Abstract
This study aimed to uncover the current major topics regarding COVID-19 vaccine, and systematically evaluate the development trends for future research. The top 100 most cited original articles on COVID-19 vaccine from January 2020 to October 2022 were identified from Web of Science Core Collection database. CiteSpace (v6.1.R3) was adopted for bibliometric analysis with statistical and visual analysis. The number of citations ranged from 206 to 5881, with a median of 349.5. The USA (n = 56), England (n = 33), and China (n = 16) ranked the top three countries/regions in terms of the number of publications. Harvard Medical School (centrality = 0.71), Boston Children’s Hospital (centrality = 0.67), and Public Health England (centrality = 0.57) were the top three institutions leading the way on COVID-19 vaccine research. The New England of medicine journal dominated with 22 articles in the 32 high-quality journals. The three most frequent keywords were immunization (centrality = 0.25), influenza vaccination (centrality = 0.21), and coronavirus (centrality = 0.18). Cluster analysis of keywords showed that the top four categories were protection efficacy, vaccine hesitancy, spike protein, and second vaccine dose (Q value = 0.535, S value = 0.879). Cluster analysis of cited references showed that top eight largest categories were Cov-2 variant, clinical trial, large integrated health system, COV-2 rhesus macaque, mRNA vaccine, vaccination intent, phase II study, and Cov-2 omicron variant (Q value = 0.672, S value = 0.794). The research on COVID-19 vaccine is currently the hottest topic in academic community. At present, COVID-19 vaccines researches have focused on vaccine efficacy, vaccine hesitancy, and the efficacy of current vaccines on omicron variants. However, how to increase vaccine uptake, focus on mutations in the spike protein, evaluate of the efficacy of booster vaccine, and how effective new vaccines under pre- and clinical development against omicron will be spotlight in the future.
Keywords: COVID-19 vaccine, Bibliometric analysis, CiteSpace, Visualization
Introduction
Coronavirus disease 2019 (COVID-19) is a contagious respiratory disease caused by the SARS-Cov-2 virus, which was first reported in Wuhan, Hubei province, China, in December 2019, and then spread rapidly around the world. On March 11, 2020, World Health Organization (WHO) declared the outbreak of the COVID-19 as a global pandemic [1]. To date, COVID-19 pandemic has caused incalculable disaster on the global medical, economy, and public health. Unfortunately, the epidemic trends of COVID-19 remain uncertain in the foreseeable future. As of October 7, 2022, exceeding 610 million confirmed cases of COVID-19 and more than 6 million deaths from the disease have been reported worldwide [2], the situation of epidemic prevention and control is still not optimistic.
Compared with drugs and other treatments, safe and effective vaccines have become the most economical way to prevent and slow down transmission of COVID-19 infection [3]. Vaccination not only decreases the disease prevalence, but also protect the unimmunized individuals of the community [4]. Vaccination, in combination with non-pharmaceutical interventions including properly wear mask, teleworking, social distancing, and isolations, was regarded as the best way to control the pandemic [5–7]. With the outbreak of the pandemic, governments and researchers immediately put the invention of COVID-19 vaccine at the top of their agenda. The wonderful news was that as of October 7, 2022, 11 vaccines were listed for emergency use by WHO, and 172 vaccines were in clinical development and 199 were in preclinical development [8]. Moreover, more than 12 billion vaccine doses have been administered globally as of October 3, 2022 [2].
Bibliometric analysis is a quantitative analysis method combining mathematics and statistics [3, 9]. It can analyze and visualize the authors, institutions, countries, keywords, and other key information of published literatures, which could quantitatively present the research highlights and development trends. Consequently, bibliometric analysis is almost extensively used in the medical research nowadays [10–12], which can promote researchers clarify ideas for scientific studies, and provide a reference for research cooperation [13, 14].
Citations are created whenever the article reference another peer-reviewed publication [15]. Therefore, the number of citations of an article can quantitatively reflect its impact on the scientific community [14]. Bibliometric analysis of high-quality and value publications can map the burgeoning research hotspots and future research tendency.
There were fewer studies using bibliometric analysis to outline the status and prospect of COVID-19 vaccine research [3, 4, 16]. However, to our knowledge, there is no bibliometric analysis to focus on the top 100 most cited articles on COVID-19 vaccine. This study analyzed the top 100 most cited original articles on COVID-19 vaccine research using bibliometric analysis, with the aim of revealing the current hotspots, then systematically predict the development direction for follow-up work.
Materials and methods
Search strategy
Web of Science (WOS) represents a comprehensive, multidisciplinary database, which contains more than 12,000 authoritative and high-impact academic journals [17].What is more, it can provide critical information of all publications, including authors, authors’ affiliated institution, keywords, the number of citations, and publishers, etc. With its powerful functions and citation reports, it can quickly target highly impactful and representative researches, thus track down the research interests concerned to domestic and international authorities, as well as capture the growing trends of the discipline [18]. Hence, WOS database has been used extensively in bibliometric analysis [4, 19, 20].
The article search was conducted on the Web of Science Core Collection (WOSCC) database. The article search strategies were TI = (corona* OR 2019-nCoV OR nCoV-19 OR SARS-CoV2 OR SARS-CoV-2 OR COVID*) AND TI = (vacc* OR immuniz*). The number of citations was provided by WOSCC database. Two investigators independently evaluate the article by reading the abstract or full text of the article to confirm whether it was related to the COVID-19 vaccine. If there was disagreement on the article, it will be discussed until reach a consensus. The articles search was completed on October 18, 2022. Documents were downloaded in plain text format.
Inclusion and exclusion criteria
Inclusion criteria:
The document type was original article;
The articles were published by English;
The time span was limited to January 2020 to October 2022.
Exclusion criteria:
The document type was review, letter, editorial material, early access, etc.;
The articles were published by non-English.
Statistical analysis and visualization
Statistical and visual analysis was performed by CiteSpace 6.1.R3 (64-bit). CiteSpace is a visualization software for bibliometric analysis based on Java platform developed by Professor Chaomei Chen [21]. The features of visualization map were described by three variables: node size, distance, and color. Each node in the map represents a country, author, institution, or keyword. The size of nodes indicates the frequency of occurrence or citation, and color of nodes denoted different years. The connection lines between nodes were regarded as the co-occurrence or co-citation relationship, the lines’ thickness represented the strength of the relationship [22, 23]. The centrality was also named betweenness centrality, which measures the percentage of shortest paths in the network to which a given node belongs [24]. Nodes with high centrality (> 0.1) were usually deemed turning points or pivotal points in this domain [23]. The threshold represents the number of occurrences. The number of variable labels displayed in the visualization map can control by adjusting the threshold. In the cluster analysis, the modularity (Q value) and the mean silhouette scores (S value) were employed to evaluate the overall structural characteristic of the network. Q value > 0.3 indicated an overall significant structure. The clustering was regarded as reasonable when S value > 0.5 and convincing when S value > 0.7 [25]. We imported the “download_*.txt” file into CiteSpace and selected “Data” to remove duplicated literatures. The CiteSpace parameters were set as follows: time-slicing was chosen from January 2020 to October 2022, the time slice is one year, and all options in the term source were selected, node types were selected one at a time, selection criteria (g-index, g2 ≤ kΣi≤g ci, k ∈ Z+, k = 25), and set the others as default values. The impact factor and quartile of the journals were obtained from Journal Citation Reports 2021.The citations of the top 10 most cited articles were analyzed and plotted using GraphPad prism v9.2.0.
Results
Characteristics of the included articles
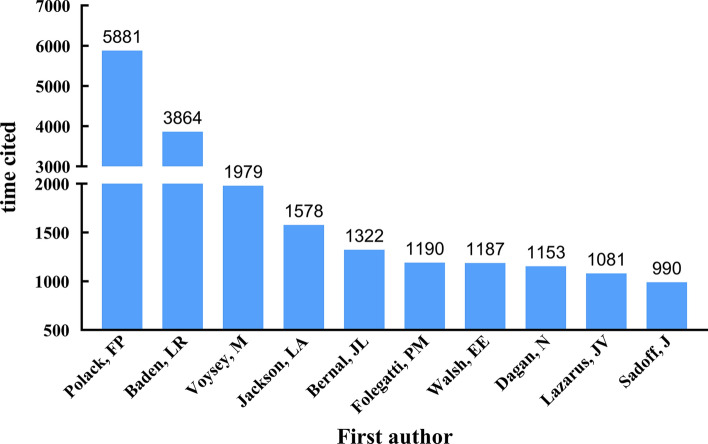
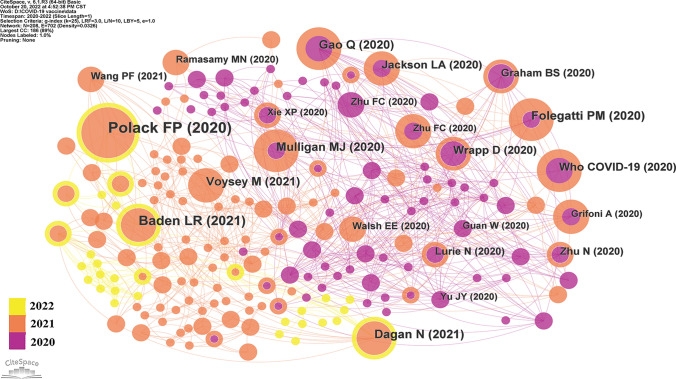
A total of 12,088 articles related to COVID-19 vaccine were retrieved from WOSCC database, while the top 100 most cited articles were determined by ranking of citations in descending order. The years of publication ranged from 2020 to 2022, with 41 articles in 2020, 57 articles in 2021, and two articles in 2022. The top 100 most cited articles on COVID-19 vaccine have been cumulatively cited 55,255 times. The median number of citations was 349.5, with a range of 206 to 5881. Only two studies were cited more than 3000 times, nine articles were cited more than 1000 times, and nearly a quarter of the articles (n = 23) were cited between 500 and 1000 times. In all, 167 authors, 48 countries/regions, 32 journals, and 133 institutions were involved. Table 1 and Fig. 1 were exhibited top 10 most cited articles on COVID-19 vaccine.
Table 1.
Top 10 most cited articles on COVID-19 vaccine
| Rank | Frist author | Article title | Journal | Publication years | Citations |
|---|---|---|---|---|---|
| 1 | Polack, FP | Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine | New England Journal of Medicine | 2020 | 5881 |
| 2 | Baden, LR | Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine | New England Journal of Medicine | 2021 | 3864 |
| 3 | Voysey, M | Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK | Lancet | 2021 | 1979 |
| 4 | Jackson, LA | An mRNA Vaccine against SARS-CoV-2-Preliminary Report | New England Journal of Medicine | 2020 | 1578 |
| 5 | Bernal, JL | Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant | New England Journal of Medicine | 2021 | 1322 |
| 6 | Folegatti, PM | Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial | Lancet | 2020 | 1190 |
| 7 | Walsh, EE | Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates | New England Journal of Medicine | 2020 | 1187 |
| 8 | Dagan, N | BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting | New England Journal of Medicine | 2021 | 1153 |
| 9 | Lazarus, JV | A global survey of potential acceptance of a COVID-19 vaccine | Nature Medicine | 2021 | 1081 |
| 10 | Sadoff, J | Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19 | New England Journal of Medicine | 2021 | 990 |
Fig. 1.
Top 10 most cited articles on COVID-19 vaccine
Analysis of countries/regions
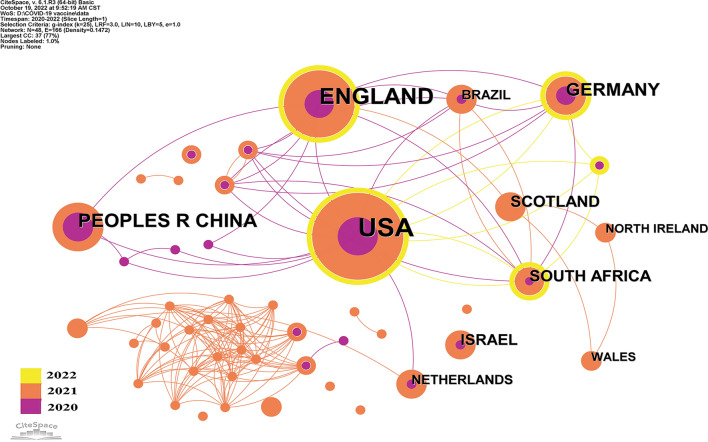
Altogether, 48 countries/regions published articles were involved in COVID-19 vaccine in the top 100 most cited articles. As depicted in Fig. 2, there were 48 nodes and 166 links in the network diagram of these countries/regions mapped by CiteSpace. The USA (n = 56), England (n = 33), and China (n = 16) ranked the top three countries/regions in terms of the number of publications. The top three countries/regions in terms of citations were the USA (n = 35,262), England (n = 22,831), and Germany (n = 13,034). Node centrality analysis demonstrated that the USA had the highest centrality (centrality = 0.63), which was relatively higher than other countries/regions, indicating that the USA play a prominent role in this field, as well as has extensive cooperation with other countries/regions. (Table 2).
Fig. 2.
Countries/regions of top 100 most cited articles on COVID-19 vaccine
Table 2.
Top 6 countries/regions of top 100 most cited articles on COVID-19 vaccine
| Rank | Country/region | Count | Centrality | Citations |
|---|---|---|---|---|
| 1 | The USA | 56 | 0.63 | 35,623 |
| 2 | England | 33 | 0.19 | 22,831 |
| 3 | Peoples R China | 16 | 0 | 6771 |
| 4 | Germany | 14 | 0 | 13,034 |
| 5 | Scotland | 10 | 0.13 | 4798 |
| 6 | South Africa | 10 | 0 | 11,966 |
Analysis of institutions
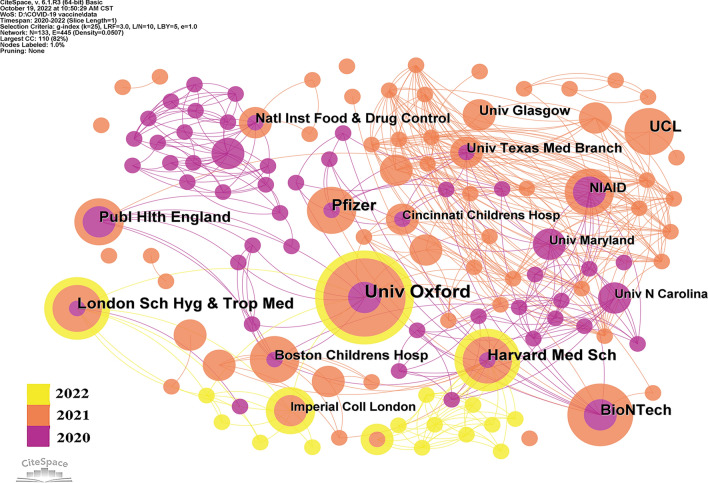
A total of 133 institutions published articles on COVID-19 vaccine in the top 100 most cited articles. The network diagram of these institutions demonstrated that there were 133 nodes and 445 links (Fig. 3). The top two institutions with the maximum number of published articles were University of Oxford (n = 14) and Pfizer Pharmaceuticals Ltd (n = 10). The top three institutions in terms of citations were Pfizer Pharmaceuticals Ltd (n = 10,357), BioNTech (n = 9698), and University of Maryland (n = 9650). However, node centrality analysis manifested that Harvard Medical School had the highest centrality (centrality = 0.71), followed by Boston Children’s Hospital (centrality = 0.67) and Public Health England (centrality = 0.57), proving that these three institutions played greater role than other institutions in COVID-19 vaccine research (Table 3). These finding demonstrated that institutions from the USA and the UK dominate the field of vaccine research.
Fig. 3.
Institutions of top 100 most cited articles on COVID-19 vaccine
Table 3.
Top 15 institutions of top 100 most cited articles on COVID-19 vaccine
| Rank | Institutions | Count | Centrality | Citations | Country |
|---|---|---|---|---|---|
| 1 | University of Oxford | 14 | 0.42 | 6318 | The UK |
| 2 | Pfizer Pharmaceuticals Ltd | 10 | 0 | 10,357 | The USA |
| 3 | Harvard Medical School | 9 | 0.71 | 4174 | The USA |
| 4 | London School of Hygiene & Tropical Medicine | 9 | 0.04 | 4616 | The UK |
| 5 | University College London | 9 | 0.03 | 2368 | The UK |
| 6 | Public Health England | 8 | 0.57 | 4471 | The UK |
| 7 | BioNTech | 8 | 0.08 | 9698 | Germany |
| 8 | Boston Children’s Hospital | 6 | 0.67 | 2807 | The USA |
| 9 | National Institute of Allergy and Infectious Disease | 6 | 0.22 | 8179 | The USA |
| 10 | National Institutes for Food and Drug Control | 6 | 0.06 | 3230 | China |
| 11 | University of Glasgow | 6 | 0 | 877 | The UK |
| 12 | University of North Carolina | 5 | 0.34 | 2544 | The USA |
| 13 | University of Maryland | 5 | 0.27 | 9650 | The USA |
| 14 | Cincinnati Children’s Hospital | 5 | 0.03 | 8519 | The USA |
| 15 | Imperial College London | 5 | 0.03 | 3552 | The UK |
Analysis of journals
The top 100 most cited articles were distributed across 32 journals. Table 4 shows that The New England of medicine journal dominated with 22 articles, more than 20% of the all journals, followed by Lancet (fifteen articles), Nature (eight articles), and Cell (six articles). It was worth pointing out that the number of articles from these four journals accounted for 51% (51/100) of the top 100 most cited articles on COVID-19 vaccine. In addition, the top three journals in terms of citations were The New England of medicine journal (n = 22,564), Lancet (n = 9626), and Lancet (n = 3531). Notably, seven of the top 10 most cited articles were from The New England of medicine journal (Table 1). The above results indicated that these top three authoritative journals will be one of the main sources of information on the latest developments in further COVID-19 vaccine research.
Table 4.
Top 5 journals with most cited articles on COVID-19 vaccine
| Rank | Journal | Count | Citations | Impact factor (2021) | Quartile (2021) | Published Country/region |
|---|---|---|---|---|---|---|
| 1 | New England Journal of Medicine | 22 | 22,564 | 176.082 | Q1 | The USA |
| 2 | Lancet | 15 | 9626 | 202.731 | Q1 | England |
| 3 | Nature | 8 | 3531 | 69.504 | Q1 | England |
| 4 | Cell | 6 | 2121 | 66.850 | Q1 | The USA |
| 5 | Lancet Infectious Diseases | 5 | 1929 | 71.421 | Q1 | The USA |
| 6 | Vaccine | 5 | 1464 | 4.169 | Q3 | Netherlands |
Analysis of authors
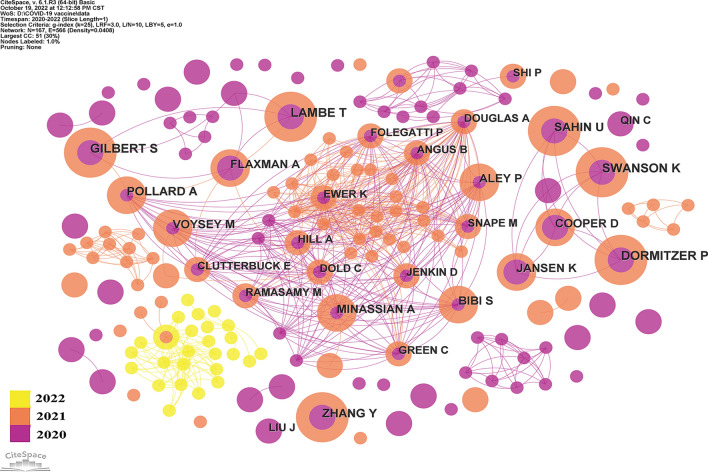
There were altogether 167 authors published papers related to COVID-19 vaccine. The network diagram of authors revealed that it was consisted of 167 nodes and 566 links (Fig. 4). Professor Lambe.T., Gilbert. S., Dormitzer. P., and Swanson. K. produced the most top-cited articles in this bibliometric analysis (n = nine, each). Professor Lambe.T. and Gilbert. S. from University of Oxford, and Professor Dormitzer. P. and Swanson. K. from Pfizer Pharmaceuticals Ltd. Significantly, these two institutions rank in the top two in the number of publications in this study (Table 3).
Fig. 4.
Authors of top 100 most cited articles on COVID-19 vaccine
Analysis of keywords
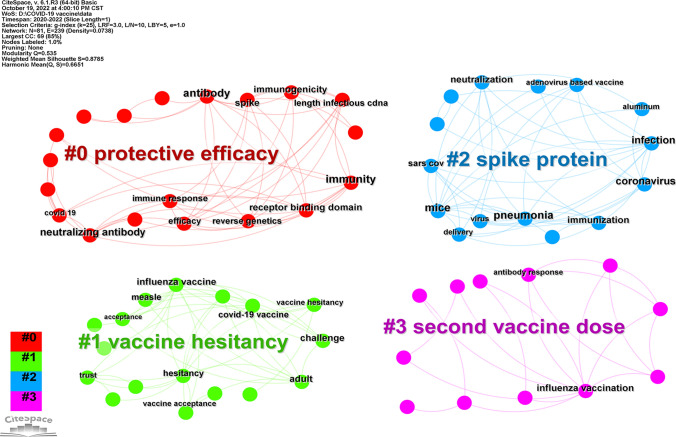
The top 100 most cited articles included in this study comprised 81 keywords. Node centrality analysis demonstrated that the top six keywords were immunization (centrality = 0.25), influenza vaccination (centrality = 0.21), coronavirus (centrality = 0.18), challenge (centrality = 0.18), infection (centrality = 0.15), and immunity (centrality = 0.15). The keywords of the network map were constructed by CiteSpace and consisted of 81 nodes and 239 links (Fig. 5). The results revealed that the immunological effect of vaccines was a primary concern for researchers.
Fig. 5.
Keywords of top 100 most cited articles on COVID-19 vaccine
A cluster analysis of the keywords displayed that 81 keywords were divided into four main clusters (Q value = 0.535, S value = 0.879): # 0 protection efficacy (red), # 1 vaccine hesitancy (green), # 2 spike protein (blue), and # 3 second vaccine dose (pink) (Fig. 6). The efficacy of vaccine and booster vaccination were the focus of vaccine research. Vaccine hesitancy studies investigated the reasons why partial population unwilling to vaccinate, so as to take appropriate action, thus to increase vaccine uptake. Additionally, the studies of spike proteins make a valuable contribution to the studies of new variants and the development of new vaccines. Consequently, these were major concerns in the field of COVID-19 vaccine research.
Fig. 6.
The clustering of keywords of top 100 most cited articles on COVID-19 vaccine (Color figure online)
Analysis of cited references
A total of 208 references were cited by the top 100 most cited articles included in this study, while these references were cited by 862 times. “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine” [26], published by Polack, F. P. was the most cited paper in the top 100 most cited article with 40 citations, followed by “Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine” [27] published by Baden LR. with 24 citations, both of them were published in The New England of medicine journal (Fig. 7). The whole citations of these two articles were ranked top two in this bibliometric analysis, with 5881 and 3864 citations, respectively (Table 1).
Fig. 7.
Cited references of top 100 most cited articles on COVID-19 vaccine
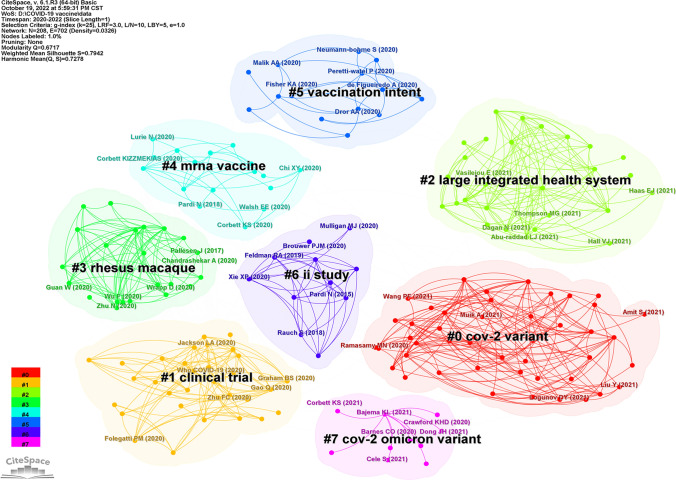
A cluster analysis of cited references demonstrated that 208 cited references were divided into eight largest clusters (Q value = 0.672, S value = 0.794): #0 Cov-2 variant (red), #1 clinical trial (yellow), #2 large integrated health system (pale green), #3 COV-2 rhesus macaque (emerald green), #4 mRNA vaccine (cyan), #5 vaccination intent (sky blue), #6 phase II study (dark blue), and #7 Cov-2 omicron variant (pink) (Fig. 8). It was showed that variants of COVID-19, rhesus macaque animal studies, and clinical trials were the focus of early COVID-19 vaccine research.
Fig. 8.
The clustering of cited reference of top 100 most cited articles on COVID-19 vaccine (Color figure online)
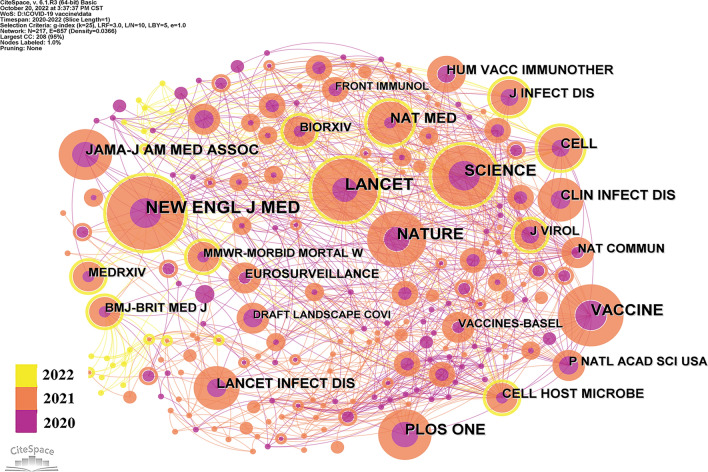
Analysis of cited journals
Publications from 217 journals were cited by the top 100 most cited articles included in this research, and these journals were cited by 1479 times. The journal co-citation network was mapped by CiteSpace, and it consisted of 217 nodes and 857 links (Fig. 9). The most cited journal was The New England of medicine journal (n = 82), followed by Lancet (n = 65) and Science (n = 60). While BMJ-British Medical Journal and Health Psychology had the highest centrality (0.17), followed by Medical Journal of Australia (0.16). To be sure, these top academic journals will continue to publish the latest major advances in COVID-19 vaccine research, which requires more attention from researchers in the future.
Fig. 9.
Cited journals of top 100 most cited articles on COVID-19 vaccine
Discussion
There was an urgent need for safe and effective COVID-19 vaccine after the emergence of SARS-CoV-2. The increasing number of research teams, and pharmaceutical companies were committed to vaccines research and development, which has contributed to an explosion of literatures on COVID-19 vaccines published in the past three years [3]. We performed this first bibliometric analysis of the top 100 most cited articles on COVID-19 vaccine to unveil the most influential authors, institutions, and journals in this field, systematically describe the research focus in the last three years, and highlight the future research direction.
Our finding revealed that the median number of citations of the top 100 most cited articles on COVID-19 vaccine was 349.5. This was lower than some fields, such as vaccine [28], bladder cancer [29], while higher than others, such as anaphylaxis [30], triple‑negative breast cancer [12]. Of note, the average citations per year of this study was remarkably higher than the above domains, suggesting that this topic is presently one of the hottest research focuses. It was indisputable that the development pace of COVID-19 vaccine will not quit, as mutation in the SARS-COV-2 was a continuous process resulting in multiple variant introductions [31]. It will remain attract intensive attention of numerous scientific researchers until free of pandemic. Harvard Medical School, Boston Children’s Hospital, and Public Health England were the top three institutions leading the way in COVID-19 vaccine research. Institutions from the USA and the UK dominated this research field, as evidenced by seven of the top 15 institutions being from the USA, and six of them from the UK. The USA, England, and China ranked the top three countries/regions in accordance with the number of publications, which meant that these three countries/regions represented the highest level in COVID-19 vaccine research worldwide. Among that, more than half of the articles (56%) had participation from American scholars, because basic research conditions in the USA are superior to other countries, with advanced laboratory equipment, adequate funding for scientific research, and first-class research teams [18]. Although China ranked third in the number of publications, the centrality value was zero (Table 2), indicating that China should strengthen international cooperation and carry out more in-depth research on COVID-19 vaccine research.
To curb this epidemic as soon as possible, the academic community lost no time in joining this “battlefield” to carried out vaccine research and development. Furthermore, medical journals with high influence such as The New England Journal of Medicine, Lancet, and British Medical Journal, have also opened special columns for COVID-19 [32], which promoted communication on the global COVID-19 research. The present study indicated that the highest-ranking journal in this field was The New England Journal of Medicine, with 22 publications and an impact factor (IF) of 176.079, and seven of the top 10 most cited articles were from this journal. It was outperformed all other journals in both quantity and quality of publications, signing its dominance on COVID-19 vaccine research. Thus, we predict that more influential and advanced researches in this field will still be published in this journal in the future.
Cluster analysis of keywords disclosed that the top four categories were protection efficacy, vaccine hesitancy, spike protein, and second vaccine dose, respectively. It demonstrated that the primary concerns of vaccine research including spike protein, protection, and vaccine hesitancy. Spike protein, one of the four major structural proteins encoded by SARS-CoV-2 [33], is a critical target for vaccine development [34]. Consequently, the studies of spike protein mutations play a pivotal role in the further COVID-19 vaccine development, because the spike protein has impact on the titers of IgG antibodies, which was correlation with vaccine efficacy [35]. Furthermore, it was proved useful to utilize the computational methods to predict the impact of the variants of concern (VOC) on the COVID-19 vaccines [36, 37]. Previous studies have been revealed that a two-dose regimen of vaccine conferred 85–95% protection against COVID-19, and safety was similar to other virus vaccines [26, 38]. Although emerging evidence indicated that the vaccines have high level of protection and sufficient safety, a segment of population remained unwilling to receive the COVID-19 vaccine [39, 40]. In addition, COVID-19 vaccine acceptance was highly heterogeneous between countries and between groups with different sociodemographic characteristics [41–43]. Hence, how to cultivate vaccine confidence and eliminate the barriers to accessing vaccination so as to increase vaccine uptake may be future trends of COVID-19 vaccine research.
In the cluster analyses, the cited references were classified into eight largest categories: Cov-2 variant, clinical trial, large integrated health system, COV-2 rhesus macaque, mRNA vaccine, vaccination intent, phase II study, and Cov-2 omicron variant. More importantly, animal studies were essential to conclusively identify of vaccine-mediated protection. The novel experimental vaccine was tested in animal before entering clinical trials. Rhesus macaques, one of non-human primates, was involved in COVID-19 vaccine experiments, and developed protective immune responses to COVID-19 [44, 45]. Given that emerging omicron subvariant, characterized by highly transmission, was the predominant variant worldwide, the protection of current COVID-19 vaccines against omicron variant has become a hot research topic. It was exciting that studies proved that the booster-dose vaccine could provide efficacy against the omicron variant [46–48]. Thus, the protection and efficacy of new vaccines under pre- and clinical development against omicron will be highlight research areas in the future. Additionally, side effects and adverse events after COVID-19 vaccination were attracted more attention for current researches [49, 50]. The development of COVID-19 antiviral drugs is equally urgent. Notably, some new bioinformatics analysis methods, such as weighted gene co-expression network analysis, play a crucial role in identifying biomarkers and potential therapeutic targets in COVID-19 [51].
We searched the articles related to COVID-19 vaccines published in several high-impact journals from January 2022 to February 2023 based on WOSCC, and found that the researches mainly focused on the following aspects: first, studies on vaccine efficacy, safety and immunogenicity, including clinical trials of new vaccines, vaccine protection against omicron, and infection protection of special populations, etc.; second, evaluation of the efficacy of third and fourth dose of vaccine; third, the investigations of vaccination willingness and vaccine hesitancy in various populations; finally, basic research involve COVID-19 virus and vaccines. Of note, these were basically consistent with our findings.
However, there were some limitations in our study. First, only two articles published in 2022 were included in the present study. Some high-quality articles published in 2022 may not be included in our study due to short publication time frame and fewer citations, which may result in bias. Second, the WOSCC database was not fully inclusive of all literature. Hence, it might not provide truly representative citation counts. Third, the number of citations might not be fully representative of article quality. Low citation number may not mean low research value, and high citation number does not necessarily indicate important guidance to this field. Fourth, the statistical methods used by CiteSpace software may have some biases, which was an inherent weakness of software. Finally, a small number of non-English articles were not included in this study due to our inclusion criteria, leading to bias in literature selection. Despite these limitations, our study offers fresh insights into the present state and development direction for further study of COVID-19 vaccine.
In conclusion, a plethora of papers on COVID-19 vaccine have been published in the last three years, indicating that this theme is currently hottest topic in academic community. The USA, the UK, and China made many contributions to COVID-19 vaccine research globally. Harvard Medical School, Boston Children’s Hospital, and Public Health England were the top three institutions leading the way on COVID-19 vaccine research. The New England Journal of Medicine was dominant in publishing high-quality COVID-19 vaccine papers. At present, COVID-19 vaccines researches have focused on vaccine efficacy, vaccine hesitancy, and the efficacy of current vaccines on omicron variants. However, how to increase vaccine uptake, focus on mutations in the spike protein, evaluate of the efficacy of booster vaccine, and how effective new vaccines under pre- and clinical development against omicron will be spotlight in the future.
Author contributions
YF and SW designed this study, WW and HW collected the data, TY, YL, and JL performed data analysis and interpretation, WW, LY, and YG drafted the article, WW, JL, YF, and SW revised the article, YF and SW approved the final version to be published. All authors reviewed the manuscript.
Funding
The study was supported by the COVID-19 Project of Shanxi Provincial Finance, and the Project of Shanxi Provincial Key Laboratory for major infectious disease response.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All data were obtained and downloaded from a publicly available database and did not involve any ethical issues requiring approval.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Weigang Wang, Email: wangweigang@sxmu.edu.cn.
Hu Wang, Email: wanghu@sxmu.edu.cn.
Tian Yao, Email: yaotian1026@sxmu.edu.cn.
Yandi Li, Email: 20180510601@b.sxmu.edu.cn.
Linzhu Yi, Email: dr20190051037@d.sxmu.edu.cn.
Ying Gao, Email: gaoying@sxmu.edu.cn.
Jia Lian, Email: lianjia@sxmu.edu.com.
Yongliang Feng, Email: yongliang.feng@sxmu.edu.cn.
Suping Wang, Email: supingwang@sxmu.edu.cn.
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 10 Oct, 2022
- 3.Chen Y, Cheng L, Lian R, Song Z, Tian J. COVID-19 vaccine research focusses on safety, efficacy, immunoinformatics, and vaccine production and delivery: a bibliometric analysis based on VOSviewer. Biosci Trends. 2021;15(2):64–73. doi: 10.5582/bst.2021.01061. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad T, Murad MA, Baig M, Hui J. Research trends in COVID-19 vaccine: a bibliometric analysis. Hum Vaccin Immunother. 2021;17(8):2367–2372. doi: 10.1080/21645515.2021.1886806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626–636. doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadj HI. Covid-19 vaccines and variants of concern: a review. Rev Med Virol. 2022;32(4):e2313. doi: 10.1002/rmv.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barranco R, Rocca G, Molinelli A, Ventura F. Controversies and challenges of mass vaccination against SARS-CoV-2 in Italy: medico-legal perspectives and considerations. Healthcare (Basel). 2021 doi: 10.3390/healthcare9091163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. COVID-19 vaccine tracker and landscape. Accessed 10 Oct, 2022.
- 9.Ellegaard O, Wallin JA. The bibliometric analysis of scholarly production: how great is the impact? Scientometrics. 2015;105(3):1809–1831. doi: 10.1007/s11192-015-1645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao RQ, Ren C, Wang JN, et al. Publication trends of research on sepsis and host immune response during 1999–2019: a 20-year bibliometric analysis. Int J Biol Sci. 2020;16(1):27–37. doi: 10.7150/ijbs.37496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devos P, Menard J. Bibliometric analysis of research relating to hypertension reported over the period 1997–2016. J Hypertens. 2019;37(11):2116–2122. doi: 10.1097/HJH.0000000000002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Chen P, Peng B, et al. The top 100 most cited articles on triple-negative breast cancer: a bibliometric analysis. Clin Exp Med. 2022 doi: 10.1007/s10238-022-00800-9. [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Tian D. Bibliometric analysis of global scientific research on COVID-19. J Biosaf Biosecur. 2021;3(1):4–9. doi: 10.1016/j.jobb.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durieux V, Gevenois PA. Bibliometric indicators: quality measurements of scientific publication. Radiology. 2010;255(2):342–351. doi: 10.1148/radiol.09090626. [DOI] [PubMed] [Google Scholar]
- 15.Kwok HT, Van M, Fan KS, Chan J. Top 100 cited articles in male breast cancer: a bibliometric analysis. Breast Dis. 2022;41(1):15–20. doi: 10.3233/BD-201024. [DOI] [PubMed] [Google Scholar]
- 16.Sarirete A. A bibliometric analysis of COVID-19 vaccines and sentiment analysis. Procedia Comput Sci. 2021;194:280–287. doi: 10.1016/j.procs.2021.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fresno-Alba S, Denche-Zamorano A, Pastor-Cisneros R, Pereira-Payo D, Franco-Garcia JM, Jimenez-Castuera R. Breast cancer and physical activity: a bibliometric analysis. Front Oncol. 2022;12:1051482. doi: 10.3389/fonc.2022.1051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia Y, Yao RQ, Zhao PY, et al. Publication trends of research on COVID-19 and host immune response: a bibliometric analysis. Front Public Health. 2022;10:939053. doi: 10.3389/fpubh.2022.939053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehmood K, Mushtaq S, Bao Y, et al. The impact of COVID-19 pandemic on air pollution: a global research framework, challenges, and future perspectives. Environ Sci Pollut Res Int. 2022;29(35):52618–52634. doi: 10.1007/s11356-022-19484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Song Y, Guo Z, et al. COVID-19 pharmacological research trends: a bibliometric analysis. Intell Med. 2022 doi: 10.1016/j.imed.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101(Suppl 1):5303–5310. doi: 10.1073/pnas.0307513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong D, Li Y, Huang Y, Hong X, Li J, Jin R. Molecular mechanisms of exercise on cancer: a bibliometrics study and visualization analysis via CiteSpace. Front Mol Biosci. 2021;8:797902. doi: 10.3389/fmolb.2021.797902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo H, Cai Z, Huang Y, et al. Study on pain catastrophizing from 2010 to 2020: a bibliometric analysis via CiteSpace. Front Psychol. 2021;12:759347. doi: 10.3389/fpsyg.2021.759347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inform Sci Technol. 2006;57(3):359–377. doi: 10.1002/asi.20317. [DOI] [Google Scholar]
- 25.Chen B, Shin S. Bibliometric analysis on research trend of accidental falls in older adults by using citespace-focused on web of science core collection (2010–2020) Int J Environ Res Public Health. 2021 doi: 10.3390/ijerph18041663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Quan L, Xiao B, Du L. The 100 top-cited studies on vaccine: a bibliometric analysis. Hum Vaccin Immunother. 2019;15(12):3024–3031. doi: 10.1080/21645515.2019.1614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mainwaring A, Bullock N, Ellul T, Hughes O, Featherstone J. The top 100 most cited manuscripts in bladder cancer: a bibliometric analysis (review article) Int J Surg. 2020;75:130–138. doi: 10.1016/j.ijsu.2020.01.128. [DOI] [PubMed] [Google Scholar]
- 30.Song Y, Zhang L, Yang Y, Sun J. The top 100 most cited articles in anaphylaxis: a bibliometric analysis. Clin Exp Med. 2022 doi: 10.1007/s10238-022-00890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araf Y, Akter F, Tang YD, et al. Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022;94(5):1825–1832. doi: 10.1002/jmv.27588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Y, Li Y, Zhang Z, et al. A bibliometric analysis using VOSviewer of publications on COVID-19. Ann Transl Med. 2020;8(13):816. doi: 10.21037/atm-20-4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ita K. Coronavirus disease (COVID-19): current status and prospects for drug and vaccine development. Arch Med Res. 2021;52(1):15–24. doi: 10.1016/j.arcmed.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik JA, Ahmed S, Mir A, et al. The SARS-CoV-2 mutations versus vaccine effectiveness: new opportunities to new challenges. J Infect Public Health. 2022;15(2):228–240. doi: 10.1016/j.jiph.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes Q, Inchakalody VP, Merhi M, et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. 2022;54(1):524–540. doi: 10.1080/07853890.2022.2031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serapian SA, Marchetti F, Triveri A, et al. The answer lies in the energy: how simple atomistic molecular dynamics simulations may hold the key to epitope prediction on the fully glycosylated SARS-CoV-2 spike protein. J Phys Chem Lett. 2020;11(19):8084–8093. doi: 10.1021/acs.jpclett.0c02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q, Qin C, Liu M, Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10(1):132. doi: 10.1186/s40249-021-00915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kricorian K, Civen R, Equils O. COVID-19 vaccine hesitancy: misinformation and perceptions of vaccine safety. Hum Vaccin Immunother. 2022;18(1):1950504. doi: 10.1080/21645515.2021.1950504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245–251. doi: 10.1016/j.puhe.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27(2):225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malik AA, McFadden SM, Elharake J, Omer SB. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26:100495. doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dube E, MacDonald NE. COVID-19 vaccine hesitancy. Nat Rev Nephrol. 2022;18(7):409–410. doi: 10.1038/s41581-022-00571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogel AB, Kanevsky I, Che Y, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592(7853):283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 45.van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586(7830):578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chenchula S, Karunakaran P, Sharma S, Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: a systematic review. J Med Virol. 2022;94(7):2969–2976. doi: 10.1002/jmv.27697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 2022;20(1):200. doi: 10.1186/s12916-022-02397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/s1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karami H, Derakhshani A, Ghasemigol M, et al. Weighted gene co-expression network analysis combined with machine learning validation to identify key modules and hub genes associated with SARS-CoV-2 infection. J Clin Med. 2021 doi: 10.3390/jcm10163567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.