Abstract
In mammalian cells, several features of the way homologous recombination occurs between transferred and chromosomal DNA are consistent with the double-strand-break repair (DSBR) model of recombination. In this study, we examined the segregation patterns of small palindrome markers, which frequently escape mismatch repair when encompassed within heteroduplex DNA formed in vivo during mammalian homologous recombination, to test predictions of the DSBR model, in particular as they relate to the mechanism of crossover resolution. According to the canonical DSBR model, crossover between the vector and chromosome results from cleavage of the joint molecule in two alternate sense modes. The two crossover modes lead to different predicted marker configurations in the recombinants, and assuming no bias in the mode of Holliday junction cleavage, the two types of recombinants are expected in equal frequency. However, we propose a revision to the canonical model, as our results suggest that the mode of crossover resolution is biased in favor of cutting the DNA strands upon which DNA synthesis is occurring during formation of the joint molecule. The bias in junction resolution permitted us to examine the potential consequences of mismatch repair acting on the DNA breaks generated by junction cutting. The combination of biased junction resolution with both early and late rounds of mismatch repair can explain the marker patterns in the recombinants.
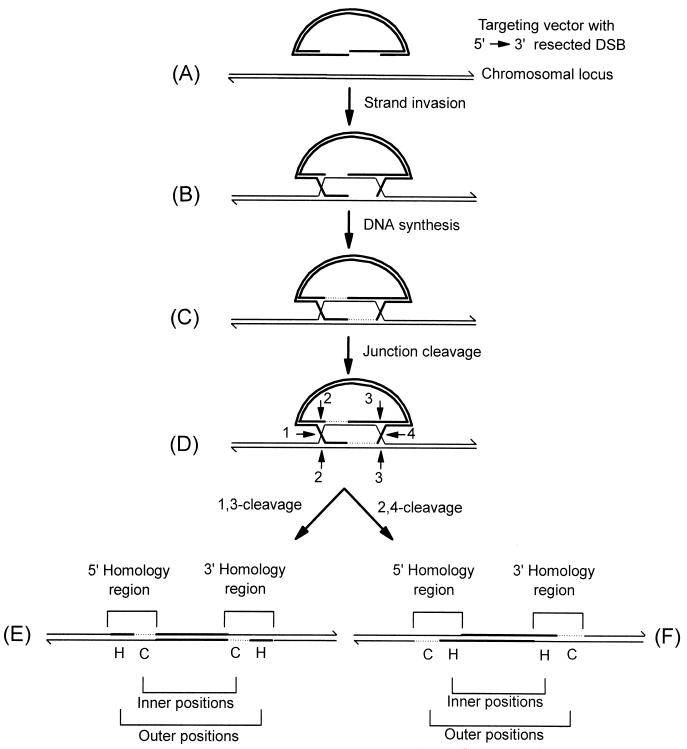
In the yeast Saccharomyces cerevisiae, transferred DNA undergoes homologous recombination with cognate chromosomal sequences (gene targeting) according to the double-strand-break repair (DSBR) model of recombination (34, 37, 44). Features of meiotic recombination in S. cerevisiae are also consistent with repair of chromosomal double-strand breaks (DSBs) by this model (15, 33, 35, 39, 40, 44). According to the canonical DSBR model (34, 37, 44) and its later revision (42), as illustrated in Fig. 1 for a typical gene targeting reaction, recombination is initiated by a DSB in the vector-borne region of homology to the chromosome. The DSB undergoes 5′→3′ resection (Fig. 1A) resulting in the formation of two 3′-ending single strands which invade cognate chromosomal sequences (Fig. 1B). The invading 3′ ends prime DNA synthesis, finally generating two Holliday junctions (Fig. 1C). Opposite-sense cleavage of the Holliday junctions in the joint molecule (Fig. 1D) results in crossover, integrating the vector into the chromosome and duplicating the region of shared homology (Fig. 1E and F).
FIG. 1.
DSBR model of recombination. The mechanism of recombination between a linearized DNA transfer vector and the homologous chromosomal locus is depicted. The targeting vector (A) is indicated by thick lines while the homologous chromosomal locus is indicated by thin lines. The 3′ ends of the DNA molecules are indicated by half arrows. After strand invasion (B), regions of newly synthesized chromosomal DNA (C) are represented by thin dotted lines. The numbered positions denoted by arrows indicate potential Holliday junction cleavage sites. Potentially, the joint molecule (D) can be cleaved in two alternate modes, resulting in vector integration into the chromosome. Cleavage at positions 1 and 3 generates the integrated structure shown in panel E, while the 2,4-cleavage mode generates the structure shown in panel F. The structures in panels E and F differ with respect to the position of gene conversion (C) and hDNA (H) tracts in the inner and outer marker positions. For further details, refer to the text.
Our laboratory has been investigating mechanisms of homologous recombination in mammalian somatic cells using a gene targeting assay as one approach. By examining the segregation patterns of small palindromic insertions, which frequently escape mismatch repair (MMR) when encompassed within heteroduplex DNA (hDNA) formed in vivo during homologous recombination, we have shown that (i) hDNA is formed on each side of the vector-borne DSB and (ii) palindrome markers in hDNA formed in each homology region reside in a trans configuration (25, 26). These and other (45) features of the mammalian gene targeting reaction are consistent with predictions of the yeast DSBR model.
In the joint molecule (Fig. 1D), crossover resolution may occur in either of the following two ways: (i) crossing strands in the left junction may be cut horizontally while noncrossing strands in the right junction are cut vertically (1,3-cleavage; Fig. 1E) or (ii) noncrossing strands at the left junction may be cut vertically while crossing strands at the right junction are cut horizontally (2,4-cleavage; Fig. 1F). The two crossover modes lead to different predicted marker configurations in the inner and outer positions in the recombinants. In the absence of MMR, the 2,4-cleavage mode is expected to generate recombinants in which hDNA is present in the inner positions, to the right and left of the DSB, while a conversion tract is present in the outer positions, to the left and right of the DSB. For the 1,3-cleavage mode, the opposite pattern is expected. Assuming no bias in strand cleavage, the two types of crossover products are expected to be recovered at an equal frequency. However, Gilbertson and Stahl (15), as discussed further by Foss et al. (14), in studying meiotic DSBR events at the ARG4 locus in S. cerevisiae reported a bias in the mode of crossover resolution that favors the generation of recombinant products that are equivalent to the recombinant structure shown in Fig. 1F. As an explanation for the bias, they propose that the dispensation of the newly synthesized DNA creates an inherent structural asymmetry in the joint molecule that dictates which strands of a Holliday junction are to be cut.
As there are similarities between DSBR in yeast and mammalian cells, it was of interest to determine whether or not DSBR in mammalian somatic cells displays a favored sense of crossover resolution. Therefore, we exploited our gene targeting assay to investigate this important question. The vector-borne DSB site was flanked with small palindromic insertions to preserve evidence of hDNA. The recovery of recombinants was performed under conditions described previously (25, 31) which ensured that all products of individual crossover events were available for molecular analysis. Our results suggest that, like meiotic recombination in S. cerevisiae, crossover events in mitotic mammalian cells are biased toward the 2,4-cleavage mode of crossover resolution illustrated in Fig. 1F. This novel finding provides support for the joint molecule depicted in Fig. 1D being an important intermediate in the mammalian gene targeting pathway. It also supports the concept that in mammalian cells a structural asymmetry exists in the joint molecule that dictates which DNA strands are to be cut. The bias in junction cutting permitted us to interpret the marker segregation patterns in the recombinants with regard to the potential consequences the junction cuts might have on end-directed MMR activities. The results presented in this study reveal important features about the DSBR process in mammalian cells.
MATERIALS AND METHODS
Recipient hybridoma cell line and targeting vector.
The wild-type hybridoma cell line Sp6/HL was used as the recipient for gene targeting. It bears a single copy of the trinitrophenyl-specific chromosomal immunoglobulin μ heavy chain gene that serves as the target for homologous recombination. The origin of this cell line along with the conditions used for cell culture have been described previously (22, 23).
The 11.4-kb insertion vector used in the gene targeting studies bears a 4.3-kb segment of homology to the wild-type Sp6/HL μ gene constant region (Cμ region) inserted into a derivative of the vector pSV2neo (41) from which the 372-bp NsiI/NdeI fragment encompassing the simian virus 40 (SV40) early region enhancer responsible for neo gene expression has been deleted. Deletion of this element creates an “enhancer-trap” vector. As reported previously (5, 30, 31), enhancer-trap vectors significantly enrich for gene targeting events because cis-acting sequences in the μ locus permit efficient neo gene expression. The vector-borne Cμ region was modified by inserting a perfect 30-bp palindrome genetic marker (5′ GTACTGTATGTGCGGCCGCACATACAGTAC 3′) into the unique BamHI and ApaI sites. The palindrome was engineered with a unique NotI site for identification (indicated in bold). To permit cohesive end ligation into the two vector-borne sites, appropriate terminal nucleotides were added to the palindrome sequence (indicated in lowercase italics in the oligonucleotide sequences below). For marker insertion at the BamHI site, the sequence 5′ gatcGTACTGTATGTGCGGCCGCACATACAGTAC 3′ was synthesized, whereas for insertion at the ApaI site, the sequence used was 5′ GTACTGTATGTGCGGCCGCACATACAGTACccgg 3′. Each oligonucleotide was self-annealed and ligated into the appropriate vector-borne Cμ region site, replacing those sites with the unique NotI site in the palindrome. Restriction enzyme mapping confirmed a single palindrome insertion at each site. The BamHI and ApaI palindrome insertion sites reside at Cμ genomic positions 724 and 1765, respectively. They flank a unique BstXI site (Cμ genomic position 1329) that, when digested, creates the vector-borne DSB. Thus, a vector-borne palindrome resides 605 and 436 bp to the left and right of the DSB, respectively. All Cμ genomic sites are numbered as reported in Goldberg et al. (16). With the exception of the NotI palindrome markers, the vector-borne and Sp6/HL Cμ regions are isogenic. To propagate plasmids containing the palindrome insertions, the palindrome-permissive Escherichia coli strain DL795 was used (kindly provided by David Leach). Vector construction and plasmid isolation was performed according to standard procedures (38).
Recovery and characterization of targeted hybridoma cells.
Transfer of vector DNA into the Sp6/HL hybridoma cell line was performed by electroporation according to conditions described previously (3). Following transfection, hybridoma cells were distributed to the individual wells of 96-well tissue culture plates at a low cell density and placed under G418 selection as described earlier (25, 26). This procedure ensures that each G418-resistant (G418r) transformant represents the progeny of a single G418r cell deposited in the culture well (25, 26, 31). Selection for G418r transformants and identification of hybridoma cell lines in which the haploid, chromosomal μ gene is modified by targeted vector insertion were performed according to procedures published elsewhere (25, 26, 31). Genomic DNA was prepared from the G418r hybridoma cell lines by the method of Gross-Bellard et al. (17). Restriction enzymes used in DNA digestion were purchased from Bethesda Research Laboratories (Gaithersburg, Md.), New England Biolabs (Beverly, Mass.), and Pharmacia Inc. (Piscataway, N.J.) and used in accordance with the manufacturers' specifications. Gel electrophoresis, transfer of DNA onto a nitrocellulose membrane, 32P-labeled probe preparation, and hybridization were all performed according to standard procedures (38). The conditions used for PCR amplification of the 5′ and 3′ Cμ regions in the targeted G418r recombinants have been described previously (31). The sequences and binding sites of primers AB9703, AB9745, and AB9438 have been reported previously (26, 31). The primer AB22339 binds to the noncoding strand of the herpes simplex virus type 1 (HSV-1) thymidine kinase (tk) gene beginning at position 1117 and has the following sequence: 5′ CCAACGGCGACCTGTATAACGTGT 3′.
RESULTS
Recombinant isolation and identification.
Our recombination system has been described previously (3, 31). In brief, it detects interactions between a gene targeting vector and the haploid, chromosomal immunoglobulin μ heavy chain locus in a mouse hybridoma cell line (Fig. 2A). In this study, mechanisms of crossover were investigated and this required that evidence of hDNA generated during recombination be preserved. As shown previously (25), a small (30 bp) palindrome insertion containing a diagnostic NotI restriction enzyme site is poorly repairable by the MMR machinery. Important information about hDNA formation during homologous recombination can be obtained from the position of sectored sites in the recombinants.
FIG. 2.
Gene targeting system. (A) This panel presents the structure of the recipient haploid chromosomal immunoglobulin μ gene in the wild-type Sp6/HL hybridoma cell line (22, 23). It is present on a 12.5-kb EcoRI (E) fragment. The location of the trinitrophenyl-specific heavy chain variable (VHTNP) region and the four Cμ region exons is indicated. The endogenous BamHI (B) and ApaI (A) restriction enzyme sites serve as diagnostic markers of the chromosomal Cμ region. The endogenous Cμ region can be amplified by PCR using the primer pair AB9703-AB9438 as described previously (31) to yield a specific 4,621-bp fragment. (B) The structure of the 11.4-kb enhancer-trap sequence insertion vector. The vector bears a 4.3-kb segment of homology derived from the Sp6/HL chromosomal Cμ region inserted into a derivative of the vector, pSV2neo (41), from which the SV40 early region enhancer responsible for neo gene expression has been removed. Although not relevant to this study, the targeting vector also contains the tk gene from HSV-1. A perfect 30-bp palindrome containing a diagnostic NotI restriction enzyme site (the palindrome sequence is presented in Materials and Methods) replaces the vector-borne BamHI (bN) and ApaI (aN) restriction enzyme sites located left (605 bp) and right (436 bp) of the unique BstXI site (position 0) that was used to introduce the DSB into the vector. With the exception of the single palindrome insertion at each site, the vector-borne and chromosomal Cμ regions are otherwise isogenic. Following cleavage with BstXI, the vector-borne Cμ region bears 1.5 and 2.8 kb of homology to the Sp6/HL chromosomal Cμ region on the left- and right-hand sides of the DSB, respectively. (C) The structure of the recombinant chromosomal μ gene. Targeted integration of a single copy of the transfer vector into the Sp6/HL chromosomal μ gene replaces the endogenous 12.5-kb EcoRI μ gene fragment with the novel 16.2- and 7.7-kb EcoRI μ fragments shown. The inner and outer Cμ region positions in the recombinant bear the symbol “?” because each recombination event has the potential to produce a different marker pattern in these regions. The primer pair AB9703-AB9745 generates a specific 4,765-bp PCR product from the 5′ Cμ region, while the primer pair AB22339-AB9438 generates a specific 5,150-bp PCR product from the 3′ Cμ region. Both primer sets bind outside of the vector-borne Cμ region. Details regarding the sequence and binding sites of the primers are given in Materials and Methods. Southern blots were hybridized with probe F, an 870-bp XbaI/BamHI fragment specific for the Cμ region. (D) Restriction enzyme mapping of the 5′ and 3′ Cμ region PCR products. As shown in panel C and at the bottom of this diagram, amplification of genomic DNA from the targeted recombinants with primer pair AB9703-AB9745 generates a specific 4,765-bp PCR product from the 5′ Cμ region, while amplification with primer pair AB22339-AB9438 generates a specific 5,150-bp PCR product from the 3′ Cμ region (in parentheses). In this diagram, the Cμ primers are depicted as oppositely oriented half arrows. The fragment sizes (in base pairs) that the indicated restriction enzymes should generate are shown without parentheses for cleavage of the 5′ Cμ region PCR product and with parentheses for cleavage of the 3′ Cμ region PCR product. The various diagrams in panels A to D are not drawn to scale.
In this study, the 11.4-kb sequence insertion vector (Fig. 2B) contained a unique BstXI site positioned near the middle of the Cμ region that was used to create the vector-borne DSB. The endogenous BamHI and ApaI sites, located 605 bp to the left and 436 bp to the right of the BstXI site, respectively, were replaced by insertion of the palindrome containing the unique NotI site. As shown in the figure, these site changes are denoted bN and aN. The positioning of the palindrome markers was judged to be far enough from the DSB to avoid potential loss by double-strand gap formation (25, 26, 31). The vector had a deletion of the SV40 early region enhancer responsible for neo expression, creating an enhancer-trap vector which, as described previously (5, 30, 31), permits efficient isolation of recombinants targeted at the chromosomal μ locus. Although not relevant to this study, the vector also contained the HSV-1 tk gene.
The vector was linearized by cleavage with BstXI and transferred to 2 × 107 recipient Sp6/HL hybridoma cells by electroporation (3), and independent recombinants were isolated according to methods described previously (25, 31). Importantly, the recovery procedures ensure that each recombinant represents the progeny of a single G418r cell and that the G418r product(s) of recombination is retained for molecular analysis. Two separate vector transfers were performed. Genomic DNA from independent G418r transformants was screened by PCR using primer pair AB9703-AB9438 for the specific 4,621-bp product that identifies the endogenous chromosomal Cμ region (Fig. 2A). Of the total of 330 independent G418r transformants that were examined, 290 cell lines contained the endogenous 4,621-bp Cμ region PCR product, but in the remaining 40 cell lines, no Cμ region product was detected. The latter hybridoma cell lines were saved as putative examples of transformants in which the targeting vector had interacted in some way with the endogenous Cμ locus. Therefore, the assay procedure is largely unbiased in detecting recombinants, as any interaction between the vector and chromosome that is sufficient to disrupt the Cμ region or change its size is recovered. Genomic DNA from the 40 hybridoma cell lines was digested with EcoRI and screened by Southern analysis (results not shown). Using 32P-labeled Cμ-specific probe F, the blots revealed the following information. Of the 40 G418r transformants, 26 bore EcoRI fragments, of 16.2 and 7.7 kb, indicative of the Cμ region duplication in recombinants generated by the targeted integration of a single copy of the transfer vector (Fig. 2C). Although not examined here, our previous studies examining targeted vector integration at the chromosomal μ locus (3, 25, 26, 30–32) reveal that the site of vector linearization is restored in the recombinants, and in Fig. 2C, the BstXI site is shown as such. Of the remaining 14 G418 transformants, 5 contained EcoRI fragments, of 16.2, 11.4, and 7.7 kb. These bands are diagnostic of a Cμ region triplication resulting from the targeted integration of two copies of the transfer vector (32). The final nine G418r transformants did not contain the EcoRI fragments indicative of targeted vector integration. Southern analysis revealed that in some of these transformants, a fragment of a size corresponding to either the 5′ or 3′ EcoRI Cμ fragment was visible but that most often they contained Cμ-hybridizing fragments of unexpected size. Hybridoma cell lines with unexpected Cμ region fragments or those that have suffered a deletion of the endogenous Cμ locus have been observed in previous gene targeting studies (4, 25, 31). Although they have not been fully characterized, these may represent cases where one arm of the vector has been degraded, forcing the cells to undergo one-sided recombination with the target locus or perhaps illegitimate recombination such as has been reported previously (4, 7, 21). In summary, of the 40 G418r transformants initially identified by the PCR screening, 26 bore the correct μ gene structure required for determination of the crossover mechanism, and therefore these hybridoma cell lines were saved for further analysis. The remaining 14 G418r transformants were deemed not to be useful for the purpose of the following study and were not included in any further analysis here.
Determination of Cμ region genetic markers.
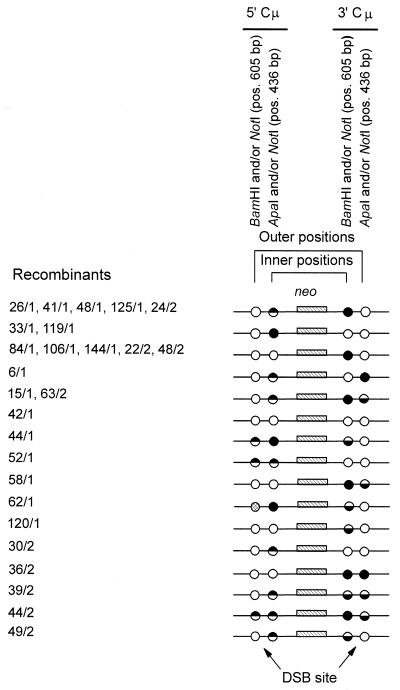
The determination of the Cμ region genetic markers in the 26 targeted recombinants was performed according to PCR and gel analysis methods described previously (31). As indicated in Fig. 2C, PCR amplification using primer pair AB9703-AB9745 generates a specific 4,765-bp product from the 5′ Cμ region while primer pair AB22339-AB9438 generates a specific 5,150-bp product from the 3′ Cμ region. Digestion of the PCR products with restriction enzymes specific for the chromosomal Cμ region sites (BamHI and ApaI) as well as with NotI (specific for the vector-borne palindrome) yields diagnostic fragments that can be resolved by standard gel electrophoresis (Fig. 2D). The results of these digests (data not shown) are summarized in Fig. 3. Recombinants identified from the two separate electroporations are indicated by the coding “/1” or “/2”, respectively. Inner positions are defined as those that lie to the right and left of the DSB in the 5′ and 3′ Cμ regions, respectively. Outer positions are defined as those residing to the left and right of the DSB in the 5′ and 3′ Cμ regions, respectively. In the 5′ Cμ region of a single recombinant (62/1), neither the vector-borne NotI palindrome nor the chromosomal BamHI site was present, suggesting that a subtle mutation had occurred at this site (marker loss indicated by the cross-hatched circle).
FIG. 3.
Inner and outer Cμ region marker patterns in the recombinants. The pSV2neo sequences separating the 5′ and 3′ Cμ regions in the recombinants are denoted neo. Inner positions are defined as those that lie to the right and left of the DSB in the 5′ and 3′ Cμ regions, respectively. Outer positions are defined as those residing to the left and right of the DSB in the 5′ and 3′ Cμ regions, respectively. Positions bearing the vector-borne NotI palindrome are indicated by a filled circle, those with a chromosomal marker are indicated by an open circle, and those that are sensitive to cleavage with restriction enzymes diagnostic of both the chromosome and vector-borne markers (i.e., sectored sites indicative of hDNA formed during homologous recombination) are indicated by half-filled circles. In a single recombinant (62/1), the outer marker position in the 5′ Cμ region contains neither the vector-borne NotI palindrome nor the chromosomal BamHI marker, suggesting a mutation at this site (indicated by a cross-hatched circle). The position of the markers relative to the site of the DSB at BstXI is indicated at the bottom of the diagram.
Biased distribution of the Cμ region genetic markers.
A total of 17 of the 26 recombinants (65%) were sectored for one or more Cμ region positions, providing strong evidence for formation of hDNA during recombination. Sectored sites were revealed by the partial sensitivity of the 5′ and/or 3′ Cμ region PCR products to cleavage with the palindrome-specific NotI enzyme and either BamHI or ApaI, which was diagnostic of chromosomal markers. Sectored sites were observed in both Cμ regions and on opposite sides of the vector-borne DSB site as reported previously (26). Where examined (data not shown), the results also confirmed previous studies (25, 26), showing that palindrome markers present in sectored sites in the 5′ and 3′ Cμ regions resided in a trans configuration as indicated for recombinants 15/1, 44/1, 39/2, 44/2, 49/2, and 63/2 in Fig. 3. That is, analysis of marker segregation patterns in individual subclones of each parental recombinant (started from a single cell) revealed two cell types. In one type, the vector-borne NotI palindrome marker residing in the 5′ Cμ region site was linked to a chromosomal marker in the 3′ Cμ region site, while in the second cell type, the chromosomal marker in the 5′ Cμ region site was linked to the NotI palindrome marker in the 3′ Cμ region site. A novel finding was the marker patterns of three of the recombinants (52/1, 39/2, and 44/2). In these cell lines, sectoring was observed on both sides of the DSB in one of the Cμ regions. With the exception of the sectored sites, the remaining Cμ region positions in the recombinants were completely sensitive to digestion with either NotI or one of the enzymes specific for the chromosomal markers. The marker frequencies for the inner and outer Cμ region positions in the recombinants are summarized in Table 1.
TABLE 1.
Frequency of genetic markers in the inner and outer Cμ region positions in recombinantsa
| Type of marker | No. of inner Cμ region positions | No. of outer Cμ region positions | Total |
|---|---|---|---|
| Chromosomal | 15 | 42 | 57 |
| Vector borne | 19 | 2 | 21 |
| Sectored | 18 | 8 | 26 |
For recombinant 62/1, the marker to the left of the DSB in the outer Cμ region is mutated as explained in the text. With this exception, the marker pattern in this recombinant is consistent with favored junction resolution and a single round of late MMR directed by the DNA end at site a, as indicated in Fig. 6. For completeness, the missing site has been included as a chromosomal marker in this table. Its inclusion here does not change the significance of the chromosomal marker distribution.
The canonical DSBR model depicts cleavage of the joint molecule in an unbiased manner generating recombinants in which the distribution of hDNA and gene conversion tracts in the inner and outer Cμ region positions is expected to be equal (Fig. 1E and F). However, as is evident from Table 1, the genetic markers in the inner and outer Cμ region positions in the recombinants in this study are not distributed evenly. In contrast to the outcome predicted by the canonical DSBR model, the outer Cμ region positions contain predominantly chromosomal markers while the inner positions consist predominantly of sites that are either sectored or bear the NotI palindrome. According to a chi-square (χ2) test, the higher frequency of chromosomal markers in the outer Cμ region positions is significant (χ2 = 12.79; P < 0.001). For the inner Cμ region positions, there is a significantly higher frequency of the vector-borne NotI palindrome (χ2 = 13.76; P < 0.001), while the higher frequency of sectored sites borders on significance (χ2 = 3.84; P = 0.05).
DISCUSSION
A nonrandom distribution of Cμ region genetic markers was evident in the 26 independent recombinants examined in this study. As indicated in Fig. 3 and summarized in Table 1, there was a significant bias toward chromosomal markers in the outer Cμ region positions. In the inner Cμ region positions, hDNA and the NotI palindrome marker predominated. The positions bearing the NotI palindrome can be explained by restorative MMR of hDNA (discussed further below). Thus, if the number of sectored sites and those bearing the NotI palindrome are combined as total evidence of hDNA, a χ2 test reveals a significantly higher frequency of hDNA in the inner Cμ region positions (χ2 = 15.51; P < 0.001).
The bias toward chromosomal markers in the outer Cμ region positions and hDNA in the inner Cμ region positions can be explained according to the DSBR model of recombination if crossover involves favored-sense cleavage of the joint molecule. The joint molecule intermediate of DSBR is illustrated in Fig. 4. The intermediate is presented as having 3′-ending, single-stranded tails in accord with studies of homologous recombination in S. cerevisiae. In this organism, DSBs are subject to resection of their 5′ termini in both meiosis (1) and mitosis (46), yielding 3′ single-stranded tails that are approximately 600 nucleotides (nt) in length (8, 9, 42). In mammalian cells, the available evidence suggests that 5′→3′ resection of the ends of transferred DNA can exceed 1,000 nt (19, 24). Therefore, as shown in Fig. 4, it is reasonable to assume that DSB resection may have frequently proceeded past the palindrome markers (denoted bN and aN), generating 3′ single-stranded tails of at least 605 nt in length. Further support for this assumption is the fact that if the DSB was subject to little or no 5′ resection, then examples of crossover at or near the DSB would have been expected. Such recombinants would bear the NotI palindrome in the inner Cμ region positions and chromosomal markers in the outer Cμ region positions, and these were not observed (Fig. 3). In principle, the joint molecule can be resolved by cutting the DNA strands of the Holliday junctions either at positions 1 and 3 or at positions 2 and 4. As hDNA and conversion tracts reside in different regions of the joint molecule, the two modes of cleavage are expected to yield recombinants with different marker patterns in the inner and outer Cμ region positions. The 1,3-cleavage mode yields a recombinant in which gene conversion is predominant in the inner Cμ region positions whereas hDNA is predominant in the outer Cμ region positions. This result is contrary to the Cμ region marker patterns that were observed in the recombinants. However, if the joint molecule is resolved according to the 2,4-cleavage mode, the outer Cμ region positions will bear predominantly chromosomal markers whereas the inner Cμ region positions will contain hDNA. This is precisely the result that was obtained. Thus, whereas the canonical DSBR model predicts that both modes of crossover resolution should occur with the same frequency, our results reveal the contrary, as crossover resolution during mammalian mitotic recombination exhibits a bias in strand cleavage.
FIG. 4.
Crossover resolution of the joint molecule. The joint molecule intermediate of DSBR is shown at the top of the diagram. For clarity in relating the structure of the joint molecule intermediate to the various recombinants, it is labeled to show the positions of the chromosomal BamHI (B) and ApaI (A) markers as well as those of the vector, namely the NotI-replaced BamHI (bN) and the NotI-replaced ApaI (aN) sites. To generate a crossover product, the joint molecule can be cleaved by cutting the DNA strands either at positions 2 and 4 or at positions 1 and 3. Following cleavage, crossover incorporates the vector (thick line) into the chromosome (thin line). The two modes of crossover resolution generate different predicted patterns for gene conversion (C) and hDNA (H) in the inner and outer Cμ region marker positions in the recombinants.
This study is the first to report results that are consistent with crossover in mitotic mammalian cells proceeding according to favored-sense cleavage of the joint molecule. The same bias in the mode of crossover resolution has been reported previously but in studies examining meiotic recombination in S. cerevisiae (13, 15, 20, 47). As an explanation for the bias in strand cleavage, Foss et al. (14), in their studies of DSBR at the ARG4 locus in S. cerevisiae, suggest that the joint molecule bears a structural asymmetry resulting from the new DNA or its synthesis that dictates which strands of the Holliday junctions are to be cut. Thus, like meiotic recombination in yeast, targeted vector integration in mitotic mammalian cells is consistent with a preferred mode of resolution that involves the cutting of those DNA strands that are newly synthesized at the junction.
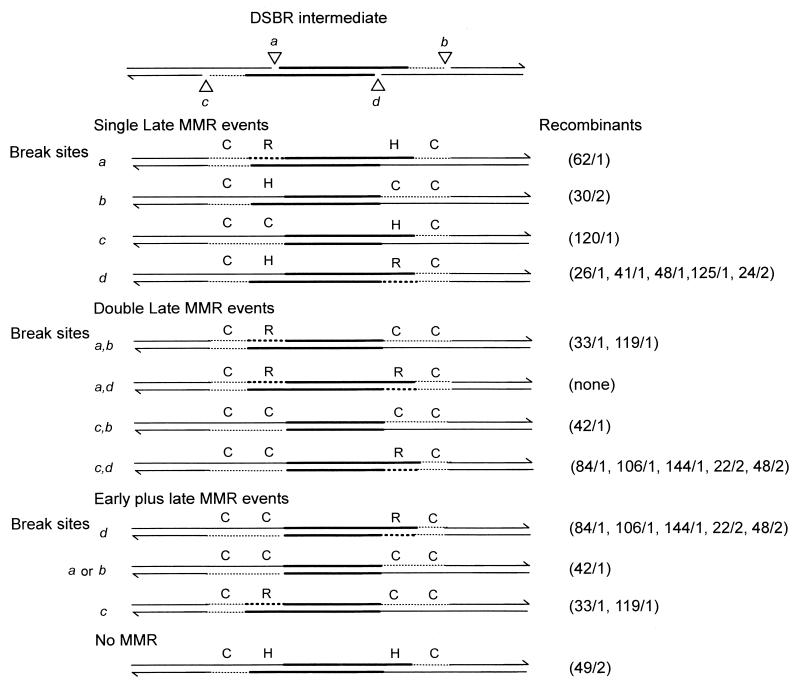
Mismatches involving the small palindrome are poorly repairable by the cellular MMR machinery in yeast (28) and mammalian cells (25). Nevertheless, the results presented here and in our previous studies (25, 26) reveal that some mismatches involving the palindrome are subject to repair. The finding that targeted vector integration results from a bias in strand cleavage permits the potential consequences of MMR acting on the recombination intermediate to be addressed. In this regard, the studies of Foss et al. (14) are relevant. As shown in Fig. 5, MMR might occur early in the recombination process, being initiated by invasion of the two 3′ single strands. Early MMR might involve DNA excision beginning from the 3′ end of one or both invading strands followed by resynthesis using the chromosomal sequence as template, generating the indicated gene conversion event. Alternatively, an invading 3′ end might direct highly biased MMR that leads to preferential correction (gene conversion) of the sequence on the interrupted strand, as suggested previously for mitotic (18, 27) and meiotic (36) recombination in S. cerevisiae. Formally, gene conversion might also result from repair of a small gap created during vector transfer, as suggested previously (31). Comparison of the predictions of Fig. 5 with the marker segregation patterns in the recombinants (Fig. 3) suggests that early MMR can account for the marker patterns in only 3 of the 26 recombinants (120/1, 42/1, and 30/2). Therefore, early MMR alone is probably not an important mechanism in targeted vector integration, a conclusion that was also reached by Foss et al. (14). This suggests that discontinuities elsewhere in the recombination intermediate might be more important for triggering MMR. Vector integration arising from Holliday junction cleavage in the favored 2,4-cleavage mode is expected to leave breaks in the DNA at positions a, b, c, and d, as indicated at the top of Fig. 6. DNA ends can trigger MMR and direct it to occur on the discontinuous strand (2). Therefore, the DNA ends generated by favored-sense resolution serve as potential sites for initiating late MMR events, resulting in partial or continuous excision of markers in cis to the strand end (2, 14, 40). MMR may involve excision from one strand end or simultaneous excision from two DNA ends involving both Cμ regions. Late MMR directed by the breaks at position a or d generates a restoration to the NotI palindrome in the inner marker positions. Alternatively, late MMR directed by breaks at position b or c which involves excision of DNA to the left of the DSB site will result in a gene conversion tract that would be indistinguishable from an early MMR event. Other patterns can arise if MMR is triggered by strand breaks in both Cμ regions (i.e., at positions a and b, positions a and d, etc.) or if MMR is triggered by a combination of both early and late MMR events. Alternatively, the recombination intermediate might escape MMR.
FIG. 5.
Early MMR followed by favored-sense crossover resolution. Early MMR is guided by the 3′ ends of the invading single-stranded vector DNA and may involve DNA excision on one or both sides of the DSB, followed by DNA synthesis to complete the joint molecule. Early MMR results in gene conversion (C) in the indicated region. The Cμ region marker patterns in three recombinants are consistent with this mechanism. H, hDNA.
FIG. 6.
Possible consequences of late MMR alone or the combination of early and late MMR. The top diagram illustrates the intermediate resulting from crossover resolution of the joint molecule in the favored sense. Holliday junction cleavage at positions 2 and 4 is expected to leave breaks in the DNA at the positions indicated by open triangles (a, b, c, and d). Late MMR may involve resection of DNA from a single break site in either the 5′ or 3′ Cμ region or from pairs of break sites in both Cμ regions. Alternatively, MMR might involve the combination of both early MMR (Fig. 5) and late MMR. Only those early MMR events for which a round of late MMR would produce a change in the marker pattern are illustrated. As indicated, the Cμ region marker patterns in several recombinants are consistent with the predicted outcomes of the various events. The recombination intermediate may also completely escape MMR, as is the case for the single recombinant 49/2. As in the previous diagrams, thick lines represent vector sequences, thin lines represent chromosomal sequences, and regions corresponding to newly synthesized chromosomal markers are presented as thin dotted lines. DNA synthesis that involves copying of the vector-borne NotI palindrome marker and generates a restoration event (R) is indicated by thick dotted lines. All other abbreviations are the same as those defined in the legend to Fig. 4.
As can be seen by comparison of Fig. 5 and 6, depending on the location of the affected mismatch relative to the cut end, the products of late MMR can produce both restorations and gene conversions, while early MMR events generate only gene conversions. Thus, there is some overlap in the products that are generated. As suggested by Foss et al. (14), late MMR directed by the junction cuts may involve a competition between the cut junctions for the likelihood of repairing a mismatch: mismatches in close proximity to a cut junction may be more likely to undergo repair directed by that junction. Therefore, from Fig. 6 it is evident that following favored-sense cleavage, a mismatch on the same side of the DSB as the cut junction would undergo restoration, whereas if the mismatch was located on the other side of the DSB from the cut junction, it would undergo gene conversion. This implies that mismatches located far from the DSB are more likely to undergo restorative-type repair than conversion-type repair.
When the Cμ region marker patterns in the recombinants are considered with respect to the possible consequences of early and/or late MMR, the following observation emerges. In one recombinant (49/2), the Cμ region marker pattern is consistent with favored junction resolution alone, with no MMR. However, the remaining 25 of the 26 recombinants can be explained on the basis of favored junction resolution in conjunction with early and/or late MMR activites. In 16 of the 25 recombinants, the Cμ region marker patterns are consistent with the occurrence of late MMR. Of these, 13 recombinants (26/1, 33/1, 41/1, 48/1, 62/1, 84/1, 106/1, 119/1, 125/1, 144/1, 22/2, 24/2, and 48/2) exhibited a restoration event to the NotI palindrome. The apparent association of late MMR with restorative-type repair during these mammalian mitotic recombination events is reminiscent of meiotic recombination in S. cerevisiae (10, 14). These authors reported that markers far from the DSB site undergo MMR that is directed by the junction cuts leading to restoration, whereas markers located close to the DSB undergo more frequent gene conversion. Further, they suggested that this makes an important contribution to the meiotic conversion gradient. In mitotic cells, most evidence suggests against a strong polarity gradient (35), although for the spontaneous recombination events discussed in that review, the nature of the recombination-initiating lesions is unknown. Where the position of the initiating DSB is known, evidence for mitotic conversion tract directionality has been obtained (27, 43). Conversion tracts are influenced by the location of initiating DSB as well as by the position of frameshift mutations in donor and recipient alleles (43). In mammalian somatic cells, a decrease in conversion frequency was observed as the distance between the genetic marker and the initiating DSB increased (11). Perhaps restorative-type MMR directed by junction cuts as proposed here makes a contribution to the conversion tract directionality in the above mitotic recombination studies.
The Cμ region marker patterns in the remaining 9 of the 26 recombinants (6/1, 15/1, 44/1, 52/1, 58/1, 36/2, 39/2, 44/2, and 63/2) are also consistent with favored junction resolution and late MMR activities. However, the presence of a sectored site or NotI palindrome in an outer Cμ region position(s) requires that 5′→3′ strand resection not remove the palindrome from at least one side of the DSB so that palindromes in both DNA strands are available for inclusion in hDNA. Thus, while the generation of the majority of recombinants is consistent with 5′ resection on both sides of the DSB, yielding 3′-ended, single-strand tails as explained above, the generation of a few recombinants can be accounted for on the basis of the formation of a slightly shorter 3′ tail on one side of the DSB.
In this study, the Cμ region marker patterns in the recombinants are compatible with MMR activities being directed by junction cuts arising from biased crossover resolution. The MMR tracts observed in recombinants described in previous studies can also be interpreted in this way (25, 26, 31). However, in some of the recombinants studied previously, MMR tracts were not always long and continuous but rather were punctuated. Recombinants with punctuated tracts were observed for poorly repaired palindrome markers as well as for simple restriction enzyme site polymorphisms that might be expected to undergo efficient MMR. This suggests that short tract repair contributes to the cellular mitotic MMR activity.
Finally, we believe that the Cμ region marker patterns of three recombinants in this study (52/1, 39/2, and 44/2) provide additional support for our interpretation of targeted vector integration according to the DSBR model. A novel feature of these recombinants is the presence of hDNA on both sides of the DSB in the same Cμ region (Fig. 3). This marker pattern is consistent with the DSBR reaction if strand invasion involves shorter 3′ tails and if Holliday junction branch migration generates symmetric hDNA on both sides of the DSB. While we have interpreted our data according to the DSBR model, it has been suggested that targeted vector integration might proceed according to alternative models, including one-sided invasion (6), which essentially is similar to synthesis-dependent strand annealing (29), and the migrating D-loop model (12). These models differ from the DSBR model in proposing (i) that an invading 3′ end generates a D-loop that fails to undergo complementary base pairing with the remaining 3′-ended, single-stranded tail and (ii) the formation of a single Holliday junction. Several points are relevant in considering whether these alternative models provide a satisfactory explanation for our data. First, with only a single Holliday junction, it is difficult to explain the formation of hDNA on both sides of the DSB in one Cμ region as was observed in the recombinants described above. Also, in its simplest form, the migrating D-loop model generates a conversion tract in an inner marker position, a feature that was infrequently observed in the present study. Second, random nicking of the unpaired D-loop has the potential to generate recombinants in which there is both a homologous and a nonhomologous junction. However, within the resolution limits defined by Southern and PCR analysis, targeted recombinants in this and our previous studies (3, 25, 26, 30–32) revealed only the correct μ gene fragment sizes expected for conservative homologous recombination. Third, it is not clear why the free D-loop would fail to undergo complementary base pairing with the second available 3′-ending single strand unless one postulates that this end is specifically blocked. Fourth, a main impetus for proposing both the one-sided invasion and migrating D-loop models was to account for observations that gene conversion and reciprocal crossover were not always associated as proposed in the canonical DSBR model. However, it is important to point out that modifications to the DSBR model can also explain this recombination data (15). In conclusion, we suggest that the simplicity of the DSBR model appears best suited to explaining our results.
ACKNOWLEDGMENT
This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR) (MOP-14416) to M.D.B.
REFERENCES
- 1.Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- 2.Alani E, Reenan R A, Kolodner R D. Interaction between mismatch repair and genetic recombination in Saccharomyces cerevisiae. Genetics. 1994;137:19–39. doi: 10.1093/genetics/137.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker M D, Pennell N, Bosnoyan L, Shulman M J. Homologous recombination can restore normal immunoglobulin production in a mutant hybridoma cell line. Proc Natl Acad Sci USA. 1988;85:6432–6436. doi: 10.1073/pnas.85.17.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker M D, Read L R. Analysis of mutations introduced into the chromosomal immunoglobulin μ gene. Somat Cell Mol Genet. 1993;19:299–311. doi: 10.1007/BF01232743. [DOI] [PubMed] [Google Scholar]
- 5.Bautista D, Shulman M J. A hit-and-run system for introducing mutations into the immunoglobulin heavy chain locus of hybridoma cells by homologous recombination. J Immunol. 1993;151:1950–1958. [PubMed] [Google Scholar]
- 6.Belmaaza A, Chartrand P. One-sided invasion events in homologous recombination at double-strand breaks. Mutat Res. 1994;314:199–208. doi: 10.1016/0921-8777(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 7.Berinstein N, Pennell N, Ottaway C A, Shulman M J. Gene replacement with one-sided homologous recombination. Mol Cell Biol. 1992;12:360–367. doi: 10.1128/mcb.12.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop D K, Park D, Xu L, Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992;69:439–456. doi: 10.1016/0092-8674(92)90446-j. [DOI] [PubMed] [Google Scholar]
- 9.Cao L, Alani E, Kleckner N. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell. 1990;61:1089–1101. doi: 10.1016/0092-8674(90)90072-m. [DOI] [PubMed] [Google Scholar]
- 10.Detloff P, White M A, Petes T D. Analysis of a gene conversion gradient at the HIS4 locus in Saccharomyces cerevisiae. Genetics. 1993;132:113–123. doi: 10.1093/genetics/132.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliot B, Richardson C, Winderbaum J, Nickoloff J A, Jasin M. Gene conversion tracts from double-strand break repair in mammalian cells. Mol Cell Biol. 1998;18:93–101. doi: 10.1128/mcb.18.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson D O, Holloman W K. Recombinational repair of gaps in DNA is asymmetric in Ustilago maydis and can be explained by a migrating D-loop model. Proc Natl Acad Sci USA. 1996;93:5419–5424. doi: 10.1073/pnas.93.11.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogel S, Mortimer R, Lusnak K, Tavares F. Meiotic gene conversion: a signal of the basic recombination event in yeast. Cold Spring Harbor Symp Quant Biol. 1979;43:1325–1341. doi: 10.1101/sqb.1979.043.01.152. [DOI] [PubMed] [Google Scholar]
- 14.Foss H M, Hillers K J, Stahl F W. The conversion gradient at HIS4 of Saccharomyces cerevisiae. II. A role for mismatch repair directed by biased resolution of the recombinational intermediate. Genetics. 1999;153:573–583. doi: 10.1093/genetics/153.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbertson L A, Stahl F W. A test of the double-strand break repair model for meiotic recombination in Saccharomyces cerevisiae. Genetics. 1996;144:27–41. doi: 10.1093/genetics/144.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg G I, Vanin E F, Zrolka A M, Blattner F R. Sequence of the gene for the constant region of the μ chain of the Balb/c mouse. Gene. 1981;15:33–42. doi: 10.1016/0378-1119(81)90102-5. [DOI] [PubMed] [Google Scholar]
- 17.Gross-Bellard M, Qudet P, Chambon P. Isolation of high-molecular weight DNA from mammalian cells. Eur J Biochem. 1973;36:32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 18.Haber J E, Ray B L, Kolb J M, White C I. Rapid kinetics of mismatch repair of heteroduplex DNA that is formed during recombination in yeast. Proc Natl Acad Sci USA. 1993;90:3363–3367. doi: 10.1073/pnas.90.8.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson G, Simons J P. Processing of DNA prior to illegitimate recombination in mouse cells. Mol Cell Biol. 1997;17:3779–3785. doi: 10.1128/mcb.17.7.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillers K J, Stahl F W. The conversion gradient at HIS4 of Saccharomyces cerevisiae. I. Heteroduplex rejection and restoration of Mendelian segregation. Genetics. 1999;153:555–572. doi: 10.1093/genetics/153.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Y, Shulman M J. Effect of vector cutting on its recombination with the chromosomal immunoglobulin gene in hybridoma cells. Somat Cell Mol Genet. 1991;17:525–536. doi: 10.1007/BF01233617. [DOI] [PubMed] [Google Scholar]
- 22.Köhler G, Potash M J, Lehrach H, Shulman M J. Deletions in immunoglobulin mu chains. EMBO J. 1982;1:555–563. doi: 10.1002/j.1460-2075.1982.tb01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Köhler G, Shulman M J. Immunoglobulin M mutants. Eur J Immunol. 1980;10:467–476. [Google Scholar]
- 24.Kumar S, Simons J P. The effects of terminal heterologies on gene targeting by insertion vectors in embryonic stem cells. Nucleic Acids Res. 1993;21:1541–1548. doi: 10.1093/nar/21.7.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Baker M D. Use of a small palindrome genetic marker to investigate mechanisms of double-strand-break repair in mammalian cells. Genetics. 2000;154:1281–1289. doi: 10.1093/genetics/154.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Baker M D. Formation and repair of heteroduplex DNA on both sides of the double-strand break during mammalian gene targeting. J Mol Biol. 2000;295:505–516. doi: 10.1006/jmbi.1999.3400. [DOI] [PubMed] [Google Scholar]
- 27.McGill C, Shafer B, Strathern J. Coconversion of flanking sequences with homothallic switching. Cell. 1989;57:459–467. doi: 10.1016/0092-8674(89)90921-5. [DOI] [PubMed] [Google Scholar]
- 28.Nag D K, White M A, Petes T D. Palindromic sequences in heteroduplex DNA inhibit mismatch repair in yeast. Nature. 1989;340:318–320. doi: 10.1038/340318a0. [DOI] [PubMed] [Google Scholar]
- 29.Nassif N, Penny J, Pal S, Engels W R, Gloor G B. Efficient copying of nonhomologous sequences from ectopic sites via P-element-induced gap repair. Mol Cell Biol. 1994;14:1613–1625. doi: 10.1128/mcb.14.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng P, Baker M D. High-efficiency, site-specific modification of the chromosomal immunoglobulin locus by gene targeting in mammalian cells. J Immunol Methods. 1998;214:81–96. doi: 10.1016/s0022-1759(98)00033-7. [DOI] [PubMed] [Google Scholar]
- 31.Ng P, Baker M D. Mechanisms of double-strand-break repair during gene targeting in mammalian cells. Genetics. 1999;151:1127–1141. doi: 10.1093/genetics/151.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng P, Baker M D. The molecular basis of multiple vector insertion by gene targeting in mammalian cells. Genetics. 1999;151:1143–1151. doi: 10.1093/genetics/151.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orr-Weaver T L, Szostak J W. Yeast recombination: the association between double strand gap repair and crossing over. Proc Natl Acad Sci USA. 1983;80:4417–4421. doi: 10.1073/pnas.80.14.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orr-Weaver T L, Szostak J W, Rothstein R J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci USA. 1981;78:6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petes T D, Malone R E, Symington L S. Recombination in yeast. In: Broach J R, Pringle J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccharomyces: genome dynamics, protein synthesis and energetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1991. pp. 407–521. [Google Scholar]
- 36.Porter S E, White M A, Petes T D. Genetic evidence that the meiotic recombination hotspot at the HIS4 locus of Saccharomyces cerevisiae does not represent a site for a symmetrically processed double-strand break. Genetics. 1993;134:5–19. doi: 10.1093/genetics/134.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resnick M A. The repair of double-strand breaks in DNA: a model involving recombination. J Theor Biol. 1976;59:97–106. doi: 10.1016/s0022-5193(76)80025-2. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Schwacha A, Kleckner N. Identification of joint molecules that form frequently between homologs but rarely between sister chromatids during yeast meiosis. Cell. 1994;76:51–63. doi: 10.1016/0092-8674(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 40.Schwacha A, Kleckner N. Identification of double Holliday junctions as intermediates in meiotic recombination. Cell. 1995;83:1–20. doi: 10.1016/0092-8674(95)90191-4. [DOI] [PubMed] [Google Scholar]
- 41.Southern P J, Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1981;1:327–341. [PubMed] [Google Scholar]
- 42.Sun H, Treco D, Szostak J W. Extensive 3′-overhanging, single-stranded DNA associated with meiosis-specific double strand breaks at the ARG4 recombination initiation site. Cell. 1991;64:1155–1161. doi: 10.1016/0092-8674(91)90270-9. [DOI] [PubMed] [Google Scholar]
- 43.Sweetser D B, Hough H, Wheldon J F, Arbuckle M, Nickoloff J A. Fine-resolution mapping of spontaneous and double-strand break-induced gene conversion tracts in Saccharomyces cerevisiae reveals reversible mitotic polarity. Mol Cell Biol. 1994;14:3863–3875. doi: 10.1128/mcb.14.6.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szostak J W, Orr-Weaver T L, Rothstein R J, Stahl F W. The double-strand-break repair model for recombination. Cell. 1983;33:25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 45.Valancius V, Smithies O. Double-strand gap repair in a mammalian gene targeting reaction. Mol Cell Biol. 1991;11:4389–4397. doi: 10.1128/mcb.11.9.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White C I, Haber J E. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J. 1990;9:663–673. doi: 10.1002/j.1460-2075.1990.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White M A, Petes T D. Analysis of meiotic recombination events near a recombination hotspot in the yeast Saccharomyces cerevisiae. Curr Genet. 1994;26:21–30. doi: 10.1007/BF00326300. [DOI] [PubMed] [Google Scholar]