Abstract
Introduction:
Immune checkpoint inhibitors (ICI), increasingly used cancer therapeutics, can cause off-target inflammatory effects called immune-related adverse events (irAEs), including ICI-induced inflammatory arthritis (ICI-induced IA) and polymyalgia rheumatica (ICI-induced PMR). There are no validated classification criteria or outcome measures for these conditions, and adaptation of treatment recommendations from corresponding rheumatic diseases may not be appropriate. We summarized clinical descriptors of ICI-induced IA and ICI-induced PMR and aggregated domains used for these conditions in order to inform the development of a core set of outcome domains.
Methods:
As the initial step of the core domain set generation process, we systemically searched Medline (Pubmed), EMBASE, Cochrane, and CINHL through March 2021 to identify all studies that provide both clinical descriptions and domains relevant to ICI-induced IA and ICI-induced PMR. Domains were mapped to core areas, such as pathophysiological manifestations, life impact, resource use, and longevity/survival, as suggested by the OMERACT 2.1 Filter.
Results:
We identified 69 publications, over a third of which utilized non-specific diagnoses of “arthritis,” “arthralgia,” and/or “PMR”. Other publications provided the number, the distribution and/or names of specific joints affected, while others labeled the irAE as the corresponding rheumatic disease, such as rheumatoid arthritis or spondyloarthritis. Most distinct domains mapped to the pathophysiology/manifestations core area (24 domains), such as signs/symptoms (13 domains), labs (6 domains), and imaging (5 domains), with harm domains of adverse effects from irAE treatment and fear of irAE treatment decreasing ICI efficacy. Forty-three publications also referenced irAE treatment and 35 subsequent response, as well as 32 tumor response.
Conclusion:
There is considerable heterogeneity in the domains used to clinically characterize ICI-induced IA and ICI-induced PMR. There were several domains mapped to the pathophysiologic manifestations core area, although several publications highlighted domains evenly distributed among the other core areas of life impact, longevity/survival and resource use.
Keywords: ICI-induced inflammatory arthritis, ICI-induced polymyalgia rheumatica, outcome measures, immune checkpoint inhibitor
1. Introduction
Immune checkpoint inhibitors (ICI) have been revolutionary for the treatment of cancer by enhancing T cell-mediated anti-tumor responses1. However, in doing so, they can cause off-target inflammation of various organ systems, which are termed immune-related adverse events (irAEs). Many irAEs resemble de novo autoimmune diseases, presenting like rheumatoid arthritis (RA) and spondyloarthritis2–4, or sicca syndrome similar to primary Sjogren’s syndrome5,6, although they differ at the pathological level with different cellular subtypes found in tissue6–8 and synovial fluid9. These rheumatic irAEs can be associated with significant morbidity, reduced quality of life, and may impact the ability of patients to continue their ICI therapy. Treatment of such an irAE often follows treatment recommendations for de novo rheumatic diseases, such as treating ICI-induced inflammatory arthritis (ICI-induced IA) as one would for RA. However, clinical characteristics and response to immunosuppression in ICI-induced IA are different than in RA, with the majority of ICI-induced IA patients having seronegative disease, higher prevalence of tenosynovitis, and often requiring higher corticosteroid doses10,11. Differences in outcomes between the two groups can also be different in terms of disease duration (sometimes shorter in ICI-IA) and longevity (most often shortened in ICI-IA)12. Thus, it is unclear if the domains and outcome measures used to evaluate disease and guide treatment of de novo rheumatic diseases, including RA, should be adapted to the treatment of irAE such as ICI-induced IA. Furthermore, the patient population used to derive the “treat-to-target” strategy in RA is vastly different than those who develop ICI-induced IA, as the latter are concurrently being treated for a malignancy. To conduct high quality observational studies or clinical trials, it is critical to have well-characterized disease definitions and identify relevant domains that should be measured longitudinally13. As a first step toward this end, we reviewed the literature to identify diagnostic features and domains that have been described for ICI-induced IA (including ICI-induced PMR), the most frequent rheumatic irAEs observed in ICI-treated patients. Therefore, in this study, we aimed to: 1) describe how ICI-induced IA and ICI-induced PMR have been categorized in the literature, and 2) identify relevant domains in ICI-induced IA/PMR that have been reported in prior clinical studies to inform the development of a core set of domains for future ICI-induced IA trials and observational studies.
2. Methods
As the initial step of the OMERACT core domain set generation process for eventual outcome measure and instrument selection13, our working group initiated a review of existing outcome domains used in ICI-induced IA and ICI-induced PMR. This scoping review was performed in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension for Scoping Review guidelines. The study protocol was pre-specified and registered in advance with the Open Science Framework (DOI 10.17605/OSF.IO/G68PE).
2.1. Eligibility criteria
We included studies describing adults (aged 18 years or older) with arthralgia, inflammatory arthritis, and/or polymyalgia rheumatica attributable to ICI that also reported at least one outcome domain. Examples of inflammatory arthritis included descriptions of rheumatoid arthritis, crystalline arthritis, spondyloarthritis, psoriatic arthritis, polymyalgia rheumatica and/or arthritis with inflammatory features (i.e., prolonged morning stiffness, joint swelling, gel effect, etc.). All published peer-reviewed, full-length, randomized-controlled trials and both prospective and retrospective observational studies, such as cohort studies, case-control studies, and cross-sectional studies involving patients with either ICI-induced IA and/or ICI-induced PMR were included. Given the relative novelty of this disease entity, we also included qualitative studies and case series of at least three patients, which we felt was an adequate number of patients to provide meaningful data for domain selection. We excluded patients with rheumatic irAEs that were not classified as ICI-induced IA/PMR PMR, as well as those with preexisting autoimmune diseases. We also excluded case reports or case series with <3 patients, editorials, guidelines, reviews without original patient data, oncologic clinical trials, preclinical animal studies, and pharmacovigilance studies.
2.2. Information Sources
To identify all available literature relevant to our aims, we performed a search in Medline (using PubMed), EMBASE, Cochrane and CINHL databases through an end date of March 19, 2021.
2.3. Search Strategy
The search protocol (see Supplementary Material for full protocol) was developed by the OMERACT (Outcome Measures in Rheumatology) irAE working group, which included clinicians, researchers, and representatives from OMERACT. Major search terms included “PD-1/PDL-1,” “CTLA-4,” “checkpoint inhibitor,” “immunotherapy,” drug names, AND “arthritis,” “arthralgia,” “polymyalgia rheumatica,” “rheumatic,” and “musculoskeletal.” Our search was restricted to humans and the English language.
2.4. Study selection
Using Covidence software, all search results were screened, and duplicates were removed. Prior to screening, the study protocol was discussed amongst members of the OMERACT irAE working group. Using the eligibility criteria, 4 screeners (NG, NC, WvB, SW) independently screened a random sample of the total search results to ensure accurate screening protocol and understanding of eligible studies. Queries were discussed prior to moving forward with review of all studies. Two of the four reviewers screened each title/abstract, and a third reviewer was responsible for resolving conflicts.
2.5. Data charting process
Two different reviewers extracted the above data from each study, and disagreements in the extracted data were resolved by discussion. One reviewer (NG) did a final review of all extractions for accuracy and completeness.
2.6. Data items
Data extracted from the selected studies included study characteristics (author, year of publication, journal, country of first author, and study design), a rheumatology co-author (yes/no), number of patients with de novo irAE, number of patients in study with pre-existing rheumatic conditions to be excluded, and patient demographics (mean age, sex distribution, race/ethnicity, cancer type/stage), ICI treatment regimen, other past therapies for cancer, information about the rheumatic irAE such as onset (weeks) and characterization, duration of follow-up, treatments, and all reported domains, both specific to the rheumatic irAE or not. Oncologic domains, if available, were also included. Instruments used to measure domains were extracted separately for future use.
2.7. Synthesis
Clinical characteristics of ICI-induced IA and ICI-induced PMR were grouped into thematic categories. The number of included publications utilizing each characterization category to describe the irAE was tabulated. Clinical descriptions not fitting one of these categories were also collected, and the number of publications using those descriptors was tallied. Domains identified from the selected publications were mapped to core areas in accordance with the OMERACT 2.1 Filter14 with some additional core areas relevant to this patient population. Some core areas were subdivided into domains such as symptoms/signs, laboratory findings, imaging, etc. The number of included publications for each reported domain was tabulated.
3. Results
3.1. Search Results
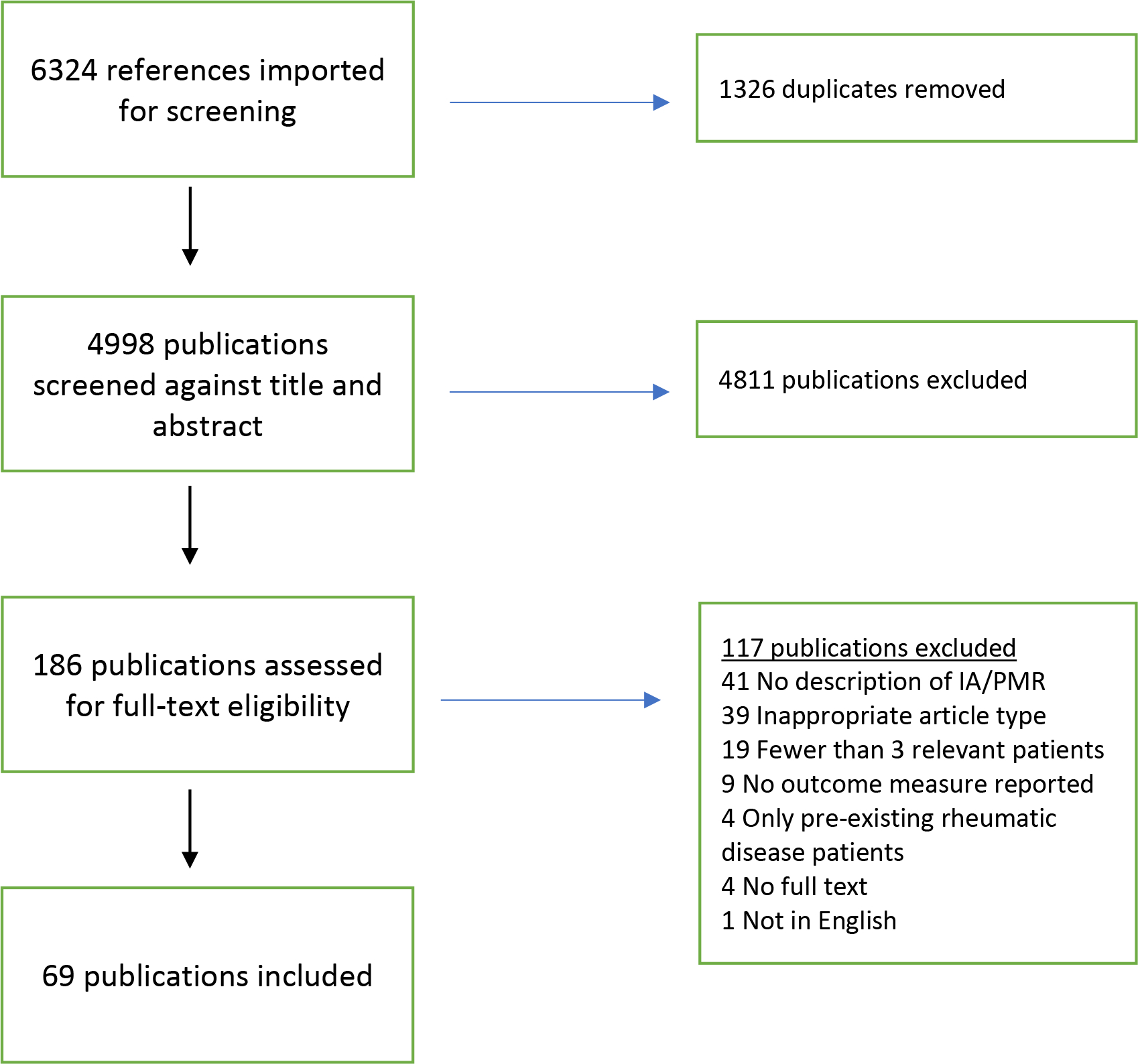
Our search strategy identified 6,324 references, and 1,326 duplicate references were removed by the software (Figure 1). Of the 4,998 titles and abstracts that were screened, 4,811 were excluded due to lack of relevance. Of the 186 publications that were assessed for full-text eligibility, 117 publications were excluded. The primary causes of ineligibility were lack of description of ICI-induced IA and ICI-induced PMR (41 publications), inappropriate article type (39 publications), <3 relevant patients (19 publications), and no outcome reported (9 publications) (Figure 1). Four publications were excluded for reporting only patients with pre-existing rheumatic disease. Overall, 69 were included in data synthesis (see Supplement for full bibliography).
Figure 1: Flow diagram for study identification.
3.2. Characteristics of publications and participants
The included publications were published between 2011 and 2021 from several countries: United States of America (31 publications), France (9 publications), Canada (6 publications), Australia (5 publications), Netherlands (4 publications), Spain (4 publications), Italy (3 publications), Germany (2 publications), China (2 publications), Greece (1 study), Japan (1 study), and the United Kingdom (1 study). Most studies were retrospective (12 case series and 45 retrospective observational studies), and 12 studies were prospective cohorts. At least one rheumatologist was a co-author in 41 (59%) publications. There was one qualitative study15. Twenty publications included some patients with pre-existing rheumatic disease along with patients developing de novo irAE, but the patients with pre-existing rheumatic disease were excluded from our analysis.
The sample sizes per publication ranged from 3 to 216, and there were 1,107 different patient descriptions, though not all patients are unique due to being included in multiple cohorts. Of the 38 publications where age could be determined, the mean age was 63.3 years (SD 6.1). Of the 33 publications where sex could be ascertained, 54% of patients were male. The three most common malignancies were melanoma (48 publications), lung cancer (42 publications), and renal/urothelial cancer (40 publications), and treatment of both stage III and IV were reported. Specific ICI treatments could be extracted from 54 publications and included anti-PD-1/PD-L1 monotherapy (22 publications), anti-CTLA-4 only (1 publication) or any combination of monotherapy, combination ICI therapy and/or sequential therapy (31 publications). No publications described patients receiving only combination therapy, and 22 publications described other cancer therapeutics including targeted agents, chemotherapies, radiation and/or surgery, either previously or concurrently with ICI. Onset of rheumatic irAE ranged from 3 to 38 weeks (median 12 weeks) after ICI initiation. Duration of follow-up ranged from 1 week to almost 5 years.
3.3. Clinical presentations
Publications were placed in one of 7 groups based on their characterization of the ICI-induced IA and/or ICI-induced PMR (Table 1). Several publications (27/69, 39%) used nonspecific terms of “arthritis”, “arthralgia”, “arthritis/arthralgia”, “arthralgia/myalgia” with or without the term “polymyalgia rheumatica,” without further descriptors. Among these 27 publications, 23 (85%) did not appear to involve rheumatology co-authors. Four publications used a more specific term of “inflammatory arthritis,” and three of these (75%) involved a rheumatology co-author. Twelve publications (17%) described arthritis based on the number of joints involved - monoarticular, oligoarticular and polyarticular - as well as a separate descriptor for PMR. Definitions for what constituted oligoarticular and polyarticular were not always provided. Seven publications (10%) listed the specific joints that were involved (i.e., involvement of the metacarpophalangeal (MCP) joints, wrist joints, and bilateral shoulder joints). Three publications (4%) used both number of joints and specific joints (i.e., monoarthritis of the left knee, polyarthritis of the MCPs, oligoarthritis of the knee/wrist/shoulder). Seven publications (10%) described joint involvement based on joint size – small, large, small and large, as well as gave the ICI-induced PMR diagnosis. Nine publications (13%) described irAEs using terms used for the corresponding de novo rheumatic disease (i.e., RA, RA-like; spondyloarthritis (SpA), SpA-like; PMR or PMR-like). Of these 9 publications, 4 publications only described ICI-induced PMR and PMR-like syndromes.
Table 1.
Categories for clinical characterization of ICI-IA/ICI-PMR.
| Category | Number of publications |
|---|---|
|
| |
| Arthritis, arthralgia, arthritis/arthralgia +/− PMR without clinical descriptors | 27 |
| Number of joints involved (monoarthritis, polyarthritis, oligoarthritis +/− PMR) | 12 |
| Diagnosis-based (RA, RA-like, PMR, Spondyloarthritis, Spondyloarthritis-like) | 9 |
| Specific joints listed (MCPs, wrists, shoulders, ankle) | 7 |
| Number of joints involved + specific joints listed (oligoarthritis of knee, ankle, wrist or monoarthritis of knee) | 3 |
| Small joints/large joint/combination small and large joint +/− PMR | 7 |
| Inflammatory arthritis | 4 |
Abbreviations: ICI = immune checkpoint inhibitor, IA = inflammatory arthritis, PMR = polymyalgia rheumatica, RA = rheumatoid arthritis, MCP = metacarpophalangeal joint
Some publications had clinical descriptions in addition to these 7 descriptive categories of inflammatory arthritis. Five publications had an additional or separate characterization of tenosynovitis or enthesopathy, 3 described an “activated osteoarthritis” phenotype which was not clearly inflammatory, and 6 publications utilized classification criteria for RA or PMR to aid in their diagnosis.
3.4. Domains
Figure 2 displays the reported domains for all included publications mapped to core areas suggested by the OMERACT Filter 2.1, adjusted for this specific patient population.
Figure 2. Concepts, Areas and Domains for Outcome Measures in ICI-Inflammatory Arthritis and ICI-PMR.
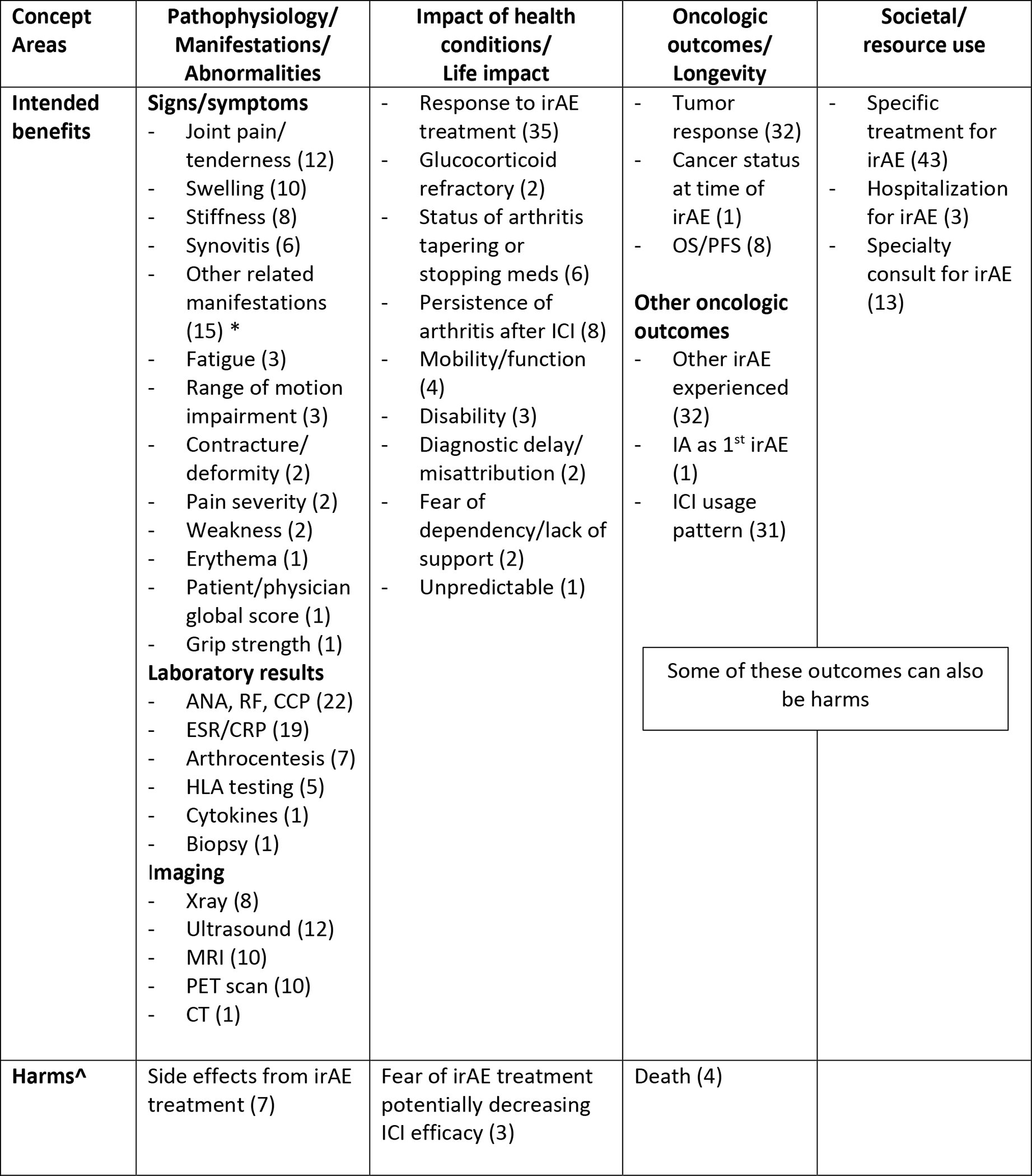
Âdverse events (harms) are a mandatory domain within OMERACT; (n) = number of studies with included outcome
*Other related manifestations: tenosynovitis, dactylitis, spondyloarthritis, remitting symmetrical seronegative synovitis with pitting edema
Legend: ANA = anti-nuclear antibody, RF = rheumatoid factor, CCP = anti-cyclic citrullinated peptide, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, HLA = human leukocyte antigen, irAE = immune-related adverse event, OS = overall survival, PFS= progression free survival, IA = inflammatory arthritis, ICI = immune checkpoint inhibitor.
3.4.1. Pathophysiologic Manifestations
This core area encompasses domains involving pathophysiology such as symptoms, signs and biomarkers, all of which can be seen as manifestations of the underlying health condition. Within signs and symptoms, the most commonly described domains were joint pain (12 publications), joint swelling (10 publications), joint stiffness (8 publications) and synovitis (6 publications). Fifteen publications also mentioned the presence or absence of clinical signs that may be associated with inflammatory arthritis, such as tenosynovitis, enthesitis, and/or dactylitis. The most common laboratory results reported were serologic investigation of anti-nuclear antibody (ANA), rheumatoid factor (RF), and/or anti-cyclic citrullinated peptide (CCP) (22 publications), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) (19 publications), followed by synovial fluid analysis (7 publications) and HLA genetic testing (5 publications). Imaging results, which comment on finding such as presence of erosions, periosteal reactions, synovitis/synovial hypertrophy, tenosynovitis, fluid, periarticular inflammation and bone marrow edema were reported for ultrasound (12 publications), MRI (10 publications), PET scan (10 publications), and X-ray (8 publications). Seven publications described harms that were attributable to specific treatments used to treat the irAE, such as steroids, disease-modifying agents, or biologics, that manifested as signs or symptoms.
3.4.2. Life impact
Life impact is identified by concepts such as well-being, health perception, utility and ability to live and function independently. Response to specific rheumatic irAE treatment, either positive or negative, as it related to life impact was the most common domain described in 35 publications, and two publications went further to describe patients as glucocorticoid-refractory. Examples of response to treatment included terms such “improvement,” “worsening,” and “resolution,” although these terms were inconsistent and not always solely specific to life impact. Eight publications described the persistence of arthritis event after ICI therapy was discontinued, and six discussed the impact that tapering or stopping immunosuppression had on the arthritis. Other life impacts published included mobility problems (4 publications), disability (3 publications), and fear of potential decreased ICI efficacy from immunosuppression for the irAE (3 publications). Only one qualitative study15 provided patient-reported outcomes and described thematic domains relating to diagnostic delay and misattribution, fear of dependency on others/lack of social support, and unpredictability of the irAE.
3.4.3. Oncologic domains/longevity
Thirty-two publications described tumor response to the ICI, which encompassed clinical responses by the oncologist (complete response, partial response, stable, progression) as influenced by oncologic criteria, such as the Response Evaluation Criteria of Solid Tumors (RECIST/iRECIST)16. Overall survival (OS), the duration of patient survival from treatment initiation, and/or progression-free survival (PFS), the time from treatment imitation until cancer worsening, was utilized in 8 publications. Death was mentioned in 4 publications. One paper discussed the status of the malignancy at the time of the irAE17. Other oncologic-related domains include description of other non-IA irAEs (32 publications) and the use of ICI after rheumatic irAE (31 publications), such as continued, temporarily held, or discontinued. While death is an obvious harm, some outcomes such as other irAEs experienced and ICI usage pattern could be seen as a benefit or harm depending on irAE severity and cancer response to therapy.
3.4.4. Societal/resource use
This core area relates to the impact on society, usually expressed as healthcare use, both directly and indirectly. The most commonly used resource were the irAE treatments themselves, such as intra-articular injections, corticosteroids, disease-modifying anti-rheumatic drugs (DMARDs) and/or the use of biologics (43 publications). Thirteen publications explicitly stated a consultation from a specialty service like rheumatology or orthopedics, and 3 publications described the hospitalization of a patient for their rheumatic irAE. All of these outcomes could be seen as a benefit (treatment of irAE) or a harm (resource utilization, possible unnecessary treatments or interventions).
3.4.5. CTCAE
Instruments used for outcome measures in rheumatologic conditions, such as RA, that were adapted for irAE were not the focus of our current investigation. However, given that irAE are adverse events from oncologic treatments, it should be noted that 31 publications utilized the Common Terminology for Cancer Adverse Events (CTCAE v5.0)18 to describe the severity of irAEs involving joints. This instrument combines descriptions of pathophysiological manifestations (e.g., swelling) with symptoms (e.g., pain), so it represents a hybrid approach to the analysis of these events. This scale ranges from grade 1 to 3 with 1 - mild pain with inflammation, erythema or joint swelling, 2 - moderate pain associated with signs of inflammation, erythema, or joint swelling limiting instrumental activities of daily living (ADL) and 3 - severe pain associated with signs of inflammation, erythema, or joint swelling; irreversible joint damage; limiting self-care ADL
3.4.6. Non-specific domains
Several domains were also noted in publications that were not specific to the irAE of interest (ICI-induced IA/PMR) but rather to larger cohorts including other irAEs. These have been listed with the number of publications for each in Supplementary Table.
4. Discussion
This is the first literature review to aggregate and characterize both clinical descriptors and outcome domains for the increasingly recognized conditions of ICI-induced IA and ICI-induced PMR. This study demonstrates the heterogeneity present in clinical descriptions of ICI-induced IA/PMR. The most common description (39%) was non-specific arthritis, arthralgia +/− PMR grouped together. Most of the publications utilizing these descriptors did not include a rheumatologist as a co-author, which could explain the lack of clinical details regarding musculoskeletal manifestations. In categories describing more detailed information regarding joint involvement, the most commonly described included number of joints (17%), the specific list of joints (10%), the differentiation of small vs. large joints (10%), or any combination of the three. Similar to the relationship between traditional, or de novo, RA and PMR, the distinction between ICI-induced IA and ICI-induced PMR can be difficult to ascertain. As such, several publications describe an inflammatory arthritis with or without features of PMR, or as primarily ICI-induced PMR with the presence of peripheral arthritis. Six publications utilized ACR/EULAR classification criteria to provide a diagnosis of ICI-induced RA or ICI-induced PMR. The heterogeneity in descriptions highlights a need for a more systematic way of reporting these irAEs, or establishing classification criteria specific to ICI-induced IA, that would assist in better studying these disorders longitudinally in the future.
As the initial step of the Core Domain Set generation process13, this review aggregated a list of relevant outcome domains that have been previously used for ICI-induced IA/PMR. The domains used in the included publications were mapped to core concepts of pathophysiology/manifestations, impact of health condition, longevity, resources (treatment), and the one included qualitative study provided several life impact domains for consideration. While some instruments that are validated in other diseases, such as those used for RA (e.g. CDAI), were reported as outcome measures for some of these patients, it is not clear if they would be appropriate for use in a consistent and universal way across all ICI-induced IA/PMR patients given the clinical differences seen between patients with irAE and de novo rheumatic disease. While the pathophysiology of irAE is beyond the scope of this paper, there have been multiple hypotheses suggested such as (1) activation of cytotoxic T cells; (2) activation of B cells and increased autoantibody production; (3) direct molecular mimicry and off-target toxicity; (4) activation of intracellular signaling and pro-inflammatory cytokine production; and (5) environmental modifiers of immune system activation19.Furthermore, currently existing instruments may not adequately reflect the heterogeneity of joint and structures involved such as enthesitis or tenosynovitis, or distribution of joints in the lower extremities and thus, were not included at this stage of outcome domain generation. Next steps in the OMERACT outcome selection process include building upon these domains with qualitative work eliciting patient-reported experiences, with the goal of eventual consensus achievement of core areas and domains through a Delphi method.
Given that this is a unique population with both an inflammatory disease and a malignancy, an adjustment to the OMERACT 2.1 Filter is proposed by adding oncologic domains to the longevity core area since cancer status and tumor response are heavily linked to longevity. Another proposal would be to add oncologic domains as a separate concept or core area. Regardless, the results of this aggregation of domains can help inform the development of an OMERACT Core Domain set for ICI-induced IA and ICI-induced PMR. This unique population also lends itself to the blurring of intended effects and harms within concept areas, as an intended benefit domain, such as the treatment of an irAE, can have harms attributed to that specific immunosuppressive treatment as well as potential harms of dampening efficacy of the ICI.
One reason for the heterogeneity in both clinical descriptions and outcome measures may relate to the specialty of the authors involved in the publication. We found that 85% of publications using non-specific clinical descriptors of rheumatic irAE did not involve a rheumatology co-author. Musculoskeletal adverse events from oncologic therapies often follow terminology used in the CTCAE dictionary, which is limited in the assessment of joint and muscle pain18. Thus, this discrepancy in clinical descriptions is likely related to the inadequacy of the CTCAE for rheumatic irAE. In addition, oncologists may focus more on oncologic domains, such as overall survival (OS) or progression-free survival (PFS) as it relates to oncologic therapies, whereas rheumatologists tend to focus more on rheumatic irAE treatment and quality of life. Thus, there is an unmet need for the input from both specialties as well as the widespread adoption and inclusion of patient-reported domains to develop specific domains within this area. There was only one qualitative study that delved more deeply into patient-reported outcomes15, and it provides a good foundation of domains that will be of relevance in this population. Upon confirmation with further qualitative work, we hope these domains will more accurately reflect the manifestations and impacts of ICI-induced IA/PMR on patients, improve disease classification, and improve measurement of treatment response when patients are followed prospectively.
A strength of this study is the broad inclusion of observational studies, including case series to cohorts, from multiple databases to determine how ICI-induced IA and ICI-induced PMR have been described and what outcome measures have been used. We excluded oncologic randomized clinical trials, which are generally of the highest quality study because these studies poorly characterize musculoskeletal adverse events20 and provide little information about relevant outcome measures due to their reliance on the CTCAE dictionary to describe rheumatic irAEs. Given the novelty of these diseases, there have been no clinical trials focusing on ICI-induced IA and ICI-induced PMR patients. We did not analyze or grade the quality of the included publications, as we were aiming to be as inclusive as possible to collect all reported outcome measures. We limited this query to patients without any pre-existing autoimmune disease (AID) in order to distinguish those patients experiencing a flare of their pre-existing disease as a result of ICI from those with a de novo irAE. Patients with pre-existing AID differ from ICI-induced IA patients pathologically6–8 and they may have different tumor responses as well depending on the use of baseline immunosuppression prior to ICI therapy21,22. Limitations of this study are the inclusion of English-only publications and the exclusion of abstracts and conference proceedings.
5. Conclusion
There is notable heterogeneity in the clinical descriptions of ICI-induced IA and ICI-induced PMR in the literature, and it is still unclear how best to describe these entities. This will become clearer with establishing classification criteria to guide physicians in proper reporting. The outcome measures reported appear to be evenly mapped across the primary core areas. However, given that a main goal for these patients is optimal treatment of the underlying cancer, we recommend the addition of oncologic domains in this specific setting to better accommodate this patient population. Further qualitative exploration of patient-reported symptoms and impacts of disease is also crucial to establishing a Delphi consensus of relevant outcome domains.
Supplementary Material
Acknowledgments:
We would like to submit this on behalf of the OMERACT irAE working group. We would also like to thank Marianne Visser, an OMERACT Patient Research Partner. In memoriam: Uwe Preckwinkel, an OMERACT Patient Research Partner. Uwe was a German scientist and a cancer patient who experienced a rheumatic immune-related adverse event. He was involved in the OMERACT working group on rheumatic immune-related adverse events since its inception.
Footnotes
Declarations of Interest: CB - Consulting: AbbVie, BMS, Eli Lilly, Janssen, Moderna, Pfizer, Sanofi; Grant support: BMS. EC - Research grants from Bio-Cancer, Biogen, Novartis, Pfizer, Roche, Sanofi and UCB, consultancy from Abbvie, Amgen, Biogen, Biocon, Chugai Pharma, Eli Lilly, Gilead, Janssen, Merck Serono, Novartis, Pfizer, Regeneron, Roche, RPharm and Sanofi, speakers fee from Abbvie, Amgen, Bristol Myer Squibbs, Chugai Pharma, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, Regeneron, RPharm, Roche, Sanofi, and UCB. JL - grant/research support from: Novartis, Pfizer; Abbvie, BMS, Gilead, Janssen, Sanofi; honoraria for consulting or speaking: Abbvie, BMS, Galapagos Janssen-Cilag, Gilead, Lilly, Medac, MSD, Novartis, Pfizer, Roche/Chugai, Sanofi, UCB. LC – Supported by AR075872 from NIAMS, Consulting: Bristol-Myers Squibb, Tremeau Pharmaceuticals, Mallinckrodt Pharmaceuticals. Research Funding: Bristol-Myers Squibb. MK - Consulting/speaker fees: Bristol-Myers Squibb, Janssen-Cilag, MSD, Novartis. KB -Consultancy and/or speaker fees and/or travel reimbursements: Abbvie, Bristol Myers Squibb (BMS), Gilead/Galapagos, Janssen, Merck Sharp & Dohme (MSD), Mundipharma, Novartis, Pfizer, Roche, Viatris, UCB. Scientific support: Medical Faculty of University of Heidelberg, Rheumaliga Baden-Württemberg e.V., AbbVie, Novartis. MH - Advisory boards: Boehringer Ingelheim, Alexion, Mallinckrodt; Research grants: Boehringer Ingelheim, Bristol Myers Squibb. CC - speaker and consulting for Sanofi/Regeneron. MSA- Pfizer, Eli Lilly, Avenue Therapeutics, Chemocentryx, Gilead, Bristol Myers Squibb, AMAG, Agile Therapeutics. SG: Grant from Novartis, Advisory board for UCB, ACR guideline subcommittee chair. MLO - National Cancer Institute and Rheumatology Research Foundation. All other co-authors have no declarations.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkhir R, Le Burel S, Dunogeant L, et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis. 2017;76(10):1747–1750. doi: 10.1136/annrheumdis-2017-211216 [DOI] [PubMed] [Google Scholar]
- 3.Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer—clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis. 2017;77:393–398. doi: 10.1136/annrheumdis-2017-212257 [DOI] [PubMed] [Google Scholar]
- 4.Ghosh N, Tiongson MD, Stewart C, et al. Checkpoint Inhibitor–Associated Arthritis. JCR J Clin Rheumatol. 2020;Publish Ah. doi: 10.1097/rhu.0000000000001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LC C, AK G, AN B, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2017;76(1 PG-43–50):43–50. doi: 10.1136/annrheumdis-2016-209595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burbelo PD, Ferré EMN, Chaturvedi A, et al. Profiling Autoantibodies against Salivary Proteins in Sicca Conditions. J Dent Res. 2019;98(7):772–778. doi: 10.1177/0022034519850564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina HA, Eickhoff J, Edison JD. Thinking Inside the Box: Synovial Tissue Biopsy in Immune Checkpoint Inhibitor–Induced Arthritis. JCR J Clin Rheumatol. Published online 2021. [DOI] [PubMed] [Google Scholar]
- 8.Murray-Brown W, Wilsdon TD, Weedon H, et al. Nivolumab-induced synovitis is characterized by florid T cell infiltration and rapid resolution with synovial biopsy-guided therapy. J Immunother Cancer. 2020;8:e000281. doi: 10.1136/jitc-2019-000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R, Singaraju A, Marks KE, et al. Clonally expanded CD38hi cytotoxic CD8 T cells define the T cell infiltrate in checkpoint inhibitor-associated arthritis. bioRxiv. Published online 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albayda J, Dein E, Shah AA, Bingham CO, Cappelli L. Sonographic Findings in Inflammatory Arthritis Secondary to Immune Checkpoint Inhibition: A Case Series. ACR open Rheumatol. 2019;1:303–307. doi: 10.1002/acr2.1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6):e002435. doi: 10.1136/jitc-2021-002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.TJ B, JR B, PM F, et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis. 2020;79(3 PG-332-338):332–338. doi: 10.1136/annrheumdis-2019-216109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaton Dorcas, Maxwell Lara, Grosskleg Shawna, Shea Beverley, Tugwell Peter, Bingham Clifton O. III, Conaghan Philip G., D’Agostino Maria-Antonietta, Hofstetter Catherine, March Lyn, Simon Lee S., Singh Jasvinder A, Vibeke Strand GW. The OMERACT Handbook.
- 14.Boers M, Beaton DE, Shea BJ, et al. OMERACT filter 2.1: Elaboration of the conceptual framework for outcome measurement in health intervention studies. J Rheumatol. 2019;46(8):1021–1027. doi: 10.3899/jrheum.181096 [DOI] [PubMed] [Google Scholar]
- 15.LC C, SM G, AA S, BCO 3rd, AM O. Immune checkpoint inhibitor-induced inflammatory arthritis: a qualitative study identifying unmet patient needs and care gaps. BMC Rheumatol. 2020;4(PG-32):32. doi: 10.1186/s41927-020-00133-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 17.Liew DFL, Leung JLY, Liu B, Cebon J, Frauman AG, Buchanan RRC. Association of good oncological response to therapy with the development of rheumatic immune-related adverse events following PD-1 inhibitor therapy. Int J Rheum Dis. 2019;22(2):297–302. doi: 10.1111/1756-185X.13444 [DOI] [PubMed] [Google Scholar]
- 18.Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Published online 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdf
- 19.Lee DJ, Lee HJ, Farmer JR, Reynolds KL. Mechanisms Driving Immune-Related Adverse Events in Cancer Patients Treated with Immune Checkpoint Inhibitors. Curr Cardiol Rep. 2021;23(8):98. doi: 10.1007/s11886-021-01530-2 [DOI] [PubMed] [Google Scholar]
- 20.Cappelli LC, Gutierrez AK, Bingham III CO, Shah AA. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: A systematic review of the literature. Arthritis Care Res (Hoboken). 2017;69(11):1751–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease. Ann Intern Med. 2018;168:121. doi: 10.7326/m17-2073 [DOI] [PubMed] [Google Scholar]
- 22.Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28:368–376. doi: 10.1093/annonc/mdw443 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


