Abstract
Objective:
Studies were performed to uncover the significance of obesity in RA and preclinical models.
Methods:
Preclinical arthritis models were utilized to examine the impact of obesity on disease onset and remission. Conditioned media from RA adipose tissues were used to investigate the mechanism contributing to joint neutrophil influx and M1 macrophage differentiation observed in early and remission phases of arthritis.
Results:
We report that mice fed with high fat diet (HFD) have an earlier onset of collagen induced arthritis (CIA) compared to mice on regular diet (RD). However, the differences in CIA joint swelling between the two diet groups are lost once disease is established. We found that early arthritis triggered by obesity is due to elevated joint MIP2/IL-8 levels detected in CIA as well as in the RA and mouse adipose tissues and the effect of this chemokine on neutrophil recruitment. Although active disease progression is similarly affected in both diet groups, arthritis resolution is accelerated in lean mice while joint inflammation is sustained in obese mice. We document that HFD can prolong toll like receptor (TLR)4 induced arthritis by increasing joint monocyte migration and further remodeling the recruited cells into M1 macrophages. Consistently, we show that adipose condition media can transform RA and wild type naïve myeloid cells into M1 macrophages; however this function is impaired by TLR4 blockade or deficiency.
Conclusions:
We conclude, that despite established disease being unaffected by obesity, the early and the resolution phases of RA are impacted by obesity through different mechanisms.
Keywords: Obesity, RA, IL-8/MIP2, neutrophils, M1 macrophage differentiation, TLR4
Rheumatoid arthritis (RA) is a chronic autoimmune disorder in which imbalanced leukocyte migration contributes to joint inflammation and bone erosion 1. A number of epidemiologic studies have demonstrated an increased incidence of RA among obese individuals 2-4. In addition, obesity has been associated with elevated disease severity and inferior response to treatment in some clinical studies 5-7. However, there are little to no experimental data to explain the mechanisms that might be at play in these epidemiologic studies.
RA patients have markedly higher prevalence of metabolic syndrome compared to the general population, yet the reasons are undefined 8. Obesity is known to be associated with metabolic syndrome components and elevated body mass index (BMI) is linked to RA onset 9. In RA, adipocytes and their surrounding macrophages in white adipose tissues produce a number of adipokines that modulate systemic inflammation 10. Interestingly, the adipokine, leptin, participates in innate immunity by increasing the TH-1 cell polarization and suppressing the T regulatory (Treg) cell differentiation 11. Recent studies reveal that joint inflammation is aggravated in collagen induced arthritis (CIA) mice fed on high fat diet (HFD) compared to regular diet (RD) due to increase in spleen TH-17 cell development 12.
Others have demonstrated that both in human and mice number of macrophages recruited into adipose tissue closely correlates with BMI and adipose size and that the expression analysis of myeloid cells validates its critical role in the inflammatory process of obesity 13. Moreover, it was shown that adipose associated macrophages in non-obese humans exhibit anti-inflammatory phenotype, while myeloid cells recruited into adipose tissue of obese individuals have a proinflammatory characteristic 14.
Studies were performed to understand the reason why increased BMI contributes to higher risk for RA development 15 and to elucidate the underlying mechanism for inferior response to anti-TNF therapy in obese compared to normal weight RA patients 5-7, 16. We uncover that neutrophil (IL-8/MIP2, CXCL1, CXCL5) and monocyte (IL-6, IL-1β, CCL2) chemoattractants are elevated in conditioned media obtained from RA and obese mice adipose tissues. We next establish that obesity affects RA inflammatory process in a distinct manner, during the early onset and the late remission phases. Employing RA specimen and preclinical arthritis models, we found that early joint inflammation is potentiated by obesity through IL-8/MIP2 induced neutrophil migration. While in the later stage of the disease, obesity sustains arthritis by remodeling the newly recruited naïve myeloid cells into proinflammatory M1 macrophages, in part via TLR4 dependent cascade. In conclusion, this novel study reveals for the first time the mechanism by which obesity initiates RA onset and further identifies the components that may be responsible for worse anti-TNF therapy response in obese RA patients.
MATERIALS AND METHODS
Study protocol for animal models
DBA/1J mice were fed a RD (19% protein) or 60% HFD (23.1% protein; Research Diet Inc., NJ) beginning at the age of 4 weeks. Subsequently, 8 week old mice were immunized with collagen type II (Chondrex) on days 0 and 21 17-19. Mice were sacrificed on days 29, 55 or 106 when they had been on RD or HFD for 8, 12 or 19 weeks.
C57BL6 mice were fed for 10 weeks on RD or 60% HFD prior to being intra-articularly (i.a.) injected with 10 μg of LPS (0111:B4) for 72h. Ankle circumference was measured by Caliper 20-22.
Adipose tissue conditioned media
Conditioned media was generated by incubating adipose tissue obtained from RA (n=14) or OA synovia (n=7) or visceral fat from 10 week fed HFD mouse (n=6) in media and filtered before use. RA was classified according to 1987 Revised Criteria 23 and the OA knee, hand and hip met the classification of 1986, 1990 and 199124-26.
Cytokine Quantification
Human and mouse TNF-α, IL-6, IL-1β, IL-17, IFN-γ, CCL2, CCL5, CXCL5, CXCL1, IL-8/MIP2, CCL3 and CCL20 (R&D Systems) were quantified by ELISA in RA and mouse adipose conditioned media as well as in CIA and LPS induced arthritis ankle homogenates.
Evaluation of adiposity and fatty liver
Lean and fat mass were calculated by the ratio of body composition and weight 27. Formalin-fixed liver samples were stained with H&E and fatty liver was scored on a 0-4 scale 28.
Flow cytometry analysis
To quantify CD3, F4/80, dual F4/80 and CD80, B220 positive cells in CIA, splenocytes were harvested on day 55. Thereafter, cells were stained with APC conjugated anti-CD3, PE labeled F4/80 or dual PE labeled F4/80 and APC conjugated CD80 as well as FITC labeled B220 (eBioscience). The frequency of TH-1 or TH-17 was quantified in CIA splenocytes harvested on day 55 and were stimulated with PMA (5 ng/ml) and ionomycin (500 ng/ml) in the presence of Brefeldin A (3 μg/ml) for 4 h. Cells were stained with APC conjugated anti-CD4 antibodies (Abs) (eBioscience). Percentage of TH-1 and TH-17 cells was determined by staining the splenocytes with FITC conjugated anti-IFN-γ or PE labeled anti-IL-17 Abs (eBioscience). RA peripheral blood (PB) monocytes 22, 29 or 7 days M-CSF driven mouse bone marrow myeloid cells were either untreated or treated with IFN-γ ± LPS, 10% RA or OA adipose conditioned media or 10-50% mouse adipose conditioned media for 24h prior to determining % PE conjugated CD127 or APC labeled CD80 positive cells.
Neutrophil isolation and chemotaxis
Polymorphonuclear cells (PMN)s were isolated from blood 30 or from mouse bone marrow progenitor cells 31. Human neutrophils were isolated using Histopaque gradient centrifugation followed by 3% Dextran separation 30. Mouse neutrophils were isolated from bone marrow cell suspensions that were laid on a three layer Percoll gradient of 78%, 69% and 52% (Sigma) 31. Neutrophil chemotaxis was performed in a Boyden chamber (Neuroprobe) using a 5μm membrane. fMLP (10 nM) was used as positive control and PBS was utilized as negative control 32.
To demonstrate that RA adipose conditioned media contributes to neutrophil migration different concentrations (5, 10 and 20%) were utilized in neutrophil chemotaxis. To identify the chemokine responsible for neutrophil migration; neutrophil chemotaxis was tested in response to 10% RA and mouse adipose media that were treated with 10 μg/ml of sham Ab or Abs to IL-8/MIP2, CXCL1 and CXCL5/LIX or IL-6.
Western blot analysis
RA and mouse adipose conditioned media were either untreated or pretreated with IgG as well as Abs to IL-8/MIP2, CXCL1, CXCL5 and/or IL-6 (R&D systems) for 1h. Neutrophils from human PB or mouse bone marrow were untreated (PBS) or stimulated with either fMLP (10 nM), 10% RA (2 min) or mouse conditioned adipose media (5 min) after being pretreated with IgG or Abs to IL-8/MIP2, CXCL1, CXCL5 and/or IL-6. Cell lysates were examined by Western blot analysis for phospho (p)-p38 (Cell Signaling; 1:1000) or for p38 (1:3000). Ankle homogenates harvested from TLR4 induced arthritis fed on RD or HFD were Western blotted for iNOS and Arginase I expression (1:1000, Santa Cruz Biotechnology) or actin equal loading (1:3000).
Real-time RT-PCR
Total RNA was extracted using TRIzol from RA monocytes 22, 29 or 7 day M-CSF (20 ng/ml) driven mouse bone marrow myeloid cells that were either untreated (PBS) or treated with IFN-γ ± LPS (100 ng/ml each), 10 to 50% de-identified RA and/or OA adipose conditioned media or 10-50% mouse adipose conditioned media for 6h. In a different experiment, 7 day M-CSF driven mouse bone marrow myeloid cells were either untreated or pretreated for 1h with 10 μg/ml IgG, anti-IL-6 (eBioscience), anti-MIP2 (R&D Systems), anti-TLR2 (Invivogen) or anti-TLR4 (eBioscience) Abs prior to being stimulated with 10% mouse conditioned adipose media. C57BL6 wild type (WT) and TLR4−/− (Jackson lab) mouse bone marrow cells cultured 7 days in M-CSF (20 ng/ml) were either untreated (PBS) or treated with IFN-γ (100 ng/ml), ultrapure LPS (100 ng/ml, binds only to TLR4) or 10% mouse conditioned adipose media for 6h. Further, 7 day M-CSF differentiated WT mouse bone cells were either untreated or pretreated with TLR4 antagonist (1 μg/ml; Invivogen) for 3h prior to stimulating the cells with PBS, LPS (10 ng/ml, binds to TLR2 and 4), ultrapure LPS (10 ng/ml) or 10% mouse conditioned media for 6h.
Abs and immunohistochemical analysis
Ankles were formalin fixed (10%), paraffin embedded and sectioned. Inflammation, synovial lining and bone erosion were determined using H&E-stained sections on a 0-5 scale 18, 20, 22, 33. Mouse ankles were immunoperoxidase-stained with diaminobenzidine as a chromogen. CIA or TLR4 induced arthritis mice fed on RD or HFD were stained with GR1 (1:50; eBioScience), F4/80 (1:100; Serotec), iNOS and Arginase I (1:200; Santa Cruz Biotechnology) or isotype IgG Ab. Positive immunostaining was scored on a 0-5 scale by two masked observers 18, 20, 22, 33.
Statistical Analysis
One way analysis of variance was used for comparisons among multiple groups, followed by Student’s post hoc 2-tailed T-test. Student’s paired and unpaired 2-tailed test were used for comparisons between 2 groups. P values less than 0.05 were considered significant.
RESULTS
HFD contributes to early CIA onset by provoking neutrophil migration through MIP2/IL-8.
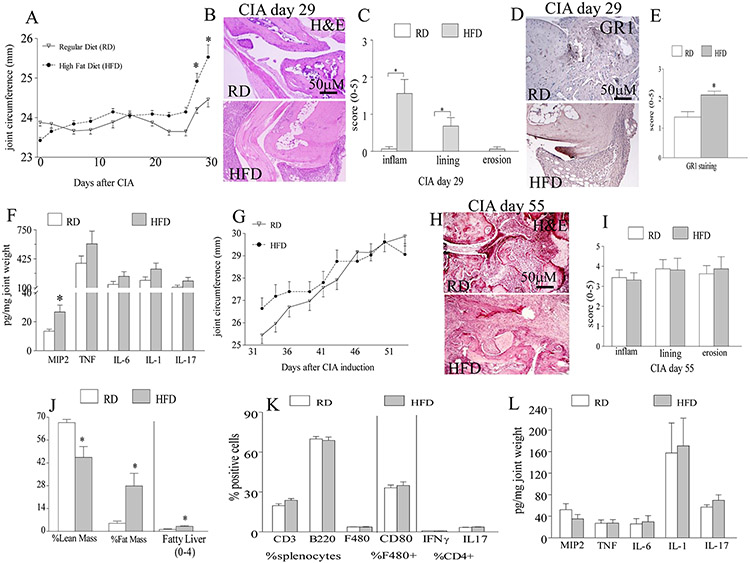
To examine the effect of obesity on RA, CIA mice were fed for 8 weeks on HFD or RD. Interestingly, CIA mice fed on HFD had a significantly earlier disease onset on days 28 and 30 (Fig. 1A). Consistent with the clinical findings, histological analysis of CIA mice sacrificed on day 29, revealed that while there were early signs of joint inflammation and lining thickness detected in HFD group, no clinical manifestations were found in mice fed on RD (Figs. 1B-C). We document that the early CIA onset observed in the HFD compared to RD is due to a 2 fold increase in MIP2 protein levels that can markedly potentiate joint neutrophil infiltration (Figs. 1D-F). In contrast, joint TNF, IL-6, IL-1 and IL-17 levels were comparable in RD and HFD mice on day 29 post CIA induction (Fig. 1F). Interestingly, we establish that the weight gain positively correlates with increased joint inflammation in CIA mice on HFD but not in mice on RD (Figs. S3-S4). These results suggest that in obese CIA mice, early disease is initiated by neutrophils infiltrating into the joint in response to MIP2.
Figure 1. Obesity impacts CIA onset but not active phase by enhancing joint MIP2 induced neutrophil migration.
A. Joint circumference were calculated in CIA mice that were fed on regular diet (RD) (n=13 mice) or 60% high fat diet (HFD) (n=12 mice) for 8 weeks. B. CIA ankles harvested on day 29 from mice fed on RD and HFD were H&E stained (orig. mag. x 200), and C. inflammation (inflam), lining thickness (lining) and bone erosion (erosion) were quantified on a 0-5 scale, n=5 mice, 10 back paws. Synovial tissues (STs) obtained from the back paws of day 29 CIA mice fed on RD and HFD were stained with anti-GR1 antibody (orig. mag. x 200) (D) and GR1 staining was quantified on a 0-5 scale, (E) n=8. F. Joint MIP2, TNF, IL-6, IL-1 and IL-17 protein levels were assessed by ELISA from the front (carpal joints) and back paws of day 29 CIA mice fed on RD and HFD and normalized by joint weight, n=8. G. Joint circumference is shown from day 31 to day 55 in CIA mice that were fed on RD or HFD for up to 12 weeks, n=12-13 mice. H. CIA ankles harvested on day 55 from mice fed on RD and HFD were H&E stained (orig. mag. x 200), and I. inflammation, lining thickness and bone erosion were quantified on a 0-5 scale, n=8. J. % lean and fat mass or H&E staining of liver samples from day 55 CIA mice on RD and HFD were measured by dual-energy x-ray absorptiometry (DXA) or scored for fatty liver on a 0-4 scale, n=12-13. K. The frequency of CD3, B220, F480, F480+CD80+ as well as TH1 and TH17 cells was assessed in splenocytes harvested on day 55 from CIA mice fed on RD and HFD, n=7. L. Changes in joint MIP2, TNF, IL-6, IL-1 and IL-17 were determined by ELISA from day 55 CIA mice fed on RD and HFD, n=7. Values are mean ± SE. * indicates p<0.05.
HFD does not affect CIA effector phase.
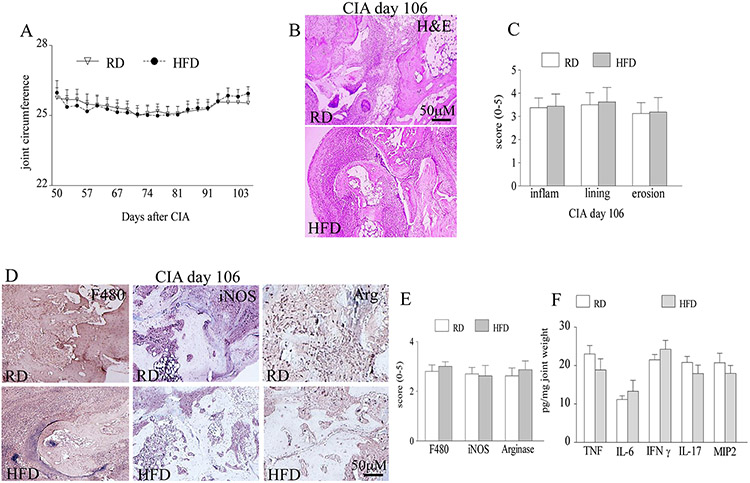
To determine the effect of obesity on the effector phase of CIA, mice were fed with HFD or RD for 12 or 19 weeks and sacrificed on days 55 and 106. Interestingly, as disease progresses beyond day 30, joint inflammation differences were lost (Figs. 1G, 2A) between the obese mice that had greater fat mass, fatty liver and weight (Fig. 1J, S1) compared to the lean mice harvested on days 55 and 106. Consistent with the clinical data, histological findings demonstrate that joint inflammation, lining thickness and bone erosion were comparable in the CIA lean and obese mice harvested on days 55 and 106 (Figs. 1H-I, 2B-C). We further uncovered that the frequency of the CIA leukocytes (CD3, B220, F480) and T cell population (TH1, TH17) as well as levels of joint monocyte (TNF, IL-1, IL-6, IL-17) and neutrophil chemoattractants (MIP2) were unaffected by obesity induced diet in mice sacrificed on day 55 (Figs. 2K-L). Interestingly, when studies were extended for 106 days, CIA disease activity did not resolve and there were no differences detected in joint monocyte infiltration, M1 or M2 macrophage differentiation as well as joint TNF, IL-6, IFN-γ, IL-17 and MIP2 protein levels in mice fed with HFD compared to RD (Figs. 2D-F). We conclude that while CIA disease initiation is exacerbated by HFD, established active disease is unaffected by this process.
Figure 2. Obesity does not impact the late stage of CIA.
A. Joint circumference was determined in CIA mice that were fed on RD (n=9) or HFD (n=8) for 19 weeks. B. CIA ankles harvested on day 106 from mice fed on RD and HFD were H&E stained (orig. mag. x 200), and C. inflammation, lining thickness and bone erosion were quantified on a 0-5 scale, n=8. D. STs harvested on day 106 from CIA mice on RD and HFD were stained with anti-F480, anti-iNOS and anti-Arginase Abs (orig. mag. x 200) and E. positive staining was quantified on a 0-5 scale, n=8. F. Joint TNF, IL-6, IFN-γ, IL-17 and MIP2 protein levels were quantified from day 106 CIA mice fed on RD and HFD by ELISA and normalized to joint weight, n=8. Values are mean ± SE. * indicates p<0.05.
In RA and mouse adipose tissues, neutrophil infiltration is fostered via IL-8/MIP2.
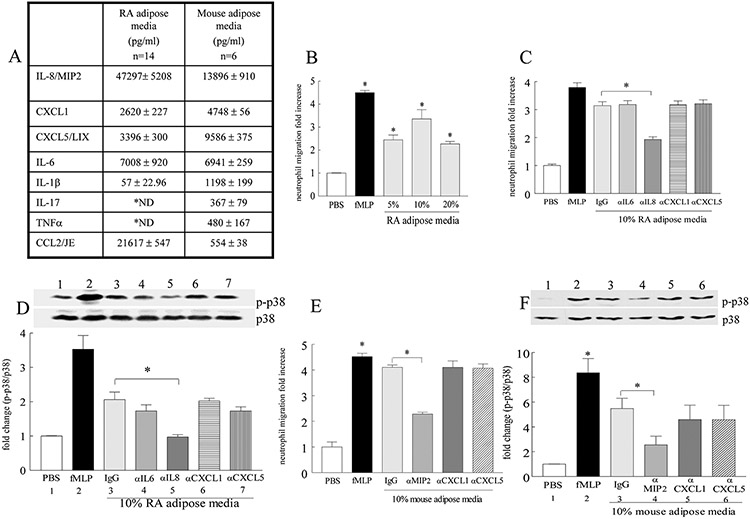
To determine whether findings in CIA are representative of RA pathogenesis, inflammatory factors were quantified in the adipose conditioned media extracted from RA synovial tissue (ST) and obese mice abdominal adipose tissue. We found that IL-8/MIP2 (47 and 14 ng/ml) levels exceeded all other inflammatory factors. In addition other neutrophil chemoattractants such as CXCL1 (2.6 and 4.7 ng/ml) and CXCL5 (3.4 and 9.6 ng/ml) were also highly expressed in RA and mouse adipose conditioned media (Fig. 3A). In contrast, CCL2 (21 and 0.5 ng/ml) and IL-6 (7 and 6.9 ng/ml) were the only monokines that were abundantly detected and IL-1β levels (0.05 and 1.2 ng/ml) were low, while TNF [not detectable (ND) and 0.5 ng/ml] and lymphokine IL-17 (ND and 0.4 ng/ml) levels were either undetectable or sparse in RA and mouse adipose conditioned media (Fig. 3A). Based on the high levels of neutrophil chemokines in the conditioned media, we asked whether RA adipose media could contribute to neutrophil migration. Our results demonstrate that 10% RA adipose media has the greatest neutrophil chemoattractant ability and this is primarily due to IL-8 but not CXCL1, CXCL5 or IL-6 (negative control) function (Figs. 3B-C). Confirming this notion, we show that similar to the positive control, fMLP, RA adipose media promotes p38 activation and that IL-8 neutralization can explicitly negate this function (Fig. 3D). Consistent with the results in RA, blockade of MIP2 (IL-8 analog) function abrogates mouse adipose media driven neutrophil chemotaxis through a p38 dependent mechanism (60-66% respectively)(Figs. 3E-F). In contrast, neutralization of CXCL1 and CXCL5 was ineffective in this process (Figs. 3E-F). These results suggest that in obese individuals, onset of RA can be triggered by joint neutrophil recruitment in response to IL-8/MIP2 induced p38 activation.
Figure 3. Elevated MIP2 detected in the conditioned media from RA and mouse adipose tissues is associated with higher neutrophil migration via p38 activation.
A. Conditioned media was generated from RA synovium (n=14) and abdominal fat from 10 week fed HFD mouse (n=6) and the proinflammatory factors were assessed by ELISA. B. Peripheral blood (PB) neutrophil chemotaxis was performed in response to 5 to 20% RA adipose conditioned media, n=3. PB neutrophils was sham depleted or neutralized with anti-IL-6, anti-IL-8, anti-CXCL1 or anti-CXCL5 Abs prior to examining (C) chemotaxis or performing (D) Western blot and densitometric analysis of the bands containing cell lysates that are probed for p38 phosphorylation in response to 10% RA adipose media, n=3-5. E. Neutrophils extracted from mouse bone marrow were either sham depleted or neutralized with anti-MIP2, anti-CXCL1 or anti-CXCL5 Abs prior to performing chemotaxis, n=4 or F. densitometric analysis of the Western blot bands for p38 phoshorylation, in response to 10% mouse adipose media, n=4. In B-F, PBS and fMLP (10nM) were used as negative and positive controls. Values are mean ± SE. * indicates p<0.05.
Obesity sustains joint inflammation through M1 macrophage polarization.
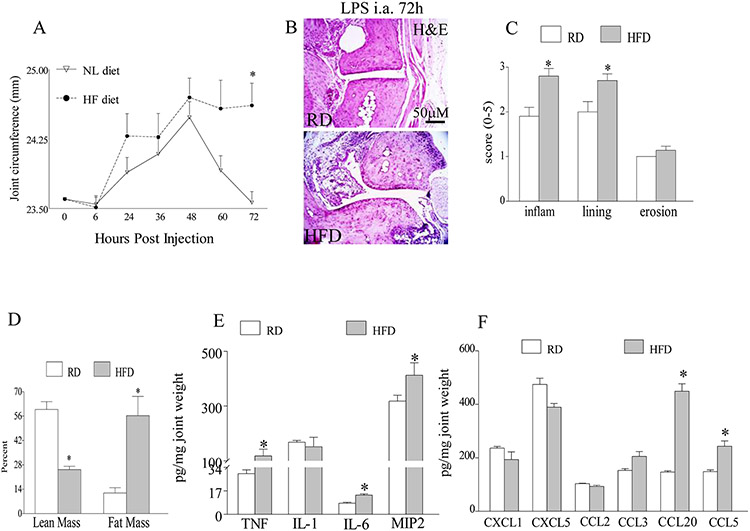
To further investigate the inhibitory effect of obesity on arthritis resolution, we chose to utilize the TLR4 induced arthritis model. This model was selected due to rapid arthritis resolution observed 72h after LPS injection; in contrast to CIA in which ankles remain inflamed 106 days post initiation (Fig. 2A) 34, 35. We found that in the first 48h, TLR4 driven joint inflammation progresses similarly in the obese and lean mice, thereafter while arthritis resolves in the lean mice, ankle swelling is sustained in the obese mice at 72h and 84h post LPS injection (Figs. 4A and S7). Histological analysis of tissues harvested at 72h confirm that mice on HFD have markedly greater joint inflammation and lining thickness with no difference in bone erosion compared to the RD group (Figs. 4B-D). Although monocyte chemoattractants such as TNF-α (4 fold), CCL20 (3 fold) and CCL5 (2 fold) were significantly upregulated in the obese arthritic mice, there were no differences detected in IL-1β, CCL3 and CCL2 levels compared to lean mice (Figs. 4E-F). On the contrary, mice on HFD and RD expressed similar levels of neutrophil chemokines, CXCL1 and CXCL5, at 72h post LPS injection (Figs. 4F). Corroborating with the higher levels of monocyte chemokines detected in the obese mice following LPS injection, we found that joint monocyte extravasation was potentiated in these HFD arthritic mice compared to RD group (Figs. 5A-B). To better understand how obesity prolongs arthritis and inhibits disease remission, joint myeloid cell phenotype was assessed in the obese and the lean arthritic mice. We uncovered that the obese arthritic mice, predominantly express iNOS+ M1 macrophages; while iNOS+ cells are reduced and Arginase+ M2 macrophages are upregulated in the lean mice (Figs. 5C-E). Our results conclude that obesity can sustain arthritis by reconstructing the newly recruited joint myeloid cells into proinflammatory M1 macrophages.
Figure 4. The obese arthritic mice demonstrate delayed remission compared to their counterpart controls.
A. Ankle circumference was determined in wild type (WT) mice that were fed on RD or 60% HFD for 10 weeks following i.a. injection with 10 μg of LPS and mice were sacrificed after 72h of LPS injection, n=10. B. RD and HFD ankles harvested after 72h of LPS injection were H&E stained (orig. mag. x 200), and C. inflammation, lining thickness and bone erosion were quantified on a 0-5 scale, n=10. D. % lean mass and fat mass were assessed by DXA in TLR4 mediated arthritis mice (72h) fed on RD and HFD, n=10. E. Protein levels of proinflammatory cytokines (TNF-α, IL-1β, IL-6 and MIP2) and F. chemokines (CXCL1, CXCL5, CCL2, CCL3, CCL20 and CCL5) were quantified by ELISA in TLR4 arthritic mice (72h) fed on RD and HFD, n=5. Values are mean ± SE. * indicates p<0.05.
Figure 5. Joint myeloid cell recruitment and M1 macrophage polarization are upregulated in obese TLR4 arthritic mice compared to lean controls.
A. STs from TLR4 arthritic mice (72h) on RD and HFD were immunostained with anti-F4/80 antibody (orig. mag. x 200) and B. macrophage staining was quantified on a 0-5 scale, n=7. C. STs from TLR4 arthritic mice (72h) on RD and HFD were immunostained with anti-iNOS and anti-Arginase antibodies (orig. mag. x 200) and D. positive staining was quantified on a 0-5 scale, n=7. E. Ankle homogenates harvested following 72h of LPS i.a. injection from mice fed on RD and HFD, were Western blotted for iNOS and Arginase expression or actin equal loading, n=5. Values are mean ± SE. * indicates p<0.05.
RA and mouse adipose conditioned media promote M1 macrophage differentiation via TLR4 dependent cascade.
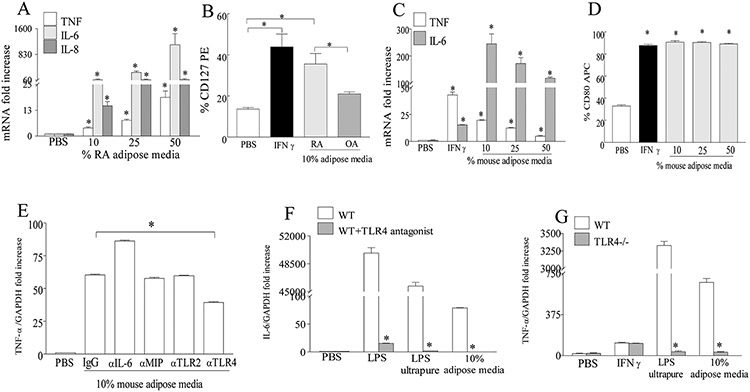
We next asked whether adipose conditioned media obtained from RA or obese mice could remodel myeloid cells into inflammatory M1 macrophages as observed in the obese arthritic mice. We show that addition of RA or mouse adipose conditioned media to RA monocytes or mouse bone marrow myeloid cells can polarize the naïve cells into M1 macrophages. The M1 differentiation process was determined by TNF-α, IL-6 and/or IL-8 transcription as well as assessing the cell surface CD127 (human) or CD80 (mouse) frequency (Fig. 6A-D). Interestingly, RA adipose conditioned media had greater capacity in transforming the naïve myeloid cells into M1 macrophages compared to those obtained from OA patients (Fig. 6B). Notably our results indicate that once the concentration of the inflammatory factors in the RA or mouse adipose conditioned media reach a specific level it no longer has a dose responsive effect on M1 differentiation as inflammatory mediators may fall in the non-linear saturated range (Figs. 6A, 6C-D). Next, to elucidate the mechanism by which adipose media promotes M1 macrophage polarization, IL-6, MIP2 and TLR2 and TLR4 pathways were blocked in 10% mouse adipose media (most optimal M1 differentiation condition; Fig. 6C). We found that although levels of IL-6 were elevated in HFD arthritic mice at 72h as well as in RA and mouse adipose conditioned media, neutralization of these cytokine did not impact the M1 polarization process (Fig. 6E). We further show that unlike TLR2 immunoneutralization, blockade or deficiency in TLR4 signaling severely impairs fat induced M1 macrophage differentiation, suggesting that TLR4 can be activated by factors available in the adipose conditioned media (Figs 6E-G). Our results suggest that interaction of TLR4+ myeloid cells in the obese joint with the inflammatory mediators released from the adipose tissues can reconstruct these naive myeloid cells into M1 proinflammatory macrophages.
Figure 6. RA and mouse adipose conditioned media can transform the RA or mouse naïve myeloid cells into M1 macrophages through TLR4 dependent function.
A. RA PB monocytes or C. 7 days M-CSF driven WT mouse bone marrow myeloid cells were treated with PBS, 10, 25 and 50% RA or mouse adipose conditioned media and/or IFN-γ for 6h and TNF-α, IL-6 and/or IL-8 mRNA expression levels were determined by real-time RT-PCR, n=4. B. RA PB monocytes or D. 7 days M-CSF driven WT bone marrow cells were treated with PBS, IFN-γ, 10% RA or OA adipose conditioned media or 10-50% mouse adipose conditioned media for 24h prior to determining % CD127 (B) or CD80 (D) by flow cytometry, n=4. E. 7 days M-CSF driven WT bone marrow cells were either untreated (PBS) or pretreated (1h) with IgG, anti-IL-6, anti-MIP2, anti-TLR2 and anti-TLR4 antibodies prior to being stimulated with 10% mouse adipose media for 6h, n=3. F. 7 days M-CSF driven WT bone marrow cells were either untreated or pretreated with TLR4 antagonist for 3h prior to stimulating the cells with PBS, LPS, ultrapure LPS or 10% mouse adipose media for 6h, n=3. G. 7 days M-CSF driven WT and TLR4−/− bone marrow cells were treated with PBS, IFN-γ, ultrapure LPS or 10% mouse adipose media for 6h, n=3. In E, F and G, TNF-α or IL-6 mRNA expression levels were determined by real-time RT-PCR. Values are mean ± SE. * indicates p<0.05.
DISCUSSION
In this novel study we show for the first time, an early and a late effect of obesity on RA. We uncover that obesity can critically impact disease initiation by fostering neutrophil migration through IL-8/MIP2 cascade. While diet induced obesity does not affect established active disease in preclinical models, it can markedly delay arthritis remission as a result of TLR4 driven M1 macrophage differentiation. Our results highlight for the first time that obesity can differentially modulate RA pathogenesis contingent on the disease status.
To shed light on the mechanism by which obesity impacts RA, diet induced obesity was provoked in CIA. In contrast to previous studies which demonstrate that obesity exacerbates CIA due to increase in the number of spleen TH-17 cells 12, our results clearly show no difference in disease activity or inflammatory parameters assessed in CIA effector phase in the obese compared to lean mice. The discrepancy in our results may be due to the differences in the mouse background (C57BL6 vs DBA/1J in our study) or the % diet fat (34.9% vs 60% in our study) between the two studies. However, we found that the obese CIA mice have an earlier disease onset (days 28-30) compared to the lean mice. On the contrary, as disease progresses beyond day 30, the differences in CIA ankle swelling are lost between the two diet groups. Examining RA and mouse adipose conditioned media, we found that IL-8/MIP2 (47 and 13 ng/ml) was the most abundantly expressed neutrophil chemokine compared to CXCL1 and CXCL5. We document that IL-8/MIP2 neutralization impairs RA and mouse adipose media induced neutrophil migration via p38 inactivation. In contrast, neutrophil chemotaxis was unaffected by blockade of CXCL1 and CXCL5 function. Interestingly, neutrophil chemokines [IL-8 (41 fold), CXCL1 (4 fold) and CXCL5 (3 fold)] expressed at significantly lower concentration in OA compared to RA adipose conditioned media can still contribute to neutrophil infiltration. We demonstrate that early onset of CIA observed in the HFD compared to RD mice is due to MIP2 dependent neutrophil trafficking which may explain why obesity is a risk factor for RA initiation. Corroborating with these findings, IL-8 levels closely correlate with the number of neutrophils in RA synovial fluid 36, 37 and others have shown that anti-MIP2 Ab treatment impairs neutrophil infiltration in preclinical models of arthritis, leading to resolution of acute disease 38. IL-8 is produced by RA ST fibroblasts, macrophages or adipocytes in response to proinflammatory cytokines such as IL-1β, TNF, IL-17 or lipoproteins, hence justifying its potential as a therapeutic target for RA 39-42. Using multi-photon real-time in vivo microscopy previous studies have described that neutrophils migrate in clusters and surround CIA joint fibroblasts but not myeloid cells at disease onset 43 suggesting that fibroblasts are the main source of MIP2/IL-8 secretion in the initial phases of CIA.
Similar to our observation in RA and mouse adipose conditioned media, circulating levels of IL-8 and IL-6 are elevated in obese individuals and these concentrations positively correlate with BMI and/or CRP 44, 45. Interestingly, joint MIP2 and IL-6 levels were upregulated during disease progression at 72h in obese TLR4 arthritic mice compared to non-obese counterparts. Previous studies demonstrate that adipose tissue express elevated levels of TLR4 46 and hence i.a injection of TLR4 ligand can contribute to adipocyte production of IL-6 and MIP2 and with increased myeloid cell migration following 72h of LPS administration, both adipocytes and macrophages can potentially elevate levels of these cytokines. Corroborating with this notion, co-culture of macrophages and adipocytes stimulated with low dose of LPS results in 100 fold higher IL-6 production compared to adipocytes alone 47.
Studies performed in knockout mice document that expression of myeloid CCR2 and CCR5 and elevation of their ligands, CCL2 and CCL5 in the adipose tissue are indispensable for obesity mediated monocyte infiltration 48, 49. Despite the increase in joint monocyte trafficking detected in the obese mice following 72h of TLR4 induced arthritis, CCL2 levels were similar in both diet groups while other monocyte chemoattractants such as CCL5, CCL20 and TNF were elevated in the HFD compared to RD mice. Consistently, others show that obesity associated monocyte recruitment is not restrained by CCL2 deficiency and the increase in cell influx can be promoted through macrophage galactose specific lectin (MAC)2 produced from adipose tissues 50, 51, suggesting that in the absence of CCL2 alternative mechanisms can maneuver this process.
It is known that macrophages are plastic cells that can alter their phenotype depending on their milieu. The M1 macrophages are “classically activated cells” that express cell surface CCR7, CD127, CD80/CD86 and can secrete TNF, IL-6, IL-8 and IL-1 upon activation with IFN-γ and/or LPS 52. We found that CIA severity did not resolve and its progression was not affected by diet induced obesity, in contrast; obese TLR4 induced arthritic mice had delayed remission compared to the lean group. We uncovered that CIA prolonged to 106 days was not impacted by obesity, since there were similar number of joint M1 and M2 macrophages in both diet groups which resulted in comparable levels of M1 associated proinflammatory factors. The lack of effect of obesity on active progression of arthritis may explain why there is a disconnection between BMI, RA joint pain and bone destruction 53.
We further show that while joint inflammation resolves in lean TLR4 arthritic mice, diet induced obesity can prolong arthritis by 24h through converting the undifferentiated myeloid cells into iNOS+ M1 macrophages that produce higher levels of proinflammatory factors. Delay in remission in the obese TLR4 arthritic mice, corroborates with lack of the anti-TNF response in obese compared to normal weight RA 5-7, 16, which may also be due to sustained joint M1 macrophages.
To examine the underlying mechanism by which adipose conditioned media transforms the undifferentiated myeloid cells into M1 macrophages, role of IL-6, MIP2, TLR2 and TLR4 was explored in this function. Interestingly, we show that only inhibition of TLR4 but not TLR2, IL-6 or MIP2 negates the development of mouse bone marrow myeloid cells into M1 macrophages. Consistently, earlier studies show that while fatty acids like palmitate degrade IκB in WT mouse peritoneal macrophages, NF-κB signaling is impaired in TLR4−/− cells 46. Notably, activation of TLR4 by free fatty acids is not restricted to macrophages since a recent paper demonstrates that RA ST fibroblasts stimulated by unsaturated and saturated fatty acids produce proinflammatory factors via TLR4 signaling 54. However, it is unclear whether fatty acids can directly bind to TLR4 or if they participate in receptor dimerization procedure 55, 56. In contrast to these findings, female TLR4−/− mice show increased obesity, yet these mice are partially protected against insulin resistance compared to WT mice 46. Our results support the conclusion that TLR4 plays an important role in obesity mediated M1 differentiation perhaps in part due to presence of fatty acids. However, our findings also suggest that M1 polarization driven by HFD is multifactorial and this process may not be explicitly due to TLR4 function. We conclude that obesity is an important risk factor that mainly dysregulates RA onset and remission and may help to explain epidemiologic studies suggesting a greater incidence of RA and poor treatment response among obese individuals.
Supplementary Material
FUNDING:
This work was supported in part by awards from Department of Veteran’s Affairs MERIT Award 1I01BX002286, the National Institutes of Health AR055240 and AR065778, grant from Within Our Reach from The American College of Rheumatology, funding provided by Department of Defense PR093477 and Arthritis Foundation Innovative award. Dr. JFB efforts were funded by VA clinical science research and development as well as VA Career Development Award IK2 CX000955.
Footnotes
COMPETING INTERESTS:
None declared.
ETHICS APPPROVAL:
Approved by local ethical committees.
LITERATURE CITED
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature 2003; 423:356–361. [DOI] [PubMed] [Google Scholar]
- 2.Wesley A, Bengtsson C, Elkan AC, et al. Association between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: results from a population-based case-control study. Arthritis Care Res (Hoboken) 2013; 65:107–112. [DOI] [PubMed] [Google Scholar]
- 3.Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol 2014; 43:843–855. [DOI] [PubMed] [Google Scholar]
- 4.Lahiri M, Luben RN, Morgan C, et al. Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register--the EPIC-2-NOAR Study). Ann Rheum Dis 2014; 73:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandberg ME, Bengtsson C, Kallberg H, et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Annals of the Rheumatic Diseases 2014; 73:2029–2033. [DOI] [PubMed] [Google Scholar]
- 6.Ellerby N, Mattey DL, Packham J, et al. Obesity and comorbidity are independently associated with a failure to achieve remission in patients with established rheumatoid arthritis. Ann Rheum Dis 2014; 73:e74. [DOI] [PubMed] [Google Scholar]
- 7.Iannone F, Fanizzi R, Notarnicola A, et al. Obesity reduces the drug survival of second line biological drugs following a first TNF-alpha inhibitor in rheumatoid arthritis patients. Joint Bone Spine 2015; 82:187–191. [DOI] [PubMed] [Google Scholar]
- 8.Kerekes G, Nurmohamed MT, Gonzalez-Gay MA, et al. Rheumatoid arthritis and metabolic syndrome. Nat Rev Rheumatol 2014; 10:691–696. [DOI] [PubMed] [Google Scholar]
- 9.Heimans L, van den Broek M, le Cessie S, et al. Association of high body mass index with decreased treatment response to combination therapy in recent-onset rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2013; 65:1235–1242. [DOI] [PubMed] [Google Scholar]
- 10.Del Prete A, Salvi V, Sozzani S. Adipokines as potential biomarkers in rheumatoid arthritis. Mediators Inflamm 2014; 2014:425068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matarese G, Procaccini C, De Rosa V, et al. Regulatory T cells in obesity: the leptin connection. Trends Mol Med 2010; 16:247–256. [DOI] [PubMed] [Google Scholar]
- 12.Jhun JY, Yoon BY, Park MK, et al. Obesity aggravates the joint inflammation in a collagen-induced arthritis model through deviation to Th17 differentiation. Exp Mol Med 2012; 44:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin B, Yang M, Fu H, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther 2015; 17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klaasen R, Wijbrandts CA, Gerlag DM, et al. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum 2011; 63:359–364. [DOI] [PubMed] [Google Scholar]
- 17.Kim SJ, Chen Z, Chamberlain ND, et al. Ligation of TLR5 Promotes Myeloid Cell Infiltration and Differentiation into Mature Osteoclasts in Rheumatoid Arthritis and Experimental Arthritis. J Immunol 2014; 193:3902–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SJ, Chen Z, Chamberlain ND, et al. Angiogenesis in Rheumatoid Arthritis Is Fostered Directly by Toll-like Receptor 5 Ligation and Indirectly Through Interleukin-17 Induction. Arthritis Rheum 2013; 65:2024–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, Kim SJ, Chamberlain ND, et al. The novel role of IL-7 ligation to IL-7 receptor in myeloid cells of rheumatoid arthritis and collagen-induced arthritis. J Immunol 2013; 190:5256–5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickens SR, Chamberlain ND, Volin MV, et al. Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum 2011; 63:2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickens SR, Chamberlain ND, Volin MV, et al. Anti-CXCL5 therapy ameliorates IL-17-induced arthritis by decreasing joint vascularization. Angiogenesis 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Kim SJ, Chamberlain ND, et al. The Novel Role of IL-7 Ligation to IL-7 Receptor in Myeloid Cells of Rheumatoid Arthritis and Collagen-Induced Arthritis. J Immunol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31:315–324. [DOI] [PubMed] [Google Scholar]
- 24.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986; 29:1039–1049. [DOI] [PubMed] [Google Scholar]
- 25.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum 1990; 33:1601–1610. [DOI] [PubMed] [Google Scholar]
- 26.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum 1991; 34:505–514. [DOI] [PubMed] [Google Scholar]
- 27.Pang J, Rhodes DH, Pini M, et al. Increased adiposity, dysregulated glucose metabolism and systemic inflammation in Galectin-3 KO mice. PLoS One 2013; 8:e57915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilat T, Leikin-Frenkel A, Goldiner I, et al. Prevention of diet-induced fatty liver in experimental animals by the oral administration of a fatty acid bile acid conjugate (FABAC). Hepatology 2003; 38:436–442. [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain ND, Vila OM, Volin MV, et al. TLR5, a novel and unidentified inflammatory mediator in rheumatoid arthritis that correlates with disease activity score and joint TNF-alpha levels. J Immunol 2012; 189:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyum A Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 1968; 97:77–89. [PubMed] [Google Scholar]
- 31.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, et al. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol 2004; 75:604–611. [DOI] [PubMed] [Google Scholar]
- 32.de Beaufort AJ, Pelikan DM, Elferink JG, et al. Effect of interleukin 8 in meconium on in-vitro neutrophil chemotaxis. Lancet 1998; 352:102–105. [DOI] [PubMed] [Google Scholar]
- 33.Pickens SR, Chamberlain ND, Volin MV, et al. Anti-CXCL5 therapy ameliorates IL-17-induced arthritis by decreasing joint vascularization. Angiogenesis 2011; 14:443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 2011; 332:478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanaba K, Hamaguchi Y, Venturi GM, et al. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol 2007; 179:1369–1380. [DOI] [PubMed] [Google Scholar]
- 36.Peichl P, Ceska M, Effenberger F, et al. Presence of NAP-1/IL-8 in synovial fluids indicates a possible pathogenic role in rheumatoid arthritis. Scand J Immunol 1991; 34:333–339. [DOI] [PubMed] [Google Scholar]
- 37.Rampart M, Herman AG, Grillet B, et al. Development and application of a radioimmunoassay for interleukin-8: detection of interleukin-8 in synovial fluids from patients with inflammatory joint disease. Lab Invest 1992; 66:512–518. [PubMed] [Google Scholar]
- 38.Harada A, Sekido N, Akahoshi T, et al. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 1994; 56:559–564. [PubMed] [Google Scholar]
- 39.Choi HM, Oh da H, Bang JS, et al. Differential effect of IL-1beta and TNFalpha on the production of IL-6, IL-8 and PGE2 in fibroblast-like synoviocytes and THP-1 macrophages. Rheumatol Int 2010; 30:1025–1033. [DOI] [PubMed] [Google Scholar]
- 40.Katz Y, Nadiv O, Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a "fine-tuning cytokine" in inflammation processes. Arthritis Rheum 2001; 44:2176–2184. [DOI] [PubMed] [Google Scholar]
- 41.Klezovitch O, Edelstein C, Scanu AM. Stimulation of interleukin-8 production in human THP-1 macrophages by apolipoprotein(a). Evidence for a critical involvement of elements in its C-terminal domain. J Biol Chem 2001; 276:46864–46869. [DOI] [PubMed] [Google Scholar]
- 42.Bruun JM, Pedersen SB, Richelsen B. Regulation of interleukin 8 production and gene expression in human adipose tissue in vitro. J Clin Endocrinol Metab 2001; 86:1267–1273. [DOI] [PubMed] [Google Scholar]
- 43.Byrne R, Rath E, Hladik A, et al. A dynamic real time in vivo and static ex vivo analysis of granulomonocytic cell migration in the collagen-induced arthritis model. PLoS One 2012; 7:e35194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 2006; 30:1347–1355. [DOI] [PubMed] [Google Scholar]
- 45.Roytblat L, Rachinsky M, Fisher A, et al. Raised interleukin-6 levels in obese patients. Obes Res 2000; 8:673–675. [DOI] [PubMed] [Google Scholar]
- 46.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116:3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamashita A, Soga Y, Iwamoto Y, et al. Macrophage-adipocyte interaction: marked interleukin-6 production by lipopolysaccharide. Obesity (Silver Spring) 2007; 15:2549–2552. [DOI] [PubMed] [Google Scholar]
- 48.Oh DY, Morinaga H, Talukdar S, et al. Increased macrophage migration into adipose tissue in obese mice. Diabetes 2012; 61:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitade H, Sawamoto K, Nagashimada M, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 2012; 61:1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inouye KE, Shi H, Howard JK, et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 2007; 56:2242–2250. [DOI] [PubMed] [Google Scholar]
- 51.Kirk EA, Sagawa ZK, McDonald TO, et al. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected]. Diabetes 2008; 57:1254–1261. [DOI] [PubMed] [Google Scholar]
- 52.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11:723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finckh A, Turesson C. The impact of obesity on the development and progression of rheumatoid arthritis. Annals of the Rheumatic Diseases 2014; 73:1911–1913. [DOI] [PubMed] [Google Scholar]
- 54.Frommer KW, Schaffler A, Rehart S, et al. Free fatty acids: potential proinflammatory mediators in rheumatic diseases. Annals of the Rheumatic Diseases 2013. [DOI] [PubMed] [Google Scholar]
- 55.Schaeffler A, Gross P, Buettner R, et al. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 2009; 126:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong SW, Kwon MJ, Choi AM, et al. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem 2009; 284:27384–27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.