Abstract
Background
Deep brain stimulation (DBS) for Parkinson's disease (PD) is generally contraindicated in persons with dementia but it is frequently performed in people with mild cognitive impairment or normal cognition, and current clinical guidelines are primarily based on these cohorts.
Objectives
To determine if moderately cognitive impaired individuals including those with mild dementia could meaningfully benefit from DBS in terms of motor and non‐motor outcomes.
Methods
In this retrospective case‐control study, we identified a cohort of 40 patients with PD who exhibited moderate (two or more standard deviations below normative scores) cognitive impairment (CI) during presurgical workup and compared their 1‐year clinical outcomes to a cohort of 40 matched patients with normal cognition (NC). The surgery targeted subthalamus, pallidus or motor thalamus, in a unilateral, bilateral or staged approach.
Results
At preoperative baseline, the CI cohort had higher Unified Parkinson's Disease Rating Scale (UPDRS) subscores, but similar levodopa responsiveness compared to the NC cohort. The NC and CI cohorts demonstrated comparable degrees of postoperative improvement in the OFF‐medication motor scores, motor fluctuations, and medication reduction. There was no difference in adverse event rates between the two cohorts. Outcomes in the CI cohort did not depend on the target, surgical staging, or impaired cognitive domain.
Conclusions
Moderately cognitively impaired patients with PD can experience meaningful motor benefit and medication reduction with DBS.
Keywords: cognitive impairment, DBS, neurocognitive screening, dementia, outcomes
Deep brain stimulation (DBS) is an effective treatment for many of the primary motor symptoms, and secondary motor fluctuations, associated with Parkinson's disease (PD). 1 , 2 , 3 This is accomplished by implantation of electrical stimulation leads in one of several subcortical targets, most commonly the subthalamic nucleus (STN) or globus pallidus interna (GPi), and less commonly ventral intermediate nucleus of thalamus (Vim). DBS candidacy is determined through a rigorous interdisciplinary workup, which includes a comprehensive neuropsychological evaluation, to inform the risk–benefit ratio of pursuing surgery.
One major goal for comprehensive cognitive evaluation in pre‐DBS workup is detection of an underlying or prodromal major neurocognitive disorder/dementia. 4 The general prevalence of dementia in persons with PD is close to 30%, with an incidence rate four to six times higher than the age‐matched general population. 5 Idiopathic PD dementia typically presents in more advanced disease, along with axial signs and symptoms (impairments of gait, balance, speech and/or swallowing) that contribute to poor quality of life and are usually refractory to DBS. 6 , 7 , 8 , 9 If found earlier in the disease, dementia may indicate an atypical parkinsonian condition for which DBS is not indicated. 10
Significant cognitive impairment is generally contraindicated for DBS as surgical risks of may outweigh potential benefits. 11 , 12 Patients may not be able to meaningfully participate in DBS procedures which could result in inaccurate lead placement or suboptimal device programming. There could be an increased difficulty in safely implanting the electrodes due to cerebral atrophy. 13 There is also concern that patients with impaired cognition may be at risk for accelerated cognitive deterioration, 14 which could worsen their quality of life more than any motor benefit would improve it. Lastly, it has been reported that patients with cognitive impairment are at increased risk for surgical complications such as postoperative confusion, 15 prolonged hospitalization post‐DBS, 16 and higher rates of nursing home admission. 17
Standards for clinical improvements after DBS and rates of complications have been established largely from controlled clinical trials; however, these studies only included patients with normal or mildly impaired cognition frequently defined by total Mattis Dementia Rating Scale (DRS‐2) 18 score above 130. 19 , 20 , 21 Mild cognitive impairment (MCI) is prevalent in the PD population, and most patients undergoing DBS have some form of MCI. 22 Patients with MCI have been shown to functionally benefit from DBS, although certain cognitive deficits may predict prolonged hospitalization. 23 Whether a single test such as DRS‐2 can predict DBS outcome has been controversial. 24 , 25
Much less is known about DBS outcomes in patients with moderate cognitive impairment, including those with mild dementia. Clinical diagnosis of dementia requires cognitive decline severe enough to impair daily life so establishing the diagnosis relies on patient or caregiver report. 26 , 27 Moderate cognitive impairment is not a clinical diagnosis, but it can be used to describe patients whose performance on neuropsychological testing is sufficiently impaired to suggest some degree of functional difficulties in daily life. 28 DBS clinical trials have typically used DRS‐2 score below 130 to define this group. 24 , 25 Montreal Cognitive Assessment below 20–23 is also indicative of possible presence of dementia in a PD population. 29 , 30
A prior study has demonstrated that there are still meaningful motor improvements in persons with moderate cognitive impairment who underwent GPi DBS. 31 , 32 This suggests that cognitive status alone should not preclude DBS candidacy, but this has not been sufficiently studied. 33 Expert consensus is that in select cases, it may even be appropriate to offer surgery to a patient with frank dementia for palliative purposes (e.g., severe dyskinesias, tremor with a high chance of improvement). 7 , 34
In the present retrospective single‐center case–control study, we report on motor and functional clinical outcomes in a cohort of patients with PD with moderate cognitive impairment (i.e., falling between MCI and frank dementia), and compare them to findings in a group of patients with PD who were cognitively normal (not MCI).
Methods
Patient Selection
The Emory Institutional Review Board approved this study, and written consent was waived.
Cognitively Impaired (CI) Cohort
We queried a clinical DBS database to identify patients with PD who had their first DBS surgery at Emory University from 2008–2019 and who had moderate cognitive impairment based on their preoperative neuropsychological battery in one or more cognitive domains (Attention, Executive, Memory, Language, Visuospatial, and Fluency). A domain was considered moderately impaired if at least one test score (or at least two for Memory) was two or more standard deviations below the normative mean. Mild impairment was defined as one to two standard deviations below the mean, and performances less than one standard deviation below the mean were considered normal. 35 Additionally, there needed to be at least two abnormal tests overall for a patient to be classified moderately impaired. We excluded patients who had poor performance due to language barrier (n = 2), intellectual disability (n = 1) or whose detailed neurocognitive scores were unavailable (n = 2). This resulted in a final cohort of 40 patients (out of 348 PD patients who received surgery during this period). On the basis of consensus of the entire DBS team, patients that did not proceed to surgery (and thus not included in this cohort) were those: (1) with frank dementia on preoperative testing (based on neuropsychologist evaluation) and (2) with moderate cognitive impairment but with poor potential for motor improvement.
For each cognitive domain, two scores were evaluated (four for Memory) and, since the batteries evolved over time, different tests were considered if the first two scores were unavailable. Tests and alternates utilized: Attention: Wechsler Adult Intelligence Scale‐IV (WAIS‐IV) Digit Span subtest—forward span (scaled score), Trail Making Test—Part A.
(T‐score), Dementia Rating Scale‐2 (DRS‐2) Attention Index (scaled score); Executive Function: Trail Making Test‐B (T‐score), Wisconsin Card Sorting Test Total Categories (raw score), DRS‐2 Conceptualization Index (scaled score); Language: Boston Naming Test‐2 Total Score (T‐score), WAIS‐IV Vocabulary subtest (scaled score), Wechsler Abbreviated Scale Intelligence‐II (WASI‐II) Vocabulary subtest (T‐score); Memory: Rey Auditory Verbal Learning Test (RAVLT) Learning Trials 1–5 (z‐score), RAVLT Delayed Free Recall (z‐score), Hopkins Verbal Learning Test‐Revised (HVLT‐R) Learning Trials 1–3 (T‐score), HVLT‐R Delayed Free Recall (T‐score), Wechsler Memory Scales‐IV (WMS‐IV) Logical Memory I (scaled score), WMS‐IV Logical Memory II (scaled score), DRS‐2 Memory Index (scaled score); Visuospatial: Judgment Of Line Orientation Total corrected score (percentile), WAIS‐IV Block Design subtest (scaled score), WASI‐II Block Design subtest (T‐score), DRS‐2 Construction Index (scaled score); Fluency: Semantic fluency—animals (T‐score), Controlled Oral Word Association Test (COWAT)—FAS (T‐score).
Normal Cognition (NC) Cohort
The NC cohort was chosen by matching patients with normal cognition to each patient in the CI cohort. Normal cognition was defined as normal clinical assessment (reviewed by C.K.B.) and no more than one test in the battery that was more than one standard deviation below a normative score (so patients who fulfilled criteria for MCI were excluded 36 ). Patients were matched on age, gender, disease duration, DBS surgical target, surgical method (awake with microelectrode recordings or asleep in interventional MRI), surgery staging (bilateral, staged or unilateral) and year of initial surgery. The matching was performed using an automated algorithm that minimized the sum of variable differences between individual CI and NC patients (continuous variables were normalized from 0–1).
Clinical Outcome Indices
A retrospective chart review was performed to collect data on demographics, disease duration, medications, preoperative baseline UPDRS subscores, the DBS surgical target (STN, GPi, Vim), staging (unilateral, bilateral, staged), surgery method (awake or asleep), and number of surgically relevant comorbidities (hypertension, diabetes, anticoagulation use, coagulopathy, immunologic conditions/treatments, seizure history, and smoking). Clinical outcomes were measured by postoperative adverse events (up to 18 months post‐surgery) and follow up UPDRS scores and medication dosages at the most comprehensive clinic visit (where medication OFF, stimulation ON examination was performed and non‐motor UPDRS scales completed; 3–18 months after first surgery). Repeat neuropsychological assessment was not routinely performed after surgery.
Patients were evaluated with one of two versions of UPDRS as clinical practice changed in 2017, and “new” Movement Disorders Society‐UPDRS (MDS‐UPDRS) scores were converted to “old” scores using published methods. 37 UPDRS subscores were analyzed separately and included: part 1 (“Mentation, behavior and mood”), part 2 (“Activities of daily living”), part 3 (“Motor examination”) and part 4 (“Complications of Therapy”). When UPDRS part II was reported for medication OFF and medication ON states, the scores were scaled by the percent time that patient reportedly spent in the medication OFF state, thereby yielding an average estimate of function during the day. This is similar to MDS‐UPDRS part II administration, thus allowing comparison. For medication OFF exams, patients withheld dopaminergic medications overnight (typically 12 hours for short formulations; long‐acting formulations were held for 24 hours). For stimulation ON exams, DBS settings may have been reprogrammed during the visit based on clinical need, and there was approximately 15‐minute wash‐in period before the documented examination. For medication ON exams, patients took their regular morning dose of PD medications, and exams were performed after the onset of action (typically 30–90 minutes). Levodopa equivalent daily dose (LEDD) was calculated using the standard conversion formulas. 38
Adverse events (within 18 months from surgery) were categorized based on type: Intracranial (including symptomatic or asymptomatic hemorrhage, symptomatic pneumocephalus, abscess, and ischemic infarct), Complete or partial hardware removal because of infection/erosion, Lead revision due to migration/misplacement or electrical malfunction, Hardware problems not resulting in interruption of therapy, Neurologic, Psychiatric and Medical. 26 Adverse events were included if it was determined (by M.P. and S.M.) that they were probably or possibly related to surgery or stimulation. Serious adverse events were defined as those that prolonged post‐operative hospitalization, required re‐hospitalization or repeat surgery (even if hardware was not removed) or resulted in permanent disability.
Statistical Analyses
Baseline demographics and clinical outcomes were compared using a two‐sample t‐test for continuous variables and Fisher exact test for categorical variables with P‐value less than 0.05 considered significant. Reported are means ± one standard deviation. For each clinical outcome (UPDRS part I, part II, part III meds OFF, part 3 with meds ON, part 4, and LEDD), separate generalized estimating equations (GEEs) with exchangeable correlation and robust standard errors were used to estimate the change between baseline and follow‐up in each cohort and to assess whether the change differed between cohorts (using interaction term between time period and cohort) while controlling for age, education, gender, target and surgery staging using the R‐package GEEpack. 39 P‐values were corrected for multiple comparisons using the Benjamini‐Hochberg method 40 and significance assessed at false discovery rate equal to 0.05 accounting for 18 comparisons (6 outcomes × 3 tests per outcome [baseline vs follow‐up in CI, baseline vs follow‐up in NC, and the interaction]).
To investigate if target choice (GPi vs STN) or surgical staging (unilateral/staged vs bilateral simultaneous) had an impact on clinical outcomes in the CI cohort, we built separate models to examine interaction effects. For target choice, we created GEEs for each of the six clinical outcomes restricted to the CI cohort with exchangeable correlation to examine the interaction between time period and target (restricted to GPi or STN given very few Vim cases) while controlling for gender, age, education and surgery staging, and accounting for 18 comparisons. We created six additional models to examine the interaction between surgery staging and time period in the CI cohort while controlling for gender, age, education and target. To examine whether clinical outcomes in the CI cohort were modified by a specific cognitive domain impairment, we defined a binary measure of impairment and created a separate model for each domain to examine an interaction between the domain and time period.
Results
Demographics
The analysis included 80 patients with PD who underwent DBS surgery at a single center: 40 patients in the moderately cognitive impaired (CI) cohort and 40 patients with normal cognition (NC; Table 1). Despite matching the NC to CI cohorts, the CI cohort was less likely to receive STN stimulation or bilateral simultaneous implantation, and there was a trend toward older age. This is consistent with our institutional approach in which we have favored GPi and staged lead placements in patients of advanced age and cognitive impairment while favoring bilateral STN in younger cognitively intact patients.
TABLE 1.
Baseline patient characteristics
| Moderate Cognitive Impairment n = 40 | Normal Cognition n = 40 | P‐value | |
|---|---|---|---|
| Age | 66.6 ± 8.0 | 62.9 ± 10.0 | 0.066 |
| Gender (M/F) | 35/5 | 33/7 | 0.756 a |
| Disease duration (yrs) | 11.1 ± 4.8 | 11.6 ± 5.6 | 0.655 |
| Education (yrs) | 13.4 ± 3.4 | 15.5 ± 2.6 | 0.002 |
| DRS‐2 score | 126.3 ± 8.7 | 139.3 ± 3.3 | <0.001 |
| UPDRS I | 3.1 ± 2.0 | 1.9 ± 1.4 | 0.004 |
| UPDRS II* | 17.8 ± 7.8 | 13.9 ± 6.7 | 0.019 |
| UPDRS III off med | 42.2 ± 12.5 | 35.6 ± 10.4 | 0.012 |
| UPDRS III on med | 18.7 ± 9.5 | 14.9 ± 8.8 | 0.078 |
| UPDRS IV | 6.2 ± 3.5 | 7.1 ± 3.1 | 0.246 |
| LEDD (mg) | 1238 ± 631 | 1410 ± 771 | 0.279 |
| UPDRS part III change with medications | 56 ± 16% | 59 ± 15% | 0.513 |
| DBS target | 0.007 a | ||
| STN | 12 (30%) | 25 (63%) | |
| GPi | 22 (55%) | 15 (37%) | |
| ViM | 6 (15%) | 0 (0%) | |
| Surgery staging | 0.013 a | ||
| Bilateral simultaneous | 5 STN, 1 GPi (15%) | 9 STN, 8 GPi (43%) | |
| Unilateral | 6 STN, 16 GPi, 4 ViM (65%) | 12 STN, 7 GPi (47%) | |
| Staged (within 1 yr) | 1 STN, 5 GPi, 2 ViM (20%) | 4 STN (10%) | |
| Surgery method | 0.494 a | ||
| Awake with MER | 22 (55%) | 26 (65%) | |
| Under anesthesia in iMRI | 18 (45%) | 14 (35%) | |
| Number of surgical risk factor comorbidities | 0.9 ± 0.8 | 0.6 ± 0.7 | 0.052 |
| Baseline to follow‐up (mo) | 15.9 ± 5.4 | 16.7 ± 4.9 | 0.487 |
| Surgery to follow‐up (mo) | 10.4 ± 4.0 | 11.1 ± 3.7 | 0.400 |
Note: p = <.01
Abbreviations: MER, microelectrode recordings; iMRI, interventional MRI.
2 × 2 Fisher exact test (STN vs GPi/ViM; Bilateral vs Unilateral/Staged).
combined score off and on medications (see Methods).
Neurocognitive Profile
Neurocognitive characterization was based on a comprehensive neuropsychological battery. As expected, the CI cohort had lower DRS‐2 total score compared to NC cohort (Table 1). In the CI cohort, a minority of patients (15%) had moderate impairment in a single cognitive domain while a majority (85%) was moderately impaired in multiple domains (Table 2). Ten patients retrospectively fulfilled criteria for dementia diagnosis given documented functional impairment reported by the patient or a caregiver during baseline neuropsychological evaluation. 27 , 41
TABLE 2.
Baseline cognitive profile of the moderately cognitively impaired cohort by domain (n = 40)
| Cognitive domain | Moderate domain impairment # (%) | Mild domain impairment # (%) | Normal domain # (%) |
|---|---|---|---|
| Executive | 34 (85%) | 3 (8%) | 3 (8%) |
| Memory | 26 (65%) | 9 (23%) | 3 (8%) |
| Attention | 23 (58%) | 8 (20%) | 9 (23%) |
| Fluency | 15 (38%) | 16 (40%) | 9 (23%) |
| Visuospatial | 9 (23%) | 12 (30%) | 19 (48%) |
| Language | 5 (13%) | 18 (45%) | 17 (43%) |
Note: p = <.01
Post‐Operative Adverse Events
The hospitalization duration was comparable between cohorts (Table 3). Overall, 26 (65%) patients in the CI cohort and 20 (50%) in the NC cohort experienced at least one surgery‐ or stimulation‐related adverse event within the first 18 months. Serious complications were reported in 16 (41%) and 14 (35%) of patients in the CI and NC cohorts, respectively. The CI cohort experienced a higher number of intracranial events and more instances of postoperative confusion and hallucinations; however, the differences were not statistically significant.
TABLE 3.
Number of patients experiencing adverse events (AEs) within 18 mo of surgery and duration of hospitalization for lead implantation surgery
| Moderate Cognitive Impairment n = 40 | Normal Cognition n = 40 | |
|---|---|---|
| Hospital stay (days) | 1.3 ± 0.6 | 1.4 ± 0.9 |
| Number of patients with any AEs | 26 (65%) | 20 (50%) |
| Number of patients with serious AEs | 16 (41%) | 14 (35%) |
| Intracranial (symptomatic or asymptomatic hemorrhage, symptomatic pneumocephalus, abscess, ischemic infarct) |
n = 6 1 symptomatic hemorrhage 3 asymptomatic hemorrhages 1 asymptomatic infarct 1 abscess |
n = 3 1 symptomatic hemorrhage 1 asymptomatic hemorrhage 1 pneumocephalus |
| Complete or partial hardware removal because of infection/erosion | n = 2 | n = 2 |
| Lead revision due to migration/misplacement or electrical malfunction | n = 3 | n = 1 |
| Hardware problems not resulting in interruption of therapy |
n = 6 0 with surgical intervention |
n = 9 3 with surgical intervention |
| Neurologic AEs, possibly or probably related to surgery or stimulation |
n = 10 1 cognitive decline 6 transient confusion 2 generalized weakness 1 speech difficulty |
n = 7 1 transient confusion 3 generalized weakness 1 transient facial weakness 1 dyskinesia 1 paresthesia |
| Psychiatric AEs, possibly or probably related to surgery or stimulation |
n = 4 new/worsened hallucinations |
n = 1 prolonged delirium |
| Medical AEs, possibly or probably related to surgery or stimulation |
n = 3 1 death at 4 mo (hospitalized for altered mental status) 1 pyelonephritis 1 pain and nausea |
n = 5 2 deaths at 9 and 17 mo (unknown causes) 2 chest pain 1 incontinence |
One patient in the CI cohort experienced clinically significant cognitive decline following surgery which was confirmed on repeat neuropsychological evaluation (DRS‐2 score declined from 132 preoperatively to 116 at 7 months postoperatively). Another patient in the CI cohort was readmitted for syncope and altered mental status following surgery and died at 4 months postoperatively (unknown cause). Six patients in the CI cohort had transient cognitive worsening in the first 1–2 postoperative months typically reported by a family member (2 improved after non‐PD medication adjustment, 1 after infection treatment, and 3 spontaneously). One patient in the NC cohort had prolonged delirium with transient cognitive worsening after discharge and died at 17 months postoperatively (unknown cause).
Changes in UPDRS Subscores and Medication Doses
The average time to follow up was approximately 1 year after surgery and comparable between the cohorts (Table 1). All patients did not have all UPDRS subscores available, thus the number of patients that contributed data is reported for each subscore (Table 4). When comparing patients who underwent more comprehensive evaluation at follow up (i.e., who filled out non‐motor scales and were examined off and on medications) compared to those who underwent limited evaluation, there were no differences in their preoperative characteristics or follow up part 3 medication OFF/stimulation ON score. Therefore, we utilized all available data for statistical comparisons between baseline and follow‐up (Fig. 1; Table 4).
TABLE 4.
UPDRS subscores and LEDD amount at baseline and at follow up for moderate cognitive impairment and normal cognition cohorts
| Moderate Cognitive Impairment n = 40 | Normal Cognition n = 40 | Interaction (CI vs NC) | |||||
|---|---|---|---|---|---|---|---|
| BL | FU | P‐value (FDS corr) | BL | FU | P‐value (FDS corr) | P‐value (FDS corr) | |
| LEDD (mg) |
1238 ± 631 n = 40 |
906 ± 515 n = 38 |
<0.001 (0.003) |
1410 ± 771 n = 40 |
882 ± 644 n = 37 |
<0.001 (<0.00) | 0.142 (0.311) |
| UPDRS I |
3.1 ± 2.0 n = 38 |
3.1 ± 1.9 n = 20 |
0.952 (0.992) |
1.9 ± 1.4 n = 40 |
2.2 ± 2.5 n = 25 |
0.383 (0.689) | 0.547 (0.881) |
| UPDRS II a |
17.9 ± 7.8 n = 38 |
17.8 ± 6.6 n = 20 |
0.792 (0.986) |
13.9 ± 6.7 n = 40 |
13.2 ± 6.8 n = 25 |
0.867 (0.986) | 0.758 (0.986) |
| UPDRS III off med b |
42.2 ± 12.5 n = 40 |
25.6 ± 10.3 n = 33 |
<0.001 (<0.001) |
35.6 ± 10.4 n = 40 |
19.2 ± 8.5 n = 36 |
<0.001 (<0.001) | 0.877 (0.986) |
| UPDRS III on med b |
18.7 ± 9.5 n = 36 |
18.6 ± 8.8 n = 22 |
0.992 (0.992) |
14.9 ± 8.8 n = 38 |
11.9 ± 7.0 n = 25 |
0.056 (0.145) | 0.156 (0.311) |
| UPDRS IV |
6.6 ± 3.5 n = 38 |
4.2 ± 3.4 n = 20 |
0.016 (0.048) |
7.1 ± 3.1 n = 40 |
4.3 ± 2.5 n = 23 |
<0.001 (<0.001) | 0.588 (0.881) |
Note: p = <.01. FDR corr. = P‐value with false discovery rate correction for multiple comparisons.
Abbreviations: BL, baseline; FU, follow‐up.
Combined score off and on medications (see Methods).
On stimulation at follow‐up. UPDRS = Unified Parkinson's Disease Rating Scale.
FIG. 1.
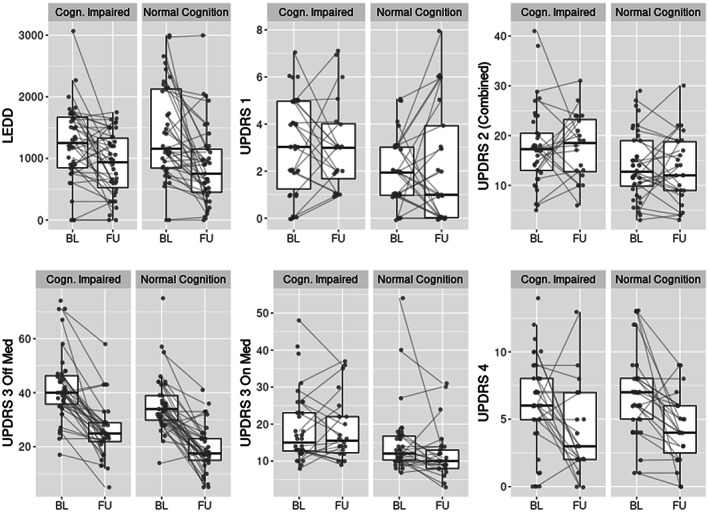
UPDRS and LEDD at baseline vs. follow up for moderately cognitively impaired and normal cognition cohorts (medians, interquartile ranges). See Table 4 for statistical comparisons. UPDRS, Unified Parkinson's Disease Rating Scale. BL, baseline. FU, follow up. LEDD, levodopa equivalent daily dose in milligrams.
Both cohorts experienced significant improvement in UPDRS part III medication OFF (stimulation ON) score and LEDD at follow up (P < =0.001 in both CI and NC, P‐values are FDR corrected) as well as a decrease in UPDRS part IV (P = 0.048 in CI, P < 0.001 in NC, Fig. 1; Table 4). The magnitude of averaged UPDRS part III score reduction was 15.4 and 15.1 points in the CI and NC cohorts, which surpasses large clinically important difference estimated in prior literature to be 10.8 points. 42 There was no significant change for UPDRS part I, part II, and part III medication ON score in either cohort. Overall, the changes in scores and LEDD were comparable between the cohorts since there were no statistically significant interaction terms (Table 4).
Predictors of Clinical Outcome in the CI Cohort
We investigated if target choice (GPi vs STN), surgical staging (unilateral/staged vs bilateral simultaneous) or type of cognitive domain impairment had an impact on clinical outcomes in the CI cohort. As expected, there was a significant reduction in LEDD for STN but not for GPi target. There were no statistically significant differences in UPDRS score outcomes between targets. Both targets reduced part 3 medication OFF score, and there was a trend toward greater reduction with the STN target (interaction term P‐value not significant after FDR correction; Table S1; Fig. S1). There were no differences in the adverse events (not shown). UPDRS part III medication OFF scores and LEDD improved comparably in patients who had unilateral/staged surgery or bilateral simultaneous surgery (Table S2; Fig. S2). There were no differences from baseline in other measures. There were also no differences in adverse events (not shown). Finally, there were no differences in clinical outcomes based on impairment in a specific cognitive domain (not shown).
Discussion
In this retrospective single‐center case–control study, we characterized clinical outcomes (i.e., adverse events, UPDRS scores, and LEDD) in patients with PD with moderate cognitive impairment and compared them to a matched normal cognition cohort following DBS surgery at a large academic center. The NC cohort was included in the analysis in order to benchmark performance of the CI cohort against a group expected to have optimal DBS outcome. The CI group showed evidence of motor improvement (lower UPDRS part III off medication and UPDRS part IV scores) as well as a medication reduction, at levels similar to patients with normal cognition. Despite the fact that the CI group had a higher cognitive burden and more severe disease at baseline, this cohort benefitted from DBS surgery and did not have significantly higher rates of surgical‐ or stimulation‐related complications—lending support toward their consideration as DBS candidates.
It is important to note that our CI group was carefully screened, and only proceeded with surgery based on team consensus that there was a strong clinical indication, i.e., motor fluctuations with a robust response to levodopa or medication‐refractory tremor. This highlights our main conclusion that poor cognitive performance should not a priori disqualify patients from DBS consideration. A quarter of the patients in our CI cohort fulfilled diagnosis for dementia 41 but the severity was considered clinically mild and patients were able to consent to the procedure. Establishing diagnosis of dementia can be particularly challenging in the setting of pre‐DBS evaluation when patients and caregivers may be reluctant to disclose full nature of functional impairments, or incorrectly assume that patient's motor symptoms alone are responsible for their inability to perform daily activities. Drawing safe selection boundaries is a challenge, with even expert consensus meetings struggling to clearly define cutoffs based on cognitive evaluation. 12 , 14 , 32
There were no major cognitive changes in the CI cohort based on stable UPDRS part I scores, but there was heterogeneity of outcomes on this measure and missing data. The effect of DBS on cognition and behavior, particularly with STN stimulation, has been controversial. 42 While large, randomized trials do not show significant detrimental changes in global cognition with DBS, 3 , 43 meta‐analyses and systematic reviews have shown adverse effects particularly in the executive cognitive domain. 44 , 45 , 46 , 47 These declines in cognition do not necessarily preclude overall improvements in quality‐of‐life post‐DBS. 48 We cannot comment on the possible interplay between cognition and functional improvement in our study as we did not systematically acquire data regarding neuropsychological outcomes.
Although the number of patients in the CI cohort who had intracranial surgical complications or reports of confusion and hallucinations was greater than in the NC cohort, this was not significant. One CI patient did experience permanent cognitive decline which could be temporally related to the surgical procedure. Previous studies have suggested that increased cognitive burden is associated with adverse surgical outcomes such as delirium 15 or prolonged hospital stay after DBS surgery, 16 but overall rates of complications are low even in cognitively impaired patients, including those with Alzheimer's and PD dementias. 31 , 49 , 50 , 51
While motor outcomes showed robust improvement, there were no significant improvements in UPDRS part II (Activities of Daily Living) on a group level. This was true for both cohorts, suggesting that higher cognitive burden was not responsible for lack of functional improvement seen on this patient‐reported metric. UPDRS part II outcomes were heterogeneous with some patients reporting significant gains while other reported worsening indicating that UPDRS part II may not be not a sensitive measure for DBS outcomes. 23 , 32 , 52 A more comprehensive outcome measure such as a quality‐of‐life scale 42 or a semi‐structured interview eliciting patients’ perceived outcome 53 may have been more sensitive, but this was not available in our study.
Another aim was to parse outcomes of patients in the CI group based on the DBS target, staging approach and type of cognitive impairment (by specific domain). In clinical practice in some centers cognitively impaired patients are recommended to undergo unilateral or staged GPi rather than bilateral simultaneous STN DBS to improve tolerability while still aiming to provide meaningful motor benefit. 54 , 55 In our study, there were comparable outcomes with both targets and approaches without differences in adverse events. But we suspect that the study was underpowered in this regard, so this remains unclear. Prior studies have reported that patients with visuospatial or attentional impairment have worse post‐surgical results, 17 , 23 but this was not demonstrated in our outcome measures.
This is the largest study reporting detailed clinical outcomes of DBS in a cohort of cognitively impaired PD patients. Strengths of this study include the case–control design and rigorous statistical analysis examining interaction terms to demonstrate differences between cohorts and multiple comparison correction. However, there were also limiting factors. First, our smaller sample size restricted statistical power in detecting differences in interaction effects particularly for sub‐analyses. Missing follow up data limited full examination of all potential cognitive and functional outcomes, and lack of repeat neuropsychological/quality of life measured and caregiver reports precluded more comprehensive analyses. Another limitation was related to the non‐randomization of DBS target and staging. Finally, our follow up extended to only approximately 1‐year post‐surgery, and differences between CI and NC cohorts may become more apparent with longer follow up. 17
In conclusion, this study suggests that moderately cognitively impaired patients can experience meaningful motor benefit and medication reduction after undergoing DBS surgery. These patients should have a clear indication for surgery such as motor fluctuations with a robust levodopa response or medication refractory tremor. We argue that this group should be identified on the basis of a comprehensive neuropsychological battery versus single composite score. Our results may inform potentially useful criteria for identifying these patients and suggest expanding cognitive cutoffs for safe selection for proceeding with DBS. Future studies should corroborate these findings and investigate possible safe selection criteria for cognitively impaired patients in a prospective randomized trial, and utilizing patient‐centered outcome measures.
Author Roles
The authors contributed to this manuscript in one or more of the following ways: research project (A: conception, B: organization; C: execution), statistical analysis (A: design, B: execution, C: review and critique), and/or manuscript preparation (A: writing of the first draft, B: review and critique). Specific author contributions:
C.K.B.: 1B, 1C, 2C, 3A, 3B.
M.P.: 1B, 1C, 3B.
B.R.: 2A, 2B.
E.S.: 1C, 3B.
D.L.: 1A, 1C, 3B.
C.D.E.: 1C, 3B.
L.S.: 1C, 3B.
L.H.: 1C, 3B.
P.A.: 1C, 3B.
M.R.D.: 1C, 3B.
T.W.: 1C, 3B.
S.A.F.: 1C, 3B.
N.A.Y.: 1C, 3B.
J.T.W.: 1C, 3B.
N.M.B.: 1C, 3B.
R.E.G.: 1C, 3B.
C.B.: 1B, 1C, 3B.
S.M.: 1A, 1B, 1C, 2C, 3B.
Disclosures
Ethical Compliance Statement: This study was conducted in accordance with all ethical principles. It was reviewed by the Emory Institutional Review Board, and based on their review consent was waived as it was not deemed necessary for this study. All authors confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: For research covered in this article, the work was supported by the American Parkinson Disease Association grant and funding from the NIH (P50 NS123103, P51 OD011132).
Financial Disclosures for the Previous 12 Months: Dr. Cady Block discloses royalties (APA Publishing). Dr. Stewart Factor discloses honoraria (Acorda, Alterity, Biogen, Lunbeck, Sunovion), royalties (Blackwell Futura, Springer, Demos, UptoDate), grants (Addex Pharma S.A., Biohaven, Boston Scientific, Impax, Medtronic, Neurocrine, Sun Michael J. Fox Foundation, NIH [U10 NS073266]), (Parkinson Foundation, Pharmaceuticals Advanced Research Company, Prilenia Therapeutics CHDI Foundation, Sunovion, Vaccinex, Voyager), and other (Signant Health [Bracket Global LLC] and CNS Ratings LLC). Dr. Robert Gross discloses fellowship support (Abbott Labs, Boston Scientific), consulting (Abbott Labs, AskBio, Bayer AG, Bluerock Therapeutics, Boston Scientific, CODA Therapeutics, Medtronic, NeuroOne, Nia Therapeutics, and Zimmer Biomet) and grants (NIH, Department of Defense). Dr. Margi Patel discloses fellowship support (Parkinson Research Foundation and Dystonia Research Medical Foundation). Dr. Benjamin Risk discloses grants (NIH [P50 NS123103]). Dr. Thomas Wichmann discloses grants (NIH/NINDS [P50 NS123103, R01 NS10098, R01 NS113746, R01 NS098441, R21 NS111765, R21 NS116438, R21 NS123487], NIH/ORP [P51 OD011132], the ASAP CN [ASAP‐010572], NSF [NSF‐1951682], the American Parkinson's Foundation) Dr. Jon Willie discloses grants (NIH/NIMH [R01 MG120194 and R01 MH120194]) and consulting (AiM Medical Robotics Inc., Clearpoint Neuro Inc., Medtronic).
Supporting information
Fig. S1. UPDRS and LEDD at baseline vs. follow up for moderately cognitively impaired cohort by target (medians, interquartile ranges).
Fig. S2. UPDRS and LEDD at baseline and at follow up for moderately cognitively impaired cohort by surgery side (medians and interquartile ranges).
Table S1. UPDRS subscores and LEDD amount at baseline and follow up for moderate cognitive impairment cohort by target.
Table S2. UPDRS subscores and LEDD amount at baseline and follow up for moderate cognitive impairment by surgery side.
Acknowledgment
We thank Shirley Triche, NP, for her assistance in collecting clinical data.
References
- 1. Deuschl G, Schade‐Brittinger C, Krack P, et al. A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med 2006;355(9):896–908. 10.1056/nejmoa060281. [DOI] [PubMed] [Google Scholar]
- 2. Rodriguez‐Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson's disease: A multicentre study with 4 years follow‐up. Brain 2005;128(10):2240–2249. 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 3. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: A randomized controlled trial. JAMA 2009;301:63–73. 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tröster AI. Some clinically useful information that neuropsychology provides patients, Carepartners, neurologists, and neurosurgeons about deep brain stimulation for Parkinson's disease. Arch Clin Neuropsychol 2017;32(7):810–828. 10.1093/arclin/acx090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Llebaria G, Pagonabarraga J, Kulisevsky J, García‐Sánchez C, Pascual‐Sedano B, Gironell A, Martínez‐Corral M. Cut‐off score of the Mattis dementia rating scale for screening dementia in Parkinson's disease. Mov Disord 2008;23(11):1546–1550. 10.1002/mds.22173. [DOI] [PubMed] [Google Scholar]
- 6. Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 1998;339(16):1105–1111. 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 7. Hariz MI, Johansson F, Shamsgovara P, Johansson E, Hariz GM, Fagerlund M. Bilateral subthalamic nucleus stimulation in a parkinsonian patient with preoperative deficits in speech and cognition: Persistent improvement in mobility but increased dependency: A case study. Mov Disord 2000;15(1):136–139. . [DOI] [PubMed] [Google Scholar]
- 8. Saint‐Cyr JA. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson's disease. Brain 2000;123(10):2091–2108. 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- 9. Jarraya B, Bonnet AM, Duyckaerts C, Houeto JL, Cornu P, Hauw JJ, Agid Y. Parkinson's disease, subthalamic stimulation, and selection of candidates: A pathological study. Mov Disord 2003;18(12):1517–1520. 10.1002/mds.10607. [DOI] [PubMed] [Google Scholar]
- 10. Artusi CA, Rinaldi D, Balestrino R, Lopiano L. Deep brain stimulation for atypical parkinsonism: A systematic review on efficacy and safety. Parkinsonism Relat Disord 2022;96:109–118. 10.1016/j.parkreldis.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 11. Bronstein JM, Tagliati M, Alterman RL, et al. Deep brain stimulation for Parkinson disease. Arch Neurol 2011;68(2):165. 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lang AE, Houeto JL, Krack P, et al. Deep brain stimulation: Preoperative issues. Mov Disord 2006;21(S14):S171–S196. 10.1002/mds.20955. [DOI] [PubMed] [Google Scholar]
- 13. Hartmann CJ, Fliegen S, Groiss SJ, Wojtecki L, Schnitzler A. An update on best practice of deep brain stimulation in Parkinson's disease. Ther Adv Neurol Disord 2019;12:1756286419838096. 10.1177/1756286419838096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trépanier LL, Kumar R, Lozano AM, Lang AE, Saint‐Cyr JA. Neuropsychological outcome of GPi Pallidotomy and GPi or STN deep brain stimulation in Parkinson's disease. Brain Cogn 2000;42(3):324–347. 10.1006/brcg.1999.1108. [DOI] [PubMed] [Google Scholar]
- 15. Pilitsis JG, Rezai AR, Boulis NM, Henderson JM, Busch RM, Kubu CS. A preliminary study of transient confusional states following bilateral subthalamic stimulation for Parkinson's disease. Stereotact Funct Neurosurg 2005;83(2–3):67–70. 10.1159/000086676. [DOI] [PubMed] [Google Scholar]
- 16. Mikos A, Pavon J, Bowers D, et al. Factors related to extended hospital stays following deep brain stimulation for Parkinson's disease. Parkinsonism Relat Disord 2010;16(5):324–328. 10.1016/j.parkreldis.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 17. Park KW, Jo S, Kim MS, et al. Cognitive profile as a predictor of the long‐term outcome after deep brain stimulation in Parkinson's disease. J Neurol Sci 2020;417:117063. [DOI] [PubMed] [Google Scholar]
- 18. Jurica P, Leitten C, Mattis S. DRS‐2: Dementia rating scale‐2. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 19. Deep‐brain stimulation of the subthalamic nucleus or the pars Interna of the Globus Pallidus in Parkinson's disease. N Engl J Med 2001;345(13):956–963. 10.1056/nejmoa000827. [DOI] [PubMed] [Google Scholar]
- 20. Schuepbach WMM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med 2013;368(7):610–622. 10.1056/nejmoa1205158. [DOI] [PubMed] [Google Scholar]
- 21. Vitek JL, Jain R, Chen L, et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current‐controlled device in Parkinson's disease (INTREPID): A multicentre, double‐blind, randomised, sham‐controlled study. Lancet Neurol 2020;19(6):491–501. 10.1016/S1474-4422(20)30108-3. [DOI] [PubMed] [Google Scholar]
- 22. Kenney L, Rohl B, Lopez FV, et al. The UF deep brain stimulation cognitive rating scale (DBS‐CRS): Clinical decision making, validity, and outcomes. Front Hum Neurosci 2020;14:578216. 10.3389/fnhum.2020.578216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abboud H, Floden D, Thompson NR, et al. Impact of mild cognitive impairment on outcome following deep brain stimulation surgery for Parkinson's disease. Parkinsonism Relat Disord 2015;21(3):249–253. 10.1016/j.parkreldis.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 24. Witt K, Daniels C, Krack P, et al. Negative impact of borderline global cognitive scores on quality of life after subthalamic nucleus stimulation in Parkinson's disease. J Neurol Sci 2011;310(1–2):261–266. 10.1016/j.jns.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 25. Floden D, Busch RM, Cooper SE, Kubu CS, Machado AG. Global cognitive scores do not predict outcome after subthalamic nucleus deep brain stimulation. Mov Disord 2015;30(9):1279–1283. 10.1002/mds.26292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engel K, Huckhagel T, Gulberti A, et al. Towards unambiguous reporting of complications related to deep brain stimulation surgery: A retrospective single‐center analysis and systematic review of the literature. PloS One 2018;13(8):e0198529. 10.1371/journal.pone.0198529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing, Washington DC; 2013. [Google Scholar]
- 28. Campos CG, Diniz BS, Firmo JO, Lima‐Costa MF, Blay SL, Castro‐Costa E. Mild and moderate cognitive impairment and mortality among Brazilian older adults in long‐term follow‐up: The Bambui health aging study. Braz J Psychiatry 2020;42(6):583–590. 10.1590/1516-4446-2019-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim HM, Nazor C, Zabetian CP, et al. Prediction of cognitive progression in Parkinson's disease using three cognitive screening measures. Clin Park Relat Disord 2019;1:91–97. 10.1016/j.prdoa.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dalrymple‐Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: Well‐suited screen for cognitive impairment in Parkinson disease. Neurology 2010;75(19):1717–1725. 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 31. Rouaud T, Dondaine T, Drapier S, et al. Pallidal stimulation in advanced Parkinson's patients with contraindications for subthalamic stimulation. Mov Disord 2010;25(12):1839–1846. 10.1002/mds.23171. [DOI] [PubMed] [Google Scholar]
- 32. Bonenfant J, Drapier S, Houvenaghel JF, Naudet F, Haegelen C, Sauleau P, Vérin M. Pallidal stimulation in Parkinson's patients with contraindications to subthalamic target: A 3 years follow‐up. Parkinsonism Relat Disord 2017;34:20–25. 10.1016/j.parkreldis.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 33. Buetefisch CM, Parsons M, Haut MW, Goldstein SR, Whiting DM, Oh MY. Safety and efficacy of deep brain stimulation in mildly demented Parkinson's disease patients: A multiple case study. Presented at: The Movement Disorder Society. Chicago, IL; 2008. [Google Scholar]
- 34. Kubu CS, Ford PJ. Clinical ethics in the context of deep brain stimulation for movement disorders. Arch Clin Neuropsychol 2017;32(7):829–839. 10.1093/arclin/acx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heaton RK, Grant I, Matthews CG. Comprehensive Norms for an Expanded Halstead‐Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources, Lutz, FL; 1991. [Google Scholar]
- 36. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society task force guidelines. Mov Disord 2012;27(3):349–356. 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): Scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170. 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 38. Schade S, Mollenhauer B, Trenkwalder C. Levodopa equivalent dose conversion factors: An updated proposal including Opicapone and safinamide. Mov Disord Clin Pract 2020;7(3):343–345. 10.1002/mdc3.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw 2006;15:1–11. [Google Scholar]
- 40. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B Methodol 1995;57(1):289–300. [Google Scholar]
- 41. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22(12):1689–1707. 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 42. Daniels C, Krack P, Volkmann J, et al. Is improvement in the quality of life after subthalamic nucleus stimulation in Parkinson's disease predictable? Mov Disord 2011;26(14):2516–2521. 10.1002/mds.23907. [DOI] [PubMed] [Google Scholar]
- 43. Witt K, Daniels C, Reiff J, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson's disease: A randomised, multicentre study. Lancet Neurol 2008;7(7):605–614. 10.1016/S1474-4422(08)70114-547. [DOI] [PubMed] [Google Scholar]
- 44. Halpern CH, Rick JH, Danish SF, Grossman M, Baltuch GH. Cognition following bilateral deep brain stimulation surgery of the subthalamic nucleus for Parkinson's disease. Int J Geriatr Psychiatry 2009;24(5):443–451. 10.1002/gps.2149. [DOI] [PubMed] [Google Scholar]
- 45. Parsons TD, Rogers SA, Braaten AJ, Woods SP, Tröster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: A meta‐analysis. Lancet Neurol 2006;5(7):578–588. 10.1016/S1474-4422(06)70475-6. [DOI] [PubMed] [Google Scholar]
- 46. Temel Y, Visser‐Vandewalle V. Targets for deep brain stimulation in Parkinson's disease. Expert Opin Ther Targets 2006;10(3):355–362. 10.1517/14728222.10.3.355. [DOI] [PubMed] [Google Scholar]
- 47. Smeding HMM, Speelman JD, Huizenga HM, Schuurman PR, Schmand B. Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson's disease. J Neurol Neurosurg Psychiatry 2011;82(7):754–760. 10.1136/jnnp.2007.140012. [DOI] [PubMed] [Google Scholar]
- 48. Wang J‐W, Yu‐Qing Z, Xiao‐Hua Z, Yun‐Peng W, Ji‐Ping L, Yong‐Jie L. Cognitive and psychiatric effects of STN versus GPi deep brain stimulation in Parkinson's disease: A meta‐analysis of randomized controlled trials. PLos One 2016;11:e0156721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lozano AM, Fosdick L, Chakravarty MM, et al. A phase II study of fornix deep brain stimulation in mild Alzheimer's disease. J Alzheimers Dis 2016;54(2):777–787. 10.3233/jad-160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuhn J, Hardenacke K, Lenartz D, et al. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer's dementia. Mol Psychiatry 2014;20(3):353–360. 10.1038/mp.2014.32. [DOI] [PubMed] [Google Scholar]
- 51. Gratwicke J, Zrinzo L, Kahan J, et al. Bilateral deep brain stimulation of the nucleus basalis of Meynert for Parkinson disease dementia: A randomized clinical trial. JAMA Neurol 2018;75(2):169–178. 10.1001/jamaneurol.2017.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zolfaghari S, Thomann AE, Lewandowski N, et al. Self‐report versus clinician examination in early Parkinson's disease. Mov Disord 2022;37(3):585–597. 10.1002/mds.28884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maier F, Lewis CJ, Horstkoetter N, et al. Patients' expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson's disease: A mixed‐method approach. J Neurol Neurosurg Psychiatry 2013;84(11):1273–1281. 10.1136/jnnp-2012-303670. [DOI] [PubMed] [Google Scholar]
- 54. Dallapiazza RF, De Vloo P, Fomenko A, et al. Considerations for patient target and selection in deep brain stimulation surgery for Parkinson's disease. In: Stoker TB, Greenland JC, eds. Parkinson's Disease: Pathogenesis and Clinical Aspects.: Codon; Brisbane, Australia; 2018:145–160. [Google Scholar]
- 55. Welter ML, Houeto JL, Tezenas du Montcel S, et al. Clinical predictive factors of subthalamic stimulation in Parkinson's disease. Brain 2002;125(3):575–583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. UPDRS and LEDD at baseline vs. follow up for moderately cognitively impaired cohort by target (medians, interquartile ranges).
Fig. S2. UPDRS and LEDD at baseline and at follow up for moderately cognitively impaired cohort by surgery side (medians and interquartile ranges).
Table S1. UPDRS subscores and LEDD amount at baseline and follow up for moderate cognitive impairment cohort by target.
Table S2. UPDRS subscores and LEDD amount at baseline and follow up for moderate cognitive impairment by surgery side.


