 26, 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Liao Y-C, Fernandopulle MS, Wang G, Choi H, Hao L, Drerup CM, Patel R, Qamar S, Nixon-Abell J, Shen Y, Meadows W, Vendruscolo M, Knowles TPJ, Nelson M, Czekalska MA, Musteikyte G, Gachechiladze MA, Stephens CA, Pasolli HA, Forrest LR, George-Hyslop PS, LippincottSchwartz J, and Ward ME (2019) RNA Granules Hitchhike on Lysosomes for Long-Distance Transport, Using Annexin A11 as a Molecular Tether. Cell 179, 147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]