Abstract
Primary mediastinal (thymic) large B-cell lymphoma (PMBCL) is a rare, aggressive subtype of non-Hodgkin lymphoma and has a complex inflammatory microenvironment. Although most patients can be cured with standard-of-care immunochemotherapy, patients who have disease relapse have an unfavorable prognosis. Pre-treatment prognostic biomarkers in PMBCL are needed. In this retrospective study, we analyzed the clinical features and outcomes of PMBCL patients and their association with immune cell subpopulations identified by multiplex immunofluorescence at initial diagnosis. Two different antibody panels were used to assess macrophages in tissue biopsy specimens collected before the initiation of induction therapy. Twelve PMBCL patients, including five patients who had disease relapse, were included in the analysis. At a median follow-up time of 32.2 months, the median progression-free and overall survival durations were not reached. Our findings suggest that a high density of PD-L1+ macrophages is associated with favorable features, such as early disease stage and the absence of B-symptoms, and indicate that a high percentage of PD-L1+ macrophages and high densities of CD30+PD-L1+ cells and CD30+ cells might be associated with a lower risk of relapse within 12 months of therapy initiation. Further studies are needed to develop a biomarker signature predictive of treatment response with therapeutic consequences for patients with newly diagnosed PMBCL.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40164-023-00396-0.
Keywords: Primary mediastinal large B-cell lymphoma, Macrophages, PD-L1, CD30, Biomarker
To the editor
Primary mediastinal (thymic) large B-cell lymphoma (PMBCL) shares several features with classic Hodgkin lymphoma, such as 9p24.1 amplification, increased programmed cell death protein 1 (PD-1) ligand and CD30 expression [1, 2]. Between 7 and 20% of PMBCL patients have relapse after frontline chemoimmunotherapy, and most have dismal outcomes in spite of intensive therapy [3–5]. Predictive studies are needed to identify PMBCL patients with inferior outcomes, who may benefit from novel therapies or enrollment in a clinical trial.
In this retrospective study, we used multiplex immunofluorescence (mIF) to analyze pretreatment samples from PMBCL patients to identify prognostic characteristics and features associated with treatment response. MIF was not previously used to analyze PMBCL samples and enables better characterization of the cellular composition of the tumor microenvironment in terms of cellular subtypes and has increased sensitivity compared to conventional immunohistochemistry [6]. Methods are described in Additional file 1.
Patient and treatment characteristics are given in Table 1. Twelve PMBCL patients were included in this study. The median age at the time of initial diagnosis was 33 years (range, 22–54 years). Of the 12 patients, 7 (58%) had bulky disease, 5 (42%) had stage I or II disease, 7 (58%) had no B-symptoms, and 8 (67%) had an IPI score of 0 or 1. Nine patients (75%) received 6 cycles of dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin [4]), as a first line of therapy. However, no patients received consolidative radiotherapy in first complete remission. Five patients had disease relapse at a median of 6 months (range, 3–8 months) and received different additional therapies. At a median follow-up time of 32.2 months (range, 18.3–65.2 months), the median PFS and OS durations were not reached. At the most recent follow-up, one patient had died of progressive disease.
Table 1.
Patient and treatment characteristics
| Deidentified number | Gender | Race | Ethnicity | Age at diagnosis | B-SX at DX | Initial stage | IPI | Bulky > 10 cm | 1st line of therapy | At least one relapse | 2nd line of therapy | 3rd line of therapy | 4th line of therapy | FU in months |
Status at last FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PML01 | Male | white | not Hispanic | 46 | No | II | 1 | Yes | 6xDA-R-EPOCH | No | 18 | Alive | |||
| PML02 | Female | Asian | not Hispanic | 30 | No | II | 1 | Yes | 6xDA-R-EPOCH | No | 32 | Alive | |||
| PML03 | Male | white | Hispanic | 36 | No | IV | 2 | No | 6xDA-R-EPOCH | No | 21 | Alive | |||
| PML04 | Male | white | not Hispanic | 54 | No | IV | 2 | Yes | 6xDA-R-EPOCH | Yes | 3xR-ICE, ASCT and XRT | 65 | Alive | ||
| PML05 | Male | American Indian | not Hispanic | 38 | No | I | 1 | Yes | 6xDA-R-EPOCH | No | 26 | Alive | |||
| PML06 | Female | Hawaiian/Pacific Islander | Hispanic | 29 | Yes | II | 1 | Yes | 6xDA-R-EPOCH | Yes | 1 × R-DHAP | 1xR-ICE, Liso-cel | 29 | Alive | |
| PML07 | Male | white | NA | 30 | Yes | III | 1 | Yes | 1xABVD + 6xDA-R-EPOCH | Yes | 3xR-DHAP, then ASCT | Selinexor and Rituximab | Rituximab + fractionated Cyclophosphamide | 15 | Dead |
| PML08 | Female | white | not Hispanic | 24 | Yes | IV | 2 | No | 6xDA-R-EPOCH | No | 38 | Alive | |||
| PML09 | Female | white | not Hispanic | 50 | Yes | IV | 3 | Yes | 6xDA-R-EPOCH | Yes | 1xR-DHAP | Tisa-cel | 7xPembrolizumab + XRT | 31 | Alive |
| PML10 | Male | white | not Hispanic | 22 | No | II | 0 | No | 4xDA-R-EPOCH | No | 35 | Alive | |||
| PML11 | Male | white | not Hispanic | 38 | Yes | III | 1 | No |
1xR-CHOP + Len, + 5xDA-R-EPOCH |
No | 37 | Alive | |||
| PML12 | Female | white | not Hispanic | 28 | No | IV | 1 | No | 5xDA-R-EPOCH | Yes | Axi-cel | 8xPembrolizumab, ASCT and XRT | 57 | Alive |
ABVD: doxorubicin hydrochloride, bleomycin sulfate, vinblastine sulfate, and dacarbazine; ASCT: autologous stem cell transplantation; axi-cel: axicabtagene ciloleucel; DA-R-EPOCH: dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; DX: diagnosis; FU: follow-up; IPI: International Prognostic Index; Len: lenalidomide; Liso-cel: lisocabtagene maraleucel; NA: not available; R-DHAP: rituximab, cytosine arabinoside, dexamethasone; R-ICE: rituximab, ifosfamide, carboplatin, and etoposide; SX: symptoms;Tisa-cel: tisagenlecleucel; x: cycles; XRT: radiotherapy of the mediastinum
Per patient, the mean tissue area analyzed was 93.66 mm2 (range, 11.6–240.0 mm2) and the mean number of cells analyzed was 9658 cells (range, 6453–12,351 cells). The presented combination of markers output (Additional file 1: Table 1) is based on the previously most commonly assessed combinations [7–9].
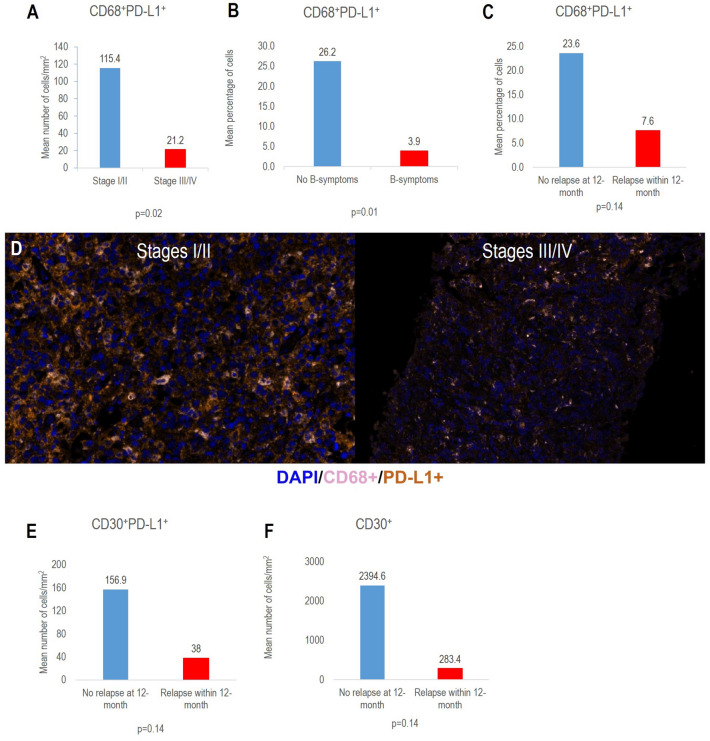
Compared with patients who had stage III or IV disease, patients who had stage I or II PMBCL had a significantly higher mean density of CD68+PD-L1+ cells (115.4 vs. 21.2 cells/mm2; p = 0.02; Fig. 1A, D). Compared with patients with B-symptoms, patients without B-symptoms had a significantly higher mean percentage of CD68+PD-L1+ cells (26.2% vs. 3.9%; p = 0.01; Fig. 1B). Compared with patients who had disease relapse within 12 months of therapy initiation, patients who did not have relapse within 12 months had a non statistically significant higher mean percentage of CD68+PD-L1+ macrophages (23.6% vs. 7.6%; p = 0.14; Fig. 1C), higher mean density of CD30+PD-L1+ cells (156.9 vs. 38.1 cells/mm2, p = 0.14; Fig. 1E, Additional file 1: Figure 1), and higher mean density of CD30+ cells (2,394.6 vs. 283.5 cells/mm2; p = 0.14; Fig. 1F).
Fig. 1.
A-F Expression of PD-L1 and CD30 by multiplex immunofluorescence on pre-treatment biopsy and their association with clinical characteristics and outcome
Our results suggest that a high density and mean percentage of PD-L1+ macrophages in pre-treatment tissue biopsy samples is associated with favorable features, including early-stage disease and the absence of B-symptoms. Moreover, our results indicate that a high percentage of PD-L1+ macrophages or high densities of CD30+PD-L1+ cells or CD30+ cells in pre-treatment biopsy samples might be associated with a lower risk of relapse within 12 months of therapy initiation.
The present study had some limitations, including its small, single-center cohort and the lack of markers to precise the phenotype of lymphoma cells and further elements from the immune microenvironment. Further studies with additional patients are necessary to evaluate potential confounding factors such as early-stage disease and the absence of B-symptoms. Besides, additional research is warranted to develop PD-L1+ macrophages density and/or percentage as a potential response signature to predict response to checkpoint inhibitors in the frontline setting [10]. We also acknowledge the limitations of our study, including the need for a precise definition of the neoplastic cells since CD30 expression is negative in around 15% of cases, can have a heterogeneous pattern [2] and can be expressed in reactive B-cells of the background. A broader assessment using high-plex technologies, which allows the association of different markers to define specific cell phenotypes, is required to better understand the immune landscape of PMBCL [11]. Several clinical trials of PD-1 inhibitors with or without brentuximab vedotin as frontline therapy for PMBCL (notably NCT04745949 and NCT04759586) are ongoing [12]. The use of targeted therapies in the frontline setting may decrease chemoresistance. However, immune checkpoint inhibitors can cause immune-related adverse events and brentuximab vedotin can induce neurotoxicity and hematotoxicity. Predictive studies to improve the personalization of PMBCL patients and avoid the unnecessary use of certain therapies and their associated risks are an unmet need.
Supplementary Information
Additional file 1: Methods; Figure S1. Representative pictures showing different cell densities of CD30+PD-L1+ cells. A-C) Multiplex immunofluorescence image of a case with low cell density of CD30+, PD-L1+, and CD30+PD-L1+ cells median: 20.46 cells/mm2, respectively. D-E) Multiplex immunofluorescence image of a case with high cell density of CD30+, PD-L1+, and CD30+PD-L1+ cells (median: 604.62 cells/mm2), respectively. Supplementary Table 1.
Acknowledgements
M.R.G. is supported by a Leukemia and Lymphoma Society Scholar award. P.S. is supported by the Leukemia Research Foundation Career Development Award, Leukemia Lymphoma Society Career Development Program, Kite Gilead Scholar in Clinical Research Award, Sabin Family Fellowship Award
Abbreviations
- IPI
International prognosis index scores
- mIF
Multiplex immunofluorescence
- OS
Overall survival
- PFS
Progression-free survival
- PMBCL
Primary mediastinal (thymic) large B-cell lymphoma
Author contributions
RES and MLM-P designed the study, analyzed the data, and wrote the manuscript; LF performed the statistical analysis and helped write the manuscript; MN collected data and helped write the manuscript; ERP, FV, JRW, SSN, PS, MRG, CRF, LMS, IIW, SA, RN, and FBH, helped write the manuscript; MLM-P performed multiplex staining. All authors have read and approved the final manuscript.
Funding
This study was supported by the Lymphoma Research Foundation and the Translational Molecular Pathology-Immunoprofiling Laboratory of MD Anderson’s Department of Translational Molecular Pathology.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of MD Anderson Cancer Center and conducted (protocol 2020–0228) in accordance with our institutional guidelines and the principles of the Declaration of Helsinki. The IRB approved the request of waiver of informed consent and a waiver of authorizations as the study does not involve therapeutic intervention or any type of direct patient contact.
Consent for publication
Not required by IRB.
Competing interests
R.E.S.: Research funding: Rafael Pharmaceuticals, BMS, GSK, and Seattle Genetics. E.R.P. has nothing to disclose. F.V. receives research funding from NCI, CRISP Therapeutics, Allogene, Geron corporation and from the Moonshot program at MDACC. L.F. has nothing to disclose. J.R.W. has received research funding from Kite, BMS, Novartis, Genentech, AstraZeneca, Morphosys/Incyte, ADC Therapeutics, Calithera, Kymera; consulting: Kite, BMS, Novartis, Genentech, AstraZeneca, Morphosys/Incyte, ADC Therapeutics, Calithera, Merck, Abbvie/GenMab, SeaGen, MonteRosa, Iksuda. S.S.N. has received research support from Kite/Gilead, BMS, Cellectis, Poseida, Allogene, Unum Therapeutics, Precision Biosciences, and Adicet Bio; served as Advisory Board Member/Consultant for Kite/Gilead, Merck, Novartis, Sellas Life Sciences, Athenex, Allogene, Incyte, Adicet Bio, BMS, Legend Biotech, Bluebird Bio, Fosun Kite, Sana Biotechnology, Caribou, Astellas Pharma, and Morphosys; has received royalty income from Takeda Pharmaceuticals; has stock options from Longbow Immunotherapy, Inc; and has intellectual property related to cell therapy. P.S. has received research support from Sobi, Astrazeneca Acerta, ADC Therapeutics, ALX Oncology. Advisory board/consultancy: Hutchinson Medipharma, Roche Genentech, Astrazeneca Acerta, ADC Therapeutics, TG Therapeutics, Kite Gilead, Sobi, Incyte Morphosys. M.R.G. reports research funding from Sanofi, Kite/Gilead, Abbvie and Allogene; consulting for Abbvie; honoraria from Tessa Therapeutics, Monte Rosa Therapeutics and Daiichi Sankyo; and stock ownership of KDAc Therapeutics. C.R.F. is consultant for Abbvie, Bayer, BeiGene, Celgene, Denovo Biopharma, Foresight Diagnostics, Genentech/Roche, Genmab, Gilead, Karyopharm, N-Power Medicine, Pharmacyclics/Janssen, SeaGen, Spectrum, has stock Options with Foresight Diagnostics, N-Power Medicine, has research Funding from 4D, Abbvie, Acerta, Adaptimmune, Allogene, Amgen, Bayer, Celgene, Cellectis EMD, Gilead, Genentech/Roche, Guardant, Iovance, Janssen Pharmaceutical, Kite, Morphosys, Nektar,Novartis, Pfizer, Pharmacyclics, Sanofi, Takeda, TG Therapeutics, Xencor, Ziopharm, Burroughs Wellcome Fund, Eastern Cooperative Oncology Group, National Cancer Institute, V Foundation, Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research. L.M.S. has nothing to disclose. I.I.W. has provided consulting or advisory roles for AstraZeneca/MedImmune, Bayer, Bristol-Myers Squibb, Genentech/Roche, GlaxoSmithKline, Guardant Health, HTG Molecular Diagnostics, Merck, MSD Oncology, OncoCyte, Jansen, Novartis, Flame Inc, and Pfizer; has received grants and personal fees from Genentech/Roche, Bristol Myers Squibb, AstraZeneca/MedImmune, HTG Molecular, Merck, and Guardant Health; has received personal fees from GlaxoSmithKline and Oncocyte, Daiichi-Sankyo, Roche, Astra Zeneca, Regeneron, Sanofi, Pfizer and Bayer; has received research funding to his institution from 4D Molecular Therapeutics, Adaptimmune, Adaptive Biotechnologies, Akoya Biosciences, Amgen, Bayer, EMD Serono, Genentech, Guardant Health, HTG Molecular Diagnostics, Iovance Biotherapeutics, Johnson & Johnson, Karus Therapeutics, MedImmune, Merck, Novartis, OncoPlex Diagnostics, Pfizer, Takeda, and Novartis. S.A. has received research support paid to her institution from Tessa Therapeutics, SeaGen, Merck, Xencor, Chimagen; Advisory committee member: Sanofi, SeaGen, Tessa Therapeutics; Consultancy: Novartis, Myeloid Therapeutics, Servier, Chimagen. R.N. has received honoraria from Incyte for speaker/preceptorship. F.B.H. has nothing to disclose. M.N. has nothing to disclose. M.L.M-P. has nothing to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Green MR, et al. Integrative analysis reveals selective 9p241 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steidl C, Gascoyne RD. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood. 2011;118(10):2659–2669. doi: 10.1182/blood-2011-05-326538. [DOI] [PubMed] [Google Scholar]
- 3.Hayden AR, et al. Outcome of primary mediastinal large B-cell lymphoma using R-CHOP: impact of a PET-adapted approach. Blood. 2020;136(24):2803–2811. doi: 10.1182/blood.2019004296. [DOI] [PubMed] [Google Scholar]
- 4.Dunleavy K, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408–1416. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casulo C, et al. Describing treatment of primary mediastinal large B cell lymphoma using rigorously defined molecular classification: a retrospective analysis. Blood. 2020;136(Supplement 1):35–36. doi: 10.1182/blood-2020-143171. [DOI] [Google Scholar]
- 6.Tan WCC, et al. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 2020;40(4):135–153. doi: 10.1002/cac2.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marques-Piubelli ML, et al. SIRPα+ macrophages are increased in patients with FL who progress or relapse after frontline lenalidomide and rituximab. Blood Adv. 2022;6(11):3286–3293. doi: 10.1182/bloodadvances.2022007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antel K, et al. CD68-positive tumour associated macrophages, PD-L1 expression, and EBV latent infection in a high HIV-prevalent South African cohort of Hodgkin lymphoma patients. Pathology. 2021;53(5):628–634. doi: 10.1016/j.pathol.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romano A, et al. The prognostic value of the myeloid-mediated immunosuppression marker Arginase-1 in classic Hodgkin lymphoma. Oncotarget. 2016;7(41):67333–67346. doi: 10.18632/oncotarget.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armand P, et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma. J Clin Oncol. 2019;37(34):3291. doi: 10.1200/JCO.19.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotlov N, et al. Clinical and biological subtypes of B-cell lymphoma revealed by microenvironmental signatures. Cancer Discov. 2021;11(6):1468–1489. doi: 10.1158/2159-8290.CD-20-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steiner RE, et al. Brentuximab vedotin and nivolumab alone and then combined with rituximab, cyclophosphamide, doxorubicin, and prednisone for frontline therapy of patients with primary mediastinal large B-cell lymphoma. J Clin Oncol. 2022;40(16):7589–7589. doi: 10.1200/JCO.2022.40.16_suppl.TPS7589. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Methods; Figure S1. Representative pictures showing different cell densities of CD30+PD-L1+ cells. A-C) Multiplex immunofluorescence image of a case with low cell density of CD30+, PD-L1+, and CD30+PD-L1+ cells median: 20.46 cells/mm2, respectively. D-E) Multiplex immunofluorescence image of a case with high cell density of CD30+, PD-L1+, and CD30+PD-L1+ cells (median: 604.62 cells/mm2), respectively. Supplementary Table 1.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.