Abstract
Neonatal invasive candidiasis (NIC) has significant morbidity and mortality. Reports have shown a different profile of those neonates affected with NIC and of fluconazole-resistant Candida spp. isolates in low- and middle-income countries (LMICs) compared to high-income countries (HICs). We describe the epidemiology, Candida spp. distribution, treatment, and outcomes of neonates with NIC from LMICs enrolled in a global, prospective, longitudinal, observational cohort study (NeoOBS) of hospitalized infants <60 days postnatal age with sepsis (August 2018–February 2021). A total of 127 neonates from 14 hospitals in 8 countries with Candida spp. isolated from blood culture were included. Median gestational age of affected neonates was 30 weeks (IQR: 28–34), and median birth weight was 1270 gr (interquartile range [IQR]: 990–1692). Only a minority had high-risk criteria, such as being born <28 weeks, 19% (24/127), or birth weight <1000 gr, 27% (34/127). The most common Candida species were C. albicans (n = 45, 35%), C. parapsilosis (n = 38, 30%), and Candida auris (n = 18, 14%). The majority of C. albicans isolates were fluconazole susceptible, whereas 59% of C. parapsilosis isolates were fluconazole-resistant. Amphotericin B was the most common antifungal used [74% (78/105)], followed by fluconazole [22% (23/105)]. Death by day 28 post-enrollment was 22% (28/127). To our knowledge, this is the largest multi-country cohort of NIC in LMICs. Most of the neonates would not have been considered at high risk for NIC in HICs. A substantial proportion of isolates was resistant to first choice fluconazole. Understanding the burden of NIC in LMIC is essential to guide future research and treatment guidelines.
Keywords: neonatal candidemia, low- and middle-income countries, Candida parapsilosis, candidiasis, Candida auris
Introduction
The World Health Organization (WHO) estimates that 2.4 million children died globally in the first month of life in 2019, with infection being the third commonest cause of death following prematurity- and intrapartum-related complications.1 The contribution of infection to deaths in the neonatal period is often underappreciated and varies according to geographic location, neonatal characteristics, and whether or not neonates are born in a medical facility.2–4
Neonatal invasive fungal infections are mostly caused by Candida spp. Reported rates of neonatal invasive candidiasis (NIC) vary significantly globally5 and are associated with a high crude mortality rate, ranging from 12% to 37% in high-income countries (HICs) and from 8.9% to 75% in low- and middle-income countries (LMICs).6 In HICs, NIC is most commonly reported in neonates <1000 gr birth weight or<28 weeks gestational age, but recent reports from LMIC neonatal units show the occurrence of NIC outside these specific groups.3,7,8 Although antifungal-resistant Candida spp. infections remain uncommon in HICs,9,10 LMICs are reporting an increasing proportion of fluconazole-resistant isolates, including C. parapsilosis,11,12C. krusei, and C. auris.13,14
The aim of this NeoOBS invasive candidiasis sub-study was to describe the epidemiology, antifungal resistance patterns, antifungal treatment, and clinical outcomes of neonates with Candida spp. bloodstream infections in LMICs. Data were collected as part of the larger NeoOBS study (https://clinicaltrials.gov/ct2/show/NCT03721302).
Materials and methods
NeoOBS study population
NeoOBS, a global, prospective, longitudinal, observational cohort study of hospitalized infants <60 days postnatal age with sepsis, was conducted at 19 hospitals in 11 countries, between August 2018 and February 2020. Hospitals were a mix of tertiary and district hospitals in Bangladesh (n = 1), Brazil (n = 2), China (n = 3), Greece (n = 1), India (n = 3), Italy (n = 1), Kenya (n = 1), South Africa (n = 3), Thailand (n = 2), Uganda (n = 1), and Vietnam (n = 1).15,16
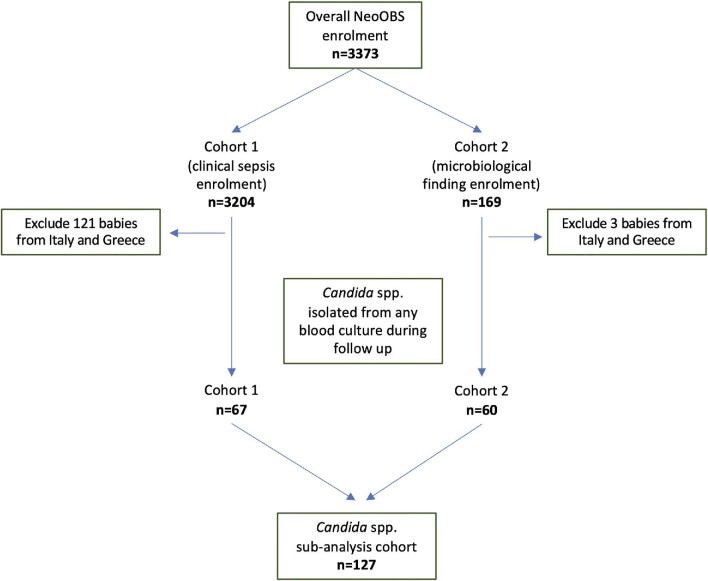
Infants could be enrolled in the study in two different ways (Fig. 1). The primary cohort of infants was enrolled with clinical sepsis meeting the diagnostic criteria of at least one clinical sign of sepsis plus one clinical or laboratory sign, with a blood culture taken prior to initiating new antimicrobial treatment (referred to as cohort 1). Up to 200 infants from each hospital were enrolled through this route. Infants were excluded if the clinical signs were subsequently deemed to be more likely related to a non-sepsis diagnosis, as determined by the treating clinician (Supplementary Table 1).
Figure 1.
Candida spp. sub-study population derived from the overall NeoOBS study. See Supplementary Figure 1 for detailed schematic of the study population indicating the two enrollment cohorts. Note: Overall NeoOBS enrollment: cohort 1 was 3204 babies from 19 hospitals in 11 countries; cohort 2 was 169 babies from 14 hospitals in 10 countries. Candida sub-analysis cohort: cohort 1 includes 67 babies from 12 hospitals in 7 countries; cohort 2 was 60 babies from 12 hospitals in 7 countries.
In addition, a secondary cohort of infants (referred to as cohort 2) was enrolled based on the isolation of a carbapenem-resistant organism or Candida spp. from blood culture or with confirmed bacterial meningitis (Supplementary Table 1). Cohort 2 was designed to better understand specific infections and capture infants with these infections who may not have been enrolled in or eligible for cohort 1. There was no minimum or maximum enrollment number for cohort 2 across hospitals. Infants already enrolled in cohort 1 were not eligible to be enrolled in cohort 2 as all microbiology findings were already captured as part of cohort 1 follow-up.
Exclusion criteria for both cohorts were significant non-infectious-related comorbidity expected to cause death within 72 h, enrollment in an interventional study or previous enrollment in this study. Hospitals were given pragmatic flexibility for enrollment time frames given variability in case numbers and staffing capacity. Full inclusion and exclusion criteria for both cohorts are described in Supplementary Table 1.
Data collection
Infants meeting the eligibility criteria were enrolled in both cohorts. Infants in cohort 1 were followed prospectively daily for the duration of hospitalization up to day 28 from the day of enrollment.
For infants in cohort 2, daily clinical and antimicrobial treatment data and any laboratory or microbiological investigations were retrospectively collected using medical notes and other available data from the day the culture was taken up to the day of enrollment, and then, prospectively collected from the day of enrollment (Supplementary Fig. 1) to 28 days from when the eligible blood culture was taken. For babies in both cohorts, clinical signs, supportive measures, and antimicrobial treatment were collected daily from the day of enrollment; blood culture, routine laboratory investigations, and other microbiology results were collected as and when conducted. At enrollment, demographics, labor and delivery details, and risk factors were collected.
All treatments and investigations were at the discretion of clinicians at the local hospital and were not determined by the study processes. At discharge or in-hospital death, information on mortality (if applicable), antimicrobial treatment, and both infection- and non-infection-related diagnoses were collected. Infants who were discharged prior to day 28 were telephoned on day 28, to assess vital status and any medical interventions since discharge. The primary outcome of the study was death by day 28, from the day the enrollment blood culture was taken. Primary and secondary causes (if applicable) of death were captured both for infants who died in hospital and those who died post-discharge before day 28.
Microbiological examinations were conducted as per local hospital procedures; however, babies must have had a blood culture taken prior to new antimicrobials being started to be eligible for enrollment in cohort 1. Blood culture results were collected as reported by local hospitals.
Study data were collected by paper case report form and entered and managed using REDCap electronic data capture tools hosted at St. George's, University of London. REDCap is a secure, web-based software platform17,18 used for the collection and management of research data.
Ethics approval from local and national bodies was received by each hospital prior to commencing recruitment. Informed consent was obtained for all patients prior to enrollment.
Candidemia study population
All infants enrolled in the NeoOBS study via cohort 1 or cohort 2 who had Candida spp. isolated from a blood culture at any point during their follow-up up to day 28 (regardless of enrollment diagnosis) were included in this analysis. Analyses were restricted to the first Candida spp. isolated from each patient. Regardless of when during follow-up the Candida spp. was taken, all infants were censored at 28 days from when the enrollment blood culture for the overall NeoOBS study was taken (see Supplementary Fig. 1) This means some infants may have contributed fewer than 28 days of follow-up from when first Candida spp. culture was taken to this candidemia sub-analysis.
Due to differing times in follow-up when Candida spp. cultures were taken for these patients, for this sub-analysis, all patients were aligned with day 0 defined as the day the positive Candida spp. blood culture was taken (Supplementary Fig. 1). All data collection tools were the same for infants in both cohorts. Analyses were restricted to infants from LMICs only, thus excluding infants from Greece (n = 3) and Italy (n = 0).
Statistical analysis
Categorical variables were described as relative frequency, and continuous variables were described as median and interquartile range (IQR). Demographic and clinical characteristics between enrollment cohorts were compared using the χ2 test. Kaplan–Meier curves and Cox proportional hazards model were used to investigate mortality. All data management and analyses were conducted in RStudio v1.4.1717 (R version 4.0.3).
Results
Study population and baseline characteristics
After excluding infants from Greece and Italy, 3083 neonates were enrolled from 17 hospitals in cohort 1, and 166 neonates were enrolled from 14 hospitals in cohort 2. Results from the overall NeoOBS study are described elsewhere.19 Overall, 127/3249 (4%) infants met the inclusion criteria for the candidemia sub-analysis (67 were from cohort 1 and 60 from cohort 2) (Fig. 1). Infants with candidemia were from 14 hospitals in eight LMICs; however, 85% (108/127) of the infants with candidemia were reported from seven hospitals in three countries (South Africa, India, and Vietnam). Forty-six percent (58/127) of infants had Candida spp. isolated during follow-up after enrollment in the overall NeoOBS study, while the remaining 54% (69/127) had Candida spp. isolated from their baseline enrollment blood culture.
At the time when the blood culture that grew Candida spp. was taken, the median postnatal age was 16 days (IQR: 10.5–21), the median gestational age at birth was 30 weeks (IQR: 28–34), and the median birth weight was 1270 gr (IQR: 990–1692). Fifty-four percent (68/127) of the infants were male. Only 19% (24/127) of the infants were born before 28 weeks of gestation, and 27% (34/127) had birth weights <1000 gr. Infants with candidemia were hospitalized for a median of 14 days (IQR: 6.5–20) prior to when the Candida spp. blood culture was taken. Eighty percent (102/127) of infants received at least one broad-spectrum antibiotic in the week prior to that blood culture. The majority of cases, 90% (114/127), were born either at the enrolling hospital or in a referral hospital and remained hospitalized from birth. Baseline characteristics of both enrollment cohorts were similar (Supplementary Table 2). Epidemiological characteristics by survival are summarized in Table 1.
Table 1.
Comparison of summary characteristics of neonates with candidemia by survival status.
| Overall (n = 127) | Survived (n = 99) | Died (n = 28) | |
|---|---|---|---|
| Sex; Female (%) | 59 (47) | 44 (44) | 15 (54) |
| Birth weight (gr) (median [IQR]) | 1270.0 [990.0, 1692.5] |
1300.00 [1022.5, 1724.5] |
955.00 [772.0, 1655.0] |
| Gestational age (weeks) (median [IQR]) | 30 [28, 34] | 30 [28, 34] | 29 [27, 33] |
| Age at Candida spp. culture (days) (median [IQR]) | 16 [10.5, 22.0] | 16.0 [12.0, 22.0] | 14.5 [8.0, 22.5] |
| Birth status Hospitalized since birth (%) |
114 (90) |
90 (91) |
24 (86) |
| Organism (n = 128) (%) | |||
| Candida albicans | 45 (35) | 35 (35) | 10 (36) |
| Candida parapsilosis | 38 (30) | 31 (31) | 7 (25) |
| Candida auris | 18 (14) | 13 (13) | 5 (18) |
| Other Candida spp.a | 27 (21) | 21 (21) | 6 (21) |
| Country (%) | |||
| India | 40 (32) | 30 (30) | 10 (36) |
| South Africa | 55 (43) | 44 (44) | 11 (39) |
| Vietnam | 13 (10) | 10 (10) | 3 (11) |
| Otherb | 19 (15) | 15 (15) | 4 (14) |
| Hospital (%) | |||
| Hospital 1 | 28 (22) | 22 (22) | 6 (21) |
| Hospital 2 | 25 (20) | 20 (20) | 5 (18) |
| Hospital 3 | 21 (17) | 17 (17) | 4 (14) |
| Hospital 4 | 13 (10) | 10 (10) | 3 (11) |
| Hospital 5 | 11 (9) | 8 (8) | 3 (11) |
| Otherc | 29 (23) | 22 (22) | 7 (25) |
aOther Candida spp. include C. famata (n = 1), C. glabrata (n = 6), C. metapsilosis (n = 1), C. pelliculosa (n = 4), C. rugosa (n = 1), undefined Candida spp. (n = 10), and C. tropicalis (n = 4).
bOther countries are comprised of five countries, each contributing <8 participants (range: 1–7 per country).
cOther sites are comprised of nine hospitals, each contributing <7 participants (range: 1–6 per site).
Microbiology findings
The most common Candida species in this study were C. albicans (n = 45, 35%), C. parapsilosis (n = 38, 30%), and C. auris (n = 18, 14%). Other species isolated were C. glabrata (n = 6), C. pelliculosa (n = 4), C. tropicalis (n = 4), C. famata (n = 1), C. metapsilosis (n = 1), and C. rugosa (n = 1). There were 10 (8%) unspecified Candida spp. (Table 1). Species distribution varied by hospital (P < .0001) and country (P < .0001) (Supplementary Table 3). One patient had two Candida spp. isolates (C. parapsilosis and C. glabrata) from the same blood culture (a total of 128 Candida spp. isolates). Sixty-one percent (11/18) of C. auris isolates were found in India, and the remaining 39% (7/18) were from South Africa.
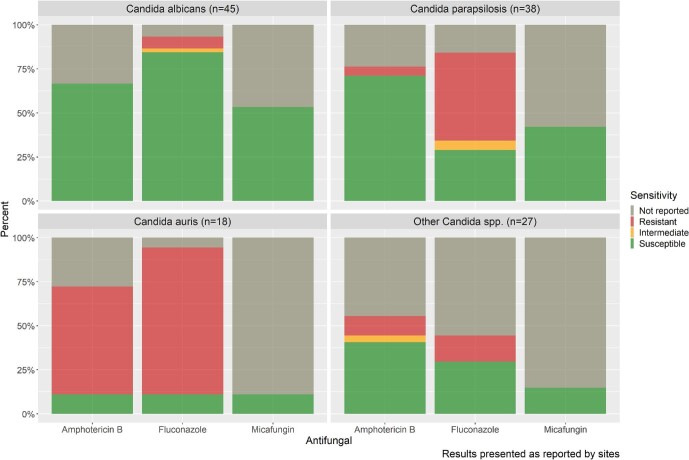
Susceptibility testing was not reported for 13% (16/128) of isolates, and for 17 isolates (13%), only fluconazole susceptibility was reported. Susceptibility results for fluconazole, amphotericin B, and an echinocandin [micafungin] were reported in 80% (103/128), 78% (87/128), and 36% (46/128) of all Candida spp. isolates, respectively (Fig. 2). Of these, overall, 41/103 (40%) were fluconazole-resistant, 16/87 (18%) were amphotericin B-resistant, and 0/46 (0%) were micafungin-resistant.
Figure 2.
Reported susceptibility profiles to amphotericin B, fluconazole, and micafungin for the most common Candida species.
Of the C. albicans isolates with susceptibility results reported, 91% (38/42) were susceptible to fluconazole, and 100% (30/30) were susceptible to amphotericin B; however, 15/45 (33%) did not have amphotericin B susceptibility reported.
Reported resistance to fluconazole in C. parapsilosis was high (19/32 resistant, 59%); however, the majority were susceptible to amphotericin B (27/29 susceptible, 93%). Almost all the fluconazole-resistant C. parapsilosis isolates (17/19, 90%) were reported from South Africa.
There was an expected high reported resistance to fluconazole (15/17, 88%) and amphotericin B (11/13, 85%) in C. auris isolates. Most C. auris isolates did not have micafungin susceptibility testing done (16/18, 89%). Resistance of C. auris isolates to voriconazole was reported in 31% (5/16). Figure 2 illustrates the susceptibility profiles of Candida spp. isolates to the three commonly used antifungal agents.
Antifungal drug use
Overall, 14% (18/127) of infants received antifungals for either prophylaxis (n = 8) or empirical treatment (n = 11) in the week preceding the positive blood culture with Candida spp. being taken. Of those, all the infants who received prophylaxis received fluconazole. Of those receiving empirical treatment prior to the blood culture, amphotericin B was received by 73% (8/11) of infants.
Overall, neonatal antifungal prophylaxis was uncommon in the infants that developed candidemia (8/127, 6%). None (0/27) of those born before 28 weeks of gestation, and only 3/34 (9%) of neonates with a birth weight <1000 gr received prophylaxis.
Ninety percent of infants (114/127) received antifungal treatment after taking the blood culture that grew Candida spp., including eight infants who continued antifungal treatment that had been started before the culture (fluconazole: n = 3 and amphotericin B: n = 5). Of the 106, who started any new antifungals after the blood culture was taken, only one had received fluconazole prophylaxis. After taking the blood culture, the median time to start a new antifungal treatment in these 105 infants was 3 days (IQR: 2–4). The first antifungal treatment of choice was amphotericin B in 74% (78/105) of the cases and fluconazole in 22% (23/105) of the cases.
Out of 88 infants, 85 (97%) received appropriate antifungal treatment based on the reported in vitro susceptibility profile of the Candida species with available susceptibility testing results. In these infants, amphotericin B (n = 45) was the most commonly prescribed antifungal, followed by fluconazole (n = 27), voriconazole (n = 10), and micafungin (n = 3). In infants with known susceptibility profile of the Candida species, the median time to appropriate antifungal treatment was 3 days from when the blood culture was taken (IQR: 2–5 days, range: 8 days prior to blood culture to 12 days after blood culture).
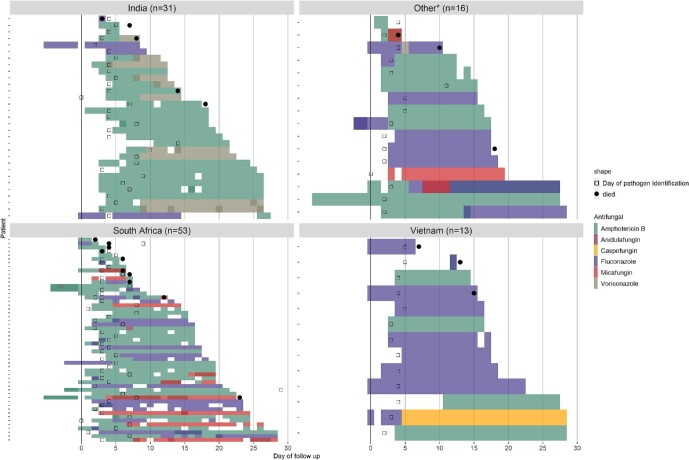
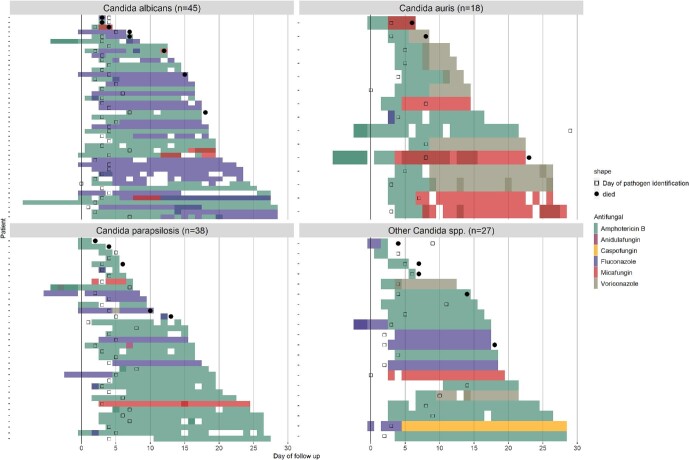
Antifungal treatment varied by country (Fig. 3) and causative Candida species (Fig. 4). Amphotericin B was used in all countries. In infants who received antifungal treatment after blood culture, a higher proportion of infants received amphotericin B in South Africa (48/53, 91%) and India (28/31, 90%) compared to Vietnam (5/13, 38%) (Fig. 3). Fluconazole was less commonly prescribed in India (4/31, 13%) and South Africa (23/53, 43%) compared to Vietnam (9/13, 69%). Voriconazole was used only in India (n = 10), micafungin was used predominantly in South Africa (n = 10), and caspofungin was used only in Vietnam (n = 1). Infants may have received more than one antifungal during their treatment.
Figure 3.
Patient-based antifungal treatment choice by country indicating the day the blood culture was taken (day 0), the day the fungal organism was identified (open squares), and mortality (solid dots). White space indicates calendar days that antifungal treatment was not given. *Other countries are comprised of five countries, each contributing < 8 participants (range: 1–7 per country).
Figure 4.
Patient-based antifungal treatment choice by causative Candida spp. Day 0 is the day the blood culture was taken that grew Candida spp. Day that the Candida spp. was identified is indicated with open squares and mortality with solid dots.
Clinical outcome
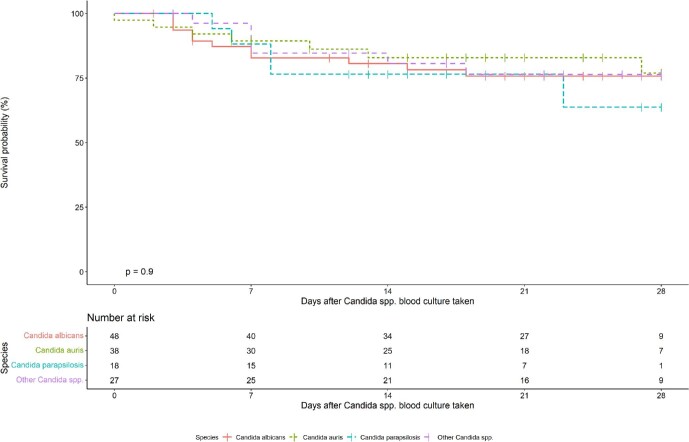
Death by day 28 post-enrollment was 22% (28/127). Sixty-four percent (81/127) of infants were still in the hospital on day 28 post-enrollment. The median length of follow-up from the day the Candida spp. culture was taken was 21 days (IQR: 11.5–27). Among infants who died, the median length of follow-up was 7 days (IQR: 4–12.25). Unadjusted mortality by species is illustrated in Fig. 5. In aunivariable Cox proportional hazards analysis, mortality was strongly associated with birthweight <1000 gr (HR: 3.83; 95% CI: 1.84–7.97) and gestational age <28 weeks (HR: 2.32; 95% CI: 1.08–4.99). There was no significant difference in mortality by species, by hospital, by country, or by study cohort (Table 2).
Figure 5.
Kaplan–Meier curve for mortality from day of culture for each Candida species.
Table 2.
Univariable hazard ratios from Cox proportional hazards analysis.
| Mortality | N (%) | Univariable hazard ratio (95% CI, P) | |
|---|---|---|---|
| Birthweight (1000 gr) | ≥1000 gr | 95 (72.5) | - |
| <1000 gr | 36 (27.5) | 3.83 (1.84–7.97, P < .001) | |
| Gestational age | ≥28 weeks | 104 (79.4) | - |
| <28 weeks | 27 (20.6) | 2.32 (1.08–4.99, P = .032) | |
| Organism | Candida albicans | 48 (36.6) | - |
| Candida parapsilosis | 38 (29.0) | 0.84 (0.32–2.16, P = .711) | |
| Candida auris | 18 (13.7) | 1.28 (0.44–3.70, P = .645) | |
| Other Candida spp. | 27 (20.6) | 0.92 (0.34–2.48, P = .868) | |
| Country | Country 1 | 40 (30.5) | - |
| Country 2 | 55 (42.0) | 0.82 (0.35–1.94, P = .658) | |
| Country 3 | 13 (9.9) | 0.77 (0.21–2.78, P = .685) | |
| Other* | 23 (17.6) | 0.80 (0.27–2.34, P = .681) | |
| Hospital | Hospital 1 | 28 (21.4) | - |
| Hospital 2 | 25 (19.1) | 0.98 (0.30–3.22, P = .977) | |
| Hospital 3 | 21 (16.0) | 1.10 (0.31–3.91, P = .883) | |
| Hospital 4 | 13 (9.9) | 0.92 (0.23–3.70, P = .911) | |
| Hospital 5 | 11 (8.4) | 1.16 (0.29–4.63, P = .837) | |
| Other* | 33 (25.2) | 1.11 (0.38–3.20, P = .851) | |
| Enrollment cohort | Cohort 1 | 69 (52.7) | - |
| Cohort 2 | 62 (47.3) | 0.60 (0.29–1.27, P = .186) |
Note: Significant covariates are bolded.
*Other should correspond to Other sites are comprised of nine hospitals, each contributing < 7 participants (range: 1–6 per site).
Discussion
To the best of our knowledge, this is the largest multi-country cohort of neonates with Candida spp. bloodstream infections in the LMIC setting. Most of the neonates, included in the NeoOBS invasive candidiasis sub-study, were outside the high-risk groups for NIC as described in HIC, with 81% born after 28 weeks gestation and 73% with a birth weight >1000 gr. Although C. albicans was the most frequent species isolated, the species distribution varied significantly between hospitals and countries. Across the Candida spp. with known in vitro susceptibility profile, 40% were fluconazole-resistant, 18% were amphotericin B-resistant, while no resistance was reported for micafungin. (Albeit many isolates were not tested.) Candida albicans was reported to be highly susceptible to fluconazole and amphotericin B. In contrast, a significant proportion of C. parapsilosis was reported to be fluconazole-resistant, driven by high resistance rates observed in South Africa.12Candida auris was the third most common species overall, but its presence varied greatly between countries. Amphotericin B was the most common empiric antifungal used (74%), followed by fluconazole (22%) with echinocandins rarely used. Antifungal prophylaxis was infrequently used in infants who developed candidemia, even for those neonates considered at high-risk. Overall, the mortality was high (22%), and it was significantly associated with low birth weight (<1000 gr) and extreme prematurity (<28 weeks).
In the cohort of neonates with NIC described here, 37% weighed >1500 gr and 38% were ≥32 weeks of gestational age. These results are similar to other studies in LMIC,11,20 and contrast dramatically with the data from HICs, where extreme prematurity and ELBW neonates are the main high-risk groups for NIC.21 In 2018, e.g., the DeNIS study reported unusually high rates of NIC in a cohort of neonates in India born outside the hospital; more than a quarter of neonates with a positive blood culture (90/339, 26.5%) had Candida spp. isolated. Remarkably, 61.5% of those neonates weighed >1500 gr, and 73.3% were born at or after 32 weeks gestation.3
Candida albicans has been reported as the most common causative species in NIC.10,22 Increasingly, a shift in epidemiology of NIC globally has been described, with a higher rate of non-albicans Candida isolates in LMICs compared to HICs.6 The rise in non-albicans Candida spp. in NIC is associated with reduced susceptibility to fluconazole. This has been described in India, where MDR strains of C. krusei and C. auris have been reported13; and in South Africa,23 where surveillance has shown an increase in the number of fluconazole-resistant C. parapsilosis.12 For example, Govender et al. reported a significant shift toward C. parapsilosis in neonates, with 53% of all C. parapsilosis isolates being fluconazole-resistant and 44% and 70% cross-resistant to voriconazole and posaconazole, respectively.12 Other South African series report similar results.7,24,25 In the cohort presented here, C. albicans and C. parapsilosis accounted for 35% and 30% of the isolates, respectively. Whereas C. albicans remained mostly susceptible to fluconazole (91% cases), 59% of the C. parapsilosis isolates were fluconazole resistant, mostly from South Africa.
Candida auris was the third most commonly reported pathogen (14% of all the cases) in this cohort, with significant variability between countries (0%–27.5%). Candida auris is a rapidly emerging, multi-drug resistant, nosocomial pathogen, with high reported resistance to fluconazole and amphotericin B.25–27 There have been scant reports focused on invasive C. auris infections in neonates28–30; most of them are from India.28 Chakabrati et al. published a multi-center prospective study from 2011 to 2012; where amongst 273 neonates from three hospitals with NIC, the proportion of C. auris isolates was 2.2%.13 More recent data,11,14,28 together with our observations, show that C. auris has quickly become one of the most commonly encountered species causing NIC in LMICs.
Reported neonatal mortality attributable to NIC in LMICs varies from 20% to as high as 50%.7,31 In the cohort described here, mortality was strongly associated with low birth weight (<1000 gr) and gestational age (<28 weeks); however, we did not find a clear association with causative species or susceptibility profiles.
Antifungal prophylaxis with fluconazole targeted to neonates <1000 gr birth weight and/or <28 weeks gestation, as well as those infants with birth weight of 1000–1500 gr with additional risk factors, is a recommended strategy in neonatal units in HICs to prevent NIC.32–36 Based on our data, NIC in LMICs affects mostly neonates with a birth weight >1500 gr, putting in doubt the relevance of HIC neonatal fungal prophylaxis guidelines for LMICs. In addition, a high prevalence of fluconazole resistance poses an important barrier to the use of fluconazole prophylaxis in these countries. Future studies determining the clinical and health economic benefit of neonatal antifungal prophylaxis in LMIC settings are needed. Fluconazole is not the only available drug to be considered; prophylaxis with nystatin, a low cost oral antifungal, which has also been proven to have an impact on neonatal mortality and is included in the essential medicines list (EML)37–39 can also be considered.
Compared with the burden of bacterial sepsis, where Klebsiella pneumoniae, Acinetobacter spp., and Escherichia coli are the most commonly reported pathogens in LMICs,40,41 the burden of fungal sepsis, particularly caused by Candida spp. has been poorly described. For this reason, it is not possible to provide an accurate estimated burden of NIC in LMICs.6,11 Reported incidence of invasive candidiasis in pediatric intensive care units is significantly higher in LMICs (42.7 cases per 1000 admissions) compared to HICs (0.043–0.47 cases per 1000 admissions).6 In general, Candida spp. are likely to be an underreported pathogen and mostly linked to healthcare-associated infections.
This study has some key limitations. First, not all neonates with Candida spp. bloodstream infections presenting at these hospitals were enrolled in the study, introducing the risk of selection bias. The aims of the NeoOBS study were to describe presentation, management, and outcomes of infants with sepsis not describe incidence of these infections, and thus we were unable to quantify incidence of NIC in this cohort due to this enrollment bias. It is also possible that the seven hospitals contributing majority of the infants to this candidemia cohort had higher repeat blood culture rates than other hospitals in the NeoOBS study. Cohort 2 enrollment may have missed some infants who died prior to a positive culture result and who were unable to be consented, potentially contributing survivor bias and lower mortality for certain Candida species. Differences in baseline characteristics and mortality between the two enrollment cohorts were explored (Supplementary Table 1), and no significant differences were found in key risk factors, Candida species, or mortality, which supported combining patients from these two cohorts into one analysis. Additionally, we used hospital-reported identification and phenotypic susceptibility testing results for this analysis, which may be less accurate than MIC values and/or molecular identification techniques; moreover, the use of interpretation guidelines of antimicrobial resistance (e.g., CLSI, EUCAST, and BSAC) may vary by hospital. Finally, there were a number of isolates that did not have any susceptibility testing done or were only tested for fluconazole. Therefore, we are unable to fully evaluate resistance and appropriateness of choice of the antifungal treatment.
In conclusion, this study demonstrates that NIC is associated with significant mortality in the LMIC setting. The optimal method of prevention and treatment of this life-threatening infection requires further targeted studies. These studies should consider the epidemiological differences of NIC in LMICs compared to HICs, with an increased incidence of NIC in neonates outside the ‘high-risk’ group (<28 weeks and/or <1000 gr) and, although with significant variability between settings, higher rates of fluconazole resistances in non-albicans Candida species. Insights into the fungal epidemiology and susceptibility profiles are of utmost relevance in order to develop management guidelines for NIC in LMICs. Diagnostics for Candida species, including susceptibility testing, need to be made available and improved. Although no micafungin resistance was observed (within the few isolates tested), the role of empiric therapy with micafungin in LMICs for NIC needs to be a research priority. Micafungin has been included in the WHO-EML for children in 2021,37 but there are still limitations for its use in neonates, such as the lack of a defined optimal dose for those cases with meningoencephalitis.42,43 Finally, studies in LMICs are required to define which neonates might benefit from antifungal prophylaxis. As our study shows the current recommendations used in HIC targeting ‘high-risk’ neonates do not entirely apply to neonates in LMICs.
Supplementary Material
Acknowledgements
In memory of Hannington Tasimwa.
Contributor Information
Aislinn Cook, Centre for Neonatal and Paediatric Infection, St. George's University of London, London, UK.
Laura Ferreras-Antolin, Centre for Neonatal and Paediatric Infection, St. George's University of London, London, UK; MRC Centre for Medical Mycology, University of Exeter, Exeter, UK.
Bethou Adhisivam, Department of Neonatology, Jawaharlal Institute of Postgraduate Medical Education & Research (JIPMER), Pondicherry, India.
Daynia Ballot, School of Clinical Medicine, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa; Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa.
James A Berkley, Clinical Research Department, KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya; Centre for Tropical Medicine & Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK; The Childhood Acute Illness & Nutrition (CHAIN) Network, Nairobi, Kenya.
Paola Bernaschi, Microbiology Unit, Bambino Gesù Children's Hospital, Rome, Italy.
Cristina G Carvalheiro, Department of Pediatrics, Ribeirão Preto Medical School, University of São Paulo, São Paulo, Brazil.
Napaporn Chaikittisuk, Queen Sirikit National Institute of Child Health, Bangkok, Thailand.
Yunsheng Chen, Clinical Laboratory, Shenzhen Children's Hospital, Shenzhen, China.
Vindana Chibabhai, Department of Clinical Microbiology & Infectious Diseases, School of Pathology, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa; NHLS Microbiology Laboratory, Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa.
Shweta Chitkara, Lady Hardinge Medical College & Associated SSK & KSC Hospitals, New Delhi, India.
Sara Chiurchiu, Academic Hospital Paediatric Department, Bambino Gesù Children's Hospital, Rome, Italy.
Elisavet Chorafa, Infectious Diseases Unit, 3rd Department of Pediatrics, School of Medicine, Faculty of Health Sciences, Aristotle University and Hippokration General Hospital, Thessaloniki, Greece.
Tran Minh Dien, Vice Director Vietnam National Children's Hospital, Hanoi, Vietnam; Department of Surgery, Vietnam National Children's Hospital, Hanoi, Vietnam.
Angela Dramowski, Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
Samantha Faria de Matos, Santa Casa de São Paulo, Sao Paulo, Brazil.
Jinxing Feng, Department of Neonatology, Shenzhen Children's Hospital, Shenzhen, China.
Daniel Jarovsky, Santa Casa de São Paulo, Sao Paulo, Brazil.
Ravinder Kaur, Lady Hardinge Medical College & Associated SSK & KSC Hospitals, New Delhi, India.
Warunee Khamjakkaew, PHPT/IRD-MIVEGEC, Chiang Mai University, Chiang Rai, Thailand.
Premsak Laoyookhong, Queen Sirikit National Institute of Child Health, Bangkok, Thailand.
Edwin Machanja, Department of Microbiology, KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya.
Marisa M Mussi-Pinhata, Department of Pediatrics, Ribeirão Preto Medical School, University of São Paulo, São Paulo, Brazil.
Flavia Namiiro, Mulago Specialised Women and Neonatal Hospital, Kampala, Uganda.
Gita Natraj, Seth G. S. Medical College & KEM Hospital, Mumbai, India.
Hakka Naziat, Child Health Research Foundation, Dhaka, Bangladesh.
Hoang Thi Bich Ngoc, Department of Microbiology, Vietnam National Children's Hospital, Hanoi, Vietnam.
Claude Ondongo-Ezhet, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Kanchana Preedisripipat, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand.
Hafizur Rahman, Child Health Research Foundation, Dhaka, Bangladesh.
Amy Riddell, Centre for Neonatal and Paediatric Infection, St. George's University of London, London, UK.
Emmanuel Roilides, Infectious Diseases Unit, 3rd Department of Pediatrics, School of Medicine, Faculty of Health Sciences, Aristotle University and Hippokration General Hospital, Thessaloniki, Greece.
Neal Russell, Centre for Neonatal and Paediatric Infection, St. George's University of London, London, UK.
Apurba S Sastry, Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education & Research (JIPMER), Pondicherry, India.
Hannington Baluku Tasimwa, Department of Mircobiology, College of Health Sciences, Makerere University, Kampala, Uganda.
Ji Tongzhen, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, China; Beijing Maternal and Child Health Care Hospital, Beijing, China.
Jeannette Wadula, National Health Laboratory Services, School of Pathology, Faculty of Health Sciences, University, of the Witwatersrand, Johannesburg, South Africa.
Yajuan Wang, Department of Neonatology, Children's Hospital, Capital Institute of Pediatrics, 2# Yabao Road, Chaoyang District, Beijing, China; Department of Neonatology, Beijing Children's Hospital, National Center for Children's Health, Capital Medical University, Beijing, China.
Andrew Whitelaw, Division of Medical Microbiology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa; National Health Laboratory Service, Tygerberg Hospital, Cape Town, South Africa.
Dan Wu, Department of Neonatology, Children's Hospital, Capital Institute of Pediatrics, 2# Yabao Road, Chaoyang District, Beijing, China.
Varsha Yadav, Seth G. S. Medical College & KEM Hospital, Mumbai, India.
Gao Yang, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, China; National Health Laboratory Services, School of Pathology, Faculty of Health Sciences, University, of the Witwatersrand, Johannesburg, South Africa.
Wolfgang Stohr, MRC Clinical Trials Unit at UCL, Institute of Clinical Trials & Methodology, University College London, London, UK.
Julia Anna Bielicki, Centre for Neonatal and Paediatric Infection, St. George's University of London, London, UK.
Sally Ellis, Global Antibiotic Research & Development Partnership (GARDP), Geneva, Switzerland.
Adilia Warris, MRC Centre for Medical Mycology, University of Exeter, Exeter, UK.
Paul T Heath, Centre for Neonatal and Paediatric Infection, St. George's University of London, London, UK.
Michael Sharland, Centre for Neonatal and Paediatric Infection, St. George's University of London, London, UK.
Funding
The study was sponsored by the Global Antibiotic Research and Development Partnership (GARDP), and additional funding was provided by the Bill and Melinda Gates Foundation, German Federal Ministry of Education and Research (BMBF), South African Medical Research Council, UK Department for Health and Social Care (DHSC), Monaco, and an in-kind donation from Indian Council for Medical Research. A.W. and L.F.A. are supported by the MRC Centre for Medical Mycology at the University of Exeter (MR/N006364/2 and MR/V033417/1).
Declaration of interest
There are no conflicts of interest to declare.
References
- 1. World Health Organization . Newborns: improving survival and well-being. World Health Organization. 2020. pp. 1–5. [Google Scholar]
- 2. World Health Organization . Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions. World Health Organization. 2020. p. 56, https://apps.who.int/iris/handle/10665/334216 [Google Scholar]
- 3. Jajoo M, Manchanda V, Chaurasia Set al. Alarming rates of antimicrobial resistance and fungal sepsis in outborn neonates in North India. PLoS One. 2018; 13: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breiman RF, Blau DM, Mutevedzi Pet al. Postmortem investigations and identification of multiple causes of child deaths: an analysis of findings from the Child Health and Mortality Prevention Surveillance (CHAMPS) network. PLoS Med. 2021; 18: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benjamin DK, Stoll BJ, Fanaroff AAet al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006; 117: 84–92. [DOI] [PubMed] [Google Scholar]
- 6. Kaur H, Chakrabarti A.. Strategies to reduce mortality in adult and neonatal candidemia in developing countries. J Fungi. 2017; 3: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ballot DE, Bosman N, Nana T, Ramdin T, Cooper PA. Background changing patterns of neonatal fungal sepsis in a developing country. J Trop Pediatr. 2013; 59: 460–464. [DOI] [PubMed] [Google Scholar]
- 8. Morkel G, Bekker A, Marais BJ, Kirsten G, van Wyk J, Dramowski A. Bloodstream infections and antimicrobial resistance patterns in a South African neonatal intensive care unit. Paediatr Int Child Health. 2014; 34: 108–114. [DOI] [PubMed] [Google Scholar]
- 9. Warris A, Pana Z, Oletto A, Lundin R.. Antifungal drug susceptibility of candida spp. in neonatal and paediatric candidaemia: a European multi-centre retrospective study (EUROCANDY) [Abstract}. European Society of Paediatric Infectious Diaseases, Malmö, 2018 May 29. [Google Scholar]
- 10. Pana ZD, Roilides E, Warris A, Groll AH, Zaoutis T.. Epidemiology of invasive fungal disease in children. J Pediatric Infect Dis Soc. 2017; 6: S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shuping L, Mpembe R, Mhlanga Met al. Epidemiology of culture-confirmed candidemia among hospitalized children in South Africa, 2012–2017. Pediatr Infect Dis J. 2021; 40: 730–737. [DOI] [PubMed] [Google Scholar]
- 12. Govender NP, Patel J, Magobo REet al. Emergence of azole-resistant Candida parapsilosis causing bloodstream infection: results from laboratory-based sentinel surveillance in South Africa. J Antimicrob Chemother. 2016; 71: 1994–2004. [DOI] [PubMed] [Google Scholar]
- 13. Chakrabarti A, Sood P, Rudramurthy SMet al. Characteristics, outcome and risk factors for mortality of paediatric patients with ICU-acquired candidemia in India: a multicentre prospective study. Mycoses. 2020; 63: 1149–1163. [DOI] [PubMed] [Google Scholar]
- 14. Van Schalkwyk E, Mpembe RS, Thomas Jet al. Epidemiologic shift in candidemia driven by Candida auris, South Africa, 2016–2017. Emerg Infect Dis. 2019; 25: 1698–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. NeoOBS _ Penta Foundation . https://penta-id.org/severe-infections-and-antimicrobial-resistance/neoobs/, (February 23, 2023, date last accessed)..
- 16. GARDP . Transforming the care of babies with sepsis. https://gardp.org/wp-content/uploads/2022/10/GARDP-Neonatal-sepsis-study-results-2022.pdf, (February 23, 2023, date last accessed).
- 17. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Minor BLet al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Russell N, Stöhr W, Plakkal Net al. Patterns of antibiotic use, pathogens and clinical outcomes in hospitalised neonates and young infants with sepsis in the NeoOBS global neonatal sepsis observational cohort study. medRxiv. https://www.medrxiv.org/content/early/2022/06/23/2022.06.20.22276674, (June 23, 2022, date last accessed). [DOI] [PMC free article] [PubMed]
- 20. Lona-Reyes JC, Gómez-Ruiz LM, Cordero-Zamora Aet al. Incidencia y factores asociados a candidiasis invasiva en una unidad de cuidados intensivos neonatales de México. An Pediatría. 2022; 97: 79–86. [DOI] [PubMed] [Google Scholar]
- 21. Steinbach WJ, Roilides E, Berman Det al. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr Infect Dis J. 2012; 31: 1252–1257. [DOI] [PubMed] [Google Scholar]
- 22. Warris A, Pana Z-D, Oletto A, Lundi R, Roilides E, EUROCANDY-Study-Group . Epidemiology and outcomes of candidaemia in neonates and children in Europe: an 10-year multinational retrospective study. 2020; 39: 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Schalkwyk E, Iyaloo S, Naicker SDet al. Large outbreaks of fungal and bacterial bloodstream infections in a neonatal unit, South Africa, 2012–2016. Emerg Infect Dis. 2018; 24: 1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pillay D, Naidoo L, Swe Swe-Han K, Mahabeer Y. Neonatal sepsis in a tertiary unit in South Africa. BMC Infect Dis. 2021; 21: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedman DZP, Schwartz IS. Emerging fungal infections: new patients, new patterns, and new pathogens. J Fungi. 2019;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lockhart SR, Etienne KA, Vallabhaneni Set al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017; 64: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lockhart SR, Guarner J. Emerging and reemerging fungal infections. Semin Diagn Pathol. 2019; 36: 177–181. [DOI] [PubMed] [Google Scholar]
- 28. Chandramati J, Sadanandan L, Kumar A, Ponthenkandath S. Neonatal Candida auris infection: management and prevention strategies––a single centre experience. J Paediatr Child Health. 2020; 56: 1565–1569. [DOI] [PubMed] [Google Scholar]
- 29. Berrio I, Caceres DH, Coronell RWet al. Bloodstream infections with Candida auris among children in Colombia: clinical characteristics and outcomes of 34 cases. J Pediatric Infect Dis Soc. 2021; 10: 151–154. [DOI] [PubMed] [Google Scholar]
- 30. Mesini A, Saffioti C, Mariani Met al. First case of Candida auris colonization in a preterm, extremely low-birth-weight newborn after vaginal delivery. J Fungi. 2021; 7: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahangarkani F, Shokohi T, Rezai MSet al. Epidemiological features of nosocomial candidaemia in neonates, infants and children: a multicentre study in Iran. Mycoses. 2020; 63: 382–394. [DOI] [PubMed] [Google Scholar]
- 32. Manzoni P, Mostert M, Latino MAet al. Clinical characteristics and response to prophylactic fluconazole of preterm VLBW neonates with baseline and acquired fungal colonisation in NICU: data from a multicentre RCT. Early Hum Dev. 2012; 88: S60–S64. [DOI] [PubMed] [Google Scholar]
- 33. Kaufman DA, Morris A, Gurka MJ, Kapik B, Hetherington S. Fluconazole prophylaxis in preterm infants: a multicenter case-controlled analysis of efficacy and safety. Early Hum Dev. 2014; 90: S87–S90. [DOI] [PubMed] [Google Scholar]
- 34. Ericson JE, Kaufman DA, Kicklighter SDet al. Fluconazole prophylaxis for the prevention of candidiasis in premature infants: a meta-analysis using patient-level data. Clin Infect Dis. 2016; 63: 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swanson JR, Vergales J, Kaufman DA, Sinkin RA. Cost analysis of fluconazole prophylaxis for prevention of neonatal invasive candidiasis. Pediatr Infect Dis J. 2016; 35: 519–523. [DOI] [PubMed] [Google Scholar]
- 36. Hope WW, Castagnola E, Groll AHet al. ESCMID * guideline for the diagnosis and management of Candida diseases 2012 : prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012; 18: 38–52. [DOI] [PubMed] [Google Scholar]
- 37. World Health Organization . World Health Organization. Model List of Essential Medicines–22nd List, 2021. 2021. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02, (February 23, 2023, date last accessed).
- 38. Kaufman DA. “Getting to Zero”: preventing invasive Candida infections and eliminating infection-related mortality and morbidity in extremely preterm infants. Early Hum Dev. 2012; 88: S45–S49. [DOI] [PubMed] [Google Scholar]
- 39. Rundjan L, Wahyuningsih R, Oeswadi CA, Marsogi M, Purnamasari A. Oral nystatin prophylaxis to prevent systemic fungal infection in very low birth weight preterm infants : a randomized controlled trial. BMC Pediatr. 2020; 20: 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Downie L, Armiento R, Subhi R, Kelly J, Clifford V, Duke T. Community-acquired neonatal and infant sepsis in developing countries: efficacy of WHO's currently recommended antibiotics––Systematic review and meta-analysis. Arch Dis Child. 2013; 98: 146–154. [DOI] [PubMed] [Google Scholar]
- 41. Russell N, Stoehr W, Plakkal N, Cook A. Analysis from the NeoOBS global neonatal sepsis prospective observational cohort study across 19 hospitals in 11 countries; clinical presentation, treatment, mortality outcomes and develpment of the NeoSEP sepsis severity score. Available at SSRN, https://ssrn.com/abstract=3864901, (June 21, 2021, date last accessed).
- 42. Taormina G, Gopinath R, Moore Jet al. A regulatory review approach for evaluation of micafungin for treatment of neonatal candidiasis. Clin Infect Dis. 2021; 73: 2335–2340. [DOI] [PubMed] [Google Scholar]
- 43. Leroux S, Jacqz-Aigrain E, Elie Vet al. Pharmacokinetics and safety of fluconazole and micafungin in neonates with systemic candidiasis: a randomized, open-label clinical trial. Br J Clin Pharmacol. 2018; 84: 1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.