Abstract
Proper nutrition, avoidance of ingesting substances that are harmful to the whole organism, and maintenance of energy homeostasis are crucial for living organisms. Additionally, mammals possess a sophisticated system to control the types and content of food that we swallow. Gustation is a vital sensory skill for determining which food stuffs to ingest and which to avoid, and for maintaining metabolic homeostasis. It is becoming apparent that there is a strong link between metabolic control and flavor perception. Although the gustatory system critically influences food preference, food intake, and metabolic homeostasis, the mechanisms for modulating taste sensitivity by metabolic hormones are just now being explored. It is likely that hormones produced in the tongue influence the amounts and types of food that we eat: the hormones that we associate with appetite control, glucose homeostasis and satiety, such as glucagon-like peptide-1, cholecystokinin, and neuropeptide Y are also produced locally in taste buds. In this report, we will provide an overview of the peptidergic endocrine hormone factors that are present or are known to have effects within the gustatory system, and we will discuss their roles, where known, in taste signaling.
1. Introduction
The intake of proper nutrition, avoidance of ingesting substances that are detrimental, and maintenance of energy homeostasis are crucial for organisms to continue their lives. In mammals, there is a complex system that provides a gateway for controlling the types of food we swallow. Gustation is a vital sensory skill for locating food sources, determining which food stuffs to ingest and maintaining metabolic homeostasis. It is becoming apparent that there is a strong link between metabolic control and “flavor perception,” and endocrine alteration in the taste system is likely to affect food intake, satiety, and general metabolism. Although the gustatory system critically influences food preference, food intake and metabolic homeostasis, the mechanisms for modulating taste sensitivity by metabolic hormones are just now being explored. It is likely that hormones produced in the tongue influence the amounts and types of food that we eat: the hormones that we associate with appetite control, glucose homeostasis, and satiety, such as cholecystokinin (CCK), glucagon-like peptide-1, neuropeptide Y (NPY), are also produced locally in taste buds. In this review, we will provide an overview of the peptidergic endocrine hormone factors that are present or to have effects within the gustatory system and we will discuss their roles, if known, in taste signaling. Table 1 lists the hormones and receptors known to be present in taste buds.
Table 1.
Location of hormones and their receptors by cell type within taste buds
| Type I | Type II | Type III | Type IV | Nerve fibers | References | |
|---|---|---|---|---|---|---|
| VIP | x | Shen et al. (2005) | ||||
| VIP receptor (VPAC1, VPAC2) | x | Martin et al. (2010) | ||||
| CCK | x | Shen et al. (2005) | ||||
| CCK receptor (CCK-1) | x | Herness et al. (2005) | ||||
| NPY | x | Zhao et al. (2005) | ||||
| NPY receptor (Y1) | x | x | Herness and Zhao (2009) | |||
| GLP-1 | x | x | Shin et al. (2008) | |||
| GLP-1 receptor | x | x | Shin et al. (2008) | |||
| Leptin receptor (Ob-Rb) | x | Shigemura et al. (2004) |
2. Hormones That Are Present in the Gustatory System
2.1. Vasoactive Intestinal Peptide
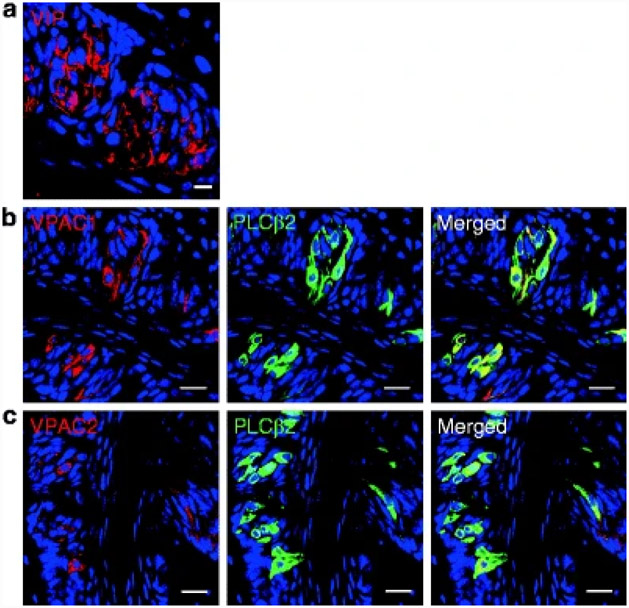
Vasoactive intestinal peptide (VIP) is a 28 amino acid peptide and a member of PACAP/glucagon superfamily that includes secretin, glucagon, and at least 11 other peptides. It was first extracted from the gut as a product of secretin purification and is widely present in the peripheral as well as the central nervous system (Said and Mutt 1970; Fahrenkrug et al. 1979; Ahrén et al. 1980; Lundberg et al. 1980; Lorén et al. 1979). It was defined by its potent smooth muscle relaxant/vasodilatory activity and as a stimulator of secretory activity (Dickson and Finlayson 2009). It serves as a ligand for two G-protein-coupled receptors (GPCRs): VPAC1 and VPAC2 (Martin et al. 2005). These receptors preferentially stimulate adenylate cyclase (AC) and increase intracellular cyclic adenosine monophosphate (cAMP). Most recently, VIP expression has been identified in the taste cells (TCs) of the rat, hamster, carp, and human (Kusakabe et al. 1998a, b; Herness 1989, 1995; Witt 1995). In mouse TCs, VIP immunoreactivity totally overlaps with PLCβ2 expression (Shen et al. 2005; Fig. 1a); any role, however, of VIP in taste appreciation had not been established prior to 2010.
Fig. 1. Expression of VIP, and coexpression of VIP receptors (VPAC1/VPAC2) with PLCβ2 in mouse circumvallate papillae (CV).
(a) VIP is expressed in taste bud. (b) VPAC1 and PLCβ2 are colocalized in a subset of PLCβ2-positive cells. (c) VPAC2 and PLCβ2 are colocalized in a subset of PLCβ2-positive cells. Scale bars, 20 μm. Blue is TO-PRO-3 nuclear stain
The majority of VIP-immunoreactive cells also colocalize with α-gustducin, while much fewer VIP-containing cells also express T1R2. α-Gustducin and T1R2 are markers for type II cells, implicating the presence of VIP as having some function in these TCs of the tongue. On the basis of its expression pattern – expression with α-gustducin but little expression with sweet taste receptors – it seemed that VIP might be involved in the transduction of bitter stimuli (Shen et al. 2005). But a recent publication presented data showing that VIP null mice exhibit enhanced taste sensitivity to sweet tastants and they also have heightened sensitivity to bitter stimuli, decreased sensitivity to sour stimuli, and no change in salt perception compared to normal mice. VIP and its receptors (VPAC1, VPAC2) are coexpressed in type II TCs (Fig. 1b and c). Even though VIP null mice had normal gross taste bud morphology, when compared to normal mice, they had significant increases in the number of cells positive for glucagon-like peptide-1 (GLP-1: its role in taste will be discussed later) and leptin receptor expression was decreased in TCs. The elevated sweet sensitivity of the VIP null mice may stem from the elevated levels of TC GLP-1 (Martin et al. 2010). In a previous study, the authors published that GLP-1’s ability to activate GLP-1 receptors in the tongue is strongly involved in the regulation of sweet taste sensitivity in mice (Shin et al. 2008). In addition, Martin et al. found that circulating levels of leptin in VIP null mice were considerably higher than those in normal mice. Therefore, despite the presence of high concentrations of this sweet taste suppressor, the reduction of TC leptin receptor expression was able to facilitate enhanced sweet sensation by preventing inhibitory leptin signaling activity in the tongue. Therefore, significant decrease in leptin receptor expression and elevated expression of GLP-1 may explain sweet hypersensitivity in VIP null mice.
2.2. Cholecystokinin
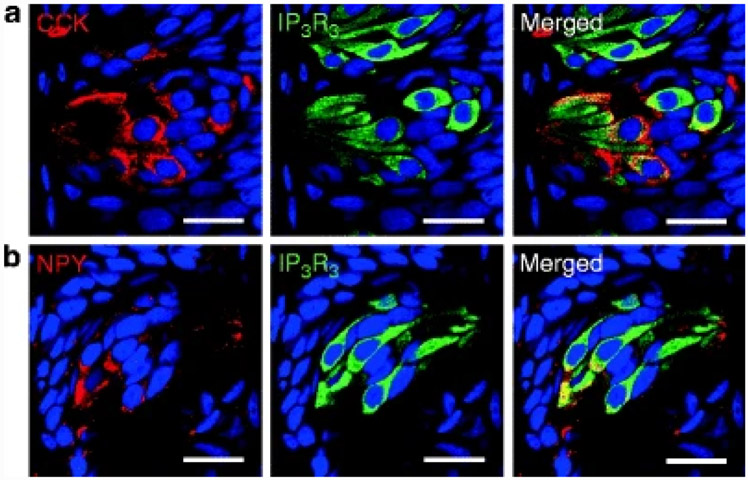
CCK is composed of varying numbers of amino acids depending on posttranslational modification of the CCK gene product, preprocholecystokinin. So, in reality, CCK is actually a family of hormones identified by the number of amino acids, e.g., CCK58, CCK33, and CCK8. It was first found to be expressed within the enteroendocrine I cells of the proximal small intestine and oral nutrient ingestion, particularly fat, was shown to induce its secretion. Stimulation of gall bladder contraction was the first function ascribed to it, but it plays a role in regulating many gastrointestinal (GI) functions, such as gastric motility and pancreatic enzyme secretion (Buffa et al. 1976; Moran and McHugh 1982). It is also a multifunctional peptide that serves as a neurotransmitter (Larsson and Rehfeld 1979) and it is present in the myenteric nerve plexus, central nervous system, pituitary corticotrophs, C cells of the thyroid, and adrenal medulla (Beinfeld et al. 1981). CCK exerts its actions through two members of the CCK-family of receptors. These receptors, named CCK-1 and CCK-2 (renamed from the original nomenclature of CCK-A and CCK-B respectively) are GPCRs that couple with the inositol trisphosphate (IP3) second messenger system (Foucaud et al. 2008; Rehfeld et al. 2007). Expression of CCK has been reported in the TCs of the rat, hamster, frog, and human (Herness et al. 2002; Kusakabe et al. 1998a, b; Fig. 2a). We have also confirmed CCK immunoreactivity in rat tongue where it completely overlaps with IP3R3 (Fig. 2a).
Fig. 2. Expression of CCK and NPY in rat circumvallate papillae (CV).
(a) CCK and IP3R3 are colocalized in a subset of IP3R3-positive cells in rats. (b) NPY and IP3R3 are colocalized in a subset of IP3R3-positive cells in rats. Scale bars, 20 μm. Blue is TO-PRO-3 nuclear stain
More that 50% of CCK-positive TCs also express α-gustducin, an essential component of the bitter pathway; however, very few CCK-containing TCs coexpress T1R2, a sweet taste receptor. Therefore, it was thought that CCK is likely involved in bitter transduction (Lu et al. 2003; Shen et al. 2005). Following this hypothesis, CCK-responsive TCs were characterized for sensitivity to two bitter stimuli, quinine or caffeine, using the calcium-sensitive dye fura-2 as a tool to study intracellular calcium. The investigators found that the same TCs that responded to CCK by increasing intracellular calcium, also responded to quinine and caffeine. The investigators were careful to show that quinine-induced elevations of intracellular calcium were not due to endogenous fluorescence of the quinine molecule. Additionally, when extracellular calcium was depleted, intracellular calcium rose in response to CCK stimulation. On the other hand, when thapsigargin, which depletes intracellular calcium stores, was added to the cells before CCK stimulation, there were minimal increases noted in intracellular calcium. These studies suggest that CCK operates through the known CCK-1 receptor in the DAG/IP3-dependent manner. Most interestingly, about 60–70% of the TCs that responded to CCK also responded to cholinergic stimulation (Lu et al. 2003). CCK was found to increase excitability of TCs by inhibiting outward- and inward-rectifying potassium currents, illustrating that CCK maintains the TCs in a depolarized state for a longer period of time (Herness et al. 2002). For practical purposes, this means that cells that presumably release neurotransmitter during an action potential should have enhanced signaling of the transmitter if CCK were also released from the same cell, which would be expected to accentuate bitter taste. CCK works in an autocrine fashion because there is complete overlap of CCK and CCK-1 receptor-expressing cells, meaning that it’s the same cells that release CCK that then has enhanced downstream signaling of the neurotransmitter (Herness et al. 2005). Other modalities besides sweet have not been tested to the same extent. On the basis of pharmacological tools, CCK-2 is not expressed on TCs (Herness et al. 2005).
2.3. Neuropeptide Y
NPY, a 36 amino acid peptide, is one of the most abundant neuropeptides in the central nervous system, where it is widely distributed. It is a member of the Y family of peptides, the other family members being pancreatic polypeptide and peptide YY. It is a potent orexigenic factor (Kalra and Kalra 2004). In addition, it plays a role in a very wide range of physiological processes, including anxiety, energy balance, feeding, vasoconstriction, immune function, reproduction, and heart disease (Gehlert 2004; McDermott and Bell 2007; Pedrazzini et al. 2003; Sperk et al. 2007; Wheway et al. 2007). NPY receptors are GPCRs and at least four Y subtypes have been cloned; Y1, Y2, Y4, and Y5 (Wraith et al. 2000). An additional receptor subtype, termed Y6, is found only in mice. NPY has high affinity for Y1, Y2, and Y5, and the NPY modulatory effect in TCs is mediated by the Y1 subtype (Zhao et al. 2005). Activation of the Y receptors causes inhibition of adenylyl cyclase, mobilization of intracellular calcium via IP3 production, activation of inward-rectifying potassium currents (the opposite to CCK-1 activation), and inhibition of potassium and calcium currents (Pedrazzini et al. 2003; Sperk et al. 2007; Sun et al. 1998; Sun and Miller 1999). In TCs, NPY is found in type II cells of the taste buds (Fig. 2b) where it completely overlaps with CCK- and VIP-expressing cells (Zhao et al. 2005), and, as expected, it completely overlaps with IP3R3 immunoreactivity (Fig. 2b). Thus, there are some TCs coexpressing CCK, NPY, and CCK-1 receptors. NPY, however, exerts inhibitory actions on single TCs that are opposite to those exerted by CCK (Zhao et al. 2005) and it acts in a paracrine fashion because, unlike CCK and its receptor, NPY and the Y1 receptor subtype are expressed in separate TCs. Using whole cell patch clamp analysis of inward-rectifying potassium channels, the magnitude of KIR currents were significantly enhanced by NPY. In the same patch clamp paradigm, voltage-dependent sodium channels and voltage-dependent outward potassium currents were not altered by NPY. Because KIR is involved in maintaining the resting membrane potential, enhancing the current would cause hyperpolarization, meaning that the cells would be less responsive to action potential-generating stimuli. BIBP3266, a Y1 antagonist, caused a highly significantly reduced response to NPY and the number of NPY-responsive TCs dropped dramatically, implying that Y1 is the receptor responsible for NPY effects in TCs. Y1 coexpresses with T1R3, a sweet receptor essential for sensing sweet taste. CCK and NPY, when they are released together with neurotransmitter from cells during ligand activation of bitter receptors, may work in concert to both upregulate the excitation of bitter-sensitive taste receptor expressing cells while concurrently inhibiting neighboring sweet-sensitive cells (For a more complete review of CCK and NPY in TCs, see Herness and Zhao 2009).
2.4. Proglucagon Fragments
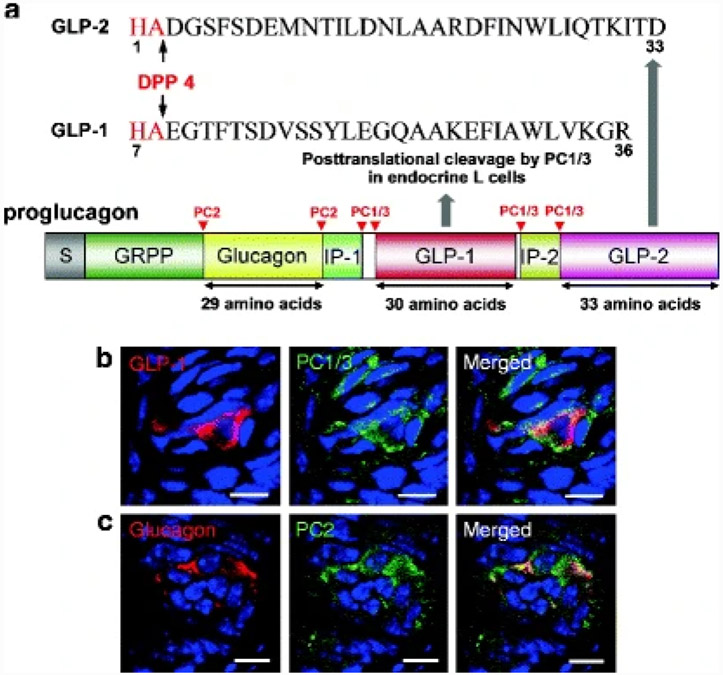
The proglucagon gene encodes glucagon and two glucagon-like peptides that have approximately 50% amino acid homology to glucagon; these are designated GLP-1 and glucagon-like peptide-2 (GLP-2). Glucagon, GLP-1 and GLP-2 are posttranslational cleavage products of proglucagon resulting from prohormone convertase PC2 and PC1/3 enzymes more usually in islet a cells and enteroendocrine L cells, respectively (Fig. 3a).
Fig. 3. Schematic representation of proglucagon, its cleavage sites and expression of prohormone convertases in mice circumvallate papillae (CV).
(a) GLP-1 and GLP-2 are posttranslational cleavage products of the proglucagon gene in enteroendocrine L cells and GLP-1 (7-36) amide is the major form of circulating biologically active GLP-1 in humans. GLP-1 and GLP-2 are rendered biologically inactive by the cleavage of their first two amino acids by dipeptidyl peptidase 4 (DPP4). Glucagon is produced primarily in pancreatic α cells. (b) GLP-1 and PC1/3 are colocalized in mice CV. (c) Glucagon and PC2 are colocalized in mice CV. Scale bars, 20 μm. Blue is TO-PRO-3 nuclear stain
Alpha cells are one of the five cells types in islet of Langerhans and enteroendocrine L cells are scattered among the enterocytes and many other enteroendocrine cells throughout the small bowel and ascending colon. However, GLP-1 and GLP-2 are also found in certain brain areas (Doyle and Egan 2007; Jang et al. 2007). In the fasting state, circulating glucagon levels gradually increase, especially in the portal blood circulation, the ultimate goal of glucagon being to protect the brain from neuroglucopenia. Activation of specific glucagon receptors, which are G-protein-coupled, on hepatocytes causes increased intracellular cAMP levels, leading to hepatic glucose production from both glycogenolysis and gluconeogenesis from gluconeogenic precursors (Williams textbook of Endocrinology).
In humans, GLP-1 exists in multiple forms. The majority (at least 80%) of circulating biologically active GLP-1 in humans is the COOH-terminally amidated form, GLP-1 (7-36) amide, with lesser amounts of the minor glycine extended form, GLP-1 (7-37), also detectable (Orskov et al. 1986). GLP-1 is one of two incretins, the other being glucose-dependent insulinotropic peptide (GIP). By definition, incretins are hormones that are produced in the gut and secreted into the blood stream in response to food: they enhance insulin secretion (insulinotropism) in a glucose-dependent manner (Creutzfeldt 1979). GLP-1’s insulinotropic effects are due to its activation of a specific G-protein-coupled receptor (GLP-1R) that is coupled to increases in intracellular cAMP and Ca2+ levels in β cells. It also inhibits gastric emptying, decreases food intake (Willms et al. 1996), inhibits glucagon secretion (Komatsu et al. 1989), and slows the rate of endogenous glucose production (Prigeon et al. 2003), all of which would lead to tight regulation of blood glucose. Additionally, it protects β cells from apoptosis (Farilla et al. 2002) and increases β-cell proliferation by upregulation of a specific β-cell transcription factor pancreatic duodenal homeobox-1 protein (PDX-1) (Perfetti and Merkel 2000; Stoffers et al. 2000), which augments insulin gene transcription leading to increases in insulin production, and upregulates glucose transporter2 (GLUT2) and glucokinase (Wang et al. 1999). Defective secretion of GLP-1 (or GIP) is not considered a cause of diabetes because its secretion after food intake is not decreased in newly diagnosed type II diabetes (T2DM). Continuous GLP-1 treatment in T2DM can normalize blood glucose, improve β-cell function, and restore first-phase insulin secretion and “glucose competence” to β cells (Holz et al. 1993; Zander et al. 2002); hence, GLP-1 analogs and GLP-1R agonists are now treatments for type II diabetes.
GLP-2 is cosecreted with GLP-1 from L cells of the gut. Like GLP-1, it transduces its effects through specific GPCRs. Pharmacological levels lead to increased gut mucosal growth, increased villi height, and increased glucose transport. Though not currently used as such, GLP-2 has been suggested as a possible treatment for short-bowel syndrome (Drucker 2005).
GLP-1 and GLP-2 are degraded within minutes of secretion by dipeptidyl peptidase (DPP4: Fig. 3a), an enzyme that is soluble in circulation and is also membrane-bound in many cells types, including endothelial cells of blood vessels and lymphocytes, where it is also known as CD26. This inactivates both peptides to the extent that their respective receptors are no longer activated by the loss of histadine and alanine on the N-terminus of the peptides.
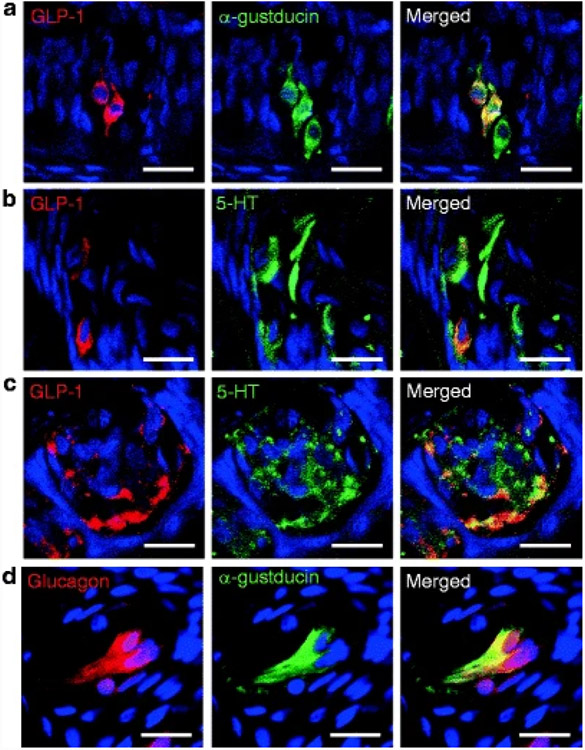
Recently, another role of GLP-1 has been added because functional GLP-1 that activates GLP-1 receptors was extracted from taste buds, where it is expressed in two distinct subsets of TCs that, as expected, also express PC1/3 (Shin et al. 2008; Feng et al. 2008; Fig. 3b). It is expressed in a subset of type II cells that coexpress T1R3 and α-gustducin (Fig. 4a), and a subset of type III cells, some of which contain 5-HT (Fig. 4b and c) and some of which contain PGP 9.5 (Shin et al. 2008).
Fig. 4. Expression of proglucagon fragments in taste buds in circumvallate papillae.
(a) GLP-1 and α-gustducin is colocalized in a subset of α-gustducin-positive cells in rats. (b) GLP-1 and 5-HT is colocalized in a subset of 5-HT-positive cells in rats. (c) GLP-1 and 5-HT is colocalized in a subset of 5-HT-positive cells in monkey. (d) Glucagon and α-gustducin is colocalized in a subset of α-gustducin-positive cells in monkey. Scale bars, 20 μm. Blue is TO-PRO-3 nuclear stain
Taste buds, like brain, are devoid of DPP 4, so the GLP-1 concentration produced in TCs should be high, giving sufficient concentration to activate GLP-1 receptors (Shin et al. 2008). Mice lacking GLP-1 receptors have significantly reduced sweet taste sensitivity to sucrose and sucralose, and they also have a significant hypersensitivity to umami taste (Martin et al. 2009). As regards bitter taste, GLP-1 receptor null mice are fairly similar to control mice (Shin et al. 2008). This work demonstrates that GLP-1 in TCs plays a role in modulating taste sensitivity through its own receptor, which is present on intragemmal nerve fibers and some PGP 9.5-positive TCs (Fig. 5a and b).
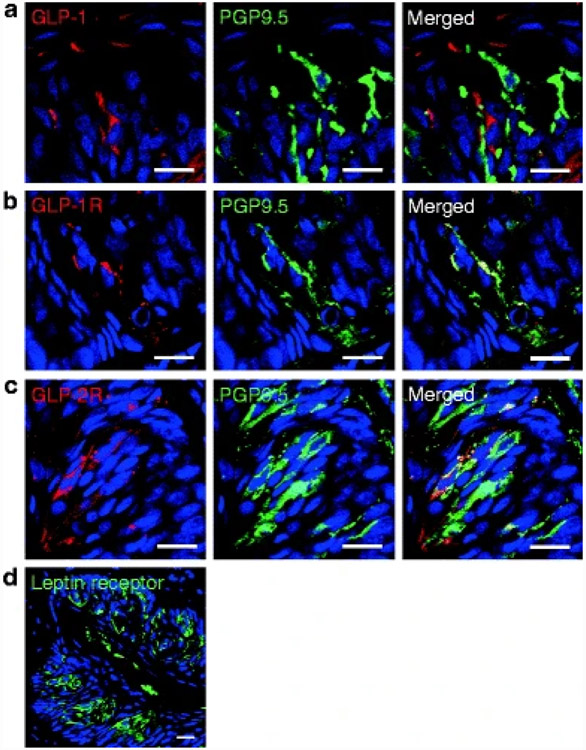
Fig. 5. Expression of GLP-1, GLP-1 and GLP-2 receptors, and leptin receptor in rat circumvallate papillae.
(a) GLP-1 is expressed in type II and III cells, some of which also stain for PGP 9.5 (see Fig. 3 for additional cell-specific markers). (b) GLP-1R and PGP 9.5 are colocalized on nerve fibers and cells. (c) GLP-2R and PGP 9.5 are colocalized on cells. (d) Leptin receptor is expressed in taste buds in mice. Scale bars, 20 μm. Blue is TO-PRO-3
Several studies have shown that many taste-related molecules are found in stomach, intestine, and pancreatic ducts. In addition, some enteroendocrine cells in small intestine (e.g., enteroendocrine L cells) secrete hormones in a T1R3- and α-gustducin-dependent manner in response of sugars (Jang et al. 2007). Our observation that a subset of TCs expressing T1R3 and α-gustducin as well as PLCβ2 and transient receptor potential M5; (Chandrashekar et al. 2006) also express and secrete GLP-1 suggests that they share other molecular mechanisms and/or physiological roles.
While glucagon immunoreactivity seems to be present in type II cells where it colocalizes with α-gustducin (along with PC2, Figs. 4d and 3c) and GLP-2 receptor (Fig. 5c), both also found in TCs, any function in taste that they may have has not yet been described in the literature.
2.5. Leptin
Leptin, 167 amino acid product of the obese (ob) gene, is a hormone primarily produced in adipocytes. In addition to white adipose tissue – the major source of leptin – it is also produced by brown adipose tissue, lymphocytes, placenta, ovaries, skeletal muscle, stomach (lower part of fundic glands), mammary epithelial cells, bone marrow, pituitary, and liver (Margetic et al. 2002). It plays a role in regulating food intake, energy expenditure, and adipose tissue mass. Its key target organ is the brain where its actions are mediated through leptin receptors in hypothalamic nuclei but leptin receptors are also present in other brain areas and some peripheral organs, including pancreatic islets (Mercer et al. 1996; Hoggard et al. 1997). Leptin acts by binding to a specific “obese receptor” (Ob-R). Several isoforms exist, which are generated as splice variants of one gene, and differ mainly in the length of the cytoplasmic domain (Takaya et al. 1996; Bjørbaek et al. 1998; Yamashita et al. 1998). The leptin receptor is encoded by the db gene (Lee et al. 1996). The db/db mouse has a point mutation of the db gene which leads to abnormal splicing of the coding region. The resulting absence of Ob-Rb, the longer form of Ob-Rs, causes leptin insensitivity, resulting in the obese, diabetic phenotype (Lee et al. 1996). Even before the discovery of leptin, studies of the genetics of taste sensitivity explored a possible role of the db gene on sweet taste responses (Sako et al. 1996; Ninomiya et al. 1995, 1998). It was found that diabetic db/db mice have greater gustatory neural sensitivities and higher behavioral preferences for various sweet substances than lean control mice. After the discovery of leptin and the finding of receptor defects in Ob-R in db/db mice, possible effect of leptin on taste responses were examined in detail. It was found that a subset of TCs is affected by peripheral leptin and that they express Ob-Rb (Fig. 5d), which indicates that TCs are an additional peripheral site of leptin action (Kawai et al. 2000). In situ hybridization (ISH) and mRNA analysis revealed that Ob-Rb mRNAs were expressed in both fungiform and circumvallate taste buds, although no such expression was evident in db/db mice.
Ob-Rb is reported to act via the STAT (signal transducers and activators of transcription) pathway of intracellular signal transduction, which is a class of transcription factors having seven members (STAT1, 2, 3, 4, 5a, 5b, and 6) (Frühbeck 2006). By in vivo and in vitro studies, leptin has been shown to activate JAK2, STAT1, STAT3, STAT5, and STAT6 (Rosenblum et al. 1996; Goíot et al. 2001), whereas in vivo studies of the mouse and rat hypothalamus, it specifically activated STAT3 without affecting other components (Vaisse et al. 1996; McCowen et al. 1998; Goíot et al. 2001). In taste papillae, mRNA of STAT members (1–6) was found and the amount of the STAT3 mRNA was higher than that of the other members by mRNA ISH analysis. But, it is still not proven conclusively that STAT3 is coexpressed with Ob-Rb in particular TCs.
Similar to leptin-mediated effects in pancreatic β cells (Harvey and Ashford 1998) and hypothalamic neurons (Spanswick et al. 1997), leptin has been shown to increase K+ conductance of TCs, which, like NPY, results in hyperpolarization and reduction of cell excitability. It has been shown that both nutritive and nonnutritive sweeteners can activate separate signaling transduction cascades, one that involves cAMP and the other that involves inositol triphosphate (IP3), in the same sweet-sensing cell in rat circumvallate taste buds (Bernhardt et al. 1996). A functional role for leptin has been established using lean and db/db mice in that leptin plays a role in modulating sweet taste perception. The obese db/db mice display enhanced neural responses and elevated behavioral preferences to sweet stimuli (Kawai et al. 2000). Administration of leptin to lean mice suppressed the responses of the peripheral taste nerves to sweet substances, but had no effect on the other taste modalities. Whole-cell patch-clamp recordings performed on TCs have shown that leptin can activate outward K+ currents, which results in a hyperpolarization of the TCs. The db/db mice showed no hyperpolarization or leptin suppression, which suggests that leptin acts as a negative modulator for sweet taste (Kawai et al. 2000; Shigemura et al. 2003, 2004). This suppression of sweet taste perception by leptin could subsequently play a role in determining food/calorie intake.
2.6. Galanin
Galanin, a neuropeptide with 29 amino acids (30 amino acids in humans), was originally isolated from porcine small intestine (Tatemoto et al. 1983). It is widely distributed in the central and peripheral nervous systems (Branchek et al. 2000; Waters and Krause 2000). Galanin is engaged in the regulation of processes such as food intake (Koegler and Ritter 1998), memory, neuroendocrine function (Mitchell et al. 1999), gut secretion, and motility (Wang et al. 1998a). Galanin mediates its effects through the activation of at least three G-protein-coupled receptor subtypes: galanin receptor (GalR)1, GalR2, GalR3 (Branchek et al. 1998, 2000; Iismaa and Shine 1999). These receptors show distinct distribution patterns and activate different second messenger pathways, depending on the cell type (Chen et al. 1992; Karelson et al. 1995; Wang et al. 1998b; Branchek et al. 2000).
Galanin expression has been observed in the sensory epithelia in the developing ear, eye, and nose (Xu et al. 1996). Later, the expression of galanin has been described for taste bud cells. RT-PCR and ISH experiments showed that mRNA of galanin and GalR2 are detected in rat TCs. Double-label studies uncovered that galanin is present in a subset of α-gustducin-, NCAM-, and PLCβ2-positive TCs, indicating that galanin-expressing TCs are type II and type III cells.
Several studies found that galanin in hypothalamic paraventricular nucleus (PVN) has a role in stimulating the consumption of ethanol and intake of a high-fat diet (Karatayev et al. 2009, 2010). Galanin-overexpressing mice using human dopamine β-hydroxylase promoter showed an increase in ethanol intake and ethanol preference, and no change in consumption of sucrose or quinine solutions in preference tests compared with control mice. In addition, GALOE mice consumed 55% more fat-rich diet during a 2-h test period (Karatayev et al. 2009). Then same group studied galanin knockout (GALKO) mice. The results revealed that GALKO mice had a decrease in ethanol intake and preference, decrease in acute intake of a fat-rich diet, no difference in consumption of sucrose or quinine solutions in preference tests, and a total loss of GAL mRNA in the PVN (Karatayev et al. 2010). These results provide strong support for a physiological role of galanin in stimulating the consumption of ethanol, as well as a fat-rich diet.
3. Hormones That Are Not Expressed in Taste Buds but Modify Taste Preference
3.1. Vasopressin
Vasopressin is nine amino acid peptide hormone. It is derived from a preprohormone precursor that is synthesized in the hypothalamus and transported to the posterior pituitary. One site of action is in the kidneys where it is necessary for the conservation of water by concentrating the urine and reducing urine volume, and it is based on this effect that it received its other name, antidiuretic hormone (ADH). It is also involved with regulation of cardiovascular processes because it is involved in pressure and osmolality regulation, which explains its second name of vasopressin and temperature regulation (Chase and Aurbach 1968; Cooper et al. 1979). Vasopressin exerts its effects through receptors that have been divided two broad classes, the V1 and V2 receptors. The receptors differ in their distribution and associated second messenger systems (Jard 1988). V1 receptors are located on blood vessels where they mediate vasopressin’s cardiovascular effects, and V2 are located on renal collecting tubules where they control water retention.
Brain release and pituitary release of vasopressin often parallel one another. For example, systemic osmotic and hypovolemic stimuli that increase circulating vasopressin levels also stimulate the release of vasopressin in the lateral septum and lateral ventricle (Demotes-Mainard et al. 1986). Manipulation that facilitates salt intake can also stimulate the systemic release of vasopressin. A prediction from vasopressin’s parallel effect is that central administration of vasopressin may actually stimulate salt intake and, indeed, intracerebroventricular (ICV) injections of vasopressin caused a dramatic decrease in NaCl intake in sodium-deficient rats and suppressed sucrose intake. Following that, ICV injection of the V1/V2 receptor antagonist and the V1 receptor antagonist significantly suppressed NaCl intake. But V1 receptor antagonist had no effect on sucrose intake. In contrast, the selective V2 receptor antagonist had no significant effect on NaCl intake. These findings suggest that endogenous vasopressin neurotransmission acting through V1 receptors plays on a role in the amount of salt that is ingested (Flynn et al. 2002).
3.2. Somatostatin
Somatostatin is a tetradecapeptide originally isolated from the hypothalamus and extensively distributed within both the peripheral and the central nerve system as well as in the pancreas, GI tract, lingual serous glands, and other sites (Johansson et al. 1984; McIntosh et al. 1978; Roberts et al. 1991). It is a classic inhibitory peptide because when it binds to its cognate receptor, it depresses spontaneous discharge rate, hyperpolarizes the postsynaptic membrane, and increases membrane conductance in electrically excitable cells (Bloom 1987; Renaud and Martin 1975). The biological effects of somatostatin are mediated by a family of G-protein-coupled receptors, which are expressed in a tissue-specific manner. Somatostatin receptors have the highest expression levels in jejunum and stomach (Yamada et al. 1993). Somatostatin plays an important role in the regulation of feeding behavior and suppression of good intake (Aponte et al. 1984; Lotter et al. 1981). The reduced feeding activity after somatostatin treatment might depend on the modification of taste. This is supported by reports that some patients treated with somatostatin complain of dysgeusia and lack of appetite (Scalera and Tarozzi 1998). Moreover, somatostatin is found at all levels in taste pathways i.e., the nucleus of the solitary tract, the parabrachial nuclear complex, the parvicellular ventral posterior medialis thalamus, hypothalamus, amygdala, and sensory cortex (Bakhit et al. 1984; De León et al. 1992; Mantyh and Hunt 1984; Moga and Gray 1985) as well as von Ebner’s glands of the tongue (Roberts et al. 1991) and saliva (Deville de Periere et al. 1988). Previous work attempted to uncover the function of somatostatin in taste perception. Scalera examined the effects of peripherally administrated somatostatin in protamine zinc to induce a relatively long-lasting effect over the period of fluid intake and taste preference testing in rats. In somatostatin-treated rats, intake of NaCl and sucrose solution decreased, the light/dark cycle of NaCl solution intake was modified, and intake of quinine-HCl and HCl solutions increased significantly (Scalera and Tarozzi 1998). Since somatostatin inhibits the release of growth hormone and the synthesis of protein, taste preference may depend on modification of taste bud morphology and/or size and, indeed, histologic examination showed that taste bud distribution on the tongue appeared to be altered after long-term treatment with somatostatin. Mice injected with somatostatin for 10 days had decreased taste bud density, decreased number of papillae, decreased size, and decreased numbers of taste buds in circumvallate papillae (Scalera and Tarozzi 1998; Scalera 2003). Somatostatin is also known to inhibit the transmission of neural traffic in the gustatory system. Thus, inhibition of the gustatory system might decrease the intake of pleasant solutions (NaCl and sucrose) but enhance that of unpleasant ones (quinine and acid). It seems unlikely that the small changes seen in taste bud morphology and topography are in any way causally or directly related to the effects of somatostatin on taste preferences. Thus, the differences in taste preferences observed after somatostatin treatments might reflect alterations in the central more than in the peripheral taste system. But, it must also be remembered that somatostatin is inhibitory to the release of just about every hormone in the body. The presence of somatostatin receptors on TCs has not yet been described but it is highly likely that somatostatin influences the release of all hormones within TCs and therefore the effect on taste perception as it relates directly to TC reactivity would be the sum of inhibition of all the hormones secreted from TCs.
3.3. Oxytocin
Like vasopressin, oxytocin in a nine amino acid peptide that is synthesized in hypothalamic neurons and transported down axons to the posterior pituitary, from where it is ultimately secreted into the bloodstream. It is also secreted within the brain and in a few other tissues, including the ovaries and testes. Oxytocin in the peripheral circulation promotes milk ejection and uterine contractility (Higuchi et al. 1985). CNS-derived oxytocin has been implicated in a variety of behaviors, including the promotion of maternal and social bonding (Insel 1990; Carter et al. 1992), the attenuation of stress and anxiety (Neumann et al. 2000; Windle et al. 1997; Mantella et al. 2003), and inhibition of ingestion (Olson et al. 1991) in rodents. The actions of oxytocin are mediated by specific, high-affinity oxytocin receptors that belong to the rhodopsin-type group of G-protein-coupled receptors, coupling specifically to Gq (Gimpl and Fahrenholz 2001). Oxytocin null mice consume significantly greater daily amounts of 10% sucrose or 0.2% saccharin solutions compared with control littermates when these sweet solutions are freely available (Amico et al. 2005; Billings et al. 2006). Even the mice exposed daily to platform shaker stress consumed more sweetened drinks. A progressive ratio operant licking procedure found that oxytocin null and control mice display a similar motivational drive to consume 10% sucrose solutions and a series of two-bottle intake tests found that oxytocin null mice consume significantly larger volumes of both sweet and non-sweet carbohydrate solutions over observed periods of time (i.e., sucrose, polycose, and cornstarch). The increased sucrose intake of oxytocin null mice seems to result from increased frequency of drinking bouts. The amount consumed during each bout did not differ between the genotypes. These findings indicate that the absence of oxytocin does not affect their appetitive drive to consume sucrose solutions, which would be palatable to the mice. Instead, satiety processes after carbohydrate intake are mediated through neural systems that likely recruit hypothalamic oxytocin neurons and the absence of oxytocin may selectively blunt satiety for carbohydrate-rich foods (Sclafani et al. 2007).
4. Hormones That Affect Conditioned Taste Aversion
Conditioned taste aversion (CTA) occurs when association of the taste of a certain food with symptoms caused by a toxic, spoiled, or poisonous substance occurs. Generally, taste aversion is caused after ingestion of the food causes nausea, sickness, or vomiting. The ability to develop a taste aversion is considered an adaptive or survival mechanism that trains the body to avoid or spit out poisonous substances (e.g., poisonous berries) before they can be absorbed and cause bodily harm. This association is meant to prevent the consumption of the same substance (or something that tastes similar) in the future, thus avoiding further poisoning. CTA was discovered when investigators realized that irradiated rats avoided solutions or food that had been present during radiation treatments (Garcia et al. 1955). When rats encountered a novel taste (the conditioning stimulus; CS) and this was followed by transient GI distress caused by low-dose radiation (the unconditioned stimulus; UCS), CTA developed. This response results in a diminished intake of CS upon subsequent presentation. Later studies found that CTA could develop following exposure to a variety of other illness-producing agents, including chemotherapeutic agents, high doses of apomorphine or amphetamine, and lithium chloride (Reilly and Bornovalova 2005). For CTA to develop, the animal must be able to detect the CS; it must be able to become ill from UCS exposure; it must be able to form an association between the UCS and CS; and, finally, it must be able to avoid the CS.
CTA is a relatively simple test to conduct, and it typically requires 2 days of combined training and testing. Mice are placed on food restriction prior to testing. Alternatively, water restriction can be used so that lithium chloride treatment can be paired with the consumption of a saccharin solution. Each animal receives the test substance paired with a flavored saccharin solution, followed by a control treatment (vehicle) paired with the same liquid with a different flavor. After one or more conditioning trials, the animals are then offered a choice between the two flavored liquids. If the animals consume relatively less of the flavored liquid paired with the anorexigenic substance, then it is assumed that the substance produces some degree of malaise.
4.1. Peptide YY3-36
Peptide YY3-36 (PYY3-36) is a polypeptide consisting of 36 amino acids and, as described above, is a member of the Y family of peptides. There are two major forms of PYY: PYY1-36 and PYY3-36. However, the most common form of circulating PYY is PYY3-36, which is a ligand for Y2 receptors (Murphy and Bloom 2006). PYY is found in enteroendocrine L cells (the same L cells that synthesize proglucagon from where it is cosecreted with GLP-1 and GLP-2) that are especially abundant in ileum and colon. It is also found in a discrete population of neurons in the brainstem, specifically localized to the gigantocellular reticular nucleus of the medulla oblongata. Like GLP-1 and GLP-2, PYY concentration in the circulation increases after food ingestion and falls during fasting (Murphy et al. 2006). It is reported to slow gastric motility and increase water and electrolyte absorption in the colon (Liu et al. 1996). It may also suppress exocrine pancreatic secretions. Several studies have shown that acute peripheral administration of PYY3-36 inhibits feeding of rodents and primates but Y2 receptor null mice have normal food intake. The anorexia produced by PYY3-36 administration could be due to taste aversion. It is observed that c-Fos immunoreactivity was significantly activated in intermediate nuclei of the solitary tract and area postrema – nuclei known to mediate the response to aversive stimuli – in a dose-dependent manner after peripheral administration of PYY3-36. This shows that PYY3-36 administration produces c-Fos activation in the same brainstem nuclei as lithium chloride. Thus, like lithium chloride, PYY3-36 may reduce food intake by causing a CTA response. In addition, previous experiment shows that 2-h intravenous infusion of PYY3-36 using higher doses in both non-food-deprived and food-deprived rats produced a dose-dependent inhibited intake of saccharin solution and a CTA (Chelikani et al. 2006). These results suggest that anorexic doses of PYY3-36 may produce a dose-dependent malaise in rats (Batterham et al. 2002; Chelikani et al. 2006), which is similar to that reported for PYY3-36 infusion in humans (Batterham et al. 2003; Le Roux et al. 2006). PYY3-36 potently inhibits gastric emptying in rats at doses that reduce their food intake (Chelikani et al. 2004), and distention of the gut can produce CTA in rats (Gyetvai and Bárdos 1999; Bárdos 2001). Thus, adverse effects caused by high dose of PYY3-36 during eating may be due in part to gastric distention caused by PYY3-36-induced slowing of gastric emptying.
5. Summary
The study of hormones produced in TCs is now in its infancy. More and more hormones are being uncovered that are produced locally in TCs. Eventually, we should have a taste bud map showing all the hormones, their cellular distribution, and their receptors. While transgenic mice null for certain hormones and their receptors are now available, none are specifically null in just taste buds and uncovering more conclusively the function of the hormones produced locally, than is now possible, will take more time. It is becoming clear, however, that the so-called “gut” hormones involved in food intake and satiety form a chain of cells extending right from colon, small bowel, stomach, taste buds, through to multiple brain areas. Almost certainly, they form a network of signaling molecules that modulates food intake, food perception, quantity and quality of food ingested, feelings of satiety, and metabolic rates.
Acknowledgements
Our research is funded by the Intramural Program of the NIA/NIH.
References
- Ahrén B, Alumets J, Ericsson M, Fahrenkrug J, Fahrenkrug L, Håkanson R, Hedner P, Lorén I, Melander A, Rerup C, Sundler F (1980) VIP occurs in intrathyroidal nerves and stimulates thyroid hormone secretion. Nature 287:343–345 [DOI] [PubMed] [Google Scholar]
- Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L (2005) Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol 289:R1798–R1806 [DOI] [PubMed] [Google Scholar]
- Aponte G, Leung P, Gross D, Yamada T (1984) Effects of somatostatin on food intake in rats. Life Sci 35:741–746 [DOI] [PubMed] [Google Scholar]
- Bakhit C, Koda L, Benoit R, Morrison JH, Bloom FE (1984) Evidence for selective release of somatostatin-14 and somatostatin-28(1-12) from rat hypothalamus. J Neurosci 4:411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárdos G (2001) Conditioned taste aversion to gut distension in rats. Physiol Behav 74:407–413 [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR (2002) Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418:650–654 [DOI] [PubMed] [Google Scholar]
- Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, Patterson M, Frost GS, Ghatei MA, Bloom SR (2003) Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab 88:3989–3992 [DOI] [PubMed] [Google Scholar]
- Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ (1981) The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res 212:51–57 [DOI] [PubMed] [Google Scholar]
- Bernhardt SJ, Naim M, Zehavi U, Lindemann B (1996) Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol 490:325–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings LB, Spero JA, Vollmer RR, Amico JA (2006) Oxytocin null mice ingest enhanced amounts of sweet solutions during light and dark cycles and during repeated shaker stress. Behav Brain Res 171:134–141 [DOI] [PubMed] [Google Scholar]
- Bjørbaek C, Elmquist JK, Michl P, Ahima RS, van Bueren A, McCall AL, Flier JS (1998) Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology 139:3485–3491 [DOI] [PubMed] [Google Scholar]
- Bloom FE (1987) Molecular diversity and cellular functions of neuropeptides. Prog Brain Res 72:213–220 [DOI] [PubMed] [Google Scholar]
- Branchek T, Smith KE, Walker MW (1998) Molecular biology and pharmacology of galanin receptors. Ann N Y Acad Sci 863:94–107 [DOI] [PubMed] [Google Scholar]
- Branchek TA, Smith KE, Gerald C, Walker MW (2000) Galanin receptor subtypes. Trends Pharmacol Sci 21:109–117 [DOI] [PubMed] [Google Scholar]
- Buffa R, Solcia E, Go VL (1976) Immunohistochemical identification of the cholecystokinin cell in the intestinal mucosa. Gastroenterology 70:528–532 [PubMed] [Google Scholar]
- Carter CS, Williams JR, Witt DM, Insel TR (1992) Oxytocin and social bonding. Ann N Y Acad Sci 652:204–211 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS (2006) The receptors and cells for mammalian taste. Nature 444:288–294 [DOI] [PubMed] [Google Scholar]
- Chase LR, Aurbach GD (1968) Renal adenyl cyclase: anatomically separate sites for parathyroid hormone and vasopressin. Science 159:545–547 [DOI] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD (2004) Comparison of the inhibitory effects of PYY (3-36) and PYY(1-36) on gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 287:R1064–R1070 [DOI] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD (2006) Dose-dependent effects of peptide YY(3-36) on conditioned taste aversion in rats. Peptides 27:3193–3201 [DOI] [PubMed] [Google Scholar]
- Chen Y, Couvineau A, Laburthe M, Amiranoff B (1992) Solubilization and molecular characterization of active galanin receptors from rat brain. Biochemistry 31:2415–2422 [DOI] [PubMed] [Google Scholar]
- Cooper KE, Kasting NW, Lederis K, Veale WL (1979) Evidence supporting a role for endogenous vasopressin in natural suppression of fever in the sheep. J Physiol 295:33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt W (1979) The incretin concept today. Diabetologia 16:75–85 [DOI] [PubMed] [Google Scholar]
- De León M, Coveñas R, Narvaéz JA, Tramu G, Aguirre JA, González-Barón S (1992) Distribution of somatostatin-28 (1-12) in the cat brainstem: an immunocytochemical study. Neuropeptides 21:1–11 [DOI] [PubMed] [Google Scholar]
- Demotes-Mainard J, Chauveau J, Rodriguez F, Vincent JD, Poulain DA (1986) Septal release of vasopressin in response to osmotic, hypovolemic and electrical stimulation in rats. Brain Res 381:314–321 [DOI] [PubMed] [Google Scholar]
- Deville de Periere D, Buys-Hillaire D, Favre de Thierrens C, Puech R, Elkaim G, Arancibia S (1988) Somatostatin-immunoreactive concentrations in human saliva and in the submandibular salivary glands of the rat. Possible sexual dependence in the human. J Biol Buccale 16:191–196 [PubMed] [Google Scholar]
- Dickson L, Finlayson K (2009) VPAC and PAC receptors: from ligands to function. Pharmacol Ther 121:294–316 [DOI] [PubMed] [Google Scholar]
- Doyle ME, Egan JM (2007) Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 113:546–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ (2005) Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat Clin Pract Endocrinol Metab 1:22–31 [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Schaffalitzky de Muckadell OB, Holst JJ, Jensen SL (1979) Vasoactive intestinal polypeptide in vagally mediated pancreatic secretion of fluid and HCO3. Am J Physiol 237:E535–E540 [DOI] [PubMed] [Google Scholar]
- Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R (2002) Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 143:4397–4408 [DOI] [PubMed] [Google Scholar]
- Feng XH, Liu XM, Zhou LH, Wang J, Liu GD (2008) Expression of glucagon-like peptide-1 in the taste buds of rat circumvallate papillae. Acta Histochem 110:151–154 [DOI] [PubMed] [Google Scholar]
- Flynn FW, Kirchner TR, Clinton ME (2002) Brain vasopressin and sodium appetite. Am J Physiol Regul Integr Comp Physiol 282:R1236–R1244 [DOI] [PubMed] [Google Scholar]
- Foucaud M, Archer-Lahlou E, Marco E, Tikhonova IG, Maigret B, Escrieut C, Langer I, Fourmy D (2008) Insights into the binding and activation sites of the receptors for cholecystokinin and gastrin. Regul Pept 145:17–23 [DOI] [PubMed] [Google Scholar]
- Frühbeck G (2006) Intracellular signalling pathways activated by leptin. Biochem J 393:7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA (1955) Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science 122:157–158 [PubMed] [Google Scholar]
- Gehlert DR (2004) Introduction to the reviews on neuropeptide Y. Neuropeptides 38:135–140 [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683 [DOI] [PubMed] [Google Scholar]
- Goíot H, Attoub S, Kermorgant S, Laigneau JP, Lardeux B, Lehy T, Lewin MJ, Bado A (2001) Antral mucosa expresses functional leptin receptors coupled to STAT-3 signaling, which is involved in the control of gastric secretions in the rat. Gastroenterology 121:1417–1427 [DOI] [PubMed] [Google Scholar]
- Gyetvai B, Bárdos G (1999) Modulation of taste reactivity by intestinal distension in rats. Physiol Behav 66:529–535 [DOI] [PubMed] [Google Scholar]
- Harvey J, Ashford ML (1998) Role of tyrosine phosphorylation in leptin activation of ATP-sensitive K + channels in the rat insulinoma cell line CRI-G1. J Physiol 510:47–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herness MS (1989) Vasoactive intestinal peptide-like immunoreactivity in rodent taste cells. Neuroscience 33(2):411–4119 [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL (2009) The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol Behav 97:581–591 [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Kaya N, Shen T, Lu SG, Cao Y (1995) Distribution of vasoactive intestinal peptide-like immunoreactivity in the taste organs of teleost fish and frog. Histochem J 27:161–165 [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Lu SG, Kaya N, Shen T (2002) Expression and physiological actions of cholecystokinin in rat taste receptor cells. J Neurosci 22:10018–10029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Kaya N, Shen T, Lu SG, Cao Y (2005) Communication routes within the taste bud by neurotransmitters and neuropeptides. Chem Senses 30(Suppl 1):i37–i38 [DOI] [PubMed] [Google Scholar]
- Higuchi T, Uchide K, Honda K, Negoro H (1985) Functional development of the oxytocin release mechanism and its role in the initiation of parturition in the rat. J Endocrinol 106:311–316 [DOI] [PubMed] [Google Scholar]
- Hoggard N, Mercer JG, Rayner DV, Moar K, Trayhurn P, Williams LM (1997) Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochem Biophys Res Commun 232:383–387 [DOI] [PubMed] [Google Scholar]
- Holz GG 4th, Kühtreiber WM, Habener JF (1993) Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37). Nature 361:362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iismaa TP, Shine J (1999) Galanin and galanin receptors. Results Probl Cell Differ 26:257–291 [DOI] [PubMed] [Google Scholar]
- Insel TR (1990) Regional changes in brain oxytocin receptors post-partum: time-course and relationship to maternal behaviour. J Neuroendocrinol 2:539–545 [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104:15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jard S (1988) Mechanisms of action of vasopressin and vasopressin antagonists. Kidney Int Suppl 26:S38–S42 [PubMed] [Google Scholar]
- Johansson O, Hökfelt T, Elde RP (1984) Immunohistochemical distribution of somatostatin-like immunoreactivity in the central nervous system of the adult rat. Neuroscience 13:265–339 [DOI] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS (2004) NPY – an endearing journey in search of a neurochemical on/off switch for appetite, sex and reproduction. Peptides 25:465–471 [DOI] [PubMed] [Google Scholar]
- Karatayev O, Baylan J, Leibowitz SF (2009) Increased intake of ethanol and dietary fat in galanin overexpressing mice. Alcohol 43:571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatayev O, Baylan J, Weed V, Chang S, Wynick D, Leibowitz SF (2010) Galanin knockout mice show disturbances in ethanol consumption and expression of hypothalamic peptides that stimulate ethanol intake. Alcohol Clin Exp Res 34:72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelson E, Laasik J, Sillard R (1995) Regulation of adenylate cyclase by galanin, neuropeptide Y, secretin and vasoactive intestinal polypeptide in rat frontal cortex, hippocampus and hypothalamus. Neuropeptides 28:21–28 [DOI] [PubMed] [Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y (2000) Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci USA 97:11044–11049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegler FH, Ritter S (1998) Galanin injection into the nucleus of the solitary tract stimulates feeding in rats with lesions of the paraventricular nucleus of the hypothalamus. Physiol Behav 63:521–527 [DOI] [PubMed] [Google Scholar]
- Komatsu R, Matsuyama T, Namba M, Watanabe N, Itoh H, Kono N, Tarui S (1989) Glucagonostatic and insulinotropic action of glucagon like peptide I-(7-36)-amide. Diabetes 38:902–905 [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Matsuda H, Gono Y, Kawakami T, Kurihara K, Tsukuda M, Takenaka T (1998a) Distribution of VIP receptors in the human submandibular gland: an immunohistochemical study. Histol Histopathol 13:373–378 [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Matsuda H, Gono Y, Furukawa M, Hiruma H, Kawakami T, Tsukuda M, Takenaka T (1998b) Immunohistochemical localisation of regulatory neuropeptides in human circumvallate papillae. J Anat 192:557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson LI, Rehfeld JF (1979) Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res 165:201–218 [DOI] [PubMed] [Google Scholar]
- le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR (2006) Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147:3–8 [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379:632–635 [DOI] [PubMed] [Google Scholar]
- Liu CD, Hines OJ, Newton TR, Adrian TE, Zinner MJ, Ashley SW, McFadden DW (1996) Cholecystokinin mediation of colonic absorption via peptide YY: foregut-hindgut axis. World J Surg 20:221–227 [DOI] [PubMed] [Google Scholar]
- Lorén I, Emson PC, Fahrenkrug J, Björklund A, Alumets J, Håkanson R, Sundler F (1979) Distribution of vasoactive intestinal polypeptide in the rat and mouse brain. Neuroscience 4:1953–1976 [DOI] [PubMed] [Google Scholar]
- Lotter EC, Krinsky R, McKay JM, Treneer CM, Porter D Jr, Woods SC (1981) Somatostatin decreases food intake of rats and baboons. J Comp Physiol Psychol 95:278–287 [DOI] [PubMed] [Google Scholar]
- Lu SG, Zhao FL, Herness S (2003) Physiological phenotyping of cholecystokinin-responsive rat taste receptor cells. Neurosci Lett 351:157–160 [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Anggård A, Fahrenkrug J, Hökfelt T, Mutt V (1980) Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: functional significance of coexisting transmitters for vasodilation and secretion. Proc Natl Acad Sci USA 77:1651–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Li X, Amico JA (2003) Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology 144:2291–2296 [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Hunt SP (1984) Neuropeptides are present in projection neurons at all levels in visceral and taste pathways: from periphery to sensory cortex. Brain Res 299:297–312 [DOI] [PubMed] [Google Scholar]
- Margetic S, Gazzola C, Pegg GG, Hill RA (2002) Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord 26:1407–1433 [DOI] [PubMed] [Google Scholar]
- Martin B, Lopez de Maturana R, Brenneman R, Walent T, Mattson MP, Maudsley S (2005) Class II G-protein-coupled receptors and their ligands in neuronal function and protection. Neuromolecular Med 7:3–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Dotson CD, Shin YK, Ji S, Drucker DJ, Maudsley S, Munger SD (2009) Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann N Y Acad Sci 1170:98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Shin YK, White CM, Ji S, Kim W, Carlson OD, Napora JK, Chadwick W, Chapter M, Waschek JA, Mattson MP, Maudsley S, Egan JM (2010) Vasoactive intestinal peptide null mice demonstrate enhanced sweet taste preference, dysglycemia and reduced taste bud leptin receptor expression. Diabetes 59:1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowen KC, Chow JC, Smith RJ (1998) Leptin signaling in the hypothalamus of normal rats in vivo. Endocrinology 139:4442–4447 [DOI] [PubMed] [Google Scholar]
- McDermott BJ, Bell D (2007) NPY and cardiac diseases. Curr Top Med Chem 7:1692–1703 [DOI] [PubMed] [Google Scholar]
- McIntosh C, Arnold R, Bothe E, Becker H, Kobberling J, Creutzfeldt W (1978) Gastrointestinal somatostatin in man and dog. Metabolism 27:1317–1320 [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PJ, Trayhurn P (1996) Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J Neuroendocrinol 8:733–735 [DOI] [PubMed] [Google Scholar]
- Mitchell V, Bouret S, Prévot V, Jennes L, Beauvillain JC (1999) Evidence for expression of galanin receptor Gal-R1 mRNA in certain gonadotropin releasing hormone neurons of the rostral preoptic area. J Neuroendocrinol 11:805–812 [DOI] [PubMed] [Google Scholar]
- Moga MM, Gray TS (1985) Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J Comp Neurol 241:275–284 [DOI] [PubMed] [Google Scholar]
- Moran TH, McHugh PR (1982) Cholecystokinin suppresses food intake by inhibiting gastric emptying. Am J Physiol 242:R491–R497 [DOI] [PubMed] [Google Scholar]
- Murphy KG, Bloom SR (2006) Gut hormones and the regulation of energy homeostasis. Nature 444:854–859 [DOI] [PubMed] [Google Scholar]
- Murphy KG, Dhillo WS, Bloom SR (2006) Gut peptides in the regulation of food intake and energy homeostasis. Endocr Rev 27:719–727 [DOI] [PubMed] [Google Scholar]
- Neumann ID, Krömer SA, Toschi N, Ebner K (2000) Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept 96:31–38 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Sako N, Imai Y (1995) Enhanced gustatory neural responses to sugars in the diabetic db/db mouse. Am J Physiol 269:R930–R937 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Imoto T, Yatabe A, Kawamura S, Nakashima K, Katsukawa H (1998) Enhanced responses of the chorda tympani nerve to nonsugar sweeteners in the diabetic db/db mouse. Am J Physiol 274:R1324–R1330 [DOI] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG (1991) Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12:113–118 [DOI] [PubMed] [Google Scholar]
- Orskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV (1986) Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 119:1467–1475 [DOI] [PubMed] [Google Scholar]
- Pedrazzini T, Pralong F, Grouzmann E (2003) Neuropeptide Y: the universal soldier. Cell Mol Life Sci 60:350–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti R, Merkel P (2000) Glucagon-like peptide-1: a major regulator of pancreatic beta-cell function. Eur J Endocrinol 143:717–725 [DOI] [PubMed] [Google Scholar]
- Prigeon RL, Quddusi S, Paty B, D’Alessio DA (2003) Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 285:E701–E707 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF, Friis-Hansen L, Goetze JP, Hansen TV (2007) The biology of cholecystokinin and gastrin peptides. Curr Top Med Chem 7:1154–1165 [DOI] [PubMed] [Google Scholar]
- Reilly S, Bornovalova MA (2005) Conditioned taste aversion and amygdala lesions in the rat: a critical review. Neurosci Biobehav Rev 29:1067–1088 [DOI] [PubMed] [Google Scholar]
- Renaud LP, Martin JB (1975) Electrophysiological studies of connections of hypothalamic ventromedial nucleus neurons in the rat: evidence for a role in neuroendocrine regulation. Brain Res 93:145–151 [DOI] [PubMed] [Google Scholar]
- Roberts IM, Solomon SE, Brusco OA, Goldberg W, Bernstein JJ (1991) Neuromodulators of the lingual von Ebner gland: an immunocytochemical study. Histochemistry 96:153–156 [DOI] [PubMed] [Google Scholar]
- Rosenblum CI, Tota M, Cully D, Smith T, Collum R, Qureshi S, Hess JF, Phillips MS, Hey PJ, Vongs A, Fong TM, Xu L, Chen HY, Smith RG, Schindler C, Van der Ploeg LH (1996) Functional STAT 1 and 3 signaling by the leptin receptor (OB-R); reduced expression of the rat fatty leptin receptor in transfected cells. Endocrinology 137:5178–5181 [DOI] [PubMed] [Google Scholar]
- Said SI, Mutt V (1970) Potent peripheral and splanchnic vasodilator peptide from normal gut. Nature 225:863–864 [DOI] [PubMed] [Google Scholar]
- Sako N, Ninomiya Y, Fukami Y (1996) Analysis of concentration-response relationship for enhanced sugar responses of the chorda tympani nerve in the diabetic db/db mouse. Chem Senses 21:59–63 [DOI] [PubMed] [Google Scholar]
- Scalera G (2003) Peptides that regulate food intake: somatostatin alters intake of amino acid-imbalanced diets and taste buds of tongue in rats. Am J Physiol Regul Integr Comp Physiol 284:R1389–R1398 [DOI] [PubMed] [Google Scholar]
- Scalera G, Tarozzi G (1998) Somatostatin administration alters taste preferences in the rat. Peptides 19:1565–1572 [DOI] [PubMed] [Google Scholar]
- Sclafani A, Rinaman L, Vollmer RR, Amico JA (2007) Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol 292:R1828–R1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Kaya N, Zhao FL, Lu SG, Cao Y, Herness S (2005) Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells. Neuroscience 130:229–238 [DOI] [PubMed] [Google Scholar]
- Shigemura N, Miura H, Kusakabe Y, Hino A, Ninomiya Y (2003) Expression of leptin receptor (Ob-R) isoforms and signal transducers and activators of transcription (STATs) mRNAs in the mouse taste buds. Arch Histol Cytol 66:253–260 [DOI] [PubMed] [Google Scholar]
- Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y (2004) Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology 145:839–847 [DOI] [PubMed] [Google Scholar]
- Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, Munger SD (2008) Modulation of taste sensitivity by GLP-1 signaling. J Neurochem 106:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML (1997) Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390:521–525 [DOI] [PubMed] [Google Scholar]
- Sperk G, Hamilton T, Colmers WF (2007) Neuropeptide Y in the dentate gyrus. Prog Brain Res 163:285–297 [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM (2000) Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 49:741–748 [DOI] [PubMed] [Google Scholar]
- Sun L, Miller RJ (1999) Multiple neuropeptide Y receptors regulate K + and Ca2+ channels in acutely isolated neurons from the rat arcuate nucleus. J Neurophysiol 81:1391–1403 [DOI] [PubMed] [Google Scholar]
- Sun L, Philipson LH, Miller RJ (1998) Regulation of K + and Ca++ channels by a family of neuropeptide Y receptors. J Pharmacol Exp Ther 284:625–632 [PubMed] [Google Scholar]
- Takaya K, Ogawa Y, Isse N, Okazaki T, Satoh N, Masuzaki H, Mori K, Tamura N, Hosoda K, Nakao K (1996) Molecular cloning of rat leptin receptor isoform complementary DNAs – identification of a missense mutation in Zucker fatty (fa/fa) rats. Biochem Biophys Res Commun 225:75–83 [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Rökaeus A, Jörnvall H, McDonald TJ, Mutt V (1983) Galanin – a novel biologically active peptide from porcine intestine. FEBS Lett 164:124–128 [DOI] [PubMed] [Google Scholar]
- Vaisse C, Halaas JL, Horvath CM, Darnell JE Jr, Stoffel M, Friedman JM (1996) Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14:95–97 [DOI] [PubMed] [Google Scholar]
- Wang S, Ghibaudi L, Hashemi T, He C, Strader C, Bayne M, Davis H, Hwa JJ (1998a) The GalR2 galanin receptor mediates galanin-induced jejunal contraction, but not feeding behavior, in the rat: differentiation of central and peripheral effects of receptor subtype activation. FEBS Lett 434:277–282 [DOI] [PubMed] [Google Scholar]
- Wang S, Hashemi T, Fried S, Clemmons AL, Hawes BE (1998b) Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry 37:6711–6717 [DOI] [PubMed] [Google Scholar]
- Wang X, Cahill CM, Piñeyro MA, Zhou J, Doyle ME, Egan JM (1999) Glucagon-like peptide-1 regulates the beta cell transcription factor, PDX-1, in insulinoma cells. Endocrinology 140:4904–4907 [DOI] [PubMed] [Google Scholar]
- Waters SM, Krause JE (2000) Distribution of galanin-1, -2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience 95:265–271 [DOI] [PubMed] [Google Scholar]
- Wheway J, Herzog H, Mackay F (2007) NPY and receptors in immune and inflammatory diseases. Curr Top Med Chem 7:1743–1752 [DOI] [PubMed] [Google Scholar]
- Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA (1996) Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab 81:327–332 [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD (1997) Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 138: 2829–2834 [DOI] [PubMed] [Google Scholar]
- Witt M (1995) Distribution of vasoactive intestinal peptide-like immunoreactivity in the taste organs of teleost fish and frog. Histochem J 27(2):161–165 [DOI] [PubMed] [Google Scholar]
- Wraith A, Törnsten A, Chardon P, Harbitz I, Chowdhary BP, Andersson L, Lundin LG, Larhammar D (2000) Evolution of the neuropeptide Y receptor family: gene and chromosome duplications deduced from the cloning and mapping of the five receptor subtype genes in pig. Genome Res 10(3):302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZQ, Shi TJ, Hökfelt T (1996) Expression of galanin and a galanin receptor in several sensory systems and bone anlage of rat embryos. Proc Natl Acad Sci USA 93:14901–14905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Kagimoto S, Kubota A, Yasuda K, Masuda K, Someya Y, Ihara Y, Li Q, Imura H, Seino S (1993) Cloning, functional expression and pharmacological characterization of a fourth (hSSTR4) and a fifth (hSSTR5) human somatostatin receptor subtype. Biochem Biophys Res Commun 195:844–852 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Murakami T, Otani S, Kuwajima M, Shima K (1998) Leptin receptor signal transduction: OBRa and OBRb of fa type. Biochem Biophys Res Commun 246:752–759 [DOI] [PubMed] [Google Scholar]
- Zander M, Madsbad S, Madsen JL, Holst JJ (2002) Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 359:824–830 [DOI] [PubMed] [Google Scholar]
- Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S (2005) Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci USA 102:11100–11105 [DOI] [PMC free article] [PubMed] [Google Scholar]