Abstract
Background
Inflammatory myofibroblastic tumor (IMT) is an ultra‐rare soft tissue neoplasm associated with fusion proteins encompassing the anaplastic lymphoma kinase (ALK) protein fused to a variety of partner proteins. Data regarding response to ALK‐targeting agents based on fusion partner is limited.
Case
A 30‐year‐old female sought emergency care after onset of abdominal and lower back pain in 2019. Computed tomography (CT) demonstrated a cystic, mesenteric mass within the pelvis measuring up to 8.9 cm. Complete laparoscopic excision of the mass from the mesentery of the right colon and terminal ileum was performed. Pathologic assessment revealed IMT with a fusion between sequestosome 1 and ALK (SQSTM1::ALK), described in only two other cases of IMT. Four months after surgery, CT revealed multi‐focal, unresectable disease recurrence. She was referred to the University of Washington/Fred Hutchinson Cancer Center and placed on therapy with alectinib, after which she experienced a partial response. Three years after IMT recurrence, disease remains under control.
Conclusion
This is the third reported case of IMT associated with the novel SQSTM1::ALK fusion protein, and the second treated with alectinib. Treatment with the ALK inhibitor alectinib appears to be active in this setting.
Keywords: alectinib, anaplastic lymphoma kinase, inflammatory myofibroblastic tumor, sequestosome 1
1. INTRODUCTION
Inflammatory myofibroblastic tumor (IMT) is a rare mesenchymal neoplasm predominantly found in children and young adults; in 75% of cases, the condition occurs in the abdominal cavity, such as the mesentery, omentum, and retroperitoneal space. 1 A variety of other sites may be affected, including head/neck, lungs, bladder, central nervous system, and ovaries/uterus. While patients may present with symptoms associated with mass effects from the primary tumor, IMT may also have a variety of vague and non‐specific signs and symptoms, reflecting its “inflammatory” behavior, such as fevers, fatigue and weight loss. 1 , 2 Laboratory assessments may demonstrate an elevated erythrocyte sedimentation rate (ESR) or C‐reactive protein, thrombocytosis, anemia, leukocytosis, and hypergammaglobulinemia, phenomena potentially related to paraneoplastic expression of interleukin‐6. 2 , 3 First‐line treatment for IMT is complete surgical resection, if possible, with the possibility of re‐resection for local recurrence. No formal clinical trials of conventional cytotoxic therapies have been conducted in IMT; retrospective analyses demonstrate some responses, although no clear pattern of benefit. 4
Despite recognition of the condition decades ago, IMT was conflated with a benign, reactive process now known as inflammatory pseudotumor, which has no malignant potential. 1 , 5 Observation of recurrent translocations at 2p23 with identification of the anaplastic lymphoma kinase (ALK) gene in fusion proteins led to the recognition that IMT cases with these alterations have intermediate malignant potential. 6 , 7 , 8 Translocations involving ALK may be present in 50%–60% of IMT cases, although there is heterogeneity. 1 , 4 , 9 In cases with absent ALK protein expression or translocation, additional target genes, such as ROS1, NTRK, RET, and PDGFR‐beta, have been identified. 9
In its native state, ALK is a transmembrane receptor tyrosine kinase that peaks in expression during the first 3 weeks of life. Its baseline physiologic role is poorly understood, with some animal models suggesting a role in fetal nervous system development. 10 , 11 A specific ligand for the receptor has not been identified. ALK activation leads to activity in several downstream pathways, including Janus kinase‐signal transducer and activator of transcription, RAS‐mitogen activated protein kinase, and the phosphoinositide‐3‐kinase‐Akt‐mammalian target of rapamycin pathways.
ALK was initially discovered as an oncogene in anaplastic large‐cell lymphoma and has been subsequently implicated in a number of malignancies, including non‐small cell lung cancer (NSCLC), large B‐cell lymphoma, and renal cell carcinoma. ALK fusion proteins contain the intracellular tyrosine kinase signaling domain, with the N‐terminal component of the fusion developing from any of a variety of fusion partners. The fusion presumably conveys constitutive activation of the kinase.
Identification of ALK translocations in IMT prompted clinical trials with crizotinib, a first‐generation ALK inhibitor. 12 , 13 These were necessarily small in size, but demonstrated clear activity of the drug in those with ALK translocation, versus markedly lesser benefit in those without ALK translocations. Crizotinib, while an effective therapy, is limited by poor penetration through the blood–brain barrier, toxicity, and development of resistance. 14 This prompted the development of second‐generation ALK inhibitors, including alectinib, designed to overcome these challenges.
To highlight the utility of second‐generation ALK inhibitors in targeting a unique fusion protein, we here present a case of a 30‐year‐old female diagnosed with IMT who subsequently developed multi‐focal disease recurrence. Tumoral cells possessed a gene fusion of the N‐terminal portion of sequestosome 1 (SQSTM1) and the C‐terminal of ALK, designated SQSTM1::ALK, which has only been reported in two other cases of IMT. 9 , 15 She demonstrated marked disease regression and prolonged disease control after treatment with alectinib.
2. CASE
A 30‐year‐old female with no significant medical comorbidities or history sought emergent care for progressive abdominal and lower back pain in 2019. She was evaluated by her primary care doctor prior to this encounter and was found to have an ESR of 81 mm/hr (normal <23 mm/hr). Computed tomography (CT) of her abdomen and pelvis demonstrated a cystic, mesenteric pelvic mass measuring up to 8.9 cm in maximal dimension (Figure 1A). Complete laparoscopic excision of the mass was performed from the mesentery of the right colon and terminal ileum (Figure 2).
FIGURE 1.
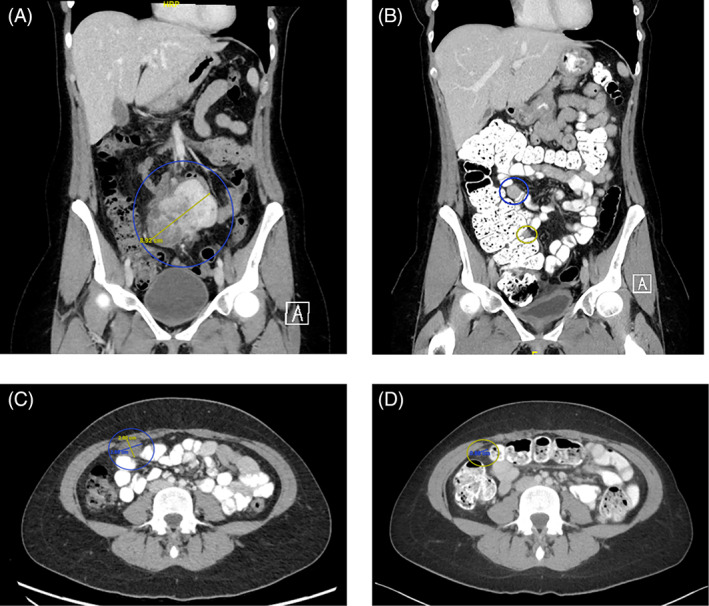
Computed tomography of the abdomen and pelvis. (A) Coronal images at time of diagnosis, demonstrating an 8.9 cm mesenteric mass. (B) Coronal images obtained 4 months after resection, with circles indicating sites of disease recurrence. (C) Axial image obtained 3 months after initiation of alectinib treatment. A 2 cm tumor mass is circled. (D) Axial image at similar level as that in image (C), obtained 14 months after initiation of alectinib treatment. The previously noted mass is now less than 1 cm maximal dimension.
FIGURE 2.
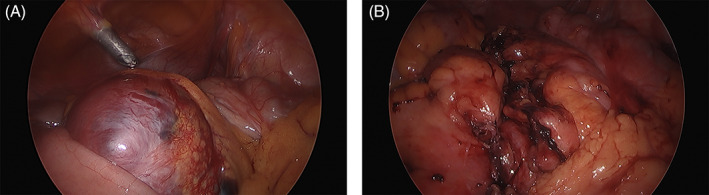
Images obtained during complete laparoscopic resection. (A) Mesenteric mass in situ. (B) Mesentery after tumor and small bowel resection.
Histologic sections showed a neoplastic proliferation of spindled‐to‐epithelioid cells with variably pleomorphic nuclei, some of which were very large with irregular contours and prominent nucleoli (Figure 3A). The cells were within a background of variably dense collagenous‐to‐myxoid stroma with an intermixed inflammatory infiltrate (Figure 3B). There was scattered mitotic activity, including occasional atypical mitoses, but no tumoral necrosis. Immunohistochemical (IHC) stains demonstrated diffusely positive cytoplasmic staining for ALK in the neoplastic cells using a D5F3 antibody clone, but only focal weak staining using an ALK1 antibody clone (Figure 3C). FISH testing was negative for MDM2 gene amplification and in situ hybridization studies were negative for kappa and lambda light chain expression and Epstein–Barr virus. Additional IHC was negative for markers of hematolymphoid differentiation in the spindled and epithelioid cells. SQSTM1::ALK fusion was detected by anchored multiplex polymerase chain reaction and RNA‐seq with custom 115‐gene FusionPlex solid tumor panel.
FIGURE 3.
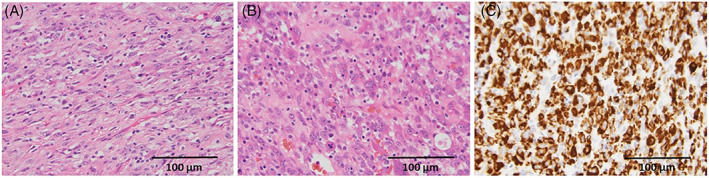
Histopathology of mesenteric mass obtained after resection. (A) Histologic appearance of the neoplasm demonstrating eosinophilic spindle cells with background mixed inflammatory infiltrate typical of inflammatory myofibroblastic tumor (H&E, 400×). (B) An area of the neoplasm demonstrating more epithelioid cells with greater nuclear pleomorphism within a somewhat myxocollagenous matrix. Mitotic figures, including an atypical tripolar mitosis, are present (H&E, 400×). (C) Immunohistochemical staining for ALK expression using a D5F3 antibody clone shows diffuse cytoplasmic expression (400×).
The predicted fusion involves a translocation of chromosome 2 involving the 3′ end of the ALK gene (NM_004304) to chromosome 5 involving the 5′ end of the SQSTM1 gene (NM_003900) (Figure 4). The SQSTM1::ALK fusion transcript is in‐frame between 3′ end of exon 5 of SQSTM1 (NM_003900) and 5′ end of exon 19 of ALK (NM_004304). The resulting fusion protein is composed of exons 1–5 of SQSTM1 (amino acids 1 to 251) in the N‐terminal and exons 19–29 of ALK (amino acids 1023 to 1620) in the C‐terminal. This 849 amino acid fusion polypeptide retained the four main functional domains of SQSTM1 (PB1, ZZ, LB, and TB domains) and the transmembrane domain and tyrosine kinase domain of ALK (Figure 4). The 5′ fusion partner, SQSTM1, is ubiquitously expressed with total median expression of 1628.86 RPKM (Figure 4A). Under SQSTM1 promoter, the SQSTM1::ALK fusion gene most likely leads to constitutive expression of the protein, with consequent activation of the ALK kinase domain.
FIGURE 4.
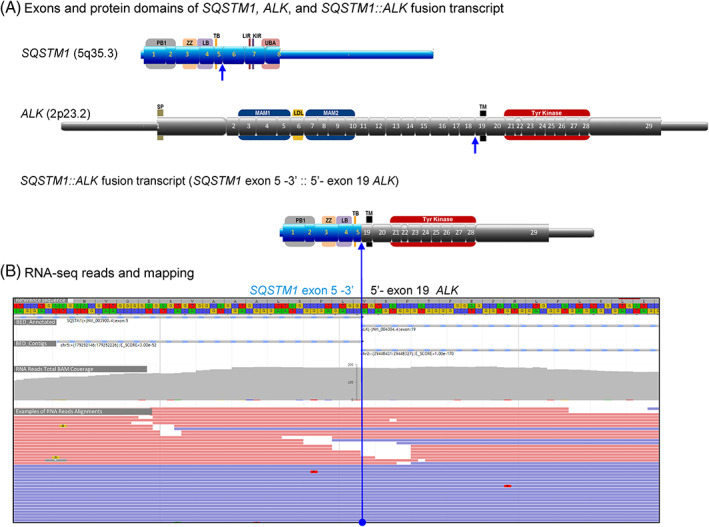
SQSTM1::ALK fusion identified by RNA‐seq. (A) The structures of SQSTM1 (blue boxes), ALK (gray boxes), and SQSTM1::ALK fusion transcript with their functional protein domains. SQSTM1::ALK fusion transcript has 3′ end of exon 5 of SQSTM1 fused (::) with 5′ end of exon 19 of ALK. Untranslated regions (5′ UTR and 3′ UTR) are shown as narrow bars. Exons are shown as boxes with numbers. The keys of protein domains are shown in the wider boxes behind exons with letters: for the domain structure of SQSTM1/p62 protein, KIR, Keap1‐interacting region; LB, LIM protein‐binding; LDL, low‐density lipoprotein receptor domain class A; LIR, LC3‐interacting region; MAM, meprin, A5 protein, and Mu domain; PB1, Phox and Bem1p; SP, signal peptide; TB, TRAF6‐binding domain; TM, transmembrane region; Tyr Kinase, tyrosine kinase domain; UBA, ubiquitin‐associated; for the domain structure of ALK protein; ZZ, Zinc finger. The blue lines and arrows indicate the breakpoints and fusion points. (B) Representative sequence reads over the breakpoints of the SQSTM1::ALK fusions by paired‐end RNA sequencing, including exon mapping of chimeric transcripts to the reference sequence at base resolution with amino acid translation in frame. Total BAM coverages of the breakpoints (~200 reads) were shown as gray bar scale. Examples of next‐generation sequencing reads over the breakpoints were shown in red bars (R1 reads) and blue bars (R2 reads).
She was referred to the University of Washington/Fred Hutchinson Cancer Center for monitoring and management. Four months after resection, the patient reported no symptoms. However, surveillance CT showed local recurrence in the mesentery and omentum, and metastatic disease in the extraperitoneal space anterior to the bladder (Figure 1B). Given the multifocal nature of the recurrence, surgical resection was judged to be infeasible and systemic therapy was recommended. Due to the identification of a unique ALK translocation in this patient, targeted therapy with alectinib was initiated at a dose of 600 mg twice daily. CT scans were obtained every 3 months thereafter to monitor for disease progression (Figure 1C).
Highlighted below, the patient's disease burden stabilized while on alectinib and showed substantial improvement at 14 months (Figure 1D). Imaging 3 years into treatment shows essentially stable disease with no new metastases. Throughout this entire time, the patient was able to tolerate treatment with no significant adverse events, other than fatigue. She continued to maintain her employment undertaking administrative work during treatment.
3. DISCUSSION
We present a case of a 30‐year‐old female patient with mesenteric IMT complicated by metastatic recurrence. In the primary resection specimen, IHC demonstrated diffuse positive staining for ALK using a D5F3 antibody clone. The presence of an ALK fusion protein, specifically SQSTM1::ALK, was documented, consistent with the IMT family of tumors. Other diagnoses considered on presentation included retroperitoneal dedifferentiated liposarcoma and a hematolymphoid neoplasm, which were excluded with negative testing for MDM2 amplification and additional IHC studies for hematolymphoid markers, respectively.
Several observations suggested that this patient might be affected by a variant of epithelioid inflammatory myofibroblastic sarcoma (EIMS). 16 , 17 , 18 These findings included its mesenteric site of origin, rapid multi‐focal recurrence after initial surgical treatment, and the focal epithelioid cytology with severe cytologic atypia and occasional atypical mitotic figures. EIMS is reported to have more aggressive clinical behavior, and has been associated, although not exclusively, with the RANBP2‐ALK fusion protein. 16 A nuclear or peri‐nuclear ALK IHC staining pattern, reported in association with the RNABP2 partner, was not present in our patient; its lack does not exclude the clinical diagnosis of EIMS.
Instead, an ALK fusion protein with an N‐terminal derived from SQSTM1 was identified. This has been reported in two other cases of IMT. 9 , 15 In one patient, the primary tumor originated in the right subclavian region and progressed slowly over the course of 5 years until margin‐negative resection. Multi‐focal recurrent disease was identified 12 months later. This patient, similar to ours, received alectinib treatment with partial response and controlled disease after 12 months. This behavior was similar to that seen in our patient and suggests that the SQSTM1::ALK fusion is associated with a more aggressive clinical course. A second patient was reported to have this fusion and was treated for 4.2 months with crizotinib, achieving stable disease. 9
At present, crizotinib is the only drug approved by the US Food and Drug Administration for treatment of ALK‐positive IMT. 19 Use of other ALK inhibitors for IMT is consistent with treatment recommendations from the National Comprehensive Cancer Network. 20 Alectinib has been compared directly to crizotinib in NSCLC. 21 , 22 In NSCLC, alectinib is associated with improved progression‐free survival and better safety/tolerability than crizotinib. Alectinib has additional theoretical advantages, including activity against ALK mutants selected during crizotinib therapy. 14 Due to its better tolerability and theoretical advantages over crizotinib, we have used alectinib in this and other IMT patients in our clinic. Crizotinib could however have been used as therapy in this condition, as reported in one other case possessing this fusion, albeit treated for only 4.2 months. 9
SQSTM1::ALK fusions have been identified in several other clinical conditions associated with ALK fusion proteins. These include non‐neural granular cell tumors, ALK‐positive B‐cell lymphoma, epithelioid fibrous histiocytoma of the skin, and NSCLC. 23 , 24 , 25 , 26 , 27 , 28 , 29 Neither the clinical nor histologic characteristics of our patient would suggest one of these alternate diagnoses.
SQSTM1 encodes a highly conserved 62 kDa protein (also known as p62) which exerts effects as a main regulator of multiple signaling pathways, including Nrf2, mTORC1, and NF‐κB. 30 It has been associated with a variety of malignancies, and non‐malignant conditions, including Paget's disease of bone, amyotrophic lateral sclerosis, and steatohepatitis and alcoholic hepatitis. Studies of the SQSTM1::ALK fusion protein in B‐cell lymphoma have suggested that the STAT3 pathway may be a viable therapeutic target. 24 As early as 2002, it was suggested that therapeutic strategies targeting ALK and its downstream effectors, including STAT3, might represent effective approaches in ALK‐driven malignancies. 31 A recent pre‐clinical report identified synergistic activity against ALK‐fusion‐dependent H2228 cells when the orally bioavailable STAT3 inhibitor YHO‐1701 was used in combination with alectinib. 32 STAT3 also has a complex relationship with endogenous SQSTM1. The addition of quercetin, a STAT3‐inhibiting flavonoid, leads to decreased accumulation and activity of SQSTM1 and decreased Epstein Barr virus‐mediated B cell immortalization. 33 It would be worth exploring whether such a combination ALK/STAT3‐inhibitor strategy may be applicable in IMT.
In conclusion, we report a patient with IMT associated with an SQSTM1::ALK fusion protein, which has been reported in only two other cases. As in one of the prior patients, the disease has responded to treatment with alectinib, and continues to do so 3 years after diagnosis of recurrent disease. This provides evidence of the utility of this therapy in this clinical setting, and potentially in other malignancies in which this rare, but recurrent fusion protein target is found.
AUTHOR CONTRIBUTIONS
Cass G. G. Sunga: Conceptualization (equal); methodology (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Michael S. Higgins: Resources (equal); writing – review and editing (equal). Robert W Ricciotti: Methodology (equal); resources (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Yajuan J Liu: Methodology (equal); resources (equal); visualization (equal); writing – review and editing (equal). Lee Cranmer: Conceptualization (equal); methodology (equal); resources (equal); supervision (lead); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ETHICS STATEMENT
The reporting of this case does not meet the definition of “research” according to 45 CFR 46.102d. Approval by Human Subjects Protection is not required. Written informed consent was obtained directly from the patient for the publication of case details and use of images. All activities were consistent with the Declaration of Helsinki. Work submitted has been performed according to Wiley's Publication Ethics Guidelines with no data fabrication or misconduct.
ACKNOWLEDGMENTS
Dr Cranmer was supported, in part, by the Curtis and Elizabeth Anderson Endowed Professorship in Sarcoma Research and by the support of Steve and Jane Urner. The authors wish to thank our patient for permission to present her clinical case and the providers and staff of the Sarcoma Clinic at the Fred Hutchinson Cancer Center and the Clinical Genomics Laboratory at University of Washington School of Medicine.
Sunga CGG, Higgins MS, Ricciotti RW, Liu YJ, Cranmer LD. Inflammatory myofibroblastic tumor of the mesentery with a SQSTM1::ALK fusion responding to alectinib. Cancer Reports. 2023;6(3):e1792. doi: 10.1002/cnr2.1792
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Siemion K, Reszec‐Gielazyn J, Kisluk J, Roszkowiak L, Zak J, Korzynska A. What do we know about inflammatory myofibroblastic tumors?—a systematic review. Adv Med Sci. 2022;67(1):129‐138. [DOI] [PubMed] [Google Scholar]
- 2. Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61(4):428‐437. [DOI] [PubMed] [Google Scholar]
- 3. Fukano R, Matsubara T, Inoue T, Gondo T, Ichiyama T, Furukawa S. Time lag between the increase of IL‐6 with fever and NF‐kappaB activation in the peripheral blood in inflammatory myofibroblastic tumor. Cytokine. 2008;44(2):293‐297. [DOI] [PubMed] [Google Scholar]
- 4. Casanova M, Brennan B, Alaggio R, et al. Inflammatory myofibroblastic tumor: the experience of the European pediatric Soft Tissue Sarcoma Study Group (EpSSG). Eur J Cancer. 2020;127:123‐129. [DOI] [PubMed] [Google Scholar]
- 5. Poh CF, Priddy RW, Dahlman DM. Intramandibular inflammatory myofibroblastic tumor—a true neoplasm or reactive lesion? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(4):460‐466. [DOI] [PubMed] [Google Scholar]
- 6. Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59(12):2776‐2780. [PubMed] [Google Scholar]
- 7. Patel AS, Murphy KM, Hawkins AL, et al. RANBP2 and CLTC are involved in ALK rearrangements in inflammatory myofibroblastic tumors. Cancer Genet Cytogenet. 2007;176(2):107‐114. [DOI] [PubMed] [Google Scholar]
- 8. Sirvent N, Hawkins AL, Moeglin D, et al. ALK probe rearrangement in a t(2;11;2)(p23;p15;q31) translocation found in a prenatal myofibroblastic fibrous lesion: toward a molecular definition of an inflammatory myofibroblastic tumor family? Genes Chromosomes Cancer. 2001;31(1):85‐90. [DOI] [PubMed] [Google Scholar]
- 9. Lee C‐J, Schöffski P, Modave E, et al. Comprehensive molecular analysis of inflammatory myofibroblastic tumors reveals diverse genomic landscape and potential predictive markers for response to crizotinib. Clin Cancer Res. 2021;27(24):6737‐6748. [DOI] [PubMed] [Google Scholar]
- 10. Brenner AK, Gunnes MW. Therapeutic targeting of the anaplastic lymphoma kinase (ALK) in neuroblastoma—a comprehensive update. Pharmaceutics. 2021;13(9):3‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aubry A, Galiacy S, Allouche M. Targeting ALK in cancer: therapeutic potential of proapoptotic peptides. Cancer. 2019;11(3):2‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Butrynski JE, D'Adamo DR, Hornick JL, et al. Crizotinib in ALK‐rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363(18):1727‐1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schöffski P, Sufliarsky J, Gelderblom H, et al. Crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumours with and without anaplastic lymphoma kinase gene alterations (European Organisation for Research and Treatment of Cancer 90101 CREATE): a multicentre, single‐drug, prospective, non‐randomised phase 2 trial. Lancet Respir Med. 2018;6(6):431‐441. [DOI] [PubMed] [Google Scholar]
- 14. Wu J, Savooji J, Liu D. Second‐ and third‐generation ALK inhibitors for non‐small cell lung cancer. J Hematol Oncol. 2016;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Honda K, Kadowaki S, Kato K, et al. Durable response to the ALK inhibitor alectinib in inflammatory myofibroblastic tumor of the head and neck with a novel SQSTM1‐ALK fusion: a case report. Invest New Drugs. 2019;37(4):791‐795. [DOI] [PubMed] [Google Scholar]
- 16. Lee J‐C, Li C‐F, Huang H‐Y, et al. ALK oncoproteins in atypical inflammatory myofibroblastic tumours: novel RRBP1‐ALK fusions in epithelioid inflammatory myofibroblastic sarcoma. J Pathol. 2017;241(3):316‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen S‐T, Lee J‐C. An inflammatory myofibroblastic tumor in liver with ALK and RANBP2 gene rearrangement: combination of distinct morphologic, immunohistochemical, and genetic features. Hum Pathol. 2008;39(12):1854‐1858. [DOI] [PubMed] [Google Scholar]
- 18. Mariño‐Enríquez A, Wang W‐L, Roy A, et al. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra‐abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35(1):135‐144. [DOI] [PubMed] [Google Scholar]
- 19. Xalkalori [package insert]. New York, NY: Pfizer, Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202570s030lbl.pdf
- 20. von Mehren M, Kane JM, Agulnik M, et al. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:815‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK‐positive non‐small‐cell lung cancer (J‐ALEX): an open‐label, randomised phase 3 trial. Lancet. 2017;390:29‐39. [DOI] [PubMed] [Google Scholar]
- 22. Nakagawa K, Hida T, Nokihara H, et al. Final progression‐free survival results from the J‐ALEX study of alectinib versus crizotinib in ALK‐positive non‐small‐cell lung cancer. Lung Cancer. 2020;139:195‐199. [DOI] [PubMed] [Google Scholar]
- 23. Cohen JN, Yeh I, Jordan RC, et al. Cutaneous non‐neural granular cell tumors harbor recurrent ALK gene fusions. Am J Surg Pathol. 2018;42(9):1133‐1142. [DOI] [PubMed] [Google Scholar]
- 24. d'Amore ESG, Visco C, Menin A, Famengo B, Bonvini P, Lazzari E. STAT3 pathway is activated in ALK‐positive large B‐cell lymphoma carrying SQSTM1‐ALK rearrangement and provides a possible therapeutic target. Am J Surg Pathol. 2013;37(5):780‐786. [DOI] [PubMed] [Google Scholar]
- 25. Iyevleva AG, Raskin GA, Tiurin VI, et al. Novel ALK fusion partners in lung cancer. Cancer Lett. 2015;362(1):116‐121. [DOI] [PubMed] [Google Scholar]
- 26. Jedrych J, Nikiforova M, Kennedy TF, Ho J. Epithelioid cell histiocytoma of the skin with clonal ALK gene rearrangement resulting in VCL‐ALK and SQSTM1‐ALK gene fusions. Br J Dermatol. 2015;172(5):1427‐1429. [DOI] [PubMed] [Google Scholar]
- 27. Kazakov DV, Kyrpychova L, Martinek P, et al. ALK gene fusions in epithelioid fibrous histiocytoma: a study of 14 cases, with new histopathological findings. Am J Dermatopathol. 2018;40(11):805‐814. [DOI] [PubMed] [Google Scholar]
- 28. Nakayama R, Togashi Y, Baba S, et al. Epithelioid cell histiocytoma with SQSTM1‐ALK fusion: a case report. Diagn Pathol. 2018;13(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takeuchi K, Soda M, Togashi Y, et al. Identification of a novel fusion, SQSTM1‐ALK, in ALK‐positive large B‐cell lymphoma. Haematologica. 2011;96(3):464‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sánchez‐Martín P, Saito T, Komatsu M. p62/SQSTM1: ‘Jack of all trades’ in health and cancer. FEBS J. 2019;286(1):8‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wasik MA. Expression of anaplastic lymphoma kinase in non‐Hodgkin's lymphomas and other malignant neoplasms. Pathol Patterns Rev. 2002;118:S81‐S92. [DOI] [PubMed] [Google Scholar]
- 32. Taniguchi K, Konishi H, Yoshinaga A, et al. Efficacy of combination treatment using YHO‐1701, an orally active STAT3 inhibitor, with molecular‐targeted agents on cancer cell lines. Sci Rep. 2021;11(1):6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Granato M, Gilardini Montani MS, Zompetta C, et al. Quercetin interrupts the positive feedback loop between STAT3 and IL‐6, promotes autophagy, and reduces ROS, preventing EBV‐driven B cell immortalization. Biomolecules. 2019;9(9):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


