Abstract
Genetic manipulation is an essential tool to investigate complex microbiological phenomena. In this chapter we describe the techniques required to transform the model hyperthermophilic, anaerobic archaeon Thermococcus kodakarensis. T. kodakarensis can support two modes of genetic manipulation, dependent either on homologous recombination into the genome or through retention of autonomously replicating plasmids. The robust genetic system developed in T. kodakarensis offers a variety of selectable and counterselectable markers for complex, accurate and iterative genetic manipulations offering greater flexibility to probe gene function in vivo.
Keywords: Transformation, Archaea, Hyperthermophile, Genetics, Anaerobic, Thermococcus
1. Introduction
Many Archaea can survive and often thrive in the environmental extremes, ranging from saturating salinities to high temperatures within hot springs and near marine vents [1–7]. Archaeal systems are increasingly employed for production of specialized and commodity bio-products, bioremediation efforts, and archaeal enzymes that function at the extremes of pH, temperature, and salinity are often used in molecular biology and biotechnological applications [5, 8–15]. Efforts to understand and take advantage of the unique characteristics of archaeal organisms demand a reliable means of genetic manipulation for rational and iterative strain construction.
Thermococcus kodakarensis is a naturally competent archaeal organism that thrives in an anaerobic, hyperthermophilic (85 °C optimal growth temperature) environment [16–19]. The genetic system for T. kodakarensis has been continuously refined through the combined efforts of multiple laboratories and these foundational and improved techniques are now regularly employed for many euryarchaeal species [20–26]. Initial efforts focused on homologous-recombination dependent manipulation of genomic sequences, with systems maturing to allow repetitive and markerless alterations. The absence of naturally occurring autonomously replicating plasmids leads to the development of novel shuttle vectors that provide a complementary method to alter the genotypes and phenotype of T. kodakarensis.
Although multiple strains of T. kodakarensis have been used as parental strains, the genetic system described here is optimized for, and reliant on strain TS559 (ΔTK2276; ΔTK0254∷TK2276; ΔTK0149; ΔTK0664) [23], which is an agmatine and tryptophan auxotroph strain of T. kodakarensis. We describe two techniques to manipulate TS559. The first is based on integration and subsequent excision of nonreplicative plasmids into the genome of TS559 to restore the agmatine biosynthesis pathway via reintroduction of TK0149, encoding a pyruvoyl-dependent arginine decarboxylase, and counterselective pressures afforded by TK0664, a hypoxanthine guanine phosphoribosyltransferase [23]. The second transformation procedure uses a shuttle vector system that restores the tryptophan biosynthesis pathway via reintroduction of TK0254, which encodes for the large subunit of anthranilate synthase, and expresses PF1848 (a hydroxymethylglutaryl coenzyme A reductase gene from Pyrococcus furiosus) providing resistance to statin-based antibiotics.
2. Materials
2.1. Microbial Culture
All solutions are sterilized by autoclaving unless noted otherwise. Solutions are made with water purified to 18 MΩ resistance. All solutions are adjusted to their final volume after the addition of dry ingredients.
2.1.1. Escherichia coli Media
Luria–Bertani (Ec-LB-Amp) liquid media (1 L): 10 g tryptone, 10 g NaCl, 5 g yeast extract, 100 μg/mL ampicillin.
LB solid media (Ec-LB-Amp plates): 10 g tryptone, 10 g NaCl, 5 g yeast extract, 20 g agar, 100 μg/mL ampicillin.
100 mg/mL ampicillin.
2.1.2. Thermococcus kodakarensis Media
KOD Vitamins (see Notes 1 and 6) (1000×) (1 L): 0.2 g niacin, 0.08 g biotin, 0.2 g pantothenate, 0.2 g lipoic acid, 0.08 g folic acid, 0.2 g thiamine, 0.2 g riboflavin, 0.2 g pyridoxine, 0.2 g cobalamin.
1 M Agmatine sulfate (1000×).
Wolfe’s Trace Minerals (200×) (1 L): 0.5 g MnSO4·H2O, 1 g CoCl2·6H2O, 0.1 g ZnSO4·7H2O, 0.01 g CuSO4·5H2O, 0.01 g AlK(SO4)2·12H2O, 0.01 g H3BO3, 0.01 g Na2MoO4·2H2O.
Artificial Sea Water (ASW)-YT (1 L): 5 g tryptone (see Note 2), 5 g yeast extract (see Note 3), 1× Wolfe’s trace mineral solution, 1× KOD vitamins, 20 g NaCl, 3 g MgCl2·6H2O, 6 g MgSO4·7H2O, 1 g (NH4)2SO4, 0.2 g NaHCO3, 0.3 g CaCl2·2H2O, 0.5 g KCl, 0.420 g KH2PO4, 0.050 g NaBr, 0.020 g SrCl2·6H2O, 0.010 g Fe(NH4)2(SO4)2·6H2O.
Elemental Sulfur (flowers of sulfur; powdered).
2× ASW (see Note 4) (1 L): 40 g NaCl, 6 g MgCl2·6H2O, 12 g MgSO4·7H2O, 2 g (NH4)2SO4, 0.4 g NaHCO3, 0.6 g CaCl2·2H2O, 1 g KCl, 0.840 g KH2PO4, 0.100 g NaBr, 0.040 g SrCl2·6H2O, 0.020 mg Fe(NH4)2(SO4)2·6H2O.
Gelzan™ CM.
Polysulfides solution (500×) (see Note 5) (15 mL): 10 g Na2S·9H2O, 3 g sulfur.
0.8× ASW (1 L): 16 g NaCl, 2.4 g MgCl2·6H2O, 4.8 g MgSO4·7H2O, 0.800 × g (NH4)2SO4, 0.160 g NaHCO3, 0.240 g CaCl2·2H2O, 0.400 g KCl, 0.336 g KH2PO4, 0.040 g NaBr, 0.016 g SrCl2·6H2O, 0.008 mg Fe (NH4)2(SO4)2·6H2O.
20 amino acid solution (20×) (see Note 6) (200 mL): 1 g cysteine, 1 g glutamic acid, 1 g glycine, 0.500 g arginine, 0.500 g proline, 0.400 g asparagine, 0.400 g histidine, 0.400 g isoleucine, 0.400 g leucine, 0.400 g lysine, 0.400 g threonine, 0.400 g tyrosine, 0.300 g alanine, 0.300 g methionine, 0.300 g phenylalanine, 0.300 g serine, 0.300 g tryptophan, 0.200 g aspartic acid, 0.200 g glutamine, 0.200 g valine.
19 amino acid solution lacking tryptophan (20×) (see Note 6) (200 mL): 1 g cysteine, 1 g glutamic acid, 0.500 g arginine, 0.500 g proline, 0.400 g asparagine, 0.400 g histidine, 0.400 g isoleucine, 0.400 g leucine, 0.400 g lysine, 0.400 g threonine, 0.400 g tyrosine, 0.300 g alanine, 0.300 g methionine, 0.300 g phenylalanine, 0.300 g serine, 0.200 g aspartic acid, 0.200 g glutamine, 0.200 g valine.
100 μM 6-methylpurine
25 mM mevinolin
T. kodakarensis solid complete media (Tk-ASW-YT): 0.5 g yeast extract, 0.5 g tryptone, 1.0 g Gelzan™, 500 μL Wolfe’s Trace minerals (200×), 200 μL polysulfide solution (500×), 5.0 mL 20 amino acid mixture (20×), 100 μL KOD vitamins (1000×), 50 mL H2O, 50 mL 2× ASW.
T. kodakarensis solid minimal media (Tk-ASW-min): 500 μL Wolfe’s trace minerals (200×), 1.0 g Gelzan™, 50 mL H2O, 50 mL 2× ASW, 200 μL polysulfide solution (500×), 100 μL KOD vitamins (1000×), 5.0 mL 20 amino acid mixture (20×).
T. kodakarensis solid minimal 19 amino acid media (TK-ASW--min-(−Trp)): 500 μL Wolfe’s trace minerals (200×), 1.0 g Gelzan™, 50 mL H2O, 50 mL 2× ASW, 200 μL polysulfide solution (500×), 100 μL KOD vitamins (1000×), 5.0 mL 19 amino acid mixture (20×).
T. kodakarensis solid minimal mevinolin media (TK-ASW-min-mev): 500 μL Wolfe’s trace minerals (200×), 1.0 Gelzan™, 50 mL H2O, 50 mL 2× ASW, 200 μL polysulfide solution (500×), 100 μL KOD vitamins (1000×), 12.5 μM mevinolin, 5.0 mL 20 amino acid mixture (20×).
2.1.3. Equipment
1 mL syringe.
Microcentrifuge.
125 mL serum bottles.
Large aluminum pot with loose-fitting lid.
Autoclave.
Anaerobic chamber (Coy Labs).
10% hydrogen, 90% nitrogen (v/v) compressed gas mix.
100% nitrogen compressed gas.
Glass Petri plates (see Note 7).
Plastic Petri plates (see Note 8).
Floor model centrifuge and compatible rotor(s).
Thermal cycler.
GasPak EZ Anaerobe Container System.
Pipettes.
Enzyme Cooler, Isotherm System.
Forced air incubator (37 °C and 85 °C).
Dry block heater.
Anaerobic canister.
1.7 mL microcentrifuge tubes.
0.2 mL PCR tubes.
Two-leg lyophilization serum bottle stoppers.
20 mm aluminum seals.
20 mm Crimper, Standard Seal.
20 mm Decapper.
Polycarbonate centrifuge tubes.
Cell spreader.
10 mL serum bottles
Face shields, lab coats, nitrile gloves, and autoclave gloves.
Paper towels.
Nonreplicative plasmid pTS700 (see Note 9).
Autonomously replicating plasmid pLC70 (see Note 9).
ZR Plasmid Miniprep™ Classic Kit.
Qubit™ ds DNA BR Assay Kit.
NucleoSpin Gel and PCR Clean-up, Mini kit.
In-Fusion® Snap Assembly.
Agilent QuikChange-II Kit.
3. Methods
3.1. Microbial Methods
T. kodakarensis transformation techniques are reliant on plasmid DNAs isolated from Escherichia coli. Standard growth and DNA preparations from E. coli yield plasmid DNA of sufficient quality for successful transformations of T. kodakarensis. Details are provided to generate (1) nonreplicative plasmids, based on pTS700, for genomic modifications and (2) autonomously replicating plasmids, based on pLC70, for ectopic expression, in Subheadings 3.2.1 and 3.2.3, respectively. The choice of E. coli strain has minimal impacts on successful plasmid isolations.
3.1.1. E. Coli Media and Cultivation
E. coli LB medium containing ampicillin: Ec-LB-Amp
Prepare Ec-LB-Amp and dispense 5 mL aliquots to sterile culture tubes.
Inoculate cultures with plasmid containing E. coli strains, incubate at 37 °C for 12–16 h with agitation (~200 rpm).
E. coli LB plate medium with ampicillin: Ec-LB-Amp plate
3.1.2. Thermococcus kodakarensis Media and Cultivation
T. kodakarensis strains are typically grown in 100 mL aliquots in nutrient-rich media (ASW-YT) (see Note 12) to provide large numbers of cells for transformations. Strain TS559 must be supplemented with agmatine even when grown in nutrient-rich media. Smaller (~3–5 mL) cultures of T. kodakarensis, prepared with the same media formulations, suffice to yield sufficient DNA from transformants for use in diagnostic PCR to confirm the genotypes of transformed cells.
Prepare T. kodakarensis complete liquid medium by combining ASW-YT and Wolfe’s Trace Minerals inside an anaerobic chamber (see Note 13). Aliquot 100 mL of mineral-supplemented ASW-YT media into 125 mL serum bottles, then seal with stoppers and aluminum seals, autoclave (see Note 14), and store sterile media at room temperature.
Immediately prior to inoculation with T. kodakarensis cultures, and inside an anaerobic chamber, add 0.2 g of elemental sulfur and 100 μL KOD vitamins (1000×). When appropriate, add 100 μL 1 M agmatine sulfate solution (1000×).
Using a syringe, withdraw 1 mL of an active culture of TS559 (or other T. kodakarensis strain), inject the inoculum into the now completely supplemented media, seal the culture with a stopper and aluminum seal (see Note 15), and incubate at 85 °C without agitation for ~13 h (cultures entering stationary phase yield the highest percentage of transformants).
Thermococcus kodakarensis complete solid medium: Tk-ASW-YT plate.
Clonal populations of transformants from the transformations must be selected on solid media. Special protocols are necessary to generate solid media that remains solid at 85 °C; solid media is prepared from two independently autoclaved solutions that are mixed and rapidly distributed (~20–30 s) into glass Petri plates before setting at room temperature. Depending on the transformation procedure and selective pressures applied, transformed cells are typically plated on nutrient-rich solid media lacking agmatine supplementation (for genomic modifications) or minimal, 19 amino acid-based solid media containing agmatine supplementation (for ectopic modifications).
Nutrient-rich T. kodakarensis solid media: Tk-ASW-YT plate
Inside an anaerobic chamber, combine 50 mL 2× ASW, 0.5 mL Wolfe’s Trace Minerals (200×), 0.5 g tryptone, and 0.5 g yeast extract in a 125 mL serum bottle. Seal the bottle with a stopper and aluminum seal, then sterilize via autoclaving.
Prepare a second 125 mL serum bottle by mixing 50 mL water and 1 g Gelzan™ CM. Seal the bottle with a stopper and aluminum seal, then sterilize via autoclaving.
Immediately after autoclaving, bring both halves of the media formulations inside the anaerobic chamber. Remove stoppers and aluminum seals from both bottles. To the bottle containing 2× ASW add 100 μL KOD vitamins (1000×) and 200 μL polysulfides (500×) (see Note 16), then immediately combine with the bottle containing the dissolved Gelzan (see Note 17). Swirl to mix then aliquot the contents into 4 glass Petri plates (~25 mL/plate). Plates will solidify within seconds and can be used immediately or stored inverted, within the anaerobic chamber at room temperature for 1–2 days (see Note 18).
Thermococcus kodakarensis minimal 20 amino acid solid medium: Tk-ASW-min plate
Inside an anaerobic chamber, combine 50 mL 2× ASW and 0.5 mL Wolfe’s Trace Minerals (200×) a in 125 mL serum bottle. Seal the bottle with a stopper and aluminum seal, then sterilize via autoclaving.
Prepare a second 125 mL serum bottle by mixing 50 mL water and 1 g Gelzan™ CM. Seal the bottle with a stopper and aluminum seal, then sterilize via autoclaving.
Immediately after autoclaving, bring both halves of the media formulations inside the anaerobic chamber. Remove stoppers and aluminum seals from both bottles. To the bottle containing 2× ASW add 100 μL KOD vitamins (1000×), 100 μL 1 M agmatine sulfate solution (1000×), 5 mL 20 amino acid solution (20×), 100 μL 100 μM 6-Methylpurine or 50 μL 25 mM mevinolin and 200 μL polysulfides (500×), then immediately combine with the bottle containing the dissolved Gelzan. Swirl to mix, then aliquot the contents into 4 glass Petri plates (~25 mL/plate). Plates will solidify within seconds and can be used immediately or stored inverted, within the anaerobic chamber at room temperature for 1–2 days (see Note 18).
Thermococcus kodakarensis minimal 19 amino acid solid medium lacking tryptophan: Tk-ASW-min-(−Trp) plate
Inside an anaerobic chamber, combine 50 mL 2× ASW and 0.5 mL Wolfe’s Trace Minerals (200×) a in 125 mL serum bottle. Seal the bottle with a stopper and aluminum seal, then sterilize via autoclaving.
Prepare a second 125 mL serum bottle by mixing 50 mL water and 1 g Gelzan™ CM. Seal the bottle with a stopper and aluminum seal, then sterilize via autoclaving.
Immediately after autoclaving, bring both halves of the media formulations inside the anaerobic chamber. Remove stoppers and aluminum seals from both bottles. To the bottle containing 2× ASW add 100 μL KOD vitamins (1000×), 100 μL 1 Magmatine sulfate solution (1000×), 5 mL 19 amino acid solution (20×), 100 μL 100 μM 6-Methylpurine and 200 μL polysulfides (500×), then immediately combine with the bottle containing the dissolved Gelzan. Swirl to mix then aliquot the contents into 4 glass Petri plates (~25 mL/plate). Plates will solidify within seconds and can be used immediately or stored inverted, within the anaerobic chamber at room temperature for 1–2 days (see Note 18).
3.2. Design and Construction of Plasmid DNAs Used to Transform Thermococcus kodakarensis
We first detail the steps to generate pTS700-based plasmids that permit desired modifications to the genome of TS559 (see Figs. 1, 2, and 4). pTS700 contains a unique SwaI cutsite that permits linearization and insertion of amplicons (see Fig. 1a). Given that many genomic targets are ultimately targeted both for deletion and modification, it is practical to generate a common plasmid (termed an “A”-plasmid; see Fig. 1) from which two unique plasmids can be generated, one for deletion of the target locus (termed a “B”--plasmid; see Fig. 2b) and another for modification of the target locus (termed either a “C”- or “D”-plasmid; see Fig. 2c, d). Standard plasmid preparations of “B”-, “C”-, and “D”-plasmids from most E. coli strains yield high-quality constructs that can be transformed into TS559 (see Fig. 4) to first generate transformants with intermediate genomes that can be resolved to generate the desired final genomic modifications (see Fig. 4b–d).
Fig. 1.
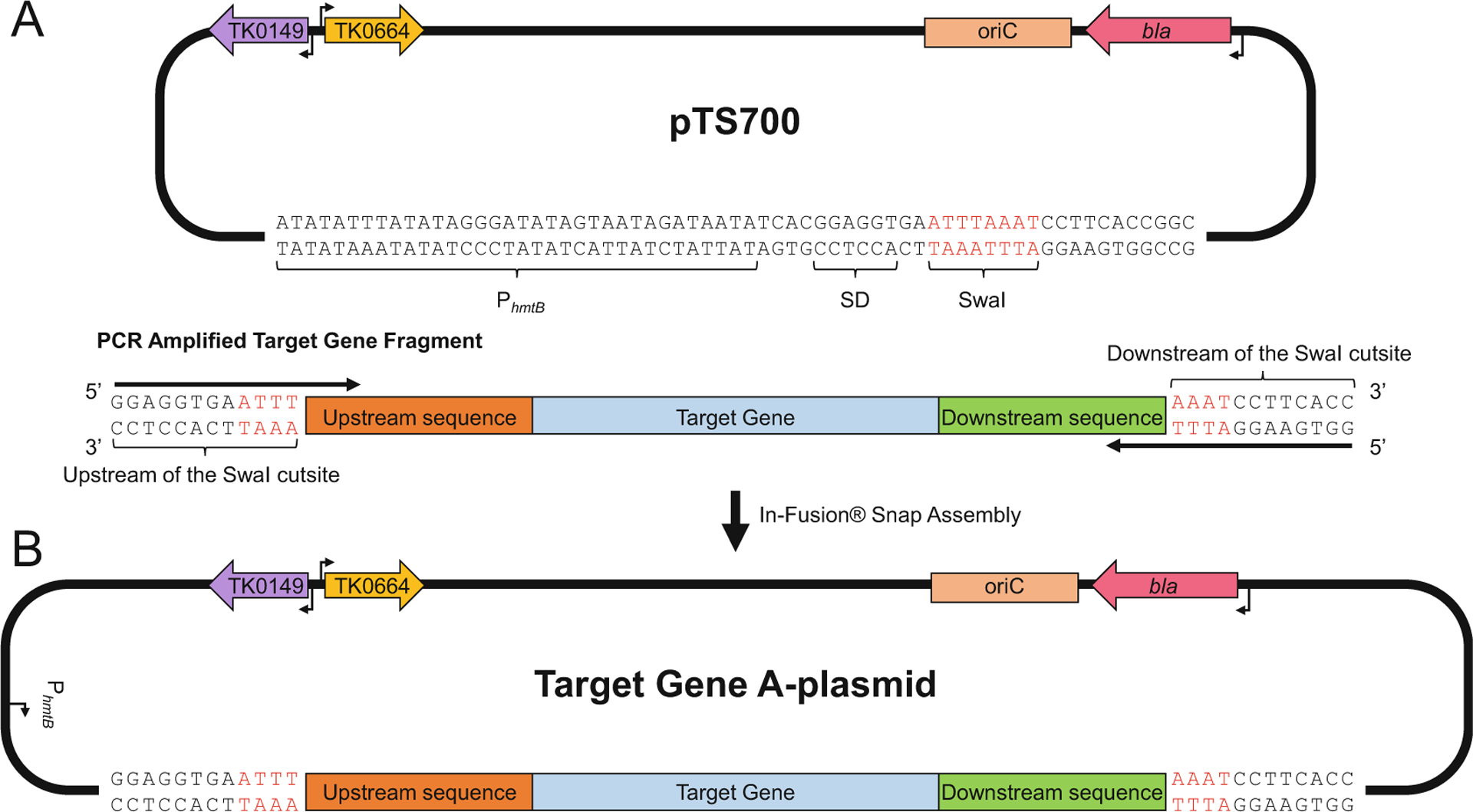
Generating an A-plasmid for the targeted T. kodakarensis genomic locus. (a, top) The pTS700 plasmid encoding TK0149, an agmatine prototrophic selectable marker (purple left arrow), TK0664, a hypoxanthineguanine-phosphoribosyltransferase, that serves as a 6-methylpurine sensitive counter selectable marker (orange right arrow), oriC, bacterial plasmid origin of replication (salmon box), and β-lactamase (bla), ampicillin resistance gene (pink left arrow). The strong constitutive archaeal promoter (PhmtB) and the Shine-Dalgarno sequence (SD) are included to promote expression in instances where recombination of the plasmid into the genome of T. kodakarensis displaces genes from their native promoters. (a, bottom) The PCR amplified target gene fragment contains 12 bp sequences complementary to both the upstream and downstream regions of the SwaI cutsite in pTS700. The amplicon contains ~700 bp of upstream sequences (red box), ~700 bp of downstream sequences (green box), and the target gene sequence (blue box). (b) In-Fusion cloning results in production of a complete A-plasmid for the target gene
Fig. 2.
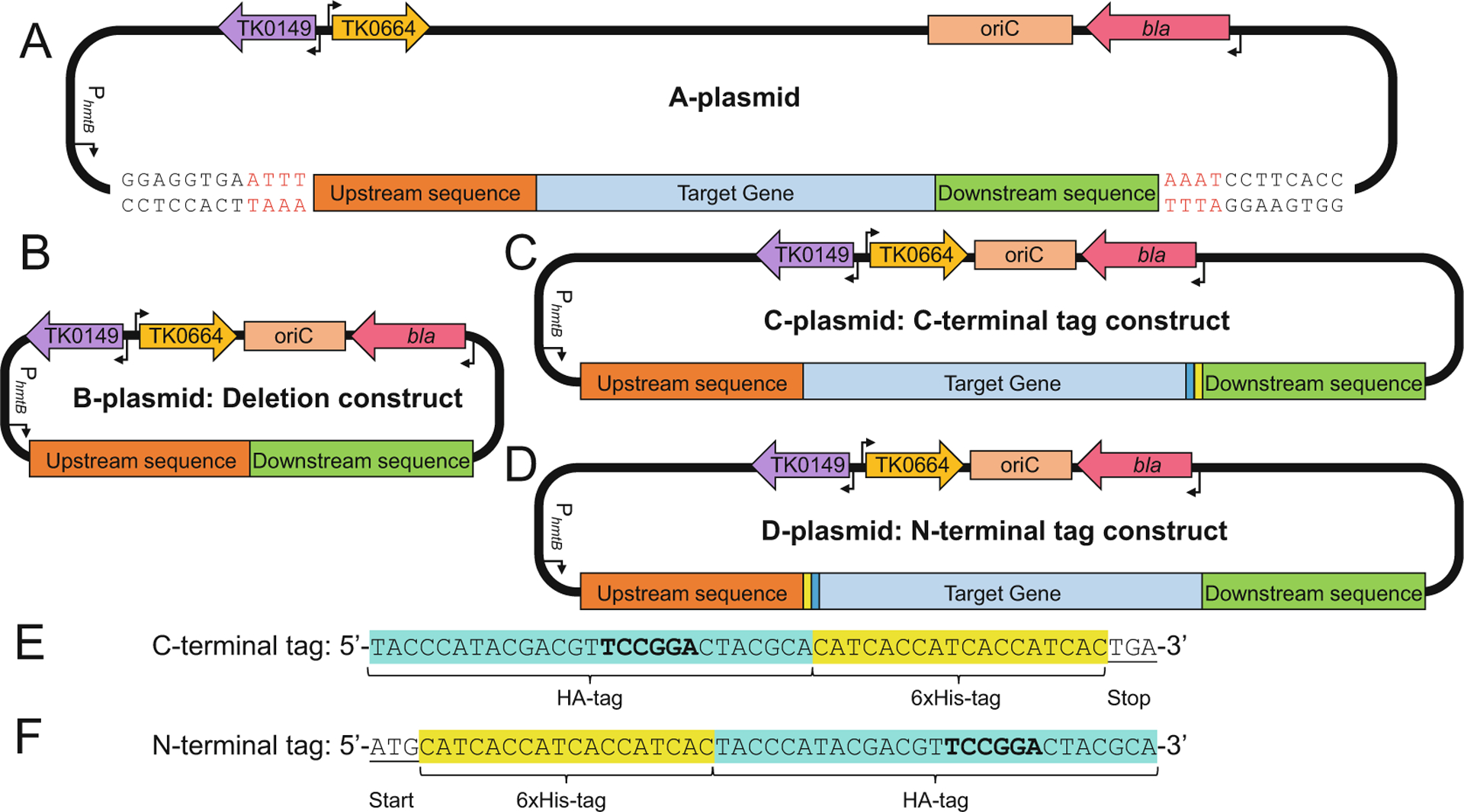
Design of integrative vectors to delete or modify genomic targets in Thermococcus kodakarensis. (a) The parental A-plasmid containing ~700 bp of upstream sequence (red box), the target gene (blue box), and ~700 bp of downstream sequence (green box). (b) B-plasmids are used to generate deletion strains of T. kodakarensis. The B-plasmid is generated from the A-plasmid by deleting the sequences encoding the target gene while retaining the upstream- and downstream-sequences. (c) & (d) The C- and D-plasmids are used to generate strains of T. kodakarensis wherein the genomic target locus is extended to encode for HA-and 6xHis-tags. The C-plasmid is generated from the A-plasmid by the inclusion of sequences encoding the HA-tag (cyan box) followed by the 6xHis-tag (yellow box) before the stop codon. The D-plasmid is generated from the A-plasmid by the inclusion of sequences encoding the 6xHis-tag (yellow box) followed by the HA-tag (cyan box) after the start codon. (e) & (f) The nucleotide sequences and positions of the C- and N-terminal tags highlighting the introduced BspEI sites (bold)
Fig. 4.
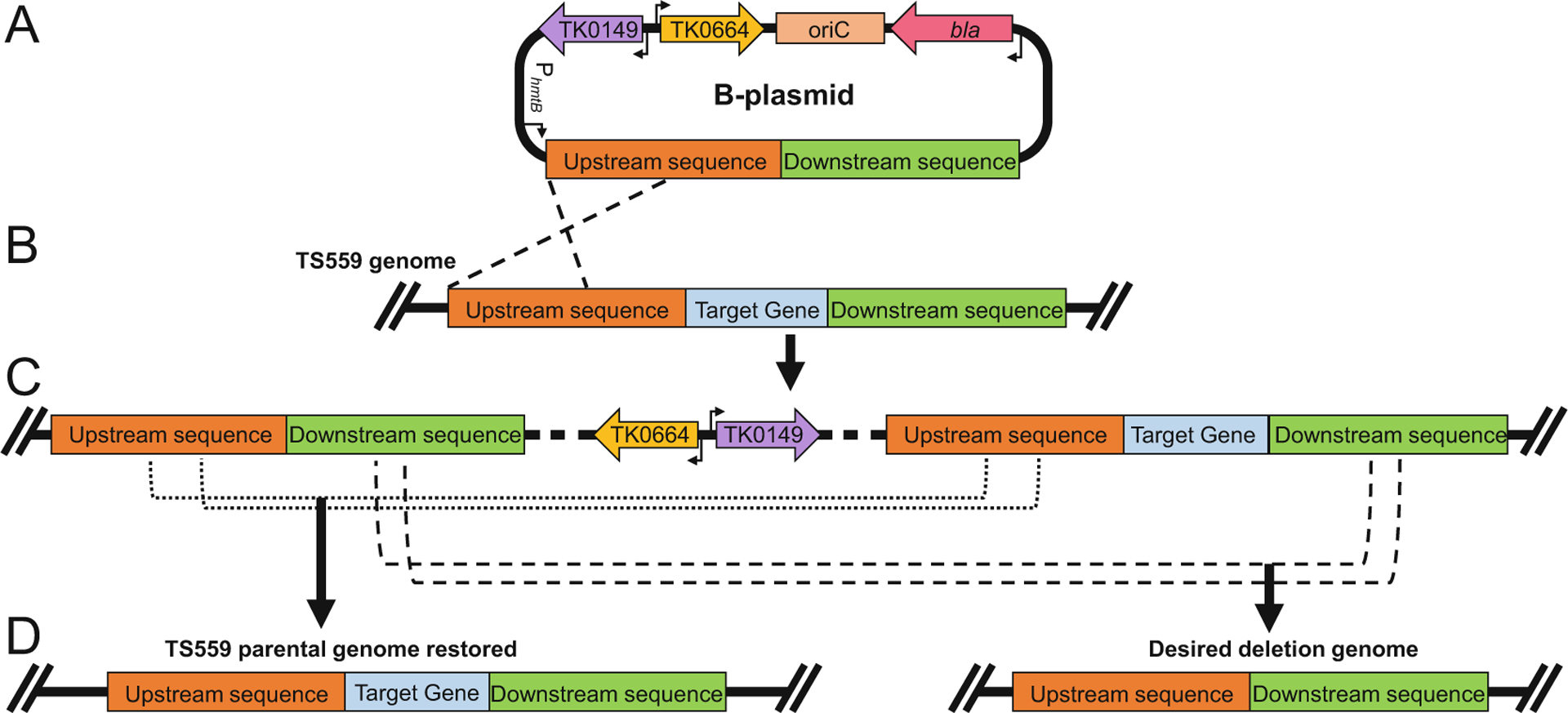
Example of target gene locus modification in Thermococcus kodakarensis using a B-plasmid to generate a deletion strain. (a) The sequence-confirmed B-plasmid of the target gene is transformed into the parental strain (TS559) and integrates into the chromosome via homologous recombination. (b) Homologous recombination (black dashed lines) of the B-plasmid into the parental (TS559) genome integrates the entire B-plasmid into the genome, resulting in restoration of agmatine prototrophy and introduction of 6-methylpurine sensitivity. Recombination through upstream sequences (red boxes) is shown, however it is equally probable that the B-plasmid will recombine with the genome via homologous recombination through downstream sequences (green boxes). (c) The genotype of the resulting intermediate strain is shown, highlighting the duplication of upstream- and downstream-sequences that ultimately permit the spontaneous excision of the integrated plasmid sequences. Transformants containing the intermediate genome are selected based on agmatine prototrophy. Intermediate strains whose genotype is confirmed by diagnostic PCR are grown with agmatine supplementation to permit for spontaneous excision of the B-plasmid sequence, then plated on media containing 6-methylpurine to select for transformants that have lost TK0664. (d) Excision of the B-plasmid from the genome can result in two potential homologous recombination events: upstream recombination (black dotted line) resulting in the restoration of the parental (TS559) genome, or downstream recombination (black dashed line) resulting in the desired deletion of the target locus in the T. kodakarensis genome. 6-methylpurine resistant strains are picked to liquid medium (ASW-YT + Agm) and their genotypes are confirmed via diagnostic PCR or whole genome sequencing
Following the use of pTS700-based vectors for genomic modifications, we then detail the use of autonomously replicating plasmids, based on the pLC70 vector, to generate transformants wherein selectable phenotypes are based on retention of and expression from ectopic vectors (see Fig. 3).
Fig. 3.
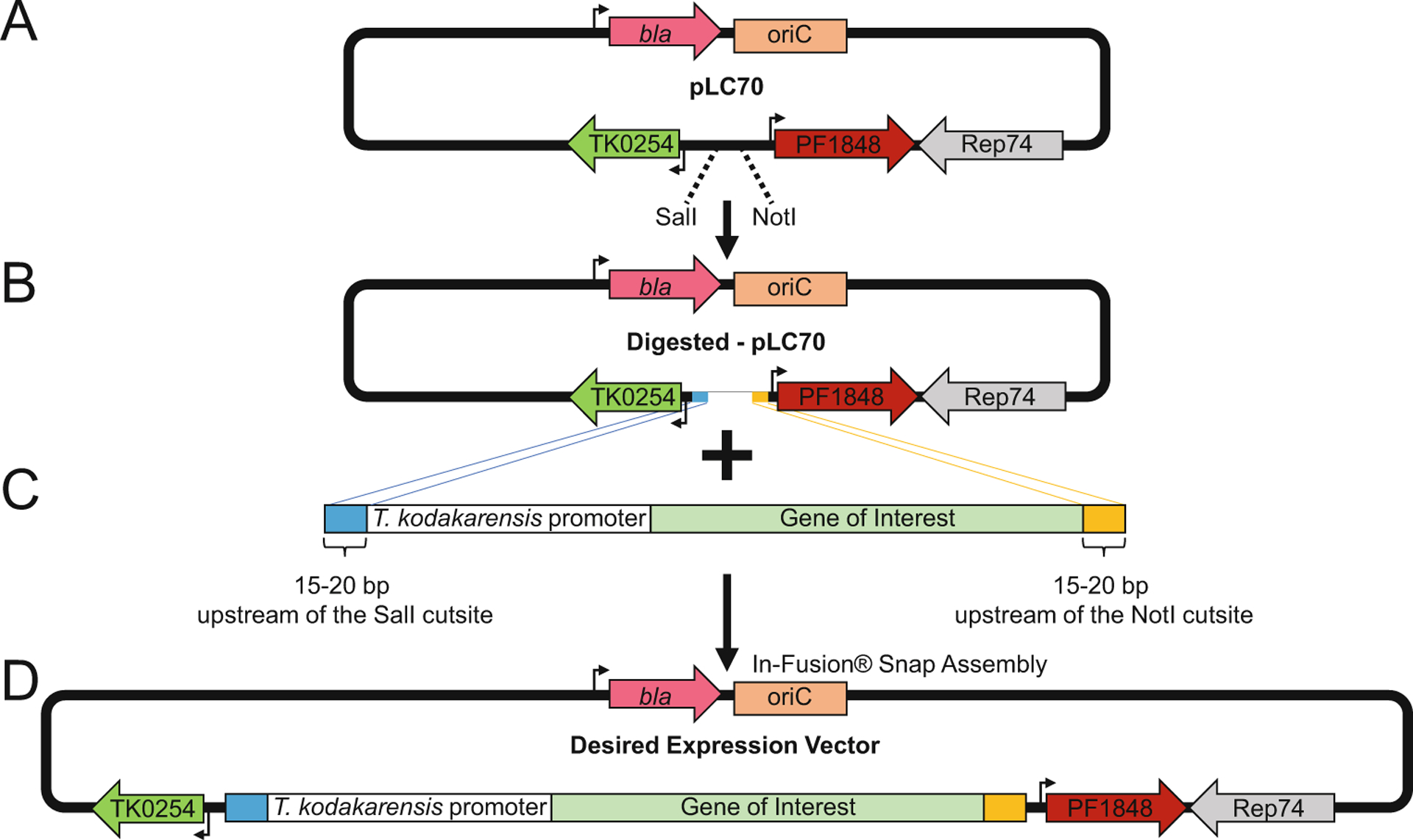
The autonomously replicating pLC70 vector permits ectopic gene expression in Thermococcus kodakarensis. (a) The pLC70 vector contains the β-lactamase (bla) ampicillin resistance gene (pink left arrow), the bacterial origin of replication, oriC (salmon box), the archaeal DNA replication protein Rep74 (gray left arrow), the Pyrococcus furiosus PF1848 gene conferring resistance to statin-based antibiotics (red right arrow), and the TK0254 gene which provides a tryptophan prototrophic selectable marker (green left arrow). (b) The pLC70 vector is linearized using SalI and NotI endonucleases to accept expression cassettes. (c) An expression cassette composed of the sequence for a T. kodakarensis promoter (white box), sequences for the target gene of interest (sage box), and terminal flanking sequences homologous to regions near the SalI cutsite (blue box) and the NotI cutsite (orange box) for insertion into the linearized pLC70 vector. (d) The complete vector with inserted expression cassette can be transformed into T. kodakarensis and selected based on restoration of tryptophan prototrophy and resistance to statin-based antibiotics
3.2.1. Generating Linearized pTS700 to Accept Amplicons for Genomic Modifications. Construction of “A”-Plasmids
Linearize ~2 μg of pTS700 plasmid using SwaI endonuclease, resolve the reactions via agarose gel electrophoresis, and purify the linear products using the NucleoSpin Gel extraction protocols (see Fig. 1a). Recovered products are quantified using the Qubit™ dsDNA BR Assay Kit. SwaI-linearized pTS700 can be prepared in bulk, purified and stored at −20 °C for use in construction of “A”-plasmids for multiple genomic targets (see Note 19).
Generate a PCR-amplicon, using T. kodakarensis genomic DNA as a template, containing the target gene with ~700 bp of upstream and downstream genomic sequences (see Fig. 1a). The primer complementary to sequences upstream of the target locus should begin with the sequence, 5′ GGAGGTGAATT (see Note 20) followed by ~25 nucleotides of complementarity to the genome. The primer complementary to sequences downstream of the target locus should begin with the sequence, 5′ GGTGAAGGATT (see Note 20) followed by ~25 nucleotides of complementarity to the genome. Resolve the reactions via agarose gel electrophoresis, and purify the linear products using the NucleoSpin Gel extraction protocols. Recovered products are quantified using the Qubit™ dsDNA BR Assay Kit.
Use In-Fusion® Snap Assembly to insert the target gene fragment into pTS700-SwaI in a 2:1 ratio (gene fragment: linearized vector) (see Fig. 1b). Transform 2.5 μL of the In-Fusion® Snap Assembly reaction into Stellar (see Note 21) competent cells and plate the cells on Ec-LB-Amp plate. Incubate at 37 °C overnight (12–14 h). Successful insertion of the target gene into pTS700 is typically confirmed by colony PCR using primers specific to pTS700 that flank the SwaI cutsite (see Note 22). Once colonies containing the presumptive “A”-plasmid are identified, they are grown in 5 mL Ec-LB-Amp overnight at 37 °C with shaking. 5 mL Ec-LB-Amp grown cultures are sufficient to yield sufficient plasmid DNAs first for sequencing and ultimately for transformation into T. kodakarensis.
Purify the target gene A-plasmid from the E. coli cells using ZR Plasmid Miniprep™—Classic and quantify the concentration of the plasmid using Qubit™ dsDNA BR Assay Kit. Store at −20 °C in a freezer. The full sequence of the amplicon should be confirmed via Sanger sequencing (or alternative sequencing techniques) prior to transformation into T. kodakarensis.
3.2.2. Generating “B”-and “C”-Plasmids from “A”-Plasmids
Mutagenic PCR for deletion of the target gene: generation of the B-plasmid (see Fig. 2a, b)
Design a primer pair that permit the deletion of the target gene sequence for use in QuikChange-II reactions. Primers typically are ~60 nt in length, with 30 nt of complementarity to sequences immediately upstream and downstream of the target locus. Use the primer pairs in a QuikChange-II reaction using the A-plasmid as the template DNA following manufacturer’s instructions, inclusive of the transformation into E. coli and plating of Ec-LB-Amp plates. Allow colonies to form during overnight incubation at 37 °C. As an alternative, the target gene sequence can be deleted via inverse PCR and ligation of the linear product.
The success of removing the target gene sequence can be evaluated via colony PCR as in Subheading 3.2.1, step 3. Once E. coli clones harboring presumptive B-plasmids are identified, growth of small cultures, plasmid recovery and sequencing should be completed as in Subheading 3.2.1, step 4. Plasmids can be stored at −20 °C for extended periods prior to transformation into T. kodakarensis.
Mutagenic PCR for tagging of the target gene: generation of C-plasmid and D-plasmids (see Fig. 2c–f).
C-plasmids are generated by addition of sequences that encode HA- and 6xHis-tags immediately prior to the stop codon of the target gene (see Fig. 2c, e). D-plasmids are generated by addition of sequences that encode HA- and 6xHis-tags immediately downstream of the start codon of the target gene (see Fig. 2d, f).
Design a primer pair for use with Quikchange-II reactions that permit the addition of the tag-encoding sequences to either the start or end of the target genes. Primers are typically ~95 nt in length, with 25 nt of complementarity immediately upstream and downstream of the insertion site (50 nt total) and an additional 45 nt encoding the 9 amino acid HA-tag and 6xHis- tags. Use the primer pairs in Quikchange-II reactions using the A-plasmid as the template DNA following manufacturer’s instructions, inclusive of transformation into E. coli and plating of Ec-LB-Amp plates. Allow colonies to form during overnight incubation at 37 °C.
The success of adding sequences to extend the target gene at the 5′ or 3′ end can be evaluated via colony PCR as in Subheading 3.2.1, step 3 using primer pairs that flank the site of the sequence insertion. Amplicons from desirable C- or D-plasmids harbored in E. coli transformants are ~45 bp longer than amplicons generated from A-plasmid that lack the tag-encoding sequences. Once E. coli clones harboring presumptive C- and D-plasmids are identified, growth of small cultures, plasmid recovery and sequencing should be completed as in Subheading 3.2.1, step 4. Plasmids can be stored at −20 °C for extended periods prior to transformation into T. kodakarensis. As an alternative method to identify successful addition of tag-encoding sequences, amplicons generated with primers that flank the site of insertion will generate a product that has a new BspEI endonuclease recognition site that will permit identification of C- and D-plasmids from A-plasmids following digestion of the amplicons with BspEI.
3.2.3. Generating pLC70-Based Expression Plasmids
pLC70 contains selectable markers to phenotypically select desired transformants (see Fig. 3a). Typically, pLC70 vectors are modified to express genes of interest by insertion of an expression cassette—a T. kodakarensis promoter and associated open reading frame—into pLC70 prior to transformation into T. kodakarensis strains (see Fig. 3b–d). The expression cassette (see Fig. 3c) can be constructed through various procedures but should ultimately be cloned into pLC70 taking advantage of unique SalI and NotI sites. Cloning procedures similar those detailed for amplicon insertion to pTS700 yield consistent results.
3.2.4. Thermococcus kodakarensis Strain Construction
Initial transformation to confirmation of final strains typically takes 2–3 weeks.
Grow strain TS559 in ASW-YT-Agm as directed in Subheading 3.1.2. Incubate at 85 °C for 12–13 h.
Harvest TS559 biomass by anaerobically transferring the culture into high-speed centrifuge tubes followed by centrifugation at ~18,500 × g for 10 min at 4 °C (see Note 23) Inside the anaerobic chamber, carefully decant the supernatant and keep the undisturbed pellet.
Directly in the anaerobic chamber, gently resuspend the TS559 cell pellet in 3 mL of ice-cold 0.8× ASW. ~200 μL of resuspended cells are typically used per transformation, and by preparing several aliquots, multiple transformations can be performed simultaneously. Aliquot 200 μL of the resuspended cells into 1.7 mL microcentrifuge tubes and incubate anaerobically on ice for 30 min (see Note 24).
Add ~2–4 μg of the transformation plasmid (either a pTS700-or pLC70-based plasmid) to the 200 μL of incubated cells and extend incubation anaerobically on ice for an additional 60 min.
Heat shock cells at 85 °C for 45 s in a dry heat block. Immediately chill on ice for 10 min following heat shock (see Note 25).
Carefully spread the now transformed cells to Tk-ASW-YT plates (for pTS700 based plasmids) or Tk-ASW-min-(−Trp) plates (for pLC70 based plasmids). Gently spread cells with a sterile cell spreader inside the anaerobic chambers. Once the transformation media has been absorbed into the plates, invert the plates and transfer to an air-tight anaerobic metal canister, packed with paper towels (see Note 26) and a Gas Pak EZ anaerobic container system packet. Seal the canister inside of the anaerobic chamber, then remove and incubate the sealed vessel in an 85 °C incubator for 48–96 h.
Return the sealed anaerobic vessel to the anaerobic chamber and remove the incubated plates, being careful to remove condensation. Identify colonies formed from successful transformations; T. kodakarensis colonies are nearly transparent and may be difficult to identify at first (see Note 27). Transformants from the transformation with pLC70-based plasmids are likely to contain the desired final genotype and phenotype but are typically replated on Tk-ASW-min-mev plates to ensure the transformants are resistant to mevinolin before being transferred to liquid media for experimental use (see Fig. 3). In contrast, colonies resultant from transformation with pTS700-based plasmids only contain an intermediate genome (see Fig. 4c) and require additional manipulations to generate the desired modified final genome.
Initial transformants from pTS700-based transformations that contain the intermediate genome wherein the entire pTS700-plasmid has integrated via homologous recombination into the TS559 genome must be picked from the plates and used to inoculate 3 mL Tk-ASW-YT liquid cultures containing agmatine in 5 mL serum bottles. Seal the serum bottles anaerobically using stoppers and aluminum seals, followed with an overnight (12–13 h) incubation at 85 °C. Growth in the presence of agmatine permits the spontaneous excision of the integrated vector (see Fig. 4d). Regardless of the nature of the excision event, the resulting genomes will lack both TK0149 and TK0664, rendering cells agmatine auxotroph that are resistant to 6-methylpurine. If the excision event captures the target gene, the resulting cells will also lack the target gene (for B-plasmid based transformations) or encode a modified target gene sequences (for C- and D-plasmid based transformations) (see Fig. 4d).
1 mL of overnight ASW-YT + Agm grown intermediate cultures should be anaerobically harvested, concentrated via centrifugation five-fold, then plated on Tk-ASW-min plates containing 6-methylpurine. Gently spread cells with a sterile cell spreader inside the anaerobic chambers. Invert plates with transformants, transfer to an air-tight anaerobic metal canister, packed with paper towels (see Note 26) and a Gas Pak EZ anaerobic container system packet. Seal the canister inside the anaerobic chamber, then remove and incubate the sealed vessel in an 85 °C incubator for 48–96 h.
Return the sealed anaerobic vessel to the anaerobic chamber and remove the incubated plates, being careful to remove condensation. Identify colonies formed from successful excision events from the genome. Colonies must be picked from the plates and used to inoculate 3 mL Tk-ASW-YT liquid cultures containing agmatine in 5 mL serum bottles. Seal the serum bottles anaerobically using stoppers and aluminum seals, followed with an overnight (12–13 h) incubation at 85 °C.
1 mL of overnight cultures is anaerobically removed to prepare genomic DNA for diagnostic PCRs and sequencing to identify strains wherein the excision event resulted in generation of the desired genotype. Sequencing of PCR amplicons provides nucleotide level confidence of the desired modifications. We also recommend whole genome sequencing of all strains to ensure that spontaneous modifications were not accidentally introduced elsewhere in the genome.
4. Notes
This solution is light-sensitive and should be protected by either wrapping a transparent tube in aluminum foil, or by storing in an opaque, amber conical tube.
T. kodakarensis requires casein peptone that is enzymatically digested using pancreatic enzymes. Other sources of tryptone are suitable for E. coli media.
AMRESCO, catalog number: J850; For E. coli media, any yeast extract is suitable, however T. kodakarensis requires this source of yeast extract.
2× ASW can be stored outside of the anaerobic chamber.
Dissolve solution using heat, it will be a deep red color when both sulfur and sodium sulfide are completely dissolved.
Autoclaving will degrade this solution.
Plastic Petri plates may melt at T. kodakarensis growth temperatures (85 °C), requiring the use of glass Petri plates.
Plastic Petri plates will not melt at E. coli growth temperature (37 °C).
Please contact corresponding author to obtain plasmid. Details of the plasmid sequences can be found at references [16, 18, 26].
Ampicillin is unstable at temperatures above 50 °C, do not add before autoclaving.
There must be enough media to cover the bottom of the plate.
Water used in the preparation of ASW-YT should be boiled before it is combined with dry ingredients. Boiling releases dissolved oxygen.
ASW-YT must be prepared anaerobically.
Autoclave liquid cycle, 30 min sterilization time.
T. kodakarensis released H2S and H2 gasses as part of its metabolism. Using a stopper alone to seal the bottle is inadequate, as the gasses produced will push the stopper out of the bottle.
Polysulfides replace elemental sulfur in solid T. kodakarensis media.
Most selective agents for solid media should be added to the ASW solution after autoclaving. Supplemented ASW-based solutions should then be poured into the autoclaved Gelzan solution before pouring plates. This order of combination ensures homogeneous solid media.
For pouring multiple sets of plates at once, keep autoclaved solutions on a hot plate to prevent premature solidification.
SwaI endonuclease creates a blunt cutsite, reducing the likelihood of the linearized vector reannealing to itself, allowing for stable long-term storage.
This sequence is homologous to regions near the SwaI cutsite.
This strain is the recommended cell line by Takara; Stellar cells do not contain a plasmid prior to transformation, increasing confidence in isolated products after transformation.
700 Forward and 700 Reverse are the primers typically used in this reaction. Further details can be found at [16, 18, 26].
Centrifuge bottles cannot be opened outside of the chamber. Ensure centrifuge bottles have a rubber seal in their cap to maintain anaerobicity. When transferring centrifuge bottles between the centrifuge and the anaerobic chamber, maintain the bottles in an inverted configuration to prevent the supernatant from washing over the pellet and reducing overall yield.
Excess cells cannot be stored for future transformations.
This step increases transformation efficiency.
Packing the anaerobic canister with paper towels reduces shifting of the canister’s contents when being moved, reducing the risk of breaking a glass Petri plate and compromising cell cultures. Additionally, condensation will build up in the canister during incubation, and paper towels serve to absorb some moisture in the canister.
T. kodakarensis colonies are transparent puncta on the surface of media; most colonies are no larger than 1 mm in diameter and appear similar to bubbles on the surface. Occasionally, salt precipitates from media will look like T. kodakarensis colonies on solid media.
Acknowledgments
We thank members of the Santangelo lab for critical reviews and improvements to the manuscript.
Funding
This work was supported with funding (to TJS) from the National Science Foundation, grant EF-2022065, the US Department of Energy, grant DE-SC0014597, the USA National Institutes of Health, GM100329 and from the USA National Aeronautics and Space Administration, Exobiology Program, 80NSSC20K0613.
References
- 1.Martínez-Espinosa RM (2020) Microorganisms and their metabolic capabilities in the context of the biogeochemical nitrogen cycle at extreme environments. Int J Mol Sci 21:4228. 10.3390/ijms21124228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quehenberger J, Shen L, Albers S-V, Siebers B, Spadiut O (2017) Sulfolobus—a potential key organism in future biotechnology. Front Microbiol 8:2474. 10.3389/fmicb.2017.02474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belilla J, Moreira D, Jardillier L, Reboul G, Benzerara K, López-García JM, Bertolino P, López-Archilla AI, López-García P (2019) Hyperdiverse archaea near life limits at the polyextreme geothermal Dallol area. Nat Ecol Evol 3:1552–1561. 10.1038/s41559-019-1005-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer F, Müller V (2014) Adaptations of anaerobic archaea to life under extreme energy limitation. FEMS Microbiol Rev 38:449–472. 10.1111/1574-6976.12043 [DOI] [PubMed] [Google Scholar]
- 5.Poli A, Finore I, Romano I, Gioiello A, Lama L, Nicolaus B (2017) Microbial diversity in extreme marine habitats and their biomolecules. Microorganisms 5:25. 10.3390/microorganisms5020025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tehei M, Zaccai G (2005) Adaptation to extreme environments: macromolecular dynamics in complex systems. Biochim Biophys Acta 1724:404–410 [DOI] [PubMed] [Google Scholar]
- 7.Efremov AK, Qu Y, Maruyama H, Lim CJ, Takeyasu K, Yan J (2015) Transcriptional repressor TrmBL2 from Thermococcus kodakarensis forms filamentous nucleoprotein structures and competes with histones for DNA binding in a salt- and DNA supercoiling-dependent manner. J Biol Chem 290:15770–15784. 10.1074/jbc.M114.626705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegazy GE, Abu-Serie MM, Abo-Elela GM, Ghozlan H, Sabry SA, Soliman NA, Abdel-Fattah YR (2020) In vitro dual (anticancer and antiviral) activity of the carotenoids produced by haloalkaliphilic archaeon Natrialba sp. M6. Sci Rep 10:5986. 10.1038/s41598-020-62663-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel AB, Shaikh S, Jain KR, Desai C, Madam-war D (2020) Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches. Front Microbiol 11:562813. 10.3389/fmicb.2020.562813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabrera MÁ, Blamey JM (2018) Biotechno-logical applications of archaeal enzymes from extreme environments. Biol Res 51:37. 10.1186/s40659-018-0186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crosby JR, Laemthong T, Lewis AM, Straub CT, Adams MW, Kelly RM (2019) Extreme thermophiles as emerging metabolic engineering platforms. Curr Opin Biotechnol 59:55–64. 10.1016/j.copbio.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 12.Straub CT, Counts JA, Nguyen DMN, Wu C-H, Zeldes BM, Crosby JR, Conway JM, Otten JK, Lipscomb GL, Schut GJ, Adams MWW, Kelly RM (2018) Biotechnology of extremely thermophilic archaea. FEMS Micro-biol Rev 42:543–578. 10.1093/femsre/fuy012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumorné K, Córdova DC, Astorga-Eló M, Renganathan P (2017) Extremozymes: a potential source for industrial applications. J Microbiol Biotechnol 27:649–659 [DOI] [PubMed] [Google Scholar]
- 14.Kanai T, Imanaka H, Nakajima A, Uwamori K, Omori Y, Fukui T, Atomi H, Imanaka T (2005) Continuous hydrogen production by the hyperthermophilic archaeon, Thermococcus kodakaraensis KOD1. J Biotechnol 116: 271–282. 10.1016/j.jbiotec.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 15.Atomi H, Sato T, Kanai T (2011) Application of hyperthermophiles and their enzymes. Curr Opin Biotechnol 22:618–626. 10.1016/j.copbio.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 16.Hileman TH, Santangelo TJ (2012) Genetics techniques for Thermococcus kodakarensis. Front Microbiol 3:195. 10.3389/fmicb.2012.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atomi H, Reeve J (2019) Microbe profile: Thermococcus kodakarensis: the modehyperthermophilic archaeon. Microbiol (United Kingdom) 165:1166–1168. 10.1099/mic.0.000839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gehring A, Sanders T, Santangelo TJ (2017) Markerless gene editing in the Hyperthermophilic archaeon Thermococcus kodakarensis. Bio Protoc 7:e2604. 10.21769/bioprotoc.2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukui T, Atomi H, Kanai T, Matsumi R, Fujiwara S, Imanaka T (2005) Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res 15:352–363. 10.1101/gr.3003105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato T, Fukui T, Atomi H, Imanaka T (2005) Improved and versatile transformation system allowing multiple genetic manipulations of the hyperthermophilic archaeon Thermococcus kodakaraensis. Appl Environ Microbiol 71: 3889–3899. 10.1128/AEM.71.7.3889-3899.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato T, Fukui T, Atomi H, Imanaka T (2003) Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J Bacteriol 185:210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumi R, Manabe K, Fukui T, Atomi H, Imanaka T (2007) Disruption of a sugar transporter gene cluster in a hyperthermophilic archaeon using a host-marker system based on antibiotic resistance. J Bacteriol 189:2683–2691. 10.1128/JB.01692-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santangelo TJ, Čuboňová L, Reeve JN (2010) Thermococcus kodakarensis genetics: Tk1827-encoded β-glycosidase, new positive-selection protocol, and targeted and repetitive deletion technology. Appl Environ Microbiol 76:1044–1052. 10.1128/AEM.02497-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farkas JA, Picking JW, Santangelo TJ (2013) Genetic techniques for the archaea. Annu Rev Genet 47:539–561 [DOI] [PubMed] [Google Scholar]
- 25.Catchpole R, Gorlas A, Oberto J, Forterre P (2018) A series of new E. coli–Thermococcus shuttle vectors compatible with previously existing vectors. Extremophiles 22:591–598. 10.1007/s00792-018-1019-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santangelo TJ, Čuboňová L, Reeve JN (2008) Shuttle vector expression in Thermococcus kodakaraensis: contributions of cis elements to protein synthesis in a hyperthermophilic archaeon. Appl Environ Microbiol 74:3099–3104. 10.1128/AEM.00305-08 [DOI] [PMC free article] [PubMed] [Google Scholar]


