Abstract
Background
The tick-borne bacterium, Neoehrlichia mikurensis (N. mikurensis) can cause severe febrile illness and thromboembolic complications in immunocompromised individuals. We investigated the presence of N. mikurensis DNA in retrospectively collected plasma from a well-characterized cohort of Danish immunocompromised patients.
Methods
Plasma samples from 239 patients with immune dysfunction related to hematological or rheumatological disease or due to immunosuppressive therapy, were retrieved from a transdisciplinary biobank (PERSIMUNE) at Rigshospitalet, Copenhagen, Denmark. Serving as immunocompetent controls, plasma samples from 192 blood donors were included. All samples were collected between 2015 and 2019. Real-time PCR targeting the groEL gene was used to detect N. mikurensis DNA. Sequencing was used for confirmation. Borrelia burgdorferi sensu lato IgG antibodies were detected by ELISA as a proxy of tick exposure. Prevalence was compared using Fisher’s exact test.
Results
Neoehrlichia mikurensis DNA was detected in 3/239 (1.3%, 95% confidence interval (CI): 0.3 – 3.6%) patients, all of whom primarily had a hematological disease. Follow-up samples of these patients were negative. N. mikurensis DNA was not detected in any of the blood donor samples. IgG antibodies against B. burgdorferi s.l. were detected with similar prevalence in immunocompromised patients and blood donors, i.e., 18/239 (7.5%, 95% CI: 4.8–11.5%) and 11/192 (5.7%, 95%: CI 3.2–10.0%).
Conclusion
In this study, patients with N. mikurensis were not identified by clinical indication and N. mikurensis may therefore be underdiagnosed in Danish patients. Further investigations are needed to explore the clinical significance and implications of this infection.
Keywords: Neoehrlichia mikurensis, Neoehrlichiosis, Biological treatment, Tick-borne disease, B-cell depleting therapy, Immunocompromised patients
Background
Lyme borreliosis is the most well-described and prevalent tick-borne infection in Europe, but other tick-borne diseases such as neoehrlichiosis are on the rise in Europe [1–3]. Neoehrlichiosis is caused by Neoehrlichia mikurensis (N. mikurensis), an obligate intracellular bacterium of the Anaplasmataceae family [1, 2, 4–8]. The prevalence of N. mikurensis in Danish ticks is estimated to be 0.17–12.1% depending on the location [2, 5–7, 9, 10].
N. mikurensis seems to have low pathogenicity but can cause severe disease in immunocompromised patients [11, 12]. The known risk factors associated with severe neoehrlichiosis are splenectomy, malignant clonal B-cell disease, and B-cell depleting therapy [4, 13, 14]. The main symptom of neoehrlichiosis is prolonged fever but vascular and thromboembolic events have also been reported [11]. Other symptoms include splenomegaly, rash, cytopenia, and fatigue, which are non-specific and may be misinterpreted as another infection or even a relapse of a primary disease [14].
The use of biological therapies, for example tumor necrosis factor (TNF)-α inhibitors and monoclonal anti-CD20 antibodies, is rapidly expanding as treatments of hematological and autoimmune diseases such as B-cell lymphomas, rheumatoid arthritis, inflammatory bowel diseases, and multiple sclerosis [15, 16]. Although highly beneficial with excellent outcomes, biological therapy leaves the recipient vulnerable to infection.
Several cases of neoehrlichiosis in individuals receiving immunosuppressive therapy have been described in Europe [11, 14]. The first and only Danish case of neoehrlichiosis was published in 2020, describing a splenectomized female receiving monoclonal anti-CD20 antibodies (rituximab) as maintenance therapy for mantle cell lymphoma [17].
N. mikurensis is not detectable using standard routine blood culture. Further, no serological assays are available and only a few laboratories offer specific real-time polymerase chain reaction (PCR) analysis for its detection. Due to inadequate diagnostic tools and the treating physicians' unawareness of neoehrlichiosis, the infection may not be correctly diagnosed.
In this retrospective study, we investigated the prevalence of N. mikurensis DNA in plasma from immunocompromised patients and a group of healthy blood donors. Immunoglobulin (Ig) G antibodies against Borrelia burgdorferi sensu lato complex (B. burgdorferi s.l.) were measured as an estimate of tick exposure.
Materials and methods
Study design and participants
The samples used in this study were retrospectively retrieved from the Centre of Excellence for Personalized Medicine for Infectious Complications in Immune Deficiency (PERSIMUNE) Biobank and Data Warehouse, Copenhagen, Denmark. A search in the PERSIMUNE Biobank for available plasma samples was made using the following inclusion criteria: adult patients (≥ 18 years); clinical course at the Department of Hematology or Department of Rheumatology between the 1st of February 2015 to the 31st of December 2019; medication code for TNF-α inhibitor, recombinant monoclonal antibodies, or recombinant antineoplastic antibodies (L04AB01, L04AB02, L04AB04, L04AB05, L04AB06, L01XC02, L01XC15, L01XC17, L01XC24). In total, 239 participants during this 4-year period were considered immunocompromised either due to their hematological or rheumatological diagnosis alone or in combination with receiving immunosuppressive therapy less than a year before blood sampling. One unique plasma sample from each participant, containing 200 µL plasma, was retrieved from the biobank. Age, gender, sample date, medication initiation date, and diagnosis were the variables retrieved from the PERIMUNE data warehouse.
Plasma samples from 192 healthy blood donors donating blood in the Capital Region of Denmark (Region Hovedstaden) were retrieved from the Danish blood bank. The blood was donated between 2016 and 2019 from March to October. Age, sex, and donation date were the only available data on the blood donors.
Since whole blood is less sensitive than plasma for the detection of N. mikurensis by real-time PCR, plasma samples were used [18].
DNA Purification
A total of 200 μL plasma was used for DNA purification by DNeasy Blood and Tissue Kit (Qiagen, Germany) following the manufacturer's instructions. All purified DNA was stored at − 20 °C for later analyses. A total of 500 μL plasma was used for DNA purification of the follow-up samples using the same method as mentioned above.
Real-time PCR
A specific TaqMan probe-based real-time PCR targeting the groEL gene was performed to detect N. mikurensis. The primers and probes used have been reported elsewhere [19]. Reactions were performed in final volumes of 50 μL using 5 μL template DNA, 25 μL Platinum® Quantitative PCR SuperMix-UDG (Invitrogen, USA), 1 μM of each primer, 0.1 μM probe, 1 μM MgCl2, 1X ROX reference dye, and 12.7 μL water. A synthetic plasmid was used as a positive control [19], and a negative control was included in all runs. The real-time PCR conditions were as follows: initial denaturation at 95 °C for 2 min, 50 cycles of denaturation at 95 °C for 15 s, and annealing and extension at 60 °C for 1 min. A positive real-time PCR was defined as a cycle threshold (Ct) value of ≤ 36 combined with a proper sigmoid curve.
Amplicon sequencing
Samples with detectable N. mikurensis DNA were further amplified by PCR with negative controls run in parallel. PCR samples were loaded on a 0.9% agarose gel stained with ethidium bromide and visualized. Amplicons were purified using QIAquick PCR Purification Kit (Qiagen, Germany) according to the manufacturer’s instructions and sequenced by Sanger sequencing by StarSEQ GmbH (Mainz, Germany) in both directions using the same primers as for the real-time PCR. The sequences were trimmed, edited, and analyzed in Geneious Prime® 2022.1.1. Forward and reverse sequences were assembled by de Novo assemble, which assembles without a reference sequence, and a consensus sequence was determined based on a threshold of 50% (bases matching at least 50% of total adjusted chromatogram qualities). Following trimming at both ends, a BLAST search was performed to confirm the identity of the sequences. A multiple alignment (MUSCLE) was performed, including groEL sequences from Anaplasma phagocytophilum (MH722254.1), Ehrlichia ruminatium (CR925678.1), Candidatus Neoehrlichia chilensis (MF805782.1), Candidatus Neoehrlichia lotoris (EF633745.1), and N. mikurensis from different geographic areas and sources (JQ359067.1, CP054597.1, KU535863.1, MG182157.1, MN701626). Genetic distances were based on the Tamura-Nei model and a phylogenetic tree was constructed according to the Neighbor-Joining method [20].
ELISA for detection of IgG antibodies against B. burgdorferi s.l.
A total of 10 μL plasma was used for the detection of IgG antibodies against B. burgdorferi s.l. All patient and control samples were analyzed with the SERION ELISA classic Borrelia burgdorferi IgG test (Virion\Serion, Germany) following the manufacturer’s instructions and cut-off levels.
Statistical analyses
Based on similar prevalence in cohorts presented in the literature, a specific power calculation was made. A sample size of 150 samples would allow the detection of a 2% or higher prevalence of microbial DNA with reasonable power (80%) and confidence level (95%) [21]. Fischer’s exact test was used to compare the prevalence of N. mikurensis DNA in the two groups. Chi-squared test for homogeneity was used to compare the seroprevalence of IgG antibodies against B. burgdorferi s.l. in the two groups. p-values < 0.05 were considered significant. All statistical analyses were performed in GraphPad Prism 9.3.1 (471).
Results
Study population
The study comprised 239 samples from immunocompromised patients. Baseline characteristics are summarized in Table 1. The male to female ratio was 1.4: 1 and the median age was 65 years, interquartile range (IQR) 51–73. The cohort of blood donors had a male to female ratio of 1.1: 1 and a median age of 33 (IQR 26–46). As expected, the blood donors were significantly younger (p < 0.0001).
Table 1.
Characteristics of 239 patients investigated for Neoehrlichia mikurensis
| Number of patients | |
|---|---|
| Age, median (IQR) | 65 (51–73) |
| Male: Female | 139:100 |
| Received immunosuppressive therapy within one year prior to blood sampling, n(%) | 91 (38) |
| Treatment | |
| Monoclonal anti-CD20 antibodies, n(%) | 197 (82.4) |
| TNF-α inhibitors1, n(%) | 27 (11.3) |
| Other2, n(%) | 15 (6.3) |
| Diagnoses | |
| Lymphoma3, n(%) | 106 (44.3) |
| Chronic lymphocytic leukemia, n(%) | 57 (23.7) |
| Acute lymphocytic leukemia, n(%) | 10 (4.2) |
| Chronic myeloid leukemia, n(%) | 8 (3.3) |
| Acute myeloid leukemia, n(%) | 8 (3.3) |
| Idiopathic thrombocytopenic purpura, n(%) | 7 (2.9) |
| Myelodysplastic syndrome, n(%) | 6 (2.5) |
| Autoimmune hemolytic anemia, n(%) | 4 (1.7) |
| Arthritis4, n(%) | 9 (3.7) |
| Waldenström macroglobulinemia | 8 (3.3) |
| Wegener’s granulomatosis, n(%) | 6 (2.5) |
| Systemic lupus erythematosus, n(%) | 4 (1.7) |
| Other5, n(%) | 7 (2.9) |
Abbreviations: IQR, interquartile range; n, number; TNF, tumor necrosis factor; GVHD, graft versus host disease.
1Adalimumab, Golimumab, Certolizumab, Eternacept
2Prednisolone, Cyclophosphamide, Methotrexate, Doxorubicin, Azacitidine, Bendamustine, Obinutuzumab, Nivolumab
3Non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, Follicular lymphoma
4Rheumatoid arthritis, Lupus arthritis, Spondyloarthritis
5Psoriasis, Graft versus host disease, Myelofibrosis
Detection of N. mikurensis DNA
In total, three of the 239 (1.3%, 95% confidence interval (CI) 0.3–3.6) plasma samples from the immunocompromised patients contained detectable N. mikurensis DNA. None of the plasma samples from the 192 blood donors contained detectable N. mikurensis DNA (0%, 95% CI 0.0–1.9). The prevalence of N. mikurensis DNA in the two groups was not statistically different (p = 0.257).
Confirmation of the detected N. mikurensis DNA by Sequencing
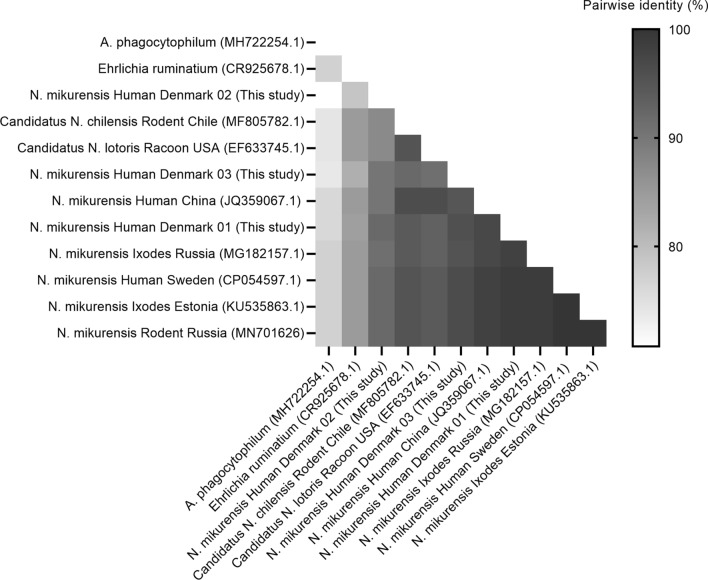
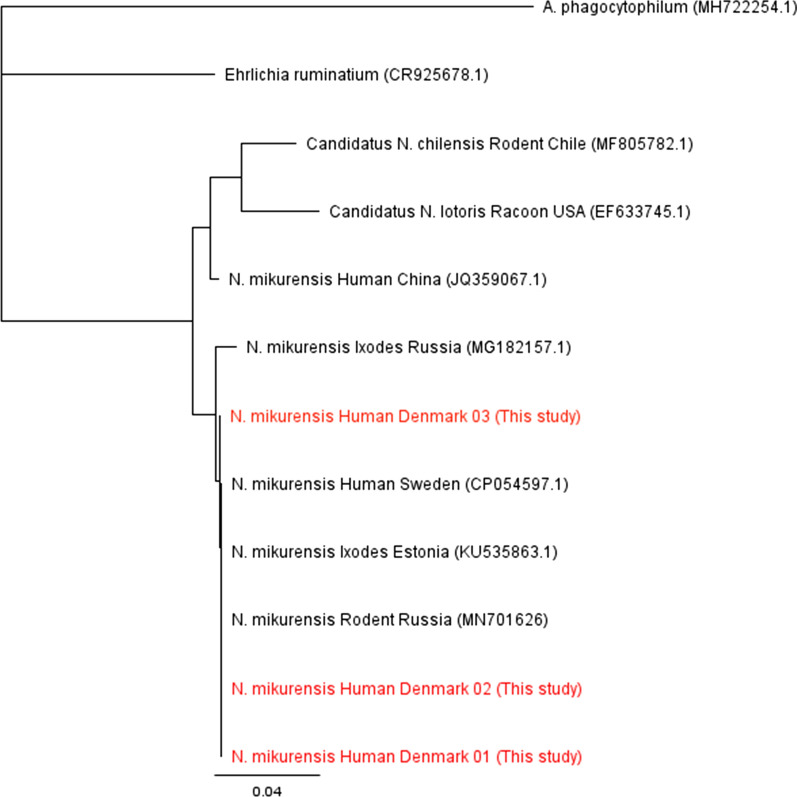
A BLAST search confirmed all three sequences to be N. mikurensis. The percentage pairwise identity of sequences from this study and N. mikurensis reference sequences ranged from 90.2–99.0% (Fig. 1). Results from the Neighbor-joining tree placed the three samples from this study in the same clade as all the included N. mikurensis sequences, except the N. mikurensis from China (JQ359067.1) (Fig. 2).
Fig. 1.
A single-color gradient pairwise nucleotide identity (%) matrix was generated from the sequences of the groEL gene from this study and selected reference sequences
Fig. 2.
Neighbor-joining phylogenetic tree based on short sequences of the groEL gene. The relationship of the sequences from this study is compared to selected reference sequences. The sequences from this study are confirmed to be from N. mikurensis
Clinical characteristics of the three cases with detectable N. mikurensis DNA
The three patients with detectable N. mikurensis DNA in a plasma sample included two females and one male (Table 2). The three patients were between 57 and 72 years at the time of blood sampling. One blood sample was collected in 2016 and two in 2019. N. mikurensis DNA was not detected in any of the follow-up samples collected and run by real-time PCR in 2022.
Table 2.
Characteristics of three patients with Neoehrlichia mikurensis detected in a plasma sample
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Sex/age | F/57 | M/72 | F/66 |
| Blood sample positive for Neoehrlichia mikurensis DNA | October, 2016 | April, 2019 | November, 2019 |
| Diagnosis above Blood sample positive for Neoehrlichia mikurensis DNA | Idiopathic thrombocytopenia | B-cell prolymphocytic leukemia | Chronic lymphocytic leukemia |
| Immunosuppressive therapy at time of blood sampling | None1 | Rituximab + Venetoclax initiated same day as blood sampling | Rituximab + bendamustine initiated same day as blood sampling |
| Time since the last dose of immunosuppressive therapy | None | Approx. 2.5 years | None |
| Symptoms | |||
| Fever | No | Yes | Yes |
| Night sweats | No | Yes | Yes |
| Fatigue | No | Yes | Yes |
| Weight loss | No | Yes | Yes |
| Thromboembolic complications | No | No | No |
| Other symptoms | Easily bruised skin | Cough | Shortness of breath during activity, tinnitus |
| Hemoglobin, reference 8.3–10.5 mmol/L (male), 7.3–9.5 mmol/L (female) | 9.7 mmol/L | 6.0 mmol/L | 5.4 mmol/L |
| Platelets, reference 145–390 × 109/L | 26 × 109/L | 144 × 109/L | 154 × 109/L |
| C-reactive protein, reference < 10 mg/L | N/A | 70 mg/L | 12 mg/L |
| Splenomegaly (CT) | N/A | Yes | Yes |
| Follow-up, May 2022 | |||
| Status of immunosuppressive therapy | Approx. 10 months since last dose | Approx. 1.5 years since last dose | Approx. 2 years since last dose |
| Follow-up real-time PCR for Neoehrlichia mikurensis DNA | Negative | Negative | Negative |
| Antibiotics registered from blood sample to follow-up sample | None |
Amoxicillin/clavulanic acid Ciprofloxacin Pivmecillinam Trimethoprim Nitrofurantoin Piperazilin/ tazobactam Azithromycin |
Piperazilin/ tazobactam Sulfamethoxazole-trimethoprim |
CT, computerized tomography; PCR, polymerase chain reaction; N/A, not applicable
1The patient did not start immunosuppressive therapy (Rituximab) until after blood sampling, the only immunosuppressive therapy prior to blood sampling was budesonide
2Only antibiotics prescribed from the hospital
Exposure to ticks by analyses of IgG antibodies against B. burgdorferi s.l.
In total, 18/239, (7.5%, 95% CI 4.8–11.6) of the plasma samples from the immunocompromised patients and 11/192 (5.7%, 95% CI 3.2–10.0) of the blood donors had detectable IgG antibodies against B. burgdorferi s.l. None of the three patients with detectable N. mikurensis DNA had detectable IgG antibodies against B. burgdorferi s.l. The seroprevalence of Borrelia-specific IgG antibodies was not significantly different (p = 0.563) in the immunocompromised patients compared with the blood donors.
Discussion
In this retrospective study, N. mikurensis DNA was detected in 1.3% (3/239) of the immunocompromised patients. All three patients had a hematological diagnosis. Follow-up samples were all negative and none of the 192 blood donor samples had detectable N. mikurensis DNA. Thus, testing for N. mikurensis in immunocompromised patients should be considered in a Danish setting. Future awareness of N. mikurensis among physicians is important to diagnose neoehrlichiosis and avoid diagnostic delay in this group of patients.
Denmark, like most of Europe and Scandinavia, is a tick endemic area, with an estimated 73.5% of the population living within 5 km of areas with tick nymphs [22]. B. burgdorferi s.l. is the most prevalent human pathogen in Danish ticks [7]. Based on the seroprevalence of Borrelia-specific IgG antibodies, tick exposure was not significantly different between the patient cohort and the blood donors (7.5% vs. 5.7%). The seroprevalence is in accordance with another recent Danish study reporting a seropositive rate of 7% in blood donors [23].
N. mikurensis has been detected in ticks from more than 18 countries in Europe with a prevalence varying from 0.3% in Poland to 25.5% in Norway [1, 24–27]. The prevalence of N. mikurensis in blood from immunocompromised patients and healthy blood donors found in this study is comparable to or slightly lower compared to our neighboring Scandinavian countries [12, 28]. This agrees with a lower prevalence of N. mikurensis in ticks collected in Denmark compared to Norway [7]. A study from southern Norway found the prevalence of N. mikurensis in a cohort of immunocompromised patients living in a tick endemic area to be 7.4% (12/163), collected in 2018 (September–December) and 2019 (March–May), and 1.2% (1/85) in a cohort of immunocompetent controls, collected in 2013/2014 [12]. A recent study from southeastern Sweden found a prevalence of 0.7% among 1006 blood donors, collected in 2019 (June–August) and 2021 (February–November) [28]. The samples from our cohort of immunocompromised patients were collected all year round, and the blood donor samples were collected from March to October. Hence, our findings may have been influenced by sampling outside “tick-season”. However, N. mikurensis has been found to persist in the bloodstream for longer periods among immunocompromised as well as in immunocompetent individuals [12, 28], although re-infection is also possible. Considerably higher prevalence was found among symptomatic individuals, with recent tick-bite exposure in Norway (10%) and Sweden (1.9%) [18, 19]. This suggests that the symptomatology of the patients under investigation and their immunological health may have a greater influence on the reported prevalence than the time of year the blood sample was collected.
The only published case of human neoehrlichiosis in Denmark describes a patient treated with rituximab, who presented with fever and a persisting rash despite antibiotic treatment [17]. Since then, three cases of neoehrlichiosis have been diagnosed at Copenhagen University Hospital, Rigshospitalet, and all three were receiving rituximab for a primary disease [29, 30]. The use of biological therapy in a variety of medical specialties is rising [15, 31, 32]. Considering the increased use of immunosuppressive therapy, the findings in the present study and the increasing number of published clinical cases around Europe, the prevalence of N. mikurensis might be higher than expected. More countries are being added to the list of published cases of neoehrlichiosis, most recently France [33], Slovenia [34], and Germany [35].
Two of three patients had symptoms attributed to neoehrlichiosis around the time N. mikurensis DNA was detected in blood, although given the retrospective nature of the study it is not definitive and could also be attributed to the hematologic malignancy or another undetected infection. No doxycycline/rifampicin treatment was documented in the three cases, but treatment could have been prescribed in a primary healthcare setting. Currently, no method for antibiotic susceptibility testing exists for N. mikurensis, and based on published cases other broad-spectrum antibiotics such as piperacillin/tazobactam, meropenem, ciprofloxacin, levofloxacin, clindamycin, cefotaxime, ceftazidime, and gentamycin are largely ineffective [4]. Since patient 2 and 3 did not receive immunosuppressive therapy for 1.5 and 2.5 years prior to the follow-up samples (Table 2), the patients may have acquired immunocompetence by the time of the follow-up, which would account for the negative follow-up samples. Patient 1 was included as a patient with assumed impaired immune system, but according to the medical record, she did not receive immunosuppressive therapy prior to the first blood sample. It is, however, not surprising as asymptomatic N. mikurensis infection has been described in both immunocompromised and immunocompetent individuals [12, 18]. Interestingly, no thromboembolic or vascular events were reported by any of the three patients, although especially thrombophlebitis and deep vein thrombosis have been associated with N. mikurensis infection in up to 63% of immunocompromised patients and 50% in immunocompetent individuals [11].
Our phylogenetic analyses of N. mikurensis DNA from Danish patients suggest a close relationship with isolates found in Sweden, Estonia, and Russia (Fig. 2), supporting that birds most likely disperse N. mikurensis infected ticks over large geographical areas as a part of their natural migration patterns [36].
Given the results from the current study and recent clinical cases from all over Europe, testing for N. mikurensis in immunocompromised patients as part of the standard investigation seems reasonable in a Danish setting as well [33].
Limitations and perspectives
The study is limited by its retrospective design, as it is not possible to ascertain that the clinical manifestations around the time of the initial blood sample in our three cases were caused by neoehrlichiosis or by another condition. No consecutive blood samples from patients with detectable N. mikurensis DNA were available in the biobank, which would have allowed us to follow the N. mikurensis bacteremia. Uncertainty remains about the time of administration of immunosuppressive therapy, some have likely received immunosuppressive therapy for the first time after the blood sampling, whereas other participants have received other types of immunosuppressives before blood sampling, and we were limited by only having data on specific immunosuppressive therapy. There was no statistically significant difference in outcome parameters between immunocompromised and blood donors. However, the analysis was underpowered, based on previous studies, we designed the study to detect a difference of 2%. A power calculation based on our newly found 1.3% among immunocompromised patients, would allow us to detect a significant difference between groups with a needed sample size of 600 in each group [21]. However, the sample size was not available for this study.
Conclusion
Neoehrlichia mikurensis DNA was detected in 1.3% of a cohort of immunocompromised patients indicating that neoehrlichiosis is likely underdiagnosed in Danish patients. Additionally, it emphasizes the likelihood of N. mikurensis being a risk in Danish patients. Therefore, prospective studies are needed to explore the clinical significance and implications in this group of patients. Screening patients receiving B-cell depleting therapy and presenting with fever for N. mikurensis could be relevant.
Acknowledgements
We thank PERSIMUNE for the opportunity to use biological material and data collected in their biobank and Data Warehouse. We thank Stine Østergaard for her useful work in the laboratory and Anna Grankvist and Christine Wennerås for useful advice on real-time PCR and for providing the positive plasmid sample. We thank the Department of Rheumatology and the Department of Hematology at Rigshospitalet, Copenhagen, Denmark for the opportunity to use biological material collected at their departments.
Abbreviations
- N. mikurensis
Neoehrlichia mikurensis
- TNF-α
tumor necrosis factor-α
- PCR
Polymerase Chain Reaction
- Ig
Immunoglobulin
- B. burgdorferi s.l.
Borrelia burgdorferi sensu lato complex
- PERSIMUNE
Personalized Medicine for Infectious Complications in Immune Deficiency
Author contributions
Material preparation, data collection, implementation of the PCR method and analysis were performed by RMMG and MFH. The first draft of the manuscript was written by RMMG and MFH. LO contributed to study conception and design. AK supervised and reviewed the writing process. RFP provided the protocol for performing the N. mikurensis real-time PCR. SRO and LH contributed with blood donor samples. SJ and UO contributed with rheumatological and hematological knowledge, respectively, and made it possible to retrieve relevant samples from the PERSIMUNE biobank. Conception, design, and supervision was performed by KAK. Conception, study design, supervision and repeated revisions of the manuscript were performed by AML and HM. All authors read and approved the final manuscript.
Funding
The project was partially supported by the Interreg North Sea Region programme, NorthTick, Grant no. J-No.: 38-2-7-19 and by Danish National Research Foundation, Grant no.: 126, Aase og Ejnar Danielsens Fond and Funding Copenhagen University Hospital, Rigshospitalet. The funding bodies had no role in the design of the study, collection, analysis, or interpretation of data nor in writing the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its additional information files].
Declarations
Ethics approval and consent to participate
The research was performed in accordance with the Declaration of Helsinki and the study protocol was approved by the Danish Regional Ethical Committee of the Capital Region (Region Hovedstaden) (j.no. H-20009322) and the Danish Data Protection Agency (approval no. P-2021-364). The patients with a blood sample with detectable N. mikurensis DNA were contacted by their treating physician to obtain written consent for the publication of clinical data and for us to contact them.
Consent for publication
The authors affirm that the three participants described in Table 2 have provided informed consent for publication of data included.
Competing interests
Anne-Mette Lebech reports speakers’ honorarium/travel grants/Advisory board activity from Gilead, speakers honorarium /travel grants from GSK, speaker’s honorarium/advisory board activity from Pfizer. Anne-Mette Lebech is supported by a grant from The Lundbeck Foundation. The other authors report no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rosa Maja Møhring Gynthersen and Mette Frimodt Hansen shared the first authorship
Contributor Information
Rosa Maja Møhring Gynthersen, Email: rosa.maja.moehring.gynthersen.01@regionh.dk.
Mette Frimodt Hansen, Email: mefrha@ruc.dk.
References
- 1.Jenkins A, Raasok C, Pedersen BN, et al. Detection of Candidatus Neoehrlichia mikurensis in Norway up to the northern limit of Ixodes ricinus distribution using a novel real time PCR test targeting the groEL gene. BMC Microbiol. 2019;19(1):199. doi: 10.1186/s12866-019-1502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klitgaard K, Kjær LJ, Isbrand A, et al. Multiple infections in questing nymphs and adult female Ixodes ricinus ticks collected in a recreational forest in Denmark. Ticks Tick-Borne Dis. 2019;10(5):1060–1065. doi: 10.1016/j.ttbdis.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Radolf JD, Strle K, Lemieux JE, et al. Lyme Disease in Humans. Curr Issues Mol Biol. 2021;42:333–384. doi: 10.21775/cimb.042.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wennerås C. Infections with the tick-borne bacterium Candidatus Neoehrlichia mikurensis. Clin Microbiol Infect. 2015;21(7):621–630. doi: 10.1016/j.cmi.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Michelet L, Delannoy S, Devillers E, et al. High-throughput screening of tick-borne pathogens in Europe. Front Cell Infect Microbiol. 2014;4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fertner ME, Mølbak L, Boye-Pihl TP, et al. First detection of tick-borne "Candidatus Neoehrlichia mikurensis" in Denmark 2011. Euro Surveill. 2012;17:8. doi: 10.2807/ese.17.08.20096-en. [DOI] [PubMed] [Google Scholar]
- 7.Kjær LJ, Klitgaard K, Soleng A, et al. Spatial patterns of pathogen prevalence in questing Ixodes ricinus nymphs in southern Scandinavia, 2016. Sci Rep. 2020;10(1):19376. doi: 10.1038/s41598-020-76334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawahara M, Rikihisa Y, Isogai E, et al. Ultrastructure and phylogenetic analysis of 'Candidatus Neoehrlichia mikurensis' in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int J Syst Evol Microbiol. 2004;54(Pt 5):1837–1843. doi: 10.1099/ijs.0.63260-0. [DOI] [PubMed] [Google Scholar]
- 9.Stensvold CR, Al Marai D, Andersen LO, et al. Babesia spp and other pathogens in ticks recovered from domestic dogs in Denmark. Parasit Vectors. 2015;8:262. doi: 10.1186/s13071-015-0843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klitgaard K, Højgaard J, Isbrand A, et al. Screening for multiple tick-borne pathogens in Ixodes ricinus ticks from birds in Denmark during spring and autumn migration seasons. Ticks Tick-Borne Dis. 2019;10(3):546–552. doi: 10.1016/j.ttbdis.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Höper L, Skoog E, Stenson M, et al. Vasculitis due to Candidatus Neoehrlichia mikurensis: a Cohort Study of 40 Swedish Patients. Clin Infect Dis. 2021;73(7):e2372–e2378. doi: 10.1093/cid/ciaa1217. [DOI] [PubMed] [Google Scholar]
- 12.Quarsten H, Salte T, Lorentzen ÅR, et al. Tick-borne pathogens detected in the blood of immunosuppressed norwegian patients living in a tick-endemic area. Clin Infect Dis. 2021;73(7):e2364–e2371. doi: 10.1093/cid/ciaa971. [DOI] [PubMed] [Google Scholar]
- 13.Andréasson K, Jönsson G, Lindell P, et al. Recurrent fever caused by Candidatus Neoehrlichia mikurensis in a rheumatoid arthritis patient treated with rituximab. Rheumatology (Oxford) 2015;54(2):369–371. doi: 10.1093/rheumatology/keu441. [DOI] [PubMed] [Google Scholar]
- 14.Grankvist A, Andersson PO, Mattsson M, et al. Infections with the tick-borne bacterium "Candidatus Neoehrlichia mikurensis" mimic noninfectious conditions in patients with B cell malignancies or autoimmune diseases. Clin Infect Dis. 2014;58(12):1716–1722. doi: 10.1093/cid/ciu189. [DOI] [PubMed] [Google Scholar]
- 15.Abbasi M, Mousavi MJ, Jamalzehi S, et al. Strategies toward rheumatoid arthritis therapy; the old and the new. J Cell Physiol. 2019;234(7):10018–10031. doi: 10.1002/jcp.27860. [DOI] [PubMed] [Google Scholar]
- 16.Bonovas S, Fiorino G, Allocca M, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2016;14(10):1385–97.e10. doi: 10.1016/j.cgh.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 17.Porskrog A, Himmelstrup BM. Tick-borne CandidatusNeoehrlichiamikurensis was the cause of fever in a haematological patient. Ugeskrift for laeger. 2020;182:40. [PubMed] [Google Scholar]
- 18.Quarsten H, Grankvist A, Høyvoll L, et al. Candidatus Neoehrlichia mikurensis and Borrelia burgdorferi sensu lato detected in the blood of Norwegian patients with erythema migrans. Ticks Tick-Borne Dis. 2017;8(5):715–720. doi: 10.1016/j.ttbdis.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Grankvist A, Sandelin LL, Andersson J, et al. Infections with Candidatus Neoehrlichia mikurensis and Cytokine Responses in 2 Persons Bitten by Ticks. Sweden Emerg Infect Dis. 2015;21(8):1462–1465. doi: 10.3201/eid2108.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 21.ClinCalc. https://clincalc.com/stats/samplesize.aspx.
- 22.Kjær LJ, Soleng A, Edgar KS, et al. Predicting and mapping human risk of exposure to Ixodes ricinus nymphs using climatic and environmental data, Denmark, Norway and Sweden, 2016. Euro Surveill. 2019;24:9. doi: 10.2807/1560-7917.ES.2019.24.9.1800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen BB, Bruun MT, Jensen PM, et al. Evaluation of factors influencing tick bites and tick-borne infections: a longitudinal study. Parasit Vectors. 2021;14(1):289. doi: 10.1186/s13071-021-04751-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen BN, Jenkins A, Paulsen KM, et al. Distribution of Neoehrlichia mikurensis in Ixodes ricinus ticks along the coast of Norway: the western seaboard is a low-prevalence region. Zoonoses Public Health. 2020;67(2):130–137. doi: 10.1111/zph.12662. [DOI] [PubMed] [Google Scholar]
- 25.Larsson C, Hvidsten D, Stuen S, et al. "Candidatus Neoehrlichia mikurensis" in Ixodes ricinus ticks collected near the Arctic Circle in Norway. Parasit Vectors. 2018;11(1):620. doi: 10.1186/s13071-018-3168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kjelland V, Paulsen KM, Rollum R, et al. Tick-borne encephalitis virus, Borrelia burgdorferi sensu lato, Borrelia miyamotoi, Anaplasma phagocytophilum and Candidatus Neoehrlichia mikurensis in Ixodes ricinus ticks collected from recreational islands in southern Norway. Ticks Tick-Borne Diseases. 2018;9(5):1098–1102. doi: 10.1016/j.ttbdis.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Portillo A, Santibáñez P, Palomar AM, et al. Candidatus Neoehrlichia mikurensis. Europe New Microbes New Infect. 2018;22:30–36. doi: 10.1016/j.nmni.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labbé Sandelin L, Olofsson J, Tolf C, et al. Detection of Neoehrlichia mikurensis DNA in blood donors in southeastern Sweden. Infect Dis (Lond) 2022;54(10):748–759. doi: 10.1080/23744235.2022.2087732. [DOI] [PubMed] [Google Scholar]
- 29.Gynthersen RMM, Mahdaoui SE, Stensvold CR, et al. Tick-borne bacterium Neoehrlichia mikurensis - an emerging safety concern during CD20-depleting therapy? – A case report. [E-poster presentation. ECTRIMS]. In press 2022.
- 30.Gynthersen RMM, Stensvold CR, Nielsen SL, et al. The first case of hemophagocytic lymphohistiocytosis triggered by the tick-borne bacterium, Neoehrlichia mikurensis. [Poster presentation. ESCMID]. In press 2022.
- 31.Miyazaki Y, Niino M. B-cell depletion therapy for multiple sclerosis. Immunological medicine. 2022;45(2):54–62. doi: 10.1080/25785826.2021.1952543. [DOI] [PubMed] [Google Scholar]
- 32.Brown JR, Cymbalista F, Sharman J, et al. The Role of Rituximab in Chronic Lymphocytic Leukemia Treatment and the Potential Utility of Biosimilars. Oncologist. 2018;23(3):288–296. doi: 10.1634/theoncologist.2017-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyer PH, Baldinger L, Degeilh B, et al. The emerging tick-borne pathogen Neoehrlichia mikurensis: first French case series and vector epidemiology. Emerging microbes & infections. 2021;10(1):1731–1738. doi: 10.1080/22221751.2021.1973347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lenart M, Simoniti M, Strašek-Smrdel K, et al. Case report: first symptomatic Candidatus Neoehrlichia mikurensis infection in Slovenia. BMC Infect Dis. 2021;21(1):579. doi: 10.1186/s12879-021-06297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumoulin B, Schriefer TC, Henes FO, et al. Neoehrlichiosis as a cause of fever of unknown origin. Z Rheumatol. 2022;81(5):427–429. doi: 10.1007/s00393-021-01156-3. [DOI] [PubMed] [Google Scholar]
- 36.Labbé Sandelin L, Tolf C, Larsson S, et al. Candidatus Neoehrlichia mikurensis in Ticks from Migrating Birds in Sweden. PLoS ONE. 2015;10(7):e0133250. doi: 10.1371/journal.pone.0133250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its additional information files].