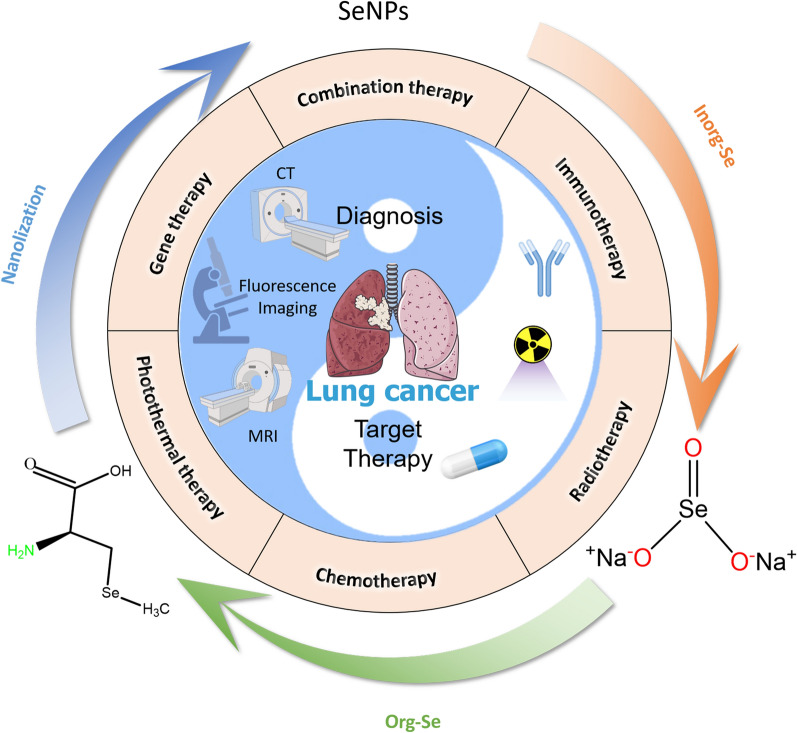
Abstract
The incidence and mortality rates of lung cancer are among the highest in the world. Traditional treatment methods include surgery, chemotherapy, and radiotherapy. Although rapid progress has been achieved in the past decade, treatment limitations remain. It is therefore imperative to identify safer and more effective therapeutic methods, and research is currently being conducted to identify more efficient and less harmful drugs. In recent years, the discovery of antitumor drugs based on the essential trace element selenium (Se) has provided good prospects for lung cancer treatments. In particular, compared to inorganic Se (Inorg-Se) and organic Se (Org-Se), Se nanomedicine (Se nanoparticles; SeNPs) shows much higher bioavailability and antioxidant activity and lower toxicity. SeNPs can also be used as a drug delivery carrier to better regulate protein and DNA biosynthesis and protein kinase C activity, thus playing a role in inhibiting cancer cell proliferation. SeNPs can also effectively activate antigen-presenting cells to stimulate cell immunity, exert regulatory effects on innate and regulatory immunity, and enhance lung cancer immunotherapy. This review summarizes the application of Se-based species and materials in lung cancer diagnosis, including fluorescence, MR, CT, photoacoustic imaging and other diagnostic methods, as well as treatments, including direct killing, radiosensitization, chemotherapeutic sensitization, photothermodynamics, and enhanced immunotherapy. In addition, the application prospects and challenges of Se-based drugs in lung cancer are examined, as well as their forecasted future clinical applications and sustainable development.
Graphical Abstract
Keywords: Lung cancer, Selenium (Se), Selenium nanomedicines, Nanotechnology, SeNPs, Theranostic applications
Introduction
According to 2020 global cancer statistics, the cancer with the highest incidence (11.4%) and mortality (18%) worldwide is lung cancer [1]. The 5-year relative survival rate for lung cancer is 19%, and that of patients with small-cell lung cancer (SCLC) is only 2% [2]. The aggressive progression of lung cancer and the high mortality rate of cancer patients have aroused great concern among scientists [3]. The major forms of therapy for lung malignancies continue to be surgery, chemotherapy, and radiotherapy (RT) [4]. However, surgery is a highly traumatic local therapy option. Chemotherapy and radiation cause side effects, such nausea, vomiting, and bone marrow suppression, thus, it difficult to employ these treatments in clinical settings. Recently, even though immunotherapies and gene mutation therapies that specifically targeted lung cancer have demonstrated excellent therapeutic effects, acquired resistance to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) is unavoidable. Most patients lose their ability to respond to TKIs after 1 year, their condition worsens quickly [5], and there is only a 20% overall response rate for immunotherapy [6]. Therefore, safer and more efficient treatment modalities are urgently needed in the therapeutic setting.
Drug research and development to diagnose and treat advanced lung cancer has advanced quickly in recent years. Examples include ALK inhibitors (crizotinib [7], ceritinib [8], lorlatinib [9]), second-generation EGFR-TKIs (afatinib [10], dacomitinib [11]), and third-generation EGFR-TKIs (osimertinib [12], olmutinib [13]). In particular, immunotherapy (nivolumab [14], pembrolizumab [15]) has been used widely and achieved remarkable results. By focusing on PD-1 on the surface of T cells, these immunotherapies aim to enhance the body's immune system and the tumor immune microenvironment (TIME). TIME consists of environmental chemicals, immune cells, and cytokines [16]. Recent research has demonstrated that peripheral chemicals contribute significantly to the tumor microenvironment. According to a recent study, cancers are not sterile environments as previously thought since they contain microorganisms that help tumor cells spread by extending their ability to survive after leaving the initial tumor [17]. TIME also involves nutritional regulation. According to studies, patients with malignant pleural effusion caused by advanced lung cancer show serum levels of selenium (Se) that are lower than those of healthy individuals [18]. This further demonstrates the significance of Se.
Se, which is known as selenoprotein, is a unique and necessary trace element [19, 20] and plays a significant role in many physiological processes in humans. Se has been studied for many years as a cancer treatment, and various studies have examined its function as a chemopreventive agent in lung cancer [21]. According to the findings of epidemiological and clinical studies, Se supplementation can considerably lower the incidence of lung cancer in people with low baseline levels of Se. Additional research verified the crucial role of Se in preventing oxidative DNA damage and increasing DNA repair, indicating its potential significant role in carcinogenesis [22, 23]. Although the importance of Se is becoming more widely acknowledged, the use of existing Se supplements (inorganic selenium [Inorg-Se] and organic selenium [Org-Se]) is constrained by issues such as poor absorption and increased toxicity. Se supplementation, at a serum level of 106 ng/mL, decreased the risk of lung cancer, but a higher Se level (121.6 ng/mL) increased the risk of lung cancer and was associated with diabetes [24]. SeMet exposure has also been shown to enhance radiosensitivity in human lung cancer cell lines without damaging normal lung cell lines. The doses used to treat cells in a study may be much higher than those needed for research in vivo [25]. Therefore, the appropriate dose for conducting in vivo studies must be determined. Creating novel systems as Se compound transporters is critical. The most recent advancements in nanotechnology have addressed this urgent problem.
Nanotechnology is a high-tech discipline that combines basic multidisciplinary research with basic applications to create materials or structures that range in size from 0.1 nm to 100 nm. Currently, nanotechnology is being used in medicine, creating the field of nanomedicine. Nanomedicine is a branch of science and technology in which molecular instruments and knowledge of the human body at the molecular level are used to identify, treat, and prevent disease and traumatic injury while reducing pain and preserving and enhancing human health [26]. As a result, the diagnosis and treatment of cancers is the most popular area of study in nanomedicine. A nanoscale drug delivery system is a device for concentrating medications and therapeutic substances in specific tissues and organs [27, 28]. Strong cell uptake, low toxicity to normal cells, and increased anticancer activity are all benefits of using nanoparticle-mediated drug delivery in cancer therapy [29–31]. Consequently, nanoscale drug delivery systems have emerged as a popular area of study. Similar to recent hot research topics, nanodrugs are increasingly becoming apparent. When treating advanced ovarian cancer, for instance, doxorubicin (DOX) hydrochloride liposome nanomedicine (Doxil) is frequently employed [32, 33]. Advanced ovarian cancer, breast cancer, and lung cancer are treated regularly with nanoparticle albumin-bound paclitaxel (Abraxane®) [34–37]. Se possesses antitumor properties as well, and SeNPs are utilized more often and are less hazardous. SeNPs that have undergone nanotechnology modifications have proven to be less harmful and more popular. To enhance immunotherapy against prostate cancer, Lai et al. [38] developed a selenium-containing ruthenium complex with natural killer cells. Liao et al. [39] investigated the use of selenium nanoparticles (SeNPs) to treat tumors by upregulating mir-16.
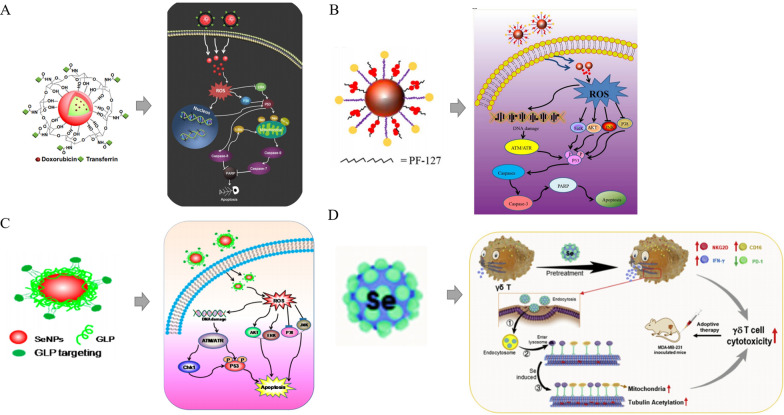
In comparison to inorganic and organic compounds (in which inorganic forms are more harmful than organic ones [40–42]), SeNPs have gained considerable attention due to their high bioavailability, strong biological activity, and low toxicity. More suitable items have been created using nanotechnology to guarantee their physiological and therapeutic effects. SeNPs have a wide variety of biological applications, have been developed for dietary supplements as well as therapeutic agents and do not exhibit noticeable side effects in lung cancer. Their impact on decreasing oxidative stress is also well documented [43, 44]. According to the findings of several researchers, SeNPs are beneficial in the chemoprevention of lung cancer [45] as a potential anticancer medication and carrier of anticancer drugs. Tian et al. demonstrated that nanoselenium combined with radiofrequency therapy significantly inhibited lung cancer cell migration and invasion; in addition, the treatment significantly inhibited the expression of the proliferation-related proteins CCND1 and c-Myc and the invasion- and migration-related proteins MMP2 and MMP9, causing lung cancer cells to undergo apoptosis. [46]. Our research group has conducted extensive research on the application of nanoselenium in the treatment of cancer, and the technology for treating lung cancer is relatively mature. SeNPs have gained much attention as potential cancer therapeutic payloads. Transferrin (Tf)-coupled SeNPs were synthesized and used in the present study to enhance cellular uptake and anticancer efficacy as cancer-targeted drugs [47] (Fig. 1A). During the research, it was also found that some SeNPs may lead to drug toxicity and produce adverse side effects for cancer patients. Multidrug resistance is among the biggest challenges in cancer treatment. The uptake of SeNPs was significantly enhanced by folate (FA) surface coupling through nystatin-dependent and clathrin-mediated endocytosis of FA receptors [48] (Fig. 1B). Second, as a surface decoration agent, Gracilaria lemaneiformis polysaccharide (GLP), a polysaccharide of Gracilaria lemaneiformis, stabilizes SeNPs and can be controlled in size. GLP − SeNPs showed high selectivity between normal and cancer cells, effectively improving cell uptake and anticancer effects [49] (Fig. 1C). In recent years, immune cell therapy has provided a paradigm for treating malignant tumors. SeNP-pretreated immune cells significantly upregulated the expression of the cytotoxicity-related molecules NKG2D, CD16, IFN-γ and other cells while downregulating the expression of PD-1 in γδ T cells. SeNPs can significantly enhance the antitumor cytotoxicity of immune cells [50] (Fig. 1D). Based on the above studies, the researchers also found that Polyporus rhinoceros water-soluble polysaccharide-protein complex (PRW) surface decoration significantly enhanced the uptake of SeNPs by cells through endocytosis. PRW-SeNPs significantly inhibited the growth of A549 cells by inducing apoptosis and G2/M phase arrest. It is possible that PRW interacts specifically with biomolecules and receptors on the cell membrane of cancer cells, thus enhancing the uptake of SeNPs by cells and increasing their cytotoxicity to A549 cells [51]. Additionally, the immunostimulatory action of SeNPs has been verified [52].
Fig. 1.
Rational design of different SeNPs for cancer treatments. A The internalized Tf-SeNPs trigger the overproduction of intracellular reactive oxygen species (ROS), thereby activating the p53 and mitogen-activated protein kinase (MAPK) pathways and promoting MCF-7 cell apoptosis [47]. Copyright 2013, Elsevier Ltd. B Internalized FA-SeNPs trigger ROS overproduction and induce apoptosis of HePG2 cells by activating the p53 and MAPK pathways [48]. Copyright 2015, Elsevier Ltd. C After the application of GLP − SeNPs, the p53, MAPK and AKT pathways are activated to promote the apoptosis of U87 cells [49]. Copyright 2014, American Chemical Society. D Schematic diagram of SeNP-induced modulation of γδ T cells [50]. Copyright 2019, Elsevier Ltd
The aim of this article was to summarize the recent progress of selenium nanomedicines in the treatment of lung cancer. The introduction will cover several forms of Se, Se compounds, SeNPs, SeNP drugs, and their roles. SeNPs and SeNP-drugs provide a fresh viewpoint on the treatment of lung cancer. In relation to targeted therapies for lung cancer, the article highlights the most significant recent advances in preclinical and clinical research. We also describe current obstacles and offer an overview of possible future perspectives as well as their potential clinical applications in this rapidly developing field.
Clinical studies of Se in respiratory disease treatment
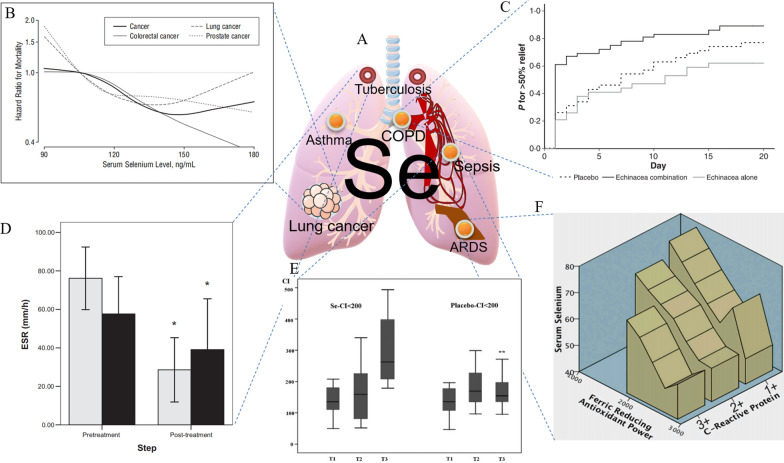
With epidemiological information on lung cancer, researchers have been interested in using Se as a vital and distinctive trace element for treating respiratory disorders (Fig. 2A). Early in 1990, Yu et al. [53] studied the use of Se for preventing lung cancer among Yunnan miners and concluded that Se exhibits an inhibitory effect on lung cancer. In 2002, Reid et al. [54] conducted a follow-up study on Se supplementation and the incidence of lung cancer, showing that Se supplementation could reduce the incidence of lung cancer and was negatively correlated with the incidence of lung cancer (Fig. 2B). Gradually, the application of Se to other respiratory diseases has been reported. A UK study evaluated the effects of Se supplementation on secondary prevention of mild to moderate adult asthma [55]. Isbaniah et al. [56] demonstrated that Se supplementation alleviates exacerbations of chronic obstructive pulmonary disease (COPD) (Fig. 2C). In terms of Se forms, Youssef et al. [57] showed that supplementation with sodium selenite provides a positive effect on the clinical prognosis of low airway respiratory diseases. Further research has shown that Se is mainly used against respiratory disease to promote immune function. Se supplementation enhances immune activity and enhances immune mechanism effects in corticosteroid-dependent asthma [58]. In tuberculosis patients, the immunomodulatory effects of Se were shown to benefit treatments and improve immunity [59], and Se supplementation activated the immune system, improved nutritional deficiency by reducing oxidative stress, and improved the clinical cure rate in patients with tuberculosis [60] (Fig. 2D). In current smokers, Se supplementation reduced the annual decline rate of forced expiratory flow [61]. In another clinical study focused on the relationship between antioxidant nutrients and lung function, Hu et al. [62] found that serum Se exhibited a strong positive correlation with lung function. The same results were found for patients with sepsis. Although research showed that Se supplementation could improve respiratory function, we must also consider that the survival rate of patients was not improved [63] (Fig. 2E). In patients with acute respiratory distress syndrome (ARDS), Mahmoodpoor et al. also found that Se supplementation could moderately regulate the inflammatory response and improve respiratory function [64] (Fig. 2F). In addition, if Se is used improperly, many side effects can occur. For example, Karp et al. [65] have shown that long-term, large amounts of Se supplementation can lead to adverse effects, such as dyspnea, weakness, nail changes, and dry skin.
Fig. 2.
Applications of Se in different respiratory diseases. A Application map of Se diseases. B Comparison of lung cancer mortality after Se supplementation [66]. Copyright 2008, American Medical Association. C Changes in remission rate after Se combined treatment of COPD [56]. Copyright 2010, Blackwell Publishing Ltd. D ESR changes in Se after treatment of pulmonary tuberculosis [60]. Copyright 2007, The Authors. E Changes in the oxygenation index after Se supplementation [63]. Copyright 2014, The Canadian Society of Clinical Chemists. F Three-dimensional description of changes in serum Se, including the effect of bronchoalveolar lavage fluid on the iron-reducing antioxidant capacity (FRAP) of C-reactive protein (CRP) [64]. Copyright 2018, Taylor & Francis
Although the Se use of is increasing, its application and the clinical studies of Se are not comprehensive, and the forms, methods and means of Se use have not been summarized. Many studies have shown that we lack effective low-drug Se. Recently, it has been encouraging to see that many Se-based drugs, especially SeNP-based drugs, have been developed for respiratory diseases, with a focus on lung cancer.
Se forms and their use in lung cancer treatment
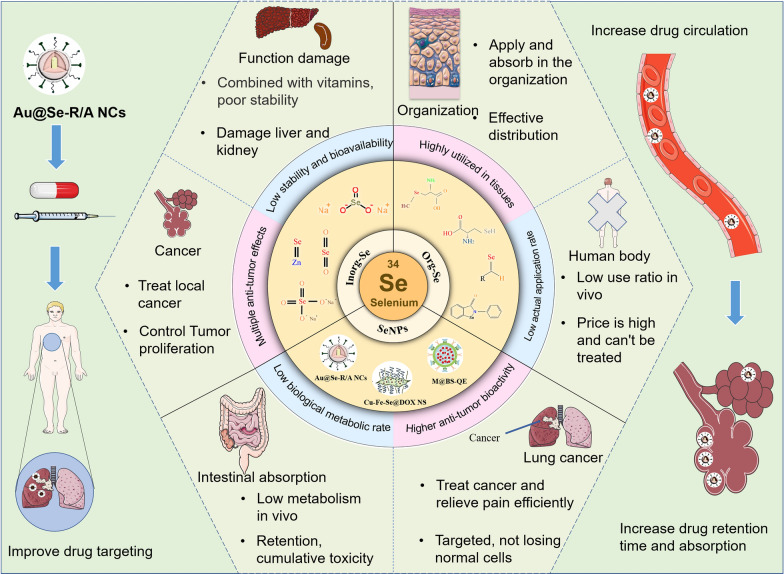
In our daily lives, different Se compounds, mostly Inorg-Se and Org-Se, can be obtained from food. Se is mostly found in organic compounds, such as selenomethionine, as well as in inorganic compounds, such as selenite and selenate. Typically, they are transformed into the metabolite hydrogen selenide (H2Se), which is the building block for selenophosphate. Glutamylmethylselenocysteine can also produce methylselenol (CH3SeH). It is possible to change H2Se and CH3SeH into one another. In contrast to natural forms of Se, compounds, such as methylseleninic acid (MSA) [67], can be synthesized in the laboratory. Different Se molecules enter the metabolic pathway at various times through the actions of various enzymes. Selenoproteins always have a biological function in cells synthesized from selenophosphate, but some effects and/or mechanisms of Se are specific to certain forms of Se [68–70]. In addition, with the development of technology, more stable and nontoxic SeNPs have been synthesized by nanotechnology (Fig. 3).
Fig. 3.
Different Se forms used in lung cancer treatments. The distinguishing degree and characteristics of the three forms of Se are shown [46, 71–77]. Copyright 2020, Tian, Wei, Zhang and Xu. Copyright 2020, Alkie et al. Copyright 2008, Elsevier B.V. Copyright 2001, Oxford University Press. Copyright 2021 by the authors. Copyright 2020, Chen, Li, Cong, Yu, Zhu, Barba, Marszalek, Puchalski and Cheng. Copyright 2000, Springer-Verlag New York Inc. Copyright 2019, Elsevier B.V
Inorg-Se in tumor treatment
Most of the early studies concentrated on Inorg-Se and have provided considerable evidence demonstrating the in vitro antitumor effects of Se. Inorg-Se agents exhibit a cytotoxic effect, which can directly kill cancer cells and inhibit their aberrant proliferation [78]. Inorg-Se also show a differentiation-promoting effect on cancer cells, which can result in reducing the invasiveness of tumors and ameliorating the prognosis of lung cancer patients [79]. In addition, Inorg-Se was shown to improve the response to chemotherapy [80] and has the ability to reduce the systemic toxicity of cancer chemotherapeutic drugs [81–83]. Other studies have shown that Se not only combats renal and cardiac toxicity by increasing intracellular superoxide dismutase (SOD) and glutathione peroxidase (GPX) levels and activities to inhibit peroxide-induced nuclear factor kappa beta (NF-κB) activation but also stimulates the production of immunoglobins and antibodies, which could improve the systemic immunity of patients [73, 84].
Overall, Inorg-Se is a promising and inexpensive antitumor agent with multiple antitumor effects, as confirmed by laboratory studies. However, Inorg-Se must bind to organic ligands in the gut before it can be absorbed by the human body, but it can also easily bind to vitamins in the body [85]. As many factors compete with Se for organic ligands in the intestinal tract, the absorption rate, stability and bioavailability of Se are low [74]. In addition, Inorg-Se is relatively toxic; if the human body overdoses locally, it can cause irreversible damage [72].
Thus, considering the good antitumor efficacy of Se but limited employment of Inorg-Se, researchers have worked to overcome the shortcomings of Inorg-Se to develop Org-Se and SeNPs. For example, Inorg-Se can be converted to Org-Se by natural transformation (through biochemical mechanisms in the body through plants, animals and microorganisms) and artificial synthesis (using chemical methods) [86, 87], SeNPs are prepared by nanotechnology [88]. Consequently, Se products featuring low toxicity and good stability have been synthesized.
Org-Se in tumor treatment
Org-Se can be stored easily in tissues, absorbed, and rapidly utilized by the human body after absorption. Org-Se has been shown to be associated with four types of cell death pathways, including cell cycle arrest, autophagy, apoptosis and necrocytosis [89], indicating its potential anticancer application. One of the best-known Org-Se compounds, ebselen—an Org-Se compound with antioxidant and anti-inflammatory properties—is a GPX mimetic and excellent peroxynitrite scavenger. Ebselen has a Se-N bond as a stimulated GPX active site, as well as a protecting Se-C bond structure to prevent Se atom release and maintain relatively low systemic toxicity compared to that of Inorg-Se. With regard to its antitumor effect, ebselen mainly acts by inhibiting thioredoxin activity in tumor cells, regulating downstream pathways and inducing tumor cell apoptosis [90]. In addition, similar to Inorg-Se, Org-Se has the ability to reduce the systemic toxicity of cancer chemotherapeutic drugs. Hu et al. observed reduced nephrotoxicity and leucopenia in solid tumor patients receiving cisplatin accompanied by seleno-kappacarrageenan, an Org-Se agent used clinically [91].
Based on previous studies, current Org-Se research in our group focuses mainly on antitumor targets and mechanisms. On the one hand, a new target has been found by using chemical biology and other techniques, which confirmed the mechanism of interaction among p53, Org-Se, and TrxR targets [92]. On the other hand, the sensitizing effect of Org-Se on RT and chemotherapy has been evaluated extensively [93]. These conclusions may enrich our knowledge of the antitumor effects of Org-Se.
Org-Se generally exists in the form of selenomethionine, which is involved in protein synthesis and is easily stored, absorbed and highly utilized in tissues. At the same time, Org-Se exhibits reduced toxicity and better biocompatibility than that of Inorg-Se, although unavoidable problems, such as potential systemic toxicity, remain [75]. In terms of safety, Org-Se does not have a very strong advantage over Inorg-Se, and research shows that both forms are subchronic toxic agents; moreover, the Org-Se production process is complex and costly, and the practical applications are fewer [76]. To solve this problem, nanosized Org-Se can be prepared by using nanotechnology to reduce toxicity and improve the safety of Org-Se [94, 95]. Therefore, virtually nontoxic and more potent SeNPs have emerged into the medical field and have become a hot topic in the cancer treatment field.
SeNPs in tumor treatment
As noted already, nanotechnology has greatly suppressed the potential toxicity associated with Inorg-Se and Org-Se while improving the targeting of drugs and realizing the development of personalized therapy. In nanomedicine, nanoparticles (NPs) have emerged as attractive carriers for intracellular delivery of drugs [96]. Due to their accumulation in tumor tissue, nanoparticles have the potential to kill tumor cells locally at high concentrations, increase the curative effect, and reduce the side effects and toxic effects of drugs. Therefore, NPs can be used to create special drug carriers. An analysis of patents and literature showed that the materials used as drug carriers mainly included metal NPs [97], inorganic nonmetallic NPs [98], biodegradable polymer NPs [99], and bioactive NPs [100]. However, the cytotoxic effects of a large number of inorganic NPs have been evaluated in cancer cells, and SeNPs were found to show the greatest potential as new antitumor drug candidates [46].
Compared with Inorg-Se and Org-Se, SeNPs were shown to have lower cytotoxicity and higher antitumor bioactivity [77]. The antitumor mechanism of NPs, particularly SeNPs, has been widely proposed, including inhibiting cell proliferation, inactivating carcinogens, stimulating the immune system and promoting cell apoptosis. The latter is the major mechanism in their antitumor effect at the molecular level [48, 77, 101, 102]. It has been well documented that oxidative stress and the formation of ROS are the major signaling pathways mediating the cytotoxicity induced by SeNPs, in which ROS can be modulated to induce intrinsic apoptosis by modifying the activity of enzymes involved in cell death pathways [48, 101, 102]. SeNPs appear to be more effective at scavenging free radicals than other forms of Se [103]. SeNPs have also been found to exhibit extraordinary effects in areas such as combined chemotherapy, radiosensitization, resistance to chemotoxicity, immunotherapy, and photothermal therapy (PTT) of cancers [104–107]. For example, Ru-MUA@Se [108], CFS@DOX NSs [109], UCNFs-Bi2Se3 [110], etc., can be applied to cancer diagnostic imaging; PEG-SeNPs [111], PHD2 [112], RBCs@Se/Av[113], FA@SeNPs [114], Se@MUN [115], TeSe [116], SeNPs@LNT [18], etc., can be applied to cancer treatment. They may act either directly or through radiochemotherapy sensitization and immunotherapy to play an anticancer role.
Overall, this review elaborates on the cutting edge of SeNPs in the field of lung cancer therapy.
Functional design and synthesis of Se-based nanomedicine for lung cancer theranostic applications
Synthesis and application of SeNPs in the treatment of lung cancer
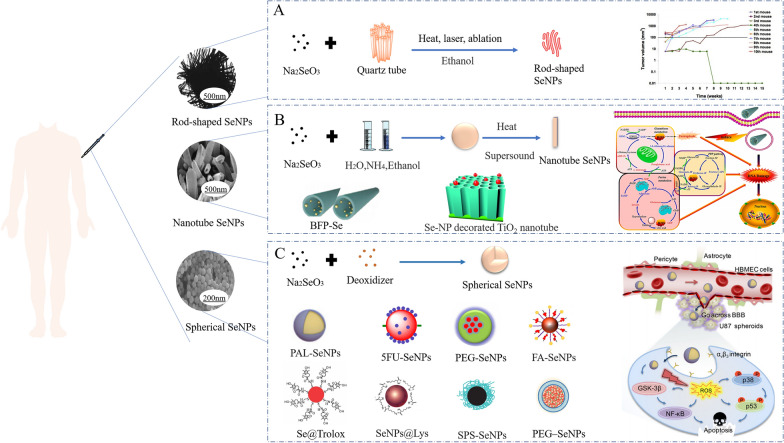
The size and morphology of SeNPs can affect their biological activity and uptake capacity of cells [127]. Therefore, it is very important to select the appropriate method for preparing the desired nano size and morphology of SeNPs (Fig. 4). In view of this, SeNPs can be synthesized with the following methods: physical synthesis, biological synthesis, and chemical synthesis (Table 1).
Fig. 4.
Morphology of different SeNPs used in cancer treatment and their biological application. A Sodium selenite and quartz tubes were treated with alcohol by heat, laser, and ablation to synthesize rod-shaped SeNPs [117, 118]. Copyright 2002, Elsevier Science B.V. Copyright 2018, IMSS. B Nanotube SeNPs were synthesized with sodium selenite, water, ammonia and ethanol by heating and sonication [119–121]. Copyright 2004, American Chemical Society. Copyright 2022, Elsevier B.V. Copyright 2019, Bilek et al. C Synthesis of spherical SeNPs from sodium selenite and potato extract [48, 104, 107, 113, 122–126]. Copyright 2015, Elsevier Inc. Copyright 2012, American Chemical Society. Copyright 2015, Elsevier B.V. Copyright 2006, The Royal Society of Chemistry. Copyright 2003, Regional SOCIETY OF CHEMISTRY. Copyright 2013, Royal Society of Chemistry. Copyright 2018, WILEY‐VCH. Copyright 2018, Royal Society of Chemistry
Table 1.
Characteristics of the three methods of SeNP synthesis for lung cancer
| Production method | SeNPs characteristics | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Physical synthesis |
• γ-radiation • Microwave radiation • Pulsed laser ablation in liquids (PLAL) |
• Rapid and uniform production • Environmentally friendly high physical properties • Controlled and not easily contaminated by chemical reagents |
• Low efficiency • Low yield • High energy consumption • Easy sample contamination • Uneven particle size |
• [128–130] |
| Biological synthesis |
• Biological synthesis uses plant extracts, fungi and bacteria to synthesize biogenic SeNPs • Microbial synthesis refers to the reduction of other forms of Se to SeNPs through microbial metabolism products |
• Safe, hypotonicity • Recyclable • Environmentally friendly • High stability |
• Conditions are very strict, not very simple • Poor quality control • SeNPs surface composition unknown |
• [131–136] |
| Chemical synthesis | • Through REDOX reaction |
• Simple process • Controllability • Stable chemical structure • Diameter of uniform |
• High cost of production • Presence of toxic byproducts |
• [50, 137–140] |
Physical synthesis and application of SeNPs
SeNPs have been prepared by physical synthesis using γ-radiation and microwave radiation [128], and pulsed laser ablation in liquids (PLAL) is a novel preparation technique for preparing pure naked SeNPs [129]. For example, Guisbiers et al. obtained SeNPs with a particle size of 115 ± 38 nm by pulsed laser ablation of pure Se pellets immersed in deionized water [130]. Physical synthesis can be used to prepare SeNPs with environmental benignity and high physical properties. However, there are some problems, such as high energy consumption, easy sample contamination, and uneven particle size.
Biological synthesis and application of SeNPs
In biological synthesis, plant extracts, fungi and bacteria are used to synthesize biogenic SeNPs. Microbial synthesis refers to the reduction of other forms of Se to SeNPs through microbial metabolism products [141, 142]. As a secondary metabolite and function of the plant extract, phenols, flavonoids, amines, alcohols, proteins and aldehydes are involved in reducing Se to SeNPs [143]. For instance, Fesharaki et al. cultured Klebsiella pneumoniae-containing SeNPs and released SeNPs with a particle size of 245 nm [142]. Biological synthesis is safe, environmentally friendly and recyclable. However, the production method and conditions are very strict and not very simple. Several parameters and steps remain to be optimized in the biosynthesis of SeNPs.
Chemical synthesis and application of SeNPs
The chemical synthesis of SeNPs is prepared through the REDOX reaction, in which selenate or Se-dioxide is often used as a source of Se. Vitamin C, sodium sulfite (Na2SO3), and sodium thiosulfate are used as reducing agents, stabilizers can be added appropriately during the process, and SeNPs can be successfully prepared. In 2010, Langi et al. reported the first chemical synthesis of SeNPs using sodium selenosulfate and 3-methylimidazolium sulfate in an ionic liquid-assisted manner [137]. Under the action of a polyvinyl alcohol stabilizer, sodium selenosulfate was used as the precursor of Se to synthesize 76–150 nm SeNPs. In addition, the molecular structure prevents SeNPs aggregation and improves its stability. Zhang et al. prepared spherical SeNPs with a particle size of 36.8 ± 4.1 nm using selenite as the Se source, ascorbic acid as a reducing agent, and β-lactoglobulin (Blg) as a stabilizer [144]. This also indicates that the reaction in ionic solution needs the addition of a certain amount of stabilizer, usually a polysaccharide such as chitosan (CS) [139], spiral algae polysaccharide (SPS) [126], polysaccharide-protein complex (PSP) [145], or sodium formaldehyde sulfonate (SFS) [146] stabilizer solution. Polysaccharides extracted from seaweed can improve the stability of SeNPs [147]. Cordyceps exopolysaccharides (EPS) [148], acacia gum, or carboxymethyl cellulose are also used as polysaccharides. Lentinan, extracted from the medicinal basidiomycetes Lentinula edodes [149], can not only be used as a stabilizer but also reduces Inorg-Se and Org-Se compounds to SeNPs. Chen et al. found that polysaccharides extracted from seaweed wakame can enhance the stability of SeNPs and form monodisperse spherical SeNPs for treating A375 human melanoma cells [147].
In view of the dual effects of lentinan, we synthesized SeNPs named SeNPs@LNT that can transform cold malignant pleural effusion (MPE) into hot MPE using LNT as the polysaccharide. Various biophysical methods, including electron microscopy, were used to characterize SeNPs after they were synthesized. As a food additive, monodisperse spherical SeNPs are very stable in solution. Chemosynthetic materials are readily available and easy to use and can be synthesized at the atomic or molecular level in sizes, shapes, and crystal types that are easily controllable. However, the high cost of production and the presence of toxic byproducts have limited the development of new methods for the synthesis of nanoparticles.
Synthesis and application of SeNP composites in the treatment of lung cancer
Nanocomposite materials are composed of nanosized inorganic particles, metals, semiconductors, rigid particles, etc., which are prepared by appropriate preparation methods. Recent research has shown that nanocomposites, including Se nanocomposites, can have medical applications, such as in cancer [150]. SeNPs exhibit unique properties, and as a drug, they possess strong penetration properties and cause little damage to the body. The Se nanocomposites currently studied include porous Se@SiO2 nanocomposites, Cu2-XSe nanocrystals coated with silica oxidized to Se quantum dots and PVP etched to form porous structures [151], which effectively inhibit the proliferation of cancer cells through the ROS-mediated mechanism. Au@Se core–shell nanostructure: a seed-mediated method was used to synthesize Au NRs after the formation and conjugation of Se shells [152]. Combined with X-ray therapy, Au NRs can induce cell apoptosis by altering the expression of p53 and dna damage genes, triggering the excessive production of intracellular ROS and greatly improving the anticancer effect. Se dioxide (SeO2) NPs and Se dioxide/titanium dioxide nanocomposites (Se/Ti (I), (II) and (III)) can also treat cancer [153]. Nanocomposites exhibit good biosafety due to the controlled release of Se, which not only ensures a beneficial effect but also reduces toxicity [151], and the prospects for their successful application are very promising.
Synthesis and application of Se-based two-dimensional nanomaterials for the treatment of lung cancer
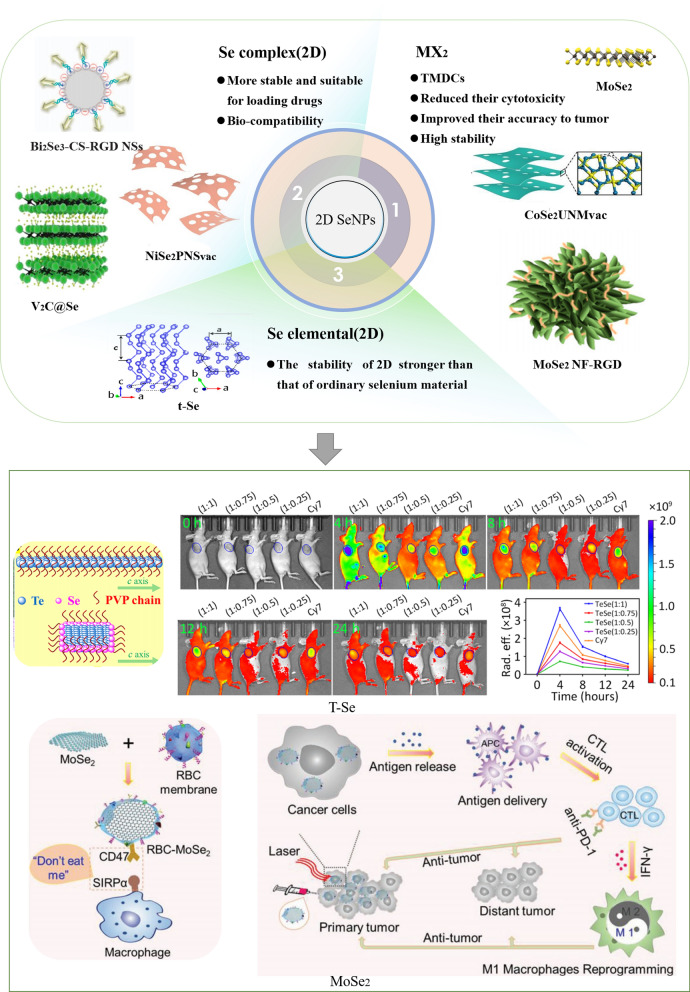
In recent years, with the progress of science and technology, two-dimensional (2D) nanomaterials have emerged, expanding from the physical field to the biological field [154, 155]. A 2D nanomaterial is a type of nanomaterial that is freestanding and sheet-like in shape, with a high ratio of lateral size to thickness [156]. A tremendous amount of interest has been generated by the unique nanosheet structures, large surface areas, and extraordinary physiochemical properties [157]. The large surface areas of 2D nanomaterials make them highly effective nanoplatforms for drug delivery. Phototherapy and RT of cancer can be enhanced by utilizing the unique optical and/or X-ray attenuation properties. 2D nanomaterials can also be engineered to serve as multimodal tumor imaging probes by integrating them with other functional nanomaterials or utilizing their inherent physical properties [158]. Jiet et al. prepared high-quality ultrathin boron nanosheets, which have great prospects in cancer diagnostic imaging and image-guided drug delivery [159]. Xie et al. researched a 2D SnS-based dual-function nano-PTT platform that proved to be effective against several cancer cell lines and xenograft tumors [160]. At present, 2D SeNPs are also the subject of intense research efforts. In the following section, we introduce 2D SeNP materials, which can be divided into the following categories: MX2, Se elemental (2D), and Se complex (2D) (Fig. 5).
Fig. 5.
Structure of 2D SeNPs for cancer. 2D SeNPs are divided into three categories (MX2, Se elemental, and Se complex) because their structures and characteristics are different [116, 161–169]. Copyright 2021, American Chemical Society. Copyright 2016, WILEY‐VCH. Copyright 2019, Royal Society of Chemistry. Copyright 2018, American Chemical Society. Copyright 2022, Elsevier B.V. Copyright 2020, The Author(s). Copyright 2017, The Author(s). Copyright 2019, WILEY‐VC. Copyright 2022, Elsevier Ltd. Copyright 2020, The Authors
Synthesis and application of MX2 for the treatment of lung cancer
Monolayer transition metal dichalcogenides (TMDCs) have layered structures similar to graphite and have attracted extensive attention because they are naturally abundant, and some TMDCs are semiconductors with considerable band gaps [170]. Due to the diversity of chemical composition and structural phases, TMDCs exhibit abundant electrical properties both in terms of band structure characteristics (metallic properties and insulation) and the appearance of related and topological phases. A single layer of 2D TMDCs (the generalized formula is MX2, in which M is a transition metal of the 4–10 group and X is a type of copper) exhibits a variety of chemical properties [171]. Wang et al. developed a novel photothermal nanocarrier, polydopamine-coated molybdenum selenide, which can load the anticancer drug DOX [172]. This not only enhanced the photothermal effect of molybdenum selenide (MoSe2) nanosheets but also reduced their cytotoxicity and improved their accuracy in the tumor. Pan et al. prepared Gd3+-doped MoSe2 (MoSe2(Gd3+)-polyethylene glycol (PEG) nanosheets [173]. The MoSe2(Gd3+)-PEG nanosheets, which exhibit high stability in physiological solution and show no obvious toxicity in vivo, can be used as a contrast agent for photoacoustic imaging (PAI). MoSe2(Gd3+)-PEG combines therapeutic and imaging capabilities to achieve cancer therapy. Dong et al. prepared Rh3Se8 NPs that exhibit many characteristics, such as high photostability, negligible adverse inflammatory effects, and low long-term toxicity; these NPs show great potential for bioimage-guided efficient photonic cancer thermotherapy for nanosystems [172]. 2D SeNPs, especially MX2, are more efficient in the space structure, effectively carrying and releasing drugs while reducing toxicity to a lower level, and future studies may find that these NPs have more advantages and wider applications.
Synthesis and application of Se (2D) for the treatment of lung cancer
The stability of 2D nanomaterials is similar to that of carbon nanotube-based nanomaterial systems and stronger than that of ordinary Se materials[173]. Qin et al. studied the anisotropic mechanical properties of individual 2D trigonal Se (t-Se) nanosheets [161]. Studies of the structure and properties of 2D t-Se have laid a good foundation for its application in biology. With the development of science and technology, 2D t-Se has become a new member of the 2D semiconducting nanomaterial family.
Synthesis and application of the Se complex (2D) for the treatment of lung cancer
In addition to the above two categories, 2D SeNPs also include the Se complex, which is more stable and suitable for loading drugs. Multifunctional 2D Bi2Se3 nanosheets can be used for antibacterial and anti-inflammatory treatment of bacterial infections [174], although the antitumor effects remain to be developed. Nevertheless, Chen et al. studied the stability and biocompatibility of the newly synthesized Se tellurium [116], which exhibits obvious tumor-targeting and antitumor effects.
More importantly, because Se can affect the TIME, 2D SeNPs possess characteristics that other 2D nanomaterials do not have, which can enhance immune cells and affect the TIME. In the near future, it is anticipated that such research may be greatly expanded, and there are high expectations for the treatment of cancer, especially lung cancer.
SeNPs for lung cancer diagnosis and imaging
SeNPs can not only be used as carriers to transport drugs to the tumor site but also as probes and contrast agents for the diagnostic imaging of lung cancer to improve the level of modern medical diagnosis. A variety of compounds have been approved for clinical use and imaging purposes [175]. Current imaging techniques used include fluorescence imaging (FRI), MR imaging (MRI), CT and PAI.
Application of SeNPs in FRI
FRI refers to the visualization of colorless and transparent cells, organs, or specific proteins that cannot be observed directly by fluorescent labeling reagents or fluorescent antibodies to observe and analyze the morphology or structure and life activities of cells and aid in the differential diagnosis of cancer [178, 179]. In preclinical research, FRI has become among the most commonly used imaging tools [180]; it is mainly used to diagnose tumors (including lung cancer) by fluorescence probes [181]. In addition, FRI can be used in the clinical diagnosis of disease by labeling tumor cells [180]. In recent years, the application of SeNPs has gradually developed. Since the NPs lack fluorescence characteristics but have an unloading effect, they can be modified with other substances with photosensitizer properties or those with high absorption of light properties to form complexes and play a photosensitive FRI role. Some examples include Ru-MUA@Se and Bi2Se3@PEG/DOX/Ce6. Sun et al. prepared bright and photostable thiol-modified SeNPs with the attached photosensitizer Ru(II)-polypyridine complex and conducted experiments at various tumor sites through FRI technology to identify different types of tumors [108] (Fig. 6A). Sun et al. concluded that Bi2Se3@PEG/DOX/Ce6 revealed the in vivo biological distribution through externally stimulated FRI, providing accurate diagnostic information for tumor treatment [176] (Fig. 6C). These results indicate that SeNPs play an important role in the fluorescence diagnosis of various cancers, including lung cancer.
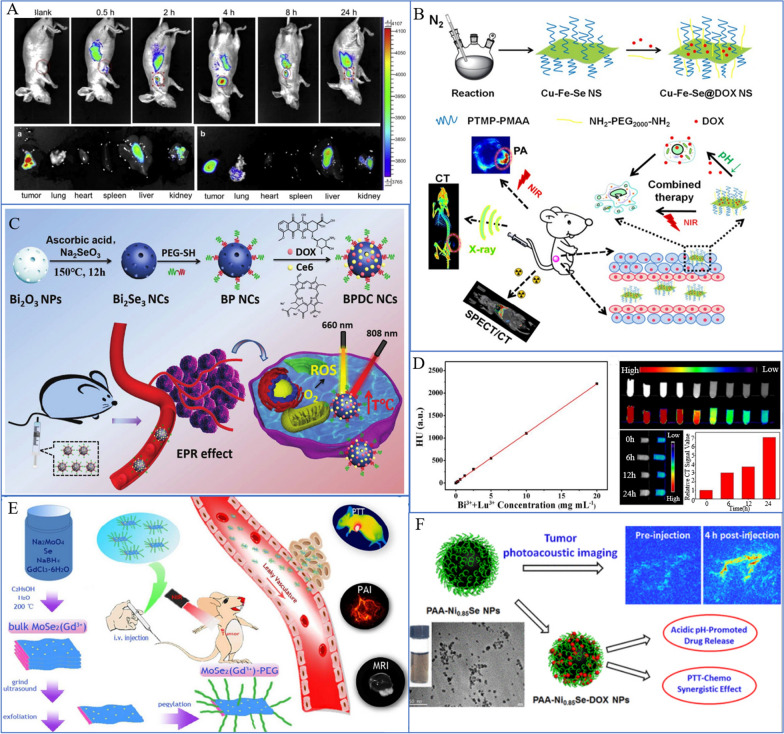
Fig. 6.
SeNPs for cancer diagnosis and imaging. A In vivo imaging of tumor-bearing mice and in vitro FRI of each tissue at different times after Ru-MUA@Se injection [108]. Copyright 2013, Elsevier Ltd. B (CFS@DOX NSs as a diagnostic probe for CT imaging and combined chemotherapy/light therapy [109]. Copyright 2018, American Chemical Society. C BPDC NCs represent a multifunctional and versatile biomedical platform for tumor diagnosis by using FRI [176]. Copyright 2019, Royal Society of Chemistry. D CT image of A549 cells incubated with UCNPS-Bi2Se3 nanoheterozygotes [110]. Copyright 2019, Wiley‐VCH. E MR-pegylated MoSe2(Gd3+) injected into mice can diagnose cancer by MR [171]. Copyright 2018, Royal Society of Chemistry. F PAA-Ni0.85Se-DOX NPs achieve photothermal-chemical cancer therapy through PAI [177]. Copyright 2017, American Chemical Society
Application of SeNPs in MRI
MR is a biological magnetic spin imaging technology that, in computer technology, uses the nuclear magnetic resonance principle through detecting electromagnetic wave imaging [182, 183]. Since 1996, pulmonary functional MR techniques have been applied continuously in the clinic [184, 185]. Currently, researchers are combining nanotechnology with MR imaging applications to improve diagnostic tumor technology. The reason why NPs can be used as contrast agents for MR imaging is that the required NPs are limited—magnetic NPs, such as SeNPs with a TMDC structure and iron diselenide (FeSe2) NPs. Pan et al. reported that MoSe2(Gd3+)-PEG with a TMDC structure can be used as a T1-weighted MRI contrast agent to diagnose tumors in vivo [171] (Fig. 6E). Fu et al. developed magnetic FeSe2 NPs and modified them with PEG to form pegylated FeSe2 NPs with a high R2 relaxation rate and strong NIR absorption rate, which can be used for MRI [186]. Magnetic nanomaterials are being further developed and will soon have many applications in medical diagnostics.
Application of SeNPs in CT imaging
CT is a relatively new clinical diagnostic method that can distinguish the difference in X-ray absorption capacity and transmittance between different tissues, input the measurement data into the computer, and reconstruct the fault plane image after the computer processes the data [187, 188]. When mixed with other materials, the negatively charged surface and multifunctional groups of composite nanomaterials can play a defining role in CT imaging, even if SeNPs alone do not have an isotope labeling function. Cu-Fe-Se ternary nanosheets have been examined by Jiang et al., and their surface and multifunctional groups allow for CT imaging diagnosis [109] (Fig. 6B). The UCNP-Bi2Se3 nanocomplex synthesized by Zhao et al. showed efficient upconversion luminescence (UCL) and reasonable CT imaging ability, highlighting the efficiency of this approach in UCL imaging and PTT [110] (Fig. 6D). Of course, whether the SeNPs can be used remains unclear.
Application of SeNPs in PAI
PAI uses substances with optical absorption to target and accumulate at the lesion site, converting the energy of the pulsed laser into heat, thus causing thermal expansion to generate ultrasonic signals, and constructs images by detecting such signals [189, 190]. Due to nickel selenide's distinctive electron configuration and relatively high catalytic activity, Wang et al. reported a multifunctional theranostic agent made of ultrasmall poly(acrylic acid)-functionalized Ni0.85Se NPs (PAA-Ni0.85Se NPs) [177]. This agent was successfully used in PAI (Fig. 6F). Although less well researched, other combinations are also being investigated in this area.
Application of SeNPs in traditional treatment of lung cancer
Application of SeNPs to directly kill tumor cells
The biological action of Se has been researched thoroughly since the late 1980s, which has led to the rapid advancement of Se-based agents. Inorg-Se agents, such as selenic acid, Se oxide, and selenite sodium, were the focus of the majority of early studies. These studies demonstrated the strong antitumor activity of Se, and in September 2003, the US Food and Drug Administration (FDA) officially recognized Se as an antitumor agent, reiterating the agent's efficacy. The primary area of our study is Se nanomedicine. Se not only exhibits fewer side effects and is biocompatible but can also target tumor cells directly. By preventing the creation of proteins and DNA, decreasing protein kinase C (PKC) activity, and encouraging the release of GSH to kill cancer cells, SeNPs increase the cytotoxic action of Se compounds. The immune system of cancer patients can also be strengthened by Se, which can enhance the production of interleukin-2 (IL-2), lymphocyte lymph cytokines, interferon, and cytokines, as well as the body's ability to produce IgG, IgM, and other antibodies that can destroy cancer cells.
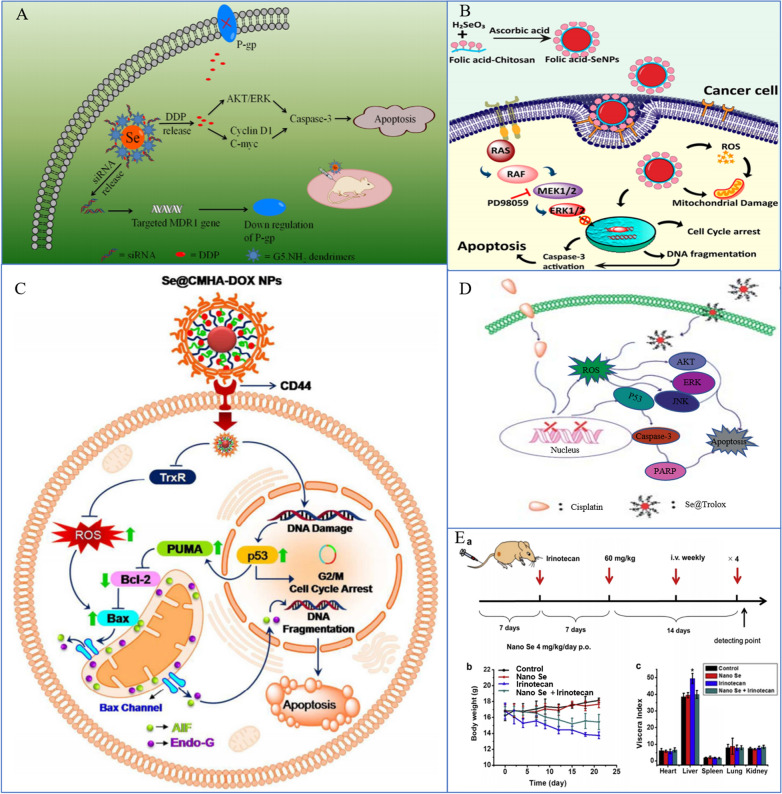
At present, Se nanomedicine contains the following drugs: DOX, 5-FU, irinotecan, cisplatin and paclitaxel [191–193]. Combining SeNPs and irinotecan, Gao et al. demonstrated greater cytotoxicity against HCT-8 tumor cells, significantly elevated p53 expression levels, and increased DNA sensitivity of HCT-8 cells to cause apoptosis [106]. The surface modification of 5-FU can dramatically increase the uptake of SeNPs by endocytosis according to studies by Liu et al. [104]. Strong selectivity and efficient growth inhibition of cancer cells are both characteristics of 5-FU@SeNPs. The mitochondria-mediated route to induce apoptosis can also cause caspase-dependent and ROS-dependent apoptosis of A375 cells. SeNPs and DOX therapy were used in a study by Xia et al. to increase the cytotoxicity of A549 cells and specifically target tumor cells to cause apoptosis [192]. To demonstrate how the release rate of paclitaxel was accelerated and how this improved the ability of A549 cells to absorb the drug and, simultaneously, reduce its toxicity [194], Zou et al. combined SeNPs with paclitaxel [195]. A549 cell apoptosis can be induced by HA-Se@PTX, which also suppresses A549 cell proliferation, migration, and invasion. The same result is also seen with cisplatin [191]. SeNPs@LNT also generates a positive therapeutic impact on lung adenocarcinoma [18] and is the focus of our research (Fig. 7).
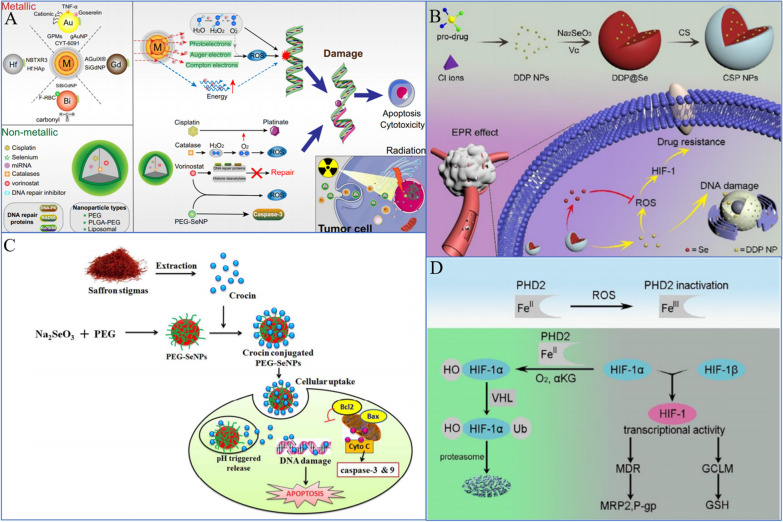
Fig. 7.
Direct cancer cell-killing activity of SeNPs. A Nonmetallic NPs encapsulated in combination with RT further induce DNA damage, prevent rapid DNA repair, and lead to more apoptosis [191]. Copyright 2020, The Author(s). C PEG-SeNPs induce apoptosis through mitochondria-mediated pathways [111]. Copyright 2019, The Royal Society of Chemistry. B, D ROS-dependent regulation of HIF-1 activity by PHD2 (chitosan-coated Se/DDP nanoparticles [CSP NPs]) [112]. Copyright 2019, Elsevier B.V
Synergistic effects of SeNPs and RT
As a proven treatment option for lung cancer, both palliative and curative RT is frequently used in conjunction with other therapies, including surgery, chemotherapy, or immunotherapy [197, 198]. In most cases, RT is combined with surgery and radiation, including preoperative radiation to shrink the tumor, preoperative radiation to facilitate surgical resection, intraoperative radiation to precisely deliver large doses of ionizing radiation (IR) to the tumor site while minimizing adverse effects on normal tissues, and postoperative radiation to decrease recurrence risk. [199–203]. In most cases, RT is a form of therapy that employs IR, which is typically used to describe high-energy photon radiation, such as X-rays and gamma (g) rays, as well as particle radiation such as alpha or beta particles, carbon ions, electron, proton, or neutron beams [204–206]. IR has the ability to disrupt biomolecules directly, including proteins, lipids, and DNA. This can stop cell division and proliferation as well as cause necrosis or apoptosis in some cells. In the meantime, the byproduct of radiolysis, ROS, can also damage biomolecules via free radicals. To target and eliminate tumor tissues, any of these radiation options can be used. This approach may be effective in reducing and eliminating cancerous cells, but it may also damage normal tissues adjacent to the site, which may result in toxic effects. Complications of RT include fatigue, anorexia, bone marrow injury, shortness of breath, cough and dyspnea. Furthermore, the degree of radiation damage to tissue cells is directly related to the rate of cell proliferation, the oxygen supply to the tissue, and the dose of irradiation. The higher the cell proliferation, the greater the oxygen supply of tissue cells, and the greater the sensitivity to radiotherapy. As a solid tumor, lung cancer contains 10% to 50% anoxic cells that are resistant to radiation, which makes RT alone ineffective at eradicating tumor cells and risks recurrence. Therefore, more precise RT techniques have emerged, such as intensity-modulated RT, which may decrease toxicity, but some patients still experience adverse reactions (e.g., bone marrow suppression, nausea, and vomiting hindrance) [196, 207, 208]. The use of RT alone to cure tumors is, however, difficult due to a variety of obstacles, such as cancer stem cells, tumor heterogeneity, angiogenesis and vasculogenesis, metabolic alterations, and complications [209–211]. A way to overcome these obstacles is to introduce radiosensitizers, which are molecules or materials that can enhance the radiosensitivity of tumor cells. With the development of nanotechnology, nanomaterials possessing good radiosensitizing effects and metabolic properties are being developed. SeNPs have attracted increasing attention in the past decade due to their high bioavailability, low toxicity and novel therapeutic properties (Fig. 8).
Fig. 8.
Synergistic effects of SeNPs and RT. A RT damaged DNA to destroy cell proliferation and gradually induced cell apoptosis over time [196]. Copyright 2019, Springer Nature Limited. B SeNPs kill cells by ROS in conjunction with RT [193]. Copyright 2019, Elsevier Masson SAS
It has been reported that the effect of SeNPs combined with RT on the proliferation of non-small cell lung cancer (NSCLC) cells was greater than that of SeNPs exposure treatment alone or irradiation alone, suggesting that SeNPs and RT exhibit a synergistic effect in inhibiting cell proliferation activity or promoting each other [46]. Furthermore, the combination of the two could also exert other anticancer activities, including inhibition of invasion and migration and promotion of apoptosis in NSCLC cells.
Therefore, we fabricated X-ray-responsive SeNPs with significant radiosensitization effects by taking advantage of PEG as a surface decorator and template. In addition, studies have been carried out to examine the application potential of PEG-SeNPs as radiosensitizers. We found that X-ray irradiation (8 Gy) or PEG-SeNPs (10 μM) alone induced only slight growth inhibition on HeLa cells, in which the cell viability was 79%, 71%, and 54% in cells treated with 20, 40 and 80 μM PEG-SeNPs, respectively. In contrast, cotreatment of the cells with PEG-SeNPs and X-ray irradiation significantly enhanced the inhibition of cell growth at the same concentration of PEG-SeNPs. In particular, cotreatment with 20 μM PEG-SeNPs and X-rays significantly suppressed cell viability to 39%. The results of microscopic examination of cells also demonstrated consistent morphological changes after combined treatment with PEG-SeNPs and X-rays, such as cell shrinkage, rounding, and the appearance of apoptotic bodies. Moreover, 20 μΜ PEG-SeNPs in combination with X-rays induced an obvious increase in the sub-G1 apoptotic fraction (41.6%) and G2/M phase arrest (21.1%) [107].
On the other hand, we further found that the nanosystem could significantly induce intracellular ROS generation in a time-dependent manner, increase oxidative stress levels, and directly bind to DNA, leading to an effective radiosensitizing effect. In lung cancer, it has also been confirmed that 20 μM PEG-SeNPs combined with X-rays generates effective anticancer effects [212]. Collectively, 20 μM PEG-SeNPs exhibited significant radiosensitization when irradiated with X-rays and synergistically enhanced the antitumor effects of radiation by inducing cell apoptosis and arresting cell cycle progression. Furthermore, we found that angiogenesis plays an important role in the growth, invasion, and metastasis of pulmonary tumors, which results in the majority of lung cancer deaths. Thus, antiangiogenesis therapy may be an effective strategy for regulating pulmonary tumor growth and metastasis [213, 214]. Bevacizumab (Avastin™, Av), as a humanized monoclonal antibody, can inhibit angiogenesis by accurately targeting vascular endothelial growth factor (VEGF), which can be used as tumor starvation (antiangiogenesis) therapy to further regulate the formation and growth of new blood vessels in and around tumor tissue [215]. The rationale for AV inhibition of tumor growth is as follows: first, it binds to VEGF secreted by angiogenic tumors; second, it inhibits AV binding to VEGF receptors in vascular endothelial cells; and finally, it inhibits VEGF-induced cell proliferation, survival, permeability, nitric oxide as well as migration and tissue factor production [216]. Therefore, to further improve the radiosensitization properties and antitumor effects of SeNPs, we rationally designed and synthesized RBCs@Se/Av that combined PEG-SeNPs and Av antibody encapsulated within RBC membrane vesicles to simultaneously enhance the efficiency of cancer RT and antiangiogenesis. In response to X-rays, the nanosystems passively accumulated within cancer cells and were then activated. In line with expectations, treatment with RBCs@Se/Av and X-ray resulted in the production of ROS and the activation of the p53 pathway, which resulted in apoptosis of cancer cells. Furthermore, RBCs@Se/Av with X-ray irradiation not only do not cause obvious histological damage to major organs but also show centrally effective anticancer efficacy [113].
This method can effectively solve systemic side effects and improve the efficiency of treatment. In addition, the study not only focuses on the treatment rate but also covers the basics of the treatment mechanism. However, the reduction in the long-term recurrence rate remains to be further studied.
Synergistic effects of SeNPs and chemotherapy
Chemotherapy is the term used to describe the administration of chemical medications to treat lung cancer. Chemotherapy is a type of systemic treatment because once anticancer medications enter the body, they are promptly dispersed throughout the body to destroy both nearby local tumors and far-off metastatic tumors. The preferred clinical therapy for NSCLC is chemotherapy coupled with radiation or immunotherapy. Chemotherapy is the main therapeutic choice for SCLC. Consequently, chemotherapy is crucial to the complete treatment of lung cancer. Antineoplastic drugs, such as DOX, paclitaxel (PTX), platinum analogs, gemcitabine, and pemetrexed, have been used widely in the treatment of lung cancer as first-line antineoplastic drugs with good results. However, most chemotherapeutic drugs are highly toxic and have side effects, which make it difficult for patients to tolerate or continue treatment completion. Furthermore, it is known that primary and acquired drug resistance in cancer cells can lead to tumor recurrence and metastasis, thus limiting the anticancer properties of chemotherapeutics [219–222]. Therefore, the chemotherapies currently available have the following primary limitations: cancer cells develop adaptive chemoresistance over time, and normal cells are subject to nonspecific toxicity [223].
Chemosensitization is the strategy used widely to enhance the activity of one drug by combining it with another drug to overcome chemoresistance. Chemotherapeutic sensitizers should be less toxic, multitargeted, and able to sensitize cancer cells to chemotherapeutic drugs by inhibiting one or more signaling pathways involved in chemoresistance, preferably multiple pathways. Therefore, the search for chemotherapeutic sensitizers has important scientific significance and application value. Numerous investigations have shown that both the tissue and cell distribution profiles of anticancer drugs can be improved by nanotechnology [224]. Nanosized anticancer drugs displayed increased antitumor efficiency and reduced serious side effects [225]. Recently, Zhou et al. synthesized SeNPs with hyaluronic acid (HA) to prepare tumor-targeted delivery vehicle HA-SeNPs and loaded PTX in HA-SeNPs to fabricate functionalized SeNPs HA-SeNPs@PTX, which showed excellent chemosensitizing activity and low toxicity [195]. Since the HA receptor CD44 expressed by A549 cells is significantly higher than that in normal human cells, HA-SeNPs@PTX has superior guided selectivity for A549 cells. HA-SeNPs@PTX can significantly improve the anticancer effect of PTX in NSCLC. The calculation of the comprehensive index value showed that HA-SeNPs@PTX enhanced the inhibition of PTX on A549 cell growth. The synergistic effect of the two is related to more significantly inducing caspase-mediated apoptosis, blocking the G2/M cell cycle and inhibiting cell proliferation. PTX is released faster under acidic conditions. This may be due to the increased protonation of HA-SeNPs under acidic conditions, which weakened the electrostatic attraction between PTX and HA-SeNPs; thus, acidic conditions facilitated the release of PTX from HA-SeNPs. Such acid-dependent drug release features of HA-SeNPs@PTX are quite favorable for cancer treatment. In addition, HA-SeNPs and HA-SeNPs@PTX showed low cytotoxicity and great biocompatibility, respectively. The results indicated that HA-Se@PTX exhibits significant potential for lung carcinoma treatment. Similarly, Xia et al. designed SeNPs that were modified with cyclic peptide (Arg–Gly–Asp–D-Phe–Cys [RGDfC]) to fabricate tumor-targeting delivery carrier RGDfC-SeNPs [192]. DOX is a very common anticancer drug used clinically [226, 227]. Nevertheless, the anticancer efficacy of DOX is not as ideal as expected, partly because of its lack of targeted specificity, poor solubility, inadequate drug accumulation in the tumor, and serious side effects. DOX was loaded onto the surface of RGDfC-SeNPs to improve the antitumor efficacy of DOX in NSCLC therapy. The anticancer mechanism of this nanosystem is similar to that of HA-SeNPs@PTX, both of which are related to caspase-mediated apoptosis and G2/M cell cycle arrest [192].
The main regulation of the cell cycle and apoptosis is ROS-mediated DNA damage, and p53 and MAPK are the main pathways of ROS-mediated DNA damage-induced apoptosis [228]. SeNPs can upregulate p53 and regulate the MAPK pathway.
In addition to its role as a transcription factor, p53 is involved in the regulation of a number of genes associated with cell cycle arrest and apoptosis [229]. The acetylation and phosphorylation of p53 play an important role in the regulation of apoptosis. For instance, phosphorylation of p53 at Ser 15 can lead to cell apoptosis caused by chemotherapeutic drugs and chemopreventive agents, especially seleno-compounds [230]. Huang et al. synthesized Tf-conjugated SeNPs and showed that they could be used as a cancer-targeted drug delivery system to achieve enhanced cellular uptake and anticancer efficacy. As a targeting ligand, Tf significantly enhances the cellular uptake of DOX-loaded SeNPs through clathrin-mediated and caveolae/lipid raft-mediated endocytosis in cancer cells overexpressing transferrin receptors and increases their selectivity between cancer and normal cells. DOX-loaded and Tf-SeNPs exhibit unprecedented, enhanced cytotoxicity toward cancer cells through the induction of apoptosis with the involvement of intrinsic and extrinsic pathways. Internalized and externalized Tf-SeNPs significantly upregulated the phosphorylation of p53 at the Ser15 site and triggered the phosphorylation of histones at the Ser139 site, indicating that Tf-SeNPs trigger cancer cell apoptosis through DNA damage-mediated p53 activation. In addition, internalized Tf-SeNPs suppressed the expression of Bcl-xl, a prosurvival member of the Bcl-2 family of proteins. Bcl-2 family proteins can regulate outer mitochondrial membrane permeability and control the on/off intrinsic apoptotic pathway. Therefore, Tf-SeNPs induce mitochondrial dysfunction, which leads to the overproduction of ROS and activates mitochondrial-mediated apoptosis. With further research, Huang et al. also found that MAPK pathways may also play an important role in Tf-SeNP-induced apoptosis [47].
MAPK is a serine/threonine protein kinase that plays a regulatory role in many cell activities, such as growth and proliferation, cell differentiation, cell movement and death [231]. Moreover, as noted earlier, MAPK is among the main pathways for ROS-mediated DNA damage to induce cell apoptosis [228]. The MAPK pathway mainly includes the following branch routes: ERK, JNK, p38 and AKT. Tf-SeNPs exhibited differential effects on the phosphorylation of p38, JNK, ERK, and AKT. Phosphorylation of the proapoptotic kinase p38 displayed a trend toward upregulation in a dose-dependent manner. In contrast, phosphorylation of the antiapoptotic ERK was effectively suppressed by Tf-SeNPs, while the phosphorylation of JNK and AKT was not affected by Tf-SeNPs. Taken together, the results indicated that internalized Tf-SeNPs trigger intracellular ROS overproduction, thus activating the p53 and MAPK pathways to promote cell apoptosis [47] (Fig. 9).
Fig. 9.
Synergistic effects of SeNPs and chemotherapy. A SeNPs double deliver MDR1 siRNA and DDP to reverse drug resistance [217]. Copyright 2014, Acta Materialia Inc. B FA@SeNPs and a MAPK-pathway inhibitor for dual targeting of cancer cells [114]. Copyright 2019, American Chemical Society. C Schematic diagram of Se@CMHA-DOX NP-induced apoptosis in cancer cells [218]. Copyright 2017, American Chemical Society. D Se@Trolox blocks cisplatin-induced signaling pathways [124]. Copyright 2013, Royal Society of Chemistry. E SeNPs combined with irinotecan can reduce irinotecan toxicity and treat mice [106]. Copyright 2014, Elsevier Ltd
In addition, SeNPs combined with chemotherapy can antagonize the side effects of chemotherapy. Cisplatin is a highly effective chemotherapeutic agent used widely in the treatment of lung cancer. However, the most common adverse effect limiting the clinical use of cisplatin is nephrotoxicity, which develops primarily in the S3 segment of the proximal tubule and impairs the patient’s quality of life; moreover, in patients with preexisting conditions, nephrotoxicity can even be life-threatening [232, 233]. Research has indicated that cisplatin-related nephrotoxicity may be mainly caused by oxidative stress [234]. Treatment with cisplatin resulted in the generation of ROS, such as superoxide anion and hydroxyl radicals, and renal lipid peroxidation [235]. Moreover, several studies have demonstrated the protective effect of natural or synthetic antioxidants against cisplatin-induced nephrotoxicity [236, 237]. 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) is a water-soluble analog of alpha-tocopherol with a chromatid ring that has been studied extensively for its protective effects against oxidative stress-related diseases. Trolox is poorly utilized due to its poor water solubility and poor stability when exposed to oxygen-containing environments due to its phenolic hydroxyl group. In recent years, due to the development of nanotechnology, the stability of Trolox can be improved effectively by nanocrystallization [238]. Chen et al. found that Trolox surface-functionalized SeNPs (Se@Trolox) could also play a similar role [124], which could effectively reduce cisplatin-induced nephrotoxicity. Through inhibition of ROS-mediated p53 phosphorylation, Se@Trolox prevented the caspase-mediated apoptosis induced by cisplatin. Intrinsically, Se@Trolox effectively protected HK-2 cells from damage by regulating the Akt and MAPK pathways. Furthermore, Chen et al. also reported a simple method for the functionalization of SeNPs by self-assembly of 11-mercapto-1-undecanol (Se@MUN) to achieve enhanced antioxidant activity and demonstrate antagonism against cisplatin-induced nephrotoxicity [115]. The mechanism for the action of Se@MUN involved significantly reducing the decreased HK-2 cell viability induced by cisplatin, including the sub-G1 peak, nuclear concentration, and DNA fragmentation. Se@MUN also effectively blocked the activation of caspase-3 in cells induced by cisplatin, and compared with SeNPs, Se@MUN showed higher free radical scavenging activity and higher cell uptake in normal human cells. Therefore, the nanocrystallization of org-Se has potential application value in preventing cisplatin-induced injury.
Therefore, SeNPs combined with chemotherapy drugs can not only enhance the efficacy of drugs but also improve the accuracy and targeting, reduce side effects, and antagonize the side effects of chemotherapy drugs, which is promising research for clinical application.
Application of SeNPs in photodynamic and photothermal tumor therapy
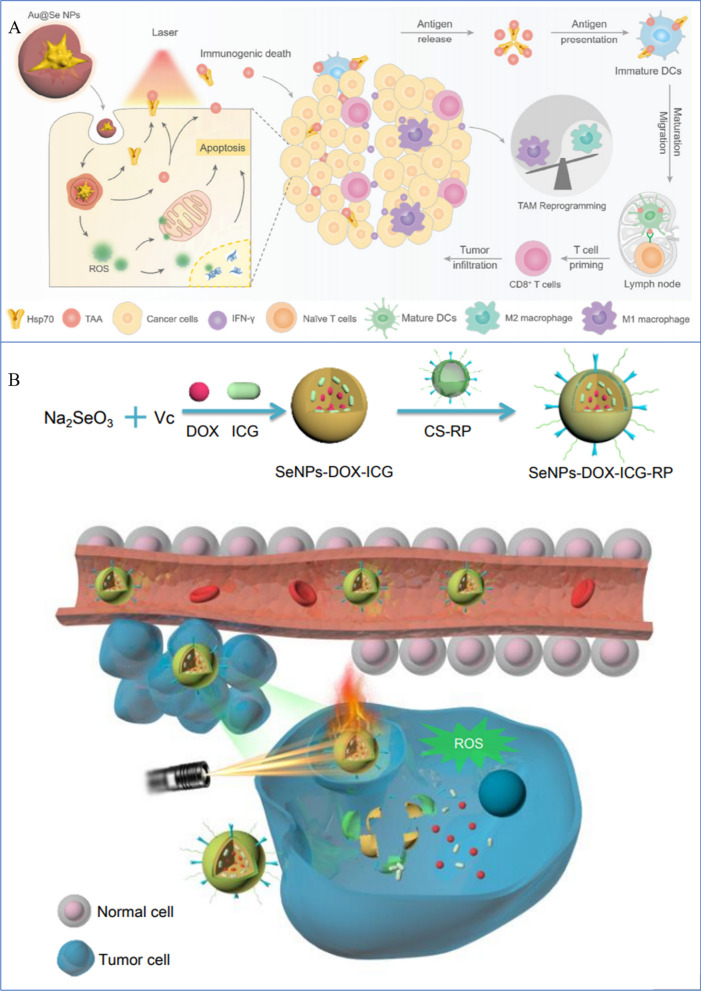
Recently, research on PTT, a newly developed and encouraging therapeutic strategy, has achieved many breakthroughs in the treatment of cancer. In PTT, photothermal transduction agents (PTAs) are used to produce heat by harvesting the light's energy and converting it into heat to increase the surrounding environment's temperature and cause cancer cells to die [241, 242]. In contrast to conventional therapy modalities, PTT exhibits unique advantages in cancer therapy, including high specificity, minimal invasiveness, and precise spatial–temporal targeting [243, 244]. PTT treatment can be used to eliminate cancer cells in a primary tumor or local metastasis in nearby lymph nodes, as well as to treat cancer cells that have metastatic spread, in conjunction with current therapeutic modalities [245, 246]. More notably, PTT is a highly effective and noninvasive therapy that is capable of eliminating various types of cancers [247]. Moreover, it has been suggested that tumors became more susceptible to chemotherapy and RT after PTT, demonstrating a synergistic effect when used in combination. The possible mechanism to explain these effects is that while PTT destroys cancer cells, it also causes a certain degree of damage to stromal cell components, including tumor vasculature, inflammatory cells, stromal fibroblasts and lymphocytes. Under these conditions, residual cancer cells may not readily adapt to the new microenvironment, and there is a window for combination therapy with pH- or hypoxia-responsive drugs [210]. On the other hand, safety is another concern for PTT against cancer. For local tissues, long-term exposure to temperatures above 43 °C is dangerous and causes irreversible, severe damage to the cells [80]. Therefore, PTT needs to be conducted at a reasonable temperature and duration to minimize damage to normal tissues.
The therapeutic efficacy of PTT is significantly dependent on PTA. An ideal PTA should have higher photothermal conversion efficiency (PCE), absorption that does not overlap with the background of the tumor, and good tumor accumulation. The occurrence of a variety of PTAs has accelerated and advances are being made in PTT studies. In particular, nano PTAs that can accumulate in tumors through enhanced permeability and retention (EPR) effects and active targeting are noteworthy [30, 248, 249]. Moreover, since nano PTAs show a higher PCE than that of small molecule PTAs, the functionalities of multiple imaging and therapy can potentially be integrated into one platform for advanced applications [250]. Chen et al. developed a stable, highly uniform in size, and nontoxic nanomaterial made of a tellurium-Se (TeSe)–based lateral heterojunction, which showed favorable photothermal stability and the potential to provide a drug carrier platform for cancer treatment [116]. To prevent off-targeting adverse effects on the surrounding tissue, which is important for its clinical translation [211], Chen et al. used 808-nm light in the near-infrared region. In all ratios studied, Te:Se at 1:1 produces uniform, oval-shaped NPs that produce high light-to-heat conversions. At this optimal ratio for maximal production of thermal energy, the Te:Se heterojunctions are completely nontoxic, which further reduces the side effects of TeSe-based PTT. Regarding safety, during the irradiation course of in vivo treatment, although the temperature was above 50 °C and skin tissue damage was observed, this kind of damage was transient and was repaired within 5 days, demonstrating that TeSe-based PTT is safe. Systemic delivery of Te@SeNPs in mice showed highly specific accumulation in tumors relative to other healthy tissues. Upon exposure to light, Te@SeNPs almost completely eradicated lung cancer in preclinical models [116].
Moreover, SeNPs can be designed as a sequentially triggered system that combines PPT with chemotherapy to achieve precise drug delivery by chemo-photothermal combination. To construct a multifunctional nanodrug delivery system (i.e., SeNPs-RP), Fang conjugated RC-12 and PG-6 to SeNPs using chitosan as the linker [240]. RC-12, which is a derivative of RGD, is a specific cancer-targeting and cell-penetrating peptide that can recognize and interact with integrin receptors that are overexpressed in various human cancer cells [251]. PG-6 (PLGALG) can be identified and cleaved by matrix metalloproteinases (MMP2 and MMP9) in the tumor microenvironment [252]. In addition, positively charged DOX molecules and negatively charged indocyanine green (ICG) molecules were loaded into the positively charged SeNPs-RP by electrostatic interactions. ICG has been reported to be harmless and an efficient therapeutic agent [253]. However, ICG, a clinical-medical diagnostic reagent that is approved by the FDA, is not an efficient PTT agent, owing to its severe photobleaching, short bloodstream circulation half-time, and low tumor accumulation rate [254]. DOX is a very common anticancer drug that is used widely in chemotherapy. Nevertheless, the anticancer efficacy of DOX is not optimal because of its lack of targeted specificity, poor solubility, inadequate drug accumulation in the tumor, and serious side effects [255]. Therefore, dual-target (RC-12 and PG-6 peptides) functionalized SeNPs loaded with both DOX and ICG would overcome the drawbacks of ICG and ensure that the chemotherapeutic drug and photothermal agent are delivered synchronously to the tumor area to produce their synergistic effect. The as-synthesized NPs exhibited good monodispersity, size stability and consistent spectral characteristics compared with ICG or DOX alone. The NPs underwent self-immolated cleavage with NIR laser irradiation and released the loaded drug due to sufficient hyperthermia. Additionally, the internalized NPs triggered intracellular ROS overproduction to induce cell apoptosis. This dual-targeted design of SeNPs loaded with both DOX and ICG might provide a feasible solution for efficient anticancer drug delivery and provide a sequentially triggered nanosystem to achieve precise drug delivery by chemo-photothermal combination (Fig. 10).
Fig. 10.
Photodynamic and photothermal tumor therapy. A Immunogenic nanotherapeutic agent Au@Se NPs are used for PTT-triggered immunotherapy [239]. Copyright 2020, Elsevier Ltd. B Mechanism of tumor targeting SeNPs by near-infrared laser irradiation [240]. Copyright 2018, Wiley‐VCH
Tumor immunotherapy based on SeNPs
Innate immunity of SeNPs in lung cancer treatment
Se supplementation is typically immunostimulatory, as shown by a variety of metrics, such as T-cell proliferation, NK cell activity, and innate immune cell activities [259]. Although Se is essential for numerous immune cell functions, there has been no conclusive evidence that Se and selenoprotein levels influence immune system development. More thorough analysis is needed to determine whether Se influences immune responses and underlying mechanisms in lung cancer.
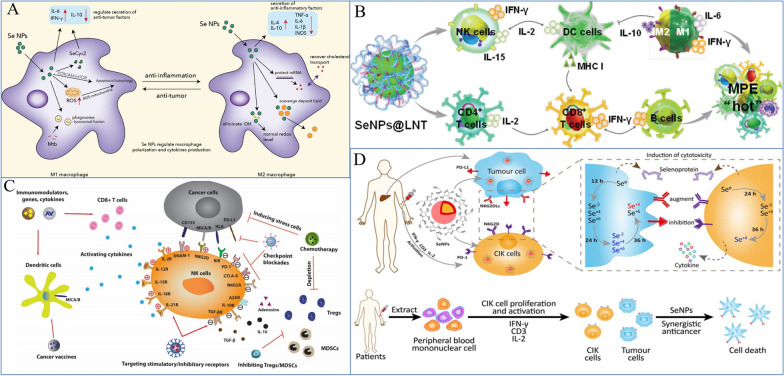
NK cells are key effector cells in tumor immunotherapy because they are a crucial part of the innate immune system [262]. Se and Se compounds can control the activation and antitumor activity of NK cells in the tumor microenvironment (TME) (Fig. 11). Se stops para fibrin from forming nonenzymatically around tumor cells, making the tumor more susceptible to immune surveillance [263, 264]. Additionally, it stimulates the NK cell population in the TME [265] (Fig. 12). With an improved EPR impact, SeNPs increased the targeted delivery of Se in target cells [191]. According to Gao et al. [266], SeNPs may act as immune checkpoint inhibitors with direct anticancer effects on lung metastasis as well as immunomodulatory activity. Their findings showed that in SeNPs, inhibiting HLA-E expression at the tumor site specifically results in the immunological augmentation of NK cells. In our previous study [18], we discovered that SeNPs@LNT increased the number of NK cells in MPE from lung adenocarcinoma (MPE-LA) compared to the control group (30.8% vs. 15.2%). SeNPs@LNT could be transformed to SeCys2 and Se(IV, ) in the immune cell lysate to regulate the secretion of cytokines by multiple immune cells (such as NK and tumor-associated macrophages [TAMs]) to reprogram the inflammatory microenvironment of MPE-LA.
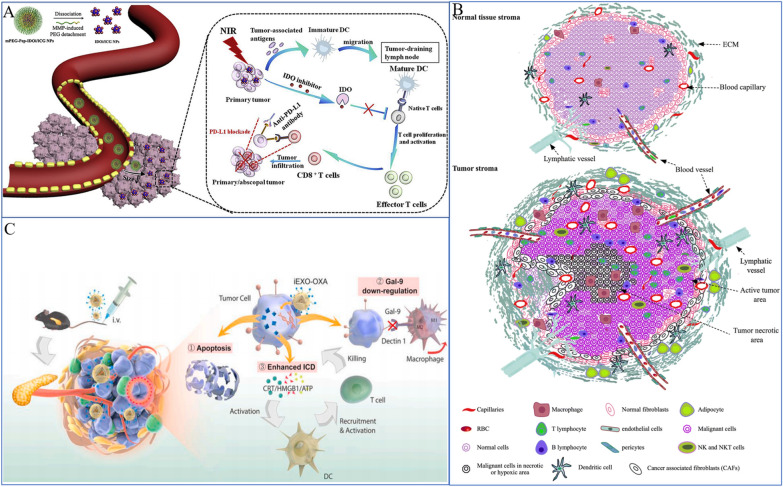
Fig. 11.
The immune microenvironment and immune cell status. The immune microenvironment is complex, including tumor cells, immune cells and the secretion of various cytokines, including a number of trace elements. A Prodrug nanodrugs are characterized by prolonged blood circulation time, enhanced tumor accumulation and deeper penetration. Combined with phototherapy, IDO inhibition and PD-L1 blocking, synergistic and effective antitumor immunotherapy can be achieved [256]. Copyright 2020, Elsevier Ltd. B Tumor microenvironments include high infiltration of immune cells, cancer cells, and CAFs/TAFs and increased deposition of ECM protein in the interstitial tissue [257]. Copyright 2018, The Author(s). C Exosomes enhance immunotherapy and reprogram the tumor microenvironment [258]. Copyright 2020, Elsevier Ltd
Fig. 12.
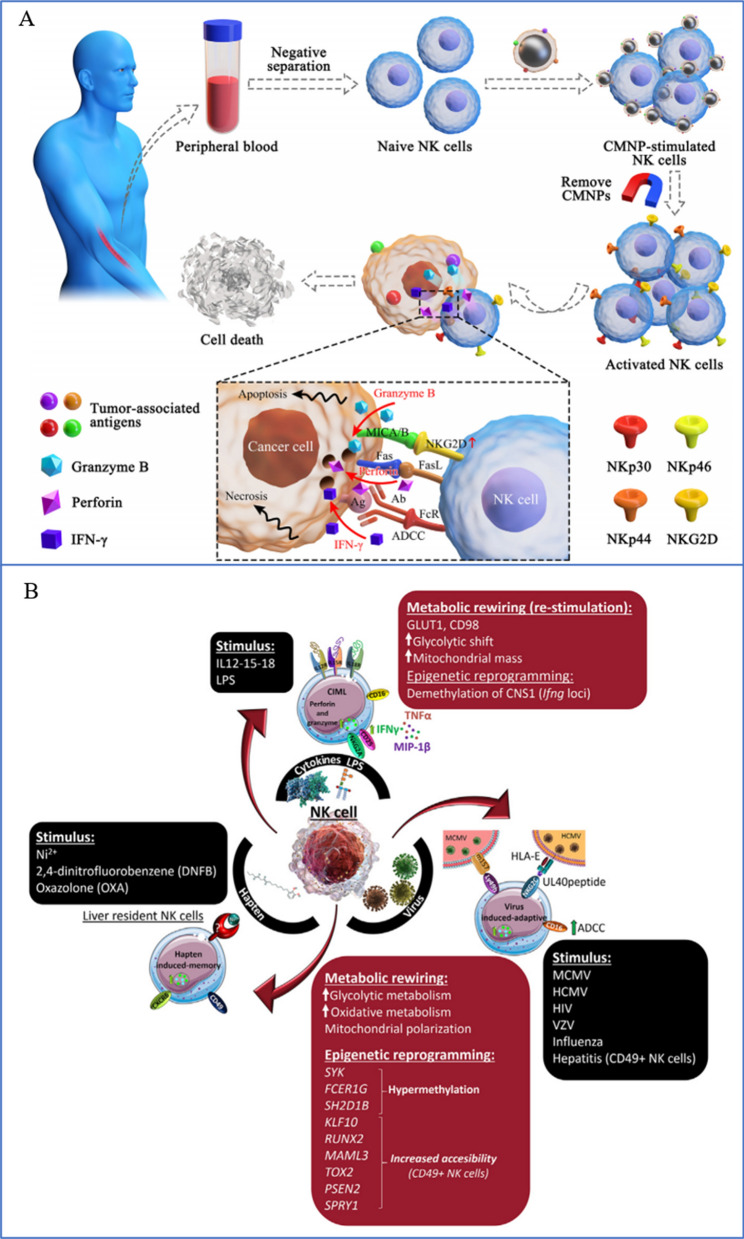
SeNPs and NPs can activate NK cell activity in vivo, release factors, and upregulate receptors to play an immunotherapeutic role. A Magnetic nanoparticles encapsulated in tumor cell-derived membranes are used to enhance NK cell-based immunotherapy [260]. Copyright 2020, Elsevier Inc. B NK cells exhibit three types of innate memory and memory-like responses depending on the initial stimulus [261]. Copyright 2021, Elsevier B.V
TAMs, as innate immune cells, also play an important role during an encounter with lung cancer cells. TAMs are heterogeneous cells that may acquire opposite functions in response to different TME signals, and they can differentiate into TAM1 or TAM2. TAM-polarized M2 macrophages inhibit cancer cell apoptosis in the MPE microenvironment, while tumoricidal M1 macrophages exhibit preferential immune activation [267]. In a previous study, we also found that treatment with SeNPs@LNT for MPE-LA helped to re-educate M2 macrophages into an M1 phenotype (Fig. 14C).
Fig. 14.
SeNPs play an immune role in coordination with other cells. A, C SeNPs and NPs enhance the activity of other cells to promote an antitumor effect [285, 286]. Copyright 2022, Chen, Yang, Fan, Jin, Liao, Li, Liu, Liang, Zhang, Xu and Pi. Copyright 2020, The Pharmaceutical Society of Korea. B SeNPs@LNT promote NK cell secretion of cytokines and macrophage transformation [18]. Copyright 2021, Wiley‐VCH D SeNPs with CIK cells for effective cancer immunotherapy [45]. Copyright 2020, Elsevier Ltd
T lymphocytes play a central role in lung cancer immunity. They kill malignant cells through T-cell receptor (TCR) recognition of specific antigenic peptides on the surface of target cells [270]. T cells secrete cytokines, including IFN-γ, TFN-α, IL-2, IL-10, IL-4, and IL-17 [271–274]. Hu et al. reported the mechanism by which lysosomal SeNPs regulate mitochondrial metabolism and biosynthesis, and at the same time, they stabilized the microtubule structure and further enhanced the cytotoxicity of γδ T cells by upregulating tubulin-α acetylation [50]. Wang et al. [239] reported that the synergistic effect between SeNP-mediated chemotherapy and AUNSS-induced PTT could support T-cell activation and kill tumors [275] (Fig. 13).
Fig. 13.
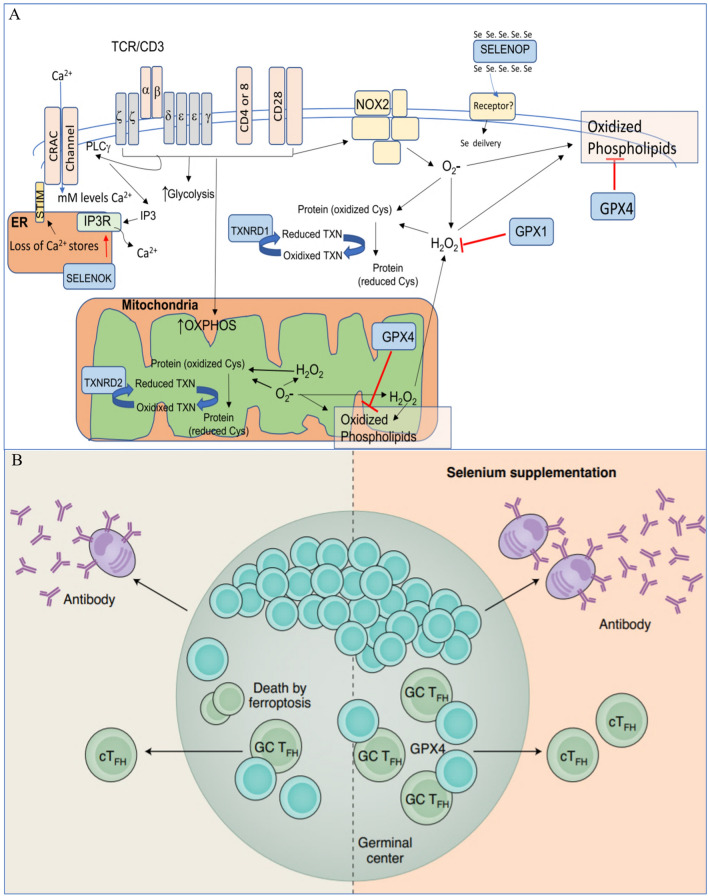
SeNPs and NPs can activate T-cell activity in vivo, release factors, and upregulate receptors to play an immunotherapeutic role. A The role of selenoproteins in T-cell biology [268]. Copyright 2021, Springer Nature America, Inc. B Selenium supplementation boosted TFH cells in mice and humans [269]. Copyright 2020, Elsevier Ltd
SeNPs regulate these innate immune cells to achieve antitumor effects, and their antitumor mechanism has been reported in the literature. However, the research involves some shortcomings, including the following: the number of immune cells may not be sufficient, so research has also shown that SeNPs regulate the proliferation of immune cells, that is, adoptive cellular immunotherapy (ACI) (Fig. 14A, B).
Application of SeNPs in ACI
ACI is a therapeutic method in which immune cells, such as NK cells and T cells, collected from tumor patients are stimulated in vitro and then transfused back into patients [276, 277]. However, the application of nanocarriers can be equivalent to in vitro amplification of immune cells, playing a therapeutic role[278]. ACI mainly includes chimeric antigen receptor (CAR) [279], cytokine-induced killer cell (CIK) [280], and tumor-infiltrating lymphocytes. However, SeNPs mainly adopt CAR and CIK [45].
ACI based on NK cells and T cells is currently an attractive approach for cancer treatment. PD-1 is a protein on T cells, and PD-1/PD-L1 is very commonly used to treat lung cancer. Therefore, NPs mainly expand T cells, secrete more PD-1, and kill tumor cells [281]. CAR-T-cell technology is limited by the inhibitory effect of the TIME and is not effective in treating solid tumors [282]. The application of nanotechnology has successfully solved these problems. NK cells, as "companion cells", can overcome the potential for serious adverse reactions in CAR-T-cell therapy for lung cancer, such as cytokine release syndrome [283].
To simultaneously deliver targeted therapy for lung cancer, Xu et al. built a therapeutic nanoplatform using a lipid aptamer nanostructure [284]. This platform can be employed in conjunction with CAR-NK immunotherapy, demonstrating that CAR-NK can benefit from the synergistic effects of nanotechnology. Additionally, SeNPs function in CAR-NK cells. For the first time, Gao et al. accomplished immunological augmentation of NK cells in the form of Se-containing NPs in metastatic lung tumor and subcutaneous tumor models by proposing a unique strategy that utilizes the ionizing radiation response features of diselenide bonds [52]. As a result, we observed the potential for using SeNPs in CAR-NK cells. In addition, Liu et al. showed a secure and successful method [45]. SeNPs can successfully increase the persistence of CIK cells in vivo in peripheral blood, enabling effective cancer immunotherapy. In addition, the combination therapy "CIK + SeNPs" can successfully encourage more NK cells to colonize malignancies (Fig. 14D).
SeNPs can play a significant role in the immunotherapy of lung cancer by working in conjunction with ACI and enhancing innate immune function in vivo. In light of the potential of ACI, more research on the use of SeNPs in lung cancer may be conducted in the future.
Conclusions and outlook
In general, Se is an important essential trace element for the human body [287]. It can interfere with many physiological processes by regulating selenoproteins and plays an antitumor role by inhibiting protein and DNA biosynthesis and PKC activity. SeNPs can also be used as carriers to deliver drugs to tumor sites to enhance antitumor efficacy. Se also shows potent application as a theranostic for lung cancer. SeNPs can play a role in lung cancer diagnosis, including fluorescence, MR, CT and photoacoustic imaging and other diagnostic methods, as well as treatment through direct killing, radiosensitization, chemotherapeutic sensitization, photothermodynamics, and enhanced immunotherapy. In the field of cancer immunotherapy, the application of Se is also a current hot spot of scientific research because it can directly inhibit tumor cells or indirectly kill tumor cells through activation of NK cells, T cells and other immune cells. However, importantly, although there are a large number of studies utilizing SeNPs in the diagnosis and treatment of lung cancer, most of them remain at the basic research stage, with only a few clinical trials. There are still many problems and challenges that must be faced from basic research to clinical application. In the future, it is necessary to further clarify the mechanism and strengthen the basic-to-clinical transformation.
Problems overcome by the application of SeNPs in the diagnosis and treatment of lung cancer
Currently, increasing numbers of nanodrugs have been developed and used in the clinical treatment of lung cancer, including polymeric micelle paclitaxel [288], liposomal cisplatin [289], CRLX–101 [290], BIND–014 [291], tecemotide [292], and other drugs that are currently in clinical trials. However, Se nanomedicine is still rarely used in the clinic, which could be due to several factors. First, we still need to find more efficient, low-toxicity, simple-structure, high stability, and high bioefficiency Se nanomaterials [293, 294]. Second, there are difficulties with technological breakthroughs from the laboratory to industrial production. Obstacles to the clinical development of nanoparticles include chemistry, manufacturing and control (CMC), good manufacturing practices (GMP), and various aspects of the process from the laboratory to clinical use and commercialization. The transition from the laboratory to the clinic is often accompanied by parameter optimization and even methodological changes, so it is important to carefully consider particle size in the early design phase of nanoparticles [295]. Third, the maturity of Se nanomedicine for oncology is another challenge. These kinds of studies must be elaborated from the aspects of tumor enrichment efficiency, tumor penetration, and how to achieve controlled release of drugs. The clinical application of Se nanomedicine in lung cancer requires more effective SeNPs to be designed based on clinical needs, achieving industrial production, and effectively solving the difficulties faced with lung cancer treatments, thus defining the direction for future development.
Future direction and challenge for Se nanomedicine in lung cancer treatment
Discovery of more definite and specific targets for lung cancer treatment
In recent decades, targeted therapy has resulted in significant progress in the clinical treatment of lung cancer patients with genetic mutations. However, TKIs are associated with notable side effects in some patients, and drug resistance is inevitable. In recent years, cancer immunotherapy has been among the most important advances in the field of cancer therapy. It has achieved obvious and lasting curative effects in the treatment of some patients with advanced lung cancer, but the overall efficacy rate remains to be improved. Therefore, further development of targeted drugs and immune checkpoint inhibitors is needed. Combined with nanomaterials, it is expected that more specific targets for the treatment of lung cancer will be discovered. Due to the high EPR effect of nanomaterials on solid tumors, lung cancer tissues can be targeted passively to improve drug delivery and accumulation [296, 297]. In addition, another major advantage of nanomaterials is their surface modification possibility, which contains as amino, sulfhydryl, carboxyl and other active groups on the surface and thus can be used to covalently connect with ligands and targeted receptor molecules to achieve active targeting of tumors and further improve the delivery efficiency of nanomaterials [298]. By taking advantage of these two characteristics, we can further improve the targeting effect of existing TKI drugs and immune checkpoint inhibitors on lung cancer, reduce side effects, and hopefully find more specific therapeutic targets for lung cancer.
Designing high-specificity Se-containing drugs
SeNPs can be used as drug carriers to deliver anticancer drugs directly to tumor sites, thus reducing toxic side effects. To date, SeNPs have been employed to carry cisplatin, DOX, paclitaxel and other drugs directly to lung cancer sites. In addition, SeNPs themselves can activate NK cells [262], T cells [270], macrophages [18] and other cells to play an immune synergistic therapeutic role. In addition, SeNPs can regulate p53 [228], MAPKs [231] and other genes to further activate ROS-related pathways and enhance the therapeutic effects. However, the specific mechanisms by which immune cells are used as therapeutic targets are not well understood, nor are the receptors on immune cells. Most importantly, these studies are limited to preclinical studies and have not yet been developed in the clinical setting. Therefore, more specific Se-containing drugs are needed to treat lung cancer, either by directly acting on tumor sites and specific genetic targets of lung cancer cells or by acting on receptors on immune cells.
Developing new treatment strategies through multimodal integration therapy
At present, nanomedicine-based treatment of lung cancer has been integrated with chemotherapy, chemo-sensitization, photodynamic therapy, immunotherapy and other treatment methods, all of which have shown great potential for lung cancer treatment. The use of immune-synergistic therapy may become the focus of future research to develop new receptor/ligand targets, new immune cell therapy models, and more new and effective therapeutic strategies through combination with physical therapy, traditional Chinese medicine, and surgery to achieve better treatment efficacy.
Acknowledgements
Not applicable.
Abbreviations
- Se
Selenium
- SCLC
Small cell lung cancer
- EGFR
Epidermal growth factor receptor
- TIME
The tumor immune microenvironment
- Inorg-Se
Inorganic selenium
- Org-Se
Organic selenium
- Doxil
Doxorubicin hydrochloride liposome nanomedicine
- SeNPs
Selenium nanoparticles
- COPD
Chronic obstructive pulmonary disease
- ARDS
Acute respiratory distress syndrome
- H2Se
Hydrogen selenide
- CH3SeH
Methylselenol
- MSA
Methylseleninic acid
- SOD
Superoxide dismutase
- NF-kB
Nuclear factor kappa beta
- GPX
Glutathione peroxidase
- NPs
Nanoparticles
- PLAL
Pulsed laser ablation in liquids
- Na2SO3
Sodium sulfite
- Blg
β Lactoglobulin
- CS
Chitosan
- SPS
Spiral algae polysaccharide
- PSP
Polysaccharide-protein complex
- SFS
Sodium formaldehyde sulfonate
- EPS
Cordyceps exopolysaccharides
- MPE
Malignant pleural effusion
- SeO2
Se dioxide
- Se/Ti (I)
(II) and (III), Se dioxide/titanium dioxide nanocomposites
- 2D
Two-dimensional
- TMDCs
Transition metal dichalcogenides
- MoSe2
Molybdenum selenide
- MoSe2(Gd3+)
Gd3+-doped MoSe2
- FRAP
Fluid on the iron-reducing antioxidant capacity
- CRP
C-reactive protein
- PEG
Polyethylene glycol
- PAI
Photoacoustic imaging
- t-Se
Trigonal Se
- FRI
Fluorescence imaging
- MRI
MR imaging
- FeSe2
Iron diselenide
- FDA
Food and Drug Administration
- IL-2
Interleukin-2
- ROS
Reactive oxygen species
- RT
Radiotherapy
- PTX
Doxorubicin, paclitaxel
- HA
Hyaluronic acid
- Tf
Transferrin
- Se@Trolox
Trolox surface functionalized SeNPs
- DOX
Doxorubicin
- PTT
Photothermal therapy
- EPR
Enhanced permeability and retention
- PTAs
Photothermal transduction agents
- TeSe
Tellurium-Se
- PCE
Photothermal conversion efficiency
- ICG
Indocyanine green
- MPE-LA
MPE from lung adenocarcinoma
- MAPK
Mitogen-activated protein kinase
- Se@MUN
SeNPs by self-assembly of 11-mercapto-1-undecanol
- RGDfC
Arg–Gly–Asp–D-Phe–Cys
- TAMs
Tumor-associated macrophages
- ACI
Adoptive cellular immunotherapy
- TME
The tumor microenvironment
- TCR
T-cell receptor
- CIK
Cytokine-induced killer cell
- CAR
Chimeric antigen receptor
Author contributions
JW and TC proposed the project; SL wrote the main manuscript text; SL and WW prepared figures; all the authors carried out reference searching and data analysis. All authors have reviewed and approved the final manuscript.
Funding
This review is supported by the Natural Science Foundation of Guangdong Province (Grant No. 2022A1515010516), the Guangzhou Science and Technology Planning Project (Grant No. 2060206).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to publish.
Competing interests
The authors declared no competing financial interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jinlin Wang, Email: wangjinlin@gird.cn.
Tianfeng Chen, Email: tchentf@jnu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17:725–737. doi: 10.1038/nrc.2017.87. [DOI] [PubMed] [Google Scholar]
- 3.Ruzycka M, Cimpan MR, Rios-Mondragon I, Grudzinski IP. Microfluidics for studying metastatic patterns of lung cancer. J Nanobiotechnol. 2019;17:71. doi: 10.1186/s12951-019-0492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Gao F, Li X, Niu G, Yang Y, Li H, et al. Tumor microenvironment-responsive fenton nanocatalysts for intensified anticancer treatment. J Nanobiotechnol. 2022;20:69. doi: 10.1186/s12951-022-01278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma A, Chopra A, Lee YW, Bharwani LD, Asmat AB, Aneez DBA, et al. Can EGFR-tyrosine kinase inhibitors (TKI) alone without Talc pleurodesis prevent recurrence of malignant pleural effusion (MPE) in lung adenocarcinoma. Curr Drug Discov Technol. 2016;13:68–76. doi: 10.2174/1570163813666160524142846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377:849–861. doi: 10.1056/NEJMra1703413. [DOI] [PubMed] [Google Scholar]
- 7.Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 8.Heigener DF, Reck M. Crizotinib. Recent Results Cancer Res. 2018;211:57–65. doi: 10.1007/978-3-319-91442-8_4. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Solomon BJ, Besse B, Bauer TM, Lin C-C, Soo RA, et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37:1370–1379. doi: 10.1200/JCO.18.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey RD, Adams VR, Beardslee T, Medina P. Afatinib for the treatment of EGFR mutation-positive NSCLC: a review of clinical findings. J Oncol Pharm Pract. 2020;26:1461–1474. doi: 10.1177/1078155220931926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y-L, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 12.Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725–737. doi: 10.1038/s41416-019-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim ES. Olmutinib: first global approval. Drugs. 2016;76:1153–1157. doi: 10.1007/s40265-016-0606-z. [DOI] [PubMed] [Google Scholar]
- 14.Reuss JE, Anagnostou V, Cottrell TR, Smith KN, Verde F, Zahurak M, et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J Immunother Cancer. 2020;8:e001282. doi: 10.1136/jitc-2020-001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 16.Fu T, Dai L-J, Wu S-Y, Xiao Y, Ma D, Jiang Y-Z, et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J Hematol Oncol. 2021;14:98. doi: 10.1186/s13045-021-01103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu A, Yao B, Dong T, Chen Y, Yao J, Liu Y, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185:1356–1372.e26. doi: 10.1016/j.cell.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Song Z, Luo W, Zheng H, Zeng Y, Wang J, Chen T. Translational nanotherapeutics reprograms immune microenvironment in malignant pleural effusion of lung adenocarcinoma. Adv Healthc Mater. 2021;10:2100149. doi: 10.1002/adhm.202100149. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Lin Z, Zhao M, Xu T, Wang C, Xia H, et al. Multifunctional selenium nanoparticles as carriers of HSP70 siRNA to induce apoptosis of HepG2 cells. Int J Nanomed. 2016;11:3065–3076. doi: 10.2147/IJN.S109822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Guo M, Lin Z, Zhao M, Xiao M, Wang C, et al. Polyethylenimine-functionalized silver nanoparticle-based co-delivery of paclitaxel to induce HepG2 cell apoptosis. Int J Nanomed. 2016;11:6693–6702. doi: 10.2147/IJN.S122666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Lin Z, Guo M, Xia Y, Zhao M, Wang C, et al. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int J Nanomed. 2017;12:5733–5743. doi: 10.2147/IJN.S140939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng X, Xu W, Li Z, Song W, Ding J, Chen X. Immunomodulatory nanosystems. Adv Sci (Weinh) 2019;6:1900101. doi: 10.1002/advs.201900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avery JC, Hoffmann PR. Selenium, selenoproteins, and immunity. Nutrients. 2018;10:E1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fritz H, Kennedy D, Fergusson D, Fernandes R, Cooley K, Seely A, et al. Selenium and lung cancer: a systematic review and meta analysis. PLoS ONE. 2011;6:e26259. doi: 10.1371/journal.pone.0026259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin SH, Yoon MJ, Kim M, Kim J-I, Lee S-J, Lee Y-S, et al. Enhanced lung cancer cell killing by the combination of selenium and ionizing radiation. Oncol Rep. 2007;17:209–216. [PubMed] [Google Scholar]
- 26.Xu Y, Luo C, Wang J, Chen L, Chen J, Chen T, et al. Application of nanotechnology in the diagnosis and treatment of bladder cancer. J Nanobiotechnol. 2021;19:393. doi: 10.1186/s12951-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Zhao J, Tan T, Liu M, Zeng Z, Zeng Y, et al. Nanoparticle drug delivery system for glioma and its efficacy improvement strategies: a comprehensive review. Int J Nanomed. 2020;15:2563–2582. doi: 10.2147/IJN.S243223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T, Lai L, Song Z, Chen T. A sequentially triggered nanosystem for precise drug delivery and simultaneous inhibition of cancer growth, migration, and invasion. Adv Funct Mater. 2016;26:7775–7790. doi: 10.1002/adfm.201604206. [DOI] [Google Scholar]
- 30.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonkusre P, Cameotra SS. Biogenic selenium nanoparticles induce ROS-mediated necroptosis in PC-3 cancer cells through TNF activation. J Nanobiotechnol. 2017;15:43. doi: 10.1186/s12951-017-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose PG, Smrekar M, Haba P, Fusco N, Rodriguez M. A phase I study of oral topotecan and pegylated liposomal doxorubicin (doxil) in platinum-resistant ovarian and peritoneal cancer. Am J Clin Oncol. 2008;31:476–480. doi: 10.1097/COC.0b013e31816a6221. [DOI] [PubMed] [Google Scholar]
- 33.Prado CMM, Baracos VE, Xiao J, Birdsell L, Stuyckens K, Park YC, et al. The association between body composition and toxicities from the combination of Doxil and trabectedin in patients with advanced relapsed ovarian cancer. Appl Physiol Nutr Metab. 2014;39:693–698. doi: 10.1139/apnm-2013-0403. [DOI] [PubMed] [Google Scholar]
- 34.Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Des Devel Ther. 2015;9:3767–3777. doi: 10.2147/DDDT.S88023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dranitsaris G, Yu B, Wang L, Sun W, Zhou Y, King J, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22:205–211. doi: 10.1177/1078155214556008. [DOI] [PubMed] [Google Scholar]
- 36.Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100:437–438. doi: 10.1016/j.ygyno.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson AM, Bhar P, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17:1263–1268. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 38.Lai H, Zeng D, Liu C, Zhang Q, Wang X, Chen T. Selenium-containing ruthenium complex synergizes with natural killer cells to enhance immunotherapy against prostate cancer via activating TRAIL/FasL signaling. Biomaterials. 2019;219:119377. doi: 10.1016/j.biomaterials.2019.119377. [DOI] [PubMed] [Google Scholar]
- 39.Liao G, Tang J, Wang D, Zuo H, Zhang Q, Liu Y, et al. Selenium nanoparticles (SeNPs) have potent antitumor activity against prostate cancer cells through the upregulation of miR-16. World J Surg Oncol. 2020;18:81. doi: 10.1186/s12957-020-01850-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fajt Z, Drabek J, Steinhauser L, Svobodova Z. The significance of pork as a source of dietary selenium - an evaluation of the situation in the Czech Republic. Neuro Endocrinol Lett. 2009;30(Suppl 1):17–21. [PubMed] [Google Scholar]
- 41.Wang H, Zhang J, Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med. 2007;42:1524–1533. doi: 10.1016/j.freeradbiomed.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Wang X, Xu T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci. 2008;101:22–31. doi: 10.1093/toxsci/kfm221. [DOI] [PubMed] [Google Scholar]
- 43.Sadeghian S, Kojouri GA, Mohebbi A. Nanoparticles of selenium as species with stronger physiological effects in sheep in comparison with sodium selenite. Biol Trace Elem Res. 2012;146:302–308. doi: 10.1007/s12011-011-9266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao S, Lin Y, Lin R, Liu J, Wang H, Hu W, et al. Traditional Chinese medicine active ingredients-based selenium nanoparticles regulate antioxidant selenoproteins for spinal cord injury treatment. J Nanobiotechnol. 2022;20:278. doi: 10.1186/s12951-022-01490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu T, Xu L, He L, Zhao J, Zhang Z, Chen Q, et al. Selenium nanoparticles regulates selenoprotein to boost cytokine-induced killer cells-based cancer immunotherapy. Nano Today. 2020;35:100975. doi: 10.1016/j.nantod.2020.100975. [DOI] [Google Scholar]
- 46.Tian J, Wei X, Zhang W, Xu A. Effects of selenium nanoparticles combined with radiotherapy on lung cancer cells. Front Bioeng Biotechnol. 2020;8:598997. doi: 10.3389/fbioe.2020.598997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y, He L, Liu W, Fan C, Zheng W, Wong Y-S, et al. Selective cellular uptake and induction of apoptosis of cancer-targeted selenium nanoparticles. Biomaterials. 2013;34:7106–7116. doi: 10.1016/j.biomaterials.2013.04.067. [DOI] [PubMed] [Google Scholar]
- 48.Liu T, Zeng L, Jiang W, Fu Y, Zheng W, Chen T. Rational design of cancer-targeted selenium nanoparticles to antagonize multidrug resistance in cancer cells. Nanomedicine. 2015;11:947–958. doi: 10.1016/j.nano.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Jiang W, Fu Y, Yang F, Yang Y, Liu T, Zheng W, et al. Gracilaria lemaneiformis polysaccharide as integrin-targeting surface decorator of selenium nanoparticles to achieve enhanced anticancer efficacy. ACS Appl Mater Interfaces. 2014;6:13738–13748. doi: 10.1021/am5031962. [DOI] [PubMed] [Google Scholar]
- 50.Hu Y, Liu T, Li J, Mai F, Li J, Chen Y, et al. Selenium nanoparticles as new strategy to potentiate γδ T cell anti-tumor cytotoxicity through upregulation of tubulin-α acetylation. Biomaterials. 2019;222:119397. doi: 10.1016/j.biomaterials.2019.119397. [DOI] [PubMed] [Google Scholar]
- 51.Wu H, Zhu H, Li X, Liu Z, Zheng W, Chen T, et al. Induction of apoptosis and cell cycle arrest in A549 human lung adenocarcinoma cells by surface-capping selenium nanoparticles: an effect enhanced by polysaccharide-protein complexes from Polyporus rhinocerus. J Agric Food Chem. 2013;61:9859–9866. doi: 10.1021/jf403564s. [DOI] [PubMed] [Google Scholar]
- 52.Gao S, Li T, Guo Y, Sun C, Xianyu B, Xu H. Selenium-containing nanoparticles combine the NK cells mediated immunotherapy with radiotherapy and chemotherapy. Adv Mater. 2020;32:e1907568. doi: 10.1002/adma.201907568. [DOI] [PubMed] [Google Scholar]
- 53.Yu S-Y, Mao B-L, Xiao P, Yu W-P, Wang Y-L, Huang C-Z, et al. Intervention trial with selenium for the prevention of lung cancer among tin miners in Yunnan, China: a pilot study. Biol Trace Elem Res. 1990;24:105–108. doi: 10.1007/BF02917199. [DOI] [PubMed] [Google Scholar]
- 54.Reid ME, Duffield-Lillico AJ, Garland L, Turnbull BW, Clark LC, Marshall JR. Selenium supplementation and lung cancer incidence: an update of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev. 2002;11:1285–1291. [PubMed] [Google Scholar]
- 55.Shaheen SO, Newson RB, Rayman MP, Wong AP-L, Tumilty MK, Phillips JM, et al. Randomised, double blind, placebo-controlled trial of selenium supplementation in adult asthma. Thorax. 2007;62:483–490. doi: 10.1136/thx.2006.071563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isbaniah F, Wiyono WH, Yunus F, Setiawati A, Totzke U, Verbruggen MA. Echinacea purpurea along with zinc, selenium and vitamin C to alleviate exacerbations of chronic obstructive pulmonary disease: results from a randomized controlled trial: Echinacea purpurea and micronutrients in COPD. J Clin Pharm Ther. 2011;36:568–576. doi: 10.1111/j.1365-2710.2010.01212.x. [DOI] [PubMed] [Google Scholar]
- 57.Youssef MA, El-khodery SA, Ibrahim HMM. Effect of selenium and vitamin C on clinical outcomes, trace element status, and antioxidant enzyme activity in horses with acute and chronic lower airway disease. A randomized clinical trial. Biol Trace Elem Res. 2013;152:333–342. doi: 10.1007/s12011-013-9636-5. [DOI] [PubMed] [Google Scholar]
- 58.Gazdik F, Horvathova M, Gazdikova K, Jahnova E. The influence of selenium supplementation on the immunity of corticoid-dependent asthmatics. Bratisl Lek Listy. 2002;103:17–21. [PubMed] [Google Scholar]
- 59.Hussain MI, Ahmed W, Nasir M, Mushtaq MH, Sheikh AA, Shaheen AY, et al. Immune modulatory and anti-oxidative effect of selenium against pulmonary tuberculosis. Pak J Pharm Sci. 2019;6. [PubMed]
- 60.Seyedrezazadeh E, Ostadrahimi A, Mahboob S, Assadi Y, Ghaemmagami J, Pourmogaddam M. Effect of vitamin E and selenium supplementation on oxidative stress status in pulmonary tuberculosis patients. Respirology. 2008;13:294–298. doi: 10.1111/j.1440-1843.2007.01200.x. [DOI] [PubMed] [Google Scholar]
- 61.Cassano PA, Guertin KA, Kristal AR, Ritchie KE, Bertoia ML, Arnold KB, et al. A randomized controlled trial of vitamin E and selenium on rate of decline in lung function. Respir Res. 2015;16:35. doi: 10.1186/s12931-015-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu G, Cassano PA. Antioxidant nutrients and pulmonary function: the Third National Health and Nutrition Examination Survey (NHANES III) Am J Epidemiol. 2000;151:975–981. doi: 10.1093/oxfordjournals.aje.a010141. [DOI] [PubMed] [Google Scholar]
- 63.Kočan L, Vašková J, Vaško L, Simonová J, Simon R, Firment J. Selenium adjuvant therapy in septic patients selected according to Carrico index. Clin Biochem. 2014;47:44–50. doi: 10.1016/j.clinbiochem.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Mahmoodpoor A, Hamishehkar H, Shadvar K, Ostadi Z, Sanaie S, Saghaleini SH, et al. The effect of intravenous selenium on oxidative stress in critically ill patients with acute respiratory distress syndrome. Immunol Invest. 2019;48:147–159. doi: 10.1080/08820139.2018.1496098. [DOI] [PubMed] [Google Scholar]
- 65.Karp DD, Lee SJ, Keller SM, Wright GS, Aisner S, Belinsky SA, et al. Randomized, double-blind, placebo-controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non-small-cell lung cancer: ECOG 5597. J Clin Oncol. 2013;31:4179–4187. doi: 10.1200/JCO.2013.49.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–410. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y-C, Sosnoski DM, Gandhi UH, Novinger LJ, Prabhu KS, Mastro AM. Selenium modifies the osteoblast inflammatory stress response to bone metastatic breast cancer. Carcinogenesis. 2009;30:1941–1948. doi: 10.1093/carcin/bgp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boudreau RTM, Conrad DM, Hoskin DW. Differential involvement of reactive oxygen species in apoptosis caused by the inhibition of protein phosphatase 2A in Jurkat and CCRF-CEM human T-leukemia cells. Exp Mol Pathol. 2007;83:347–356. doi: 10.1016/j.yexmp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Jiang C, Ganther H, Lu J. Monomethyl selenium–specific inhibition of MMP-2 and VEGF expression: implications for angiogenic switch regulation. Mol Carcinog. 2000;29:236–250. doi: 10.1002/1098-2744(200012)29:4<236::AID-MC1006>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 70.Park J-M, Kim A, Oh J-H, Chung A-S. Methylseleninic acid inhibits PMA-stimulated pro-MMP-2 activation mediated by MT1-MMP expression and further tumor invasion through suppression of NF-kappaB activation. Carcinogenesis. 2007;28:837–847. doi: 10.1093/carcin/bgl203. [DOI] [PubMed] [Google Scholar]
- 71.Alkie TN, de Jong J, Moore E, DeWitte-Orr SJ. Phytoglycogen nanoparticle delivery system for inorganic selenium reduces cytotoxicity without impairing selenium bioavailability. Int J Nanomed. 2020;15:10469–10479. doi: 10.2147/IJN.S286948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pedrero Z, Madrid Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: a review. Anal Chim Acta. 2009;634:135–152. doi: 10.1016/j.aca.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 73.Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001;131:1010S–S1015. doi: 10.1093/jn/131.3.1010S. [DOI] [PubMed] [Google Scholar]
- 74.Ye R, Huang J, Wang Z, Chen Y, Dong Y. Trace element selenium effectively alleviates intestinal diseases. Int J Mol Sci. 2021;22:11708. doi: 10.3390/ijms222111708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen X, Li S, Cong X, Yu T, Zhu Z, Barba FJ, et al. Optimization of Bacillus cereus fermentation process for selenium enrichment as organic selenium source. Front Nutr. 2020;7:543873. doi: 10.3389/fnut.2020.543873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson VJ, Tsunoda M, Sharma RP. Increased production of proinflammatory cytokines by murine macrophages following oral exposure to sodium selenite but not to seleno-l-methionine. Arch Environ Contam Toxicol. 2000;39:243–250. doi: 10.1007/s002440010101. [DOI] [PubMed] [Google Scholar]
- 77.Cruz LY, Wang D, Liu J. Biosynthesis of selenium nanoparticles, characterization and X-ray induced radiotherapy for the treatment of lung cancer with interstitial lung disease. J Photochem Photobiol B. 2019;191:123–127. doi: 10.1016/j.jphotobiol.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 78.Selenius M, Fernandes AP, Brodin O, Björnstedt M, Rundlöf A-K. Treatment of lung cancer cells with cytotoxic levels of sodium selenite: effects on the thioredoxin system. Biochem Pharmacol. 2008;75:2092–2099. doi: 10.1016/j.bcp.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 79.Xu W, Ma W-W, Zeng H-H. Synergistic effect of ethaselen and selenite treatment against A549 human non-small cell lung cancer cells. Asian Pac J Cancer Prev. 2014;15:7129–7135. doi: 10.7314/APJCP.2014.15.17.7129. [DOI] [PubMed] [Google Scholar]
- 80.Asfour IA, Fayek M, Raouf S, Soliman M, Hegab HM, El-Desoky H, et al. The impact of high-dose sodium selenite therapy on Bcl-2 expression in adult non-Hodgkin’s lymphoma patients: correlation with response and survival. Biol Trace Elem Res. 2007;120:1–10. doi: 10.1007/s12011-007-0029-5. [DOI] [PubMed] [Google Scholar]
- 81.Ohkawa K, Tsukada Y, Dohzono H, Koike K, Terashima Y. The effects of co-administration of selenium and cis-platin (CDDP) on CDDP-induced toxicity and antitumour activity. Br J Cancer. 1988;58:38–41. doi: 10.1038/bjc.1988.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vermeulen NP, Baldew GS, Los G, McVie JG, De Goeij JJ. Reduction of cisplatin nephrotoxicity by sodium selenite. Lack of interaction at the pharmacokinetic level of both compounds. Drug Metab Dispos. 1993;21:30–6. [PubMed] [Google Scholar]
- 83.Rao M, Rao MN. Protective effects of selenomethionine against cisplatin-induced renal toxicity in mice and rats. J Pharm Pharmacol. 1998;50:687–691. doi: 10.1111/j.2042-7158.1998.tb06906.x. [DOI] [PubMed] [Google Scholar]
- 84.Baldew GS, Mol JG, de Kanter FJ, van Baar B, de Goeij JJ, Vermeulen NP. The mechanism of interaction between cisplatin and selenite. Biochem Pharmacol. 1991;41:1429–1437. doi: 10.1016/0006-2952(91)90558-M. [DOI] [PubMed] [Google Scholar]
- 85.Fernández-Martínez A, Charlet L. Selenium environmental cycling and bioavailability: a structural chemist point of view. Rev Environ Sci Biotechnol. 2009;8:81–110. doi: 10.1007/s11157-009-9145-3. [DOI] [Google Scholar]
- 86.Huang S, Yang W, Huang G. Preparation and activities of selenium polysaccharide from plant such as Grifola frondosa. Carbohydr Polym. 2020;242:116409. doi: 10.1016/j.carbpol.2020.116409. [DOI] [PubMed] [Google Scholar]
- 87.Yang W, Huang G, Huang H. Preparation and structure of polysaccharide selenide. Ind Crops Prod. 2020;154:112630. doi: 10.1016/j.indcrop.2020.112630. [DOI] [Google Scholar]
- 88.Yan J-K, Qiu W-Y, Wang Y-Y, Wang W-H, Yang Y, Zhang H-N. Fabrication and stabilization of biocompatible selenium nanoparticles by carboxylic curdlans with various molecular properties. Carbohydr Polym. 2018;179:19–27. doi: 10.1016/j.carbpol.2017.09.063. [DOI] [PubMed] [Google Scholar]
- 89.Moreno E, Doughty-Shenton D, Plano D, Font M, Encío I, Palop JA, et al. A dihydroselenoquinazoline inhibits S6 ribosomal protein signalling, induces apoptosis and inhibits autophagy in MCF-7 cells. Eur J Pharm Sci. 2014;63:87–95. doi: 10.1016/j.ejps.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 90.Chen Z, Lai H, Hou L, Chen T. Rational design and action mechanisms of chemically innovative organoselenium in cancer therapy. Chem Commun (Camb) 2019;56:179–196. doi: 10.1039/C9CC07683B. [DOI] [PubMed] [Google Scholar]
- 91.Hu YJ, Chen Y, Zhang YQ, Zhou MZ, Song XM, Zhang BZ, et al. The protective role of selenium on the toxicity of cisplatin-contained chemotherapy regimen in cancer patients. Biol Trace Elem Res. 1997;56:331–341. doi: 10.1007/BF02785304. [DOI] [PubMed] [Google Scholar]
- 92.Chen T, Wong Y-S, Zheng W, Liu J. Caspase- and p53-dependent apoptosis in breast carcinoma cells induced by a synthetic selenadiazole derivative. Chem Biol Interact. 2009;180:54–60. doi: 10.1016/j.cbi.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 93.Liang Y-W, Zheng J, Li X, Zheng W, Chen T. Selenadiazole derivatives as potent thioredoxin reductase inhibitors that enhance the radiosensitivity of cancer cells. Eur J Med Chem. 2014;84:335–342. doi: 10.1016/j.ejmech.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 94.Nonsuwan P, Puthong S, Palaga T, Muangsin N. Novel organic/inorganic hybrid flower-like structure of selenium nanoparticles stabilized by pullulan derivatives. Carbohydr Polym. 2018;184:9–19. doi: 10.1016/j.carbpol.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 95.Hu T, Li H, Zhao G, Guo Y. Selenium enriched Hypsizygus marmoreus, a potential food supplement with improved Se bioavailability. LWT. 2021;140:110819. doi: 10.1016/j.lwt.2020.110819. [DOI] [Google Scholar]
- 96.Wang Y, Deng Y, Luo H, Zhu A, Ke H, Yang H, et al. Light-responsive nanoparticles for highly efficient cytoplasmic delivery of anticancer agents. ACS Nano. 2017;11:12134–12144. doi: 10.1021/acsnano.7b05214. [DOI] [PubMed] [Google Scholar]
- 97.Kuchur OA, Tsymbal SA, Shestovskaya MV, Serov NS, Dukhinova MS, Shtil AA. Metal-derived nanoparticles in tumor theranostics: potential and limitations. J Inorg Biochem. 2020;209:111117. doi: 10.1016/j.jinorgbio.2020.111117. [DOI] [PubMed] [Google Scholar]
- 98.Dastjerdi R, Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids Surf B Biointerfaces. 2010;79:5–18. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 99.Lu X-Y, Wu D-C, Li Z-J, Chen G-Q. Polymer nanoparticles. Prog Mol Biol Transl Sci. 2011;104:299–323. doi: 10.1016/B978-0-12-416020-0.00007-3. [DOI] [PubMed] [Google Scholar]
- 100.Surendran SP, Moon MJ, Park R, Jeong YY. Bioactive nanoparticles for cancer immunotherapy. Int J Mol Sci. 2018;19:3877. doi: 10.3390/ijms19123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y, Li X, Huang Z, Zheng W, Fan C, Chen T. Enhancement of cell permeabilization apoptosis-inducing activity of selenium nanoparticles by ATP surface decoration. Nanomedicine. 2013;9:74–84. doi: 10.1016/j.nano.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y, Chen P, Zhao G, Sun K, Li D, Wan X, et al. Inverse relationship between elemental selenium nanoparticle size and inhibition of cancer cell growth in vitro and in vivo. Food Chem Toxicol. 2015;85:71–77. doi: 10.1016/j.fct.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 103.Zhu C, Zhang S, Song C, Zhang Y, Ling Q, Hoffmann PR, et al. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J Nanobiotechnol. 2017;15:20. doi: 10.1186/s12951-017-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu W, Li X, Wong Y-S, Zheng W, Zhang Y, Cao W, et al. Selenium nanoparticles as a carrier of 5-fluorouracil to achieve anticancer synergism. ACS Nano. 2012;6:6578–6591. doi: 10.1021/nn202452c. [DOI] [PubMed] [Google Scholar]
- 105.Tan L, Jia X, Jiang X, Zhang Y, Tang H, Yao S, et al. In vitro study on the individual and synergistic cytotoxicity of adriamycin and selenium nanoparticles against Bel7402 cells with a quartz crystal microbalance. Biosens Bioelectron. 2009;24:2268–2272. doi: 10.1016/j.bios.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 106.Gao F, Yuan Q, Gao L, Cai P, Zhu H, Liu R, et al. Cytotoxicity and therapeutic effect of irinotecan combined with selenium nanoparticles. Biomaterials. 2014;35:8854–8866. doi: 10.1016/j.biomaterials.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 107.Yu B, Liu T, Du Y, Luo Z, Zheng W, Chen T. X-ray-responsive selenium nanoparticles for enhanced cancer chemo-radiotherapy. Colloids Surf B Biointerfaces. 2016;139:180–189. doi: 10.1016/j.colsurfb.2015.11.063. [DOI] [PubMed] [Google Scholar]
- 108.Sun D, Liu Y, Yu Q, Qin X, Yang L, Zhou Y, et al. Inhibition of tumor growth and vasculature and fluorescence imaging using functionalized ruthenium-thiol protected selenium nanoparticles. Biomaterials. 2014;35:1572–1583. doi: 10.1016/j.biomaterials.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 109.Jiang X, Han Y, Zhang H, Liu H, Huang Q, Wang T, et al. Cu-Fe-Se ternary nanosheet-based drug delivery carrier for multimodal imaging and combined chemo/photothermal therapy of cancer. ACS Appl Mater Interfaces. 2018;10:43396–43404. doi: 10.1021/acsami.8b15064. [DOI] [PubMed] [Google Scholar]
- 110.Zhao S, Tian R, Shao B, Feng Y, Yuan S, Dong L, et al. UCNP-Bi2 Se3 upconverting nanohybrid for upconversion luminescence and CT imaging and photothermal therapy. Chemistry. 2020;26:1127–1135. doi: 10.1002/chem.201904586. [DOI] [PubMed] [Google Scholar]
- 111.Mary TA, Shanthi K, Vimala K, Soundarapandian K. PEG functionalized selenium nanoparticles as a carrier of crocin to achieve anticancer synergism. RSC Adv R Soc Chem. 2016;6:22936–49. doi: 10.1039/C5RA25109E. [DOI] [Google Scholar]
- 112.Zhang X, He C, Yan R, Chen Y, Zhao P, Li M, et al. HIF-1 dependent reversal of cisplatin resistance via anti-oxidative nano selenium for effective cancer therapy. Chem Eng J. 2020;380:122540. doi: 10.1016/j.cej.2019.122540. [DOI] [Google Scholar]
- 113.Liu T, Shi C, Duan L, Zhang Z, Luo L, Goel S, et al. A highly hemocompatible erythrocyte membrane-coated ultrasmall selenium nanosystem for simultaneous cancer radiosensitization and precise antiangiogenesis. J Mater Chem B. 2018;6:4756–4764. doi: 10.1039/C8TB01398E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bidkar AP, Sanpui P, Ghosh SS. Combination therapy with MAPK-pathway-specific inhibitor and folic-acid-receptor-targeted selenium nanoparticles induces synergistic antiproliferative response in BRAF mutant cancer cells. ACS Biomater Sci Eng Am Chem Soc. 2019;5:2222–34. doi: 10.1021/acsbiomaterials.9b00112. [DOI] [PubMed] [Google Scholar]
- 115.Li Y, Li X, Wong Y-S, Chen T, Zhang H, Liu C, et al. The reversal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-mercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials. 2011;32:9068–9076. doi: 10.1016/j.biomaterials.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 116.Chen S, Xing C, Huang D, Zhou C, Ding B, Guo Z, et al. Eradication of tumor growth by delivering novel photothermal selenium-coated tellurium nanoheterojunctions. Sci Adv. 2020;6:eaay6825. doi: 10.1126/sciadv.aay6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jiang Z-Y, Xie Z-X, Xie S-Y, Zhang X-H, Huang R-B, Zheng L-S. High purity trigonal selenium nanorods growth via laser ablation under controlled temperature. Chem Phys Lett. 2003;368:425–429. doi: 10.1016/S0009-2614(02)01918-8. [DOI] [Google Scholar]
- 118.Shahverdi AR, Shahverdi F, Faghfuri E, Reza Khoshayand M, Mavandadnejad F, Yazdi MH, et al. Characterization of folic acid surface-coated selenium nanoparticles and corresponding in vitro and in vivo effects against breast cancer. Arch Med Res. 2018;49:10–17. doi: 10.1016/j.arcmed.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 119.Zhang H, Yang D, Ji Y, Ma X, Xu J, Que D. Selenium nanotubes synthesized by a novel solution phase approach. J Phys Chem B. 2004;108:1179–1182. doi: 10.1021/jp036168z. [DOI] [Google Scholar]
- 120.Cai L, Zhou S, Yu B, Zhou E, Zheng Y, Ahmed NSI, et al. The composites of triple-helix glucan nanotubes/selenium nanoparticles target hepatocellular carcinoma to enhance ferroptosis by depleting glutathione and augmenting redox imbalance. Chem Eng J. 2022;446:137110. doi: 10.1016/j.cej.2022.137110. [DOI] [Google Scholar]
- 121.Bilek O, Fohlerova Z, Hubalek J. Enhanced antibacterial and anticancer properties of Se-NPs decorated TiO2 nanotube film. PLoS ONE. 2019;14:e0214066. doi: 10.1371/journal.pone.0214066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu X, Yu JC, Li Q. Synthesis of surface-functionalized t-Se microspheres via a green wet-chemical route. J Mater Chem. 2006;16:748–751. doi: 10.1039/B514605D. [DOI] [Google Scholar]
- 123.Feng Y, Su J, Zhao Z, Zheng W, Wu H, Zhang Y, et al. Differential effects of amino acid surface decoration on the anticancer efficacy of selenium nanoparticles. Dalton Trans. 2014;43:1854–1861. doi: 10.1039/C3DT52468J. [DOI] [PubMed] [Google Scholar]
- 124.Li Y, Li X, Zheng W, Fan C, Zhang Y, Chen T. Functionalized selenium nanoparticles with nephroprotective activity, the important roles of ROS-mediated signaling pathways. J Mater Chem B. 2013;1:6365–6372. doi: 10.1039/c3tb21168a. [DOI] [PubMed] [Google Scholar]
- 125.Chen M, Huang Y, Zhu X, Hu X, Chen T. Efficient overcoming of blood–brain barrier by functionalized selenium nanoparticles to treat glioma. Adv Therapeut. 2018;1:1800074. doi: 10.1002/adtp.201800074. [DOI] [Google Scholar]
- 126.Yang F, Tang Q, Zhong X, Bai Y, Chen T, Zhang Y, et al. Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int J Nanomed. 2012;7:835–844. doi: 10.2147/IJN.S28278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhai X, Zhang C, Zhao G, Stoll S, Ren F, Leng X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J Nanobiotechnol. 2017;15:4. doi: 10.1186/s12951-016-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.El-Batal AI, Mosallam FM, Ghorab MM, Hanora A, Gobara M, Baraka A, et al. Factorial design-optimized and gamma irradiation-assisted fabrication of selenium nanoparticles by chitosan and Pleurotus ostreatus fermented fenugreek for a vigorous in vitro effect against carcinoma cells. Int J Biol Macromol. 2020;156:1584–1599. doi: 10.1016/j.ijbiomac.2019.11.210. [DOI] [PubMed] [Google Scholar]
- 129.Geoffrion LD, Hesabizadeh T, Medina-Cruz D, Kusper M, Taylor P, Vernet-Crua A, et al. Naked selenium nanoparticles for antibacterial and anticancer treatments. ACS Omega. 2020;5:2660–2669. doi: 10.1021/acsomega.9b03172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guisbiers G, Wang Q, Khachatryan E, Mimun LC, Mendoza-Cruz R, Larese-Casanova P, et al. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int J Nanomed. 2016;11:3731–6. doi: 10.2147/IJN.S106289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pyrzynska K, Sentkowska A. Biosynthesis of selenium nanoparticles using plant extracts. J Nanostruct Chem. 2022;12:467–480. doi: 10.1007/s40097-021-00435-4. [DOI] [Google Scholar]
- 132.Shoeibi S, Mozdziak P, Golkar-Narenji A. Biogenesis of selenium nanoparticles using green chemistry. Top Curr Chem (Z) 2017;375:88. doi: 10.1007/s41061-017-0176-x. [DOI] [PubMed] [Google Scholar]
- 133.Xu Q, Song Y, Lin Z, Bañuelos G, Zhu Y, Guo Y. The small RNA chaperone Hfq is a critical regulator for bacterial biosynthesis of selenium nanoparticles and motility in Rahnella aquatilis. Appl Microbiol Biotechnol. 2020;104:1721–1735. doi: 10.1007/s00253-019-10231-4. [DOI] [PubMed] [Google Scholar]
- 134.El-Sayed E-SR, Abdelhakim HK, Ahmed AS. Solid-state fermentation for enhanced production of selenium nanoparticles by gamma-irradiated Monascus purpureus and their biological evaluation and photocatalytic activities. Bioprocess Biosyst Eng. 2020;43:797–809. doi: 10.1007/s00449-019-02275-7. [DOI] [PubMed] [Google Scholar]
- 135.Yang F, Huang J, Liu H, Lin W, Li X, Zhu X, et al. Lentinan-functionalized selenium nanosystems with high permeability infiltrate solid tumors by enhancing transcellular transport. Nanoscale. 2020;12:14494–14503. doi: 10.1039/D0NR02171G. [DOI] [PubMed] [Google Scholar]
- 136.Ramamurthy C, Sampath KS, Arunkumar P, Kumar MS, Sujatha V, Premkumar K, et al. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst Eng. 2013;36:1131–1139. doi: 10.1007/s00449-012-0867-1. [DOI] [PubMed] [Google Scholar]
- 137.Langi B, Shah C, Singh K, Chaskar A, Kumar M, Bajaj PN. Ionic liquid-induced synthesis of selenium nanoparticles. Mater Res Bull. 2010;45:668–671. doi: 10.1016/j.materresbull.2010.03.005. [DOI] [Google Scholar]
- 138.Zheng L, Li C, Huang X, Lin X, Lin W, Yang F, et al. Thermosensitive hydrogels for sustained-release of sorafenib and selenium nanoparticles for localized synergistic chemoradiotherapy. Biomaterials. 2019;216:119220. doi: 10.1016/j.biomaterials.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 139.Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Peng Q, et al. Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomed. 2018;13:2107–2128. doi: 10.2147/IJN.S157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xia Y, Wang C, Xu T, Li Y, Guo M, Lin Z, et al. Targeted delivery of HES5-siRNA with novel polypeptide-modified nanoparticles for hepatocellular carcinoma therapy. RSC Adv. 2018;8:1917–1926. doi: 10.1039/C7RA12461A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hunter WJ, Manter DK. Bio-reduction of selenite to elemental red selenium by Tetrathiobacter kashmirensis. Curr Microbiol. 2008;57:83–88. doi: 10.1007/s00284-008-9160-6. [DOI] [PubMed] [Google Scholar]
- 142.Fesharaki PJ, Nazari P, Shakibaie M, Rezaie S, Banoee M, Abdollahi M, et al. Biosynthesis of selenium nanoparticles using Klebsiella pneumoniae and their recovery by a simple sterilization process. Braz J Microbiol. 2010;41:461–466. doi: 10.1590/S1517-83822010000200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Husen A, Siddiqi KS. Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanobiotechnol. 2014;12:28. doi: 10.1186/s12951-014-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang J, Teng Z, Yuan Y, Zeng Q-Z, Lou Z, Lee S-H, et al. Development, physicochemical characterization and cytotoxicity of selenium nanoparticles stabilized by beta-lactoglobulin. Int J Biol Macromol. 2018;107:1406–1413. doi: 10.1016/j.ijbiomac.2017.09.117. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Z, Du Y, Liu T, Wong K-H, Chen T. Systematic acute and subchronic toxicity evaluation of polysaccharide-protein complex-functionalized selenium nanoparticles with anticancer potency. Biomater Sci. 2019;7:5112–5123. doi: 10.1039/C9BM01104H. [DOI] [PubMed] [Google Scholar]
- 146.Xia Z-M, Liu Y-N, Huang Z, Qin L-Z, Lin H, Li Q. A facile green approach for synthesis of selenium nanowires with visible light photocatalytic properties. J Nanosci Nanotechnol. 2019;19:156–162. doi: 10.1166/jnn.2019.16461. [DOI] [PubMed] [Google Scholar]
- 147.Chen T, Wong Y-S, Zheng W, Bai Y, Huang L. Selenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloids Surf B Biointerfaces. 2008;67:26–31. doi: 10.1016/j.colsurfb.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 148.Xiao Y, Huang Q, Zheng Z, Guan H, Liu S. Construction of a Cordyceps sinensis exopolysaccharide-conjugated selenium nanoparticles and enhancement of their antioxidant activities. Int J Biol Macromol. 2017;99:483–491. doi: 10.1016/j.ijbiomac.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 149.Vetchinkina E, Loshchinina E, Kursky V, Nikitina V. Reduction of organic and inorganic selenium compounds by the edible medicinal basidiomycete Lentinula edodes and the accumulation of elemental selenium nanoparticles in its mycelium. J Microbiol. 2013;51:829–835. doi: 10.1007/s12275-013-2689-5. [DOI] [PubMed] [Google Scholar]
- 150.Feldman D. Polymer nanocomposites in medicine. J Macromol Sci Part A. 2016;53:55–62. doi: 10.1080/10601325.2016.1110459. [DOI] [Google Scholar]
- 151.Liu X, Deng G, Wang Y, Wang Q, Gao Z, Sun Y, et al. A novel and facile synthesis of porous SiO2-coated ultrasmall Se particles as a drug delivery nanoplatform for efficient synergistic treatment of cancer cells. Nanoscale. 2016;8:8536–8541. doi: 10.1039/C6NR02298G. [DOI] [PubMed] [Google Scholar]
- 152.Chang Y, He L, Li Z, Zeng L, Song Z, Li P, et al. Designing core–shell gold and selenium nanocomposites for cancer radiochemotherapy. ACS Nano. 2017;11:4848–4858. doi: 10.1021/acsnano.7b01346. [DOI] [PubMed] [Google Scholar]
- 153.Ahmed HH, Aglan HA, Mabrouk M, Abd-Rabou AA, Beherei HH. Enhanced mesenchymal stem cell proliferation through complexation of selenium/titanium nanocomposites. J Mater Sci Mater Med. 2019;30:24. doi: 10.1007/s10856-019-6224-z. [DOI] [PubMed] [Google Scholar]
- 154.Chhowalla M, Shin HS, Eda G, Li L-J, Loh KP, Zhang H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat Chem. 2013;5:263–275. doi: 10.1038/nchem.1589. [DOI] [PubMed] [Google Scholar]
- 155.Wang C, Bai J, Liu Y, Jia X, Jiang X. Polydopamine coated selenide molybdenum: a new photothermal nanocarrier for highly effective chemo-photothermal synergistic therapy. ACS Biomater Sci Eng. 2016;2:2011–2017. doi: 10.1021/acsbiomaterials.6b00416. [DOI] [PubMed] [Google Scholar]
- 156.Chen Y, Wu Y, Sun B, Liu S, Liu H. Two-dimensional nanomaterials for cancer nanotheranostics. Small. 2017;13:1603446. doi: 10.1002/smll.201603446. [DOI] [PubMed] [Google Scholar]
- 157.Liu S, Pan X, Liu H. Two-dimensional nanomaterials for photothermal therapy. Angew Chem Int Ed Engl. 2020;59:5890–5900. doi: 10.1002/anie.201911477. [DOI] [PubMed] [Google Scholar]
- 158.Guo Z, Ouyang J, Kim NY, Shi J, Ji X. Emerging two-dimensional nanomaterials for cancer therapy. ChemPhysChem. 2019;20:2417–2433. doi: 10.1002/cphc.201900551. [DOI] [PubMed] [Google Scholar]
- 159.Ji X, Kong N, Wang J, Li W, Xiao Y, Gan ST, et al. A novel top-down synthesis of ultrathin 2D boron nanosheets for multimodal imaging-guided cancer therapy. Adv Mater. 2018;30:e1803031. doi: 10.1002/adma.201803031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xie Z, Wang D, Fan T, Xing C, Li Z, Tao W, et al. Black phosphorus analogue tin sulfide nanosheets: synthesis and application as near-infrared photothermal agents and drug delivery platforms for cancer therapy. J Mater Chem B. 2018;6:4747–4755. doi: 10.1039/C8TB00729B. [DOI] [PubMed] [Google Scholar]
- 161.Qin J-K, Sui C, Qin Z, Wu J, Guo H, Zhen L, et al. Mechanical anisotropy in two-dimensional selenium atomic layers. Nano Lett. 2021;21:8043–8050. doi: 10.1021/acs.nanolett.1c02294. [DOI] [PubMed] [Google Scholar]
- 162.Yang Y, Li X, Wen M, Hacopian E, Chen W, Gong Y, et al. Brittle fracture of 2D MoSe2. Adv Mater. 2017;29:1604201. doi: 10.1002/adma.201604201. [DOI] [PubMed] [Google Scholar]
- 163.Zhang Y, Zhang C, Guo Y, Liu D, Yu Y, Zhang B. Selenium vacancy-rich CoSe2 ultrathin nanomeshes with abundant active sites for electrocatalytic oxygen evolution. J Mater Chem A R Soc Chem. 2019;7:2536–40. doi: 10.1039/C8TA11407B. [DOI] [Google Scholar]
- 164.Chang A, Zhang C, Yu Y, Yu Y, Zhang B. Plasma-assisted synthesis of NiSe2 ultrathin porous nanosheets with selenium vacancies for supercapacitor. ACS Appl Mater Interfaces. 2018;10:41861–41865. doi: 10.1021/acsami.8b16072. [DOI] [PubMed] [Google Scholar]
- 165.Lv W, Wu G, Li X, Li J, Li Z. Two-dimensional V2C@Se (MXene) composite cathode material for high-performance rechargeable aluminum batteries. Energy Storage Mater. 2022;46:138–146. doi: 10.1016/j.ensm.2022.01.019. [DOI] [Google Scholar]
- 166.Zhu C, Yu M, Zhou J, He Y, Zeng Q, Deng Y, et al. Strain-driven growth of ultra-long two-dimensional nano-channels. Nat Commun. 2020;11:772. doi: 10.1038/s41467-020-14521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Song Z, Chang Y, Xie H, Yu X-F, Chu PK, Chen T. Decorated ultrathin bismuth selenide nanosheets as targeted theranostic agents for in vivo imaging guided cancer radiation therapy. NPG Asia Mater Nature Publ Group. 2017;9:e439–e439. doi: 10.1038/am.2017.167. [DOI] [Google Scholar]
- 168.He L, Nie T, Xia X, Liu T, Huang Y, Wang X, et al. Designing bioinspired 2D MoSe2 nanosheet for efficient photothermal-triggered cancer immunotherapy with reprogramming tumor-associated macrophages. Adv Func Mater. 2019;29:1901240. doi: 10.1002/adfm.201901240. [DOI] [Google Scholar]
- 169.Jiang W, Zhang Z, Ye M, Pan S, Huang G, Chen T, et al. Morphology-directed radiosensitization of MoSe2 nanoplatforms for promoting cervical cancer radiotherapy. Nano Today. 2022;46:101598. doi: 10.1016/j.nantod.2022.101598. [DOI] [Google Scholar]
- 170.You J, Hossain MD, Luo Z. Synthesis of 2D transition metal dichalcogenides by chemical vapor deposition with controlled layer number and morphology. Nano Converg. 2018;5:26. doi: 10.1186/s40580-018-0158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pan J, Zhu X, Chen X, Zhao Y, Liu J. Gd3+-Doped MoSe2 nanosheets used as a theranostic agent for bimodal imaging and highly efficient photothermal cancer therapy. Biomater Sci. 2018;6:372–387. doi: 10.1039/C7BM00894E. [DOI] [PubMed] [Google Scholar]
- 172.Dong L, Sun L, Li W, Jiang Y, Zhan Y, Yu L, et al. Degradable and excretable ultrasmall transition metal selenide nanodots for high-performance computed tomography bioimaging-guided photonic tumor nanomedicine in NIR-II biowindow. Adv Funct Mater. 2021;31:2008591. doi: 10.1002/adfm.202008591. [DOI] [Google Scholar]
- 173.Qu L, Dai L, Stone M, Xia Z, Wang ZL. Carbon nanotube arrays with strong shear binding-on and easy normal lifting-off. Science. 2008;322:238–242. doi: 10.1126/science.1159503. [DOI] [PubMed] [Google Scholar]
- 174.Ouyang J, Wen M, Chen W, Tan Y, Liu Z, Xu Q, et al. Multifunctional two dimensional Bi2Se3 nanodiscs for combined antibacterial and anti-inflammatory therapy for bacterial infections. Chem Commun (Camb) 2019;55:4877–4880. doi: 10.1039/C9CC01129C. [DOI] [PubMed] [Google Scholar]
- 175.Li X, Yu Y, Chen Q, Lin J, Zhu X, Liu X, et al. Engineering cancer cell membrane-camouflaged metal complex for efficient targeting therapy of breast cancer. J Nanobiotechnol. 2022;20:401. doi: 10.1186/s12951-022-01593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sun L, Li Q, Zhang L, Chai H, Yu L, Xu Z, et al. Stimuli responsive PEGylated bismuth selenide hollow nanocapsules for fluorescence/CT imaging and light-driven multimodal tumor therapy. Biomater Sci. 2019;7:3025–3040. doi: 10.1039/C9BM00351G. [DOI] [PubMed] [Google Scholar]
- 177.Wang X, Li F, Yan X, Ma Y, Miao Z-H, Dong L, et al. Ambient aqueous synthesis of ultrasmall Ni0.85Se nanoparticles for noninvasive photoacoustic imaging and combined photothermal-chemotherapy of cancer. ACS Appl Mater Interfaces. 2017;9:41782–93. doi: 10.1021/acsami.7b15780. [DOI] [PubMed] [Google Scholar]
- 178.Wang Q, Hopgood JR, Finlayson N, Williams GOS, Fernandes S, Williams E, et al. Deep learning in ex-vivo lung cancer discrimination using fluorescence lifetime endomicroscopic images. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:1891–1894. doi: 10.1109/EMBC44109.2020.9175598. [DOI] [PubMed] [Google Scholar]
- 179.Kim H, Goh S-H, Choi Y. Quenched cetuximab conjugate for fast fluorescence imaging of EGFR-positive lung cancers. Biomater Sci. 2021;9:456–462. doi: 10.1039/D0BM01148G. [DOI] [PubMed] [Google Scholar]
- 180.Etrych T, Lucas H, Janoušková O, Chytil P, Mueller T, Mäder K. Fluorescence optical imaging in anticancer drug delivery. J Control Release. 2016;226:168–181. doi: 10.1016/j.jconrel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 181.Kamiya M, Urano Y. Rapid and sensitive fluorescent imaging of tiny tumors in vivo and in clinical specimens. Curr Opin Chem Biol. 2016;33:9–15. doi: 10.1016/j.cbpa.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 182.Key J, Leary JF. Nanoparticles for multimodal in vivo imaging in nanomedicine. Int J Nanomedicine. 2014;9:711–726. doi: 10.2147/IJN.S53717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Christophe C, Dan B. Magnetic resonance imaging cranial and cerebral dimensions: is there a relationship with Chiari I malformation? A preliminary report in children. Eur J Paediatr Neurol. 1999;3:15–23. doi: 10.1053/ejpn.1999.0174. [DOI] [PubMed] [Google Scholar]
- 184.Ciliberto M, Kishida Y, Seki S, Yoshikawa T, Ohno Y. Update of MR imaging for evaluation of lung cancer. Radiol Clin North Am. 2018;56:437–469. doi: 10.1016/j.rcl.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 185.Hatabu H, Tadamura E, Levin DL, Chen Q, Li W, Kim D, et al. Quantitative assessment of pulmonary perfusion with dynamic contrast-enhanced MRI. Magn Reson Med. 1999;42:1033–1038. doi: 10.1002/(SICI)1522-2594(199912)42:6<1033::AID-MRM7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 186.Fu T, Chen Y, Hao J, Wang X, Liu G, Li Y, et al. Facile preparation of uniform FeSe2 nanoparticles for PA/MR dual-modal imaging and photothermal cancer therapy. Nanoscale. 2015;7:20757–20768. doi: 10.1039/C5NR06840A. [DOI] [PubMed] [Google Scholar]
- 187.Cavanaugh D, Johnson E, Price RE, Kurie J, Travis EL, Cody DD. In vivo respiratory-gated micro-CT imaging in small-animal oncology models. Mol Imaging. 2004;3:55–62. doi: 10.1162/153535004773861723. [DOI] [PubMed] [Google Scholar]
- 188.Badea C, Hedlund LW, Johnson GA. Micro-CT with respiratory and cardiac gating. Med Phys. 2004;31:3324–3329. doi: 10.1118/1.1812604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Attia ABE, Balasundaram G, Moothanchery M, Dinish US, Bi R, Ntziachristos V, et al. A review of clinical photoacoustic imaging: current and future trends. Photoacoustics. 2019;16:100144. doi: 10.1016/j.pacs.2019.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Beard P. Biomedical photoacoustic imaging. Interface Focus. 2011;1:602–631. doi: 10.1098/rsfs.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jin J, Zhao Q. Engineering nanoparticles to reprogram radiotherapy and immunotherapy: recent advances and future challenges. J Nanobiotechnol. 2020;18:75. doi: 10.1186/s12951-020-00629-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xia Y, Chen Y, Hua L, Zhao M, Xu T, Wang C, et al. Functionalized selenium nanoparticles for targeted delivery of doxorubicin to improve non-small-cell lung cancer therapy. Int J Nanomed. 2018;13:6929–6939. doi: 10.2147/IJN.S174909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C. Therapeutic applications of selenium nanoparticles. Biomed Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 194.Zhang D, You Y, Xu Y, Cheng Q, Xiao Z, Chen T, et al. Facile synthesis of near-infrared responsive on-demand oxygen releasing nanoplatform for precise MRI-guided theranostics of hypoxia-induced tumor chemoresistance and metastasis in triple negative breast cancer. J Nanobiotechnol. 2022;20:104. doi: 10.1186/s12951-022-01294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zou J, Su S, Chen Z, Liang F, Zeng Y, Cen W, et al. Hyaluronic acid-modified selenium nanoparticles for enhancing the therapeutic efficacy of paclitaxel in lung cancer therapy. Artif Cells Nanomed Biotechnol. 2019;47:3456–3464. doi: 10.1080/21691401.2019.1626863. [DOI] [PubMed] [Google Scholar]
- 196.De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Dis Primers. 2019;5:13. doi: 10.1038/s41572-019-0064-5. [DOI] [PubMed] [Google Scholar]
- 197.Polgár C, Ott OJ, Hildebrandt G, Kauer-Dorner D, Knauerhase H, Major T, et al. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:259–268. doi: 10.1016/S1470-2045(17)30011-6. [DOI] [PubMed] [Google Scholar]
- 198.Barton MB, Jacob S, Shafiq J, Wong K, Thompson SR, Hanna TP, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112:140–144. doi: 10.1016/j.radonc.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 199.Riet FG, Fayard F, Arriagada R, Santos MA, Bourgier C, Ferchiou M, et al. Preoperative radiotherapy in breast cancer patients: 32 years of follow-up. Eur J Cancer. 2017;76:45–51. doi: 10.1016/j.ejca.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 200.Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu C, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18:336–346. doi: 10.1016/S1470-2045(17)30086-4. [DOI] [PubMed] [Google Scholar]
- 201.Nussbaum DP, Rushing CN, Lane WO, Cardona DM, Kirsch DG, Peterson BL, et al. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case–control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016;17:966–975. doi: 10.1016/S1470-2045(16)30050-X. [DOI] [PubMed] [Google Scholar]
- 202.Spiotto MT, Jefferson G, Wenig B, Markiewicz M, Weichselbaum RR, Koshy M. Differences in survival with surgery and postoperative radiotherapy compared with definitive chemoradiotherapy for oral cavity cancer: a national cancer database analysis. JAMA Otolaryngol Head Neck Surg. 2017;143:691–699. doi: 10.1001/jamaoto.2017.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pilar A, Gupta M, Ghosh Laskar S, Laskar S. Intraoperative radiotherapy: review of techniques and results. Ecancermedicalscience. 2017;11:750. doi: 10.3332/ecancer.2017.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Asavei T, Bobeica M, Nastasa V, Manda G, Naftanaila F, Bratu O, et al. Laser-driven radiation: biomarkers for molecular imaging of high dose-rate effects. Med Phys. 2019;46:e726–e734. doi: 10.1002/mp.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Imai R, Kamada T, Araki N. Working Group for Bone and Soft Tissue Sarcomas. Carbon ion radiation therapy for unresectable sacral chordoma: an analysis of 188 cases. Int J Radiat Oncol Biol Phys. 2016;95:322–7. doi: 10.1016/j.ijrobp.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 206.Liu H, Wang H, Ni D, Xu Y. Lactic acid modified rare earth-based nanomaterials for enhanced radiation therapy by disturbing the glycolysis. J Nanobiotechnol. 2022;20:490. doi: 10.1186/s12951-022-01694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27:v119–v133. doi: 10.1093/annonc/mdw270. [DOI] [PubMed] [Google Scholar]
- 208.Wang Q, Ye T, Chen H-L, Zhang X-G, Zhang L-Z. Correlation between intensity modulated radiotherapy and bone marrow suppression in breast cancer. Eur Rev Med Pharmacol Sci. 2016;20:75–81. [PubMed] [Google Scholar]
- 209.Marie-Egyptienne DT, Lohse I, Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxia. Cancer Lett. 2013;341:63–72. doi: 10.1016/j.canlet.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 210.Krause M, Dubrovska A, Linge A, Baumann M. Cancer stem cells: radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv Drug Deliv Rev. 2017;109:63–73. doi: 10.1016/j.addr.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 211.Lalla RV, Treister N, Sollecito T, Schmidt B, Patton LL, Mohammadi K, et al. Oral complications at 6 months after radiation therapy for head and neck cancer. Oral Dis. 2017;23:1134–1143. doi: 10.1111/odi.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang H, Sun Q, Tong L, Hao Y, Yu T. Synergistic combination of PEGylated selenium nanoparticles and X-ray-induced radiotherapy for enhanced anticancer effect in human lung carcinoma. Biomed Pharmacother. 2018;107:1135–1141. doi: 10.1016/j.biopha.2018.08.074. [DOI] [PubMed] [Google Scholar]
- 213.Sato H, Nagashima H, Akiyama M, Ito T, Hashimoto T, Saikawa H, et al. Analysis of bevacizumab treatments and metastatic sites of lung cancer. Cancer Treat Res Commun. 2021;26:100290. doi: 10.1016/j.ctarc.2020.100290. [DOI] [PubMed] [Google Scholar]
- 214.Roviello G, Sobhani N, Generali D. Bevacizumab in small cell lung cancer. Ann Transl Med. 2017;5:361. doi: 10.21037/atm.2017.06.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mukherji SK. Bevacizumab (Avastin) AJNR Am J Neuroradiol. 2010;31:235–236. doi: 10.3174/ajnr.A1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 217.Zheng W, Cao C, Liu Y, Yu Q, Zheng C, Sun D, et al. Multifunctional polyamidoamine-modified selenium nanoparticles dual-delivering siRNA and cisplatin to A549/DDP cells for reversal multidrug resistance. Acta Biomater. 2015;11:368–380. doi: 10.1016/j.actbio.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 218.Purohit MP, Verma NK, Kar AK, Singh A, Ghosh D, Patnaik S. Inhibition of thioredoxin reductase by targeted selenopolymeric nanocarriers synergizes the therapeutic efficacy of doxorubicin in MCF7 human breast cancer cells. ACS Appl Mater Interfaces. 2017;9:36493–36512. doi: 10.1021/acsami.7b07056. [DOI] [PubMed] [Google Scholar]
- 219.Bordoloi D, Roy NK, Monisha J, Padmavathi G, Kunnumakkara AB. Multi-targeted agents in cancer cell chemosensitization: what we learnt from curcumin thus far. Recent Pat Anticancer Drug Discov. 2016;11:67–97. doi: 10.2174/1574892810666151020101706. [DOI] [PubMed] [Google Scholar]
- 220.Drápela S, Bouchal J, Jolly MK, Culig Z, Souček K. ZEB1: a critical regulator of cell plasticity, DNA damage response, and therapy resistance. Front Mol Biosci. 2020;7:36. doi: 10.3389/fmolb.2020.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kunnumakkara AB, Bordoloi D, Sailo BL, Roy NK, Thakur KK, Banik K, et al. Cancer drug development: the missing links. Exp Biol Med (Maywood) 2019;244:663–689. doi: 10.1177/1535370219839163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sailo BL, Banik K, Girisa S, Bordoloi D, Fan L, Halim CE, et al. FBXW7 in cancer: what has been unraveled thus far? Cancers (Basel) 2019;11:E246. doi: 10.3390/cancers11020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Król M, Pawłowski KM, Majchrzak K, Szyszko K, Motyl T. Why chemotherapy can fail? Pol J Vet Sci. 2010;13:399–406. [PubMed] [Google Scholar]
- 224.Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6:865–78. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- 225.Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res. 2004;10:2561–2569. doi: 10.1158/1078-0432.CCR-03-0268. [DOI] [PubMed] [Google Scholar]
- 226.Sun H, Cao D, Wu H, Liu H, Ke X, Ci T. Development of low molecular weight heparin based nanoparticles for metastatic breast cancer therapy. Int J Biol Macromol. 2018;112:343–355. doi: 10.1016/j.ijbiomac.2018.01.195. [DOI] [PubMed] [Google Scholar]
- 227.Wang Z, He Q, Zhao W, Luo J, Gao W. Tumor-homing, pH- and ultrasound-responsive polypeptide-doxorubicin nanoconjugates overcome doxorubicin resistance in cancer therapy. J Control Release. 2017;264:66–75. doi: 10.1016/j.jconrel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 228.Chen T, Wong YS. Selenocystine induces apoptosis of A375 human melanoma cells by activating ROS-mediated mitochondrial pathway and p53 phosphorylation. Cell Mol Life Sci. 2008;65:2763–2775. doi: 10.1007/s00018-008-8329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Meulmeester E, Jochemsen AG. p53: a guide to apoptosis. Curr Cancer Drug Targets. 2008;8:87–97. doi: 10.2174/156800908783769337. [DOI] [PubMed] [Google Scholar]
- 230.Jiang C, Hu H, Malewicz B, Wang Z, Lü J. Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apoptosis in LNCaP human prostate cancer cells. Mol Cancer Ther. 2004;3:877–884. doi: 10.1158/1535-7163.877.3.7. [DOI] [PubMed] [Google Scholar]
- 231.Cheung S, Jain P, So J, Shahidi S, Chung S, Koritzinsky M. p38 MAPK inhibition mitigates hypoxia-induced AR signaling in castration-resistant prostate cancer. Cancers (Basel) 2021;13:831. doi: 10.3390/cancers13040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tseng C-L, Su W-Y, Yen K-C, Yang K-C, Lin F-H. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials. 2009;30:3476–3485. doi: 10.1016/j.biomaterials.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 233.Aryal S, Hu C-MJ, Zhang L. Polymer–cisplatin conjugate nanoparticles for acid-responsive drug delivery. ACS Nano. 2010;4:251–8. doi: 10.1021/nn9014032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sung MJ, Kim DH, Jung YJ, Kang KP, Lee AS, Lee S, et al. Genistein protects the kidney from cisplatin-induced injury. Kidney Int. 2008;74:1538–1547. doi: 10.1038/ki.2008.409. [DOI] [PubMed] [Google Scholar]
- 235.Jiang M, Pabla N, Murphy RF, Yang T, Yin X-M, Degenhardt K, et al. Nutlin-3 protects kidney cells during cisplatin therapy by suppressing Bax/Bak activation. J Biol Chem. 2007;282:2636–2645. doi: 10.1074/jbc.M606928200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ajith TA, Nivitha V, Usha S. Zingiber officinale Roscoe alone and in combination with alpha-tocopherol protect the kidney against cisplatin-induced acute renal failure. Food Chem Toxicol. 2007;45:921–927. doi: 10.1016/j.fct.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 237.Lee J-H, Lee H-J, Lee H-J, Choi W-C, Yoon S-W, Ko S-G, et al. Rhus verniciflua Stokes prevents cisplatin-induced cytotoxicity and reactive oxygen species production in MDCK-I renal cells and intact mice. Phytomedicine. 2009;16:188–197. doi: 10.1016/j.phymed.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 238.Han L, Du L-B, Kumar A, Jia H-Y, Liang X-J, Tian Q, et al. Inhibitory effects of trolox-encapsulated chitosan nanoparticles on tert-butylhydroperoxide induced RAW264.7 apoptosis. Biomaterials. 2012;33:8517–28. doi: 10.1016/j.biomaterials.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 239.Wang J, Chang Y, Luo H, Jiang W, Xu L, Chen T, et al. Designing immunogenic nanotherapeutics for photothermal-triggered immunotherapy involving reprogramming immunosuppression and activating systemic antitumor responses. Biomaterials. 2020;255:120153. doi: 10.1016/j.biomaterials.2020.120153. [DOI] [PubMed] [Google Scholar]
- 240.Fang X, Li C, Zheng L, Yang F, Chen T. Dual-targeted selenium nanoparticles for synergistic photothermal therapy and chemotherapy of tumors. Chem Asian J. 2018;13:996–1004. doi: 10.1002/asia.201800048. [DOI] [PubMed] [Google Scholar]
- 241.Beik J, Abed Z, Ghoreishi FS, Hosseini-Nami S, Mehrzadi S, Shakeri-Zadeh A, et al. Nanotechnology in hyperthermia cancer therapy: from fundamental principles to advanced applications. J Control Release. 2016;235:205–221. doi: 10.1016/j.jconrel.2016.05.062. [DOI] [PubMed] [Google Scholar]
- 242.Deng X, Liu H, Xu Y, Chan L, Xie J, Xiong Z, et al. Designing highly stable ferrous selenide-black phosphorus nanosheets heteronanostructure via P-Se bond for MRI-guided photothermal therapy. J Nanobiotechnol. 2021;19:201. doi: 10.1186/s12951-021-00905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Shanmugam V, Selvakumar S, Yeh C-S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem Soc Rev. 2014;43:6254–6287. doi: 10.1039/C4CS00011K. [DOI] [PubMed] [Google Scholar]
- 244.Zhang Z, Wang J, Chen C. Near-infrared light-mediated nanoplatforms for cancer thermo-chemotherapy and optical imaging. Adv Mater. 2013;25:3869–3880. doi: 10.1002/adma.201301890. [DOI] [PubMed] [Google Scholar]
- 245.Zhou F, Li X, Naylor MF, Hode T, Nordquist RE, Alleruzzo L, et al. InCVAX—a novel strategy for treatment of late-stage, metastatic cancers through photoimmunotherapy induced tumor-specific immunity. Cancer Lett. 2015;359:169–177. doi: 10.1016/j.canlet.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wang D, Xu Z, Yu H, Chen X, Feng B, Cui Z, et al. Treatment of metastatic breast cancer by combination of chemotherapy and photothermal ablation using doxorubicin-loaded DNA wrapped gold nanorods. Biomaterials. 2014;35:8374–8384. doi: 10.1016/j.biomaterials.2014.05.094. [DOI] [PubMed] [Google Scholar]
- 247.Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48:2053–2108. doi: 10.1039/C8CS00618K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Matsumoto Y, Nichols JW, Toh K, Nomoto T, Cabral H, Miura Y, et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat Nanotechnol. 2016;11:533–538. doi: 10.1038/nnano.2015.342. [DOI] [PubMed] [Google Scholar]
- 249.Setyawati MI, Tay CY, Chia SL, Goh SL, Fang W, Neo MJ, et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat Commun. 2013;4:1673. doi: 10.1038/ncomms2655. [DOI] [PubMed] [Google Scholar]
- 250.Wang Z, Huang P, Jacobson O, Wang Z, Liu Y, Lin L, et al. Biomineralization-inspired synthesis of copper sulfide-ferritin nanocages as cancer theranostics. ACS Nano. 2016;10:3453–3460. doi: 10.1021/acsnano.5b07521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Casal JI, Bartolomé RA. RGD cadherins and α2β1 integrin in cancer metastasis: a dangerous liaison. Biochim Biophys Acta Rev Cancer. 2018;1869:321–332. doi: 10.1016/j.bbcan.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 252.Mondal S, Adhikari N, Banerjee S, Amin SA, Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: a minireview. Eur J Med Chem. 2020;194:112260. doi: 10.1016/j.ejmech.2020.112260. [DOI] [PubMed] [Google Scholar]
- 253.Chen R, Wang X, Yao X, Zheng X, Wang J, Jiang X. Near-IR-triggered photothermal/photodynamic dual-modality therapy system via chitosan hybrid nanospheres. Biomaterials. 2013;34:8314–8322. doi: 10.1016/j.biomaterials.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 254.Zheng C, Zheng M, Gong P, Jia D, Zhang P, Shi B, et al. Indocyanine green-loaded biodegradable tumor targeting nanoprobes for in vitro and in vivo imaging. Biomaterials. 2012;33:5603–5609. doi: 10.1016/j.biomaterials.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 255.Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 256.Liu Y, Lu Y, Zhu X, Li C, Yan M, Pan J, et al. Tumor microenvironment-responsive prodrug nanoplatform via co-self-assembly of photothermal agent and IDO inhibitor for enhanced tumor penetration and cancer immunotherapy. Biomaterials. 2020;242:119933. doi: 10.1016/j.biomaterials.2020.119933. [DOI] [PubMed] [Google Scholar]
- 257.Rijal G, Li W. Native-mimicking in vitro microenvironment: an elusive and seductive future for tumor modeling and tissue engineering. J Biol Eng. 2018;12:20. doi: 10.1186/s13036-018-0114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546. doi: 10.1016/j.biomaterials.2020.120546. [DOI] [PubMed] [Google Scholar]
- 259.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16:705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wu D, Shou X, Zhang Y, Li Z, Wu G, Wu D, et al. Cell membrane-encapsulated magnetic nanoparticles for enhancing natural killer cell-mediated cancer immunotherapy. Nanomedicine. 2021;32:102333. doi: 10.1016/j.nano.2020.102333. [DOI] [PubMed] [Google Scholar]
- 261.Mikelez-Alonso I, Magadán S, González-Fernández Á, Borrego F. Natural killer (NK) cell-based immunotherapies and the many faces of NK cell memory: a look into how nanoparticles enhance NK cell activity. Adv Drug Deliv Rev. 2021;176:113860. doi: 10.1016/j.addr.2021.113860. [DOI] [PubMed] [Google Scholar]
- 262.Han X, Shen S, Fan Q, Chen G, Archibong E, Dotti G, et al. Red blood cell-derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci Adv. 2019;5:eaaw6870. doi: 10.1126/sciadv.aaw6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lipinski B. Sodium selenite as an anticancer agent. Anticancer Agents Med Chem. 2017;17:658–661. doi: 10.2174/1871520616666160607011024. [DOI] [PubMed] [Google Scholar]
- 264.Diwakar BT, Korwar AM, Paulson RF, Prabhu KS. The regulation of pathways of inflammation and resolution in immune cells and cancer stem cells by selenium. Adv Cancer Res. 2017;136:153–172. doi: 10.1016/bs.acr.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium augments the functions of natural killer and lymphokine-activated killer cells. Biol Trace Elem Res. 1996;52:227–239. doi: 10.1007/BF02789164. [DOI] [PubMed] [Google Scholar]
- 266.Gao S, Li T, Guo Y, Sun C, Xianyu B, Xu H. Selenium-containing nanoparticles combine the NK cells mediated immunotherapy with radiotherapy and chemotherapy. Adv Mater. 2020;32:1907568. doi: 10.1002/adma.201907568. [DOI] [PubMed] [Google Scholar]
- 267.Kaczmarek M, Rubis B, Frydrychowicz M, Nowicka A, Brajer-Luftmann B, Kozlowska M, et al. Pleural macrophages can promote or inhibit apoptosis of malignant cells via humoral mediators depending on intracellular signaling pathways. Cancer Invest. 2018;36:264–278. doi: 10.1080/07357907.2018.1477158. [DOI] [PubMed] [Google Scholar]
- 268.Ma C, Hoffmann PR. Selenoproteins as regulators of T cell proliferation, differentiation, and metabolism. Semin Cell Dev Biol. 2021;115:54–61. doi: 10.1016/j.semcdb.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Linterman MA, Denton AE. Selenium saves ferroptotic TFH cells to fortify the germinal center. Nat Immunol. 2021;22:1074–1076. doi: 10.1038/s41590-021-01007-y. [DOI] [PubMed] [Google Scholar]
- 270.Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F. Recent advances in Targeting CD8 T-cell immunity for more effective cancer immunotherapy. Front Immunol. 2018;9:14. doi: 10.3389/fimmu.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhou L, Chong MMW, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 272.Sekiya T, Yoshimura A. In vitro Th differentiation protocol. Methods Mol Biol. 2016;1344:183–191. doi: 10.1007/978-1-4939-2966-5_10. [DOI] [PubMed] [Google Scholar]
- 273.Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 274.Zambricki E, Shigeoka A, Kishimoto H, Sprent J, Burakoff S, Carpenter C, et al. Signaling T-cell survival and death by IL-2 and IL-15. Am J Transplant. 2005;5:2623–2631. doi: 10.1111/j.1600-6143.2005.01075.x. [DOI] [PubMed] [Google Scholar]
- 275.Nagoshi M, Goedegebuure PS, Burger UL, Sadanaga N, Chang MP, Eberlein TJ. Successful adoptive cellular immunotherapy is dependent on induction of a host immune response triggered by cytokine (IFN-gamma and granulocyte/macrophage colony-stimulating factor) producing donor tumor-infiltrating lymphocytes. J Immunol. 1998;160:334–344. doi: 10.4049/jimmunol.160.1.334. [DOI] [PubMed] [Google Scholar]
- 276.Lee DA. Cellular therapy: adoptive immunotherapy with expanded natural killer cells. Immunol Rev. 2019;290:85–99. doi: 10.1111/imr.12793. [DOI] [PubMed] [Google Scholar]
- 277.Ruella M, Kalos M. Adoptive immunotherapy for cancer. Immunol Rev. 2014;257:14–38. doi: 10.1111/imr.12136. [DOI] [PubMed] [Google Scholar]
- 278.Nawaz W, Xu S, Li Y, Huang B, Wu X, Wu Z. Nanotechnology and immunoengineering: How nanotechnology can boost CAR-T therapy. Acta Biomater. 2020;109:21–36. doi: 10.1016/j.actbio.2020.04.015. [DOI] [PubMed] [Google Scholar]
- 279.Liu M, Wang X, Li W, Yu X, Flores-Villanueva P, Xu-Monette ZY, et al. Targeting PD-L1 in non-small cell lung cancer using CAR T cells. Oncogenesis. 2020;9:72. doi: 10.1038/s41389-020-00257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mesiano G, Todorovic M, Gammaitoni L, Leuci V, Giraudo Diego L, Carnevale-Schianca F, et al. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther. 2012;12:673–684. doi: 10.1517/14712598.2012.675323. [DOI] [PubMed] [Google Scholar]
- 281.Wu Y, Gu W, Li J, Chen C, Xu ZP. Silencing PD-1 and PD-L1 with nanoparticle-delivered small interfering RNA increases cytotoxicity of tumor-infiltrating lymphocytes. Nanomedicine (Lond) 2019;14:955–967. doi: 10.2217/nnm-2018-0237. [DOI] [PubMed] [Google Scholar]
- 282.Zeltsman M, Dozier J, McGee E, Ngai D, Adusumilli PS. CAR T-cell therapy for lung cancer and malignant pleural mesothelioma. Transl Res. 2017;187:1–10. doi: 10.1016/j.trsl.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Siegler EL, Zhu Y, Wang P, Yang L. Off-the-Shelf CAR-NK cells for cancer immunotherapy. Cell Stem Cell. 2018;23:160–161. doi: 10.1016/j.stem.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 284.Xu M, Xue B, Wang Y, Wang D, Gao D, Yang S, et al. Temperature-feedback nanoplatform for NIR-II penta-modal imaging-guided synergistic photothermal therapy and CAR-NK immunotherapy of lung cancer. Small. 2021;17:e2101397. doi: 10.1002/smll.202101397. [DOI] [PubMed] [Google Scholar]
- 285.Chen G, Yang F, Fan S, Jin H, Liao K, Li X, et al. Immunomodulatory roles of selenium nanoparticles: novel arts for potential immunotherapy strategy development. Front Immunol. 2022;13:956181. doi: 10.3389/fimmu.2022.956181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Phung CD, Tran TH, Kim JO. Engineered nanoparticles to enhance natural killer cell activity towards onco-immunotherapy: a review. Arch Pharm Res. 2020;43:32–45. doi: 10.1007/s12272-020-01218-1. [DOI] [PubMed] [Google Scholar]
- 287.Bai K, Hong B, Hong Z, Sun J, Wang C. Selenium nanoparticles-loaded chitosan/citrate complex and its protection against oxidative stress in D-galactose-induced aging mice. J Nanobiotechnol. 2017;15:92. doi: 10.1186/s12951-017-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Smith AD. Big Moment for Nanotech: Oncology Therapeutics Poised for a Leap. 2013 [cited 2022 Feb 28]; Available from: https://www.onclive.com/view/big-moment-for-nanotech-oncology-therapeutics-poised-for-a-leap.
- 289.Stathopoulos GP, Antoniou D, Dimitroulis J, Michalopoulou P, Bastas A, Marosis K, et al. Liposomal cisplatin combined with paclitaxel versus cisplatin and paclitaxel in non-small-cell lung cancer: a randomized phase III multicenter trial. Ann Oncol. 2010;21:2227–2232. doi: 10.1093/annonc/mdq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.NewLink Genetics Corporation. A Randomized, Phase 2, Study to Assess the Safety and Activity of CRLX101, a Nanoparticle Formulation of Camptothecin, in Patients With Advanced Non-Small Cell Lung Cancer Who Have Failed One or Two Previous Regimens of Chemotherapy [Internet]. clinicaltrials.gov; 2020 May. Report No.: NCT01380769. Available from: https://clinicaltrials.gov/ct2/show/NCT01380769.
- 291.BIND Therapeutics. An Open Label, Multicenter, Phase 2 Study to Determine the Safety and Efficacy of BIND-014 (Docetaxel Nanoparticles for Injectable Suspension) as Second-line Therapy to Patients With Non-Small Cell Lung Cancer [Internet]. clinicaltrials.gov; 2016 Apr. Report No.: NCT01792479. Available from: https://clinicaltrials.gov/ct2/show/NCT01792479.
- 292.EMD Serono. A Multi-center Phase III Randomized, Double-blind Placebo-controlled Study of the Cancer Vaccine Stimuvax® (L-BLP25 or BLP25 Liposome Vaccine) in Non-small Cell Lung Cancer (NSCLC) Subjects With Unresectable Stage III Disease. [Internet]. clinicaltrials.gov; 2015 Oct. Report No.: NCT00409188. Available from: https://clinicaltrials.gov/ct2/show/NCT00409188
- 293.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond) 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kievit FM, Zhang M. Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc Chem Res. 2011;44:853–862. doi: 10.1021/ar2000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252–1276. doi: 10.1016/j.msec.2019.01.066. [DOI] [PubMed] [Google Scholar]
- 297.Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25–37. doi: 10.1007/978-1-60761-609-2_3. [DOI] [PubMed] [Google Scholar]
- 298.Durymanov MO, Rosenkranz AA, Sobolev AS. Current approaches for improving intratumoral accumulation and distribution of nanomedicines. Theranostics. 2015;5:1007–1020. doi: 10.7150/thno.11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.